Abstract
The main burden of SARS-CoV-2 falls on the lungs but neurological manifestations, the most disabling of which are strokes and which correlate with disease severity, are common. We proffer a novel mechanism for acute COVID-19 stroke whereby pulmonary vein clots developing within the characteristic pulmonary intravascular thrombotic lesions can embolize to the brain. Appreciation of this mechanism requires an understanding of the tricompartmental model of lung parenchyma oxygenation (the alveolus, the bronchial artery, and the pulmonary artery), all of which are compromised in COVID-19. Of these 3 sources, the bronchial artery plays a crucial role in COVID-19 stroke because the unique collaterals from bronchial artery to pulmonary vein which exist under normal physiological conditions (and which maintain venous patency when the pulmonary artery is blocked by embolus) are occluded, thus leading to venular thrombosis in the presence of hypercoagulability. Dislodgement of clots from this source translocates the pathology to the brain and is a disease mechanism, formerly rare, which may account for many cases of large vessel occlusion stroke in COVID-19. This mechanism extends the concept of cardioembolic stroke from endocardium retrogradely into the pulmonary circulation with which the left cardiac chambers lie in direct continuity, and which is an accepted stroke mechanism under other circumstances such as lung lobectomy, where surgical ligation of the pulmonary vein creates a blind sac from which thrombi can embolize. The proposed model is supported by postmortem studies which have demonstrated venular thrombosis and by case reports of pulmonary vein thrombosis in COVID-19. This concept provides a more plausible cause for COVID-19 associated large vessel occlusion stroke than other putative mechanisms, such as cerebral endotheliitis, cytokine storm, and hypercoagulopathy, although it is acknowledged that the latter mechanism contributes to the genesis of pulmonary vein clots. Recognizing that extrapulmonary manifestations including stroke arise within thrombosed pulmonary veins is key to understanding of neurological manifestations of SARS-CoV-2 infection.
Keywords: bronchial artery, COVID-19, embolism and thrombosis, pulmonary artery, pulmonary vein
COVID-19 is caused by the SARS-CoV-2 and typically manifests with pneumonia.1–12 In severe COVID-19, respiratory failure accounts for most deaths, but neurological complications, most commonly acute stroke, develop in tandem with increasing severity of pneumonia, the pathological basis of which is poorly understood.13–30 In the absence of a clear mechanism linking acute neurological syndromes and severe lung abnormalities in COVID-19, explanations including cytokine storm syndrome, severe hypoxemia, treatment-related complications from COVID-19 therapies and intensive care-related complications, cerebral vasculitis, multifocal cerebral thrombotic microangiopathy, ACE (angiotensin-converting enzyme)-2 receptor-mediated endothelial cell infection and central nervous system neuroinvasion have been invoked.19,31–41
Existing theories of causation fail to account for the fact that stroke in COVID-19 is radiologically identical to stroke unassociated with COVID-19 being associated with a high incidence of large vessel occlusion (LVO) suggesting an embolic cause.32,33,42–47 In this article, we describe how disruption of the lung tricompartment in the presence of inflammatory cytokines, hypoxia, endotheliopathy, and hypercoagulability leads to in situ thrombosis within the draining pulmonary venules, from which emboli may dislodge into the systemic circulation, causing stroke.9 Immunothrombotic disruption of the alveolar-capillary barrier results in not only dislodgement of immune-rich emboli but also discharge of RNA which is extremely thrombogenic (RNAaemia) and other viral proteins into the circulation, which could exacerbate immunothrombosis in the cerebral vasculature in a manner similar to that occurring in the lungs.48,49
COVID-19 Pneumonia, Pulmonary Vein Physiology, and Pulmonary Venular Thrombosis: THE Substrate for Acute Ischemic Stroke in COVID-19
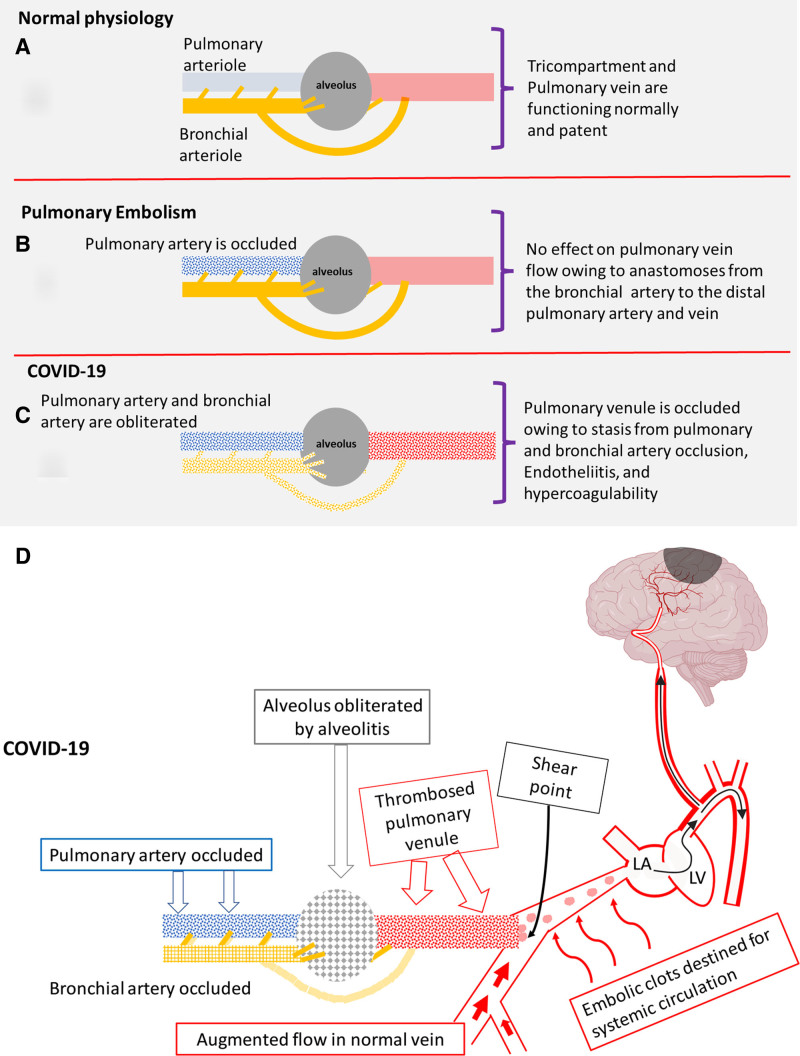
