Abstract
In this study, magnesium and strontium-doped β-tricalcium phosphates were synthesized to understand dopant impact on substrate chemistry and morphology, and proliferation and osteogenic differentiation of mesenchymal stem cells. Under solid-state synthesis, magnesium doping stabilized the β-phase in tricalcium phosphate, with 22% less α-phase content than control. Strontium doping increased α-phase formation by 17%, and also resulted in greater surface porosity, leading to greater crystal precipitation in vitro. Magnesium also significantly enhanced the proliferation of stem cells (P < 0.05) and differentiation into osteoblasts with increased alkaline phosphatase production (P < 0.05) at all time points. These results indicated that magnesium stabilizes β-tricalcium phosphate in vitro and enhanced early and late-time-point osteoconduction and osteoinduction of mesenchymal stem cells.
Keywords: Bone, Biomaterial, Dopant, osteogenic differentiation
Graphical Abstract
Magnesium-doped tricalcium phosphate significantly enhances stem cell proliferation and osteogenic differentiation while strontium-doped tricalcium phosphate greatly increases crystallite formation over β-tricalcium phosphate.
Introduction
The current trend in bone tissue engineering demands that biocompatible materials be not only osteoconductive but also osteoinductive.1 Autologous bone grafts, the gold standard for bone tissue replacement, are both osteoconductive and osteoinductive. Native bone tissue is laden with cells and growth factors responsible for facilitating migration, proliferation, recruitment, and differentiation of human mesenchymal stem cells (hMSCs) into osteoblasts.2 As a biocomposite, bone consists of both organic material including cells, bone morphogenic proteins, and collagen and inorganic material like carbonated calcium phosphate. Thus, synthetic calcium phosphate ceramics (CaPs) have been the inspiration for the majority of bone substitute materials.3–5 Although chemically similar to bone and osteoconductive, calcium phosphates possess no tangible osteoinductivity.4,5 Osteoinduction can be enhanced by the incorporation of biomolecules and growth factors such as bone morphogenic proteins (BMPs).6 Incorporating elements such as magnesium (Mg), iron (Fe), strontium (Sr), and silicon (Si) in trace amounts is another promising approach to enhance osteogenic and angiogenic properties of CaPs through their effects on crystallinity, surface morphology, and cell signaling.7–9 During osteogenesis, Mg is present to a high degree reaching ~ 5% of the content of newly formed bone and then decreasing significantly during maturation.10 Mg plays a crucial role in bone turnover through its inhibitory effect on osteoclastogenesis.11 Sr is another element that has been explored extensively to reduce the effects of osteoporosis by stimulating osteoblastogenesis while inhibiting osteoclastogenesis.12,13 When doped with Sr, bioglass nanoparticles used for drug delivery and their dissolution products have been shown to induce osteogenic maturation, increase cell survival, and enhance mineralization.12 The mechanism of Sr-induced osteoblastogenesis in human mesenchymal stem cells is the activation of the Wnt/β-catenin signaling pathway. Activation by Sr results in a greater expression of β-catenin and Wnt signaling ligands, and subsequent upregulation of osteogenic genes like RUNX2, osteocalcin, and bone sialoprotein.14
Controlling the recruitment and osteogenic differentiation of hMSCs through CaPs is critical to the success of bone tissue engineering scaffolds. Reports have shown increased proliferation and osteogenic differentiation of hMSCs cultured on hydroxyapatite and biphasic calcium phosphates15–17 and further demonstrated that grafting hMSCs enhances the osteogenic properties of CaPs in vitro and in vivo.18–20 In vivo, hMSCs typically differentiate into the appropriate cell lineage in the defect site21, while in vitro, the differentiation of hMSCs to osteoblasts can be directed by the addition of ascorbic acid, β-glycerophosphate, and dexamethasone.22 Reports also show osteogenic differentiation in the absence of osteogenic factors of doped CaPs by tailoring the structure of scaffolds.23 Previous studies of these dopants focused on osteoblast cultures; in contrast, the focus of the current research is the effect of the Sr and Mg dopants on the TCP surface morphology and properties, and how they affect the interaction with hMSCs and osteoinduction. TCPs doped with Sr and Mg were prepared using a solid-state synthesis method. The effects of these dopants on phase composition, surface properties, and in vitro proliferation and differentiation of hMSCs were measured. We hypothesized that incorporation of Sr and Mg would alter the hMSCs interaction with TCP through the changes in present phases and surface properties of the ceramics.
Results
Phase Analysis and Microstructure Evaluation
The XRD spectra for β-TCP, Mg-TCP, and Sr-TCP samples (Fig. 1) are shown after sintering at 1250 °C. β-TCP was the primary phase present for all compositions, but α-TCP was also visible in the β-TCP control and Sr-TCP samples post sintering. Total α-TCP was increased by 17% with the addition of Sr and decreased by 22% with the addition of Mg compared to control β-TCP. No apparent α-TCP peaks were found in Mg-TCP. SEM of samples (Fig. 2) showed a slight granular structure in sintered β-TCP that was less apparent in Sr-TCP, yet more pronounced in Mg-TCP. All samples showed microporous surfaces, with the largest and most abundant pores found in Sr-TCP and minimal surface porosity found in Mg-TCP.
Figure 1.
