Abstract
A healthy diet is an important protective factor to prevent cardiometabolic disease. Traditional face-to-face dietary interventions are often episodic, expensive, and may have limited effectiveness, particularly among older adults and people living in rural areas. Telehealth-delivered dietary interventions have proven to be a low-cost and effective alternative approach to improve dietary behaviors among adults with chronic health conditions. In this study, we developed a validated agent-based model of cardiometabolic health conditions to project the impact of expanding telehealth-delivered dietary interventions among older adults in the state of Georgia, a state with a large rural population. We projected the incidence of major cardiometabolic health conditions (type 2 diabetes, hypertension, and high cholesterol) with the implementation of telehealth-delivered dietary interventions versus no intervention among all older adults and 3 subpopulations (older adults with diabetes, hypertension, and high cholesterol, separately). The results showed that expanding telehealth-delivered dietary interventions could avert 22,774 (95% confidence interval [CI]: 22,091–23,457) cases of type 2 diabetes, 19,732 (19,145–20,329) cases of hypertension, and 18,219 (17,672–18,766) cases of high cholesterol for 5 years among older adults in Georgia. The intervention would have a similar effect in preventing cardiometabolic health conditions among the 3 selected subpopulations. Therefore, expanding telehealth-delivered dietary interventions could substantially reduce the burden of cardiometabolic health conditions in the long term among older adults and those with chronic health conditions.
Keywords: telehealth, aging, cardiovascular disease, food and nutrition
Introduction
Cardiometabolic health conditions such as diabetes, hypertension, and cardiovascular disease have large health and economic burdens in the United States.1,2 In 2020, more than one third of total deaths in the United States could be attributed to cardiometabolic health conditions.3 Currently, about 108 million US adults live with hypertension and 30.2 million with diabetes, with higher rates among minorities and low-income groups.1,2 The cost of diabetes alone in the United States is about $327 billion each year, including $237 billion in direct medical costs and $90 billion in lost productivity.4
A healthy diet is an important protective factor for cardiometabolic health.5–7 Low fruit and vegetable consumption and high sodium intake, for example, are independently associated with increased cardiometabolic risk.8–11 Diets low in sodium and high in fruit and vegetable intake, such as the Dietary Approaches to Stop Hypertension (DASH) diet, have been shown to lower blood pressure, with an effect comparable with that of interventional drug therapy.12 A simulation study estimated that 280,000–500,000 deaths for the next 10 years could be averted in the United States if population-wide reductions in sodium consumption were achieved.13
Despite the importance of maintaining a healthy diet, traditional face-to-face dietary interventions are often not effective due to a high rate of nonattendance and noncompliance, especially among older adults and those with chronic health conditions.14 Also, people living in rural areas are less likely to receive in-person dietary interventions (eg, nutrition classes and consultations) due to transportation or financial barriers.15
As an alternative, telehealth-delivered dietary interventions have been increasingly implemented thanks to the fast growth of information technology.16,17 According to the World Health Organization, telehealth refers to “the delivery of health services from a distance synchronously by use of information and communication technologies to exchange health information.”18 Telehealth-delivered dietary interventions can be provided to either individuals or groups remotely through computers, telephones, or mobile applications.18 Existing literature has documented the short-term effects of telehealth-delivered dietary interventions on changes in dietary behaviors.16,17
The recent Centers for Disease Control and Prevention guidelines to using telehealth to expand access to essential health services during and beyond the COVID-19 pandemic have recommended the use of telehealth to deliver weight management and nutrition counseling that supported patients managing chronic health conditions.19 However, few studies have examined the effect of these interventions on long-term health outcomes, partly due to a lack of data on long-term patient follow-ups, as well as a lack of current evidence to support widespread implementation.
In this study, we used an agent-based model to assess the long-term effects of a telehealth-delivered dietary intervention on cardiometabolic health conditions among older adults in Georgia, a state with a large population of rural residents and older adults with limited access to in-person health care. Georgia also has higher prevalence rates of hypertension, obesity, and heart disease than the national averages.20 We assessed the impact of the intervention among all older adults in Georgia and by specific cardiometabolic health conditions. Our modeling study provides policymakers with evidence to inform the implementation of telehealth-delivered dietary interventions.
