Significance
Organisms face many new threats in the Anthropocene Epoch. We examine exposure to elevated concentrations of heavy metals as a paradigmatic threat, using fruit flies as a model organism. Release of metals into the biosphere has increased sharply due to human activities. We found that flies are capable of using their gustatory system to avoid feeding or laying eggs on nine metal ions. Such avoidance is conserved in the mosquito Aedes albopictus, which is a vector of disease. We define molecular and cellular underpinnings of the avoidance response. Our results suggest that mechanisms of taste avoidance provide a first line of defense against these stressors in the Anthropocene.
Keywords: Drosophila, metal, taste, Anthropocene
Abstract
The Anthropocene Epoch poses a critical challenge for organisms: they must cope with new threats at a rapid rate. These threats include toxic chemical compounds released into the environment by human activities. Here, we examine elevated concentrations of heavy metal ions as an example of anthropogenic stressors. We find that the fruit fly Drosophila avoids nine metal ions when present at elevated concentrations that the flies experienced rarely, if ever, until the Anthropocene. We characterize the avoidance of feeding and egg laying on metal ions, and we identify receptors, neurons, and taste organs that contribute to this avoidance. Different subsets of taste receptors, including members of both Ir (Ionotropic receptor) and Gr (Gustatory receptor) families contribute to the avoidance of different metal ions. We find that metal ions activate certain bitter-sensing neurons and inhibit sugar-sensing neurons. Some behavioral responses are mediated largely through neurons of the pharynx. Feeding avoidance remains stable over 10 generations of exposure to copper and zinc ions. Some responses to metal ions are conserved across diverse dipteran species, including the mosquito Aedes albopictus. Our results suggest mechanisms that may be essential to insects as they face challenges from environmental changes in the Anthropocene.
Life on Earth faces great challenges in the Anthropocene Epoch (1–5). Animal, plant, and microbial life are increasingly exposed to stresses they have not experienced before in their evolutionary history (5–7). Their ability to adapt to these stresses will have a major influence on the future of the biome (3, 8–10).
Among these stresses is the exposure of organisms to compounds at higher levels than occur naturally in their environments (4, 11, 12). The land, air, and water of the planet now abound with such compounds due to the agricultural, mining, and manufacturing industries (4, 12–14). Many of these compounds, organic and inorganic, are toxic at high levels. A critical problem for many species is to detect the elevated levels of these compounds and respond adaptively.
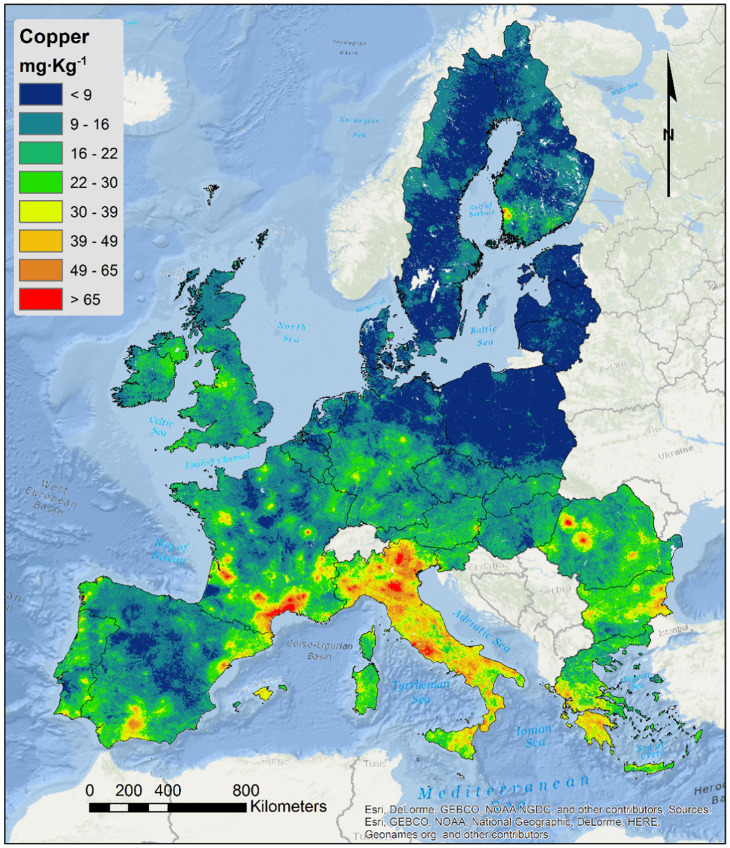
Metals provide a paradigmatic case for investigating how animals sense and respond to anthropogenic chemical threats. Although metals occur naturally in the Earth’s crust, they have been released into the biosphere at rapidly growing levels due to mining, metal-based industry, and the widespread use of metal-based agricultural products (Fig. 1) (12, 13, 15). Metal ions are essential to many physiological processes but are toxic at high concentrations (16, 17).
Fig. 1.
Copper concentrations in European topsoil. Reprinted from ref. 15, which is licensed under CC-BY-4.0.
How do organisms detect metals at unnaturally high concentrations? Do the organisms respond with adaptive behaviors? We chose to examine this problem in Drosophila, whose chemosensory systems have been studied in detail at the molecular and cellular levels (18–20). We examined a variety of heavy metals, with a particular focus on copper and zinc. Both of these metals have become major environmental pollutants from mining, manufacturing, and agriculture, where they are components of pesticides and fungicides (13–15).
Whereas humans detect metal ions in part via a G protein–coupled bitter taste receptor, TAS2R7 (21, 22), and TRPV (transient receptor potential vanilloid) channels have been implicated in copper sensation in Caenorhabditis elegans (23) and mice (24), none of these receptors appear to have orthologs that function as taste receptors in Drosophila. Rather, taste in Drosophila is mediated largely by a family of 60 Gustatory receptors (Grs), which are predicted to contain seven transmembrane domains, and by a family of Ionotropic receptors (Irs); other receptors have been implicated as well (20, 25, 26).
Taste receptors in Drosophila are expressed in gustatory receptor neurons (GRNs) that are contained in taste sensilla (27). Each sensillum has a pore at the tip through which tastants can pass and then activate GRNs within (28). Sensilla contain up to four GRNs, with different GRNs responding to sugars, bitter compounds, or other taste stimuli (29, 30). Taste sensilla are distributed on several organs. The labellum, the major taste organ of the fly head, contains 31 taste sensilla of three morphological classes: short (S), intermediate (I), and long (L) (30, 31). Taste sensilla are also located on the legs, the pharynx, and the wing (27).
Here, we found that different subsets of taste receptors contribute to the detection of nine metal ions when present at high concentrations that the flies experienced rarely, if ever, until the Anthropocene. We documented the avoidance of metal ions in both feeding and egg-laying decisions, and we identified taste organs, neurons, and receptors that contribute to this avoidance. We found that metal ions activate some taste neurons and inhibit others. Receptor mutations have different effects on different avoidance behaviors. Some responses to metal ions are conserved across diverse dipteran species, including a mosquito species. Our results suggest mechanisms that may be essential to the survival of insects as they face environmental stresses of the Anthropocene.
Results
Taste Avoidance of Copper and Zinc Ions.
A variety of metal ions are critical for biological functions but toxic at high concentrations. We first measured the ability of flies to detect high concentrations of copper and zinc ions, which are both anthropogenic stressors in the environment but which differ in many chemical properties (17). We initially tested the responses of flies to these metal ions in two taste paradigms, one that measures feeding preference and one that measures egg-laying preference.
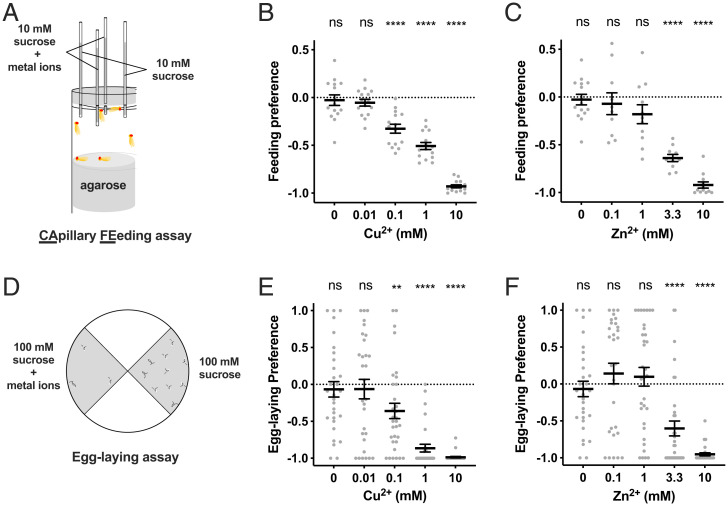
Feeding preference was measured in a capillary feeding assay (CAFE assay; Fig. 2A) (32), in which flies were offered a choice between two sucrose solutions, one containing metal ions and the other a control. Flies showed a strong feeding avoidance of both CuSO4 and ZnSO4 at high (10 mM) concentrations (Fig. 2 B and C). Flies were more sensitive to CuSO4, which was aversive at concentrations as low as 0.1 mM.
