Abstract
Background and Objectives
Cerebrovascular disease (CBVD) is frequently comorbid with autopsy-confirmed Alzheimer disease (AD), but its contribution to the clinical presentation of AD remains unclear. We leveraged the National Alzheimer's Coordinating Center (NACC) uniform and neuropathology datasets to compare the cognitive and functional trajectories of AD+/CBVD+ and AD+/CBVD− brain donors.
Methods
The sample included NACC brain donors with autopsy-confirmed AD (Braak stage ≥3, Consortium to Establish a Registry for Alzheimer's Disease score ≥2) and complete Uniform Data Set (UDS) evaluations between 2005 and 2019, with the most recent UDS evaluation within 2 years of autopsy. CBVD was defined as moderate to severe arteriosclerosis or atherosclerosis. We used propensity score weighting to isolate the effects of comorbid AD and CBVD. This method improved the balance of covariates between the AD+/CBVD+ and AD+/CBVD− groups. Longitudinal mixed-effects models were assessed with robust bayesian estimation. UDS neuropsychological test and the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) scores were primary outcomes.
Results
Of 2,423 brain donors, 1,476 were classified as AD+/CBVD+. Compared with AD+/CVBD− donors, the AD+/CBVD+ group had accelerated decline (i.e., group × time effects) on measures of processing speed (β = −0.93, 95% CI −1.35, −0.51, Bayes factor [BF] 130.75), working memory (β = 0.05, 95% CI 0.02, 0.07, BF 3.59), verbal fluency (β = 0.10, 95% CI 0.04, 0.15, BF 1.28), naming (β = 0.09, 95% CI 0.03, 0.16, BF = 0.69), and CDR-SB (β = −0.08, 95% CI −0.12, −0.05, BF 18.11). Effects ranged from weak (BFs <3.0) to strong (BFs <150). We also found worse performance in the AD+/CBVD+ group across time on naming (β = −1.04, 95% CI −1.83, −0.25, BF 2.52) and verbal fluency (β = −0.73, 95% CI −1.30, −0.15, BF 1.34) and more impaired CDR-SB scores (β = 0.45, 95% CI 0.01, 0.89, BF 0.33).
Discussion
In brain donors with autopsy-confirmed AD, comorbid CBVD was associated with an accelerated functional and cognitive decline, particularly on neuropsychological tests of attention, psychomotor speed, and working memory. CBVD magnified effects of AD neuropathology on semantic-related neuropsychological tasks. Findings support a prominent additive and more subtle synergistic effect for comorbid CBVD neuropathology in AD.
Detection, diagnosis, and management of neurodegenerative diseases can be challenging due to heterogeneous clinical and neuropathologic presentations. The clinical presentation of Alzheimer disease (AD) traditionally involves initial impairments in episodic memory and expressive language with subsequent declines in executive functioning and visuospatial abilities.1 There are gradual onset and progressive worsening of cognitive symptoms.1,2 Approximately 25% (estimates vary) of individuals with neuropathologically confirmed AD do not have this traditional presentation.3 Other clinical syndromes in AD have been identified, characterized primarily by initial and predominant impairments in language, visuospatial skills, or executive functioning.4-6 The rates of clinical progression can vary within and between subtypes.7 These atypical presentations may be related to differences in the location and density of AD neuropathologic changes and neurodegeneration.5 Alternatively, there is increasing focus on the contribution of comorbid neuropathologies.8,9
Cerebrovascular disease (CBVD) is among the most common comorbid neuropathologies with AD; up to 80% of participants with autopsy-confirmed AD neuropathology have CBVD.10 CBVD refers to disorders that affect large and small vessels of the brain, including atherosclerosis, arteriolosclerosis, infarcts, microinfarcts, microhemorrhages, and white matter rarefaction.11 In vivo MRI studies have long shown that markers of CBVD (e.g., white matter hyperintensities, infarcts, microbleeds) are associated with subtle cognitive changes,12-14 especially frontal-mediated functions (e.g., processing speed and executive functioning), with accelerated cognitive decline.15,16 These findings have been instrumental in our understanding of the independent effects of CBVD on cognitive health. Yet, the effects of comorbid CBVD on the clinical presentation of AD (e.g., rate of decline, phenotypic expression) are less well understood and often debated.17-19
Clinical-pathologic studies are the gold standard for the characterization of disease expression. The National Alzheimer's Coordinating Center (NACC) Uniform Data Set (UDS) and Neuropathology Data Set (NDS) are publicly available datasets that were established to promote collaborative research into AD and AD-related dementias. Two studies used the NACC UDS and NDS to examine the combined effects of AD and CBVD on cognitive and functional decline. The first study examined 2,046 brain donors and showed that donors with both AD and CBVD neuropathology had a slower rate of functional decline, as assessed by the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB),20 compared to those who had AD neuropathology only.21 The second study showed a slower rate of decline for language measures in the AD+/CBVD+ donors, with trend-level effects for attentional measures.22 In both, the authors focused on macroinfarcts and microinfarcts.
The aforementioned studies have played a key role in our understanding of the clinical consequences of comorbid AD and CBVD. However, the slower rate of decline observed was somewhat unexpected, suggesting greater nuance in this relationship than previously believed. The second study defined cognitive domains using averaged z scores for pairs of neuropsychological tests instead of using a structural model or individual tests.23 In addition, both studies noted that higher levels of AD neuropathology tended to overwhelm any effect of comorbid conditions, and the sample tended toward higher AD neuropathology in those without CBVD. These studies also assessed multiple comorbid conditions in the same model. While the authors used propensity score weighting to address confounds related to individuals who agreed to brain donation, no studies have used propensity scores to isolate the effects of mixed AD and CBVD from other forms of neuropathology.
