Abstract
Reoccurring seasonal flu epidemics and occasional pandemics are among the most severe threats to public health. Current seasonal influenza vaccines provide limited protection against drifted circulating strains and no protection against influenza pandemics. Next-generation influenza vaccines, designated as universal influenza vaccines, should be safe, affordable, and elicit long-lasting cross-protective influenza immunity. Nanotechnology plays a critical role in the development of such novel vaccines. Engineered nanoparticles can incorporate multiple advantageous properties into the same nanoparticulate platforms to improve vaccine potency and breadth. These immunological properties include virus-like biomimicry, high antigen-load, controlled antigen release, targeted delivery, and induction of innate signaling pathways. Many nanoparticle influenza vaccines have shown promising results in generating potent and broadly protective immune responses. This review will summarize the necessity and characteristics of next-generation influenza vaccines and the immunological correlates of broad influenza immunity and focus on how cutting-edge nanoparticle technology contributes to such vaccine development. The review will give new insights into the rational design of nanoparticle universal vaccines to combat influenza epidemics and pandemics.
Keywords: Universal influenza vaccines, nanoparticles, vaccine delivery, cross-protection
Graphical Abstract
Nanotechnology plays a critical role in the development of next-generation universal influenza vaccines that produce long-lasting cross-protective influenza immunity. Here we summarize the necessity and characteristics of next-generation influenza vaccines and the immunological correlates of broadly protective influenza immunity, emphasizing how cutting-edge nanoparticle strategies contribute to the development of such vaccines to combat influenza epidemics and pandemics.
1. Introduction
Influenza is one of the most severe and contagious respiratory infectious diseases. Influenza virus infection causes annual epidemics and occasional pandemics, resulting in enormous health and economic burden worldwide. During the 2019-2020 flu season, CDC estimates that flu caused 38 million illnesses, including 18 million medical visits, 405,000 hospitalizations, and 22,000 deaths in the United States.[1] Influenza viruses are enveloped RNA viruses of the Orthomyxoviridae family. Because the RNA polymerases lack the proof-reading function, influenza viruses undergo constant antigenic drift and shift within animal and human reservoirs.[2] Influenza A (IAVs) and influenza B viruses (IBVs) are the main circulating viruses that affect human health. So far, 18 hemagglutinin (HA) subtypes belonging to two HA phylogenetic groups and 11 neuraminidase (NA) subtypes have been identified for IAVs.[3] The IAVs H1N1 and H3N2 currently cause most epidemic diseases in humans. IBVs form a single antigenic group with two distinct lineages: Victoria and Yamagata.
Seasonal vaccination is the most cost-effective method to combat influenza infection. However, current seasonal flu vaccines induce strain-specific immunity that rapidly wanes and displays low efficiencies against mismatched circulating strains and no effect on pandemics. The vaccines need annual reformulation based on the prediction by the WHO Global Influenza Surveillance and Response System to counter antigenic variations.[4] This uncertainty makes mismatches occur, resulting in suboptimal immunity. Fewer influenza cases occurred in the past 2020-2021 flu season in the United States than in any on record,[5] probably due to the widespread use of face masks and social distance to control COVID-19. The lack of exposure to the flu may make the population more susceptible to the virus when it returns.[6] Parallelly, predicting which strains will be circulating in the upcoming flu season and selected for vaccine manufacturing will be more challenging than ever.
A next-generation universal influenza vaccine that elicits long-lasting cross-protective immunity against variant influenza strains will overcome the limitations of the current flu vaccines.[7-8] Nanotechnology plays an indispensable role in the development of such vaccines.[9-11] Influenza viruses are nanosized particles enveloped by lipid membranes inserted with surface glycoprotein spikes. Nanoparticle vaccines can optimally mimic the influenza virus nanostructure and high antigen loads, facilitate targeted delivery and controlled antigen release, and synergize with molecular adjuvants to improve vaccine potency and breadth. Moreover, nanoparticle vaccines can facilitate rapid and scalable production, minimize cold-chain dependence, incorporate antigens in a flexibly modular fashion, and be affordably produced. Up to date, a variety of nanoparticle formulations have shown promising results in generating cross-reactive influenza immunity.
In this review, we will first discuss the necessity and characteristics of next-generation universal influenza vaccines and the immunological correlates of cross-protective influenza immunity. Then, we will focus on how engineered nanoparticles contribute to improving the breadth of current influenza vaccines and developing universal influenza vaccines. We will also discuss the challenges and outlooks of nanoparticle influenza vaccines. Our review will give new insights into the rational design of nanoparticle universal vaccines to combat recurring and pandemic influenza and other respiratory infectious diseases threats.
2. Pathway to a Universal Influenza Vaccine
2.1. Evolutionary Trends in Influenza Vaccine Development
Seasonal influenza vaccines have saved countless lives since their introduction in the 1940s. Currently licensed vaccines include inactivated influenza virus vaccines (split or subunit), recombinant HA-based vaccines, and live-attenuated virus vaccines, containing three or four strains (two influenza A and one or two influenza B viruses).[12] However, the vaccine effectiveness (VE) varies every year depending on the degrees of antigenic divergence.[13] During the past decade, the adjusted overall VE is up to 60% (2010-2011 flu season) and as low as 19% (2014-2015 flu season). Moreover, seasonal influenza vaccines show low efficacy in the young (< 5-year-old (y/o)), elderly (>65 y/o), immunocompromised, pregnant, and those with co-morbidities such as obesity and smoking.[14]
To limit the public health consequences of seasonal and pandemic influenza, developing a vaccine that provides durable cross-protection against multiple influenza strains becomes a high priority. In 2018, the National Institute of Allergy and Infectious Diseases (NIAID) unveiled its strategic plan for developing a universal influenza vaccine.[15] The plan suggested that a universal influenza vaccine should be at least 75% effective for all age groups against HA phylogenic groups I and II influenza A viruses and have durable protection for at least one year. Incremental steps with increasing vaccine protection breadth, ranging from strain-specific to subtype-specific, multi-subtype, pan-group/lineage, type A, types A and B, and universal coverage, were proposed to guide the development of such vaccines. Since then, scientists have made extensive efforts to improve current seasonal vaccines and create new cross-protective influenza vaccines through designing novel influenza antigens and developing potent adjuvants and delivery vehicles.
2.2. Conserved Immune Targets for Vaccine Development
Induction of protective immunity against influenza requires recognition of viral components by the host immune system.[16] Seasonal vaccination induces strain-specific antibody responses to the highly variable immune determinants on the HA head. Such antibodies are often neutralizing and effective in combating virus infection but suffer from immune evasion. Recent advances in influenza immunology and vaccinology indicate that universal influenza vaccines targeting conserved immune determinants and providing broad protection against variant strains are feasible, though challenges exist.[12, 17] Designing and characterizing novel immune targets as immunogens are essential to universal influenza vaccine development.
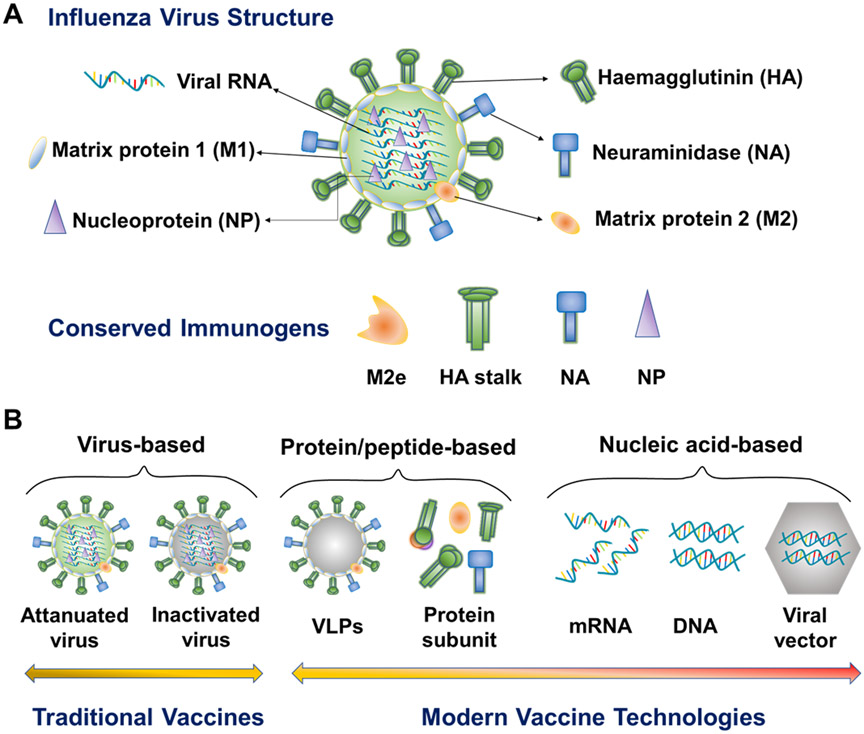
Influenza viruses are enveloped single-stranded RNA viruses (Figure 1A).[18] The Influenza lipid membrane and its three integral membrane proteins (HA, NA and M2) overlay matrix protein 1 (M1). Internal viral proteins include M1, NP, the polymerases (PB1, PB2, and PA), and NEP/NS2. Broadly reactive antibody-recognized epitopes have been identified mainly from HA, NA, and M2 ectodomain (M2e). T lymphocyte-recognized epitopes have been characterized from both internal and surface proteins.
Figure 1.
Schematic illustration of influenza virus structure, conserved influenza immunogens, and modern influenza vaccine types. M2e, M2 extracellular domain. VLP, virus-like particle.
HA is the major antigenic surface glycoprotein of the influenza virus. Two structurally defined conserved antigenic regions targeted by broadly neutralizing antibodies (bnAbs) have been identified in HA, the receptor-binding site (RBS) inside the globular head region and the hydrophobic groove surrounding Trp21HA2 in the HA stem.[19-22] Kanekiyo and colleagues identified a bnAb against conserved RBS supersites from mice immunized with mosaic nanoparticles displaying diverse hemagglutinin receptor-binding domains from multiple strains.[23]
Compared with the variable head domain, the HA stalk domain is less flexible and more conserved among different influenza subtypes.[24] Some studies have characterized various bnAbs from infected humans that recognize the HA stalk region,[25-27] demonstrating that the human immune system can sense and respond to conserved immune determinants in this region.[28-29] However, significant challenges have been encountered in designing an HA-stalk based universal influenza vaccine: (i) the difficulty in constructing and expressing the HA stalk antigens apart from other HA sequences[30]; (ii) the reduced immunogenicity of subunit vaccines as compared with particulate antigens; and (iii) the relatively short duration of the immunity induced. Nevertheless, several studies, including ours, have demonstrated that HA stalk region sequences particularly presented on nanoparticle vaccines can be applied as immunogens in developing broadly protective influenza vaccines.[31-33]
Influenza NA can cleave the HA-sialic acid tether with host cell membranes to release new virions. NA-reactive antibodies can block NA cleavage function, hampering viral spread. NA has relatively poor immunogenicity in nature but experiences slower antigenic drift in comparison to HA. As antigenic drift and shift of NA occur independently of HA, NA-specific antibodies may help prevent against variant strains in which HA but not NA changes. A universally conserved NA epitope between amino acid residues 222 and 230 induced NA-inhibiting antibodies against all influenza types,[34] driving NA a potential immunogen candidate for universal influenza vaccines. Recent advances claimed the potential of NA as a standalone or a synergistic component of universal influenza vaccines.[35]
M2 is a membrane ion channel protein essential for influenza-host cell membrane fusion by acidifying endosomes during virus infection and new virion budding from infected cells. In comparison with HA and NA, M2 is less variable. M2e is a highly conserved universal vaccine antigen but is weakly immunogenic. To increase M2e immunogenicity, several M2e constructs incorporating multiple M2e repeats,[36] stabilizing M2e conformation,[37] or fusing with innate signaling receptor ligands,[38-41] and various nanoparticle vaccine formulations including M2e nanoclusters,[42] self-assembling M2e nanoparticles,[43] M2e outer membrane vesicles,[44] and M2e virus-like particles (VLPs)[36, 45] have been developed. M2e can mediate broad influenza protection through Fc-mediated effector functions and T cell responses.[46] Previously, we have shown that skin vaccination with dissolving microneedle patches (MNPs) encapsulating 4M2e-flagellin broadened the protective efficacy of conventional influenza vaccines.[39-40] M2e nanoclusters induced cross-reactive M2e antibodies recognizing diverse M2e variants, including human influenza M2e consensus, A/California/7/2009 (H1N1, p09) M2e, A/Vietnam/1203/2004 (H5N1, Vtn) M2e, and A/Shanghai/2/2013 (H7N9, SH) M2e.[42, 47]
Distinct from antibody responses, influenza-specific T cells, particularly CD8 T cells, typically recognize peptides derived from the highly conserved internal viral proteins (M1 and NP), thus mediating heterotypic influenza protection.[48-51] NP has been recognized as a significant target for T cell-based universal vaccine development. Various immunization strategies, including protein/peptide-based and DNA vaccines, have generated NP-specific immune responses.[47, 52-53] Combination of these conserved antigens is a promising strategy for developing next-generation vaccines with cross-protection against different viruses.
