Abstract
Endocytosis in Saccharomyces cerevisiae is inhibited by concentrations of ethanol of 2 to 6% (vol/vol), which are lower than concentrations commonly present in its natural habitats. In spite of this inhibition, endocytosis takes place under enological conditions when high concentrations of ethanol are present. Therefore, it seems that yeast has developed some means to circumvent the inhibition. In this work we have investigated this possibility. We identified two stress conditions under which endocytosis was resistant to inhibition by ethanol: fermentation during nitrogen starvation and growth on nonfermentable substrates. Under these conditions, yeast accumulates stress protectors, primarily trehalose and Hsp104, a protein required for yeast to survive ethanol stress. We found the following. (i) The appearance of ethanol resistance was accompanied by trehalose accumulation. (ii) Mutant cells unable to synthesize trehalose also were unable to develop resistance. (iii) Mutant cells that accumulated trehalose during growth on sugars were resistant to ethanol even under this nonstressing condition. (iv) Mutant cells unable to synthesize Hsp104 were able to develop resistance. We conclude that trehalose is the major factor in the protection of endocytosis from ethanol. Our results suggest another important physiological role for trehalose in yeast.
Saccharomyces spp. are resistant to alcohol and can grow in the presence of 8 to 12% (vol/vol) alcohol and survive exposure to up to 15% alcohol (19). However, in spite of this high resistance to ethanol, there is at least one pathway, endocytosis, in yeast cells that is remarkably sensitive to ethanol. Endocytosis in Saccharomyces spp. is strongly inhibited by 2 to 6% (vol/vol) concentrations of ethanol, which are well below those often present in their natural habitats (28). This remarkable sensitivity to ethanol raises the question of the physiological relevance of endocytosis in yeast.
Endocytosis is the mechanism whereby eukaryotic cells internalize their own plasma membrane proteins and macromolecules from the external environment (for a review, see reference 13). In animal cells, endocytosis is involved in functions such as nutrition, hormone response, immune defense, and membrane conservation. In yeast cells, endocytosis is involved in mating, which is initiated by binding and internalization of the secreted a- and α-factors to receptors located on the plasma membrane (for a review, see reference 30), and the reconstruction of the plasma membrane, which is initiated by changes in nutritional conditions that trigger internalization and degradation of plasma membrane proteins, usually unneeded nutrient transporters (15, 18, 20, 31, 40, 41, 53). As both functions are important for yeast survival, endocytosis is probably an important process in yeast and, therefore, this organism should have developed some means to circumvent the inhibition of endocytosis by ethanol.
Our objective in this study was to identify this avoidance mechanism by determining the conditions under which endocytosis is resistant to ethanol and the factor(s) responsible for the resistance. We used the maltose transporter as the main experimental model.
Under enological conditions, yeast fermentation slows down when the nitrogen source is consumed and the medium still contains high sugar concentrations. This slowdown is accompanied by endocytosis and degradation of the sugar transporters (18, 20, 26, 27, 37, 40, 46). We hypothesize that endocytosis is resistant to ethanol under this stress condition. Our results confirmed this possibility and also showed that endocytosis is resistant to ethanol during growth on nonfermentable substrates. Under stress conditions, Saccharomyces accumulates small organic compounds and heat shock proteins (Hsps) to protect cells against damage (39, 45). In Saccharomyces cerevisiae, Hsp104 and the disaccharide trehalose are the two main stress protectors (9, 11, 48). Trehalose preserves the integrity of biological membranes (8), stabilizes proteins in their native state (52), and suppresses the aggregation of denatured proteins, maintaining them in a partially folded state from which they can be reactivated by Hsp104 (52). We investigated the role of these two stress protectors in the tolerance of endocytosis to ethanol by using mutants deficient in the synthesis or degradation of trehalose or in the synthesis of Hsp104. Our results suggest another important physiological role for trehalose in yeast.
MATERIALS AND METHODS
Reagents.
d-[U-14C]maltose, d-[U-14C]galactose, and enhanced chemoluminescence reagents were from Amersham International (Little Chalfont, United Kingdom). The goat anti-rabbit antibody–peroxidase conjugate was from Biosource International (Caramillo, Calif.). The scintillation cocktail was from Fisher Chemicals (Loughborough, Leicester, United Kingdom). The yeast nitrogen base (YNB) media were from Difco Laboratories (Detroit, Mich.). All other reagents were of analytical grade.
Plasmids.
Two plasmids were used: pA33hygB#1, which is an integrative plasmid carrying the MAL61 gene under the control of the ADH1 promoter (provided by Paul Klaassen, Gist-brocades, Delft, The Netherlands), and pRM1-1, which is a multicopy plasmid carrying the inducible MAL1 locus (43).
Yeast strains.
