Abstract
Maternal food restriction (MFR) during pregnancy leads to pulmonary dysplasia in the newborn period and increases susceptibility to diseases, such as asthma and chronic lung disease, later in life. Previous studies have shown that maternal electro-acupuncture (EA) applied to “Zusanli” (ST 36) could prevent the abnormal expression of key lung developmental signaling pathways and improve the lung morphology and function in perinatal nicotine exposed offspring. There is a significant overlap in lung developmental signaling pathways affected by perinatal nicotine exposure and MFR during pregnancy; however, whether maternal EA at ST 36 also blocks the MFR-induced lung phenotype is unknown. Here, we examined the effects of EA applied to maternal ST 36 on lung morphology and function and the expression of key lung developmental signaling pathways, and the hypercorticoid state associated with MFR during pregnancy. These effects were compared with those of metyrapone, an intervention known to block MFR-induced offspring hypercorticoid state and the resultant pulmonary pathology. Like metyrapone, maternal EA at ST 36 blocked the MFR-induced changes in key developmental signaling pathways and protected the MFR-induced changes in lung morphology and function. These results offer a novel and safe, non-pharmacologic approach to prevent MFR-induced pulmonary dysplasia in offspring.
Keywords: Intrauterine growth restriction, Maternal food restriction, Lung development, Electro-acupuncture, “Zusanli” acupoint
1. Introduction
Intrauterine growth restriction (IUGR) is a common pregnancy complication associated with significant adverse perinatal outcomes [1]. It is an important cause of perinatal morbidity and death [2], affecting 10–15% of pregnant women worldwide [3]. IUGR is closely related to malnutrition during pregnancy, infectious diseases, pregnancy-induced hypertension, substance abuse, and teratogen exposures [1], among which malnutrition during pregnancy is the most common cause of IUGR [3,4]. Maternal food restriction (MFR) during pregnancy leads to insufficient fetal nutrition, resulting in IUGR and an associated pulmonary phenotype characterized by an increased susceptibility to bronchopulmonary dysplasia and hyperresponsive airways [5–12]. Although the exact mechanism(s) underlying the respiratory consequences of MFR-associated IUGR remain unknown, alterations in key lung developmental signaling pathways (summarized below) have been demonstrated [12]. Moreover, MFR up-regulates the hypothalamic-pituitary-adrenal (HPA) axis of the offspring, resulting in a chronic hypercorticoid state, which can also potentially interfere with mechanisms underlying normal lung development [5,6]. Importantly, there is no clinically effective therapeutic intervention that prevents the MFR-associated pulmonary consequences.
Parathyroid hormone-related protein/Peroxisome proliferator-activated receptor γ (PTHrP/PPARγ) and Wnt/β-catenin signaling pathways play significant roles in MFR-induced pulmonary dysplasia in offspring [5,6]. Parathyroid hormone-related protein is secreted and regulated by alveolar type II cells [12], which up-regulates the expression of adipocyte differentiation-related protein, promoting the uptake and storage of neutral lipids by lipofibroblasts, transporting these to alveolar type II cells [13,14]. PPARγ, expressed by various alveolar cell types, including the lung fibroblasts, determines the differentiation of fibroblasts into lipofibroblasts [15,16], thus maintaining their lipogenic phenotype [17]. On the contrary, the Wnt/β-catenin signaling is a key factor in the differentiation of fibroblasts into myofibroblasts, determining their myogenic phenotype [5,6]. Since myofibroblasts do not support alveolar type II cells’ differentiation and growth, it results in failed alveolarization and surfactant deficiency. MFR down-regulates the PTHrP/PPARγ signaling and up-regulates the Wnt/β-catenin signaling in offspring lung [5,6,16], driving fibroblasts to differentiate into myofibroblasts, thus impairing normal lung development.
The transforming growth factor (TGF)-β1/Smads signaling may also be involved in MFR-induced pulmonary dysplasia in offspring. The TGF-β signaling is initiated by binding the TGF-β ligand to the type II TGF-β receptor, forming a complex with either the type I receptor or activin A receptor type II-like 1. The type I receptor transmits signals within the cell via second-messenger Smad proteins, namely Smad1-Smad4, or by Smad-independent pathways. This signaling is negatively regulated by Smad6/7 [18,19]. As a multifunctional cytokine, TGF-β1 plays a vital role in various biological processes such as cell proliferation, differentiation, and apoptosis. The activation/differentiation of lung fibroblasts into lung myofibroblasts and the production and deposition of extracellular matrix are also regulated by the TGF-β1/Smads signaling [20]. Previous studies have shown that MFR can significantly increase the expression of elastin and myofibroblast marker α-smooth muscle actin (α-SMA) in the lung tissue of offspring rats, accompanied by altered lung morphology and function [16]; TGF-β1/Smads signaling activation following MFR is also supported by the increased connective tissue growth factor and collagen I and IV expression in offspring myocardial tissue in other MFR models [21].
Electro-acupuncture (EA) is a modification of acupuncture that stimulates acupoints with low-frequency pulsed electrical current. Biologically, it is a combination of acupuncture stimulation and its consequent electrophysiological effects. “Zusanli” (ST 36) is one of the 365 classical acupoints. It is an acupoint of the “Stomach Meridian”, located at the posterolateral part of the knee joint, about 5 mm below the fibulae capitulum. It is one of the most frequently used acupoints. It is easily reproducible and, in addition to its effects on the digestive system, it is effective in multiple other system diseases, including the respiratory system [22–25]. We have previously demonstrated that EA applied to maternal ST 36 blocks alterations in key lung developmental signaling pathways and the resulting offspring lung phenotype following perinatal nicotine exposure [22–25]. However, it is unknown whether it can also block the MFR-induced changes in lung developmental signaling pathways and the resultant lung phenotype. Here, we determine the effect of EA applied to maternal ST 36 on the MFR-induced modifications in the expression of key PTHrP/PPARγ, Wnt/β-catenin, and TGF-β1/Smads signaling pathway intermediates in offspring lung and the resultant lung phenotype. To explain the possible mechanism underlying EA’s effect, we also explored whether EA blocks the MFR-induced chronic hypercorticoid state.
