Abstract
Background and Objectives
The aims of this work were to compare rates of longitudinal change in neurologic and neuropsychological test performance between the logopenic progressive aphasia (LPA) and posterior cortical atrophy (PCA) variants of atypical Alzheimer disease (AD) and to use unbiased principal component analysis to assess heterogeneity in patterns of change and relationships to demographics and concurrent brain atrophy.
Methods
Patients with PCA or LPA who were positive for amyloid and tau AD biomarkers and had undergone serial neurologic and neuropsychological assessments and structural MRI were identified. Rates of change in 13 clinical measures were compared between groups in a case-control design, and principal component analysis was used to assess patterns of clinical change unbiased by clinical phenotype. Components were correlated with rates of regional brain atrophy with tensor-based morphometry.
Results
Twenty-eight patients with PCA and 27 patients with LPA were identified. Those with LPA showed worse baseline performance and faster rates of decline in naming, repetition, and working memory, as well as faster rates of decline in verbal episodic memory, compared to those with PCA. Conversely, patients with PCA showed worse baseline performance in tests of visuospatial and perceptual function and on the Clinical Dementia Rating Scale and faster rates of decline in visuoperceptual function compared to those with LPA. Principal component analysis showed that patterns of clinical decline were highly heterogeneous across the cohort, with 10 principal components required to explain >90% of the variance. The first principal component reflected overall severity, with higher scores in LPA than PCA reflecting faster decline in LPA, and was related to left temporoparietal atrophy. The second and third principal components were not related to clinical phenotype but showed some relationship to regional atrophy. No relationships were identified between the principal components and age, sex, disease duration, amyloid PET findings, or apolipoprotein genotype.
Discussion
Longitudinal patterns of clinical decline differ between LPA and PCA but are heterogeneous and related to different patterns of topographic spread. PCA is associated with a more slowly progressive course than LPA.
Alzheimer disease (AD) is a heterogeneous disorder in which patients can present with a wide range of clinical symptoms during life that are related to patterns of neurodegeneration. In young-onset patients with AD, neurodegeneration heavily targets the cortex, and this cortical involvement gives rise to atypical clinical phenotypes of AD. The 2 most common atypical AD phenotypes are posterior cortical atrophy (PCA)1,2 and logopenic progressive aphasia (LPA).3 These 2 syndromes are relatively distinct from each other early in the disease course, with PCA associated with early visuospatial and perceptual deficits and LPA associated with early word finding deficits and difficulties with sentence repetition. The PCA syndrome results from neurodegeneration predominantly in posterior cortices of the brain,4 while LPA results from involvement of the left temporoparietal cortex.5,6 Over the course of the disease, patterns of neurodegeneration spread to involve more of the cortex in both PCA and LPA.7-9 Similarly, the characteristic clinical symptoms of each syndrome worsen, and other clinical and neuropsychological deficits can develop.7,10-15 These findings may suggest a gradual overlapping in clinical symptoms in PCA and LPA, although little is known about how patterns of clinical progression compare between these 2 atypical AD phenotypes.
The goal of this study was to assess longitudinal change in neurologic and neuropsychological performance in a large cohort of patients with PCA or LPA. We aimed to determine the degree to which PCA and LPA differ at baseline and longitudinally. We then aimed to perform a proof-of-concept analysis using unbiased principal component analysis to determine whether there was evidence for patterns of clinical change that may be unrelated to clinical syndrome and to determine how longitudinal decline relates to demographic features and concurrent change in brain atrophy. We hypothesize that, as neurodegeneration in both syndromes spreads throughout the brain, PCA and LPA may become less distinct longitudinally than they are at baseline, with some variability in longitudinal decline unrelated to clinical syndrome.
Methods
Patient Recruitment
Patients with PCA or LPA were recruited by the Neurodegenerative Research Group from the Department of Neurology, Mayo Clinic, Rochester MN, into an NIH-funded grant (R01-AG50603) between April 1, 2016, and October 22, 2020. All participants underwent detailed neurologic assessments by a behavioral neurologist (K.A.J., J.G.-R.), neuropsychological assessments that were performed by a psychometrist and overseen by a neuropsychologist (M.M.M, 3T head MRI, Pittsburgh compound B (PiB) PET, and 18F-flortaucipir PET. For this study, we selected all participants who met clinical criteria for either PCA1 or LPA,3 had evidence for both β-amyloid deposition on PiB-PET and tau deposition on flortaucipir PET, and had undergone 2 serial visits ≈1 year apart. All participants underwent identical clinical and neuroimaging assessments at follow-up. Other inclusion criteria included age >21 years and presence of an informant to provide independent evaluation of functioning. Patients were excluded from the study if they met clinical criteria for amnestic Alzheimer dementia,16 the semantic or agrammatic/nonfluent variants of primary progressive aphasia,3 primary progressive apraxia of speech,17 or corticobasal syndrome18 or if they did not have biomarker evidence of AD. APOE genotyping was performed for all patients.
