Abstract
Nonhuman terrestrial mammals sniff themselves and each other to decide who is friend or foe. Humans also sniff themselves and each other, but the function of this is unknown. Because humans seek friends who are similar to themselves, we hypothesized that humans may smell themselves and others to subconsciously estimate body odor similarity, which, in turn, may promote friendship. To test this, we recruited nonromantic same-sex friend dyads and harvested their body odor. We found that objective ratings obtained with an electronic nose, and subjective ratings obtained from independent human smellers converged to suggest that friends smell more similar to each other than random dyads. Last, we recruited complete strangers, smelled them with an electronic nose, and engaged them in nonverbal same-sex dyadic interactions. We observed that dyads who smelled more similar had more positive dyadic interactions. In other words, we could predict social bonding with an electronic nose. We conclude that there is indeed chemistry in social chemistry.
An electronic nose can predict whether two strangers will have a positive social interaction.
INTRODUCTION
Human dyadic same-sex nonromantic friendships are a critical pillar of psychological health (1, 2). Such friendships can develop slowly over time, but occasionally, in so-called click friendships, a strong sense of bonding can form almost instantaneously (3). Because similarity within a dyad is a strong positive predictor of friendship (4–8), which acts at the very early phase of interaction (7), one may assume that similarity also plays a role in forming such click friendships. Some of the known similarities that predict friendship are expected, such as age, race, education, religion, and, indeed, physical appearance (9, 10). There are also more complex similarities such as personality (11, 12) and values (13) and even in measures such as patterns of neural activity (14, 15) and genetic makeup (16–19).
Nonhuman terrestrial mammals constantly sniff themselves and each other and, based on this, decide who is friend or foe (20). Humans also constantly sniff themselves (21, 22) and each other (22–25). Similarity in the sniffed body odor can infer kinship with self (26, 27) or between strangers (26, 28). Moreover, growing evidence implies that humans can infer from body odor alone emotional states in conspecifics, ranging from fear (29) to happiness (30). Given that a friend’s body odor and one’s own body odor induce similar patterns of brain activity, yet exposure to a stranger’s body odor induces a very different limbic fear–type brain response (31), we hypothesized that similarity in body odor may contribute to rapid friendship formation. To test this, we first asked whether click friends indeed smell alike. After recruiting same-sex nonromantic click friends and harvesting their body odor, we found that both an analytical device (an electronic nose) and independent human smellers converged to rate the body odors of click friends as more similar than those of random dyads. Moreover, we next used the electronic nose (eNose) to predict social interactions between strangers. We found that strangers whose body odor was more similar were more prone to later positive dyadic social interaction. Thus, we conclude that similarity in human body odor is related to a mechanism involved in friendship formation.
RESULTS
Defining click friendships
We hypothesized that if body odor similarity plays a role in friendship formation, then this should be particularly pronounced in click friendships, where a sense of friendship was formed before extensive biographical information was exchanged (3). Although clicking is indeed a term used in the context of friendship, we are unaware of a formal definition for it in the literature. To define click friendship, in study 1, 235 participants (135 women, aged between 20 and 43 years, M = 26.35 ± 4.166) were asked to define what click friendship is in their own words. Only 10 of 235 participants said they did not know what click friendship is. That 225 of 235 participants had a clear notion of what we were asking about further supports that click friendship is a real social event, despite the lack of formal definition. Moreover, we observed high consistency across individuals, whereby the 225 participants spontaneously converged to use only 42 broadly different statements to define click friendship (the specific wording varied), such as “friendship that is formed immediately when meeting,” “matching,” “good friendship that is rapidly formed,” and “chemistry.” These statements and their frequency of application are shown in table S1, and we used the top 20 descriptors as our definition of click friendships.
The body odors of click friends are more similar than expected by chance
We conducted a 6-month-long social media–centered recruitment effort in search of friends who mutually described their initial encounter as a click. After phone interviews and questionnaires, this culminated in 20 same-sex nonromantic click friend dyads who met the strict participation criteria (10 male and 10 female dyads, aged between 22 and 39 years, M ± SD = 24.757 ± 3.388, mean friendship duration = 6.185 ± 5.793 years), who were from all over Israel. These dyads mutually self-reported that their friendship began as a click friendship, and each affirmed all 20 top criteria of table S1. These participants donated body odor using a strict body odor donation protocol (see Materials and Methods). To ask whether there is similarity in the body odor chemical fingerprint across members of click dyads, in study 2, we first sampled all body odors with an eNose (Fig. 1A). This particular eNose (PEN3, Airsense Analytics, Schwerin, Germany) has 10 metal oxide sensors, each coated with a different material conferring chemical specificity. Thus, each sample is potentially made of 10 responses that combine to generate a specific pattern associated with an odor. We observed that only five of the sensor subtypes responded to body odors (sensor nos. 2, 6, 7, 8, and 10). We thus represented each of the 40 body odors as a five-dimensional vector (fig. S1; all study 2 raw eNose results are available in data file S1). We then calculated the Euclidean distance between the two body odors from each dyad within the five-dimensional space. We observed that mean Euclidean distance between click friends was 5.075 ± 4.947 arbitrary units (AU) within the five-dimensional space. In turn, we used the same 40 individuals and randomly shuffled the participant’s ID numbers 10,000 times, generating 20 same-sex random dyads in each iteration and thus obtaining a distribution of the mean Euclidean distances between 20 random dyads. We observed that mean Euclidean distance between such random same-sex dyads was 6.535 ± 0.55 AU (note that click dyads’ SD is for 20 Euclidean distances, yet random dyads’ SD is for 20 distances, 10,000 times). Using a permutation test, we find that these values are significantly different (mean clicks = 5.075 ± 4.947, mean random dyads = 6.535 ± 0.55, permuted P = 0.006, Cohen’s d = 0.415), or in other words, the chemical signature from body odors of click friends is significantly more similar than the chemical signature from body odors of random dyads (Fig. 1B) (alternatives to Euclidean distance are explored and shown in fig. S2).
Fig. 1. The body odors of click friends are more similar than expected by chance.
(A) A PEN3 eNose was used to measure the volatiles that accumulated above a T-shirt in a jar (i.e., to measure the jar headspace). Photo credit: Inbal Ravreby, Weizmann Institute of Science. (B) Histogram showing 10,000 iterations of the average Euclidean distance (ED) between 20 same-sex random dyads in the eNose space. The distance between click friends is denoted by the red dashed line. (C) Histogram showing 10,000 iterations of the average ED between 20 same-sex random dyads in perceptual rating space. The distance between click friends is denoted by the red dashed line. (D) Pearson correlation between the difference in perceptual ratings and triangle test accuracy. The black line is the linear regression, and the gray area marks the confidence interval (CI) of the regression line. Each point is a click dyad (n = 20). (E) Histogram showing 10,000 iterations of the average difference in sniff duration when sniffing 20 same-sex random dyads. The distance between click friends is denoted by the red dashed line. (F) Perceived perceptual odor similarity for click dyads (x axis) versus random dyads (y axis). Each point is a rater (n = 25), and the point reflects the average of their 40 ratings. The diagonal line reflects the unit slope line (x = y), such that if points accumulate under the line, then the values are greater for click dyad similarity, and if they accumulate above the line, then the values are greater for random dyad similarity. The associated bar graph is the average perceived similarity. (G) Identical to (F) but for visual rather than olfactory similarity data.