Fig. 2.
Feeding and egg-laying avoidance of copper and zinc ions. (A) Schematic drawing of CAFE assay. (B) Feeding preference to 10 mM sucrose mixed with copper at indicated concentrations compared with sucrose alone. n = 15. (C) Feeding preference to 10 mM sucrose mixed with zinc at indicated concentrations compared with sucrose alone. n = 10 to 15. (D) Schematic drawing of egg-laying assay. (E) Egg-laying preference to 100 mM sucrose mixed with copper at indicated concentrations compared with sucrose alone. n = 30 to 32. (F) Egg-laying preference to 100 mM sucrose mixed with zinc at indicated concentrations compared with sucrose alone. n = 30 to 33. Asterisks indicate significant differences from 0 (**P < 0.01, ****P < 0.0001) using Wilcoxon signed rank test. Error bars are SEM. ns, not significant.
To determine whether the avoidance was due to the Cu2+ and Zn2+ cations as opposed to the anions, we first tested CuCl2 and Cu(NO3)2 and found the same results as with CuSO4 (SI Appendix, Fig. S1A). Likewise, ZnCl2 elicited the same response as ZnSO4. As a second test, we examined MgCl2 and found no response (SI Appendix, Fig. S1A). Next, since metal solutions are acidic, we tested the response to an HCl solution at pH 4, slightly more acidic than the 10 mM metal solutions, and found no response (SI Appendix, Fig. S1B). Finally, we replaced sucrose with three other sugar solutions and found that the response to 10 mM CuSO4 was unaffected (SI Appendix, Fig. S1C), even though the amount of sugar solution consumed varied (SI Appendix, Fig. S1D). Taken together, these results support the interpretation that flies avoid feeding on high concentrations of Cu2+ and Zn2+ ions.
An aversion to feeding on these metal ions is likely to be beneficial, since flies maintained on high concentrations of these ions showed a decreased survival rate (SI Appendix, Figs. S1 E and F). Survival rate was not affected when animals were maintained on culture medium at pH 4 (SI Appendix, Fig. S1G).
Egg-laying preference was also deterred by Cu2+ and Zn2+ ions. When given a choice between two substrates, one containing metal ions, flies avoided high concentrations of both CuSO4 and ZnSO4 (Fig. 2 D–F). As in feeding avoidance, flies were more sensitive to CuSO4, which was aversive at concentrations as low as 0.1 mM. Flies showed an equivalent egg-laying avoidance of CuCl2, Cu(NO3)2, and ZnCl2 (SI Appendix, Fig. S1H). Egg-laying preference was not reduced on a substrate containing 10 mM MgCl2, nor was it reduced on a substrate at pH 4 (SI Appendix, Fig. S1I), indicating that the egg-laying aversion is due to the metal cations.
An aversion to egg-laying on these metal ions is also beneficial, in that eggs laid on substrates containing Cu2+ or Zn2+ ions showed impaired development: Few if any eggs developed to the pupal stage at 10 mM concentrations of either metal ion (SI Appendix, Fig. S1J). Animals that survived development were allowed to mate and lay eggs in culture medium of the same concentration. Pupation rates of this second generation were comparable to those of the first generation (SI Appendix, Fig. S1K).
Different Taste Receptors Are Required for Taste Avoidance of Different Metal Ions.
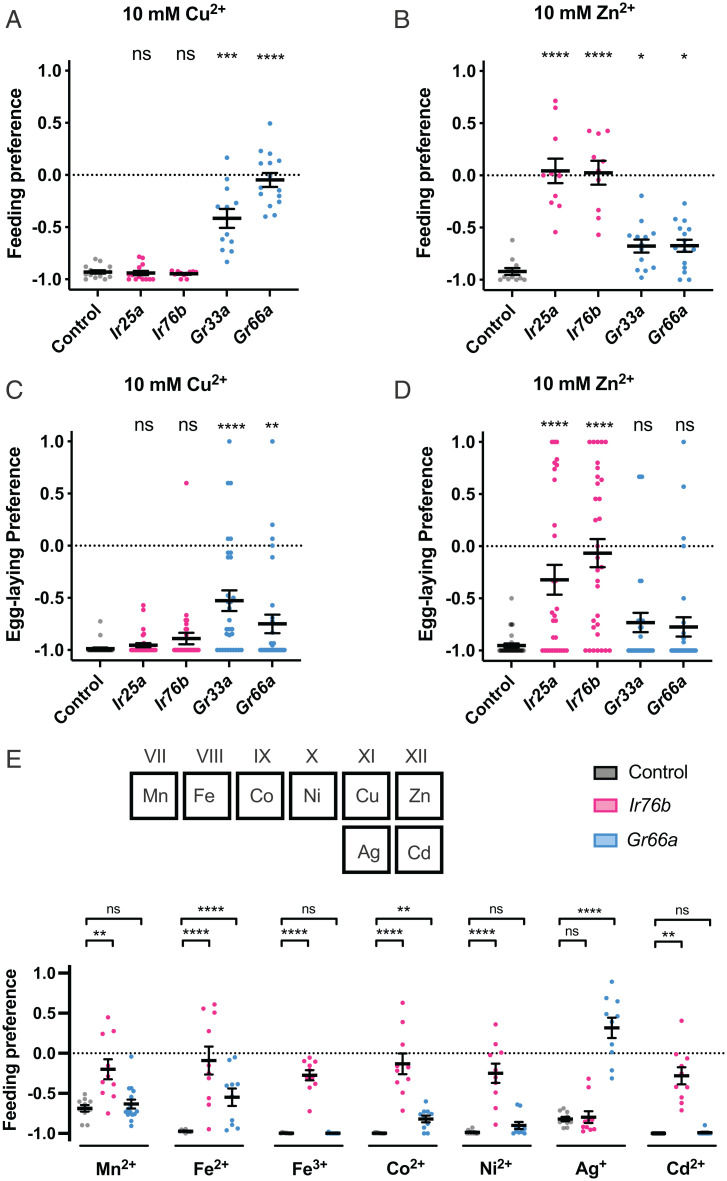
Taste avoidance of high concentrations of sodium, potassium, and calcium depends on Ir76b, a coreceptor for Irs (33–36). We tested the avoidance of copper and zinc ions in mutants of Ir76b and another Ir coreceptor, Ir25a, as well as in two Gr mutants, Gr33a2 and Gr66a1, which were defective in responses to most bitter compounds tested in a study of taste physiology (37).
Surprisingly, feeding avoidance of 10 mM Cu2+ was normal in both Ir mutants (Fig. 3A). By contrast, preferences were greatly reduced in each of the two Gr mutants. Dependence of the feeding avoidance of 10 mM Zn2+ was strikingly different from that of Cu2+ avoidance: Preferences were completely abolished in both of the Ir mutants (Fig. 3B). Preferences were altered by both Gr mutations, but only modestly.
Fig. 3.
Different requirements of receptors for taste avoidance of different metal ions. (A–D) Mutants of four receptors showed different phenotypes for CuSO4 (A and C) and ZnSO4 (B and D) in feeding (A and B) and egg-laying (C and D) behaviors. (E) Feeding avoidance of other metals (MnSO4, FeSO4, Fe2(SO4)3, CoSO4, NiSO4, AgNO3, CdSO4) showed various dependence on Ir76b and Gr66a. n = 10 to 15 in feeding assay and n = 30 to 32 in egg-laying assay. Asterisks indicate significant differences from control (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) using Mann–Whitney U test. Error bars are SEM. ns, not significant.
Egg-laying preference showed similar dependence to that of feeding preference, in that it was normal in the Ir mutants in the case of Cu2+ (Fig. 3C) but was abolished or greatly reduced in the case of Zn2+ (Fig. 3D). The Gr mutations affected preference to Cu2+ but not to Zn2+ in egg-laying preferences.
The simplest interpretation of these results is that the response to anthropogenic metal ions in the environment is mediated through multiple classes of taste receptor, which vary in their responses to different metal ions. To test this interpretation, we expanded our analysis to include additional metal ions: manganese, iron, cobalt, nickel, silver, and cadmium, all of which are considered transition metal ions based on their positions in the periodic table, but which have diverse chemical properties (17). We tested two oxidation states of iron, Fe2+ and Fe3+.
All of these metal ions elicited strong feeding avoidance (Fig. 3E). They could be divided into two groups based on their sensitivity to mutation of Ir76b: Six metal ions were like Zn2+ in that the response they elicited was severely diminished in the Ir76b mutant. By contrast, Ag+ was like Cu2+ in that it elicited a robust avoidance response in an Ir76b mutant. Responses to Fe2+, Co2+, and Ag+ were sensitive to Gr66a1; responses to the others were not. These results support the notion that responses to different metal ions are mediated via different receptors.
