Abstract
Obsessive-Compulsive Disorder (OCD) is a debilitating illness causing marked distress and functional impairment. While advances in behavioral and pharmacotherapies have been effective for a majority of patients with OCD, 10-30% remain treatment refractory and severely impaired. For a subset of treatment-resistant individuals with the most severe and disabling (intractable) illness, gamma ventral capsulotomy (GVC) appears effective in reducing OCD symptoms and functional impairment. However, the effects of the ventral internal capsule lesion via GVC surgery on executive function in everyday life have been minimally investigated. Examining behavioral outcomes of GVC also provides a rare opportunity to probe the functional importance of the ventral prefrontal-subcortical connections of the internal capsule white matter tract in a relatively homogenous sample of patients with comparable white matter lesions. The present study investigated changes in frontally-mediated behaviors, measured by the Frontal Systems Behavior Scale (FrSBe), following GVC in 45 individuals with severe and otherwise intractable OCD, as rated by patients themselves and family members. Linear mixed effects models revealed a significant improvement in patient self-ratings on the FrSBe after surgery, while family ratings did not significantly change. Interestingly, improvement on the FrSBe for both self and family raters was significantly correlated with improvement in OCD symptomatology post-surgery, as measured by the Yale-Brown Obsessive Compulsive Scale (Y-BOCS). At the group level, we found no evidence of decline in frontally-mediated behaviors assessed by the FrSBe as a result of focal white matter disconnection via GVC. However, we cannot rule out the possibility that placebo effects or compromised patient self-awareness or insight contributed to the significant improvement in self-ratings. Our measures may also have limited sensitivity to more selective impairments that could result from a small lesion to the ventral internal capsule. The present study demonstrates the need for detailed investigation of cognitive and behavioral changes as important factors when considering GVC as a viable treatment option for patients with refractory OCD.
Keywords: gamma ventral capsulotomy, frontal lobe functions, executive function, obsessive compulsive disorder, internal capsule lesion, ventromedial prefrontal cortex
1. Introduction
Obsessive-Compulsive Disorder (OCD) is a debilitating illness and is associated with significant disability worldwide (Mancebo et al., 2008; Veale & Roberts, 2014; World Health Organization, 2008, 2017). OCD is characterized by recurrent and persistent intrusive or unwanted thoughts, images, or urges (i.e., obsessions). These intrusions cause marked distress, and individuals may deploy specific behaviors or mental acts (i.e., compulsions) to resist obsessions or alleviate distress (American Psychiatric Association, 2013). Individuals with severe OCD can have considerable difficulty dismissing obsessions and disengaging from compulsions, often associated with significant functional impairment.
While advances in pharmacological and psychotherapeutics are effective for a majority of patients with OCD, 10-30% of patients are estimated to remain treatment refractory and severely functionally impaired (Hirschtritt, 2017; Husted & Shapira, 2004; Jakubovski et al., 2013; Lopes et al., 2004; McKay et al., 2015). In select cases of individuals with highly refractory OCD for whom minimal treatment alternatives remain, gamma ventral capsulotomy (GVC) has been shown to be effective in reducing OCD symptoms (D'Astous et al., 2013; McLaughlin et al., 2021; Miguel et al., 2019; Rasmussen et al., 2018).
Stemming from empirically derived methods (Greenberg et al., 2010; Rauch, 2003), surgical approaches in the extant literature have varied with regard to the specific target location within the region of the internal capsule, lesion size, methods used to induce lesions, and number of surgical operations conducted, unsurprisingly leading to heterogeneity of findings. In contrast to older ventral capsulotomies that ablated the entire internal capsule in the coronal plane, modern gamma ventral capsulotomy procedures target the ventral subregion (bottom ½ to ⅓) of the anterior limb of the internal capsule via gamma knife stereotactic radiosurgical techniques, focusing on ventral cortico-subcortical connections thought most important to improvement of OCD symptomatology (Lopes et al., 2004; Rasmussen et al., 2018). Similar procedures using radiofrequency thermolesions or laser ablations have also been performed on the same target location (McLaughlin et al., 2021; Nyman & Mindus, 1995; Rück et al., 2008), resulting in OCD symptom reduction. However, there is inter-individual variability in the latency of clinical response, with reports of clinically meaningful change ranging from 6-36 months post-surgery (Miguel et al., 2019; Rasmussen et al., 2018), indicating the importance of multiple long-term follow-ups when evaluating the consequences of GVC for clinical response and daily functioning. Nonetheless, prior studies report OCD symptom improvement following GVC and related surgical procedures, with variable reports of 37-80% of individuals demonstrating full treatment response (typically characterized as ≥ 35% reduction in the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score) at varying long-term follow-up intervals (Brown et al., 2016).
The internal capsule is a bidirectional white matter pathway with the anterior limb containing projection fibers connecting the thalamus, caudate, and ventral striatum to frontal cortex. Specifically, the ventral subregion contains fibers from ventromedial prefrontal (vmPFC), lateral (lOFC) and medial orbitofrontal (mOFC), and anterior cingulate (ACC) cortices (Lehman et al., 2011), while the dorsal subregion includes white matter connections to the dorsolateral prefrontal cortex (dlPFC). Abnormal connections via white matter microstructure of the cingulum bundle and anterior limb of the internal capsule have been reported in OCD relative to healthy comparisons (Cannistraro et al., 2007). By targeting the ventral subregion of the anterior limb of the internal capsule, GVC aims to disrupt aberrant fronto-subcortical circuitry implicated in the maintenance of OCD symptomatology (Greenberg et al., 2010; Pauls et al., 2014), while likely sparing more dorsal connections between subcortical structures and dlPFC. Functional magnetic resonance imaging (fMRI) studies in individuals with OCD corroborate these findings by demonstrating hyperactivity of cortico-striatal pathways in the brain at rest, including orbitofrontal and anterior cingulate cortices (Greenberg et al., 2010; Harrison et al., 2009).
While there is evidence of therapeutic benefit in reducing OCD symptoms (D'Astous et al., 2013; Gong et al., 2018; McLaughlin et al., 2021; Miguel et al., 2019; Rasmussen et al., 2018), neuropsychological outcomes are less well described. The few extant capsulotomy studies of neuropsychological function primarily report preserved cognitive and intellectual functioning (Batistuzzo et al., 2015; Csigó et al., 2010; Gong et al., 2018; Taub et al., 2009), with modest evidence of domain-specific improvements in learning and memory, visuospatial skills, and reasoning (Batistuzzo et al., 2015; Gong et al., 2018; Taub et al., 2009), though mixed findings are reported in the domain of executive functioning (Batistuzzo et al., 2015; Csigó et al., 2010; Gong et al., 2018; Nyman et al., 2001; Nyman & Mindus, 1995; Rück et al., 2008; Taub et al., 2009).
Executive functions, or higher-order cognitive abilities such as inhibition, switching, organization, planning, and mental flexibility rely on the integrity of neural structure and function among frontal and distributed brain areas specifically encompassing ACC, OFC, and vmPFC (Dias et al., 1996; Fellows & Farah, 2003; Mansouri et al., 2020; Rudebeck et al., 2017; Rudebeck et al., 2013), regions directly disconnected as a result of GVC. Executive function outcomes following GVC have been inconsistent (Batistuzzo et al., 2015; Csigó et al., 2010; Gong et al., 2018; Nyman et al., 2001; Nyman & Mindus, 1995; Rück et al., 2008; Taub et al., 2009) and are likely confounded by small sample sizes as well as heterogeneity of patient sample characteristics, lesion methodologies, measures employed, and follow-up time periods. Older studies of samples with more extensive capsular lesions have reported post-surgical declines in task-based measures of executive function (e.g., Wisconsin Card Sorting Test (WCST)) and evidence of apathy, disinhibition, and dysexecutive behaviors despite improved OCD symptoms (Nyman & Mindus, 1995; Rück et al., 2008). While recent studies using the more modern, circumscribed lesion employed here have generally reported no change (Batistuzzo et al., 2015; Nyman et al., 2001; Taub et al., 2009) or improved performance (Csigó et al., 2010; Gong et al., 2018) on task-based measures of executive functioning following GVC, mild declines in select cases were also reported (Csigó et al., 2010; Taub et al., 2009).
Nevertheless, the broader behavioral consequences of lesioning this tract connecting multiple regions of ventral prefrontal cortex to deep subcortical targets remain unclear. The sole study that has directly investigated frontal lobe behaviors in daily life including apathy, disinhibition, and dysexecutive behaviors after capsulotomy for OCD reported either stability or greater dysfunction upon follow-up, though greater dysfunction was found in patients with larger lesions of the internal capsule (Rück et al., 2008). Notably, when informant report was available for patients with treatment refractory OCD, discordant frontal behavior ratings between informant- and self-report have led some studies to question the role of patient insight (Batistuzzo et al., 2009; Rück et al., 2008), posing a challenge to interpreting measures that are dependent on self-report. In fact, in patients with more extensive frontal lobe damage due to non-traumatic acquired insult (e.g., aneurysm, tumor), discrepancies between self- and informant-report were found such that patients underestimated their impairments relative to informant ratings (Hoerold et al., 2013). Other work has found that damage to the ventromedial frontal lobe caused by stroke, tumor, or traumatic brain injury resulted in reduced self-awareness, impaired insight (Barrash et al., 2000; Beer et al., 2006), and a reduction in accuracy of metacognitive judgments (Fleming et al., 2014; Janowsky et al., 1989). Abundant fMRI evidence also indicates that vmPFC is specially involved in self-referential processing (Denny et al., 2012; Qin & Northoff, 2011), and metacognitive processes such as decision confidence and mentalizing (Vaccaro & Fleming, 2018). Taken together, these data raise concern about how disconnection of ventromedial prefrontal regions from subcortical targets might impact insight and self-awareness of GVC surgical patients, and thereby influence their ability to assess their own functioning.
As the consequences of GVC on executive function and other frontal lobe symptoms remain ambiguous, testing the effects of this procedure on frontal behaviors with well-validated measures is of both clinical and basic scientific significance. Examining this population is also likely to be informative for understanding how frontal white matter tracts support executive functions, given the rarity of focal deep white matter lesions that connect several regions of ventral, orbital, medial, and lateral frontal cortex with thalamus and striatum. Whereas most studies of the behavioral consequences of white matter lesions rely on stroke and brain tumor patients where damage is diffuse and heterogeneous (Foulon et al., 2018; Gleichgerrcht et al., 2017; Urbanski et al., 2008), GVC provides a rare opportunity to investigate the functional importance of a white matter tract in a relatively homogenous niche population (i.e., all patients with treatment refractory OCD) with an extremely focal and consistent white matter lesion. As such, we attempt to address both the clinically relevant impact of GVC on daily functioning in OCD, as well as the broader role of focal white matter disconnection on brain and behavior relationships from a cognitive neuroscience perspective.
The present study investigated changes in behaviors associated with frontally-mediated neural circuitry following gamma ventral capsulotomy (GVC) in individuals with treatment refractory OCD. We used the Frontal Systems Behavior Scale (FrSBe) to evaluate longitudinal behavior change in frontal lobe functions following GVC. The FrSBe has been shown to be an ecologically valid and sensitive measure of changes in frontally-mediated behaviors (including dysexecutive behavior, disinhibition, and apathy) following damage to the frontal lobes across a range of populations (Grace & Malloy, 2001; Grace et al., 1999), including within a non-surgical treatment refractory OCD sample (Batistuzzo et al., 2009). In addition, the FrSBe enables us to explore the role of patient insight by comparing patient self-report with informant reports when evaluating change in frontal lobe behaviors. In analyses of these data, we will (1) examine how family and self-reported ratings of everyday frontal lobe functions change after GVC, (2) clarify associations of OCD symptomatology with daily frontal lobe functioning before and after GVC, and (3) assess whether an objective neuropsychological measure of executive function, the Wisconsin Card Sorting Task (WCST; Heaton, 1993), relates to self- and family-rated daily frontal lobe behaviors.
We hypothesize that if GVC causes frontal lobe dysfunction, patient and family members will rate frontally-mediated behaviors as worsened post-surgery. If GVC impacts patient insight and awareness, we expect that patients will underestimate their impairments relative to informants, and that family behavior ratings will correlate more strongly than self ratings with performance on a task-based measure of executive functioning as well as with OCD symptom severity.
2. Materials and methods
2.1. Participants and study design
The analyses reported here were carried out by authors MTK, OL, ARV, and DB on data shared with them by co-authors NCRM and SAR. The authors carrying out these analyses (MTK, OL, ARV, DB) were not involved in the surgical procedures, design of this study, collection of data or in the treatment and care of enrolled patients and are independent of the group using these procedures as part of patient care. Here, we provide details regarding the study population, surgery, design, and data collection procedures that formed the analyzed dataset that was shared. Detailed information about participants and study design have been previously described by Rasmussen et al. (2018).
Over a 20-year period, a cohort of 55 adult patients (mean age = 33.6, SD = 10.5) with treatment refractory OCD underwent either single shot repeated (n=15) or double shot (n=40) GVC (detailed in section 2.2 Radiosurgical Procedure) (Rasmussen et al., 2018). A subset of patients completed the FrSBe and neuropsychological measures including the WCST at baseline and across multiple follow-up time points. Primary clinical outcomes, detailed sample characteristics, and study eligibility criteria are previously reported by Rasmussen et al. (2018). In brief, patients with a formal diagnosis of OCD for at least 5 years were eligible for GVC only if they were considered treatment-refractory, defined by failure to respond (i.e., continued severe to extreme OCD symptoms) to multiple adequate trials of pharmacological and psychotherapeutic interventions per consensus guidelines (Nuttin et al., 2014). Additional eligibility criteria included a baseline Y-BOCS score of ≥ 28, severe impairment in social and occupational functioning, and ability to provide informed consent (see Rasmussen et al., 2018 for complete description of inclusion/exclusion criteria for GVC surgical candidacy). Potential candidates were each assessed by a psychiatrist (SAR or BDG), neurologist, and neuropsychologist (PFM or NCRM) to obtain a comprehensive psychiatric history via direct interview with the patient, patient family, and treatment providers, as well as review of all available patient records. Candidate suitability was reviewed by an independent board whose members included an ethicist, two psychiatrists, a minister, a lawyer, a nurse, and a member of the National Alliance for the Mentally Ill (Rasmussen et al., 2018).