Chemical similarity inferred by eNose does not imply human perceived olfactory similarity. To ask whether the eNose results are mirrored in human perception, in study 3, we recruited 24 smellers (13 females, aged between 22 and 39 years, M ± SD = 27 ± 4.63). We designed the perceptual study such that it would tap both explicit and implicit classifications. To probe explicit classification, we used a modified triangle test. On each trial, the participants were presented with a body odor triplet, where two odorants were from members of a click dyad, and the third distractor odorant was from an unrelated same-sex body odor donor. Participants were asked to select the odorant outlier. Each participant completed 20 trials (intertrial interval = 25 s), one for each click dyad. We note that this is a variation of the triangle test, as in a traditional triangle test two odorants are identical, yet here, two odorants are at best more similar to each other. In an ensuing task, participants saw two face-torso photographs of a click dyad, and the third distractor face-torso photograph belonged to an unrelated same-sex body odor donor. Participants were asked to select the outlier photograph. Next, to probe implicit classification, participants smelled the 40 click friend body odors one by one, randomly ordered, and rated them using visual analog scales (VASs) for pleasantness, intensity, sexual attraction, competence, and warmth (temperament). Whereas the former two descriptors were used because they reflect the primary dimensions of odor (32), the latter two descriptors were used because they reflect primary dimensions in social interaction (33). Last, given that sniffing patterns provide an added implicit measure of olfactory perception (34), throughout tasks, participants wore a nasal cannula linked to a spirometer, providing a precise measure of nasal airflow (all study 3 raw data are available in data file S2).
Significant classification is typically attributed to a d prime (d′) score of “1” or higher (35, 36), and in the triangle test where chance = 33.33%, d′ = 1 is at 41.8% accuracy (19, 20). We observe that, overall, for the group of click dyads, mean accuracy was 35.91 ± 14.54% [t(19) = 0.894, P = 0.382, Cohen’s d = 0.177], reflecting a mean d′ score of 0.693 ± 0.69 [one-sample two-tailed t test of d′ values against d′ = 1: t(19) = 1.99, P = 0.061, Cohen’s d = 0.445]. In other words, at the group level, participants failed to explicitly classify click dyads based on body odor. Similarly, despite a trend, participants failed to explicitly classify click dyads based on photographs in a triangle test [mean accuracy = 42.29 ± 20.6%, t(19) = 2.017, P = 0.058, Cohen’s d = 0.435, reflecting a mean d′ score of 1.131 ± 0.979, t(19) = 0.598, P = 0.57, Cohen’s d = 0.134]. This vision-based null result is in contrast to previous findings (10).
To examine implicit perceived body odor similarity, we compared the similarity within click dyads versus random dyads in the five-dimensional VAS space. This was done by comparing the average distance between the 20 click dyads to the probability distribution of the average distance between 20 same-sex random dyads, 10,000 times. We found that the Euclidean distances were significantly lower between click dyads compared to random dyads (mean clicks = 0.233 ± 0.153, mean random = 0.277 ± 0.019, permuted P = 0.019, Cohen’s d = 0.40) (Fig. 1C). To ask whether this effect was carried by any particular descriptor, we repeated the analysis for each descriptor alone [false discovery rate (FDR) corrected]. We found that click dyads were rated as significantly more similar than random dyads in body odor attractiveness (mean clicking = 0.096 ± 0.106 AU, mean random = 0.136 ± 0.013 AU, permuted P = 0.009, Benjamini-Hochberg P = 0.045, Cohen’s d = 0.45) (fig. S3). Moreover, despite no overall group effect in the previous triangle test, we observed that the better a given click dyad was explicitly classified in the triangle test, the more similar their body odors were in the implicit rating study (Pearson r = −0.785 and P < 0.001) (Fig. 1D). Last, given that sniff duration is modulated in accordance with odorant content (37), we compared sniff duration across samples in the implicit test. We observed significantly greater similarity in sniff duration when sniffing members of a click dyad versus sniffing random dyads (mean clicks’ duration difference = 0.171 ± 0.173 s, mean random = 0.222 ± 0.023, permuted P = 0.024, Cohen’s d = 0.413) (Fig. 1E), i.e., the smellers exhibited smaller difference in their sniff durations for click dyads relative to the difference in sniff duration for random dyads.
Whereas the above implicit measures suggested that click friends indeed smell alike, the previous explicit triangle test did not. This triangle test, however, entails an inherent memory component that may complicate the comparison of body odors. As noted, this is particularly the case in the current iteration, as the two intended “same” body odors in the triangle test were from two different people (the click friends), so the cognitive load of this test entailed three odorants. To address this, in study 4, we conducted a different explicit test, where now 25 participants (19 females, aged between 21 and 38 years, M = 25.76 ± 4.075) explicitly rated the perceptual similarity of pairs of body odors. Each participant rated the perceptual similarity of 40 dyads, 20 click dyads, and 20 random same-sex dyads, along a VAS ranging from similar to different. An additional 25 participants (13 females, aged between 21 and 37 years, M = 26.56 ± 5.091) conducted a similar study using face-torso photographs rather than body odors (all study 4 raw data are available in data file 3). We observed that the body odors of click friends were significantly more explicitly similar to each other than the body odors of random dyads [mean clicks = 0.483 ± 0.097, mean random = 0.442 ± 0.105, two-tailed paired t test: t(24) = 2.206, P = 0.037, Cohen’s d = 0.443] (Fig. 1F and figs. S4A and S5). Consistent with previous reports (10), we also observed remarkably greater visual similarity between click friends using this paradigm [mean click = 0.447 ± 0.116 VAS units, mean random = 0.351 ± 0.11 VAS units, two-tailed paired t test: t(24) = 4.797, P < 0.001, Cohen’s d = 0.965] (Fig. 1G and figs. S3B and S6). In other words, unlike the triangle test, this explicit test converged with the implicit measures to suggest that click dyads smell alike, beyond random dyads.
Both eNose similarity and human perceptual similarity were higher for click friends versus random dyads. This outcome may imply that either the eNose provides a good reflection of human perception or, in turn, that each measurement type, eNose and perception, captured a different portion of the chemical variance underlying this link. To address these alternatives, we asked whether eNose-derived similarity was correlated with perceptual similarity across the 20 dyads. We observed no sign of such correlation (Pearson r = −0.16 and P = 0.497, and after removing an outlier, r = −0.29 and P = 0.231) (fig. S7), implying that the chemical cues used by the eNose were likely not those used by human raters. We note that given the many thousands of molecules present in body odor (38), that the human nose and a machine ended up using different cues is not entirely unexpected.
An eNose can predict social interaction
The above results suggest that click friends have greater similarity in body odor chemistry and in body odor perceived smell in comparison to random dyads. There are at least three alternative explanations for this: First, consistent with our hypothesis, this similarity may be related to the root causes of friendship. Second, and alternatively, this similarity may somehow be a consequence of long-term friendship, following common body odor–shaping experiences, e.g., living in the same area, eating together, etc. Last, we acknowledge that this similarity may be related to some independent unknown factor and that this same unknown factor may, in turn, be driving friendship. To disentangle the two initial alternatives, in study 5, we tested whether similarity in body odor as determined by eNose can predict the quality of social interaction between complete strangers. We recruited 17 strangers (10 females, ages between 20 and 37 years, M = 26.2 ± 4.56) and collected their body odors as before. To force nonverbal dyadic interaction, we used the Mirror Game (39). In this paradigm, two participants stand facing each other 50 cm apart (i.e., a close distance allowing body odor exposure) and, for 2 min, try to mirror each other’s hand motion (Fig. 2A). Participants were not allowed to talk throughout the study. This paradigm provides for several potential measures on the quality of dyadic interaction. First, using motion energy analysis (MEA) (40), we can calculate the extent or accuracy in mirroring. Second, after each 2-min interaction, participants rated their partner using a continuous version of the “inclusion of other in the self” (IOS) scale (41), where participants place two on-screen circles to graphically represent the quality of “overlap” with their partner. Third, the participants rated the quality of interaction with their partner along 12 VASs that were chosen following the definition of click friendship (table S1) and relevant to the Mirror Game (Fig. 3). Fourth, participants were requested to indicate whether they clicked with their partner or not (this binary indication is of course not taken to imply that they here became genuine click friends). We conducted two sessions of a within-sex round-robin design such that each participant played with each of the other same-sex participants, providing for 66 unique dyads, 21 males and 45 females (all study 5 raw data are available in data file S4). eNose analyses were performed after completion of the study, rendering all interactions double blind. We calculated as before the eNose-derived chemical similarity between all 66 possible same-sex dyads.