Methods
We used an agent-based model of cardiometabolic health conditions to conduct the simulation analyses. The model has been validated and used to study programs such as smoking cessation and nutrition promotion.21,22
Agent-based modeling is a computational approach used to simulate the behaviors and health outcomes of autonomous individuals (agents) nested within environments.23 Recent literature reviews demonstrated that, compared with other systems science and simulation modeling approaches (eg, system dynamics model and discrete-event simulation), agent-based modeling provides a natural description of a complex system and is uniquely positioned to capture heterogeneous individual characteristics, dietary decision making, and population health outcomes.24–27 These characteristics of an agent-based model make it an ideal tool to assess the effect of dietary interventions on population health outcomes.26
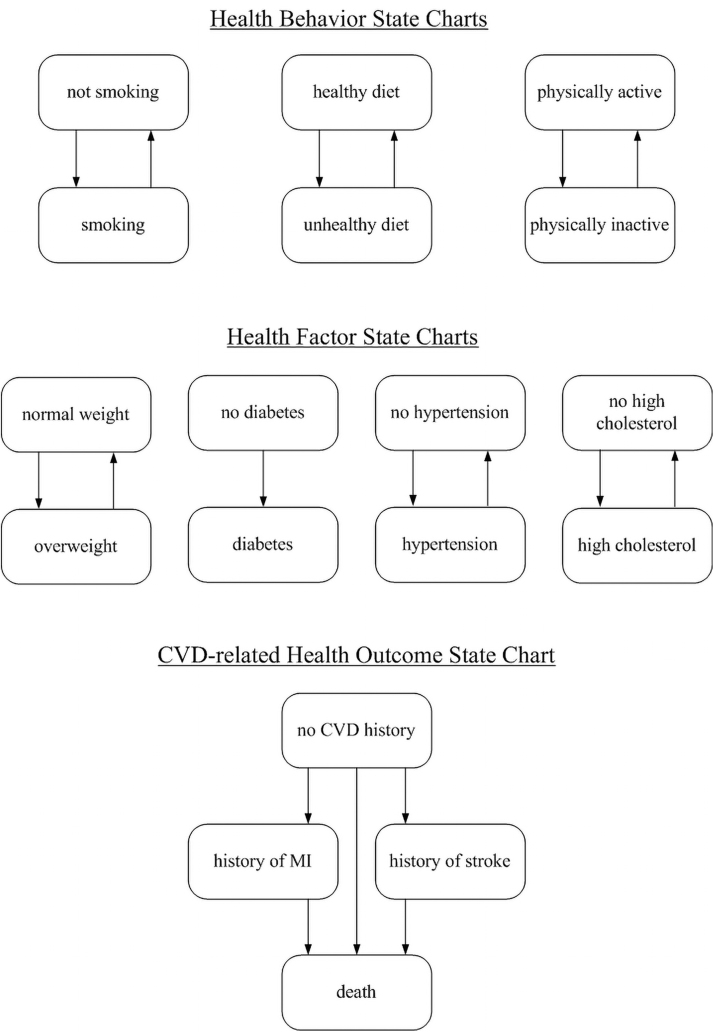
In our simulation model, each agent (person) is defined according to 7 behavioral and health factors (smoking, physical activity, healthy diet, healthy weight, cholesterol, blood pressure, and blood glucose) and by age, gender, and race/ethnicity. These factors were defined following Life's Simple Seven based on the concept of ideal cardiovascular health developed by the American Heart Association.28 Figure 1 shows a simplified model structure in which changes in health behaviors, factors, and health outcomes are represented in 8 parallel state charts. As presented in our previous study, each agent's behavior and health factors evolve simultaneously and interactively as time progresses in the model.21
FIG. 1.
Eight parallel state charts capturing individual health progression.