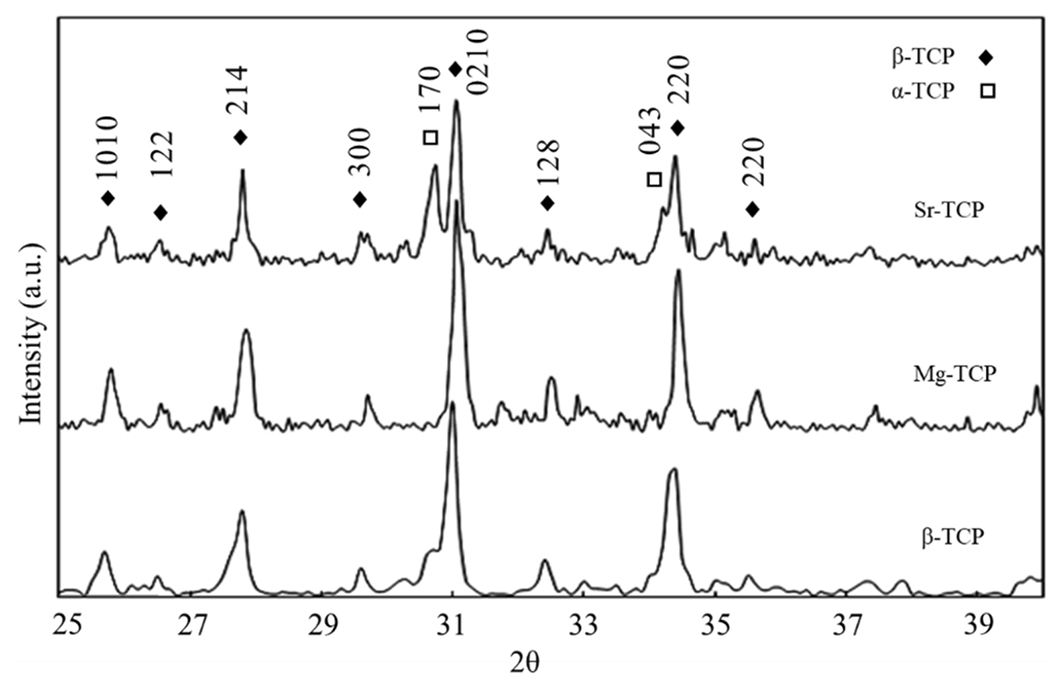
XRD spectra of Sr-TCP, Mg-TCP, and β-TCP control samples sintered at 1250 °C. The α-TCP peak at 30.7° visible in β-TCP and Sr-TCP is not present in Mg-TCP. β-TCP and α-TCP peaks identified according to ICDD 09-0169 and 09-0348, respectively.
Figure 2.
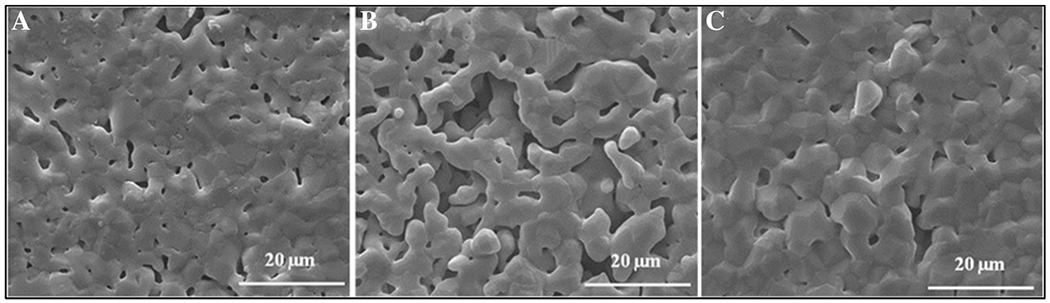
Microstructural changes observed under SEM of (A) β-TCP control, (B) Sr-TCP, and (C) Mg-TCP disks sintered at 1250 °C. Increased surface porosity compared to the control and Mg-TCP noted in Sr-TCP compositions.
In Vitro Culture of Mesenchymal Stem Cells
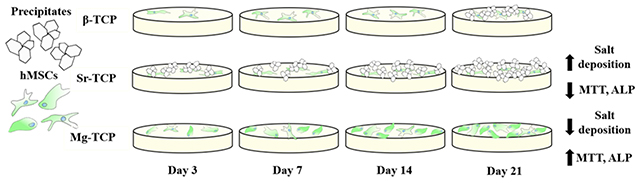
The morphology of cells was analyzed after 3, 7, 14, and 21 days of culture to understand the interaction between TCP compositions and hMSCs during proliferation and osteogenic differentiation (Fig. 3). After 3 days of culture, cells were well attached on all three compositions. Morphology was similar on Mg-TCP and Sr-TCP samples and appeared more well flattened and spread than on β-TCP with plate-like salt crystals on the surface of Sr-TCP. Similar morphology was found on day 7, and a larger area of Mg-TCP surface was covered with cells compared to β-TCP and Sr-TCP. Through day 21 cells increasingly spread on β-TCP and Mg-TCP with fewer apparent cells on the surface of Sr-TCP. Plate-like structures were formed on β-TCP but absent on Mg-TCP, while these crystals almost entirely covered Sr-TCP.
Figure 3.
