Abstract
Purpose
To investigate ON-pathway versus OFF-pathway dysfunction in glaucoma using handheld electroretinography (ERG) with a temporally modulated sinusoidal flicker stimulus.
Design
Cross-sectional study.
Participants
Fifty-nine participants accounting for 104 eyes, comprised of 19 control eyes, 26 glaucoma suspect eyes, and 59 glaucoma eyes.
Methods
Participants underwent portable ERG testing, which included the photopic flash, photopic flicker, photopic negative response stimulus, ON-OFF stimulus, and a custom-written sinusoidal flicker stimulus that was modulated from 50 to 0.3 Hz.
Main Outcome and Measures
The ERG response amplitudes were measured by the handheld ERG. For the custom-written sinusoidal flicker stimulus, we derived and compared the log10 first harmonic frequency response amplitudes. Patient discomfort and fatigue after ERG testing were rated on a scale from 1 to 5
Results
Baseline demographics were not significantly different between groups, except for ocular characteristics. Analysis was performed adjusting for participant age, sex, race, and dilation status, and the sinusoidal frequency responses were stratified at 10 Hz because higher frequencies are associated with the OFF-pathway, whereas lower frequencies are associated with the ON-pathway. After stratification, glaucoma eyes showed an adjusted decrease of 32.1% at frequencies of more than 10 Hz (95% confidence interval [CI], −51.8% to −4.1%; P = 0.03). For 10 Hz stimulus frequencies or less, an adjusted 11.5% reduction was found (95% CI, −39.5% to 29.1%; P = 0.50). Glaucoma suspect eyes did show a decreased response, but this was not significant at either frequency range. When comparing handheld ERG with traditional visual field assessments, participants found the handheld ERG to result in much less discomfort and fatigue.
Conclusions
Our finding that glaucoma participants showed greater decreases in ERG response at higher frequencies supports the hypothesis that the OFF-pathway may be more vulnerable in human glaucoma. Using a handheld ERG device with a sinusoidal flicker stimulus may provide an objective assessment of visual function in glaucoma.
Keywords: Electroretinography, Glaucoma, OFF-pathway, ON-pathway, Retinal ganglion cell, Sinusoidal flicker
Abbreviations and Acronyms: CI, confidence interval; ERG, electroretinography; HVF, Humphrey visual field; PERG, pattern electroretinography; PhNR, photopic negative response; RGC, retinal ganglion cell; RNFL, retinal nerve fiber layer; SEM, standard error of the mean
Glaucoma is a set of progressive optic neuropathies characterized by dysfunction and subsequent death of the retinal ganglion cells (RGCs), the neurons whose axons comprise the optic nerve. One major challenge in ameliorating disease burden is that patients often do not recognize their vision loss, because approximately 50% of people with glaucoma do not even know it.1, 2, 3, 4 Furthermore, the current gold standard for visual function assessment involves standard automated perimetry to quantify the degree of visual field loss. This test is prone to subjectivity because it relies on the patient’s ability to respond reliably to visual stimuli and to suppress their foveation reflex.5,6 Additionally, standard automated perimetry integrates responses not just from the retina, but also higher-order visual systems. As a result, increasing attention has been brought to developing electroretinography (ERG) measures to assess visual function in glaucoma, because it measures the electric potential specifically across the retina in response to a visual stimulus.7
Growing evidence from animal models of experimental glaucoma suggests that differences exist in RGC type susceptibility.8, 9, 10, 11, 12, 13, 14, 15 Functionally, OFF-pathway RGCs increase their spiking rate to decrements in light stimulus, whereas ON-pathway RGCs spike in response to increments of light in their receptive field center. Several lines of evidence from ERG studies support the hypothesis that the OFF-pathway may be more vulnerable. Transient pattern electroretinography (PERG) induces a positive response at 50 ms and a negative response at 95 ms. Evidence in both mouse and nonhuman primate models suggests that each wave has contributions primarily from the ON- and OFF-pathway, respectively, although contributions to each wave overlap.16,17 Glaucoma suspect patients and those with preperimetric glaucoma were shown to have greater reductions in the negative PERG peak at 95 ms (N95 peak) compared with the positive PERG peak at 50 ms (P50 peak).18,19 Moreover, studies measuring the ERG response from a photopic negative response (PhNR) stimulus and a sawtooth stimulus found greater reductions with the stimulus correlating with the OFF-pathway versus the ON-pathway.20,21 Finally, glaucoma participants exhibit decreased temporal contrast sensitivity and flicker visual evoked potential losses at higher frequencies.22, 23, 24 Thus, human glaucoma may be associated with greater OFF-pathway susceptibility.
A flicker-type stimulus with varying temporal frequencies is a promising method whereby lower frequencies are associated with the ON-pathway and higher frequencies are associated with the OFF-pathway.25, 26, 27 In the DBA/2J model of experimental glaucoma, ERG responses were decreased most notably at higher flicker frequencies.28 Despite this ability to evaluate the ON- and OFF-pathways, to our knowledge, no studies have modulated the temporal frequency as an approach to measure changes in these pathways in the setting of glaucoma.
The purpose of this study was to determine whether a sinusoidal flicker stimulus modulated between 0.3 and 50 Hz in glaucoma could detect defects in the ON- versus OFF-pathways in glaucoma, glaucoma suspect, and normal participants. We used a handheld ERG device to measure the response amplitudes to a frequency-modulated sinusoidal flicker stimulus. The data were stratified from 10 Hz or less (greater ON-pathway contributions) and more than 10 Hz (greater OFF-pathway contributions) to examine changes further at lower versus higher frequencies.
Methods
Participants
This was a cross-sectional study approved by the Institutional Review Board of the Department of Ophthalmology, University of California, San Francisco. Participants were recruited from June 2019 through November 2019 at the University of California, San Francisco, ophthalmology clinics. For sample size calculations, because limited studies have measured ERG response amplitudes with a flicker stimulus on a handheld device, we based our calculations on outcome standard deviations from a previous handheld ERG study measuring PhNR.29 The number of control and glaucoma participants needed for an α error of 0.05 and a power of 0.85 was 17 and 49, respectively. Written informed consent was obtained from all participants, and testing was carried out the same day of the patient’s scheduled clinic appointment. This study was conducted in accordance with the tenets of the Declaration of Helsinki for research involving human subjects.
