Abstract
Purpose
To retrospectively collect and analyze demographic information as well as symptoms, laboratory results, endoscopic and pathologic findings, and treatment of ulcerative colitis (UC) and Crohn’s disease (CD) patients in Wuhan, China.
Methods
Patients who were diagnosed as inflammatory bowel disease (IBD) and hospitalized from January 2012 to December 2017 were enrolled in this study. The clinical characteristics including symptoms, laboratory results, and treatment were reviewed and analyzed.
Results
Totally 821 cases were screened, and finally 430 UC patients and 286 CD patients were selected and enrolled in this study. The most common symptom in UC patients was bloody stool (90.7%) followed by diarrhea (87.7%), mucus in stool (72.1%), and abdominal pain (66.3%), which were significantly different from those of CD patients (P < 0.01). In contrast, the most common symptom in CD patients was abdominal pain (80.0%) followed by diarrhea (58.4%), bloody stool (27.6%), and fever (18.2%). Erythrocyte sedimentation, C-reactive protein, and platelets were significantly increased, while hemoglobin was decreased, in the moderately or highly active IBD. The percentage of positive perinuclear anti-neutrophil cytoplasmic antibody was significantly higher in UC patients (31.1%) than that in CD patients (4.8%, P < 0.001), while the percentage of positive anti-intestinal goblet cell antibody was significantly higher in CD patients (23.1%) than that in UC patients (14.9%, P = 0.037).
Conclusion
The findings of the current study may provide evidence-based information for Chinese gastroenterologists to treat IBD more effectively in the future.
Keywords: inflammatory bowel disease, Crohn’s disease, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD), namely ulcerative colitis (UC) and Crohn’s disease (CD), is a group of chronic and progressive inflammatory diseases predominantly affecting the gastrointestinal tract.1,2 While its pathogenesis is unclear, IBD develops from a combination of genetic susceptibility and environmental factors that lead to a remitting and relapsing course of chronic inflammation. IBD affects approximately 1.4 million individuals in the USA and 2.2 million in Europe,3 which account for up to 0.5% of the general population in the Western world.4,5 The prevalence of IBD in China varies depending on regions, and is higher in the coastal areas such as Guangdong (3.14 per 100,000 population), Hong Kong, and Macao than that in the inland area.6,7
IBD has significant effects on the quality of life and workplace performance.8,9 Although it can occur at any age, IBD primarily affects population between the age of 20 and 40 years.10 The diagnosis of IBD is established based on symptoms such as persistent or recurrent bloody diarrhea with abdominal pain and frequent bowel movements, characteristic findings of IBD in the physical examination, laboratory test, and endoscopic as well as pathologic findings typical of IBD.
Currently, there is no curative treatment for IBD; thus, the main medical therapy for IBD is remission induction by alleviating symptoms, promoting mucosal healing, and preventing intestinal complications to avoid surgical resection.11 In this regard, the evidence-based consensus and guidelines on the diagnosis and treatment of IBD have been published and updated around the world.8,12–16 According to these guidelines, corticosteroids, 5-aminosalicylates (5-ASA), immunomodulators and biological agent have been the mainstay of IBD medical therapy.
The incidence of IBD continues to rise and the burden of IBD care will increase globally, especially, in the newly industrialized countries.5 With rapid economic development and industrialization in China, we anticipate that the prevalence of IBD will increase rapidly in China during the next decade; thus, it is important for gastroenterologists to be aware of the rising number of IBD cases and to deliver efficient, cost-effective and innovative treatment for IBD patients. Therefore, in the current study, we retrospectively compared and analyzed demographic data as well as symptoms, laboratory test, endoscopic and pathologic findings, and treatment of UC and CD, who visited our hospital from January 2012 through December 2017. We expect that the findings of the current study could provide evidence-based information for gastroenterologists to treat IBD more effectively in the future in China.
Methods
Patient Enrollment
In this retrospective study, demographic data and clinical history of patients with IBD who visited the Department of Gastroenterology, Zhongnan Hospital, Wuhan University from January 2012 through December 2017 were collected and analyzed. The inclusion criteria were as follows. The patients were diagnosed with IBD according to the “Chinese Consensus on Diagnosis and Treatment in Inflammatory Bowel Disease (2018, Beijing)”.17 Specifically, the clinical manifestations, laboratory examination results, colonoscopy and pathology of the biopsies, and radiological studies met the diagnostic criteria of IBD. We excluded patients with malignant tumor, HIV infection, hepatitis B, and autoimmune diseases such as systemic lupus erythematosus, psoriasis, rheumatoid arthritis, and Behcet’s disease.
The study was approved by the ethics committee of Zhongnan Hospital of Wuhan University (No. 2022035K). Patient consent was not required given the retrospective nature of the study. Our experiments were performed in accordance with the Declaration of Helsinki.