Of the 55 patients who underwent GVC, 48 had at least one FrSBe assessment (see Table 1). Of these 48, three patients who received the single shot repeated procedure had FrSBe assessments only in between the first and second GVC surgeries, thus no valid pre- or post-surgical assessments were available. The FrSBe assessment data from these three patients were therefore excluded, given the lack of clarity to interpretation if combined with patient data following completed single shot repeated or double shot surgeries. For the remaining 45 patients, 31 completed a pre-surgical self-assessment, and 28 had pre-surgical assessments rated by a family member or close associate if no family member was available (i.e., family-assessment). Twenty-nine patients had at least one post-surgical self-assessment and 23 patients had at least one post-surgical family-assessment. Twenty patients had self-assessments both pre- and post-surgery, while 14 patients had pre- and post-surgical family-assessments. Ten patients had both self- and family-rated assessments pre- and post-surgery; however, one patient did not have sufficient items completed for adequate calculation of a FrSBe total score, per criteria outlined in the FrSBe manual (Grace & Malloy, 2001). Thus, a total of 9 patients had complete data for both self and family FrSBe ratings before and after surgery. A notable limitation, the FrSBe was under development and not yet published when data collection for the present study began in 1993 at the same site (Butler Hospital) and was less consistently administered as part of the general clinical battery. Follow-up assessments were conducted approximately 6, 12, 24 and 60 months after the initial surgery. In certain cases, follow-up assessments were also completed between 5-10 years or after 10 years post-surgery.
Table 1.
Number of patients with self- and family-rated FrSBe assessments at each time point
| Pre-surgical Baseline |
Any Available Post-surgical Follow-up* |
Both Pre-surgery and Any Post-surgical Follow-up |
|
|---|---|---|---|
| Self | 31 | 29 | 20 |
| Family | 28 | 23 | 14 |
| Both Self and Family | 20 | 19 | 9 |
Patients had at least 1 FrSBe assessment collected at any of the post-surgical follow-up visits (typically 6, 12, 24, and 60 months, with occasional longer term following beyond 5 years).
Note. 9 patients had both self- and family-rated assessments pre- and post-surgery.
2.2. Radiosurgical procedure
Details of the radiosurgical procedures are described by Rasmussen et al., 2018: The first 37 patients were treated with the Leksell Gamma Knife model U, while the remainder were treated with the model C. Per the initial study hypothesis that a single bilateral lesion in the anterior limb of the internal capsule would be equally efficacious to previously studied larger lesions, 15 patients received a single shot GVC procedure in the initial study phase. One patient demonstrated significant improvement and did not require a second procedure. A second patient withdrew from the study after experiencing no improvement following the first shot. Due to the lack of response to the first procedure, the remaining 13 patients completed a second-stage procedure (single shot repeated). For the single shot repeated group (n=13), in the coronal plane, the initial bilateral single target was located centrally in the capsule, one-third of the distance dorsally from the capsule’s most ventral extension and in the axial plane adjusted so the posterior part of the 20% isodose line transected the genu of the capsule. The second stage target (single shot repeated) was defined immediately ventral to the first stage shot in the center of the capsule. Individual variation in the anatomy of the anterior limb of the internal capsules as they coursed through the corpus striatum necessitated minor alterations in targeting. The 40 patients subsequently enrolled were treated with two shots bilaterally in one session (double shot), targeted to cover the ventral third of the anterior capsule/ventral striatum, that were consistently placed 8 to 10 mm anterior to the posterior border of the anterior commissure (see Figure 1). For all treatments in all patients, the radiation dose used was 180Gy to the maximum (100%) at the center of the target with a prescription dose of 90Gy to the 50% isodose line intended to correspond to the periphery of the volume of necrosis (lesion) created by the treatment. A collimator size of 4 mm was used in all cases. Target locations and details about the distribution of lesion locations are available in Rasmussen et al. (2018). For the purpose of this study, data collected between the first and second surgeries for single shot repeated patients were excluded from all analyses. For these patients, analyses used only the measures completed before the first surgery to represent pre-surgical data and only measures completed after the second surgery (single shot repeated) as post-surgical measurement references. Consistent with standard treatment protocols, patients continued to receive behavioral and pharmacological intervention post-surgery.
Figure 1.
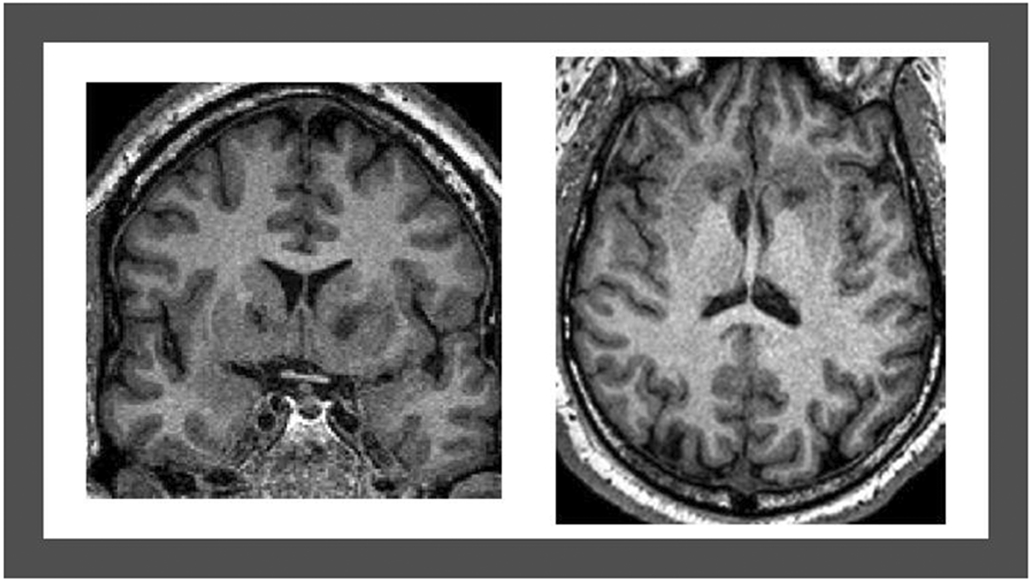
T1-weighted magnetic resonance imaging acquired 12 months post-surgery showing double shot GVC bilateral lesions of the ventral portion of the internal capsule, visualized in the coronal (left) and axial (right) planes.
2.3. Measures
2.3.1. Frontal Systems Behavior Scale
The Frontal Systems Behavior Scale (FrSBe) is an internally reliable, validated brief rating scale designed to measure behaviors associated with frontal lobe damage (Grace & Malloy, 2001; Grace et al., 1999; Stout et al., 2003). Two complementary forms include a self-rated assessment for patient report and an analogous form rated by family members for collateral report. Each scale consists of 46 items describing behaviors that can be judged based on their frequency of occurrence (e.g., ‘Says one thing, then does another’). Participants rate each item on a 5-point Likert scale indicating the frequency of each behavior. After reverse scoring relevant items, a total score (range 46-230) is computed by taking the sum of the individual item scores; the scale can also be parsed into three subscales comprised of specific items: apathy (14 items), disinhibition (15 items) and executive dysfunction (17 items). Scoring procedures stipulate that accurate calculation of the measure cannot be completed if ≥ 5 items comprising the total or ≥ 4 items from a given subscale have missing ratings (Grace & Malloy, 2001). Standardized T-scores for the FrSBe total and subscales were computed to adjust for patient age and education level at assessment, patient gender, and assessment rater (self or family), based on normative data from a sample of healthy individuals (Grace & Malloy, 2001). Higher T-score values indicate greater impairment. Standardized T-scores for the FrSBe total and subscales were used in all analyses.
Participants received either the current version of this scale (FrSBe), or a previous version (Frontal Lobe Personality Scale (FLOPS); (Grace et al., 1999)) earlier in the course of this study. While the specific items and content are unchanged, the versions of the scale differ principally in format with modified item order and coding direction for a subset of items. Thus, all items were reordered and re-coded where necessary, to match the current FrSBe scale version. Two research assistants manually entered the paper assessments into a computer software program and each item was double-entered to reconcile potential entry errors.
2.3.2. Wisconsin Card Sorting Test
Patients were administered the 128-card version of the Wisconsin Card Sorting Test (WCST) (Heaton, 1993). In this task, patients are asked to sort cards according to an undisclosed abstract rule that changes over the course of administration. Patients receive deterministic feedback about whether their response for each card is correct according to the current prevailing rule, and therefore must flexibly adjust to examiner feedback provided with each trial. Perseverative errors on the WCST have been shown to index frontal lobe dysfunction (Dias et al., 1996; Gläscher et al., 2019; Milner, 1963) and serve as a measure of interest in the current study. Perseverative errors indicate a persistence on a response set within a particular sorting principle for which the patient has already received negative feedback. Higher perseverative errors are hypothesized to result from inflexibility and failures to shift attentional set, reduced sensitivity to negative feedback, or failures of response inhibition. The number of perseverative errors was selected as a measure of behavioral flexibility, which was converted to a normed standard score, with higher standard scores indicating better performance or fewer perseverative errors. Additionally, given that reduced concept formation has been suggested as a possible neurocognitive endophenotype highlighting the need to explore more nuanced measures of executive function in OCD (Abramovitch et al., 2021), and particularly within a sample demonstrating largely intact performance at pre-surgical baseline, the WCST learning-to-learn raw score was also selected to index conceptual learning efficiency. The learning-to-learn score measures average change in learning efficiency across consecutive categories, with positive scores indicating better performance or fewer percent errors made over time within a given category, presumably reflecting greater conceptual learning efficiency.
2.3.3. Yale-Brown Obsessive Compulsive Scale
The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) is a 10-item validated measure of symptom severity in OCD (Goodman et al., 1989b; Goodman et al., 1989a). The scale is clinician-rated, based on an interview with the patient and family member(s) if available. Each item is rated on a scale of 0 (no symptoms) to 4 (extreme symptoms). A total score reflecting the degree of OCD symptom severity is calculated by taking the sum of the 10 items (range 0-40).
2.3.4. Hamilton Depression Rating Scale
The Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960) is a widely used, well-validated, clinician-rated measure of depressive symptom severity. Items rated on a scale of 0 (absence of symptoms) to 4 (extreme symptoms) are summed into a total score with higher total scores indicating greater depressive symptom severity.
2.4. Statistical Analyses
A series of linear mixed effects (LME) models were conducted in MATLAB (Mathworks, Natick, MA) to assess the effects of GVC surgery on self- and family-rated frontal lobe behaviors (FrSBe) and their association with performance on a neuropsychological measure of executive function (WCST) as well as with obsessive-compulsive symptom severity (Y-BOCS). For all models, the most complex model with parameters for all possible random effects was first attempted; however, if this model could not converge, a simpler model was fit to the data with random effects that could not be estimated removed from the model. Standardized T-scores for the FrSBe total and subscales were used in all analyses. Analyses exclusively evaluating change in self- and family-ratings were performed on FrSBe total scores and each subscale (apathy, disinhibition, executive dysfunction). For simplicity, only total FrSBe scores were plotted in the figures, except for the effect of surgery on FrSBe ratings in patients with complete data (i.e., Figure 3).
2.4.1. Effect of GVC Surgery on Self and Family Rated Frontal Lobe Functions
An initial LME model examined the effect of surgery on frontal lobe functions within the subset of patients for whom pre- and post-surgery data were available from both self and family raters for the same individual (n=9). FrSBe T-scores constituted the dependent variable. Predictors entered into the model included categorical variables for surgery (pre- or post-), rater (self or family), and the interaction of surgery and rater, allowing for direct examination of within-subjects effects of rater by surgery in this patient subsample. This LME model was constrained to the first follow-up after surgery (mean days after surgery = 274, SD = 90, range = 155 - 405), for which the most data were available.
Given the limited number of patients with complete data, a subsequent LME analysis sought to contextualize these subsample analyses within the entire dataset and leveraged all available FrSBe assessment data, including self and family ratings, both pre- and post-surgery, regardless of whether multiple corresponding time points or raters were available for a given patient. Surgery (pre- or post-), rater (self or family), and their interaction were entered as predictors of FrSBe T-scores, with number of days before and after surgery (relative date) included as a covariate. Although this allowed for use of data from all patients with FrSBe assessments at any time point, many individuals had FrSBe ratings either only at a pre- or post-surgical visit and both self and family ratings were not always available for a given time point. As such, within-subjects comparisons of rater by surgery in these analyses of the entire sample are difficult to interpret at the individual patient level.
Given these limitations on our ability to interpret within-subjects comparisons of rater by surgery due to inconsistent FrSBe assessments available in the entire patient sample, two distinct LME models using all available data for self- and family-rated assessments were conducted in order to investigate the effect of surgery on self and family FrSBe ratings separately, and included relative date as a covariate.