In total, 60 participants meeting our inclusion criteria were recruited to participate in this study; 1 participant was excluded from analysis because of excessive blinking artifact during the examination. Inclusion criteria were participants 18 years of age or older, best-corrected visual acuity of 20/60 or better, and a spherical refraction within ±6.0 diopters. We recruited participants regardless of dilation status so as to integrate this study within the normal clinic workflow. Glaucoma participants were defined as patients with open-angle glaucoma, including normal-tension glaucoma, pseudoexfoliation, and pigmentary glaucoma. Participants included had mild, moderate, and severe open-angle glaucoma, as evidenced by optic nerve damage by either optic disc or retinal nerve fiber layer (RNFL) structural abnormalities or reliable and reproducible visual field abnormalities consistent with RNFL damage. Visual field defects included persistent scotoma on at least 2 consecutive standard automated perimetry tests with less than 33% false-positive results, false-negative results, and fixation losses. Abnormal disc appearance included neuroretinal rim thinning, localized or diffuse RNFL defects, disc hemorrhages, or progressive narrowing of the neuroretinal rim with increased cupping, observed with slit-lamp biomicroscopy and a handheld lens or with spectral-domain OCT imaging (Optovue, Inc).
A diagnosis of open-angle glaucoma suspect was established by the presence of a consistently elevated intraocular pressure of more than 21 mmHg, also known as ocular hypertension, or a suspicious optic nerve or RNFL in one or both eyes without visual field defects. Finally, control participants were recruited primarily from comprehensive ophthalmology and optometry practices undergoing cataract evaluation or follow-up and were defined by normal cup-to-disc ratio without evidence of glaucomatous cupping, normal visual field test results, or both. Although some control participants had not undergone a visual field test, we did not exclude them if they showed normal optic nerves and did not meet other exclusion criteria.
Electrophysiologic Recording
The RETeval (LKC Technologies), a small, handheld, nonmydriatic full-field flicker ERG recording system, has been used to generate visual stimuli and to record ERG responses.29, 30, 31, 32, 33, 34, 35 Electroretinography signals were recorded via a skin electrode array (Sensor Strip; LKC Technologies) placed on the orbital rim of the lower eyelid. The electrical potentials are DC amplified and digitized (sampling rate, 2 kHz; 0.3-Hz high-pass filters and 300-Hz low-pass filters). For each patient, we first used Nuprep gel (Weaver and Company) as a skin abrasive to improve conductivity, and an alcohol swab was used to remove the gel before placement of the electrode. These skin RETeval electrodes were shown to be a robust method to measure retinal potential.30
Patients underwent 5 stimulation protocols, the first 4 of which are based on the International Standard for Clinical Electrophysiology of Vision protocol with minor modifications,36, 37, 38 in the following order. First, photopic flash was carried out with 30 responses averaged per eye. Second, photopic flicker (30-Hz frequency) using a square-wave stimulus was carried out. Third, PhNR stimulus was carried out using a 38-Troland seconds (Td·s) red flash on a 380-Troland blue background at 3.4 Hz, which was preceded by 1 minute of blue light adaptation similar to previous studies29,30; 200 responses were averaged per eye.39 For the first 3 stimuli, the pupil size was measured automatically in real time to keep a constant flash retinal illuminance (Troland seconds [Td·s]).40 The handheld ERG device was able to compensate for pupil diameters ranging from 1.3 to 9 mm. Fourth, ON-OFF square-wave stimulus using a red stimulus (560 cd/m2 for 209 ms) over green background (160 cd/m2) was carried out; 200 responses were averaged per eye. Fifth, a custom-written sinusoidal flicker stimulus (300 cd/m2 peak luminance, 100% contrast), modulated from 50 to 0.3 Hz (50, 45, 40, 35, 30, 25, 20, 15, 10, 7, 5, 3, 2, 1, 0.7, 0.5, and 0.3 Hz), was carried out. Responses were averaged 50 to 9 times depending on frequency. Both eyes were tested (right eye first) unless 1 eye met exclusion criteria or if the participant declined testing because of time constraints or fatigue. For protocols 1 through 3, on occasion the participant pupil size could not be measured by the handheld ERG device, and as a result, that test could not be performed. For protocols 4 and 5 (ON-OFF square-wave and sinusoidal flicker stimulus), pupil size was not measured.
Data Variables
Data from the handheld ERG device was processed using custom-written MATLAB software (MathWorks, Inc). The generated code is available on GitHub at https://github.com/UCSFVisionResearch/RETevalTools. Parameters measured included the a-wave and b-wave for the photopic flash stimulus; the average amplitude in the time domain and the amplitude of the first harmonic in the frequency domain (calculated transforming data via the discrete Fourier transform) for the photopic flicker; the PhNR amplitude, defined as the amplitude from the peak b-wave to the peak, negative PhNR waveform; and the a-wave, b-wave, and d-wave for the ON-OFF stimulus. The first harmonic frequency response amplitudes for the sinusoidal flicker stimulus were calculated. After the completion of the ERG tests, participants completed a survey to determine their discomfort and fatigue. Participants were asked to rate their discomfort and fatigue for Humphrey visual field (HVF) testing, OCT, and handheld ERG on a Likert scale from 1 to 5, where 1 was low discomfort or fatigue and 5 was high discomfort or fatigue.
