Abstract
Prostate cancer (PCa) affects millions of men globally. Due to advances in understanding genomic landscapes and biological functions, the treatment of PCa continues to improve. Recently, various new classes of agents, which include next-generation androgen receptor (AR) signaling inhibitors (abiraterone, enzalutamide, apalutamide, and darolutamide), bone-targeting agents (radium-223 chloride, zoledronic acid), and poly(ADP-ribose) polymerase (PARP) inhibitors (olaparib, rucaparib, and talazoparib) have been developed to treat PCa. Agents targeting other signaling pathways, including cyclin-dependent kinase (CDK)4/6, Ak strain transforming (AKT), wingless-type protein (WNT), and epigenetic marks, have successively entered clinical trials. Furthermore, prostate-specific membrane antigen (PSMA) targeting agents such as 177Lu-PSMA-617 are promising theranostics that could improve both diagnostic accuracy and therapeutic efficacy. Advanced clinical studies with immune checkpoint inhibitors (ICIs) have shown limited benefits in PCa, whereas subgroups of PCa with mismatch repair (MMR) or CDK12 inactivation may benefit from ICIs treatment. In this review, we summarized the targeted agents of PCa in clinical trials and their underlying mechanisms, and further discussed their limitations and future directions.
Subject terms: Cancer therapy, Urological cancer
Introduction
Prostate cancer (PCa) is the second most common cancer, and it is the fifth leading cause of cancer-related death among men.1 The incidence rates of PCa are 37.5 per 100,000 in developed countries and 11.3 per 100,000 in developing countries, while mortality rates are 8.1 per 100,000 in developed countries and 5.9 per 100,000 in developing countries.1 Approximately 10 million men are presently diagnosed with PCa. PCa causes more than 400,000 deaths annually worldwide, and by 2040, the mortality rate is expected to reach more than 800,000 deaths annually.1–4 Prostate-specific antigen (PSA) testing and digital rectal examinations (DRE) facilitate the diagnosis of PCa in most men at early stages of the disease.5,6 Androgen receptor (AR) signaling plays an essential role in PCa initiation and disease progression,7 and androgen deprivation therapy (ADT) has been a backbone of treatment for patients with advanced disease.8,9 Generally, localized PCa is managed by deferred treatment or active local therapy (such as radical prostatectomy or radiation therapy) with or without ADT. For metastatic PCa, ADT with gonadotropin-releasing hormone (GnRH) antagonists/agonists followed by treatment with docetaxel plus prednisolone and continued ADT after disease progression has become the standard treatment.10 However, patient responses to ADT vary, and most patients eventually develop castration-resistant prostate cancer (CRPC).11 In the past decade, significant progress has been made in the treatment of CRPC; this progress has been aided by the approval of effective AR-targeting agents, including abiraterone, enzalutamide, apalutamide, and darolutamide.12–16 The agents newly approved in the 21st century for PCa treatment and diagnosis are summarized in Table 1.
Table 1.
Theranostic agents approved for PCa in the 21st century
| International non-proprietary name (INN) | Brand name | Pharmacotherapeutic group | EMA approval date | FDA approval date | China NMPA approval date |
|---|---|---|---|---|---|
| Zoledronic acid | Zometa | Bone-targeting therapy | Mar 2001 | Feb 2002 | Dec 2018 |
| Degarelix | Firmagon | Endocrine therapy | Feb 2009 | Dec 2008 | July 2019 |
| Sipuleucel-T | Provenge | Immunotherapy | Sept 2013 | Apr 2010 | / |
| Cabazitaxel | Jevtana | Antineoplastic agents | Mar 2011 | Jun 2010 | / |
| Denosumab | Prolia/Xgeva | Bone-targeting therapy | July 2011 | Nov 2010 | May 2019 |
| Abiraterone | Zytiga | Endocrine therapy | Sept 2011 | Apr 2011 | Dec 2019 |
| Enzalutamide | Xtandi | Endocrine therapy | Jun 2013 | Aug 2012 | Nov 2019 |
| Radium-223 dichloride | Xofigo | Therapeutic radiopharmaceuticals | Nov 2013 | May 2013 | Aug 2020 |
| Fluciclovine (18F) | Axumin | Diagnostic radiopharmaceuticals | May 2017 | May 2016 | / |
| Pembrolizumab | Keytruda | Immunotherapy | / | May 2017 | / |
| Padeliporfin | Tookad | Antineoplastic agents | Sept 2017 | / | / |
| Darolutamide | Nubeqa | Endocrine therapy | Mar 2020 | Jul 2019 | Feb 2021 |
| Rucaparib | Rubraca | Antineoplastic agents | / | May 2020 | / |
| Olaparib | Lynparza | Antineoplastic agents | Nov 2020 | May 2020 | Jun 2021 |
| Relugolix | Orgovyx | Endocrine therapy | Mar 2022 | Dec 2020 | / |
| Piflufolastat F 18 | Pylarify | Diagnostic radiopharmaceuticals | / | May 2021 | / |
| Dostarlimab-gxly | Jemperli | Immunotherapy | / | Aug 2021 | / |
| 177Lu-PSMA-617 | Pluvicto | Therapeutic radiopharmaceuticals | / | Mar 2022 | / |
| 68Ga-PSMA-11 | Locametz | Diagnostic radiopharmaceuticals | / | Mar 2022 | / |
Bone metastasis is a major concern in patients with CRPC. Successful therapeutic strategies for the treatment of bone metastases include radium 223, bisphosphonates, and receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor denosumab.17–22 Treatments targeting genomic alterations in DNA repair pathways have been increasingly validated in clinical settings. Poly(ADP-ribose) polymerase (PARP) inhibitors, including olaparib, rucaparib, and talazoparib, are being evaluated in phase 2/3 trials for metastatic castration-resistant prostate cancer (mCRPC).23–27 Moreover, early clinical studies on agents that target immune checkpoints, such as cytotoxic T-lymphocyte associated protein 4 (CTLA4), programmed cell death protein 1 (PD1) or programmed death-ligand 1 (PD-L1) have been evaluated in clinics.28–32 Prostate-specific membrane antigen (PSMA) is highly expressed in PCa cell membranes.33,34 Thus, PSMA-targeting small molecules or antibodies labeled with radionuclides or cytostatic agents have been evaluated in several clinical studies.35–45 Moreover, multifarious cell growth and survival pathways, including phosphatidylinositol-3-kinase (PI3K)/Ak strain transforming (AKT)/mechanistic target of rapamycin (mTOR), interact with AR signaling, and are involved in PCa progression. Single-agent treatment with PI3K/AKT/mTOR specific inhibitors or combination approaches with AR signaling inhibitors have been investigated in clinical studies.46–52 Alterations of epigenetic modifications, such as histone methylation and acetylation, as well as DNA methylation, are ubiquitous in PCa.53,54 Therefore, compounds concerning epigenetic targets, such as lysine methyltransferase (KMT), histone lysine demethylase (KDM), histone acetyltransferase (HAT), bromodomain and extra-terminal (BET), histone deacetylase (HDAC) or DNA methyltransferase (DNMT), have entered clinical trials.54–62 Agents targeting other PCa-related signaling pathways, including cyclin-dependent kinase (CDK)4/6, p53, wingless-type protein (WNT) signaling, vascular endothelial growth factor (VEGF), endothelin A receptor (ETAR), fibroblast growth factor receptor (FGFR), epidermal growth factor receptor (EGFR), receptor tyrosine kinases (RTKs), transforming growth factor beta (TGFβ), proto-oncogene tyrosine-protein kinase Src (SRC), and mitogen-activated protein kinase kinase (MEK), have also entered clinical trials.63–74 Alternative splicing affects genes (such as FGFR, ERG, VEGFA, and AR) that are clearly linked to the etiology of PCa; therefore, developing novel targeted therapies that modulate alternative splicing for the treatment of PCa are warranted.75 In this review, we have discussed strategies for targeting signaling pathways in PCa, as well as their mechanisms and related clinical trials.
Genomic landscape and therapeutic targets
Inflammation and chronic prostatic diseases, which are possibly associated with diet, chemical injury, and microbial infection, are believed to drive prostate carcinogenesis through DNA damage and mutagenesis.76,77 The initiation and progression of PCa are linked to complex interactions between acquired somatic gene alterations and microenvironmental factors.4,76,77 Both hereditary and environmental factors can increase the risk of PCa. Estimations of the hereditary risk of PCa have been partly explained in the Nordic Twin Study of Cancer (which included 80,309 monozygotic and 123,382 same-sex dizygotic twins), which found that ~60% cases of PCa are influenced by genetic factors.78 Of environmental factors, smoking, alcohol consumption, and infections (such as gonorrhea and HPV) increase the risk of developing PCa.79–82 Furthermore, obesity and diet (such as the consumption of saturated animal fat and meat) are also associated with increased risk of PCa.83–85 Interestingly, incidence rates of PCa have large international geographical variations (for example, Australia/New Zealand has the highest incidence of PCa, almost 25 times higher than that in areas such as South-Central Asia), while immigrants moving from countries with lower PCa incidence to countries with higher PCa rates soon acquire higher risks,86,87 suggesting complex mechanisms for the etiologies of PCa.
Nevertheless, with the development of next-generation sequencing techniques, substantial advances have been made in understanding genomic alterations in PCa (Fig. 1a, b).88–94 In the early stage of PCa, frequent genomic alterations include TMPRSS2-ERG fusions in 40–60% of patients and SPOP mutations in 5–15% of patients.88,90 Interestingly, Asian patients with PCa have fewer TMPRSS2-ERG fusions, whereas genomic alterations in FOXA1, ZNF292, and CHD1 are observed in more than 40% of these patients.95 Aberrations in AR are infrequent in the early stage of PCa, but AR pathway alterations and increased AR signaling commonly occur in advanced PCa via amplification, gain-of-function mutations, or overexpression or increased transcription of AR (Fig. 1a, b).88,90,92 Genomic alteration of PTEN and TP53 often occurs across different stages of PCa (Fig. 1a, b).92 The proportion of PTEN and TP53 deletions or mutations is 10–20% in localized PCa, but increases to nearly 40% in mCRPC (Fig. 1a, b).88,90,92 Oncogene MYC amplification or WNT signaling activation via APC loss and CTNNB1 amplification are also frequent, occurring in approximately 10–30% of all mCRPC cases (Fig. 1a, b).88,90,92 RB1 loss is seen in approximately 10% of cases in mCRPC, and has been associated with poor prognoses (Fig. 1a, b).88,90,92 The concurrence of PTEN deletion, TP53 mutations, and RB1 loss are correlated with lineage plasticity and neuroendocrine prostate cancer (NEPC), which is highly treatment-refractory.88,89,96,97 Aberrations in DNA damage response genes, such as BRCA1, BRCA2, ATM, CHEK2, and CDK12, occur in approximately 20% of metastatic PCa (Fig. 1a, b).88,90,92 The alterations in DNA damage response genes as well as mismatch repair (MMR) genes have led to efficiently targeted approaches, which will be discussed later. Given that genomic alterations and signaling activation are diverse in different stages of the disease as well as in individual patients, multiple approaches have been developed to target various pathways and practiced in clinical trials (Fig. 1c).
Fig. 1.
Overview of genetic alterations and therapeutic strategies in PCa. a Genetic alterations in localized PCa, metastatic castration-sensitive PCa, and metastatic castration-resistant PCa.90–93 b Common somatic mutations at different disease stages of PCa.4 c Overview of therapeutic targeting strategies for the treatment of PCa
Targeting AR signaling
Androgen-signaling axis
The androgen-signaling axis plays a crucial role in PCa progression.7 Androgen synthesis is tightly regulated by the hypothalamic–pituitary–gonadal axis (Fig. 2).98,99 When bound by androgens such as testosterone and dihydrotestosterone (DHT), AR releases from the heat shock protein complex, translocates into the nucleus, and promotes gene transcription to accelerate tumor progression, as well as maintains normal prostate cell maturation (Fig. 2).100,101 ADT has become essential in the treatment of PCa and metastatic disease since Huggins and Hodges first discovered the central role of the androgen-signaling axis in PCa after finding that orchiectomy significantly suppresses tumor progression.102 The aim of ADT by either orchiectomy or chemical castration is to suppress serum testosterone to castration levels and thus block the activation of the AR.103 So far, the most effective strategy to treat PCa is still to target the androgen-signaling axis, which includes multiple approaches, such as targeting GnRH to prevent luteinizing hormone release, targeting cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1) to restrain androgen synthesis, or directly targeting AR to inhibit AR transcriptional activity (Fig. 2).103–107
Fig. 2.
AR signaling pathway and targeted therapeutic approaches in PCa. Androgen synthesis is regulated by the hypothalamic–pituitary–gonadal axis. When androgens such as testosterone (T) and DHT bind to AR, AR releases itself from the heat shock protein complex, translocates into the nucleus, and promotes gene transcription to accelerate tumor progression. Targeting the androgen-signaling axis includes multiple approaches, such as targeting GnRH to prevent luteinizing hormone release, targeting CYP17A1 to restrain androgen synthesis, or directly targeting AR to inhibit AR transcription activity. Parts of images generated from BioRender (https://biorender.com/)
AR
AR, a steroid receptor transcriptional factor composed of 919 amino acids and encoded by the gene located on chromosome X (Xq11-12), is composed of an N-terminal domain (NTD) encoded by exon 1, a DNA-binding domain (DBD) encoded by exons 2–3, hinge region encoded by exon 4, and a ligand-binding domain (LBD) encoded by exons 5–6.100,108 AR plays an important role in PCa pathogenesis, and its expression has been found in most primary and metastatic PCa.109,110 The intensity of AR staining in the nucleus of bone mCRPC is associated with a worse outcome.111,112 The activation of AR signaling supports the survival and growth of PCa cells.7,113 Mechanically, in the absence of ligands such as DHT and testosterone, the AR is located in the cytoplasm and complexes with chaperone proteins such as HSP90. When the ligands bind to the LBD of AR, they translocate into the nucleus to form a homodimer, and the AR dimer interacts with its coregulatory proteins to recognize cognate DNA response elements located in the proximal or distal intragenic and intergenic regions of androgen target genes, thereby regulating gene expression (e.g., KLK3, NKX3.1, FKBP5, TMPRSS2-ERG).105
AR inhibitors (bicalutamide, flutamide, and nilutamide),114–120 which bind to the LBD of AR and result in the inhibition of androgen binding to LBD, reduce the serum level of PSA (encoded by the KLK3 gene) and alleviate symptoms in PCa patients.106 Recently, several novel AR inhibitors have been developed and used in clinical settings (Table 2). Enzalutamide (also known as MDV3100),121 approved by the FDA in 2012, is a second-generation AR inhibitor with a high affinity for the LBD of AR. Multiple clinical trials have confirmed that enzalutamide significantly prolongs the overall survival of patients with metastatic or nonmetastatic CRPC.13,14,122–124 Apalutamide (also known as ARN-509)125 has a greater efficacy than enzalutamide and was approved for treatment of nonmetastatic CRPC by the FDA in 2018. Apalutamide inhibits the nuclear localization and DNA binding of AR in PCa cells.125 A clinical study showed that apalutamide administration significantly lengthened metastasis-free survival in patients with nonmetastatic CRPC.15
Table 2.
Selective clinical trials of AR signaling inhibitors
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| Degarelix | GnRHR | PCa | Completed | 3 | NCT00451958 |
| Degarelix | GnRHR | PCa | Completed | 3 | NCT01071915 |
| Degarelix | GnRHR | PCa | Completed | 3 | NCT02015871 |
| Relugolix | GnRHR | PCa | Recruiting | 3 | NCT05050084 |
| Relugolix | GnRHR | PCa | Completed | 3 | NCT03085095 |
| Abarelix | GnRHR | PCa | Completed | 3 | NCT00841113 |
| Leuprolide | GnRHR | PCa | Completed | 4 | NCT00220194 |
| Leuprolide | GnRHR | PCa | Recruiting | 3 | NCT04914195 |
| Goserelin | GnRHR | PCa | Completed | 3 | NCT00439751 |
| Goserelin | GnRHR | PCa | Not yet recruiting | 4 | NCT03971110 |
| Triptorelin | GnRHR | PCa | Completed | 3 | NCT01715129 |
| Triptorelin | GnRHR | PCa | Completed | 3 | NCT00104741 |
| Histrelin | GnRHR | Metastatic PCa | Recruiting | 2/3 | NCT04787744 |
| Histrelin | GnRHR | PCa | Completed | 3 | NCT01697384 |
| Buserelin | GnRHR | PCa | Completed | 3 | NCT00003653 |
| Buserelin | GnRHR | PCa | Recruiting | 3 | NCT05050084 |
| Enzalutamide | AR | CRPC | Completed | 3 | NCT00974311 |
| Enzalutamide | AR | mCRPC | Completed | 4 | NCT02116582 |
| Enzalutamide | AR | mCRPC | Completed | 4 | NCT02485691 |
| Apalutamide | AR | PCa | Completed | 2 | NCT01790126 |
| Apalutamide | AR | Nonmetastatic CRPC | Recruiting | 3 | NCT04108208 |
| Darolutamide | AR | PCa | Not yet recruiting | 3 | NCT02799602 |
| Darolutamide | AR | Nonmetastatic CRPC | Completed | 3 | NCT02200614 |
| ARV-110 | AR | mCRPC | Recruiting | 1/2 | NCT03888612 |
| Abiraterone | CYP17A1 | mCRPC | Completed | 3 | NCT04056754 |
| Abiraterone | CYP17A1 | mCRPC | Completed | 3 | NCT00887198 |
| Abiraterone | CYP17A1 | mCRPC | Completed | 3 | NCT02111577 |
| Seviteronel | CYP17A1 | CRPC | Completed | 2 | NCT02445976 |
| Orteronel | CYP17A1 | mCRPC | Completed | 3 | NCT01193257 |
| Galeterone | CYP17A1 | CRPC | Completed | 2 | NCT01709734 |
Notably, AR gene mutations and amplifications occur in ~60% of mCRPC (Fig. 1a). AR mutations are predominant in LBD, limiting binding affinity of AR inhibitors.126–129 Darolutamide (also named ODM-201),130,131 approved for treatment of nonmetastatic CRPC by the FDA in 2019, is a novel AR inhibitor that antagonizes mutated AR, such as F877L and T878A, which confers resistance to enzalutamide and apalutamide.132–134 Phase 3 trial studies have shown that darolutamide significantly prolongs metastasis-free survival for high-risk nonmetastatic CRPC.16,135 Furthermore, the latest AR protein degrader ARV-110, an oral proteolysis targeting chimera (PROTAC),136 specifically degrades more than 95% of AR and overcomes enzalutamide resistance in xenograft models.137,138 ARV-110 is presently under evaluation in clinical trials (Table 2). Current AR-targeted therapies primarily target LBD. However, AR variants such as AR-V7 and ARv567es lack the entire LBD or a functional LBD, but retain their ability to bind DNA in the absence of androgens and display constitutive activity, thus conferring drug resistance to next-generation AR inhibitors.139–143
GnRH
GnRH, also known as luteinizing hormone-releasing hormone (LHRH),144,145 is a hypothalamic peptide (pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2) that plays a central role in controlling the hypothalamic–pituitary-axis in mammals.146–148 GnRH binds to the GnRH receptor (GnRHR), which belongs to the rhodopsin-like G protein coupled receptor (GPCR) family, and induces the release of luteinizing hormone (LH), which then arrives to the Leydig cells of the testes to stimulate testosterone synthesis.148–151 Schally and Guillemin (1982) first developed a synthetic GnRH agonist (also known as GnRH analog) to manipulate the hypothalamic–pituitary–gonadal axis.152 Mechanically, the GnRH agonists induce sustained stimulation of the pituitary gland to induce the downregulation and desensitization of GnRHR, resulting in the reduction of LH release and suppression of testosterone production to castration levels.103,148,153 An early study by Tolis et al. found that patients with advanced PCa treated daily with GnRH agonists experienced a 75% suppression in serum testosterone levels, resulting in a decreased prostate size and reduction in tumor-associated bone pain.152 Several synthetic GnRH agonists have been developed for clinical use since the 1980s, including leuprolide, triptorelin, and buserelin.154–166 Many clinical studies of GnRH agonists are completed or ongoing (Table 2). Although long-acting GnRH agonists suppress the release of LH and testosterone, GnRH agonists initially produce a rapid and transient increase in LH and testosterone levels, which is called the “flare-up” phenomenon, and may lead to side effects, such as bone pain and cardiovascular complications.103,154
In contrast to GnRH agonists, GnRH antagonists bind competitively to the GnRHR in the pituitary to rapidly prevent LH production, thereby suppressing testosterone to castration levels, which reduces the risk of the “flare-up” phenomenon.103,167 Abarelix was the first GnRH antagonist approved by the FDA, but it was discontinued from the market because of severe hypersensitivity reactions.168–171 Degarelix, which was approved by the FDA in 2008, is the most widespread GnRH antagonist used in clinical practice.172–177 Relugolix, which is a novel oral GnRH antagonist, was approved by the FDA in 2020.178,179 Numerous promising clinical trials on GnRH antagonists in combination with radiotherapy or chemotherapy have been completed or are still ongoing (Table 2). However, the use of these agents remains controversial. For example, a clinical study revealed that neoadjuvant degarelix is related to the upregulation of DHT in tumors.180 Other studies have found that the administration of GnRH agonists or antagonists can decrease lean body mass and increase fat mass.181–183 Further studies will provide critical evidence to address whether GnRH agonists or antagonists are safe for patients with cardiovascular disease.
