Abstract
Objective:
While immune cells were originally thought to only play a role in maternal tolerance of the semiallogenic fetus, an active role in pregnancy establishment is becoming increasingly apparent. Uterine natural killer (uNK) cells are of specific interest because of their cyclic increase in number during the window of implantation. As a distinct entity from their peripheral blood counterparts, understanding the biology and function of uNK cells will provide the framework for understanding their role in early pregnancy establishment and adverse pregnancy outcomes.
Evidence Review:
This review discusses unique uNK cell characteristics and presents clinical implications resulting from their dysfunction. We also systematically present existing knowledge about uNK cell function in three processes critical for successful human embryo implantation and placentation: stromal cell decidualization, spiral artery remodeling, and extravillous trophoblast invasion. Finally, we review the features of uNK cells that could help guide future investigations.
Results:
It is clear the uNK cells are intimately involved in multiple facets of early pregnancy. This is accomplished directly, through the secretion of factors that regulate stromal cells and trophoblast function; and indirectly, via interaction with other maternal cell types present at the maternal-fetal interface. Current work also suggests that uNK cells are a heterogenous population, with subsets that potentially accomplish different functions.
Conclusion:
Establishment of pregnancy through successful embryo implantation and placentation requires crosstalk between multiple maternal cell types and invading fetal trophoblast cells. Defects in this process have been associated with multiple adverse perinatal outcomes including hypertensive disorders of pregnancy, placenta accreta, and recurrent miscarriage though the mechanism underlying development of these defects remain unclear. Abnormalities in NK cell number and function which would disrupt physiological maternal-fetal crosstalk, could play a critical role in abnormal implantation and placentation. It is therefore imperative to dissect the unique physiological role of uNK cells in pregnancy and use this knowledge to inform clinical practice by determining how uNK cell dysfunction could lead to reproductive failure.
Keywords: Implantation, placentation, pregnancy outcomes, uterine natural killer cell
Multiple adverse pregnancy outcomes, including early pregnancy loss, preeclampsia, preterm birth and intrauterine growth restriction, have been associated with disordered placentation (1–5). For proper pregnancy establishment, specialized invasive fetal trophoblast cells known as extravillous trophoblasts (EVTs) must invade through the uterus and migrate toward maternal spiral arteries. These arteries are then remodeled into low-resistance vessels to ensure appropriate uteroplacental perfusion (6–9). Maternal uterine cells play a critical role in this process, ensuring appropriate control of EVT invasion. In support of this, an abnormal maternal uterine environment, as well as preconception exposures that change this environment, can increase the risk of adverse perinatal outcomes (10–20). To develop interventions to prevent these adverse outcomes, it is critical to characterize how specific maternal cells function during early pregnancy.
It is becoming increasingly clear that uterine immune cells are active players in establishing and supporting pregnancy throughout gestation (21). Disturbances in the immune cell population have been linked to several adverse pregnancy outcomes including infertility, preterm birth, preeclampsia, intrauterine growth restriction, and early pregnancy loss (22). Uterine natural killer (uNK) cells are the most abundant immune cell in the endometrium. During the menstrual cycle, uNK cells are enriched in the late secretory phase, when embryo implantation occurs. If conception occurs, this increase in uNK cell number lasts throughout EVT invasion (23–26). These population fluctuations indicate a role for uNK cells in processes significant to early pregnancy establishment. Indeed, studies in animal models and in vitro experiments utilizing uNK cells from first trimester termination tissue suggest roles in the regulation of EVT invasion and spiral artery remodeling (27). This suggests that uNK cells are critical to ensuring healthy birth outcomes.
In this review, we discuss the role of uNK cells with specific attention to their dynamics during the menstrual cycle and pregnancy, origin, and heterogeneity. In addition, we discuss the clinical evidence linking uNK cell number and functions to clinical adverse pregnancy outcomes. Additionally, we describe existing knowledge about human uNK function in three processes critical for embryo implantation and placentation: stromal cell decidualization, where endometrial stromal cells are transformed into specialized cells capable of supporting pregnancy; EVT invasion, where fetal trophoblasts invade through the maternal decidua and into maternal spiral arteries; and spiral artery remodeling, where maternal arteries are physiologically transformed. Human placentation is unique; therefore, this review focuses on studies specific to human uNK cells with some references to key murine studies that fill gaps in knowledge. Such animal models are designated as such within the text; otherwise, the remainder of studies are performed utilizing human cells.
Most studies examining the characteristics and function of human uNK cells use cells isolated from first trimester pregnancy tissue. However, uNK cells in preimplantation maternal secretory endometrium may have unique functions compared with those of early pregnancy. We present evidence supporting this hypothesis and propose that it is critical to distinguish these two cell types and examine their functions in parallel. This is significant to keep in mind especially since key functional studies thus far have been performed in murine animal models, where terminally differentiated uNK cells are only present after embryo arrival (28–30). In this review, we use the terms “prepregnancy NK cells” to discuss cells isolated from a nonpregnant woman during the luteal phase of menstrual cycle and “pregnancy NK cells” to discuss cells obtained from early pregnancy tissues. The term uNK cells is used to encompass both populations of cells.
WHAT ARE uNK CELLS?
Peripheral NK Cells vs. uNK Cells
Natural killer cells are innate lymphocytes well known to be critical for host defense against viruses and tumors (31). They express an array of activating and inhibitory receptors, the balance of which governs production of cytokines and cytolysis by NK cells. Natural cytotoxicity receptors and killer immunoglobulin-like receptors expressed by NK cells are germline-encoded and recognize certain activating ligands and major histocompatibility complexes, respectively. In contrast, adaptive immune lymphocytes express individually rearranged T and B cell receptors that allow the host to react against virtually any antigen. Further, while adaptive immune lymphocytes predominate in such traditional lymphoid tissues as lymph nodes and the spleen, NK cells are overrepresented in the uterus.
Natural killer cells present in uterine tissue both before and during pregnancy are unique cell populations with divergent characteristics compared with their peripheral blood counterparts. Peripheral blood NK (pb NK) cells are cytotoxic in nature and play an integral role in the clearance of virally infected and tumor cells (32). Uterine NK cells display distinct differences in surface marker expression—while pb NK cells are predominantly CD56dim and CD16bright, uNK cells are CD56bright and CD16dim (33). While these cells contain cytolytic molecules, their cytotoxic effect is not applied to classical target cells in vitro, playing a permissive, rather than a defensive, role (27, 34, 35).
Most data exploring uNK cells have been generated utilizing pregnancy uNK cells isolated from first trimester termination tissue (Tables 1 and 2). Fewer studies have examined the number and function of prepregnancy uNK cells, present in the endometrium before trophoblast arrival (36). This is a significant distinction as the immune cells that regulate early EVT invasion and placental establishment are likely those that accumulate in the endometrium during the secretory phase and may differ from those present in the first trimester. Supporting this, a study by Kopcow et al. (37) showed distinct gene expression differences between NK cells from cycling endometrium and first trimester NK cells, and both of these cell types differed from circulating peripheral NK cells. Another study by Manaster et al. (35) demonstrated phenotypic and functional differences between the prepregnancy and pregnancy uNK cell populations: prepregnancy NK cells possessed a different repertoire of chemokine receptors and were not cytotoxic until activated by interleukin (IL)-15. These investigators go on to speculate that prepregnancy NK cells are nonfunctional but poised to act after pregnancy. However, the investigators in this study focused specifically on cytotoxicity and the cytokines known to be expressed by NK cells obtained during pregnancy. Current data suggest a much broader secretome from these NK cells, and a wider study of the function of prepregnancy NK cells is still needed.
TABLE 1.
Cell sources in background reference literature.
| Author | Year | Experiment/key findings | Cell source |
|---|---|---|---|
| King et al. (24) | 1989 | Increase in uNK cells during the secretory phase of menstrual cycle | Endometrium from routine hysterectomies |
| Bulmer et al. (39) | 1991 | Increase in uNK cells seen in late secretory endometrium and is sustained through the first trimester | 8–10-week decidua, hysterectomy specimens |
| Flynn et al. (38) | 2000 | Increase in uNK cells during the secretory phase of menstrual cycle | Hysterectomy specimens and EMB at time of tubal ligation |
| Sentman (55) | 2004 | Estradiol and progesterone promotion of chemokine expression to attract peripheral blood NK cells to the endometrium | Hysterectomy specimens (included women on hormone therapy and postmenopausally, 46 ± 11 years)–> created uNK clones |
| Hannan et al. (42) | 2004 | Progesterone-dependent chemotactic recruitment of leukocytes by endometrial cells | Endometrial biopsies from normal, fertile women |
| Jones et al. (43) | 2004 | Chemokines capable of leukocyte recruitment are expressed by epithelial and stromal endometrial cells and upregulated during the implantation window | Endometrial tissue obtained by dilation and curettage from women undergoing gynecological surgical procedures |
| Taylor et al (47) | 2004 | Donor HLA-type leukocytes found after bone marrow transplant suggesting in situ origin of uNK cells | Endometrial biopsies |
| Matsura-Sawada (49) | 2005 | Transplantation of human endometrium into mice lacking NK cells showed an increase in uNK cells | Hysterectomy samples from women with benign gynecologic disease |
| Ordi et al (40) | 2006 | Increase in uNK cells in decidual endometrium independent of the presence of an embryo | EMB from ectopic pregnancy patients |
| Lash et al. (61) | 2006 | Production of angiogenic factors by uNK cells differs by gestational age | 8–10-week decidua, 12–14-week decidua |
| Keskin et al. (45) | 2007 | Exposure of peripheral NK cells to stromal cell conditioned media leads to uNK cell-surface marker expression | 6–12-week decidua |
| Manaster (35) | 2008 | Secretory-phase NK cells are 30% of endometrial lymphocytes vs. 5%–15% of peripheral blood lymphocytes; have a unique receptor repertoire and are cytotoxic and produce cytokines only after IL-15 stimulation | Proliferative- and secretory-phase endometrium |
| Vacca et al (51) | 2011 | Hematopoietic precursors identified that differentiated into cells with uNK cell-surface marker phenotype after coculture with stromal cells | 9–12-week decidua |
| Robson et al. (62) | 2012 | Gestational age-dependent functions of uNK cells | 8–10-week decidua, 12–14-week decidua |
| Szereday et al. (52) | 2012 | Hematopoietic progenitor phenotype cells found in decidual tissue that were dysregulated in women with early spontaneous abortions | 7–12-week decidua |
| Cerdeira et al. (46) | 2013 | Exposure of peripheral NK cells to cocktail of factors converts them to uNK cell phenotype | First trimester decidua |
| Fu (60) | 2017 | “Growth factor-producing” NK cell subset identified that produces factors critical for fetal development | Decidua from elective pregnancy terminations |
| Vento-Tormo et al. (56) | 2018 | Three distinct subpopulations of uNK cells | First trimester decidua (6–14 weeks) |
| Gamliel et al. (59) | 2018 | Existence of “pregnancy trained” uNK cells in repeated pregnancies capable of producing increased levels of interferon gamma and VEGF-alpha, precursors found in endometrium | 6–14-week decidua |
| Suryawanshi et al. (57) | 2018 | Single-cell RNA sequencing indicates “resting” and “proliferative” uNK cell subsets | First trimester decidua |
| Huhn et al. (58) | 2020 | Mass cytometry confirmation of dNK1, dNK2, and dNK3 subsets and chemokine production differences indicating potential functional heterogeneity | 7–12-week decidua |
Note: EMB = endometrial biopsy; HLA = human leukocyte antigen; IL = interleukin; uNK = uterine natural killer; VEGF = vascular endothelial growth factor.