Changes in health behaviors and factors can then influence the probability of developing a cardiometabolic health condition for the simulated agent. The equations for calculating the initial and transition probabilities of developing cardiometabolic health conditions were derived from the Framingham Heart Study.29,30 The model can track the health conditions of each agent and then calculate the cumulative incidence of each health outcome of interest. The model was programmed in the software AnyLogic v8.4, a specialized simulation model development platform.31
We used data from the 2018 and 2019 Behavioral Risk Factor Surveillance System (BRFSS) to identify the health and demographic profile of the older adult population in Georgia to be simulated within the agent-based model.32,33 The BRFSS is a telephone survey conducted annually among community-dwelling adults 18 years of age and older in the United States. The survey includes standard core questions related to preventive health practices and chronic health conditions. We extracted demographic characteristics and health profiles for adults aged 65 years and over and 3 subpopulations (older adults with diabetes, hypertension, and high cholesterol, separately).
We selected these populations because they are groups of interest to decision makers in health care organizations focused on delivering effective telehealth-based primary care.34 The variables selected included age, gender, race/ethnicity, body mass index, physical activity, dietary behaviors, diabetes, hypertension, and high cholesterol. Using the BRFSS data, we estimated the mean and standard deviation of the age for older adults in Georgia and the proportion of each category for all other variables.
We used the agent-based model to assess health outcomes over time for adults 65 years and over in Georgia and by specific cardiometabolic health conditions. The health outcomes of the model resulting from normal health progression were compared with the health outcomes obtained from implementing a telehealth-delivered dietary intervention designed to increase the consumption of fruits and vegetables and reduce sodium intake. The effect size of the intervention was estimated based on a recent systematic review and meta-analysis of telehealth-delivered dietary interventions.17 These telehealth-delivered dietary interventions were designed to provide dietary education by a qualified health care professional through a telehealth approach to adults with an established diet-related chronic disease such as diabetes, hypertension, high cholesterol, and heart disease.
Findings from the systematic review and meta-analysis showed that telehealth-delivered dietary interventions could increase the consumption of fruits and vegetables by 1.04 servings per day (95% confidence interval [CI]: 0.46–1.62 servings per day) and reduce sodium intake on urinary and/or self-assessed scores by 0.39 (95% CI: 0.20–0.58).17 These effect sizes were incorporated into the model to project future health outcomes. We did not consider the effect of other interventions in the modeling because introducing other interventions may confound the simulation results.
For each of the simulation experiments, we generated 100,000 simulated individuals based on the demographic characteristics and health profiles of the population group. We then calculated the cumulative numbers of people with diabetes, hypertension, and high cholesterol in 1, 3, and 5 years, and reported the results for all older adults and each of the subpopulations in Georgia. Although our model could be used to predict health outcomes such as myocardial infarction and stroke, we only focused on diabetes, hypertension, and high cholesterol as outcomes because our longest simulation timeframe was 5 years. This study is exempted from IRB review because only de-identified, publicly available data were used.
Results
Table 1 reports the demographic characteristics and health profiles of older adults in Georgia estimated from the BRFSS data and the 3 subpopulations described in the previous section. The mean age of our overall sample was 72.9 years. The proportion of the population who had a healthy diet was between 42.3% and 53.7% for older adults and the 3 subpopulations.28 The proportion of nonsmokers was between 89.9% and 91.0% and the proportion physically active (spending >150 minutes per week doing moderate physical activity) was between 42.3% and 53.7% for older adults and the 3 subpopulations. The table shows that older adults with one or more cardiometabolic health conditions in Georgia were, in general, less likely to engage in a healthy behavior compared with those without cardiometabolic health conditions.
Table 1.