Finally, to examine whether patient and family members had similar evaluations of patient behavior pre- and post-surgery, a Pearson’s correlation coefficient between self- and family-rated FrSBe total scores was calculated both pre- and post-surgery, and aggregating data over pre- and post-surgery. For each patient, each self rating was matched with a corresponding family rating from the same assessment date. If no exact date match could be found, we matched it with the closest family rating in time, so long as the ratings were less than 1 year apart. Since Pearson’s correlation assumes all data points are independent, all post-surgery assessments for a given patient were averaged to yield a single post-surgery score per patient. In order to account for multiple samples from a given patient, we also fit a LME model that predicts self ratings as a function of family ratings, with random slopes for family ratings, grouped by patient. Finally, an additional LME was conducted to test for change in the self and family relationship after surgery. Self ratings constituted the dependent variable, while family ratings, surgery, and the interaction between surgery and family ratings were included as predictors, with random slopes for family ratings grouped by patient.
2.4.2. Self and Family Rated Frontal Lobe Functions and Task-based Executive Function
To examine the relationship between self- and family-rated frontal lobe function with performance on a task-based measure of executive function, two distinct LME models were calculated using either self- or family-rated FrSBe total scores to predict perseverative errors on the WCST. In each LME model, FrSBE total scores, surgery, and their interaction were entered as predictors of perseverative errors on the WCST. Additionally, two analogous LME analyses were also conducted to predict the WCST learning-to-learn score and similarly included as predictors the FrSBe total scores (self- or family-assessments entered separately), surgery, and their interaction.
2.4.3. Self and Family Rated Frontal Lobe Functions and OCD Symptom Severity
Two linear mixed-effects models were also used to assess the relationship between self- and family-rated frontal lobe functioning (FrSBe) with OCD symptom severity (Y-BOCS), both pre- and post-surgery. The first LME was performed on FrSBe self ratings, while the second LME was performed on family ratings. Y-BOCS total score was the dependent variable, with the following predictors: FrSBe total scores from either self- or family-assessments, surgery, and the interaction of surgery with FrSBe total score.
2.4.4. Frontal Lobe Functions and Depressive Symptoms
To ensure that changes in frontal lobe functions after surgery were not potentially driven by changes in depressive symptoms, we conducted a control analysis to account for the potential contribution of depression severity (HDRS) on changes in FrSBe ratings pre- and post-surgery. LME models included the following predictors of FrSBe scores: HDRS scores, surgery, and the interaction of surgery with HDRS scores.
2.4.5. Multiple Comparisons Correction
The threshold for statistical significance was corrected for multiple comparisons within each specific hypothesis using the Bonferroni method. Given the potential for deleterious effects of surgery on frontally-mediated behaviors (FrSBe) and the need for sensitive analyses within a potentially vulnerable population in which any statistically significant effects might be of great clinical significance and thus should not be ignored, primary analyses of the effects of surgery on FrSBe total scores were not corrected for multiple comparisons. These primary analyses include the effect of surgery and rater on FrSBe total scores in the 9 patients with complete data (Table 3, Model 1) and in the full patient sample (Table 3, Model 2), as well as the effect of surgery on self ratings (Table 3, Model 3) and family ratings (Table 3, Model 4) in the full sample. Additional tests of the effects of surgery on FrSBe subscales were corrected for multiple comparisons (resulting in a significance level of alpha = 0.05 / 3 = 0.017 for the three subscales). Analyses assessing potential changes in insight in the 9 patients with complete data, including FrSBe Item 13 and differences between self and family ratings for total scores and subscales, were corrected for the 5 tests performed (alpha = 0.05 / 5 = 0.01). Pearson correlations between self and family FrSBe ratings (Results section 3.3) were corrected across two time points (before and after surgery), yielding alpha = 0.05 / 2 = 0.025. LME models testing whether surgery changed the correlation between self and family ratings (Table 3, Model 5) were corrected across total scores and three subscales, yielding alpha = 0.05 / 4 = 0.0125.
For analyses examining associations between FrSBe ratings and WCST performance, multiple comparisons corrections were applied within rater for the two WCST performance variables evaluated (perseverative errors and learning-to-learn), resulting in a significance level of alpha = 0.05 / 2 = 0.025. Analyses of self and family FrSBe ratings associated with Y-BOCS scores were treated as separate hypothesis tests; however, additional analyses of the relationships between Y-BOCS with FrSBe subscales were corrected for multiple comparisons (alpha = 0.05 / 3 = 0.017, for three subscales), as were Pearson correlations between FrSBe total scores and Y-BOCS scores across multiple timepoints (alpha = 0.05 / 3 = 0.017, for three time points). For control analyses accounting for depression severity (HDRS) in statistical tests of the effects of surgery on FrSBe ratings, multiple comparisons correction was applied across the three FrSBe subscales (alpha = 0.05 / 3 = 0.017 for three subscales). In the Results section, the Bonferroni-corrected significance threshold is indicated whenever multiple comparisons corrections were performed, except when the uncorrected effects were not significant to begin with.
3. Results
3.1. Patient Sample Characteristics
To assess the degree of clinically significant frontal lobe behavioral symptoms patients exhibited before and after surgery, the mean FrSBe T-scores and percentage of patients in the clinically elevated range (T-score ≥ 65) at baseline and across multiple post-surgical follow-up time points are reported in Table 2. Of note, the precise patients represented at each time-point differ depending on the availability of assessment data. At baseline, more than half of patients exhibited FrSBe total scores in the clinically elevated range, based on both self (61.3%) and family (57.1%) ratings; baseline sample mean performance on the WCST was intact in the broad average range (5.6% impaired on perseverative errors, 11.8% of scores were <16th percentile on learning-to-learn); and 78.9% of patients exhibited comorbid depressive symptomatology at baseline in at least a moderate severity range, HDRS total ≥ 20 (Table 2).
Table 2.
Longitudinal assessment of patient frontal lobe function and OCD symptomatology
| Scale | Pre-surgical baseline |
6-month follow-up |
1-year follow-up |
2–4-year follow-up |
≥ 5-year follow-up* |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Systems Behavior Scale (FrSBe) | |||||||||||
| Self | Family | Self | Family | Self | Family | Self | Family | Self | Family | ||
| Total | T-score (mean, SD) | 71.87 (14.50) | 71.07 (17.53) | 60.23 (12.92) | 67.00 (15.54) | 64.80 (16.66) | 62.77 (16.71) | 57.11 (21.31) | 55.00 (16.69) | 67.50 (19.42) | 61.06 (16.80) |
| % elevated (n elevated/total) | 61.3 (19/31) | 57.1 (16/28) | 29.4 (5/17) | 66.7 (8/12) | 53.3 (8/15) | 46.1 (6/13) | 33.3 (3/9) | 30.0 (3/10) | 50.0 (3/6) | 33.3 (3/9) | |
| AP | T-score (mean, SD) | 85.06 (17.32) | 81.36 (18.72) | 76.35 (19.13) | 79.33 (18.72) | 77.13 (24.12) | 73.83 (25.18) | 66.67 (24.66) | 66.80 (21.13) | 73.11 (23.90) | 66.55 (21.73) |
| % elevated (n elevated/total) | 93.5 (29/31) | 78.6 (22/28) | 76.5 (13/17) | 75.0 (9/12) | 66.67 (10/15) | 58.3 (7/12) | 44.44 (4/9) | 40.0 (4/10) | 50.0 (3/6) | 44.4 (4/9) | |
| DI | T-score (mean, SD) | 59.90 (13.85) | 51.46 (14.50) | 48.53 (12.24) | 47.25 (16.44) | 54.37 (14.19) | 47.23 (11.19) | 51.33 (17.58) | 42.20 (8.32) | 57.94 (12.84) | 48.28 (16.55) |
| %elevated (n elevated/total) | 35.4 (11/31) | 10.7 (3/28) | 11.8 (2/17) | 16.7 (2/12) | 31.2 (5/16) | 7.7 (1/13) | 22.2 (2/9) | 0.0 (0/10) | 33.3 (2/6) | 11.1 (1/9) | |
| ED | T-score (mean, SD) | 65.42 (13.46) | 70.29 (17.36) | 56.29 (10.43) | 69.42 (16.63) | 59.06 (12.40) | 61.00 (15.50) | 52.89 (16.26) | 55.60 (18.37) | 64.89 (19.26) | 63.56 (14.24) |
| % elevated (n elevated/total) | 51.6 (16/31) | 60.7 (17/28) | 17.6 (3/17) | 66.7 (8/12) | 37.5 (6/16) | 46.1 (6/13) | 33.3 (3/9) | 40.0 (4/10) | 50.0 (3/6) | 33.3 (3/9) | |
| Wisconsin Card Sorting Test (WCST) | |||||||||||
| PE | Standard score (mean, SD) | 101.31 (21.94) | 104.22 (20.30) | 105.83 (23.68) | 108.25 (27.02) | 99.40 (15.36) | |||||
| % impaired (≤ 2 SD) (n impaired/total) | 5.6 (2/36) | 5.6 (1/17) | 11.1 (2/18) | 8.3 (1/12) | 0.0 (0/10) | ||||||
| LL | Raw score (mean, SD) | −0.80 (2.77) | −0.82 (4.15) | 0.52 (2.08) | −2.00 (4.20) | −1.11 (5.87) | |||||
| Yale-Brown Obsessive Compulsive Scale (Y-BOCS) | |||||||||||
| Total | (mean, SD) | 33.60 (3.93) | 22.68 (7.87) | 18.63 (6.93) | 14.29 (7.78) | 15.55 (10.72) | |||||
| % elevated (total ≥ 28) (n impaired/total) | 94.7 (36/38) | 21.0 (4/19) | 5.3 (1/19) | 0.0 (0/12) | 11.1 (1/9) | ||||||
| Hamilton Depression Rating Scale (HDRS) | |||||||||||
| Total | (mean, SD) | 25.66 (8.25) | 21.06 (11.00) | 16.21 (9.43) | 13.50 (7.20) | 12.78 (10.99) | |||||
| % elevated (total ≥ 20) (n elevated/total) | 78.9 (30/38) | 61.11 (11/18) | 31.6 (6/19) | 25.0 (3/12) | 33.33 (3/9) | ||||||
Includes averaged values for 1 patient with multiple assessments in ≥ 5 years.
Note. AP = Apathy, DI = Disinhibition, ED = Executive Dysfunction subscales of the FrSBe; PE = perseverative errors, LL = learning-to-learn on the WCST. Clinical elevation threshold on FrSBe is T-Scores ≥ 65 for total and subscale scores.
3.2. Effect of GVC surgery on Self and Family Rated Frontal Lobe Functions
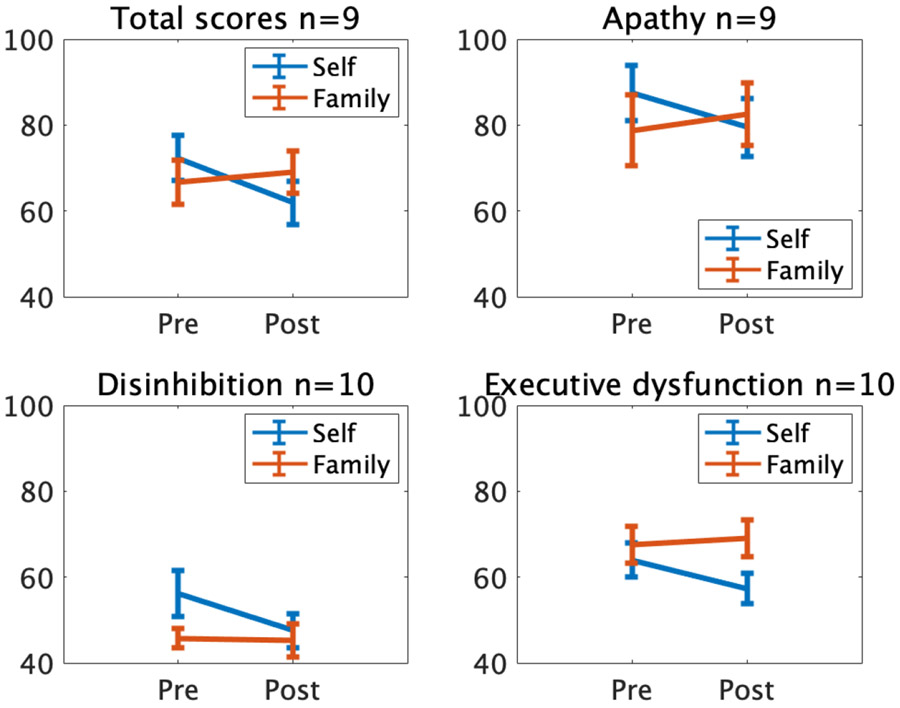
Given that many patients within the broader sample did not have corresponding pre- and post-surgical assessments from both self- and family raters, an initial LME analysis was performed with the 9 patients for whom within-subjects comparisons were possible, as both self and family ratings assessed at baseline and post-surgical follow-up were available for these patients. The analysis was constrained to the first follow-up after surgery available in all 9 patients. This allowed for direct within-subjects comparison of self and family ratings pre- and post-surgically. For total scores, a significant surgery (pre vs. post) by rater (self vs. family) interaction was found, t (32) = −2.04, p = .049, suggesting that the effect of surgery on self-ratings was significantly different from the effect of surgery on family ratings (Table 3, Figure 2). No main effects of rater or surgery were evident (all p > 0.5). While subscale analyses revealed a similar pattern of results qualitatively, the interaction between surgery and rater was not significant for any of the FrSBe subscales: apathy, t (32) = −1.86, p = 0.07; disinhibition, t (36) = −1.74, p = 0.09; executive dysfunction, t (36) = −1.61, p = 0.12 (Figure 2). Additionally, no significant main effects of surgery or rater were observed for the FrSBe subscales (all p > .05).
