Abstract
Objective
To determine the effect of a user centered clinical decision support tool versus usual care on rates of initiation of buprenorphine in the routine emergency care of individuals with opioid use disorder.
Design
Pragmatic cluster randomized controlled trial (EMBED).
Setting
18 emergency department clusters across five healthcare systems in five states representing the north east, south east, and western regions of the US, ranging from community hospitals to tertiary care centers, using either the Epic or Cerner electronic health record platform.
Participants
599 attending emergency physicians caring for 5047 adult patients presenting with opioid use disorder.
Intervention
A user centered, physician facing clinical decision support system seamlessly integrated into user workflows in the electronic health record to support initiating buprenorphine in the emergency department by helping clinicians to diagnose opioid use disorder, assess the severity of withdrawal, motivate patients to accept treatment, and complete electronic health record tasks by automating clinical and after visit documentation, order entry, prescribing, and referral.
Main outcome measures
Rate of initiation of buprenorphine (administration or prescription of buprenorphine) in the emergency department among patients with opioid use disorder. Secondary implementation outcomes were measured with the RE-AIM (reach, effectiveness, adoption, implementation, and maintenance) framework.
Results
1 413 693 visits to the emergency department (775 873 in the intervention arm and 637 820 in the usual care arm) from November 2019 to May 2021 were assessed for eligibility, resulting in 5047 patients with opioid use disorder (2787 intervention arm, 2260 usual care arm) under the care of 599 attending physicians (340 intervention arm, 259 usual care arm) for analysis. Buprenorphine was initiated in 347 (12.5%) patients in the intervention arm and in 271 (12.0%) patients in the usual care arm (adjusted generalized estimating equations odds ratio 1.22, 95% confidence interval 0.61 to 2.43, P=0.58). Buprenorphine was initiated at least once by 151 (44.4%) physicians in the intervention arm and by 88 (34.0%) in the usual care arm (1.83, 1.16 to 2.89, P=0.01).
Conclusions
User centered clinical decision support did not increase patient level rates of initiating buprenorphine in the emergency department. Although streamlining and automating electronic health record workflows can potentially increase adoption of complex, unfamiliar evidence based practices, more interventions are needed to look at other barriers to the treatment of addiction and increase the rate of initiating buprenorphine in the emergency department in patients with opioid use disorder.
Trial registration
ClinicalTrials.gov NCT03658642.
Introduction
The emergency department is a critical access point for many patients seeking care related to opioid use. An estimated 0.5-1.2 million visits annually to the emergency department for opioid use disorder has been reported in the US.1 Although clinicians in the emergency department have a clear role in caring for patients with opioid overdose or withdrawal, interest in initiating treatment for opioid use disorder in the emergency department is rising.2 In particular, buprenorphine-naloxone (combined formulation, subsequently referred to as buprenorphine alone) is one of the most effective treatments for opioid use disorder in the emergency department. Buprenorphine controls symptoms of withdrawal and cravings, and is associated with reduced use of illicit drugs and mortality.3 4 5 A 2015 randomized clinical trial of 329 patients presenting to the emergency department with opioid use disorder showed that starting buprenorphine in the emergency department doubled engagement in outpatient treatment for addiction and reduced self-reported use of illicit opioids sixfold compared with usual care and brief intervention groups.6
Despite the evidence in favor of the use of buprenorphine in the emergency department, adoption has been slow.7 8 9 10 11 Currently, only about 19% of eligible people with opioid use disorder receive medication for opioid use disorder (“medication for opioid use disorder” is deliberately worded to reduce stigma and negative bias when discussing addiction).8 A 2021 mixed methods study of 268 emergency department clinicians reported that only 56 (20.9%) felt ready to start buprenorphine for patients presenting to the emergency department with opioid use disorder, citing concerns with training, experience, linkage to outpatient care, and scarce time and resources in the emergency department.11 Clinical decision support (computerized patient specific assessments and recommendations) offers one potential solution to deal with clinicians unfamiliar with this treatment and increase the initiation of buprenorphine in the emergency care of patients with opioid use disorder. Clinical decision support tools face their own challenges to successful implementation, however, including alert fatigue, cognitive load, and appropriate timing in the electronic health record workflow.12 13 14 15 16 Up to 96% of interruptive alerts are dismissed,17 leading to more task switching which, in turn, increases risk to patient safety from greater cognitive load and task abandonment.18 19
To deal with these issues, we developed and implemented a non-interruptive, user centered (that is, seamlessly integrated into digital workflows to meet users’ needs) clinical decision support intervention called EMBED (EMergency department initiated BuprenorphinE for opioid use Disorder).20 21 22 23 24 An interrupted times series that implemented EMBED at one emergency department found that the intervention was feasible, acceptable, and associated with increased rates of initiating buprenorphine in the emergency department among 906 patients with opioid use disorder.25 We now report the findings of a pragmatic cluster randomized trial on the implementation of EMBED across five healthcare systems in the US.
Methods
Study design
From November 2019 to May 2021, we conducted a pragmatic, parallel, cluster randomized superiority trial in 18 clusters across five healthcare systems in five states, representing the north east, south east, and western regions of the US. The 18 clusters included 21 emergency departments, with three sites paired with a second emergency department within the same healthcare system because of low patient volume or high physician crossover rates, or both, between the two sites. Randomization was performed in a 1:1 ratio with clusters assigned to receive the intervention or usual care. The primary hypothesis was that the rate of initiating buprenorphine in the emergency department would be higher for patients at the intervention sites. Site specific changes and ongoing clinical quality improvement initiatives that could create temporal trends or otherwise affect the trial were tracked (supplementary material).26
All data were captured via the electronic health record (with exceptions noted below), collated by local information technology teams, and shared with the data coordination team. To protect the identity of patients and clinicians, visit dates were reported as days since implementation, with the exact trial start date not reported to the investigators or data coordinating center. The full study protocol was previously reported.20 27 Our reporting followed the CONSORT (Consolidated Standards of Reporting Trials) checklist for randomized trials.28
Covid-19 pandemic trial considerations
The covid-19 pandemic started in the fourth month of the trial and continued until the end of the trial. No major deviations from the trial protocol or changes to the intervention because of the pandemic were necessary (supplementary material).20 All of the emergency departments in this study and their community referral sites were open throughout the pandemic. At the beginning of the pandemic, clinicians at all study sites were encouraged to prescribe longer courses of buprenorphine because of concerns for delayed follow-up. At the study sites, visits to the emergency department dropped during the first pandemic wave29 with a subsequent rise in visits for non-fatal opioid overdose.30 Therefore, more data on visits to the emergency department and covid-19 admissions were collected to allow analysis of the effects of trends related to covid-19 on the findings of the trial.
Participants
The study sample was derived from all patient visits to the participating emergency departments during the study period. Participating emergency departments included sites in Alabama, Colorado, Connecticut, Massachusetts, and North Carolina, with 3-5 emergency departments per health system. The sites used the Epic (Epic Systems Corporation, Verona, WI) or Cerner (Cerner Corporation, Kansas City, MO) electronic health record, and served a diverse mix of rural, urban, and suburban communities. Eligible patient visits were identified with a validated electronic health record phenotype that included two algorithms (one based on ICD-10 (international classification of diseases, 10th revision) diagnostic codes related to opioid use and the second on chief complaints related to substance use but with no alternative diagnosis related to the use of alcohol or benzodiazepines; supplementary material, structured query language (SQL) query for emergency department opioid use disorder electronic health record phenotype) to identify patients aged 18 years or older with probable opioid use disorder who were discharged from the emergency department, not pregnant, and not currently receiving medication for opioid use disorder, as reported in the electronic health record.31 For patients with multiple visits to the emergency department during the trial, only the first visit was included in the primary analysis.
