The authors evaluated the effect of biochemical parameters on the clinical course of coronavirus disease 2019 in patients treated with tocilizumab for cytokine storm. Medical documents of patients with coronavirus disease 2019 were searched retrospectively.
Key Words: COVID-19, cytokine storm, electrolyte, tocilizumab
Abstract
Objectives
Patients who develop cytokine storm while they have coronavirus disease 2019 (COVID-19) experience more severe symptoms. This article aims to evaluate the effect of biochemical parameters on the clinical course of the disease in patients treated with tocilizumab (TCZ) due to cytokine storm.
Methods
Medical documents of patients with COVID-19 were searched retrospectively. Patients who entered cytokine storm were classified as group 1 and divided into two subgroups as patients who were followed up in the ward and in the intensive care unit (ICU). Less severe COVID-19 patients who did not enter cytokine storm were included in the control group as group 2.
Results
A total of 522 patients with COVID-19 infection were included in the study. The mean age was 62.0 ± 15.6 years, and the majority were male (64.4%). Hypertension and diabetes mellitus were the two most common diseases, seen in 50.8% and 29.9%, respectively. There were 392 patients with TCZ application (group 1) and 130 patients without TCZ (group 2). Significantly higher serum glucose, magnesium, and sodium and lower calcium levels were present in group 1 than in group 2 (<0.001). Hypocalcemia, hypernatremia, hypermagnesemia, and hyperkalemia were more frequently detected in the ICU compared with the patients treated in the wards (P = 0.001, P < 0.001, P = 0.039, and P < 0.001, respectively).
Conclusions
Following up closely electrolyte disturbances may support patient survival and decrease the probability of ICU necessity. This approach should be taken before the development of important disorders to be effective in the treatment process of the main disease.
Key Points
Significantly higher serum glucose, magnesium, and sodium and lower calcium levels were present in coronavirus disease 2019 patients than in the control group.
Hypocalcemia, hypernatremia, hypermagnesemia and, hyperkalemia were more frequently detected in patients treated in the intensive care unit compared with the patients treated in the wards.
Following up closely the electrolyte disturbances may support patient survival and decrease the probability of intensive care unit necessity.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is an important problem that is mainly asymptomatic or characterized by mild findings and affects in particular the respiratory system.1 Patients with coronavirus disease 2019 (COVID-19) may develop severe respiratory distress resulting from cytokine release. Patients who develop cytokine storm may experience more severe symptoms as a result of the overproduction of inflammatory cytokines, including interleukin-6.2 Tocilizumab (TCZ) is an interleukin-6 receptor antagonist that may be useful for severe COVID-19 when cytokine storm is suspected.2 Many factors such as demographic characteristics, comorbid diseases, and/or laboratory values affect the prognosis of the disease.3 Electrolyte imbalance has been reported as a potential biomarker to predict disease severity and mortality in COVID-19.4,5 In the studies performed, biochemistry parameters such as sodium, potassium, magnesium, calcium, and fasting blood sugar were measured during the COVID-19 disease process.6–8 The progress of the disease may be highly dependent on the abnormality of the parameters listed above.
The aim of this study was to evaluate the effect of biochemical parameters on the clinical course of the disease in patients treated with TCZ for cytokine storm. These parameter values are important to better understand the impact of COVID-19 on patients. As such, the results of these parameters and monitoring patients are important.
Methods
Study Participants and Ethics Approval
The medical documents of patients who tested positive for COVID-19 by polymerase chain reaction test were searched retrospectively. The demographic characteristics of the participants such as age, sex, and comorbid diseases (congestive heart failure, coronary artery disease, diabetes mellitus [DM], hypertension [HTN], asthma, chronic obstructive pulmonary disease, cancer, and pulmonary thromboemboli) of the participants were recorded. A total of 522 patients with COVID-19 infection were included in the study. Data regarding demographics and laboratory test results were documented and analyzed.
Group 1 (study group) consisted of the patients who entered cytokine storm and received TCZ treatment. This group was divided into two subgroups: the patients who were followed up in the ward and the patients who were followed up in the intensive care unit (ICU). Group 2 (control group) consisted of the COVID-19 patients who did not enter cytokine storm (as less severe COVID-19) and received COVID-19 treatment other than TCZ. The day they were admitted to the hospital was considered day 0. The patients were studied until they were discharged and/or died.