The current investigation leveraged the NACC UDS and NDS to examine the clinical course and presentation of deceased individuals with neuropathologically confirmed AD and CBVD. Among 2,423 deceased individuals with AD neuropathology who completed serial antemortem neuropsychological testing, we used bayesian mixed-effects modeling to assess group differences in test performance and functional status for brain donors with and without CBVD, defined as cerebral large and small vessel disease, as well as differences in rates of decline over time (i.e., progression of impairment). We chose arteriosclerosis and atherosclerosis as metrics of CBVD because other measures were rated inconsistently across NDS versions, because past work has examined macroinfarcts and microinfarcts in the NACC dataset, and because arteriosclerosis and atherosclerosis represent global indices of CBVD. We hypothesized that AD brain donors with comorbid CBVD (i.e., CBVD+) would have worse dementia severity and neuropsychological test performance and a faster rate of decline on tests of processing speed and executive functioning compared with donors who did not have CBVD (i.e., CBVD−).
Methods
Participants and Design
The sample included brain donors with autopsy-confirmed AD from the NACC UDS and NDS who completed UDS visits between 2005 and 2019 and completed a UDS evaluation within at least 2 years of autopsy (N = 2,423). Since 2005, ≈30 Alzheimer's Disease Research Centers (ADRCs) have contributed to the NACC UDS, which is a database of standardized cognitive, behavioral, and functional participant evaluations (a full description is provided elsewhere24,25). A subset of NACC UDS participants agree and consent to brain donation and neuropathologic examination; these individuals form the NACC NDS. Brain donors were also excluded who did not meet classification criteria for autopsy-confirmed AD (see below; n = 1,992). Brain donors were also excluded who lacked autopsy data on atherosclerosis and arteriolosclerosis (NACCAVAS and NACCARTE; n = 293) or lacked data on covariates (see propensity score weighting; n = 140).
Standard Protocol Approvals, Registrations, and Patient Consents
The NACC database was approved by the University of Washington Institutional Review Board. Informed consent from brain donors was obtained at each individual ADC.
AD and CBVD Neuropathology
For the NACC NDS, neuropathologic data are recorded with a standardized Neuropathology Form and Coding Guidebook.26 Following previous studies,27,28 we classified individuals as having AD neuropathologic changes (i.e., AD+) who had evidence of at least moderate neuropathology for both plaques and tangles, i.e., donors with Braak stage for neurofibrillary degeneration (B score; NACCBRAA) ≥3 and density of neocortical neuritic plaques (Consortium to Establish a Registry for Alzheimer's Disease C score; NACCNEUR) ≥ 2. Of 4,445 eligible brain donors, 2,423 met these criteria. We defined CBVD neuropathology (i.e., CBVD+) as moderate to severe arteriosclerosis (i.e., concentric hyaline thickening of the media arterioles; NACCARTE) or atherosclerosis (i.e., fibrofatty atheromatous plaques in the large arteries of the circle of Willis; NACCAVAS). On the basis of the above criteria, 2 groups were created: AD+/CBVD+ and AD+/CBVD−. Consistent with previous research in the NACC sample that served as the foundation for this study,21,22 we dichotomized CBVD indices into absence or presence. While this approach does not allow us to examine the impact of disease severity (as reviewed in the section discussing limitation), it was necessary to balance confounds across groups and to accurately model relationships of interest. We did not include an AD−/CBVD− group because of the following: (1) the objective of this study was to investigate the isolated, additive effects of CBVD in AD; (2) we were unable to conduct our propensity score analyses as planned (see below); and (3) we did not regard the NACC NDS as an optimal healthy control sample because of factors related to selection. Of note, CBVD has been rated inconsistently across the NDS versions, with the exception of arteriolosclerosis and atherosclerosis. These variables were thus used as a primary CBVD outcome variable to maximize data availability and to minimize inconsistencies of assessment across versions. Information on other forms of CBVD in the sample, including macroinfarcts and microinfarcts, can be found in eTable 1 (links.lww.com/WNL/B976).
Neuropsychological Testing and Dementia Severity
Brain donors were administered the UDS neuropsychological battery as part of their annual visit at each ADRC.29,30 There are 3 versions of the UDS, with the most recent version (UDS version 3.0) being released in March 2015.29 For UDS version 3.0, a new neuropsychological test battery was adopted, and a subset of instruments were replaced with similar but nonproprietary tests. Harmonization between versions was achieved with results from the Crosswalk study.30 The specific tests examined included the Mini-Mental State Examination, Short Form of the Boston Naming Test (BNT), Animal Fluency and Vegetable Fluency, Trail Making Test (TMT) Parts A and B, Wechsler Adult Intelligence Scale-IV Digit Span Forward and Digit Span Backward (DSB), and Logical Memory Immediate and Delayed Recall. These assessed the following neuropsychological functions: global cognitive status, confrontation naming, semantic fluency, processing speed, set shifting, auditory attention, working memory, and learning and episodic memory, respectively. Raw scores were used, and lower scores reflect worse performance for all tests except for TMT-A and TMT-B, for which lower scores reflect better performance.
Dementia severity was assessed with the CDR-SB Dementia Staging Instrument.20,31 The CDR stages dementia severity on the basis of informant report of orientation, memory, judgment/problem solving, home and hobbies, community affairs, and personal care. Higher scores indicate increased severity of dementia.