Because the intervention is physician facing, attending physicians in the emergency department caring for patients who met the electronic health record phenotype criteria were the primary study subjects. Patients were assigned to one attending of record based on practices specific to each site. However, all clinicians in the emergency department practicing at intervention sites had access to EMBED for all of the patients in their care at that site. To prevent contamination from crossover use or influence of the intervention, usual care site visits with an attending physician who practiced at both the intervention and usual care sites during the trial were excluded. Non-clinical physician characteristics and outcomes, including age, sex, and X waiver status, were obtained locally from departmental records, cross checked with the Substance Abuse and Mental Health Administration’s (SAMHSA) practitioner lookup website,32 and de-identified before sharing with the investigative team.
Intervention
EMBED is a user centered, clinician facing clinical decision support system integrated into the electronic health record workflow to facilitate initiating buprenorphine in the emergency department by: diagnosing opioid use disorder with a checklist based on the diagnostic criteria of the DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, fifth edition),33 assessing the severity of withdrawal with the Clinical Opioid Withdrawal Scale (COWS),34 motivating patients to accept treatment with a scripted brief negotiation interview,6 35 and automating the electronic health record workflow, including clinical and after visit documentation, order entry, prescribing, and referral for ongoing treatment in the community (supplementary fig).22 Unlike an interruptive alert, EMBED is entirely user initiated and flexible, allowing use for any patient as little or as often as needed. Buprenorphine can also be started without launching the intervention. EMBED has no pop-ups, alerts, or hard stops. Participating healthcare systems could make pragmatic modifications to EMBED according to local security protocols, system based messaging standards, and cost requirements. Modifications were required to maintain fidelity with the intervention’s primary features.21 22 23 All study sites (regardless of study arm) received education on opioid use disorder and initiating buprenorphine in the emergency department, along with a posting of the buprenorphine clinical algorithm in the clinical work area. Intervention sites trained their clinicians on how to launch and use EMBED following clinician training practices specific to each site, such as staff meetings, group emails, just-in-time in-person training, or a combination of these.
Outcomes
Outcomes were calculated at the patient and physician levels. The primary outcome was the patient level rate of initiating buprenorphine in the emergency department, defined as whether an eligible patient was given buprenorphine in the emergency department or prescribed buprenorphine on discharge from the emergency department. Secondary implementation outcomes were also assessed to determine the effect of EMBED compared with usual care based on the reach, effectiveness, adoption, implementation, maintenance (RE-AIM) framework.36 37 These outcomes included: number of unique physicians who initiated buprenorphine at least once during the trial; launching the intervention; referral for ongoing medication for opioid use disorder reported in the electronic health record; prescription for naloxone at discharge from the emergency department; and obtaining a Drug Addiction Treatment Act of 2000 X waiver to prescribe buprenorphine during the trial. Although the protocol included a plan to capture whether discharge instructions specific to opioids were provided, electronic health record data on this outcome were too unreliable and thus excluded from the analyses. Similarly, past medical and psychiatric history, recent medical or psychiatric admissions to hospital, recent enrollment in treatment for addiction, and active prescriptions for other opioids were inconsistent across the systems and were excluded from our analysis. We did not collect data on length of time using EMBED. Although clinicians listed their own time constraints as the main barrier to adopting initiation of buprenorphine in the emergency department in their practice,11 EMBED offers a one click option for full automation of an appropriate care pathway and optional decision support that takes no more than 1-5 minutes to navigate.21
Sample size
We used the National Institutes of Health randomized sample size calculator38 to determine the target number of sites to be randomized. Twelve clusters with a two sided type I error of 0.05, a conservative intraclass correlation of 0.03, and an expected average of 200 participants per cluster would provide 90% power to detect a 9% difference in initiating buprenorphine in the emergency department from 1% to 10%.20 Six more clusters were added to the sample to help with variability in enrollment across sites, with the formula described by Eldridge et al.39
Randomization, allocation, and blinding
The 18 clusters (representing 21 emergency departments) were allocated in a 1:1 ratio with constrained randomization40 to balance cluster level characteristics that could confound estimates of the effect size of the intervention.41 Potential confounders that were determined a priori20 and used to adequately balance the clusters were: healthcare system, electronic health record vendor, practice setting (academic v community), urbanicity, annual patient volume, presence of additional providers in the emergency department (such as emergency medicine residents), other resources for opioid use disorder in the emergency department (such as addiction counselors or funds for waiver training), and presence of any attending physicians with a waiver to prescribe buprenorphine at the start of the trial.
The allocation sequence and constrained randomization processes were independently performed by a statistician from the data coordinating center (FL), and the allocation sequence was concealed from the investigators until the final allocation was determined. Participants were assigned to the intervention if they were patients seeking care at an intervention site or a physician practicing at an intervention site. Participants could not be blinded to their allocation because the clinical decision support was launched by clinicians in the electronic health record and could be used when the patient was present. Clinicians decided whether to inform patients of the use of the clinical decision support consistent with their usual practice. All study sites posted information in the emergency department informing patients of the study. All parties responsible for data collection were aware of the assigned allocation of sites to study arms but had no role in the decision of whether to use the intervention or to give or prescribe buprenorphine in the emergency department.
Statistical methods
Comparisons between the intervention and usual care arms are reported as odds ratios with 95% confidence intervals. All analyses were performed in SAS version 9.4 (Cary, NC). The primary outcome, initiating buprenorphine in the emergency department, was assessed for all eligible patients. Differences in the intervention (clinical decision support v usual care) for this dichotomous outcome were examined with generalized estimating equations. A patient level generalized estimating equations model (binomial family with logistic link) had an indicator for intervention (clinical decision support v usual care) and was specified with an exchangeable working correlation structure to account for correlation of responses within the emergency department clusters. The model also included the cluster level covariates used in the constrained randomization process and patient level covariates that might be associated with the delivery of buprenorphine (age, sex, race, ethnicity, insurance type and status, opioid use disorder on the problem list, naloxone prescription in the past 24 months, results of urine drug screen, and time (months) since the start of the trial).
We performed sensitivity analyses to assess whether inpatient volume during the covid-19 pandemic influenced the effect of the intervention on the primary outcome. Secondary patient level outcomes (referral for ongoing treatment and prescription for naloxone at discharge from the emergency department) and physician level outcomes (provision of any initiation of buprenorphine in the emergency department during the trial, provision of any referral for ongoing medication for opioid use disorder during the trial, and receipt of waiver training) were evaluated with generalized estimating equations, as described above. The primary outcome was compared in the intervention phase to the maintenance phase in the intervention arm with the Wilcoxon signed rank test at the cluster level.
Patient and public involvement
The development of the research question and outcome measures was led by emergency physician investigators, the primary population being studied. Formal user design sessions and focus groups were conducted with both attending and resident emergency physicians to ensure the clinical decision support would be useful and would not interfere with patient care or place an undue burden on clinicians’ time.21 Patients did not participate in the design of the study and were not involved in recruitment or conducting the study.