Standard Protocol Approvals/Patient Consents
The study was approved by the Mayo Institutional Review Board. All participants consented for enrollment into the study.
Neurologic and Neuropsychological Measures
For this study, we identified a group of 13 neurologic and neuropsychological tests that captured different domains of impairment and had <15% of missing data across our cohort. To assess cognition, this dataset included the Montreal Cognitive Assessment Battery (MoCA)19 to measure general cognitive severity; the Auditory Verbal Learning Test (AVLT)20 to assess verbal episodic memory; the Wechsler Memory Scale (WMS)-III21 Visual Reproduction percent retention to assess visual memory; the WMS-III Digit Span to assess attention/working memory; the Boston Naming Test (BNT)22 to assess confrontational naming; the Boston Diagnostic Aphasia Examination subtest to assess sentence repetition (BDAE-R)23; the Visual Object and Space Perception (VOSP)24 incomplete letter and cubes tests to assess visuoperceptual and visuospatial functioning, respectively; and the Rey-Osterrieth (Rey-O) Complex Figure Copy20 to assess visuoconstruction. For the AVLT, we calculated the AVLT recognition percent correct (AVLT RCP), which is the number of true-positive and true-negative responses, expressed as a proportion of the total number of possible correct responses. The dataset also included the Clinical Dementia Rating Scale sum of boxes (CDR-SB)25 score to assess global functional impairment; the Cambridge Behavioral Inventory (CBI)26 to assess severity of cognitive, behavioral, and affective symptoms; the Movement Disorders Society–sponsored revision of the Unified Parkinson's Disease Rating Scale part III (MDS-UPDRS III)27 to assess parkinsonism; and the Western Aphasia Battery (WAB)28 praxis subtest to assess ideomotor apraxia.
Imaging Analysis
All patients in the study underwent a volumetric 3T MRI at baseline and follow-up using a standardized protocol on a GE scanner (GE Healthcare, Chicago, IL) that included a magnetization-prepared rapid gradient echo (MPRAGE) sequence (repetition time/echo time/inversion time 2,300/3/900 milliseconds; flip angle 8°; 26-cm field of view; 256 × 256 in-plane matrix with a phase field of view of 0.94 and slice thickness of 1.2 mm). All MPRAGE images were corrected for intensity inhomogeneity. An in-house–developed version of tensor-based morphometry using symmetric normalization29 was used to assess rates of gray matter atrophy. The baseline and follow-up MPRAGE images of each patient were coregistered to their common mean. Using Advanced Normalization Tools, we computed and applied the symmetric normalization30 deformation from the late to the early image and vice versa and averaged the deformed image with the stationary image to generate synthetic early and late images. We saved the image log of the determinant of the jacobian for the deformations and divided them by the scan interval to get an annualized log jacobian image. All MPRAGE images were normalized to the Mayo Clinic Adult Lifespan Template (MCALT)31 and segmented using MCALT priors and settings in SPM12. The annualized log jacobian images were spatially normalized to the MCALT and segmented using the MPRAGE segmentations, and the gray matter jacobian images were blurred with an 8-mm full width at half-maximum kernel. Mean annualized log jacobian values (which can be thought of as annualized percent change in gray matter volume) were also calculated for specific regions of interest using the MCALT atlas.
All participants had also undergone PiB-PET and 18F-flortaucipir PET at baseline. PET scans were acquired with a PET/CT scanner (GE Healthcare) while operating in 3-dimensional mode. For PiB-PET, patients were injected with PiB of ≈628 MBq (range 385–723 MBq), and after a 40- to 60-minute uptake period, a 20-minute PiB scan was obtained. For tau-PET, patients were injected with ≈370 MBq (range 333–407 MBq) 18F-flortaucipir, followed by a 20-minute PET acquisition performed 80 minutes after injection. Emission data were reconstructed into a 256 × 256 matrix with a 30-cm field of view. A global PiB standard uptake value ratio (SUVR) was calculated as previously described,32 and PiB-PET positivity was defined as a global PiB SUVR >1.48.32 Flortaucipir SUVR images were generated using the cerebellar crus gray matter as the reference region, and patterns of uptake were evaluated visually for positivity.