Fig. 2. Body odor similarity is related to clicking in the Mirror Game.
(A) A dyad playing the Mirror Game (a frame from a video recording taken by the hidden alarm clock camera that was on the table during the experiment). (B) Histogram showing 10,000 iterations of the average ED between 22 same-sex nonmutual clicking dyads who played the Mirror Game, represented in the eNose space. The distance between the 22 dyads who reported mutual clicking in the game is denoted by the red dashed line. (C) eNose-derived ED between all male dyads (n = 21) who played the Mirror Game. Dyads who reported a mutual click are outlined in black. (D) eNose-derived ED between all female dyads (n = 45) who played the Mirror Game. Dyads who reported a mutual click are outlined in black.
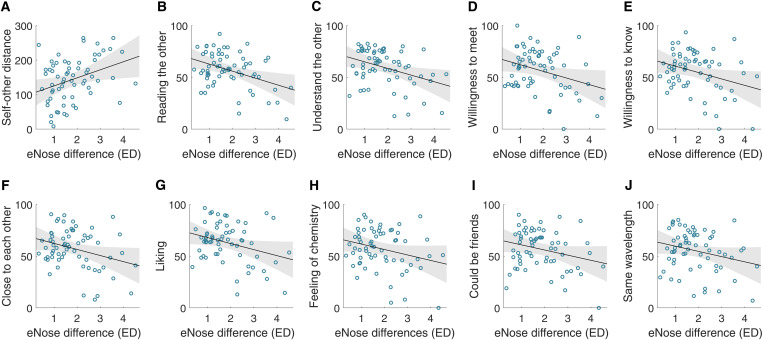
Fig. 3. Body odor similarity is related to the quality of interaction in strangers.
Each panel is the Pearson correlation between the chemical differences in a dyad as determined by eNose versus 1 of the 13 measures of social interaction. Each blue circle is 1 of the 66 dyads who played the Mirror Game. The black line is the linear regression line, and the gray area marks the CI of the regression. The 13 measures are as follows: (A) including the other in the self as was measured in the IOS, (B) reading the partner’s mind, (C) understanding the partner, (D) willingness to meet again with the partner, (E) willingness to get to know the partner, (F) feeling close to the partner, (G) liking the partner, (H) feeling chemistry with the partner, (I) thinking that they could be good friends, and (J) feeling on the same wavelength.
We observe that 22 dyads (8 males and 14 females) reported a mutual click (this self-report had some reflection in motion synchronization; fig. S8). We compared the eNose distance between these 22 dyads to the eNose distance between 10,000 random selections of 22 (of the 44) same-sex nonmutual clicking dyads (8 males and 14 females) and observed that dyads who reported clicking were significantly more chemically similar than dyads who did not report clicking (mean clicking = 1.592 ± 0.803 AU, mean random = 2.003 ± 0.1559 AU, permuted P = 0.003, Cohen’s d = 0.711) (Fig. 2B and fig. S9). This was expressed in reduced eNose distance between mutual click dyads (Fig. 2, C and D). Moreover, we observed that the eNose-derived chemical similarity between dyad members was significantly correlated (FDR corrected) with IOS scores, as well as with 9 of the remaining 12 measures of interaction provided by participants, which were averaged within dyad to obtain dyadic scores (these 9 measures were also significantly correlated with each other; table S2) (42–44). Because each participant was present in multiple dyads, we also used a permutation test to compare the correlation coefficients to chance (also FDR corrected). For this, each eNose vector was randomly shuffled across the same-sex participants, and then we correlated the permuted eNose-derived chemical similarity between the dyads with each of the subjective ratings. This was done 10,000 times to obtain a null distribution and assess the significance level (Table 1, Fig. 3, and fig. S10). In turn, we did not observe a link between eNose-derived chemical similarity and the quality of mirroring as estimated by MEA, whether applied to the entire interaction or to the difference between the beginning and the end of the Mirror Game (fig. S11).
Table 1. Pearson correlations between the 13 measures for quality of interaction and body odor chemical similarity as derived by the eNose.
Column 1 is the quality of interaction measure. Column 2 is the Pearson correlation. Column 3 is the P value of the Pearson correlation. Column 4 is the FDR-corrected P value. Column 5 is the P value obtained in a permutation test to account for the fact that each participant was present in multiple dyads. Column 6 is the FDR-corrected P value of the permutation tests.
| Quality of interaction measure | r | P | Benjamini-Hochberg P | Permuted P |
Benjamini-Hochberg
permuted P |
| IOS | 0.35 | 0.005 | 0.02 | 0.003 | 0.010 |
| Reading the partner’s mind | −0.40 | 0.001 | 0.013 | 0.0005 | 0.003 |
| Understanding the partner | −0.36 | 0.003 | 0.018 | 0.0003 | 0.003 |
| Willingness to meet again with the partner | −0.32 | 0.009 | 0.025 | 0.004 | 0.010 |
| Willingness to get to know the partner | −0.32 | 0.011 | 0.025 | 0.005 | 0.011 |
| Feeling close to the partner | −0.31 | 0.012 | 0.025 | 0.004 | 0.010 |
| Liking the partner | −0.31 | 0.014 | 0.025 | 0.006 | 0.011 |
| Feeling chemistry with the partner | −0.27 | 0.032 | 0.047 | 0.011 | 0.018 |
| Thinking that they could be good friends | −0.26 | 0.033 | 0.047 | 0.017 | 0.022 |
| Feeling on the same wavelength | −0.26 | 0.036 | 0.047 | 0.015 | 0.022 |
| Feeling as if they already knew the partner | −0.21 | 0.099 | 0.116 | 0.063 | 0.074 |
| Feeling comfortable to share personal issues with the partner |
−0.20 | 0.11 | 0.122 | 0.095 | 0.095 |
| Feeling that the partner was friendly | −0.15 | 0.25 | 0.25 | 0.074 | 0.080 |
In combination, the above results imply that the more chemically similar the dyad body odors were, the better they interacted (by most measures other than motion energy). Given these relationships, we asked whether a classifier could use eNose data to predict social interaction. Using a weighted K nearest neighbors (KNN) classifier, we contrasted the 22 dyads who mutually reported clicking, with the other 44 dyads (13 males and 31 females) who either mutually reported not clicking (19 dyads) or had one-sided click (25 dyads). A leave-one-out cross-validation gave rise to a meaningful receiver operator curve (ROC) (Fig. 4). The area under the curve was 0.67, which is significantly different from chance (U = 2.811 and P = 0.005). This reflects a cross-validation accuracy of 71.21% (binomial P < 0.001 and Cohen’s g = 21.21%), permitting correct identification in 17 of 22 mutual click reports and 30 of 44 reports of no mutual click (77.27% sensitivity and 68.18% specificity).
Fig. 4. Classifying mutual click dyads by eNose-derived body odor similarity.

An ROC classifying dyads who mutually clicked (n = 22, 14 females) or did not mutually click (n = 44, 31 females). The blue dot marks the performance of the leave-one-out cross-validation weighted KNN classifier. The dashed black line represents chance performance.
Last, as to the third alternative explanation for our results, namely, that similarity in body odor is related to some independent unknown factor and that this same unknown factor may, in turn, be driving friendship, we cannot rule this out, as we cannot test everything. However, from the measures we obtained, we note that chemical similarity between click dyads, obtained both in study 2 and study 5, is not explained by race, country of birth, mother tongue, values, level of education, marital status, smoking status, caffeine consumption, chronic health issues, dominant hand, profession, and glasses; and in women, usage of contraceptive pill, regular or irregular periods, and the day of the menstrual cycle (figs. S12 and S13). We note that only similarity in age was significantly positively correlated with similarity in eNose distance in experiment 2, yet it was marginally negatively correlated in experiment 5 (figs. S12 and S13). In other words, unlike our main result that was consistent across these experiments, this alternative explanation was not.