Results
Enrollment
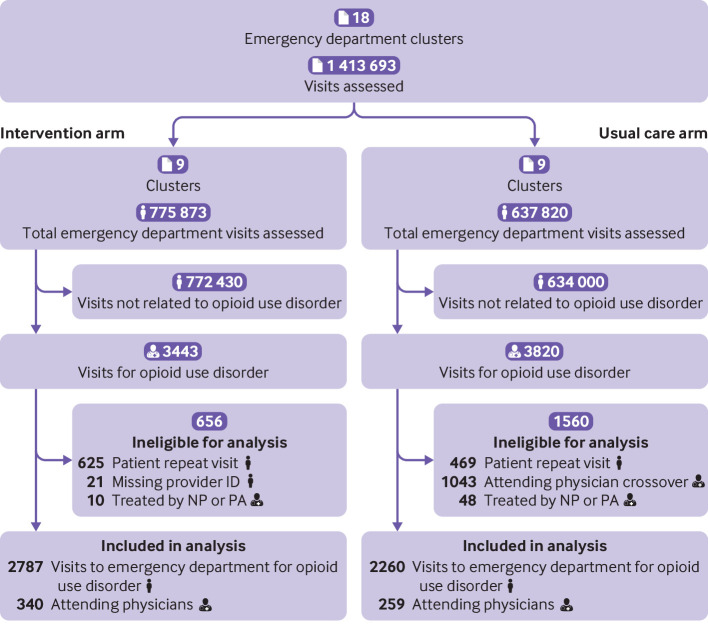
A total of 1 413 693 visits to the emergency department at the study sites from November 2019 to May 2021 were assessed for eligibility (fig 1). At the intervention sites, 3078 and 365 visits met the phenotype criteria for algorithms 1 and 2, respectively. At the usual care sites, 3307 and 513 visits met the phenotype criteria for algorithms 1 and 2, respectively. The median and mean number of visits per cluster were 239 and 310 in the intervention arm and 114 and 251 in the usual care arm, respectively. These visits were associated with 635 attending physicians of record (352 intervention, 338 usual care). Of these attending physicians, 56 assigned to the intervention arm crossed over from intervention to usual care sites. Visits to the usual care sites associated with these physicians (n=1043) were excluded from the analysis because these physicians were assigned to the intervention arm. We excluded 625 intervention and 469 usual care visits because of repeat patient visits, leaving 2818 patients in the intervention arm and 2308 in the usual care arm. After applying all of the enrollment criteria, 5047 patients (2787 intervention, 2260 usual care) and their first visits were eligible for analysis along with the 599 attending physicians who cared for them (340 in the intervention arm and 259 in the usual care arm).
Fig 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram. Note that repeat visits do not equate to patients, and therefore sums of patient numbers might not seem complete. NP=nurse practitioner; PA=physician associate
Participant characteristics
Median patient age was 36.0 years (interquartile range 29.0-47.0), and 1730 (34.3%) patients were women (table 1). Most patients were white (n=3613, 71.6%) and not Hispanic or Latino (n=4100, 81.2%). The most common insurance type was Medicaid (n=1899, 38.1%). We found differences in baseline values for several patient characteristics (ethnicity, insurance, naloxone given in the emergency department, naloxone prescribed before the emergency department visit, and opioid use disorder on the problem list or in the past medical history) that were not included in the constrained randomization. The median age category of the attending physicians was 35-44 years (n=206, 39.3%), and 173 (33.0%) physicians were women. At the start of the trial, 154 (29.4%) physicians had a waiver to prescribe buprenorphine. Most physicians cared for 5-20 patients with opioid use disorder during the trial (n=277, 46.2%).
Table 1.
Participant characteristics. Data are number (%) of participants unless stated otherwise
| Characteristics | Intervention | Usual care | Total |
|---|---|---|---|
| Patients | |||
| Total (No) | 2787 | 2260 | 5047 |
| Median (IQR) age (years) | 36.0 (29.0-48.0) | 36.0 (29.0-46.0) | 36.0 (29.0-47.0) |
| Sex: | |||
| Men | 1870 (67.1) | 1447 (64.0) | 3317 (65.7) |
| Women | 917 (32.9) | 813 (36.0) | 1730 (34.3) |
| Race: | |||
| Black or African American | 452 (16.2) | 406 (18.0) | 858 (17.0) |
| White | 2048 (73.5) | 1565 (69.2) | 3613 (71.6) |
| Other | 196 (7.1) | 219 (9.8) | 415 (8.2) |
| Unknown or not reported | 91 (3.3) | 70 (3.1) | 161 (3.2) |
| Ethnicity: | |||
| Hispanic or Latino | 505 (18.1) | 196 (08.7) | 701 (13.9) |
| Not Hispanic or Latino | 2166 (77.7) | 1934 (85.6) | 4100 (81.2) |
| Unknown or patient refused | 116 (4.2) | 130 (5.8) | 246 (4.9) |
| Insurance: | |||
| Medicare | 313 (11.4) | 274 (12.3) | 587 (11.8) |
| Medicaid | 1113 (40.4) | 786 (35.3) | 1899 (38.1) |
| Private or HMO | 770 (28.0) | 347 (15.6) | 1117 (22.4) |
| Self-pay | 468 (17.0) | 762 (34.2) | 1230 (24.7) |
| Other | 57 (2.1) | 44 (2.0) | 101 (2.0) |
| Unknown or not reported | 32 (1.2) | 14 (0.6) | 46 (0.9) |
| Opioid use disorder phenotype: | |||
| Algorithm 1 | 2443 (87.7) | 1984 (87.8) | 4427 (87.7) |
| Algorithm 2 | 344 (12.3) | 276 (12.2) | 620 (12.3) |
| Naloxone given in emergency department | 266 (9.6) | 105 (4.7) | 371 (7.4) |
| Naloxone prescribed in past 24 months | 606 (22.4) | 132 (5.9) | 738 (14.9) |
| Opioid use disorder previously on problem list | 409 (14.7) | 194 (8.6) | 603 (11.9) |
| Physicians | |||
| Total (No) | 340 | 259 | 599 |
| No of missing demographics | 26 (7.6) | 49 (19.9) | 75 (12.5) |
| Sex: | |||
| Men | 210 (66.9) | 141 (67.1) | 351 (67.0) |
| Women | 104 (33.1) | 69 (32.9) | 173 (33.0) |
| Age group (years): | |||
| <35 | 68 (21.7) | 41 (19.5) | 109 (20.8) |
| 35-44 | 124 (39.5) | 82 (39.0) | 206 (39.3) |
| 45-54 | 84 (26.8) | 56 (26.7) | 140 (26.7) |
| 55-64 | 29 (9.2) | 24 (11.4) | 53 (10.1) |
| ≥65 | 9 (2.9) | 7 (3.3) | 16 (3.1) |
| X waiver trained before trial: | |||
| No | 161 (51.4) | 128 (61.0) | 289 (55.3) |
| Yes | 102 (32.6) | 52 (24.8) | 154 (29.4) |
| No of patients with opioid use disorder during trial: | |||
| <5 | 145 (42.6) | 119 (45.9) | 264 (44.1) |
| 5-20 | 165 (48.5) | 112 (43.2) | 277 (46.2) |
| 20+ | 30 (8.8) | 28 (10.8) | 58 (9.7) |
IQR=interquartile range; HMO=health maintenance organization.
Outcomes
The intervention did not affect patient level rates of initiating buprenorphine in the emergency department (primary outcome 12.5% and 12.0% in the intervention and usual care arms, respectively; adjusted odds ratio 1.22, 95% confidence interval 0.61 to 2.43, P=0.58, table 2). The proportion of attending physicians who initiated buprenorphine in the emergency department at least once was higher at the intervention sites than at the usual care sites (44.4% v 34.0%, adjusted odds ratio 1.83, 95% confidence interval 1.16 to 2.89, P=0.01). This difference was still present in a sensitivity analysis with removal of all attending physicians who crossed over between the intervention and control arms.