TABLE 2.
Cell sources and experimental findings in functional studies of uNK cells.
| Author | Year | Experiment/key findings | Cell source |
|---|---|---|---|
| Endometrial decidualization | |||
| Gong et al. (105) | 2014 | uNK cell conditioned medium induces gene expression associated with decidualization | 7–8 week decidual tissue |
| Zhang (106) | 2018 | Coculture of uNK cells and endometrial stromal cells resulted in decidualization | First trimester decidua |
| Spiral artery remodeling | |||
| Croy et al. (118) | 1997 | No spiral artery remodeling seen in mice without uNK cells | N/A |
| Craven et al. (117) | 1998 | Early structural changes in spiral arteries occur before extravillous trophoblast arrival | First trimester elective termination samples (mean gestational age, 9 weeks); late secretory phase endometrium from surgical pathology archives, postovulatory day 10 ± 2 |
| Guimond et al. (121) | 1998 | Features of spiral artery remodeling seen in uNK cell-deficient mice after reconstitution of uNK cells through bone marrow transplants | n/a |
| Eriksson et al. (33) | 2004 | Secretomics of uNK cells | Hysterectomy specimens (included women on hormone therapy and postmenopausally, 46 ± 11 years)—created uNK clones |
| Lash et al. (61) | 2006 | uNK cells secrete angiogenic factors; higher levels secreted by uNK cells isolated from 8–10-week decidua vs. 12–14-week decidua | 8–10 week decidua, 12–14 week decidua |
| Hanna et al. (111) | 2006 | uNK cells release angiogenic factors that support vascularization in vivo | First trimester decidua |
| Smith et al. (115) | 2009 | uNK cells infiltrate vascular smooth muscle layers and secrete MMPs known to participate in artery remodeling | 8–12-week decidua |
| Robson et al. (62) | 2012 | Supernatant from uNK cells isolated from 8–10-week decidua induces disruption of vascular smooth muscle cells and breakdown of ECM components; 12–14-week uNK cells do not have this effect | First trimester decidua |
| Fraser et al. (92) | 2012 | uNK cells from first trimester pregnancies with elevated uterine artery resistance indices do not induce EC apoptosis, while those from pregnancies with normal-resistance indices do | 9–14-week decidua |
| Trophoblast invasion | |||
| Hanna et al. (111) | 2006 | uNK cells release chemoattractants that promote EVT invasion in vitro and in vivo | First trimester decidua |
| Hu (114) | 2006 | uNK cells inhibit EVT invasion through secretion of interferon gamma | 6–12-week decidua |
| Lash et al. (107) | 2010 | 12–14-week uNK cell supernatant promotes EVT invasion; 8–10-week uNK cells do not have this effect | 8–10-week decidua, 12–14-week decidua |
| De Oliveira et al. (108) | 2010 | Promotion of EVT invasion by uNK cells is partially mediated by IL-8 | 8–10-week decidua, 12–14-week decidua |
| Fraser et al. (92) | 2012 | Treatment of EVTs with uNK cell conditioned medium isolated from first trimester pregnancies with elevated uterine artery resistance indices leads to lower mobility than from pregnancies with normal artery resistance | 9–14-week decidua |
| Xiong et al. (112) | 2013 | uNK cells secrete factors that enhance EVT invasion | 7–12-week decidua |
| Zhang et al. (113) | 2013 | Downregulation of uNK cell VEGF expression abrogates EVT migration | 6–20-week decidua |
| Wallace et al. (93) | 2015 | uNK cells from pregnancies with high umbilical artery resistance have decreased expression of HLA-binding cell-surface receptors | 9–14-week decidua |
| Tilburgs (143) | 2015 | A single EVT forms synapses with several uNK cells | 6–12-week decidua |
| Ma (109) | 2017 | uNK cell conditioned medium promotes trophoblast invasion and endothelial cell tube formation | 6–10-week decidua |
Note: ECM = extracellular matrix; EVT = extravillous trophoblast; HLA = human leukocyte antigen; IL = interleukin; MMPs = matrix metalloproteinases; uNK = uterine natural killer; VEGF = vascular endothelial growth factor.
UterineNK Cell Dynamics
Uterine NK cells are not a static population of cells but undergo cyclic shifts in abundance during the menstrual cycle, with the population enriching during the window of implantation and during early pregnancy, when trophoblast invasion occurs. In nonpregnant endometrium, uNK cells increase drastically between the proliferative and secretory phases, going from 38.9% of all lymphocytes to 83.2% of all lymphocytes by the late secretory phase (38). If conception occurs, this increase in uNK cell number lasts through the first trimester of pregnancy and then decreases in the late second and early third trimester after cessation of trophoblast invasion around 20 weeks (23–26, 38, 39) (Fig. 1). Enrichment of prepregnancy uNK cells is independent of the presence of an embryo, as evidenced by biopsies in nonpregnant decidualized endometrium as well as in sampling of decidualized endometrium from women with ectopic pregnancy (40). Notably, if pregnancy is not established during a given cycle, the uNK cells simply apoptose (41). These cyclic changes in population have led to the study of their function in endometrial preparation for pregnancy as well as early pregnancy implantation.
FIGURE 1.
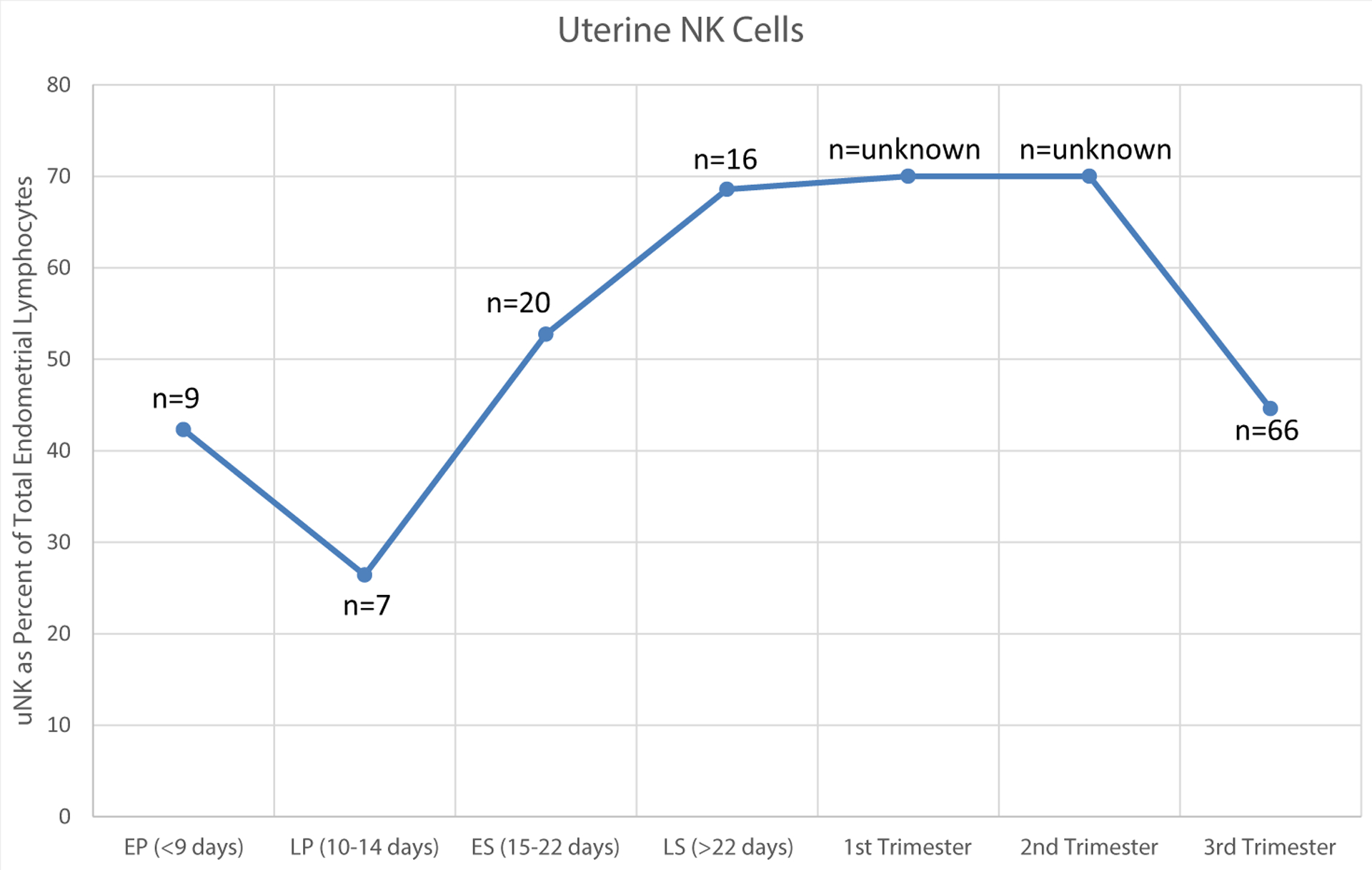
Change in human endometrial uterine natural killer cell abundance through the menstrual cycle and early pregnancy. EP = early proliferative; ES = early secretory; LP = late proliferative; LS = late secretory. (Bulmer et al. (39); S. K. Lee et al. (23), (144); (Rieger et al. (25); Flynn et al. (38)]
Uterine NK Cell Origin
The origin of uNK cells is an area of intense scrutiny. Although no clear consensus currently exists, it remains unclear whether uNK cells originate from pb NK cells that migrate into the uterus or whether they originate in situ. In support of recruitment from the peripheral circulation, cytokines and chemokines secreted by endometrial cells have been suggested to function in recruitment of peripheral NK cells. Specific factors such as CXCL12, CXCL9, CXCL10, CCL3, and CCL4 are produced by decidual stromal cells, uterine endothelial cells, and fetal trophoblast cells that induce chemotactic responses in pb NK cells (42–44). In vitro, exposure of pb NK cells to factors such as transforming growth factor beta 1, hypoxia, or stromal cell conditioned medium leads to pb NK cell acquisition of phenotypic and functional characteristics of uNK cells (45, 46). Examination of bone marrow transplant recipients additionally demonstrated the presence of donor-derived cells capable of generating endometrium (47). More recent literature from uterus transplant recipients demonstrates uNK cells after transplantation identified as recipient in origin on the basis of human leukocyte antigen (HLA) expression, indicating that these cells may be replenished from peripheral circulation over time (48).