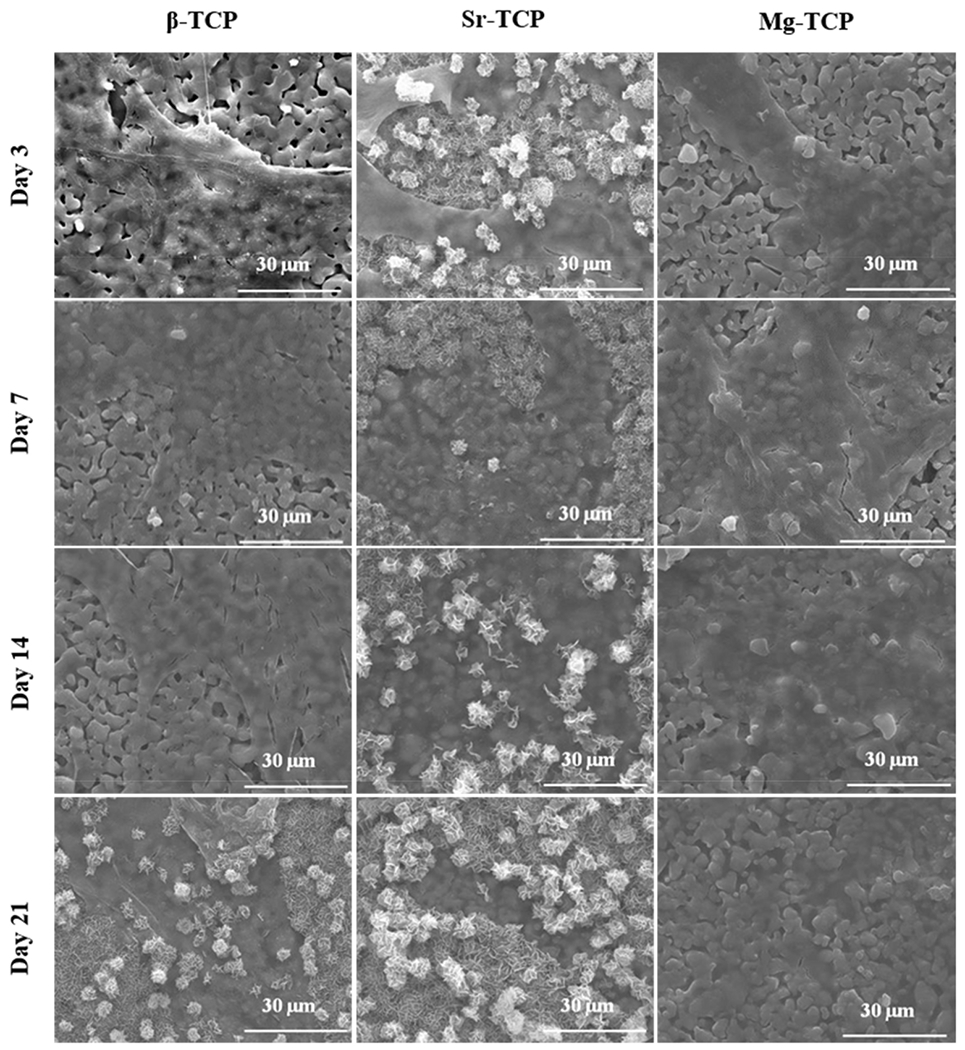
SEM images of sample surfaces after 3, 7, 14, and 21 days of culture. Cellular morphology on samples after 3, 7, 14, and 21 days of culture exhibited greater flattening and spreading on Sr and Mg-TCP over control. Greater amounts of salt formation was present at all time points of Sr-TCP and day 21 of β-TCP.
The results of the MTT assay (Fig. 4) were used to quantify the viability of the cells. Early time points showed similar optical density (OD) on Sr-TCP and Mg-TCP samples were significantly higher than β-TCP. Both β-TCP and Mg-TCP OD increased through days 14 and 21 while Sr-TCP decreased. The OD of Mg-TCP remained significantly higher than all other compositions at all time points. The degree of osteogenic differentiation of hMSCs was quantified using the ALP assay (Fig. 5). The results coincide with MTT data showing higher ALP activity in both Sr-TCP and Mg-TCP on day 7, followed by a decrease in Sr-TCP and a significantly higher expression in Mg-TCP at all time points.
Figure 4.
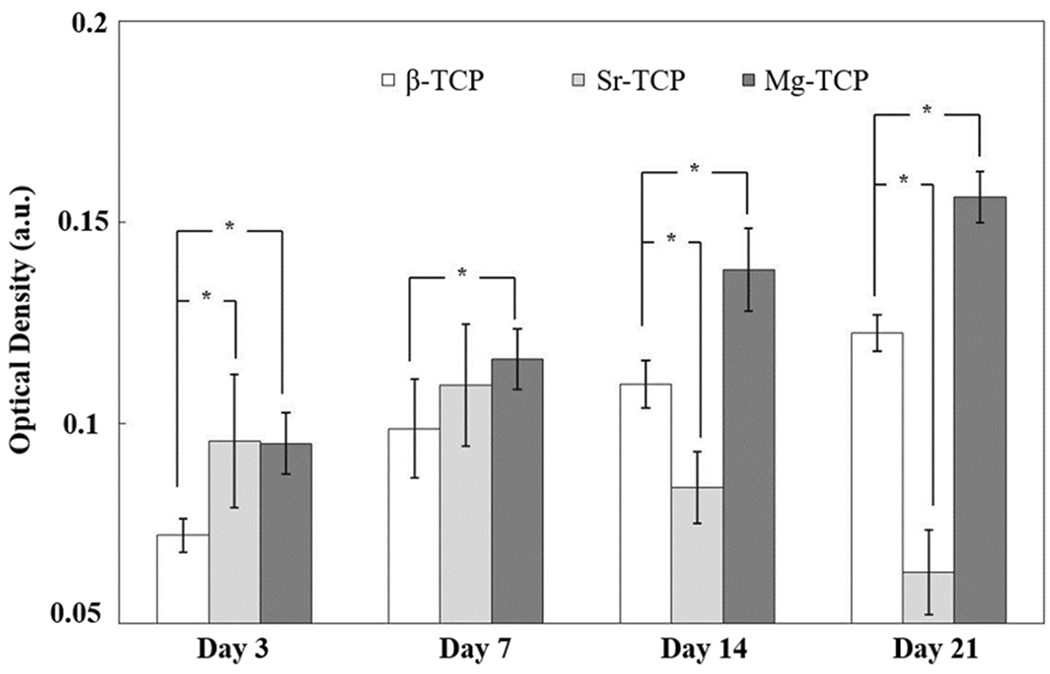
MTT optical density after 3, 7, 14 and 21 days of culture (* P < 0.05) showed increased cellular activity on Mg-TCP over control and Sr-TCP samples.