Genotypes of the strains used are given in Table 1. Synthesis of the galactose transporter requires the presence of galactose (23). We used a gal80 mutant to constitutively express the galactose transporter. The TPS1 gene encodes the small subunit of the trehalose-6-phosphate synthase–trehalose-6-phosphatase complex (3), and disruption of this gene blocks trehalose synthesis. tps1Δ mutant cells are deficient in trehalose accumulation and do not catabolize glucose because of a disorder in the glycolytic flux. This disorder is probably related to the lack of trehalose-6-phosphate, which is an inhibitor of hexokinase II (5). Deletion of the gene encoding hexokinase II restores the ability of tps1Δ cells to use glucose (17). Glucose is required as an energy source for endocytosis, and the double-mutant strain tps1Δ hxk2 was used to meet this requirement. Hydrolysis of trehalose is catalyzed by an acid trehalase located in the vacuole, Ath1p, and two neutral trehalases located in the cytosol, Nth1p and Nth2p (33). Nth1p is the major enzyme responsible for trehalose degradation in vivo, and nth1Δ mutant cells contain high levels of trehalose even under nonstressing conditions (34, 35). HSP104 encodes Hsp104, which is highly expressed under some stressing conditions (24) and is required for yeast to survive ethanol stress (49).
TABLE 1.
Strains used in this study
| Strain (reference) | Genotype |
|---|---|
| ATCC 42407 | MATa suc− MAL GAL |
| WCG4a (16) | MATa mal his3-11,15 leu2-3,112 ura3-D GAL+ Cans |
| X106-3D (32) | MATa gal80 his1 ura3 |
| WHC (5) | MATa mal can1-100 ade2-1 his3-11,15 leu2-3,13 trp1-1 ura3-1 hxk2::LEU2 TPS1 |
| WHDC (44) | MATa mal can1-100 ade2-1 his3-11,15 leu2-3,13 trp1-1 ura3-1 hxk2::LEU2 tps1::HIS3 |
| W303-1A | MATa mal can1-100 ade2-1 his3-11,15 leu2-3,13 trp1-1 ura3-1 |
| hsp104Δ-LEU (52) | MATa mal can1-100 ade2-1 his3-11,15 leu2-3,13 trp1-1 ura3-1 hsp104::LEU2 |
| YS18 (34) | MATa mal his3-11, 15 leu2-3, 112 ura3D5 CanR gal− |
| YSN1 (34) | MATa mal his3-11, 15 leu2-3, 112 ura3D5 CanR gal− nth1::LEU2 |
Growth conditions.
ATCC 42407 and hsp104Δ-LEU were transformed with the integrative plasmid pA33hygB#1. The transformed cells transported maltose at the same rates as did mal+ wild-type cells. These cells, as well as the gal80 mutant strain X106-3D, were grown at 30°C in a rotatory shaker (200 rpm) in a rich medium containing 2% peptone and 1% yeast extract. As indicated, 2% glucose, 2% ethanol, 2% glycerol, or 2% glucose in the presence of 2% ethanol was used as the carbon and energy source. All other strains were transformed with the multicopy plasmid pRM1-1. The transformed strains that grew and transported maltose at the same rates as did mal+ wild-type strains were grown in the presence of 2% maltose and 5 mM antimycin A in rich medium or in YNB medium with ammonium sulfate without amino acids, supplemented with the required amino acids (200 mg/liter, except for leucine, which was used at 200 mg/liter). Antimycin A (5 mM) was added to inhibit respiration. This inhibition favors plasmid expression by forcing the cells to ferment maltose. Cell growth was monitored by measuring optical density at 640 nm.
Conditions for endocytosis in the presence or absence of ethanol.
To trigger endocytosis, the cells were harvested by centrifugation during exponential growth (about 0.7 mg [dry weight]/ml), washed with water, and suspended to about 0.2 mg/ml in YNB medium without ammonium sulfate and without amino acids (7) in the presence of 2% glucose, which is an efficient energy source for endocytosis (36), and 250 μg of tetracycline chlorohydrate/ml. The antibiotic was added to avoid bacterial contamination during handling of the cells. Unless otherwise indicated, the suspension was immediately divided into aliquots and ethanol was added to the final concentration. After incubation at 30°C in a rotatory shaker (200 rpm), samples of the suspension were taken and endocytosis was measured. In some experiments, the entire cellular suspension was incubated at 30°C for 3 h. Then it was divided into aliquots, ethanol was added, and the cells were treated as described above. By the end of the experiments, the glucose remaining in the medium was >1%.
Endocytosis measurements.
Internalization of the maltose and galactose transporters, the first step of endocytosis, was measured by monitoring the decrease in the rate of transport activity with radioactive sugars (18, 28). Endocytosis was also measured by monitoring the disappearance of the transporters from the plasma membrane by immunoblotting purified plasma membrane preparations (28). It is well established that under the used experimental conditions, i.e., in the absence of de novo protein synthesis, the decrease in transporter content is due to endocytosis (18, 28).
Transport activity measurements.