We asked whether any of eight physical properties of the nine ions (e.g., ionic radius, rion) correlated with these phenotypes. The strongest correlation was between the dependence on Gr66a of a metal ion and its absolute hardness, (hard generally implies small and nonpolarizable): Gr66a1 mutants tended to show greater phenotypes with softer metal ions (SI Appendix, Fig. S2). The strong relationship between the Gr66a1 phenotype and suggests that Cu+, which is softer and more common in cellular environments than Cu2+, might be a more effective activator of Gr66a than Cu2+; however, this hypothesis is difficult to test since the stability of Cu+ is limited in aqueous solutions.
Physiological Responses of GRNs to Metal Ions.
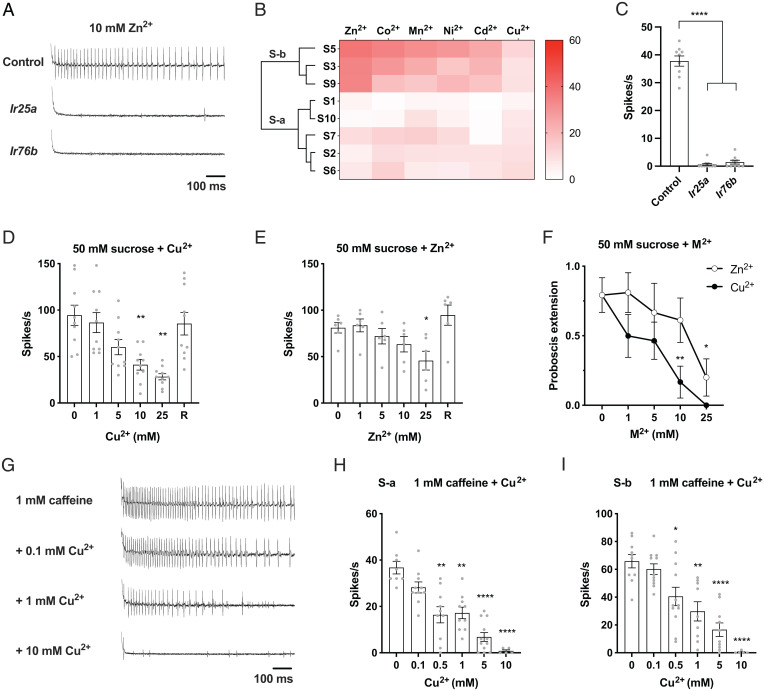
To investigate the neural basis of taste avoidance of metal ions, we carried out electrophysiological recordings from the taste sensilla of the labellum, the main taste organ of the head. We focused on two classes of S sensilla, the S-a sensilla (S1, S2, S6, S7, S10) and the S-b sensilla (S3, S5, S9), which are convenient to record from and which gave the most reproducible and robust responses to metal ions in a preliminary analysis. The S-a and S-b sensilla contain a neuron that responds to bitter compounds and produces an action potential that is readily identifiable by its large amplitude (38). Bitter-sensing neurons (also referred to as “B” neurons in ref. 20) of the S-a class show the same response profile to a set of bitter compounds and express the same Grs; sensilla of the S-b class show a different response profile and express a different subset of Grs (37, 38).
S-b sensilla gave trains of action potentials from the bitter neuron to a variety of metal ions (Fig. 4 A and B). The response frequencies varied among metal ions, with Zn2+ eliciting the greatest mean responses. S-a gave weaker responses than S-b.
Fig. 4.
Physiological responses of taste sensilla in the labellum to metal ions. (A) Representative recordings of responses to 10 mM zinc ions in S-b sensilla of control flies and Ir25a and Ir76b mutants. (B) Action potentials induced by different metal ions at 10 mM in S-a and S-b sensilla. n = 4 to 12. (C) Responses to 10 mM zinc ions of S-b sensilla of control flies and Ir25a and Ir76b mutants. n = 8 to 10. ****P < 0.0001, Mann–Whitney U test. (D and E) Effects of different concentrations of copper or zinc on sugar responses in L sensilla. Mixtures of 50 mM sucrose with increasing concentrations of metal ions were applied to the same L sensilla at 2-min intervals. R denotes “recovery” (i.e., the response to 50 mM sucrose after the sensilla were stimulated with sucrose-metal mixtures). (D) Responses to sucrose and sucrose-copper mixtures from L sensilla. n = 10. **P < 0.01, Wilcoxon test. (E) Responses to sucrose and sucrose-zinc mixtures from L sensilla. n = 6. *P < 0.05, Wilcoxon test. (F) PERs to 50 mM sucrose alone or mixtures containing different concentrations of copper or zinc ions. n = 5 to 8. *P < 0.05, **P < 0.01, Mann–Whitney U test. (G–I) Effects of different concentrations of copper on responses to 1 mM caffeine in S sensilla. (G) Representative recordings of responses to 1 mM caffeine alone or caffeine-copper mixtures in S-b sensilla. (H) Responses to caffeine and caffeine-copper mixtures in S-a sensilla. n = 5 to 12. **P < 0.01, ****P < 0.001, Wilcoxon test. (I) Responses to caffeine and caffeine-copper mixtures in S-b sensilla. n = 5 to 12. *P < 0.05, **P < 0.01, ****P < 0.001, Wilcoxon test. Error bars are SEM.
The strong physiological response to Zn2+ in S-b sensilla was severely reduced by mutations of Ir25a and Ir76b (Fig. 4 A and C). These results are consistent with the dramatic reduction in feeding and egg-laying responses to Zn2+ by Ir25a and Ir76b mutations (Fig. 3 B and D).
We note that an intrinsic property of the metal solutions used in these experiments is that they are slightly acidic and cannot be neutralized without affecting the metal ions. HCl solutions more acidic than those used here elicit some, but fewer, spikes than the more effective metal solutions (39, 40). The simplest interpretation of these results is that the strong physiological responses we observed to certain metal solutions are due in part to their acidity but primarily to the metal ions they contain.
Interestingly, Cu2+ did not elicit strong responses from any of these S-a or S-b sensilla, so we explored another parameter of physiological response. In addition to activating bitter GRNs, some bitter compounds inhibit the activation of sugar GRNs (referred to as “A” neurons in ref. 20) (41, 42). We asked whether Cu2+ or other metal ions inhibited the response of the sugar neuron in L sensilla. We chose L sensilla because they contain no bitter-sensing neurons, thereby simplifying the analysis.
The activation of the sugar neuron was greatly reduced by Cu2+ (Fig. 4D). The reduction depended on the dose of Cu2+. The response was reduced from 94 ± 11 spikes/s (n = 10) to 28 ± 3 spikes/s (n = 10) at the highest Cu2+ concentration tested. The sugar neuron recovered most or all of its sugar response after a brief interval (“R” in Fig. 4D), showing that the reduction was not due to irreversible toxicity.
Other metal ions also showed inhibition of the sugar response. Zn2+, Mn2+, Co2+, and Ni2+ all showed reductions at concentrations of 5 mM, 10 mM, or 25 mM, and all showed recovery thereafter (Fig. 4E and SI Appendix, Fig. S3 A–C). We also tested Mg2+, which is not a transition metal ion, and found that MgCl2 did not show a reduction (SI Appendix, Fig. S3D).
Does the inhibition of the sugar neuron by metal ions have behavioral consequences? As an initial test of this hypothesis, we measured the proboscis extension response (PER), in which a sucrose solution is applied to the labellum. This contact elicits a robust extension of the proboscis, but the PER can be inhibited by the addition of bitter compounds to the sugar solution (41). We found that both Cu2+ and Zn2+ severely inhibit the PER in a dose-dependent fashion (Fig. 4F).
Having found that Cu2+ did not show strong activation of bitter neurons and that it inhibited the activation of sugar neurons, we next asked whether it might inhibit the activation of bitter neurons. We found that the activation of neurons in S sensilla by caffeine, a well-studied bitter compound, is in fact inhibited by Cu2+ (Fig. 4G). The activation depended on the dose of Cu2+ and was observed in both S-a and S-b neurons at concentrations as low as 0.5 mM (Fig. 4 H and I). There is little if any precedent for similar inhibition of a bitter response, to our knowledge. We note that we have not quantified the recovery of bitter-sensing neurons following exposure to Cu2+, but preliminary analysis suggests that Cu2+ may impair subsequent responses of these neurons for some period of time (43).
In summary, we found that i) Zn2+, but not Cu2+, elicits a robust response from certain bitter neurons; ii) both Zn2+ and Cu2+, as well as other metal ions, inhibit the response of certain sugar neurons to sucrose; and iii) Cu2+ inhibits the response of bitter neurons to caffeine.
These results suggest that the avoidance of metal ions observed in our initial two-choice feeding preference and egg-laying preference assays could have either of two mechanistic origins. First, the activation of bitter neurons by Zn2+ could shift the preference away from the substrate with Zn2+. Second, the reduction in sugar signaling by Zn2+ and Cu2+ could, in principle, shift the preference toward the sugar substrates that do not contain metal ions.
The necessity of Ir25a and Ir76b for Zn2+, but not Cu2+, avoidance could reflect the role of these receptors in the activation of bitter neurons we observed by Zn2+, but not Cu2+. To investigate the neural circuitry underlying the avoidance of Cu2+, we extended our analysis to include other taste organs.
