In the past three decades, imaging techniques that offer glimpses of the living, working brain have redefined the study of human cognition. Functional magnetic resonance imaging, or fMRI, has led the way. Based on changes in blood flow and oxygenation, these high-resolution measures present a compelling view of the mechanisms of mental function. But MRI’s requirements—in particular, that a subject remain still for an extended period within the confines of a large, noisy magnet—limit the types of behaviors that can be examined. And its considerable infrastructure means that fMRI is only accessible at a dedicated facility.
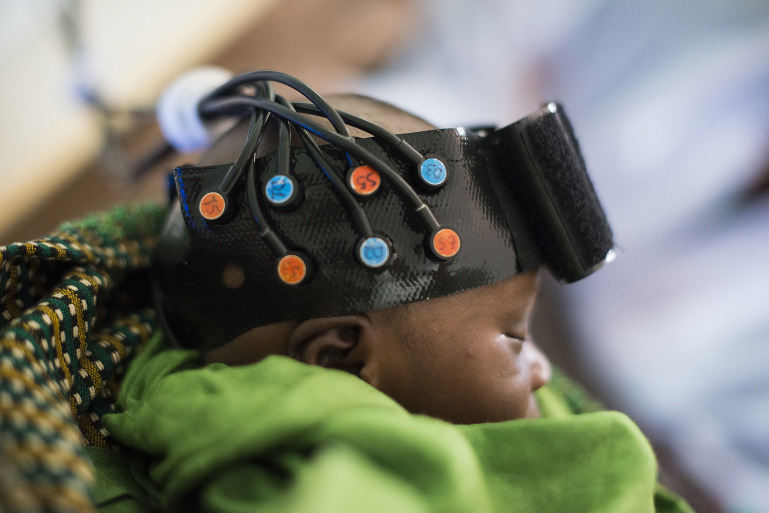
Researchers are using fNIRS to explore brain activity in populations and places once thought to be inaccessible. Image credit: Ciara Crocker (photographer).
Functional near-infrared spectroscopy, or fNIRS, affords a view into the brain based on blood oxygenation without the need for a big, immobile scanner. This optical imaging technique detects changes in how hemoglobin absorbs near-infrared light—usually wavelengths between 750 and 1,200 nanometers. And like fMRI, fNIRS provides an indirect measure of localized brain activity (1, 2). In the last 40 years, researchers have explored the technique’s potential to augment studies of brain function. Now, fNIRS has advanced from relatively simple measures of blood-oxygen changes to a sophisticated method of recording real-time brain responses associated with a wide variety of activities and cognitive tasks.
Although fNIRS has lower spatial resolution than fMRI, fNIRS does have a big advantage: It allows subjects to freely move, speak, and interact with people and environments while being scanned. The light sources and detectors of fNIRS can be mounted on a wearable cap, much like electroencephalography (EEG) electrodes, making a portable, lightweight, inexpensive array that can be worn in a variety of settings. Armed with this freedom, researchers are using fNIRS to explore brain activity in populations and places once thought to be inaccessible. The hope: to answer questions about how the brain functions during dynamic, real-world interactions.
“The next frontier is studying the human brain function that makes us human,” says David Boas, a biomedical engineer who directs the Neurophotonics Center at Boston University (BU), MA; “social interactions, movements, and how we perceive the world around us.”
New View on the Brain
In 1977, Frans Jöbsis, a physiologist at Duke University in Durham, NC, described the optical properties of brain tissue that make it relatively transparent to near-infrared light (3). Because the oxygenated and deoxygenated forms of hemoglobin absorb these wavelengths differently, measuring patterns of near-infrared absorption with an external detector could noninvasively monitor real-time changes in blood oxygenation levels in the cortex of a cat.
By the mid-1980s, Marco Ferrari, then a researcher at the Italian National Institute of Health in Rome, adapted near-infrared spectroscopy for humans with a detector that could measure changes in prefrontal cortex oxygenation in both adults and infants with possible brain injuries (4). In 1993, multiple research groups published the first evidence correlating NIRS signals with regional blood flow in the cerebral cortex during cognitive tasks, producing the first “functional” NIRS measurements in humans (1).
Since then, fNIRS has advanced as a research technique, although largely in the shadow of rapidly developing fMRI capabilities, which built on the established clinical use of structural NMR imaging. fNIRS was never expected to compete with fMRI, Boas points out, but to guide interpretation of blood oxygenation signals and complement fMRI’s use. Light scattering limits fNIRS signals to the outer two centimeters of the brain, with a spatial resolution of about two to three centimeters—lower than fMRI but higher than EEG. And although somewhat limited by the time lag of the blood-flow responses, which occur on a scale of seconds rather than the millisecond-scale of neural impulses, fNIRS offers much better temporal resolution than fMRI (2, 5). Moreover, technological advances have improved both detector hardware and signal processing software, allowing researchers to develop denser arrays that capture NIRS signals at multiple wavelengths and across a larger area of the brain.
fNIRS often isn’t used in isolation, however. Many studies still partner fNIRS with other modalities to take advantage of complementary strengths. Combining fNIRS with wearable EEG systems, for example, “can give us information both about the spatial and the temporal aspects of neural activation,” explains Judit Gervain, a professor of developmental psychology at the University of Padua, Italy, and researcher at the Integrative Neuroscience and Cognition Center in Paris.