Demographic Characteristics and Health Profiles of Older Adults (65+ Years) in Georgia Based on Behavioral Risk Factor Surveillance System 2019
| All | Subpopulation with diabetes | Subpopulation with hypertension | Subpopulation with high cholesterol | |
|---|---|---|---|---|
| Age (mean, SD) | 72.9 (0.1) | 73.1 (0.2) | 72.7 (0.2) | 72.6 (0.2) |
| Female (%) | 56.0 | 54.7 | 54.6 | 56.3 |
| No currently smoking (%) | 90.4 | 89.8 | 90.2 | 91.0 |
| BMI <25 (%) | 33.9 | 23.3 | 27.7 | 29.4 |
| Physically active (%) | 53.7 | 42.3 | 51.1 | 53.4 |
| Have healthy diet (%) | 53.7 | 48.1 | 50.3 | 51.4 |
| No diabetes (%) | 75.0 | 68.8 | 70.3 | |
| No hypertension (%) | 35.1 | 18.9 | 24.4 | |
| No high cholesterol (%) | 48.6 | 39.1 | 40.7 | |
| History of MI (%) | 11.7 | 15.0 | 15.5 | 14.5 |
| History of stroke (%) | 9.5 | 12.8 | 11.8 | 12.0 |
BMI, body mass index; MI, myocardial infarction; SD, standard deviation.
The proportion of older adults in Georgia with no diabetes, no hypertension, and no high cholesterol was 75.0%, 35.1%, and 48.6%, respectively. There was a high prevalence of comorbidities. Among people with diabetes, the proportion of the population with no hypertension and no high cholesterol was 18.9% and 39.1%, respectively. Similarly, among people with hypertension or high cholesterol, the prevalence of the other cardiometabolic health conditions was higher compared with the prevalence for the general population.
Table 2 reports the simulated results of the 3 cardiometabolic health conditions for older adults and the 3 subpopulations in Georgia. We compared the normal progression of these health conditions with the telehealth-delivered dietary intervention.
Table 2.
Projected Effects of a Telehealth Dietary Intervention on Chronic Health Outcomes Among Older Adults (65+ Years) in Georgia
| Diabetes (%) |
Hypertension (%) |
High cholesterol (%) |
||||
|---|---|---|---|---|---|---|
| No intervention | Telehealth | No intervention | Telehealth | No intervention | Telehealth | |
| All | ||||||
| Year 1 | 25.6 (25.1–26.1) | 25.0 (24.4–25.5) | 68.9 (69.3–68.5) | 68.4 (68.0–68.8) | 58.3 (57.9–58.7) | 57.9 (57.5–58.3) |
| Year 3 | 28.7 (28.2–29.2) | 27.7 (27.2–28.1) | 71.0 (70.6–71.4) | 70.2 (69.8–70.5) | 63.9 (63.5–70.2) | 63.0 (62.6–63.4) |
| Year 5 | 31.3 (30.9–31.8) | 29.8 (29.3–30.2) | 72.9 (72.5–73.3) | 71.6 (71.2–72.0) | 68.1 (67.7–68.5) | 66.9 (66.5–67.3) |
| Subpopulation with diabetes | ||||||
| Year 1 | 76.6 (77.0–76.2) | 76.4 (76.2–77.0) | 67.4 (67.0–67.8) | 67.0 (66.6–67.4) | ||
| Year 3 | 78.0 (77.7–78.4) | 77.4 (77.1–77.8) | 76.3 (76.0–76.7) | 75.4 (75.1–75.8) | ||
| Year 5 | 79.9 (79.5–80.3) | 78.3 (77.9–78.7) | 82.0 (81.6–82.4) | 80.9 (80.5–81.3) | ||
| Subpopulation with hypertension | ||||||
| Year 1 | 31.7 (31.2–32.1) | 31.2 (30.7–31.7) | 65.1 (64.7–65.5) | 64.8 (64.4–65.2) | ||
| Year 3 | 34.3 (34.0–34.8) | 33.6 (33.1–34.0) | 73.7 (73.3–74.1) | 72.9 (72.5–73.3) | ||
| Year 5 | 37.1 (36.7–37.6) | 36.1 (35.7–36.6) | 78.8 (78.2–79.2) | 78.1 (77.7–78.5) | ||
| Subpopulation with high cholesterol | ||||||
| Year 1 | 29.5 (29.0–29.9) | 29.3 (28.8–29.8) | 73.4 (73.0–73.7) | 73.1 (72.7–73.5) | ||
| Year 3 | 33.1 (32.6–33.6) | 32.3 (31.8–32.8) | 74.9 (74.5–75.2) | 74.2 (73.9–74.6) | ||
| Year 5 | 35.3 (34.8–35.8) | 34.0 (33.5–34.5) | 76.3 (76.0–76.7) | 74.9 (74.5–75.3) | ||
Ninety-five percent confidence intervals are reported in the parenthesis.