2.3. Employment of Modern Vaccine Types
The evolution of influenza vaccine development has shown a trend from traditional egg-dependent virus-based vaccines to safer, productive, and affordable modern vaccine technologies,[54-55] such as recombinant protein/peptide- and nucleic acid-based vaccine strategies (Figure 1B).
Recombinant protein/peptide-based vaccines have attracted enormous attention for vaccine development owing to their high safety profiles and quick, scalable, cost-effective, and egg-independent production.[12, 56-57] They can eliminate the potential toxicity, egg allergies, and unfavorable egg-adapted mutations associated with traditional virus-based vaccines. Moreover, recombinant protein technology has facilitated the development of a variety of promising recombinant proteins that direct the immune responses toward conserved epitopes, thus eliciting broadly cross-reactive responses and overcoming the immunodominance of traditional virus-based vaccines.[58] Various HA-based recombinant proteins, including HA fusion proteins, headless HA stalk, chimeric HAs, computationally optimized HAs, mosaic HA receptor-binding domains (RBDs), and hyperglycosylated HA heads, have been developed.[30, 58] We have generated recombinant 4M2e containing four tandem copies of M2e from human, swine, avian and domestic fowl viruses and demonstrated its potential to prevent pandemic influenza strains.[33, 47]
However, most conserved immune determinants suffer from inherent poor immunogenicity. Nanotechnology has been employed to overcome this challenge. To this end, VLP, a multiprotein nanostructure that mimics the organization and conformation of native viruses and can be produced in various cell types (insect, mammalian, and plants cells), has been extensively studied. The virus-mimicking nanostructure and morphological features endow VLPs with intrinsic immunoenhancing properties. In addition, increasing diversity of new nanoplatforms mimicking the virion size, the repetitive antigen presenting pattern, and native antigen conformation are emerging, which we will discuss later.
Nucleic acid (mRNA and DNA) vaccine technology has achieved the latest advancement and has been a recent focus for vaccine development.[59-61] Nucleic acid vaccines are non-infectious and can use host cellular machinery to produce the encoded protein antigens to stimulate potent immune responses. The rapid egg/cell-free and scalable generic DNA/mRNA manufacturing and flexible sequence modification processes make nucleic acid vaccines easily adaptable to changing pathogen strains, fastening the vaccine production and distribution for recurring and pandemic influenza viruses. Specifically, mRNA vaccines are safer than DNA vaccines because they can be naturally degraded and not integrate into the host's genome. RNA production techniques such as the application of modified nucleosides have increased the resistance of mRNA to RNases. Self-amplifying replicon RNA could achieve sustained antigen expression.[62] The remarkable success of the COVID-19 mRNA vaccines encourages the development of mRNA vaccines against other infectious diseases, including influenza.
Self-replicable and non-replicable viral vector vaccines can deliver protein antigen-encoding nucleic acids into host cells.[63-65] Adenovirus (Ad) vectors are among the most commonly employed viral vectors. However, the viral vectors are potentially associated with inherent shortcomings, such as potential genomic insertion risks, difficulties in controlling gene expression, and immunological side effects. Recent advances in nanotechnology and material sciences have yielded promising nanoparticle alternatives that facilitate targeted delivery of nucleic acid vaccines in a safe and non-invasive manner.
3. Immunological Correlates of Broadly Cross-Protective Influenza Immunity
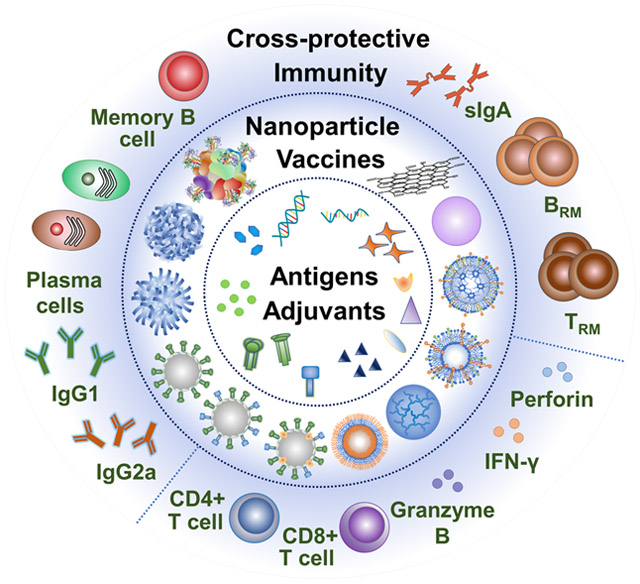
Understanding the immunological correlates of broadly cross-protective influenza immunity is critical to developing next-generation universal influenza vaccines. In this section, we will review recent recognition of these correlates — cross-reactive antibody responses, cellular responses, and mucosal immunity patrolling the vulnerable respiratory tracts (Figure 2). We then discuss the key factors determining these targeted immune responses.
Figure 2.
The immunological correlates of broadly cross-protective influenza immunity for next-generation influenza vaccines. (A). Induction of broadly reactive antibody responses and memory B cells via GC reactions. (B) Generation of cross-reactive T cell responses. (C) Immunity in vulnerable respiratory tracts. SHM, somatic hypermutation. DC, dendritic cell. FDC, follicular dendritic cell. TFH, T follicular helper cell. ADCC & ADCP, antibody-dependent cellular cytotoxicity & phagocytosis. TCR, T-cell receptor. MHC, major histocompatibility complex. M cell, microfold cell. TRM, resident memory T cell. BRM, resident memory B cell.
3.1. Broadly Neutralizing Antibody (bnAb) Responses
bnAbs have been recognized as a critical correlate of influenza immune protection and the holy grail of a broadly protective influenza vaccine.[66-69] Passive application of bnAbs protected recipients against variant influenza infections.[70]
An extended germinal center (GC) reaction is critical to the generation of bnAbs post-infection or vaccination (Figure 2A).[71-72] Progressive antibody responses are achieved from GC B cell affinity maturation under helps by follicular helper T (TFH) cells.[73] Through B cell receptor (BCR) somatic hypermutation (SHM) and selection, GC B cells acquire fitness and enhanced affinity for antigens and differentiate into high-affinity antibody-secreting B cells and memory B (Bmem) cells. Conserved antigen-specific Bmem plays a crucial role in protecting future variant virus reencounters.[74] Although conserved influenza epitopes are intrinsically not immunogenic, extended GC reactions can drive stepwise selections after multiple round SHM from B cell precursors, facilitating the production of high-affinity bnAbs.
As antigen persistence in GCs is required for BCR affinity maturation after initial antigen exposure,[75] an optimal vaccine platform with sustained antigen release should trigger robust bnAb responses. Innovative delivery technologies to control the release of vaccine antigens would substantially boost antibody responses. Durable antigen availability can enhance antibody response potency through multiple potential mechanisms, including shifting B cell recognition away from non-neutralizing immunodominant epitopes, altered kinetics of immune complex deposition, improved TFH cell responses, enhanced affinity maturation, and enhanced development of Bmem.[72]
3.2. Antibodies through Fc-mediated Effector Mechanisms
Fc receptors (FcRs) have demonstrated numerous roles in broadly protective influenza immunity.[76] While strain-specific neutralizing Abs do not require FcR interactions for potent protective activity, the protective potency of the broadly reactive anti-HA stalk and anti-NA antibodies depends on the activation and engagement of FcRs.[77-79] Non-neutralizing antibodies also provide potent antiviral activity in vivo through Fc-FcRs mediated effector mechanisms, such as antibody-dependent cellular cytotoxicity & phagocytosis (ADCC & ADCP).[80-81] HA stalk nanoclusters have induced broad protection against different virus challenges through ADCC and ADCP.[33]
Vaccine-induced IgG isotypes play separate but important roles in controlling influenza infection.[82] Murine IgG2a and IgG2b can more efficiently activate the Fc-mediated effector functions than IgG1. Murine IgG2a—with the greatest ability to activate FcRs pathways among all murine IgG isotypes— is more efficient at clearing influenza virus infections than the IgG1 isotype.[83-85] Like mice, human IgG subtypes that efficiently activate the FcRs pathway can provide adequate Fc-mediated immune protection.[76]
Conserved influenza immunogens (HA stalk, NA, and M2e)-binding antibodies are potent inducers of ADCC and protect against divergent influenza strains.[79-80] Therefore, programming antibody phenotype production to boost Fc-mediated effector mechanisms by directing GC B cell class-switching is an important strategy in developing a universal influenza vaccine.
3.3. Cross-Reactive T Cell Responses
T cell responses are critical for combating intracellular pathogens such as influenza (Figure 2B). Although T cell responses cannot prevent infection, they substantially promote virus clearance, reduce pathology, and mediate rapid host recovery from mild and severe influenza diseases.[14] T cell-mediated immunity plays a vital role in improving the influenza vaccine durability, potency, and breadth, and mediating cross-protection against heterologous and heterosubtypic influenza strains, particularly in the absence of neutralizing antibodies.[50-51]
Antigen-presenting cells (APCs) internalize extracellular antigens through endocytosis and pinocytosis, process the antigens, and present antigenic peptides with MHC II molecules to activate CD4 T cells. Most protein vaccine antigens enter the CD4 rather than CD8 T cell activation pathway due to their poor cytosolic delivery efficiencies. Some antigens may escape APC endosome/lysosomes into cytosols and are presented with MHC I molecules to activate CD8 T cells (antigen cross-presentation).[86] Promoting antigen cross-presentation in APCs is a promising strategy for protein antigens to induce balanced CD4 and CD8 T cell responses.