2. Materials and methods
2.1. Animals
This experimental protocol was approved by the Ethics Committee of Beijing University of Chinese Medicine (approval number: BUCM-4–2019090101-3041) and it strictly complied with the “Guide for Care and Use of Laboratory Animals” advocated by the National Institutes of Health. Thirty female and ten male specific pathogen free nine-week old Sprague-Dawley rats without mating history were acquired from Beijing Vital River Laboratory Animal Technology Co., Ltd. (production license number: SCXK (Beijing) 2016–0006). The rearing conditions were as follows: room temperature (23 ± 1)°C, relative humidity (45 ± 5)%, and 12:12 h light-dark cycle.
2.2. Experimental protocol
The dams were randomly divided into control (“C”) group, control plus EA (“C+EA”) group, model (“M”) group, model plus EA (“M+EA”) group, and model plus metyrapone (“M+MTP”) group, with six rats in each group. Using our previously described rat model of MFR during pregnancy that induces intrauterine growth restricted offspring [5,6,26],, from embryonic day 10 until delivery, the dams in the food restriction groups were provided a 50% food-restricted diet, which was determined by the normal food intake of the “C” group every 24 hours. The “C+EA” group dams were provided unrestricted diet similar to that of the “C” group. The “C+EA” and “M+EA” groups were administered EA to bilateral ST 36 acupoints, and the “M+MTP” group water was supplemented with an endogenous glucocorticoid synthesis inhibitor metyrapone (MTP; 0.5mg/mL; Catalog#: 856525, Sigma-Aldrich), using the previously described dosing regimen [5,6]. To minimize a bias towards selecting either heavier or lighter pups, on postnatal day (PD) 0, all pups from each litter were marked and weighed, and six pups closest to the median body weight were included in the study. On PD7, PD14, and PD21, the pups were weighed. On PD21, pulmonary function testing was performed. Subsequently, pups were sacrificed for lungs and serum collections.
2.3. Electro-acupuncture treatment
In line with the description in “Experimental Acupuncture Science”, acupuncture site ST 36 was selected posterolateral to the knee joint about 5mm from the fibular head. As described previously [22–25], the acupuncture needles (0.20 mm×13 mm, Catalog#: 1552484411, Hwato) were perpendicularly pierced into ST 36 bilaterally with a depth of 7 mm, connecting the negative pole of EA, and horizontally pierced into the skin 2 mm below ST 36, connecting the positive pole of EA. The EA parameters were as follows: frequency 2/15 Hz, intensity 1 mA, duration 20 min, and administered once a day between 10 AM and Noon.
2.4. Pulmonary function testing
The pulmonary function of pups was measured using a plethysmograph (Buxco, USA). On PD21 (end of the alveolarization process [27]), the pups were weighed and deeply anesthetized with 2% pentobarbital sodium (55 mg/kg), tracheostomized, intubated, and then connected with a small animal ventilator for plethysmography. Lung resistance (RL) and dynamic compliance (Cdyn) were recorded after stable breathing.
2.5. Lung morphometry
At sacrifice, offspring lungs were perfused in situ with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). After the trachea was ligated, the lungs were immediately transferred to 4% PFA and fixed at 4°C for about 4 hours. The lungs were transferred to PBS containing 30% sucrose at 4°C until fully equilibrated. Subsequently, the lungs were embedded in paraffin and sliced with a thickness of 5 μm and stained according to the hematoxylin-eosin staining kit’s instructions (Catalog#: ab245880, abcam). Finally, lung morphometry was objectively evaluated by measuring alveolar number, mean linear intercept, and septal thickness, as described previously [5,6].
2.6. Western Blotting assay for PPARγ, α-SMA, β-catenin and Smad2 proteins of the offspring
Western blotting was performed according to the previously described methods [23]. The specific primary antibodies used included PPARγ (1:1,500; Catalog#: 16643–1-AP, Proteintech), α-SMA (1:1,000; Catalog#: 55135–1-lg, Proteintech), β-catenin (1:3,000; Catalog#: 66379–1-lg, Proteintech), Smad2 (1:5,000; Catalog#: 67343–1-lg, Proteintech), and GAPDH (1:25,000; Catalog#: 60004–1-lg, Proteintech).
2.7. Real time PCR assay for lung PPARγ, β-catenin, α-SMA, LEF-1, TGF-β1, and Smad2 mRNAs of the offspring
The total RNA extraction, concentration and purity determination, structural integrity assessment, reverse transcription and PCR reaction system have been described previously [22,25]. The polymerase was activated at 95°C for 5 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 1 min. The relative quantification for target gene expression was performed using the 2-ΔΔCT method based on cycle threshold (CT) values. The RT-PCR probes used included-rat PPARγ: F-5’-CCAAGTGACTCTGCTCAAGTATGG-3’ and R-5’-CATGAATCCTTGTCCCTCTGATATG-3’ (106 bp); rat β-catenin: F-5’-GTGCAATTCCTGAGCTGACC-3’ and R-5’-CGGGCTGTTTCTACGTCATT-3’ (184 bp); rat α-SMA: F-5’-TATCCGATAGAACACGGCATCA-3’ and R-5’-CACGCGAAGCTCGTTATAGAAG-3’ (84 bp); rat LEF-1: F-5’-GAGCACGAACAGAGAAAGGAACA-3’ and F-5’-TTGATAGCTGCGCTCTCCTTTA-3’ (137 bp); rat TGF-β1: F-5’-CTAATGGTGGACCGCAACAAC-3’ and F-5’-CACTGCTTCCCGAATGTCTGA-3’ (99 bp); rat Smad2: F-5’-TTACAGATCCATCGAACTCGGAGA-3’ and F-5’-CACTTAGGCACTCGGCAAACAC-3’ (150 bp); rat GAPDH: F-5’-GACATGCCGCCTGGAGAAAC-3’ and F-5’-AGCCCAGGATGCCCTTTAGT-3’ (92 bp).