Statistical Methods
For demographic data, we used a 1-way analysis of variance to compare continuous values among control, glaucoma suspect, and glaucoma groups. We used a chi-square test for categorical data. For unadjusted comparisons between different eye types in log10 ERG response, we estimated means and differences stratified by frequency along with percentile-based 95% confidence intervals (CIs) using a nonparametric bootstrap that resampled patients with replacement (1000 iterations). The log10 ERG response was used to account for the higher magnitude of response at the lower frequency ranges. We estimated differences in response between eye types stratified by frequencies of more than and less than 10 Hz and formally tested for interaction using an interaction term between eye type and more than 10 Hz frequency in a generalized linear model with robust standard errors clustered on the patient to account for both eyes tested.41,42 We stratified the data at 10 Hz because this inflection point represents where the phases of the ON and OFF components cancel out.43 Although the ON-pathway may affect the ERG response at a higher frequency, a greater OFF-pathway contribution occurs at higher frequencies and greater ON-pathway contribution occurs at lower frequencies. Adjusted models included participant age, sex, race, and dilation status. We used the same modeling approach to compare eyes in photopic flash, flicker, PhNR, and ON-OFF stimuli. Finally, a Mann–Whitney U test was used to compare participant-rated discomfort and fatigue. We used P < 0.05 for significance. Data analyses were performed with R software version 4.0.2 (R Foundation for Statistical Computing).
Results
Overall, 59 participants were included for testing and analysis, and 18 participants only had 1 eye tested. Of these 18 participants, 1 requested only 1 eye be tested, and the other participants had 1 eye that met our exclusion criteria, most commonly because of poor best-corrected visual acuity, evidence of other retinal diseases, and unreliable visual field testing. Additionally, 3 participants, accounting for 5 glaucoma eyes, ended the test early because of time constraints. In total, 104 eyes were tested and analyzed, which included 19 control, 26 glaucoma suspect, and 59 glaucoma eyes. All 59 glaucoma eyes were of the primary open-angle glaucoma type, except for 4 eyes, 2 of which were of the pseudoexfoliation type and 2 of which were of the pigmentary type. Because of the device’s inability to measure some pupils and some participants not finishing the testing protocol owing to time constraints, not all 104 eyes completed each test (Fig 1). Demographic and ocular data are summarized in Table 1. No significant differences were found among groups except for intraocular pressure, mean deviation, RNFL thickness, and dilated eye status.
Figure 1.
Electroretinography (ERG) stimulus paradigms and response. Participants enrolled underwent 5 ERG tests in the order of: (A) photopic flash, (B) flicker, (C) photopic negative response (PhNR), (D) photopic ON-OFF, and (E) a sinusoidal flicker stimulus that started at 50 Hz and decreased incrementally down to 0.3 Hz. The light stimulus was administered with a handheld ERG device, and the electrical potentials were recorded with a skin electrode. The number of eyes tested for each protocol is listed.
Table 1.
Baseline Characteristics of Control, Glaucoma Suspect, and Glaucoma Groups
| Control Group (n = 19 Eyes) | Glaucoma Suspect Group (n = 26 Eyes) | Glaucoma Group (n = 59 Eyes) | P Value | |
|---|---|---|---|---|
| Age (yrs) | ||||
| Mean (SD) | 69.4 (11.4) | 70.9 (10.8) | 70.3 (12.8) | 0.91∗ |
| Range | 52–92 | 43–89 | 29–90 | |
| Female sex (%) | 52.6 | 69.2 | 47.5 | 0.18† |
| Race (%) | 0.18† | |||
| White | 84.2 | 57.7 | 57.6 | |
| Asian | 5.3 | 26.9 | 32.2 | |
| Black | 10.5 | 15.4 | 10.2 | |
| HTN (%) | 36.8 | 57.7 | 54.2 | 0.33† |
| DM (%) | 5.3 | 30.8 | 13.6 | 0.05† |
| IOP (mmHg) | ||||
| Mean (SD) | 13.6 (2.6) | 16.9 (3.6) | 13.6 (3.3) | 0.001∗ |
| Range | 9–17 | 10–27 | 8–24 | |
| BCVA (logMAR) | ||||
| Mean (SD) | 0.117 (0.142) | 0.074 (0.102) | 0.097 (0.126) | 0.51∗ |
| Range | 0–0.398 | 0–0.301 | 0–0.477 | |
| Spherical refraction (diopters) | ||||
| Mean (SD) | –0.95 (1.99) | –0.09 (1.67) | –1.02 (2.25) | 0.28∗ |
| Range | –5.12–1.25 | –4.50 to 2.50 | –5.63 to 2.75 | |
| Mean deviation (dB) | ||||
| Mean (SD) | –1.85 (3.15) | –5.41 (5.77) | 0.02∗ | |
| Range | –11.3 to 1.22 | –26.49 to 0.94 | ||
| RNFL thickness (μm) | ||||
| Mean (SD) | 87.3 (8.6) | 75.5 (14.1) | <0.001∗ | |
| Range | 72–104 | 35–105 | ||
| Dilation status (%) | 73.7 | 42.3 | 20.3 | <0.001† |
BCVA = best-corrected visual acuity; DM = diabetes mellitus; HTN = hypertension; IOP = intraocular pressure; logMAR = logarithm of the minimum angle of resolution; RNFL = retinal nerve fiber layer; SD = standard deviation; Bolded P values < 0.05.
Analysis of variance.
Chi-square test.
Sinusoidal Flicker Stimulus
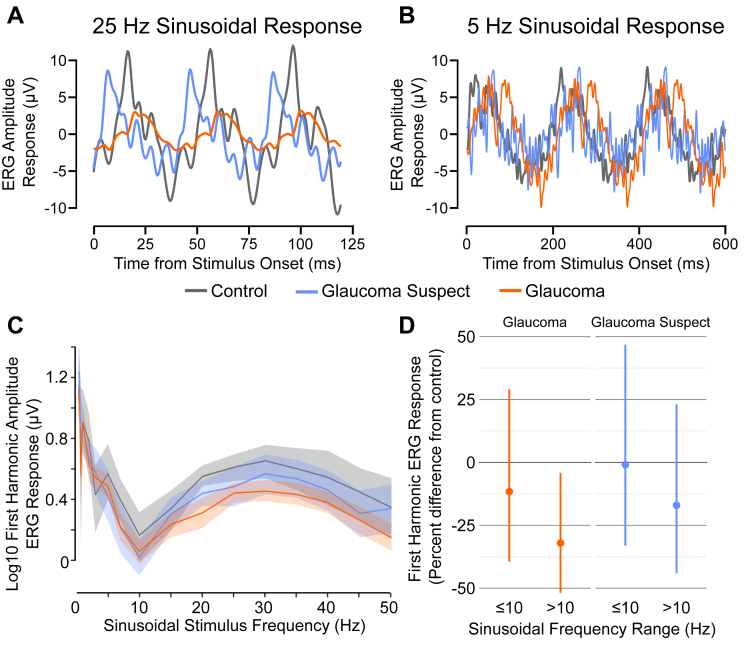
The log10 ERG response amplitudes were measured as the sinusoidal flicker stimulus ranged from 0.3 to 50 Hz (Fig 2A–C). We observed a bimodal curve with an inflection at 10 Hz and a local maximum near 30 Hz. When comparing the mean difference of the overall curves while accounting for both eyes tested in the same patient, glaucoma participants showed a 24.7% decrease (95% CI, −44.4% to 2.1%; P = 0.07) in ERG amplitude compared with control participants. When adjusting further for age, sex, race, and dilation status, this difference was attenuated slightly with a decrease of 21.8% (95% CI, −44.3% to 9.6%; P = 0.14). Although glaucoma suspect eyes also demonstrated decreased amplitudes with an adjusted decrease of 8.8% (95% CI, −36.3% to 30.3%; P = 0.60), differences were not statistically significant.