CYP17A1
CYP17A1, a membrane-bound monooxygenase, is a pivotal enzyme for androgen synthesis.184 CYP17A1 is composed of 508 amino acids with four structural domains, including a substrate-binding domain, a catalytic activity area, a heme-binding region, and a redox-partner binding site.185,186 CYP17A1 has both 17α-hydroxylase and 17,20-lyase catalytic activities, and is essential in the production of both androgens and glucocorticoids.185,186 CYP17A1 predominantly localizes at the endoplasmic reticulum in the adrenal glands, testicular Leydig cells, and ovarian thecal cells.187 The 17α-hydroxylase activity of CYP17A1 is required for the hydroxylation of pregnenolone and progesterone at the C17 position, which generates 17α-hydroxypregnenolone and 17α-hydroxyprogesterone.188 The 17,20-lyase activity of CYP17A1 is essential for the cleavage of 17α-hydroxypregnenolone or 17α-hydroxyprogesterone, which form dehydroepiandrosterone (DHEA) and androstenedione, respectively, and is a critical step for testosterone synthesis.105,188 Importantly, CYP17A1 also confers to intratumoral androgen biosynthesis in CRPC.189–192 Low levels of androgen are still found in the serum during ADT; thus, many CYP17A1 inhibitors have been tested in clinics (Table 2).
Abiraterone, a selective inhibitor of 17α-hydroxylase and 17,20-lyase,193 was first approved by the FDA for treatment of mCPRC in 2011.194,195 Administration of abiraterone induces a significant decline in PSA levels, improves overall survival, and alleviates pain in both chemotherapy-naive and docetaxel-resistant patients.12,196–198 Although the suppression of 17α-hydroxylase activity leads to overproduction of mineralocorticoids, which can result in adverse events (such as hypokalaemia, fluid retention, hypertension, and cardiac disorders), these side effects are largely prevented by the co-administration of glucocorticoid prednisone.199
Other CYP17A1 inhibitors, such as orteronel (TAK-700) and galeterone (TOK-001), have also been developed.200–202 Orteronel is a nonsteroidal selective inhibitor of 17,20-lyase, while galeterone has multiple mechanisms of action, including CYP17A1 inhibition, AR antagonism, and a reduction in both full-length AR and AR-V7 levels.192,200–203 Orteronel preferentially inhibits 17,20-lyase over 17α-hydroxylase, leading to a reduction in the risk of overproduction of mineralocorticoids.184,192,201 Results from phase 1/2 studies have indicated that patients had an approximately 60% PSA response rate at 12 weeks after the administration of orteronel twice daily.204 A phase 1 study in patients with CRPC observed that ~50% of men had a PSA decline after 12 weeks of treatment with galeterone, and no adrenal mineralocorticoid excess was noted.205 Therefore, orteronel and galeterone are potentially attractive drugs for longer duration therapy and overcoming drug resistance, although a clinical study showed that orteronel did not meet the primary endpoint of overall survival.206 Clinical studies of galeterone compared to enzalutamide in mCRPC expressing AR-V7 have been conducted, but the result do not meet the primary endpoint.207 Additionally, seviteronel (VT-464), a newly developed drug used as a CYP17A1 inhibitor and AR antagonist, selectively inhibits 17,20-lyase and greatly decreases AR transactivation and offers an advantage over abiraterone because it does not require combination with prednisone.208 Notably, abiraterone treatment markedly increases intratumoral expression of CYP17A1 in tumor biopsies from CRPC patients, and many patients ultimately become resistant to CYP17 inhibitors.143,190,209
Targeting bone microenvironment
Bone microenvironment of PCa
Bone metastasis in PCa is a highly frequent event that occurs in up to 90% of patients with advanced disease.210–212 The bone microenvironment is a dynamic compartment that provides a milieu in which metastatic cancer cells can colonize and grow.213,214 The “vicious cycle” hypothesis is an appropriate model with which to explain the process of cancer cells metastasizing to the bone (Fig. 3).213,215 Tumor cells in bone induce osteoclast-mediated bone resorption, while osteoclasts release bone-stored factors that stimulate tumor cell proliferation, establishing a vicious cycle. Bone metastasis is driven by the cooperation among metastatic tumor cells, bone-forming osteoblasts, bone-dissolving osteoclasts, and other cell populations.212 Physiologically, mature osteoblasts, osteocytes, and osteoclasts regulate the dynamic remodeling of bone tissue.216 The increased levels of parathyroid hormone-related peptide (PTHrP) from osteoclasts can induce bone resorption by upregulating the receptor activator of RANKL, which promotes the release of various growth factors (such as ionized calcium and TGFβ) into the bone microenvironment to support cancer cell implantation and transformation.217–221 Invading tumor cells secrete osteolytic cytokines, such as granulocyte-macrophage colony-stimulating factor, matrix metalloproteinases, interleukin (IL)-6, insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), endothelin 1, growth differentiation factor 15 (GDF15), dickkopf-1 (DKK-1), and WNTs.222–226 These osteolytic cytokines stimulate preosteoblast differentiation and promote osteoclast maturation to accelerate bone resorption.217,227,228 Meanwhile, osteoblasts release IL-6 and RANKL to accelerate the maturation of osteoclasts, further secreting growth factors to facilitate tumor cell growth (Fig. 3).212,217,229 Thus, various approaches that target the bone microenvironment, such as bone-targeting agents, are effective for managing bone metastases in PCa (Fig. 3).
Fig. 3.
The vicious cycle of bone metastasis and targeting strategies in PCa. Tumor cells in bone secrete osteolytic cytokines such as RANKL, PTHrP, GM-CSF, MMPs, IL-6, IGFs, FGFs, endothelin 1, GDF15, DKK-1, and WNTs to induce osteoclast-mediated bone resorption, while osteoclasts release bone-stored factors such as TGFβ, IGFs, and Ca2+ that stimulate tumor cell proliferation, establishing a vicious cycle. Targeting the bone microenvironment (such as bone-targeted radioisotopes, bisphosphonates, and RANKL inhibitors) is effective to manage bone metastases in PCa
Bone-targeted radioisotopes
Bone-seeking therapeutic radioisotopes are distinct among anticancer therapies, because they target calcium hydroxyapatite in the bone instead of tumor cells. Bone metastases often contain osteosclerotic lesions, with increased osteoblastic bone formation. Thus, ionizing radiation is selectively delivered to bone with increased osteoblastic activity to simultaneously target multiple metastases,230 enabling the delivery of high-energy radiation to bone metastases while limiting toxicity to other normal cells. Calcium-mimetic radiopharmaceuticals, such as the first generation of primarily β-emitting radioisotopes strontium-89 and samarium-153, have been approved early by the FDA based on successful endpoints of bone pain palliation.231–235 More recently, the bone-targeted radioisotope radium-223 was also approved by the FDA for patients with mCRPC and painful bone metastases.17,18,236–238 Unlike strontium-89 and samarium-153, radium-223 is predominately an α-emitter with short tissue penetration range,230 which potentially reduces bone marrow toxicity and limits undue exposure.237 Radium-223 targets bone as a calcium-mimetic and preferentially uptakes into areas of increased bone formation, resulting in a highly localized antitumor effect on adjacent bone metastases.239 In a phase 3 clinical trial involving 921 CRPC patients with bone pain symptoms, administration of radium-223 reduced bone pain and significantly prolonged overall survival compared to patients administered placebo.17 Treatment with radium-223 was tolerated, although the frequency of thrombocytopenia was increased.240 Moreover, no patients with leukemia or other cancers were identified during long-term surveillance.241 Many promising results have been found in clinical settings, and further clinical trials of bone-targeted radioisotopes are still ongoing (Table 3).
Table 3.
Selective clinical trials of drugs targeting bone microenvironment
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| Radium-223 | Calcium-mimetic α-emitting | Bone-metastatic CRPC | Completed | 3 | NCT00699751 |
| Radium-223 | Calcium mimetic α-emitting | Bone-metastatic CRPC | Completed | 3 | NCT01618370 |
| Radium-223 | Calcium mimetic α-emitting | Bone-metastatic CRPC | Completed | 3 | NCT01810770 |
| Radium-223 | Calcium mimetic α-emitting | Bone-metastatic CRPC | Recruiting | 3 | NCT03432949 |
| Strontium-89 | Calcium mimetic β-emitting | Bone-metastatic CRPC | Completed | 3 | NCT00002503 |
| Samarium-153 | Calcium mimetic β-emitting | Bone-metastatic CRPC | Completed | 3 | NCT00365105 |
| Zoledronic acid | FDPS | Bone-metastatic CRPC | Completed | 3 | NCT00242567 |
| Zoledronic acid | FDPS | Bone-metastatic CRPC | Completed | 3 | NCT00079001 |
| Zoledronic acid | FDPS | Bone-metastatic CRPC | Completed | 3 | NCT00321620 |
| Zoledronic acid | FDPS | Bone-metastatic CRPC | Completed | 4 | NCT00242554 |
| Clodronic acid | FDPS | PCa | Completed | Unknown | NCT01198457 |
| Risedronic acid | FDPS | PCa | Completed | 3 | NCT00426777 |
| Alendronic acid | FDPS | Metastatic PCa | Terminated | 2 | NCT00019695 |
| Ibandronic acid | FDPS | Metastatic PCa | Completed | 3 | NCT00082927 |
| Denosumab | RANKL | CRPC | Completed | 3 | NCT01824342 |
| Denosumab | RANKL | CRPC | Completed | 3 | NCT00286091 |
| Denosumab | RANKL | CRPC | Completed | 3 | NCT00838201 |
| Denosumab | RANKL | PCa | Completed | 3 | NCT00925600 |
| Mipsagargin | SERCA | Metastatic PCa | Withdrawn | 2 | NCT01734681 |
| SOR-C13 | TRPV6 | Advanced cancers | Recruiting | 1 | NCT03784677 |
Bisphosphonates
The antitumor effects of bisphosphonates might be attributable to their anti-osteoclast activity.212 Bisphosphonates preferentially bind to hydroxyapatite of bone, resulting in increased uptake of bisphosphonates by osteoclasts during the osteoclastic resorption process.242–244 Because bisphosphonates are accumulated in bone, they are internalized selectively by osteoclasts rather than other cell types.245 Following uptake by osteoclasts, bisphosphonates intracellularly inhibit farnesyl diphosphate synthase (FDPS), which is a key enzyme for cholesterol biosynthesis. As a result, osteoclasts accumulate intracellular isopentenyl pyrophosphates, which form cytotoxic ATP analogs that induce the apoptosis of osteoclasts.246 In contrast, by the inhibitory effect on FDPS, bisphosphonates also inhibit the function of Rho GTPases by disrupting prenylation-dependent signaling,244 thus leading to the apoptosis of osteoclasts due to the impaired mobility and adhesion of these cells.247 Therefore, the exposure of osteoclasts to bisphosphonates leads to less bone resorption and lower release of bone-stored factors, breaking the “vicious cycle” between tumors and bone.247 The clinical value of bisphosphonate-based drugs (such as ibandronate,248,249 clodronate,250,251 pamidronate,252,253 and zoledronate19,22,254–256) has been shown in numerous trials of PCa (Table 3).
The third-generation bisphosphonate zoledronic acid has the highest affinity for bone and it was approved by the FDA to prevent skeletal-related events (SREs) in patients with mCRPC in 2002.257,258 A phase 3 study demonstrated that the zoledronic acid-treated group had fewer SREs compared to those in the placebo group (44.2% versus 33.2%, p = 0.021).258 Zoledronic acid also reduced the ongoing risk of SREs by 36% (risk ratio = 0.64, p = 0.002).259 However, bisphosphonates are also associated with adverse events. For example, a few patients receiving bisphosphonates developed hypocalcemia, nausea, emesis, diarrhea, gastric pain, esophagitis, gastrointestinal bleeding, or ulcers.260,261 In particular, intravenous administration of bisphosphonates is associated with an increased risk of renal impairment.261–265
RANKL
RANKL, together with its receptor RANK and the decoy receptor osteoprotegerin, are key factors that regulate osteoclast development and bone metabolism.266–269 RANKL/RANK signaling induces preosteoclast differentiation and maintains the survival and function of osteoclasts.270–273 RANKL plays important role in bone metastases; therefore, a specific RANKL antibody denosumab that neutralizes the activity of RANKL has been developed.274 Denosumab has shown significant efficacy in inducing osteoclast apoptosis and impairing osteoclast activity.275 Accordingly, denosumab has been approved by the FDA for the treatment of diseases driven by a high activity of osteoclasts, including cancer with bone metastases as well as osteoporosis.276–279 Many clinical trials of denosumab have been conducted in PCa (Table 3). Denosumab significantly reduced SREs such as pathologic fractures and hypercalcemia. A phase 3 study enrolled 1432 men with nonmetastatic CRPC and a high risk of bone metastasis, and demonstrated that treatment of denosumab significantly improved bone-metastasis-free survival compared with placebo group (median 29.5 versus 25.2 months; p = 0.028), although it did not improve overall survival.280 Another trial involving 1904 patients found that denosumab treatment increased the median time to first on-study SREs compared with the results of zoledronic acid (20.7 versus 17.1 months; p = 0.008).22 Because denosumab treatment is associated with life-threatening hypocalcemia, proactive treatment of calcium and calcitriol should be considered when using denosumab.281
Calcium channels
Cytosolic calcium (Ca2+) signaling plays an important role in the bone metastasis of PCa.219 Elevated Ca2+ stimulates PTHrP secretion and activates RANKL/RANK signaling in osteoclasts, which promotes bone resorption and calcium release, in turn promoting tumor cell proliferation and maintaining PCa cell homing to bone.219 Targeting calcium signaling could be a promising strategy for managing PCa bone metastasis. New agents for targeting calcium signaling include calcium-ATPase inhibitors, voltage-gated calcium channel inhibitors, transient receptor potential (TRP) channel inhibitors, and Orai inhibitors, although most of these agents are still in the early stages of studies.282 For instance, mipsagargin (G-202), a SERCA inhibitor, was tested in mCRPC in a phase 2 clinical trial; however, the study was withdrawn without results posted (Table 3). The TRPV6 inhibitor, SOR-C13, is currently under evaluation in patients with advanced tumors, including PCa, in a phase 1 clinical trial (Table 3). Because calcium channels are also critical for numerous cellular homeostasis and physiological functions under normal conditions,283 future calcium-based therapies should specifically target PCa cells to decrease normal tissue toxicity.
Targeting PSMA
PSMA and PSMA-targeted ligands
PSMA is a type II transmembrane glycoprotein that includes activities of folate hydrolase and N-acetyl-α-linked acidic dipeptidase and consists of 750 amino acids located in three domains, including the intracellular domain, which contains 19 amino acids, the transmembrane domain, which consists of 24 amino acids, and the extracellular domain, which contains 707 amino acids.284,285 PSMA is expressed at a very low level in normal prostatic tissues and nonprostatic tissues, but its expression in PCa tissues increases by 100–1000 times compared to that in normal tissues.286 Thus, PSMA is a theranostic target for imaging diagnostics and targeted radionuclide therapy for PCa and its metastases.287–289
Three main types of ligands are used to target PSMA: monoclonal antibodies, aptamers, and small-molecule inhibitors. PSMA monoclonal antibodies can be classified into two types: intracellular domain antibodies (7E11, PM2J004.5) and extracellular domain antibodies (J591, J533, J415).284,290,291 Importantly, the humanized monoclonal antibody J591, which targets the extracellular domain of PSMA, has an impressive application prospect in the diagnosis and treatment of PCa. Aptamers of PSMA (such as xPSM-A9, xPSM-A10, A10-3.2, and A9g) are nucleotides or deoxynucleotides that can selectively recognize PSMA.292–294 Small-molecule inhibitors that can interact with PSMA, including 123I-MIP-1072, 123I-MIP-1095, PSMA-I&T, PSMA-I&S, and PSMA-617, have become the preferred choice for molecular imaging probes and targeted therapy for PCa.295,296
PSMA-based diagnostic imaging
The early PSMA-targeted imaging agent was ProstaScint (also known as 111In-capromab pendetide), a mouse monoclonal antibody (7E11) linked to 111In for SPECT (single photon emission computed tomography) imaging. However, ProstaScint was only able to bind to the intracellular epitope of PSMA, and cannot be accessed in viable tumor cells, thus limiting its clinical performance.297,298 68Ga-PSMA-11 (also called PSMA-HBED-CC) for PET is probably the tracer most often used for PCa.299–301 Several 68Ga-labeled PSMA ligands have been developed as theranostic agents, including 68Ga-PSMA-617 and 68Ga-PSMA-I&T.302–304 The 18F-labeled agents include 18F-DCFBC, 18F-DCFPyL, and 18F-PSMA-1007, which exhibit many advantages such as a lower positron range and longer half-life compared with those of 68Ga-labeled agents.305–308 PSMA-based imaging has shown improved sensitivity and diagnostic accuracy in PCa. For example, a study in 96 patients with PCa demonstrated that 18F-PSMA-1007 PET had a sensitivity of 85.9% and a specificity of 99.5% in a patient-based analysis for detecting positive lymph nodes larger than 3 mm.309 Furthermore, the 99mTc-labeled PSMA ligand 99mTc-MIP-1404 is in a phase 3 clinical trial designed to evaluate its sensitivity and specificity to detect PCa (Table 4). Standardized criteria of the PSMA ligand PET are evolving and will facilitate its use in clinical practice; several prospective trials (Table 4) are ongoing to support final market approval.310
Table 4.