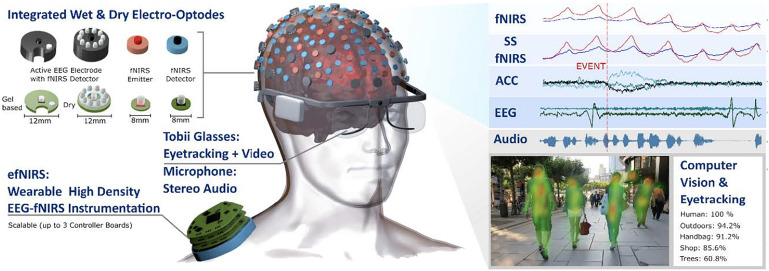
Looking to leverage the advantages of fNIRS with other technologies, a group of researchers is working on something they call hybrid wearable high-density fNIRS-EEG instrumentation (efNIRS), combined with eye-tracking. They expect they’ll achieve long-duration continuous monitoring of normal and altered brain activity during movement, perception, and social interaction in real time. Image credit: Reprinted from Ref. 6, with permission from Elsevier.
Out of the Scanner
The portability of fNIRS systems is allowing researchers to scrutinize the brain activity of subjects who are on the move. Boas and his team at BU are designing whole-head, portable systems for a project they call Neuroscience of the Everyday World, which aims to understand how the brain works in dynamic, multisensory, real-world settings (6). “We’ll have people walking around campus, and we’ll be measuring their brain activity,” Boas says. The system can simultaneously collect EEG, fNIRS, and eye tracking data, as well as recordings of the visual and auditory environment. And associate professor of occupational therapy Simone Gill, working with autistic children, is using fNIRS to understand the workload in brain areas associated with kids keeping their balance, especially while doing other tasks such as using an object or answering a question. “Maybe, if we understand the relationship between motor function and cognition, there are specific interventions that we could use to target both,” says Gill, who’s affiliated with the BU Neurophotonics Center.
But the technology is proving especially valuable for exploring the underpinnings of language in the brain. Researchers are investigating, for example, how, in the wake of a stroke or other injury, undamaged parts of a patient’s brain can compensate to recover the ability to speak. fMRI poses challenges when studying communication, says Swathi Kiran, a professor of speech, language, and hearing sciences at the BU Neurophotonics Center. “Every time we try to have [patients] speak, the scanner detects motion, and we don't get good data.” There are technical workarounds, she adds, but most are harder to use for stroke patients.
Language production requires coordinated activity across multiple brain regions, Kiran notes, and fNIRS excels at discerning these types of temporal patterns. Stroke patients struggle to describe simple objects from a picture (e.g., a blue couch, something to sit on), Kiran says; their brain signals are delayed or of much lower amplitude than in healthy individuals. Her group has found that during language tasks, stroke patients’ brains engage areas outside typical language processing networks (7). These abnormal patterns are “a good starting point for us to see if that can change as a function of rehabilitation,” she says.
Probing the Youngest Brains
The portability and flexibility of fNIRS makes it possible to study language in even the youngest humans. Looking to understand auditory cues that infants use to detect and understand language, Gervain, in Padua, studies newborns, just one to three days old, while they are still in the hospital. At this age, “their behavioral repertoire is quite limited,” she says. But their auditory cortex is active, even during sleep.
The researchers can bring a wearable array containing both fNIRS and EEG sensors right to the babies’ hospital rooms. “We just snap the cap on their heads,” she says, then record brain responses to a series of auditory cues for 20 to 30 min as the newborn sleeps. “It's extremely baby-friendly.”
Babies learn the rhythm and intonation of speech patterns in the womb, Gervain says. Even at birth, the same brain regions used by adults—parts of the left frontotemporal cortex including Broca’s area—are responsive to language structure, differences in syllable sounds, and functional exchanges between speakers (8, 9). A clearer understanding of the stages of language development after birth could help to identify children who would benefit from early interventions. “By the time kids get to school and problems come up, language has already developed and the critical period is essentially over,” Gervain says.
The early-development applications of fNIRS extend to global health projects. With funding from the Gates Foundation, Clare Elwell, a professor of medical physics at University College London, UK, and colleague Sarah Lloyd-Fox took an fNIRS system to the Gambia in western Africa to study infants’ responses to auditory and visual stimuli. “We were able to arrive at the field station and set up the equipment and get our first study within two hours,” Elwell recalls. Working with Gambian researchers, they now run the Brain Imaging for Global Health (BRIGHT) study, a longitudinal cohort study of children’s cognitive development from one month of age up to five years in the Gambia and two years in the United Kingdom (10).