CD4 T cells play a central role in organizing an appropriate protective influenza immunity by providing helps in the activation of B cells and CD8 T cells.[51] Memory CD4 T cells can mediate the induction of an antiviral state of cells close to virus-infected cells[87] and the rapid initiation of the innate antiviral responses in the lung[88]. Moreover, multifunctional CD4 effector cells expressing IFN-γ and perforin were reported exhibiting direct cytolytic activity and mediating protective recovery from influenza virus infection.[89-90] Recently, Nelson and colleagues reported that intranasal vaccination with a self-assembled nanolipoprotein particle linked to conserved influenza NP and an adjuvant elicited a persistent polyfunctional CD4 T cell response in the murine airway and lung tissues. These polyfunctional CD4 cells produced significantly more effector cytokines IFN-γ and TNF-α as well as cytotoxic functionality. Adoptive transfer of these CD4 T cells to naïve recipients mediates 100% survival from lethal H1N1 influenza virus challenge.[91] Thus, a comprehensive CD4 and CD8 T cell immune response favor the pursuit of a cross-protective universal influenza vaccine.
3.4. Mucosal Immunity in Vulnerable Respiratory Tracts—the Viral Entry Portals
Mucosal immunity is an essential immunological correlate of cross-protection for preventing respiratory viruses such as influenza (Figure 2C). Mucosal secretory IgAs (sIgAs) are the primary humoral mediator of mucosal immunity. sIgAs are secreted by plasma cells adjacent to the mucosal epithelial cells, the site where infection occurs, thus potentially preventing virus infection at the portal of entry. sIgAs have unique structural and functional features distinct from other antibody classes. Anti-HA IgA display increased avidity than IgG antibodies, likely owing to their polymeric quaternary structures and multivalency.[92] This advantage may be significant for heterosubtypic immunity.
Moreover, tissue-resident memory cells patrol nonlymphoid organs and act as the first line of defense against infectious pathogens that commonly infect mucosal and barrier tissues, such as influenza.[93] Resident memory T (TRM) cells in the lung parenchyma (LP) and airways provide heterologous influenza protection.[94] The specific niches for lung TRM cells at virus primary-infection sites are necessary for long-term protection against secondary influenza infections.[95] Reports showed that lung TRM responses require local antigen-presentation, like respiratory tract infection of influenza viruses or mucosal vaccination.[96-98] In parallel with TRM, resident memory B (BRM) cells in the lung, but not systemic memory B cells, contributed to early plasmablast responses following challenge infection.[99] Thus, a vaccine delivery platform suitable for the harsh environment of respiratory tract immunization is significant to a universal influenza vaccine to induce cross-protective local memory immune responses.
Mucosal (intranasal or pulmonary) administration can be a competitive and preferential approach for next-generation influenza vaccine development. In comparison with conventional intramuscular vaccination that generates mainly systemic immunity, mucosal immunization can effectively establish both systemic and local mucosal immunity. Furthermore, needle-free mucosal vaccines possess superior logistical advantages over traditional injected vaccines, such as easy administration with high acceptance for recipients and avoidance of biohazardous sharps waste.
Mucosal vaccine formulations should be adapted to the harsh mucosal environment of the respiratory tracts to induce long-lasting cross-protective local immunity. Antigens alone generally display poor immunogenicity in a mucosal immunization route owing to their relative inability to cross the mucosal barrier and the rapid elimination by mucociliary clearance. Mucosal vaccines have a higher requirement for adjuvant activity due to the tolerogenic mucosal environment.[100] Most current adjuvants that function well in injected vaccines are not optimal for a mucosal route. Nanoparticle platforms have shown advantages in overcoming the shortage of conventional vaccines in inducing robust mucosal immune responses.[101-104] More advances regarding nanotechnology contributing to mucosal immunity will be further commented below.
4. Nanotechnology Contributes to Next-Generation Influenza Vaccines Development
Nanoparticle vaccine platform is one of the most promising innovative technologies for next-generation influenza vaccine development.[105-108] Nanoparticles can accommodate various vaccine components (proteins, peptides, DNA, mRNA, and molecular adjuvants) in a modular, tunable, and flexible way and facilitate the development of affordable vaccines by promoting logistically simplified vaccine manufacturing, storage, and distribution, and administration. Tailored nanoparticle vaccines can program the induction of potent broadly protective immune responses and allow antigen dose-sparing. In this section, we will briefly summarize the general characteristics of diverse nanoparticle vaccine platforms and then focus on how engineered nanoparticles vaccines program and direct the immune correlates of broadly cross-protective influenza immunity, providing insights into the rational design of next-generation universal nanoparticulate influenza vaccines.
4.1. Diverse Nanoparticle Vaccine Platforms
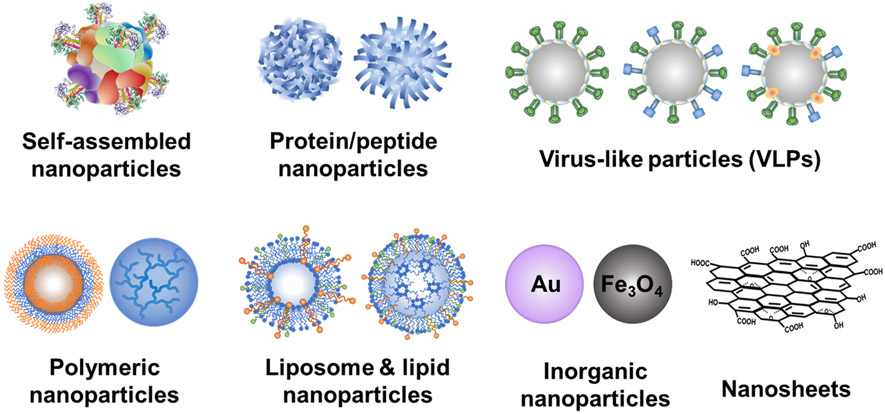
Various cutting-edge nanoparticle vaccine platforms have demonstrated broad-spectrum protection in laboratory animal models and early phase clinical trials.[109]. These nanoplatforms include self-assembled nanoparticles, protein nanoparticles, VLPs, polymeric nanoparticles, lipid nanoparticles or liposomes, and inorganic nanoparticles (Au, Fe3O4, carbon nanomaterials, etc.) (Figure 3). Herein, we will summarize the characteristics of these nanoplatforms.
Figure 3.
Various nanoparticle platforms for vaccine development.
Self-assembled protein nanoparticles take advantage of some natural proteins' intrinsic self-assembling functions (such as ferritin and fibrillizing peptides) to form a symmetrical spatial nanostructure.[110] Well-designed self-assembled protein nanoparticles can present antigens in a native steric configuration that is favorable to induce protective immune responses. Kanekiyo and colleagues used self-assembling ferritin to display influenza HA.[111] The HA-ferritin fusion protein was self-assembled into 24-unit nanoparticles with eight threefold axes. Each three-fold axis stabilized a trimeric HA. Self-assembled HA/HA stalk,[31, 111-112] and M2e nanoparticles[43, 113] have demonstrated improved immunogenicity and cross-protection.
However, self-assembled nanoparticles may induce unfavorable off-target immune responses to the assembling sequences, obstructing subsequent vaccine immunizations containing the same off-target domains. Furthermore, the small number of naturally occurring scaffolds limited the tunability of self-assembling protein nanoparticles. Recent advances in computational protein design have driven the generation of more synthetic scaffold candidates (e.g., T33, E2p, I3-01, I53-50, etc.) that can form self-assembled nanoparticles with controllable antigen valency, spacing, and location, as well as the assembly process.[114-117]
Proteins or peptides can aggregate into nanoscale structures upon exposure to desolvation reagents such as organic solvents (methanol, ethanol, acetone, etc.) and neutral salts. The exclusive protein/peptide composition ensures high safety profiles. Desolvated protein nanoparticles have displayed an extended antigen release and induced robust, long-lasting protective immunity.[47] We have prepared protein/peptide nanoparticles incorporating influenza immunogens such as full-length HA, HA stalk, M2e, NP, or NP polypeptides to enhance their immunogenicity.[33, 47, 118]
VLP is another optimal carrier for surface proteins with native-like antigen presentation. VLPs are multiprotein structures produced by the expression of the viral envelope or capsid proteins and self-assembled in the cell surface-adjacent area (like the budding release) or membrane-enveloped intracellular spaces. VLPs lack the viral genomes, thus cannot replicate or recombine in the vaccinated hosts, potentially yielding relatively safe vaccine candidates. The virus-mimicking features grant VLPs with high immunogenicity versus soluble antigenic proteins. VLPs displaying M2e[36, 119], HA[120-122], HA stalk[123], long alpha-helix (LAH) on conserved HA stalk[124], or computationally optimized, broadly reactive antigen[125] have been developed and demonstrated cross-reactive immunity against variant influenza virus challenges. Moreover, protein-based adjuvants (flagellin,[45] GM-CSF,[126] and CCL28[103-104]) can be co-incorporated with immunogens into the VLPs to boost immunogenicity. Encouraged by the success of VLP-based vaccines against hepatitis B virus and human papillomavirus, many researchers are investing effort in VLP-based universal influenza vaccines.