2.8. ELISA assay for lung and serum CORT and GR levels
On PD21, the pups were deeply anesthetized by intraperitoneal injection of 2% pentobarbital sodium (200 mg/kg), blood drawn from the abdominal aorta, and the serum was stored at −80°C after centrifugation. The lungs were collected and immediately frozen in liquid nitrogen, and then transferred to −80°C until processing. The levels of corticosterone (CORT, Catalog#: m1002893-J, Shanghai Enzyme-linked Biotechnology Co., Ltd.) and glucocorticoid receptor (GR, Catalog#: m1003239-J, Shanghai Enzyme-linked Biotechnology Co., Ltd.) were detected according to the instructions of the ELISA kit.
2.9. Statistical analysis
Data were analyzed using the statistical software (version 22.0; IBM SPSS, USA). Data are expressed as mean ± SD. Multiple groups were compared using two-way analysis of variance, with the maternal food restriction and EA or metyrapone treatment as independent variables, followed by Tukey’s test. A p value of less than 0.05 was considered significant.
3. Results
3.1. Effect of maternal EA on MFR-induced changes in offspring body and lung weights and pulmonary function
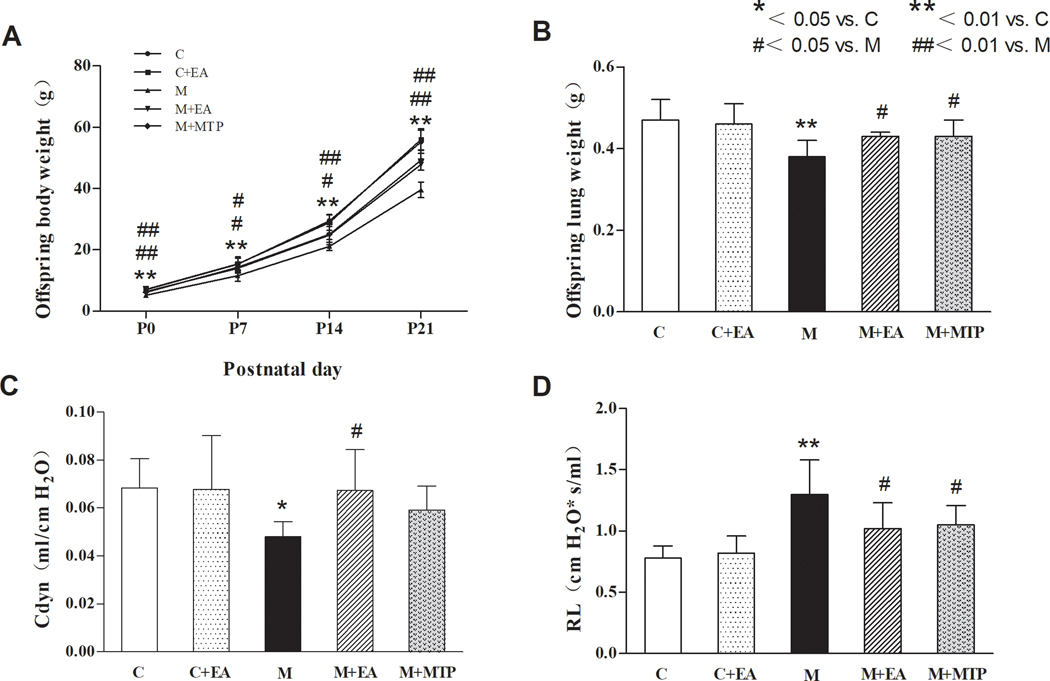
Effect on body weight:
On PD 0, 7, 14, and 21, compared with the “C” group, pup body weight in the “M” group decreased significantly (p < 0.01), but there was no significant difference in the “C+EA” group (p > 0.05). Compared with the “M” group, the body weight in the “M+EA” and “M+MTP” groups increased significantly (p < 0.05, P < 0.01, respectively). Notably, there was no significant difference in body weight between the “M+EA” and “M+MTP” groups (p > 0.05) (Figure 1A).
Figure 1.
Effect of maternal EA on MFR-induced changes in offspring body and lung weights. (A) Body weight. (B) Lung weight. (C) Cdyn. (D) RL. Values are mean ± SD; n=6 for each group.
Effect on lung weight:
Compared with the “C” group, the lung weight in the “M” group decreased significantly (p < 0.01), but there was no significant difference in the “C+EA” group (p > 0.05). Compared with the “M” group, the lung weight in the “M+EA” and “M+MTP” groups increased significantly (p < 0.05). Similar to the pattern seen with body weight, there was no significant difference in lung weight between the “M+EA” and “M+MTP” groups (p > 0.05) (Figure 1B).