Selective clinical trials of PSMA-targeted agents
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| 177Lu-PSMA-617 | PSMA | mCRPC | Unknown | 2 | NCT03392428 |
| 177Lu-PSMA-617 | PSMA | mCRPC | Recruiting | 3 | NCT04689828 |
| 225Ac-PSMA-617 | PSMA | PCa, CRPC | Recruiting | 1 | NCT04597411 |
| 177Lu-J591 | PSMA | Metastatic PCa | Completed | 2 | NCT00195039 |
| MLN2704 | PSMA | mCRPC | Completed | 1/2 | NCT00070837 |
| PSMA-MMAE | PSMA | mCRPC | Completed | 2 | NCT01695044 |
| MEDI3726 | PSMA | mCRPC | Completed | 1 | NCT02991911 |
| BIND-014 | PSMA | mCRPC | Completed | 2 | NCT01812746. |
| 68Ga-PSMA-11 | PSMA | PCa | Completed | 3 | NCT03803475 |
| 68Ga-PSMA-617 | PSMA | PCa | Completed | 2 | NCT03604757 |
| 18F-PSMA-1007 | PSMA | PCa | Completed | 3 | NCT04102553 |
| 99mTc-MIP-1404 | PSMA | PCa | Completed | 3 | NCT02615067 |
| CART-PSMA-TGFβRDN | PSMA | mCRPC | Not yet recruiting | 1 | NCT04227275 |
| PD1-PSMA-CART | PSMA | CRPC | Recruiting | 1 | NCT04768608 |
| LIGHT-PSMA-CART | PSMA | CRPC | Suspended | 1 | NCT04053062 |
| P-PSMA-101 CAR-T | PSMA | PCa | Recruiting | 1 | NCT04249947 |
PSMA-targeted radionuclide therapy (PSMA-TRT)
In contrast to conventional external radiotherapy, targeted radionuclide therapy (TRT) is a treatment conducted by injecting a radionuclide-labeled ligand into the body to specifically target cancer cells. The radionuclide then releases α-particles, β-particles, or auger electrons to produce free radicals that induce DNA damage, thus specifically promoting apoptosis or necrosis of targeted cells.284,311 Conjugations of PSMA-targeted ligands (antibodies or small molecules) with radionuclides such as β-emitters (most commonly 177Lu) or α-emitters (commonly 225Ac) produce PSMA-TRT agents, including 177Lu-PSMA-617, 225Ac-PSMA-617, and 177Lu-J591 (Table 4).312–314 More importantly, in a recent phase 2 study comparing 177Lu-PSMA-617 and cabazitaxel in mCRPC, 177Lu-PSMA-617 led to a higher PSA response (66% versus 44% by intention-to-treat analysis; p = 0.0016) and fewer adverse events, indicating that 177Lu-PSMA-617 is a potential alternative therapy to cabazitaxel in patients with mCRPC.315 Lu-PSMA-617 was approved by the FDA for the treatment of mCRPC in 2021. In addition to 177Lu-PSMA-617, 225Ac-PSMA-617 and 177Lu-J591 have been studied in clinical trials (Table 4). Ongoing clinical trials of PSMA-TRT will further explore the optimal sequencing of this therapy in earlier disease settings, as well as novel combinations. Overall, PSMA expression at different metastatic sites among different patients and the selection of optimal patients remain to be defined.
PSMA-antibody-drug conjugates (PSMA-ADC)
PSMA-specific antibodies have been used to bind cytotoxic drugs via different chemical bonds to obtain PSMA-ADC. PSMA-ADC avoids systemic medication and reduces the toxicity to non-target organs compared to traditional cytotoxic drugs. Many applications of monoclonal antibody-based PSMA-ADC have entered clinical trials (Table 4), including that of MLN2704, which links to the antimicrotubule agent maytansinoid-1;316 PSMA-MMAE (monomethyl auristatin E), which connects to the microtubule disrupting agent MMAE;317 MEDI3726, which combines with the pyrrolobenzodiazepine-based linker-drug tesirine;318 and BIND-014, which conjugates with docetaxel.40 MLN2704 was discontinued after a phase 1/2 study because of the instability of the bond between the antibody and the drug.319,320 The first clinical trial of MEDI3726 observed a high incidence of treatment-related adverse events.321 A phase 2 clinical trial demonstrated that PSMA-MMAE showed some activity with regards to PSA decline and circulating tumor cell reduction in patients with mCRPC, but it also included significant treatment-related toxicities, such as neutropenia and neuropathy.317 Interestingly, the phase 2 clinical trial of BIND-014 (a novel PSMA-ADC) in patients with chemotherapy-naive mCRPC suggest that BIND-014 is well tolerated and patients are likely to benefit from the treatment.40 Optimization of dose administration, conjugation of more appropriate drugs, and patient selection should be considered to improve the efficacy of PSMA-ADC in the future.
PSMA-based chimeric antigen receptor (CAR)-T cells therapy
CAR-T cells are genetically engineered T cells that express an artificial T-cell receptor, endowing T-cell populations with the ability to target tumors independently of major histocompatibility-complex (MHC) engagement.322 CAR-T-cell therapy has gained momentum in PCa treatment in clinical trials. CAR-T cells are activated when the antigen is recognized by CAR, thus stimulating the release of cytotoxins, such as perforin and granzyme, into tumor cells to induce apoptosis. PSMA is considered to be a reliable target for CAR-T-cell therapy. First-generation CAR-T cells targeting PSMA were constructed with a chimeric anti-PSMA immunoglobulin-T-cell receptor gene based on the monoclonal antibody 3D8.323 Second-generation CAR-T cells were constructed by inserting the CD28 signal domain into first-generation CAR-T cells.324 Recently, many new PSMA-based CAR-T cells, such as CART-PSMA-TGFβRDN, have been evaluated in phase 1 clinical trials in CRPC patients.42 Meanwhile, other clinical trials are underway and will test the safety and efficacy of PSMA-targeted CAR-T cells for the treatment of PCa (Table 4). Additionally, side effects such as cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and cytopenia are commonly observed in patients receiving CAR-T-cell therapy; therefore, additional treatment with corticosteroids should be considered when using CAR-T-cell treatment.325
Targeting DNA repair pathways
PARP function in DNA repair and synthetic lethality
PARP is a family of enzymes involved in DNA repair and transcriptional regulation.326 Activation of PARP1/2 is important for recruiting the key effectors of DNA repair (Fig. 4).327 DNA damage in cells, including single-strand breaks (SSB) and double-strand breaks (DSB), can be induced by exposure to chemicals (such as chemotherapy), physical agents (such as radiotherapy), or endogenous reactive metabolites (such as reactive oxygen and nitrogen species) (Fig. 4). Effective DNA repair is essential for cellular survival. Mechanisms of SSB repair include base-excision repair, nucleotide excision repair, and mismatch excision repair, whereas DSB repair includes homologous recombination (HR) and non-homologous end-joining (NHEJ).327 The primary mechanism for inhibiting PARP in cancer therapy is synthetic lethality, which indicates two genomic alteration events that are each relatively innocuous individually but become lethal when they occur together.328 When PARP1/2 is pharmacologically inhibited, the accumulation of SSB by PARP inhibition can progress to DSB, which is usually repaired through HR. The DSBs can be fixed if the DNA repair system is intact in cells; however, PARP inhibition would lead to lethality if a cell lacks HR repair capacity (mutations of BRCA1, BRCA2, or ATM).329 PARP inhibition would not induce cell death in normal cells due to efficient DSB repair mechanisms; however, PARP inhibition would be lethal for tumor cells with deficient HR, such as BRCA1/2 mutations.330–332 Furthermore, PARP inhibition would result in fork collapse and would transform into DSB, since PARP1 is involved in the restart of stalled forks.333,334 If the function of the BRCA (breast cancer susceptibility protein) is deficient, these DSB would not be repaired, thus causing synthetic lethality. Up to 30% of mCRPC tumors harbor DNA damage repair gene aberrations,24 which can be therapeutically used with PARP inhibitors to induce synthetic lethality. However, the interpretation of PARP inhibition-related mechanisms of synthetic lethality may be incomplete. PARP inhibitors may also induce cytotoxic effects by inhibition of SSB repair, as well as other mechanisms.335 Moreover, genomic alterations, such as TMPRSS2-ERG fusion, SPOP mutation, PTEN loss, and CHD1 deletion, are linked to an impaired DNA damage response phenotype, which might increase the therapeutic effectiveness of PARP inhibition.336 DNA damage response genes are regulated by AR; consequently, the ADT response is also influenced by DNA repair deficiency.337 Functional inactivation of DNA repair pathways also enhances sensitivity to chemotherapy and radiotherapy, and this effect is further enhanced by inhibitors of the targeting DNA repair pathways that induce synthetic sensitivity or lethality in DNA repair-deficient cancers (Fig. 4).336
Fig. 4.
Inhibition of PARP mediates synthetic lethality in PCa. When PARP1/2 are pharmacologically inhibited, the accumulation of SSBs by PARP inhibition can progress to DSBs, which are usually repaired through HR. The DSBs can be fixed if the DNA repair system is intact in cells; however, PARP inhibition can lead to lethality if a cell is lacking HR repair capacity (mutations of BRCA1, BRCA2, or ATM). BCL2 overexpression, TMPRSS2-ERG fusion, SPOP mutation, PTEN loss, and CHD1 deletion are also linked with an impaired DNA damage response phenotype, which might increase the therapeutic effectiveness of PARP inhibition
PARP inhibitors
Several PARP inhibitors have been evaluated in clinical trials (Table 5). In 2020, olaparib was approved by the FDA for the treatment of mCRPC with deficient HR genes. The first clinical data from a phase 2 study demonstrated that 88% of patients with a homozygous deletion or mutation in gene-associated DNA repair responded to olaparib.23 Responses were observed in patients with deletions or mutations of BRCA1, BRCA2, FANCA, CHEK2, and PALB2. The overall survival in patients with BRCA1/2 alterations are more favorable than in those who were negative (13.8 versus 7.5 months; p = 0.05).23 Rucaparib was approved by the FDA in 2020 based on a recent clinical study, which involved 78 mCRPC patients with DNA repair gene alterations, including ATM (n = 49), CDK12 (n = 15), CHEK2 (n = 12), and other genes (n = 14).338 A PSA response was seen in 54.8% of all patients, and those that had PALB2, FANCA, BRIP1, and RAD51B mutations showed a better response compared with those with ATM alterations, while the objective response rate and PSA responses in patients with ATM, CHEK2, and CDK12 mutations were low compared to those with BRCA mutated tumors.338 The efficacy of rucaparib is currently being evaluated in a phase 3 trial (Table 5). A phase 2 study demonstrated that the objective response rate of talazoparib was seen in 29.8% (31 of 104) of patients, whereas serious treatment-related adverse events were reported in 43 (34%) patients.27 In addition, PARP1/2 selective inhibitors, including niraparib and pamiparib, are currently being tested in phase 2/3 trials of PCa (Table 5). Finally, trials combining PARP inhibitors with other drugs, such as AR-targeting agents and radium-223, have gained momentum based on the concept of cross-sensitivity. For example, a randomized trial combining veliparib and abiraterone determined that the subgroup patients (27%) with aberrations in DNA repair genes showed better response rates to the combination group compared to those with abiraterone alone.339 However, the identification of prognostic and predictive biomarkers of responses in combination trials remains difficult.
Table 5.
Selective clinical trials of PARP inhibitors
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| Olaparib (AZD2281) | PARPs | PCa | Completed | 1 | NCT02324998 |
| Olaparib (AZD2281) | PARPs | mCRPC | Not yet recruiting | 3 | NCT03732820 |
| Olaparib (AZD2281) | PARPs | mCRPC | Not yet recruiting | 3 | NCT02987543 |
| Olaparib (AZD2281) | PARPs | mCRPC | Not yet recruiting | 3 | NCT03834519 |
| Rucaparib (AG014699) | PARPs | HR deficient mCRPC | Completed | 2 | NCT02952534 |
| Rucaparib (AG014699) | PARPs | mCRPC | Not yet recruiting | 3 | NCT02975934 |
| Veliparib (ABT-888) | PARPs | mCRPC | Completed | 2 | NCT01576172 |
| Talazoparib (BMN 673) | PARPs | HR deficient mCRPC | Not yet recruiting | 2 | NCT03148795 |
| Talazoparib (BMN 673) | PARPs | mCRPC | Recruiting | 3 | NCT03395197 |
| Niraparib (MK-4827) | PARP1/2 | mCRPC | Completed | 1 | NCT02924766 |
| Niraparib (MK-4827) | PARP1/2 | mCRPC | Recruiting | 3 | NCT04497844 |
| Pamiparib (BGB-290) | PARP1/2 | mCRPC | Terminated | 2 | NCT03712930 |
| Pamiparib (BGB-290) | PARP1/2 | mCRPC | Not yet recruiting | 1 | NCT03150810 |
Targeting immune checkpoints
MMR defect and immunotherapy response
Tumors with defects in MMR genes or microsatellite instability (MSI) often have an enhanced antitumor immune response, displaying a higher density of tumor-infiltrating lymphocytes (TILs).340,341 This phenomenon is attributed to high rates of mutations and increased levels of neoantigens in MMR-deficient tumors, which occur through different mechanisms, including mutant peptides, frameshift mutations, and indels in coding microsatellites.342,343 These neoantigens are presented on the cell surface by MHCI molecules, facilitating T-cell-mediated tumor cell killing (Fig. 5). In mammalian cells, MutL homolog 1 (MLH1), MutS homologue 2 (MSH2), MutS homologue 6 (MSH6) and PMS1 homologue 2 (PMS2) are the main proteins of the DNA MMR system, which are critical for recognizing and repairing erroneous insertion, deletion, and misincorporation of bases during DNA replication or DNA recombination.344 Approximately 3–5% of PCa cases are associated with deficiency of MMR genes, such as MSH2, MSH6, PMS2, and MLH1, resulting in hypermutation and MSI.345 Mutation of MMR genes in PCa is highly associated with increased expression of neoantigens and accumulation of TILs.346
Fig. 5.
Mechanisms of elicitation of T-cell-mediated cancer killing in MMR- or CDK12-deficient cancer cells. A dysfunctional MMR system or CDK12 generate neoantigens through mutant peptides, or frameshift mutations and indels in coding microsatellites. These neoantigens are presented to the cell surface by MHCI molecules, thus facilitating T-cell-mediated tumor cell killing, which can be enhanced by ICIs, such as CTLA4 inhibitors, PD1 inhibitors, and PD-L1 inhibitors
Immune checkpoint inhibitors (ICIs)
Several clinical studies have evaluated the efficacy of ICIs, including PD-L1 (nivolumab, atezolizumab, durvalumab, and avelumab), PD1 (pembrolizumab and nivolumab), and CTLA4 (ipilimumab) antibodies (Fig. 5 and Table 6). Early studies showed that ICIs exhibit limited anticancer activity.347,348 It is currently accepted that the selection of patients with deficiency in MMR genes is important, because this subset of patients is potentially responsive to ICIs.345 The anti-PD1 antibody pembrolizumab is approved by the FDA to treat cancers, including PCa with MMR mutations or MSI.349 The responses of ICIs in MMR mutations or MSI PCa are not universal. For example, ~54% (6 of 11) CRPC patients with MMR mutations or MSI-high tumors achieved a 50% reduction in PSA level after treatment with pembrolizumab.345 It remains unclear why some patients with MMR loss/MSI-high do not respond to ICI therapy in PCa. Despite these disappointing results, the interest in combining ICIs with other therapies remains high. Promising results were observed from a phase 1/2 clinical trial of pembrolizumab plus docetaxel, AR inhibitors, or PARP inhibitors in patients with mCRPC.350 Moreover, several phase 3 clinical trials to evaluate the efficacy of pembrolizumab combined with docetaxel, enzalutamide, and olaparib are ongoing (Table 6).
Table 6.
Selective clinical trials of immune checkpoint inhibitors
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| Ipilimumab | CTLA4 | mCRPC | Completed | 2 | NCT02279862 |
| Ipilimumab | CTLA4 | mCRPC | Completed | 3 | NCT01057810 |
| Ipilimumab | CTLA4 | mCRPC | Completed | 3 | NCT00861614 |
| Atezolizumab | PD-L1 | mCRPC | Completed | 1 | NCT03024216 |
| Atezolizumab | PD-L1 | mCRPC | Recruiting | 3 | NCT04446117 |
| Durvalumab | PD-L1 | mCRPC | Completed | 2 | NCT03204812 |
| Avelumab | PD-L1 | NEPC | Completed | 2 | NCT03179410 |
| Pembrolizumab | PD1 | mCRPC | Recruiting | 1/2 | NCT02861573 |
| Pembrolizumab | PD1 | mCRPC | Completed | 2 | NCT03473925 |
| Pembrolizumab | PD1 | Hormone-sensitive PCa | Recruiting | 3 | NCT04934722 |
| Pembrolizumab | PD1 | mCRPC | Recruiting | 3 | NCT03834493 |
| Pembrolizumab | PD1 | mCRPC | Recruiting | 3 | NCT04907227 |
| Nivolumab | PD1 | mCRPC | Completed | 2 | NCT02601014 |
| Nivolumab | PD1 | mCRPC | Recruiting | 3 | NCT04100018 |
| Ipilimumab + Nivolumab | CTLA4, PD1 | mCRPC with CDK12 mutations | Recruiting | 2 | NCT03570619 |
Notably, up to 10% of mCRPC patients present with CDK12 aberration (Fig. 1a), which is associated with the response to ICIs.351 CDK12, which forms a complex with cyclin K, is critical for DNA repair during gene translation (Fig. 5). Inactivation of CDK12 leads to focal tandem duplications that increase gene fusions or mutations, thus enhancing neoantigen production and the tumor immune response (Fig. 5).351 Two of four mCRPC patients with CDK12 mutations had obvious PSA responses after administration of a PD1 inhibitor.351 A phase 2 clinical trial to evaluate the efficacy of ipilimumab and nivolumab for CDK12-mutated mCRPC is ongoing (Table 6).
Targeting the cell cycle
CDK4/6 and cell cycle
Hyperproliferation is a hallmark of cancer development. The cell cycle can be divided into four ordered phases: G1 (gap 1), S (DNA synthesis), G2 (gap 2), and M (mitosis), which are precisely controlled by molecules such as CDKs. The key regulatory checkpoints in the G1 and G2 phases determine whether cells enter the S phase and mitosis. CDK4 and CDK6, two serine/threonine kinases, are crucial for governing the transition from the G1 to S phase.352 CDK4/6 is activated by the binding of cyclin D1/2/3 during the early G1 phase in response to mitogenic stimuli. The cyclin D-CDK4/6 complexes subsequently phosphorylate and inactivate the retinoblastoma (RB) tumor suppressor protein.353,354 RB proteins typically bind to the transcription factor E2Fs, such as E2F1, to limit the expression of many E2F target genes that are involved in the cell cycle and mitotic progression.355,356 Phosphorylation by CDK4/6 reduces the binding affinity of RB to E2F, leading to transactivation of E2F transcription factors such as E2F1 (Fig. 1c). Activated E2F1 recruits RNA-POLII to induce the transcription of CDK2, E-type cyclins, and other cell cycle-related proteins that further phosphorylate RB and promote the G1-to-S-phase cell cycle transition.357,358 CDK4/6 also phosphorylates other substrates and plays an important role in differentiation and metabolism.359,360
CDK4/6 inhibitors
Currently, three new CDK4/6 inhibitors, palbociclib (PD0332991), ribociclib (LEE011), and abemaciclib (LY2835219), have entered early phase trials for PCa (Table 7). A recent phase 2 clinic study evaluated the efficacy of ADT (including bicalutamide, goserelin, and leuprolide) plus palbociclib in patients with RB-positive metastatic hormone-sensitive PCa. The primary PSA endpoint was met in 80% of patients in both the ADT alone and ADT plus palbociclib groups (16/20 versus 32/40; p = 0.87), while 1-year biochemical progression-free survival (PFS) was 69% in the ADT alone group and 74% in the ADT plus palbociclib group.361 Although these important clinical data are still not sufficient, this study suggests that co-targeting of AR signaling and the cell cycle is possible. Furthermore, abiraterone plus abemaciclib was evaluated in a phase 2/3 trial, and ribociclib plus enzalutamide or docetaxel are under investigation in different trials for the treatment of mCRPC (Table 7). PCa has shown limited response to immunotherapy because of its cold tumor environment.348 Notably, CDK4/6 inhibitors have been shown to increase the tumor immune response and TILs,362,363 which supports the potential synergistic effects of CDK4/6 inhibitors and ICIs. Additional studies are trying to identify the population of appropriate patients and synergistic combinations that would make these agents more efficacious.
Table 7.