Figure 5.
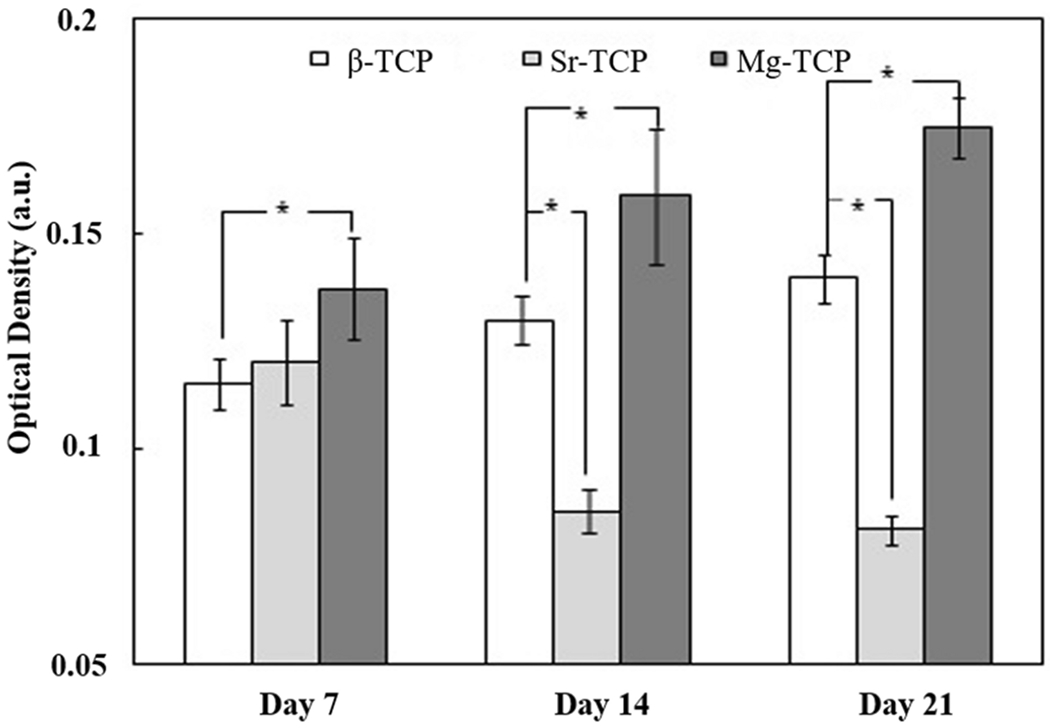
Relative ALP production after 7, 14 and 21 days of culture (* P < 0.05) showed greater osteogenic differentiation on Mg-TCP over Sr-TCP and control samples.
Discussion
Understanding the effects of doped CaPs on hMSC proliferation and osteoblastic differentiation is critical to creating superior bone substitutes. The interaction between the host tissue and CaP can be manipulated by tailoring the physical structure and chemical composition of the bioceramic.24 Mg and Sr are naturally present in bone, dentine, and enamel, and substitution with these trace elements in the cation sites of CaPs can be used to enhance their bioactivity and alter their biodegradability.25 The effects of Sr and Mg as single or binary dopants in CaP systems on in vitro proliferation of osteoblasts, proliferation and differentiation of mouse-derived osteoclasts, and in vivo osteogenesis in rat and rabbit have been previously reported.9,11,26,27 The novelty of this study lay in the examination of the effects that Mg and Sr have on the β-TCP phase, surface morphology and crystallite formation, and their overall impact on osteogenic differentiation of hMSCs in vitro.
The major phase in all three samples was β-TCP, as indicated by the XRD results (Fig. 1). In the β-TCP control and Sr-TCP samples, α-TCP peaks were distinctly visible and grew more prominent with the addition of Sr. Total α-TCP content in Sr-TCP was 17% higher than control β-TCP, and 22% lower in Mg-TCP. While the β-TCP phase is stable through 1125 °C, between 1125-1430 °C phase transformation from β-TCP to α-TCP occurs. Although the α-TCP phase formation is expected at a sintering temperature of 1250 °C, no related peak was found in Mg-TCP samples, which is associated with the effect of Mg on β-TCP stability.28 Ca and Mg have ionic radii of 0.99 Å and 0.69 Å, respectively. During the substitution of Mg in Ca sites into the β-TCP structure, the size of the unit cell decreases. This results in lattice contraction and stabilizes the β-TCP structure. Sr has an ionic radius of 1.13Å, and lattice expansion due to substitution in Ca sites destabilizes the β-TCP structure lowering the phase transformation temperature required for α-TCP formation.9,29,30 Microstructure of samples (Fig. 2) shows a dense structure containing microporosity. Compared to β-TCP and Mg-TCP, the addition of Sr to TCP results in slight liquid phase formation, which has been linked to a decrease in mechanical strength and an increase in the dissolution rate of Sr-TCP.27
Our results showed the significant effect of Sr and Mg, as the two most abundant trace elements in bone, on attachment, proliferation, and differentiation of hMSCs on β-TCP. SEM images (Fig. 3) showed that cells were well attached on all three compositions at day 3. hMSCs displayed an elongated structure on β-TCP and Sr-TCP; however, their morphology was more flattened on Mg-TCP. The hMSCs are anchorage-dependent cells, and their differentiation and final fate strongly depend on initial cellular adhesion. Consequently, surface chemistry and topography alter the initial attachment of cells, and that change in surface roughness alters the integrin activation and rearrangement of cytoskeletons in titanium surfaces.31 The difference in cellular adhesion in Mg-TCP can be attributed to several factors, such as activating specific signaling pathways that govern the interaction between Mg and hMSCs, stability of the β-TCP structure, and decrease in resorption rate in Mg-TCP. It is worth to mention that samples were hydrated using the MSCGM prior to cell seeding to prevent rapid drying of cells through seeding procedure and adjust the temperature of samples, as hMSCs are temperature-sensitive. The viability of cells, quantified using MTT assay (Fig. 4), showed enhanced proliferation of cells in both doped samples at early time points and confirmed the positive effect of dopants on cell attachment and proliferation. The effective role of these dopants on the proliferation of osteoblast cells has been reported elsewhere; however, their effect on the proliferation of hMSCs on TCP has not been well studied. At day 3 of culture, MSCGM was substituted with osteogenic differentiation medium including ascorbate, β-glycerophosphate, and dexamethasone, which facilitate the differentiation of hMSCs to osteoblast lineage cells.22 After 7 days of culture, larger areas of the disks were layered with cells. In addition, cells were embedded