Table 3.
Linear Mixed Effects results: Effect of surgery on self and family rated FrSBe total scores
| Analysis | t (df) | p | Beta | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| FrSBe Ratings | |||||
| Model 1: Rater by Surgery (within-subjects, n=9) | |||||
| Main Effect of Surgery | 0.50 (32) | .617 | 1.17 | −3.54 | 5.88 |
| Main Effect of Rater | −0.15 (32) | .885 | −0.72 | −10.79 | 9.35 |
| Interaction of Surgery by Rater | −2.04 (32) | .049 | −6.39 | −12.76 | −0.02 |
| Model 2: Rater by Surgery* | |||||
| Main Effect of Surgery | −1.77 (148) | .078 | −7.4 | −15.65 | 0.85 |
| Main Effect of Rater | 0.054 (148) | .957 | 0.22 | −7.77 | 8.21 |
| Interaction of Surgery by Rater | −0.30 (148) | .765 | −1.35 | −10.26 | 7.56 |
| Model 3: Self Ratings* | |||||
| Main Effect of Surgery | −3.29 (77) | .002 | −9.64 | −15.47 | −3.80 |
| Model 4: Family Ratings* | |||||
| Main Effect of Surgery | −1.86 (70) | .068 | −8.55 | −17.74 | 0.63 |
| Model 5: Self: Family by Surgery* | |||||
| Main Effect of Family Ratings | 1.37 (49) | .178 | 0.25 | −0.12 | 0.62 |
| Main Effect of Surgery | −0.54 (49) | .593 | −7.46 | −35.36 | 20.43 |
| Interaction of Family by Surgery | 0.13 (49) | .895 | 0.03 | −0.37 | 0.42 |
Includes all patients across all time points with available data.
Note. FrSBe = Frontal Systems Behavior Scale; CI = Confidence Interval. Model 1: Effect of surgery (pre vs. post) and rater (self vs. family) on FrSBe ratings in 9 patients with both self and family ratings pre-surgery and at first post-surgical follow-up; LME formula: TotalScore ~ 1 + Rater*Surgery + (1 + Rater + Surgery ∣ PatientID). Model 2*: Effect of surgery (pre vs. post) by rater (self vs. family) on FrSBe ratings; LME formula: TotalScore ~ 1 + Date + Rater*Surgery + (1 + Rater*Surgery ∣ PatientID) + (Date ∣ PatientID). Model 3*: Effect of surgery (pre vs. post) on FrSBe self-ratings; LME formula: SelfTotalScore ~ 1 + Date + Surgery + (1 ∣ PatientID). Model 4*: Effect of surgery (pre vs. post) on FrSBe family-ratings; LME formula: FamilyTotalScore ~ 1 + Date + Surgery + (Date ∣ PatientID) + (1 + Surgery ∣ PatientID). Model 5*: Effect of Family ratings and surgery (pre vs. post) on Self-ratings; LME formula: SelfTotal ~ 1 + FamilyTotal*Surgery + (1 + FamilyTotal ∣ PatientID). Relative date (number of days before or after surgery) was included as a covariate in models 2, 3 and 4.
Figure 2.
Mean FrSBe scores at baseline and the first follow-up after surgery, for the 10 patients for whom within-subject comparisons were possible. Error bars represent standard errors of the mean across the 10 patients. One of the patients had too many missing items in the Apathy subscale, which also prevented calculation of a Total Score for that patient (n=9).
This interaction between rater and surgery indicated that patient ratings of frontal lobe symptoms differed from those of their family members after surgery. As this could be a sign of a failure of patient insight into their own behavior after surgery, we sought to more specifically test if surgery was associated with a change in patient insight after surgery within this subset of patients where within-subject comparison of self and family ratings pre- and post-surgery was possible (n=9). We first examined item 13 of the FrSBe (‘Denies having problems or is unaware of problems or mistakes’), which we reasoned would more explicitly measure patient insight and awareness. As these FrSBe item 13 scores were not normally distributed, we calculated the difference between post- and pre-surgery ratings for both self and family raters and submitted the difference scores to a non-parametric Wilcoxon signed rank test. The test revealed a significant effect, such that family ratings of FrSBe item 13 increased after surgery, whereas self ratings did not (see Supplementary Figure 1), though this did not survive multiple comparisons correction (p = 0.03 uncorrected; corrected alpha = 0.01). In other words, family members rated patients as more unaware of deficits after surgery. We also compared the difference in family and self-rated FrSBe total scores in this same subset of patients at baseline and in first post-surgery follow-up. While there was a trend toward patients rating themselves as less impaired compared to family member ratings, this was not statistically significant (Within-subjects t-test: t (8) = 1.93, p = 0.09; Supplementary Figure 2). Similarly, there were no differences in this measure for the FrSBe subscales (all t ≤ 1.75, p ≥ 0.1, uncorrected). Thus, within this subset of patients (n=9), there were only trending effects to indicate a deficit in patient insight.
In order to quantify and contextualize the effect of surgery on FrSBe ratings within the entire patient sample, we examined all available data across all time points, regardless of whether a pre- and post-surgery self- and family-rated assessment were each available for a given patient. LME models including all longitudinal FrSBe assessment data were performed, with surgery (pre vs. post), rater (self vs. family), and their interaction to predict FrSBe scores for total scale and subscales separately. For total scores, results revealed no main effect of surgery or rater, nor an interaction of surgery by rater (all p > .05; Table 3, Figure 3). A similar pattern of results was evident for the apathy and executive dysfunction subscales, (all p > .05); however, a main effect of rater (self vs. family) was observed for the disinhibition subscale (t(149) = 3.22, p = 0.002), such that patients rated themselves as significantly more disinhibited than family member ratings, which remained significant after correction for multiple comparisons (corrected alpha = 0.017 for the three subscales).
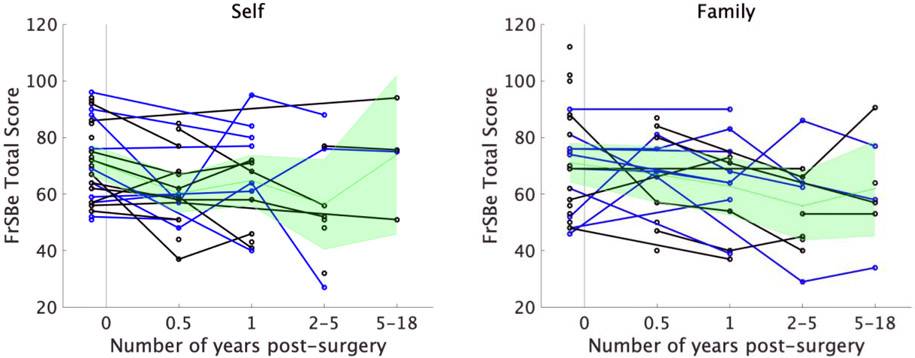
Figure 3.
Change in self-rated (left panel) and family-rated (right panel) FrSBe total scores over time for each patient. The 9 patients who had both self and family ratings pre- and post-surgery are represented with blue lines. For visualization purposes only, when multiple assessments were available within a time bin for a given patient, the assessments were averaged to yield a single score per patient per time bin. The mean and 95% confidence intervals are depicted in green.
Given the previously noted discrepancy between self and family ratings post-surgery in the subsample of 9 patients with complete data, we wanted to ensure that any potential effects of surgery in the full patient sample were not obscured by including both self and family raters in the same model. To investigate this, two separate LME models for self and family ratings were computed. All patients and time points were included in these models regardless of whether they had both pre- and post-surgical ratings. A linear mixed effects model with surgery as a predictor of self-rated FrSBe total scores, and number of days post-surgery as a covariate, revealed a significant decrease in self-ratings after surgery, t(77) = −3.29, p = .0015 (Table 3, Figure 2). Similarly, the reduction in self-ratings after surgery was significant for the apathy (t(77) = −2.51, p = 0.014), disinhibition (t(78) = −3.01, p = 0.0035), and executive dysfunction (t(78) = −2.84, p = 0.006) subscales, after correction for multiple comparisons (corrected alpha = 0.017 for the three subscales). In contrast to self ratings, an analogous LME model for family-rated FrSBe total scores indicated no significant difference in family ratings as a result of surgery, t(70) = −1.86, p = 0.068 (Table 3, Figure 3). Examination of the FrSBe subscales similarly indicated no significant change in family ratings for apathy t(69) = −1.23, p = 0.22; disinhibition: t(70) = −1.46, p = 0.15; and executive dysfunction: t(70) = −1.62, p = 0.11. Taken together, patients rated themselves as having significantly improved frontal lobe behaviors after GVC surgery, whereas no significant change in patient frontal lobe functions was apparent based on collateral reports of family members.
3.3. Association between Self and Family Ratings of Frontal Lobe Functions
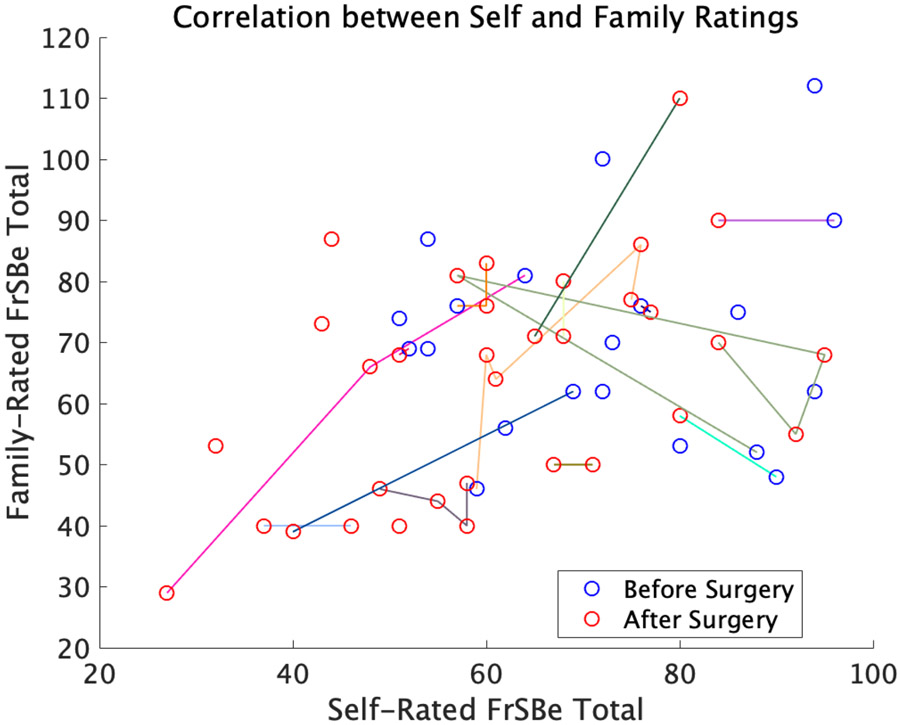
Given the significant effect of surgery on self, but not family, FrSBe ratings, we assessed whether self and family ratings became decorrelated after surgery. If the improvement in self-reports after surgery was due to lack of insight, then we hypothesized that self-ratings would become decorrelated from family ratings after surgery. Instead, for total scores, we found no correlation between self and family ratings at baseline (r = 0.09, p = 0.71, n = 20), and a significant positive correlation between self and family ratings after surgery (r = 0.51; p = 0.02, n = 33; Figure 4; corrected alpha = 0.025 for the two time points tested). In fact, the correlation between self and family ratings across all data points, both before and after surgery, was significant at r = 0.35, p = 0.03, n = 53. This pattern of results was qualitatively similar across subscales, with the correlation between self and family ratings numerically higher after surgery compared to before surgery.
Figure 4.
Correlation between self and family FrSBe ratings across all time points, r = 0.35, p = 0.03. Pre-surgical assessments are depicted with blue circles (Pearson’s r = 0.09, p = 0.71, n = 20), while post-surgery assessments are depicted with red circles (Pearson’s r = 0.51; p = 0.02, n = 33). Samples from the same patient over time are connected by colored lines.
To test whether GVC surgery significantly changed the relationship between self and family ratings, we fit a LME model to predict self ratings as a function of family ratings, surgery, and the interaction between surgery and family ratings. There was no significant interaction between surgery and family ratings on self ratings for total scores or any of the subscales (total scores: t(49) = 0.13, p = 0.90; apathy: t(49) = 1.93, p = 0.06; disinhibition: t(50) = −1.67, p = 0.10; executive dysfunction: t(50) = 0.19, p = 0.85; Table 3). Thus, we did not find evidence that the GVC surgery changed the relationship between self and family ratings.
3.4. Self and Family Rated Frontal Lobe Functions and Task-based Executive Function
We next tested whether self or family-rated FrSBe total scores were associated with performance on the WCST, a task-based measure of frontal lobe function (Milner, 1963; Nyhus & Barceló, 2009). Results of separate LME models for self- and family-rated FrSBe total scores revealed no significant effects of FrSBe total scores on perseverative errors in the WCST, nor an interaction between FrSBe total scores and surgery (all p > .05; Table 4). Results of analogous LMEs to predict WCST learning-to-learn scores were likewise not significant for FrSBe total scores, surgery, or their interaction (all p > .05; Table 4). Thus, there was no association between FrSBe ratings and WCST perseverative errors or learning-to-learn scores.
Table 4.