Propensity Score Weighting
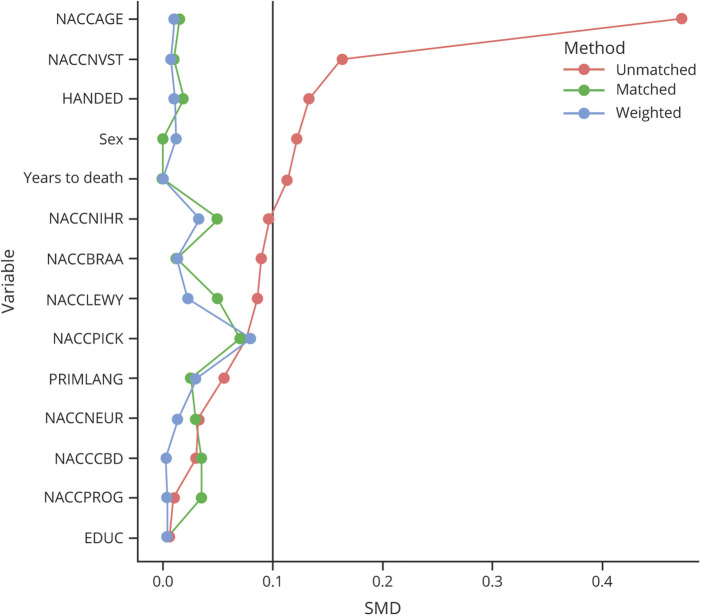
Propensity scores reduce bias on causal estimates in the absence of randomized experiments.32 These methods adjust for confounding as an alternative to traditional covariate adjustment, with several theoretical and practical advantages (e.g., flexibility of estimation and assessment of balance). In this study, we wanted to isolate the impact of CBVD on the cognitive trajectories of individuals with AD neuropathologic changes by balancing the severity of AD pathology between the CBVD+ and CBVD− groups, as well as other comorbid neuropathology and relevant demographic and visit variables (Table 1). We regarded AD neuropathology as a confound because it was unbalanced between groups; we wanted to assess the specific additive effect of CBVD in donors with AD. Similarly, we considered other neuropathologies to be confounds for our particular research question, even though they are causal factors or moderators for overall cognitive health. The propensity score model used a logit function with age, biological sex, racial identity, primary language, education, handedness, number of in-person visits, years from final in-person visit to death, Lewy body disease, Pick disease, corticobasal degeneration, progressive supranuclear palsy, and AD pathology variables included as predictors (i.e., NACCBRAA, NACCNEUR). Covariates of neuropathology were selected to optimize data availability and to encompass major classes of neuropathology. Variables related to CBVD were not selected for the propensity score because this was our primary comparison of interest. Several included covariates were unbalanced in the sample as assessed by standardized mean difference (Table 1). Inverse probability weighting (IPW) was used, as well as caliper matching.33 Both methods provided adequate balance for these variables in the final sample across the AD+/CBVD+ and AD+/CBVD− groups (Figure 1). However, IPW was favored to maintain consistency with past studies21,22 and to include all donors in the sample. Examination of the region of common support revealed sufficient overlap in the propensity score distributions between the 2 groups.34 Of note, weighting based on autopsy selection was not used because both previous studies involving this sample reported negligible effects from this procedure.21,22
Table 1.
Demographic and Neuropathologic Covariates of Brain Donors With AD by Cerebrovascular Group
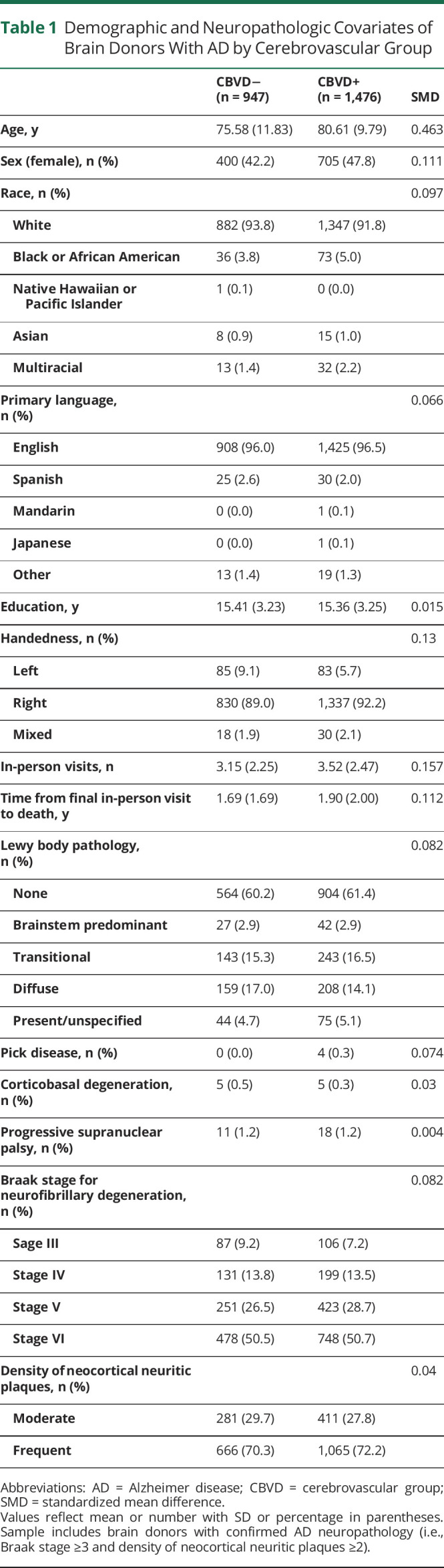
Figure 1. Balance of Demographic and Neuropathologic Covariates Among Cerebrovascular Groups Before and After Propensity Score Analysis.
EDUC = years of education; HANDED = handedness; NACCAGE = age at final in-person visit; NACCBRAA = Braak stage for neurofibrillary degeneration; NACCCBD = corticobasal degeneration; NACCLEWY = Lewy body pathology; NACCNEUR = density of neocortical neuritic plaques; NACCNIHR = race; NACCNVST = number of in-person visits; NACCPICK = Pick disease; NACCPROG = progressive supranuclear palsy; PRIMLANG = primary language; SEX = biological sex; SMD = standardized mean difference; YearstoDeath = years from final in-person visit to death.