beneath the crystalline structure in Sr-TCP samples. The deposition of these salts on Sr-TCP was likely due to a higher α-phase presence, and thus, increased degradation rate.27 With a solubility product of 3.16*10−26 α-TCP is less stable in aqueous environments than β-TCP, which has a solubility product of 1.25*10−29. A higher solubility product leads to greater local ion concentration as well as faster resorption rates in the context of a biological environment.32 Despite this, the formation of biological apatite is a positive indicator of good bioactivity in Sr-TCP. Starting on day 7, ALP activity was evaluated as an indicator of hMSC osteogenic differentiation (Fig. 5). The results show the superior effect of dopants on the differentiation of hMSCs. Although the increase in ALP activity was not significant in Sr-TCP, higher ALP expression was found in Mg-TCP compared to the other compositions indicating a greater degree of osteoblastogenesis. On day 14, a higher number of cells were observed on β-TCP and Mg-TCP compared to that of Sr-TCP, which corresponded to the MTT data. The MTT OD decreased significantly in Sr-TCP compared to day 7, indicating reduced cellular activity. At the same time, increased salt formation on Sr-TCP samples resulted in the start of the cells being embedded. Similar MTT results were found on day 21 with an increase in OD in β-TCP and Mg-TCP and a significant decrease in the Sr-TCP samples. Crystal formation on the β-TCP samples is likely related to higher solubility due to the presence of more α-TCP compared to Mg-TCP. Like MTT, the ALP activity increased for both β-TCP and Mg-TCP, while the lowest ALP expression was found in Sr-TCP. Previously, the addition of Sr has been shown to increase salt deposition, and increasing amounts of Sr eventually led to decreased cell viability.12 Here, the Sr-TCP sample surfaces had greater porosity than β-TCP and Mg-TCP as well as higher α-phase content, which lead to higher apatite formation. We believe that apatite formation and resultant cell embedding is the main reason for decrease in cell density, as they prevent the formazan crystal formation and later dissolution during the MTT assay. Similar to the MTT assay, current ALP assay includes cell detachment from the surface of the samples. Apatite formation on the surface of the Sr-TCP samples acts as a barrier, thus, less ALP has been detected.
It has been shown that the Ca/P ratio, crystallinity, surface charge, and solubility have significant effects on adhesion, proliferation, and differentiation of cells through selective adsorption or structural organization of ECM proteins.33 Calcium phosphate ceramics support the proliferation and osteogenic differentiation of hMSCs by mimicking the CaP-rich microenvironments and phosphate metabolism, which alters adenosine signaling.20 The effects of Sr and Mg dopants in different CaPs on osteoblastogenesis and osteoclastogenesis have been reported previously.11,34 Higher osteogenic differentiation has been shown in magnesium doped HA-collagen composites compared to collagen is attributed to mimicking the osteoinductive environment for cells.35 The presence of Sr in a binary system with Si in HA coatings enhanced the proliferation and differentiation of hMSCs in medium supplemented with osteogenic factors.16 Additionally, enhanced differentiation of hMSCs in Sr doped calcium phosphate bone cement was also attributed to the release of Sr2+ as well as the stabilization of the cell culture medium pH.36 In the current work, the presence of both dopants did not change the color of the medium compared to the β-TCP during the 21 days of the experiment, indicating a similar pH for all cultured systems. Similar MTT and ALP activity in Mg-TCP and Sr-TCP at earlier time points suggest that Sr may have a positive effect on proliferation and differentiation of hMSCs through day 7. Moreover, enhanced proliferation and ALP expression on Mg-TCP compared to Sr-TCP indicated significant and lasting pro-osteogenic effects of Mg.
This study serves as a foundational paper for future work with bone tissue engineering scaffolds, elucidating the effect of Mg and Sr on the physical properties of β-TCP and the implications they have on the proliferation and differentiation of hMSCs. The effects of dopants on CaP systems for bone tissue engineering applications go beyond the roles of ions in cell signaling to include changes in phase and morphology, dissolution kinetics, and surface topography.