Effect on pulmonary function:
Compared with the “C” group, the Cdyn in the “M” group decreased significantly (p < 0.05), while the RL increased significantly (p < 0.01); however, there were no significant differences in these parameters in the “C+EA” group (p > 0.05). Compared with the “M” group, the Cdyn in the “M+EA” group increased, while RL decreased (p < 0.05 for both). On the other hand, compared with the “M” group, “M+MTP” group exhibited no significant change in Cdyn (although there was increasing trend, it did not reach statistical significance, p > 0.05), while it blocked the MFR-induced increase in RL (p < 0.05). Notably, there were no significant differences in Cdyn and RL values between the “M+EA” and “M+MTP” groups (p > 0.05) (Figure 1C and 1D).
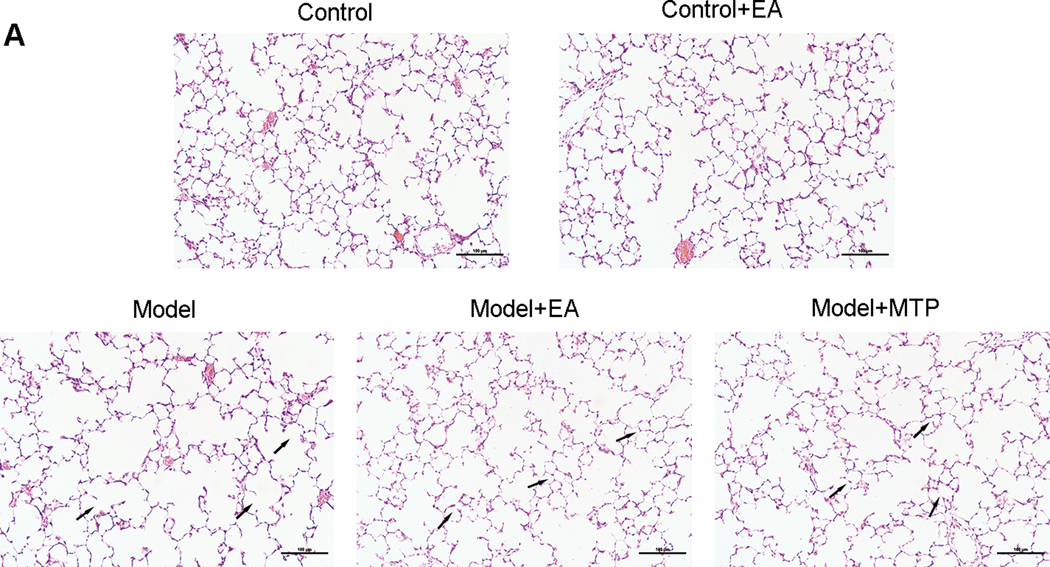
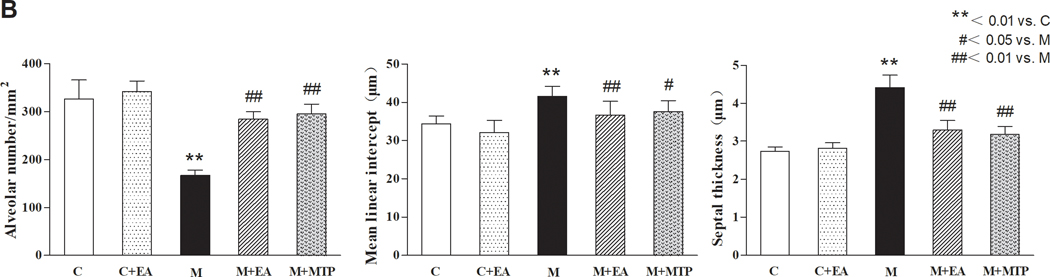
3.2. Effect of maternal EA on MFR-induced changes in offspring lung morphology
Compared with the “C” group, the alveolar number in the “M” group decreased, while the mean linear intercept and septal thickness increased significantly (p < 0.01 for all); however, there were no significant differences in the “C+EA” group in these parameters (p > 0.05). Compared with the “M” group, the alveolar number in the “M+EA” and “M+MTP” groups increased significantly (p < 0.01), and the mean linear intercept and septal thickness decreased significantly (p < 0.05 and p < 0.01, respectively). There were no significant differences in these parameters between the “M+EA” and “M+MTP” groups (p > 0.05) (Figure 2).
Figure 2.
Effect of maternal EA on MFR-induced changes in offspring lung morphology. (A) Representative H&E-stained lung sections. Magnification ×20; arrows point to the integrity and/or rupture of alveolar walls. (B) Alveolar number, mean linear intercept and septal thickness. Values are mean ± SD; n=6 for each group.
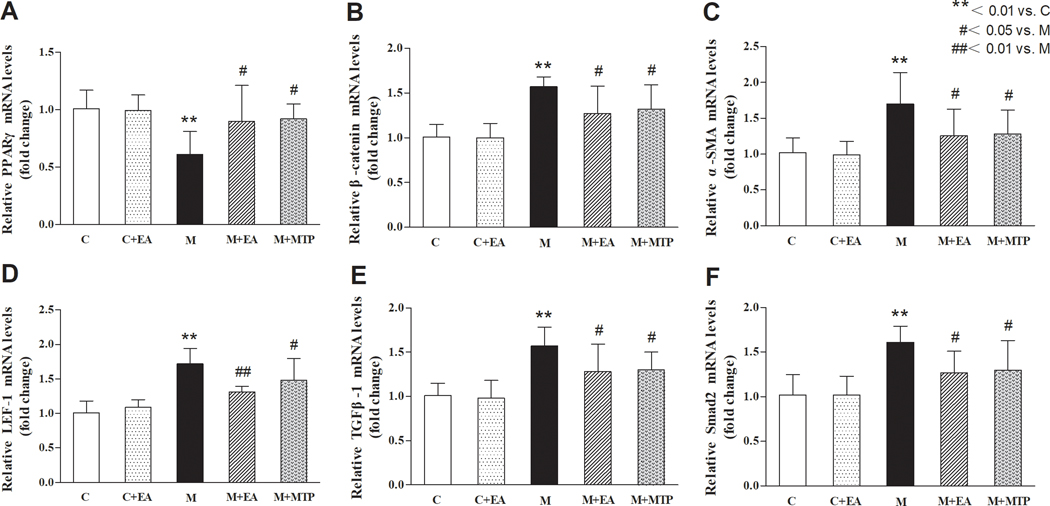
3.3. Effects of maternal EA on MFR-induced changes in key developmental signaling pathways in offspring lung
Real-time PCR assay showed that compared with “C” group, the lung PPARγ mRNA levels in the “M” group decreased significantly (p < 0.01), and the levels of β-catenin, α-SMA, LEF-1, TGF-β1, and Smad2 mRNAs significantly increased (p < 0.01 for all), but there were no significant differences in these markers in the “C+EA” group (p > 0.05). Compared with the “M” group, the levels of PPARγ mRNA in the “M+EA” and “M+MTP” groups increased significantly (p < 0.05), and the levels of β-catenin, α-SMA, LEF-1, TGF-β1, and Smad2 mRNAs decreased significantly (p < 0.05, p < 0.01, respectively). There were no significant differences in the expression of these genes between the “M+EA” and “M+MTP” groups (p > 0.05) (Figure 3).