Simulation results showed that in 1 year after the intervention was implemented, the prevalence of diabetes, hypertension, and high cholesterol among older adults could be reduced by 0.6%, 0.5%, and 0.4%, respectively. The benefit of the intervention increased with the number of years after it was implemented. For example, in 5 years, the prevalence of diabetes, hypertension, and high cholesterol among older adults could be reduced by 1.5%, 1.3%, and 1.2%, respectively. Given that the number of older adults in Georgia was 1,518,291 in 2018, we estimated that 22,774 cases of diabetes, 19,732 cases of hypertension, and 18,219 cases of high cholesterol could be averted over 5 years if telehealth-based dietary interventions were implemented in Georgia for older adults.
The telehealth-delivered dietary intervention would also reduce the prevalence of cardiometabolic health conditions among people with one or more health conditions. For example, among people with diabetes, the intervention could reduce the prevalence of hypertension from 79.9% to 78.3% and the prevalence of cholesterol from 82.0% to 80.9% in 5 years. We observed similar reductions among people with hypertension and high cholesterol.
Discussion
This study showed that telehealth-delivered dietary interventions could reduce the prevalence of diabetes, hypertension, and high cholesterol among older adults in Georgia. Given that Georgia has a large rural population who have limited access to in-person nutrition counseling and other health care services, our findings suggest that the expansion of telehealth-delivered dietary services in Georgia and other states with similar demographics may result in substantial benefits in reducing cardiovascular risk for older adults.
Our findings complement those from several other studies in the literature. For example, using data from rural Upstate New York, researchers found that telehealth-delivered nutrition counseling interventions among older adults with diabetes improved their knowledge base, practices, and behaviors regarding diet.35 In a randomized controlled trial that evaluated a nurse-led e-mail reminder program for improving diet, participants in the intervention group reported significantly lower rates of uncontrolled hypertension and high cholesterol compared with those in the control group.36 Another randomized controlled trial showed that a text messaging-based telehealth intervention could significantly reduce systolic blood pressure and cholesterol levels among patients with coronary heart disease in 6 months.37
The COVID-19 pandemic has created an opportunity for health care providers and patients to embrace telehealth more than ever before. The shelter-in-place and social distancing measures imposed during the pandemic strongly discouraged patients from seeking in-person care, especially for chronic disease prevention and management purposes. As a result, the use of telehealth has increased substantially over the past year.38 This transition to telehealth was also supported and accelerated by sweeping changes in telehealth reimbursement policies by both public and private payers.39 These social and policy changes all support a wider adoption of telehealth-delivered dietary interventions, particularly among older adults who have higher health care and social needs than the general population. Findings from this study provide additional evidence to support a wider adoption of telehealth even in the postpandemic era.19
This study has several limitations. First, similar to all other simulation models, our model is a simplification of the real world and has important assumptions that have to be fully understood before it can be used to inform decision making. For example, we assumed all the older adults in Georgia adopted the telehealth-delivered dietary intervention in our simulation experiment, which certainly will not be the case in practice. However, although effort is needed to provide technology support to this subpopulation, recent literature showed that the majority (>60%) of elderly patients have access to telehealth services.40 Our simulation modeling results provide estimates on the potential effects of the intervention; the simulation estimates can be improved as more data become available.
Second, although racial and ethnic disparities in telehealth access have been reported elsewhere,41 we did not study the impact of the intervention on different racial and ethnic subpopulations because we do not have data on how telehealth utilization would differ across racial and ethnic groups. Once data become available for the populations of interest, we could conduct additional analyses to investigate how the intervention could influence racial and ethnic disparities. Finally, the model was validated based on national data from our previous studies because we do not have local longitudinal data to conduct additional model validation. Such validation will be needed when data become available to strengthen the validity of the results.