Selective clinical trials of CDK4/6 inhibitors or p53-targeted agents
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| Palbociclib (PD0332991) | CDK4/6 | RB-positive metastatic PCa | Completed | 2 | NCT02059213 |
| Palbociclib (PD0332991) | CDK4/6 | mCRPC | Not yet recruiting | 2 | NCT02905318 |
| Ribociclib (LEE011) | CDK4/6 | mCRPC | Completed | 2 | NCT02494921 |
| Ribociclib (LEE011) | CDK4/6 | RB-positive metastatic PCa | Recruiting | 2 | NCT02555189 |
| Abemaciclib (LY2835219) | CDK4/6 | mCRPC | Recruiting | 2/3 | NCT03706365 |
| Abemaciclib (LY2835219) | CDK4/6 | Locally advanced PCa | Recruiting | 2 | NCT04298983 |
| Abemaciclib (LY2835219) | CDK4/6 | mCRPC | Not yet recruiting | 2 | NCT04408924 |
| APR-246 | Mutant p53 | Refractory PCa | Completed | 1 | NCT00900614 |
| Arsenic trioxide | Mutant p53 | Stage IV PCa | Completed | 2 | NCT00004149 |
p53 and the targeting approaches
The tumor suppressor p53 is widely known as the “genome guardian.” Activated p53 binds to a specific DNA sequence as a tetramer to promote gene expression (such as CDKN1A, BAX, PUMA, and NOXA), thus inducing cell apoptosis and cell-cycle arrest.364 The TP53 gene, which encodes the p53 protein, is frequently mutated in PCa, especially in neuroendocrine-like mCRPC. The combined alteration of RB and TP53 occurs in 74% of neuroendocrine-like mCRPC.365 p53 mutations are predominantly distributed throughout the DBD, and these p53 mutations either lose their DNA-binding ability or form a heterodimer complex with wild-type p53 to attenuate wild-type p53 functions, thus disrupting the tumor-suppressive functions of p53.366 Moreover, many mutant p53 proteins acquire gain-of-function activities, which enable them to deactivate the other p53 family members, specifically p73 and p63.367 Under normal conditions, p53 has a half-life of less than 20 min owing to the feedback regulation of proteasome-mediated p53 degradation by E3 ubiquitin-protein ligase MDM2.368
p53 alteration fosters more lethal PCa; thus, targeting p53 is an attractive therapeutic strategy for aggressive PCa. Approaches for targeting p53 can be summarized as follows: first, compounds such as idasanutlin (RG7388) and RG7112 were developed to prevent degradation of wild-type p53 by blocking p53-MDM2 interactions,369,370 thus maintaining their ability to suppress tumor formation. Although no clinical studies exist for PCa, multiple p53-MDM2 antagonists are undergoing clinical trials; of these, idasanutlin is the most advanced and is under testing in a phase 3 clinical trial in patients with refractory acute myeloid leukemia.371 Second, pharmacological reactivation of mutant p53 uses small molecules like APR-246, COTI-2, and arsenic trioxide (Table 7), which bind to mutant p53 and convert the protein to a p53 wild-type-like conformation, thus restoring wild-type DNA-binding properties.372–374 Based on the results of a phase 1b/2 trial for myelodysplastic syndrome,375 the FDA granted Fast Track designation to APR-246 for the treatment of myelodysplastic syndrome in patients with a p53 mutation.376 APR-246 was tested in PCa, and showed a favorable pharmacokinetic profile in a phase 1 trial (Table 7).377 Third, mutant p53 neoantigens can elicit intratumoral T-cell responses;378 therefore, p53 neoantigens are considered to be promising targets. For example, a recent study generated a T-cell-based therapy that links T cells to cancer cells using a novel antibody that specifically binds to the p53R175H peptide-MHC complex, lysing cancer cells depending on the presence of the neoantigen.379 Clinical studies targeting mutant p53 neoantigens in PCa are rare. More clinical studies on p53-targeted agents in PCa are warranted, and it is projected that at least several of these molecules will prove effective in the future.
Targeting the PI3K/AKT/mTOR signaling axis
PTEN/PI3K/AKT/mTOR signaling
Inactivation of PTEN (phosphatase and tensin homologue) by deletion or mutation has been identified in ~20% of primary PCa and approximately 35% of CPRC cases (Fig. 1a).380 PTEN, a dual-specificity phosphatase, converts phosphatidylinositol-3,4,5-trisphosphate (PIP3) into phosphatidylinositol 4,5-bisphosphate (PIP2),381 causing PTEN to function as a direct antagonist of the activity of class I PI3K, which converts PIP2 to PIP3. As a result, PTEN loss leads to aberrant accumulation of PIP3 on cell membranes, which leads to the recruitment of PDK1 to phosphorylate its substrate AKT. Phosphorylated AKT subsequently regulates several downstream signaling cascades, including mTOR, which is crucial for protein synthesis, autophagy, cellular proliferation, and metabolism.382 PTEN acts as a gatekeeper of the PI3K/AKT/mTOR pathway. Therefore, PTEN deletions or mutations are strongly associated with activation of PI3K/AKT/mTOR signaling and poor prognosis in advanced PCa.380
PI3K, ATK, mTOR, and PIM (proviral-integration site for Moloney-murine leukemia virus) inhibitors
PI3K, a plasma membrane-associated protein kinase, is formed by two functional subunits: a catalytic subunit (p110α, p110β, or p110δ) and a regulatory subunit (p85α, p55α, p50α, p85β, or p55γ isoform).383 The catalytic subunit p110β is believed to be the most relevant isoform for PCa progression.384,385 PI3K inhibitors, such as BKM120 and PX866, target the catalytic subunits of all three isoforms (p110α, p110β, and p110δ). BKM120 showed a partial response in a phase 1 study.386 PX866, a derivative of wortmannin, was well tolerated in patients with recurrent mCRPC.387,388 However, monotherapy with PI3K inhibitors is limited in the clinic.
AKT, a serine/threonine protein kinase, is the main downstream effector of PI3K, and is fully activated when both Thr308 and Ser473 sites are phosphorylated.389 Activated AKT phosphorylates several targets, such as mTOR, GSK3, FOXO, and AMPK, which are involved in multiple cellular processes.390,391 Thus far, the AKT inhibitors that have reached the clinical phase include allosteric inhibitors (such as perifosine and MK-2206) and ATP-competitive inhibitors (such as capivasertib, ipatasertib, and uprosertib). Of note, the combination of capivasertib with docetaxel resulted in a greater than 50% reduction in PSA levels in 70% of men with mCRPC in a phase 1 study.392 Ipatasertib prolonged PSA progression-free interval and overall survival compared to the placebo group in a phase 2 trial.49 A phase 3 trial to evaluate the efficacy of abiraterone plus ipatasertib for the treatment of mCRPC is ongoing (Table 8).
Table 8.
Selective clinical trials of PI3K, AKT and mTOR inhibitors
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| Buparlisib (BKM120) | PI3K | CRPC | Completed | 1 | NCT01634061 |
| Buparlisib (BKM120) | PI3K | CRPC | Terminated | 2 | NCT01385293 |
| Dactolisib (BEZ235) | PI3K | mCRPC | Terminated | 1/2 | NCT01717898 |
| Samotolisib (LY3023414) | PI3K | CRPC | Completed | 2 | NCT02407054 |
| AZD8186 | PI3K | CRPC | Completed | 1 | NCT01884285 |
| GSK2636771 | PI3K | CRPC | Completed | 1 | NCT02215096 |
| Perifosine (KRX-0401) | AKT | mCRPC | Completed | 2 | NCT00060437 |
| MK2206 | AKT | Advanced cancers | Completed | 1 | NCT01295632 |
| Ipatasertib (GDC-0068) | AKT | Locally advanced PCa | Recruiting | 1/2 | NCT04737109 |
| Ipatasertib (GDC-0068) | AKT | mCRPC | Not yet recruiting | 3 | NCT03072238 |
| Capivasertib (AZD5363) | AKT | CRPC | Completed | 1 | NCT04087174 |
| Sirolimus | mTOR | Locally advanced PCa | Completed | 1/2 | NCT00311623 |
| Ridaforolimus (MK8669) | mTOR | mCRPC | Completed | 2 | NCT00777959 |
| Temsirolimus (CCI-779) | mTOR | CRPC | Completed | 2 | NCT00919035 |
| Everolimus (RAD001) | mTOR | CRPC | Completed | 2 | NCT00814788 |
| Sapanisertib (MLN0128) | mTOR | mCRPC | Completed | 2 | NCT02091531 |
| Vistusertib (AZD2014) | mTOR | PCa | Completed | 1 | NCT02064608 |
| Apitolisib (GDC-0980) | PI3K, mTOR | CRPC | Not yet recruiting | 1/2 | NCT01485861 |
| CC-115 | mTOR, DNA-PK | CRPC | Not yet recruiting | 1 | NCT02833883 |
The serine/threonine protein kinase mTOR, the major downstream effector of AKT signaling, interacts with different proteins and forms two distinct complexes, mTORC1 and mTORC2.393 Several types of mTOR inhibitors exist, including mTORC1 inhibitors (such as rapamycin, everolimus, and temsirolimus), mTORC1/2 dual inhibitors (such as sapanisertib and vistusertib), and dual PI3K-mTORC1/2 inhibitors (such as apitolisib and BEZ235). Clinical trials using single mTORC1 inhibitors showed predictable toxicity with no favorable clinical responses in mCRPC.394,395 Sapanisertib was previously tested in a phase 2 study in advanced CRPC but showed limited clinical efficacy.47 Vistusertib was tested in men with high-risk PCa and was administered prior to radical prostatectomy in a phase 1 trial (Table 8). Currently, apitolisib plus abiraterone is being tested for CRPC in phase 1/2 clinical trials (Table 8). A novel mTOR and DNA-PK (DNA-dependent protein kinase) dual inhibitor, CC-155, was evaluated in a phase 1 study (Table 8).
PIM kinases have been found to sustain the PI3K/AKT/mTOR pathway.396,397 Increased expression of PIM family members has been detected in PCa, and PIM confers resistance not only to PI3K/AKT inhibitors, but also to chemotherapy and radiotherapy.398 Therefore, co-targeting PIM and PI3K/AKT/mTOR could offer superior clinical outcomes relative to targeting either of these alone. The combination of the PIM inhibitor AZD1208 and the PI3K/mTOR inhibitor BEZ235 (dactolisib) has been investigated in clinical trials.399 A novel and highly efficient triple PIM/PI3K/mTOR inhibitor AUM302 elicited a superior functional outcome compared to the effects of the combination of AZD1208 and BEZ235; these results may help reduce treatment toxicity in future trials.399 Overall, the clinical application of PI3K/AKT/mTOR inhibitors is still limited in PCa, and further studies that can identify new biomarkers for patient selection or improve co-targeting strategies are still required to enhance their therapeutic effects.
Targeting epigenetic marks
Epigenetic modifications
Epigenetic traits are heritable phenotypes attributable to changes in chromosomes or DNA modifications without alterations in the DNA sequence.400 In addition to genomic changes, epigenetic alterations (such as histone modifications and DNA methylation) have been reported to be associated with PCa progression.401–403 Epigenetic modifications, including acetylation, methylation, ubiquitination, and phosphorylation, play critical roles in transcription, DNA repair, and replication.404 Epigenetic regulation is a dynamic and reversible process that adds epigenetic marks onto either histones or DNA by epigenetic writers, recognizes or recruits epigenetic marks by epigenetic readers, and removes epigenetic marks by epigenetic erasers (Fig. 6). Aberrant histone modifications may upregulate oncogenes or reduce the expression of tumor suppressor genes. Importantly, histone methylation/acetylation and DNA methylation play a central role in controlling gene expression, thus promoting the progression and metastasis of PCa.405,406
Fig. 6.
Schematic of major histone or DNA modification and the key modifiers implicated in PCa. Aberrant histone (such as acetylation, methylation, phosphorylation, and ubiquitination) or DNA modifications (such as methylation) might upregulate oncogenes or reduce tumor suppression genes; thus, targeting these epigenetic modifications is an attractive strategy to treat PCa. Several compounds (such as EZH2 inhibitors, LSD1 inhibitor, BET inhibitors, HDAC inhibitors and DNMT inhibitors) based on epigenetic targets have entered clinical trials in succession
Histone methylation
Histones are methylated by the addition of one, two, or three methyl groups from S-adenosylmethionine to the side chains of the arginine, lysine, and histidine residues. Histone methylations such as H3K4me1, H3K9me2, and H3K9me3 have been reported to be reduced in PCa tissues compared to those in non-malignant tissues;407 however, in comparison with localized PCa and normal prostate tissues, H3K27me3 marks at promoter regions of tumor suppressor genes were strongly enriched in metastatic PCa.403,408 Overexpression of EZH2, a histone methyltransferase, is a major reason for the increased genomic distribution of H3K27me3 in metastatic PCa.409 EZH2 plays an important role in promoting lineage plasticity and differentiation changes, which are highly associated with NEPC.410 Thus, EZH2 is an attractive target, and many EZH2 inhibitors (lirametostat, tazemetostat, valemetostat, PF-06821497, and SHR2554) have emerged in early clinical studies (Table 9). The effectiveness of EZH2 inhibitors alone, in combination with AR inhibitors, or in combination with immunotherapy for the treatment of PCa is currently under evaluation in clinical trials (Table 9).
Table 9.
Selective clinical trials of epigenetic inhibitors
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| Lirametostat (CPI-1205) | EZH2 | mCRPC | Not yet recruiting | 1/2 | NCT03480646 |
| PF-06821497 | EZH2 | CRPC | Recruiting | 1 | NCT03460977 |
| Tazemetostat (EPZ-6438) | EZH2 | mCRPC | Recruiting | 1 | NCT04846478 |
| Tazemetostat (EPZ-6438) | EZH2 | mCRPC | Recruiting | 1/2 | NCT04179864 |
| SHR2554 | EZH2 | CRPC | Terminated | 1/2 | NCT03741712 |
| Valemetostat (DS-3201) | EZH2 | mCRPC | Recruiting | 1 | NCT04388852 |
| CC-90011 | LSD1 | mCRPC | Recruiting | 1 | NCT04628988 |
| CCS1477 | p300/CBP | mCRPC | Recruiting | 1/2 | NCT03568656 |
| FT-7051 | p300/CBP | mCRPC | Recruiting | 1 | NCT04575766 |
| Birabresib (MK-8628) | BET bromodomain | CRPC | Completed | 1 | NCT02259114 |
| Mivebresib (ABBV-075) | BET bromodomain | PCa | Completed | 1 | NCT02391480 |
| Molibresib (GSK525762) | BET bromodomain | CRPC | Completed | 1 | NCT03150056 |
| ZEN-3694 | BET bromodomain | mCRPC | Completed | 1/2 | NCT02711956 |
| ZEN-3694 | BET bromodomain | mCRPC | Recruiting | 2 | NCT04471974 |
| PLX2853 | BET bromodomain | mCRPC | Not yet recruiting | 1/2 | NCT04556617 |
| Entinostat (MS-275) | HDACs | CRPC | Terminated | 1 | NCT03829930 |
| Panobinostat (LBH589) | HDACs | CRPC | Completed | 1 | NCT00878436 |
| Pracinostat (SB939) | HDACs | CRPC | Completed | 2 | NCT01075308 |
| Vorinostat (SAHA) | HDACs | Metastatic PCa | Completed | 2 | NCT00330161 |
| Azacitidine (5-Aza) | DNMT | PCa | Completed | 2 | NCT00384839 |
| Decitabine (NSC127716) | DNMT | PCa | Withdrawn | 1/2 | NCT03709550 |
In contrast, histone demethylase catalyzes the removal of methyl groups from histones. Multiple histone demethylases, such as LSD1 (also known as KDM1A), are overexpressed in patients with advanced PCa.411 LSD1 demethylates H3K4me1 and H3K4me2.412 LSD1 co-operates with AR and activates AR-dependent transcription or a subset of cell-cycle gene expression.413,414 A clinical trial with a novel LSD1 inhibitor CC-90011 was recently launched (Table 9).415
Histone acetylation
Histone acetylation is achieved by the addition of an acetyl group to the lysine residues of histones, and is linked to open and active chromatin. Histone acetylation is usually correlated with activating transcription, whereas histone deacetylation is commonly associated with gene silencing.416 Super-enhancers, a cluster of enhancers marked by high H3K27ac levels, play a key role as an oncogenic driver in cancer cells.417,418 Activation of histone acetyltransferases, such as p300 and CBP (CREB-binding protein), is highly associated with increased modification of H3K27ac.419 Furthermore, p300 and CBP play crucial roles in regulating key genes in PCa, including AR target genes.420 Recently, p300 and CBP inhibitors (such as CCS1477, A-485, and FT-7051) have been developed.421,422 Clinical trials to test the efficacy of CCS1477 and FT-7051 in PCa have recently begun (Table 9).
In contrast, HDACs can remove acetylation of histones. Eighteen different types of HDACs have been identified in humans,423 and overexpression of HDACs occurs in different malignancies, including PCa.424 HDAC1 and HDAC2 expression is positively correlated with higher Gleason scores of PCa, while the expressions of HDAC1, HDAC2, and HDAC3 are positively associated with the proliferative marker Ki67.425 HDAC inhibitors are potential therapeutic options because the expression of HDACs is associated with poor clinical outcomes.425 There are five classes of HDAC inhibitors, including hydroxamic acids, cyclic tetrapeptides, short chain carboxylic acids, benzamides, and keto-derivatives.426 Several HDAC inhibitors, including vorinostat, pracinostat, panobinostat, and romidepsin, have been tested in phase 2 clinical trials for PCa (Table 9); however, most patients exhibited either toxicity from these agents or disease progression.427 Clinical trials involving HDAC inhibitors have not achieved significant success because of poor oral bioavailability, non-selectivity of the drugs, or other mechanisms that remain to be explored.427
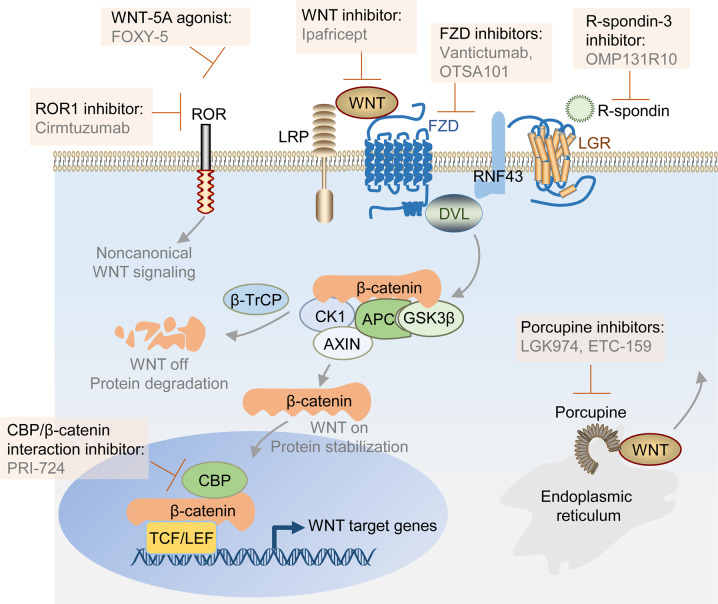
BET protein
Acetylated lysines are recognized by a class of proteins containing bromodomains, such as the BET proteins BRD2, BRD3, BRD4, and BRDT.428 Acetylated lysine residues in histones can be bound by BET proteins via BD1 and BD2 bromodomains, which is the key step in regulating transcription.428 Importantly, the expression of BRD4 is significantly associated with poor clinical outcomes.429,430 BET proteins, such as BRD4, are tightly linked to AR signaling activation and drug resistance in SPOP mutant PCa.431–433 Numerous BET inhibitors, including pan-BET bromodomain inhibitors (such as JQ1, I-BET151, birabresib, mivebresib, and ZEN-3694) and selective inhibitors (such as ABBV-744, molibresib, and PLX2853), have been demonstrated to have antitumor effects in preclinical models. Birabresib (MK-8628) and mivebresib (ABBV-075) were tested in patients with solid tumors, including CRPC, but neither birabresib nor mivebresib demonstrated significant antitumor activity in CRPC patients (Table 9).59,61 However, a clinical trial at phase 1/2 demonstrated that enzalutamide plus ZEN-3694 prolonged the PFS in a subset of patients with mCRPC resistant to enzalutamide and/or abiraterone.62 A new study found molibresib (GSK525762) was well tolerated in patients.434 Recently, phase 1 or phase 2 clinical trials of ZEN-3694 and PLX2853, in combination with AR inhibitors (enzalutamide or abiraterone) have been launched in CRPC patients (Table 9). Ongoing clinical studies of BET inhibitors are needed to demonstrate their safety profiles and the role of pharmacodynamic regulation of AR signaling in patients.