The hallmark of severe COVID-19 pneumonia is diffuse alveolar damage and microvascular immunothrombosis (pulmonary intravascular coagulopathy [PIC]), which is distinct from the disseminated intravascular coagulation that accompanies cytokine storm and macrophage activation syndrome. Recently, we published a pathological model explaining the severe, widespread lung infarcts in COVID-19 based on a tricompartmental model under which normal lung parenchyma receives oxygen from 3 sources—directly from the alveolus, from the pulmonary artery, and from the bronchial artery (Figure 1A and 1B).50,51 Obliteration of all 3 sources by alveolitis and immunothrombosis results in the classical lung infarcts observed at computed tomography (CT) and at autopsy (Figure 1C), but we further propose that the unique COVID-19 pulmonary pathophysiology also causes pulmonary vein thrombosis with the potential for embolic stroke.
Figure 1.
Oxygenation in normal physiology, pulmonary embolism, and COVID-19. A, Normal: Pulmonary artery, bronchial artery, and alveolus are normal. Pulmonary vein flow is uninterrupted. B, Pulmonary embolism: Pulmonary artery is occluded, but the bronchial artery remains patent. Alveolus is normal (no interference with ventilation). Pulmonary vein remains patent despite loss of the major blood supply (the pulmonary artery) owing to flush-through from profuse anastomoses from bronchial artery to the pulmonary venules. C, COVID-19: The pulmonary and bronchial arteries are occluded by immunothrombosis, and the alveolus is occluded by alveolitis. The pulmonary vein is also occluded because of endotheliitis, hypercoagulability, and loss of the flush-through from the profuse bronchial artery-pulmonary vein anastomoses. D, Dislodgement of small clots at confluence points with patent pulmonary vein segments, leads to clots accessing the systemic circulation which cause strokes. Immunothrombosis in COVID-19 areas leads to augmented flow in adjacent normal areas with 2 important effects. First, central clot propagation is limited apart from the most severe cases; second, increased shear forces on thrombus protruding into patent segments at confluence points (curved arrow) leads to systemic emboli (in a manner similar to the stump emboli theory of stroke in extracranial carotid occlusion).53 LA indicates left atrium; and LV, left ventricle.
Understanding pulmonary vein physiology is key to understanding strokes in COVID-19. Pulmonary vein thrombosis is extremely rare apart from occasional cases following lung lobectomy where surgical ligation of the pulmonary vein creates a blind sac which leads to in situ thrombosis and embolic stroke.52
The reason that pulmonary vein thrombosis is rare is multifactorial but attributable to factors including extremely high blood flow (each lung receives 50% of cardiac output), high blood flow velocity, short length compared with their systemic counterparts, absence of venous tortuosity/varicosity and the fact that respiration causes a rhythmic compression of the veins which are also subject to the Bernoulli effect from the cardiac cycle, which collectively limit pulmonary venular in situ thrombosis. Another crucial, overlooked reason relates to the second independent arterial input from the systemic bronchial arteries which not only prevents lung infarction in most cases of pulmonary embolism owing to collateral flow to the distal pulmonary arterioles and capillaries but because of profuse anastomoses to the pulmonary venules maintains sufficient venous flow to prevent venous thrombosis when the pulmonary arteries are occluded (Figure 1B).50,51
Because of extensive arborization of small pulmonary arterioles, we believe that COVID-19 immunothrombosis in one area leads to redistribution of blood from diseased to normal segments which limit thrombosis to small venules only (Figure 1D) (apart from the most severe cases), rendering it extremely difficult to detect on CT.10–12 Diverted blood maintains high flow rates in normal venules which accentuates shear forces on friable thrombi protruding into patent veins at confluence points thus leading to systemic emboli, similar to the stump theory of stroke in patients with extracranial carotid occlusion.53
Severe COVID-19 Pneumonia Is a Marker for Acute Neurological Conditions
Our model proposes a mechanism linking increasing severity of COVID-19 pneumonia with neurological complications as highlighted by many authors. Mao et al54 reported neurological manifestations in 36.4% of patients, more commonly in those with severe pulmonary infection. Lang et al55 reported hospitalization duration, likelihood of ICU admission, and requirement for mechanical ventilation were significantly greater in the 26% of patients with acute neuroimaging findings who, in turn, had higher radiological severity scores than those without neurology. Mahammedi et al56 reported that a high CT lung severity score threshold of 0.8 had 74% sensitivity and 65% specificity for acute findings on neuroimaging.