To measure transport activity, the cells were harvested, washed, and resuspended at 40 mg (dry weight) of yeast/ml in 100 mM tartaric acid adjusted to pH 4.2 with Tris in the case of the maltose transporter or 50 mM potassium phosphate (pH 6.0) in the case of the galactose transporter. Aliquots of 50 ml, containing 2 mg (dry weight) of yeast, were added to 5 ml of either 45 mM labeled maltose or galactose (0.5 mCi/mmol) and were incubated at 20°C for 15 s. Sugar uptake was stopped by the addition of 10 ml of chilled water (4°C). After rapid filtration, the cells and filters were washed with 10 ml of chilled water and immediately submerged in liquid scintillation cocktail and the radioactivity was counted. As previously described, internalization of these transporters follows first-order kinetics (18, 28, 40).
Plasma membrane purification and immunoblotting.
Crude extracts and crude membrane preparations were obtained as previously described (28). Plasma membrane preparations containing less than 5% of other cellular membranes were purified by applying the crude membrane preparation to a discontinuous sucrose gradient (28). Samples of purified plasma membrane preparations, containing 7 or 3 mg of protein, were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (26), and the maltose transporter was detected by using antiserum against the maltose transporter (diluted 1/3,000 in blocking buffer) and against the goat anti-rabbit peroxidase conjugate (diluted 1/10,000) as primary and secondary antibodies, respectively (38). The peroxidase conjugate was revealed with an enhanced chemoluminescence detection kit. The H+-ATPase was used as a marker protein for the plasma membrane (50). This protein is very stable and shows almost no internalization under the conditions that we used (4, 28). The intensity of the band corresponding to the maltose transporter was quantified by scanning densitometry as previously described (26).
Trehalose measurement.
The amount of trehalose was determined by measuring the glucose released after treatment of cellular extracts with acid trehalase as previously described (6).
Protein measurement.
The amount of protein was measured by using the method of Lowry et al. (25) after precipitation with trichloroacetic acid.
RESULTS
Endocytosis in nitrogen-starved cells.
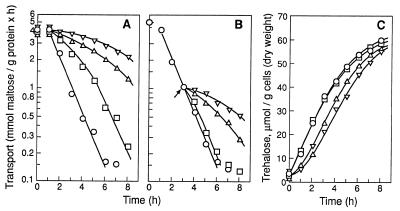
We shifted exponentially growing cells to media lacking a nitrogen source but containing glucose and different concentrations of ethanol (Fig. 1A). We found that after about 1 h of nitrogen starvation, the maltose transporter was inactivated at rates that were inversely proportional to the concentration of ethanol present, i.e., the higher the ethanol concentration, the lower the rate of inactivation. This protein is inactivated by its internalization during endocytosis (40), so these results show, as found previously (28), that endocytosis of this transporter was inhibited by ethanol. The effect of ethanol tended to disappear as the starvation period increased. Endocytosis of the transporter in the absence of ethanol occurred at a constant rate (Fig. 1A). In the presence of ethanol, however, the rate of endocytosis appeared to accelerate until, in the case of 2% ethanol, it became equal to the rate observed in the absence of alcohol (Fig. 1A).
FIG. 1.
Appearance of tolerance to ethanol in nitrogen-starved cells. Strain WCG4a was grown in rich medium with maltose. (A and C) Cells were suspended in ammonium-free medium containing 2% glucose in the absence (○) or presence of 2 (□), 4 (▵), or 6% ethanol (▿). (B) Cells were suspended in ammonium-free medium containing 2% glucose. After 3 h of incubation at 30°C, the suspension was divided into aliquots and 0 (○), 2 (□), 4 (▵), or 6% ethanol (▿) was added. After incubation at 30°C for the indicated times, maltose transport activity (A and B) and trehalose content (C) were measured. Data are mean values for two experiments. Differences between the two values were <10%.
These results show that nitrogen-starved cells incubated in the presence of ethanol developed a tolerance of endocytosis to ethanol. We also incubated cells for 3 h in the absence of ethanol before testing the ethanol effect (Fig. 1B) and found that in the absence of alcohol, the cells still developed tolerance. This result was particularly evident in the case of 2% ethanol, since after 3 to 4 h of nitrogen starvation, in either the presence (Fig. 1A) or absence of ethanol (Fig. 1B), endocytosis appeared to be completely tolerant to this concentration of ethanol.
Tolerance to ethanol in growing cells.