DISCUSSION
“We sometimes encounter people, even perfect strangers, who begin to interest us at first sight, somehow suddenly, all at once, before a word has been spoken.”
Fyodor Dostoevsky, Crime and Punishment, 1866
Here, we investigated an alternative hypothesis, namely, that perfect strangers may begin to interest us at first sniffs rather than at first sight alone. Across studies, our data converged to imply that the body odors of same-sex click friends are more similar to each other than the body odors of same-sex random dyads. General similarity within a dyad is indeed a strong positive predictor of friendship (4–8), which acts at the very early phase of interaction (7). Here, we add the observation that body odor similarity may also support rapid friendship formation.
Olfaction is a dominant sensory input underlying social interaction (20). This statement is largely acknowledged with respect to other terrestrial mammals, but it is often rejected with respect to humans. In humans, the role of olfaction has been denigrated (45) in part because of various social taboos (45), culminating in the view that olfaction is unimportant for human sociality (46). Recent evidence, however, implies a significant role for olfaction in human social interaction. Whereas in some cultures, odor processing is very explicit (47, 48), in Western culture, social olfaction is largely without conscious awareness. Humans are constantly but mostly subconsciously sniffing themselves (21) and their conspecifics (23). These odors then have a host of effects and may carry a host of information. For example, sniffing women’s body odors may coordinate women’s menstrual cycles (49) [although this effect remains debated (50)]. Sniffing women’s tears lowers testosterone in men (51, 52), which potentially alters behavior. Sniffing one particular molecule expressed in body odor (androstadienone) raises levels of cortisol in women (53), and sniffing a different particular molecule expressed in body odor (hexadecanal), blocks aggression in men but triggers aggression in women (54). Humans can infer a state of disease in body odor (55) and can smell aggression (56), fear (29), stress (57), depression (58), and happiness (30) in conspecifics. Body odor also serves as a cue for human kinship (26–28) and influences human mate choice (59). In the current study, we add to this by finding that humans may use olfactory information to guide preferences in same-sex nonromantic dyadic interactions. This notion is consistent with findings that a friend’s body odor and one’s own body odor induce similar patterns of brain activity, yet exposure to a stranger’s body odor induces a very different limbic fear–type brain response (31). Moreover, this notion dovetails with reports on impaired sociality in human congenital anosmia (60) and on altered social chemosignaling in autism spectrum disorder (61). In the current study, we did not directly probe for a brain mechanism underlying our observations. We can speculate, however, that in self-sampling (21), humans form a body odor template of themselves and then subconsciously compare to this template. Previous body odor studies indeed support the idea that self-referent processing may mediate body odor identification in humans (62, 63), as it does in other primates (64, 65).
This study has several limitations we would like to acknowledge. First, as previously noted, whereas our hypothesis and results support the notion that body odor similarity promotes friendship, an alternative is that some unidentified factor independently promotes similarity in both body odor and friendship independently. We conducted any analysis we could to address this alternative in our data (figs. S12 and S13) and failed to identify any such independent factor. As noted, age emerged as a possibility in experiment 2 but was negated in experiment 5. Nevertheless, the possibility that some unknown factor underlies the relationships observed in this study remains, and age remains a candidate. Second, we uncovered these effects in click friends. Whether this pattern is unique to click friendships, or is similarly evident in friendships that did not begin with a click, remains to be tested. Third, we acknowledge the two null results obtained in this study: In the first, perceived body odor similarity was not different across click and random dyads in the triangle test of study 3. We think that the odor memory–related difficulty of this task underlies this null result, and we can add that visual similarity, despite it being a well-known friendship similarity cue (9), was also not related to friendship when using the triangle paradigm. Despite this mitigating factor, this null result, combined with our reliance on permutation techniques, implies limited effect magnitude. The second null result was in the MEA of the Mirror Game in study 5. This would have provided a valuable objective measure in this task, and in its absence, we rely on subjective measures alone to analyze the Mirror Game results. This in contrast to study 3, for example, where sniff patterns provided for an objective similarity measure to bolster subjective perception. Also, although not a limitation per se, we would like to emphasize that the chemical similarity that was inferred by eNose was unrelated to the perceptual similarity inferred by human raters (fig. S7). In other words, these measures captured independent sources of chemical variance, and this keeps us further away from identifying what components of body odor ultimately contribute to the observed effects. Although the eNose is a convenient tool, its information is limited and hard to generalize. For example, although eNose similarity was related to the quality of dyadic interaction in two separate studies, we observe that the absolute eNose values were very different across these two studies that were conducted more than a year apart. Thus, the eNose can report on chemical similarity within a study but remains largely uninformative beyond this. We stress that this is not a limitation of this study alone or this eNose alone. Olfactory perceptual space and eNose space are not correlated. In other words, eNose signal similarity or dissimilarity does not infer perceptual similarity or dissimilarity. Solving this limitation will allow digitization of olfaction, a highly sought goal in science and technology. Last, several of our limitations may have been addressed with added experiments, yet these are currently limited by coronavirus disease 2019. The data of this manuscript were collected before the pandemic. Currently, one cannot conduct experiments that bring unmasked strangers into close interaction. Moreover, we speculate that social chemosignaling behavior may have shifted during the pandemic. Whereas previously individuals may have subconsciously sniffed others (23), now, people consciously avoid inhaling when in close proximity to strangers. Thus, we speculate a genuine shift in this behavior, yet given the significant role we attribute to chemosignaling in human sociality, we further speculate a return of these behaviors in the long run.
Despite all these limitations, this study also has specific strengths and, primarily, the recurrence of the link between body odor similarity and quality of same-sex dyadic interaction across multiple studies and study designs. This link was particularly convincing in the predictive eNose model applied to the round-robin interaction between strangers in study 5. One may ask, however, whether these effects play any role in life outside a laboratory experiment. Our round-robin study diverged from natural conditions in that participants were not allowed to speak with each other. In natural behavior, humans use complex language to interact, and it is in this that we are indeed most different from other terrestrial mammals in our social interactions. Thus, the significance of body odor similarity in our paradigm may have been greater than it is in everyday behavior. Nevertheless, we think our results imply that we may also be more like other terrestrial mammals in this respect than we typically appreciate. This message is important, because beyond a deeper understanding of human behavior, it may point toward novel olfaction-based paths to intervention in social impairment. We conclude in reiterating that several studies converged to suggest that human same-sex nonromantic click friends smell more similar to each other than expected by chance and that complete strangers who smell more similar to each other as determined by an eNose have better dyadic interactions. Thus, there is indeed chemistry in social chemistry.
MATERIALS AND METHODS
Participants
All participants provided written informed consent to procedures approved by the Wolfson Hospital Helsinki Committee (protocol reference number 0035-16-WOMC). All participants were screened for self-reported lack of nasal congestion or olfactory dysfunction and then participated for monetary reward. Distribution of participants across studies was as follows.
Study 1—Define click friendship
We recruited 235 participants online, 135 females and 100 males aged between 20 and 42 years (M = 26.35 ± 4.166).
Study 2—Harvesting click dyads’ body odor
To find click friends, we posted extensively on campus billboards and on social media. Respondents were first phone-interviewed and then interviewed by questionnaire to verify that they satisfied click friend criteria. This recruitment effort lasted 6 months and entailed collecting body odors from participants across the entire country. In total, we harvested body odor from 20 pairs of same-sex close friends (half males and half females) who reported that their friendship began as a click friendships, aged between 22 and 39 years (M = 24.757 ± 3.388 and mean friendship duration = 6.185 ± 5.793 years).