While the aforementioned data support peripheral immune cell recruitment, an elegant study by Matsuura-Sawada et al. (49) showed an increase in human uNK cells when human proliferative endometrium was transplanted subcutaneously into immunodeficient mice lacking NK cells. This suggests that uNK cells could arise in the endometrium and proliferate from in situ hematopoietic precursors. Studies show that nonpregnant endometrium and first trimester decidua both contain these precursors (50–52), and NK cell differentiation factors are produced by the endometrium in a cyclic manner that coincides with uNK cell abundance (53). Regardless of uNK cell origin, it seems clear that hormonal regulation plays a significant role in NK cell accumulation. In vitro, pregnancy uNK cells obtained during the first trimester demonstrate increased migration in response to estrogen (54). Using in vitro cultured human endometrial tissue samples, estradiol and progesterone promote chemotactic marker expression, supporting a role for these hormones in uNK cell accumulation (55).
Uterine NK Cell Heterogeneity
Complicating the distinction between prepregnancy and pregnancy uNK cells even further, a recent study profiling first trimester maternal decidua discovered multiple coexisting uNK cell subtypes. Using single-cell RNA sequencing, Vento-Tormo et al. (56) defined dNK1, dNK2, and dNK3 populations in the decidua on the basis of unique surface marker expression, with each cell type demonstrating a unique transcriptome. While the function of each subset is unknown, dNK1 cells express transcripts that suggest a role in EVT recognition and interaction; dNK2 cells potentially have anti-inflammatory functions; and dNK3 cells could play a role in EVT regulation. A second study that similarly profiled the transcriptome of maternal–fetal interface separated uNK cells present in the first trimester into “resting” NK cells, which represented most uNK cells present, and “proliferative” uNK cells that were nearly sevenfold fewer and were enriched in genes involved in cell cycle processes and cell proliferation (57). The earlier described subsets were corroborated by a recent study that distinguished these populations using protein surface markers via mass cytometry (58).
In addition, other groups have described heterogeneity in the first trimester uNK cell population: pregnancy trained decidual NK cells that confer “trained memory” were recently described, existing only in multigravid women (59). These cells produce increased levels of factors supporting vascularization, and the investigators speculate that they originate from precursors present in the endometrium during the menstrual cycle. Fu et al. (60) identified a subset of first trimester uNK cells that express CD49a and Eomes and produce growth-promoting factors such as pleiotrophin, osteoglycin, and osteopontin that play a role in fetal development. Patients with recurrent pregnancy loss (RPL) contain fewer growth-promoting factor+ uNK cells, and lack of these cells results in fetal growth restriction in mice.
Another significant characteristic of uNK cells is the impact of gestational age on their function, although few studies have explored this phenomenon, possibly owing to the difficulty in obtaining human tissue. Angiogenic factors such as Ang1, Ang2, and vascular endothelial growth factor (VEGF)-C are secreted at higher levels by uNK cells obtained between 8 and 10 weeks of pregnancy compared with 12–14 weeks (61). This is accompanied by corresponding functional differences: vascular smooth muscle cell disorganization, a hallmark of spiral artery remodeling, was only observed using supernatant from 8–10-week uNK cells and not 12–14-week uNK cells in a chorionic plate artery in vitro model (62). Interestingly, examining first trimester uNK cell gestational age effects on EVT invasion and apoptosis yielded the opposite conclusion: 12–14-week uNK cell supernatant increased EVT invasion compared with 8–10-week uNK cell supernatant. These data suggest that uNK cell function during pregnancy changes throughout gestation. This could reflect changing requirements for extent of trophoblast invasion and/or artery remodeling required at different stages of pregnancy and is a significant variable to consider in analyzing data and future studies.
The heterogeneity identified by the aforementioned studies could truly represent distinct uNK cell subpopulations or depict progressive stages in uNK cell development, represent multiple functions of a single population, or arise from regional differences in localization within the decidua. It remains to be seen if these subsets exist before conception.
ASSOCIATIONS BETWEEN uNK ABUNDANCE AND FUNCTION WITH PREGNANCY OUTCOMES
The clinical relevance of uNK cell abundance and function to infertility and adverse pregnancy outcomes remains in dispute. This may be due largely in part to heterogeneity of the available studies (Table 3). For example, some studies demonstrate a decrease in uNK cells on examination of endometrial biopsies in the secretory phase of women with infertility (63–65) and a decrease in CD56 bright uNK cells in women with miscarriage after assisted reproductive technology (ART) compared with those with live birth after ART (66). However, other data demonstrate no difference in uNK abundance (67, 68) or increased uNK progenitor cells in the endometrium of infertile women (50) on the basis of endometrial biopsy at time of elective surgery.
TABLE 3.
Human studies of uNK cell abundance and function with pregnancy outcomes.
| Author | Year | Study population | Timing of Bx/path sample | Quantification technique for uNK cells | Conclusions | Category | |
|---|---|---|---|---|---|---|---|
| Klentzeris et al. (63) | 1994 | 24 unexplained infertility; 24 fertile controls | Secretory endometrial biopsies (LH surge +4, +7, +10, and +13) | Immunohistochemistry | Significantly decreased numbers of CD56+ cells in infertile population compared with fertile controls | Infertility | |
| Fukui et al. (66) | 1999 | 76 women undergoing IVF | Midsecretory endometrial biopsy | Flow cytometry | No difference in % uNK cells in women with infertility who failed to get pregnant and those who became pregnant after IVF and between miscarriage and live birth in those who were pregnant Higher subpopulation of CD56+ uNK cells in women who had live birth compared with miscarriage No normal range reported |
Infertility | |
| Lynch et al. (50) | 2007 | 12 infertile women undergoing surgical tubal patency assessment, 7 fertile women undergoing elective surgery | Endometrial biopsy at time of elective surgery | Flow cytometry | Increased proportion of NK progenitors in endometrium of infertile women | Infertility | |
| McGrath et al. (67) | 2009 | 18 women with unexplained infertility; 10 parous control women | Endometrial biopsy at time of elective surgery | Flow Cytometry | Increased secretory-phase expression of CD94 and CD158b and proliferative-phase expression of CD158a by uNK cells in infertile women No difference in the number of uNK cells between fertile and infertile women across the menstrual cycle |
Infertility | |
| Kofod et al. (65) | 2017 | 41 infertile (hydrosalpinx, history of salpingectomy, or unexplained), 20 control (Caucasian only) | Midsecretory endometrial biopsy (LH surge + 7 days) | Immunohistochemistry | Decreased number of CD56+ uNK cells in infertile women compared with fertile controls Increased number and percentage of CD56+ uNK cells predictive of pregnancy in future IVF treatment cycle |
Infertility | |
| Recurrent implantation failure | Ledee-Bataille (69) | 2004 | 15 women with >2 IVF cycle failures undergoing natural IVF | Midsecretory endometrial biopsy | Immunohistochemistry | No difference in number of uNK cells in women with infertility who failed to get pregnant and those who became pregnant after IVF | Infertility, RIF |
| Matteo et al. (70) | 2007 | 10 women with unexplained infertility and RIF (>3 IVF failures with unsuccessful embryo transfers of at least 2 high-grade blastocysts), 25 historical controls undergoing gynecologic surgery | Midsecretory endometrial biopsy (d22–26) | Flow cytometry | Uterine NK cell percentage did not differ between infertile women and historical controls | Infertility, RIF | |
| LeDee (71) | 2016 | 394 women with RIF (no ongoing pregnancy > 10 weeks despite multiple embryo transfers with a total of at least 6 embryos transferred on day 3 or 5), 26 fertile controls with male factor infertility and successful pregnancy after IVF | Midsecretory endometrial biopsy | Immunohistochemistry | No difference in uNK abundance between infertile and fertile populations but substantial subpopulation of both overactivation and low activation of uNK cells | Infertility, RIF | |
| Donoghue et al. (72) | 2019 | 14 women with RIF, 9 women with potential implantation failure, 11 controls with implantation success | Midsecretory endometrial biopsy (LH surge + 6–8 days) | Immunohistochemistry | No difference in cell density of CD56+ or CD16+ uNK cells in RIF compared with women with implantation success or potential RIF Significant reduction in uNK density in parous women compared with nulliparous, regardless of current implantation status |
Infertility, RIF | |
| Tohma et al. (73) | 2020 | 16 women with RIF (3 unsuccessful IVF/ICSI treatments despite transfer of good-quality embryos), 25 infertile patients without RIF | Midsecretory uterine lavage | Flow cytometry | Increased percentage of uNK cells in controls vs. study group (P = .026) | Infertility, RIF | |
| Recurrent pregnancy loss | Chen et al. (74) | 2017 | 97 women with recurrent miscarriage, 34 women with RIF, 84 fertile controls | Midsecretory endometrial biopsy (LH surge + 7 days) | Immunohistochemistry | Women with infertility fell both above and below the reference range for uNK % compared with fertile controls Significant increase in percentage of uNK cells in both recurrent miscarriage (P= .042) as well as RIF (P = .048) compared with fertile controls |
Infertility, RIF, RPL |
| Marron (75) | Feb, 2019 | 178 women with RIF (>2 unsuccessful embryo transfers of high-grade blastocysts), 155 women with RPL (>2 clinically detectable consecutive or nonconsecutive miscarriages), 130 women with primary infertility, 114 women with secondary infertility, 35 control women with male factor infertility < 38 years of age | Endometrial biopsy following standard hormone replacement therapy protocol after 5 days of vaginal progesterone | Flow cytometry | Patients with history of RIF had significantly higher numbers of uNK cells compared with controls and patients with RPL (P< .0001) | Infertility, RIF, RPL | |
| Marron (76) | March, 2019 | 149 women with RIF (>2 unsuccessful embryo transfers of high-grade blastocysts), 121 women with RPL (>2 clinically detectable consecutive or nonconsecutive miscarriages), 76 women with primary infertility, 80 women with secondary infertility, 29 control women with male factor infertility < 38 years of age | Endometrial biopsy following standard hormone replacement therapy protocol after 5 days of vaginal progesterone | Flow cytometry | Infertile populations with increased concentrations of peripheral-type NK cells (P = .016) | Infertility, RIF, RPL | |
| Parkin et al. (64) | 2011 | 24 women with unexplained RPL, 31 women with unexplained infertility, 10 controls with no history of infertility | Midsecretory endometrial biopsy | Immunohistochemistry | CD56+ uNK cells significantly lower in unexplained infertility compared with controls (P= .002) as well as in unexplained RPL compared with controls (P= .035) Differences between study groups for CD56+ uNK cells not significant nor were there significant differences in CD16 or NKG2a-positive cells | Infertility, RPL | |