Figure 2.
Electroretinography (ERG) amplitude response to the sinusoidal flicker stimulus. A handheld ERG device was used to generate the ERG amplitude response curve. A, B, Representative raw ERG response tracings for control, glaucoma suspect, and glaucoma eyes at 25 Hz and 5 Hz. C, Log10 response amplitudes (in microvolts) of the principal harmonic were plotted with 95% confidence intervals against the sinusoidal frequencies (hertz), which ranged from 0.3 to 50 Hz. D, The authors stratified the data at 10 Hz, and a greater reduction was found in the ERG response at frequencies of more than 10 Hz for glaucoma patients, while adjusting for participant age, sex, race, and dilation status. Glaucoma suspects showed no difference at either frequency range.
The mean amplitudes were stratified further above and below 10 Hz (Fig 2D). When adjusting for patient characteristics, glaucoma eyes (0.630 ± 0.027 log10 μV at ≤ 10 Hz; 0.342 ± 0.015 log10 μV at > 10 Hz) showed a significant reduction in ERG amplitude response compared with control eyes (0.699 ± 0.047 log10 μV at ≤ 10 Hz; 0.525 ± 0.024 log10 μV at > 10 Hz) for stimulus frequencies of more than 10 Hz, with a 32.1% decrease (95% CI, −51.8% to −4.1%; P = 0.03). For 10 Hz stimulus frequencies or less, an 11.5% reduction (95% CI, −39.5% to 29.1%; P = 0.50) was found, which was not statistically significant. The interaction P value for this stratification, which asks whether amplitude differences between groups at 10 Hz or less versus more than 10 Hz are different, was 0.12. For glaucoma suspects (0.691 ± 0.041 log10 μV at ≤ 10 Hz; 0.441 ± 0.023 log10 μV at > 10 Hz), no significant reduction was found in ERG amplitude response compared with control participants at either range with a 17.0% decrease at more than 10 Hz (95% CI, −44.0% to 23.0%; P = 0.35) and a 0.9% decrease at 10 Hz or less (95% CI, −33.2% to 46.9%; P = 0.96). The interaction P value for glaucoma suspect stratification was P = 0.35.
Within each eye type, no significant differences were found in the ERG response by dilation status. Compared with undilated eyes, the ERG response amplitudes of dilated eyes were 4.7% increased (95% CI, −36.9% to 73.8%; P = 0.82), 8.8% decreased (95% CI, −47.5% to 62.2%; P = 0.76), and 4.7% increased (95% CI, −38.3% to 73.8%; P = 0.85) for control, glaucoma suspect, and glaucoma eyes, respectively. When comparing the pupil size recorded in the first 3 protocols, the pupil size was not significantly different among the dilated control, dilated glaucoma suspect, and dilated glaucoma groups (P = 0.71). This is true for the undilated group as well (P = 0.08).
When comparing phakic and pseudophakic status, 78.9% of the control group was pseudophakic compared with 42.3% and 52.5% of the glaucoma suspect and glaucoma groups. Nonetheless, the lens status did not alter the ERG response significantly for the control group (18.7% less for phakic eyes; 95% CI, −81.8% to 255%; P = 0.52), glaucoma group (24.1% less for phakic eyes: 95% CI, −52.1% to 16.8%; P = 0.21), or glaucoma group (20.6% less for phakic eyes; 95% CI, −75.5% to 63.0%; P = 0.63).
Photopic Flash, Flicker, Photopic Negative Response, and ON-OFF Flash
Although both glaucoma suspect and glaucoma patients demonstrated decreased ERG responses for the photopic flash, photopic flicker, PhNR, and ON-OFF stimuli, none of these differences were statistically significant after adjusting for participant characteristics (Table 2).
Table 2.
Recorded Electroretinography Response Amplitudes for the Photopic Flash, Photopic Flicker, Photopic Negative Response, and Long-Duration ON-OFF Stimuli
| Electroretinography Type/Measurement | Control Amplitude Response (μV) | Glaucoma Suspect Eyes |
Glaucoma Eyes |
||
|---|---|---|---|---|---|
| Amplitude Response (μV) | P Value | Amplitude Response (μV) | P Value | ||
| Photopic flash | |||||
| a-wave (SEM) | 6.53 (0.73) | 5.63 (0.44) | 0.63 | 4.77 (0.27) | 0.35 |
| b-wave (SEM) | 24.39 (2.61) | 24.59 (2.01) | 0.74 | 19.65 (1.05) | 0.66 |
| Photopic flicker | |||||
| Average amplitude (SEM) | 24.01 (2.65) | 20.46 (1.81) | 0.73 | 18.44 (1.06) | 0.62 |
| First harmonic amplitude (SEM) | 8.51 (0.71) | 7.32 (0.54) | 0.55 | 6.64 (0.38) | 0.40 |
| Full-field PhNR | |||||
| PhNR (SEM) | 18.39 (1.34) | 17.87 (1.26) | 0.92 | 15.63 (0.87) | 0.57 |
| ON-OFF stimulus | |||||
| a-wave (SEM) | 6.53 (0.52) | 5.63 (0.40) | 0.71 | 4.77 (0.24) | 0.81 |
| b-wave (SEM) | 11.11 (0.88) | 10.91 (0.78) | 0.99 | 10.57 (0.56) | 0.99 |
| d-wave (SEM) | 6.93 (1.00) | 5.87 (0.54) | 0.60 | 5.67 (0.42) | 0.92 |
PhNR = photopic negative response; SEM = standard error of the mean.