Statistical Analyses
Through IPW, we assessed 2 groups (i.e., AD+/CBVD− and AD+/CBVD+) who were matched on AD neuropathologic severity, comorbid neuropathology, and demographic and other relevant characteristics. We used robust bayesian estimation of linear mixed-effects models to assess differences in neuropsychological test performance and CDR-SB score for CBVD+ and CBVD− brain donors with AD. Outcome variables included neuropsychological test raw scores and CDR-SB score. Predictors included neuropathology group, time (i.e., years to death at each visit), and a group × time interaction. Models also included a random intercept for participant and ADRC. Full information on our statistical analysis plan can be found in the eMethods (links.lww.com/WNL/B976). Effects were estimated with a 95% credible interval (CI35) and interpreted with Bayes factors (BFs). The following reference ranges were used for BFs: <1 indicates evidence against an effect, between 1 and 3 indicates weak evidence for an effect, between 3 and 20 indicates positive evidence for an effect, between 20 and 150 indicates strong evidence for an effect, and >150 indicates very strong evidence for an effect. Null findings were interpreted when BFs and CIs were in agreement.36 Unlike frequentist models, bayesian inference rests on cumulative evidence for or against a hypothesis, rather than a preset decision rule. Bayesian CIs reflect estimation of population values for model effects rather than a means for rejecting a null hypothesis. Thus, bayesian inference does not require traditional corrections for multiple comparisons (see eMethods). We chose to maintain dense and conservative estimation of the data to reduce the probability of false-positive findings. Our methodology also contains an inherent component of regularization (i.e., a tendency toward underestimated effects), avoidance of random overfitting, and resistance to outliers. Bayesian methodologies have historically been less likely to produce type I errors. Given our bayesian approach and optimal partial pooling of all available information, we did not exclude brain donors on the basis of the number of follow-up visits (i.e., we included donors with any follow-up visits in estimation of longitudinal effects). Analyses were conducted with R (version 4.1.0, R Core Team, Vienna, Austria) in the brms library.37
Data Availability
All anonymized data from this article are publicly accessible and available through the NACC datasets.
Results
Participant Characteristics
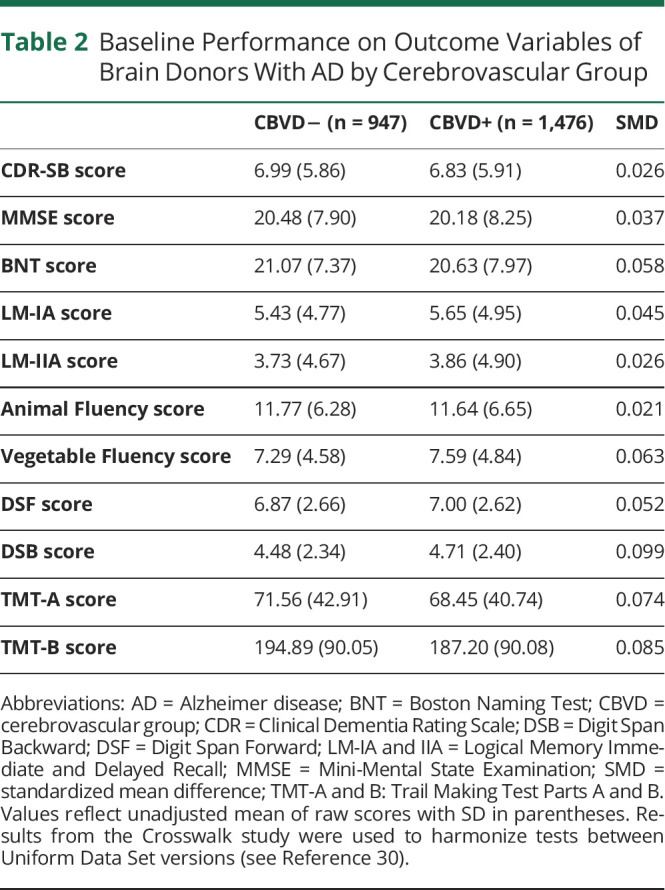
This study included 2,423 brain donors. Of these, 2,229 identified as non-Hispanic White (92.0%), and 1,105 identified as female (45.6%). Table 1 includes full demographic and descriptive information on the sample. Average age at death was 80.7 (SD 11.7) years, and the mean interval between death and last UDS evaluation was 9.0 (SD 7.9) months. The mean follow-up time from each visit to the initial visit was 3.0 (SD 2.7) years, and the mean follow-up time between the initial visit and final visit was 6.1 (SD 2.9) years. Brain donors were evaluated approximately once a year with an average of 396.2 (SD 25.1) days between visits. In the sample, 1,476 donors were classified as AD+/CBVD+ (60.9%). After matching, there were no group differences on any of the propensity score covariates. Baseline performance on outcome variables for each participant's initial visit is summarized in Table 2. There were no group differences in baseline neuropsychological test performance (standardized mean differences <0.10). Performance at the brain donor's final visit can be found in eTable 2 (links.lww.com/WNL/B976). Group differences in neuropsychological test performance at the final visit were most pronounced for donors ≥80 years of age (eTable). At baseline, 269 donors received a clinical diagnosis of normal cognition (11.1%), 34 donors were cognitively impaired but did not meet criteria for mild cognitive impairment (MCI) (1.4%), 337 donors were diagnosed with amnestic or nonamnestic MCI (13.9%), and 1,783 donors were diagnosed with dementia (73.6%). At their final visit, 90 donors received a clinical diagnosis of normal cognition (3.7%), 18 donors were cognitively impaired but did not meet criteria for MCI (0.7%), 136 donors were diagnosed with amnestic or nonamnestic MCI (5.6%), and 2,179 donors were diagnosed with dementia (89.9%). Clinical diagnoses grouped by AD+/CBVD+ and AD+/CBVD− brain donors can be found in eTable 4.