Data Retrieval
Specifically, the following data was collected: age, gender, age when IBD was diagnosed, days of hospital stay, type of inflammatory disease (UC or CD), location of bowel inflammation (UC: rectum, left colon, and whole colon; CD: ileal, ileocolonic, colonic, and isolated upper gastrointestinal Crohn’s disease), activity status of UC by Mayo score (remission, mildly active, moderately active, highly active), clinical behavior of CD (inflammatory, stricturing, penetrating, or perianal disease) by Crohn’s Disease Activity Index (CDAI), symptoms and complications, results of laboratory test including erythrocyte sedimentation rate (ESR), C-reactive protein, (CRP), platelet, and hemoglobin, findings of colonoscopy, pathologic findings of the biopsies, and treatment.
Statistical Analysis
All data was analyzed using IBM SPSS 23 software. Categorical variables were compared using Chi square test. Continuous variables with normal distribution were expressed by mean ± SD and compared between the two groups using Student’s t test and Wilcoxon two samples test was used for non-normally distributed data. P < 0.05 was considered statistically significant.
Results
Demographic Information of the Patients
Total 821 cases were initially screened in this study. Of them, 716 cases were enrolled in this study and the remaining 105 cases were excluded due to the following reasons: 9 cases were not diagnosed as IBD; 38 cases had compromised immunity or autoimmune disease (including 3 cases of malignant tumor, 1 case of HIV infection, 31 cases of hepatitis B, 2 cases of rheumatoid arthritis, and 1 case of systemic lupus erythematosus), and 58 cases had incomplete medical history.
As shown in Table 1, of the 716 patients who were enrolled in this retrospective study, 430 cases had UC and 286 cases had CD. The incidence of both UC and CD was predominantly higher in male patients (UC: 60.7% vs 39.3%, P<0.01; CD: 66.8% vs 33.2%, P<0.01). In addition, the mean age of patients with UC (39.3 ± 13.8 y) was significantly higher than that of patients with CD (29.5 ± 12.4 y, P<0.01). There was no significant difference in the mean duration of hospital stay between the two types of IBD.
Table 1.
Demographic Information of the IBD Patients
| Ulcerative Colitis (N = 430) | Crohn’s Disease (N = 286) | P value | |
|---|---|---|---|
| Gender | |||
| Male | 261 (60.7%)* | 191 (66.8%)* | |
| Female | 169 (39.3%) | 95 (33.2%) | |
| Age (y) | |||
| Average | 39.3 ± 13.8 | 29.5 ± 12.4 | < 0.01 |
| < 17 | 5 (1.2%) | 29 (10.1%) | |
| 17~40 | 243 (56.5%) | 204 (71.4%) | |
| >40 | 182 (42.3%) | 53 (18.5%) | < 0.01 |
| Average length of hospitalization (day) | |||
| 10.9 ± 7.4 | 11.3 ± 6.1 | > 0.05 |
Note: * P < 0.01 compared to female.
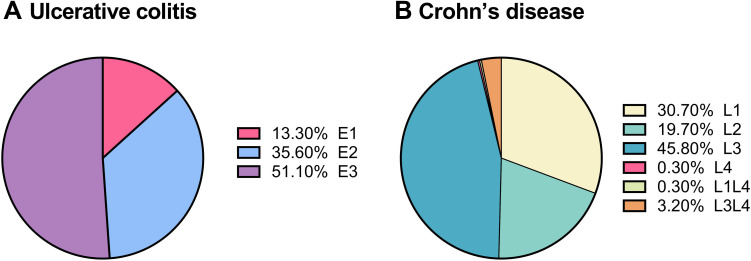
Analysis of the lesions of IBD indicated that over half of the UC patients had pan-colon lesion (E3: 51.1%) followed by left-colon involvement (E2: 35.6%) and rectal lesion (E1: 13.3%, Figure 1A); meanwhile, the most common location of CD lesion was the ileocolon (L3: 45.8%) followed by the ileum (L1: 30.7%) and colon (L2: 19.7%, Figure 1B).
Figure 1.
Lesion locations of inflammatory bowel diseases. (A) Ulcerative disease. E1: Rectus, E2: left-colon; E3: pan-colon. (B) Crohn’s disease. L1: ileum, L2: colon; L3: ileo-colon, L4: isolated upper gastric tract.
Comparison of Symptoms and Complications
As shown in Table 2, for patients with UC, bloody stool was the most common symptom (90.7%) followed by diarrhea (87.7%), mucus in stool (72.1%), and abdominal pain (66.3%), which were significantly different from those of patients with CD (P < 0.01). In contrast, for patients with CD, the most common symptom was abdominal pain (80.0%) followed by diarrhea (58.4%), bloody stool (27.6%), and fever (18.2%). In addition, patients with CD had a higher incidence of complications including intestinal obstruction (27.3%), perianal lesion (25.5%), bowel perforation (7.7%), and fistula (5.9%), which was significantly higher than that of patients with UC (intestinal obstruction: 0.7%; perianal lesion: 1.6%; bowel perforation: 0; fistula: 0.2%; P < 0.001).