Conclusion
While the addition of Mg inhibited the transformation of β-TCP to α-TCP, Sr doping increased α-TCP phase content significantly. This change to a less stable phase, along with the increased surface porosity, led to a greater dissolution of tricalcium phosphate and precipitation of crystals to the Sr-TCP samples’ surfaces. MTT OD increased gradually in β-TCP and Mg-TCP samples until day 21, while the highest viability was found in Mg-TCP at all time points. Osteogenic differentiation of hMSCs evaluated using an ALP assay showed a significant increase in ALP expression in Mg-TCP samples compared to β-TCP at all time points, and Sr-TCP at days 14 and 21. Mg-TCP showed overall greater cell activity and osteogenic differentiation than Sr-TCP and control samples. While the addition of Sr did have a significant effect on proliferation and differentiation of hMSCs through day 7, significant decreases in both activities were found at later time points. These results, summarized in figure 6, show the substantial impact that individual dopants may have on TCP composition and surface morphology, and how significant an effect these characteristics, as well as the dopants themselves, have on the differentiation and maturation of osteoblasts from hMSCs.
Figure 6.
(A) Processing of TCP disks and (B) the trends observed in the hMSC cultures resulting from β-TCP, Sr-TCP, and Mg-TCP compositions at each time point.
Materials and Methods
Calcium Phosphate Ceramics fabrication
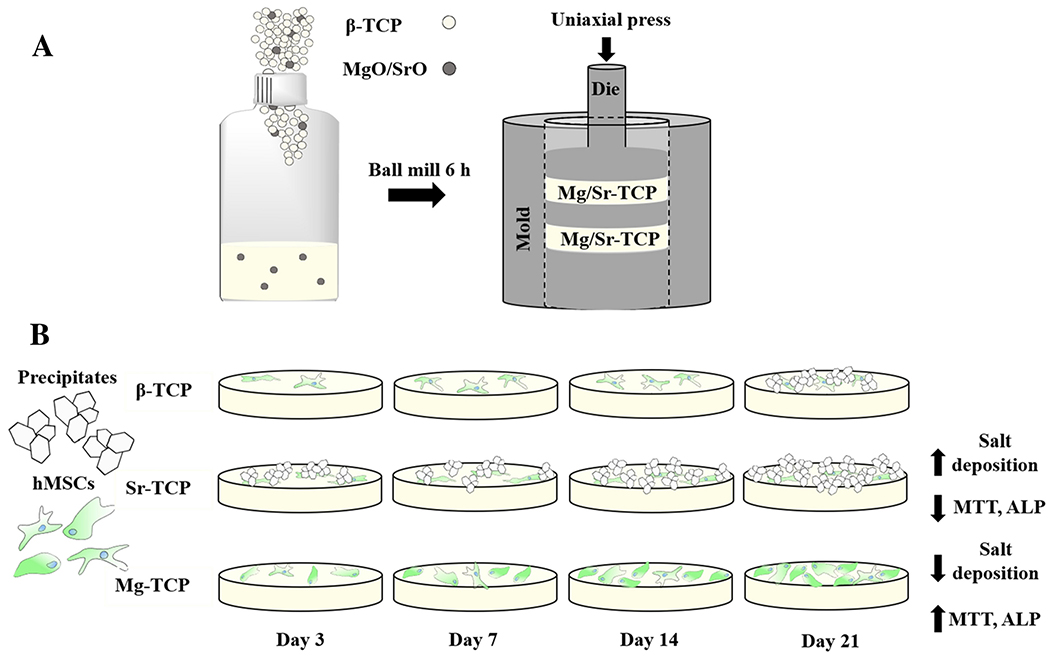
A solid-state synthesis method was used to prepare pure and doped CaP powders. Calcium carbonate and calcium phosphate dibasic powders were mixed for 2 hours, followed by calcination at 1050 °C. TCP powders doped with Mg (1 wt%) or Sr (1 wt%) were then prepared by mixing the calcined TCP with the relative amount of SrO and MgO in ethanol for 6 h, followed by drying for 72 h at 70 °C. These concentrations were selected based on our previously published results.9,11,26,37 Powders were pressed using uniaxial press at 145 MPa to create 12 mm disks followed by sintering in a furnace for 2h at 1250 °C. 11,26,38 Undoped and doped TCP samples will be referred to as β-TCP, Sr-TCP, and Mg-TCP, respectively.
Phase Analysis and Microstructure Evaluation
An X-ray diffractometer (XRD) was used to identify phases using Cu Kα radiation over a 2θ range of 25-40 ° at a 0.05° step size and 0.1 sec per step count time. The α-TCP percentage was calculated from the relative ratio of the intensities of the major peak of α-TCP to all the phases.39 Sample surface morphology was evaluated using a field emission scanning electron microscope (FESEM, FEI 200F, FEI Inc., OR, USA).
Cell Culture
Culturing of Human Bone Marrow-Derived Mesenchymal Stem Cells and Cell Seeding
The hMSCs were purchased from Lonza (Walkersville, MD). After thawing, they were added to pre-warmed mesenchymal stem cell growth medium (MSCGM™), including mesenchymal stem cell basal medium supplemented with l-glutamine, gentamicin, and amphotericin provided by Lonza and incubated at 37 °C in a 5% CO2 atmosphere. Upon 90 % confluency, trypsin/EDTA solution was used to detach cells for seeding.
Prior to seeding, the disks were autoclaved at 121 °C for 1 h. The samples were placed in 24-well plates, MSCGM™ was added to wet the samples, and the plates were incubated for 45 min. The cells were aspirated in media and seeded directly onto the samples (12,000 cells/sample), then incubated for 15 min to facilitate cell attachment. MSCGM™ was then added at 1 mL per well, and cultures were kept at 37 °C for the rest of the study. On day 3, the MSCGM™ was substituted with osteogenic induction medium, purchased from Lonza as a kit. The medium kit provided hMSC differentiation basal medium, dexamethasone, L-glutamine, ascorbate, β-glycerophosphate, Mesenchymal Cell Growth Supplement (MCGS™), and penicillin/streptomycin. This differentiation media was changed every 2-3 days.