Statistical Analysis
Our primary analysis aimed to compare baseline performance and longitudinal rates of change on a battery of 13 clinical tests covering a broad range of cognitive domains between PCA and LPA. We used linear mixed-effect models to predict each clinical score as the outcome using syndrome, time from baseline, the interaction between syndrome and time, and age at baseline as the independent variables. Each model included a random person-specific intercept to account for common variability within person across measures and to allow the inclusion of multiple time points per person in each model. These models allow us to estimate LPA and PCA average performance at baseline, as well as the average annual change on each measure in each syndrome, while controlling for differences in age at baseline, sex, and CDR-SB score at baseline and accounting for person-specific variability in overall performance (baseline CDR-SB score was not included in our modeling of CDR-SB score). Linear mixed-effect models use all available data in each clinical test without requiring complete data (i.e., the models can handle sparsely missing data) while using the idea of partial pooling to stabilize parameter estimates.33
In a second analysis, we wanted to explore and describe latent dimensions in how these patients change over time in their clinical presentation. We used principal component analysis, an unsupervised, data-driven dimensions reduction technique, to describe independent modes of variation in change across these 13 clinical measures (i.e., this second analysis is blinded to clinical syndrome). To do this, we computed a matrix of annualized change per person and per measure by subtracting the baseline measurement from the follow-up measurement and dividing by the time between visits before performing the principal component analysis. This analysis allows us to explore the independent patterns of clinical decline across both syndromes. Principal component analyses require complete data (no missing values); to fill in the sparsely missing values in this dataset, we used multiple imputation by chained equations (MICE). In essence, MICE uses all available data within a visit to predict any missing clinical scores by identifying patterns using the rest of the cohort. Five imputed datasets were generated using random forest imputation that implemented the Brelman random forest algorithm using 10 trees per imputed dataset. After imputation, these 5 datasets were averaged to generate a single, complete imputed dataset across all clinical measures. This is a slight imprecision; analyses typically would be performed on each imputation and the results would be pooled. There is no method for pooling principal component decompositions; thus, we accepted this potential introduction of a small bias because we are imputing <4% of the total data (49 missing data points of 1,430 total data points).
Associations between principal component loadings (projecting patients into the principal component space) and clinical and demographic measures were quantified across the entire cohort using Pearson correlations or, when syndrome-specific associations were investigated, Spearman correlations. Pearson correlations were also implemented at the voxel level between gray matter annualized jacobians and principal component scores.
These analyses were performed with the statistical software R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) in conjunction with the lme4 package34 version 1.1-27 and mice package35 version 3.13.0.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Comparisons Between LPA and PCA
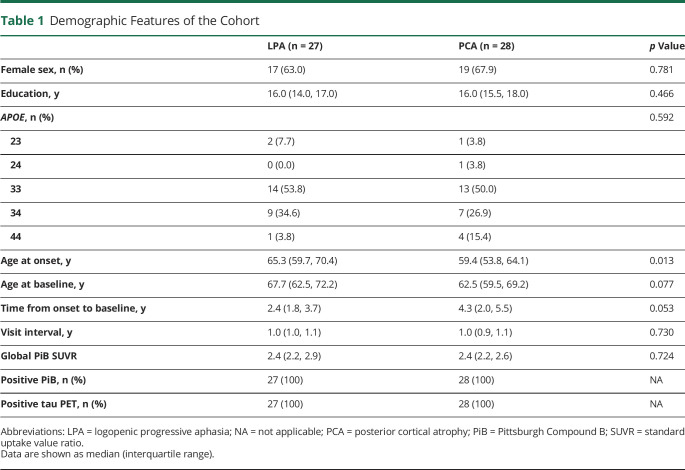