Table 2.
Patient and physician level outcomes
| Outcomes | Implementation outcome type (RE-AIM) | Counts (No (%)) | Unadjusted (odds ratio (95% CI))† | Adjusted (odds ratio (95% CI))‡ | |||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Effect size | P value | Effect size | P value | ||||
| Patient level outcomes | |||||||||
| Total (No) | — | 2787 | 2260 | — | — | — | — | ||
| EMBED launched | Reach | 261 (9.4) | — | — | — | — | — | ||
| Buprenorphine administered in emergency department or prescribed at discharge* | Effectiveness | 347 (12.5) | 271 (12.0) | 1.52 (0.72 to 3.23) | 0.27 | 1.22 (0.61 to 2.43) | 0.58 | ||
| Referral for ongoing medication for opioid use disorder | Effectiveness | 367 (13.2) | 226 (10.0) | 1.03 (0.43 to 2.47) | 0.95 | 0.91 (0.52 to 1.59) | 0.74 | ||
| Naloxone prescription at discharge | Effectiveness | 517 (18.6) | 135 (6.0) | 1.62 (0.81 to 3.22) | 0.17 | 1.17 (0.45 to 3.10) | 0.74 | ||
| Buprenorphine administered or prescribed with EMBED | Implementation | 161/261 (61.7) | — | — | — | — | — | ||
| Maintenance: buprenorphine initiated after trial | Maintenance | 108/763 (14.2) | — | — | — | — | — | ||
| Physician level outcomes | |||||||||
| Total (No) | 340 | 259 | — | — | — | — | |||
| Unique attending physicians who launched EMBED | Adoption | 130 (38.2) | — | — | — | — | — | ||
| Initiated buprenorphine (administered or prescribed) | Adoption | 151 (44.4) | 88 (34.0) | 1.55 (1.11 to 2.16) | 0.01 | 1.83 (1.16 to 2.89) | 0.01 | ||
| Obtained X waiver during trial | Adoption | 50 (14.7) | 30 (11.6) | 1.32 (0.81 to 2.14) | 0.27 | 1.31 (0.69 to 2.48) | 0.42 | ||
| Provided referral for ongoing medication for opioid use disorder | Adoption | 141 (41.5) | 100 (38.6) | 1.13 (0.91 to 1.57) | 0.48 | 0.86 (0.57 to 1.32) | 0.49 | ||
| Prescribed naloxone at discharge from emergency department | Adoption | 177 (52.1) | 71 (27.4) | 2.88 (2.03 to 4.07) | <0.001 | 5.30 (3.33 to 8.44) | <0.001 | ||
| Initiated buprenorphine using EMBED | Implementation | 92/130 (70.7) | — | — | — | — | — | ||
RE-AIM=reach, effectiveness, adoption, implementation, and maintenance framework; EMBED=EMergency department initiated BuprenorphinE for opioid use Disorder.
Primary outcome.
Obtained from a generalized estimating equations model accounting for clustering by site.
Obtained from a generalized estimating equations model accounting for clustering by site and these covariates: healthcare system, electronic health record vendor, practice setting, urbanicity, annual patient volume, presence of additional providers in the emergency department, other resources for opioid use disorder in the emergency department, presence of any attending physicians with a waiver to prescribe buprenorphine before the start of the trial, age, sex, race, ethnicity, insurance type and status, naloxone prescription in past 24 months, opioid use disorder on problem list, urine drug screen results, and time (months) since the start of the trial.
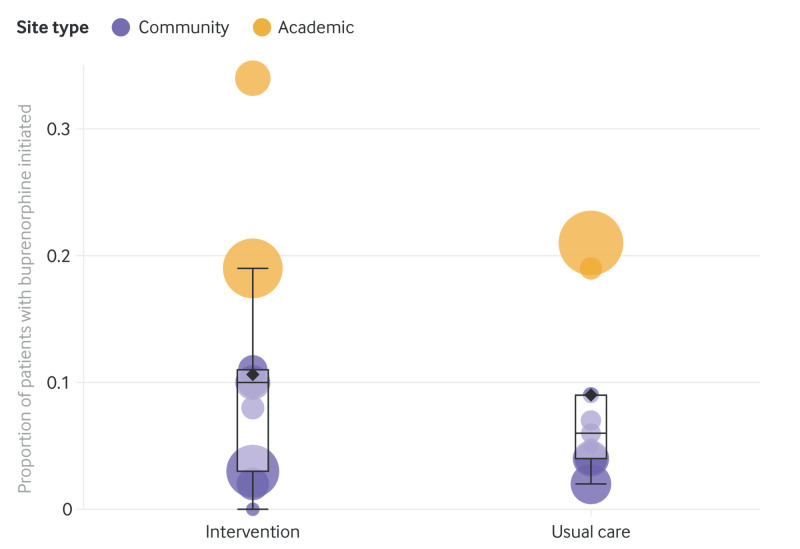
In the intervention arm, EMBED was launched by 130 (38.2%) physicians for 261 of 2787 (9.4%) patients with opioid use disorder. When EMBED was launched, buprenorphine was ordered in 161 patients (61.7% of 261 visits where EMBED was launched). For both study arms, we found wide variability in the number of patients with opioid use disorder per site (intervention range 41-802, usual care 57-940) and rates for initiating buprenorphine by site (intervention range 0.0-33.6%, usual care 1.9-21.3%), with the highest rates at academic sites and more variability in rates at community sites in the intervention arm (fig 2).
Fig 2.