This study was retrospective and cross-sectional, conducted by evaluating the medical documents of COVID-19 patients studied in a Konya tertiary care hospital between May 2020 and January 2021. The Institutional Ethics Committee for Clinical Research approved the study, which was in accordance with the Helsinki Declaration and Good Clinical Practices Guideline. All of the individuals gave written consent preparticipation.
Statistical Analysis
Descriptive statistics were given as means ± standard deviations and medians with minimum-maximum values for continuous variables, depending on their distribution. Numbers and percentages were used for categorical variables. The normal distribution of the numerical variables was analyzed by the Shapiro-Wilk, Kolmogorov-Smirnov, and Anderson-Darling tests.
The independent samples t test was used in comparing two independent groups in which numerical variables had a normal distribution. For variables without normal distribution, the Mann-Whitney U test was applied. Pearson χ2 and Fisher exact tests were used in 2 × 2 tables to compare the differences between categorical variables. For the comparison of differences between categorical variables, the Fisher-Freeman-Halton test was used in R × C tables.
For the statistical analysis, Jamovi project (2020), Jamovi (version 1.8.4.0; https://www.jamovi.org), and JASP (version 0.14.1.0; https://jasp-stats.org) were used. The significance level (P value) was set at 0.05 in all of the statistical analyses.
Results
A total of 522 patients with COVID-19 infection were included in the study. The mean age was 62.0 ± 15.6 years, and the majority were male (64.4%). In 371 patients (71.1%), there was at least one type of comorbidity. HTN and DM were the two most common diseases seen in 265 (50.8%) and 156 patients (29.9%), respectively. There were 392 patients with TCZ application (group 1, the study group) and 130 patients without TCZ (group 2, the control group). The patients were significantly older (65.2 vs 52.6 years, P < 0.001) and more frequently were male (70.9% vs 44.6, P < 0.001) in group 1 as compared with group 2 (Table 1). The rate of comorbidity was significantly higher in group 1 than in group 2 (77.8% vs 50.8%; P < 0.001).
Table 1.
Demographic and clinical characteristics of the patients
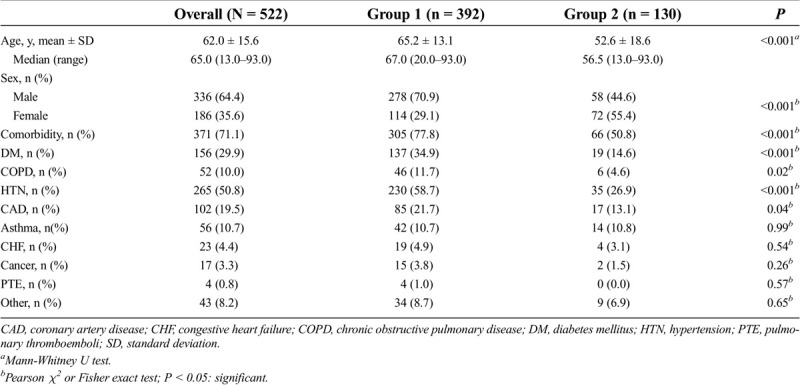
The median length of hospital stay was significantly higher in group 1 than in group 2 (12 vs 7 days; P < 0.001). In group 1, the necessity of treatment in the ICU and the overall mortality rate was 58.2% and 51.0%, respectively. There was no ICU follow-up or mortality in group 2.
A comparison of the laboratory values and the groupings based on the laboratory ranges is given in Table 2. Those whose laboratory values were not recorded in the file at the beginning of hospitalization were not compared in this respect. Significantly higher serum glucose levels, magnesium, and sodium and lower calcium levels were present in group 1 than in group 2 (P < 0.001 for all).
Table 2.