Figure 3.
Effects of maternal EA on MFR-induced changes in key developmental mRNAs in offspring lung. (A) PPARγ mRNA. (B) β-catenin mRNA. (C) α-SMA mRNA. (D) LEF-1 mRNA. (E) TGF-β1 mRNA. (F) Smad2 mRNA. Values are mean ± SD; n=6 for each group.
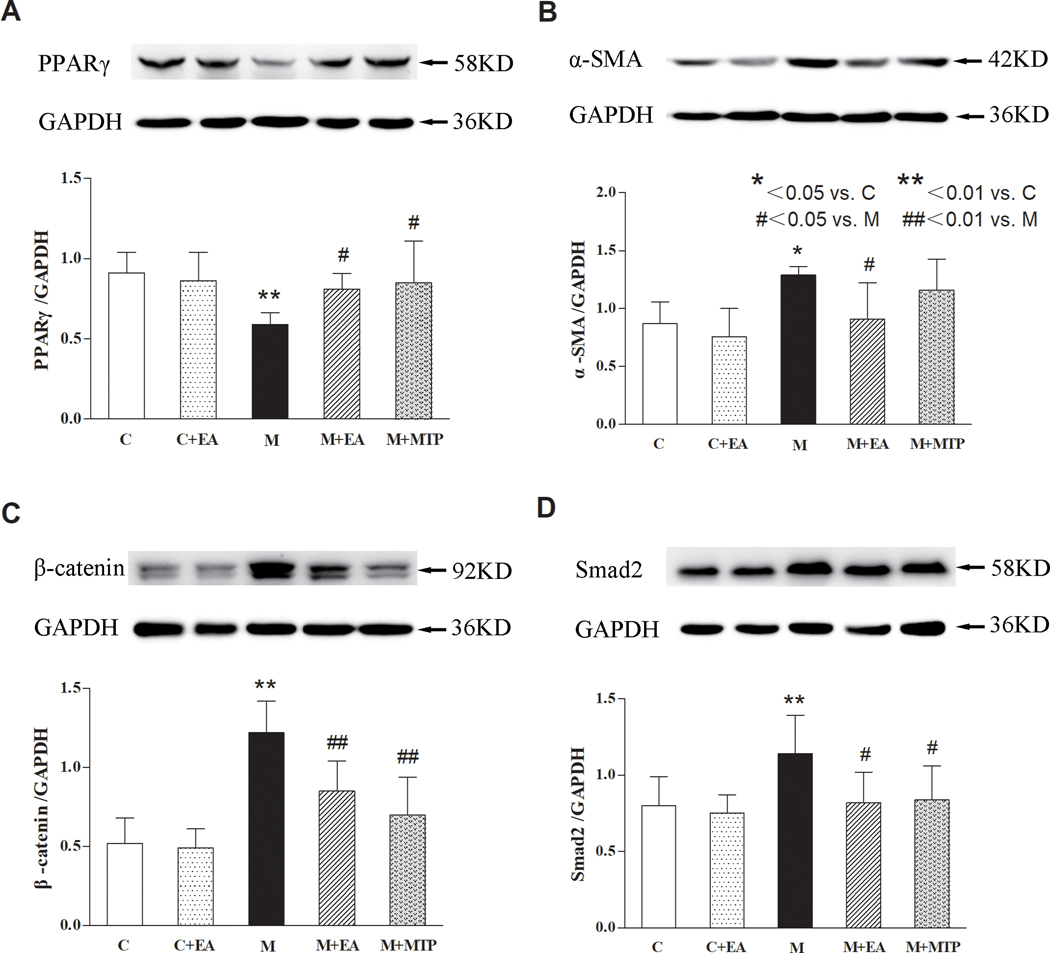
Next, using western analysis, we determined if lung lysate protein levels of PPARγ, α-SMA, β-catenin, and Smad2 reflect changes in the mRNA levels of these proteins. Compared with the “C” group, the lung PPARγ protein levels in the “M” group decreased significantly (p < 0.01), the lung α-SMA, β-catenin, and Smad2 protein levels in the “M” group increased significantly (p < 0.01 for all), but there were no significant differences in these markers in the “C+EA” group (p > 0.05). Compared with the “M” group, the lung PPARγ protein levels in the “M+EA” and “M+MTP” groups increased (p < 0.05), the lung β-catenin and Smad2 protein levels decreased (p < 0.05 and p < 0.01, respectively). On the other hand, compared with the “M” group, the α-SMA protein levels in the “M+EA” group decreased (p < 0.05), but there was no significant difference in the M+MTP group (p > 0.05). Notably, there were no significant differences in the expression of these proteins between the “M+EA” and “M+MTP” groups (p > 0.05) (Figure 4).