Morphology of Cells
To study cellular morphology on the samples, cultured specimens were transferred to separate wells then immediately fixed in paraformaldehyde (2%) / glutaraldehyde (2%) in phosphate buffer (0.1 M ) at 4 °C overnight at 3, 7, 14, and 21 days. Phosphate buffer (0.1 M) was used to rinse the samples, and osmium tetroxide (OsO4, 2%) was added as a fixative. Samples were kept at room temperature for 2 h and rinsed once more with phosphate buffer. The dehydration of samples was performed using a graduated ethanol series (30-100 % ethanol) and finally dried with hexamethyldisilane (HMDS).
MTT Assay
Cell viability was evaluated using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay after 3, 7, 14, and 21 days of culture. MTT solution (100 μL) made from MTT powder (5 mg) in filter-sterilized PBS (1 ml) was pipetted onto each sample. Culture media (900 μL) was added to each well then incubated for 2 h at 37 °C. Afterward, the media was vacuumed out and solubilizer solution (600 μL) made from Triton X-100 (10 % v/v) and HCl (0.1N) in isopropanol was used to dissolve the formazan crystals. Aliquots of solution (100 μL) were pipetted in triplicate to a 96-well plate and adsorption was measured at 570 nm using a UV–Vis spectrometer (BioTek).
ALP Assay
The relative osteogenic differentiation of hMSCs between compositions was evaluated at 7, 14, and 21 days using an alkaline phosphatase (ALP) colorimetric assay kit (Abcam, Cambridge, MA). The samples were moved to new wells, cells were detached from the sample surfaces, and assay buffer solution (400 μL) was added to each well. The buffer was collected and centrifuged (13000 rpm) for 3 min to remove the insoluble material. The supernatant (80 μL) was pipetted in triplicate to a 96-well plate followed by the addition of provided p-nitrophenyl phosphate (pNPP) solution (5 mM, 50 μL). The well plate was protected from light and left for 1 h at 25 °C. The provided stop solution (20 μL) was added to each well and absorption was measured using UV–Vis spectroscopy at 405 nm.
Statistical Analysis
MTT and ALP assays were analyzed in triplicate of samples. Measurements of each were also performed in three independent samples. Data is presented as mean ± standard deviation. Statistical Analysis was performed using a two way ANOVA and Tukey method with 95 % confidence and P<0.025 was considered statistically significant.
Acknowledgments
The authors would like to thank the financial support from the National Institutes of Health, NIH National Institute of Dental and Craniofacial Research (NIDCR-R01-DE-029204-A1, PI: S.Bose), and NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS-R01-AR-066361, PI: S. Bose). The authors also would like to acknowledge the Franceschi Microscopy and Imaging Center at Washington State University. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Data Availability Statement
All relevant data has been used in this manuscript.
References
- 1.Kasir R, Vernekar VN, and Laurencin CT: Inductive biomaterials for bone regeneration. J. Mater. Res 32(6), 1047 (2017). [Google Scholar]
- 2.Giannoudis PV, Dinopoulos H, and Tsiridis E: Bone substitutes: An update. Injury 36(3), S20 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Bruyas A, Lou F, Stahl AM, Gardner M, Maloney W, Goodman S, and Yang YP: J. Mater. Res 33, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogata K, Imazato S, Ehara A, Ebisu S, Kinomoto Y, Nakano T, and Umakoshi Y: Comparison of osteoblast responses to hydroxyapatite and hydroxyapatite/soluble calcium phosphate composites. J. Biomed. Mater. Res. A 72A(2), 127 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Tarafder S and Bose S: Polycaprolactone-Coated 3D Printed Tricalcium Phosphate Scaffolds for Bone Tissue Engineering: In Vitro Alendronate Release Behavior and Local Delivery Effect on In Vivo Osteogenesis. ACS Appl. Mater. Interfaces 6(13), 9955 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S-L, Lou J, Wright NM, Lai CF, Avioli LV, and Riew KD: In Vitro andIn Vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif. Tissue Int 68(2), 87 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Bracci B, Torricelli P, Panzavolta S, Boanini E, Giardino R, and Bigi A: Effect of Mg2+, Sr2+, and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J. Inorg. Biochem 103(12), 1666 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Hing KA, Revell PA, Smith N, and Buckland T: Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 27(29), 5014 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Tarafder S, Davies NM, Bandyopadhyay A, and Bose S: 3D printed tricalcium phosphate bone tissue engineering scaffolds: effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater. Sci 1(12), 1250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bruijn JD, Klein CPAT, De Groot K, and Van Blitterswijk CA: The ultrastructure of the bone-hydroxyapatite interface in vitro. J. Biomed. Mater. Res 26(10), 1365 (1992). [DOI] [PubMed] [Google Scholar]