Comparison of the laboratory values between groups 1 and 2
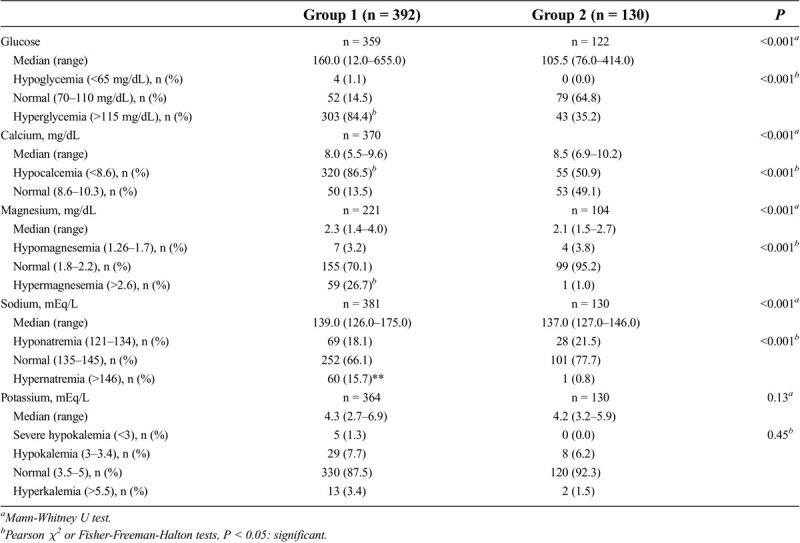
Table 3 presents the comparison of the outcomes of the patients in the study group treated in the wards and in the ICU. In total, 58.2% of patients in the study group were treated in the ICU. The median length of hospital stay was significantly longer in the patients treated in the wards than in the ICU (13 vs 11 days; P < 0.001). A total of 31 patients (13.6%) were discharged after the treatment in the ICU. The mortality rate in the ICU was significantly higher than that in the ward (86.4% vs 1.8%; P < 0.001).
Table 3.
Comparison of the outcomes of the patients in group 1 treated in the wards and ICU
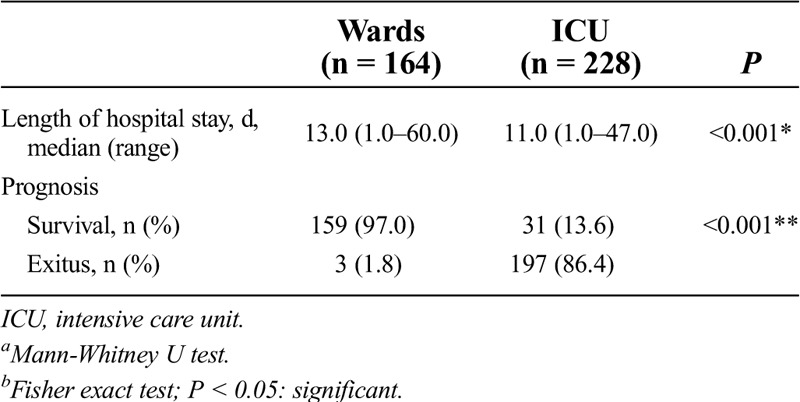
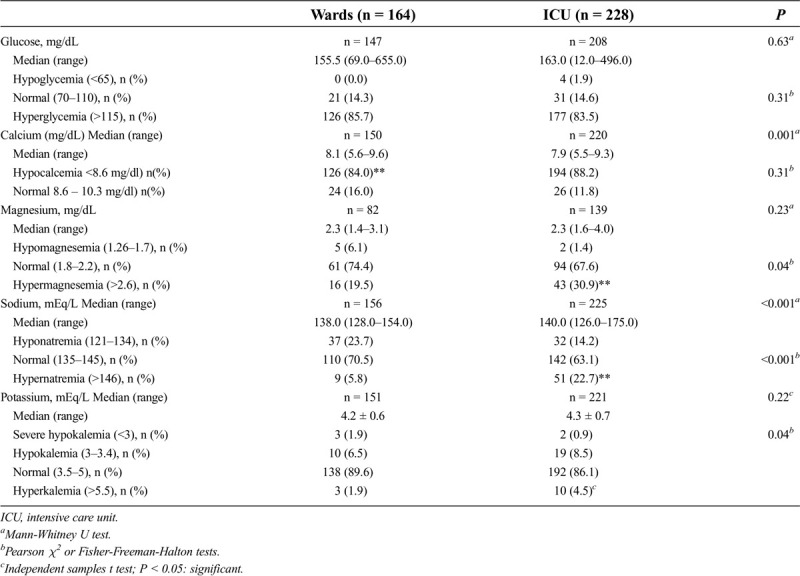
Table 4 summarizes the comparison of the laboratory investigations of the patients in the study group treated in the wards and in the ICU. Those whose laboratory values were not recorded in the file at the beginning of hospitalization were not compared in this respect. Hypocalcemia, hypernatremia, hypermagnesemia, and hyperkalemia were more frequently detected in patients in the ICU compared with the patients treated in the wards (P = 0.001, P < 0.001, P = 0.039, and P < 0.001, respectively).
Table 4.
