Abstract
Ultrasound-guided percutaneous core-needle biopsy is an excellent diagnostic tool for solid pancreatic lesions. It allows for identifying neoplastic pancreatic tumors with nearly 100% sensitivity, specificity and accuracy. Unresectable tumor assessment prior to planned palliative treatment is the primary indication for percutaneous pancreatic tumor biopsy. In the case of potential tumors eligible for radical surgery, endosonography-guided biopsy is used, if clinically necessary, to avoid the peritoneal spread of tumor cells during puncture. The possibility of obtaining a specimen for a detailed microscopic assessment during an easily accessible and simple procedure is the main advantage of core-needle biopsy over the low, yet higher when compared to other biopsy techniques, risk of complications. Obtaining tissue samples for molecular analysis is essential for palliative targeted therapy in pancreatic cancer and may become the main indication for the common core-needle biopsy of inoperable pancreatic tumors in the near future. The present paper describes the indications and the technique for core-needle biopsy in pancreatic tumors. Based on the studies published to date, the safety of the procedure, significant complications, including bleeding in particular, and the diagnostic sensitivity and specificity, also compared to other biopsy techniques, have been summarized. The present paper may contribute to the introduction of core-needle biopsy of pancreatic masses into clinical practice.
Keywords: ultrasound, pancreatic cancer, core needle biopsy, percutaneous biopsy
Introduction
The diagnosis of pancreatic diseases, solid focal lesions in particular, still poses a clinical challenge. Pancreatic adenocarcinoma, which has an exceptionally poor prognosis with a five-year survival of no more than 8%(1) and the time from diagnosis to death of usually less than a year, is the greatest health risk. Other pancreatic pathologies include less common neoplasms, e.g. neuroendocrine cancer, lymphoma, pancreatic metastases (kidney, breast, lung, and colorectal cancers, melanoma), as well as benign tumors, such as focal inflammation, especially autoimmune, and very rare cases of tuberculosis or sarcoidosis. Microscopic confirmation of adenocarcinoma is not needed in the case of resectable foci(2,3), and it becomes necessary in the group of patients with advanced disease, before palliative or neoadjuvant chemo/radiotherapy(4). In these cases, ultrasound-guided thin-needle biopsy is most often performed although it is not indicated in patients with resectable tumors and/or an unclear pancreatic lesions due to the risk of very rare complications of the procedure and cell spread along the peritoneal biopsy canal, which make surgical treatment impossible. If there is a clinical need for pathological diagnosis in these patients, endoscopic ultrasound (EUS) guided biopsy is recommended(4). Percutaneous fine needle biopsy of pancreatic tumors has high sensitivity and specificity of over 98%, which helps diagnose pancreatic cancer with nearly 100% accuracy(5), comparable to endosonography-guided fine-needle biopsy(6), with the rates of adverse events of approximately 0.8%. Theoretically, core-needle biopsy, in which a histological sample is obtained, should have even better diagnostic parameters; however this has not been confirmed in the available research(7). On the other hand, complications are more common. The advantage of core-needle biopsy therefore lies primarily in the possibility of histological evaluation based on specific staining, e.g. in doubtful inflammatory cases, Ki-67 quantification, as well as receptor evaluation or identifying tumor mutations prior to targeted treatment onset. In the light of available reports, e.g. on the efficacy of pembrolizumab in tumors with microsatellite instability, it seems that the latter indications may become the main reason for the extensive use of core-needle biopsy in unresectable pancreatic hyperplastic lesions(8,9).
Technique
As in the case of fine-needle biopsy, after ultrasonographic identification of a pancreatic lesion, the shortest biopsy path that avoids other organs, such as the liver, gallbladder, vessels (including medium-sized vessels, in which case Doppler imaging is helpful) and the transverse colon, as their puncture may lead to bleeding, peritonitis or abscess formation, is selected. Extending the needle through the gastric wall is necessary in some cases. Transducer pressure against the abdominal wall often reduces the needle path and helps avoid puncture of hollow organs. In a pancreatic lesion, a solid portion with a diameter of at least 1–2 cm is selected for biopsy (depending on the length of the tissue column collected by the needle), avoiding necrosis, small fluid spaces or a dilated pancreatic duct. Figure 1 shows an ultrasound image of a pancreatic head tumor (A) with a Doppler assessment of blood flow (B) and biopsy puncture of the lesion (C). Prior to puncture, the skin and subcutaneous tissues are usually anesthetized with a local anesthetic, e.g. 2% lidocaine. It is recommended to perform a minimum number of punctures to reduce the risk of possible complications (usually 1–2 samples were collected in the available studies). Patient’s lack of cooperation and uncontrollable bleeding disorders are a contraindication for invasive diagnosis of solid lesions. According to the recommendations of the Societies of Interventional Radiology, percutaneous solid organ biopsy is considered a high bleeding risk procedure, requiring a platelet count of ≥50 × 109/L and an INR of ≥1.5–1.8(10). The above guidelines also discuss in detail the perioperative management in patients receiving anticoagulants and/or antiplatelets(10).