Polymeric and lipid-based nanoparticles have also attracted intensive attention in vaccine development due to their rich chemistry (flexible chemical conjugation), tailorability, and biocompatibility.[105] Various polymer-based nanostructures have been developed as influenza vaccine candidates, including particles, nanogels, micelles, and polymersomes. These nanoparticles can be engineered to incorporate multiple immunological factors mimicking pathogenic microbes or viruses' biophysical and biochemical characteristics (e.g. size and controlled antigen release) to improve vaccine potency. Moreover, polymers can be integrated within other nanoparticle formulations such as lipid and inorganic nanoparticles to tune physicochemical characteristics. Lipid-based nanoparticles (LNPs) have many advantages such as highly customizable compositions, biocompatibility, biodegradability, tailorability for loading and controlled release of both hydrophilic and lipophilic drugs, rapid large-scale production, flexible adaption to emerging pathogens, and self-adjuvating properties, and are safer and promising alternatives to viral vectors for the delivery of nucleic acids.[127-128]
Inorganic nanoparticles include gold nanoparticles (AuNPs), metal oxide nanoparticles, mesoporous silica nanoparticles, graphene oxide (GO) nanoparticles, etc.[129-131] Inorganic materials generally have improved stability and are immunogenically inertial, avoiding the induction of carrier-specific off-target immunity following repeated administration. AuNPs appear to be one of the most attractive inorganic nanoparticle platforms due to their facile fabrication, tunable size and shape, good biocompatibility, and flexible surface functionalization.[132-133] Functionalized AuNPs incorporating antigens and adjuvants in a repetitive, oriented configuration have elicited significantly more robust immune responses than soluble antigen and adjuvant mixtures.[102] Two-dimensional (2-D) sheet-like nanoparticles such as GO nanoparticles also have great potential as a novel vaccine platform due to their high antigen-loading capacities, inherent immunoenhancing properties, and intriguing cytosolic delivery profiles.[134-135] GO-based influenza HA nanoparticles significantly enhanced the immunogenicity of HA in an intranasal route.[101]
4.2. Engineered Nanoparticle Vaccines Boost Cross-Protective Influenza Immunity
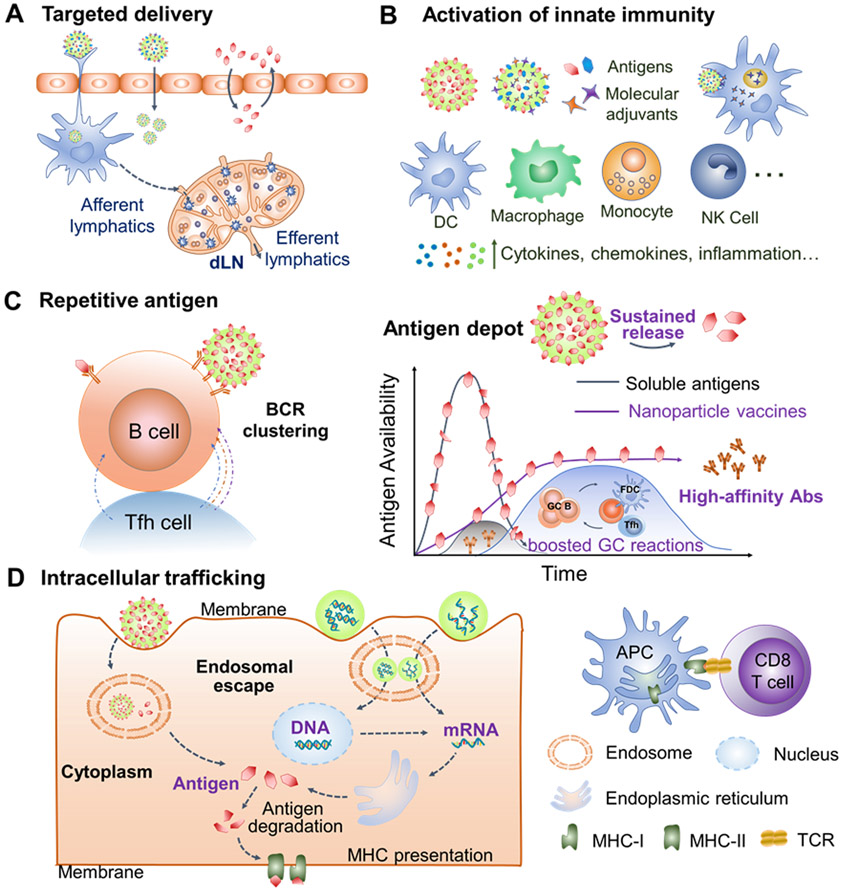
Targeted induction of immunological correlates of broad cross-protection is the primary rule in engineering nanoparticle universal influenza vaccines. Engineered nanoparticle vaccine platforms have intriguing characteristics that could improve all the immunological correlates (discussed in Session 3) required for universal influenza vaccines (Figure 4).
Figure 4.
Intriguing characteristics of engineered nanoparticle vaccine platforms. (A) Nanoparticles facilitate targeted antigen/adjuvant delivery to dLNs and immune cells. (B) Nanoparticles cooperate with molecular adjuvants to induce innate immunity. (C) The nanosize, high antigen loads, and controlled antigen releases enhance B cell activation and GC reactions, producing high-affinity antibody responses. (D) Nanoparticles trafficking cargos into the cytoplasm facilitate cross-presentation of exogenous antigens and delivery of nucleic acid vaccines. dLNs, draining lymph nodes. GC, germinal center. Abs, antibodies. bnAbs, broadly neutralizing antibodies. NK, natural killer. MHC, major histocompatibility complex. TCR, T-cell receptor.
4.2.1. Engineered Nanoparticle Vaccines Boost Broadly Reactive Antibody Responses
Antibody responses to conserved immune determinants are critical to universal influenza vaccines. The production of long-lasting, high-affinity, and cross-reactive antibodies, particularly bnAbs, requires persistent antigen supply to maintain an extended GC reaction, as reviewed above [136-137]. An optimal nanoparticle vaccine will protect antigens from degradation and function as an antigen-depot for sustained antigen availability to synchronize with the GC reactions.
BnAbs have been induced from precisely designed nanoparticle vaccines, such as self-assembled HA-ferritin nanoparticles.[111] The HA-ferritin nanoparticles elicited HAI antibody titers > 10-fold higher than licensed inactivated vaccines and neutralizing antibodies to highly conserved vulnerable HA structures, conferring broad cross-protection. An HA-nanoparticle (HA from 1999 H1N1 virus) vaccination elicited antibodies neutralizing H1N1 viruses from 1934 to 2007 and protected ferrets against an unmatched 2007 H1N1 virus. In a follow-up study, structure-based HA stem-ferritin nanoparticles generated broadly cross-reactive antibodies, providing heterosubtypic protection in mice and ferrets.[31] Later, Kanekiyo and colleagues developed a modular self-assembling ferritin nanoparticle system that presents an array of homotypic and heterotypic antigens on the surface.[23] The mosaic heterotypic influenza HA RBDs nanoparticles promoted the activation of B cells expressing a cross-reactive BCR and produced broader antibody responses than those induced by an admixture of homotypic nanoparticles. Such mosaic nanoparticles generated bnAbs neutralizing H1N1 viruses spanning over 90 years. Thus, self-assembled nanoparticles are innovative nanoplatforms for mosaic antigen presentation and can subvert monotypic immunodominance to otherwise subdominant cross-reactive B cell responses.
Mechanism studies indicated that self-assembling nanoparticles expanded GC activity, deposited more antigen colocalizing with follicular dendritic cells (FDCs) in dLNs, and drove memory B cell maturation.[112] The antigen integrity, orientation, and presentation on the nanoparticles maximized the HA immunogenicity. Vu and colleagues oriented His-tagged HA on liposome surfaces via stable nitrilotriacetic acid (NTA) metal chelation.[138] The HA-liposomal vaccine elicited increased antibody titers than HA and HA+liposomes by enhancing antigen deposition into GCs and driving B cell and TFH cell responses, providing robust protection against highly pathogenic virus challenges in mice.
We have shown that nanoparticles' antigen depot effect increased antigen immunogenicity.[33, 47, 118] We found that protein nanoparticles maintained long-lasting antigen retention at the injection site and in secondary lymphoid organs, contributing to enhanced antibody responses.[47] In a follow-up study, double-layered NA-flagellin/M2e nanoparticles induced larger CD95 and GL7 double-positive GC B cell populations in dLNs.[139] MNP skin vaccination with these nanoparticles further boosted the GC reactions, significantly increasing the antigen-specific antibody levels, the numbers of GC B cells, and IL-4 positive splenocytes, and protecting mice against homologous and heterosubtypic influenza viruses.
Nanoparticle vaccines can also boost antibody potency and breadth through Fc-mediated effector mechanisms beyond neutralization. Double-layered M2e-hrHA protein nanoclusters conferred cross-protection through ADCC and ADCP.[33] H1 HA-stem nanoparticles generated heterosubtypic protection against lethal H5N1 challenges in the absence of detectable neutralizing activity.[31] Additionally, nanoparticle incorporation of polarizing molecular adjuvants (e.g., CpG) can enhance Fc-mediated protection effects by inducing Th1-type antibodies with optimized Fc-FcγRIII affinity and cytokine secretion to activate effector cells. CpG increased the percentage of activated NK cells and boosted NK-mediated ADCC.[140-141] Influenza vaccine formulations containing liposomal CpG nanoparticles demonstrated significantly higher cross-reactive Th1-type antibody levels than free ODN, contributing to superior cross-protection against influenza.[142-143] A comprehensive antibody response should include bnAbs and Abs mediating protection through non-neutralization mechanisms.
Therefore, controlling the antigen presentation, release behavior, and co-incorporation with adjuvants in nanoparticles are of great importance to optimal humoral immune responses.
4.2.2. Engineered Nanoparticle Vaccines Elicit Cross-Protective Cellular Responses
Robust T cell responses are important to eliminate infected cells and stop a spreading influenza infection. Memory T cell responses are critical for long-lasting influenza protection. Nanoparticles were proven multifunctional for accommodating T cell epitopes and triggering T cell responses. Nanoparticles can enhance targeted recognization by immune cells, including CD8a+ DCs in dLNs.[144-145] Engineered nanoparticles can mediate endosomal/lysosome escape through endosomal swelling, membrane fusion, destabilization or rupture, to increase cytoplasmic antigen localization, a prerequisite for cross-presentation of extracellular antigens to induce CD8 T cell responses.
Reduction- or pH-sensitive nanovaccines facilitate endosomal membrane fusion or destabilization, leading to enhanced CD8 T cell responses.[146-147] Endosomally reduction responsive disassemblable nanoparticle conjugation of ovalbumin (OVA) with CpG induced a three- and ten-fold increase of antigen-specific CD8 T cells in spleens and lungs respectively than OVA and CpG mixture following pulmonary vaccination, and completely protected mice from morbidity following influenza-OVA infection.[147] This nanoparticle recruited a long-lasting pool of protective memory CD8 T cells to the lung. Knight and colleagues prepared a pH-responsive nanoparticle vaccine for cytosolic antigen delivery, and showed that a single intranasal dose of this vaccine elicited robust airway- and lung-resident CD8 TRM cells, protecting mice against lethal influenza virus challenges for up to 9 weeks post-immunization.[148] By contrast, a structurally analogous non-pH-responsive control carrier elicited fewer lung-resident CD8 T cells, indicating the essential role of the pH-responsive activity. Co-delivery of antigen and CpG on the same nanoparticle substantially enhanced the magnitude, functionality, and longevity of the lung-resident CD8 TRM responses than the antigen and adjuvant mixtures. Moreover, the nanoparticles are superior in generating CD8 TRM responses via the intranasal route v.s. the subcutaneous route.
Some specific nanoparticles also displayed intracellular cytosolic delivery functions. A typical example is graphene oxide (GO) nanoparticle, a 2-D sheet-like nanoparticle that can destabilize intracellular vesicle lipid membranes.[149-150] GO nanoparticles are promising vaccine delivery vehicles with intriguing features, including an ultra-large surface area for high-density antigen association, abundant chemical groups for flexible surface modification, and noncovalent antigen loading via electrostatic adsorption, hydrogen bonding, hydrophobic, and π–π stacking interactions. Various GO nanoparticle vaccines have shown improved cellular immune responses.[151-152] We recently fabricated a polyethyleneimine-functionalized GO (GP) influenza vaccine that significantly boosted influenza HA immunogenicity following intranasal immunization.[101] This GP-HA nanoparticle immunization induced robust antibody and cellular responses in mice, protecting mice against homologous and heterologous influenza viruses.
Besides, polycationic polymer and lipid nanoparticles can facilitate endosomal escape by inducing endosomal swelling and rupture via the “proton sponge effect”. Cell-penetrating peptides can promote the release of cargos trapped in endosomes into the cytosol.[153] Co-delivery of immune-modulators (e.g., TLR agonists) with antigens can upregulate costimulating molecules and cytokines to prime APC maturation and enhance antigen cross-presentation.[154-155]
4.2.3. Mucosal Nanoparticle Vaccines Induce Local Immune Responses
Induction of long-lasting mucosal immunity in the virus entry portals is a preferential option for preventing influenza and is the only approach functioning before the virus invasion into the host cells. Antigen-specific mucosal responses initiate in organized mucosa-associated lymphoid tissues (MALTs), including nasal-associated lymphoid tissues (NALTs) and bronchus-associated lymphoid tissues (BALTs). Mucosally administered free antigens tend to be rapidly cleared by the swinging mucosal cilia. Recent studies have shown that well-designed nanoparticles can tolerate the harsh respiratory mucosal environment, penetrate mucin-abundant mucus layer and mucosal epithelium to engage the mucosal immune system, and induce robust influenza mucosal responses. These nanoparticles can increase antigen stability, bioavailability, and targeting to underlying lymphoid tissue and immune cells.