Table 2.
Baseline Performance on Outcome Variables of Brain Donors With AD by Cerebrovascular Group
Group Differences Across Time
Neuropsychological test performance and CDR-SB score declined over time in all models (BFs >1,000; eTable 5 gives full model results, links.lww.com/WNL/B976). For dementia severity, CDR-SB scores were worse across time in the AD+/CBVD+ compared with the AD+/CBVD− group but with a small effect (βMean = 0.45, 95% CI 0.01, 0.89, BF 0.33). There were few differences in neuropsychological test performance between the AD+/CBVD+ and AD+/CBVD− groups in the models. The CBVD+ group performed worse across time on the BNT (βMean = −1.04, 95% CI −1.81, −0.25, BF 2.52) and animal fluency (βMean = −0.73, 95% CI −1.30, −0.15, BF 1.34), with weak effects. In contrast, there was evidence against group differences on other neuropsychological tests (BFs <1).
Trajectory of Decline Across Time: Group × Time Results
Brain donors showed accelerated functional decline and dementia progression (CDR-SB score) over time (i.e., years to death at each visit) (βMean = −0.92, 95% CI −0.95, −0.89, BF >1,000). As years to death decreased (i.e., brain donor closer to death), CDR-SB score increased. There was also a positive effect for the group × time interaction, suggesting a faster rate of dementia progression in the AD+/CBVD+ group compared with the AD+/CBVD− group (βMean = −0.08, 95% CI −0.12, −0.05, BF 18.11).
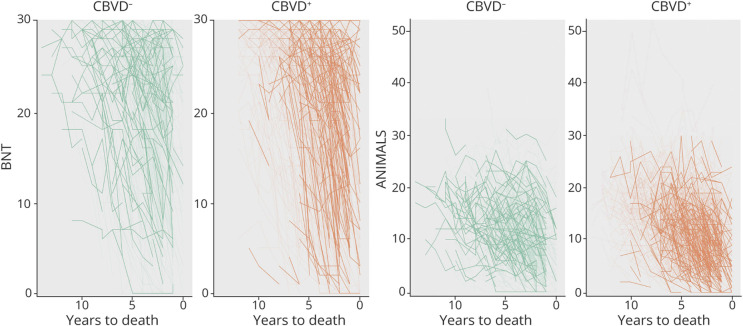
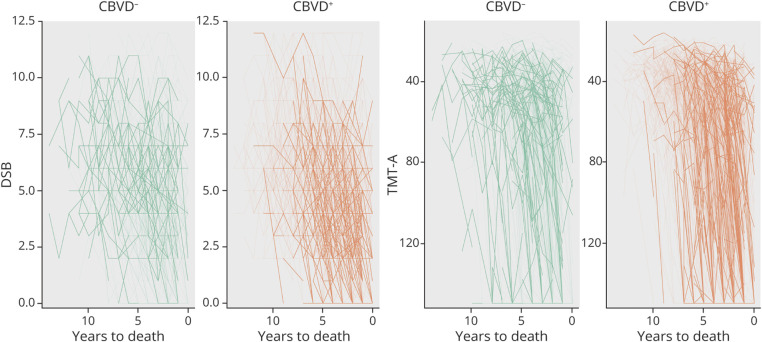
Performance on DSB also declined over time for all brain donors, with a very strong effect (βMean = 0.27, 95% CI 0.25, 0.28, BF >1,000), particularly for the AD+/CBVD+ group (i.e., there was a positive effect for the group × time interaction) (βMean = 0.05, 95% CI 0.02, 0.07, BF 3.59). Likewise, TMT-A performance declined over time for all brain donors (βMean = −5.59, 95% CI −5.91, −5.28, BF >1,000), particularly for the AD+/CBVD+ group, with a strong effect for the group × time interaction (βMean = −0.93, 95% CI −1.35, −0.51, BF 130.75). Performance on animal fluency declined over time (βMean = 1.00, 95% CI 0.96, 1.05, BF >1,000), particularly for the AD+/CBVD+ group, with a strong effect for the group × time interaction (βMean = 0.10, 95% CI 0.04, 0.15, BF 1.28). Representations of individual cognitive trajectories for BNT, Animal Fluency, DSB, and TMT-A scores are shown in Figures 2 and 3.
Figure 2. Individual Cognitive Trajectories on the BNT and Animal Fluency.
Highlighted lines reflect test performances over time for the upper 20% of brain donors weighted on the propensity score for the CBVD− and CBVD+ groups. Denser and more directly downward trajectory of the CBVD+ group indicates a faster rate of decline. ANIMALS = Animal Fluency; BNT = Boston Naming Test; CBVD = cerebrovascular group.
Figure 3. Individual Cognitive Trajectories on DSB and TMT-A.
Highlighted lines reflect test performances over time for the upper 20% of brain donors weighted on the propensity score for the CBVD− and CBVD+ groups. Denser and more directly downward trajectory of the CBVD+ group indicates a faster rate of decline. CBVD = cerebrovascular group; DSB = Digit Span Backward; TMT-A = Trail Making Test Part A.