Fifty-five participants met our inclusion criteria, including 28 participants with PCA and 27 with LPA. The demographic features of the LPA and PCA groups are shown in Table 1. The groups did not differ in sex, education, APOE ε4 frequency, or global PiB SUVR. However, the PCA group was younger at onset than the LPA group, with trends for younger age at baseline and longer time from onset to baseline.
Table 1.
Demographic Features of the Cohort
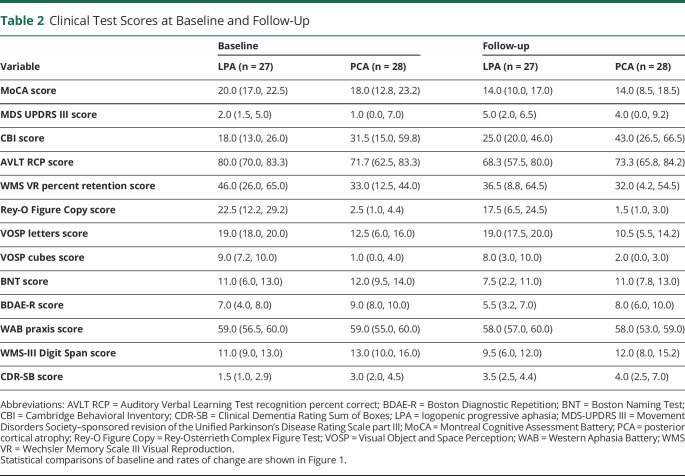
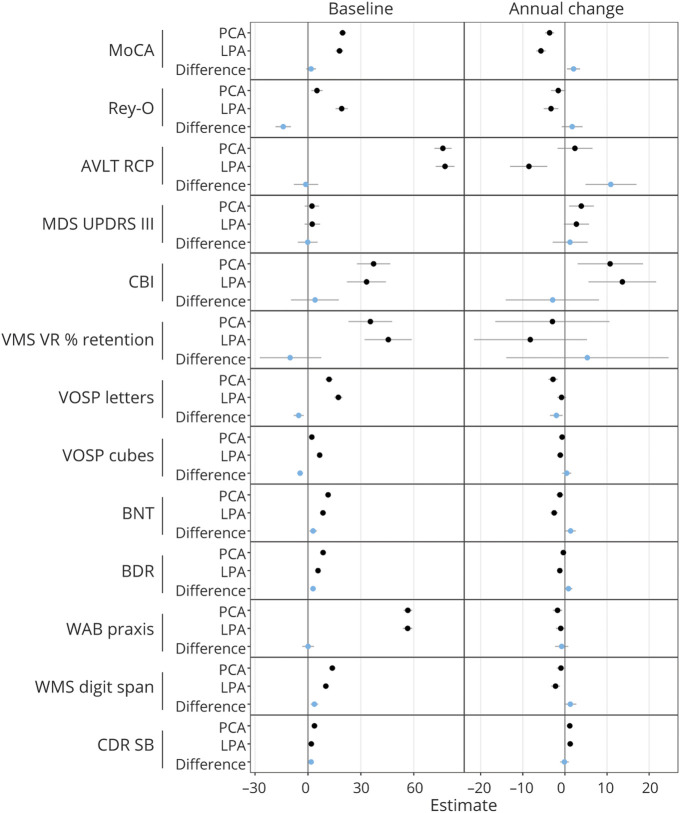
Performance on the clinical tests at baseline and follow-up is shown in Table 2, and the statistical analyses comparing the LPA and PCA groups are shown in Figure 1 and eTable 1 (links.lww.com/WNL/B918). At baseline, those with PCA performed worse on the Rey-O Figure Copy (p < 0.001), VOSP letters (p < 0.001), VOSP cubes (p < 0.001), and CDR-SB (p = 0.007) compared to participants with LPA. Conversely, participants with LPA performed worse at baseline on BDAE-R (p < 0.001), BNT (p = 0.003), and WMS digit span (p < 0.001) compared to participants with PCA. No differences between individuals with LPA and PCA were observed at baseline in MoCA, AVLT RCP, MDS-UPDRS III, CBI, WMS-III Visual Reproduction percent retention, and WAB praxis scores. Longitudinally, those with LPA showed faster rates of decline in MoCA (p = 0.005), AVLT RCP (p < 0.001), BNT (p = 0.014), BDAE-R (p = 0.034), and WMS digit span (p = 0.036) scores compared to individuals with PCA. Conversely, participants with PCA showed faster rates of decline in VOSP letters scores compared to those with LPA (p = 0.004). No differences between participants with LPA and PCA were observed longitudinally in Rey-O Figure Copy, MDS-UPDRS III, CBI, WMS-III Visual Reproduction percent retention, VOSP cubes, WAB praxis, and CDR-SB scores.
Table 2.
Clinical Test Scores at Baseline and Follow-Up
Figure 1. Baseline and Longitudinal Linear Mixed-Effects Model Results Assessing Differences Between PCA and LPA.
Baseline and annualized change estimates are shown for logopenic progressive aphasia (LPA) and posterior cortical atrophy (PCA) (black dots), and then the shift in estimates between LPA and PCA are shown as a difference (blue dot). AVLT RCP = Auditory Verbal Learning Test recognition percent correct; BDAE-R = Boston Diagnostic Repetition; BNT = Boston Naming Test; CBI = Cambridge Behavioral Inventory; CDR-SB = Clinical Dementia Rating Sum of Boxes; MDS-UPDRS III = Movement Disorders Society–sponsored revision of the Unified Parkinson's Disease Rating Scale part III; MoCA = Montreal Cognitive Assessment Battery; Rey-O Figure Copy = Rey-Osterrieth Complex Figure Test; VOSP = Visual Object and Space Perception; WAB = Western Aphasia Battery; WMS VR % = Wechsler Memory Scale III Visual Reproduction percent retention.
Principal Component Analysis Results
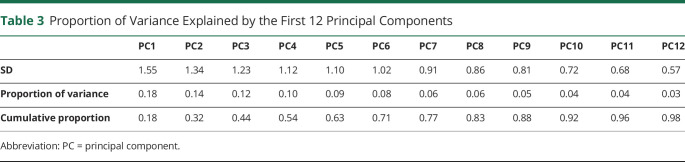
The cohort of 55 participants had at least 1 missing score on a clinical measure in 27 of the 110 total visits; hence, data at these 27 visits were imputed with MICE (eFigure 1, links.lww.com/WNL/B918), and the principal component analysis was run using a complete dataset. Longitudinal patterns of clinical decline in the 13 tests were highly heterogeneous across the cohort, with 10 principal components required to explain >90% of the variance (Table 3). The first principal component represented 18% of the variability in how the cohort changed over time and captured overall severity of decline, with negative loadings across all tests (Figure 2). The tests with the greatest loadings and thus most prominently indicating overall severity were the MoCA, WMS-III Digit Span, and CBI. Faster decline across tests was associated with faster rates of atrophy in the left lateral temporal lobe and supramarginal gyrus, as well as faster rates of ventricular expansion (Figure 3).
Table 3.