Table 2.
Comparison of Symptoms and Complications
| Symptoms | Ulcerative Colitis (%) | Crohn’s Disease (%) | P value |
|---|---|---|---|
| Abdominal pain | 285 (66.3) | 223 (80.0) | <0.001 |
| Diarrhea | 377 (87.7) | 167 (58.4) | <0.0001 |
| Abdominal bloating | 35 (8.1) | 36 (12.6) | 0.051 |
| Bloody stool | 388 (90.7) | 79 (27.6) | <0.0001 |
| Mucus stool | 310 (72.1) | 49 (17.1) | <0.0001 |
| Nausea, vomiting | 16 (3.7) | 18 (6.3) | 0.113 |
| Fever | 35 (8.1) | 52 (18.2) | <0.0001 |
| Tenesmus | 32 (7.4) | 4 (1.4) | <0.0001 |
| Intestinal obstruction | 3 (0.7) | 78 (27.3) | <0.0001 |
| Bowel perforation | 0 (0) | 22 (7.7) | <0.0001 |
| Perianal lesion | 7 (1.6) | 73 (25.5) | <0.0001 |
| Fistula | 1 (0.2) | 17 (5.9) | <0.0001 |
| Others | 8 (1.9) | 2 (0.7) | 0.195 |
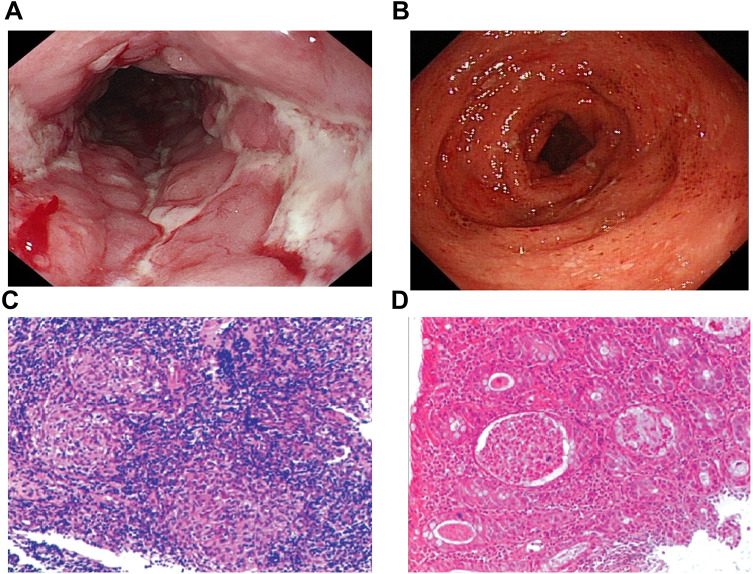
Endoscopic examination of UC showed severe UC as defined by friable colonic mucosa, spontaneous bleeding and ulcers (Figure 2A), and histological (microscopic) lesion of crypt abscess with infiltration of inflammatory cells (Figure 2B). Meanwhile, cobblestone appearance and longitudinal ulcers were observed under endoscopy in CD patients (Figure 2C), and histologic feature of noncaseating granulomas under microscopy (Figure 2D).
Figure 2.
Endoscopic and pathologic features of IBD. (A and C) Endoscopic (A) and histopathologic (C) appearance of Crohn’s disease. (B and D) Endoscopic (B) and histopathologic (D) appearance of ulcerative colitis. Magnification of C and D x 100.
Laboratory Biomarkers and Its Application in Evaluating the Status of IBD Through ROC Curve Analysis
To assess the activity status of IBD, laboratory parameters including ESR, CRP, platelet counts, and hemoglobin were examined and analyzed by receiver operating characteristic (ROC) curve and the area under the curve (AUC). As shown in Table 3, ESR significantly increased in the moderately (21.5 ± 20.3 mm/h) and highly (36.2 ± 27.1 mm/h) active cases of UC compared to cases at remission (17.4± 32.2 mm/h, P < 0.001) although patients at mildly active status had a lower ESR (13.1 ± 15.1 mm/h). In addition, CRP and platelet counts gradually and significantly increased, while hemoglobin content was significantly reduced in the mildly, moderately and highly active UC (P< 0.001, Table 3).
Table 3.
Parameters of Laboratory Test in Ulcerative Colitis at Various Activity Status
| Remission | Mildly Active | Moderately Active | Highly Active | P value | |
|---|---|---|---|---|---|
| ESR (mm/h) | 17.4 ± 32.2 | 13.1 ± 15.1 | 21.5 ± 20.3 | 36.2 ± 27.1 | < 0.001 |
| CRP (mg/L) | 2.5 ± 1.8 | 7.0 ± 16.0 | 13.7 ± 20 | 33.3 ± 40.5 | < 0.001 |
| PLT (109/L) | 205.7±70.0 | 224.7±197.9 | 249.4±90 | 300.0±106.6 | < 0.001 |
| HGB (g/L) | 122.4±25.6 | 129.3±15.9 | 120.6±20.4 | 105.3±25.5 | < 0.001 |
Abbreviations: ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PLT, platelet; HGB, hemoglobin.