| Giuliani et al. (68) | 2014 | 21 women with unexplained RPL, 30 women with unexplained infertility, 10 controls without history of infertility, RPL, or endometriosis | Midsecretory endometrial biopsy | Immunohistochemistry | No significant difference of CD56+ uNK cells in women with unexplained RPL or unexplained infertility compared with fertile women Ratio of NKp46+:CD56+ cells higher in women with unexplained RPL and unexplained infertility compared with fertile patients |
Infertility, RPL | |
| Hill et al. (79) | 1995 | 20 women with RPL (>4) Controls are 20 elective terminations |
Placental tissue | Immunohistochemistry | No difference in CD56+ cells | RPL | |
| LaChapelle et al. (80) | 1996 | 20 women with RPL (>2), idiopathic | Midsecretory endometrial biopsy (days 18–25) | Flow cytometry | No difference in % total uNK cells in women with miscarriage and ongoing pregnancy No normal range reported |
RPL | |
| Vassiliadou and Bulmer (84) | 1996 | 40 women with SA Controls are 19 elective terminations |
Placental tissue | Immunohistochemistry | 50% had significantly increased numbers of “classic” CD57 NK cells compared with normal human pregnancy | RPL | |
| Clifford et al. (85) | 1999 | 29 women with RPL (>3) Controls are 10 parous women |
Midsecretory endometrial biopsy (days 20–23) | Immunohistochemistry | Increased CD56+ uNK cells in RPL over control group (P= .001) When stratified by miscarriage type, difference maintained only if history of early pregnancy loss |
RPL | |
| Kwak (77) | 1999 | 71 women with RPL (>3) Controls are 20 elective terminations |
Placental tissue | Immunohistochemistry | 29.6% (P= .03) demonstrated elevated CD57+ uNK cells at the implantation site | RPL | |
| Quenby et al. (86) | 1999 | 22 women with RPL (>3) Controls are 9 fertile women (2 or more prior pregnancies, no miscarriages) |
Midsecretory endometrial biopsy (day 19–23) | Immunohistochemistry | 8/22 had few CD57+ uNK cells vs. none in the controls Women with miscarriages had significantly more CD4+, CD14+, CD16+, and CD56+ (uNK) leukocytes than either those who had live births or women with proven fertility |
RPL | |
| Quack et al. (81) | 2001 | 17 women with RPL of normal male pregnancy Controls are 20 elective terminations and 21 unexplained RPL with trisomy 16 |
Placental tissue | Immunohistochemistry | No difference in CD56+ uNK cells Decreased CD56:CD45 ratio in women with RPL of normal male pregnancy compared with normal elective abortion |
RPL | |
| Emmer et al. (87) | 2002 | 9 unexplained RPL (>1 consecutive miscarriage before 16 weeks), 9 controls with healthy pregnancy at time of CVS sampling, 2 controls of hysterectomies with 12–13-week pregnancies | Tissue collected after miscarriage/curettage of nonvital pregnancy | Immunohistochemistry | Increased expression of CD56+ and CD16+ uNK cells in miscarriage tissue compared with healthy pregnancy | RPL | |
| Michimata et al. (83) | 2002 | 17 women with RPL (≥2 with normal karyotype) Controls are 15 women with male factor in fertility |
Midsecretory endometrial biopsy (day 18–21) |
Immunohistochemistry | No difference in CD56+ or CD16+ uNK cells No normal range reported |
RPL | |
| Shimada et al. (82) | 2004 | 20 women with unexplained RPL (>1 miscarriage) Controls are 17 fertile women (history of 1 or more normal live birth, no history of miscarriage or ectopic pregnancy) |
Midsecretory endometrial biopsy | Flow cytometry | No significant difference in uNK cell percentages between women with RPL and fertile controls. Evaluated CD56+, CD56+CD16+, and CD56+CD16- | RPL | |
| Tuckerman et al. (78) | 2007 | 87 women with RPL 10 normal controls |
Midsecretory endometrial biopsy (LH surge + 7–9 days) |
Immunohistochemistry | Significantly higher CD56+ uNK cells in women with RPL vs. control No difference in % uNK cells in women who conceived following biopsy with respect to pregnancy outcome (miscarriage vs. live birth) |
RPL | |
| Hosseini et al. (88) | 2014 | 15 women with recurrent spontaneous abortion (2 or more successive miscarriages < 20 weeks), 15 healthy fertile controls (at least 1 prior live birth, no history of abortion) | Menstrual blood collection | Flow cytometry | Higher percentage of CD56+CD3−CD45RO+ uNK cells in menstrual blood of fertile women compared with infertile. No other significant difference in menstrual blood for other uNK subtypes | RPL | |
| Preeclampsia | Fraser et al. (92) | 2012 | Elective terminations screened by uterine artery Doppler ultrasound to categorize into high arterial resistance vs. normal arterial resistance | 9–14-week termination tissue | Immunohistochemistry | No difference in uNK cell abundance in decidua from high- vs. low-uterine-artery-resistance pregnancies Decreased trophoblast motility when treated with supernatant of uNK cells from high-resistance pregnancies than supernatant of uNK cells from normal-resistance pregnancies In elevated resistance pregnancies, uNK cells secrete fewer proinvasive factors, fail to induce vascular apoptosis, and secrete fewer apoptotic factors |
Preeclampsia |
| Wallace et al. (93) | 2015 | Elective terminations screened by uterine artery Doppler ultrasound to categorize into high arterial resistance vs. normal arterial resistance | First trimester termination tissue | Flow cytometry | Reduction in receptor expression for HLA-C and HLA-G on trophoblast in uNK cells from high-resistance pregnancies uNK cells with reduced receptor expression for HLA-G has altered production of two cytokines known to be significant in uNK-trophoblast interactions |
Preeclampsia | |
| Accreta | Laban et al. (97) | 2014 | 10 patients with unseparated placenta accreta, 16 patients with separated placenta accreta, 25 patients with placenta previa, 25 patients with normal placentation | Decidual biopsies at time of cesarean section | Immunohistochemistry | Possible quantitative difference in pregnancy NK cells in placenta accrete vs. normal placentation with decreased uNK score associated with cases of morbidly adherent placenta accreta | Placenta accreta |
Note: ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilization; LH = luteinizing hormone; RIF = recurrent implantation failure; RPL = recurrent pregnancy loss; SA = semen analysis; uNK = uterine natural killer.
For those studies focusing on recurrent implantation failure (RIF) after in vitro fertilization, most studies demonstrate no difference in abundance of uNK cells in women with RIF compared with those with history of RIF with subsequent pregnancy (69), historical controls (70), or fertile women (71, 72). However, one recent analysis of a relatively small cohort of women demonstrated a lower number of secretory uNK cells when obtained by uterine lavage in women with RIF compared with infertile women without RIF (73). To add further confusion to the subject, two more studies demonstrated an increase in uNK abundance in women with RIF (74, 75) with another demonstrating an increase in peripheral-type NK cells within the uterus (76).
The greatest volume of literature with regard to evaluating uNK cell abundance and early pregnancy outcomes exists in the literature studying RPL. Although most of these studies are relatively small, with 20 or fewer subjects per arm, there are two larger studies investigating 71 and 87 women with RPL compared with 20 and 10 controls, respectively (77, 78). The first of these larger studies demonstrates an increase in uNK cells, but this was quantified in placental tissue utilizing immunohistochemistry at the implantation site after the demise of the pregnancy as opposed to prepregnancy uNK cells (77). The second of these studies evaluated prepregnancy uNK cells by midsecretory endometrial biopsy and similarly found an increase in uNK abundance in women with RPL vs. normal controls (78). In examining smaller studies evaluating this population, most conclude that there is no difference in uNK cell abundance in RPL (68, 79–83). Of those studies remaining, most demonstrate an increase in uNK abundance in RPL (84–87) although all but one of these is on the basis of placental tissue as opposed to endometrial tissue. Two additional studies demonstrate lower uNK cells in this population, although one of these studies evaluated uNK abundance in menstrual blood (64, 88). Finally, none of these studies examined uNK cell subtypes, and it remains unknown whether alterations in uNK cell abundance can be predictably correlated to early adverse pregnancy outcomes.
Aberrant NK cell number and function have been associated not only with pregnancy establishment but also with disorders of placentation that may manifest later on in pregnancy such as preeclampsia, intrauterine growth restriction, and placenta accreta. Among these, the most well studied is preeclampsia. Although the symptoms of preeclampsia develop toward the end of gestation, it has been suggested that abnormal trophoblast invasion during the first trimester is responsible for this phenotype. Histologic examination of placental bed biopsies demonstrates spiral artery changes of the placental bed in hypertensive pregnancies (89, 90). Placental bed biopsies performed at time of cesarean section in normotensive patients as well as those with preeclampsia with severe features demonstrated trophoblast invasion in 100% of the decidual spiral arteries in the normotensive patients but only 44% in those with severe preeclampsia (91). Several studies have attempted to correlate these changes with immune cell number and function. Late first trimester pregnancies with higher uterine artery resistance indices, implicated in the pathophysiology of preeclampsia, show uNK malfunction and decreased expression of HLA-binding cell-surface receptors integral to normal EVT invasion (92, 93).
Although it is difficult to demonstrate a causative relationship between immune cell function and hypertensive disorders, animal data has supported this hypothesis. Data from BPH/5 mice, which develop late gestational hypertension and proteinuria that resolve on delivery, among other features of preeclampsia, showed a causative relationship between regulatory factors that controlled immune cell abundance, NK cell abundance, and the development of placentation abnormalities (94). Others have demonstrated that decreasing pregnancy-related factors known to promote recruitment and activation of uNK in the mouse leads to failure of spiral artery remodeling and reendothelialization, hallmarks of the pathophysiology of preeclampsia, in the setting of reduced uNK numbers (95).
Other disorders of placentation, such as placenta accreta, which is associated with excessive invasion of trophoblasts, have additionally been linked with disordered trophoblast invasion and immune cell function. Placenta accreta specimens demonstrate a multitude of abnormalities including imbalance of cytotrophoblasts and decidua, failure of spiral artery remodeling, and predominance of immature-appearing EVTs (96). A study of gravid hysterectomy cases found that accreta, increta, and percreta cases demonstrated fewer remodeled spiral arteries as well as many with incomplete remodeling (4). In addition, this study noted increased depth of vascular remodeling as well as deeper invasion of interstitial trophoblasts in the uterine wall (4). Early human data demonstrates a possible quantitative difference in pregnancy NK cells in placenta accreta spectrum compared with normal placentation (97). More recent evidence demonstrates a link between uNK dysfunction and disordered placentation via Grb2-associated binding protein 3 (Gab3). Gab3 has known function in NK cell priming and expansion; therefore, Sliz et al. (98) utilized a Gab3-deficient mouse model to evaluate pregnancy implications. The resultant impairment in uNK expansion in these mice led not only to increased trophoblast invasion but also to adverse pregnancy outcomes including retained placenta, maternal hemorrhage, and undelivered fetoplacental units at term (98).
HOW COULD uNK CELLS IMPACT PLACENTATION?