For the remaining measures, an interaction was present, but the evidence did not reach the threshold to be interpretable. Performance on the Mini-Mental State Examination declined over time for all brain donors (βMean = 1.24, 95% CI 1.20, 1.28, BF >1,000), particularly for the AD+/CBVD+ group, but without even a weak group × time interaction effect (βMean = 0.07, 95% CI 0.01, 0.13, BF 0.06). Likewise, BNT performance declined over time for all brain donors (βMean = 1.00, 95% CI 0.96, 1.05, BF >1,000), particularly for the AD+/CBVD+ group, but without even a weak group × time interaction effect (βMean = 0.09, 95% CI 0.03, 0.16, BF 0.69). Last, performance on vegetable fluency declined over time (βMean = 0.75, 95% CI 0.72, 0.78, BF >1,000), particularly for the AD+/CBVD+ group, but without even a weak group × time interaction effect (βMean = 0.06, 95% CI 0.02, 0.10, BF 0.25). There was evidence against group × time interactions on other neuropsychological tests.
Discussion
CBVD is frequently comorbid with AD and contributes to the clinical and neuropathologic pathogenesis of AD.38,39 Many previous investigations into the contribution of CBVD to the clinical presentation of AD have been cross-sectional or without autopsy confirmation of disease, limiting inference on clinical trajectory. In this study of 2,423 brain donors with neuropathologically confirmed AD from the NACC NDS sample, we compared the clinical and cognitive trajectories of AD+/CBVD+ and AD+/CBVD− donors. We used propensity score weighting to balance numerous covariates between these groups, including age, biological sex, race, primary language, education, handedness, number of visits, years from final in-person visit to death, and presence and severity of neuropathology. This method, along with our use of robust bayesian linear mixed-effects modeling, allowed us to improve on limitations in the existing literature. We found that the presence of CBVD was associated with an accelerated functional and cognitive decline in donors with AD, particularly on neuropsychological tests of attention, psychomotor speed, and working memory. CBVD also magnified the effects of AD neuropathology on semantic-related neuropsychological tasks; however, our strongest findings suggested a faster rate of decline on tests of frontal-executive systems in the AD+/CBVD+ group. This supports a prominent additive effect and a more subtle synergistic effect for comorbid CBVD neuropathology in AD.
Previous studies using the NACC NDS sample found unexpected antagonistic,21,22 rather than additive, effects of CBVD neuropathology on cognitive decline and dementia severity as shown by this study. In both studies, the authors assessed similar mixed-effects models but with AD neuropathology, severity of Lewy body disease, and vascular brain injury (operationalized as presence of gross infarcts or cortical microinfarcts) included in the primary models. This methodology has advantages (e.g., the inclusion of brain donors without AD and examination of multiple comorbid pathologies). However, neither study isolated the effects of AD and CBVD through balancing covariates between groups. In addition, secondary analyses suggested that higher levels of AD neuropathology in brain donors without vascular brain injury may have accounted for some of the effects. The authors also noted the need for further investigation of specific vessel disorders such as arteriosclerosis and atherosclerosis as opposed to focus on infarcts and microinfarcts.21
The differences between our findings and past research with the NACC NDS are likely due to these methodologic factors (i.e., the use of propensity score weighting to isolate AD+/CBVD+ effects in this study). That is, understanding the association between AD and CBVD depends on the nuances of measuring confounds. When accounting for covariates (including the severity of AD neuropathology) and using arteriolosclerosis and atherosclerosis to define CBVD, we found that CBVD was associated with a faster rate of functional and cognitive decline on several neuropsychological measures in AD. Domain-specific effects were most prominent for tests of attention, processing speed, and working memory in brain donors with AD and CBVD. Unexpected findings were that there was no effect for TMT-B and there are no other measures of executive function as part of the UDS. Extant literature links in vivo markers of CBVD and cardiovascular risk factors with cognitive decline in frontal-executive functions and progression of AD pathology.15,16,40 However, neuroimaging studies also posit that AD and CBVD are independent predictors of cognitive decline.41,42 The trajectories of cognitive decline in AD can be heterogeneous, including a subset who have a prominent dysexecutive variant.43,44 The presence of comorbid CBVD neuropathology might contribute to this presentation.
Although there was evidence for a faster rate of decline in AD+/CBVD+ donors, there were minimal group differences across neuropsychological measures of attention, information processing speed, working memory, and executive function. In this, our unique sample of brain donors with neuropathologically confirmed AD appears to diverge from previous research on healthy samples.12-14,45 There were, however, overall group differences on tests related to semantic brain networks (confrontation naming and semantic fluency), with worse performance in the AD+/CBVD+ group over time and faster decline in semantic fluency.46 This could result from CBVD accelerating AD pathology and worsening its impact on AD-related cognitive networks (i.e., a synergistic effect such that the impact of comorbidity is greater than the sum of the parts40). It is also possible that these performance differences reflect dysexecutive retrieval difficulties. One cross-sectional study found that the severity of white matter hyperintensities fully mediated performance on semantic tasks.47 The authors concluded that these findings reflect a role for axonal integrity in lexical-semantic retrieval. We did not find group differences on tests of episodic memory. However, the NACC UDS is limited in the assessment of episodic memory, which is typically affected first in AD and CBVD, and does not include list learning word tasks. On the basis of unadjusted performance, we also found some indication that the effects of AD and CBVD may be more pronounced in older brain donors (≥80 years of age).
The nature of the relationship between AD and CBVD remains uncertain. CBVD might have a mechanistic role in AD pathophysiology,17,48 an additive role, and/or a synergistic role.18,19,41 The directionality of the effects cannot be concluded from the findings from this postmortem study. Prospective studies with serial in vivo assessments of amyloid, tau, and CBVD will be instrumental for addressing causal pathways to dementia. It is important to note that we used propensity scores to balance confounding effects and to assess whether the presence of CBVD alters the cognitive presentation and trajectory of brain donors with AD, rather than making inferences regarding causality. CBVD is associated with aging, and together (i.e., aging + CBVD), they might precipitate neurodegenerative disease processes. CBVD from cardiovascular disease or other risk factors might also act as a parallel path to already present neurodegenerative processes and exert independent effects on cognition. Last, CBVD could be a downstream consequence of neurodegenerative disease. Amyloidosis and tau aggregation, as central pathologies of AD, may precipitate both neurodegeneration and CBVD.