Feeding Avoidance of Cu2+ Is Driven by Neurons of the Pharynx.
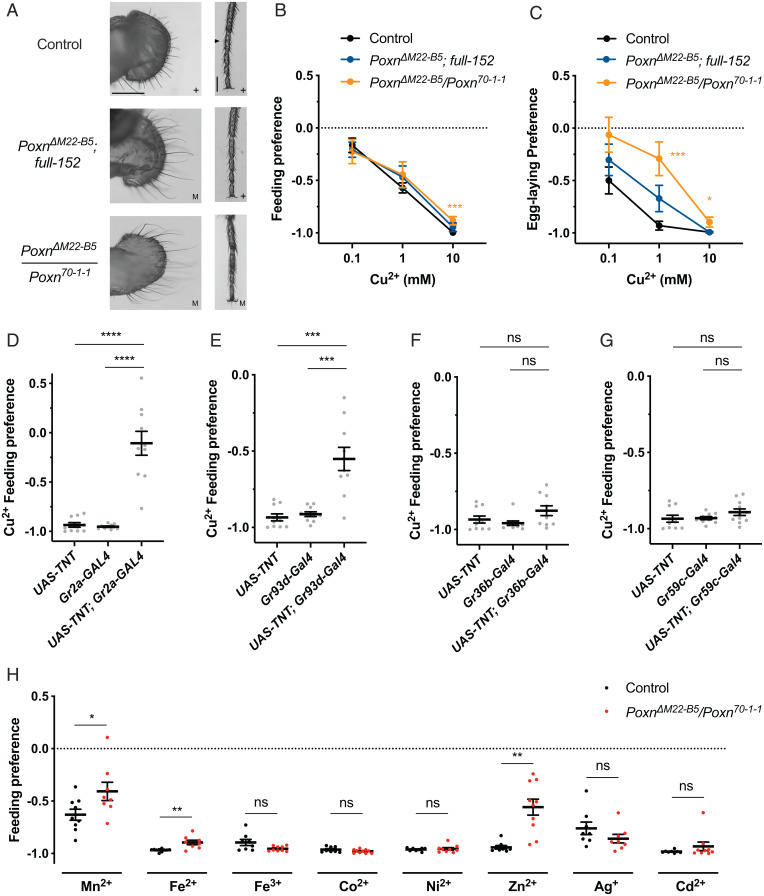
We first examined behavioral preferences to Cu2+ in Pox neuro (Poxn) mutants, which lack external taste neurons because external taste sensilla are transformed into mechanosensory sensilla. We compared two mutants: PoxnΔM22-B5/Poxn70-1-1, which lacks taste neurons from external taste sensilla on the labellum, legs, and wings, and PoxnΔM22-B5; full-152, a less severe mutant that lacks taste neurons only from the labellum (Fig. 5A) (44, 45).
Fig. 5.
Robust taste avoidance of copper and other metal ions in Poxn mutants. Control flies were w, Berlin to match the genetic background of Poxn mutants. (A) Bright-field images of labellum and foreleg of control female flies and Poxn null and partial rescue mutants. + denotes normal anatomy with functional chemosensory hairs. M denotes abnormal anatomy with chemosensory hairs transformed into mechanosensory hairs, which are longer. Triangle marks a chemosensory hair on leg. Scale bar, 100 µm. (B) Feeding preferences to copper of control flies and Poxn mutants. n = 8 to 10. Asterisks indicate significant differences from control. ***P < 0.001, Mann–Whitney U test. (C) Egg-laying preferences to copper of control flies and Poxn mutants. n = 30 to 34. Asterisks indicate significant differences from control (*P < 0.05, ***P < 0.001), Mann–Whitney U test. (D–G) Feeding preferences to 10 mM copper ions with neurons silenced by different GAL4 drivers. n = 10 or 11. ***P < 0.001, ****P < 0.0001, Mann–Whitney U test. (H) Feeding preferences to different metal ions of control and a Poxn mutant. n = 8 to 10. *P < 0.05, **P < 0.01, Mann–Whitney U test. Error bars are SEM. ns, not significant.
Surprisingly, feeding avoidance of both mutants was remarkably similar to that of the control (Fig. 5B). In egg-laying tests, the lack of neurons from labellar sensilla only (PoxnΔM22-B5; full-152) resulted in a modest reduction in Cu2+ avoidance (Fig. 5C), and lack of neurons from all external sensilla (PoxnΔM22-B5/Poxn70-1-1) resulted in a more severe defect, but the flies still avoided laying eggs in the substrate with Cu2+. The simplest interpretation of these results is that neurons of the pharynx, the principal internal taste organ, drive feeding avoidance to Cu2+ and make an important contribution to egg-laying avoidance.
To test the hypothesis that feeding avoidance of Cu2+ is driven primarily by pharyngeal neurons, we used GAL4 drivers to express a UAS-TNT transgene (Tetanus Toxin Light Chain, which cleaves synaptobrevin to block synaptic transmission) (46) in several classes of GRN. We first used Gr2a-GAL4, which drives expression in L7-3, a presumed bitter neuron of the labral sense organ (LSO) of the pharynx, but little if any expression in taste sensilla of the labellum, leg, or wing (47, 48). Flies containing both Gr2a-GAL4 and UAS-TNT showed a dramatic decrease in feeding avoidance of Cu2+ compared with the parental controls containing Gr2a-GAL4 or UAS-TNT alone (Fig. 5D). The same result was found for Gr93d-GAL4 (Fig. 5E), which is expressed in the same pharyngeal neuron with Gr2a-GAL4 (as well as in two other pharyngeal neurons) (47). By contrast, feeding preference was not affected when blocking was driven by Gr36b-GAL4 (Fig. 5F), a driver that is expressed in S-a sensilla of the labellum, or Gr59c-GAL4 (Fig. 5G), which is expressed in both S-a and a class of I sensilla (I-a) on the labellum (38). Similar results were obtained when the same GAL4 constructs were used to drive UAS-Kir2.1, a channel that silences neurons (SI Appendix, Fig. S4 A–D) (49).
Use of Gr5a-GAL4, which drives expression in many sugar-sensing neurons of the labellum and leg (50), did not affect feeding preference (SI Appendix, Fig. S4E). We also used four other constructs to drive UAS-TNT in the other major classes of labellar GRNs as specified by the analysis of Jaeger et al. (33): Ir94e-GAL4, ppk28-GAL4, ppk23-GAL4, and Gr66a-GAL4. The only driver that produced a phenotype was Gr66a-GAL4, which is also expressed in the L8 and L9 neurons of the LSO of the pharynx, as well as in three other pharyngeal neurons (SI Appendix, Fig. S4 F–I) (47). The simplest interpretation of all these results taken together is that the feeding response to Cu2+ is driven largely by bitter-sensing neurons of the pharynx.
Finally, we systematically tested the feeding responses to a series of other transition metal ions to determine whether they also depended largely on the pharynx, as in the case of Cu2+. We found that the responses to Fe2+, Fe3+, Co2+, Ni2+, Cd2+, and Ag+ were also unaffected by the loss of external taste sensilla and are thus likely to be driven largely by neurons of the pharynx. Only Zn2+ and Mn2+ were less aversive to flies lacking these sensilla (Fig. 5H).
Avoidance of Metal Ions Is Conserved in Other Drosophila Species and a Mosquito.
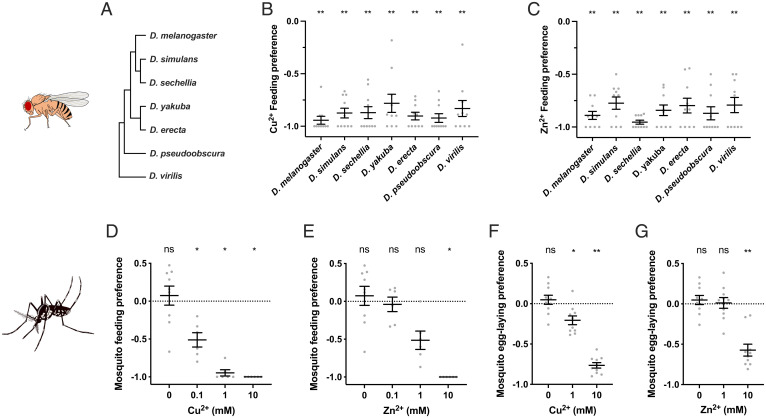
We asked whether the strong feeding avoidance to Cu2+ and Zn2+ shown by Drosophila melanogaster is conserved among other Drosophila species. We found that all of seven other Drosophila species tested avoided feeding on both of these metal ions at 10-mM concentrations (Fig. 6 A–C).
Fig. 6.