Fig. 1.
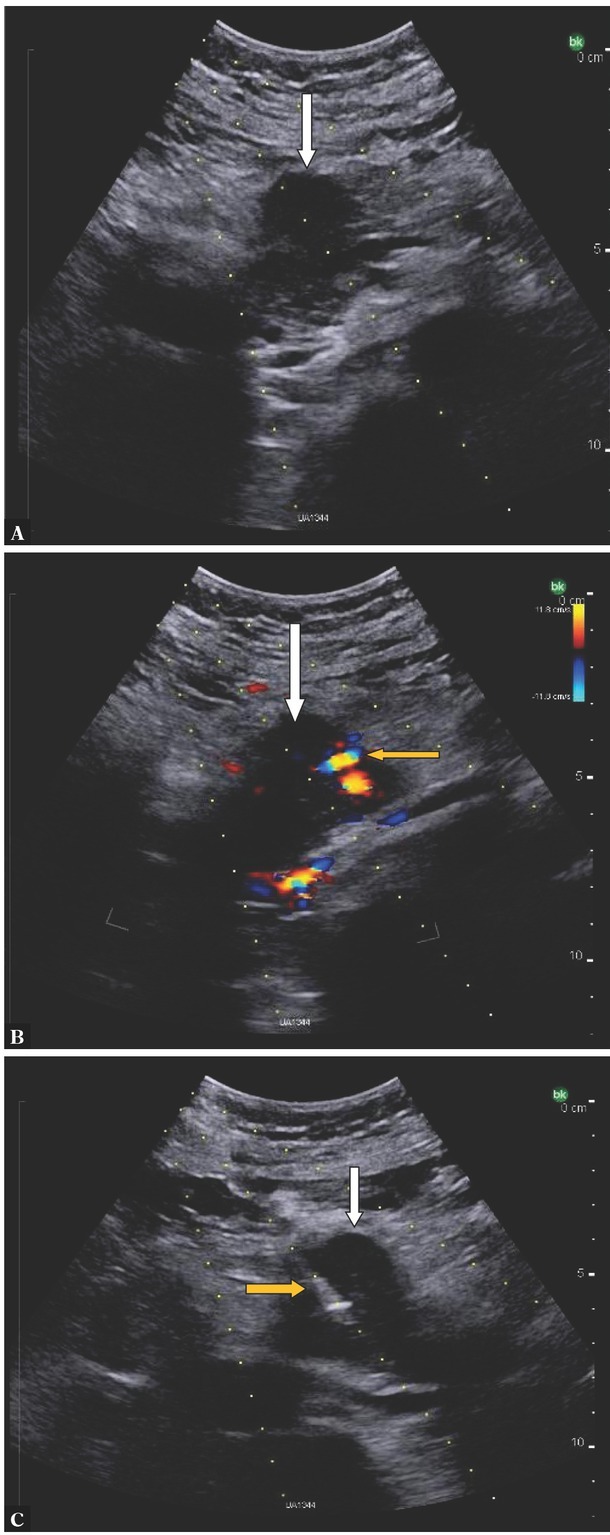
Pancreatic head tumor (white arrow). A. Ultrasound B-mode imaging. B. The same tumor in Doppler imaging; blood vessels in the projection of the lesion (yellow arrow). C. The same tumor – core needle biopsy, biopsy needle in the tumor (yellow arrow)(courtesy of Dr Kamil Jakubowicz)
Equipment
Core biopsy needles used in research had a size of 14G to 20G(11), with 18G needles most commonly used(11). A study on the biopsy of a transplanted pancreas showed a slightly higher efficacy of a 20G vs. 18G needle in collecting specimens adequate for pathological analysis, with a slightly lower rate of adverse effects for the thinner needle(12). However, sufficient data to conclude that needle size has a significant impact on the risk of complications is missing(5).
Different types of needles are used to obtain histological specimens from pancreatic lesions. Both cutting needles, which require forward and backward movement in the tumor to obtain a tissue biopsy, and sets of automatic cutting needles, the so-called biopsy gun, are used. Also, a coaxial needle set is often used that includes an outer cannula with an inner stylet, which is first inserted into the lesion and then, after the inner stylet is removed, a smaller cutting needle is inserted into the cannula for biopsy. Limited tissue trauma and the possibility of collecting several specimens from one puncture by changing the angle of the inserted needle may be considered an advantage of this method. A recent retrospective study comparing the coaxial technique with a conventional core-needle biopsy showed that the coaxial approach was associated with better pancreatic sample quality with a lower complication rate and shorter procedure time(13). Some authors also point to a lower risk of peritoneal tumor dissemination during biopsy collection using the coaxial method due to the separation of the surrounding tissue from the needle(11,14).