The findings from our study provide weak support for a synergistic relationship between CBVD and AD. That is, we found weak support for an impact of CBVD on naming and fluency performance. Given the classic presentation of these 2 syndromes, we would not expect this change with increased CBVD pathology but rather by increasing the impact of AD on cognition (although an executive component cannot be discounted47,49). However, there was stronger support for an effect of CBVD on dementia severity, processing speed, and working memory, which is congruent with the additive effect that we would expect, given the classic presentation of CBVD. Either of these effects could help explain the heterogeneous and complex presentation of mixed pathology in individuals with AD, which may become more pronounced with age.
There are several limitations to our findings. Neuropathology was confirmed postmortem, and cognitive performance does not necessarily reflect the burden of pathology at the time of each visit. While we accounted for the interaction between CBVD and amyloid and tau indirectly via AD pathology postmortem, we did not specifically examine amyloid and tau severity ratings and their contribution to cognitive and functional decline. Along these lines, we dichotomized CBVD indices such that we could easily balance confounding effects. However, it is unclear whether cognitive trajectories depend on severity of pathology or only its presence. Future studies should consider alternate methodologic approaches that are able to examine severity (e.g., multinomial propensity scores). We used arteriosclerosis and atherosclerosis to operationalize CBVD+ because these variables have been consistently assessed and recorded across NACC NDS versions. While they serve as global indicators of CBVD, they might not encompass all forms of CBVD and are not granular assessments of vessel pathology. There was greater incidence of multiple CBVD neuropathologies in our AD+/CBVD+ group, providing support for our decision (eTable 1, links.lww.com/WNL/B976). We considered stratifying the analyses by diagnostic group in secondary models (i.e., normal cognition, MCI, and dementia). However, this would have increased our number of comparisons 3-fold. Because cognitive performance declined over time (our most reliable finding), we assessed a similar question in these models. The additive effect of CBVD is prominent as brain donors age toward death and cognitive performance declines (i.e., those same donors more likely to be diagnosed with MCI or dementia). It is also possible that individuals with a slower course of AD had more time to develop severe forms of CBVD (e.g., stroke) or other comorbid conditions associated with aging (as evidenced by the older mean age of the AD+/CBVD+ group). Alternatively, it is possible that those who had a more rapid course had more severe CBVD and died younger compared with those who were more resilient and had the slower course (i.e., survival bias). We attempted to address these issues with the propensity scores that balanced both age and AD severity in statistical models. These findings have limited generalizability, including this being an autopsy sample of research participants with limited ethnic and racial diversity who agreed to brain donation. Last, and as alluded to previously, the UDS neuropsychological battery is limited in scope in the assessment of both episodic memory and executive function.
In this study, we examined 2,423 deceased individuals with neuropathologically confirmed AD from the NACC UDS and NDS sample. We used bayesian mixed-effects modeling and propensity score weighting to assess group differences and rates of decline for test performance and functional status in brain donors with and without CBVD. Brain donors with AD and CBVD declined faster over time on several measures, most prominently on measures related to the classic presentation of CBVD (i.e., processing speed and working memory).
Acknowledgment
The NACC database is funded by National Institute on Aging (NIA)/NIH grant U24 AG072122. NACC data are contributed by the NIA-funded ADRCs: P30 AG019610 (principal investigator [PI] Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Glossary
- AD
Alzheimer disease
- ADRC
Alzheimer's Disease Research Center
- BF
Bayes factor
- BNT
Boston Naming Test
- CBVD
cerebrovascular disease
- CDR-SB
Clinical Dementia Rating Scale Sum of Boxes
- CI
credible interval
- DSB
Digit Span Backward
- IPW
inverse probability weighting
- MCI
mild cognitive impairment
- NACC
National Alzheimer's Coordinating Center
- NACCARTE
NACC arteriolosclerosis
- NACCAVAS
NACC atherosclerosis of the circle of Willis
- NDS
Neuropathology Data Set
- TMT
Trail Making Test
- UDS
Uniform Data Set
Appendix. Authors
Study Funding
This work was supported by grants from the NIH (P30AG072978; K23NS102399). This publication was supported by the National Center for Advancing Translational Sciences, NIH, through BU-CTSI grant 1UL1TR001430. The content is solely the responsibility of the authors.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crutch SJ, Schott JM, Rabinovici GD, et al. Shining a light on posterior cortical atrophy. Alzheimers Dement. 2013;9(4):463-465. [DOI] [PubMed] [Google Scholar]
- 5.Rohrer JD, Rossor MN, Warren JD. Alzheimer's pathology in primary progressive aphasia. Neurobiol Aging. 2012;33(4):744-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson BC, Wolk DA,Alzheimer’s Disease Neuroimaging Initiative. Dysexecutive versus amnesic phenotypes of very mild Alzheimer's disease are associated with distinct clinical, genetic and cortical thinning characteristics. J Neurol Neurosurg Psychiatry. 2011;82(1):45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risacher SL, Anderson WH, Charil A, et al. Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology. 2017;89(21):2176-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ Study. Neurology. 2015;85(6):535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toledo JB, Arnold SE, Raible K, et al. . Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136(9):2697-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119(3):277-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsiger S, Koppelmans V, Mérillat S, et al. . Executive functions in healthy older adults are differentially related to macro- and microstructural white matter characteristics of the cerebral lobes. Front Aging Neurosci. 2017;9:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockhart SN, Roach AE, Luck SJ, et al. White matter hyperintensities are associated with visual search behavior independent of generalized slowing in aging. Neuropsychologia. 