DNA methylation
DNA methylation is achieved when a methyl group is added to the C5 position of cytosine residues in the CpG dinucleotides, and is linked to gene silencing.435 DNMT enzymes catalyze 5-methyl cytosine (5mC) in DNA, whereas these marks can be removed by the DNA demethylases TET (ten-eleven translocation) family.436 Approximately 60% of all promoters colocalize with CpG islands437; therefore, aberrant DNA hypermethylation at CpG islands can lead to gene silencing, such as inactivation of tumor suppressor genes.88,438 A previous studied showed that 22% of tumors were associated with hypermethylation in PCa.439 DNMT inhibitors azacytidine (5-Aza) and decitabine have been developed to target aberrant DNA hypermethylation. Azacitidine and decitabine (NSC127716) were clinically evaluated for PCa (Table 9). A phase 1/2 study of azacitidine in combination with docetaxel in mCRPC demonstrated a PSA response in 52% (10/19) of patients, without exhibiting dose-limiting toxicity.57
Targeting WNT signaling
WNT/β-catenin signaling
The canonical WNT/β-catenin pathway is activated in late-stage PCa, and promotes tumor cell growth and drug resistance in PCa.440 The binding of WNT ligands to their receptors in cell surface activates signaling pathways that regulate cell differentiation and proliferation.441 In the absence of WNT ligands, cytoplasmic β-catenin is rapidly degraded by a destruction complex, whose components contain adenomatous polyposis coli protein (APC), AXIN, casein kinase 1 (CK1), β-transducin-repeat-containing protein (β-TrCP), and glycogen synthase kinase 3 (GSK3) (Fig. 7).65,441,442 When WNT ligands bind to frizzled (FZD) receptors and co-receptors LRP5/6, LRP5/6 are phosphorylated by CK1 and GSK3, and then the signal is transduced to activate the cytoplasmic phosphoprotein dishevelled (DVL). Phosphorylated DVL recruits the destruction complex to the plasma membrane. This inhibits GSK3 and prevents phosphorylation of β-catenin, thereby resulting in the stabilization and accumulation of β-catenin proteins. Sequentially, β-catenin proteins translocate into nucleus, form a complex with T-cell factors (TCFs)/lymphoid enhancer-binding factor 1 (LEF1), recruit transcription factors and co-activators, such as the CBP/p300, and activate the transcription of downstream target genes, including ABCB1, MYC, MYCN, NEUROG1, NEUROD1, SOX2, SUZ12, TWIST, and YAP (Fig. 7).65,441–443 Importantly, activating mutations of the WNT/β-catenin pathway genes were enriched in metastatic PCa (19%) compared to those in primary PCa (6%).444 An increasing number of studies have indicated that the activation of WNT/β-catenin signaling is highly linked to cell proliferation, invasion, bone metastasis, drug resistance, and neuroendocrine differentiation in the late stage of PCa.445–455
Fig. 7.
The WNT signaling pathway and targeting therapeutic strategies. WNT ligands bind to FZD and LRP5/6 receptors to phosphorylate DVL, and then phosphorylated DVL recruits the destruction complex to the plasma membrane. This inhibits GSK3 and prevents phosphorylation of β-catenin, resulting in the stabilization and accumulation of β-catenin proteins to form a complex with TCF/LEF in the nucleus, thereby activating the transcription of downstream target genes. There are several targeting strategies that prevent the activation of WNT signaling, such as targeting WNT ligands and their receptors, inhibiting the WNT secretion by targeting porcupine, and disrupting the interaction between CBP and β-catenin
WNT signaling inhibitors
Numerous agents that target different components of the WNT pathway have been developed and tested in clinical trials (Fig. 7), although only a few have been associated with PCa. Several strategies can prevent the activation of WNT signaling. First, targeting WNT ligands and their receptors with monoclonal antibodies and small molecules is an attractive therapeutic strategy. The WNT ligand WNT-5A was targeted using a WNT-5A-mimicking peptide named Foxy-5.456 Foxy-5 was evaluated in a phase 1 clinical trial in patients with solid malignant tumors including PCa (Table 10). An R-spondin-3 antibody (OMP-131R10) was tested in a phase 1 clinical trial for advanced solid tumors.65 The antibody OMP-18R5 (vantictumab) targets FZD1, FZD2, FZD5, FZD7, and FZD8.65 OTSA101 is a radiolabeled antibody against FZD10.457 Pafricept (OMP-54F28) is a decoy WNT receptor that has been tested in a phase 1a clinical trial and has demonstrated evidence of WNT pathway inhibition,458 but its development has been terminated due to bone-related toxicity.459 Second, inhibition of WNT secretion by targeting porcupine, a membrane-bound O-acetyltransferase, is another optional strategy. Porcupine is important for WNT palmitoylation, which is essential for the secretion of WNT proteins.460 Several porcupine inhibitors, such as IWP-2, WNT-C59, LGK974 (WNT974), and ETC-159, also have been developed65,461, and of these, LGK974 (discontinued) and ETC-159 (phase 1) have entered trials in patients with advanced solid tumors.462 Third, the interaction between cofactor CBP and β-catenin is critical for transcriptional activation of β-catenin.65 Thus, disruption of the interaction between CBP and β-catenin provides a new strategy to prevent WNT signaling. ICG-001 and its derivative PRI-724 were found to inhibit the binding of β-catenin to CBP.463 PRI-724, which has an acceptable toxicity profile, was tested in phase 2 clinical trials for metastatic colorectal cancer (but was withdrawn owing to drug supply issues) and advanced myeloid malignancies (completed).463
Table 10.
Selective clinical trials of other signaling pathway inhibitors
| Drug | Target | Condition | Status | Phase | NCT identifier |
|---|---|---|---|---|---|
| FOXY-5 | WNT-5A receptors | PCa | Completed | 1 | NCT02020291 |
| Bevacizumab | VEGF-A | PCa | Completed | 3 | NCT00110214 |
| Zibotentan (ZD4054) | ETAR | mCRPC | Completed | 3 | NCT00554229 |
| Erdafitinib (JNJ-42756493) | FGFR | DN-PCa | Suspended | 2 | NCT03999515 |
| Cetuximab | EGFR | mCRPC | Completed | 2 | NCT00728663 |
| Gefitinib (ZD1839) | EGFR | PCa | Completed | 2 | NCT00265070 |
| Dovitinib (TKI258) | FGFR, VEGFR, PDGFR | CRPC | Completed | 2 | NCT01741116 |
| Sunitinib (SU11248) | VEGFR, PDGFR | mCRPC | Terminated | 3 | NCT00676650 |
| Cabozantinib (XL184) | VEGFR, c-MET, c-KIT | mCRPC | Completed | 3 | NCT01605227 |
| Masitinib (AB1010) | KIT, PDGFR, FGFR | mCRPC | Completed | 3 | NCT03761225 |
| Galunisertib (LY2157299) | TGFβR1 | mCRPC | Recruiting | 2 | NCT02452008 |
| M7824 | TGFβ, PD-L1 | Metastatic PCa | Recruiting | 1/2 | NCT04633252 |
| Dasatinib (BMS-354825) | SRC, c-KIT | CRPC | Completed | 3 | NCT00744497 |
| Saracatinib (AZD0530) | SRC | CRPC | Completed | 2 | NCT00513071 |
| Trametinib (GSK1120212) | MEK | mCRPC | Not yet recruiting | 2 | NCT02881242 |
Although targeting WNT signaling is very attractive for treating late-stage PCa, WNT signaling inhibitors are in early stages of development and application, and several limitations remain. First, WNT signaling is complicated because there are 19 WNT-secreted glycoproteins and more than 15 types of WNT receptors in humans,464,465 which activate different downstream pathways. Second, variations in and balances between canonical and noncanonical WNT signaling is elusive,466 making it even more difficult to target the WNT pathway. Third, WNT signaling plays a fundamental role in the homeostasis of the intestine, hair follicles, and hematopoietic system;464 thus, blocking the WNT pathway could cause systemic toxicity. Prospective studies include: (I) the combination of WNT inhibitors with other cancer drugs or immunotherapies to limit toxicities or improve therapeutic efficacy, (II) the classification of disease subtypes to distinguish methods of WNT activation among different stages of disease, and (III) the expansion of models to more clearly understand mechanism of action between the canonical and noncanonical WNT pathways and thus, discover novel therapeutic approaches.
Targeting other pathways
VEGF
VEGF is a prominent factor involved in angiogenesis and is highly associated with tumor growth, including in PCa.66,467 Angiogenesis is essential for tumor growth, because the newly formed blood vessels are important to sustain adequate energy and oxygen.468,469 The binding of VEGFs (VEGFA, VEGFB, VEGFC, and VEGFD) to cell surface receptors (VEGFR1, VEGFR2, VEGFR3) activates signaling pathways that play important roles in cell growth and motility in PCa.468 Of note, VEGFA is the founding member of the vascular permeability factor, and is frequently overexpressed in PCa.470,471 Overexpression of VEGFA in PCa is associated with angiogenesis, recurrence, and advanced disease stages among patients.472,473 Importantly, bevacizumab, a humanized monoclonal antibody against VEGFA, is used to treat several different cancers.474 A phase 3 clinical study in mCRPC patients demonstrated that the combination of bevacizumab and docetaxel did not result in a significant increase in median overall survival versus docetaxel alone (22.6 versus 21.5 months; p = 0.181); however, improvements were found in median PFS (9.9 versus 7.5 months; p < 0.001) and major PSA response (69.5% versus 57.9%; P < 0.001).475
ETAR
Activation of ETAR by endothelin-1 is involved in tumor progression through angiogenesis, invasion, apoptosis, and the effect of the bone microenvironment.476,477 Activated ETAR signaling promotes osteoblast proliferation and new bone formation, which is highly associated with bone metastasis in PCa.478,479 Thus, the ETAR inhibitors zibotentan (ZD4054) and atrasentan have been tested in men with mCRPC. In a phase 2 trial, zibotentan prolonged overall survival from 17.3 to 24.5 months in patients with mCRPC,480 but a phase 3 trial did not result in a statistically significant improvement in overall survival in the patients.481 Similarly, atrasentan delayed PFS and PSA progression in a phase 2 trial,482 but a phase 3 trial demonstrated that atrasentan did not significantly reduce the disease progression time in CRPC patients.483 However, a phase 3 trial in mCRPC demonstrated that patients with the highest level of bone metabolism markers (such as pyridinoline and alkaline phosphatase) obtained a survival benefit from atrasentan compared to that from placebo (13 versus 5 months; p = 0.005).484
TGFβ
TGF-β is a cytokine that regulates many cellular functions, such as cell differentiation and migration. Binding of TGFβ to TGFβR2 (TGFβ receptor 2) phosphorylates and activates TGFβR1 (TGFβ receptor 1). Subsequently, activated TGFβR1 phosphorylates SMAD2 and SMAD3 proteins, leading to SMAD2/SMAD3 complexes with SMAD4. Next, these complexes are translocated into the nucleus and stimulate target gene expression.485,486 Increased TGFβ or its target genes in PCa are associated with a more aggressive disease, metastasis, and poor prognosis.487–489. TGFβ also plays an important role in the context of the bone microenvironment and supports the progression of bone metastasis in PCa.490–492 Furthermore, TGFβ facilitates tumor growth through its immunosuppressive function.493,494 To target TGFβ signaling, galunisertib (LY2157299), an oral small-molecule inhibitor of TGFβR1, has been developed.495 A clinical study of galunisertib plus enzalutamide in mCRPC has been initiated (Table 10). Furthermore, a clinical trial of M7824,496 targeting both TGFβ and PD-L1, also has been launched for the treatment of metastatic PCa (Table 10).
RTKs and nonreceptor tyrosine kinases (NRTKs)
Both RTKs (such as FGFR, EGFR, PDGFR, and VEGFR) and NRTKs (such as SRC) are very important in the carcinogenesis and progression of PCa, and are potential targets for the treatment of PCa.497,498 Signal transductions through ligands binding with RTKs or stimulation of unique NRTKs lead to cross-phosphorylation of specific tyrosine residues, which activate downstream signaling such as PI3K/AKT, phospholipase C, and Janus tyrosine kinase.499,500 The signaling subsequently regulates the transcription of genes involved in proliferation, survival and differentiation.499
Fibroblast growth factor (FGF) receptors (FGFR1/4) and their ligands (FGF1/2/4/8/17) are overexpressed in PCa.501–504 Enhanced FGF signaling leads to tumor progression, angiogenesis, epithelial-to-mesenchymal transition (EMT), and upregulation of AR.505–507 Moreover, FGF/FGFR activation is highly correlated with AR-null PCa and drug resistance.508 Therefore, inhibition of the FGF axis may be a viable strategy for the treatment of PCa. Erdafitinib (JNJ-42756493),509 a small oral molecule that works against all four FGFR family members, is being tested in a phase 2 clinical study (Table 10) of AR-null and neuroendocrine-null PCa, termed double-negative prostate cancer (DNPC).
EGFR mutation or overexpression leads to malignant progression in many cancer types.510 In PCa, increased expression of EGFR correlates with a high Gleason score and advanced stage disease,511 and activation of EGFR promotes metastatic progression and recurrence.512 EGFR inhibitors such as gefitinib (a small-molecule compound) and cetuximab (a monoclonal antibody) have been widely used in metastatic colorectal cancer and non-small cell lung cancer. In a phase 2 clinical trial, gefitinib did not exhibit any response to PSA or objective measurable disease in patients with CRPC.510 However, in another trial, cetuximab resulted in an obvious PSA decline in many patients, and improved PFS was found in patients with EGFR overexpression.513
The SRC signaling pathway has many effects on tumorigenesis and tumor progression.73,498 In PCa, SRC activity is highly increased in bone metastasis.73,514–516 Encouraging results in preclinical studies of PCa led to several clinical studies of SRC inhibitors, such as dasatinib (BMS-354825)517,518 and saracatinib (AZD0530).519,520 However, a phase 3 study of docetaxel plus dasatinib did not improve the overall survival in patients with mCRPC, despite the fact that the PSA progression time was delayed.518 Similar results were found in the trials of abiraterone plus dasatinib.517,521 A phase 2 study of saracatinib also failed to achieve satisfactory oncological outcomes, while adverse events were observed, such as dehydration, thrombocytopenia, and weakness.519
Moreover, multitargeted tyrosine kinase inhibitors, such as dovitinib (TKI258),522 sunitinib (SU11248),523,524 cabozantinib (XL184),525 and masitinib (AB1010)526, have entered clinical trials for PCa (Table 10). For example, dovitinib, an oral multitargeted RTK inhibitor, potently inhibits FGFR1/2/3, VEGFR1/2/3, PDGFR, and KIT.522 A phase 2 clinical study found that dovitinib showed a better response in chemotherapy-naive patients and patients with high serum VEGFR2.522 Finally, cabozantinib,525 a small-molecule inhibitor of c-MET and VEGFR2, has been evaluated in two phase 3 clinical trials, which reported no survival benefit of cabozantinib, suggesting that further rational development may be justified.527–529
MEK
Aberrant activation of mitogen-activated protein kinase (MAPK) signaling resulting from upstream activating mutations, such as the rat sarcoma viral oncogene homolog (RAS), v-raf-1 murine leukemia viral oncogene homolog (RAF), and growth factors, converges with MEK. Increased activation of the RAS/RAF/MEK/extracellular signal-regulated kinase (ERK) pathway has been associated with poor prognosis and androgen independence in advanced PCa.508,530,531 Increased expression of MAPK pathway members and high levels of phosphorylated ERK1/2 were observed in mCRPCs.532–535 Pharmacological targeting of the MEK/ERK pathway may be a viable strategy for patients with mCRPC, and the MEK1/2 inhibitor trametinib (GSK1120212) is currently being tested in a phase 2 trial of patients with mCRPC (Table 10).
Modulation of alternative splicing
The vast majority of human genes (>95%) are alternatively spliced by the spliceosome, which enables genes to express splice isoforms that often exhibit distinctive functions.75,536 Alternative splicing affects genes (such as FGFR, ERG, VEGFA, and AR) that are clearly linked to the etiology of PCa, allowing us to develop novel targeted therapies that modulate alternative splicing for the treatment of PCa. For example, alternative splicing of FGFR2 leads to the expression of FGFR2(IIIb) and FGFR2(IIIc) isoforms, whereas the IIIb isoform is specific to epithelial cells, while the IIIc isoform is exclusively expressed in most mesenchymal cells. Therefore, this splicing switch is a sensor of the EMT that results in tumor growth and/or metastasis.537 Using the FGFR2-based splicing reporter as a readout, a recent preclinical study identified compounds such as nemadipine-A (a T-type calcium channel inhibitor), NNC-55-0396 dihydrochloride (a L-type Ca channel inhibitor), and naltrexone hydrochloride (an opioid antagonist) that change FGFR2 splicing and induce an epithelial phenotype in PCa cells.538 Moreover, the ERG oncogene is fused with the TMPRSS2 promoter in 50% of PCa cases, and the fusion most often occurs between the ERG exon 4 and exons 1 or 2 of TMPRSS2.539 Interestingly, a novel oligonucleotide-based agent that targets ERG by inducing skipping of its constitutive exon 4 resulted in a reduction in ERG protein levels and tumor growth in mice.540 Although these agents have not entered clinical practice yet, splicing events are a plausible mechanism for treating PCa, and further research is warranted.
Future directions and conclusions
The arsenal of drugs available to treat advanced PCa has expanded significantly in recent years. Despite these advances, current treatment options have many limitations (Table 11), and more personalized treatment strategies and targeted agents are required for novel therapeutic options. In most cases, AR continues to be the primary molecular driver in CRPC patients, while effective therapies targeting AR are not always curative.541 AR overexpression, AR amplification, AR mutations, and AR variants (AR-Vs) are important mechanisms that contribute to drug resistance.143,542,543 Of note, drug resistance mediated by AR-Vs continues to be an issue, as it is very difficult to directly target AR-Vs.541,544 Despite the loss of AR-LBD, AR-Vs contain AR-DBD and are capable of transcriptional regulation. To date, AR-V7 is the type of AR-V most frequently detected in CRPC.545,546 AR-V7 was found to contribute to resistance to enzalutamide and abiraterone in PCa patients.547 In addition, other studies have demonstrated that other AR-Vs such as AR-V1, AR-V3, AR-V9, and ARv567es confer anti-AR drug resistance.548,549 Thus, further development of AR-V-targeted drugs such as AR-DBD or AR-NTD is required,550,551 since these domains are shared between full-length AR, LBD-mutant AR, and AR-Vs.
Table 11.