Similarly, as shown in Table 4, patients with active CD had gradually increased ESR (mild: 23.2 ± 16.8 mm/h; moderate: 36.8 ± 24.6 mm/h; high: 50.1 ± 32.8 mm/h), which was significantly higher than that of patients at remission (19.9 ± 22.2 mm/h, P<0.001). CRP was significantly higher in patients with active CD (mild: 20.4 ± 19.9 mg/L; moderate: 32.2 ± 28.2 mg/L; high: 42.6 ± 31.8 mg/L) compared to that of patients at remission (14.7 ± 23.3 mg/L, P<0.001). The platelet counts were significantly higher in patients with active CD (mild: 269.4 ± 86.1 x109/L; moderate: 298.4 ± 96.1 x109/L; high: 345.5 ± 111.6 x109/L) compared to that of patients at remission (240.3 ± 75.9 x109/L, P < 0.001). Hemoglobin significantly decreased in patients with active CD (mild: 111.4 ± 21.4 g/L; moderate: 105.2 ± 22.5 g/L; high: 95.2 ± 23.0 g/L) compared to that of patients at remission (117.8 ± 22.2 g/L, P<0.001, Table 4). However, albumin level was not analyzed in this study.
Table 4.
Parameters of Laboratory Test in Crohn’s Disease at Various Activity Status
| Remission | Mildly Active | Highly Active | Moderately Active | P value | |
|---|---|---|---|---|---|
| ESR (mm/h) | 19.9 ± 22.2 | 23.2 ± 16.8 | 36.8 ± 24.6 | 50.1 ± 32.8 | < 0.001 |
| CRP (mg/L) | 14.7 ± 23.3 | 20.4 ± 19.9 | 32.2 ± 28.2 | 42.6 ± 31.8 | < 0.001 |
| PLT (109/L) | 240.3±75.9 | 269.4±86.1 | 298.4±96.2 | 345.5±111.6 | < 0.001 |
| HGB (g/L) | 117.8 ± 22.2 | 111.4 ± 21.4 | 105.2 ± 22.5 | 95.2 ± 23.0 | < 0.001 |
Abbreviations: ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PLT, platelet; HGB, hemoglobin.
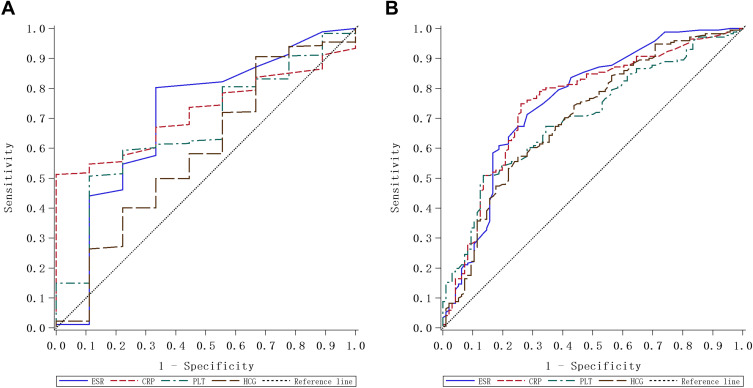
We carried out ROC curve and AUC analysis to assess the sensitivity and specificity of the aforementioned laboratory parameters in evaluating the activity status of IBD. As shown in Figure 3A and Table 5, for evaluating UC activity, when the CRP cut-off was set at 5.2 mg/L, CRP had a specificity of 100% for UC activity was 100%, which was statistically significant (P < 0.001), while it had a sensitivity of 51.3%. Similarly, the platelet count, at a cut-off of 238.5 x 109/L, had a sensitivity of 50.7%, but a high specificity (88.9%) for evaluating UC activity with statistical significance (P = 0.047). However, neither ESR, nor hemoglobin was statistically significant for evaluating UC activity.
Figure 3.
Receiver-operating characteristic (ROC) analysis of ESR, CRP and platelet counts for evaluating IBD. (A) ROC curve of ESR, CRP and platelet for evaluating UC. (B) ROC curve of ESR, CRP and platelet for evaluating CD.
Table 5.