Changes to the maternal environment even before conception can affect early placentation. For example, obese patients are known to have an increased risk of preeclampsia, gestational hypertension, preterm birth, and perinatal death (99). Animal studies utilizing embryo transfer have demonstrated that in mice, the maternal environment is responsible for many of the placental changes associated with obesity (16,100). In addition, the supraphysiologic hormonal milieu created by ARTs leads to an increased risk of placental abnormalities as seen in a number of mouse models (14–16, 101) as well as demonstrated adverse sequelae in humans including low birth weight (13), preeclampsia (10), placenta previa (11), and placental abruption (10). Although the mechanisms contributing to these outcomes are poorly understood, recent evidence suggests that the maternal uterine immune cell population is a significant contributor to adverse perinatal outcomes. Immune cell population disturbances have been found in multiple adverse pregnancy outcomes including fetal growth restriction and preeclampsia (22). In addition, these complications have been associated with abnormal placentation (1–4), suggesting a role for maternal immune cells in processes significant for the establishment of the placenta. In this section, we examine how uNK cells could impact three processes essential for placentation—decidualization, trophoblast invasion, and spiral artery remodeling.
Role of uNK Cells in Endometrial Decidualization
Endometrial decidualization is an imperative process that begins before the arrival of the embryo, altering the structure of the endometrium to allow for embryo implantation. These predominantly hormone-mediated structural changes involve transformation of endometrial stromal fibroblasts into secretory, epithelioid-like decidual cells, differentiation of glandular elements, and increased tortuosity of the spiral arteries (102–104). With massive uNK cell influx into the environment at this time, it is suspected that they may play a critical role in this process.
Although current literature demonstrates that uNK cells are present in abundance at time of decidualization, their role in decidualization of endometrial stromal cells is not yet clear. The initiation of an increase in uNK cells begins 3 days after the luteinizing hormone surge (41), before arrival of the embryo. The first stromal changes, however, are not noted until approximately 8 days after the luteinizing hormone surge (41). This temporal appearance suggests that prepregnancy NK cells play a role in the changes to the endometrial stroma that are necessary for successful implantation. This is supported by data showing that adding supernatant from cultured uNK cells to endometrial stromal cells cocultured with uterine epithelial cells led to significant changes in expression of genes known to be markers of decidualization (105). In addition, this coculture system demonstrated the presence of a positive feedback loop where uNK cells promoted stromal cell production of factors such as IL-15 involved in uNK cell proliferation and localization to the endometrium. In addition, another study reinforced the presence of this positive feedback loop, demonstrating that pregnancy uNK cells additionally secreted factors such as IL-25 required for decidualized stromal cell proliferation (106). In addition, this work tested the functional influence of pregnancy uNK cells on stromal cells and found that decidualization was accelerated in the presence of uNK cells, as measured by prolactin secretion and upregulation of IL-25 production (106). While these studies provide compelling evidence for a role for uNK cells in decidualization, both utilized uNK cells isolated from first trimester tissue after the onset of pregnancy (Table 2). In humans, stromal cell decidualization takes place before pregnancy, and examining how prepregnancy uNK cells interact with stromal cells is, thus, imperative. However, these data including temporal relationship of uNK abundance increase before decidualization as well as evidence of crosstalk between uNK and stromal cells with functional evidence of uNK importance in endometrial decidualization (Fig. 2A), integral to human pregnancy establishment.
FIGURE 2.
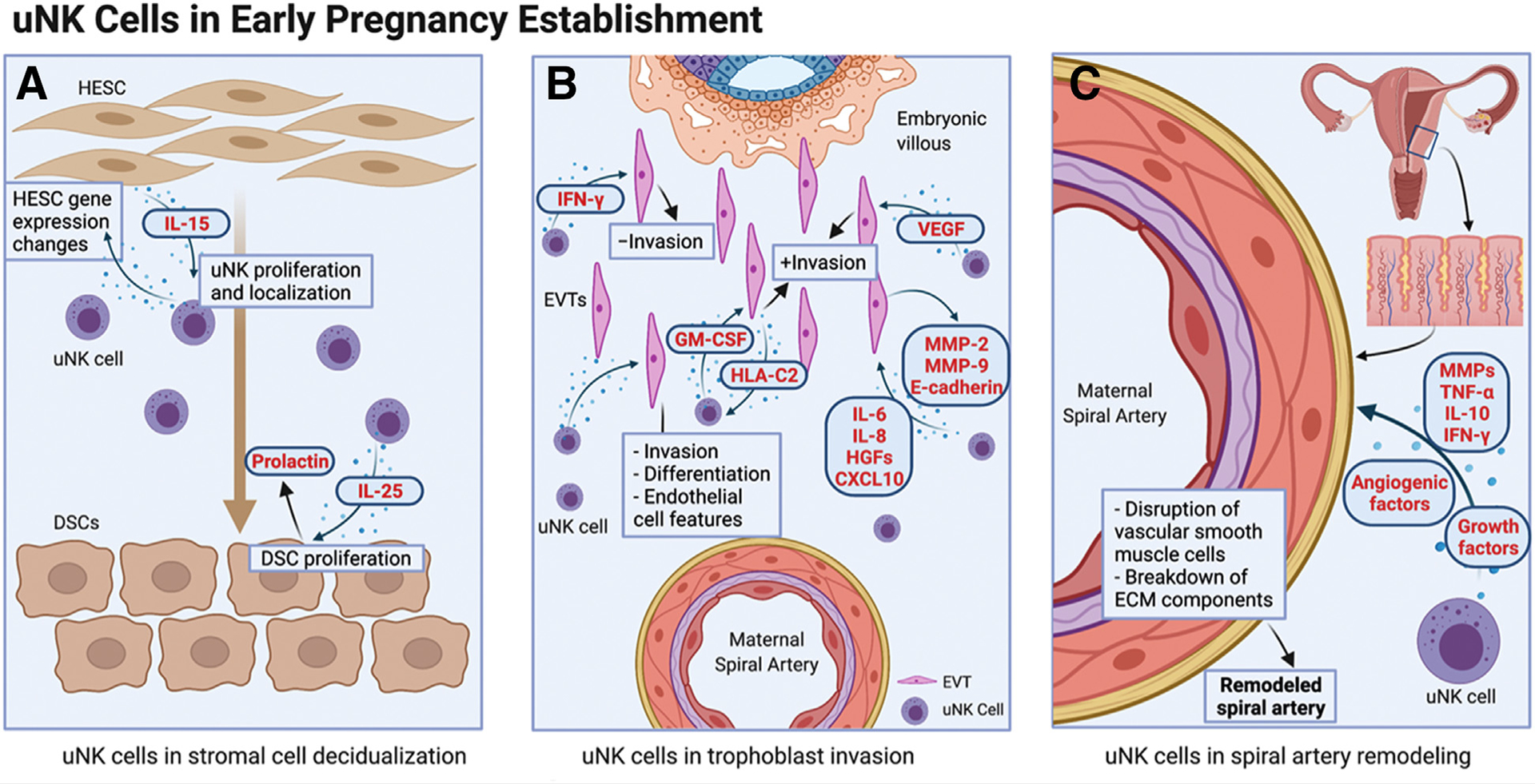
Uterine natural killer cells in early pregnancy establishment. (A) Influence of uNK cells on stromal cell decidualization. (B) Uterine natural killer cells in regulation of trophoblast invasion. (C) Uterine natural killer promotion of spiral artery remodeling. CXCL = c-x-c motif chemokine ligand; DSC = decidualized stromal cell; IL = interleukin; IFN-γ = interferon gamma; GM-CSF = granulocyte macrophage colony-stimulating factor; HESC = human endometrial stromal cell; HLA-C2 = human leukocyte antigen C2; HGF = hepatocyte growth factor; MMP = matrix metalloproteinase; TNF-α = tumor necrosis factor alpha. uNK = uterine natural killer cell; VEGF = vascular endothelial growth factor. Created with BioRender.com.
Role of uNK Cells in Trophoblast Invasion
During the process of implantation and establishment of the placenta, there are two routes of EVT invasion. The first occurs through the decidua, giving rise to interstitial EVTs (61). The second is up the lumen of the spiral arteries, giving rise to endovascular EVTs (61). This second group of EVTs takes on characteristics of endothelial cells and replaces maternal endothelial cells within the dilated spiral arteries. Extravillous trophoblast invasion is essential for establishment of the placental bed for the purpose of oxygen and nutrient exchange between the mother and developing fetus. Uterine NK cells have been shown to assist in regulation of this process.
Uterine NK cells produce cytokines that regulate EVT invasion. This has been demonstrated through several mechanisms. Supernatant from cultured uNK cells obtained from late first trimester abortion tissue stimulates EVT invasion from placental explants through a Matrigel layer (107, 108). In addition, uterine NK cell conditioned medium is able to promote trophoblast differentiation and invasion of cultured primary cytotrophoblasts and an EVT cell line through Matrigel. Additionally, this conditioned medium enables trophoblasts to acquire endothelial cell features (109).
Specific agents of chemoattraction have additionally been proffered as a mechanism of influence via uNK release of hepatocyte growth factors, IL-8, CXCL10, and IL-6 (110, 111). Further investigation of the role of IL-8 in this process demonstrates high levels of IL-8 protein and mRNA in uNK cells, stimulation of EVT invasion with exogenous IL-8, upregulation of matrix metalloproteinase (MMP)-2 with exposure to IL-8, and finally significant reduction in EVT invasion with exposure to IL-8 neutralizing antibody (108).
In vitro, granulocyte macrophage colony-stimulating factor is known to enhance primary trophoblast invasion. Granulocyte macrophage colony-stimulating factor production by pregnancy NK cells increases when activated by HLA-C2 (expressed by trophoblasts), thereby demonstrating positive regulation of invasion by the NK cells (112). In addition, vascular endothelial growth factor expression by uNK cells appears to play a role as coculture of uNK cells treated with a sphingosine-I-phosphate analog and receptor modulator, leading to decreased VEGF expression results in decreased EVT migration compared with untreated uNK cell coculture (113).
Other studies have demonstrated uNK cell inhibition of EVT invasion. Exposure of first trimester trophoblasts to concordant first trimester uNK cells demonstrates contact-independent inhibition of EVT invasion. On further investigation, changes in protease expression (MMP-2 and MMP-9) and E-cadherin expression were documented in the EVTs. Finally, interferon gamma (IFN-gamma) has been shown as a potential mediator of this process with demonstrated partial reversal of EVT invasion inhibition with introduction of anti-IFN-gamma (114).
These data demonstrate the functional effect of various secreted products of uNK cells on EVT invasion, thereby supporting the role of uNK cells in the regulation of trophoblast invasion (Fig. 2B). This multimodal influence of uNK cells on trophoblast invasion may again have implications for dysfunctional placentation and subsequent adverse perinatal outcomes.