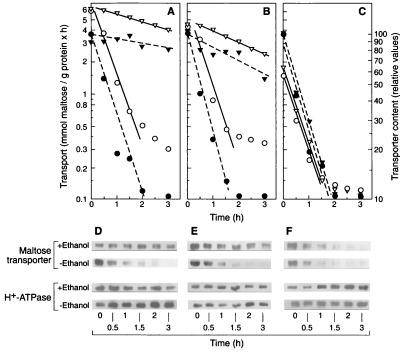
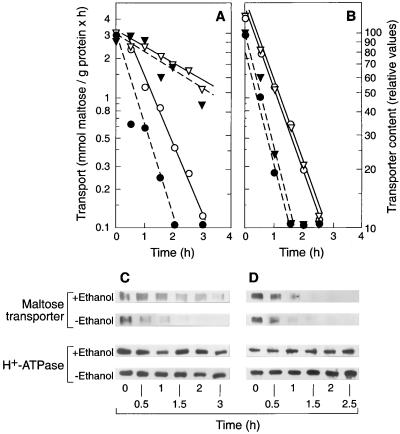
We grew cells on glucose, ethanol, or glucose plus ethanol, transferred them to ammonium-free medium with and without 6% ethanol, and measured endocytosis. We found that 6% ethanol inhibited endocytosis of the transporter by more than 90% in cells grown on glucose (Fig. 2A and D) or on glucose in the presence of ethanol (Fig. 2B and E) but that no inhibition occurred in cells grown on ethanol (Fig. 2C and F). The intensity of the H+-ATPase band, the control protein, remained constant in all tested conditions (Fig. 2D through F).
FIG. 2.
Tolerance to ethanol of endocytosis of the maltose transporter in growing cells. Strain ATCC 42407 grown in rich medium containing 2% glucose (A and D), 2% glucose plus 2% ethanol (B and E), or 2% ethanol (C and F) was harvested, suspended in ammonium-free medium containing 2% glucose in the absence (○, ●) or presence of 6% ethanol (▿, ▾), and incubated at 30°C. At the indicated times, measurements of transport activity (open symbols) (A through C) and immunoblots of the transporter using purified plasma membrane preparations (D through F) were performed. The intensity of the transporter band was plotted in arbitrary units (dark symbols) (A through C). The experiment was repeated three times. Differences between the values were <10%. Representative data from one experiment are shown.
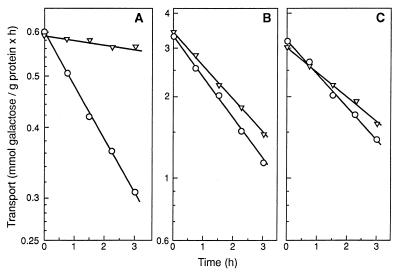
We also used glycerol as the carbon source and measured endocytosis of the maltose and galactose transporters. The galactose transporter is another plasma membrane protein marker for endocytosis (28). As in the case of the maltose transporter, 6% ethanol inhibited endocytosis of the galactose transporter in cells grown on glucose by more than 90% (Fig. 3A), but almost no inhibition was seen in cells grown on ethanol (Fig. 3B) or on glycerol (Fig. 3C and data not shown). The low rate of galactose transport in glucose-grown cells (Fig. 3A) can be explained by the repressor effect of glucose on the expression of the galactose transporter (23).
FIG. 3.
Tolerance to ethanol of endocytosis of the galactose transporter in growing cells. Strain X106-3D was harvested during growth in rich medium containing 2% glucose (A), 2% ethanol (B), or 2% glycerol (C), suspended in ammonium-free medium containing 2% glucose in the absence (○) or presence of 6% ethanol (▿), and incubated at 30°C. Data are mean values of two experiments. Differences between the two values were <10%.
These results show that growing cells also may develop tolerance of endocytosis to ethanol, although in this case, the appearance of tolerance required utilization of a nonfermentable substrate.
Trehalose levels in growing and nitrogen-starved cells.
The appearance of ethanol tolerance in nitrogen-starved cells (Fig. 1A) was accompanied by accumulation of trehalose (Fig. 1C). The high tolerance to ethanol observed in ethanol- (Fig. 2C) and glycerol-grown cells (Fig. 3C) was also accompanied by a high concentration of trehalose (255 to 300 μmol/g [dry weight] of cells), whereas the low tolerance observed in glucose- (Fig. 2A and 3A) or glucose-plus-ethanol-grown cells (Fig. 2B) was accompanied by a low concentration of trehalose (5 to 10 μmol/g of cells).
Endocytosis in a mutant strain unable to accumulate trehalose.
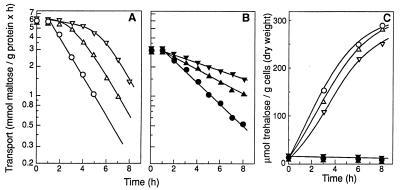
In a tps1Δ mutant with completely blocked trehalose synthesis (5), the inhibition of endocytosis by ethanol remained constant for at least 8 h under nitrogen starvation (Fig. 4B). In the isogenic TPS1 strain, which accumulates large amounts of trehalose, about 300 μmol of trehalose/g (dry weight) of cells (Fig. 4C), the inhibition disappeared in less than 5 h under starvation (Fig. 4A).
FIG. 4.
Inhibition of endocytosis by ethanol in a mutant strain unable to accumulate trehalose. Strains WHC (open symbols) (A and C) and WHDC (closed symbols) (B and C) were harvested during growth in rich medium with maltose and suspended in ammonium-free medium with 2% glucose in the absence (○, ●) or presence of 3 (▵, ▴) or 6% ethanol (▿, ▾) and incubated at 30°C. Maltose transport activity (A and B) and trehalose content of the cells (C) were measured. Data are mean values of two experiments. Differences between the two values were <10%.