Bubble plot by study arm (intervention or usual care) showing proportion of patients with opioid use disorder receiving buprenorphine by cluster site (academic and community). Each bubble represents one cluster. Bubble area is proportional to the number of patients with opioid use disorder. Vertical position represents the proportion of these patients with buprenorphine initiated. Box lines indicate median, top, and bottom quartile values of the primary outcome by cluster, whiskers indicate the last value within 1.5 interquartile ranges, and the diamond indicates the mean value of the primary outcome by cluster. An interactive version of this graphic is available at https://bit.ly/39aojsT
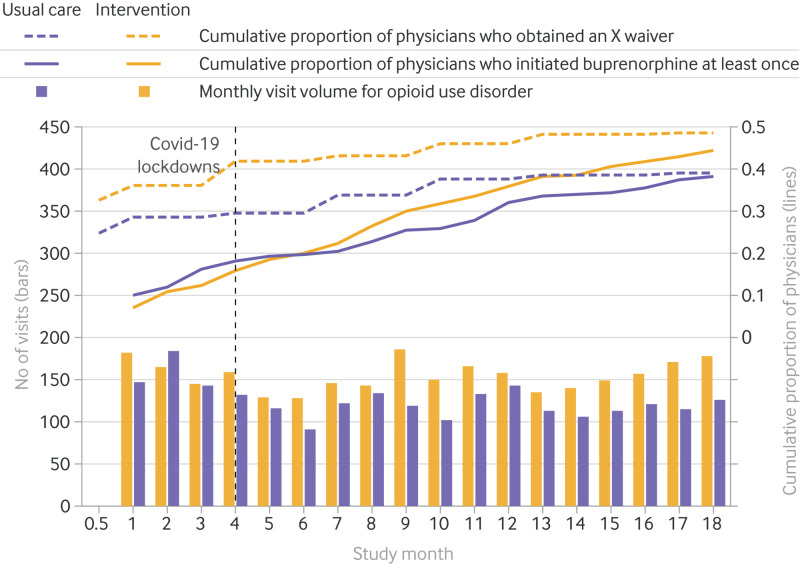
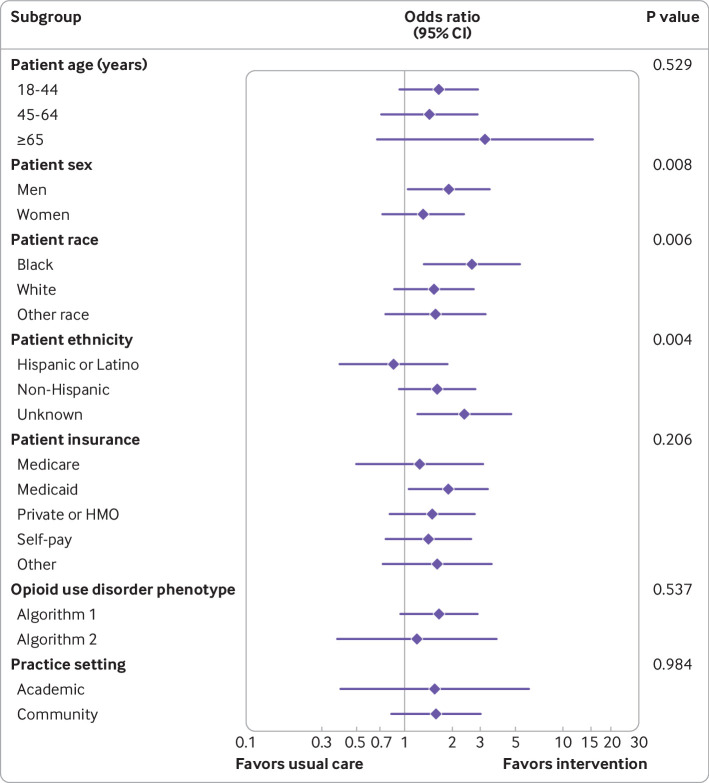
Examination of temporal trends showed higher cumulative proportions in the intervention arm for physicians who initiated buprenorphine at least once during the trial and who obtained an X waiver (fig 3). Subgroup analysis for rates of initiating buprenorphine by study arm suggested that men benefited more from the intervention than women (P=0.008) and black people more than other races (P=0.006), whereas Hispanic or Latino individuals benefited less compared with other ethnicities (P=0.004, fig 4). Differences between the intervention and usual care arms were not significantly modified by patient age, insurance, electronic health record phenotype algorithm, or practice setting (academic or community). In sensitivity analyses assessing whether the volume of inpatients during the covid-19 pandemic affected the primary outcome, we found no difference in the rates of initiating buprenorphine after controlling for inpatient volume during the covid-19 pandemic at the site level.
Fig 3.
Temporal trends in visits to the emergency department for opioid use disorder and cumulative proportion of physicians who initiated buprenorphine at least once during the trial and obtained an X waiver to prescribe buprenorphine by study arm (intervention or usual care). Monthly visits for opioid use disorder is charted by study arm. An interactive version of this graphic is available at https://bit.ly/3NvGuIj
Fig 4.
Forest plot of subgroup analyses showing the effect of patient baseline characteristics and practice setting on the primary outcome for the usual care and intervention arms. Odds ratios, 95% confidence intervals, and P values are from analyses adjusted for site level variables included in the constrained randomization and account for clustering. HMO=health maintenance organization
We found no significant changes in the secondary outcomes at the patient level for referral for ongoing medication for opioid use disorder (13.2% v 10.0% in the intervention and usual care arms, respectively, odds ratio 1.03, 95% confidence interval 0.43 to 2.47, P=0.95) and prescription of naloxone at discharge from the emergency department (18.6% v 6.0% in the intervention and usual care arms, respectively, 1.62, 0.81 to 3.22, P=0.17). Maintenance rates for initiating buprenorphine in the emergency department persisted after the end of the trial, with buprenorphine administered to 14.2% (108/763) of patients at intervention sites in the 120 days after the trial ended (P=0.38 compared with trial primary outcome). No serious adverse events or unintended effects were reported in the study arms.
Discussion
Principal findings
The EMBED intervention did not affect the rate of patients with opioid use disorder receiving buprenorphine in the emergency department (primary outcome) compared with usual care in this pragmatic trial across five diverse healthcare systems in the US (adjusted generalized estimating equations odds ratio 1.22, P=0.58). This finding is consistent with previous results across 122 clinical decision support clinical trials in a 2020 meta-analysis. The meta-analysis found that the effectiveness of clinical decision support on patient outcomes is typically small,42 reinforcing the potentially limited role of clinical decision support as a standalone intervention to change clinical practice and supporting the need for comprehensive implementation strategies. Given the barriers to adoption of initiating buprenorphine in the emergency department, including clinicians’ desires for integrated, automated electronic pathways,9 11 benefit is likely from interventions such as EMBED. More than 60% of administration of buprenorphine in the intervention arm was initiated with EMBED, suggesting that the intervention is acceptable for use in routine practice. Overall, the rate of administration of buprenorphine by physicians, like the patient initiation rate, was similar in the two study arms. EMBED was associated with a higher proportion of physicians initiating buprenorphine at least once during the trial in the intervention arm compared with the usual care arm (44.4% v 34.0%), however. About a third of attending physicians in the intervention arm (130/340, 38.2%) launched EMBED at least once. Among these 130 physicians, 92 (70.7%) initiated buprenorphine with the clinical decision support at least once. We could not attribute the use of clinical decision support to situations where a physician might have launched EMBED without ultimately prescribing buprenorphine, however; these situations might include patients who were ineligible for buprenorphine (eg, if their opioid use disorder was not severe enough to warrant medication) and those who were not ready to start treatment. We found some evidence that EMBED might have increased evidence based care beyond buprenorphine administration, as shown by the significant (P<0.001) increase in unique physicians who prescribed naloxone at discharge in the intervention arm compared with the usual care arm (52.1% v 27.4%).
Strengths and weaknesses of the study
The pragmatic implementation of the trial in a wide variety of emergency departments allows generalizable interpretation of the real world effectiveness of EMBED across multiple regions, electronic health record systems, and practice settings. The parallel group randomized design also minimized confounding from temporal trends that could have affected administration of buprenorphine, which was particularly important given the timing of the covid-19 pandemic.43 Also, collecting outcomes related to the intervention’s reach, adoption, implementation, and maintenance gave a better understanding of the effect of the implementation itself.36 Unlike most other clinical decision support systems, EMBED was non-interruptive,15 developed with a user centered design,21 integrated into existing electronic health record workflows,22 and automated multiple electronic health record activities, decreasing the burden of using the electronic health record.44 45 Although the launch rate of EMBED of 9.4% might seem low, this rate of use could be considered relatively high given that up to 96% of interruptive alerts are dismissed17 and that buprenorphine was administered 61.7% of the time when EMBED was launched. Use rates as high as 27% have been reported for other non-interruptive alerts used in the emergency department46 which could mean that implementation of EMBED can be further improved. Assuming a continued rate of administration of buprenorphine of 61.7% when EMBED is used, to achieve the a priori determined clinically significant increase in patient rates of buprenorphine in the emergency department of 9% (12.0-21.0%) in the intervention arm, EMBED launch rates would need to increase to 34.0%.