Role of uNK Cells in Spiral Artery Remodeling
The spiral arteries of the endometrium are a transient component of the uterine architecture, providing blood supply to the endometrium during the luteal phase of the menstrual cycle. Remodeling of these arteries must occur to allow successful implantation of a human embryo and integration with the maternal vascular supply. This process involves loss of the musculoelastic structure of the arteries with fibrinoid material replacement of the vascular media (61, 110, 115). During this time, the endothelium swells with resultant desquamation and sloughing into the lumen (116). In addition, the arteries dilate to decrease resistance to blood flow (61). The process continues into early pregnancy with completion of the remodeling of the decidual portion of the spiral arteries by 10–12 weeks’ gestation and the myometrial portion of these vessels by 14–16 weeks’ gestation (61). These structural changes have both trophoblast-independent and trophoblast-dependent stages (62). This has been demonstrated in the setting of ectopic pregnancy with notable structural changes to the endometrium without the presence of an intrauterine gestation (117). This complex process is integral for successful placentation in early pregnancy. There are a number of influences at play in guiding this process.
Uterine NK cells can be found in the vicinity of the spiral arteries during spiral artery remodeling, suggesting a role in this process (115). Culture media from uNK cells can induce disruption of vascular smooth muscle cells and breakdown of extracellular matrix components as required for spiral artery remodeling (62). Additionally, uNK cells are known to secrete MMPs, which are required for disruption and loss of cohesion between layers of vascular smooth muscle (115). In addition to MMPs, uNK cells secrete cytokines such as tumor necrosis factor alpha, IFN-gamma, and IL-10; growth factors such as granulocyte macrophage colony-stimulating factor and placental growth factor; and angiogenic factors such as VEGF-C, angiopoietins 1 and 2, and transforming growth factor beta 1, all of which have been implicated in spiral artery remodeling (33, 61, 62, 110–112). Notably, the production of angiogenic growth factors by uNK cells during pregnancy decreases with increasing gestational age, further supporting a unique temporal role of these cells during spiral artery remodeling (61).
To further explore how uNK cells could functionally impact early pregnancy, several knockout mouse models have been developed that are deficient in NK cells. Such studies demonstrate lack of spiral artery remodeling as well as vacuolated decidua. This results in no development of metrial glands, imperative for ongoing pregnancy in the mouse, as well as markedly decreased placental surface areas compared with normal controls. Pregnancies in these mice demonstrate adverse outcomes including decreased litter size, increased rates of intrauterine demise, intrauterine growth restriction, and offspring with persistent weight deficit into adulthood with severity of such outcomes dependent on the knockout approach (118, 119). In another model of mice severely deficient in NK cells, <1% of normal frequency additionally demonstrated implantation site histopathological abnormalities with subsequent fetal death or growth restriction (120). A subsequent study then transplanted bone marrow from mice with preserved NK cells and found that spiral artery remodeling was restored leading to improvement of fetal viability as well as increase in placenta size (121). Later literature supported these findings utilizing a mouse NK-depleted model to demonstrate delayed spiral artery development (122). This group further linked this finding back to trophoblast invasion with demonstration of decreased oxygen tension at site of placentation with subsequent effect on trophoblast lineage decisions (122).
This literature supports the role of uNK cells in spiral artery remodeling through human studies demonstrating not only their location adjacent to spiral arteries but also a number of soluble factors that regulate this process (Fig. 2C), while murine studies suggest functional implications.
DISCUSSION
Uterine NK cells dominate the maternal uterine immune cell environment and accumulate in the uterus at timepoints critical for embryo implantation and placentation. As discussed in this review, emerging studies point to an active involvement of these cells in processes necessary for appropriate placentation: stromal cell decidualization, trophoblast invasion, and spiral artery remodeling. In addition, there has been considerable evidence suggesting that disturbances in uNK cell number and/or function play a role in disorders of placentation. Prematurely, clinical tests have already emerged to measure NK cell abundance as a marker for successful pregnancy outcome (123, 124). It is critical that we first understand the potentially changing role of NK cells throughout pregnancy to thoroughly comprehend the biologic bases of these pathologies and eventually translate these to clinical practice.
Uterine NK cells are one of many significant maternal cell types that interact with and potentially regulate EVTs during early pregnancy (125). To properly investigate their function in a physiologically relevant manner, we must use systems that incorporate other maternal as well as fetal cell types. These studies, however, prove challenging because of a lack of animal and in vitro models that accurately replicate embryo implantation and early human placentation as well as ethical considerations around in vivo human studies. Animal models currently used, including mice, rats, guinea pigs, and primates, are unable to recapitulate the depth of invasion and cellular complexity seen in human placentation (126–131). In vitro studies most often examine uNK cells with just one other fetal or maternal cell type, failing to mimic the complex interplay between EVTs and the multicellular maternal environment (132–134). Human tissue studies often use the term placenta, which has a significant cellular difference; placental bed biopsies, which do not include all relevant cell types; or hysterectomized uteri, which often have underlying pathology (2, 135). It is critical for us to develop novel methods for studying early placentation to expand our understanding of the cells and factors that regulate this critical process.
While these issues are common to other studies examining implantation and placentation, the current work studying uNK cells involves specific limitations as well. Foremost is the extrapolation of behavior from peripheral immune cells despite unique characteristics of uNK cell populations (136, 137). Additionally, uNK cells from animal models show distinct developmental, phenotypic, and functional differences compared with human uNK cells, reducing the translatability of findings in animal models (137). In addition, functional readouts when studying uNK cells are unclear, since cytotoxicity assays such as chromium release assay, considered the “gold standard” in testing NK cell function, may not be relevant to the function of uNK cells (138). Finally, obtaining nonpregnant human tissue during the window of implantation and first trimester termination tissue is challenging. Without human tissue, the study of secretory-phase NK cells becomes extremely limited because mouse uteri, the most commonly used animal model in the study of early pregnancy, do not contain mature NK cells before implantation (139). Current studies should be interpreted keeping in mind these difficulties, and developing a physiologically relevant in vitro alternative to study uNK cell function is a critical next step.
Although this review brings to light a body of evidence indicating a significant role of uNK cells in early pregnancy establishment, the remainder of the immune cell environment merits attention. Comparison of the immune environment between late proliferative and midsecretory endometrium demonstrates upregulation of immune-related genes. This includes genes related to not only proliferation of uNK cells but also inhibition of T cell growth and the classical complement pathway (140). Studies have demonstrated that, like NK cells, T regulatory cells additionally increase in numbers within the endometrium before embryo implantation and during pregnancy (141). Others have shown that the overall number of T regulatory cells is decreased in women with recurrent miscarriage and numbers may in addition be linked to the development of preeclampsia (142). These findings remind us of the complexity of crosstalk required for normal pregnancy establishment and ongoing pregnancy, with a need for expansion to study the entirety of the immune cell environment within the uterus.
In conclusion, early pregnancy implantation is a key element of healthy pregnancy establishment, which remains incompletely understood. Literature points to a likely essential role of the immune system in this process, particularly as seen here with the unique subpopulation of uNK cells. Evidence suggests that these uNK cells are involved in endometrial decidualization, spiral artery remodeling, and trophoblast invasion, all of which are essential for normal placentation. Shortcomings in each of these essential steps for implantation can lead to adverse perinatal outcomes such as hypertensive disorders of pregnancy, intrauterine growth restriction, and RIF. Therefore, determining failures with regard to uNK influence on these processes may provide new targets for medical intervention. Further research is needed to clarify the mechanism of action of uNK cells in this domain to guide such intervention. To accomplish this, special care must be taken to not generalize uNK cells as one homogenous population and distinguish between NK cell subtypes in any future investigation. Finally, this field would greatly benefit from in vitro model systems that accurately capture the complexities of the maternal–fetal interface.
ESSENTIAL POINTS.
Uterine natural killer (uNK) cells are a critical component of maternal-fetal crosstalk required for normal placentation with evidence of playing a role in endometrial decidualization, spiral artery remodeling, and trophoblast invasion.
Available data with regard to changes in uNK cell abundance in adverse pregnancy outcomes demonstrates mixed results possibly because of variability in sampling and quantification techniques.
Evidence of aberrant uNK cell function as a contributing factor to adverse pregnancy outcomes in humans is present but limited in the literature, attributable to the difficulties of performing such analyses on human tissues.
Understanding of the function of uNK cells in pregnancy establishment and outcomes would be furthered substantially with a multicellular in vitro model system with the ability to accurately capture the complexity of the maternal–fetal interface.
Acknowledgments
J.R.K. reports grants from the National Center for Advancing Translational Sciences of the National Institutes of Health during the conduct of the study. S.M. has nothing to disclose. S.M.G. has nothing to disclose. M.M. has nothing to disclose.
Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880. The content is solely the responsibility of the investigators and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Goldman-Wohl D, Yagel S. Regulation of trophoblast invasion: from normal implantation to pre-eclampsia. Mol Cell Endocrinol 2002;187: 233–8. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 2003;69:1–7. [DOI] [PubMed] [Google Scholar]
- 3.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2003;189: 1063–9. [DOI] [PubMed] [Google Scholar]
- 4.Tantbirojn P, Crum CP, Parast MM. Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008;29:639–45. [DOI] [PubMed] [Google Scholar]
- 5.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med 2001;345:1400–8. [DOI] [PubMed] [Google Scholar]
- 7.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest 2004;114:744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitley GS, Cartwright JE. Cellular and molecular regulation of spiral artery remodelling: lessons from the cardiovascular field. Placenta 2010;31:465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 2006;27:939–58. [DOI] [PubMed] [Google Scholar]
- 10.Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol 2005;106:1039–45. [DOI] [PubMed] [Google Scholar]
- 11.Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjærven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod 2006;21:2353–8. [DOI] [PubMed] [Google Scholar]
- 12.Weinerman R, Ord T, Bartolomei MS, Coutifaris C, Mainigi M. The super-ovulated environment, independent of embryo vitrification, results in low birthweight in a mouse model. Biol Reprod 2017;97:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalra SK, Ratcliffe SJ, Coutifaris C, Molinaro T, Barnhart KT. Ovarian stimulation and low birth weight in newborns conceived through in vitro fertilization. Obstet Gynecol 2011;118:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril 2014;102:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Waal E, Vrooman LA, Fischer E, Ord T, Mainigi MA, Coutifaris C, et al. The cumulative effect of assisted reproduction procedures on placental development and epigenetic perturbations in a mouse model. Hum Mol Genet 2015;24:6975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasson IE, Vitins AP, Mainigi MA, Moley KH, Simmons RA. Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice. Diabetologia 2015;58:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol 2007;36:412–9. [DOI] [PubMed] [Google Scholar]
- 18.Radulescu L, Munteanu O, Popa F, Cirstoiu M. The implications and consequences of maternal obesity on fetal intrauterine growth restriction. J Med Life 2013;6:292–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds LP, Borowicz PP, Palmieri C, Grazul-Bilska AT. Placental vascular defects in compromised pregnancies: effects of assisted reproductive technologies and other maternal stressors. Adv Exp Med Biol 2014;814:193–204. [DOI] [PubMed] [Google Scholar]
- 20.Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol 2016;4:1025–36. [DOI] [PubMed] [Google Scholar]
- 21.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017;17:469–82. [DOI] [PubMed] [Google Scholar]
- 22.Triggianese P, Perricone C, Chimenti MS, De Carolis C, Perricone R. Innate immune system at the maternal-fetal interface: mechanisms of disease and targets of therapy in pregnancy syndromes. Am J Reprod Immunol 2016; 76:245–57. [DOI] [PubMed] [Google Scholar]
- 23.Lee JY, Lee M, Lee SK. Role of endometrial immune cells in implantation. Clin Exp Reprod Med 2011;38:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King A, Wellings V, Gardner L, Loke YW. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol 1989;24: 195–205. [DOI] [PubMed] [Google Scholar]
- 25.Rieger L, Segerer S, Bernar T, Kapp M, Majic M, Morr AK, et al. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia–a prospective observational study. Reprod Biol Endocrinol 2009;7:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol 2009;82:24–31. [DOI] [PubMed] [Google Scholar]
- 27.Kwak-Kim J, Gilman-Sachs A. Clinical implication of natural killer cells and reproduction. Am J Reprod Immunol 2008;59:388–400. [DOI] [PubMed] [Google Scholar]
- 28.Bianco J, Stephenson K, Yamada AT, Croy BA. Time-course analyses addressing the acquisition of DBA lectin reactivity in mouse lymphoid organs and uterus during the first week of pregnancy. Placenta 2008; 29:1009–15. [DOI] [PubMed] [Google Scholar]
- 29.Croy BA, Zhang J, Tayade C, Colucci F, Yadi H, Yamada AT. Analysis of uterine natural killer cells in mice. Methods Mol Biol 2010;612:465–503. [DOI] [PubMed] [Google Scholar]
- 30.Paffaro VA, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 2003;24: 479–88. [DOI] [PubMed] [Google Scholar]
- 31.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008;9:503–10. [DOI] [PubMed] [Google Scholar]
- 32.Matson BC, Caron KM. Uterine natural killer cells as modulators of the maternal-fetal vasculature. Int J Dev Biol 2014;58:199–204. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson M, Meadows SK, Wira CR, Sentman CL. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J Leukoc Biol 2004;76:667–75. [DOI] [PubMed] [Google Scholar]
- 34.Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens 2004;63:1–12. [DOI] [PubMed] [Google Scholar]
- 35.Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, et al. Endometrial NK cells are special immature cells that await pregnancy. J Immunol [Internet] 2008;181(3):1869–1876. Available at: http://10.0.15.209/jimmunol.181.3.1869. Accessed July 29, 2019. [DOI] [PubMed] [Google Scholar]
- 36.Manaster I, Mandelboim O. The unique properties of human NK cells in the uterine mucosa. Placenta 2008;29(Suppl A):S60–6. [DOI] [PubMed] [Google Scholar]
- 37.Kopcow HD, Eriksson M, Mselle TF, Damrauer SM, Wira CR, Sentman CL, et al. Human decidual NK cells from gravid uteri cells and NK cells from cycling endometrium are distinct NK cell subsets. Placenta 2010;31:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flynn L, Byrne B, Carton J, Kelehan P, O’Herlihy C, O’Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from nonpregnant human endometrium. Am J Reprod Immunol 2000;43:209–17. [DOI] [PubMed] [Google Scholar]
- 39.Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod 1991;6:791–8. [DOI] [PubMed] [Google Scholar]
- 40.Ordi J, Casals G, Ferrer B, Creus M, Guix C, Palacín A, et al. Uterine (CD56+) natural killer cells recruitment: association with decidual reaction rather than embryo implantation. Am J Reprod Immunol 2006;55:369–77. [DOI] [PubMed] [Google Scholar]
- 41.King A Uterine leukocytes and decidualization. Hum Reprod Update 2000; 6:28–36. [DOI] [PubMed] [Google Scholar]
- 42.Hannan NJ, Jones RL, Critchley HO, Kovacs GJ, Rogers PAW, Affandi B, et al. Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestin-only contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J Clin Endocrinol Metab 2004;89:6119–29. [DOI] [PubMed] [Google Scholar]
- 43.Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab 2004;89:6155–67. [DOI] [PubMed] [Google Scholar]
- 44.Kitaya K, Yamaguchi T, Yasuo T, Okubo T, Honjo H. Post-ovulatory rise of endometrial CD16(−) natural killer cells: in situ proliferation of residual cells or selective recruitment from circulating peripheral blood? J Reprod Immunol 2007;76:45–53. [DOI] [PubMed] [Google Scholar]
- 45.Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A 2007;104:3378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol 2013;190:3939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004;292:81–5. [DOI] [PubMed] [Google Scholar]
- 48.Strunz B, Bister J, Jönsson H, Filipovic I, Crona-Guterstam Y, Kvedaraite E, et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci Immunol 2021;6:eabb7800. [DOI] [PubMed] [Google Scholar]
- 49.Matsuura-Sawada R, Murakami T, Ozawa Y, Nabeshima H, Akahira JI, Sato Y, et al. Reproduction of menstrual changes in transplanted human endometrial tissue in immunodeficient mice. Hum Reprod 2005;20: 1477–84. [DOI] [PubMed] [Google Scholar]
- 50.Lynch L, Golden-Mason L, Eogan M, O’Herlihy C, O’Farrelly C. Cells with haematopoietic stem cell phenotype in adult human endometrium: relevance to infertility? Hum Reprod 2007;22:919–26. [DOI] [PubMed] [Google Scholar]
- 51.Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U S A 2011;108:2402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szereday L, Miko E, Meggyes M, Barakonyi A, Farkas B, Varnagy A, et al. Commitment of decidual haematopoietic progenitor cells in first trimester pregnancy. Am J Reprod Immunol 2012;67:9–16. [DOI] [PubMed] [Google Scholar]
- 53.Bulmer JN, Lash GE. Uterine natural killer cells: time for a re-appraisal? F1000Res 2019;8:F1000, Faculty Rev-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson DA, Greaves E, Critchley HO, Saunders PT. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum Reprod 2015;30:1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sentman CL, Meadows SK, Wira CR, Eriksson M. Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J Immunol 2004;173:6760–6. [DOI] [PubMed] [Google Scholar]
- 56.Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018;563:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suryawanshi H, Morozov P, Straus A, Sahasrabudhe N, Max KEA, Garzia A, et al. A single-cell survey of the human first-trimester placenta and decidua. Sci Adv 2018;4:eaau4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huhn O, Ivarsson MA, Gardner L, Hollinshead M, Stinchcombe JC, Chen P, et al. Distinctive phenotypes and functions of innate lymphoid cells in human decidua during early pregnancy. Nat Commun 2020;11:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R, et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity 2018;48:951–62.e5. [DOI] [PubMed] [Google Scholar]
- 60.Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z, et al. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity 2017;47:1100–13.e6. [DOI] [PubMed] [Google Scholar]
- 61.Lash GE, Schiessl B, Kirkley M, Innes B, Cooper A, Searle R, et al. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol 2006;80:572–80. [DOI] [PubMed] [Google Scholar]
- 62.Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, et al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012;26:4876–85. [DOI] [PubMed] [Google Scholar]
- 63.Klentzeris LD, Bulmer JN, Warren MA, Morrison L, Li TC, Cooke ID. Lymphoid tissue in the endometrium of women with unexplained infertility: morphometric and immunohistochemical aspects. Hum Reprod 1994;9:646–52. [DOI] [PubMed] [Google Scholar]
- 64.Parkin KL, Lessey BA, Young SL, Fazleaba AT. Comparison of NK cell phenotypes in the endometrium of patients with recurrent pregnancy loss versus unexplained infertility. Am J Reprod Immunol 2011;65:43. [Google Scholar]
- 65.Kofod L, Lindhard A, Bzorek M, Eriksen JO, Larsen LG, Hviid TVF. Endometrial immune markers are potential predictors of normal fertility and pregnancy after in vitro fertilization. Am J Reprod Immunol 2017;78. [DOI] [PubMed] [Google Scholar]
- 66.Fukui A, Fujii S, Yamaguchi E, Kimura H, Sato S, Saito Y. Natural killer cell subpopulations and cytotoxicity for infertile patients undergoing in vitro fertilization. Am J Reprod Immunol 1999;41:413–22. [DOI] [PubMed] [Google Scholar]
- 67.Mcgrath E, Ryan EJ, Lynch L, Golden-Mason L, Mooney E, Eogan M, et al. Changes in endometrial natural killer cell expression of CD94, CD158a and CD158b are associated with infertility. Am J Reprod Immunol 2009;61: 265–76. [DOI] [PubMed] [Google Scholar]
- 68.Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 2014;72:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lédée-Bataille N, Dubanchet S, Kadoch J, Castelo-Branco A, Frydman R, Chaouat G. Controlled natural in vitro fertilization may be an alternative for patients with repeated unexplained implantation failure and a high uterine natural killer cell count. Fertil Steril 2004;82:234–6. [DOI] [PubMed] [Google Scholar]
- 70.Matteo MG, Greco P, Rosenberg P, Mestice A, Baldini D, Falagario T, et al. Normal percentage of CD56bright natural killer cells in young patients with a history of repeated unexplained implantation failure after in vitro fertilization cycles. Fertil Steril 2007;88:990–3. [DOI] [PubMed] [Google Scholar]
- 71.Lédée N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol 2016;75:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donoghue JF, Paiva P, Teh WT, Cann LM, Nowell C, Rees H, et al. Endometrial uNK cell counts do not predict successful implantation in an IVF population. Hum Reprod 2019;34:2456–66. [DOI] [PubMed] [Google Scholar]