2014;52:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer Disease Neuroimaging Initiative. Arch Neurol. 2010;67(11):1370-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alosco ML, Sugarman MA, Besser LM, et al. . A clinicopathological investigation of white matter hyperintensities and Alzheimer's disease neuropathology. J Alzheimers Dis. 2018;63(4):1347-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangen KJ, Preis SR, Delano-Wood L, et al. Baseline white matter hyperintensities and hippocampal volume are associated with conversion from normal cognition to mild cognitive impairment in the Framingham Offspring Study. Alzheimer Dis Assoc Disord. 2018;32(1):50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer's disease. Alzheimer’s Demen Diagn Assess Dis Monit. 2015;1(2):144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brickman AM, Zahodne LB, Guzman VA, et al. . Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging 2015;36(1):27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60(7):1082-1088. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 21.Brenowitz WD, Hubbard RA, Keene CD, et al. Mixed neuropathologies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement. 2017;13(6):654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenowitz WD, Hubbard RA, Keene CD, et al. Mixed neuropathologies and associations with domain-specific cognitive decline. Neurology. 2017;89(17):1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moeller J. A word on standardization in longitudinal studies: don't. Front Psychol. 2015;6:1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beekly DL, Ramos EM, Lee WW, et al. . The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Associated Disord. 2007;21(3):249-258. [DOI] [PubMed] [Google Scholar]
- 25.Weintraub S, Salmon D, Mercaldo N, et al. . The Alzheimer's Disease Centers' Uniform Data Set (UDS): the Neuropsychologic Test Battery. Alzheimer Dis Associated Disord. 2009;23(2):91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besser LM, Kukull WA, Teylan MA, et al. . The revised National Alzheimer's Coordinating Center's Neuropathology Form: available data and new analyses. J Neuropathol Exp Neurol. 2018;77(8):717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugarman MA, McKee AC, Stein TD, et al. . Failure to detect an association between self-reported traumatic brain injury and Alzheimer's disease neuropathology and dementia. Alzheimers Dement. 2019;15(5):686-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaffert J, LoBue C, White CL III, et al. . Traumatic brain injury history is associated with an earlier age of dementia onset in autopsy-confirmed Alzheimer's disease. Neuropsychology. 2018;32(4):410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besser L, Kukull W, Knopman DS, et al. . Version 3 of the national Alzheimer's Coordinating Center's Uniform Data Set. Alzheimer Dis Associated Disord. 2018;32(4):351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monsell SE, Dodge HH, Zhou XH, et al. . Results from the NACC Uniform Data Set Neuropsychological Battery Crosswalk study. Alzheimer Dis Assoc Disord. 2016;30(2):134-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cedarbaum JM, Jaros M, Hernandez C, et al. . Rationale for use of the Clinical Dementia Rating Sum of Boxes as a primary outcome measure for Alzheimer's disease clinical trials. Alzheimers Dement. 2013;9(1 suppl):S45–S55. [DOI] [PubMed] [Google Scholar]
- 32.Thoemmes FJ, Kim ES. A systematic review of propensity score methods in the social sciences. Multivariate Behav Res. 2011;46(1):90-118. [DOI] [PubMed] [Google Scholar]
- 33.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King G, Zeng L. When can history be our guide? The pitfalls of counterfactual inference. Int Stud Q. 2007;51(1):183-210. [Google Scholar]
- 35.Wagenmakers EJ, Marsman M, Jamil T, et al. Bayesian inference for psychology, part I: theoretical advantages and practical ramifications. Psychon Bull Rev. 2018;25(1):35-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dienes Z. Using Bayes to get the most out of non-significant results. Front Psychol. 2014;5:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bürkner PC. Brms: an R package for bayesian multilevel models using stan. J Stat Softw. 2017;80(1):1-28. [Google Scholar]
- 38.Grinberg LT, Nitrini R, Suemoto CK, et al. Prevalence of dementia subtypes in a developing country: a clinicopathological study. Clinics (Sao Paulo). 2013;68(8):1140-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6(9):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer's disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimers Demen Diagn Assess Dis Monit. 2017;7:69-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015;138(pt 3):761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye BS, Seo SW, Kim GH, et al. Amyloid burden, cerebrovascular disease, brain atrophy, and cognition in cognitively impaired patients. Alzheimers Dement. 2015;11(5):494–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godefroy O, Martinaud O, Verny M, et al. . The dysexecutive syndrome of Alzheimer's disease: the GREFEX study. J Alzheimers Dis. 2014;42(4):1203-1208. [DOI] [PubMed] [Google Scholar]
- 44.Townley RA, Graff-Radford J, Mantyh WG, et al. . Progressive dysexecutive syndrome due to Alzheimer's disease: a description of 55 cases and comparisons to other clinical AD phenotypes. Alzheimers Dement. 2020;16(S6):e040622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology. 2012;79(5):442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apostolova LG, Lu P, Rogers S, et al. . 3D mapping of language networks in clinical and pre-clinical Alzheimer's disease. Brain Lang. 2008;104(1):33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupont PS, Bocti C, Joannette M, et al. Amyloid burden and white matter hyperintensities mediate age-related cognitive differences. Neurobiol Aging. 2020;86:16-26. [DOI] [PubMed] [Google Scholar]
- 48.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12(12):723-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heyanka DJ, Mackelprang JL, Golden CJ, Marke CD. Distinguishing Alzheimer's disease from vascular dementia: an exploration of five cognitive domains. Int J Neurosci. 2010;120(6):409-414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All anonymized data from this article are publicly accessible and available through the NACC datasets.