Figure 4.
Effects of maternal EA on MFR-induced changes in key developmental proteins in offspring lung. (A) PPARγ protein. (B) α-SMA protein. (C) β-catenin protein. (D) Smad2 protein. Values are mean ± SD; n=4 or 6 for each group.
3.4. Effects of maternal EA on MFR-induced changes in offspring serum and lung CORT and GR levels
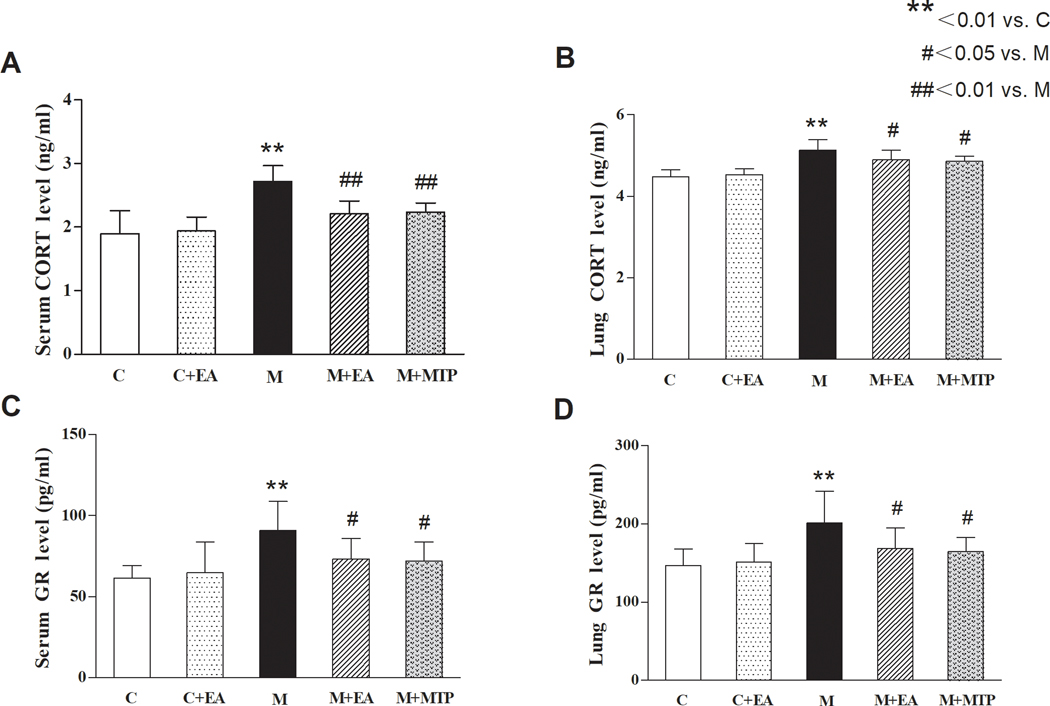
ELISA showed that compared with the “C” group, serum and lung CORT and GR levels in the “M” group increased significantly (p < 0.01 for both), but there was no significant difference in the “C+EA” group (p > 0.05). Compared with the “M” group, serum and lung CORT and GR levels in the “M+EA” and “M+MTP” groups decreased significantly (p < 0.05 and p < 0.01, respectively), while there was no significant difference between the “M+EA” and “M+MTP” groups (p > 0.05) (Figure 5).
Figure 5.
Effects of EA on MFR-induced changes in offspring serum and lung CORT and GR levels. (A) Serum CORT levels. (B) Lung CORT levels. (C) Serum GR levels. (D) Lung GR levels. Values are mean ± SD; n=6 for each group.
4. Discussion
Exposure to several maternal, placental, or fetal risk factors, including but limited to maternal malnutrition, can lead to IUGR [1] structural and functional impairment of organs in offspring, and increase the susceptibility to bronchopulmonary dysplasia, asthma, and other chronic lung diseases [7–10]. Malnutrition is the most common cause of IUGR, with nutritional insufficiency being the most common contributor in developing countries [3,4]. The essence of IUGR is the adaptive protection of the fetus against insufficient nutrient supply, with the potential of long-term effects on organs such as the lung [11,28,29]. The adverse effects on lung development have been widely addressed in previous studies [5,6,16]. However, there is no specific clinical intervention to prevent MFR-induced pulmonary dysplasia in offspring [1]. However, some animal studies have shown that the glucocorticoid synthesis inhibitor MTP can effectively improve the lung morphology and function in MFR-exposed offspring rats [5,6]. Due to lack of clinical data, possibly related to MTP’s expected side effects, this treatment has not translated into clinical practice. Therefore, further work to explore a safe and effective treatment is warranted.
As an integral part of the Chinese medical system, acupuncture has the advantage of reliable safety and efficacy in preventing and treating many clinical entities. ST 36 is one of the most frequently used acupoints in the TCM. In addition to improving general well-being, it is known to exert beneficial effects on diseases of the digestivee [30], motor [31], and immune systems [32]. And via its impact on the HPA axis, it exerts a protective effect on the respiratory system [22–25].