A positive nanoparticle surface charge can facilitate antibody responses following mucosal immunization.[156-157]. Cationic nanoparticles can bind anionic cell receptors such as heparan sulfate proteoglycans, syndecans, and β-glycans from the glycosaminoglycans family, promoting transmucosal antigen delivery and cellular uptake by DCs in dLNs.[157] Cationic OVA-conjugated hydrogel nanoparticles induced more robust GC B-cell expansion and CD4 T-cell activation in lung dLNs, leading to robust systemic and lung antibody titers to anionic particles.[156] Dombu and colleagues found that cationic maltodextrins nanoparticles bond to anionic receptors on human bronchial epithelial cells [158]. Polycationic PEI targeted epithelial cells and microfold cells that express high levels of heparan sulfate proteoglycans.[159] A single intranasal dose of PEI-complexed influenza HA or herpes simplex virus type-2 (HSV-2) glycoprotein nanoparticles elicited robust antibody-mediated protection via an Irf-3-dependent signaling pathway from otherwise lethal infection.[159] PEI-functionalized GP-HA nanoparticles demonstrated potent cross-reactive immune responses at systemic and mucosal sites, providing homo- and heterologous immune protection in mice.[101]
Engineering nanoparticle mucoadhesivity is another effective approach to boost mucosal immunity through prolonging the particle adhesion and retention on mucosal surfaces and increasing antigen availability. Mucoadhesive polymers, including chitosan (CS), polysaccharides, alginate, polyvinyl pyrrolidone, polyacrylic acid copolymer, polyvinyl alcohol copolymers, and cellulose derivatives, have attracted particular interest.[160-162] Chitosan and its derivates significantly enhanced intranasal influenza vaccine efficacy in various studies.[163-165] Polysaccharide nanoparticle Supra Molecular Biovector (SMBV) is a supramolecular structure with a spherical cationic polysaccharide particle core associated with proteins/peptides surrounded by a phospholipid/cholesterol layer.[166] SMBV stimulated both Th1 and Th2 immune responses, induced serosal and mucosal humoral and cellular immunity, and is under clinical trials for intranasal influenza vaccination. Additionally, mucus-penetrating polymers such as polyethylene glycol, poly(vinyl alcohol), and zwitterionic polymers can also facilitate efficient delivery to the underlying epithelium.[167-168] Microfold (M) cell-targeting motifs can promote M cell-mediated transport (via transcytosis) across the nasal epithelium.[169]
Virus-mimic nanoparticles are also promising mucosal vaccine candidates. Intranasal vaccination with chimeric VLPs containing influenza HA antigen and GPI-CCL28 induced long-lasting mucosal immunity against H3N2 virus infection.[103-104] Co-delivery of recombinant influenza HA and TLR5 agonist flagellin in an oriented way on AuNPs elicited strong mucosal and systemic immune responses, protecting hosts against lethal influenza challenges.[102] Furthermore, liposomes that mimic the properties of a respiratory pathogen and contain highly conserved influenza peptides elicited localized mucosal airway innate and virus-specific adaptive memory T-cell responses, providing immediate and durable protection in mice against challenges with lethal influenza viruses of diverse genetic backgrounds.[170] The T cell-dependent immunity uniquely offers a practical means of achieving multistrain cross-protection.
4.2.4. Nanoparticulate Adjuvants Magnify Immonostimulating Effect
Adjuvants can trigger early and proper innate immune responses to aid in the generation of robust and long-lasting adaptive immune responses and are crucial for next-generation influenza vaccine development. Adjuvants program the dimension and magnitude of immune responses by modulating cytokine and chemokine production, recruiting and activating immune cells, and enhancing antigen uptake, processing and presentation by APCs. Several adjuvants, including aluminum salts (Alum), oil-in-water emulsions (MF59, AS03, and AF03), and virosomes, have been licensed in influenza vaccines.[171] However, these adjuvants were mostly developed using empirical methods, emphasize mainly antibody responses, fail to elicit Th1 or cytotoxic T lymphocytes (CTLs) responses, and are not optimal for universal influenza vaccines. Nanoparticulate adjuvants that can direct innate immunity in a programmable way are of great significance.
Nanoparticulate adjuvants may generate immunoenhancing profiles distinct from and superior to traditional adjuvants, like nanoAlum vs. traditional Alum.[172-175] Orr and colleagues reported that poly(acrylic acid)-stabilized nanoAlum could provide superior antigen immunogenicity and elicit robust Th1 immune responses, including antigen-specific CD4 T cells expressing IFN-γ and TNF and high IgG2 titers, improving protection efficacy against lethal influenza challenge.[172] Jiang and colleagues reported that turning the traditional AlOH adjuvant from gel to nanoparticles can enhance antigen accumulation in lymph nodes and cellular uptake by DCs, resulting in amplified antigen cross-presentation efficiency and CD8 T cell responses[175]. Thus, physicochemical modifications, such as particle size reduction to the nanometer scale, will significantly influence adjuvant immune response patterns.
Nanoparticles protect and target molecular adjuvants to appropriate immune cells for optimal stimulation and minimum adverse effects.[142, 147, 176] Toll-like receptor (TLR) agonists represent promising vaccine adjuvants as potent DC activators to augment T cell responses.[177-178] Specific TLR agonists have been included in licensed adjuvant combinations like MPL in AS04 and MPL and CpG1018 in AS01.[179] However, TLR agonists alone demonstrated poor targeting ability and suboptimal performance. Polymeric nanoparticle-conjugated CpG ODNs led to dual-targeting of adjuvants and antigens in cross-presenting DCs, resulting in enhanced DC maturation, Th1 cytokine secretion, effector CD8 T cell activation, and more robust memory recall responses.[176] This nanoparticle demonstrated an antigen dose sparing effect. Conjugation of D-type CpG ODNs into LNPs enhanced the protective breadth of conventional influenza split vaccines.[142] With a naturally occurring phosphodiester backbone, D-type CpG ODNs are prone to degradation by nucleases. LNP-CpGs significantly promoted cytokine secretion and co-stimulatory molecule expression by mouse DCs. LNP-CpGs adjuvanted vaccines improved cross-reactive antibody and T-cell responses, protecting mice against homologous, heterologous, and heterosubtypic challenges, while naked CpG adjuvanted vaccines only protected mice against homologous challenge.
Cytokines and chemokines are a class of vaccine immunopotentiators to recruit, activate, migrate, or home different immune cells to manipulate immune responses in direction, magnitude, and site.[180] Cytokines and chemokines in nanoparticle forms have shown superior immunostimulating properties. GIFT4 (a fusion cytokine of GM-CSF and IL-4) anchored on VLPs induced significantly increased sera antibody responses with higher binding avidity and improved neutralizing breadth and potency to multiple influenza strains, as well as higher IgG and IgA levels at mucosal sites.[126] GPI-anchored CCL28 (mucosal-associated epithelial chemokine) on influenza HA VLPs acted as a superior immunostimulator to boost cross-protection against heterologous viruses across a considerable phylogenic distance in mice.[103-104] Particularly, nanoparticulate CCL28 significantly attracted IgA-producing cells to mucosal sites and induced high mucosal antibody responses, contributing to the cross-protective influenza immunity.[104]
2',3'-cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) is a potent agonist of the stimulator of interferon genes (STING) and an ideal adjuvant to boost T cell responses in respiratory tracks by stimulating type I IFNs expression.[181-183] However, delivery of the small and water-soluble cGAMP into the cytosol of alveolar macrophages (AMs) and alveolar epithelial cells (AECs, vital responding cells for STING activation) without breaching the pulmonary surfactant (PS) layer integrity is a substantial challenge. Biomimetic nanoparticles can deliver cargos to sites generally inaccessible to adjuvants or immunogens, opening novel approaches to boost immunity in such vulnerable sites, e.g., lung alveolar epithelium.[184-186] Recently, Wang and colleagues developed a PS-biomimetic liposome nanoparticle mimicking the lipid composition and charge of PS constituents.[184] This nanoparticle successfully delivered cGAMP to AMs followed by transfer to AECs and substantially broadened the breadth of traditional inactivated influenza vaccines by inducing robust and durable lung CD8 TRM cells, while non-PS biomimetic nanoparticles did not. The potentiated heterosubtypic influenza immunity lasted for at least six months. This work further emphasized the importance of the rational nanoparticle design for site-targeted adjuvant delivery for broad influenza immunity.
To summarize, nanoparticles can function synergistically with molecular adjuvants to modulate the induction of innate and adaptive immunity. A deep understanding of adjuvant mechanisms of action, including responder cells (DCs, macrophages, T cells, or NKT cells), receptor locations (transmembrane or cytoplasmic), and the downstream pathways, is critical to rational nanoparticle vaccine design.[187] The differently localized compartments of pattern-recognition receptors (PRRs) determine the optimal nanoparticle trafficking approaches.[187] TLRs 1, 2, 4, 5, and 6 agonists should be conjugated on the nanoparticle surface to target cell surface receptors, while delivery to endosomal compartments is required to activate TLRs 7, 8, and 9 signaling pathways. Intracellular trafficking and delivery to the cytosolic PRRs are required to activate the cytosolic signaling pathways, such as double-stranded DNA (dsDNA) sensor cGAMP-STING. In addition, delivery in strong responder cells but not in weak responder cells is preferred for optimal immune responses.[183-184, 187] Moreover, molecular adjuvants' physicochemical properties, such as hydrophobicity/hydrophilicity, surface charge, and stability, will also guide the selection and rational design of delivery nanoparticles.
4.3. Challenges and Outlooks
Nanoparticle vaccines have demonstrated intriguing characteristics and great potentials in the development of next-generation influenza vaccines. However, limitations and challenges exist to the successful research & development of nanoparticle vaccines.
Many emerging nanoparticles lack a proven track record of safety profiles, hampering their translation into clinical trials. In this regard, rigorous safety and mechanisms studies underlying the improved immunity of nanoparticle vaccines are necessary for both basic and translational immunology. Controllable, reproducible, and scale-up preparation of homogenous nanoparticles remains a problem for most particle formulations, hindering their industrial scale-up production. As particle attributes significantly influence the nanoparticle vaccines' immune responses, more studies are necessary to establish the precise nanostructure/activity relationship. Furthermore, a deep understanding of antigen/adjuvant-immune system interactions regarding the timing of exposure, routes, retention, and innate microenvironment will further contribute to the rational design of nanoparticle vaccines.
In recent years, various nanoparticle strategies have been vigorously explored for next-generation influenza vaccine development. Some nanoparticle formulations such as HA-stem-ferritin and nucleic acid-LNPs have entered clinical trials.[12] With the increasing emergence of clinical nanoparticle vaccines, we are entering a bright future of translating nanovaccine from benchtop to clinic.
5. Conclusions
The development of universal influenza vaccines that are safe, productive, affordable, and elicit long-lasting cross-protective immunity against variant influenza strains is an unprecedented task for scientists and vaccine enterprises. Innovative and cutting-edge engineered nanoparticle vaccine platforms have demonstrated high potential in improving all the immune characteristics required for universal influenza vaccines, but challenges in terms of safety and scale-up reproducible preparation exist. Nevertheless, a safe, effective, and affordable nanoparticle-based universal influenza vaccine is feasible. We anticipate more nanoparticle vaccine candidates will enter clinical studies in the next few years.