Sensitivity and Specificity of the Parameters on Evaluating Ulcerative Colitis Activity Status by ROC Curve Analysis
| Cut-Off | Sensitivity | Specificity | AUC (95% CI) | P value | |
|---|---|---|---|---|---|
| ESR (mm/h) | 6.5 | 80.2% | 66.7% | 0.705 (0.498–0.8912) | =0.0526 |
| CRP (mg/L) | 5.2 | 51.3% | 100% | 0.721 (0.618–0.822) | < 0.001 |
| PLT (109/L) | 238.5 | 50.7% | 88.9% | 0.670 (0.502–0.837) | =0.0468 |
| HGB (g/L) | 104.4 | 90.9% | 24.9% | 0.588 (0.374–0.802) | =0.4187 |
Abbreviations: ROC, receiver-operating characteristics; AUC, area under the curve; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PLT, platelet; HGB, hemoglobin.
As shown in Figure 3B and Table 6, for evaluating CD activity, when the best threshold of ESR was set at 19.5 mm/h, the sensitivity was 71.0%, and the specificity was 72.7% (P<0.01). When the best threshold of CRP was set at 13.6 mg/L, the sensitivity was 75.0%, and the specificity was 73.7% (P<0.01). When the best threshold of platelet count was set at 304.5 x 109/L, the sensitivity was 50.6%, and the specificity was 86.9% (P<0.01). Furthermore, when the best threshold of hemoglobin was set at 106.8 g/L, the sensitivity was 74.4%, and the specificity was 57.7% (P<0.01).
Table 6.
Sensitivity and Specificity of the Parameters on Evaluating Crohn’s Disease Activity Status by ROC Curve Analysis
| Optimal Threshold | Sensitivity | Specificity | AUC (95% CI) | P value | |
|---|---|---|---|---|---|
| ESR (mm/h) | 19.5 | 71.0% | 72.7% | 0.762 (0.700–0.823) | < 0.01 |
| CRP (mg/L) | 13.6 | 75.0% | 73.7% | 0.758 (0.697–0.819) | < 0.01 |
| PLT (109/L) | 304.5 | 50.6% | 86.9% | 0.702 (0.697–0.764) | < 0.01 |
| HGB (g/L) | 106.8 | 74.4% | 57.7% | 0.703 (0.639–0.767) | < 0.01 |
Abbreviations: ROC, receiver-operating characteristics; AUC, area under the curve; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PLT, platelet; HGB, hemoglobin.
Next, serum levels of antibodies against a variety of antigens including anti-perinuclear anti-neutrophil cytoplasmic antibody (pANCA), anti-intestinal goblet cell antibody (GAB), anti-pancreatic exocrine gland antibody (PAB), anti-Saccharomyces cerevisiae cell antibody (ASCA), and anti- ANCA were examined in patients with IBD. As shown in Table 7, the percentage of positive anti-pANCA was significantly higher in UC patients (31.1%) compared to that in CD patients (4.8%, P<0.001). Meanwhile, the percentage of positive anti-GAB was significantly higher in patients with CD (23.1%) than that in patients with UC (14.9%, P = 0.037).
Table 7.
Positivity of Antibodies Against Various Antigens in Ulcerative Colitis and Crohn’s Disease
| Ulcerative Colitis (%) N=262 | Crohn’s Disease (%) N=147 | P value | |
|---|---|---|---|
| pANCA | 82 (31.3) | 7 (4.8) | <0.001 |
| GAB | 39 (14.9) | 34 (23.1) | 0.037 |
| PAB | 0 (0) | 5 (3.4) | |
| ASCA | 0 (0) | 4 (2.7) | |
| ANCA | 15 (5.7) | 2 (1.4) |
Abbreviations: pANCA, perinuclear anti-neutrophil cytoplasmic antibody; GAB, anti-intestinal goblet cell antibody; PAB, anti-pancreatic exocrine gland antibody; ASCA, anti-Saccharomyces cerevisiae cell antibody; ANCA, anti-neutrophil cytoplasmic antibodies.
Comparison of Medicines Used for IBD Treatment
For IBD, 5-ASA, steroids, immunomodulators, and humanized monoclonal antibody such as infliximab are often used. In addition, enteral nutrition is also used as supportive therapy. In the current study, as shown in Table 8, the majority of UC patients (90.5%) and over half of CD patients (51.2%) were treated with 5-ASA. Meanwhile, only a small percentage of UC patients were treated with infliximab (2.6%) or underwent surgery (1.9%), infliximab was used in 49.7% of CD patients and surgery was applied in 46.9% of CD patients. In addition, 23.4% of CD patients and only 4.7% of UC patients received enteral nutrition. The number of patients treated with steroid was similar in UC patients (23.3%) and CD patients (24.2%).
Table 8.
Summary of the Treatment for Ulcerative Colitis and Crohn’s Disease
| Medicine | Ulcerative Colitis (%) N = 430 | Crohn’s Disease (%) N = 286 |
|---|---|---|
| 5-ASA | 389 (90.5) | 146 (51.2) |
| Steroid | 100 (23.3) | 69 (24.2) |
| Immune suppressor | 6 (1.4) | 84 (29.4) |
| Infliximab | 11 (2.6) | 142 (49.7) |
| Enteral nutrition | 20 (4.7) | 67 (23.4) |
| Surgery | 8 (1.9) | 143 (46.9) |
Abbreviation: 5-ASA, 5-aminosalicylic acid.