Despite these limitations, our study is the first to show the potential for long-term impact of a telehealth-delivered dietary intervention on cardiometabolic health, providing important evidence to support state-level decision making on the potential expansion of telehealth services at a time when the use of telehealth is growing rapidly and evidence of its potential impact is very limited.
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research study was supported by a grant from the National Institute on Minority Health and Health Disparities (R01MD013886) and a grant from the National Heart, Lung, and Blood Institute (R01HL141427) of the National Institutes of Health (NIH).
References
- 1. Virani SS, Alonso A, Benjamin EJ, et al. . Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. National diabetes statistics report, 2020. Atlanta GA: Centers for Disease Control and Prevention; U.S. Department of Health and Hum and Services, 2020:12–15. [Google Scholar]
- 3. Flagg LA, Anderson RN. Unsuitable underlying causes of death for assessing the quality of cause-of-death reporting. National Vital Statistics Reports; Hyattsville, MD: National Center for Health Statistics. 2021;69(14):1–25. [PubMed] [Google Scholar]
- 4. Riddle MC, Herman WH. The cost of diabetes care—an elephant in the room. Diabetes Care 2018;41:929–932. [DOI] [PubMed] [Google Scholar]
- 5. Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 2006;136:2588–2593. [DOI] [PubMed] [Google Scholar]
- 6. Montonen J, Knekt P, Härkänen T, et al. . Dietary patterns and the incidence of type 2 diabetes. Am J Epidemiol 2005;161:219–227. [DOI] [PubMed] [Google Scholar]
- 7. Epstein LH, Gordy CC, Raynor HA, Beddome M, Kilanowski CK, Paluch R. Increasing fruit and vegetable intake and decreasing fat and sugar intake in families at risk for childhood obesity. Obes Res 2001;9:171–178. [DOI] [PubMed] [Google Scholar]
- 8. Cook NR, Obarzanek E, Cutler JA, et al. . Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med 2009;169:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayalath VH, de Souza RJ, Ha V, et al. . Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr 2015;102:914–921. [DOI] [PubMed] [Google Scholar]
- 10. Keller A, Heitmann BL, Olsen N. Sugar-sweetened beverages, vascular risk factors and events: a systematic literature review. Public Health Nutr 2015;18:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xi B, Huang Y, Reilly KH, et al. . Sugar-sweetened beverages and risk of hypertension and CVD: a dose–response meta-analysis. Br J Nutr 2015;113:709–717. [DOI] [PubMed] [Google Scholar]
- 12. Sacks FM, Svetkey LP, Vollmer WM, et al. . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 13. Coxson PG, Cook NR, Joffres M, et al. . Mortality benefits from US population-wide reduction in sodium consumption projections from 3 modeling approaches. Hypertension 2013;61:564–570. [DOI] [PubMed] [Google Scholar]
- 14. Paterson BL, Charlton P, Richard S. Non-attendance in chronic disease clinics: a matter of non-compliance? J Nurs Healthc Chronic Illn 2010;2:63–74. [Google Scholar]
- 15. Marcin JP, Shaikh U, Steinhorn RH. Addressing health disparities in rural communities using telehealth. Pediatr Res 2016;79:169–176. [DOI] [PubMed] [Google Scholar]
- 16. Goode AD, Reeves MM, Eakin EG. Telephone-delivered interventions for physical activity and dietary behavior change: an updated systematic review. Am J Prev Med 2012;42:81–88. [DOI] [PubMed] [Google Scholar]
- 17. Kelly JT, Reidlinger DP, Hoffmann TC, Campbell KL. Telehealth methods to deliver dietary interventions in adults with chronic disease: a systematic review and meta-analysis. Am J Clin Nutr 2016;104:1693–1702. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. Telemedicine: opportunities and developments in Member States. Report on the Second Global Survey on EHealth. World Health Organization, 2010. [Google Scholar]
- 19. CDC. Using Telehealth to Expand Access to Essential Health Services during the COVID-19 Pandemic. Centers for Disease Control and Prevention, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html Accessed January 10, 2021.