Conserved taste avoidance of copper and zinc ions in other drosophilids and the mosquito species Ae. albopictus. (A) Phylogenetic tree of drosophilid species included in this study. (B and C) Feeding preferences to 10 mM copper (B) or zinc (C) of different drosophilids. n = 10. Asterisks indicate significant differences from 0 (**P < 0.01), Wilcoxon signed rank test. (D and E) Feeding preferences to copper (D) or zinc (E) of mosquitoes. n = 9 for 0 mM; n = 6 for all other concentrations. Asterisks indicate significant differences from 0 (*P < 0.05), Wilcoxon signed rank test. (F and G) Egg-laying preferences to copper (F) or zinc (G) of mosquitoes. n = 10. Asterisks indicate significant differences from 0 (*P < 0.05, **P < 0.01), Wilcoxon signed rank test. Error bars are SEM. ns, not significant.
We also tested the mosquito Aedes (Ae.) albopictus, which diverged from D. melanogaster ∼260 million years ago (51). Ae. albopictus is a disease vector native to Southeast Asia that has spread in the past few decades to many countries, including countries of North America, South America, Europe, and Africa. We found that this mosquito also showed a clear taste avoidance to both Cu2+ and Zn2+ in feeding assays (Fig. 6 D and E). It also avoided both metal ions in egg-laying assays (Fig. 6 F and G).
Physiological Effects of Exposure to High Levels of Copper and Zinc Ions.
What effects do high environmental levels of copper and zinc have on Drosophila? We showed above that these metal ions reduce viability and impair development (SI Appendix, Fig. S1 E, F, J, and K). We then decided to examine in more depth the effects of exposing flies to copper or zinc (SI Appendix, Fig. S5A).
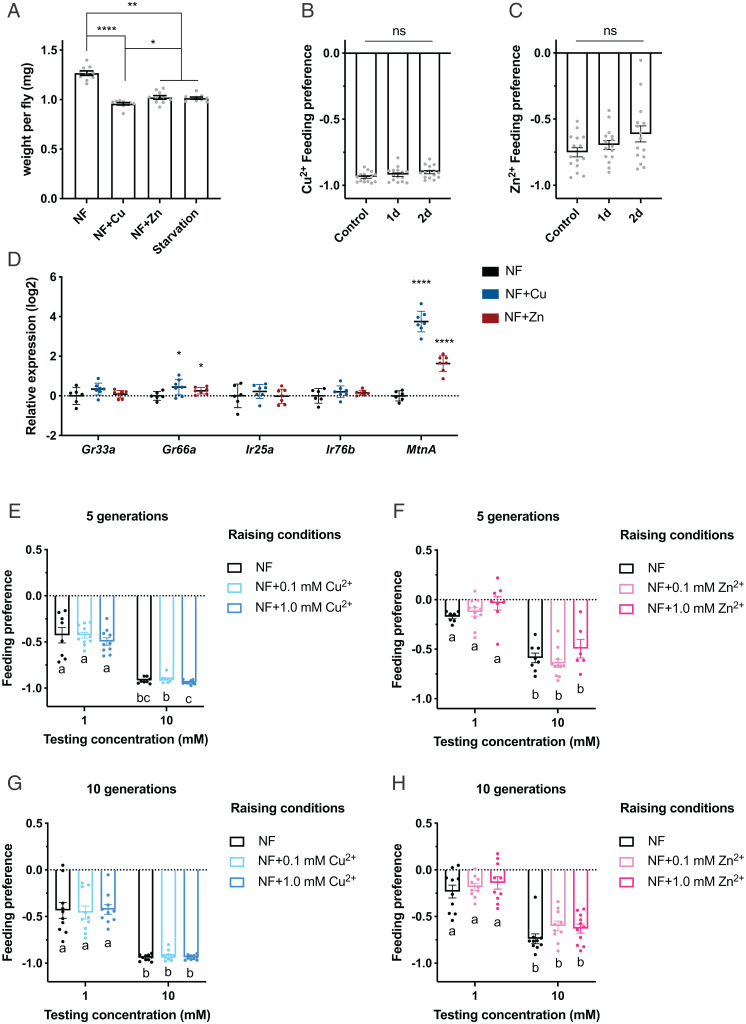
Female flies exposed to 10 mM copper or zinc for 1 d showed a severe loss of body mass: 24% in the case of CuSO4 and 19% in the case of ZnSO4 (Fig. 7A) compared with controls maintained on normal fly food. These reductions are comparable to those of flies that are starved for the same period of time, consistent with the interpretation that flies maintained on these metal ions consume little if any food. Male flies showed the same effect, although less pronounced (SI Appendix, Fig. S5B), which is likely caused by a similar feeding avoidance to these metal ions (SI Appendix, Fig. S5C).
Fig. 7.
Taste preferences to copper and zinc ions after short- or long-term exposure. (A) Weight per female fly after short exposure to copper or zinc or starvation. NF denotes normal food. n = 10. *P < 0.05, **P < 0.01, ****P < 0.0001, Kruskal–Wallis test followed by uncorrected Dunn’s test. (B and C) Feeding preferences to 10 mM CuSO4 (B) or 10 mM ZnSO4 (C) following 1-d or 2-d exposure to 10-mM concentrations of the corresponding metal ions. n = 15. Mann–Whitney U test. (D) Relative messenger RNA levels of Gr33a, Gr66a, Ir25a, Ir76b, and MtnA in fly heads upon exposure to 10 mM CuSO4 or ZnSO4 for 1 d. n = 6 or 7. Asterisks indicate significant differences from NF control (*P < 0.05, ****P < 0.0001), Welch’s t test. (E–H) Feeding preferences to copper or zinc after 5 or 10 generations raised on media as indicated. n values are 8 to 11 (E), 6 to 10 (F), 10 (G), and 10 or 11 (H). Groups with different preferences are marked with different letters (a, b, c). Kruskal–Wallis test followed by uncorrected Dunn’s test. Error bars are SEM. ns, not significant.
Does chronic exposure to these levels of metal alter their feeding preferences (i.e., do they adapt to the presence of metals)? We found that after 1 or 2 d on high levels of copper or zinc, flies showed the same feeding avoidance as control flies (Fig. 7 B and C).
Are there effects of exposure on gene expression? We tested by qRT-PCR the levels of the four genes that are required for responses to either 10 mM copper or zinc following 1 d of exposure. The expression of one of these genes, Gr66a, was elevated following exposure to both copper and zinc, but the effect size was small; a much larger effect was observed for Metallothionein A (MtnA), which encodes a small cysteine-rich protein that protects against heavy metal toxicity, in agreement with previous results (Fig. 7D) (52, 53).
We also exposed flies to 0.1 mM or 1 mM concentrations of Cu2+ and Zn2+ continually over the course of 5 or 10 generations. We found that feeding avoidance remained stable (Fig. 7 E–H). For both metal ions, 1 mM concentrations were toxic (SI Appendix, Figs. S1 J and K and S5 D–F), and the level of toxicity, as measured by the number of eggs that developed to the pupal stage, remained relatively stable. Thus, the presence of metals in the environment presented a threat that flies were not able to overcome over the course of 10 generations.
Discussion
We examined the responses of Drosophila to high levels of nine metal ions as a paradigm for the responses of organisms to unnatural levels of environmental toxins, a major threat of the Anthropocene. We analyzed responses at the behavioral, physiological, and molecular levels and found that strong effects of metal ions on behaviors were mediated by subsets of taste neurons and multiple classes of taste receptors.
Metal concentrations in the environment have increased dramatically as a result of human activities, and declines in arthropod densities have been widely observed at sites of high metal contamination (5, 54). Metal ion concentrations are increased still further by acidification of the environment, either at a macroscale via anthropogenic acid rain or at a microscale via organismal metabolism (55–57); such acidification releases metal ions from metal-containing compounds.
Copper, zinc, and other metal pollutants contaminate a wide variety of plants, at levels comparable to those used in our experiments in some cases (58–61). Insects have mechanisms for detoxifying metals (62–64), but high concentrations impair development and viability (52, 65), as confirmed in our study. The responses of the taste system we have studied here act to reduce ingestion of high metal concentrations, but since the system presumably did not evolve under selective pressure to detect such unnaturally high concentrations, the mechanisms are of particular scientific interest as well as ecological significance.
We found that responses to different metal ions were mediated via different mechanisms. Previous studies have focused on Irs in the taste avoidance of calcium, an alkaline earth metal, and zinc, a transition metal (35, 66). We have confirmed the requirement for Irs and extended it to include a total of seven transition metal ions. We have now in addition implicated a second class of taste receptors, the Grs, in the response to Cu2+, Zn2+, Fe2+, and Ag+.
It is striking that the response to some metal ions depends on both Ir and Gr genes (Fig. 3E). Genes of these two families are coexpressed in many GRNs (20, 29). Interestingly, normal taste response to lactic acid requires receptors of both classes, with the response onset depending on Ir25a and offset depending on Gr64a-f genes (67). In humans, the bitter taste of some metal ions is mediated by a G protein–coupled taste receptor (21, 22), whereas their astringent taste is likely mediated at least in part by TRPV1 (24, 68, 69).