Endocytosis in a mutant deficient in neutral trehalase.
We grew nth1Δ cells, which lack neutral trehalase, in maltose and found that these cells, which contained 100 μmol of trehalose/g (dry weight) of cells, had a substantially lower inhibition of endocytosis by ethanol than did the isogenic NTH1 cells, which contained only 7 μmol of trehalose/g of cells (Table 2).
TABLE 2.
Effect of ethanol on the internalization rate of the maltose transporter in NTH1 wild-type cells and nth1Δ mutant cellsa
| Ethanol concn (%) | Inhibition by ethanol (%) of the internalization rate in:
|
|
|---|---|---|
| NTH1 cells | nth1Δ cells | |
| 0 | 0 | 0 |
| 2 | 55 | 13 |
| 4 | 70 | 28 |
| 6 | 90 | 37 |
Strains YS18 and YSN1 were harvested during growth in YNB medium containing 2% maltose and the required amino acids, suspended in ammonium-free medium in the absence or presence of ethanol at the indicated concentrations, and incubated at 30°C. The rate of internalization, which in these strains started immediately after suspension in ammonium-free medium, was monitored over the first 2 h by measuring maltose transport activity in samples of the suspension taken every 20 min. Data are mean values of two experiments. Differences between the two values were <10%.
Endocytosis in a mutant strain unable to accumulate Hsp104.
We measured endocytosis in an hsp104Δ mutant, which lacks Hsp104. We found that, as occurred in HSP104 wild-type cells (Fig. 2), endocytosis was inhibited by ethanol in the hsp104Δ strain when cells were grown on glucose (Fig. 5A and C) and that, in spite of the lack of Hsp104, endocytosis was resistant to inhibition when cells were grown on ethanol (Fig. 5B and D). As demonstrated before (Fig. 2), H+-ATPase levels remained constant (Fig. 5C and D). The levels of trehalose in hsp104Δ cells were 2 and 190 μg/g (dry weight) of cells during growth on glucose and ethanol, respectively.
FIG. 5.
Inhibition of endocytosis by ethanol in a mutant strain unable to accumulate Hsp104. Strain hsp104Δ-LEU grown in rich medium containing 2% glucose (A and C) or 2% ethanol (B and D) was harvested and suspended in ammonium-free medium with 2% glucose in the absence (○, ●) or presence of 6% ethanol (▿, ▾) and incubated at 30°C. Measurements of transport activity (open symbols) (A and B) and immunoblotting of the transporter using purified plasma membrane preparations (C and D) were achieved. The intensity of the transporter band was plotted in arbitrary units (A and B) (dark symbols). The experiment was repeated twice. Differences between the values were <10%. Data from one representative experiment are shown.
DISCUSSION
Endocytosis in yeast is inhibited by low concentrations of ethanol. In spite of this inhibition, endocytosis appears to take place under enological conditions when high concentrations of ethanol are present. We identified two conditions under which endocytosis is resistant to ethanol: fermentation in the absence of a nitrogen source and growth on a nonfermentable substrate. These nutritional conditions are stress conditions under which yeast accumulates stress protectors, primarily trehalose and Hsp104 (9, 11, 48).
We found that in all cases examined, high resistance of endocytosis to ethanol inhibition was accompanied by a high intracellular level of trehalose and that low resistance was accompanied by a low level of this disaccharide. These results suggest that trehalose may play a role in the resistance. For this hypothesis to be correct, two predictions must be satisfied: first, a mutant unable to synthesize trehalose should be unable to resist ethanol and second, a mutant deficient in the hydrolysis of trehalose, which accumulates trehalose during growth on sugars, should resist ethanol even under this nonstressing condition. The results obtained with tps1Δ and nth1Δ strains satisfied both predictions, indicating that trehalose is a major factor in the protection of endocytosis from ethanol. Results obtained with a mutant strain which lacks Hsp104 ruled out a role for this protein in ethanol protection. Unlike trehalose, which acts as both a protein and membrane protector (1, 52), Hsp104, in concert with other Hsps, acts only as a protein protector (14). These differences in their functions are probably responsible for the different effects on endocytosis of the two factors.
Water plays an important role in the structure of biological membranes by penetrating the lipid bilayer and forming hydrogen bonds with the polar groups of phospholipids (for review, see reference 8). Ethanol may substitute for water in this role and, in doing so, alter the structure of the membrane, the interactions between lipids and proteins, and the positioning of these molecules in the membranes (2, 8, 19). These changes may affect membrane functions, with endocytosis being particularly sensitive to these changes (28). Trehalose can displace water and ethanol in yeast membranes. However, in contrast to the situation for ethanol, the formation of hydrogen bonds between the hydroxyl groups of trehalose and the polar groups of lipids stabilizes the membrane (8).