Outcomes
A summary of over a dozen of the largest studies shows very high mean sensitivity, specificity and diagnostic accuracy of percutaneous core-needle biopsy of focal pancreatic lesions, namely approximately 94% (range 90 –100%), 98% (range 95 –100%) and 96% (range 91–100%), respectively(11). These parameters are comparable with the analogous data reported for ultrasound-guided fine-needle biopsy and endosonography(5,6). Although the lack of a cytopathologist intraoperatively assessing the quality of collected specimens reduces the value of fine-needle biopsy(15), it still remains very high(5). An opportunity to obtain a histological specimen for further detailed analysis, which is necessary in doubtful cases, rare tumors, and especially before targeted anticancer treatment, is both an advantage of and the primary indication for a core-needle pancreatic biopsy. Furthermore, percutaneous core-needle biopsy is simpler, less expensive and more accessible than endosonographic procedures. Fig. 2 and Fig. 3 show typical microscopic images: cytology, tumor cells from an aspirate collected during fine-needle biopsy, and a histopathological image obtained from cutting biopsy of pancreatic adenocarcinoma.
Fig. 2.
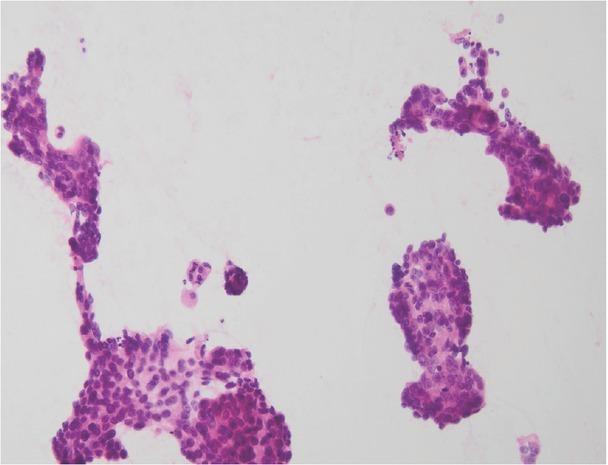
A microscopic image from cytological biopsy of pancreatic adenocarcinoma (courtesy of Prof. Andrzej Mróz)
Fig. 3.
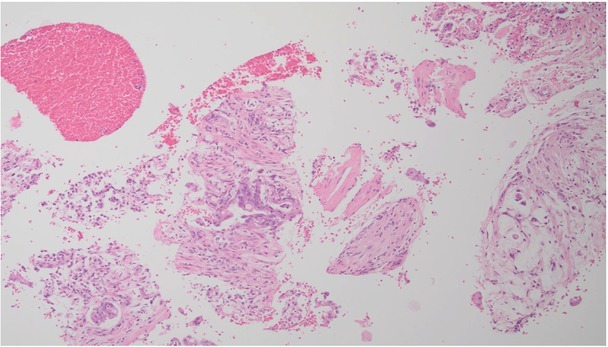
A microscopic image from histological biopsy of pancreatic adenocarcinoma (courtesy of Prof. Andrzej Mróz)
Complications
Based on an analysis of 14 studies with a total of over 1,500 samples, it was estimated that the average incidence of major complications of percutaneous core-needle biopsy of solid focal pancreatic lesions is
approximately 2.08% (range 6.1–0% in separate studies)(11). It is higher than in the case of percutaneous ultrasound-guided fine-needle biopsy (approximately 0.8%)(5) and comparable (2.44%) with the incidence after fine-needle EUS biopsy observed in prospective studies(16). The most common serious sequelae included hemorrhagic complications (bleeding, abdominal hematomas, pseudoaneurysm of the gastroduodenal artery in one case) requiring medical intervention of a varying degree, as well as pancreatitis, severe abdominal pain, accidental puncture of other organs, and pancreatic fistula. Theoretically, both the higher number of punctures and the larger size of the biopsy needle may be factors that increase the risk of complications; however, data to support this is not sufficient. Operator experience and efficiency are probably important elements influencing the safety of the procedure. Although the risk of peritoneal dissemination of tumor cells during percutaneous core-needle biopsy has not been assessed, it should be remembered that these procedures are performed in patients with already advanced, unresectable proliferative disease.