We found three effects of metal ions on taste neurons. First, several metal ions activated certain bitter neurons of the labellum. Different metal ions elicited different levels of firing from bitter neurons of S-a and S-b sensilla, with Zn2+ showing the greatest activation, of S-b neurons, and Cu2+ showing the least (Fig. 4B). These responses to Zn2+ depended completely on Ir25a and Ir76b, as did the feeding and egg-laying behaviors induced by Zn2+ (Figs. 3 B and D and 4 A and C). Moreover, blocking or silencing of bitter neurons in the pharynx reduced the feeding avoidance of Cu2+ (Fig. 5). Taken together, these results support a model in which the activation of bitter neurons contributes to behavioral responses to high levels of environmental metals.
Second, Cu2+, Zn2+, and several other metal ions inhibited the response of sugar neurons to sucrose (Fig. 4 D and E and SI Appendix, Fig. S3). In principle, the reduction in sugar neuron firing could contribute to the feeding and egg-laying preferences for substrates that contain sucrose alone over those that contain sucrose and metal ions. Blocking of sugar neurons did not affect the feeding preference to Cu2+ (SI Appendix, Fig. S4F). However, Cu2+ and Zn2+ both reduced the PER elicited by sucrose (Fig. 4F), and it is possible that inhibition of sucrose neurons contributes to this reduction.
Third, Cu2+ inhibited the response of bitter neurons to caffeine, and the extent of inhibition depended on the dose of Cu2+ (Fig. 4 G–I). We have not quantified the recovery of neurons following exposure to Cu2+, but preliminary analysis suggests that Cu2+ may impair subsequent neuronal responses. If so, this would represent a direct threat of environmental metal pollution to sensory neurons.
It will be interesting to determine at high resolution the molecular mechanisms by which metal ions are detected by taste receptors. We do not know whether metal ions bind to the ligand-binding pockets of taste receptors and thereby activate or inhibit them. Some metal ions have been shown to alter the activity of certain ion channels by acting as allosteric modulators or channel blockers (70–74) and could have comparable effects on Grs, Irs, or other components of the taste system.
We found that the effect of Cu2+ on feeding avoidance is mediated largely through the pharynx, which also contributes to egg-laying avoidance of Cu2+. These findings are in agreement with a major role for the pharynx in egg-laying responses to lobeline and feeding responses to L-canavanine, two bitter compounds (45, 75).
The Cu2+ and Zn2+ feeding preferences we have analyzed here are conserved across a variety of Drosophila species and extend to the mosquito species Ae. albopictus as well (Fig. 6). Moreover, the egg-laying preferences are conserved in the mosquito. These results suggest that elevated metal levels elicit responses from the taste systems of a broad range of dipteran insects. The results also suggest the possibility of novel applications of metals in vector control.
The three taste behaviors considered in this study, feeding, egg-laying, and PER, are all strongly affected by metal ions whose levels are increasing in many environmental locations. We found that feeding responses to Cu2+ or Zn2+ do not habituate quickly, either during short-term exposure (Fig. 7B) or long-term exposure over the course of 10 generations (Fig. 7 E–H), even though a metallothionein-mediated detoxification program was initiated (Fig. 7D).
In summary, the challenge posed by toxic metals in the environment exemplifies a broad challenge currently faced by life on Earth. Organisms face many novel threats, and they may either respond adaptively or die. The taste system of Drosophila detects high levels of nine metal ions via multiple classes of receptors, neurons, and taste organs. Metal ions activate bitter neurons, inhibit sugar responses, and inhibit bitter responses, with different metal ions detected via different mechanisms. Collectively, the system drives avoidance behaviors that protect the animal and its offspring from ingestion of many toxic metal ions. The workings of these mechanisms are essential to the survival of flies and many related species to the perils of the Anthropocene.
Materials and Methods
Drosophila Strains.
Flies were reared on glucose media (Archon Scientific) at 25 °C and 60% relative humidity, in a 12-h light:12-h dark cycle. Control flies were w1118 Canton-S unless otherwise mentioned. Canton-S flies were used for short- and long-term exposure experiments. The following lines were obtained from the Drosophila Bloomington Stock Center: Ir25a (41737), Ir76b (51309), UAS-TNT (28838), and UAS-Kir2.1 (6596). Gr33a and Gr66a are described in ref. 37. Gr2a-GAL4, Gr5a-GAL4, Gr36b-GAL4, Gr59c-GAL4, Gr66a-GAL4, Gr93d-GAL4, and Ir94e-GAL4 are described in refs. 38 and 76. Ppk23-GAL4 and ppk28-GAL4, described in refs. 77 and 78, were provided by K. Scott (University of California, Berkeley). PoxnΔM22-B5/CyO and PoxnΔM22-B5; full-152 with the corresponding genetic background control w1118 Berlin were described in refs. 44 and 45, and Poxn70-1-1/CyO was described in refs. 79 and 80. Drosophila erecta (14021-0224.01), Drosophila pseudoobscura (15030-1161.03), Drosophila sechellia (14021-0248.27), Drosophila simulans (14021-0251.001), Drosophila virilis (15010-1051.00), and Drosophila yakuba (14021‐0261.40) were obtained from the Drosophila Species Stock Center.
Mosquito Strain.
Laboratory strain (Foshan) wild-type Ae. albopictus were reared in a 12-h light:12-h dark photocycle at 26 to 28 °C, 70 to 80% relative humidity. The same rearing conditions were maintained for mosquito behavioral assays. Larvae were fed TetraMarine Saltwater Granules fish food ad libitum. Adult mosquitoes were housed in a mixed-sex cage and fed 10% sucrose ad libitum. Mated adult females were blood-fed on defibrillated sheep blood via a membrane-feeding device or on a human arm.
Chemicals.
Cadmium sulfate (20920), caffeine (C1778), cobalt sulfate (C6768), copper chloride (222011), copper nitrate (467855), copper sulfate (209198), iron(II) sulfate (215422), iron(III) sulfate (307718), magnesium chloride (M2670), manganese sulfate (M7634), nickel sulfate (203890), potassium chloride (409316), potassium nitrate (P8394), silver nitrate (916404), sucrose (S7903), zinc chloride (746355), and zinc sulfate (Z4750) were purchased from Sigma-Aldrich. Agarose (AB00972) was purchased from AmericanBio. Hydrochloric acid (JT9535) was purchased from J. T. Baker.
CAFE Assay.
CAFE assays were performed as described in ref. 81, with slight modifications. A chamber was prepared by filling a 50-mL Falcon conical tube with 35 mL 1% agarose. Four glass capillary tubes (Drummond Scientific Company, Catalog #2–000-001) were inserted through the cap and secured by 200-μL pipette tips (not shown in Fig. 2A). Two tubes were filled with 10 mM sucrose, and the other two were filled with 10 mM sucrose mixed with metal ions. In SI Appendix, Fig. S1 C and D, sucrose was substituted with other sugars as indicated.
For the assay, 10 mated female flies (3 to 5 d old) were starved for 20 to 24 h and introduced into the CAFE chamber without anesthesia. The amount of consumption within 3 h was measured, and a preference index (P.I.) was calculated as (consumption from capillaries containing sucrose and metal ions − consumption from capillaries with sucrose alone)/(total consumption). Rarely, the total consumption volume in an individual Falcon conical tube was less than 0.50 μL, and these tubes were excluded from the analysis.
Egg-Laying Assay.
Egg-laying assays were performed as described in ref. 82. Briefly, two of four quadrants were filled with 0.5% (wt/vol) agarose with 100 mM sucrose in Petri dishes (Dot Scientific, catalog #557684). One of the two quadrants contained metal ions, and the other served as a control. Metal solutions were added to agarose solutions that had been cooled to 55 to 58 °C to reduce hydrolysis of metal ions.
Single mated female flies (4 to 6 d old) were introduced into one of the two empty quadrants without anesthesia and were allowed 20 to 24 h to lay eggs in a dark room (25 °C, 60% relative humidity). An egg-laying P.I. was then calculated as (number of eggs on test quadrant − number of eggs on control quadrant)/(total number of eggs). A very small fraction of dishes contained fewer than five eggs and were excluded from the assay.
Survival Assay.
Survival assays (SI Appendix, Fig. S1 E–G) were performed similarly as described in ref. 35. Newly eclosed flies were collected in groups of 10 females and 3 males and cultured on standard media for 3 d. Then, female flies were transferred into vials containing 1% agarose and either 100 mM sucrose alone or 100 mM sucrose mixed with different concentrations of copper or zinc as indicated. The viability of flies was monitored for 10 d. Flies were transferred to new vials with the same media every 12 h, with the number of live flies recorded.
Tip Recording.
Tip recordings were performed as in ref. 37 with mated female flies (3 to 6 d old). The reference tungsten electrode was inserted into the eye. A fine glass pipette filled with a tastant solution was used as the recording electrode, and 1 mM KCl was used as an electrolyte in recordings with sucrose or caffeine. Signals were amplified (10× Syntech Universal AC/DC Probe), filtered (100 to 3,000 Hz with 50/60-Hz suppression), digitized with IDAC-4 (Syntech), and further analyzed with Auto Spike 32 software. Response was quantified as the number of spikes in the first 500 ms after contact.
PER Assay.