Study 3—Triangle test and ratings
We recruited 24 naive participants, 13 females, aged between 22 and 39 years (M = 27 ± 4.63)
Study 4—Explicit similarity ratings
We recruited for olfactory similarity ratings 25 participants, 19 females, aged between 21 and 38 years (M = 25.76 ± 4.075). For visual similarity ratings, we recruited another 25 participants, 13 females, aged between 21 and 37 years (M = 26.56 ± 5.091).
Study 5—The Mirror Game
We recruited a total of 17 naive healthy participants that did not know each other personally, 10 females, aged between 20 and 37 years (M = 26.2 ± 4.56). No prior acquaintance between dyads was verified through detailed questionnaires where it was uncovered that 2 of the 21 male dyads and 5 of the 45 female dyads had attended common undergraduate large-scale courses, but they had no personal interaction and proclaimed not to know each other.
Paradigms
Study 1—Define click friendship
The participants were asked to define click friendship in their own words (in Hebrew). Ten participants responded that they cannot, retaining 225 respondents.
Study 2—Harvesting body odor from click dyads
Donors were provided with nonperfumed soap to shower with each evening before wearing provided 100% cotton T-shirts to wear on two consecutive nights. Donors were instructed to use only the items provided to them and avoid other soaps and the use of lotions, deodorants, antiperspirants, perfumes, colognes, etc. They were instructed to wear the shirts at least 6 hours each night and prevent other humans or pets from sleeping on, or using, the bed during the testing period. Moreover, they were asked to avoid foods that strongly influence body odor such as curry, amchoor, fenugreek, asparagus, and garlic. In the morning after the first night, donors placed their shirts in a plastic zip-lock bag to prevent absorption of other odors and to keep the participants’ body odor in the shirts. After the second night, the donors were instructed to store the bagged shirts in the freezer to minimize loss of odor. The T-shirts were collected (typically on the same day) and stored in laboratory at −20°C in designated glass jars. Each donor also completed a general questionnaire and Schwartz’s value survey (66).
Study 2—eNose similarity test
To examine whether there is chemical similarity between click dyads’ body odor, we used a PEN3 eNose (Airsense Analytics GmbH, Schwerin, Germany). The PEN3 is a compact (92 mm by 190 mm by 255 mm) lightweight (2.3 kg) device, consisting of a gas sampling unit and a sensor array. The sensor array is composed of 10 different thermoregulated metal oxide sensors, positioned in a stainless-steel chamber (volume, 1.8 ml; temperature, 110°C). Each sensor is uniquely coated, rendering it particularly sensitive to a restricted class of chemical compounds. When a compound interacts with the sensor, this results in an oxygen exchange that leads to a change in electrical conductivity (67). We used the PEN3 with its native sampling software (WinMuster) and the following settings: chamber flow = 400 ml/min, flush time = 100 s, zero-point trim time = 10s, and measurement time = 80s. T-shirts were first thawed for 1 hour at room temperature. Then, to measure the headspace, we covered each jar with parafilm sheet and waited for another 1 hour. Next, we used the eNose to measure the headspace of each jar at room temperature (see Fig. 1A).
Study 3—Triangle test
The 40 T-shirts of the click dyads were thawed at room temperature 1 hour before the smelling study. We used a previously described shirt sniffing device (SSD) (68) to standardize body odor sampling. The SSD consists of a glass jar containing the T-shirt, with an air intake port via soda lime filter and air sampling port via one-way flap valve into individual-use airtight nose mask (68). Using the SSD assured that environmental odors and/or other participant odors did not contaminate the sample. To probe explicit classification, we used a triangle test. On each trial, the participants were presented with a body odor triplet: Two odorants were from a click dyad, and the third distractor odorant was from an unrelated same-sex body odor donor. Participants were asked to select the odorant outlier. Each participant completed 20 trials (intertrial interval = 25 s), one for each click dyad. The triangle smell test was followed by a control triangle visual test, in which participants were asked to select the outlier picture, according to pictures of the same three people, randomly ordered. To match the odor test, in which the odors in each triplet were smelled one after another, the pictures were presented one by one rather than simultaneously side by side. During sampling, we measured nasal airflow using a nasal cannula (1103, Teleflex Medical) placed at the nares and attached to a spirometer (spirometer FE141 ADInstruments). The nasal airflow was sampled at 1 kHz and recorded using a Power-Lab 16SP Monitoring System (ADInstruments, Australia). Airflow data were later displayed, stored, reduced, and analyzed using LabChart 7 software (ADInstruments).
Study 3—Descriptor ratings
Each participant smelled the 40 body odors one by one, randomly ordered, and rated them using VASs for “pleasantness,” “intensity,” “sexual attraction,” “competence,” and “warmth” (temperament). Nasal airflow was monitored throughout this task as before.
Study 4—Explicit similarity ratings
Each participant was presented with 40 dyads, consisting of 20 click friends and 20 random dyads. They rated the odors of each dyad using a VAS ranging from “different” to “similar.”
Study 5—The Mirror Game
The participants were asked to avoid use of perfume or deodorant and to avoid foods known to influence body odor (as detailed previously). The study took place in two different round-robin sessions, one for females and one for males. In each session, the participants were requested to split into pre-assigned dyads in each round. All pairs were tested simultaneously in separate identically arranged study rooms. In each round the dyads were instructed to stand facing each other, at a distance of 50 cm, which was marked on the floor. This minimal distance ensured exposure to conspecific volatiles. Then, the dyads were asked to play the full-body Mirror Game, in which they had to move their hands coordinately while keeping their legs at the starting point, with no designated leader or follower (Fig. 2A). The participants were not allowed to speak with each other during the entire study. In this way, the impression formation was not influenced by voice or by a conversation content but only by the nonverbal interaction. During the game, the participants were filmed using a hidden camera. Each game round lasted 2 min, and each participant played the Mirror Game with all the other same sex participants, culminating in 45 female dyads and 21 male dyads. After each round, the participants were asked to use an on-screen indicator where they freely moved circles that denoted themselves and their partners toward or away from each other, according to their feelings of “closeness” to and “overlapping” with their partner in the Mirror Game. This was used to estimate closeness in terms of self-other boundaries, an adaptation of IOS scale (41). The distance between the other and the self was calculated by extracting the distance (in pixels) between the centers of the two circles. In addition to the IOS scale, the participants were requested to indicate whether they had a “click” with their partner or not. In addition, each participant was asked to indicate on a VAS of 1 to 100 the following aspects regarding clicking: how much they read their partner’s mind, understood their partner, would like to meet again with their partner, wanted to know their partner, felt close to their partner, liked their partner, felt chemistry with their partner, thought that they could be good friends, felt on the same wavelength, had a feeling that they already knew their partner, felt comfortable to share personal issues with their partner, and felt that their partner was friendly toward them. In addition, each participant completed a general questionnaire and Schwartz’s value survey (66).
Study 5—Click dyad classification by eNose
We used a leave-one-out weighted KNN classifier to classify dyads who reported mutual click versus dyads who did not report mutual click. The classifier was applied to the difference between the five activated sensors (nos. 2, 6, 7, 8, and 10) of each member in a dyad. To have a consistent geometry, we kept the first component of the vector positive (i.e., multiply the vector by minus one if the first component was negative). We then used principal component analysis, keeping 95% of the variance, and thus reduced the five dimensions into two. We then classified the resulting data with the following parameters: The number of neighbors was 13, the distance metric was Euclidean distance, and the distance weight was squared inverse. To correct for the imbalanced dataset (22 dyads who mutually clicked versus 44 dyads who did not mutually click), we used cost-sensitive learning during the training. The cost was equal to the inverse of the proportion between mutual click dyads and no mutual click dyads.