Novel techniques
The use of contrast enhanced ultrasound (CEUS) during biopsy may increase its accuracy due to better visualization of the size, borders, and structure of a pancreatic focal lesion. Preliminary results indicate that CEUS facilitates percutaneous biopsy of poorly visible tumors in conventional ultrasound (B-mode) imaging(17), as well as helps select the most optimal site for specimen collection, bypassing fluid and necrotic components(13).
Footnotes
Conflict of interest
The author does not report any financial or personal connections with other persons or organizations which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Author contributions
Original concept of study and writing of manuscript: AZR.
References
- 1.Cancer Statistics Center. 2018. https://cancerstatisticscenter.cancer.org. https://cancerstatisticscenter.cancer.org American Cancer Society. In. Available at. [26.05.2018]
- 2.National Comprehensive Cancer Network. NCCN Guidelines Version 2.2018 Pancreatic Adenocarcinoma. http://www.nccn.org http://www.nccn.org Available at.
- 3.Takaori K, Bassi C, Biankin A, Brunner TB, Cataldo I, Campbell F. International Association of Pancreatology (IAP)/European Pancreatic Club (EPC) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology. 2016;16:14–27. doi: 10.1016/j.pan.2015.10.013. et al. [DOI] [PubMed] [Google Scholar]
- 4.Winter K, Talar-Wojnarowska R, Dąbrowski A, Degowska M, Durlik M, Gąsiorowska A. Diagnostic and therapeutic recommendations in pancreatic ductal adenocarcinoma. Recommendations of the Working Group of the Polish Pancreatic Club. Prz Gastroenterol. 2019;14:1–18. doi: 10.5114/pg.2019.83422. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Onofrio M, De Robertis R, Barbi E, Martone E, Manfrin E, Gobbo S. Ultrasound-guided percutaneous fine-needle aspiration of solid pancreatic neoplasms: 10-year experience with more than 2,000 cases and a review of the literature. Eur Radiol. 2016;26:1801–1807. doi: 10.1007/s00330-015-4003-x. et al. [DOI] [PubMed] [Google Scholar]
- 6.Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas. 2013;42:20–26. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 7.Kahriman G, Ozcan N, Dogan S, Ozmen S, Deniz K. Percutaneous ultrasound-guided core needle biopsy of solid pancreatic masses: results in 250 patients. J Clin Ultrasound. 2016;44:470–473. doi: 10.1002/jcu.22362. [DOI] [PubMed] [Google Scholar]
- 8.Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24:1326–1336. doi: 10.1158/1078-0432.CCR-17-3099. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG. Society of interventional radiology consensus guidelines for periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiology Society of Europe. J Vasc Interv Radiol. 2019;30:1168–1184. doi: 10.1016/j.jvir.2019.04.017. et al. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Shi J, Chen Y-Y, Li K. Ultrasound-guided percutaneous core needle biopsy for the diagnosis of pancreatic disease. Ultrasound Med Biol. 2018;44:1145–1154. doi: 10.1016/j.ultrasmedbio.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Lee BC, McGahan JP, Perez RV, Boone JM. The role of percutaneous biopsy in detection of pancreatic transplant rejection. Clin Transplant. 2000;14:493–498. doi: 10.1034/j.1399-0012.2000.140508.x. [DOI] [PubMed] [Google Scholar]
- 13.Xin Y, Chen Y, Wang Y, Cao X-J, Zhou X. safety and efficacy of ultrasound-guided percutaneous coaxial core biopsy of pancreatic lesions: a retrospective study. Journal of Ultrasound. 2021;24:269–277. doi: 10.1007/s40477-020-00487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006;187:1184–1187. doi: 10.2214/AJR.05.1347. et al. [DOI] [PubMed] [Google Scholar]
- 15.Ecka RS, Sharma M. Rapid on-site evaluation of EUS-FNA by cytopathologist: an experience of tertiary hospital. Diagn Cytopathol. 2013;41:1075–1080. doi: 10.1002/dc.23047. [DOI] [PubMed] [Google Scholar]
- 16.Wang KX, Ben QW, Jin ZD, Du Y-Q, Zhou D-W, Liao Z. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–290. doi: 10.1016/j.gie.2010.10.045. et al. [DOI] [PubMed] [Google Scholar]
- 17.Wei Y, Yu X-L, Liang P, Cheng Z-G, Han Z-Y, Liu F-Y. Guiding and controlling percutaneous pancreas biopsies with contrast-enhanced ultrasound: target lesions are not localized on B-mode ultrasound. Ultrasound Med Biol. 2015;41:1561–1569. doi: 10.1016/j.ultrasmedbio.2015.01.015. et al. [DOI] [PubMed] [Google Scholar]