Acknowledgments
The authors acknowledge financial support from the US National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) under grants R01AI101047, R01AI116835, and R01AI143844 to B.-Z.W.
Biography

Dr. Chunhong Dong is a postdoctoral fellow in the Center for Inflammation, Immunity & Infection, Institute for Biomedical Sciences, Georgia State University. She obtained her Ph.D. in Materials Science from the School of Materials Science and Engineering, Tianjin University in 2016. Her research focuses on developing functional nanovaccines/nanomedicines for respiratory infectious disease prevention and tumor theranostics.

Dr. Bao-Zhong Wang is a professor at Georgia State University Institute for Biomedical Sciences. He earned his Ph.D. in Molecular Biology and Biochemistry from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, in 2003. His research focuses on the interaction of viral pathogens with the host immune system. He studies how viral antigens trigger immune responses, with specific emphasis on the crossroad of vaccines and bioengineering through applications of structure-based antigen designs, nanotechnology, and controlled releases in vaccine development.
References
- [1].Centers for Disease Control and Prevention, Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States — 2019–2020 Influenza Season, https://www.cdc.gov/flu/about/burden/2019-2020.html, accessed: September, 2021.
- [2].Flerlage T, Boyd DF, Meliopoulos V, Thomas PG, Schultz-Cherry S, Nat Rev Microbiol 2021, 19, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bullard BL, Weaver EA, Vaccines 2021, 9, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].World Health Organization Global Influenza Surveillance and Response System (GISRS), https://www.who.int/initiatives/global-influenza-surveillance-and-response-system, accessed: September, 2021.
- [5].Centers for Disease Control and Prevention, 2020-2021 Flu Season Summary, https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm#anchor_1627000307956, accessed: September, 2021.
- [6].The New York Times, The Flu Vanished During Covid. What Will Its Return Look Like? https://www.nytimes.com/interactive/2021/04/22/science/flu-season-coronavirus-pandemic.html, accessed: September, 2021.
- [7].Vogel OA, Manicassamy B, Front Microbiol 2020, 11, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, Paules CI, Graham BS, Fauci AS, J Infect Dis 2018, 218, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kirtane AR, Verma M, Karandikar P, Furin J, Langer R, Traverso G, Nat. Nanotechnol 2021, 16, 369. [DOI] [PubMed] [Google Scholar]
- [10].Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, Wirth DM, Chen A, Sack M, Pokorski JK, Steinmetz NF, Nat. Nanotechnol 2020, 15, 646. [DOI] [PubMed] [Google Scholar]
- [11].Deng L, Wang BZ, ACS Infect Dis 2018, 4, 1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wei CJ, Crank MC, Shiver J, Graham BS, Mascola JR, Nabel GJ, Nat. Rev. Drug Discovery 2020, 19, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Centers for Disease Control and Prevention, Past Seasons Vaccine Effectiveness Estimates, https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html#2021, accessed: Oct, 2021.
- [14].Grant EJ, Quinones-Parra SM, Clemens EB, Kedzierska K, Curr Opin Virol 2016, 16, 132. [DOI] [PubMed] [Google Scholar]
- [15].Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, Paules CI, Graham BS, Fauci AS, J. Infect. Dis 2018, 218, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bouvier NM, Palese P, Vaccine 2008, 26 Suppl 4, D49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doherty PC, Turner SJ, Webby RG, Thomas PG, Nat. Immunol 2006, 7, 449. [DOI] [PubMed] [Google Scholar]
- [18].Nelson MI, Holmes EC, Nat Rev Genet 2007, 8, 196. [DOI] [PubMed] [Google Scholar]
- [19].Whittle JR, Zhang R, Khurana S, King LR, Manischewitz J, Golding H, Dormitzer PR, Haynes BF, Walter EB, Moody MA, Kepler TB, Liao HX, Harrison SC, Proc. Natl. Acad. Sci. U. S. A 2011, 108, 14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ekiert DC, Kashyap AK, Steel J, Rubrum A, Bhabha G, Khayat R, Lee JH, Dillon MA, O'Neil RE, Faynboym AM, Horowitz M, Horowitz L, Ward AB, Palese P, Webby R, Lerner RA, Bhatt RR, Wilson IA, Nature 2012, 489, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yamashita A, Kawashita N, Kubota-Koketsu R, Inoue Y, Watanabe Y, Ibrahim MS, Ideno S, Yunoki M, Okuno Y, Takagi T, Yasunaga T, Ikuta K, Biochem Biophys Res Commun 2010, 393, 614. [DOI] [PubMed] [Google Scholar]
- [22].Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A, Science 2011, 333, 850. [DOI] [PubMed] [Google Scholar]
- [23].Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, Leung K, Yang ES, Boyoglu-Barnum S, Georgiev IS, Tsybovsky Y, Prabhakaran MS, Andersen H, Kong WP, Baxa U, Zephir KL, Ledgerwood JE, Koup RA, Kwong PD, Harris AK, McDermott AB, Mascola JR, Graham BS, Nat. Immunol 2019, 20, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krammer F, Palese P, J Infect Dis 2019, 219, S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA, Nat. Struct. Mol. Biol 2009, 16, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA, Science 2009, 324, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, Vogels R, Brakenhoff JP, Kompier R, Koldijk MH, Cornelissen LA, Poon LL, Peiris M, Koudstaal W, Wilson IA, Goudsmit J, Science 2011, 333, 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Science 2011, 333, 850. [DOI] [PubMed] [Google Scholar]
- [29].Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, Lee JH, Metlagel Z, Bujny MV, Jongeneelen M, van der Vlugt R, Lamrani M, Korse HJ, Geelen E, Sahin O, Sieuwerts M, Brakenhoff JP, Vogels R, Li OT, Poon LL, Peiris M, Koudstaal W, Ward AB, Wilson IA, Goudsmit J, Friesen RH, Science 2012, 337, 1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nachbagauer R, Palese P, Curr. Opin. Immunol 2018, 53, 51. [DOI] [PubMed] [Google Scholar]
- [31].H. M. Yassine, Boyington JC, McTamney PM, Wei CJ, Kanekiyo M, Kong WP, Gallagher JR, Wang L, Zhang Y, Joyce MG, Lingwood D, Moin SM, Andersen H, Okuno Y, Rao SS, Harris AK, Kwong PD, Mascola JR, Nabel GJ, Graham BS, Nat. Med 2015, 21, 1065. [DOI] [PubMed] [Google Scholar]
- [32].Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, Science 2015, 349, 1301. [DOI] [PubMed] [Google Scholar]
- [33].Deng L, Mohan T, Chang TZ, Gonzalez GX, Wang Y, Kwon YM, Kang SM, Compans RW, Champion JA, Wang BZ, Nat. Commun 2018, 9, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen YQ, Wohlbold TJ, Zheng NY, Huang M, Huang Y, Neu KE, Lee J, Wan H, Rojas KT, Kirkpatrick E, Henry C, Palm AE, Stamper CT, Lan LY, Topham DJ, Treanor J, Wrammert J, Ahmed R, Eichelberger MC, Georgiou G, Krammer F, Wilson PC, Cell 2018, 173, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Skarlupka AL, Bebin-Blackwell AG, Sumner SF, Ross TM, J. Virol 2021, 95, e0075921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim MC, Song JM, O E, Kwon YM, Lee YJ, Compans RW, Kang SM, Mol Ther 2013, 21, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Andersson AM, Hakansson KO, Jensen BA, Christensen D, Andersen P, Thomsen AR, Christensen JP, PLoS One 2012, 7, e46395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bernasconi V, Bernocchi B, Ye L, Le MQ, Omokanye A, Carpentier R, Schon K, Saelens X, Staeheli P, Betbeder D, Lycke N, Front Immunol 2018, 9, 2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhu W, Li S, Wang C, Yu G, Prausnitz MR, Wang BZ, Front Immunol 2018, 9, 1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhu W, Pewin W, Wang C, Luo Y, Gonzalez GX, Mohan T, Prausnitz MR, Wang BZ, J Control Release 2017, 261, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang BZ, Gill HS, He C, Ou C, Wang L, Wang YC, Feng H, Zhang H, Prausnitz MR, Compans RW, J Control Release 2014, 178, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang L, Hess A, Chang TZ, Wang YC, Champion JA, Compans RW, Wang BZ, Nanomedicine 2014, 10, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Qi M, Zhang XE, Sun X, Zhang X, Yao Y, Liu S, Chen Z, Li W, Zhang Z, Chen J, Cui Z, Small 2018, 14, e1703207. [DOI] [PubMed] [Google Scholar]
- [44].Rappazzo CG, Watkins HC, Guarino CM, Chau A, Lopez JL, DeLisa MP, Leifer CA, Whittaker GR, Putnam D, Vaccine 2016, 34, 1252. [DOI] [PubMed] [Google Scholar]
- [45].Wang BZ, Gill HS, Kang SM, Wang L, Wang YC, Vassilieva EV, Compans RW, Clin Vaccine Immunol 2012, 19, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kolpe A, Schepens B, Fiers W, Saelens X, Expert Rev. Vaccines 2017, 16, 123. [DOI] [PubMed] [Google Scholar]
- [47].Deng L, Chang TZ, Wang Y, Li S, Wang S, Matsuyama S, Yu G, Compans RW, Li JD, Prausnitz MR, Champion JA, Wang BZ, Proc. Natl. Acad. Sci. U. S. A 2018, 115, E7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rajao DS, Perez DR, Front Microbiol 2018, 9, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Koutsakos M, Illing PT, Nguyen THO, Mifsud NA, Crawford JC, Rizzetto S, Eltahla AA, Clemens EB, Sant S, Chua BY, Wong CY, Allen EK, Teng D, Dash P, Boyd DF, Grzelak L, Zeng W, Hurt AC, Barr I, Rockman S, Jackson DC, Kotsimbos TC, Cheng AC, Richards M, Westall GP, Loudovaris T, Mannering SI, Elliott M, Tangye SG, Wakim LM, Rossjohn J, Vijaykrishna D, Luciani F, Thomas PG, Gras S, Purcell AW, Kedzierska K, Nat. Immunol 2019, 20, 613. [DOI] [PubMed] [Google Scholar]
- [50].La Gruta NL, Turner SJ, Trends Immunol 2014, 35, 396. [DOI] [PubMed] [Google Scholar]
- [51].Altenburg AF, Rimmelzwaan GF, de Vries RD, Vaccine 2015, 33, 500. [DOI] [PubMed] [Google Scholar]
- [52].Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu MA, Donnelly JJ, Caulfield MJ, J. Virol 1998, 72, 5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. , Science 1993, 259, 1745. [DOI] [PubMed] [Google Scholar]