PER assays were performed as in ref. 83, with some modifications. Three- to five-day-old female flies were starved for 24 h and immobilized in 200-μL pipette tips. Flies were tested with water before every tastant (50 mM sucrose alone or mixed with copper or zinc as indicated) and were water-satiated (i.e., the fly was given access to water until a negative response was seen). Each fly was tested with only a single solution, three times, at 2-min intervals. The PER was thus calculated as the fraction of full proboscis extension to the total number of tastant presentations (0, 0.33, 0.67, or 1). Flies were tested with 100 mM sucrose afterward as a positive control; flies with no response were excluded from the analysis. An exception was made for 50 mM sucrose mixed with 25 mM copper stimuli, after which flies rarely gave any PER responses to 100 mM sucrose; we have indicated the PER as 0 to reflect the lack of response to 25 mM copper.
Two-Color Choice Feeding Assay.
The feeding preferences of different drosophilids were tested in a two-color choice feeding assay as in ref. 75. Briefly, 10 female flies (3 to 5 d old) were starved for 24 h and then placed in Petri dishes (Falcon, Catalog #35–1006) following short anesthesia on ice. D. virilis were starved for 48 h and were tested in a larger Petri dish (Falcon, Catalog #35–1001). Tastants were mixed with either 0.25 mg/mL indigo carmine (Sigma-Aldrich, I8130) or 0.5 mg/mL sulforhodamine B (Sigma-Aldrich, 230162). Each tastant was paired the same number of times with each dye to offset any dye preference. Flies were allowed to feed for 3 h in a dark, humidified chamber at 25 °C. The color of their abdomen was scored following dissection of the gut, which made scoring more precise. The P.I. was calculated as PI = (Nred − Nblue)/(Nred + Nblue + Npurple) or PI = (Nblue − Nred)/(Nred + Nblue + Npurple), depending on the dye/tastant combinations. PIs of 1.0 and −1.0 indicate complete preferences for one solution or the other. A PI of 0 indicates no preference. Trials with <50% colored abdomens were excluded.
Weight Measurement.
Adult flies 3 to 4 d old were exposed for 1 d or 2 d to 10 mM CuSO4, 10 mM ZnSO4, or ddH2O (double distilled H2O). Following light anesthesia with CO2, 10 to 20 flies were then weighed together on a balance with a precision of 0.1 mg.
RNA Quantification.
After flies (3 to 4 d old) were exposed to fly media mixed with 10 mM CuSO4, 10 mM ZnSO4, or ddH2O for 24 h, fly heads were collected using forceps and placed immediately into collection tubes kept cold in liquid nitrogen. Head tissues were then ground in 500 μL RLT lysis buffer (Qiagen) on ice. RNA was extracted with acid phenol and Direct-zol RNA microprep kits (Zymo Research) according to the manufacturer’s protocol. Complementary DNA was synthesized with EpiScript (Lucigen) and added to the iTaq Universal SYBR Green (Bio-Rad) system for qPCR. Target gene expression was normalized to the level of RP49 transcripts. Primers are provided below, with sequences adopted from refs. 84–86:
RP49fwd (CCAAGCACTTCATCCGCCACC)
RP49rev (GCGGGTGCGCTTGTTCGATCC)
Gr33afwd (CCACCATCG CGGAAAATAC)
Gr33arev (ACACACTGTGGTCCAAACTC)
Gr66afwd (ACAGGAATCAG TCTGCACAA)
Gr66arev (AATGTTTCCATGTCCAGGGT)
Ir25afwd (CAATCCACTCAGCCATTCAA)
Ir25arev (ACCAGAGGCACTCCTTCAGA)
Ir76bfwd (CAGCGCAGCTTCGTCTACTA)
Ir76brev (CACAAAGTGCTTGTTCTTCG)
MtnAfwd (ACTGCGGATCTGACTGCAAG)
MtnArev (AAGATGCAGCGCCTCTACTC)
Mosquito Two-Choice Feeding Assay.
Adult female mosquitoes, mated and 4 to 7 d old, were starved by giving them access to water but not food for ∼24 h. Twenty female mosquitoes were introduced by mouth aspiration (no anesthesia) into the cage (24.5 cm × 24.5 cm × 24.5 cm), which contained two feeding wicks. One wick contained 100 mM sucrose, with the red dye 0.05% amaranth (Sigma-Aldrich, A1016), and the other contained 100 mM sucrose mixed with metal substrate and the blue dye 0.05% indigo carmine (Sigma-Aldrich, 131164). Mosquitoes were allowed to feed for ∼24 h, after which abdomens were scored as red, blue, purple, or uncolored. The P.I. was calculated as PI = (Nblue − Nred)/(Nred + Nblue + Npurple). P.I.s of 1.0 and −1.0 indicate complete preferences for one solution or the other. A P.I. of 0 indicates no preference. Trials with <50% colored abdomens were excluded. Control experiments showed there was no preference for either of the two dyes (Fig. 6).
Mosquito Two-Choice Egg-Laying Assay.
Adult female mosquitoes, mated and 4 to 7 d old, were blood-fed on a human arm. Blood-fed and gravid females were immediately selected and housed in a cage with ad libitum access to 10% sucrose for ∼96 h until the egg-laying assays were started. For the egg-laying assay, each cage contained two egg-laying cups filled with 100 mL deionized water or metal solution at the indicated concentrations. Ten gravid females were introduced by mouth aspiration and allowed to lay eggs for ∼24 h. The number of eggs in each egg-laying cup was counted, and the P.I. was calculated as (number of eggs in metal cup − number of eggs in control cup)/(total number of eggs).
Supplementary Material
Acknowledgments
We thank Zina Berman for support. This work was supported by NIH Grants R01 DC02174, R01 DC04729, and R01 DC11697 (to J.R.C.) and F32DC019250 (to L.S.B.).
Footnotes
Reviewers: W.L., University of California, Davis; and C.M., University of California Santa Barbara.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2204238119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Crutzen P. J., “The Anthropocene” in Earth System Science in the Anthropocene, Ehlers E., Krafft T., Eds. (Springer-Verlag, 2006), pp. 13–18. [Google Scholar]
- 2.Laurance W. F., The Anthropocene. Curr. Biol. 29, R953–R954 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Pelletier F., Coltman D. W., Will human influences on evolutionary dynamics in the wild pervade the Anthropocene? BMC Biol. 16, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockström J., et al. , A safe operating space for humanity. Nature 461, 472–475 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Wagner D. L., Insect declines in the Anthropocene. Annu. Rev. Entomol. 65, 457–480 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Dirzo R., et al. , Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Turvey S. T., Crees J. J., Extinction in the Anthropocene. Curr. Biol. 29, R982–R986 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Carroll S. P., Hendry A. P., Reznick D. N., Fox C. W., Evolution on ecological time-scales. Funct. Ecol. 21, 387–393 (2007). [Google Scholar]
- 9.Hoffmann A. A., Willi Y., Detecting genetic responses to environmental change. Nat. Rev. Genet. 9, 421–432 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Sullivan A. P., Bird D. W., Perry G. H., Human behaviour as a long-term ecological driver of non-human evolution. Nat. Ecol. Evol. 1, 65 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Bernhardt E. S., Rosi E. J., Gessner M. O., Synthetic chemicals as agents of global change. Front. Ecol. Environ. 15, 84–90 (2017). [Google Scholar]
- 12.Hou D., et al. , Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 1, 366–381 (2020). [Google Scholar]
- 13.Clemens S., Ma J. F., Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 67, 489–512 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Tang F. H. M., Lenzen M., McBratney A., Maggi F., Risk of pesticide pollution at the global scale. Nat. Geosci. 14, 206–210 (2021). [Google Scholar]
- 15.Ballabio C., et al. , Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 636, 282–298 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Chang C. J., Searching for harmony in transition-metal signaling. Nat. Chem. Biol. 11, 744–747 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Lippard S. J., Berg J. M., Principles of Bioinorganic Chemistry (University Science Books, 1994). [Google Scholar]
- 18.Chen Y. D., Dahanukar A., Recent advances in the genetic basis of taste detection in Drosophila. Cell. Mol. Life Sci. 77, 1087–1101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph R. M., Carlson J. R., Drosophila chemoreceptors: A molecular interface between the chemical world and the brain. Trends Genet. 31, 683–695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montell C., Drosophila sensory receptors: A set of molecular Swiss Army knives. Genetics 217, 1–34 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behrens M., Redel U., Blank K., Meyerhof W., The human bitter taste receptor TAS2R7 facilitates the detection of bitter salts. Biochem. Biophys. Res. Commun. 512, 877–881 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., et al. , Metal ions activate the human taste receptor TAS2R7. Chem. Senses 44, 339–347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilliard M. A., et al. , In vivo imaging of C. elegans ASH neurons: Cellular response and adaptation to chemical repellents. EMBO J. 24, 63–72 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riera C. E., Vogel H., Simon S. A., Damak S., le Coutre J., Sensory attributes of complex tasting divalent salts are mediated by TRPM5 and TRPV1 channels. J. Neurosci. 29, 2654–2662 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B., Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clyne P. J., Warr C. G., Carlson J. R., Candidate taste receptors in Drosophila. Science 287, 1830–1834 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Stocker R. F., The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 275, 3–26 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Shanbhag S. R., Park S. K., Pikielny C. W., Steinbrecht R. A., Gustatory organs of Drosophila melanogaster: Fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 304, 423–437 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Freeman E. G., Dahanukar A., Molecular neurobiology of Drosophila taste. Curr. Opin. Neurobiol. 34, 140–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liman E. R., Zhang Y. V., Montell C., Peripheral coding of taste. Neuron 81, 984–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiroi M., Marion-Poll F., Tanimura T., Differentiated response to sugars among labellar chemosensilla in Drosophila. Zool. Sci. 19, 1009–1018 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Park J., Carlson J. R., Physiological responses of the Drosophila labellum to amino acids. J. Neurogenet. 32, 27–36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaeger A. H., et al. , A complex peripheral code for salt taste in Drosophila. eLife 7, e37167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee M. J., et al. , Ionotropic receptor 76b is required for gustatory aversion to excessive Na+ in Drosophila. Mol. Cells 40, 787–795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y., Poudel S., Kim Y., Thakur D., Montell C., Calcium taste avoidance in Drosophila. Neuron 97, 67–74.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDowell S. A. T., Stanley M., Gordon M. D., A molecular mechanism for high salt taste in Drosophila. bioRxiv [Preprint] (2022). 10.1101/2022.02.25.481885 (Accessed 2 March 2022). [DOI] [PubMed]