Comparison of the laboratory investigations of the patients in group 1 treated in the wards and ICU
Discussion
High frequencies of severe cases were observed in older adult patients, especially with comorbid diseases.9,10 Also in the present study the patients were older; the incidences of HTN, DM, chronic obstructive pulmonary disease, and coronary artery diseases were the most common comorbid diseases; there was a male predominance in patients with cytokine storm.
We found that hyperglycemia, hypocalcemia, hypermagnesemia, and hypernatremia were significantly higher in patients with cytokine storm (group 1) than in group 2.
In the studies conducted during the 2003 SARS epidemic, hyperglycemia was reported in patients without a previous diagnosis of DM. When pancreatic tissue was stained by immunohistochemical staining, angiotensin-converting enzyme 2 (ACE2) receptors were seen in pancreatic tissue. It was believed that through ACE2 receptors, the coronavirus damages islet cells by entering these cells, leading to DM.11–13 Meanwhile, in the acute phase of COVID-19 infection, the virus captures CD4+ T and CD8+ T lymphocytes, leading to cell apoptosis.14 In addition, an increase in blood sugar increases SARS-CoV-2 replication in monocytes, which leads to a decrease in T cell response.15 It has been reported that a history of DM or impaired glucose metabolism complicates and negatively affects the treatment of COVID-19 disease.16,17
In the present study, 34.9% of patients had DM, but the incidence of hyperglycemia was detected more often (84.4% of group 1). As such, hyperglycemia may be part of the disease or an increase in blood sugar can be expected because of stress factors.18–20
The coronavirus infects cells by binding to ACE2 receptors, which are found in the kidneys and gastrointestinal (GI) tract.21–23 As a result, virus-induced renal and GI tract impairment can be seen in patients. This may cause acute renal failure and considerable disorders in the gut. COVID-19 in particular damages podocyte and proximal tubule cells in the kidneys. Acute renal failure is one of the important reasons that can increase the risk of mortality in COVID-19 patients.22 Fluid and electrolyte disturbance are serious problems when the kidneys and GI tract are involved in COVID-19. Among these disturbances, hyponatremia, hypernatremia, hypocalcemia, hypercalcemia, hypochloremia, hypovolemia and hypervolemia occur very frequently. Even minor changes in electrolyte balance can lead to major dysfunctions in the body. The treatment of these disorders is of critical importance, and the risk of mortality increases as these impairments continue and are not treated.21,23
Hypocalcemia has been shown to be associated with severe illness and intensive care. It increases morbidity and mortality in COVID-19, and studies have reported that hypocalcemia is especially high in critically ill patients.24–27 At the same time, in another study conducted on mild and moderate COVID-19 patients, the rate of hypocalcemia also was commonly found in these patients.28 The hypocalcemia seen in COVID-19 may be caused by the direct effect of SARS-CoV-2, as well as by problems in parathyroid hormone and 25-hydroxyvitamin D.29 The receptor for the SARS-CoV-2, ACE2, is expressed in the proximal renal tubule, and renal 1α-hydroxylase enzyme is also expressed in the proximal tubules. As such, defects in 1α-hydroxylase activity lead to decreased calcitriol production, resulting in hypocalcemia.28 Similarly, in the present study, hypocalcemia was highly prevalent in group 2 (50.9%), but it was higher in group 1 (86.5%) as compared with group 2, and this rate increased to 88.2% in critically ill patients treated in the ICU.
When the pathophysiology of SARS-CoV-2 is examined, it is known that the virus binds to the ACE2 receptor and decreases ACE2 protein expression. As a result, it can lead to hypokalemia by increasing the excretion of potassium from the kidney.30 Overall, potassium disorders have been associated with poor prognosis. Because it can be detected incidentally, it is more often associated with underlying diseases.31,32 The prevalence of hypokalemia was found to be high in COVID-19 patients, and the degree of hypokalemia was shown to accelerate some COVID-19 symptoms.30 In a study of 306 patients with COVID-19 pneumonia, hypokalemia was shown to be common, which may be an indication of the need for mechanical ventilation, and it may be an important parameter for the poor course of the disease.33 In this study, hypokalemia and severe hypokalemia were seen in 5 (1.3%) and 29 (7.7%) patients, but this number was not significantly different from group 2. Although there was no difference in the frequency of hypokalemia between the groups in our study, hyperkalemia was more frequently detected in the patients in the ICU compared with the patients treated in the wards (4.5% vs 1.9%). In a previous study,31 hyperkalemia was found to be extremely high in deceased patients as a result of COVID-19 (37%), but in another study,32 this rate was similar to our study (4.15% vs 4.5%) in critically ill patients.