Proportion of Variance Explained by the First 12 Principal Components
Figure 2. Illustration of the Relationship Between the First 3 PCs and Decline in the 10 Clinical Tests.
(A) Loadings from the clinical tests color-coded, with darker red representing more positive loadings and darker blue representing more negative loadings for each principal component (PC). Hence, each PC should be thought of as a red-vs-blue contrast. (B) Scatterplots illustrating the relationship between the patient-level PC scores and an important clinical test in each component. AVLT RCP = Auditory Verbal Learning Test Recognition Percent Correct; BDAE-R = Boston Diagnostic Repetition; BNT = Boston Naming Test; CBI = Cambridge Behavioral Inventory; CDR-SB = Clinical Dementia Rating Sum of Boxes; MDS-UPDRS III = Movement Disorders Society–sponsored revision of the Unified Parkinson's Disease Rating Scale part III; MoCA = Montreal Cognitive Assessment Battery; Rey-O Figure Copy = Rey-Osterrieth Complex Figure Test Copy Trial; VOSP = Visual Object and Space Perception; WAB = Western Aphasia Battery; WMS VR III PCT RET RAW = Wechsler Memory Scale-III Visual Reproduction percent retention.
Figure 3. Correlations Between Rates of Atrophy and the First 3 PCs.
Renderings show voxel-level Pearson correlations with the loadings from each principal component (PC) and the entire cohort. Pearson correlations of R ≥ 0.38 are shown (p < 0.01).
The second principal component represented 14% of the variability in how the cohort changed over time and was a parkinsonian/praxis and naming/repetition vs functional impairment/memory component (Figure 2). The tests contributing most to this component were the WAB praxis, MDS-UPDRS III, BNT, and BDAE-R, which had positive loadings, and the CDR-SB, WMS III Visual Reproduction percent retention, AVLT RCP, and CBI, which had negative loadings. Faster rates of decline in the naming/repetition and parkinsonian/praxis tests were related to faster rates of atrophy in the parietal, posterior frontal, and lateral temporal cortices (Figure 3).
The third principal component represented 12% of the variability in how the cohort changed over time and was a parkinsonian/praxis vs visuospatial/perceptual component (Figure 2). The tests contributing most to this component were the MDS-UPDRS III and WAB praxis, which had positive loadings, and the VOSP letters and cubes and Rey-O figure copy, which had negative loadings. Faster rates of decline in visuospatial/perceptual function were related to faster rates of atrophy in the dorsolateral frontal and lateral temporal lobes, particularly on the left, while faster rates of decline in parkinsonian/praxis were related to faster rates of atrophy in the occipital lobes (Figure 3).
Only the first principal component was related to diagnosis, with slower decline across tests observed in PCA (Wilcoxon rank-sum p = 0.015). Baseline MoCA score was associated with the third principal component in PCA only (Spearman rank correlation 0.74, p < 0.001), with lower MoCA score associated with faster decline in visuospatial/perceptual performance (eFigure 2, links.lww.com/WNL/B918). There was no clear relationship between the any of the principal components and age, sex, disease duration, global PiB SUVR, or APOE ε4 status (eFigure 2).
Discussion
This study highlights that patterns of clinical change over time are heterogeneous across these 2 common variants of atypical AD. The expected differences in the core clinical features of LPA and PCA were observed at baseline, with many, but not all, of these features also differing longitudinally. However, additional variance in patterns of clinical change was observed, unrelated to clinical syndrome, showing high variability in how clinical presentation changes over time in this cohort.
The LPA group showed worse baseline performance and faster rates of decline in the characteristic features of naming and sentence repetition, as well as working memory, compared to the PCA group. Working memory deficits resulting from damage to the phonologic loop,36-38 as well as disruption to the working memory network,39 have previously been observed in LPA. The 2 groups performed comparably at baseline in verbal and visual memory performance, but individuals with LPA showed faster decline in verbal memory compared to those with PCA over time. Although episodic memory typically is relatively spared in both LPA and PCA early in the disease, it has been observed to develop,38,40 and our finding suggests that it may be more likely to become a feature of LPA. In this study, we assessed the AVLT RCP because it is more resistant to floor effects than the AVLT delayed recall. The PCA group showed worse baseline performance in tests of visuospatial and perceptual performance compared to the LPA group, as expected. However, longitudinally, we observed faster rates of decline in the PCA group only in the VOSP letters test, with no difference in rate of decline observed between those with PCA and LPA in the VOSP cubes and Rey-O figure copy tests. This could suggest that visuospatial difficulties are developing in the patients with LPA over time. Previous studies have similarly observed the development of visuospatial abnormalities in patients with LPA,13,15,41 with 1 study finding a similar longitudinal rate of decline in a judgment of line orientation task in both individuals with LPA and patients with PCA.41 Another explanation, however, could be that, because the patients with PCA performed so poorly at baseline, the tests may lack sensitivity to detect much further change in PCA. There was some evidence that faster decline in visuospatial/perceptual performance in PCA was related to lower baseline MoCA score, suggesting that low MoCA score could be useful prognostically in PCA to predict those patients who may decline faster in visuospatial/perceptual performance over time.