Linear mixed effects results: Association between WCST perseverative errors and learning-to-learn with self and family rated FrSBe scores
| Analysis | t (df) | p | Beta | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| FrSBe Ratings to predict WCST-PE | |||||
| Self FrSBe Ratings | |||||
| Main Effect of Surgery | 1.90 (70) | .062 | 31.8 | −1.66 | 65.26 |
| Main Effect of FrSBe Ratings | 1.64 (70) | .105 | 0.37 | −0.08 | 0.82 |
| Interaction of Surgery by FrSBe Ratings | −1.57 (70) | .120 | −0.36 | −0.81 | 0.10 |
| Family FrSBe Ratings | |||||
| Main Effect of Surgery | 0.004 (62) | .997 | 0.06 | −36.54 | 36.67 |
| Main Effect of FrSBe Ratings | −0.88 (62) | .380 | −0.20 | −0.65 | 0.25 |
| Interaction of Surgery by FrSBe Ratings | −0.26 (62) | .792 | −0.07 | −0.59 | 0.45 |
| FrSBe Ratings to predict WCST-LL | |||||
| Self FrSBe Ratings | |||||
| Main Effect of Surgery | −1.04 (66) | .304 | −3.53 | −10.34 | 3.28 |
| Main Effect of FrSBe Ratings | 0.82 (66) | .414 | 0.03 | −0.05 | 0.11 |
| Interaction of Surgery by FrSBe Ratings | 1.36 (66) | .179 | 0.07 | −0.03 | 0.16 |
| Family FrSBe Ratings | |||||
| Main Effect of Surgery | 0.80 (58) | .429 | 2.42 | −3.66 | 8.51 |
| Main Effect of FrSBe Ratings | 1.68 (58) | .098 | 0.06 | −0.01 | 0.12 |
| Interaction of Surgery by FrSBe Ratings | −0.95 (58) | .346 | −0.04 | −0.12 | 0.04 |
Note. FrSBe = Frontal Systems Behavior Scale; WCST-PE = Wisconsin Card Sorting Test perseverative errors; WCST-LL = Wisconsin Card Sorting Test learning-to-learn; CI = Confidence Interval.
3.5. Self and Family Rated Frontal Lobe Functions and OCD Symptom Severity
We next assessed the relationship between frontal lobe behavior ratings and obsessive-compulsive symptom severity. Including all patients and time points, two distinct LME models were conducted for self and family ratings, with FrSBe total scores, surgery, and their interaction as predictors of Y-BOCS scores.
The LME model for self-rated FrSBe scores revealed a significant main effect of surgery on Y-BOCS (t(72) = −6.07, p < .0001), and a significant interaction of surgery with FrSBe total scores (t(72) = 3.91, p = .0002), such that the correlation between self-rated FrSBe and Y-BOCS was significantly stronger after surgery (Table 5). Subscale results similarly demonstrated significant main effects of surgery on Y-BOCS and interactions of surgery with FrSBe scores for apathy (t(72) = 3.12, p = .003), disinhibition (t(72) = 2.73, p = .008), and executive dysfunction (t(72) = 3.93, p < .001), corrected for multiple comparisons (alpha = 0.017 for the three subscales).
Table 5.
Linear mixed effects results: Association between OCD symptom severity (Y-BOCS) and self and family rated FrSBe scores
| Analysis | t (df) | p | Beta | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| FrSBe Ratings to predict Y-BOCS | |||||
| Self FrSBe Ratings | |||||
| Main Effect of Surgery | −6.07 (72) | <.0001 | −35.87 | −47.64 | −24.09 |
| Main Effect of FrSBe Ratings | −0.61 (72) | .543 | −0.04 | −0.18 | 0.09 |
| Interaction of Surgery by FrSBe Ratings | 3.91 (72) | <.001 | 0.33 | 0.16 | 0.50 |
| Family FrSBe Ratings | |||||
| Main Effect of Surgery | −5.06 (65) | <.0001 | −29.05 | −40.51 | −17.59 |
| Main Effect of FrSBe Ratings | 0.91 (65) | .368 | 0.06 | −0.07 | 0.19 |
| Interaction of Surgery by FrSBe Ratings | 2.70 (65) | .009 | 0.23 | 0.06 | 0.40 |
Note. FrSBe = Frontal Systems Behavior Scale; Y-BOCS = Yale-Brown Obsessive-Compulsive Scale; CI = Confidence Interval.
The LME model for family-rated FrSBe total scores similarly yielded a significant main effect of surgery on Y-BOCS scores (t(65) = −5.06, p < .0001) and a significant interaction of surgery with family-rated FrSBe scores, such that FrSBe and Y-BOCS scores were significantly correlated after surgery (t(65) = 2.70, p = .009; Table 5). These results were qualitatively similar for each of the subscales, although the interaction between surgery and family-rated FrSBe did not survive multiple comparisons correction (corrected alpha = .017).
When comparing the two separate models, the overlap of the 95% confidence intervals (CI) for the interaction between surgery and FrSBe self (95% CI: 0.16 - 0.50) and family (95% CI: 0.06 - 0.40) ratings suggest that the relationship between Y-BOCS and FrSBe did not differ for self compared to family ratings. The same was evident in each of the subscales.
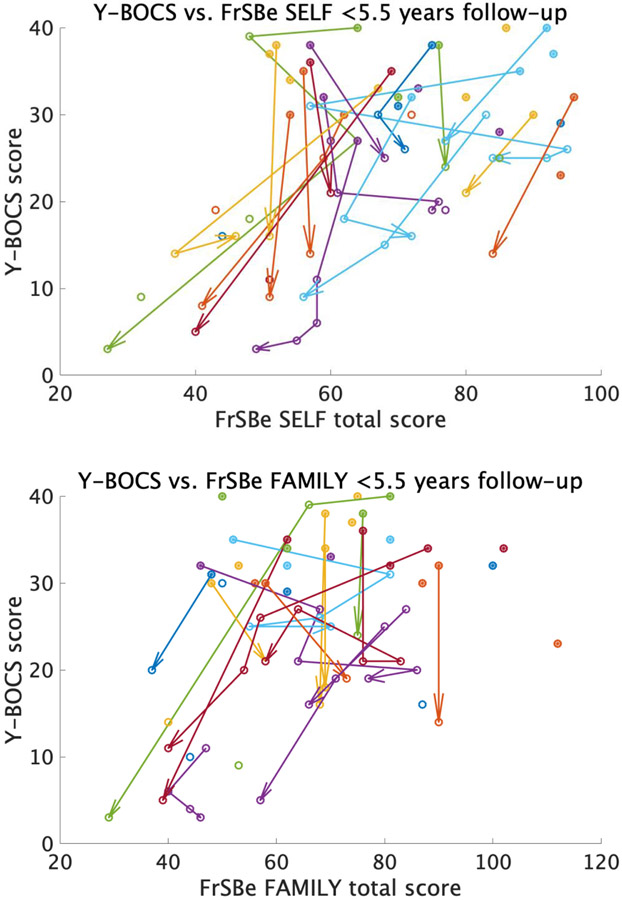
Post hoc Pearson’s correlations were subsequently computed to evaluate the association of FrSBe ratings and Y-BOCS scores at distinct time windows (pre-surgery, <1.5 years and 1.5-5.5 years post-surgery). For patients who had both Y-BOCS and FrSBe scores at baseline, no significant correlation between the two measures was detected, either for self-rated (r = −0.20, p = .30, n = 30) or family-rated (r = −0.13, p = .51, n = 28) FrSBe scores (Figure 5A). However, within 1.5 years after surgery, there was a significant positive correlation between Y-BOCS and self-rated FrSBe scores (r = 0.53, p = .01, n = 23), though only a non-significant positive relationship for family ratings (r = 0.29, p = .25, n = 18; Figure 5B). For subsequent follow-ups (between 1.5-5.5 years) the positive correlation between Y-BOCS and FrSBe scores was significant for both self (r = 0.82, p < .01), and family (r = 0.71, p = .01, n = 11; Figure 5C) ratings (corrected alpha = 0.017 for the three time points tested). The significant correlation between family-rated FrSBe and Y-BOCS after surgery in the mixed-effects model, but not in the simple Pearson’s correlations for the 1.5-year follow-up, may be due to the ability of the mixed effects model to capture within-subject effects. In fact, while Figure 5B shows a non-significant correlation between Y-BOCS and FrSBe family ratings across patients, Figure 6 shows that the correlation within patients over time was significant, such that as Y-BOCS scores decreased over time for a given patient, FrSBe self and family ratings tended to decrease as well.
Figure 5.
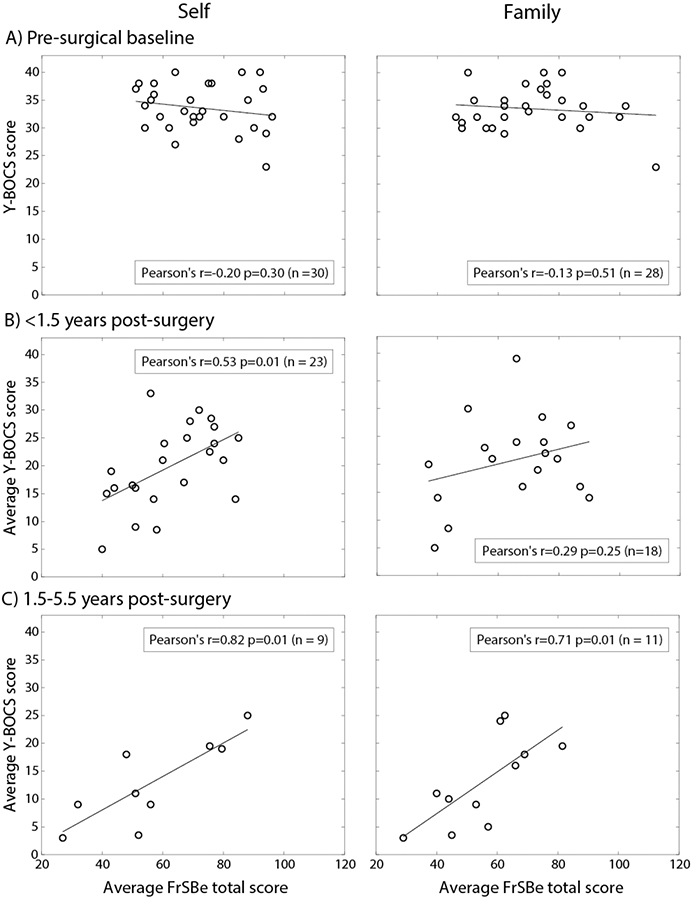
Correlation between Y-BOCS and FrSBe scores at (A) baseline, (B) <1.5 years after surgery, and (C) 1.5 - 5.5 years after surgery, for self (left column) and family (right column) ratings. When multiple assessments were performed within a time window, they were averaged to yield a single score per patient.
Figure 6.
Within- and across-patient correlation between Y-BOCS and FrSBe total scores for both self (top) and family (bottom) ratings. Filled-in circles represent scores at baseline, while empty circles represent post-surgery scores. The arrows show within-patient change over time.
Exploratory analyses were conducted to assess whether baseline FrSBe was a predictor of change in Y-BOCS after surgery. The relationships between pre-surgical self- and family-rated FrSBe scores with percent change in Y-BOCS were assessed using Pearson’s correlations for total and subscale scores. Lower pre-surgical self-rated disinhibition (r = 0.36, p = .05) and FrSBe total scores (r = 0.36, p = .05) were marginally associated with greater reduction in Y-BOCS scores post-surgery; however, these effects did not survive correction for multiple comparisons (corrected alpha = 0.0125 for the total and 3 subscale tests; see Supplementary Figure 3). No such effects were found for family ratings (see Supplementary Figure 4).
In sum, while Y-BOCS and FrSBe scores were not correlated at baseline, significant positive correlations emerged after surgery for both self and family ratings, indicating that those patients who had reduced OCD symptom severity post-surgery also showed improvement on both self-rated and family-rated daily behaviors associated with frontal lobe function.
3.6. Frontal Lobe Functions and Depressive Symptoms
Given the frequent comorbidity of depressive symptoms with OCD (Lochner et al., 2014; Pallanti et al., 2011) and high comorbidity rate observed in our sample, additional analyses were performed to ensure that the significant reduction in self-rated FrSBe impairments after surgery were not solely driven by a concomitant reduction in depression. As such, we examined whether the reduction of self-rated FrSBe scores after surgery remained significant when accounting for depression severity in the model. Including all patients and time points, an LME model with HDRS scores, surgery, and their interaction were entered as predictors of FrSBe self-rated total scores. Results revealed a main effect of surgery (t(70) = −3.14, p = .002) and a significant interaction of surgery by HDRS depression scores (t(70) = 2.77, p = .007) such that the correlation between self-rated FrSBe and HDRS increased after surgery and no main effect of HDRS was found (Table 6; Supplementary Figure 5) together suggesting that changes in depression severity as a result of surgery did not account for the improved self-ratings on the FrSBe post-surgery. Notably, the same pattern of results was evident for the apathy (main effect of surgery, t(70) = −2.50, p = .015; interaction of surgery by HDRS, t(70) = 2.70, p = .009; no main effect of HDRS, t(70) = 1.17, p = .245) and executive dysfunction (main effect of surgery, t(70) = −2.70, p = .009; interaction of surgery by HDRS, t(70) = 2.22, p = .030; no main effect of HDRS, t(70) = 0.76, p = .448) subscales, similarly indicating that improvements in self-rated apathy and executive dysfunction were not accounted for by reduced depression severity post-surgery (corrected alpha = 0.017 for the three subscales). No significant findings were observed for the disinhibition subscale (no main effect of surgery, t(70) = −1.90, p = .062; no interaction of surgery by HDRS, t(70) = 0.67, p = .504; no main effect of HDRS, t(70) = 0.62, p = .539).
Table 6.