This study had sampling bias and misclassification bias related to limitations in data collection. Except for data about physician and site level characteristics, all of the study data were collected from existing clinical data in the electronic health record. This pragmatic approach increased the real world significance of the findings of the study but limited the reliability of the data related to whether: a patient had untreated opioid use disorder and was thus a candidate for initiation of buprenorphine because methadone maintenance is not reliably captured in the electronic health record; a patient was motivated or ready to start buprenorphine if offered47; EMBED was launched; a referral for ongoing medication for opioid use disorder was made outside of the formal electronic health record process23; and naloxone was dispensed at discharge without an order placed in the electronic health record. All of these limitations might have resulted in systematic differences in the intervention’s reach, adoption, implementation, and effect size. Other limitations that could have changed estimates of effect size were that: only the first emergency department visit was included for each patient (possibly patients were more likely to engage in treatment in subsequent visits); the electronic health record phenotype only identified visits related to the use of opioids and could have missed patients with opioid use disorder presenting with diagnoses unrelated to opioid use; physicians who became comfortable initiating buprenorphine after initial support did not use EMBED for subsequent patients; and inclusion of community sites that were not yet ready to adopt the practice of initiating buprenorphine in the emergency department because of barriers not related to information technology, such as limited community referral options and stigma.11
Major temporal events during the trial that could have affected the study results were the covid-19 pandemic and ongoing clinical quality improvement initiatives related to opioid use disorder at the study sites (fig 3 and supplementary material). Although covid-19 was associated with an increased proportion of visits to the emergency department for opioid overdose,30 48 the pandemic also caused marked changes in clinical practice in the emergency department.29 The pandemic also changed the landscape of outpatient treatment for addiction with some programs closing physical locations and restricting in-person visits.49 Therefore, despite the increased proportion of visits for opioid overdose, initiating buprenorphine during the pandemic might have faced extra barriers. Conversely, at the time of the initial power calculation, baseline rates of initiating buprenorphine were 0-2%20 but during the trial the rate in the usual care arm was 12.0%, reflecting a temporal trend in the rate of adoption of initiating buprenorphine in the emergency department. Although this higher event rate decreased the power to detect a difference between the study arms, the sample size provided 80% power to detect a difference of 9% with a baseline event rate of 12.0%. Furthermore, given the ongoing quality improvement initiatives related to opioid use disorder, in particular at academic sites, EMBED might have offered less incremental benefit over other initiatives.
We found differences in patient and physician characteristics across the study arms. Because randomization in a group randomized trial is at the site level (not the patient level), differences in patient characteristics (ethnicity, insurance, naloxone given in the emergency department or prescribed at discharge) were not unexpected, and adjusted analyses accounted for potential confounders defined a priori. The similarity in physician characteristics (the target of the intervention) was reassuring and expected because of the site level randomization. We found a higher proportion of waivered physicians at the beginning of the trial in the intervention arm but the difference was not significant. Although the rate of growth of the proportion of physicians with a waiver was similar across the study arms, the rate of increase in the proportion of physicians who initiated buprenorphine at least once during the trial in the intervention arm was greater than the rate of growth in the usual care arm.
Strengths and weaknesses in relation to other studies
Despite the established clinical effectiveness of initiating buprenorphine in the emergency department in an explanatory trial,2 6 implementation into routine emergency care has been slow. Educational interventions alone have had no effect on increasing adoption of initiating buprenorphine in the emergency department.50 Only 20% of emergency physicians have expressed high levels of readiness to initiate buprenorphine.11 Given a mortality rate close to 5% at 12 months after a non-fatal opioid overdose,5 it is concerning that historically <5% of patients in the emergency department with non-fatal opioid overdose receive a buprenorphine prescription.51
Initiating buprenorphine in the emergency department is a process that requires multiple actions at the patient, clinician, and healthcare system levels before, during, and after the visit to the emergency department.50 Comprehensive programs for opioid use disorder in the emergency department have achieved high rates of initiation of buprenorphine and engagement in outpatient follow-up; a program in South Carolina reported a buprenorphine rate in the emergency department of 45.4% in 509 patients, and a program in California reported a rate of 59.7% in 12 009 patients.52 53 Although these multifaceted programs confirm that initiating buprenorphine in the emergency department is feasible, they included dedicated funding, staff, and hospital based outpatient resources. In contrast, the EMBED trial evaluated the effect of one technology based intervention across a variety of settings. Given that EMBED increased the proportion of physicians who initiated buprenorphine, EMBED could play a role in changing practice as part of a more comprehensive implementation strategy. For example, multiple recent studies have begun to explore strategies to change behavior, such as nudge interventions,54 55 56 that as part of a more comprehensive strategy could guide clinicians to EMBED resources.
Meaning of the study
The benefit of EMBED could be in increasing adoption of this potentially lifesaving practice by clinicians. Although EMBED does not overcome all barriers to implementing initiation of buprenorphine in the emergency department, such as stigma and limited community referral options,11 50 57 it offers a major opportunity to increase adoption by offering a simple, automated, rapid solution to initiating treatment and referral for people with opioid use disorder. Targeted prompts and interventions might be needed to increase universal implementation of initiation of buprenorphine in the routine care of eligible patients with opioid use disorder visiting the emergency department, however. EMBED was designed as a user initiated tool, to support and guide clinicians in the emergency department after they had identified patients who could potentially benefit from initiation of buprenorphine. Although not associated with changes in patient rates of initiating buprenorphine, EMBED increased adoption of initiating buprenorphine in the emergency department and prescribing naloxone at discharge by attending physicians, was scalable over a diverse group of sites, and was adaptable to the unique aspects of each healthcare system. User centered clinical decision support that makes initiating buprenorphine in the emergency department easier to adopt into routine practice is not sufficient for universal implementation, however. Instead, more holistic broader implementation strategies, such as local champions acting as resources and role models, and quality improvement, including audit and feedback, are necessary to promote change and look at other barriers to changes in behavior, including the motivation of clinicians and patients, and the social context.
Unanswered questions and future research
Understanding the experiences of EMBED users during the trial could help to refine the intervention to expand its dissemination outside of the trial. Subgroup analysis suggested that the EMBED intervention might have benefited men more than women, had a greater effect in black people, and Hispanic or Latino people benefitted less than other ethnicities. Secondary analyses are underway to explore these potential differences. A qualitative study is ongoing to identify barriers and facilitators to adoption and maintenance of EMBED among key trial stakeholder groups. Because the use of EMBED required clinicians to identify patients who would benefit from buprenorphine, future research could study a targeted, non-interruptive alert for patients in the emergency department who are eligible for buprenorphine as well as more granular analysis of the effectiveness of individual components of the intervention or the effect of the intervention on patient safety outcomes. Given our findings, the recent literature on barriers to implementation,11 50 the role of comprehensive buprenorphine programs in the emergency department,52 53 and new changes in the requirements for waiver training,58 future research could also study the effect of EMBED combined with other implementation facilitators to assess whether EMBED could contribute to increased rates of initiating buprenorphine in the emergency department, engagement with treatment, and other downstream outcomes for patients with opioid use disorder.
Conclusion
In this pragmatic trial, the EMBED clinical decision support tool did not increase rates of initiating buprenorphine in the emergency department. EMBED was used for a small proportion of eligible patients and was not used by a large proportion of physicians. When used, EMBED was associated with high rates of initiation of buprenorphine. EMBED also increased the number of unique physicians that provided initiation of buprenorphine in the emergency department and prescribed naloxone. Clinical decision support that streamlines and automates electronic workflows can increase physician adoption of complex, unfamiliar evidence based practices. Combined with interventions that deal with external barriers to administration of buprenorphine, the EMBED intervention can help to improve the consistency and quality of care for patients in the emergency department with opioid use disorder.