- 20. Rollins L, Akintobi TH, Hermstad A, et al. . Community-based approaches to reduce chronic disease disparities in Georgia. J Ga Public Health Assoc 2017;6(4):402–410. [Google Scholar]
- 21. Li Y, Kong N, Lawley M, Pagán JA. Assessing lifestyle interventions to improve cardiovascular health using an agent-based model. In: Proceedings of the 2014 Winter Simulation Conference. IEEE Press, New York, NY: USA, 2014:1221–1232. [Google Scholar]
- 22. Li Y, Kong N, Lawley M, Weiss L, Pagán JA. Advancing the use of evidence-based decision-making in local health departments with systems science methodologies. Am J Public Health 2015;105(S2):S217–S222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc Natl Acad Sci 2002;99(suppl 3):7280–7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luke DA, Stamatakis KA. Systems science methods in public health: dynamics, networks, and agents. Annu Rev Public Health 2012;33:357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Lawley MA, Siscovick DS, Zhang D, Pagán JA. Agent-based modeling of chronic diseases: a narrative review and future research directions. Prev Chronic Dis 2016;13:150561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Berenson J, Gutiérrez A, Pagán JA. Leveraging the food environment in obesity prevention: the promise of systems science and agent-based modeling. Curr Nutr Rep 2016;5:245–254. [Google Scholar]
- 27. Nianogo RA, Arah OA. Agent-based modeling of noncommunicable diseases: a systematic review. Am J Public Health 2015;105:e20–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lloyd-Jones DM, Hong Y, Labarthe D, et al. . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 29. Wilson PW, Castelli WP, Kannel WB. Coronary risk prediction in adults (the Framingham Heart Study). Am J Cardiol 1987;59:G91–G94. [DOI] [PubMed] [Google Scholar]
- 30. D'Agostino Sr. RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 31. Borshchev A. The big book of simulation modeling: multimethod modeling with anylogic 6, AnyLogic, Oakbrook Terrace, IL: USA, 2013.
- 32. Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System: Overview BRFSS 2007. http://www.cdc.gov/brfss/annual_data/annual_2007.htm Accessed March 7, 2014.
- 33. Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System: Overview BRFSS 2012. http://www.cdc.gov/brfss/annual_data/2012/pdf/Overview_2012.pdf Accessed March 7, 2014.
- 34. Jones A, Hedges-Chou J, Bates J, Loyola M, Lear SA, Jarvis-Selinger S. Home telehealth for chronic disease management: selected findings of a narrative synthesis. Telemed E-Health 2014;20:346–380. [DOI] [PubMed] [Google Scholar]
- 35. Kaufman DR, Pevzner J, Hilliman C, et al. . Redesigning a telehealth diabetes management program for a digital divide seniors population. Home Health Care Manag Pract 2006;18:223–234. [Google Scholar]
- 36. Cicolini G, Simonetti V, Comparcini D, et al. . Efficacy of a nurse-led email reminder program for cardiovascular prevention risk reduction in hypertensive patients: a randomized controlled trial. Int J Nurs Stud 2014;51:833–843. [DOI] [PubMed] [Google Scholar]
- 37. Chow CK, Redfern J, Hillis GS, et al. . Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314:1255–1263. [DOI] [PubMed] [Google Scholar]
- 38. Koonin LM, Hoots B, Tsang CA, et al. . Trends in the use of telehealth during the emergence of the COVID-19 pandemic—United States, January–March 2020. Morb Mortal Wkly Rep 2020;69:1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomas EE, Haydon HM, Mehrotra A, et al. . Building on the momentum: sustaining telehealth beyond COVID-19. J Telemed Telecare 2020. [Epub ahead of print]; DOI: 10.1177/1357633X20960638 [DOI] [PubMed] [Google Scholar]
- 40. Lam K, Lu AD, Shi Y, Covinsky KE. Assessing telemedicine unreadiness among older adults in the United States during the COVID-19 pandemic. JAMA Intern Med 2020;180:1389–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jain V, Al Rifai M, Lee MT, et al. . Racial and geographic disparities in internet use in the US among patients with hypertension or diabetes: implications for telehealth in the era of COVID-19. Diabetes Care 2021;44:e15–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]