Taquet et al57 conclusively showed increase in ischemic stroke in 236 379 survivors of COVID-19, compared with 2 matched controls (influenza and any chest infection). They showed stepwise increased incidence of stroke in 4 COVID-19 groups stratified for increasing severity as follows: mild, nonhospitalized cases (n=190 077) 1.3%, hospitalized cases (n=46 302) 4.38%, ICU admitted patients (n=8945) 6.92%, and encephalopathic patients (n=6229) 9.35%. Similar increases were noted for first ischemic stroke, that is, those with fewer expected risk factors (0.43%, 1.60%, 2.82%, and 3.28%, respectively). Risk of stroke was increased even in mild infection (nonhospitalized) indicating that no group was exempt.
LVO in Acute COVID-Related Stroke
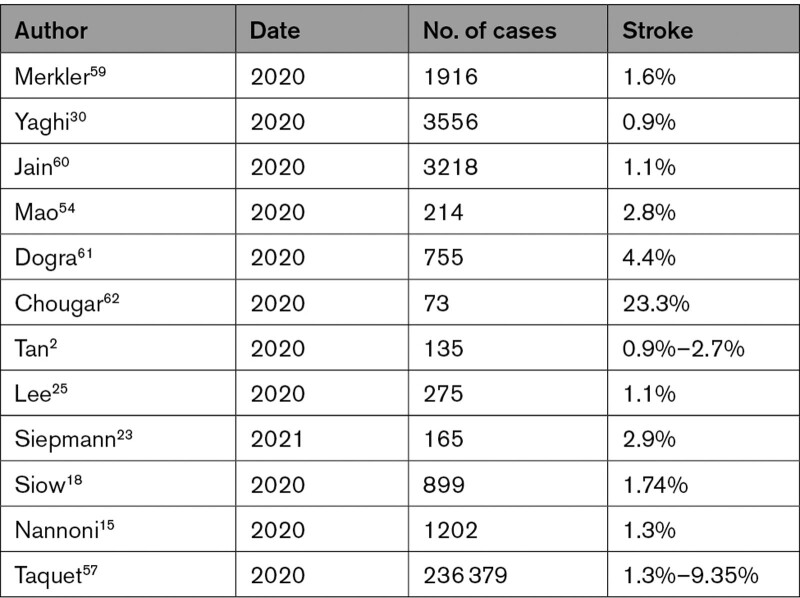
Acute ischemic stroke is most commonly thromboembolic from a source within the supplying arteries or heart.58 Published data gives an incidence of acute cerebrovascular disease in COVID-19 of 0.9% to 9.35%2,14,15,17,18,23–26,28 (Table 1).
Table 1.
Stroke Incidence in COVID-19
LVO similar to that of non-COVID-19 stroke is a consistent feature across multiple series, being reported in 79.6% in one series and in 50% of stroke in the NYC COVID-19 outbreak which had a 2-fold increase in LVO over baseline, but prior authors did not explanations the discordance between proposed mechanisms invoking endothelial injury, direct brain infection and hypercoagulability, and the recurring feature of embolic vessel occlusion, often within multiple vascular territories.20,42–45,62–66
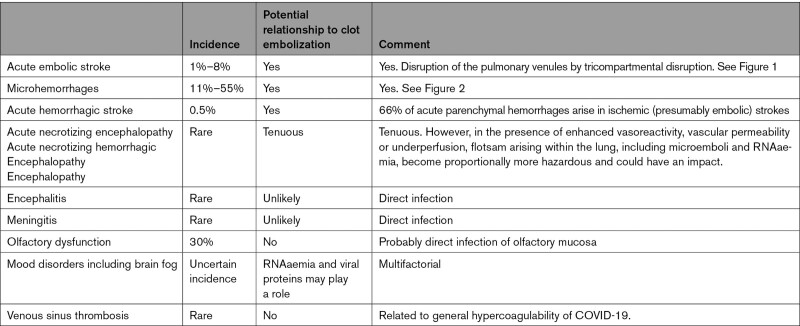
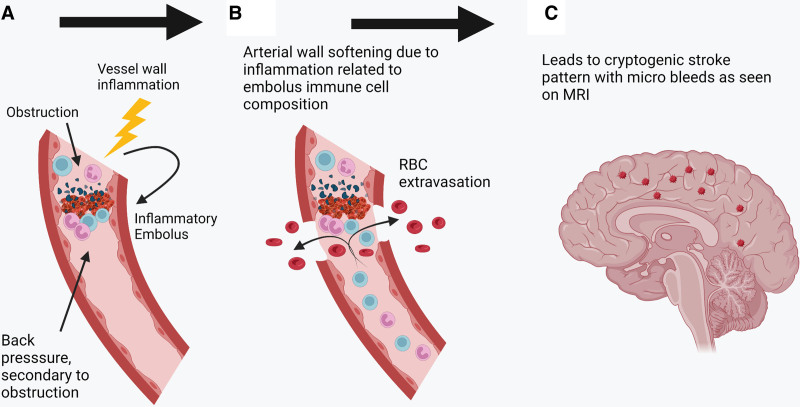
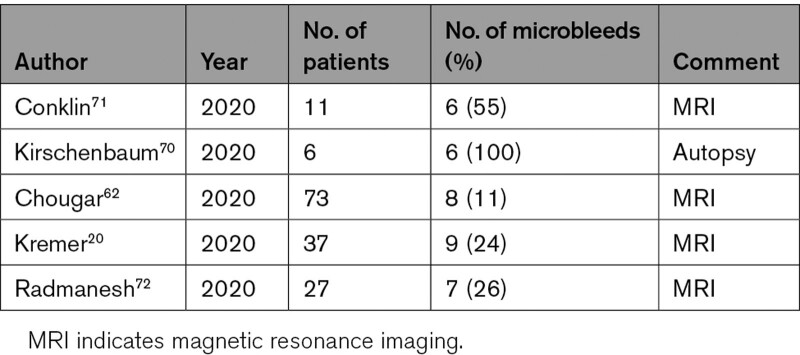
Our hypothesis explains the fact that strokes in COVID-19 are radiologically similar to non-COVID-19 strokes and accounts for the frequent detection of LVO on cerebral CT, angiography, and autopsy.28,43,45,46 Our model addresses significant gaps in knowledge and rationalizes existing theories of causation under a unifying central tenet of immune disruption of the alveolus leading to pulmonary vein thrombosis, which provides a nidus from which emboli dislodge and which can potentially contribute to multiple cerebral pathologies in COVID-19 (Table 2). It also accounts for the fact that anterior circulation strokes are much more common in COVID-19 which should not be the case if factors such as hypercoagulability were primarily responsible as these factors would presumably act equally in all areas, for the fact that infarcts are often within multiple vascular territories (similar to cardioembolic strokes, whereas strokes arising from carotid plaque are unilateral), and often repeated.28,43,45,46 It also accounts for the correlation with severity of pneumonia, as more extensive lung disease implies more extensive pulmonary vein thrombosis.56,67,68 Also, the hypothesis emphasizes the inflammatory nature of embolic clots of COVID-19, which can lead to hemorrhagic complications.69 We propose that microemboli laden with inflammatory cells lodge within the distal intracerebral arterioles and incite an inflammatory response within the vessel wall, which results in microhemorrhage, which can be detected on magnetic resonance imaging, and which mirrors the hemorrhagic change seen in the lungs on histology (Figure 2).20,62,70 We note similarities between microhemorrhages in COVID-19 and those of infective endocarditis, and the pattern of widespread microbleeds in COVID-19 fits with an embolic/hematogenous source, in comparison to microhemorrhages in other diseases which tend to be site-specific (eg, subcortical posterior regions in amyloid angiopathy and basal ganglia in hypertension; Table 3).62,70–72 The same mechanism of inflammatory softening of arteries can explain hemorrhagic infarction.