What is already known on this topic
The emergency department is a critical access point for many patients seeking care related to opioid use
Buprenorphine, one of the most effective treatments for opioid use disorder, can be safely initiated in the emergency department, but adoption of this practice has been slow
What this study adds
The EMBED (EMergency department initiated BuprenorphinE for opioid use Disorder) intervention did not increase rates of initiation of buprenorphine in the emergency department but the number of unique physicians that provided buprenorphine in the emergency department and prescribed naloxone increased in the intervention arm
Streamlining and automating electronic health record workflows can potentially increase adoption of complex, unfamiliar evidence based practices
More interventions are needed to deal with other barriers to treatment of addiction and increase the rate of initiating buprenorphine in the emergency department among patients with opioid use disorder
Acknowledgments
We thank the project managers of this study: Cheryl Napier (Colorado), Shams Rayad (UNC); IT analysts, Carolyn Williams and Dale Johnson (UAB), Bill K Ross (UNC), Haiping Li (Baystate), Terrence Dew, Michelle DeWitt, Kristina Follo, Cheryl Brophy, and Tim Cooney (Yale-New Haven); Yauehni Solad (Yale-New Haven); Sean Michael (Colorado); Ashley Deutsch (Baystate); Jessica Ray (Yale); Cynthia Brandt (Yale), Harini Bathulapalli and Charles Lu (Yale); and medical students Wesley Holland and Osama Ahmed (Yale), and Jodi Mao (EVMS).
Web extra.
Extra material supplied by authors
Web appendix 1: Supplementary materials
Web appendix 2: Structured query language (SQL) query for emergency department opioid use disorder electronic health record phenotype
Contributors: ERM and GD conceived of the work. ERM, MMJ, JDD, EPH, TFP-M, and GD designed the study. MMJ, TFP-M, EPH, GD, and ERM developed the intervention. ERM, JAH, MMJ, EPH, TFP-M, HP, BN, MFC, WES, RMS, LAW, and GD are responsible for the implementation of the study. ERM, MFC, HP, HR, WES, JAS, RMS, LAW, MDP, and TFP-M acquired the data. ERM, BN, JDD, MFC, SVC, MMJ, and WES drafted the initial manuscript. FL performed the statistical analysis. All authors analyzed the data and revised the manuscript and approved the final version submitted for publication. ERM takes responsibility for and is guarantor of all aspects of the work. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work is supported within the US National Institutes of Health (NIH) Health Care Systems Research Collaboratory by the NIH Common Fund through cooperative agreement U24AT009676 from the Office of Strategic Coordination within the Office of the NIH Director and cooperative agreement (UG3DA047003/ UH3DA047003) from the National Institute on Drug Abuse of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institute on Drug Abuse of the National Institutes of Health for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Patient consent: The study protocol was granted a waiver of informed consent under the Common Rule (45 code of federal regulations (CFR) 46.116).
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results will be published in open access, peer reviewed journals, presented at national meetings and shared with the clinicians at participating sites via a broadcast email notification of publications. The full results will be published for public access; access to the participant level dataset will be made in accordance with NIH policy after safeguarding that the datasets are fully de-identified at the site, provider, and patient level.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The full study protocol was approved by the Western Institutional Review Board (study No 1189765) with reliance agreements by the individual institutions’ institutional review boards.
Data availability statement
A de-identified participant dataset with an associated data dictionary will be publicly available at www.icpsr.umich.edu/web/pages/NAHDAP/index.html.
References
- 1. Jackson G, Brown AM, DeFrances C. Opioid-involved emergency department visits in the National Hospital Care Survey and the National Hospital Ambulatory Medical Care Survey. Natl Health Stat Report 2020;1-15. [PubMed] [Google Scholar]
- 2. D’Onofrio G, Chawarski MC, O’Connor PG, et al. Emergency department-initiated buprenorphine for opioid dependence with continuation in primary care: outcomes during and after intervention. J Gen Intern Med 2017;32:660-6. 10.1007/s11606-017-3993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sullivan LE, Fiellin DA. Narrative review: buprenorphine for opioid-dependent patients in office practice. Ann Intern Med 2008;148:662-70. 10.7326/0003-4819-148-9-200805060-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014;(2):CD002207. 10.1002/14651858.CD002207.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018;169:137-45. 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA 2015;313:1636-44. 10.1001/jama.2015.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larochelle MR, Stopka TJ, Xuan Z, Liebschutz JM, Walley AY. Medication for opioid use disorder after nonfatal opioid overdose and mortality. Ann Intern Med 2019;170:430-1. 10.7326/L18-0685 [DOI] [PubMed] [Google Scholar]
- 8. Wu L-T, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the United States. Drug Alcohol Depend 2016;169:117-27. 10.1016/j.drugalcdep.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: A physician survey. Am J Emerg Med 2019;37:1787-90. 10.1016/j.ajem.2019.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Onofrio G, McCormack RP, Hawk K. Emergency departments - A 24/7/365 Option for combating the opioid crisis. N Engl J Med 2018;379:2487-90. 10.1056/NEJMp1811988. [DOI] [PubMed] [Google Scholar]
- 11. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and facilitators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open 2020;3:e204561. 10.1001/jamanetworkopen.2020.4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765. 10.1136/bmj.38398.500764.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sittig DF, Wright A, Osheroff JA, et al. Grand challenges in clinical decision support. J Biomed Inform 2008;41:387-92. 10.1016/j.jbi.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Melnick ER, Nielson JA, Finnell JT, et al. Delphi consensus on the feasibility of translating the ACEP clinical policies into computerized clinical decision support. Ann Emerg Med 2010;56:317-20. 10.1016/j.annemergmed.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 15. Powers EM, Shiffman RN, Melnick ER, Hickner A, Sharifi M. Efficacy and unintended consequences of hard-stop alerts in electronic health record systems: a systematic review. J Am Med Inform Assoc 2018;25:1556-66. 10.1093/jamia/ocy112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melnick ER, Harry E, Sinsky CA, et al. Perceived electronic health record usability as a predictor of task load and burnout among US physicians: mediation analysis. J Med Internet Res 2020;22:e23382. 10.2196/23382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phansalkar S, Desai AA, Bell D, et al. High-priority drug-drug interactions for use in electronic health records. J Am Med Inform Assoc 2012;19:735-43. 10.1136/amiajnl-2011-000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benda NC, Meadors ML, Hettinger AZ, Ratwani RM. Emergency physician task switching increases with the introduction of a commercial electronic health record. Ann Emerg Med 2016;67:741-6. 10.1016/j.annemergmed.2015.07.514 [DOI] [PubMed] [Google Scholar]
- 19. Westbrook JI, Raban MZ, Walter SR, Douglas H. Task errors by emergency physicians are associated with interruptions, multitasking, fatigue and working memory capacity: a prospective, direct observation study. BMJ Qual Saf 2018;27:655-63. 10.1136/bmjqs-2017-007333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melnick ER, Jeffery MM, Dziura JD, et al. User-centred clinical decision support to implement emergency department-initiated buprenorphine for opioid use disorder: protocol for the pragmatic group randomised EMBED trial. BMJ Open 2019;9:e028488. 10.1136/bmjopen-2018-028488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ray JM, Ahmed OM, Solad Y, et al. Computerized clinical decision support system for emergency department-initiated buprenorphine for opioid use disorder: user-centered design. JMIR Hum Factors 2019;6:e13121. 10.2196/13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melnick ER, Holland WC, Ahmed OM, et al. An integrated web application for decision support and automation of EHR workflow: a case study of current challenges to standards-based messaging and scalability from the EMBED trial. JAMIA Open 2019;2:434-9. 10.1093/jamiaopen/ooz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed OM, Mao JA, Holt SR, et al. A scalable, automated warm handoff from the emergency department to community sites offering continued medication for opioid use disorder: Lessons learned from the EMBED trial stakeholders. J Subst Abuse Treat 2019;102:47-52. 10.1016/j.jsat.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melnick ER, Nath B, Ahmed OM, et al. Progress report on EMBED: A pragmatic trial of user-centered clinical decision support to implement EMergency Department-Initiated BuprenorphinE for Opioid Use Disorder. J Psychiatr Brain Sci 2020;5:e200003. 10.20900/jpbs.20200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holland WC, Nath B, Li F, et al. Interrupted time series of user-centered clinical decision support implementation for emergency department-initiated buprenorphine for opioid use disorder. Acad Emerg Med 2020;27:753-63. 10.1111/acem.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuzzio L, Meyers CM, Dember LM, et al. Accounting for quality improvement during the conduct of embedded pragmatic clinical trials within healthcare systems: NIH collaboratory case studies. Healthc (Amst) 2021;8(Suppl 1):100432. 10.1016/j.hjdsi.2020.100432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical Decision Support to Implement ED-initiated Buprenorphine for OUD - Full Text View - ClinicalTrials. Gov. https://clinicaltrials.gov/ct2/show/NCT03658642 (accessed 9 Aug 2021).