Our results also suggested that the patients with LPA may be declining faster than the patients with PCA across tests. The first principal component identified in the principal component analysis reflected a severity component, with negative loadings across most tests, and faster decline was observed in the patients with LPA compared to the patients with PCA. This component was driven particularly by tests of general disease severity, attention/working memory, naming, sentence repetition, and memory. This fits with the fact that slow clinical progression and long disease course have been described in patients with PCA.2,42,43 The MRI analysis showed that faster decline in this component was associated with concurrent atrophy of the left temporoparietal lobes, which also concurs with the finding that the participants with LPA are most likely to progress rapidly. LPA is characterized by lateral temporal and parietal volume loss on MRI,5,6 with longitudinal studies showing atrophy in these same regions.8,9 Cross-sectionally, aphasia severity has been related to temporoparietal volume in LPA,39,44 and naming and repetition deficits have been related to volume and tau PET uptake in lateral temporal regions.45-47
However, our results have the caveat that we are assessing only a 1-year window in the disease, and it is possible that the patients with LPA and patients with PCA may be at a different stage in their disease. The patients with PCA had a longer time from disease onset to baseline assessment and performed worse on the CDR-SB at baseline compared to the patients with LPA. We included baseline CDR-SB score as a covariate in our clinical comparisons of LPA and PCA to try to correct for this imbalance. However, assessing disease stage in 2 very different clinical syndromes is challenging given differences in the sensitivity and difficulty level across tests, potential performance differences and confounds related to each syndrome, and difficulty with the accurate estimation of disease onset in 2 diseases with very different symptoms. Future studies assessing a longer window of clinical change will be needed to determine the influence of disease stage on longitudinal clinical decline in these syndromes.
We did not observe any differences between PCA and LPA in degree of parkinsonism or ideomotor apraxia, at baseline or longitudinally. Both groups showed mild worsening of parkinsonism on the MDS-UPDRS III but performed within normal limits at both time points. Similarly, performance on the WAB praxis test was within normal limits at both time points for most patients. The slight increases in MDS-UPDRS III score over time could be age-related worsening rather than a disease-related symptom in most patients, although we cannot rule out the fact that some patients may have disease-related parkinsonism. For example, an association between these atypical AD syndromes and Lewy body disease has been observed in autopsy-confirmed cases.48 It is notable that neither MDS-UPDRS III or WAB praxis score was implicated in the first disease-severity principal component, supporting the view that changes in these 2 clinical features were unrelated to change in the predominant disease-specific LPA and PCA symptoms. Both tests did, however, feature in the second and third principal components, suggesting that after variation in decline in general disease severity was removed, there is some variance remaining related to change in MDS-UPDRS III and WAB praxis scores, although these principal components explained only a relatively small amount of the overall variance.
The principal component analysis was a proof-of-concept analysis, and because we did not have a replication cohort, it is possible that the specific tests identified in the components may not generalize to other cohorts or other disease stages. However, despite these limitations, this analysis did show that there was a great deal of heterogeneity in individual-level patterns of decline in atypical AD, with 10 components needed to explain 90% of the variability. Furthermore, only the first principal component was related to clinical syndrome (i.e., LPA vs PCA), suggesting that much of the variance in how patients change over time clinically is unrelated to syndrome. There was some evidence that the different patterns of clinical change are related to different patterns of brain atrophy, and hence, it is likely that heterogeneous spread of neurodegeneration may lead to differing individual-level patterns of clinical decline. However, patterns of clinical change did not appear to be related to age, sex, disease duration, global PiB SUVR, or APOE ε4 status. It is possible that variability in clinical decline may also be related to different patterns of tau progression. We have previously shown variability in patterns of tau accumulation over time in atypical AD; however, rates of atrophy showed a stronger relationship to clinical syndrome than rate of tau accumulation.8
A strength of this study was that we used a set of clinical tests that had relatively complete data available for analysis at both time points, and we used imputation to deal with the small amount of missing data. Unfortunately, we were not able to assess executive function, although executive function does contribute to performance on the Rey-O Figure Copy. Other limitations of the study include potential confounds in test performance due to patients' difficulties with language or visuospatial/perceptual function, that only 2 time points were available for analysis, and that the 2 groups may differ in relative disease stage, as discussed above. Although all patients were β-amyloid and tau positive, other copathologies could be present that may drive the rate of decline; autopsy validation would be needed to test this theory. Future studies with more follow-up will help to further elucidate patterns of clinical progression in atypical AD. Other atypical variants of AD, including the dysexecutive49 and behavioral50 variants of AD, have now been recognized, and it will be important to determine whether our findings generalize to these other variants. Previous studies have shown that clinical disease progression differs between both LPA and PCA and the typical amnestic variant of AD,41,51 but a cohort of typical AD with these same clinical tests is not available to include in this study.