Table 2.
Potential Role of Venous Clot Embolization in COVID-19 Neuropathology
Figure 2.
Effect of emboli on end-arteriole in the brain. A, Immune-rich inflammatory embolus obstructs the arterial wall lumen. B, Arterial wall infiltration by inflammatory mediators leads to extravasation of blood products. C, This gives rise to the characteristic appearance of blooming on magnetic resonance imaging (MRI) owing to iron content. RBC indicates red blood cell.
Table 3.
Incidence of Microbleeds in COVID-19
The proposed model explains the higher incidence of neurological complications over other systemic manifestations simply owing to a higher proportion of the cardiac output going to the brain (25%) than to any other organ.4,73 The model potentially also explains pathology in the gut, kidney, and skin where ischemic changes are reported.73–75
Pulmonary Intravascular Coagulopathy: Diagnosis and Therapeutic Implications
Postmortem studies have identified the hallmark of severe COVID-19 as marked pulmonary intravascular coagulopathy (PIC), which is clinically characterized by normal liver function with normal or increased fibrinogen and elevated D-dimer with associated thrombolysis pathway activation. Under normal conditions, endothelial cells contain dedicated intracellular organelles known as Weibel Palade bodies. Weibel Palade bodies store a series of proteins, including VWF (von Willebrand Factor), VWFpp (VWF propeptide), P-selectin, OPG (osteoprotegerin), and Ang-2 (angiopoietin 2). Following acute endothelial cell activation, these Weibel Palade bodies contents are actively secreted and act to promote and modulate local hemostasis, inflammation, and angiogenesis.76,77 Several recent publications have demonstrated that plasma levels of VWF, VWFpp, OPG, and Ang-2 correlate with disease severity and are elevated in acute COVID-19 to higher levels than those observed in other types of severe sepsis.76,77
Recent studies suggest that anticoagulation has a role in management of acute COVID-19 with beneficial effects of therapeutic low molecular weight heparin in patients with moderately severe COVID-19. However, therapeutic heparin in patients with COVID-19 requiring ICU support was ineffective and associated with increased bleeding.78 These findings suggest there may be a sweet spot for anticoagulation in COVID-19, but further studies are required to define subgroups with exaggerated PIC responses (eg, with D-dimers or biomarkers of endothelial cell activation) in whom earlier intervention with therapeutic anticoagulation (and other therapies) might be effective in attenuating the immunothrombotic positive feedback loop.76,77
Other Considerations and Limitations of the Tricompartmental Hypothesis
Determining the cause of COVID-19 stroke is complicated by the confounding influence of inter-related pathologies such as endothelial dysfunction, vasculitis, cardiomyopathy, hypercoagulability, and cytokine storm, and the fact that ventilated ICU patients are at risk of cerebral hypoperfusion, hypertensive bleed and stress-induced cardiomyopathy, all of which can lead to or facilitate thrombosis.