In line with previous studies, birthweight, the main criterion to diagnose IUGR, was significantly lower in the “M” group compared with that in the “C” group [5,6]. Similarly, as expected, MFR resulted in decreased lung weight, alveolar number, and Cdyn, increased mean linear intercept, and RL in offspring rats, which are all consistent with previous studies [5,6]. Electro-acupuncture applied to maternal ST 36 normalized the MFR-induced changes of birth weight, lung weight, and lung morphology and function. Furthermore, it blocked the decrease of PPARγ, and the increase of β-catenin, α-SMA, and LEF-1 in offspring rat lungs, induced by MFR, with this effect equivalent to that of MTP. In line with our previous work, these findings suggest that the protection against MFR-induced offspring pulmonary dysplasia by EA applied to maternal ST 36 is likely mediated via the upregulation of the PTHrP/PPARγ signaling and the downregulation of the Wnt/β-catenin signaling [5,6,16].
This study also showed that MFR significantly up-regulated the expression of TGF-β1 and Smad2, which are key modulators of the myogenic marker α-SMA, suggesting the transformation of fibroblasts to myofibroblasts in MFR offspring lungs. Electro-acupuncture at maternal ST 36 normalized these MFR-induced changes, and the beneficial effects were equivalent to those of MTP. Therefore, a reduction in the expression of myogenic extracellular matrix-regulating pathway markers (TGF-β1 and Smad2) and the maintenance of the lung’s lipogenic phenotype (increased PTHrP-PPARγ levels) as a result of maternal EA likely significantly contribute to protection against MFR-induced pulmonary dysplasia.
The previous work has also shown that MFR-induced chronic hypercorticoid state is related to HPA upregulation, which specifically impairs normal lung development and it can be effectively blocked by MTP [5,6]. The present study also showed that the serum and lung CORT and GR levels of MFR offspring were significantly up-regulated, suggesting that the MFR-induced pulmonary dysplasia, at least in part, maybe related to glucocorticoid overexposure. EA applied to maternal ST 36 effectively blocked the overexpression of CORT and GR, ensuring the normal development of the lung, and this protective effect was equivalent to that of MTP. Taken together, our data offer a novel and safe, non-pharmacologic approach to prevent MFR-induced pulmonary dysplasia in offspring, a condition for which, as yet, there is no effective preventive or therapeutic intervention.
Acknowledgment
This research was funded by grants from the National Natural Sciences Foundation of China (NO. 81674059), the Tobacco-Related Disease Research Program (23RT-0018, 27IP-0050, and T29IR0737), and the National Institutes of Health (HL151769).
Footnotes
Conflict of interests:
The authors declare that there are no conflict of interests.
Data Availability:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].ACOG Practice Bulletin No. 204: Fetal Growth Restriction. Obstet Gynecol, 2019, 133(2): e97–e109. [DOI] [PubMed] [Google Scholar]
- [2].Biesiada L, Sakowicz A, Grzesiak M, Borowiec M, Lisowska M, Pietrucha T, Von Kaisenberg C, Lewandowski K. Identification of placental genes linked to selective intrauterine growth restriction (IUGR) in dichorionic twin pregnancies: gene expression profiling study. Hum Genet, 2019, 138(6): 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Armengaud JB, Yzydorczyk C, Siddeek B, Peyter AC, Simeoni U. Intrauterine growth restriction: clinical consequences on health and disease at adulthood. Reprod Toxicol, 2020. [DOI] [PubMed] [Google Scholar]
- [4].Sharma D, Sharma P, Shastri S. Genetic, metabolic and endocrine aspect of intrauterine growth restriction: an update. J Matern Fetal Neonatal Med, 2017, 30(19): 2263–2275. [DOI] [PubMed] [Google Scholar]
- [5].Paek DS, Sakurai R, Saraswat A, Li Y, Khorram O, Torday JS, Rehan VK. Metyrapone alleviates deleterious effects of maternal food restriction on lung development and growth of rat offspring. Reprod Sci, 2015, 22(2): 207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rehan VK, Li Y, Corral J, Saraswat A, Husain S, Dhar A, Sakurai R, Khorram O, Torday JS. Metyrapone blocks maternal food restriction-induced changes in female rat offspring lung development. Reprod Sci, 2014, 21(4): 517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lv Y, Fu L, Zhang Z, Gu W, Luo X, Zhong Y, Xu S, Wang Y, Yan L, Li M, et al. Increased Expression of MicroRNA-206 Inhibits Potassium Voltage-Gated Channel Subfamily A Member 5 in Pulmonary Arterial Smooth Muscle Cells and Is Related to Exaggerated Pulmonary Artery Hypertension Following Intrauterine Growth Retardation in Rats. J Am Heart Assoc, 2019, 8(2): e010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu XF, Hu QY, Liang LF, Wu L, Gu WZ, Tang LL, Fu LC, Du LZ. Epigenetics of hyper-responsiveness to allergen challenge following intrauterine growth retardation rat. Respir Res, 2014, 15: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lio A, Rosati P, Pastorino R, Cota F, Tana M, Tirone C, Aurilia C, Ricci C, Gambacorta A, Paladini A, et al. Fetal Doppler velocimetry and bronchopulmonary dysplasia risk among growth-restricted preterm infants: an observational study. BMJ Open, 2017, 7(7): e015232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Looi K, Kicic A, Noble PB, Wang KCW. Intrauterine growth restriction predisposes to airway inflammation without disruption of epithelial integrity in postnatal male mice. J Dev Orig Health Dis, 2020: 1–9. [DOI] [PubMed] [Google Scholar]