Lipids are important for both the structure and function of yeast membranes and are differently distributed in the two halves of the lipid bilayer (10). Concerning endocytosis, synthesis of ergosterol and ceramide has been shown to be required for the internalization step in yeast cells (42). In addition, many proteins involved in endocytosis have lipid-binding domains (for review, see reference 42). Therefore, the ability of trehalose to displace ethanol from plasma membrane lipids and to stabilize its structure (8) might be responsible for its role in the tolerance of endocytosis to ethanol and possibly in the tolerance to other types of stress which affect the plasma membrane (9, 12, 51).
Endocytosis in yeast may be inhibited during exponential growth on fermentable carbon sources even when only moderate concentrations of ethanol are present. That this inhibition is not deleterious to growth suggests that the two functions involving endocytosis in S. cerevisiae, mating and turnover of plasma membrane proteins, are dispensable under these nutritional conditions. The available information supports this hypothesis (4, 21). However, under field conditions, yeast cells spend most of their time not in logarithmic growth but in the stationary phase, starving for one or several nutrients (54, 55). Under these conditions, mating, which allows sporulation (30), and turnover of plasma membrane proteins, which allows adaptation to new nutrients (15, 18, 20, 31, 40, 41, 53), could be critical for survival and effective competition with other organisms. Therefore, trehalose accumulated during nutrient starvation in yeast cells could be essential for the occurrence of endocytosis and its related functions, i.e., mating and plasma membrane turnover, when these two functions are required and high concentrations of ethanol are present in the medium.
ACKNOWLEDGMENTS
We thank M. Herweijer and R. Serrano for the polyclonal antibodies against the maltose transporter and the plasma membrane ATPase; C. Gancedo, H. Holzer, S. Lindquist, and R. Rodicio for plasmids and strains; A. Fernández and J. Pérez for help in the preparations of figures; and C. Fernández, O. Zaragoza, D. R. Jones, and J. M. Gancedo for critically reading the manuscript.
This work was supported by the Spanish Dirección General Científica y Técnica (PB97-1213-CO2-01) and by the European Commission (BIO4-CT95-01).
REFERENCES
- 1.Attfield P V. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat Biotechnol. 1997;15:1351–1357. doi: 10.1038/nbt1297-1351. [DOI] [PubMed] [Google Scholar]
- 2.Barry J A, Gawrisch K. Effects of ethanol on bilayers containing cholesterol, gangliosides, and sphingomyelin. Biochemistry. 1995;34:8852–8860. doi: 10.1021/bi00027a037. [DOI] [PubMed] [Google Scholar]
- 3.Bell W, Klaasen P, Ohnacker M, Boller T, Herweijer M, Shoppink P, Van der Zee P, Wiemkem A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem. 1992;209:951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- 4.Benito B, Moreno E, Lagunas R. Half-life of the plasma membrane ATPase and its activating system in resting yeast cells. Biochim Biophys Acta. 1991;1063:265–268. doi: 10.1016/0005-2736(91)90381-h. [DOI] [PubMed] [Google Scholar]
- 5.Blazquez M A, Lagunas R, Gancedo C, Gancedo J M. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 1993;329:51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- 6.Blázquez M A, Stucka R, Feldman H, Gancedo C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J Bacteriol. 1994;176:3895–3902. doi: 10.1128/jb.176.13.3895-3902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busturia A, Lagunas R. Catabolite inactivation of the glucose transport system in Saccharomyces cerevisiae. J Gen Microbiol. 1986;132:379–385. doi: 10.1099/00221287-132-2-379. [DOI] [PubMed] [Google Scholar]
- 8.Crowe J H, Crowe L M. Effects of dehydration on membranes and membrane stabilization at low water activities. In: Chapman D, editor. Biological membranes. Vol. 5. London, United Kingdom: Academic Press Ltd.; 1984. pp. 58–103. [Google Scholar]
- 9.De Virgilio C, Hottinger T, Dominguez J, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem. 1994;219:179–186. doi: 10.1111/j.1432-1033.1994.tb19928.x. [DOI] [PubMed] [Google Scholar]
- 10.Dixit B L, Gupta C M. Role of actin cytoskeleton in regulating the outer phosphatidylethanolamine levels in yeast plasma membrane. Eur J Biochem. 1998;254:202–206. doi: 10.1046/j.1432-1327.1998.2540202.x. [DOI] [PubMed] [Google Scholar]