ACE2 receptor expression occurs in the proximal tubule in the kidneys. Hyponatremia is believed to be caused by the increased expression of this receptor.34 In these studies, the correlation between sodium level and the severity of COVID-19 was taken into account.35,36 It was shown that hyponatremia was more common in outpatients than in severely ill patients, but all cases of hypernatremia were observed in patients with severe disease.36 In this study, hyponatremia was observed in 18.1% and 21.5% in group 1 and group 2, respectively, but the difference was not meaningful between the groups. Conversely, similar to the previous study,36 in this study, there also was a meaningful amount of hypernatremia (22.7%) in patients observed in the ICU. When sodium imbalance is corrected quickly, it can lead to serious brain damage.21 As such, it is necessary to be cautious when treating electrolyte imbalance.
Magnesium is a critically important substance in the immune system and in the protection of vascular and pulmonary tissue. In the presence of hypomagnesemia, there is an increase in airway hyperreactivity, susceptibility to upper respiratory tract infections and the risk of developing fibrosis in the lung tissue.37 As such, magnesium-related disorders may affect sensitivity and response to SARS-CoV-2. The reduction in magnesium is believed to exacerbate virus-induced inflammation and cause a cytokine storm.38 In a study, the risk of mortality in COVID-19 was found increasing in the presence of hypomagnesemia.39 In another study, it was found that in moderate COVID-19 patients, the magnesium level was low, and hypomagnesemia was more common in patients with more severe disease.39,40 It was observed that the frequency of hypermagnesemia was higher in patients in the ICU, however.40 In these previous studies, patients generally had comorbid disease; therefore, they already were prone to COVID-19 disease and have a higher risk of experiencing changes in magnesium levels. For this purpose, the magnesium levels were investigated in those among the COVID-19 patients who did not have a comorbid disease.36 It also was noted that both hypomagnesemia and hypermagnesemia were reported in different periods, meaning changes in magnesium levels occur frequently, especially in patients in the ICU. In the present study, the rate of hypomagnesemia was low in both group 1 (3.2%) and group 2 (3.8%). Conversely, hypermagnesemia was found to be higher than hypomagnesemia. The rate of hypermagnesemia was found as 26.7% in group 1 while it was higher (30.9%) in patients treated in the ICU. This high rate is similar to the previous study.36
Hyperglycemia, hypocalcemia, hypermagnesemia, and hypernatremia were higher in all of the patients with cytokine storm in group 1. At the same time, all of the parameters except hyperglycemia also were found to be significantly higher, especially in critically ill patients treated in the ICU. The overall mortality rate of group 1 was 51.0%, whereas this rate increased to 86.4% among the patients treated in the ICU. There were many factors affecting mortality; however, electrolyte imbalance should be considered one of the most important factors because of its influence on morbidity and mortality. As such, this situation should be taken into account to improve the response to the main treatment of the disease.
Our study has limitations. Because of the rapid development of the COVID-19 pandemic and many unknown factors related to the disease, the research was conducted retrospectively. The relation between comorbid diseases and biochemistry parameters was not examined. The initial laboratory values of the patients were compared between each group. The study is based on the initial measurement of parameters and it is missing later measurements for comparison. To better understand the effectiveness of the treatment, a comparison should be performed prospectively with a larger study that includes multiple results spread across a time during the disease.
Conclusions
The COVID-19 pandemic continues to affect our lives in different aspects, but especially our health. To address this situation, it is critical to initiate treatment in the early stages of the disease.21,37 In general, hyperglycemia or other electrolyte disturbances occurred comorbidly, especially in severe cases. Electrolyte imbalance is common and increases the risk of mortality, especially among patients hospitalized in the ICU. These patients should receive more attention and be studied carefully. Furthermore, when these disorders are observed, exact and urgent treatment should be initiated immediately. Early correction of the disorder or prevention of its development may support patient survival and decrease the probability of admittance to the ICU. This approach should be undertaken before the development of important disorders, so that it can be effective in the treatment process of the main disease and severe complications of the disease can be prevented. Because our study was a comprehensive study, we believe that the results will be meaningful and therefore can help guide the literature.
Footnotes
The authors did not report any finanical relationships or conflicts of interest.
Contributor Information
Cengiz Burnik, Email: cburnik@gmail.com.
Mehmet Mermer, Email: drmmermer@gmail.com.
Serkan Yavuz, Email: serkanyavuz14@hotmail.com.