Discussion
In this study, two types of IBD, UC and CD were retrospectively compared and analyzed in the following respects: symptoms and complications, laboratory parameters (ESR, CRP, platelet counts, and hemoglobin) at various statuses of disease activity, ROC curve and AUC, serum level of antibodies against specific antigens (pANCA, GAB, PAB, ASGA, and ANCA), and commonly used medicines for the treatment of IBD. It was found that male patients were predominant in both UC and CD, that the average age of patients with UC was significantly older than that of patients with CD. Furthermore, the symptoms of bloody stool, diarrhea, mucus in stool, and abdominal pain were more common in UC patients than those in patients with CD. In addition, patients with CD had a higher incidence of complications including intestinal obstruction, perianal lesion, bowel perforation, and fistula. Moreover, ESR, CRP and platelet counts were significantly increased in patients with moderately or highly active disease.Anti-pANCA antibody was higher in UC patients while anti-GAB antibody was higher in patients with CD. In addition, 5-ASA was commonly used as medical therapy for both UC and CD, and nearly 50% of CD patients were treated with infliximab or surgery.
IBD is a chronic, incurable disease with low mortality. While precise etiology of IBD is unknown, it is associated with endocrine abnormalities. In this regard, studies have demonstrated that IBD susceptibility, severity and progression were associated with the level of sex hormones.18–20 Consistently, the prevalence of UC and CD was significantly higher in male patients in this study. In addition, consistent with previous studies on age difference between CD and UC patients,21 the current study found that the average age of CD patients was younger than that of UC patients.
IBD patients may live with a considerable symptom burden and a high incidence of complications despite medical treatment. A variety of symptoms and signs are presented in patients with IBD, including abdominal pain, diarrhea, abdominal bloating, bloody stool, mucus stool, nausea, vomiting, fever, tenesmus, intestinal obstruction, and bowel perforation. In the current study, we found that bloody stool was the most common symptom in UC patients and abdominal pain was the most common symptom in CD patients. In addition, CD patients had a higher incidence of complications such as intestinal obstruction, perianal lesion, and fever than that in UC patients.
Laboratory parameters such as ESR, CRP and platelet counts have been studied extensively in IBD patients. However, the specificity and sensitivity of these parameters in assessing UC and CD are largely different. For instance, CRP, which is not widely used, was not as useful in UC as it was in CD for the assessment of disease activity.22–24 In the current study, we found that elevated ESR, CRP and platelet counts were more significantly associated with the activity of CD than that of UC. While albumin was not analyzed in this study, we found that hemoglobin level was negatively associated with IBD activity, and the prognosis of patients with low hemoglobin levels was poor following colectomy.25 In addition, ROC curve and AUC analysis of CRP in the current study indicated that the sensitivity and specificity of CRP were over 70% when the best threshold of CRP was set at 13.6 mg/L. Although none of the aforementioned laboratory markers is highly specific and sensitive, changes in these parameters in IBD might be useful in assisting diagnosis of UC and CD. In this regard, the guideline of the European Crohn’s and Colitis Organization (ECCO) suggests that in patients receiving parenteral steroids, CRP > 45 mg/L within 48 to 72 h following hospital admission for severe colitis together with three to eight stools a day is highly predictive for colectomy.12
In addition to the aforementioned laboratory parameters, serum biomarkers have been used for the diagnosis and differential diagnosis of IBD. In this regard, pANCAs and ASCAs have been widely investigated. Studies indicated that pANCAs were detected in up to 65% of the patients with UC and in less than 10% of the patients with CD.26–28 In the current study, in addition to pANCA and ASCA, the positivity of anti-GAB antibody and anti-pancreatic exocrine antibody (ANCA) in UC and CD patients was also analyzed. However, given the limited positivity of these serological biomarkers in UC (30% for pANCA) or CD (23.1% for GAB) patients, routine use of these biomarkers for the diagnosis of UC or CD is not suggested.
Endoscopic examination is the gold standard for the diagnosis of IBD as well as for surveillance of disease progression or remission. The most common endoscopic feature of UC is continuous, confluent colonic involvement with clear demarcation of inflammation and rectal involvement. The current study demonstrated that pan-colonic ulceration was the most common type of UC and typical features of UC such as friable colonic mucosa, spontaneous bleeding and ulcerations as well as crypt abscess were observed under endoscopy and microscopy; typical features of CD such as cobblestone appearance and longitudinal ulcer as well as noncaseating granulomas were observed in CD patients.