- [54].Chen J, Wang J, Zhang J, Ly H, Front Immunol 2021, 12, 711997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Brisse M, Vrba SM, Kirk N, Liang Y, Ly H, Front Immunol 2020, 11, 583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Milian E, Kamen AA, Biomed Res Int 2015, 2015, 504831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dunkle LM, Izikson R, Patriarca P, Goldenthal KL, Muse D, Callahan J, Cox MMJ, Team PSCS, N. Engl. J. Med 2017, 376, 2427.28636855 [Google Scholar]
- [58].Mathew NR, Angeletti D, Front Immunol 2019, 10, 2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pardi N, Parkhouse K, Kirkpatrick E, McMahon M, Zost SJ, Mui BL, Tam YK, Kariko K, Barbosa CJ, Madden TD, Hope MJ, Krammer F, Hensley SE, Weissman D, Nat. Commun 2018, 9, 3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yan J, Morrow MP, Chu JS, Racine T, Reed CC, Khan AS, Broderick KE, Kim JJ, Kobinger GP, Sardesai NY, Weiner DB, Vaccine 2018, 36, 3079. [DOI] [PubMed] [Google Scholar]
- [61].Pardi N, Hogan MJ, Porter FW, Weissman D, Nat. Rev. Drug Discovery 2018, 17, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H, Reece ST, Sahin U, Tregoning JS, Mol Ther 2018, 26, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Demminger DE, Walz L, Dietert K, Hoffmann H, Planz O, Gruber AD, von Messling V, Wolff T, EMBO Mol. Med 2020, 12, e10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kerstetter LJ, Buckley S, Bliss CM, Coughlan L, Front Immunol 2020, 11, 607333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ferrara F, Del Rosario JMM, da Costa KAS, Kinsley R, Scott S, Fereidouni S, Thompson C, Kellam P, Gilbert S, Carnell G, Temperton N, Front Immunol 2021, 12, 661379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nath Neerukonda S, Vassell R, Weiss CD, Vaccines (Basel) 2020, 8, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Isakova-Sivak I, Stepanova E, Mezhenskaya D, Matyushenko V, Prokopenko P, Sychev I, Wong PF, Rudenko L, Expert Rev. Vaccines 2021, 20, 1097. [DOI] [PubMed] [Google Scholar]
- [68].Pappas L, Foglierini M, Piccoli L, Kallewaard NL, Turrini F, Silacci C, Fernandez-Rodriguez B, Agatic G, Giacchetto-Sasselli I, Pellicciotta G, Sallusto F, Zhu Q, Vicenzi E, Corti D, Lanzavecchia A, Nature 2014, 516, 418. [DOI] [PubMed] [Google Scholar]
- [69].Corti D, Cameroni E, Guarino B, Kallewaard NL, Zhu Q, Lanzavecchia A, Curr Opin Virol 2017, 24, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Biswas M, Yamazaki T, Chiba J, Akashi-Takamura S, Vaccines (Basel) 2020, 8, 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Robert PA, Marschall AL, Meyer-Hermann M, Curr. Opin. Biotechnol 2018, 51, 137. [DOI] [PubMed] [Google Scholar]
- [72].Cirelli KM, Crotty S, Curr. Opin. Immunol 2017, 47, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kubo M, Miyauchi K, Trends Immunol 2020, 41, 394. [DOI] [PubMed] [Google Scholar]
- [74].Takahashi Y, Kelsoe G, Curr. Opin. Immunol 2017, 45, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Havenar-Daughton C, Carnathan DG, Torrents de la Pena A, Pauthner M, Briney B, Reiss SM, Wood JS, Kaushik K, van Gils MJ, Rosales SL, van der Woude P, Locci M, Le KM, de Taeye SW, Sok D, Mohammed AUR, Huang J, Gumber S, Garcia A, Kasturi SP, Pulendran B, Moore JP, Ahmed R, Seumois G, Burton DR, Sanders RW, Silvestri G, Crotty S, Cell Rep 2016, 17, 2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Thulin NK, Wang TT, Vaccines (Basel) 2018, 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].He W, Tan GS, Mullarkey CE, Lee AJ, Lam MM, Krammer F, Henry C, Wilson PC, Ashkar AA, Palese P, Miller MS, Proc. Natl. Acad. Sci. U. S. A 2016, 113, 11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].DiLillo DJ, Palese P, Wilson PC, Ravetch JV, J Clin Invest 2016, 126, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].DiLillo DJ, Tan GS, Palese P, Ravetch JV, Nat. Med 2014, 20, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jegaskanda S, Reading PC, Kent SJ, J Immunol 2014, 193, 469. [DOI] [PubMed] [Google Scholar]
- [81].Vanderven HA, Kent SJ, Immunol Cell Biol 2020, 98, 253. [DOI] [PubMed] [Google Scholar]
- [82].Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA, Clin Vaccine Immunol 2006, 13, 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W, J. Virol 1997, 71, 4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K, Immunol. Rev 1997, 159, 95. [DOI] [PubMed] [Google Scholar]
- [85].Nimmerjahn F, Ravetch JV, Science 2005, 310, 1510. [DOI] [PubMed] [Google Scholar]
- [86].Du G, Sun X, Curr. Opin. Biotechnol 2020, 66, 113. [DOI] [PubMed] [Google Scholar]
- [87].Swain SL, McKinstry KK, Strutt TM, Nat. Rev. Immunol 2012, 12, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL, Nat. Med 2010, 16, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Brown DM, Lee S, Garcia-Hernandez Mde L, Swain SL, J. Virol 2012, 86, 6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Brown DM, Dilzer AM, Meents DL, Swain SL, J Immunol 2006, 177, 2888. [DOI] [PubMed] [Google Scholar]
- [91].Nelson SA, Dileepan T, Rasley A, Jenkins MK, Fischer NO, Sant AJ, J. Virol 2021, JVI. 00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Muramatsu M, Yoshida R, Yokoyama A, Miyamoto H, Kajihara M, Maruyama J, Nao N, Manzoor R, Takada A, PLoS One 2014, 9, e85582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Uddback I, Cartwright EK, Scholler AS, Wein AN, Hayward SL, Lobby J, Takamura S, Thomsen AR, Kohlmeier JE, Christensen JP, Mucosal Immunol 2021, 14, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].McMaster SR, Wein AN, Dunbar PR, Hayward SL, Cartwright EK, Denning TL, Kohlmeier JE, Mucosal Immunol 2018, 11, 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, Miyazawa M, J Exp Med 2016, 213, 3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Takamura S, Roberts AD, Jelley-Gibbs DM, Wittmer ST, Kohlmeier JE, Woodland DL, J Exp Med 2010, 207, 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zens KD, Chen JK, Farber DL, JCI Insight 2016, 1, e85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS, J Leukoc Biol 2014, 95, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Allie SR, Bradley JE, Mudunuru U, Schultz MD, Graf BA, Lund FE, Randall TD, Nat. Immunol 2019, 20, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Woodrow KA, Bennett KM, Lo DD, Annu. Rev. Biomed. Eng 2012, 14, 17. [DOI] [PubMed] [Google Scholar]
- [101].Dong C, Wang Y, Gonzalez GX, Ma Y, Song Y, Wang S, Kang SM, Compans RW, Wang BZ, Proc. Natl. Acad. Sci. U. S. A 2021, 118, e2024998118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang C, Zhu W, Luo Y, Wang BZ, Nanomedicine 2018, 14, 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mohan T, Kim J, Berman Z, Wang S, Compans RW, Wang BZ, J Control Release 2016, 233, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mohan T, Berman Z, Luo Y, Wang C, Wang S, Compans RW, Wang BZ, Sci. Rep 2017, 7, 40226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wibowo D, Jorritsma SHT, Gonzaga ZJ, Evert B, Chen S, Rehm BHA, Biomaterials 2021, 268, 120597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhou X, Jiang X, Qu M, Aninwene GE 2nd, Jucaud V, Moon JJ, Gu Z, Sun W, Khademhosseini A, ACS Nano 2020, 14, 12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Feng X, Xu W, Li Z, Song W, Ding J, Chen X, Adv Sci 2019, 6, 1900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sia ZR, Miller MS, Lovell JF, Mol Pharm 2021, 18, 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kim JK, Kang S-M, Compans RW, Wang B-Z, Expert Opin. Drug Discovery 2021, 16, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Lopez-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ, Comput. Struct. Biotechnol. J 2016, 14, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, Rao SS, Kong WP, Wang L, Nabel GJ, Nature 2013, 499, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kelly HG, Tan HX, Juno JA, Esterbauer R, Ju Y, Jiang W, Wimmer VC, Duckworth BC, Groom JR, Caruso F, Kanekiyo M, Kent SJ, Wheatley AK, JCI Insight 2020, 5, e136653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wang Q, Zhang Y, Zou P, Wang M, Fu W, She J, Song Z, Xu J, Huang J, Wu F, Front Microbiol 2020, 11, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].He LL, Lin XH, Wang Y, Abraham C, Sou C, Ngo T, Zhang Y, Wilson IA, Zhu J, Sci. Adv 2021, 7, eabf1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Brouwer PJM, Brinkkemper M, Maisonnasse P, Dereuddre-Bosquet N, Grobben M, Claireaux M, de Gast M, Marlin R, Chesnais V, Diry S, Allen JD, Watanabe Y, Giezen JM, Kerster G, Turner HL, van der Straten K, van der Linden CA, Aldon Y, Naninck T, Bontjer I, Burger JA, Poniman M, Mykytyn AZ, Okba NMA, Schermer EE, van Breemen MJ, Ravichandran R, Caniels TG, van Schooten J, Kahlaoui N, Contreras V, Lemaitre J, Chapon C, Fang RHT, Villaudy J, Sliepen K, van der Velden YU, Haagmans BL, de Bree GJ, Ginoux E, Ward AB, Crispin M, King NP, van der Werf S, van Gils MJ, Le Grand R, Sanders RW, Cell 2021, 184, 1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, Aye P, Navarro MJ, Lai L, Edara VV, Roltgen K, Rogers K, Shirreff L, Ferrell DE, Wrenn S, Pettie D, Kraft JC, Miranda MC, Kepl E, Sydeman C, Brunette N, Murphy M, Fiala B, Carter L, White AG, Trisal M, Hsieh CL, Russell-Lodrigue K, Monjure C, Dufour J, Spencer S, Doyle-Meyers L, Bohm RP, Maness NJ, Roy C, Plante JA, Plante KS, Zhu A, Gorman MJ, Shin S, Shen X, Fontenot J, Gupta S, O'Hagan DT, Van Der Most R, Rappuoli R, Coffman RL, Novack D, McLellan JS, Subramaniam S, Montefiori D, Boyd SD, Flynn JL, Alter G, Villinger F, Kleanthous H, Rappaport J, Suthar MS, King NP, Veesler D, Pulendran B, Nature 2021, 594, 253. [DOI] [PubMed] [Google Scholar]
- [117].King NP, Bale JB, Sheffler W, McNamara DE, Gonen S, Gonen T, Yeates TO, Baker D, Nature 2014, 510, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang L, Chang TZ, He Y, Kim JR, Wang S, Mohan T, Berman Z, Tompkins SM, Tripp RA, Compans RW, Champion JA, Wang BZ, Nanomedicine 2017, 13, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W, Nat. Med 1999, 5, 1157. [DOI] [PubMed] [Google Scholar]