Sample sizes
We collected the body odor of 20 click dyads (i.e., 40 participants) for two reasons. First, because of habituation, there is a limit to the number of dyads smellers could later smell in studies 3 and 4. We limit these studies to about 1 hour because of fatigue and introduce an interstimulus interval of at least 30 s. Thus, 20 separate dyads reflect an upper limit. Second, collecting this material was challenging. Participation hinged on agreeing to sleep separated from life partners for two consecutive nights (to prevent body odor contamination from partner) and to follow a strict 3-day diet and hygiene protocol. Moreover, both members of the dyad had to independently agree to these terms. Given these restrictions, it took a 6-month geographically unlimited effort to obtain 40 participants. For the human rater studies, the sample sizes were in accordance with previous studies that asked participants to rate body odors (23, 61, 69), taking into account that the body odors gradually evaporate when smelling them, and thus to have rating for the same (or almost the same body odors), the number of smellers is restricted. Following the small to medium effect sizes we obtained in studies 2 to 4, to choose the sample size for the Mirror Game (study 5), we used power analysis for Pearson correlation, assuming correlations of about 0.35 between the eNose-derived body odor similarity and the self-reports regarding the interaction quality. Correlation of r = 0.35 corresponds to a sample size of 62 dyads. Accordingly, we recruited participants allowing for 66 dyads in the round robin designs.
Statistics and inclusion/exclusion criteria
Data analyses software
All data analyses were performed using MATLAB R2018a and JASP (version 0.13.1.0).
Define click friendship
Statistics
The frequencies of the statements are reported.
Exclusion
Ten participants of the 235 participants (4.26%) did not know what “click friendship” means and thus were excluded.
Triangle test
Statistics
Statistics were calculated using one-sample two-tailed t test of the d′ scores against d′ = 1.
Exclusion
There were six trials of missing data (of 480 trials overall, i.e., 1.25%), in which the participants mistakenly inserted odor numbers that did not exist.
Descriptor ratings
Statistics
To account for individual differences in use of scales, each participant’s data were normalized by first subtracting the minimal value applied by the participant and then dividing by the maximal remaining value. This generated a normalized range between 0 and 1. To obtain a five-dimensional VASs ratings’ vector, we used the average rating in each descriptor for each body odor of the 20 click dyads and then calculated the Euclidean distances. We used the 40 individuals in the click dyads cohort to randomly generate 10,000 iterations of 20 nonclick same-sex dyads. In each iteration, we averaged the Euclidean distances of the 20 random dyads, to obtain a distribution of the mean Euclidean distances between 20 random dyads, 10,000 times. We then evaluated where the mean Euclidean distance of the 20 click dyads falls in the distribution. The click dyads’ mean and SD are the mean and SD of the 20 click dyads’ Euclidean distances, and the random dyads’ mean and SD are the mean and SD for the distribution of the mean Euclidean distances between 20 random dyads, 10,000 times. We adopted Cohen’s d to calculate the effect size based on the above means and SDs. We stress that this Cohen’s d value is assigned to the P value of the permutation test. It is not the effect size of the phenomena per se, as it is influenced by the low SD afforded by 10,000 repetitions.
Exclusion
One participant of the 24 dropped out mid-study.
Sniffing duration difference
Statistics
We used the 40 individuals in the click dyads cohort to randomly generate 10,000 iterations of 20 nonclick same-sex dyads. In each iteration, we averaged the difference in sniff duration for sniffing each of the 20 random dyads, to obtain a distribution of the mean sniff duration difference between 20 random dyads, 10,000 times. We then evaluated where the mean sniff duration difference for sniffing each of the 20 click dyads falls in the distribution.
Exclusion
One participant of the 24 dropped out mid-study. In three participants, movement of the nasal cannula prevented nasal airflow analysis.
Explicit similarity ratings
Statistics
The ratings between different and similar were first transformed to a scale between 0 and 100. Then, to account for individual differences in use of scales, we normalized the data and compared between the click dyads and random dyads the same way as we did for the five descriptor ratings.
Exclusion
There were no exclusions.
The Mirror Game
Statistics
We asked a naive judge to watch the videos of the Mirror Game one by one, to verify that participants indeed played as if they tried to mirror each other. To test whether the 22 dyads who mutually reported clicking with each other have a body odor that is chemically more similar than the body odor of the other 44 dyads, we compared the eNose distance between these 22 dyads to the eNose distance between 10,000 random selections of 22 (of the 44) same-sex nonmutual clicking dyads (8 males and 14 females). The click dyads’ mean and SD are the mean and SD of the 22 click dyads Euclidean distances, and the random dyads’ mean and SD are the mean and SD for the distribution of the mean Euclidean distances between 22 random dyads, 10,000 times. We used Cohen’s d to calculate the effect size based on the above means and SDs.
To get dyadic reports of the 13 self-reports, we averaged each self-report of the two partners in a same-sex dyad, as was done in previous experiments (42–44). We used a Pearson correlation to examine the relationship between various aspects of social interaction quality and the chemical body odor similarity, FDR corrected. Because each participant was present in multiple dyads, we also used a permutation test to compare the correlation coefficients to random chance (FDR corrected as well). For this, we applied a permutation test that shuffles the relationship between the eNose Euclidean distance and the subjective ratings for social interaction quality in each round-robin session. This was done 10,000 times, to obtain a null distribution of the r values. To assess the significance level, we compared the actual r value to the null distribution.
Exclusion
In the correlation analyses, the exclusion criteria threshold was 2.5 SD from the regression line (2.5 root mean square error). Accordingly, of the 66 dyads, two dyads (3%) were excluded from the IOS, one from reading the partner’s mind, one from understanding the partner, one from willingness to meet again, one from willingness to know the partner, one from feeling close to the partner, two from liking the partner, one from feeling that there is chemistry with the partner, one from thinking that they could be good friends, and one from feeling that the partner was friendly.
We filmed the games using a hidden camera and quantified each player’s motions using MEA (70). The motion energy was measured using frame-by-frame differences in pixel color between consecutive video frames. We performed video noise reduction using automatic detectors for time series of raw pixel change (40) and then z-scored the MEA signal of each participant to account for differences in the players’ height and hand size. To measure the synchronization level, we used the maximum cross-correlation of the z-scored MEA signals of each dyad. We performed two-sample t test to compare the overall synchronization of mutual click dyads compared to no mutual click dyads during the Mirror Game. Because each player participated in multiple dyads, there is dependency between some of the dyads. We therefore also applied a permutation test to assess the significance level. This was done by dividing the maximum cross-correlation values randomly into two groups, one of 22 dyads and the other of 44 dyads. We then performed two-sample t tests. To obtain a null distribution of the t values, this was done 10,000 times. Last, we assessed the significance level by comparing the actual t value to the null distribution. Furthermore, to test whether difference in synchronization during early time points and late time points is related to clicking, for each dyad, we first calculated the difference in the synchronization level, as measured by the maximum cross-correlation, during the 10 first and last seconds of the Mirror Game. We used two-sample t tests to compare the difference in the synchronization level in mutual click dyads and no mutual click dyads, followed by permutation tests.
eNose classification of click dyads
Statistics
We used a Mann-Whitney U test to examine whether the area under the ROC is significantly different from chance. A binomial test was used to examine whether the classification accuracy was different from chance. To estimate the success of the classifier, we also calculated sensitivity and specificity.
Exclusion
There were no exclusions.
Acknowledgments
We thank L. Rozenkrantz for insightful discussions.
Funding: This work was funded by an ISF grant (714103) awarded to N.S. I.R. is funded by the Ariane de Rothschild Women’s Doctoral Program.
Author contributions: Conceived the idea: I.R. Designed studies: I.R and N.S. Ran studies: I.R. Analyzed data: I.R., N.S., and K.S. Wrote the first draft: I.R. Edited the final draft: I.R., K.S., and N.S.