This study provides important insight into how disease progression compares between LPA and PCA and highlights heterogeneity in progression that is unrelated to clinical syndrome. Our findings will improve prognostic estimates provided to patients and their families by aiding clinicians in predicting rates and patterns of disease progression. The findings will also have implications for diagnosis of these syndromes because diagnosis may become more challenging with disease progression. Our findings also highlight the neuroanatomic underpinnings of clinical progression in atypical AD that will aid in the development of appropriate disease biomarkers in these cohorts.
Glossary
- AD
Alzheimer disease
- AVLT
Auditory Verbal Learning Test
- AVLT RPC
AVLT recognition percent correct
- BDAE-R
Boston Diagnostic Repetition
- BNT
Boston Naming Test
- CBI
Cambridge Behavioral Inventory
- CDR-SB
Clinical Dementia Rating Scale sum of boxes
- LPA
logopenic progressive aphasia
- MCALT
Mayo Clinic Adult Lifespan Template
- MDS-UPDRS III
Movement Disorders Society–sponsored revision of the Unified Parkinson's Disease Rating Scale part III
- MICE
multiple imputation by chained equations
- MoCA
Montreal Cognitive Assessment Battery
- MPRAGE
magnetization-prepared rapid gradient echo
- PCA
posterior cortical atrophy
- PiB
Pittsburgh compound B
- Rey-O
Rey-Osterrieth
- SUVR
standard uptake value ratio
- VOSP
Visual Object and Space Perception
- WAB
Western Aphasia Battery
- WMS
Wechsler Memory Scale
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
This study was funded by NIH grants R01-AG050603, U01 AG046193, and R01 AG061796.
Disclosure
J. Whitwell receives research support from the NIH. P.R. Martin reports no disclosures relevant to the manuscript. J. Graff-Radford and M. Machulda receive research support from the NIH. I. Sintini and M. Buciuc report no disclosures relevant to the manuscript. M.L. Senjem holds stocks in Align Technology, Inc, Inovio Pharmaceuticals Inc, LHC Group, Inc, Mesa Laboratories, Inc, Natus Medical Inc, and Varex Imaging Corp. C.G. Schwarz, H. Botha, M.M. Carrasquillo, and N. Ertekin-Taner receive research support from the NIH. V.J. Lowe consults for Bayer Schering Pharma, Piramel Life Sciences, and Merck Research and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH. C.R. Jack has consulted for Biogen and Eli Lilly, served as a speaker for Eisai Co Inc, and serves on an independent data monitoring board for Roche but receives no personal compensation from any commercial entity. He receives research support from the NIH. K.A. Josephs receives research support from the NIH. Go to Neurology.org/N for full disclosures.
References
- 1.Crutch SJ, Schott JM, Rabinovici GD, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13(8):870-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168-1174. [DOI] [PubMed] [Google Scholar]
- 3.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitwell JL, Jack CR Jr., Kantarci K, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2007;28(7):1051-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madhavan A, Whitwell JL, Weigand SD, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer's type. PLoS One. 2013;8(4):e62471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorno‐Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Firth NC, Primativo S, Marinescu RV, et al. Longitudinal neuroanatomical and cognitive progression of posterior cortical atrophy. Brain. 2019;142(7):2082-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sintini I, Graff-Radford J, Senjem ML, et al. Longitudinal neuroimaging biomarkers differ across Alzheimer's disease phenotypes. Brain. 2020;143(7):2281-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brambati SM, Amici S, Racine CA, et al. Longitudinal gray matter contraction in three variants of primary progressive aphasia: a tenser-based morphometry study. Neuroimage Clin. 2015;8:345-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panegyres PK, Goh J, McCarthy M, Campbell AI. The nature and natural history of posterior cortical atrophy syndrome: a variant of early-onset Alzheimer disease. Alzheimer Dis Assoc Disord. 2017;31:295-306. [DOI] [PubMed] [Google Scholar]
- 11.Chan LT, Lynch W, De May M, Horton JC, Miller BL, Rabinovici GD. Prodromal posterior cortical atrophy: clinical, neuropsychological, and radiological correlation. Neurocase. 2015;21(1):44-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goethals M, Santens P. Posterior cortical atrophy: two case reports and a review of the literature. Clin Neurol Neurosurg. 2001;103(2):115-119. [DOI] [PubMed] [Google Scholar]
- 13.Funayama M, Nakagawa Y, Nakajima A, Takata T, Mimura Y, Mimura M. Dementia trajectory for patients with logopenic variant primary progressive aphasia. Neurol Sci. 2019;40(12):2573-2579. [DOI] [PubMed] [Google Scholar]
- 14.Etcheverry L, Seidel B, Grande M, et al. The time course of neurolinguistic and neuropsychological symptoms in three cases of logopenic primary progressive aphasia. Neuropsychologia. 2012;50(7):1708-1718. [DOI] [PubMed] [Google Scholar]
- 15.Foxe D, Irish M, Hu A, et al. Longitudinal cognitive and functional changes in primary progressive aphasia. J Neurol. 2021;268(5):1951-1961. [DOI] [PubMed] [Google Scholar]
- 16.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(pt 5):1522-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 20.Rey A. L’examen clinique en psychologie. Presses Universitaires de France; 1964. [Google Scholar]
- 21.Wechsler DA. Wechsler Memory Scale Revised. Psychological Corp; 1987. [Google Scholar]
- 22.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test, 2nd ed. Pro-ed; 2001. [Google Scholar]
- 23.Goodglass H, Kaplan E, Barresi B. The Boston Diagnostic Aphasia Examination (BDAE). Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- 24.Warrington EK, James M. The Visual Object and Space Perception Battery. Thames Valley Test Co; 1991. [Google Scholar]
- 25.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566-572. [DOI] [PubMed] [Google Scholar]
- 26.Wear HJ, Wedderburn CJ, Mioshi E, et al. The Cambridge behavioural inventory revised. Dement Neuropsychol. 2008;2:102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. [DOI] [PubMed] [Google Scholar]
- 28.Kertesz A. Western Aphasia Battery (Revised). Psychological Corp; 2007. [Google Scholar]
- 29.Jack CR Jr., Wiste HJ, Knopman DS, et al. Rates of beta-amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82:1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NITRC. Mayo Clinic Adult Lifespan Template and Atlas. Accessed April 2021. nitrc.org/projects/mcalt/.