Statistical analysis was carried adjusting for age, sex, race, and eye dilation status.
Patient Satisfaction
Participants ranked the handheld ERG device as having much less discomfort (Fig 3), with an average Likert score of 1.72 (standard error of the mean [SEM], 0.13) compared with 2.75 (SEM, 0.18) for the HVF (P < 0.001). Patients rated the handheld ERG device to be much less fatiguing, with an average score of 1.74 (SEM, 0.12) compared with 2.91 (SEM, 0.19) for HVF testing (P < 0.001).
Figure 3.
Bar graphs showing participant-rated discomfort and fatigue. Participants rated their (A) discomfort and (B) fatigue after Humphrey visual field (HVF) testing and handheld electroretinography (ERG) on a Likert scale from 1 to 5 after the handheld ERG test. Scores of 4 or 5 were combined to signify a large amount, whereas a rating of 3, 2, or 1 represented moderate, little, or no discomfort or fatigue, respectively. P < 0.001.
Discussion
We identified an asymmetric ERG response in glaucoma patients compared with control participants, in which glaucoma patients showed a decreased response at higher temporal frequencies of more than 10 Hz, whereas the ERG response was similar to that of control participants at 10 Hz or less. This asymmetric response suggests that greater OFF-pathway susceptibility may occur in glaucoma because the OFF-pathway has been shown to be correlated with higher frequencies. Although previous studies have suggested that humans may have greater OFF-pathway vulnerability,20,21 these studies did not modulate the stimulus frequency. Consequently, this study supports the hypothesis of OFF-pathway vulnerability in glaucoma participants using a temporally modulated sinusoidal flicker stimulus with a handheld ERG device.
Evidence from animal studies suggests that when using a flicker stimulus, the ON-pathway is associated with lower frequencies and the OFF-pathway is associated with higher frequencies. In nonhuman primates, the addition of 2-amino-4-phosphonobutyric acid, a noncompetitive glutamate analog that hyperpolarizes ON-pathway bipolar cells, altered the ERG response at lower frequencies.26 The addition of cis-2,3-piperidinedicarboxylic acid after the addition of 2-amino-4-phosphonobutyric acid, which inhibits OFF-pathway activity and higher-order neurons, affected the ERG response at higher frequencies.26 In addition, mouse models with deficits in the OFF cone bipolar cells showed diminished ERG responses at higher frequencies of more than 18 Hz.25 This line of thought was supported further in a genetic mouse model of experimental glaucoma in which researchers found a greater deficit of the ERG response amplitude at higher frequencies.28 Our study similarly showed preferential reduction at higher frequencies, although further studies to characterize the specific frequency ranges that correlate with the ON- and OFF-pathways in humans are warranted.
The greater ERG amplitude reduction at higher frequencies also is consistent with other studies that incorporated a sinusoidal flicker stimulus in various psychophysical and electroretinographic methods. Although several studies using flicker stimuli focused on parameters such as higher-order harmonics,44 several groups found contrast sensitivity loss at higher frequencies in glaucoma patients.22,45 Holopigian et al46 similarly showed greater reductions for flicker ERG and visual evoked potential responses at frequencies of 10, 30, 40, and 50 Hz in glaucoma patients. Additional flicker visual evoked potential studies showed response attenuation to be directly proportional to the severity of visual field loss.23,24 These studies corroborate our findings of greater OFF-pathway vulnerability at higher frequencies, although at the time these studies were carried out, it was not known that OFF ganglion cells are more vulnerable in experimental glaucoma.
Although evidence exists that PhNR may be sensitive at detecting early functional changes,18,20,47, 48, 49 we did not find significant decreases in amplitude in the glaucoma participants. This may be because the amplitudes are attenuated in a handheld ERG device, because previous studies showed that the handheld device was able to detect PhNR changes better for moderate to advanced glaucoma, defined as a mean deviation of –6 dB or worse.30,50 Most of the current glaucoma patients had mild disease with a mean deviation of more than –6 dB. Thus, the fact that we did not find a significant reduction in PhNR could imply that measuring the PhNR with a handheld ERG device is not sensitive enough to pick up subtle changes in mild glaucoma, although it is also possible that the interstimulus frequency in our PhNR protocol can be optimized further to identify these differences.31,51 Additionally, further modifications to the PhNR stimulus, such as using a long-step flash, may provide better separation of ON and OFF responses.20,52
Interestingly, we did not identify a difference between glaucoma suspect and control participants in this study. Although glaucoma suspects did show decreased ERG responses, these differences were not significant. Previous flicker ERG studies also did not show a significant change in the ERG response for participants with ocular hypertension.46 Other stimulus methods such as PERG are able to show differences in glaucoma suspects18,19 and that changes on PERG may precede visual field or RNFL thickness changes.53,54 This may illustrate further a limitation of the handheld ERG device and possibly the sinusoidal flicker stimulus paradigm. Future work should investigate how alternative stimuli such as PERG may provide better sensitivity for detecting differences in glaucoma suspects.
The results from the photopic flash, photopic flicker, and ON-OFF stimulus largely were consistent with previous studies. The a-wave and b-wave have been shown to be no different between glaucoma patients and control participants using both traditional and handheld ERG in humans,20,31,55,56 and the photopic flicker was not shown to be sensitive to changes in glaucoma using multifocal ERG.57 Evidence exists that the d-wave from the ON-OFF stimulus is derived from the OFF-pathway,58,59 and the d-wave was diminished in a nonhuman primate model of glaucoma.60 Nevertheless, in humans, the d-wave has been shown to have both ON- and OFF-pathway contributions,27 and Horn et al20 demonstrated that the d-wave amplitude actually increased in glaucoma patients. We demonstrated that the d-wave amplitude was not decreased significantly in glaucoma versus control eyes, but future work is needed to determine if the d-wave is altered in more advanced stages of glaucoma.