Although it is likely that no single mechanism accounts for all strokes in COVID-19, we present our hypothesis for LVO stroke arising within the pulmonary venous circulation against a background of several anecdotal case reports of pulmonary vein thrombosis in COVID-19, and the fact that pulmonary vein thrombosis has been reported at autopsy.6 However, we recognize that determining the exact source of emboli in patients with LVO stroke is fraught with difficulty, although previous authors emphasized cryptogenic cause, cardioembolic causes, and paradoxical embolism. The high proportion of cryptogenic cases might relate to under investigation in ill patient and to resources overstretched by the pandemic. However, a label of cardioembolic stroke has been made in 45% of patients with LVO stroke and although myocardial injury (myocardial infarction, myocarditis, or stress and inflammatory mediated myocardial suppression) and endocardial dysfunction (direct ACE-2 mediated viral effect, left ventricular dysfunction, and arrhythmia) frequently occur in severe COVID-19, the assumption that impaired cardiac function in COVID-19 stroke implied an origin within the heart (in the absence of demonstration of intracardiac clot), is flawed.2,15,18,79,80 Also, prior authors have attributed high-intensity transient signals (indicating microemboli) on transcranial Doppler to paradoxical embolism across a PFO, facilitated by increased pulmonary pressures resulting from, for example, severe COVID pneumonitis although the assumption that this is responsible for most cases is flawed as the NAVIGATE ESUS (embolic stroke of unknown source) trial that specifically addressed cryptogenic strokes found PFOs as a putative cause in <10% of cases so it is unlikely to be the major contributor to stroke overall or in COVID-19.81,82 Also, the detection of high-intensity transient signals implies no information as to the source, and we add the pulmonary veins to the potential list of sources which also includes paradoxical emboli and sources within the heart. Likewise, the high detection rate of microbubbles in ventilated patients with severe COVID undergoing contrast-enhanced transcranial Doppler simply reflects the underlying process of severe PIC where thrombosis of dual arterial supplies and concomitant venous thrombosis stimulates distal lung collaterals and promotes A-V shunting through the pulmonary circulation.83,84
We acknowledge that our hypothesis lacks definitive proof, although this is also true of other competing theories of origin of LVO clots from the heart or lower extremity via paradoxical embolism. However, LVO stroke from pulmonary clot embolism is well established postlung lobectomy,52 and there have been several case reports of pulmonary vein thrombosis since onset of COVID-19, which represents a notable increase in incidence of a very rare disease.9–12 Additionally, pulmonary venous thrombosis is part of the PIC complex on autopsy examination.6 Therefore, a causative link between pulmonary vein thrombosis and stroke has already been validated both in non-COVID and COVID stroke, but we acknowledge the limitation that pulmonary vein thrombosis has not been widely reported on CT pulmonary angiography which is frequently performed in patients with COVID-19, but there are several valid reasons why this is so. First, redistribution of flow from abnormal to normal areas augments venous return within veins draining normal segments which limits thrombosis to small, hard to visualize, venules (Figure 1D). Second, CT pulmonary angiography is timed to the pulmonary arteries and uses lower contrast volume and lower radiation dose which impairs pulmonary vein visualization.85 Thirdly, CT pulmonary angiography is only performed in the small subset of patients with COVID-19 associated stroke in whom concomitant pulmonary embolism is suspected, meaning that most patients in whom this finding might be present never undergo CT pulmonary angiography. Likewise, despite pulmonary vein thrombosis being part of the PIC process, it has only been mentioned in passing in patients succumbing to COVID-19, and the possibility that it might have significance rather than simply representing collateral damage in the inflammatory process centered on the distal airways does not appear to have been considered.6,86
Conclusions
Our hypothesis of pulmonary vein clot which extends the model of cardioembolism from endocardial surface and heart valves, retrogradely into the pulmonary veins which are continuous with the left cardiac chambers, is based on sound pulmonary physiology, unifies many of the existing theories of causation of stroke neuropathology in COVID-19, and is underpinned by reports of stroke complicating non-COVID-19 and COVID-19 related pulmonary vein thrombosis. We do not propose this as the sole mechanism of COVID-19 neurology but suggest that the flotsam originating in the disrupted lung tricompartment including larger clots or microclots leads to LVO strokes and cerebral microhemorrhages, and potentially also to other systemic complications. This model embraces other proposed mechanisms, such as hypercoagulability, endothelial damage, and hypoxemia, and holds that all mechanisms act in concert to cause tissue damage and infarction depending on location. Our model links severe COVID-19 pneumonia with brain diseases through lung tricompartment disruption and venous thrombosis, supports the increasingly recognized notion that lung microvascular pathology drives both the pulmonary and systemic manifestations of severe COVID-19 and offers a more plausible model for COVID-19 associated LVO strokes, than other proposed mechanisms.56,86
Article Information
Sources of Funding
None.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ACE
- angiotensin-converting enzyme
- Ang-2
- angiopoietin 2
- CT
- computed tomography
- LVO
- large vessel occlusion
- OPG
- osteoprotegerin
- PIC
- pulmonary intravascular coagulopathy
- VWF
- von Willebrand Factor
- VWFpp
- VWF propeptide
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Sources of Funding and Disclosures, see page 2417.
Contributor Information
Charles Bridgewood, Email: c.d.bridgewood@leeds.ac.uk.