- [11].Abbas G, Shah S, Hanif M, Shah A, Rehman AU, Tahir S, Nayab K, Asghar A. The frequency of pulmonary hypertension in newborn with intrauterine growth restriction. Sci Rep, 2020, 10(1): 8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Torday JS, Sanchez-Esteban J, Rubin LP. Paracrine mediators of mechanotransduction in lung development. Am J Med Sci, 1998, 316(3): 205–8. [DOI] [PubMed] [Google Scholar]
- [13].Schultz CJ, Torres E, Londos C, Torday JS. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol, 2002, 283(2): L288–96. [DOI] [PubMed] [Google Scholar]
- [14].Torday J, Rehan V. Neutral lipid trafficking regulates alveolar type II cell surfactant phospholipid and surfactant protein expression. Exp Lung Res, 2011, 37(6): 376–86. [DOI] [PubMed] [Google Scholar]
- [15].Jiang JS, Chou HC, Yeh TF, Chen CM. Maternal nicotine effects on vascular endothelial growth factor expression and morphometry in rat lungs. Early Hum Dev, 2012, 88(7): 525–9. [DOI] [PubMed] [Google Scholar]
- [16].Rehan VK, Sakurai R, Li Y, Karadag A, Corral J, Bellusci S, Xue YY, Belperio J, Torday JS. Effects of maternal food restriction on offspring lung extracellular matrix deposition and long term pulmonary function in an experimental rat model. Pediatr Pulmonol, 2012, 47(2): 162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mcgowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol, 1997, 59: 43–62. [DOI] [PubMed] [Google Scholar]
- [18].Massagué J. TGF-beta signal transduction. Annu Rev Biochem, 1998, 67: 753–91. [DOI] [PubMed] [Google Scholar]
- [19].Alejandre Alcázar MA, Morty RE, Lendzian L, Vohlen C, Oestreicher I, Plank C, Schneider H, Dötsch J. Inhibition of TGF-β signaling and decreased apoptosis in IUGR-associated lung disease in rats. PLoS One, 2011, 6(10): e26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Louzada RA, Corre R, Ameziane El Hassani R, Meziani L, Jaillet M, Cazes A, Crestani B, Deutsch E, Dupuy C. NADPH oxidase DUOX1 sustains TGF-β1 signalling and promotes lung fibrosis. Eur Respir J, 2020. [DOI] [PubMed] [Google Scholar]
- [21].Menendez-Castro C, Fahlbusch F, Cordasic N, Amann K, Münzel K, Plank C, Wachtveitl R, Rascher W, Hilgers KF, Hartner A. Early and late postnatal myocardial and vascular changes in a protein restriction rat model of intrauterine growth restriction. PLoS One, 2011, 6(5): e20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu Y, Ji B, Zhao G, Su H, Ge Y, Dai J, Lu Y, Sakurai R, Rehan VK. Protective effect of electro-acupuncture at maternal different points on perinatal nicotine exposure-induced pulmonary dysplasia in offspring based on HPA axis and signal transduction pathway. Biochem Biophys Res Commun, 2018, 505(2): 586–592. [DOI] [PubMed] [Google Scholar]
- [23].Ji B, Zhao GZ, Sakurai R, Cao Y, Zhang ZJ, Wang D, Yan MN, Rehan VK. Effect of Maternal Electroacupuncture on Perinatal Nicotine Exposure-Induced Lung Phenotype in Offspring. Lung, 2016, 194(4): 535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dai J, Ji B, Zhao G, Lu Y, Liu Y, Mou Q, Sakurai R, Xie Y, Zhang Q, Xu S, et al. Developmental Timing Determines the Protective Effect of Maternal Electroacupuncture on Perinatal Nicotine Exposure-Induced Offspring Lung Phenotype. Biomed Res Int, 2020, 2020: 8030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu Y, Ji B, Zhao G, Dai J, Sakurai R, Liu Y, Mou Q, Xie Y, Zhang Q, Xu S, et al. Comparison of Protective Effects of Electroacupuncture at ST 36 and LU 5 on Pulmonary and Hypothalamic Pituitary Adrenal Axis Changes in Perinatal Nicotine-Exposed Rats. Biomed Res Int, 2020, 2020: 3901528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sreekantha S, Wang Y, Sakurai R, Liu J, Rehan VK. Maternal food restriction-induced intrauterine growth restriction in a rat model leads to sex-specific adipogenic programming. Faseb j, 2020, 34(12): 16073–16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zana-Taieb E, Pham H, Franco-Montoya ML, Jacques S, Letourneur F, Baud O, Jarreau PH, Vaiman D. Impaired alveolarization and intra-uterine growth restriction in rats: a postnatal genome-wide analysis. J Pathol, 2015, 235(3): 420–30. [DOI] [PubMed] [Google Scholar]
- [28].Terstappen F, Lely AT. Long-term renal disease after prematurity or fetal growth restriction: who is at risk? Nephrol Dial Transplant, 2020, 35(7): 1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gilchrist CP, Cumberland AL, Kondos-Devcic D, Hill RA, Khore M, Quezada S, Reichelt AC, Tolcos M. Hippocampal neurogenesis and memory in adolescence following intrauterine growth restriction. Hippocampus, 2020: e23291. [DOI] [PubMed] [Google Scholar]
- [30].Bao CH, Wang CY, Li GN, Yan YL, Wang D, Jin XM, Wu LY, Liu HR, Wang XM, Shi Z, et al. Effect of mild moxibustion on intestinal microbiota and NLRP6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome. World J Gastroenterol, 2019, 25(32): 4696–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu YP, Luo ZR, Wang C, Cai H, Zhao TT, Li H, Shao SJ, Guo HD. Electroacupuncture Promoted Nerve Repair After Peripheral Nerve Injury by Regulating miR-1b and Its Target Brain-Derived Neurotrophic Factor. Front Neurosci, 2020, 14: 525144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang Z, Yi T, Long M, Ding F, Ouyang L, Chen Z. Involvement of the Negative Feedback of IL-33 Signaling in the Anti-Inflammatory Effect of Electro-acupuncture on Allergic Contact Dermatitis via Targeting MicroRNA-155 in Mast Cells. Inflammation, 2018, 41(3): 859–869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.