Linear mixed effects results: Effect of surgery on self-rated FrSBe scores when accounting for depression symptom severity
| Analysis | t (df) | p | Beta | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Self FrSBe Total Ratings | |||||
| Main Effect of Surgery | −3.14 (70) | .002 | −23.90 | −39.08 | −8.71 |
| Main Effect of HDRS | 0.92 (70) | .360 | 0.23 | −0.27 | 0.73 |
| Interaction of Surgery by HDRS | 2.77 (70) | .007 | 0.82 | 0.23 | 1.41 |
Note. FrSBe = Frontal Systems Behavior Scale; HDRS = Hamilton Depression Rating Scale; CI = Confidence Interval.
4. Discussion
The present study sought to investigate changes in behaviors associated with frontally-mediated neural circuitry following gamma ventral capsulotomy (GVC) in individuals with treatment refractory OCD. We compared patient self-reported ratings of frontal lobe functions with reports of family members using the FrSBe scale. We also examined whether self and family ratings of frontal lobe behaviors predicted performance on the Wisconsin Card Sorting Test (WCST), a task-based measure of executive functioning. Finally, we investigated the association between OCD symptomatology and frontal lobe behaviors. Our results indicated that GVC did not cause worsening of frontally-mediated behaviors in daily life, as measured by the FrSBe. Rather, patients rated themselves as exhibiting less frontal behavior dysfunction post-surgically, even after accounting for changes in depression severity. This self-reported improvement in frontally-mediated behaviors was related to decreased obsessive-compulsive severity. OCD symptom severity improved after surgery, consistent with prior studies (D'Astous et al., 2013; McLaughlin et al., 2021; Miguel et al., 2019; Rasmussen et al., 2018). However, family ratings of frontal lobe functions (FrSBe) and performance on a task-based measure of executive function (WCST) did not change significantly following surgery. In summary, we did not find evidence that focal ablation to the anterior limb of the internal capsule had deleterious effects on frontally-mediated behaviors in patients with treatment refractory OCD. We did, however, find a difference in self-reported versus family-reported frontal lobe functioning. That is, we found a significant difference in the effect of surgery on self-ratings compared to family-ratings in the subsample of patients for whom both self and family ratings were available before and after surgery. We consider the implications of this observation below.
First, this discrepancy between the effects of surgery on self and family ratings was only evident in a subset of patients. In the full sample, family ratings of frontal lobe dysfunction also trended toward improvement after surgery and over the long term. However, fully characterizing this interaction was only possible in this subset of patients who had complete data. Further, as this discrepancy points to the potential for change in insight or awareness following GVC, we believe that continued research into these effects is essential. Multiple interpretations to account for this difference in self and family ratings after surgery are plausible and we consider each in turn below.
One possibility is that patients may perceive an improvement in their behavior due to a desire for the treatment to work, akin to a placebo effect. However, a subset of patients who were initially treated with only a single shot of gamma radiation hypothesized to be efficacious showed no change in Y-BOCS scores after this surgery, and thus did not appear to demonstrate a placebo effect (Rasmussen et al., 2018). Additionally, using an identical double shot GVC surgical procedure as in our study, Lopes et al. (2014) also found that improvements in Y-BOCS scores were greater after GVC for treatment-refractory OCD compared to a sham control group. Thus, a placebo effect influencing patients’ perception of improvement in their behavior is an unlikely explanation.
Two additional accounts of this difference in self and family ratings may relate to functional implications of the deep frontal white matter connections targeted by this surgery, though additional work will be required to distinguish between these accounts as the present study unfortunately lacks sufficient data and specificity of measures administered to comprehensively address potential changes in patient insight and awareness. One possibility is that patients have diminished insight into their behavior and daily functioning. Indeed, fMRI studies of metacognitive processing and self-awareness have heavily implicated ventromedial prefrontal cortex (Denny et al., 2012; Qin & Northoff, 2011; Vaccaro & Fleming, 2018), and frontal lobe damage, particularly in the ventromedial aspect, has been associated with impairments of insight (Barrash et al., 2000; Beer et al., 2006; Wilson, 1996). Prior work in a non-surgical sample of treatment refractory OCD has shown a similar pattern of discordant patient self and family-ratings, with family members reporting greater frontally-mediated behavior dysfunction relative to patient self-report cross-sectionally (Batistuzzo et al., 2009). Notably, self and family ratings did not differ in the healthy comparison group, leading the authors to conclude that treatment-refractory OCD patients may have limited insight into their behaviors (Batistuzzo et al., 2009). In contrast to that study, our data did not show a difference between self and family ratings at baseline, suggesting that limited insight was not a likely factor pre-surgically. Rather, the ratings only differed post-surgically, with a decrease in the self ratings and no change in the family ratings. Rück and colleagues (2008) similarly described discrepancies between patients’ subjective evaluation of improvement in daily functioning and the report of patient relatives; however, methodological concerns such as retrospective collection of behavioral and functional measures notably limit interpretation of their findings.
While our findings in the full sample largely argue against the interpretation that patient insight decreased after surgery, the evidence in the subset of patients with complete data is mixed and therefore this possibility warrants consideration. In this patient subset, analysis of the sole FrSBe item that directly probes patient insight and awareness indicated a decrement after surgery that was detectable by family members, but not by patients. However, this effect did not survive multiple comparisons correction. Additionally, direct comparison of the discrepancy of patient and family ratings of the full FrSBe scale in this subset also trended toward patients perceiving less frontal behavior dysfunction post-surgery compared to family members, though this result was not statistically significant. Previous studies have found that lack of insight correlates with other frontal lobe deficits, particularly disrupted socio-emotional functioning in patients with vmPFC lesions (Barrash et al., 2021; Barrash et al., 2018). Given this pattern, there would be reason to suspect that a loss of patient insight might also be associated with evidence of worsening frontal lobe symptoms in family ratings on the FrSBe. However, in our study, given that family members did not report decline in patient frontal lobe behaviors overall, it is difficult to cite reduced patient insight stemming from frontal damage as the primary driver of the change in self ratings. Moreover, if patients entirely lacked insight after surgery, we would expect either no correlation or a negative correlation between self and family ratings. Instead, we found a significant positive correlation between self and family ratings after surgery. As mentioned above, performance remained stable on the WCST, a task-based measure of executive functioning, though this finding may likely be associated with intact dlPFC connection in the more focal ablative procedure used in our study. Further, OCD symptom severity measured by the Y-BOCS improved after surgery. Improvement on the Y-BOCS was highly correlated with improvement in self-reported frontal behavior ratings, which suggests that these ratings tapped into a general improvement in functioning. Of note, the Y-BOCS is clinician-rated based on a patient interview, and it is thus possible that clinician ratings reflect a general sense of improvement conveyed by patients without measurable behavioral improvements in everyday life. However, the correlation observed between Y-BOCS and family ratings of frontal lobe behaviors renders this interpretation less likely and may instead indicate that the two measures tracked genuine improvement in behavior assessed by clinicians and family members post-surgery.
Considering the above, it is possible that self-ratings reflect a genuine improvement in frontally-mediated behaviors, and that the lack of immediate improvement in family-ratings results from less sensitivity of family members to patients’ daily behavioral functioning, as some of the items on the FrSBe scale are more self-referential and may not be accessible to family members (e.g., ‘Is interested in sex’). This may have led to a delay in family members detecting improvement, resulting in the non-significant trend (p < .068) toward improved frontal lobe functioning in family ratings, observed in our full sample. While all three accounts are possible, the evidence is less consistent with a change in patient insight or a placebo effect for the entire sample, and seems more consistent with reduced sensitivity of family members relative to self reports. Nonetheless, given the findings in the subset of patients with complete data we cannot confidently rule out that patient insight is not limited as a consequence of GVC.
In addition to comparing self and family ratings of frontal lobe behaviors via the FrSBe, we also examined the association between the FrSBe ratings and OCD symptom severity measured by the Y-BOCS. We found a significant correlation between Y-BOCS and FrSBe ratings after surgery, but no correlation at baseline. The lack of a correlation at baseline may have been due to the restricted range of most Y-BOCS scores being at ceiling in this sample based on inclusion criteria, thereby reducing the variance and limiting detection of a correlation. Batistuzzo et al. (2009) did not observe a correlation between FrSBe and Y-BOCS scores in a non-surgical, albeit similar, refractory OCD sample. Akin to our sample at baseline, ceiling effects on Y-BOCS in their sample may have also limited the ability to detect a correlation with FrSBe scores. However, it is also possible that the Y-BOCS and FrSBe scales capture different aspects of pathology and that these two measures only become correlated after surgery because patients and family members are reporting general improvement in functioning. A similar pattern was observed for correlations between FrSBe and depressive symptoms measured by the HDRS, which became significant only after surgery, lending further support to this interpretation. In addition, exploratory analyses showed that lower self-rated FrSBe total and disinhibition scores at baseline marginally correlated with greater reduction in Y-BOCS symptom severity after surgery; however, these effects did not survive multiple comparisons correction. Further replication of these results in a separate sample is needed to draw conclusions about the value of baseline FrSBe as a predictor of obsessive-compulsive symptom improvement following ventral capsulotomy.
Finally, we found no correlation between either self or family behavior ratings and performance on the WCST, as measured by the number of perseverative errors and the learning-to-learn score. The lack of a correlation between behavior ratings that probe executive function in daily life and the task-based WCST may not be surprising, since these measures from the WCST are only a small component of the types of behaviors the FrSBe questionnaire is designed to assess. In fact, neuropsychological tests of executive functioning have limited ecological validity in predicting questionnaires that measure real-world behaviors and daily functioning (Burgess et al., 2006; Chan et al., 2008; Chaytor & Schmitter-Edgecombe, 2003; Chaytor et al., 2006), suggesting that they capture different sources of variance.
Additionally, in our sample, we did not find significant impairment in WCST performance either before or after surgery. Prior meta-analyses in non-surgical OCD samples with varying levels of severity have found lower performance on multiple task-based measures of executive function relative to healthy comparisons, although findings of impairment in WCST performance have been mixed (Shin et al., 2013; Bora 2020; Abramovitch et al., 2013). However, the surgical sample in the current study likely differs from the majority of OCD samples recruited from the general population for comparison with healthy controls on task-based measures of cognition.
Past work has found evidence of significant frontal lobe dysfunction and personality change following capsulotomy in a subset of patients (Mindus et al., 1999; Rück et al., 2008). Notably, the lesions in these studies were larger and more dorsal within the internal capsule compared to those in the GVC procedure described in our study (Miguel et al., 2019; Rasmussen et al., 2018). Likely, the procedures that created larger lesions caused damage within fibers originating from dlPFC and lOFC — whereas the GVC procedure used here, where the lesions were smaller and more ventral, likely primarily affected fibers originating from vmPFC and mOFC (Miguel et al., 2019; Rasmussen et al., 2018). Furthermore, prior studies demonstrating performance decline on the WCST after GVC surgery were also limited by larger capsular lesions (Nyman & Mindus, 1995; Rück et al., 2008). Given that our patient sample was not impaired on WCST at baseline, these findings of stable performance with dlPFC connections intact suggest that prior reports of post-surgical decline in task-based measures of executive function were likely related to disrupted dlPFC connections due to larger capsular lesions. Thus, while it is possible that the effects of the GVC procedure described here had minimal deleterious impact on frontally-mediated behaviors and executive functions, we cannot rule out that our measures were not sensitive enough to detect an impact of these lesions, perhaps in other functional domains not assessed by WCST or FrSBe.
Large scale studies of patients with focal frontal lobe lesions have generally implicated the anterior cingulate, dlPFC and underlying white matter in WCST performance, with relatively minimal impact for lesions in vmPFC or OFC in WCST performance (Gläscher et al., 2012; Gläscher et al., 2019; Milner, 1963). Damage to the ventromedial frontal lobe may also cause more specific changes in personality and behavior related to socio-emotional functioning, decision-making, insight, and initiative (Barrash et al., 2021; Barrash et al., 2000; Yu et al., 2020). While items interrogating these functions are contained within the FrSBe, the total score and subscales may not be particularly sensitive to domain-specific dysfunction. Examining the impact of GVC on social and decision-making behavior with more precisely tailored ecological and task-based measures would be fruitful to establish whether GVC surgery spares these functions. Greater depth and breadth of neuropsychological testing and ecologically valid behavioral measures with higher construct precision are needed. For example, specific measures probing metacognitive processes and insight in experimental settings (Fleming et al., 2014), as well as scales aimed at evaluation of self-awareness and insight in daily functioning (Barrash et al., 2000; Beck et al., 2004), and careful comparison of self and informant accounts, will all be needed in future work.
There are other significant limitations to this study worth noting. While the sample size of this study was comparatively large for a capsulotomy psychiatric neurosurgical population (Brown et al., 2016; Csigó et al., 2010; D'Astous et al., 2013; Kondziolka et al., 2011; Liu et al., 2008; Lopes et al., 2009; Oliver et al., 2003; Rück et al., 2008; Sheehan et al., 2013), FrSBe assessments were inconsistently collected over the course of this study. Thus, there were only a small number of cases where these data were available pre- and post-surgery with both self and family assessments, making it challenging to draw strong conclusions about differences in how raters perceived changes in frontal lobe behaviors over time. In part, this reflects the challenges of administering long-term follow-up assessments for patients who were spread out across the United States, necessitating travel for the highly specialized GVC procedure initially and for follow-up evaluations. Thus, patients may have been lost to follow-up at various time points due to difficulty re-contacting patients or patient family members, and variable interest in returning for further assessment, among other potential barriers. Nonetheless, greater consistency in the administration of follow-up assessments from multiple raters will be necessary to evaluate the efficacy and risks of this procedure in future studies.