Competing Interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Data supporting the findings of this study are deposited in https://osf.io/9aeqh/?view_only=2e2a19c581e24a79a20d87fbfb1110da. The videos of the Mirror Game containing information that could compromise the privacy of research participants are not publicly available.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S13
Table S1 to S2
References
Other Supplementary Material for this manuscript includes the following:
Data files S1 to S6
REFERENCES AND NOTES
- 1.Winstead B. A., Derlega V. J., Benefits of same-sex friendships in a stressful situation. J. Soc. Clin. Psychol. 3, 378–384 (1985). [Google Scholar]
- 2.Demir M., Davidson I., Toward a better understanding of the relationship between friendship and happiness: Perceived responses to capitalization attempts, feelings of mattering, and satisfaction of basic psychological needs in same-sex best friendships as predictors of happiness. J. Happiness Stud. 14, 525–550 (2013). [Google Scholar]
- 3.S. Degges-White, C. Borzumato-Gainey, Friends Forever: How Girls and Women Forge Lasting Relationships (Rowman & Littlefield, 2011), p. 296. [Google Scholar]
- 4.Byrne D., Gouaux C., Griffitt W., Lamberth J., Murakawa N., Prasad M., Prasad A., Ramirez M., The ubiquitous relationship: Attitude similarity and attraction: A cross-cultural study. Hum. Relat. 24, 201–207 (1971). [Google Scholar]
- 5.Granovetter M. S., The strength of weak ties. Am. J. Sociol. 78, 1360–1380 (1973). [Google Scholar]
- 6.Huston T. L., Levinger G., Interpersonal attraction and relationships. Annu. Rev. Psychol. 29, 115–156 (1978). [DOI] [PubMed] [Google Scholar]
- 7.D. Krackhardt, The Strength of Strong Ties: The Importance of Philos in Organizations, in Networks in the Knowledge Economy (Oxford University Press, 2003); 10.1093/oso/9780195159509.001.0001/isbn-9780195159509-book-part-8. [DOI]
- 8.Zeggelink E., Evolving friendship networks: An individual-oriented approach implementing similarity. Soc. Netw. 17, 83–110 (1995). [Google Scholar]
- 9.DeBruine L. M., Facial resemblance increases the attractiveness of same–sex faces more than other–sex faces. Proc. R. Soc. Lond. B. 271, 2085–2090 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hehman E., Flake J. K., Freeman J. B., The faces of group members share physical resemblance. Pers. Soc. Psychol. Bull. 44, 3–15 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Berkowitz W. R., Perceived height, personality, and friendship choice. Psychol. Rep. 24, 373–374 (1969). [DOI] [PubMed] [Google Scholar]
- 12.Goodreau S. M., Kitts J. A., Morris M., Birds of a feather, or friend of a friend? Using exponential random graph models to investigate adolescent social networks. Proc. Natl. Acad. Sci. U.S.A. 46, 103–125 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPherson M., Smith-Lovin L., Cook J. M., Birds of a feather: Homophily in social networks. Annu. Rev. Sociol. 27, 415–444 (2001). [Google Scholar]
- 14.Parkinson C., Kleinbaum A. M., Wheatley T., Similar neural responses predict friendship. Nat. Commun. 9, 332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyon R., Youm Y., Kim J., Chey J., Kwak S., Parkinson C., Similarity in functional brain connectivity at rest predicts interpersonal closeness in the social network of an entire village. Proc. Natl. Acad. Sci. U.S.A. 117, 33149–33160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christakis N. A., Fowler J. H., Friendship and natural selection. Proc. Natl. Acad. Sci. U.S.A. 111, 10796–10801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domingue B. W., Belsky D. W., Fletcher J. M., Conley D., Boardman J. D., Harris K. M., The social genome of friends and schoolmates in the National Longitudinal Study of Adolescent to Adult Health. Proc. Natl. Acad. Sci. U.S.A. 115, 702–707 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler J. H., Settle J. E., Christakis N. A., Correlated genotypes in friendship networks. Proc. Natl. Acad. Sci. U.S.A. 108, 1993–1997 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philippe Rushton J., Genetic similarity in male friendships. Ethol. Sociobiol. 10, 361–373 (1989). [Google Scholar]
- 20.Eisenberg J. F., Kleiman D. G., Olfactory communication in mammals. Annu. Rev. Ecol. Evol. Syst. 3, 1–32 (1972). [Google Scholar]
- 21.Perl O., Mishor E., Ravia A., Ravreby I., Sobel N., Are humans constantly but subconsciously smelling themselves? Philos. Trans. R. Soc. Lond. B. Biol. Sci. 375, 20190372 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts S. C., Havlíček J., Schaal B., Human olfactory communication: Current challenges and future prospects. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 375, 20190258 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frumin I., Perl O., Endevelt-Shapira Y., Eisen A., Eshel N., Heller I., Shemesh M., Ravia A., Sela L., Arzi A., Sobel N., A social chemosignaling function for human handshaking. eLife 4, e05154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schäfer L., Sorokowska A., Sauter J., Schmidt A. H., Croy I., Body odours as a chemosignal in the mother–child relationship: New insights based on an human leucocyte antigen-genotyped family cohort. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 375, 20190266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaal B., Saxton T. K., Loos H., Soussignan R., Durand K., Olfaction scaffolds the developing human from neonate to adolescent and beyond. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 375, 20190261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter R. H., Olfaction and human kin recognition. Genetica 104, 259–263 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Weisfeld G. E., Czilli T., Phillips K. A., Gall J. A., Lichtman C. M., Possible olfaction-based mechanisms in human kin recognition and inbreeding avoidance. J. Exp. Child Psychol. 85, 279–295 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Roberts S. C., Gosling L. M., Spector T. D., Miller P., Penn D. J., Petrie M., Body odor similarity in noncohabiting twins. Chem. Senses 30, 651–656 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Chen D., Haviland-Jones J., Human olfactory communication of emotion. Percept. Mot. Skills 91, 771–781 (2000). [DOI] [PubMed] [Google Scholar]
- 30.de Groot J. H. B., Smeets M. A. M., Rowson M. J., Bulsing P. J., Blonk C. G., Wilkinson J. E., Semin G. R., A sniff of happiness. Psychol. Sci. 26, 684–700 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Lundström J. N., Boyle J. A., Zatorre R. J., Jones-Gotman M., Functional neuronal processing of body odors differs from that of similar common odors. Cereb. Cortex 18, 1466–1474 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Khan R. M., Luk C.-H., Flinker A., Aggarwal A., Lapid H., Haddad R., Sobel N., Predicting odor pleasantness from odorant structure: Pleasantness as a reflection of the physical world. J. Neurosci. 27, 10015–10023 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiske S. T., Cuddy A. J. C., Glick P., Xu J., A model of (often mixed) stereotype content: Competence and warmth respectively follow from perceived status and competition. J. Pers. Soc. Psychol. 82, 878–902 (2002). [PubMed] [Google Scholar]
- 34.Arzi A., Rozenkrantz L., Holtzman Y., Secundo L., Sobel N., Sniffing patterns uncover implicit memory for undetected odors. Curr. Biol. 24, R263–R264 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Ennis D. M., The power of sensory discrimination methods. J. Sens. Stud. 8, 353–370 (1993). [Google Scholar]
- 36.Ennis J. M., Ennis D. M., Yip D., O’Mahony M., Thurstonian models for variants of the method of tetrads. Br. J. Math. Stat. Psychol. 51, 205–215 (1998). [Google Scholar]
- 37.Sobel N., Khan R. M., Hartley C. A., Sullivan E. V., Gabrieli J. D., Sniffing longer rather than stronger to maintain olfactory detection threshold. Chem. Senses 25, 1–8 (2000). [DOI] [PubMed] [Google Scholar]