Promises and limitations of therapeutic targets for PCa
| Target | Promises | Limitations |
|---|---|---|
| AR signaling |
• Backbone of systemic therapy • Multiple lines of treatment options available • Survival benefits |
• Inevitability of castration resistance • Sexual dysfunction • Other complications such as increase fat mass |
| Bone microenvironment | • Prevention of skeletal-related events |
• No survival benefits • Dental complications • Risk of hypocalcemia |
| PSMA |
• High specificity • Diagnostic and therapeutic options available |
• Issues of accessibility • PSMA PET might lead to inappropriate changes in PCa management or trial participation |
| DNA repair | • Promising response for selected patients |
• Low incidence of DNA repair gene alterations in PCa • Unsubstantiated benefit for DNA repair gene alterations other than BRCA1/2 and ATM • Lack of evidence for OS benefit |
| Immune checkpoints | • Possibility for long-term cancer remission |
• Generally poor response due to immunologically ‘cold’ tumor microenvironment • Eligible for very few PCa patients |
| Cell cycle | • Opportunity for synergistic success |
• Limited clinical trials • Questionable safety profile |
| PI3K/AKT/mTOR signaling | • Opportunity for synergistic success |
• Limited clinical trials • Biomarkers needed for patient selection |
| Epigenetic marks | • Mechanistically novel and promising |
• Questionable safety profile • Limited efficacy |
| WNT signaling | • Mechanistically promising for late stage or refractory PCa |
• Bone-related toxicity • Limited clinical trials |
Remarkably, the number of patients with distant metastasis is rapidly increasing and shows no signs of cessation. Poor clinical outcomes are often observed following prolonged use of potent AR-targeted therapy, which is highly associated with treatment-induced neuroendocrine PCa (NEPC).552 Most NEPCs exhibit AR-indifference, drug resistance, and poor clinical outcomes553, as well as have high levels of genomic alterations, such as loss of function mutations of PTEN, RB1, and TP53.554,555 Moreover, other phenotypes, such as the double-negative (AR-null and NE-null) PCa or AR noncanonical function-dependent PCa also occur in patients,508,556 prompting investigators to examine oncogenic signaling pathways that drive these progressive and drug-resistant PCa. Several new pathways and key molecules have been identified, including GR, GATA2, IGF2, ONECUT2, POM121, AURKA, N-Myc, HP1α, PEG10, SRRM4, BRN2, SOX2, and PRMT5, which could be effective therapeutic targets for PCa.365,557–566
Notably, the pioneer factor FOXA1 mutation and overexpression could be an important factor in PCa.406,567–573 Gain-of-function mutations of FOXA1 frequently occur in up to 9% and 13% of primary PCa and mCRPC cases, respectively.569,573 A previous study identified FOXA1 single nucleotide variants in approximately 25% of the NEPC cases.574 Interestingly, a whole genome sequence study of PCa found that the rate of FOXA1 mutation in Asian populations (~40%) is significantly higher than in western cohorts (~8%).95 Importantly, FOXA1 overexpression also suppresses the immune response, resulting in therapeutic resistance of ICIs in PCa.348 While it is well known that FOXA1 is very difficult to target, the proteolysis targeting chimeras (PROTACs), such as oligonucleotide-based PROTACs (O’PROTACs),575 present a new opportunity to develop novel anticancer agents targeting FOXA1 and its mutations.
More prospective clinical trials are urgently required for the optimization of treatment sequences and drug combinations that can delay resistance or decrease drug toxicities. Interestingly, a phase 2 study demonstrated that the use of abiraterone followed by enzalutamide led to a longer PSA progression-free interval compared with the reverse sequence.576 Results from a phase 1b trial that evaluated the combination of cabozantinib (a multityrosine kinase inhibitor) and atezolizumab (a PD-L1 inhibitor) in high-risk PCa are encouraging.577 Therefore, more prospective clinical studies regarding treatment sequences and drug combinations are required, including a combination of the new approved agent 177Lu-PSMA-617 either with PARP inhibitors or with AR signaling inhibitors, as well as with chemotherapy in the PSMA-positive population. Moreover, future studies should include a combination of EZH2 inhibitors with PARP inhibitors, tyrosine kinase inhibitors, CDK4/6 inhibitors, CDK7/9 inhibitors, AKT inhibitors, MEK inhibitors, CBP/p300 inhibitors, AURKA inhibitors, or chemotherapy for aggressive AR-negative PCa or NEPC.
PCa is an immune “cold” tumor that remains unresponsive to ICIs. Future directions also include the development of new immunotherapy agents or combinations that improve the efficacy of immunotherapy in PCa, including the combination of ICIs with inhibitors that target myeloid-derived suppressor cells (MDSC),578,579 cancer-associated fibroblasts (CAF),580 hypoxia,581 or other cancer immune response suppression factors such as KDM5B, PTPN2, SOCS1, ADAR1, MYC, and integrin αvβ6.361,582–587 PCa remains a complex and severe health issue globally, but technological advances, such as genomic sequencing and predictive algorithms, are constantly improving our understanding of the biology of this disease, thus facilitating the discovery of drugs with the best function and minimal toxicity. These advances are expected to promote precision medicine and improve clinical outcomes of PCa in the future.
Acknowledgements
This work was supported by grants from the Science and Technology Commission of Shanghai Municipality (18410750200 to R.S.), National Natural Science Foundation of China (82125025 to R.S., 81872105 to R.S.), and National Major R&D Program (2017YFC0908002).
Author contributions
H.Y. and R.S. were responsible for the search strategy and overall structure of the paper. H.Y. and X.Y.-T. performed the literature search and wrote the paper. H.Y. plotted the figures. R.S., X.W., G.D., and H.H. contributed to the content of the paper and revised the paper. All authors have read and approved the final paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yundong He, Weidong Xu, Yu-Tian Xiao
Contributor Information
Yundong He, Email: ydhe@bio.ecnu.edu.cn.
Di Gu, Email: sveong@163.com.
Shancheng Ren, Email: renshancheng@gmail.com.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Disease GBD, Injury I, Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foreman KJ, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandhu S, et al. Prostate cancer. Lancet. 2021;398:1075–1090. doi: 10.1016/S0140-6736(21)00950-8. [DOI] [PubMed] [Google Scholar]
- 5.Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J. Am. Board Fam. Pract. 2003;16:95–101. doi: 10.3122/jabfm.16.2.95. [DOI] [PubMed] [Google Scholar]
- 6.Halpern JA, et al. Use of digital rectal examination as an adjunct to prostate specific antigen in the detection of clinically significant prostate cancer. J. Urol. 2018;199:947–953. doi: 10.1016/j.juro.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972;22:232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 9.Ku SY, Gleave ME, Beltran H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 2019;16:645–654. doi: 10.1038/s41585-019-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 11.Klotz L, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J. Clin. Oncol. 2015;33:1151–1156. doi: 10.1200/JCO.2014.58.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan CJ, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 14.Beer TM, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MR, et al. Apalutamide treatment and Metastasis-free Survival in Prostate Cancer. N. Engl. J. Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 16.Fizazi K, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 17.Parker C, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 18.Saad F, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–1316. doi: 10.1016/S1470-2045(16)30173-5. [DOI] [PubMed] [Google Scholar]
- 19.James ND, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vale CL, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17:243–256. doi: 10.1016/S1470-2045(15)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MR, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fizazi K, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateo J, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bono J, et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020;382:2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 25.Hussain M, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2020;383:2345–2357. doi: 10.1056/NEJMoa2022485. [DOI] [PubMed] [Google Scholar]
- 26.Abida W, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J. Clin. Oncol. 2020;38:3763–3772. doi: 10.1200/JCO.20.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Bono JS, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22:1250–1264. doi: 10.1016/S1470-2045(21)00376-4. [DOI] [PubMed] [Google Scholar]
- 28.Beer TM, et al. Randomized, double-blind, phase III trial of Ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 29.Sharma P, et al. Nivolumab plus Ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell. 2020;38:489–499.e483. doi: 10.1016/j.ccell.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Shenderov E, et al. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: a phase-2 nonrandomized clinical trial. Prostate. 2021;81:326–338. doi: 10.1002/pros.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karzai F, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer. 2018;6:141. doi: 10.1186/s40425-018-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan EM, et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur. Urol. 2022;81:253–262. doi: 10.1016/j.eururo.2021.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Silver DA, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 34.Perner S, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007;38:696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Schmidkonz C, et al. (99m) Tc-MIP-1404-SPECT/CT for the detection of PSMA-positive lesions in 225 patients with biochemical recurrence of prostate cancer. Prostate. 2018;78:54–63. doi: 10.1002/pros.23444. [DOI] [PubMed] [Google Scholar]
- 36.Hofman MS, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 37.Rahbar K, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 38.Baum RP, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J. Nucl. Med. 2016;57:1006–1013. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 39.Hofman MS, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 40.Autio KA, et al. Safety and rfficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 2018;4:1344–1351. doi: 10.1001/jamaoncol.2018.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thang SP, et al. Clinical outcomes of 177lutetium-prostate-specific membrane antigen therapy in advanced prostate cancer-a prospective pilot study in an Asian population. Nucl. Med Commun. 2020;41:618–628. doi: 10.1097/MNM.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 42.Kloss CC, et al. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 2018;26:1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calais J, et al. (18)F-fluciclovine PET-CT and (68)Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–1294. doi: 10.1016/S1470-2045(19)30415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Violet J, et al. Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of (177)Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J. Nucl. Med. 2020;61:857–865. doi: 10.2967/jnumed.119.236414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tagawa ST, et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 ((177) Lu-J591) for metastatic castration-resistant prostate cancer. Cancer. 2019;125:2561–2569. doi: 10.1002/cncr.32072. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong AJ, et al. A phase II trial of temsirolimus in men with castration-resistant metastatic prostate cancer. Clin. Genitourin. Cancer. 2013;11:397–406. doi: 10.1016/j.clgc.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Graham L, et al. A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer. Investig. N. Drugs. 2018;36:458–467. doi: 10.1007/s10637-018-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chow H, et al. A phase 2 clinical trial of everolimus plus bicalutamide for castration-resistant prostate cancer. Cancer. 2016;122:1897–1904. doi: 10.1002/cncr.29927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Bono JS, et al. Randomized phase II study evaluating akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 2019;25:928–936. doi: 10.1158/1078-0432.CCR-18-0981. [DOI] [PubMed] [Google Scholar]
- 50.Massard C, et al. Phase Ib dose-finding study of abiraterone acetate plus buparlisib (BKM120) or dactolisib (BEZ235) in patients with castration-resistant prostate cancer. Eur. J. Cancer. 2017;76:36–44. doi: 10.1016/j.ejca.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Wei XX, et al. A phase I study of abiraterone acetate combined with BEZ235, a dual PI3K/mTOR inhibitor, in metastatic castration resistant prostate cancer. Oncologist. 2017;22:503–e543. doi: 10.1634/theoncologist.2016-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narayan V, et al. Phase 1 trial of everolimus and radiation therapy for salvage treatment of biochemical recurrence in prostate cancer patients following prostatectomy. Int J. Radiat. Oncol. Biol. Phys. 2017;97:355–361. doi: 10.1016/j.ijrobp.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Nowacka-Zawisza M, Wisnik E. DNA methylation and histone modifications as epigenetic regulation in prostate cancer (Review) Oncol. Rep. 2017;38:2587–2596. doi: 10.3892/or.2017.5972. [DOI] [PubMed] [Google Scholar]
- 54.Kumaraswamy A, et al. Recent advances in epigenetic biomarkers and epigenetic targeting in prostate cancer. Eur. Urol. 2021;80:71–81. doi: 10.1016/j.eururo.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rathkopf DE, et al. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2013;72:537–544. doi: 10.1007/s00280-013-2224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheler JJ, et al. Phase I study of anti-VEGF monoclonal antibody bevacizumab and histone deacetylase inhibitor valproic acid in patients with advanced cancers. Cancer Chemother. Pharmacol. 2014;73:495–501. doi: 10.1007/s00280-014-2384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singal R, et al. Phase I/II study of azacitidine, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel-based therapy. Clin. Genitourin. Cancer. 2015;13:22–31. doi: 10.1016/j.clgc.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Eigl BJ, et al. A phase II study of the HDAC inhibitor SB939 in patients with castration resistant prostate cancer: NCIC clinical trials group study IND195. Investig. N. Drugs. 2015;33:969–976. doi: 10.1007/s10637-015-0252-4. [DOI] [PubMed] [Google Scholar]
- 59.Lewin J, et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J. Clin. Oncol. 2018;36:3007–3014. doi: 10.1200/JCO.2018.78.2292. [DOI] [PubMed] [Google Scholar]
- 60.Ferrari AC, et al. Epigenetic therapy with panobinostat combined with bicalutamide rechallenge in castration-resistant prostate cancer. Clin. Cancer Res. 2019;25:52–63. doi: 10.1158/1078-0432.CCR-18-1589. [DOI] [PubMed] [Google Scholar]
- 61.Piha-Paul SA, et al. First-in-human study of mivebresib (ABBV-075), an oral pan-inhibitor of bromodomain and extra terminal proteins, in patients with relapsed/refractory solid tumors. Clin. Cancer Res. 2019;25:6309–6319. doi: 10.1158/1078-0432.CCR-19-0578. [DOI] [PubMed] [Google Scholar]
- 62.Aggarwal RR, et al. A phase Ib/IIa study of the Pan-BET inhibitor ZEN-3694 in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2020;26:5338–5347. doi: 10.1158/1078-0432.CCR-20-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kase AM, Copland Iii JA, Tan W. Novel therapeutic strategies for CDK4/6 inhibitors in metastatic castrate-resistant prostate cancer. Onco Targets Ther. 2020;13:10499–10513. doi: 10.2147/OTT.S266085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumari S, et al. Therapeutic potential of p53 reactivation in prostate cancer: Strategies and opportunities. Eur. J. Pharmacol. 2022;919:174807. doi: 10.1016/j.ejphar.2022.174807. [DOI] [PubMed] [Google Scholar]
- 65.Murillo-Garzon V, Kypta R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017;14:683–696. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- 66.Melegh Z, Oltean S. Targeting angiogenesis in prostate cancer. Int. J. Mol. Sci. 2019;20:2676. doi: 10.3390/ijms20112676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fizazi K, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2013;31:1740–1747. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- 68.Corn PG, Wang F, McKeehan WL, Navone N. Targeting fibroblast growth factor pathways in prostate cancer. Clin. Cancer Res. 2013;19:5856–5866. doi: 10.1158/1078-0432.CCR-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin SR, Yeh HL, Liu YN. Interplay of epidermal growth factor receptor and signal transducer and activator of transcription 3 in prostate cancer: beyond androgen receptor transactivation. Cancers. 2021;13:3452. doi: 10.3390/cancers13143452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chau V, Madan RA, Aragon-Ching JB. Protein kinase inhibitors for the treatment of prostate cancer. Expert Opin. Pharmacother. 2021;22:1889–1899. doi: 10.1080/14656566.2021.1925250. [DOI] [PubMed] [Google Scholar]
- 71.Odero-Marah V, Hawsawi O, Henderson V, Sweeney J. Epithelial-mesenchymal transition (EMT) and prostate cancer. Adv. Exp. Med Biol. 2018;1095:101–110. doi: 10.1007/978-3-319-95693-0_6. [DOI] [PubMed] [Google Scholar]
- 72.Ahel J, Hudorovic N, Vicic-Hudorovic V, Nikles H. Tgf-beta in the natural history of prostate cancer. Acta Clin. Croat. 2019;58:128–138. doi: 10.20471/acc.2019.58.01.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varkaris A, et al. Src signaling pathways in prostate cancer. Cancer Metastasis Rev. 2014;33:595–606. doi: 10.1007/s10555-013-9481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nickols NG, et al. MEK-ERK signaling is a therapeutic target in metastatic castration resistant prostate cancer. Prostate Cancer Prostatic Dis. 2019;22:531–538. doi: 10.1038/s41391-019-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paschalis A, et al. Alternative splicing in prostate cancer. Nat. Rev. Clin. Oncol. 2018;15:663–675. doi: 10.1038/s41571-018-0085-0. [DOI] [PubMed] [Google Scholar]
- 76.Sfanos KS, Yegnasubramanian S, Nelson WG, De Marzo AM. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018;15:11–24. doi: 10.1038/nrurol.2017.167. [DOI] [PubMed] [Google Scholar]
- 77.de Bono JS, et al. Prostate carcinogenesis: inflammatory storms. Nat. Rev. Cancer. 2020;20:455–469. doi: 10.1038/s41568-020-0267-9. [DOI] [PubMed] [Google Scholar]
- 78.Mucci LA, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA. 2016;315:68–76. doi: 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am. J. Public Health. 2010;100:693–701. doi: 10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao J, Stockwell T, Roemer A, Chikritzhs T. Is alcohol consumption a risk factor for prostate cancer? A systematic review and meta-analysis. BMC Cancer. 2016;16:845. doi: 10.1186/s12885-016-2891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al Moustafa AE. Involvement of human papillomavirus infections in prostate cancer progression. Med Hypotheses. 2008;71:209–211. doi: 10.1016/j.mehy.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 82.Taylor ML, Mainous AG, 3rd, Wells BJ. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam. Med. 2005;37:506–512. [PubMed] [Google Scholar]
- 83.Parekh N, et al. Obesity and prostate cancer detection: insights from three national surveys. Am. J. Med. 2010;123:829–835. doi: 10.1016/j.amjmed.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aronson WJ, et al. Growth inhibitory effect of low fat diet on prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J. Urol. 2010;183:345–350. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schultz C, Meier M, Schmid HP. Nutrition, dietary supplements and adenocarcinoma of the prostate. Maturitas. 2011;70:339–342. doi: 10.1016/j.maturitas.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 86.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 87.Shimizu H, et al. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br. J. Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fraser M, et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature. 2017;541:359–364. doi: 10.1038/nature20788. [DOI] [PubMed] [Google Scholar]
- 89.Blee AM, et al. TMPRSS2-ERG controls luminal epithelial lineage and antiandrogen sensitivity in PTEN and TP53-mutated prostate cancer. Clin. Cancer Res. 2018;24:4551–4565. doi: 10.1158/1078-0432.CCR-18-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cancer Genome Atlas Research, N. The Molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stopsack KH, et al. Oncogenic genomic alterations, clinical phenotypes, and outcomes in metastatic castration-sensitive prostate cancer. Clin. Cancer Res. 2020;26:3230–3238. doi: 10.1158/1078-0432.CCR-20-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abida W, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA. 2019;116:11428–11436. doi: 10.1073/pnas.1902651116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, et al. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature. 2020;580:93–99. doi: 10.1038/s41586-020-2135-x. [DOI] [PubMed] [Google Scholar]
- 96.Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;162:454. doi: 10.1016/j.cell.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 97.Beltran H, et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin. Cancer Res. 2019;25:6916–6924. doi: 10.1158/1078-0432.CCR-19-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016;12:452–466. doi: 10.1038/nrendo.2016.70. [DOI] [PubMed] [Google Scholar]
- 99.Corradi PF, Corradi RB, Greene LW. Physiology of the hypothalamic pituitary gonadal axis in the male. Urol. Clin. North Am. 2016;43:151–162. doi: 10.1016/j.ucl.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 100.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr. Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 101.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J. Clin. Oncol. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 102.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J. Urol. 2002;168:9–12. doi: 10.1016/S0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 103.Moussa M, et al. Current and emerging gonadotropin-releasing hormone (GnRH) antagonists for the treatment of prostate cancer. Expert Opin. Pharmacother. 2021;22:2373–2381. doi: 10.1080/14656566.2021.1948012. [DOI] [PubMed] [Google Scholar]
- 104.Davies AH, Zoubeidi A. Targeting androgen receptor signaling: a historical perspective. Endocr. Relat. Cancer. 2021;28:T11–T18. doi: 10.1530/ERC-21-0116. [DOI] [PubMed] [Google Scholar]