Another issue, common to studies of psychiatric neurosurgery, is that there was no comparable group of refractory OCD patients to serve as a control for the effects of GVC. The lack of such a control group makes it difficult to differentiate the effects of GVC on frontal lobe function from a possible general reduction in symptom severity with time or aging. Our results could also reflect a regression toward the mean — as Y-BOCS scores are definitionally near or at ceiling in these patients pre-surgically, their symptom severity would be more likely to drop over time, if only due to the range of this measure. However, it is unclear whether assessments of frontal lobe functions would also exhibit regression to the mean given that FrSBe scores were elevated though not at ceiling. One challenge to including a control group is that withholding a potential treatment for refractory OCD poses ethical challenges thereby limiting opportunities to study refractory OCD patients longitudinally. Other work that has examined refractory OCD patients with sham surgery over the course of a year found a trend toward improvement in OCD symptoms that was smaller than the improvement in the GVC group tracked over the same period (Lopes et al., 2014). However, there is less information about the expected trend for OCD symptomatology or frontal lobe functioning in GVC patients for longer term follow-ups akin to those examined in this study.
Complete rule out of the possibility of a placebo effect driving the improvement in patient self-report would require sham surgery and double-blinding in future studies. While there is a need for sham control groups, conducting such studies are immense undertakings and ethical considerations of withholding treatment would preclude long-term follow-up assessments with a sham control. However, blinding non-neurosurgery clinicians involved in assessing surgical outcomes to the stage of care (pre- or post-surgery) can help to prevent bias in the evaluation of change in OCD symptomatology. Preferably, clinicians involved in evaluating study outcomes would be independent of those involved in the design and implementation of the psychiatric neurosurgery.
In conclusion, we found no evidence that GVC impaired frontal lobe functions as assessed by self and family ratings of everyday behaviors on the FrSBe in patients with refractory OCD. Indeed, frontally-mediated behaviors appeared to improve, or at least not substantially change, as rated by the patients themselves versus their family. However, we cannot conclusively rule out the possibility that a placebo effect or impairment in patient insight may have contributed to improvements in self-ratings of behavior post-surgery. Our results indicate the need for future work that systematically examines the effects of ventral capsulotomy on patient insight and uses placebo-controls and blinding of clinician assessments to rule out these possible alternative explanations. While gamma ventral capsulotomy may provide an effective treatment for refractory OCD, assessing the effects of this procedure on frontal lobe functions will require future work that examines the longitudinal consequences of this surgery with greater systematic detail.
Supplementary Material
Highlights.
Ventral capsulotomy is a treatment for intractable obsessive-compulsive disorder
Impact of gamma ventral capsulotomy on frontal behaviors remains unclear
No significant declines in frontal lobe functioning found post-surgery
Self but not informant reports of frontal behaviors improved post-surgery
Assessment of metacognition after ventral capsulotomy may be beneficial
Acknowledgements:
We thank Emily Waters and Isabel Reyes for their contributions to this project in data processing.
Funding:
This work was supported by the National Institute of Mental Health [F-32 MH116592 (A.V.), K-23 MH200607 (N.C.R.M.)]; the National Institute of General Medical Sciences [5 P-20 GM130452-02 (N.C.R.M., S.A.R.)], and Center for Neurorestoration and Neurotechnology, Providence, RI [VA ORD N9228TC (B.D.G.)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: This manuscript is based on work supported, in part, by the Department of Veterans Affairs. Views expressed in this article are those of the authors and are not necessarily those of the Department of Veterans Affairs or the United States Government.
Declarations of interest: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: One contributor (Malloy) is an author of the original scale used to assess frontal lobe behaviors (FrSBe) comprising the analyses conducted. The FrSBe is published by Psychological Assessment Resources and Dr. Malloy receives royalties on the sale of the measure. However, the first three authors (Kassel, Lositsky, Vaidya) primarily were responsible for conducting analyses and interpretation. No biases occurred as a result of this author’s involvement.
ClinicalTrials.gov: Safety and Effectiveness of Gamma Capsulotomy in Intractable OCD; https://clinicaltrials.gov/ct2/show/NCT01849809; NCT01849809.
CRediT Author Statement
Michelle T. Kassel: Conceptualization; Methodology; Software; Formal analysis; Data Curation; Writing - Original Draft; Writing - Review & Editing; Visualization
Olga Lositsky: Conceptualization; Methodology; Software; Formal analysis; Data Curation; Writing - Original Draft; Writing - Review & Editing; Visualization
Avinash R. Vaidya: Conceptualization; Methodology; Software; Formal analysis; Data Curation; Writing - Original Draft; Writing - Review & Editing; Visualization
David Badre: Conceptualization; Methodology; Supervision; Writing - Review & Editing; Resources
Paul F. Malloy: Conceptualization; Investigation; Writing- Review and Editing
Benjamin D. Greenberg: Writing- Review and Editing
Richard Marsland: Supervision; Investigation; Project administration; Data Curation; Writing-Review and Editing
Georg Noren: Investigation; Writing-Review and Editing
Anna Sherman: Data Curation; Writing-Review and Editing
Steven A. Rasmussen: Conceptualization; Investigation; Supervision; Funding Acquisition; Writing-Review and Editing
Nicole C. R. McLaughlin: Conceptualization; Methodology; Supervision; Writing - Review & Editing; Resources; Project administration; Investigation
5. References
- Abramovitch A, Abramowitz JS, & Mittelman A (2013). The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clinical psychology review, 33(8), 1163–1171. 10.1016/j.cpr.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Abramovitch A, De Nadai AS, & Geller DA (2021, Aug 30). Neurocognitive endophenotypes in pediatric OCD probands, their unaffected parents and siblings. Prog Neuropsychopharmacol Biol Psychiatry, 110, 110283. 10.1016/j.pnpbp.2021.110283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5 ed.). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Barrash J, Bruss J, Anderson SW, Kuceyeski A, Manzel K, Tranel D, & Boes AD (2021). Lesions in different prefrontal sectors are associated with different types of acquired personality disturbances. Cortex. 10.1016/j.cortex.2021.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Stuss DT, Aksan N, Anderson SW, Jones RD, Manzel K, & Tranel D (2018, Sep). "Frontal lobe syndrome"? Subtypes of acquired personality disturbances in patients with focal brain damage. Cortex, 106, 65–80. 10.1016/j.cortex.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Tranel D, & Anderson SW (2000). Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol, 18(3), 355–381. 10.1207/S1532694205Barrash [DOI] [PubMed] [Google Scholar]
- Batistuzzo MC, Hoexter MQ, Taub A, Gentil AF, Cesar RC, Joaquim MA, D'Alcante CC, McLaughlin NC, Canteras MM, Shavitt RG, Savage CR, Greenberg BD, Norén G, Miguel EC, & Lopes AC (2015, Jul). Visuospatial Memory Improvement after Gamma Ventral Capsulotomy in Treatment Refractory Obsessive-Compulsive Disorder Patients. Neuropsychopharmacology, 40(8), 1837–1845. 10.1038/npp.2015.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistuzzo MC, Taub A, Nakano E, D'Alcante CC, de Mathis ME, Hoexter MQ, Miguel EC, & Lopes AC (2009). Performance of patients with refractory obsessive-compulsive disorder in the Frontal Systems Behavior Scale. Neurocase, 15(2), 157–162. 10.1080/13554790802709047 [DOI] [PubMed] [Google Scholar]
- Beck AT, Baruch E, Balter JM, Steer RA, & Warman DM (2004, Jun 1). A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res, 68(2-3), 319–329. 10.1016/s0920-9964(03)00189-0 [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, & Knight RT (2006). Orbitofrontal Cortex and Social Behavior: Integrating Self-monitoring and Emotion-Cognition Interactions. Journal of Cognitive Neuroscience, 18(6), 871–879. 10.1162/jocn.2006.18.6.871 [DOI] [PubMed] [Google Scholar]
- Bora E (2020). Meta-analysis of neurocognitive deficits in unaffected relatives of obsessive-compulsive disorder (OCD): Comparison with healthy controls and patients with OCD. Psychological Medicine, 50(8), 1257–1266. 10.1017/S0033291720001634 [DOI] [PubMed] [Google Scholar]
- Brown LT, Mikell CB, Youngerman BE, Zhang Y, McKhann GM 2nd, & Sheth SA (2016, Jan). Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. J Neurosurg, 124(1), 77–89. 10.3171/2015.1.Jns14681 [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Forbes C, Costello A, Coates LM, Dawson DR, Anderson ND, Gilbert SJ, Dumontheil I, & Channon S (2006, Mar). The case for the development and use of "ecologically valid" measures of executive function in experimental and clinical neuropsychology. J Int Neuropsychol Soc, 12(2), 194–209. 10.1017/s1355617706060310 [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, Kennedy DN, & Rauch SL (2007). A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depress Anxiety, 24(6), 440–446. 10.1002/da.20246 [DOI] [PubMed] [Google Scholar]
- Chan RCK, Shum D, Toulopoulou T, & Chen EYH (2008, 2008/March/01/). Assessment of executive functions: Review of instruments and identification of critical issues. Archives of Clinical Neuropsychology, 23(2), 201–216. 10.1016/j.acn.2007.08.010 [DOI] [PubMed] [Google Scholar]
- Chaytor N, & Schmitter-Edgecombe M (2003, 2003/December/01). The Ecological Validity of Neuropsychological Tests: A Review of the Literature on Everyday Cognitive Skills. Neuropsychology Review, 13(4), 181–197. 10.1023/B:NERV.0000009483.91468.fb [DOI] [PubMed] [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M, & Burr R (2006). Improving the ecological validity of executive functioning assessment. Archives of Clinical Neuropsychology, 21(3), 217–227. 10.1016/j.acn.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Csigó K, Harsányi A, Demeter G, Rajkai C, Németh A, & Racsmány M (2010, Oct). Long-term follow-up of patients with obsessive-compulsive disorder treated by anterior capsulotomy: a neuropsychological study. J Affect Disord, 126(1-2), 198–205. 10.1016/j.jad.2010.02.127 [DOI] [PubMed] [Google Scholar]
- D'Astous M, Cottin S, Roy M, Picard C, & Cantin L (2013, Nov). Bilateral stereotactic anterior capsulotomy for obsessive-compulsive disorder: long-term follow-up. J Neurol Neurosurg Psychiatry, 84(11), 1208–1213. 10.1136/jnnp-2012-303826 [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, & Ochsner KN (2012, Aug). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci, 24(8), 1742–1752. 10.1162/jocn_a_00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, & Roberts AC (1996, 1996/March/01). Dissociation in prefrontal cortex of affective and attentional shifts. Nature, 380(6569), 69–72. 10.1038/380069a0 [DOI] [PubMed] [Google Scholar]
- Fellows LK, & Farah MJ (2003, Aug). Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain, 126(Pt 8), 1830–1837. 10.1093/brain/awg180 [DOI] [PubMed] [Google Scholar]
- Fleming SM, Ryu J, Golfinos JG, & Blackmon KE (2014, Oct). Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain, 137(Pt 10), 2811–2822. 10.1093/brain/awu221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnéhun S, Levy R, Rosso C, Urbanski M, Volle E, & Thiebaut de Schotten M (2018). Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. GigaScience, 7(3). 10.1093/gigascience/giy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, & Tranel D (2012). Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences, 109(36), 14681. 10.1073/pnas.1206608109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, & Tranel D (2019, 2019/January/03). Model-based lesion mapping of cognitive control using the Wisconsin Card Sorting Test. Nature Communications, 10(1), 20. 10.1038/s41467-018-07912-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichgerrcht E, Fridriksson J, Rorden C, & Bonilha L (2017, 2017/January/01/). Connectome-based lesion-symptom mapping (CLSM): A novel approach to map neurological function. NeuroImage: Clinical, 16, 461–467. 10.1016/j.nicl.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong F, Li P, Li B, Zhang S, Zhang X, Yang S, Liu H, & Wang W (2018, Feb). A study of cognitive function in treatment-refractory obsessive-compulsive disorder treated with capsulotomy. J Neurosurg, 128(2), 583–595. 10.3171/2016.9.Jns152494 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, & Charney DS (1989b, Nov). The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry, 46(11), 1012–1016. 10.1001/archpsyc.1989.01810110054008 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, & Charney DS (1989a). The Yale-Brown Obsessive Compulsive Scale: I. Development, Use, and Reliability. Archives of General Psychiatry, 46(11), 1006–1011. 10.1001/archpsyc.1989.01810110048007 [DOI] [PubMed] [Google Scholar]