To our knowledge, this is the first study to incorporate a frequency-modulated sinusoidal flicker stimulus with a handheld ERG device to measure the ON- and OFF-pathway in glaucoma. Previous studies using the handheld ERG device primarily focused on using PhNR to assess glaucoma status.29, 30, 31,50 We therefore were able not only to distinguish the ON and OFFcomponents better, but we also provided a proof of concept that a portable, handheld device could be implemented into a normal clinic flow with participants being tested either shortly before or after being seen by a provider. Participants also found the handheld ERG device to result in less discomfort and fatigue compared with HVF testing, suggesting that a handheld ERG device with a sinusoidal flicker stimulus may be implemented readily in future clinical studies to assess its potential as a possible screening or diagnostic tool.
Limitations of our study include the disparity in the number of dilated patients in each group. Our study emphasized integrating the handheld ERG device into the flow of a busy clinic. We enrolled participants regardless of dilation status, resulting in an imbalance of dilated participants between groups, which could affect result interpretation significantly. Although the handheld ERG device could account for pupil size to give constant luminescence for some of the protocols,40 dilated pupils may increase the ERG response amplitude artificially. However, we accounted for dilation status in our statistical model, and adjustment for dilation along with other covariates did not markedly change the estimates. Furthermore, no significant difference in the ERG amplitude based on dilation status alone was found within each eye type. The ERG response amplitudes also are attenuated when using the skin electrodes compared with traditional ERG electrodes.30 However, this makes the difference found at higher ERG sinusoidal frequencies more meaningful because the handheld ERG remained capable of identifying these changes, thus supporting our hypothesis that the OFF-pathway is more affected in glaucoma. In addition, this finding was observed in a mixed group of dilated and undilated participants. Finally, regarding the sinusoidal flicker stimulus, the lower frequencies require longer testing to complete a full cycle. This introduces a greater chance of blink artifacts and foveation defects that may distort the ERG responses collected, especially at lower frequency stimuli, where greater variability in the responses exists. This could explain the attenuation of the interaction P value between glaucoma patients and control participants, which warrants further investigation with a larger sample.
In summary, we used a handheld ERG device and a sinusoidal flicker stimulus to demonstrate that glaucoma patients show greater reductions in ERG amplitudes at higher-frequency stimuli. This corroborates a growing body of evidence in animal models suggesting the same, and this asymmetric response potentially reflects greater OFF-pathway vulnerability in glaucoma. Nevertheless, further investigations are needed to characterize better the specific frequency ranges that correlate with the ON- and OFF-pathways in humans, which could result in the development of improved objective visual function assessment in glaucoma.
Acknowledgments
The authors thank Drs Neeti Parikh, Taras Litvin, Emily Mak, and Jennifer Currier for their help in recruiting participants for this study.
Manuscript no. D-21-00003.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supported in part by the National Eye Institute, National Institutes of Health, Bethesda, Maryland (grant nos.: R01EY028148 [Y.O.] and P30 EY002162 [Core Grant for Vision Research]); the Glaucoma Research Foundation (Y.O.); the BrightFocus Foundation (Y.O.); That Man May See (L.D.S. and Y.O.); the University of California, San Francisco, Dean’s Office Medical Student Research Program, San Francisco, California (A.W.K.); and Research to Prevent Blindness, Inc., New York, New York (unrestricted grant). LKC Technologies, Gaithersburg, Maryland, provided an in-kind donation of sensor strips. The sponsors or funding organizations had no role in the design or conduct of this research.
HUMAN SUBJECTS: Human subjects were included in this study. The human ethics committees at the University of California, San Francisco, approved the study. All research adhered to the tenets of the Declaration of Helsinki. All participants provided informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Kong, Chan, Della Santina, Ou
Analysis and interpretation: Kong, Chan, Arnold, Della Santina, Ou
Data collection: Kong, Turner, Stamper, Ou
Obtained funding: Kong, Della Santina, Ou
Overall responsibility: Kong, Chan, Arnold, Della Santina, Ou
References
- 1.Tielsch J.M., Sommer A., Katz J., et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 2.Mitchell P., Smith W., Attebo K., Healey P.R. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103(10):1661–1669. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 3.Leske M.C., Connell A.M., Schachat A.P., Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112(6):821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 4.Wensor M.D., McCarty C.A., Stanislavsky Y.L., et al. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105(4):733–739. doi: 10.1016/S0161-6420(98)94031-3. [DOI] [PubMed] [Google Scholar]
- 5.Jampel H.D., Singh K., Lin S.C., et al. Assessment of visual function in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(5):986–1002. doi: 10.1016/j.ophtha.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z., Medeiros F.A. Recent developments in visual field testing for glaucoma. Curr Opin Ophthalmol. 2018;29(2):141–146. doi: 10.1097/ICU.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 7.Kong A.W., Santina L.D., Ou Y. Probing ON and OFF retinal pathways in glaucoma using electroretinography. Transl Vis Sci Technol. 2020;9(11):14. doi: 10.1167/tvst.9.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Della Santina L., Inman D.M., Lupien C.B., et al. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci Off J Soc Neurosci. 2013;33(44):17444–17457. doi: 10.1523/JNEUROSCI.5461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou Y., Jo R.E., Ullian E.M., et al. Selective vulnerability of specific retinal ganglion cell types and synapses after transient ocular hypertension. J Neurosci Off J Soc Neurosci. 2016;36(35):9240–9252. doi: 10.1523/JNEUROSCI.0940-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Danaf R.N., Huberman A.D. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci Off J Soc Neurosci. 2015;35(6):2329–2343. doi: 10.1523/JNEUROSCI.1419-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puyang Z., Gong H.-Q., He S.-G., et al. Different functional susceptibilities of mouse retinal ganglion cell subtypes to optic nerve crush injury. Exp Eye Res. 2017;162:97–103. doi: 10.1016/j.exer.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Daniel S., Clark A.F., McDowell C.M. Subtype-specific response of retinal ganglion cells to optic nerve crush. Cell Death Discov. 