References
- 1.di Filippo L Doga M Frara S, et al. Hypocalcemia in COVID-19: prevalence, clinical significance and therapeutic implications. Rev Endocr Metab Disord 2021;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knorr JP Colomy V Mauriello CM, et al. Tocilizumab in patients with severe COVID-19: a single-center observational analysis. J Med Virol 2020;92:2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal R, Bhadada SK. COVID-19 and non-communicable diseases. Postgrad Med J 2020;96:429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med 2020;76:97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 2020;58:1131–1134. [DOI] [PubMed] [Google Scholar]
- 6.Guan WJ Ni ZY Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C Wang Y Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sever MY Uzun N Ceritli S, et al. Hyponatremia in COVID-19 patient using angiotensin type 1 receptor (AT1R) blocker and diuretic: a case report. J Res Clin Med 2020;8:22. [Google Scholar]
- 9.Guan WJ Liang WH Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B Li R Lu Z, et al. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JK Lin SS Ji XJ, et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 2010;47:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004;25:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W Moore MJ Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angelidi AM, Belanger MJ, Mantzoros CS. Commentary: COVID-19 and diabetes mellitus: what we know, how our patients should be treated now, and what should happen next. Metabolism 2020;107:154245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Codo AC Davanzo GG Monteiro LB, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab 2020;32:437–446.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima-Martínez MM Carrera Boada C Madera-Silva MD, et al. COVID-19 and diabetes: a bidirectional relationship. Clin Investig Arterioscler 2021;33:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppelli A Giannarelli R Aragona M, et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 Study. Diabetes Care 2020;43:2345–2348. [DOI] [PubMed] [Google Scholar]
- 18.Sardu C D’Onofrio N Balestrieri ML, et al. Outcomes in patients With hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care 2020;43:1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuetz P, Castro P, Shapiro NI. Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care 2011;34:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G Wu D Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pourfridoni M Abbasnia SM Shafaei F, et al. Fluid and electrolyte disturbances in COVID-19 and their complications. Biomed Res Int 2021;2021:6667047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valizadeh R Baradaran A Mirzazadeh A, et al. Coronavirus-nephropathy; renal involvement in COVID-19. J Renal Inj Prev 2020;9:e18. [Google Scholar]
- 23.Duan Y Prasad R Feng D, et al. Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res 2019;125:969–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J Han P Wu J, et al. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health 2020;13:1224–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem 2020;57:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun JK Zhang WH Zou L, et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (Albany NY) 2020;12:11287–11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Filippo L Formenti AM Rovere-Querini P, et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine 2020;68:475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal R Ram S Zohmangaihi D, et al. High prevalence of hypocalcemia in non-severe COVID-19 patients: a retrospective case-control study. Front Med (Lausanne) 2021;7:590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieto-Torres JL DeDiego ML Verdiá-Báguena C, et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog 2014;10:e1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D Li X Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open 2020;3:e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T Wu D Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noori M Nejadghaderi SA Sullman MJM, et al. Epidemiology, prognosis and management of potassium disorders in Covid-19. Rev Med Virol 2021;32:e2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-P O Leon-Ramirez JM Fuertes-Kenneally L, et al. COVID19-ALC Research Group . Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID-19 pneumonia: a case series of 306 Mediterranean patients. Int J Infect Dis 2020;100:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim JH Jung HY Choi JY, et al. Hypertension and electrolyte disorders in patients with COVID-19. Electrolyte Blood Press 2020;18:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W Lu S Zhang M, et al. Correlation between hyponatremia and the severity of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:774–778. [DOI] [PubMed] [Google Scholar]
- 36.Sarvazad H Cahngaripour SH Eskandari Roozbahani N, et al. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. New Microbes New Infect 2020;38:100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapani V Rosanoff A Baniasadi S, et al. The relevance of magnesium homeostasis in COVID-19. Eur J Nutr 2022;61:625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iotti S Wolf F Mazur A, et al. The COVID-19 pandemic: is there a role for magnesium? Hypotheses and perspectives. Magnes Res 2020;33:21–27. [DOI] [PubMed] [Google Scholar]
- 39.Alamdari NM Afaghi S Rahimi FS, et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J Exp Med 2020;252:73–84. [DOI] [PubMed] [Google Scholar]
- 40.Quilliot D Bonsack O Jaussaud R, et al. Dysmagnesemia in Covid-19 cohort patients: prevalence and associated factors. Magnes Res 2020;33:114–122. [DOI] [PubMed] [Google Scholar]