IBD treatment strategy largely depends on the severity of the disease and treatment history. Currently, the goal of IBD treatment is to achieve not only clinical remission but also macroscopic remission under endoscopy. The most commonly used medicines for IBD include 5-ASA, steroids, immune suppressors, and biologics. Studies also indicated that thiopurine and methotrexate could be used for maintenance of remission in CD,29,30 and that infliximab was effective in the treatment of perianal lesion such as fistulizing CD.31 In the current study, the majority of UC patients and over half of CD patients were treated with 5-ASA, and nearly half of the CD patients were treated with infliximab. Surgery is also considered if conservative treatment fails. Studies indicated that nearly half of IBD patients received surgical therapy within 10 years after diagnosis, one third of them underwent surgery multiple times, and 14% of the severe patients (especially with rectal involvement) had a permanent stoma.32 Consistent with previous reports, in the current study, nearly half of CD patients underwent surgery, while less than 2% of UC patients had surgery. In addition to medicine and surgery, enteral nutrition support is also crucial for CD patients, especially for patients with malnutrition or for the management of pediatric CD.33 In the current study, 23.4% of CD patients and 4.7% of UC patients received enteral nutrition support.
There are limitations in the current study. First, it has been reported that smoking was associated with IBD34 and a high risk for postoperative recurrence in CD.35 However, smoking history in IBD patients was not collected and analyzed in this study. Second, immunogenicity of infliximab in CD is an important cause for diminished clinical response, which may lead to discontinuation of treatment with infliximab.36,37 While nearly half of CD patients were treated with infliximab in this study, the level of anti-infliximab antibodies in these patients was not assessed. Third, postoperative recurrence in IBD remains a problem in these patients. In randomized trials, it has been reported that the rate of first year postoperative recurrence was 10–38%, while the rate of endoscopic recurrence in the first year after surgery was 35–85%.38 In this study, nearly half of CD patients went through surgical resection. However, postoperative recurrence rate in these patients was not analyzed.
Taken together, in the current study, we retrospectively compared UC and CD patients’ symptoms and complications, specificity and sensitivity of laboratory parameters including CRP, ESR, platelet and hemoglobin in assessing the activity of UC and CD, and commonly used medicines for IBD. It was found that IBD prevalence was higher in males compared to females; the average age of CD patients was younger than that of UC patients; bloody stool, mucus stool and diarrhea were more common in UC patients while abdominal pain and fever were more common in CD patients. ESR, CRP, platelet count and hemoglobin could be used for assessment of CD activity while for evaluating UC activity, CRP and platelet, but not ESR and hemoglobin, could be used as biomarkers. Furthermore, pANCA might be useful for UC diagnosis. In addition, 5-ASA was commonly used in both UC and CD patients, and steroids and enema were commonly used in UC patients while immunomodulator, biologics and enteral nutrition support were more commonly used in CD patients. The findings of the current may assist clinicians to differentially diagnose UC and CD and to treat IBD patients more efficiently in the future.
Funding Statement
No specific funding was received for this study.
Abbreviations
5-ASA, 5-aminosalicylates; CD, Crohn’s disease; CRP, C-reactive protein; ESR, Erythrocyte sedimentation; IBD, Inflammatory bowel disease; GAB, anti-intestinal goblet cell; pANCA, perinuclear anti-neutrophil cytoplasmic antibody; UC, ulcerative colitis.
Ethics Approval and Consent to Participate
The study was approved by the ethics committee of the Zhongnan Hospital of Wuhan University. All study participants gave written informed consent before being recruited.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roda G, Chien NS, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers. 2020;6(1):22. doi: 10.1038/s41572-020-0156-2 [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063 [DOI] [PubMed] [Google Scholar]
- 4.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 5.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150 [DOI] [PubMed] [Google Scholar]
- 6.Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013;28(7):1148–1153. doi: 10.1111/jgh.12164 [DOI] [PubMed] [Google Scholar]
- 7.Ng SC, Leung WK, Shi HY, et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis. 2016;22(8):1954–1960. doi: 10.1097/MIB.0000000000000846 [DOI] [PubMed] [Google Scholar]
- 8.Cohn HM, Dave M, Loftus EV Jr. Understanding the cautions and contraindications of immunomodulator and biologic therapies for use in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(8):1301–1315. doi: 10.1097/MIB.0000000000001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lonnfors S, Vermeire S, Greco M, Hommes D, Bell C, Avedano L. IBD and health-related quality of life – discovering the true impact. J Crohns Colitis. 2014;8(10):1281–1286. doi: 10.1016/j.crohns.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 10.Ekbom A. The changing epidemiology of IBD. In: Cohen RD, editor. Inflammatory Bowel Disease. Springer Science + Business Media; 2011:17–26. [Google Scholar]