Joseph Harbison, Email: jharbiso@tcd.ie.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan YK, Goh C, Leow AST, Tambyah PA, Ang A, Yap ES, Tu TM, Sharma VK, Yeo LLL, Chan BPL, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50:587–595. doi: 10.1007/s11239-020-02228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, Zhang J, Zhao C. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902–1914. doi: 10.1002/jmv.25884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021;3:e224–e233. doi: 10.1016/S2665-9913(20)30420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D, Bridgewood C, Meaney JFM. A tricompartmental model of lung oxygenation disruption to explain pulmonary and systemic pathology in severe COVID-19. Lancet Respir Med. 2021;9:665–672. doi: 10.1016/S2213-2600(21)00213-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Tafforeau P, Wagner WL, Walsh CL, Werlein C, Kühnel MP, Länger FP, Disney C, Bodey AJ, Bellier A, et al. The bronchial circulation in COVID-19 pneumonia. Am J Respir Crit Care Med. 2022;205:121–125. doi: 10.1164/rccm.202103-0594IM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goddard SA, Tran DQ, Chan MF, Honda MN, Weidenhaft MC, Triche BL. Pulmonary vein thrombosis in COVID-19. Chest. 2021;159:e361–e364. doi: 10.1016/j.chest.2020.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasha AK, Rabinstein A, McBane RD, 2nd. Pulmonary venous thrombosis in a patient with COVID-19 infection. J Thromb Thrombolysis. 2021:51:985–988. doi: 10.1007/s11239-021-02388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goette A, Patscheke M, Henschke F, Hammwöhner M. COVID-19-induced cytokine release syndrome associated with pulmonary vein thromboses, atrial cardiomyopathy, and arterial intima inflammation. TH Open. 2020;4:e271–e279. doi: 10.1055/s-0040-1716717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kruijsdijk RC, de Jong PA, Abrahams AC. Pulmonary vein thrombosis in COVID-19. BMJ Case Rep. 2020;13:e239986. doi: 10.1136/bcr-2020-239986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg MF, Goldberg MF, Cerejo R, Tayal AH. Cerebrovascular disease in COVID-19. AJNR Am J Neuroradiol. 2020;41:1170–1172. doi: 10.3174/ajnr.A6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghannam M, Alshaer Q, Al-Chalabi M, Zakarna L, Robertson J, Manousakis G. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol. 2020;267:3135–3153. doi: 10.1007/s00415-020-09990-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spence JD, de Freitas GR, Pettigrew LC, Ay H, Liebeskind DS, Kase CS, Del Brutto OH, Hankey GJ, Venketasubramanian N. Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 2020;49:451–458. doi: 10.1159/000509581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamakawa M, Kuno T, Mikami T, Takagi H, Gronseth G. Clinical characteristics of stroke with COVID-19: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2020;29:105288. doi: 10.1016/j.jstrokecerebrovasdis.2020.105288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A, Young B. Stroke as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes and predictors. J Stroke Cerebrovasc Dis. 2021;30:105549. doi: 10.1016/j.jstrokecerebrovasdis.2020.105549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremer S, Lersy F, de Sèze J, Ferré JC, Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G, Martin-Blondel G, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:E242–E251. doi: 10.1148/radiol.2020202222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wijeratne T, Gillard Crewther S, Sales C, Karimi L. COVID-19 pathophysiology predicts that ischemic stroke occurrence is an expectation, not an exception-a systematic review. Front Neurol. 2020;11:607221. doi: 10.3389/fneur.2020.607221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, Jayaseelan DL, Kumar G, Raftopoulos RE, Zambreanu L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siepmann T, Sedghi A, Simon E, Winzer S, Barlinn J, de With K, Mirow L, Wolz M, Gruenewald T, Schroettner P, et al. Increased risk of acute stroke among patients with severe COVID-19: a multicenter study and meta-analysis. Eur J Neurol. 2021;28:238–247. doi: 10.1111/ene.14535 [DOI] [PubMed] [Google Scholar]
- 24.E Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KW, Yusof Khan AHK, Ching SM, Chia PK, Loh WC, Abdul Rashid AM, Baharin J, Inche Mat LN, Wan Sulaiman WA, Devaraj NK, et al. Stroke and novel coronavirus infection in humans: a systematic review and meta-analysis. Front Neurol. 2020;11:579070. doi: 10.3389/fneur.2020.579070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nepal G, Rehrig JH, Shrestha GS, Shing YK, Yadav JK, Ojha R, Pokhrel G, Tu ZL, Huang DY. Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020;24:421. doi: 10.1186/s13054-020-03121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27.e1. doi: 10.1016/j.cell.2020.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraiman P, Godeiro Junior C, Moro E, Cavallieri F, Zedde M. COVID-19 and cerebrovascular diseases: a systematic review and perspectives for stroke management. Front Neurol. 2020;11:574694. doi: 10.3389/fneur.2020.574694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valderrama EV, Humbert K, Lord A, Frontera J, Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020;51:e124–e127. doi: 10.1161/STROKEAHA.120.030153 [DOI] [PubMed] [Google Scholar]