- 105.Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017;7:a030452. doi: 10.1101/cshperspect.a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin. Cancer Res. 2011;17:3876–3883. doi: 10.1158/1078-0432.CCR-10-2815. [DOI] [PubMed] [Google Scholar]
- 107.Velho PI, Bastos DA, Antonarakis ES. New approaches to targeting the androgen receptor pathway in prostate cancer. Clin. Adv. Hematol. Oncol. 2021;19:228–240. [PubMed] [Google Scholar]
- 108.Fujita K, Nonomura N. Role of androgen receptor in prostate cancer: a review. World J. Mens. Health. 2019;37:288–295. doi: 10.5534/wjmh.180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–5511. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saylor PJ. Prostate cancer: the androgen receptor remains front and centre. Nat. Rev. Clin. Oncol. 2013;10:126–128. doi: 10.1038/nrclinonc.2013.14. [DOI] [PubMed] [Google Scholar]
- 111.Crnalic S, et al. Nuclear androgen receptor staining in bone metastases is related to a poor outcome in prostate cancer patients. Endocr. Relat. Cancer. 2010;17:885–895. doi: 10.1677/ERC-10-0059. [DOI] [PubMed] [Google Scholar]
- 112.Ruizeveld de Winter JA, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am. J. Pathol. 1994;144:735–746. [PMC free article] [PubMed] [Google Scholar]
- 113.Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat. Clin. Pr. Oncol. 2007;4:236–244. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 114.McLeod DG, et al. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU Int. 2006;97:247–254. doi: 10.1111/j.1464-410X.2005.06051.x. [DOI] [PubMed] [Google Scholar]
- 115.Iversen P, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. J. Urol. 2000;164:1579–1582. doi: 10.1016/S0022-5347(05)67032-2. [DOI] [PubMed] [Google Scholar]
- 116.Akaza H, et al. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115:3437–3445. doi: 10.1002/cncr.24395. [DOI] [PubMed] [Google Scholar]
- 117.Eisenberger MA, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N. Engl. J. Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 118.Fossa SD, et al. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. J. Clin. Oncol. 2001;19:62–71. doi: 10.1200/JCO.2001.19.1.62. [DOI] [PubMed] [Google Scholar]
- 119.Dijkman GA, Janknegt RA, De Reijke TM, Debruyne FM. Long-term efficacy and safety of nilutamide plus castration in advanced prostate cancer, and the significance of early prostate specific antigen normalization. International Anandron Study Group. J. Urol. 1997;158:160–163. doi: 10.1097/00005392-199707000-00051. [DOI] [PubMed] [Google Scholar]
- 120.Kassouf W, Tanguay S, Aprikian AG. Nilutamide as second line hormone therapy for prostate cancer after androgen ablation fails. J. Urol. 2003;169:1742–1744. doi: 10.1097/01.ju.0000057795.97626.66. [DOI] [PubMed] [Google Scholar]
- 121.Tran C, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hussain M, et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Davis ID, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 124.Sternberg CN, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 2020;382:2197–2206. doi: 10.1056/NEJMoa2003892. [DOI] [PubMed] [Google Scholar]
- 125.Clegg NJ, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van de Wijngaart DJ, et al. Systematic structure-function analysis of androgen receptor Leu701 mutants explains the properties of the prostate cancer mutant L701H. J. Biol. Chem. 2010;285:5097–5105. doi: 10.1074/jbc.M109.039958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goldstein A, et al. Detection fidelity of AR mutations in plasma derived cell-free DNA. Oncotarget. 2017;8:15651–15662. doi: 10.18632/oncotarget.14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Romanel A, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci. Transl. Med. 2015;7:312re310. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.LaTulippe E, et al. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002;62:4499–4506. [PubMed] [Google Scholar]
- 130.Shore ND. Darolutamide (ODM-201) for the treatment of prostate cancer. Expert Opin. Pharmacother. 2017;18:945–952. doi: 10.1080/14656566.2017.1329820. [DOI] [PubMed] [Google Scholar]
- 131.Moilanen AM, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci. Rep. 2015;5:12007. doi: 10.1038/srep12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gottlieb B, et al. The androgen receptor gene mutations database: 2012 update. Hum. Mutat. 2012;33:887–894. doi: 10.1002/humu.22046. [DOI] [PubMed] [Google Scholar]
- 133.Rathkopf DE, et al. Androgen receptor mutations in patients with castration-resistant prostate cancer treated with apalutamide. Ann. Oncol. 2017;28:2264–2271. doi: 10.1093/annonc/mdx283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borgmann H, et al. Moving towards precision urologic oncology: targeting enzalutamide-resistant prostate cancer and mutated forms of the androgen receptor using the novel inhibitor darolutamide (ODM-201) Eur. Urol. 2018;73:4–8. doi: 10.1016/j.eururo.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 135.Fizazi K, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N. Engl. J. Med. 2020;383:1040–1049. doi: 10.1056/NEJMoa2001342. [DOI] [PubMed] [Google Scholar]
- 136.Gadd MS, et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat. Chem. Biol. 2017;13:514–521. doi: 10.1038/nchembio.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun X, Rao Y. PROTACs as potential therapeutic agents for cancer drug resistance. Biochemistry. 2020;59:240–249. doi: 10.1021/acs.biochem.9b00848. [DOI] [PubMed] [Google Scholar]
- 138.Mullard A. Targeted degraders clear first safety hurdles. Nat. Rev. Drug Discov. 2020;19:435. doi: 10.1038/d41573-020-00109-w. [DOI] [PubMed] [Google Scholar]
- 139.Hu R, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang X, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS ONE. 2011;6:e27970. doi: 10.1371/journal.pone.0027970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun S, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Investig. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Y, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Antonarakis ES, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schally AV, et al. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173:1036–1038. doi: 10.1126/science.173.4001.1036. [DOI] [PubMed] [Google Scholar]
- 145.Guillemin R, Burgus R. The hormones of the hypothalamus. Sci. Am. 1972;227:24–33. doi: 10.1038/scientificamerican1172-24. [DOI] [PubMed] [Google Scholar]
- 146.Conn PM, Crowley WF., Jr. Gonadotropin-releasing hormone and its analogs. Annu Rev. Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 147.Tzoupis H, Nteli A, Androutsou ME, Tselios T. Gonadotropin-releasing hormone and GnRH receptor: structure, function and drug development. Curr. Med Chem. 2020;27:6136–6158. doi: 10.2174/0929867326666190712165444. [DOI] [PubMed] [Google Scholar]
- 148.Fontana F, et al. Gonadotropin-releasing hormone receptors in prostate cancer: molecular aspects and biological functions. Int. J. Mol. Sci. 2020;21:9511. doi: 10.3390/ijms21249511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stopa EG, et al. Computer-assisted mapping of immunoreactive mammalian gonadotropin-releasing hormone in adult human basal forebrain and amygdala. Endocrinology. 1991;128:3199–3207. doi: 10.1210/endo-128-6-3199. [DOI] [PubMed] [Google Scholar]
- 150.Seeburg PH, Mason AJ, Stewart TA, Nikolics K. The mammalian GnRH gene and its pivotal role in reproduction. Recent Prog. Horm. Res. 1987;43:69–98. doi: 10.1016/b978-0-12-571143-2.50008-3. [DOI] [PubMed] [Google Scholar]
- 151.Maggi R, et al. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum. Reprod. Update. 2016;22:358–381. doi: 10.1093/humupd/dmv059. [DOI] [PubMed] [Google Scholar]
- 152.Tolis G, et al. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc. Natl Acad. Sci. U.S.A. 1982;79:1658–1662. doi: 10.1073/pnas.79.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Engel JB, Schally AV. Drug Insight: clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:157–167. doi: 10.1038/ncpendmet0399. [DOI] [PubMed] [Google Scholar]
- 154.Bolla M, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N. Engl. J. Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 155.Stolley PD, et al. Three-area epidemiological study of geographic differences in stroke mortality. II. Results Stroke. 1977;8:551–557. doi: 10.1161/01.STR.8.5.551. [DOI] [PubMed] [Google Scholar]
- 156.Ahmann FR, et al. Zoladex: a sustained-release, monthly luteinizing hormone-releasing hormone analogue for the treatment of advanced prostate cancer. J. Clin. Oncol. 1987;5:912–917. doi: 10.1200/JCO.1987.5.6.912. [DOI] [PubMed] [Google Scholar]
- 157.in LiverTox: Clinical and Research Information on Drug-Induced Liver Injury (2012). [PubMed]
- 158.Schlegel PN, et al. Effective long-term androgen suppression in men with prostate cancer using a hydrogel implant with the GnRH agonist histrelin. Urology. 2001;58:578–582. doi: 10.1016/S0090-4295(01)01293-6. [DOI] [PubMed] [Google Scholar]
- 159.Schlegel PN, Histrelin Study, G. Efficacy and safety of histrelin subdermal implant in patients with advanced prostate cancer. J. Urol. 2006;175:1353–1358. doi: 10.1016/S0022-5347(05)00649-X. [DOI] [PubMed] [Google Scholar]
- 160.Chertin B, et al. An implant releasing the gonadotropin hormone-releasing hormone agonist histrelin maintains medical castration for up to 30 months in metastatic prostate cancer. J. Urol. 2000;163:838–844. doi: 10.1016/S0022-5347(05)67816-0. [DOI] [PubMed] [Google Scholar]
- 161.Shore N, Cookson MS, Gittelman MC. Long-term efficacy and tolerability of once-yearly histrelin acetate subcutaneous implant in patients with advanced prostate cancer. BJU Int. 2012;109:226–232. doi: 10.1111/j.1464-410X.2011.10370.x. [DOI] [PubMed] [Google Scholar]
- 162.Roila F. Buserelin in the treatment of prostatic cancer. Biomed. Pharmacother. 1989;43:279–285. doi: 10.1016/0753-3322(89)90009-7. [DOI] [PubMed] [Google Scholar]
- 163.Kuhn JM, et al. Prevention of the transient adverse effects of a gonadotropin-releasing hormone analogue (buserelin) in metastatic prostatic carcinoma by administration of an antiandrogen (nilutamide) N. Engl. J. Med. 1989;321:413–418. doi: 10.1056/NEJM198908173210701. [DOI] [PubMed] [Google Scholar]
- 164.Klign JG, de Voogt HJ, Schroder FH, de Jong FH. Combined treatment with buserelin and cyproterone acetate in metastatic prostatic carcinoma. Lancet. 1985;2:493. doi: 10.1016/S0140-6736(85)90415-5. [DOI] [PubMed] [Google Scholar]
- 165.Volmer MC, Lascano A, Poggi U, Arguelles AE. Lung metastases of prostate carcinoma cleared by intranasal GRH analogue. Lancet. 1985;1:1507. doi: 10.1016/S0140-6736(85)92279-2. [DOI] [PubMed] [Google Scholar]
- 166.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001–1006. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]
- 167.Frampton JE, Lyseng-Williamson KA. Degarelix. Drugs. 2009;69:1967–1976. doi: 10.2165/10484080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 168.McLeod D, et al. A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology. 2001;58:756–761. doi: 10.1016/S0090-4295(01)01342-5. [DOI] [PubMed] [Google Scholar]
- 169.Garnick MB, Mottet N. New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. BJU Int. 2012;110:499–504. doi: 10.1111/j.1464-410X.2011.10708.x. [DOI] [PubMed] [Google Scholar]
- 170.Trachtenberg J, et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J. Urol. 2002;167:1670–1674. doi: 10.1097/00005392-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 171.Moul JW. Utility of LHRH antagonists for advanced prostate cancer. Can. J. Urol. 2014;21:22–27. [PubMed] [Google Scholar]
- 172.Klotz L, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 173.Crawford ED, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J. Urol. 2011;186:889–897. doi: 10.1016/j.juro.2011.04.083. [DOI] [PubMed] [Google Scholar]
- 174.Sawazaki H, Araki D, Kitamura Y, Yagi K. Metabolic changes with degarelix vs leuprolide plus bicalutamide in patients with prostate cancer: a randomized clinical study. World J. Urol. 2020;38:1465–1471. doi: 10.1007/s00345-019-02937-x. [DOI] [PubMed] [Google Scholar]
- 175.Damber JE, et al. The effect of baseline testosterone on the efficacy of degarelix and leuprolide: further insights from a 12-month, comparative, phase III study in prostate cancer patients. Urology. 2012;80:174–180. doi: 10.1016/j.urology.2012.01.092. [DOI] [PubMed] [Google Scholar]
- 176.Boccon-Gibod L, et al. Degarelix as an intermittent androgen deprivation therapy for one or more treatment cycles in patients with prostate cancer. Eur. Urol. 2014;66:655–663. doi: 10.1016/j.eururo.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 177.Tombal B, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur. Urol. 2010;57:836–842. doi: 10.1016/j.eururo.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 178.Shore ND, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N. Engl. J. Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 179.Dearnaley DP, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur. Urol. 2020;78:184–192. doi: 10.1016/j.eururo.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 180.Sayyid RK, et al. A phase II, randomized, open-label study of neoadjuvant degarelix versus LHRH agonist in prostate cancer patients prior to radical prostatectomy. Clin. Cancer Res. 2017;23:1974–1980. doi: 10.1158/1078-0432.CCR-16-1790. [DOI] [PubMed] [Google Scholar]
- 181.Levine GN, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smith MR, et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J. Clin. Oncol. 2012;30:3271–3276. doi: 10.1200/JCO.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cereda V, et al. Hormonal prostate cancer therapies and cardiovascular disease: a systematic review. Heart Fail. Rev. 2022;27:19–134. doi: 10.1007/s10741-020-09984-2. [DOI] [PubMed] [Google Scholar]
- 184.Kmetova Sivonova M, et al. The role of CYP17A1 in prostate cancer development: structure, function, mechanism of action, genetic variations and its inhibition. Gen. Physiol. Biophys. 2017;36:487–499. doi: 10.4149/gpb_2017024. [DOI] [PubMed] [Google Scholar]
- 185.Auchus RJ, Miller WL. Molecular modeling of human P450c17 (17alpha-hydroxylase/17,20-lyase): insights into reaction mechanisms and effects of mutations. Mol. Endocrinol. 1999;13:1169–1182. doi: 10.1210/mend.13.7.0326. [DOI] [PubMed] [Google Scholar]
- 186.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol. Metab. Clin. North Am. 2001;30:101–119. doi: 10.1016/S0889-8529(08)70021-5. [DOI] [PubMed] [Google Scholar]
- 187.Missaghian E, et al. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J. Endocrinol. 2009;202:99–109. doi: 10.1677/JOE-08-0353. [DOI] [PubMed] [Google Scholar]
- 188.Yoshimoto FK, Auchus RJ. The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1) J. Steroid Biochem Mol. Biol. 2015;151:52–65. doi: 10.1016/j.jsbmb.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Locke JA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 190.Cai C, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reid AH, Attard G, Barrie E, de Bono JS. CYP17 inhibition as a hormonal strategy for prostate cancer. Nat. Clin. Pract. Urol. 2008;5:610–620. doi: 10.1038/ncpuro1237. [DOI] [PubMed] [Google Scholar]
- 192.Alex AB, Pal SK, Agarwal N. CYP17 inhibitors in prostate cancer: latest evidence and clinical potential. Ther. Adv. Med Oncol. 2016;8:267–275. doi: 10.1177/1758834016642370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vasaitis TS, Bruno RD, Njar VC. CYP17 inhibitors for prostate cancer therapy. J. Steroid Biochem Mol. Biol. 2011;125:23–31. doi: 10.1016/j.jsbmb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Parker C, Sartor O. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011;365:767. doi: 10.1056/NEJMc1107198. [DOI] [PubMed] [Google Scholar]
- 195.Antonarakis ES, Eisenberger MA. Expanding treatment options for metastatic prostate cancer. N. Engl. J. Med. 2011;364:2055–2058. doi: 10.1056/NEJMe1102758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fizazi K, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 197.James ND, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fizazi K, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700. doi: 10.1016/S1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 199.Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69:4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 200.Kaku T, et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg. Med Chem. 2011;19:6383–6399. doi: 10.1016/j.bmc.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 201.Yamaoka M, et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J. Steroid Biochem Mol. Biol. 2012;129:115–128. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 202.Njar VC, Brodie AM. Discovery and development of Galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancer. J. Med Chem. 2015;58:2077–2087. doi: 10.1021/jm501239f. [DOI] [PubMed] [Google Scholar]
- 203.Kwegyir-Afful AK, et al. Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo. Oncotarget. 2015;6:27440–27460. doi: 10.18632/oncotarget.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu H, Garcia JA. Targeting the adrenal gland in castration-resistant prostate cancer: a case for orteronel, a selective CYP-17 17,20-lyase inhibitor. Curr. Oncol. Rep. 2013;15:105–112. doi: 10.1007/s11912-013-0300-1. [DOI] [PubMed] [Google Scholar]
- 205.Montgomery B, et al. Androgen receptor modulation optimized for response (ARMOR) phase I and II Studies: galeterone for the treatment of castration-resistant prostate cancer. Clin. Cancer Res. 2016;22:1356–1363. doi: 10.1158/1078-0432.CCR-15-1432. [DOI] [PubMed] [Google Scholar]
- 206.Fizazi K, et al. Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5. J. Clin. Oncol. 2015;33:723–731. doi: 10.1200/JCO.2014.56.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Taplin ME, et al. Androgen receptor modulation optimized for response-splice variant: a phase 3, randomized trial of galeterone versus enzalutamide in androgen receptor splice variant-7-expressing metastatic castration-resistant prostate cancer. Eur. Urol. 2019;76:843–851. doi: 10.1016/j.eururo.2019.08.034. [DOI] [PubMed] [Google Scholar]
- 208.Toren PJ, et al. Anticancer activity of a novel selective CYP17A1 inhibitor in preclinical models of castrate-resistant prostate cancer. Mol. Cancer Ther. 2015;14:59–69. doi: 10.1158/1535-7163.MCT-14-0521. [DOI] [PubMed] [Google Scholar]
- 209.Mostaghel EA, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Saxby H, Mikropoulos C, Boussios S. An update on the prognostic and predictive serum biomarkers in metastatic prostate cancer. Diagnostics. 2020;10:549. doi: 10.3390/diagnostics10080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mollica V, et al. Bone targeting agents in patients with metastatic prostate cancer: state of the art. Cancers. 2021;13:546. doi: 10.3390/cancers13030546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hofbauer LC, et al. Novel approaches to target the microenvironment of bone metastasis. Nat. Rev. Clin. Oncol. 2021;18:488–505. doi: 10.1038/s41571-021-00499-9. [DOI] [PubMed] [Google Scholar]
- 213.Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat. Rev. Cancer. 2016;16:373–386. doi: 10.1038/nrc.2016.44. [DOI] [PubMed] [Google Scholar]
- 214.Wong SK, et al. Prostate cancer and bone metastases: the underlying mechanisms. Int. J. Mol. Sci. 2019;20:2587. doi: 10.3390/ijms20102587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Berish RB, et al. Translational models of prostate cancer bone metastasis. Nat. Rev. Urol. 2018;15:403–421. doi: 10.1038/s41585-018-0020-2. [DOI] [PubMed] [Google Scholar]
- 216.Datta HK, et al. The cell biology of bone metabolism. J. Clin. Pathol. 2008;61:577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- 217.Roodman GD. Biology of osteoclast activation in cancer. J. Clin. Oncol. 2001;19:3562–3571. doi: 10.1200/JCO.2001.19.15.3562. [DOI] [PubMed] [Google Scholar]
- 218.Msaouel P, Nandikolla G, Pneumaticos SG, Koutsilieris M. Bone microenvironment-targeted manipulations for the treatment of osteoblastic metastasis in castration-resistant prostate cancer. Expert Opin. Investig. Drugs. 2013;22:1385–1400. doi: 10.1517/13543784.2013.824422. [DOI] [PubMed] [Google Scholar]
- 219.Liao J, Schneider A, Datta NS, McCauley LK. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 2006;66:9065–9073. doi: 10.1158/0008-5472.CAN-06-0317. [DOI] [PubMed] [Google Scholar]
- 220.Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr. Rev. 2009;30:178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- 221.Msaouel P, et al. Targeting the bone microenvironment in metastatic castration-resistant prostate cancer. Curr. Drug Targets. 2016;17:276–289. doi: 10.2174/1389450116666150420143932. [DOI] [PubMed] [Google Scholar]
- 222.Koutsilieris M, Rabbani SA, Bennett HP, Goltzman D. Characteristics of prostate-derived growth factors for cells of the osteoblast phenotype. J. Clin. Investig. 1987;80:941–946. doi: 10.1172/JCI113186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fizazi K, et al. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin. Cancer Res. 2003;9:2587–2597. [PubMed] [Google Scholar]
- 224.Zhang J, et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J. Clin. Investig. 2001;107:1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hall CL, et al. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 226.Coleman RE, et al. Bone metastases. Nat. Rev. Dis. Prim. 2020;6:83. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- 227.Ell B, Kang Y. SnapShot: bone metastasis. Cell. 2012;151:690–690. doi: 10.1016/j.cell.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 228.Ren G, Esposito M, Kang Y. Bone metastasis and the metastatic niche. J. Mol. Med. 2015;93:1203–1212. doi: 10.1007/s00109-015-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hofbauer LC, Rachner TD, Coleman RE, Jakob F. Endocrine aspects of bone metastases. Lancet Diabetes Endocrinol. 2014;2:500–512. doi: 10.1016/S2213-8587(13)70203-1. [DOI] [PubMed] [Google Scholar]
- 230.Harrison MR, Wong TZ, Armstrong AJ, George DJ. Radium-223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag. Res. 2013;5:1–14. doi: 10.2147/CMAR.S25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lewington VJ, et al. A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur. J. Cancer. 1991;27:954–958. doi: 10.1016/0277-5379(91)90257-E. [DOI] [PubMed] [Google Scholar]
- 232.Porter AT, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1993;25:805–813. doi: 10.1016/0360-3016(93)90309-J. [DOI] [PubMed] [Google Scholar]