- 11.Dave M, Loftus EV Jr. Mucosal healing in inflammatory bowel disease-a true paradigm of success? Gastroenterol Hepatol (N Y). 2012;8(1):29–38. [PMC free article] [PubMed] [Google Scholar]
- 12.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670. doi: 10.1093/ecco-jcc/jjx008 [DOI] [PubMed] [Google Scholar]
- 13.Olivera PA, Zuily S, Kotze PG, et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(12):857–873. doi: 10.1038/s41575-021-00492-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489–526. doi: 10.1007/s00535-021-01784-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JL, Tse F, Carroll MW, et al. Canadian association of gastroenterology clinical practice guideline for immunizations in patients with Inflammatory Bowel Disease (IBD)-Part 2: Inactivated vaccines. J Can Assoc Gastroenterol. 2021;4(4):e72–e91. doi: 10.1093/jcag/gwab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benchimol EI, Tse F, Carroll MW, et al. Canadian association of gastroenterology clinical practice guideline for immunizations in patients with inflammatory bowel disease (IBD)-Part 1: Live vaccines. J Can Assoc Gastroenterol. 2021;4(4):e59–e71. doi: 10.1093/jcag/gwab015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group IB, Chinese Medical Association. Beijing: Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). Chin J Dig. 2018;38(5):292–311. [Google Scholar]
- 18.Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-based differences in incidence of inflammatory bowel diseases-pooled analysis of population-based studies from Western Countries. Gastroenterology. 2018;155(4):1079–1089 e1073. doi: 10.1053/j.gastro.2018.06.043 [DOI] [PubMed] [Google Scholar]
- 19.Khalili H, Granath F, Smedby KE, et al. Association between long-term oral contraceptive use and risk of Crohn’s disease complications in a nationwide study. Gastroenterology. 2016;150(7):1561–1567 e1561. doi: 10.1053/j.gastro.2016.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortizo R, Lee SY, Nguyen ET, Jamal MM, Bechtold MM, Nguyen DL. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur J Gastroenterol Hepatol. 2017;29(9):1064–1070. doi: 10.1097/MEG.0000000000000915 [DOI] [PubMed] [Google Scholar]
- 21.Duricova D, Burisch J, Jess T, Gower-Rousseau C, Lakatos PL. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis. 2014;8(11):1351–1361. doi: 10.1016/j.crohns.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 22.Fagan EA, Dyck RF, Maton PN, et al. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur J Clin Invest. 1982;12(4):351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x [DOI] [PubMed] [Google Scholar]
- 23.Solem CA, Loftus EV Jr., Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707–712. doi: 10.1097/01.MIB.0000173271.18319.53 [DOI] [PubMed] [Google Scholar]
- 24.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029 [DOI] [PubMed] [Google Scholar]
- 25.Ho GT, Mowat C, Goddard CJ, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19(10):1079–1087. doi: 10.1111/j.1365-2036.2004.01945.x [DOI] [PubMed] [Google Scholar]
- 26.Riis L, Vind I, Vermeire S, et al. The prevalence of genetic and serologic markers in an unselected European population-based cohort of IBD patients. Inflamm Bowel Dis. 2007;13(1):24–32. doi: 10.1002/ibd.20047 [DOI] [PubMed] [Google Scholar]
- 27.Joossens S, Daperno M, Shums Z, et al. Interassay and interobserver variability in the detection of anti-neutrophil cytoplasmic antibodies in patients with ulcerative colitis. Clin Chem. 2004;50(8):1422–1425. doi: 10.1373/clinchem.2004.032318 [DOI] [PubMed] [Google Scholar]
- 28.Vermeire S, Peeters M, Vlietinck R, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm Bowel Dis. 2001;7(1):8–15. doi: 10.1097/00054725-200102000-00002 [DOI] [PubMed] [Google Scholar]
- 29.Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK, Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;10:CD000067. doi: 10.1002/14651858.CD000067.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N, Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;8:CD006884. doi: 10.1002/14651858.CD006884.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–885. doi: 10.1056/NEJMoa030815 [DOI] [PubMed] [Google Scholar]
- 32.Cosnes J, Bourrier A, Nion-Larmurier I, Sokol H, Beaugerie L, Seksik P. Factors affecting outcomes in Crohn’s disease over 15 years. Gut. 2012;61(8):1140–1145. doi: 10.1136/gutjnl-2011-301971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179–1207. doi: 10.1016/j.crohns.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 34.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. doi: 10.4065/81.11.1462 [DOI] [PubMed] [Google Scholar]
- 35.Vuitton L, Koch S, Peyrin-Biroulet L. Preventing postoperative recurrence in Crohn’s disease: what does the future hold? Drugs. 2013;73(16):1749–1759. doi: 10.1007/s40265-013-0128-x [DOI] [PubMed] [Google Scholar]
- 36.Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11(4):444–447. doi: 10.1016/j.cgh.2012.10.020 [DOI] [PubMed] [Google Scholar]
- 37.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348(7):601–608. doi: 10.1056/NEJMoa020888 [DOI] [PubMed] [Google Scholar]
- 38.Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012;35(6):625–633. doi: 10.1111/j.1365-2036.2012.05002.x [DOI] [PubMed] [Google Scholar]