- 37.Dweck H. K. M., Carlson J. R., Molecular logic and evolution of bitter taste in Drosophila. Curr. Biol. 30, 17–30.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss L. A., Dahanukar A., Kwon J. Y., Banerjee D., Carlson J. R., The molecular and cellular basis of bitter taste in Drosophila. Neuron 69, 258–272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganguly A., et al. , Requirement for an Otopetrin-like protein for acid taste in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 118, e2110641118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlu S., Wisotsky Z., Medina A., Dahanukar A., Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat. Commun. 4, 2042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.French A. S., et al. , Dual mechanism for bitter avoidance in Drosophila. J. Neurosci. 35, 3990–4004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeong Y. T., et al. , An odorant-binding protein required for suppression of sweet taste by bitter chemicals. Neuron 79, 725–737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dethier V. G., The physiology and histology of the contact chemoreceptors of the blowfly. Q. Rev. Biol. 30, 348–371 (1955). [DOI] [PubMed] [Google Scholar]
- 44.Boll W., Noll M., The Drosophila Pox neuro gene: Control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129, 5667–5681 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Joseph R. M., Heberlein U., Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics 192, 521–532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweeney S. T., Broadie K., Keane J., Niemann H., O’Kane C. J., Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Chen Y. D., Dahanukar A., Molecular and cellular organization of taste neurons in adult Drosophila pharynx. Cell Rep. 21, 2978–2991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon J. Y., Dahanukar A., Weiss L. A., Carlson J. R., A map of taste neuron projections in the Drosophila CNS. J. Biosci. 39, 565–574 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baines R. A., Uhler J. P., Thompson A., Sweeney S. T., Bate M., Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523–1531 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chyb S., Dahanukar A., Wickens A., Carlson J. R., Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. U.S.A. 100 (suppl. 2), 14526–14530 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X.-G., et al. , Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl. Acad. Sci. U.S.A. 112, E5907–E5915 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balamurugan K., et al. , Copper homeostasis in Drosophila by complex interplay of import, storage and behavioral avoidance. EMBO J. 26, 1035–1044 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lastowski-Perry D., Otto E., Maroni G., Nucleotide sequence and expression of a Drosophila metallothionein. J. Biol. Chem. 260, 1527–1530 (1985). [PubMed] [Google Scholar]
- 54.Olivares-Castro G., Cáceres-Jensen L., Guerrero-Bosagna C., Villagra C., Insect epigenetic mechanisms facing anthropogenic-derived contamination, an overview. Insects 12, 780 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh A., Agrawal M., Acid rain and its ecological consequences. J. Environ. Biol. 29, 15–24 (2008). [PubMed] [Google Scholar]
- 56.Shanableh A., Omar M., Bio-acidification and leaching of metals, nitrogen, and phosphorus from soil and sludge mixtures. Soil Sediment Contam. Int. J. 12, 565–589 (2003). [Google Scholar]
- 57.Krebs W., Brombacher C., Bosshard P. P., Bachofen R., Brandl H., Microbial recovery of metals from solids. FEMS Microbiol. Rev. 20, 605–617 (1997). [Google Scholar]
- 58.Kumar V., et al. , A tabulated review on distribution of heavy metals in various plants. Environ. Sci. Pollut. Res. Int. 24, 2210–2260 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Khan A., Khan S., Khan M. A., Qamar Z., Waqas M., The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. Int. 22, 13772–13799 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Cheng S., Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. Int. 10, 192–198 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Malaisse F., Gregoire J., Brooks R. R., Morrison R. S., Reeves R. D., Aeolanthus biformifolius De Wild.: A hyperaccumulator of copper from Zaire. Science 199, 887–888 (1978). [DOI] [PubMed] [Google Scholar]
- 62.Egli D., et al. , A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol. Cell. Biol. 26, 2286–2296 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maroni G., Lastowski-Perry D., Otto E., Watson D., Effects of heavy metals on Drosophila larvae and a metallothionein cDNA. Environ. Health Perspect. 65, 107–116 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Navarro J. A., Schneuwly S., Copper and zinc homeostasis: Lessons from Drosophila melanogaster. Front. Genet. 8, 223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Momani F. A., Massadeh A. M., Effect of different heavy-metal concentrations on Drosophila melanogaster larval growth and development. Biol. Trace Elem. Res. 108, 271–277 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Luo R., et al. , Molecular basis and homeostatic regulation of zinc taste. Protein Cell 13, 462–469 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanley M., Ghosh B., Weiss Z. F., Christiaanse J., Gordon M. D., Mechanisms of lactic acid gustatory attraction in Drosophila. Curr. Biol. 31, 3525–3537.e6 (2021). [DOI] [PubMed] [Google Scholar]
- 68.Ecarma M. J. Y., Nolden A. A., A review of the flavor profile of metal salts: Understanding the complexity of metallic sensation. Chem. Senses 46, bjab043 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Riera C. E., Vogel H., Simon S. A., le Coutre J., Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R626–R634 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Coddou C., Yan Z., Obsil T., Huidobro-Toro J. P., Stojilkovic S. S., Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 63, 641–683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horning M. S., Trombley P. Q., Zinc and copper influence excitability of rat olfactory bulb neurons by multiple mechanisms. J. Neurophysiol. 86, 1652–1660 (2001). [DOI] [PubMed] [Google Scholar]
- 72.Lacinová L., T-type calcium channel blockers - New and notable. Gen. Physiol. Biophys. 30, 403–409 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Srivastava S., et al. , Histidine phosphorylation relieves copper inhibition in the mammalian potassium channel KCa3.1. eLife 5, e16093 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarzycka B., Zaidi S. A., Roth B. L., Katritch V., Harnessing ion-binding sites for GPCR pharmacology. Pharmacol. Rev. 71, 571–595 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y. D., Park S. J., Joseph R. M., Ja W. W., Dahanukar A. A., Combinatorial pharyngeal taste coding for feeding avoidance in adult Drosophila. Cell Rep. 29, 961–973.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koh T.-W., et al. , The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron 83, 850–865 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thistle R., Cameron P., Ghorayshi A., Dennison L., Scott K., Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 149, 1140–1151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cameron P., Hiroi M., Ngai J., Scott K., The molecular basis for water taste in Drosophila. Nature 465, 91–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Awasaki T., Kimura K., pox-neuro is required for development of chemosensory bristles in Drosophila. J. Neurobiol. 32, 707–721 (1997). [DOI] [PubMed] [Google Scholar]
- 80.He Z., Luo Y., Shang X., Sun J. S., Carlson J. R., Chemosensory sensilla of the Drosophila wing express a candidate ionotropic pheromone receptor. PLoS Biol. 17, e2006619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delventhal R., Carlson J. R., Bitter taste receptors confer diverse functions to neurons. eLife 5, e11181 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao S., Sun J. S., Carlson J. R., Robust olfactory responses in the absence of odorant binding proteins. eLife 8, e51040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shiraiwa T., Carlson J. R., Proboscis extension response (PER) assay in Drosophila.JoVE, 10.3791/193 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Croset V., et al. , Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6, e1001064 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rass M., Oestreich S., Guetter S., Fischer S., Schneuwly S., The Drosophila fussel gene is required for bitter gustatory neuron differentiation acting within an Rpd3 dependent chromatin modifying complex. PLoS Genet. 15, e1007940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tatomer D. C., et al. , The integrator complex cleaves nascent mRNAs to attenuate transcription. Genes Dev. 33, 1525–1538 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.