- 30.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guzik TJ, Mohiddin SA, Dimarco A, Patel V, Savvatis K, Marelli-Berg FM, Madhur MS, Tomaszewski M, Maffia P, D’Acquisto F, et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur S, Bansal R, Kollimuttathuillam S, Gowda AM, Singh B, Mehta D, Maroules M. The looming storm: blood and cytokines in COVID-19. Blood Rev. 2021;46:100743. doi: 10.1016/j.blre.2020.100743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Román GC, Spencer PS, Reis J, Buguet A, Faris MEA, Katrak SM, Láinez M, Medina MT, Meshram C, Mizusawa H, et al. ; WFN Environmental Neurology Specialty Group. The neurology of COVID-19 revisited: a proposal from the environmental neurology specialty group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci. 2020;414:116884. doi: 10.1016/j.jns.2020.116884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanafi R, Roger PA, Perin B, Kuchcinski G, Deleval N, Dallery F, Michel D, Hacein-Bey L, Pruvo JP, Outteryck O, et al. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am J Neuroradiol. 2020;41:1384–1387. doi: 10.3174/ajnr.A6651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, Hernandez T, Stock A, Zhao Z, AlRasheed MR, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanou EM, Coutinho JM, Shannon P, Kiehl TR, Levi MM, Wilcox ME, Aviv RI, Mandell DM. Critical illness-associated cerebral microbleeds. Stroke. 2017;48:1085–1087. doi: 10.1161/STROKEAHA.116.016289 [DOI] [PubMed] [Google Scholar]
- 38.Fara MG, Stein LK, Skliut M, Morgello S, Fifi JT, Dhamoon MS. Macrothrombosis and stroke in patients with mild COVID-19 infection. J Thromb Haemost. 2020;18:2031–2033. doi: 10.1111/jth.14938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JY, Lee HK, Park JH, Cho SJ, Kwon M, Jo C, Koh YH. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem Biophys Res Commun. 2020;528:413–419. doi: 10.1016/j.bbrc.2020.05.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yachou Y, El Idrissi A, Belapasov V, Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41:2657–2669. doi: 10.1007/s10072-020-04575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sweid A, Hammoud B, Bekelis K, Missios S, Tjoumakaris SI, Gooch MR, Herial NA, Zarzour H, Romo V, DePrince M, et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;15:733–742. doi: 10.1177/1747493020937189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kihira S, Schefflein J, Mahmoudi K, Rigney B, N Delman B, Mocco J, Doshi A, Belani P. Association of coronavirus disease (COVID-19) with large vessel occlusion strokes: a case-control study. AJR Am J Roentgenol. 2021;216:150–156. doi: 10.2214/AJR.20.23847 [DOI] [PubMed] [Google Scholar]
- 44.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majidi S, Fifi JT, Ladner TR, Lara-Reyna J, Yaeger KA, Yim B, Dangayach N, Oxley TJ, Shigematsu T, Kummer BR, et al. Emergent large vessel occlusion stroke during New York City’s COVID-19 outbreak: clinical characteristics and paraclinical findings. Stroke. 2020;51:2656–2663. doi: 10.1161/STROKEAHA.120.030397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escalard S, Chalumeau V, Escalard C, Redjem H, Delvoye F, Hébert S, Smajda S, Ciccio G, Desilles JP, Mazighi M, et al. Early brain imaging shows increased severity of acute ischemic strokes with large vessel occlusion in COVID-19 patients. Stroke. 2020;51:3366–3370. doi: 10.1161/STROKEAHA.120.031011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belani P, Schefflein J, Kihira S, Rigney B, Delman BN, Mahmoudi K, Mocco J, Majidi S, Yeckley J, Aggarwal A, et al. COVID-19 is an independent risk factor for acute ischemic stroke. AJNR Am J Neuroradiol. 2020;41:1361–1364. doi: 10.3174/ajnr.A6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGonagle D, Kearney MF, O’Regan A, O’Donnell JS, Quartuccio L, Watad A, Bridgewood C. Therapeutic implications of ongoing alveolar viral replication in COVID-19. Lancet Rheumatol. 2022;4:e135–e144. doi: 10.1016/S2665-9913(21)00322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magro CM, Mulvey J, Kubiak J, Mikhail S, Suster D, Crowson AN, Laurence J, Nuovo G. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann Diagn Pathol. 2021;50:151645. doi: 10.1016/j.anndiagpath.2020.151645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deffebach ME, Charan NB, Lakshminarayan S, Butler J. The bronchial circulation. Small, but a vital attribute of the lung. Am Rev Respir Dis. 1987;135:463–481. doi: 10.1164/arrd.1987.135.2.463 [DOI] [PubMed] [Google Scholar]
- 51.Walker CM, Rosado-de-Christenson ML, Martínez-Jiménez S, Kunin JR, Wible BC. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics. 2015;35:32–49. doi: 10.1148/rg.351140089 [DOI] [PubMed] [Google Scholar]
- 52.Ohtaka K, Hida Y, Kaga K, Kato T, Muto J, Nakada-Kubota R, Sasaki T, Matsui Y. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg. 2013;95:1924–1928. doi: 10.1016/j.athoracsur.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 53.Hashimoto G, Wada S, Morita T, Tomohara S, Hara K, Kumabe M, Matsushima T, Kadowaki M, Hamaguchi M, Kuwashiro T, et al. Ischemic stroke caused by carotid stump at the common carotid artery. Intern Med. 2020;59:3071–3074. doi: 10.2169/internalmedicine.5021-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lang M, Li MD, Jiang KZ, Yoon BC, Mendoza DP, Flores EJ, Rincon SP, Mehan WA, Jr, Conklin J, Huang SY, et al. Severity of chest imaging is correlated with risk of acute neuroimaging findings among patients with COVID-19. AJNR Am J Neuroradiol. 2021;42:831–837. doi: 10.3174/ajnr.A7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahammedi A, Ramos A, Bargalló N, Gaskill M, Kapur S, Saba L, Carrete H, Jr, Sengupta S, Salvador E, Hilario A, et al. Brain and lung imaging correlation in patients with COVID-19: could the severity of lung disease reflect the prevalence of acute abnormalities on neuroimaging? A global multicenter observational study. AJNR Am J Neuroradiol. 2021;42:1008–1016. doi: 10.3174/ajnr.A7072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mello S, Judge C, Kelly R, Bradley D, Harbison J. A systematic review of the causes and management of nonthrombotic embolic stroke of tissue origin. Stroke Res Treat. 2018;2018:8092862. doi: 10.1155/2018/8092862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1–7. doi: 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, Bilaloglu S, Hochman K, Raz E, Galetta S, Horwtiz L. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S, Lewis A, Melmed K, Hochman K, Horwitz L, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chougar L, Shor N, Weiss N, Galanaud D, Leclercq D, Mathon B, Belkacem S, Ströer S, Burrel S, Boutolleau D, et al. ; CoCo Neurosciences Study Group. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology. 2020;297:E313–E323. doi: 10.1148/radiol.2020202422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kihira S, Schefflein J, Pawha P, Rigney B, Delman BN, Xuan D, Fifi J, Doshi AH, Belani P. Neurovascular complications that can be seen in COVID-19 patients. Clin Imaging. 2021;69:280–284. doi: 10.1016/j.clinimag.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anzalone N, Castellano A, Scotti R, Scandroglio AM, Filippi M, Ciceri F, Tresoldi M, Falini A. Multifocal laminar cortical brain lesions: a consistent MRI finding in neuro-COVID-19 patients. J Neurol. 2020;267:2806–2809. doi: 10.1007/s00415-020-09966-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gulko E, Oleksk ML, Gomes W, Ali S, Mehta H, Overby P, Al-Mufti F, Rozenshtein A. MRI brain findings in 126 patients with COVID-19: initial observations from a descriptive literature review. AJNR Am J Neuroradiol. 2020;41:2199–2203. doi: 10.3174/ajnr.A6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, Kaya Y, Yildirim D, Tuzuner F, Yildirim MS, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297:E232–E235. doi: 10.1148/radiol.2020201697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.South K, McCulloch L, McColl BW, Elkind MS, Allan SM, Smith CJ. Preceding infection and risk of stroke: an old concept revived by the COVID-19 pandemic. Int J Stroke. 2020;15:722–732. doi: 10.1177/1747493020943815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009;11:417–426. doi: 10.1007/s12028-009-9242-8 [DOI] [PubMed] [Google Scholar]
- 69.Chernysh IN, Nagaswami C, Kosolapova S, Peshkova AD, Cuker A, Cines DB, Cambor CL, Litvinov RI, Weisel JW. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep. 2020;10:5112. doi: 10.1038/s41598-020-59526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirschenbaum D, Imbach LL, Rushing EJ, Frauenknecht KBM, Gascho D, Ineichen BV, Keller E, Kohler S, Lichtblau M, Reimann RR, et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol Appl Neurobiol. 2021;47:454–459. doi: 10.1111/nan.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conklin J, Frosch MP, Mukerji SS, Rapalino O, Maher MD, Schaefer PW, Lev MH, Gonzalez RG, Das S, Champion SN, et al. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J Neurol Sci. 2021;421:117308. doi: 10.1016/j.jns.2021.117308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, Borja MJ, Zan E, Fatterpekar GM. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297:E223–E227. doi: 10.1148/radiol.2020202040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estébanez A, Pérez-Santiago L, Silva E, Guillen-Climent S, García-Vázquez A, Ramón MD. Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol. 2020;34:e250–e251. doi: 10.1111/jdv.16474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Donnell JS, Peyvandi F, Martin-Loeches I. Pulmonary immuno-thrombosis in COVID-19 ARDS pathogenesis. Intensive Care Med. 2021;47:899–902. doi: 10.1007/s00134-021-06419-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ward SE, Curley GF, Lavin M, Fogarty H, Karampini E, McEvoy NL, Clarke J, Boylan M, Alalqam R, Worrall AP, et al. ; Irish COVID-19 Vasculopathy Study (ICVS) Investigators. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br J Haematol. 2021;192:714–719. doi: 10.1111/bjh.17273 [DOI] [PubMed] [Google Scholar]
- 78.Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Ní Áinle F, Alomran F, Alayed K, Alsheef M, AlSumait F, et al. Heparin for moderately ill patients with COVID-19. medRxiv. Preprint posted online Jul 12, 2021: doi: 10.1111/2021.07.08.21259351. [Google Scholar]
- 79.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jäger HR, Losseff NA, Perry RJ, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fifi JT, Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol. 2020;19:713–715. doi: 10.1016/S1474-4422(20)30272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ameriso SF, Amarenco P, Pearce LA, Perera KS, Ntaios G, Lang W, Bereczki D, Uchiyama S, Kasner SE, Yoon BW, et al. Intracranial and systemic atherosclerosis in the NAVIGATE ESUS trial: recurrent stroke risk and response to antithrombotic therapy. J Stroke Cerebrovasc Dis. 2020;29:104936. doi: 10.1016/j.jstrokecerebrovasdis.2020.104936 [DOI] [PubMed] [Google Scholar]
- 82.Veltkamp R, Pearce LA, Korompoki E, Sharma M, Kasner SE, Toni D, Ameriso SF, Mundl H, Tatlisumak T, Hankey GJ, et al. Characteristics of recurrent ischemic stroke after embolic stroke of undetermined source: secondary analysis of a randomized clinical trial. JAMA Neurol. 2020;77:1233–1240. doi: 10.1001/jamaneurol.2020.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reynolds AS, Lee AG, Renz J, DeSantis K, Liang J, Powell CA, Ventetuolo CE, Poor HD. Pulmonary vascular dilatation detected by automated transcranial doppler in COVID-19 pneumonia. Am J Respir Crit Care Med. 2020;202:1037–1039. doi: 10.1164/rccm.202006-2219LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cherian R, Chandra B, Tung ML, Vuylsteke A. Positive bubble study in severe COVID-19 indicates the development of anatomical intrapulmonary shunts in response to microvascular occlusion. Am J Respir Crit Care Med. 2021;203:263–265. doi: 10.1164/rccm.202008-3186LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aldosari S, Sun Z. A systematic review of double low-dose CT pulmonary angiography in pulmonary embolism. Curr Med Imaging Rev. 2019;15:453–460. doi: 10.2174/1573405614666180813120619 [DOI] [PubMed] [Google Scholar]
- 86.McGonagle D, O’ Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2:e437–445. doi: 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]