- 38.de Lacy Costello B., Amann A., Al-Kateb H., Flynn C., Filipiak W., Khalid T., Osborne D., Ratcliffe N. M., A review of the volatiles from the healthy human body. J. Breath Res. 8, 014001 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Feniger-Schaal R., Hart Y., Lotan N., Koren-Karie N., Noy L., The body speaks: Using the mirror game to link attachment and non-verbal behavior. Front. Psychol. 9, 01560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramseyer F., Tschacher W., Nonverbal synchrony in psychotherapy: Coordinated body movement reflects relationship quality and outcome. J. Consult. Clin. Psychol. 79, 284–295 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Aron A., Aron E. N., Smollan D., Inclusion of other in the self scale and the structure of interpersonal closeness. J. Pers. Soc. Psychol. 63, 596–612 (1992). [Google Scholar]
- 42.Bernieri F. J., Coordinated movement and rapport in teacher-student interactions. J. Nonverbal Behav. 12, 120–138 (1988). [Google Scholar]
- 43.McEllin L., Knoblich G., Sebanz N., Synchronicities that shape the perception of joint action. Sci. Rep. 10, 15554 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vacharkulksemsuk T., Fredrickson B. L., Strangers in sync: Achieving embodied rapport through shared movements. J. Exp. Soc. Psychol. 48, 399–402 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGann J. P., Poor human olfaction is a 19th-century myth. Science 356, eaam7263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.A. Harrington, V. Rosario, Olfaction and the Primitive: Nineteenth-Century Medical Thinking on Olfaction, in Science of Olfaction, M. J. Serby, K. L. Chobor, Eds. (Springer, 1992), pp. 3–27; 10.1007/978-1-4612-2836-3_1. [DOI]
- 47.Burenhult N., Majid A., Olfaction in aslian ideology and language. Senses Soc. 6, 19–29 (2011). [Google Scholar]
- 48.Majid A., Human olfaction at the intersection of language, culture, and biology. Trends Cogn. Sci. 25, 111–123 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Stern K., McClintock M. K., Regulation of ovulation by human pheromones. Nature 392, 177–179 (1998). [DOI] [PubMed] [Google Scholar]
- 50.Strassmann B. I., Menstrual synchrony pheromones: Cause for doubt. Hum. Reprod. 14, 579–580 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Gelstein S., Yeshurun Y., Rozenkrantz L., Shushan S., Frumin I., Roth Y., Sobel N., Human tears contain a chemosignal. Science 331, 226–230 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Oh T. J., Kim M. Y., Park K. S., Cho Y. M., Effects of chemosignals from sad tears and postprandial plasma on appetite and food intake in humans. PLOS ONE 7, e42352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyart C., Webster W. W., Chen J. H., Wilson S. R., McClary A., Khan R. M., Sobel N., Smelling a single component of male sweat alters levels of cortisol in women. J. Neurosci. 27, 1261–1265 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishor E., Amir D., Weiss T., Honigstein D., Weissbrod A., Livne E., Gorodisky L., Karagach S., Ravia A., Snitz K., Karawani D., Zirler R., Weissgross R., Soroka T., Endevelt-Shapira Y., Agron S., Rozenkrantz L., Reshef N., Furman-Haran E., Breer H., Strotmann J., Uebi T., Ozaki M., Sobel N., Sniffing the human body volatile hexadecanal blocks aggression in men but triggers aggression in women. Sci. Adv. 7, eabg1530 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regenbogen C., Axelsson J., Lasselin J., Porada D. K., Sundelin T., Peter M. G., Lekander M., Lundström J. N., Olsson M. J., Behavioral and neural correlates to multisensory detection of sick humans. Proc. Natl. Acad. Sci. U.S.A. 114, 6400–6405 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pause B. M., Storch D., Lübke K. T., Chemosensory communication of aggression: Women’s fine-tuned neural processing of male aggression signals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 375, 20190270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mujica-Parodi L. R., Strey H. H., Frederick B., Savoy R., Cox D., Botanov Y., Tolkunov D., Rubin D., Weber J., Chemosensory cues to conspecific emotional stress activate amygdala in humans. PLOS ONE 4, e6415 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Croy I., Hummel T., Olfaction as a marker for depression. J. Neurol. 264, 631–638 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Jacob S., McClintock M. K., Zelano B., Ober C., Paternally inherited HLA alleles are associated with women’s choice of male odor. Nat. Genet. 30, 175–179 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Schäfer L., Schriever V. A., Croy I., Human olfactory dysfunction: Causes and consequences. Cell Tissue Res. 383, 569–579 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Endevelt-Shapira Y., Perl O., Ravia A., Amir D., Eisen A., Bezalel V., Rozenkrantz L., Mishor E., Pinchover L., Soroka T., Honigstein D., Sobel N., Altered responses to social chemosignals in autism spectrum disorder. Nat. Neurosci. 21, 111–119 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Lundström J. N., Boyle J. A., Zatorre R. J., Jones-Gotman M., The neuronal substrates of human olfactory based kin recognition. Hum. Brain Mapp. 30, 2571–2580 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pause B. M., Krauel K., Sojka B., Ferstl R., Body odor evoked potentials: A new method to study the chemosensory perception of self and non-self in humans. Genetica 104, 285–294 (1998). [DOI] [PubMed] [Google Scholar]
- 64.Boulet M., Charpentier M. J., Drea C. M., Decoding an olfactory mechanism of kin recognition and inbreeding avoidance in a primate. BMC Evol. Biol. 9, 281 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mateo J. M., Johnston R. E., Kin recognition and the ‘armpit effect’: Evidence of self–referent phenotype matching. Proc. Biol. SCi. 267, 695–700 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.S. H. Schwartz, Universals in the Content and Structure of Values: Theoretical Advances and Empirical Tests in 20 Countries, in Advances in Experimental Social Psychology, M. P. Zanna, Ed. (Academic Press, 1992), vol. 25, pp. 1–65; www.sciencedirect.com/science/article/pii/S0065260108602816.
- 67.Baietto M., Wilson A., Bassi D., Ferrini F., Evaluation of three electronic noses for detecting incipient wood decay. Sensors 10, 1062–1092 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozenkrantz L., Weissgross R., Weiss T., Ravreby I., Frumin I., Shushan S., Gorodisky L., Reshef N., Holzman Y., Pinchover L., Endevelt-Shapira Y., Mishor E., Soroka T., Finkel M., Tagania L., Ravia A., Perl O., Furman-Haran E., Carp H., Sobel N., Unexplained repeated pregnancy loss is associated with altered perceptual and brain responses to men’s body-odor. eLife 9, e55305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perl O., Ravia A., Rubinson M., Eisen A., Soroka T., Mor N., Secundo L., Sobel N., Human non-olfactory cognition phase-locked with inhalation. Nat. Hum. Behav. 3, 501–512 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Feniger-Schaal R., Schönherr D., Altmann U., Strauss B., Movement synchrony in the mirror game. J. Nonverbal Behav. 45, 107–126 (2021). [Google Scholar]
- 71.Lemay E. P. Jr., Clark M. S., How the head liberates the heart: Projection of communal responsiveness guides relationship promotion. J. Pers. Soc. Psychol. 94, 647–671 (2008). [DOI] [PubMed] [Google Scholar]
- 72.McAuley E., Duncan T., Tammen V. V., Psychometric properties of the intrinsic motivation inventory in a competitive sport setting: A confirmatory factor analysis. Res. Q. Exerc. Sport 60, 48–58 (1989). [DOI] [PubMed] [Google Scholar]
- 73.Mendelson M. J., Aboud F. E., Measuring friendship quality in late adolescents and young adults: McGill friendship questionnaires. Can. J. Behav. Sci. 31, 130–132 (1999). [Google Scholar]
- 74.Rossignac-Milon M., Bolger N., Zee K. S., Boothby E. J., Higgins E. T., Merged minds: Generalized shared reality in dyadic relationships. J. Pers. Soc. Psychol. 120, 882–911 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S13
Table S1 to S2
References
Data files S1 to S6