- 11.Roy M and Bose S: Osteoclastogenesis and osteoclastic resorption of tricalcium phosphate: Effect of strontium and magnesium doping. J. Biomed. Mater. Res. A 100A(9), 2450 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naruphontjirakul P, Porter AE, and Jones JR: In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater 66, 67 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Zhao F, Huang D, Fu X, Li X, and Chen X: Strontium-Substituted Submicrometer Bioactive Glasses Modulate Macrophage Responses for Improved Bone Regeneration. ACS Appl. Mater. Interfaces 8(45), 30747 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Cui X, Zhang Y, Wang J, Huang C, Wang Y, Yang H, Liu W, Wang T, Wang D, Wang G, Ruan C, Chen D, Lu WW, Huang W, Rahaman MN, and Pan H: Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/β-catenin signaling pathway. Bioact. Mater 5(2), 334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu C-C, Lin S-S, Chen W-J, Liu S-J, Chen L-H, Yang C-Y, Wang C-J, Yuan L-J, Chen P-H, and Cheng H-Y: Benefits of biphasic calcium phosphate hybrid scaffold-driven osteogenic differentiation of mesenchymal stem cells through upregulated leptin receptor expression. J. Orthop. Surg 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Valencia C, Pereiro I, Pirraco RP, López-Álvarez M, Serra J, González P, Marques AP, and Reis RL: Human mesenchymal stem cells response to multi-doped silicon-strontium calcium phosphate coatings. J. Biomater. Appl 28(9), 1397 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Maji K and Dasgupta S: Characterization and in vitro evaluation of gelatin–chitosan scaffold reinforced with bioceramic nanoparticles for bone tissue engineering. J. Mater. Res 34(16), 2807 (2019). [Google Scholar]
- 18.Singh SS, Roy A, Lee BE, Ohodnicki J, Loghmanian A, Banerjee I, and Kumta PN: A study of strontium doped calcium phosphate coatings on AZ31. Mater. Sci. Eng. C 40, 357 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Gamblin A-L, Brennan MA, Renaud A, Yagita H, Lézot F, Heymann D, Trichet V, and Layrolle P: Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: The local implication of osteoclasts and macrophages. Biomaterials 35(36), 9660 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Shih Yu-Ru V., Hwang Yongsung, Phadke Ameya, Kang Heemin, Hwang Nathaniel S., Caro Eduardo J., Nguyen Steven, Siu Michael, Theodorakis Emmanuel A., Gianneschi Nathan C., Vecchio Kenneth S., Chien Shu, Lee Oscar K., and Varghese Shyni: Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci 111(3), 990 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chamberlain G, Fox J, Ashton B, and Middleton J: Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. STEM CELLS 25(11), 2739 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, and Marshak DR: Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 284(5411), 143 (1999). [DOI] [PubMed] [Google Scholar]
- 23.De Godoy RF, Hutchens S, Campion C, and Blunn G: Silicate-substituted calcium phosphate with enhanced strut porosity stimulates osteogenic differentiation of human mesenchymal stem cells. J. Mater. Sci. Mater. Med 26(1), 54 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Samavedi S, Whittington AR, and Goldstein AS: Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater 9(9), 8037 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Boanini E, Gazzano M, and Bigi A: Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater 6(6), 1882 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Bandyopadhyay A, Bernard S, Xue W, and Bose S: Calcium Phosphate-Based Resorbable Ceramics: Influence of MgO, ZnO, and SiO2 Dopants. J. Am. Ceram. Soc 89(9), 2675 (2006). [Google Scholar]
- 27.Bandyopadhyay A, Petersen J, Fielding G, Banerjee S, and Bose S: ZnO, SiO2, and SrO doping in resorbable tricalcium phosphates: Influence on strength degradation, mechanical properties, and in vitro bone–cell material interactions. J. Biomed. Mater. Res. B Appl. Biomater 100B(8), 2203 (n.d.). [DOI] [PubMed] [Google Scholar]
- 28.Salma-Ancane K: Development of Mg-containing porous β-tricalcium phosphate scaffolds for bone repair. Ceram. Int 41(3), 4996 (2015). [Google Scholar]
- 29.Yin X, Stott MJ, and Rubio A: α - and β -tricalcium phosphate: A density functional study. Phys. Rev. B 68(20), <xocs:firstpage xmlns:xocs=“”/> (2003). [Google Scholar]
- 30.Banerjee SS, Tarafder S, Davies NM, Bandyopadhyay A, and Bose S: Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of β-TCP ceramics. Acta Biomater 6(10), 4167 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Khang D, Choi J, Im Y-M, Kim Y-J, Jang J-H, Kang SS, Nam T-H, Song J, and Park J-W: Role of subnano-, nano- and submicron-surface features on osteoblast differentiation of bone marrow mesenchymal stem cells. Biomaterials 33(26), 5997 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Carbajal L, Serena S, Caballero A, Saínz MA, Detsch R, and Boccaccini AR: Role of ZnO additions on the β/α phase relation in TCP based materials: Phase stability, properties, dissolution and biological response. J. Eur. Ceram. Soc 34(5), 1375 (2014). [Google Scholar]
- 33.Müller P, Bulnheim U, Diener A, Lüthen F, Teller M, Klinkenberg E-D, Neumann H-G, Nebe B, Liebold A, Steinhoff G, and Rychly J: Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J. Cell. Mol. Med 12(1), 281 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fielding GA, Smoot W, and Bose S: Effects of SiO2, SrO, MgO, and ZnO dopants in tricalcium phosphates on osteoblastic Runx2 expression.(Report). J. Biomed. Mater. Res. A 102(7), 2417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minardi S, Corradetti B, Taraballi F, Sandri M, Van Eps J, Cabrera FJ, Weiner BK, Tampieri A, and Tasciotti E: Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 62, 128 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Schumacher M, Lode A, Helth A, and Gelinsky M: A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater 9(12), 9547 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Tarafder S, Dernell WS, Bandyopadhyay A, Bose S: SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. B Appl. Biomate 103 (3), 679 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Tarafder S, Banerjee S, Bandyopadhyay A, Bose S: Electrically polarized biphasic calcium phosphates: adsorption and release of bovine serum albumin. Langmuir 26 (22), 16625 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pattanayak DK, Dash R, Prasad RC, Rao BT, and Rama Mohan TR: Synthesis and sintered properties evaluation of calcium phosphate ceramics. Mater. Sci. Eng. C 27(4), 684 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data has been used in this manuscript.