2018;4(1):1–16. doi: 10.1038/s41420-018-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel S., Meyer K.J., Clark A.F., et al. Effect of ocular hypertension on the pattern of retinal ganglion cell subtype loss in a mouse model of early-onset glaucoma. Exp Eye Res. 2019;185:107703. doi: 10.1016/j.exer.2019.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran N.M., Shekhar K., Whitney I.E., et al. Single-cell profiles of retinal ganglion cells differing in resilience to injury reveal neuroprotective genes. Neuron. 2019;104(6):1039–1055. doi: 10.1016/j.neuron.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H., Zhao Y., Liu M., et al. Progressive degeneration of retinal and superior collicular functions in mice with sustained ocular hypertension. Invest Ophthalmol Vis Sci. 2015;56(3):1971–1984. doi: 10.1167/iovs.14-15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura G., Wang M.H., Ivers K.M., Frishman L.J. Retinal pathway origins of the pattern ERG of the mouse. Exp Eye Res. 2009;89(1):49–62. doi: 10.1016/j.exer.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X., Frishman L.J. Retinal pathway origins of the pattern electroretinogram (PERG) Invest Ophthalmol Vis Sci. 2011;52(12):8571–8584. doi: 10.1167/iovs.11-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cvenkel B., Sustar M., Perovšek D. Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc Ophthalmol Adv Ophthalmol. 2017;135(1):17–28. doi: 10.1007/s10633-017-9595-9. [DOI] [PubMed] [Google Scholar]
- 19.Jung K.I., Jeon S., Shin D.Y., et al. Pattern electroretinograms in preperimetric and perimetric glaucoma. Am J Ophthalmol. 2020;215:118–126. doi: 10.1016/j.ajo.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Horn F.K., Gottschalk K., Mardin C.Y., et al. On and off responses of the photopic fullfield ERG in normal subjects and glaucoma patients. Doc Ophthalmol Adv Ophthalmol. 2011;122(1):53–62. doi: 10.1007/s10633-011-9258-1. [DOI] [PubMed] [Google Scholar]
- 21.Pangeni G., Lämmer R., Tornow R.P., et al. On- and off-response ERGs elicited by sawtooth stimuli in normal subjects and glaucoma patients. Doc Ophthalmol Adv Ophthalmol. 2012;124(3):237–248. doi: 10.1007/s10633-012-9323-4. [DOI] [PubMed] [Google Scholar]
- 22.Breton M.E., Wilson T.W., Wilson R., et al. Temporal contrast sensitivity loss in primary open-angle glaucoma and glaucoma suspects. Invest Ophthalmol Vis Sci. 1991;32(11):2931–2941. [PubMed] [Google Scholar]
- 23.Schmeisser E.T., Smith T.J. High-frequency flicker visual-evoked potential losses in glaucoma. Ophthalmology. 1989;96(5):620–623. doi: 10.1016/s0161-6420(89)32839-9. [DOI] [PubMed] [Google Scholar]
- 24.Schmeisser E.T., Smith T.J. Flicker visual evoked potential differentiation of glaucoma. Optom Vis Sci. 1992;69(6):458–462. doi: 10.1097/00006324-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Tanimoto N., Sothilingam V., Kondo M., et al. Electroretinographic assessment of rod- and cone-mediated bipolar cell pathways using flicker stimuli in mice. Sci Rep. 2015;5:10731. doi: 10.1038/srep10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo M., Sieving P.A. Post-photoreceptoral activity dominates primate photopic 32-Hz ERG for sine-, square-, and pulsed stimuli. Invest Ophthalmol Vis Sci. 2002;43(7):2500–2507. [PubMed] [Google Scholar]
- 27.Khan N.W., Kondo M., Hiriyanna K.T., et al. Primate retinal signaling pathways: suppressing on-pathway activity in monkey with glutamate analogues mimics human CSNB1-NYX genetic night blindness. J Neurophysiol. 2005;93(1):481–492. doi: 10.1152/jn.00365.2004. [DOI] [PubMed] [Google Scholar]
- 28.Harazny J., Scholz M., Buder T., et al. Electrophysiological deficits in the retina of the DBA/2J mouse. Doc Ophthalmol Adv Ophthalmol. 2009;119(3):181–197. doi: 10.1007/s10633-009-9194-5. [DOI] [PubMed] [Google Scholar]
- 29.Tang J., Hui F., Coote M., et al. Baseline detrending for the photopic negative response. Transl Vis Sci Technol. 2018;7(5):9. doi: 10.1167/tvst.7.5.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang J., Hui F., Hadoux X., et al. A comparison of the RETeval Sensor Strip and DTL electrode for recording the photopic negative response. Transl Vis Sci Technol. 2018;7(6):27. doi: 10.1167/tvst.7.6.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hui F., Tang J., Hadoux X., et al. Optimizing a portable ERG device for glaucoma clinic: the effect of interstimulus frequency on the photopic negative response. Transl Vis Sci Technol. 2018;7(6):26. doi: 10.1167/tvst.7.6.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuo M., Kondo M., Hirose A., et al. Screening for diabetic retinopathy using new mydriasis-free, full-field flicker ERG recording device. Sci Rep. 2016;6:36591. doi: 10.1038/srep36591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasuda S., Kachi S., Ueno S., et al. Flicker electroretinograms before and after intravitreal ranibizumab injection in eyes with central retinal vein occlusion. Acta Ophthalmol (Copenh) 2015;93(6):e465–e468. doi: 10.1111/aos.12674. [DOI] [PubMed] [Google Scholar]
- 34.Maa A.Y., Feuer W.J., Davis C.Q., et al. A novel device for accurate and efficient testing for vision-threatening diabetic retinopathy. J Diabetes Complications. 2016;30(3):524–532. doi: 10.1016/j.jdiacomp.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura G., Nakamura Y., Sato E., Yamamoto S. Effects of cataracts on flicker electroretinograms recorded with RETevalTM system: new mydriasis-free ERG device. BMC Ophthalmol. 2016;16:22. doi: 10.1186/s12886-016-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCulloch D.L., Kondo M., Hamilton R., et al. ISCEV extended protocol for the stimulus-response series for light-adapted full-field ERG. Doc Ophthalmol Adv Ophthalmol. 2019;138(3):205–215. doi: 10.1007/s10633-019-09685-8. [DOI] [PubMed] [Google Scholar]
- 37.Frishman L., Sustar M., Kremers J., et al. ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol. 2018;136(3):207–211. doi: 10.1007/s10633-018-9638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sustar M., Holder G.E., Kremers J., et al. ISCEV extended protocol for the photopic On–Off ERG. Doc Ophthalmol. 2018;136(3):199–206. doi: 10.1007/s10633-018-9645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Z., Hadoux X., Hui F., et al. Photopic negative response obtained using a handheld electroretinogram device: determining the optimal measure and repeatability. Transl Vis Sci Technol. 2016;5(4):8. doi: 10.1167/tvst.5.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kato K., Kondo M., Sugimoto M., et al. Effect of pupil size on flicker ERGs recorded with RETeval System: new mydriasis-free full-field ERG system. Invest Ophthalmol Vis Sci. 2015;56(6):3684–3690. doi: 10.1167/iovs.14-16349. [DOI] [PubMed] [Google Scholar]
- 41.Imbens G.W., Kolesár M. Robust standard errors in small samples: some practical advice. Rev Econ Stat. 2015;98(4):701–712. [Google Scholar]
- 42.Blair G., Cooper J., Coppock A., et al. Estimatr: fast estimators for design-based inference. 2020. https://CRAN.R-project.org/package=estimatr Available at: Accessed 31.07.20.