- 32.Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenland S. Principles of multilevel modelling. Int J Epidemiol. 2000;29(1):158-167. [DOI] [PubMed] [Google Scholar]
- 34.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1-48. [Google Scholar]
- 35.van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1-67. [Google Scholar]
- 36.Meyer AM, Snider SF, Campbell RE, Friedman RB. Phonological short-term memory in logopenic variant primary progressive aphasia and mild Alzheimer's disease. Cortex. 2015;71:183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foxe D, Irish M, Roquet D, et al. Visuospatial short-term and working memory disturbance in the primary progressive aphasias: neuroanatomical and clinical implications. Cortex. 2020;132:223-237. [DOI] [PubMed] [Google Scholar]
- 38.Mendez MF, Monserratt LH, Liang LJ, et al. Neuropsychological similarities and differences between amnestic Alzheimer's disease and its non-amnestic variants. J Alzheimers Dis. 2019;69(3):849-855. [DOI] [PubMed] [Google Scholar]
- 39.Whitwell JL, Jones DT, Duffy JR, et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer's dementia. Neurobiol Aging. 2015;36(3):1245-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens TE, Machulda MM, Duffy JR, et al. Patterns of neuropsychological dysfunction and cortical volume changes in logopenic aphasia. J Alzheimers Dis. 2018;66(3):1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips JS, Da Re F, Irwin DJ, et al. Longitudinal progression of grey matter atrophy in non-amnestic Alzheimer's disease. Brain. 2019;142(6):1701-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kujovic M, Malikovic A, Jochum S, Margittai Z, Lange-Asschenfeldt C, Supprian T. Longitudinal progression of posterior cortical atrophy over 11 years: relationship between lesion topology and clinical deficits. J Clin Exp Neuropsychol. 2019;41(8):875-880. [DOI] [PubMed] [Google Scholar]
- 43.Areza-Fegyveres R, Caramelli P, Porto CS, Ono CR, Buchpiguel CA, Nitrini R. The syndrome of progressive posterior cortical dysfunction: a multiple case study and review. Dement Neuropsychol. 2007;1:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machulda MM, Whitwell JL, Duffy JR, et al. Identification of an atypical variant of logopenic progressive aphasia. Brain Lang. 2013;127(2):139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Win KT, Pluta J, Yushkevich P, et al. Neural correlates of verbal episodic memory and lexical retrieval in logopenic variant primary progressive aphasia. Front Neurosci. 2017;11:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogalski E, Cobia D, Harrison TM, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31(9):3344-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tetzloff KA, Graff-Radford J, Martin PR, et al. Regional distribution, asymmetry, and clinical correlates of tau uptake on [18F]AV-1451 PET in atypical Alzheimer's disease. J Alzheimers Dis. 2018;62:1713-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buciuc M, Whitwell JL, Kasanuki K, et al. Lewy body disease is a contributor to logopenic progressive aphasia phenotype. Ann Neurol. 2021;89(3):520-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Townley RA, Graff-Radford J, Mantyh WG, et al. Progressive dysexecutive syndrome due to Alzheimer's disease: a description of 55 cases and comparison to other phenotypes. Brain Commun. 2020;2(1):fcaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ossenkoppele R, Singleton EH, Groot C. Research Criteria for the Behavioral Variant of Alzheimer Disease: A Systematic Review and Meta-analysis. JAMA Neurol 2022;79(1):48-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Firth NC, Primativo S, Brotherhood E, et al. Sequences of cognitive decline in typical Alzheimer's disease and posterior cortical atrophy estimated using a novel event-based model of disease progression. Alzheimers Dement. 2020;16(7):965-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.