- 28. Schulz KF, Altman DG, Moher D, CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 2011;9:672-7. 10.1016/j.ijsu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 29. Jeffery MM, D’Onofrio G, Paek H, et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID-19 pandemic in the US. JAMA Intern Med 2020;180:1328-33. 10.1001/jamainternmed.2020.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soares WE, 3rd,Melnick ER, Nath B, et al. Emergency department visits for nonfatal opioid overdose during the COVID-19 pandemic across six US health care systems. Ann Emerg Med 2022;79:158-67. 10.1016/j.annemergmed.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chartash D, Paek H, Dziura JD, et al. Identifying opioid use disorder in the emergency department: multi-system electronic health record-based computable phenotype derivation and validation study. JMIR Med Inform 2019;7:e15794. 10.2196/15794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BupePharm. https://www.samhsa.gov/bupe/lookup-form (accessed 4 Feb 2022).
- 33. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub, 2013. [Google Scholar]
- 34. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003;35:253-9. 10.1080/02791072.2003.10400007 [DOI] [PubMed] [Google Scholar]
- 35. Pantalon MV, Dziura J, Li F-Y, Owens PH, O’Connor PG, D’Onofrio G. An interventionist adherence scale for a specialized brief negotiation interview focused on treatment engagement for opioid use disorders. Subst Abus 2017;38:191-9. 10.1080/08897077.2017.1294548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322-7. 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glasgow RE, Lichtenstein E, Marcus AC. Why don’t we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health 2003;93:1261-7. 10.2105/AJPH.93.8.1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Group- or Cluster-Randomized Trials (GRTs). https://researchmethodsresources.nih.gov/methods/grt (accessed 12 Aug 2021).
- 39. Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol 2006;35:1292-300. 10.1093/ije/dyl129 [DOI] [PubMed] [Google Scholar]
- 40. Raab GM, Butcher I. Balance in cluster randomized trials. Stat Med 2001;20:351-65. [DOI] [PubMed] [Google Scholar]
- 41. Li F, Lokhnygina Y, Murray DM, Heagerty PJ, DeLong ER. An evaluation of constrained randomization for the design and analysis of group-randomized trials. Stat Med 2016;35:1565-79. 10.1002/sim.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwan JL, Lo L, Ferguson J, et al. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ 2020;370:m3216. 10.1136/bmj.m3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: a review of recent methodological developments. Am J Public Health 2004;94:423-32. 10.2105/AJPH.94.3.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc 2016;91:836-48. 10.1016/j.mayocp.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 45. Melnick ER, Dyrbye LN, Sinsky CA, et al. The association between perceived electronic health record usability and professional burnout among US physicians. Mayo Clin Proc 2020;95:476-87. 10.1016/j.mayocp.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 46. Dhaliwal JS, Goss F, Whittington MD, et al. Reduced admission rates and resource utilization for chest pain patients using an electronic health record-embedded clinical pathway in the emergency department. J Am Coll Emerg Physicians Open 2020;1:1602-13. 10.1002/emp2.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coupet E, Jr, D’Onofrio G, Chawarski M, et al. Emergency department patients with untreated opioid use disorder: A comparison of those seeking versus not seeking referral to substance use treatment. Drug Alcohol Depend 2021;219:108428. 10.1016/j.drugalcdep.2020.108428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slavova S, Rock P, Bush HM, Quesinberry D, Walsh SL. Signal of increased opioid overdose during COVID-19 from emergency medical services data. Drug Alcohol Depend 2020;214:108176. 10.1016/j.drugalcdep.2020.108176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Becker WC, Fiellin DA. When epidemics collide: coronavirus disease 2019 (COVID-19) and the opioid crisis. Ann Intern Med 2020;173:59-60. 10.7326/M20-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schoenfeld EM, Soares WE, Schaeffer EM, Gitlin J, Burke K, Westafer LM. “This is part of emergency medicine now”: A qualitative assessment of emergency clinicians’ facilitators of and barriers to initiating buprenorphine. Acad Emerg Med 2022;29:28-40. 10.1111/acem.14369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kilaru AS, Xiong A, Lowenstein M, et al. Incidence of treatment for opioid use disorder following nonfatal overdose in commercially insured patients. JAMA Netw Open 2020;3:e205852. 10.1001/jamanetworkopen.2020.5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bogan C, Jennings L, Haynes L, et al. Implementation of emergency department-initiated buprenorphine for opioid use disorder in a rural southern state. J Subst Abuse Treat 2020;112S:73-8. 10.1016/j.jsat.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med 2021;78:759-72. 10.1016/j.annemergmed.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 54. Butler K, Chavez T, Wakeman S, et al. Nudging emergency department initiated addiction treatment. J Addict Med 2021;12. 10.1097/ADM.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 55. Margaret L, McFadden R, Dina A-R, et al. Redesign of opioid use disorder screening and treatment in the ED. NEJM Catal 2022;3. 10.1056/CAT.21.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lowenstein M, Perrone J, Xiong RA, et al. Sustained implementation of a multicomponent strategy to increase emergency department-initiated interventions for opioid use disorder. Ann Emerg Med 2022;79:237-48. 10.1016/j.annemergmed.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dong KA, Lavergne KJ, Salvalaggio G, et al. Emergency physician perspectives on initiating buprenorphine/naloxone in the emergency department: A qualitative study. J Am Coll Emerg Physicians Open 2021;2:e12409. 10.1002/emp2.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the X-waiver requirement now. Ann Emerg Med 2021;78:220-2. 10.1016/j.annemergmed.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Supplementary materials
Web appendix 2: Structured query language (SQL) query for emergency department opioid use disorder electronic health record phenotype
Data Availability Statement
A de-identified participant dataset with an associated data dictionary will be publicly available at www.icpsr.umich.edu/web/pages/NAHDAP/index.html.