- [120].Wang BZ, Xu R, Quan FS, Kang SM, Wang L, Compans RW, PLoS One 2010, 5, e13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Donaldson B, Lateef Z, Walker GF, Young SL, Ward VK, Expert Rev. Vaccines 2018, 17, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schwartzman LM, Cathcart AL, Pujanauski LM, Qi L, Kash JC, Taubenberger JK, MBio 2015, 6, e01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P, MBio 2010, 1, e00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kazaks A, Lu IN, Farinelle S, Ramirez A, Crescente V, Blaha B, Ogonah O, Mukhopadhyay T, de Obanos MP, Krimer A, Akopjana I, Bogans J, Ose V, Kirsteina A, Kazaka T, Stonehouse NJ, Rowlands DJ, Muller CP, Tars K, Rosenberg WM, BMC Biotechnol 2017, 17, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Giles BM, Crevar CJ, Carter DM, Bissel SJ, Schultz-Cherry S, Wiley CA, Ross TM, J Infect Dis 2012, 205, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Feng H, Zhang H, Deng J, Wang L, He Y, Wang S, Seyedtabaei R, Wang Q, Liu L, Galipeau J, Compans RW, Wang BZ, Sci. Rep 2015, 5, 11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Nat. Rev. Mater 2021, 6, 99. [Google Scholar]
- [128].Hou X, Zaks T, Langer R, Dong Y, Nat Rev Mater 2021. 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhou H, Ge J, Miao Q, Zhu R, Wen L, Zeng J, Gao M, Bioconjug Chem 2020, 31, 315. [DOI] [PubMed] [Google Scholar]
- [130].Yang G, Phua SZF, Bindra AK, Zhao Y, Adv Mater 2019, 31, e1805730. [DOI] [PubMed] [Google Scholar]
- [131].Hess KL, Medintz IL, Jewell CM, Nano Today 2019, 27, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Marques Neto LM, Kipnis A, Junqueira-Kipnis AP, Front Immunol 2017, 8, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Salazar-Gonzalez JA, Gonzalez-Ortega O, Rosales-Mendoza S, Expert Rev. Vaccines 2015, 14, 1197. [DOI] [PubMed] [Google Scholar]
- [134].Yue H, Wei W, Gu Z, Ni D, Luo N, Yang Z, Zhao L, Garate JA, Zhou R, Su Z, Ma G, Nanoscale 2015, 7, 19949. [DOI] [PubMed] [Google Scholar]
- [135].Cao W, He L, Cao W, Huang X, Jia K, Dai J, Acta Biomater 2020, 112, 14. [DOI] [PubMed] [Google Scholar]
- [136].Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ, Proc. Natl. Acad. Sci. U. S. A 2016, 113, E6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wang Y, Huang G, Wang J, Molina H, Chaplin DD, Fu YX, Eur. J. Immunol 2000, 30, 2226. [DOI] [PubMed] [Google Scholar]
- [138].Vu MN, Kelly HG, Tan HX, Juno JA, Esterbauer R, Davis TP, Truong NP, Wheatley AK, Kent SJ, Adv. Healthcare Mater 2021, 10, e2002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang Y, Li S, Dong C, Ma Y, Song Y, Zhu W, Kim J, Deng L, Denning TL, Kang SM, Prausnitz MR, Wang BZ, ACS Appl. Bio Mater 2021, 4, 4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Roda JM, Parihar R, Carson WE 3rd, J Immunol 2005, 175, 1619. [DOI] [PubMed] [Google Scholar]
- [141].Hayashi T, Momota M, Kuroda E, Kusakabe T, Kobari S, Makisaka K, Ohno Y, Suzuki Y, Nakagawa F, Lee MSJ, Coban C, Onodera R, Higashi T, Motoyama K, Ishii KJ, Arima H, Front Immunol 2018, 9, 2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Shirai S, Shibuya M, Kawai A, Tamiya S, Munakata L, Omata D, Suzuki R, Aoshi T, Yoshioka Y, Front Immunol 2019, 10, 3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Joseph A, Louria-Hayon I, Plis-Finarov A, Zeira E, Zakay-Rones Z, Raz E, Hayashi T, Takabayashi K, Barenholz Y, Kedar E, Vaccine 2002, 20, 3342. [DOI] [PubMed] [Google Scholar]
- [144].Irvine DJ, Swartz MA, Szeto GL, Nat Mater 2013, 12, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Howard GP, Verma G, Ke X, Thayer WM, Hamerly T, Baxter VK, Lee JE, Dinglasan RR, Mao HQ, Nano Res. 2019, 12, 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Xu J, Wang H, Xu L, Chao Y, Wang C, Han X, Dong Z, Chang H, Peng R, Cheng Y, Liu Z, Biomaterials 2019, 207, 1. [DOI] [PubMed] [Google Scholar]
- [147].Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA, Proc. Natl. Acad. Sci. U. S. A 2011, 108, E989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Knight FC, Gilchuk P, Kumar A, Becker KW, Sevimli S, Jacobson ME, Suryadevara N, Wang-Bishop L, Boyd KL, Crowe JE Jr., Joyce S, Wilson JT, ACS Nano 2019, 13, 10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Li H, Fierens K, Zhang Z, Vanparijs N, Schuijs MJ, Van Steendam K, Feiner Gracia N, De Rycke R, De Beer T, De Beuckelaer A, De Koker S, Deforce D, Albertazzi L, Grooten J, Lambrecht BN, De Geest BG, ACS Appl. Mater. Interfaces 2016, 8, 1147. [DOI] [PubMed] [Google Scholar]
- [150].Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, Liu Z, Huang Q, Fan C, Fang H, Zhou R, Nat. Nanotechnol 2013, 8, 594. [DOI] [PubMed] [Google Scholar]
- [151].Cao YH, Ma YF, Zhang MX, Wang HM, Tu XL, Shen H, Dai JW, Guo HC, Zhang ZJ, Adv. Funct. Mater 2014, 24, 6963. [Google Scholar]
- [152].Meng C, Zhi X, Li C, Li C, Chen Z, Qiu X, Ding C, Ma L, Lu H, Chen D, Liu G, Cui D, ACS Nano 2016, 10, 2203. [DOI] [PubMed] [Google Scholar]
- [153].Endoh T, Ohtsuki T, Adv Drug Deliv Rev 2009, 61, 704. [DOI] [PubMed] [Google Scholar]
- [154].Alloatti A, Kotsias F, Pauwels AM, Carpier JM, Jouve M, Timmerman E, Pace L, Vargas P, Maurin M, Gehrmann U, Joannas L, Vivar OI, Lennon-Dumenil AM, Savina A, Gevaert K, Beyaert R, Hoffmann E, Amigorena S, Immunity 2015, 43, 1087. [DOI] [PubMed] [Google Scholar]
- [155].Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, Overholtzer M, Roche PA, Tampe R, Brown BD, Amsen D, Whiteheart SW, Blander JM, Cell 2014, 158, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Fromen CA, Robbins GR, Shen TW, Kai MP, Ting JP, DeSimone JM, Proc. Natl. Acad. Sci. U. S. A 2015, 112, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Dombu CY, Betbeder D, Biomaterials 2013, 34, 516. [DOI] [PubMed] [Google Scholar]
- [158].Dombu CY, Kroubi M, Zibouche R, Matran R, Betbeder D, Nanotechnology 2010, 21, 355102. [DOI] [PubMed] [Google Scholar]
- [159].Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho LP, Lambe T, Puthia M, Svanborg C, Scherer EM, Krashias G, Williams A, Blattman JN, Greenberg PD, Flavell RA, Moghaddam AE, Sheppard NC, Sattentau QJ, Nat Biotechnol 2012, 30, 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Li Z, Xiong F, He J, Dai X, Wang G, Eur. J. Pharm. Biopharm 2016, 109, 24. [DOI] [PubMed] [Google Scholar]
- [161].Amidi M, Romeijn SG, Verhoef JC, Junginger HE, Bungener L, Huckriede A, Crommelin DJ, Jiskoot W, Vaccine 2007, 25, 144. [DOI] [PubMed] [Google Scholar]
- [162].Singh B, Maharjan S, Cho KH, Cui L, Park IK, Choi YJ, Cho CS, Int J Biol Macromol 2018, 110, 54. [DOI] [PubMed] [Google Scholar]
- [163].Sawaengsak C, Mori Y, Yamanishi K, Srimanote P, Chaicumpa W, Mitrevej A, Sinchaipanid N, Int J Pharm 2014, 473, 113. [DOI] [PubMed] [Google Scholar]
- [164].Read RC, Naylor SC, Potter CW, Bond J, Jabbal-Gill I, Fisher A, Illum L, Jennings R, Vaccine 2005, 23, 4367. [DOI] [PubMed] [Google Scholar]
- [165].Dabaghian M, Latifi AM, Tebianian M, NajmiNejad H, Ebrahimi SM, Vaccine 2018, 36, 2886. [DOI] [PubMed] [Google Scholar]
- [166].von Hoegen P, Adv. Drug Delivery Rev 2001, 51, 113. [DOI] [PubMed] [Google Scholar]
- [167].Khutoryanskiy VV, Adv Drug Deliv Rev 2018, 124, 140. [DOI] [PubMed] [Google Scholar]
- [168].Xu Q, Ensign LM, Boylan NJ, Schon A, Gong X, Yang JC, Lamb NW, Cai S, Yu T, Freire E, Hanes J, ACS Nano 2015, 9, 9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Rajapaksa TE, Stover-Hamer M, Fernandez X, Eckelhoefer HA, Lo DD, J Control Release 2010, 142, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Tai W, Roberts L, Seryshev A, Gubatan JM, Bland CS, Zabriskie R, Kulkarni S, Soong L, Mbawuike I, Gilbert B, Kheradmand F, Corry DB, Mucosal Immunol 2011, 4, 197. [DOI] [PubMed] [Google Scholar]
- [171].Zhu W, Dong C, Wei L, Wang BZ, Pharmaceutics 2021, 13, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Orr MT, Khandhar AP, Seydoux E, Liang H, Gage E, Mikasa T, Beebe EL, Rintala ND, Persson KH, Ahniyaz A, Carter D, Reed SG, Fox CB, npj Vaccines 2019, 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Sun B, Ji Z, Liao YP, Wang M, Wang X, Dong J, Chang CH, Li R, Zhang H, Nel AE, Xia T, ACS Nano 2013, 7, 10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Li X, Aldayel AM, Cui Z, J Control Release 2014, 173, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Jiang H, Wang Q, Li L, Zeng Q, Li H, Gong T, Zhang Z, Sun X, Adv Sci 2018, 5, 1700426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA, Proc. Natl. Acad. Sci. U. S. A 2013, 110, 19902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Shirota H, Klinman DM, Expert Rev. Vaccines 2014, 13, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Kuai R, Sun X, Yuan W, Ochyl LJ, Xu Y, Hassani Najafabadi A, Scheetz L, Yu MZ, Balwani I, Schwendeman A, Moon JJ, J Control Release 2018, 282, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Del Giudice G, Rappuoli R, Didierlaurent AM, Semin Immunol 2018, 39, 14. [DOI] [PubMed] [Google Scholar]
- [180].Mohan T, Zhu W, Wang Y, Wang BZ, Immunobiology 2018, 223, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ, Science 2013, 341, 1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Gutjahr A, Papagno L, Nicoli F, Kanuma T, Kuse N, Cabral-Piccin MP, Rochereau N, Gostick E, Lioux T, Perouzel E, Price DA, Takiguchi M, Verrier B, Yamamoto T, Paul S, Appay V, JCI Insight 2019, 4, e125107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Ritchie C, Li L, Biochemistry 2020, 59, 1713. [DOI] [PubMed] [Google Scholar]
- [184].Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, Sun Z, Jiang S, Lu L, Wu MX, Science 2020, 367, 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Herold S, Sander LE, Science 2020, 367, 852. [DOI] [PubMed] [Google Scholar]
- [186].Wang L, Wang X, Yang F, Liu Y, Meng L, Pang Y, Zhang M, Chen F, Pan C, Lin S, Nano Today 2021, 40, 101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A, Nat. Immunol 2005, 6, 769. [DOI] [PMC free article] [PubMed] [Google Scholar]