- 11.Elliot B, Haltiwanger R S, Futcher B. Synergy between trehalose and Hsp104 for thermotolerance in Saccharomyces cerevisiae. Genetics. 1996;144:923–933. doi: 10.1093/genetics/144.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii S, Iwahashi H, Obuchi K, Fujii T, Komatsu Y. Characterization of a barotolerant mutant of the yeast Saccharomyces cerevisiae: importance of trehalose content and membrane fluidity. FEMS Microbiol Lett. 1996;141:97–101. doi: 10.1111/j.1574-6968.1996.tb08369.x. [DOI] [PubMed] [Google Scholar]
- 13.Geli M I, Riezman H. Endocytic internalization in yeast and animal cells: similar and different. J Cell Sci. 1998;111:1031–1037. doi: 10.1242/jcs.111.8.1031. [DOI] [PubMed] [Google Scholar]
- 14.Glover J R, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 15.Hein C, Springael J Y, Volland C, Haguenauer-Tsapis R, André B. NPI1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin-protein ligase. Mol Microbiol. 1995;18:77–87. doi: 10.1111/j.1365-2958.1995.mmi_18010077.x. [DOI] [PubMed] [Google Scholar]
- 16.Heinemeyer W, Gruhler A, Mohrle V, Mahé Y, Wolf D H. PRE2, highly homologous to the human major histocompatibility complex-linked RING10 gene, codes for a yeast proteasome subunit necessary for chymotryptic activity and degradation of ubiquitinated proteins. J Biol Chem. 1993;268:5115–5120. [PubMed] [Google Scholar]
- 17.Hohmann S, Neves M J, de Koning W, Alijo R, Ramos J, Thevelein J M. The growth and signalling defects of the ggs1(fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr Genet. 1993;23:281–289. doi: 10.1007/BF00310888. [DOI] [PubMed] [Google Scholar]
- 18.Horak J, Wolf D H. Catabolite inactivation of the galactose transporter in the yeast Saccharomyces cerevisiae: ubiquitination, endocytosis, and degradation in the vacuole. J Bacteriol. 1997;179:1541–1549. doi: 10.1128/jb.179.5.1541-1549.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingram L O, Buttke T M. Effects of alcohols on micro-organisms. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- 20.Krampe S, Stamm O, Hollenberg C P, Boles E. Catabolite inactivation of the high hexose transporters Hxt6 and Hxt7 of Saccharomyces cerevisiae occurs in the vacuole after internalization by endocytosis. FEBS Lett. 1998;441:343–347. doi: 10.1016/s0014-5793(98)01583-x. [DOI] [PubMed] [Google Scholar]
- 21.Kupiec M, Byers B, Esposito R E, Mitchell A P. Meiosis and sporulation in Saccharomyces cerevisiae. In: Pringel J R, Broach J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces. 3. Cell cycle and cell biology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 889–1036. [Google Scholar]
- 22.Lagunas R. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast. 1986;2:221–228. doi: 10.1002/yea.320020403. [DOI] [PubMed] [Google Scholar]
- 23.Lagunas R. Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;104:229–242. doi: 10.1016/0378-1097(93)90598-v. [DOI] [PubMed] [Google Scholar]
- 24.Lindquist S, Kim G. Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc Natl Acad Sci USA. 1996;93:5301–5306. doi: 10.1073/pnas.93.11.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Lucero P, Herweijer M, Lagunas R. Catabolite inactivation of the yeast maltose transporter is due to proteolysis. FEBS Lett. 1993;333:165–168. doi: 10.1016/0014-5793(93)80397-d. [DOI] [PubMed] [Google Scholar]
- 27.Lucero P, Lagunas R. Catabolite inactivation of the yeast maltose transporter requires ubiquitin-ligase npi1/rsp5 and ubiquitin-hydrolase npi2/doa4. FEMS Microbiol Lett. 1997;147:273–277. doi: 10.1111/j.1574-6968.1997.tb10253.x. [DOI] [PubMed] [Google Scholar]
- 28.Lucero P, Peñalver E, Moreno E, Lagunas R. Moderate concentrations of ethanol inhibit endocytosis of the yeast maltose transporter. Appl Environ Microbiol. 1997;63:3831–3836. doi: 10.1128/aem.63.10.3831-3836.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucero P, Peñalver E, Vela L, Lagunas R. Monoubiquitination is sufficient to signal internalization of the maltose transporter in Saccharomyces cerevisiae. J Bacteriol. 2000;182:241–243. doi: 10.1128/jb.182.1.241-243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marsh L, Neiman A M, Herskowitz I. Signal transduction during pheromone response in yeast. Annu Rev Cell Biol. 1991;7:699–728. doi: 10.1146/annurev.cb.07.110191.003411. [DOI] [PubMed] [Google Scholar]
- 31.Medintz I, Jiang H, Han E K, Cui W, Michels C A. Characterization of the glucose-induced inactivation of the maltose permease in Saccharomyces cerevisiae. J Bacteriol. 1996;178:2245–2254. doi: 10.1128/jb.178.8.2245-2254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nevado J, Heredia C F. Galactose induces in Saccharomyces cerevisiae sensitivity of the utilization of hexokinases to inhibition by d-glucosamine. Can J Microbiol. 1996;42:6–11. doi: 10.1139/m96-002. [DOI] [PubMed] [Google Scholar]