- 43.Kondo M., Sieving P.A. Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Invest Ophthalmol Vis Sci. 2001;42(1):305–312. [PubMed] [Google Scholar]
- 44.Porciatti V., Moretti G., Ciavarella P., Falsini B. The second harmonic of the electroretinogram to sinusoidal flicker: spatiotemporal properties and clinical application. Doc Ophthalmol. 1993;84(1):39–46. doi: 10.1007/BF01203281. [DOI] [PubMed] [Google Scholar]
- 45.Horn F.K., Jonas J.B., Korth M., et al. The full-field flicker test in early diagnosis of chronic open-angle glaucoma. Am J Ophthalmol. 1997;123(3):313–319. doi: 10.1016/s0002-9394(14)70126-6. [DOI] [PubMed] [Google Scholar]
- 46.Holopigian K., Seiple W., Mayron C., et al. Electrophysiological and psychophysical flicker sensitivity in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 1990;31(9):1863–1868. [PubMed] [Google Scholar]
- 47.Viswanathan S., Frishman L.J., Robson J.G., et al. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40(6):1124–1136. [PubMed] [Google Scholar]
- 48.Kim H.D., Park J.Y., Ohn Y.-H. Clinical applications of photopic negative response (PhNR) for the treatment of glaucoma and diabetic retinopathy. Korean J Ophthalmol KJO. 2010;24(2):89–95. doi: 10.3341/kjo.2010.24.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hara Y., Machida S., Ebihara S., et al. Comparisons of photopic negative responses elicited by different conditions from glaucomatous eyes. Jpn J Ophthalmol. 2020;64(2):114–126. doi: 10.1007/s10384-019-00711-5. [DOI] [PubMed] [Google Scholar]
- 50.Kita Y., Holló G., Saito T., et al. RETeval portable electroretinogram parameters in different severity stages of glaucoma. J Glaucoma. 2020;29(7):572–580. doi: 10.1097/IJG.0000000000001509. [DOI] [PubMed] [Google Scholar]
- 51.Tang J., Hui F., Hadoux X., et al. Short-term changes in the photopic negative response following intraocular pressure lowering in glaucoma. Invest Ophthalmol Vis Sci. 2020;61(10):16. doi: 10.1167/iovs.61.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prencipe M., Perossini T., Brancoli G., Perossini M. The photopic negative response (PhNR): measurement approaches and utility in glaucoma. Int Ophthalmol. 2020;40(12):3565–3576. doi: 10.1007/s10792-020-01515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bode S.F.N., Jehle T., Bach M. Pattern electroretinogram in glaucoma suspects: new findings from a longitudinal study. Invest Ophthalmol Vis Sci. 2011;52(7):4300–4306. doi: 10.1167/iovs.10-6381. [DOI] [PubMed] [Google Scholar]
- 54.Banitt M.R., Ventura L.M., Feuer W.J., et al. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Invest Ophthalmol Vis Sci. 2013;54(3):2346–2352. doi: 10.1167/iovs.12-11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colotto A., Falsini B., Salgarello T., et al. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000;41(8):2205–2211. [PubMed] [Google Scholar]
- 56.Wilsey L., Gowrisankaran S., Cull G., et al. Comparing three different modes of electroretinography in experimental glaucoma: diagnostic performance and correlation to structure. Doc Ophthalmol Adv Ophthalmol. 2017;134(2):111–128. doi: 10.1007/s10633-017-9578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Todorova M.G., Palmowski-Wolfe A.M., Orguel S., Flammer J. 30 Hz-flicker mfERG in primary open-angle glaucoma patients: 30 Hz-flicker-mfERG in POAG. Doc Ophthalmol Adv Ophthalmol. 2006;113(1):11–20. doi: 10.1007/s10633-006-9008-y. [DOI] [PubMed] [Google Scholar]
- 58.Sieving P.A. Photopic ON- and OFF-pathway abnormalities in retinal dystrophies. Trans Am Ophthalmol Soc. 1993;91:701–773. [PMC free article] [PubMed] [Google Scholar]
- 59.Ueno S., Kondo M., Ueno M., et al. Contribution of retinal neurons to d-wave of primate photopic electroretinograms. Vision Res. 2006;46(5):658–664. doi: 10.1016/j.visres.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 60.Liu K.-G., Peng X.-Y., Zhang Z., et al. Reduction on OFF-responses of electroretinogram in monkeys with long-term high intraocular pressure. Chin Med J (Engl) 2017;130(22):2713–2719. doi: 10.4103/0366-6999.218021. [DOI] [PMC free article] [PubMed] [Google Scholar]