- 73.Tohma YA, Musabak U, Gunakan E, Akilli H, Onalan G, Zeyneloglu HB. The role of analysis of NK cell subsets in peripheral blood and uterine lavage samples in evaluation of patients with recurrent implantation failure. J Gynecol Obstet Hum Reprod 2020;49:101793. [DOI] [PubMed] [Google Scholar]
- 74.Chen X, Mariee N, Jiang L, Liu Y, Wang CC, Li TC, et al. Measurement of uterine natural killer cell percentage in the periimplantation endometrium from fertile women and women with recurrent reproductive failure: establishment of a reference range. Am J Obstet Gynecol 2017;217:680.e1–6. [DOI] [PubMed] [Google Scholar]
- 75.Marron K, Walsh D, Harrity C. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J Assist Reprod Genet 2019;36:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marron K, Harrity C. Endometrial lymphocyte concentrations in adverse reproductive outcome populations. J Assist Reprod Genet 2019;36:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwak JY, Beer AE, Kim SH, Mantouvalos HP. Immunopathology of the implantation site utilizing monoclonal antibodies to natural killer cells in women with recurrent pregnancy losses. Am J Reprod Immunol 1999; 41:91–8. [DOI] [PubMed] [Google Scholar]
- 78.Tuckerman E, Laird SM, Prakash A, Li TC. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum Reprod 2007;22:2208–13. [DOI] [PubMed] [Google Scholar]
- 79.Hill JA, Melling GC, Johnson PM. Immunohistochemical studies of human uteroplacental tissues from first-trimester spontaneous abortion. Am J Obstet Gynecol 1995;173:90–6. [DOI] [PubMed] [Google Scholar]
- 80.Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol 1996;156:4027–34. [PubMed] [Google Scholar]
- 81.Quack KC, Vassiliadou N, Pudney J, Anderson DJ, Hill JA. Leukocyte activation in the decidua of chromosomally normal and abnormal fetuses from women with recurrent abortion. Hum Reprod 2001;16:949–55. [DOI] [PubMed] [Google Scholar]
- 82.Shimada S, Kato EH, Morikawa M, Iwabuchi K, Nishida R, Kishi R, et al. No difference in natural killer or natural killer T-cell population, but aberrant T-helper cell population in the endometrium of women with repeated miscarriage. Hum Reprod 2004;19:1018–24. [DOI] [PubMed] [Google Scholar]
- 83.Michimata T, Ogasawara MS, Tsuda H, Suzumori K, Aoki K, Sakai M, et al. Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortion. Am J Reprod Immunol 2002;47:196–202. [DOI] [PubMed] [Google Scholar]
- 84.Vassiliadou N, Bulmer JN. Immunohistochemical evidence for increased numbers of “classic” CD57+ natural killer cells in the endometrium of women suffering spontaneous early pregnancy loss. Hum Reprod 1996; 11:1569–74. [DOI] [PubMed] [Google Scholar]
- 85.Clifford K, Flanagan AM, Regan L. Endometrial CD56+ natural killer cells in women with recurrent miscarriage: a histomorphometric study. Hum Reprod 1999;14:2727–30. [DOI] [PubMed] [Google Scholar]
- 86.Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, et al. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod 1999;14:2386–91. [DOI] [PubMed] [Google Scholar]
- 87.Emmer PM, Steegers EA, Kerstens HM, Bulten J, Nelen WL, Boer K, et al. Altered phenotype of HLA-G expressing trophoblast and decidual natural killer cells in pathological pregnancies. Hum Reprod 2002;17:1072–80. [DOI] [PubMed] [Google Scholar]
- 88.Hosseini S, Zarnani AH, Asgarian-Omran H, Vahedian-Dargahi Z, Eshraghian MR, Akbarzadeh-Pasha Z, et al. Comparative analysis of NK cell subsets in menstrual and peripheral blood of patients with unexplained recurrent spontaneous abortion and fertile subjects. J Reprod Immunol 2014;103:9–17. [DOI] [PubMed] [Google Scholar]
- 89.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 1991;98:648–55. [DOI] [PubMed] [Google Scholar]
- 90.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol 1981;88:876–81. [DOI] [PubMed] [Google Scholar]
- 91.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–74. [DOI] [PubMed] [Google Scholar]
- 92.Fraser R, Whitley GS, Johnstone AP, Host AJ, Sebire NJ, Thilaganathan B, et al. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol 2012;228:322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallace AE, Whitley GS, Thilaganathan B, Cartwright JE. Decidual natural killer cell receptor expression is altered in pregnancies with impaired vascular remodeling and a higher risk of pre-eclampsia. J Leukoc Biol 2015;97:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sones JL, Cha J, Woods AK, Bartos A, Heyward CY, Lob HE, et al. Decidual Cox2 inhibition improves fetal and maternal outcomes in a preeclampsia-like mouse model. JCI Insight 2016;1:e75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li M, Schwerbrock NM, Lenhart PM, Fritz-Six KL, Kadmiel M, Christine KS, et al. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest 2013;123:2408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khong TY, Robertson WB. Placenta creta and placenta praevia creta. Placenta 1987;8:399–409. [DOI] [PubMed] [Google Scholar]
- 97.Laban M, Ibrahim EA, Elsafty MS, Hassanin AS. Placenta accreta is associated with decreased decidual natural killer (dNK) cells population: a comparative pilot study. Eur J Obstet Gynecol Reprod Biol 2014;181: 284–8. [DOI] [PubMed] [Google Scholar]
- 98.Sliz A, Locker KCS, Lampe K, Godarova A, Plas DR, Janssen EM, et al. Gab3 is required for IL-2- and IL-15-induced NK cell expansion and limits trophoblast invasion during pregnancy. Sci Immunol 2019;4:eaav3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16:621–38. [DOI] [PubMed] [Google Scholar]
- 100.Stuart TJ, O’Neill K, Condon D, Sasson I, Sen P, Xia Y, et al. Diet-induced obesity alters the maternal metabolome and early placenta transcriptome and decreases placenta vascularity in the mouse. Biol Reprod 2018;98: 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod 2001;16:221–5. [DOI] [PubMed] [Google Scholar]
- 102.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 2007;25:445–53. [DOI] [PubMed] [Google Scholar]
- 103.Moffett A, Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest 2014;124:1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 1999;140:4809–20. [DOI] [PubMed] [Google Scholar]
- 105.Gong X, Liu Y, Chen Z, Xu C, Lu Q, Jin Z. Insights into the paracrine effects of uterine natural killer cells. Mol Med Rep 2014;10:2851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Wang Y, Wang XH, Zhou WJ, Jin LP, Li MQ. Crosstalk between human endometrial stromal cells and decidual NK cells promotes decidualization in vitro by upregulating IL-25. Mol Med Rep 2018;17:2869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lash GE, Otun HA, Innes BA, Percival K, Searle RF, Robson SC, et al. Regulation of extravillous trophoblast invasion by uterine natural killer cells is dependent on gestational age. Hum Reprod 2010;25:1137–45. [DOI] [PubMed] [Google Scholar]
- 108.De Oliveira LG, Lash GE, Murray-Dunning C, Bulmer JN, Innes BA, Searle RF, et al. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta 2010;31:595–601. [DOI] [PubMed] [Google Scholar]
- 109.Ma L, Li G, Cao G, Zhu Y, Du MR, Zhao Y, et al. dNK cells facilitate the interaction between trophoblastic and endothelial cells via VEGF-C and HGF. Immunol Cell Biol 2017;95:695–704. [DOI] [PubMed] [Google Scholar]
- 110.Faas MM, De Vos P. Innate immune cells in the placental bed in healthy pregnancy and preeclampsia. Placenta 2018;69:125–33. [DOI] [PubMed] [Google Scholar]
- 111.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006;12:1065–74. [DOI] [PubMed] [Google Scholar]
- 112.Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, et al. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest 2013;123:4264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang J, Dunk CE, Lye SJ. Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum Reprod 2013;28: 3026–37. [DOI] [PubMed] [Google Scholar]
- 114.Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R, von Dadelszen P. Decidual NK cells alter in vitro first trimester extravillous cytotrophoblast migration: a role for IFN-gamma. J Immunol 2006;177:8522–30. [DOI] [PubMed] [Google Scholar]
- 115.Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 2009;174:1959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hazan AD, Smith SD, Jones RL, Whittle W, Lye SJ, Dunk CE. Vascular-leukocyte interactions: mechanisms of human decidual spiral artery remodeling in vitro. Am J Pathol 2010;177:1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta 1998;19:241–52. [DOI] [PubMed] [Google Scholar]
- 118.Croy BA, Ashkar AA, Foster RA, DiSanto JP, Magram J, Carson D, et al. Histological studies of gene-ablated mice support important functional roles for natural killer cells in the uterus during pregnancy. J Reprod Immunol 1997;35:111–33. [DOI] [PubMed] [Google Scholar]
- 119.Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 2003;171:2937–44. [DOI] [PubMed] [Google Scholar]
- 120.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod 1997;56:169–79. [DOI] [PubMed] [Google Scholar]
- 121.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med 1998;187: 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci USA 2011;108: 16295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alecsandru D, García-Velasco JA. Immune testing and treatment: still an open debate. Hum Reprod 2015;30:1994. [DOI] [PubMed] [Google Scholar]
- 124.Moffett A, Shreeve N. First do no harm: uterine natural killer (NK) cells in assisted reproduction. Hum Reprod 2015;30:1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pollheimer J, Vondra S, Baltayeva J, Beristain AG, Knöfler M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front Immunol 2018;9:2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med 2010;28:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carter AM, Mess AM. Mammalian placentation: implications for animal models. In: McManus LM, RNM, editors. Pathobiology of human disease: a dynamic encyclopedia of disease mechanisms; 2014:2423–42. [Google Scholar]
- 128.Grigsby PL. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med 2016;34:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carter AM. Animal models of human placentation–a review. Placenta 2007;28(Suppl A):S41–7. [DOI] [PubMed] [Google Scholar]
- 130.Swanson AM, David AL. Animal models of fetal growth restriction: considerations for translational medicine. Placenta 2015;36:623–30. [DOI] [PubMed] [Google Scholar]
- 131.Silva JF, Serakides R. Intrauterine trophoblast migration: a comparative view of humans and rodents. Cell Adh Migr 2016;10:88–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod 2010; 25:862–73. [DOI] [PubMed] [Google Scholar]
- 133.James JL, Cartwright JE, Whitley GS, Greenhill DR, Hoppe A. The regulation of trophoblast migration across endothelial cells by low shear stress: consequences for vascular remodelling in pregnancy. Cardiovasc Res 2012;93:152–61. [DOI] [PubMed] [Google Scholar]
- 134.Campbell S, Rowe J, Jackson CJ, Gallery EDM. In vitro migration of cytotrophoblasts through a decidual endothelial cell monolayer: the role of matrix metalloproteinases. Placenta 2003;24:306–15. [DOI] [PubMed] [Google Scholar]
- 135.Priming Lyall F. and remodelling of human placental bed spiral arteries during pregnancy–a review. Placenta 2005;26(Suppl A):S31–6. [DOI] [PubMed] [Google Scholar]
- 136.Faas MM, de Vos P. Uterine NK cells and macrophages in pregnancy. Placenta 2017;56:44–52. [DOI] [PubMed] [Google Scholar]
- 137.Gaynor LM, Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front Immunol 2017; 8:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kopcow HD, Allan DSJ, Chen X, Rybalov B, Andzelm MM, Ge B, et al. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A 2005;102:15563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev 2006;214:161–85. [DOI] [PubMed] [Google Scholar]
- 140.Lobo SC, Huang ST, Germeyer A, Dosiou C, Vo KC, Tulac S, et al. The immune environment in human endometrium during the window of implantation. Am J Reprod Immunol 2004;52:244–51. [DOI] [PubMed] [Google Scholar]
- 141.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 2009;15:517–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsuda S, Nakashima A, Shima T, Saito S. New paradigm in the role of regulatory T cells during pregnancy. Front Immunol 2019;10:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tilburgs T, Evans JH, Crespo ÂC, Strominger JL. The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface. Proc Natl Acad Sci USA 2015;112:13312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee SK, Kim CJ, Kim D-J, Kang J. Immune cells in the female reproductive tract. Immune Netw 2015;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]