- 33.Nwaka S, Holzer H. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol. 1998;58:197–237. doi: 10.1016/s0079-6603(08)60037-9. [DOI] [PubMed] [Google Scholar]
- 34.Nwaka S, Kopp M, Holzer H. Expression and function of the trehalase genes NTH1 and YBR0106 in Saccharomyces cerevisiae. J Biol Chem. 1995;270:10193–10198. doi: 10.1074/jbc.270.17.10193. [DOI] [PubMed] [Google Scholar]
- 35.Nwaka S, Mechler B, Destruelle M, Holzer H. Phenotypic features of trehalase mutants in Saccharomyces cerevisiae. FEBS Lett. 1995;360:286–290. doi: 10.1016/0014-5793(95)00105-i. [DOI] [PubMed] [Google Scholar]
- 36.Peñalver É, Lucero P, Moreno E, Lagunas R. Catabolite inactivation of the maltose transporter in nitrogen-starved cells could be due to the stimulation of the general protein turnover. FEMS Microbiol Lett. 1998;167:317–324. doi: 10.1111/j.1574-6968.1998.tb13907.x. [DOI] [PubMed] [Google Scholar]
- 37.Peñalver É, Lucero P, Moreno E, Lagunas R. Clathrin and two components of the COPII complex, Sec23p and Sec24p, could be involved in endocytosis of the Saccharomyces cerevisiae maltose transporter. J Bacteriol. 1999;181:2555–2563. doi: 10.1128/jb.181.8.2555-2563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peñalver É, Ojeda L, Moreno E, Lagunas R. Role of the cytoskeleton in endocytosis of the yeast maltose transporter. Yeast. 1997;13:541–549. doi: 10.1002/(SICI)1097-0061(199705)13:6<541::AID-YEA112>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Piper P. Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;11:339–359. doi: 10.1111/j.1574-6976.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 40.Riballo E, Herweijer M, Wolf D H, Lagunas R. Catabolite inactivation of the yeast maltose transporter occurs in the vacuole after internalization by endocytosis. J Bacteriol. 1995;177:5622–5627. doi: 10.1128/jb.177.19.5622-5627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riballo E, Lagunas R. Involvement of endocytosis in catabolite inactivation of the K+ and glucose transport systems in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1994;121:77–80. doi: 10.1111/j.1574-6968.1994.tb07078.x. [DOI] [PubMed] [Google Scholar]
- 42.Riezman H, Woodman P G, van Meer G, Marsh M. Molecular mechanisms of endocytosis. Cell. 1997;91:731–738. doi: 10.1016/s0092-8674(00)80461-4. [DOI] [PubMed] [Google Scholar]
- 43.Rodicio R. Insertion of non-homologous DNA sequences into a regulatory gene causes a constitutive maltase synthesis in yeast. Curr Genet. 1986;11:235–241. doi: 10.1007/BF00420612. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez C, Gancedo J M. Glucose signalling in yeast is partially mimicked by galactose and does not require Tps1 protein. Mol Cell Biol Res Commun. 1999;1:52–58. doi: 10.1006/mcbr.1999.0112. [DOI] [PubMed] [Google Scholar]
- 45.Ruis H, Schüller C. Stress signaling in yeast. Bioessays. 1995;17:959–965. doi: 10.1002/bies.950171109. [DOI] [PubMed] [Google Scholar]
- 46.Salmon J M. Effect of sugar transport inactivation in Saccharomyces cerevisiae on sluggish and stuck enological fermentations. Appl Environ Microbiol. 1989;55:953–958. doi: 10.1128/aem.55.4.953-958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmon J M, Vicent O. Sugar transport inactivation in Saccharomyces cerevisiae is a major limiting factor during enological fermentations. Am J Enol Vitic. 1993;44:56–64. [Google Scholar]
- 48.Sanchez Y, Lindquist S. HSP104 required for inducer thermotolerance. Science. 1990;248:1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez Y, Taulien J, Borkovich K A, Lindquist S. Hsp104 is required for tolerance to many forms of stress. EMBO J. 1992;11:2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serrano R. H+-ATPase from plasma membrane of Saccharomyces cerevisiae and Avena sativa roots. Purification and reconstitution. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]
- 51.Sharma S C. A possible role of trehalose in osmotolerance and ethanol tolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1997;152:11–15. doi: 10.1111/j.1574-6968.1997.tb10402.x. [DOI] [PubMed] [Google Scholar]
- 52.Singer M A, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 53.Volland C, Urban-Grimal D, Géraud G, Haguenauer-Tsapis R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J Biol Chem. 1994;269:9833–9841. [PubMed] [Google Scholar]
- 54.Werner-Washburne M, Brown E L, Crawford M E, Peck V M. Stationary phase in Saccharomyces cerevisiae. Mol Microbiol. 1996;19:1159–1166. doi: 10.1111/j.1365-2958.1996.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 55.Werner-Washburne M, Brown E, Johnston G C, Singer R A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1993;57:383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]