- Grace J, & Malloy PF (2001). Frontal Systems Behavior Scale (FrSBe): Professional Manual. Psychological Assessment Resources. [Google Scholar]
- Grace J, Stout JC, & Malloy PF (1999, Sep). Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment, 6(3), 269–284. 10.1177/107319119900600307 [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Rauch SL, & Haber SN (2010, Jan). Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology, 35(1), 317–336. 10.1038/npp.2009.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960, Feb). A rating scale for depression. J Neurol Neurosurg Psychiatry, 23, 56–62. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14399272 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC495331/pdf/jnnpsyc00273-0060.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, Deus J, Alonso P, Yücel M, Pantelis C, Menchon JM, & Cardoner N (2009, Nov). Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry, 66(11), 1189–1200. 10.1001/archgenpsychiatry.2009.152 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, & Curtiss G (1993). Wisconsin Card Sorting Test Manual: Revised and Expanded. Psychological Assessment Resources. [Google Scholar]
- Hirschtritt ME, Bloch MH, & Mathews CA (2017). Obsessive-Compulsive Disorder: Advances in Diagnosis and Treatment. Jama, 317(13), 1358–1367. 10.1001/jama.2017.2200 [DOI] [PubMed] [Google Scholar]
- Hoerold D, Pender NP, & Robertson IH (2013, 2013/February/01/). Metacognitive and online error awareness deficits after prefrontal cortex lesions. Neuropsychologia, 51(3), 385–391. 10.1016/j.neuropsychologia.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Husted DS, & Shapira NA (2004, Nov). A review of the treatment for refractory obsessive-compulsive disorder: from medicine to deep brain stimulation. CNS Spectr, 9(11), 833–847. 10.1017/s109285290000225x [DOI] [PubMed] [Google Scholar]
- Jakubovski E, Diniz JB, Valerio C, Fossaluza V, Belotto-Silva C, Gorenstein C, Miguel E, & Shavitt RG (2013, Aug). Clinical predictors of long-term outcome in obsessive-compulsive disorder. Depress Anxiety, 30(8), 763–772. 10.1002/da.22013 [DOI] [PubMed] [Google Scholar]
- Janowsky J, Shimamura A, & Squire L (1989). Memory and metamemory: Comparisons between patients with frontal lobe lesions and amnesic patients. Psychobiology, 17, 3–11. [Google Scholar]
- Kondziolka D, Flickinger JC, & Hudak R (2011, Jan). Results following gamma knife radiosurgical anterior capsulotomies for obsessive compulsive disorder. Neurosurgery, 68(1), 28–32; discussion 23-23. 10.1227/NEU.0b013e3181fc5c8b [DOI] [PubMed] [Google Scholar]
- Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, & Haber SN (2011). Rules Ventral Prefrontal Cortical Axons Use to Reach Their Targets: Implications for Diffusion Tensor Imaging Tractography and Deep Brain Stimulation for Psychiatric Illness. The Journal of Neuroscience, 31(28), 10392. 10.1523/JNEUROSCI.0595-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Zhang H, Liu C, Guan Y, Lang L, Cheng Y, Sun B, Wang H, Zuo C, Pan L, Xu H, Li S, Shi L, Qian J, & Yang Y (2008, Jun). Stereotactic treatment of refractory obsessive compulsive disorder by bilateral capsulotomy with 3 years follow-up. J Clin Neurosci, 15(6), 622–629. 10.1016/j.jocn.2007.07.086 [DOI] [PubMed] [Google Scholar]
- Lochner C, Fineberg NA, Zohar J, van Ameringen M, Juven-Wetzler A, Altamura AC, Cuzen NL, Hollander E, Denys D, Nicolini H, Dell‘Osso B, Pallanti S, & Stein DJ (2014, 2014/October/01/). Comorbidity in obsessive–compulsive disorder (OCD): A report from the International College of Obsessive–Compulsive Spectrum Disorders (ICOCS). Comprehensive Psychiatry, 55(7), 1513–1519. 10.1016/j.comppsych.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Lopes AC, de Mathis ME, Canteras MM, Salvajoli JV, Del Porto JA, & Miguel EC (2004, 2004/March//). [Update on neurosurgical treatment for obsessive compulsive disorder]. Revista brasileira de psiquiatria; (Sao Paulo, Brazil: 1999), 26(1), 62–66. 10.1590/s1516-44462004000100015 [DOI] [PubMed] [Google Scholar]
- Lopes AC, Greenberg BD, Canteras MM, Batistuzzo MC, Hoexter MQ, Gentil AF, Pereira CAB, Joaquim MA, de Mathis ME, D'Alcante CC, Taub A, de Castro DG, Tokeshi L, Sampaio LANPC, Leite CC, Shavitt RG, Diniz JB, Busatto G, Norén G, Rasmussen SA, & Miguel EC (2014). Gamma Ventral Capsulotomy for Obsessive-Compulsive Disorder: A Randomized Clinical Trial. JAMA Psychiatry, 71(9), 1066–1076. 10.1001/jamapsychiatry.2014.1193 [DOI] [PubMed] [Google Scholar]
- Lopes AC, Greenberg BD, Norén G, Canteras MM, Busatto GF, de Mathis ME, Taub A, D'Alcante CC, Hoexter MQ, Gouvea FS, Cecconi JP, Gentil AF, Ferrão YA, Fuentes D, de Castro CC, Leite CC, Salvajoli JV, Duran FL, Rasmussen S, & Miguel EC (2009, Fall). Treatment of resistant obsessive-compulsive disorder with ventral capsular/ventral striatal gamma capsulotomy: a pilot prospective study. J Neuropsychiatry Clin Neurosci, 21(4), 381–392. 10.1176/jnp.2009.21.4.381 [DOI] [PubMed] [Google Scholar]
- Mancebo MC, Greenberg B, Grant JE, Pinto A, Eisen JL, Dyck I, & Rasmussen SA (2008, Jan-Feb). Correlates of occupational disability in a clinical sample of obsessive-compulsive disorder. Comprehensive Psychiatry, 49(1), 43–50. 10.1016/j.comppsych.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Freedman DJ, & Buckley MJ (2020, 2020/November/01). Emergence of abstract rules in the primate brain. Nature Reviews Neuroscience, 21(11), 595–610. 10.1038/s41583-020-0364-5 [DOI] [PubMed] [Google Scholar]
- McKay D, Sookman D, Neziroglu F, Wilhelm S, Stein DJ, Kyrios M, Matthews K, & Veale D (2015, 2015/February/28/). Efficacy of cognitive-behavioral therapy for obsessive–compulsive disorder. Psychiatry Research, 225(3), 236–246. 10.1016/j.psychres.2014.11.058 [DOI] [PubMed] [Google Scholar]
- McLaughlin NCR, Lauro PM, Patrick MT, Pucci FG, Barrios-Anderson A, Greenberg BD, Rasmussen SA, & Asaad WF (2021, May 13). Magnetic Resonance Imaging-Guided Laser Thermal Ventral Capsulotomy for Intractable Obsessive-Compulsive Disorder. Neurosurgery, 88(6), 1128–1135. 10.1093/neuros/nyab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel EC, Lopes AC, McLaughlin NCR, Norén G, Gentil AF, Hamani C, Shavitt RG, Batistuzzo MC, Vattimo EFQ, Canteras M, De Salles A, Gorgulho A, Salvajoli JV, Fonoff ET, Paddick I, Hoexter MQ, Lindquist C, Haber SN, Greenberg BD, & Sheth SA (2019, Feb). Evolution of gamma knife capsulotomy for intractable obsessive-compulsive disorder. Mol Psychiatry, 24(2), 218–240. 10.1038/s41380-018-0054-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B (1963). Effects of Different Brain Lesions on Card Sorting: The Role of the Frontal Lobes. JAMA Neurology, 9, 90–100. [Google Scholar]
- Mindus P, Edman G, & Andréewitch S (1999, Jan). A prospective, long-term study of personality traits in patients with intractable obsessional illness treated by capsulotomy. Acta Psychiatr Scand, 99(1), 40–50. 10.1111/j.1600-0447.1999.tb05383.x [DOI] [PubMed] [Google Scholar]
- Nuttin B, Wu H, Mayberg H, Hariz M, Gabriëls L, Galert T, Merkel R, Kubu C, Vilela-Filho O, Matthews K, Taira T, Lozano AM, Schechtmann G, Doshi P, Broggi G, Régis J, Alkhani A, Sun B, Eljamel S, Schulder M, Kaplitt M, Eskandar E, Rezai A, Krauss JK, Hilven P, Schuurman R, Ruiz P, Chang JW, Cosyns P, Lipsman N, Voges J, Cosgrove R, Li Y, & Schlaepfer T (2014). Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. Journal of neurology, neurosurgery, and psychiatry, 85(9), 1003–1008. 10.1136/jnnp-2013-306580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus E, & Barceló F (2009, Dec). The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain Cogn, 71(3), 437–451. 10.1016/j.bandc.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Nyman H, Andréewitch S, Lundbäck E, & Mindus P (2001). Executive and cognitive functions in patients with extreme obsessive-compulsive disorder treated by capsulotomy. Appl Neuropsychol, 8(2), 91–98. 10.1207/s15324826an0802_3 [DOI] [PubMed] [Google Scholar]
- Nyman H, & Mindus P (1995). Neuropsychological correlates of intractable anxiety disorder before and after capsulotomy. Acta Psychiatrica Scandinavica, 91(1), 23–31. 10.1111/j.1600-0447.1995.tb09737.x [DOI] [PubMed] [Google Scholar]
- Oliver B, Gascón J, Aparicio A, Ayats E, Rodriguez R, Maestro De León JL, García-Bach M, & Soler PA (2003). Bilateral anterior capsulotomy for refractory obsessive-compulsive disorders. Stereotact Funct Neurosurg, 81(1-4), 90–95. 10.1159/000075110 [DOI] [PubMed] [Google Scholar]
- Pallanti S, Grassi G, Cantisani A, Sarrecchia E, & Pellegrini M (2011, 2011-December-21). Obsessive–Compulsive Disorder Comorbidity: Clinical Assessment and Therapeutic Implications [Review]. Frontiers in Psychiatry, 2. 10.3389/fpsyt.2011.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls DL, Abramovitch A, Rauch SL, & Geller DA (2014, Jun). Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci, 15(6), 410–424. 10.1038/nrn3746 [DOI] [PubMed] [Google Scholar]
- Qin P, & Northoff G (2011, Aug 1). How is our self related to midline regions and the default-mode network? Neuroimage, 57(3), 1221–1233. 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Noren G, Greenberg BD, Marsland R, McLaughlin NC, Malloy PJ, Salloway SP, Strong DR, Eisen JL, Jenike MA, Rauch SL, Baer L, & Lindquist C (2018, Sep 1). Gamma Ventral Capsulotomy in Intractable Obsessive-Compulsive Disorder. Biol Psychiatry, 84(5), 355–364. 10.1016/j.biopsych.2017.11.034 [DOI] [PubMed] [Google Scholar]
- Rauch SL (2003, Apr). Neuroimaging and neurocircuitry models pertaining to the neurosurgical treatment of psychiatric disorders. Neurosurg Clin N Am, 14(2), 213–223, vii-viii. 10.1016/s1042-3680(02)00114-6 [DOI] [PubMed] [Google Scholar]
- Rück C, Karlsson A, Steele JD, Edman G, Meyerson BA, Ericson K, Nyman H, Asberg M, & Svanborg P (2008, Aug). Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Arch Gen Psychiatry, 65(8), 914–921. 10.1001/archpsyc.65.8.914 [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Lundgren DA, & Murray EA (2017, Aug 30). Specialized Representations of Value in the Orbital and Ventrolateral Prefrontal Cortex: Desirability versus Availability of Outcomes. Neuron, 95(5), 1208–1220.e1205. 10.1016/j.neuron.2017.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Saunders RC, Prescott AT, Chau LS, & Murray EA (2013, 2013/August/01). Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nature Neuroscience, 16(8), 1140–1145. 10.1038/nn.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JP, Patterson G, Schlesinger D, & Xu Z (2013, Nov). γ knife surgery anterior capsulotomy for severe and refractory obsessive-compulsive disorder. J Neurosurg, 119(5), 1112–1118. 10.3171/2013.5.Jns13201 [DOI] [PubMed] [Google Scholar]
- Shin N, Lee T, Kim E, & Kwon J (2014). Cognitive functioning in obsessive-compulsive disorder: A meta-analysis. Psychological Medicine, 44(6), 1121–1130. 10.1017/S0033291713001803 [DOI] [PubMed] [Google Scholar]
- Stout JC, Ready RE, Grace J, Malloy PF, & Paulsen JS (2003, Mar). Factor analysis of the frontal systems behavior scale (FrSBe). Assessment, 10(1), 79–85. 10.1177/1073191102250339 [DOI] [PubMed] [Google Scholar]
- Taub A, Lopes AC, Fuentes D, D’Alcante CC, de Mathis ME, Canteras MM, Siviero M, Greenberg BD, Norén G, Batistuzzo M, Hoexter MQ, Cecílio S, Savage C, Rasmussen S, & Miguel EC (2009, Fall). Neuropsychological outcome of ventral capsular/ventral striatal gamma capsulotomy for refractory obsessive-compulsive disorder: a pilot study. J Neuropsychiatry Clin Neurosci, 21(4), 393–397. 10.1176/jnp.2009.21.4.393 [DOI] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touzé E, Chokron S, Méder J-F, Lévy R, Dubois B, & Bartolomeo P (2008). Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. Journal of Neurology, Neurosurgery & Psychiatry, 79(5), 598–601. 10.1136/jnnp.2007.126276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro AG, & Fleming SM (2018). Thinking about thinking: A coordinate-based meta-analysis of neuroimaging studies of metacognitive judgements. Brain and Neuroscience Advances, 2, 2398212818810591. 10.1177/2398212818810591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale D, & Roberts A (2014). Obsessive-compulsive disorder. BMJ : British Medical Journal, 348, g2183. 10.1136/bmj.g2183 [DOI] [PubMed] [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie HC and Evans JJ (1996). The Behavioural Assessment of the Dysexecutive Syndrome. [Google Scholar]
- World Health Organization. (2008). The global burden of disease : 2004 update. [Google Scholar]
- World Health Organization. (2017). Depression and other common mental disorders: global health estimates CC BY-NC-SA 3.0 IGO). https://apps.who.int/iris/handle/10665/254610 [Google Scholar]
- Yu LQ, Kan IP, & Kable JW (2020, Feb-Mar). Beyond a rod through the skull: A systematic review of lesion studies of the human ventromedial frontal lobe. Cogn Neuropsychol, 37(1-2), 97–141. 10.1080/02643294.2019.1690981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.