For the Gambian babies, the primary risk is malnutrition. Layered on to that are other effects of poverty, such as low-quality health care, high levels of infection, or lack of early education [see also "The neuroscience of poverty" (11)]. Brain imaging over time can give the clearest idea of which regions are vulnerable to these risks and which are more resilient. One emerging finding: Gambian babies appear to have weaker brain responses to novel stimuli compared with UK infants, a result that Elwell says may reflect an impact on executive functions related to working memory (12). These comparisons can help to pinpoint how environmental factors affect the children’s neural development and inform targeted interventions. “One of the main aims of the BRIGHT project was to develop reference curves for brain function for these infants, in the same way that you might be familiar with growth curves for babies,” she says.
Using fNIRS and working with Gambian researchers as part of the BRIGHT project, UK researchers are seeking to better understand cognitive development by following a longitudinal cohort of children ages one month to five years. Image credit: Clare Elwell and The Bill and Melinda Gates Foundation.
Do You See What I See?
The attributes of fNIRS allow for insights not only into brain development and the foundations of language but also into the basis of nonverbal communication. fNIRS is pushing frontiers in social interaction studies via so-called hyperscanning experiments that examine two people as they interact. Researchers look for correlations between neural activity in known locations across the two brains. “The hypothesis is that this interbrain synchrony represents a kind of sharing of information,” says Joy Hirsch, who runs the Brain Function Laboratory at Yale University in New Haven, CT.
Her group has found a strong degree of synchrony between the brains of healthy participants engaged in live interpersonal interactions, such as eye contact (13). Newer work with people with autism suggests that they process eye gaze using different neural systems than in neurotypical individuals. “It's not a deficit in the normal system,” she says. “It is a different system.”
Hirsch is now exploring the transmission of emotional information via facial expressions. As one study participant (the sender) watches short video clips designed to evoke an emotive facial expression—frolicking puppies, creeping spiders, and so on—a receiving individual who cannot see the video watches the sender’s face. Similar to the eye gaze experiments, Hirsch expects to find synchrony between the sending and receiving brains, indicative of “emotional contagion.”
Getting to the Clinic
Growing interest in the research applications of fNIRS has spurred production of a new generation of wearable devices, propelling fNIRS further into mainstream use. But despite what Ferrari sees as a potential “unique advantage in clinics,” he says, it is still not clear which clinical applications would benefit most from fNIRS.
Amid that expansion, it is critical for researchers to remain question-driven rather than getting caught up in technical capabilities, Elwell says. “I think we're at an interesting crossroads now with the technology.” There’s the potential to tap into the burgeoning field of personalized consumer health—an extension, perhaps, of tracking one’s sleep patterns with a smartwatch or oxygen saturation with a fingertip sensor. But the science isn’t yet able to provide adequate guidance for interpreting fNIRS data in such settings, she says.
“There's a lot of ‘Silicon Valley’ hype,” says Hirsch. “Mucking about with signals that don’t carry a lot of meaning.” Rigorous experimental design will continue to be essential for validating fNIRS results, she notes. “But there is a real trend toward democratizing the science, which fascinates me.”
References
- 1.Almajidy R. K., Mankodiya K., Abtahi M., Hofmann U. G.,A newcomer’s guide to functional near infrared spectroscopy experiments. IEEE Rev. Biomed. Eng. 13, 292–308 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Pinti P., et al. , The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 1464, 5–29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jöbsis F. F., Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198, 1264–1267 (1977). [DOI] [PubMed] [Google Scholar]
- 4.Ferrari M., Giannini I., Sideri G., Zanette E., Continuous non invasive monitoring of human brain by near infrared spectroscopy. Adv. Exp. Med. Biol. 191, 873–882 (1985). [DOI] [PubMed] [Google Scholar]
- 5.Logothetis N. K., The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 1003–1037 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Lühmann A., et al. , Towards Neuroscience of the Everyday World (NEW) using functional near-infrared spectroscopy. Curr. Opin. Biomed. Eng. 18, 100272 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmore N., Yücel M. A., Li X., Boas D. A., Kiran S., Investigating language and domain-general processing in neurotypicals and individuals with aphasia - A functional near-infrared spectroscopy pilot study. Front. Hum. Neurosci. 15, 728151 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forgács B., Tauzin T., Gergely G., Gervain J., The newborn brain is sensitive to the communicative function of language. Sci. Rep. 12, 1220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera L., Gervain J., Speech perception at birth: The brain encodes fast and slow temporal information. Sci. Adv. 6, eaba7830 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global fNIRS: Brain imaging for global health. https://www.globalfnirs.org/ Accessed 10 April 2022.
- 11.Katsnelson A., The neuroscience of poverty. Proc. Natl. Acad. Sci. U.S.A. 112, 15530–15532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Fox S., et al. ; BRIGHT Project Team, Habituation and novelty detection fNIRS brain responses in 5- and 8-month-old infants: The Gambia and UK. Dev. Sci. 22, e12817 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noah J. A., et al. , Real-time eye-to-eye contact is associated with cross-brain neural coupling in angular gyrus. Front. Hum. Neurosci. 14, 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]