- 233.Quilty PM, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother. Oncol. 1994;31:33–40. doi: 10.1016/0167-8140(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 234.Sartor O, et al. Samarium-153-Lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology. 2004;63:940–945. doi: 10.1016/j.urology.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 235.Manna F, et al. Metastases in prostate cancer. Cold Spring Harb. Perspect. Med. 2019;9:a033688. doi: 10.1101/cshperspect.a033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sartor O, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–746. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- 237.Gallicchio R, et al. Radium-223 for the treatment of bone metastases in castration-resistant prostate cancer: when and why. Tumori. 2019;105:367–377. doi: 10.1177/0300891619851376. [DOI] [PubMed] [Google Scholar]
- 238.Smith AW, Greenberger BA, Den RB, Stock RG. Radiopharmaceuticals for Bone Metastases. Semin Radiat. Oncol. 2021;31:45–59. doi: 10.1016/j.semradonc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 239.Targeted Alpha Therapy Working, G. et al. Targeted alpha therapy, an emerging class of cancer agents: a review. JAMA Oncol. 2018;4:1765–1772. doi: 10.1001/jamaoncol.2018.4044. [DOI] [PubMed] [Google Scholar]
- 240.Hoskin P, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15:1397–1406. doi: 10.1016/S1470-2045(14)70474-7. [DOI] [PubMed] [Google Scholar]
- 241.Parker CC, et al. Three-year safety of Radium-223 Dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases from phase 3 randomized Alpharadin in symptomatic prostate cancer trial. Eur. Urol. 2018;73:427–435. doi: 10.1016/j.eururo.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 242.Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J. Clin. Investig. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/S8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 244.Caraglia M, et al. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr. Relat. Cancer. 2006;13:7–26. doi: 10.1677/erc.1.01094. [DOI] [PubMed] [Google Scholar]
- 245.Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 246.Monkkonen H, et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br. J. Pharmacol. 2006;147:437–445. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Clezardin P. Bisphosphonates’ antitumor activity: an unravelled side of a multifaceted drug class. Bone. 2011;48:71–79. doi: 10.1016/j.bone.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 248.Hoskin P, et al. A Multicenter randomized trial of ibandronate compared with single-dose radiotherapy for localized metastatic bone pain in prostate cancer. J. Natl Cancer Inst. 2015;107:djv197. doi: 10.1093/jnci/djv197. [DOI] [PubMed] [Google Scholar]
- 249.Coleman RE, et al. Double-blind, randomised, placebo-controlled, dose-finding study of oral ibandronate in patients with metastatic bone disease. Ann. Oncol. 1999;10:311–316. doi: 10.1023/A:1008386501738. [DOI] [PubMed] [Google Scholar]
- 250.Mason MD, et al. Oral sodium clodronate for nonmetastatic prostate cancer−results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873) J. Natl Cancer Inst. 2007;99:765–776. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 251.Dearnaley DP, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial) J. Natl Cancer Inst. 2003;95:1300–1311. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 252.Small EJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J. Clin. Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 253.Smith MR, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 254.Henry DH, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 255.Himelstein AL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317:48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mitsiades CS, et al. Randomized controlled clinical trial of a combination of somatostatin analog and dexamethasone plus zoledronate vs. zoledronate in patients with androgen ablation-refractory prostate cancer. Anticancer Res. 2006;26:3693–3700. [PubMed] [Google Scholar]
- 257.Dunford JE, et al. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharm. Exp. Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 258.Saad F, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 259.Saad F, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 260.Bartl R, Bartl C, Gradinger R. [Use of bisphosphonates in orthopedic surgery] Orthopade. 2008;37:595–613. doi: 10.1007/s00132-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 261.Gartrell BA, et al. Toxicities following treatment with bisphosphonates and receptor activator of nuclear factor-kappaB ligand inhibitors in patients with advanced prostate cancer. Eur. Urol. 2014;65:278–286. doi: 10.1016/j.eururo.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Macherey S, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst. Rev. 2017;12:CD006250. doi: 10.1002/14651858.CD006250.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones. 2009;8:96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 264.Miller PD, et al. Renal safety in patients treated with bisphosphonates for osteoporosis: a review. J. Bone Min. Res. 2013;28:2049–2059. doi: 10.1002/jbmr.2058. [DOI] [PubMed] [Google Scholar]
- 265.Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74:1385–1393. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 266.Anderson DM, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 267.Dell’Aquila E, et al. Denosumab for cancer-related bone loss. Expert Opin. Biol. Ther. 2020;20:1261–1274. doi: 10.1080/14712598.2020.1814731. [DOI] [PubMed] [Google Scholar]
- 268.Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 269.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. U.S.A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Udagawa N, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Min. Metab. 2021;39:19–26. doi: 10.1007/s00774-020-01162-6. [DOI] [PubMed] [Google Scholar]
- 271.Lorenzo J. The many ways of osteoclast activation. J. Clin. Investig. 2017;127:2530–2532. doi: 10.1172/JCI94606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Li C, Chung CJ, Hwang CJ, Lee KJ. Local injection of RANKL facilitates tooth movement and alveolar bone remodelling. Oral. Dis. 2019;25:550–560. doi: 10.1111/odi.13013. [DOI] [PubMed] [Google Scholar]
- 273.Ming J, Cronin SJF, Penninger JM. Targeting the RANKL/RANK/OPG axis for cancer therapy. Front Oncol. 2020;10:1283. doi: 10.3389/fonc.2020.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Pageau SC. Denosumab. MAbs. 2009;1:210–215. doi: 10.4161/mabs.1.3.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Saad F, et al. The role of bisphosphonates or denosumab in light of the availability of new therapies for prostate cancer. Cancer Treat. Rev. 2018;68:25–37. doi: 10.1016/j.ctrv.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 276.Raje N, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 277.Bone HG, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 278.Dempster DW, et al. Effects of long-term denosumab on bone histomorphometry and mineralization in women with postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 2018;103:2498–2509. doi: 10.1210/jc.2017-02669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Leder BZ, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386:1147–1155. doi: 10.1016/S0140-6736(15)61120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Smith MR, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lau LH, et al. Hypocalcaemia following denosumab in prostate cancer: a clinical review. Clin. Endocrinol. 2020;92:495–502. doi: 10.1111/cen.14169. [DOI] [PubMed] [Google Scholar]
- 282.Ardura JA, Alvarez-Carrion L, Gutierrez-Rojas I, Alonso V. Role of calcium signaling in prostate cancer progression: effects on cancer hallmarks and bone metastatic mechanisms. Cancers. 2020;12:1071. doi: 10.3390/cancers12051071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Terrar DA. Calcium signaling in the heart. Adv. Exp. Med. Biol. 2020;1131:395–443. doi: 10.1007/978-3-030-12457-1_16. [DOI] [PubMed] [Google Scholar]
- 284.Wang F, et al. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2022;25:11–26. doi: 10.1038/s41391-021-00394-5. [DOI] [PubMed] [Google Scholar]
- 285.Jones W, Griffiths K, Barata PC, Paller CJ. PSMA Theranostics: review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers. 2020;12:1367. doi: 10.3390/cancers12061367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kinoshita Y, et al. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006;30:628–636. doi: 10.1007/s00268-005-0544-5. [DOI] [PubMed] [Google Scholar]
- 287.Cimadamore A, et al. New prostate cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane antigen. Front Oncol. 2018;8:653. doi: 10.3389/fonc.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wester HJ, Schottelius M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin Nucl. Med. 2019;49:302–312. doi: 10.1053/j.semnuclmed.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 289.Eiber M, et al. Prostate-specific membrane antigen ligands for imaging and therapy. J. Nucl. Med. 2017;58:67S–76S. doi: 10.2967/jnumed.116.186767. [DOI] [PubMed] [Google Scholar]
- 290.Pastorino S, et al. Toward the discovery and development of PSMA targeted inhibitors for nuclear medicine applications. Curr. Radiopharm. 2020;13:63–79. doi: 10.2174/1874471012666190729151540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bander NH, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J. Urol. 2003;170:1717–1721. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- 292.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62:4029–4033. [PubMed] [Google Scholar]
- 293.Dassie JP, et al. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat. Biotechnol. 2009;27:839–849. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Rockey WM, et al. Rational truncation of an RNA aptamer to prostate-specific membrane antigen using computational structural modeling. Nucleic Acid Ther. 2011;21:299–314. doi: 10.1089/nat.2011.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hillier SM, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932–6940. doi: 10.1158/0008-5472.CAN-09-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bouchelouche K, Turkbey B, Choyke PL. PSMA PET and radionuclide therapy in prostate cancer. Semin Nucl. Med. 2016;46:522–535. doi: 10.1053/j.semnuclmed.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wilkinson S, Chodak G. The role of 111indium-capromab pendetide imaging for assessing biochemical failure after radical prostatectomy. J. Urol. 2004;172:133–136. doi: 10.1097/01.ju.0000132138.02846.08. [DOI] [PubMed] [Google Scholar]
- 298.Bander NH. Technology insight: monoclonal antibody imaging of prostate cancer. Nat. Clin. Pract. Urol. 2006;3:216–225. doi: 10.1038/ncpuro0452. [DOI] [PubMed] [Google Scholar]
- 299.Afshar-Oromieh A, et al. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur. J. Nucl. Med Mol. Imaging. 2012;39:1085–1086. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 300.Afshar-Oromieh A, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med Mol. Imaging. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 301.Afshar-Oromieh A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med Mol. Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schollhammer R, et al. 68Ga-PSMA-617 compared With 68Ga-RM2 and 18F-FCholine PET/CT for the initial staging of high-risk prostate cancer. Clin. Nucl. Med. 2019;44:e535–e536. doi: 10.1097/RLU.0000000000002672. [DOI] [PubMed] [Google Scholar]
- 303.Liu D, et al. PET/CT using (68) Ga-PSMA-617 versus (18) F-fluorodeoxyglucose to differentiate low- and high-grade gliomas. J. Neuroimaging. 2021;31:733–742. doi: 10.1111/jon.12856. [DOI] [PubMed] [Google Scholar]
- 304.Schmuck S, et al. Multiple time-point 68Ga-PSMA I&T PET/CT for characterization of primary prostate cancer: value of early dynamic and delayed imaging. Clin. Nucl. Med. 2017;42:e286–e293. doi: 10.1097/RLU.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 305.Cho SY, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 2012;53:1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Giesel FL, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med Mol. Imaging. 2017;44:678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Rowe SP, et al. PSMA-based [(18)F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol. Imaging Biol. 2016;18:411–419. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Schwarzenboeck SM, et al. PSMA ligands for PET imaging of prostate cancer. J. Nucl. Med. 2017;58:1545–1552. doi: 10.2967/jnumed.117.191031. [DOI] [PubMed] [Google Scholar]
- 309.Sprute K, et al. Diagnostic accuracy of (18)F-PSMA-1007 PET/CT imaging for lymph node staging of prostate carcinoma in primary and biochemical recurrence. J. Nucl. Med. 2021;62:208–213. doi: 10.2967/jnumed.120.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA theranostics: current status and future directions. Mol. Imaging. 2018;17:1536012118776068. doi: 10.1177/1536012118776068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Dash A, Knapp FF, Pillai MR. Targeted radionuclide therapy—an overview. Curr. Radiopharm. 2013;6:152–180. doi: 10.2174/18744710113066660023. [DOI] [PubMed] [Google Scholar]
- 312.Sun M, et al. Review of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Cureus. 2020;12:e8921. doi: 10.7759/cureus.8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kratochwil C, et al. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 314.Vallabhajosula S, et al. Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin. Cancer Res. 2005;11:7195s–7200s. doi: 10.1158/1078-0432.CCR-1004-0023. [DOI] [PubMed] [Google Scholar]
- 315.Hofman MS, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 316.Milowsky MI, et al. Phase 1/2 multiple ascending dose trial of the prostate-specific membrane antigen-targeted antibody drug conjugate MLN2704 in metastatic castration-resistant prostate cancer. Urol. Oncol. 2016;34:530 e515–530.e521. doi: 10.1016/j.urolonc.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Petrylak DP, et al. PSMA ADC monotherapy in patients with progressive metastatic castration-resistant prostate cancer following abiraterone and/or enzalutamide: efficacy and safety in open-label single-arm phase 2 study. Prostate. 2020;80:99–108. doi: 10.1002/pros.23922. [DOI] [PubMed] [Google Scholar]
- 318.Huang Y, et al. Multifaceted bioanalytical methods for the comprehensive pharmacokinetic and catabolic assessment of MEDI3726, an anti-prostate-specific membrane antigen pyrrolobenzodiazepine antibody-drug conjugate. Anal. Chem. 2020;92:11135–11144. doi: 10.1021/acs.analchem.0c01187. [DOI] [PubMed] [Google Scholar]
- 319.Galsky MD, et al. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2008;26:2147–2154. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 320.Baskin AS, et al. Low self-efficacy is associated with decreased emergency department use in underserved men with prostate cancer. Urol. Oncol. 2016;34:3 e15–21. doi: 10.1016/j.urolonc.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 321.de Bono JS, et al. Phase I Study of MEDI3726: a prostate-specific membrane antigen-targeted antibody-drug conjugate, in patients with mCRPC after failure of abiraterone or enzalutamide. Clin. Cancer Res. 2021;27:3602–3609. doi: 10.1158/1078-0432.CCR-20-4528. [DOI] [PubMed] [Google Scholar]
- 322.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ma Q, et al. Anti-prostate specific membrane antigen designer T cells for prostate cancer therapy. Prostate. 2004;61:12–25. doi: 10.1002/pros.20073. [DOI] [PubMed] [Google Scholar]
- 324.Ma Q, Gomes EM, Lo AS, Junghans RP. Advanced generation anti-prostate specific membrane antigen designer T cells for prostate cancer immunotherapy. Prostate. 2014;74:286–296. doi: 10.1002/pros.22749. [DOI] [PubMed] [Google Scholar]
- 325.Schubert ML, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann. Oncol. 2021;32:34–48. doi: 10.1016/j.annonc.2020.10.478. [DOI] [PubMed] [Google Scholar]
- 326.Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mateo J, et al. DNA repair in prostate cancer: biology and clinical implications. Eur. Urol. 2017;71:417–425. doi: 10.1016/j.eururo.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 328.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34:360–394. doi: 10.1101/gad.334516.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 331.McCabe N, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 332.Murai J, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014;13:433–443. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ying S, Hamdy FC, Helleday T. Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res. 2012;72:2814–2821. doi: 10.1158/0008-5472.CAN-11-3417. [DOI] [PubMed] [Google Scholar]
- 334.Petermann E, et al. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol. Cell. 2010;37:492–502. doi: 10.1016/j.molcel.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc. Natl Acad. Sci. U.S.A. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Burdak-Rothkamm S, Mansour WY, Rothkamm K. DNA damage repair deficiency in prostate cancer. Trends Cancer. 2020;6:974–984. doi: 10.1016/j.trecan.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 337.Asim M, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017;8:374. doi: 10.1038/s41467-017-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Abida W, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the phase II TRITON2 study. Clin. Cancer Res. 2020;26:2487–2496. doi: 10.1158/1078-0432.CCR-20-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hussain M, et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J. Clin. Oncol. 2018;36:991–999. doi: 10.1200/JCO.2017.75.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Turajlic S, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18:1009–1021. doi: 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 341.Willis JA, et al. Immune activation in mismatch repair-deficient carcinogenesis: more than just mutational rate. Clin. Cancer Res. 2020;26:11–17. doi: 10.1158/1078-0432.CCR-18-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Schumacher TN, Scheper W, Kvistborg P. Cancer neoantigens. Annu. Rev. Immunol. 2019;37:173–200. doi: 10.1146/annurev-immunol-042617-053402. [DOI] [PubMed] [Google Scholar]
- 343.Maby P, et al. Correlation between density of CD8+ T-cell infiltrate in microsatellite unstable colorectal cancers and frameshift mutations: a rationale for personalized immunotherapy. Cancer Res. 2015;75:3446–3455. doi: 10.1158/0008-5472.CAN-14-3051. [DOI] [PubMed] [Google Scholar]
- 344.Jiricny J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 345.Abida W, et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019;5:471–478. doi: 10.1001/jamaoncol.2018.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nava Rodrigues D, et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Investig. 2018;128:4441–4453. doi: 10.1172/JCI121924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Rescigno P, de Bono JS. Immunotherapy for lethal prostate cancer. Nat. Rev. Urol. 2019;16:69–70. doi: 10.1038/s41585-018-0121-y. [DOI] [PubMed] [Google Scholar]
- 348.He, Y. et al. FOXA1 overexpression suppresses interferon signaling and immune response in cancer. J. Clin. Investig. 131, e147025 (2021). [DOI] [PMC free article] [PubMed]
- 349.Boyiadzis MM, et al. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J. Immunother. Cancer. 2018;6:35. doi: 10.1186/s40425-018-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yu, E. Y. et al. Pembrolizumab Plus Docetaxel and Prednisone in Patients with Metastatic Castration-resistant Prostate Cancer: Long-term Results from the Phase 1b/2 KEYNOTE-365 Cohort B Study. Eur. Urol. 82, 22–30 (2022). [DOI] [PubMed]
- 351.Wu YM, et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173:1770–1782.e1714. doi: 10.1016/j.cell.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 353.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 354.Malumbres M. Cyclin-dependent kinases. Genome Biol. 2014;15:122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Ren B, et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 358.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/S1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 359.Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016;17:280–292. doi: 10.1038/nrm.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Salazar-Roa M, Malumbres M. Fueling the cell division cycle. Trends Cell Biol. 2017;27:69–81. doi: 10.1016/j.tcb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 361.Palmbos PL, et al. A randomized phase II study of androgen deprivation therapy with or without palbociclib in RB-positive metastatic hormone-sensitive prostate cancer. Clin. Cancer Res. 2021;27:3017–3027. doi: 10.1158/1078-0432.CCR-21-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Deng J, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zhang J, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat. Rev. Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 365.Mu P, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum. Mutat. 2014;35:672–688. doi: 10.1002/humu.22552. [DOI] [PubMed] [Google Scholar]
- 367.Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Moll UM, Petrenko O. The MDM2-p53 interaction. Mol. Cancer Res. 2003;1:1001–1008. [PubMed] [Google Scholar]
- 369.Ding Q, et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J. Med Chem. 2013;56:5979–5983. doi: 10.1021/jm400487c. [DOI] [PubMed] [Google Scholar]
- 370.Vu B, et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med Chem. Lett. 2013;4:466–469. doi: 10.1021/ml4000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Montesinos P, et al. MIRROS: a randomized, placebo-controlled, Phase III trial of cytarabine +/- idasanutlin in relapsed or refractory acute myeloid leukemia. Future Oncol. 2020;16:807–815. doi: 10.2217/fon-2020-0044. [DOI] [PubMed] [Google Scholar]
- 372.Perdrix, A. et al. PRIMA-1 and PRIMA-1(Met) (APR-246): from mutant/wild type p53 reactivation to unexpected mechanisms underlying their potent anti-tumor effect in combinatorial therapies. Cancers9, 172 (2017). [DOI] [PMC free article] [PubMed]
- 373.Salim KY, Maleki Vareki S, Danter WR, Koropatnick J. COTI-2, a novel small molecule that is active against multiple human cancer cell lines in vitro and in vivo. Oncotarget. 2016;7:41363–41379. doi: 10.18632/oncotarget.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Chen S, et al. Arsenic trioxide rescues structural p53 mutations through a cryptic allosteric site. Cancer Cell. 2021;39:225–239. doi: 10.1016/j.ccell.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 375.Sallman DA, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J. Clin. Oncol. 2021;39:1584–1594. doi: 10.1200/JCO.20.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Duffy MJ, Synnott NC, O’Grady S, Crown J. Targeting p53 for the treatment of cancer. Semin Cancer Biol. 2022;79:58–67. doi: 10.1016/j.semcancer.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 377.Lehmann S, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J. Clin. Oncol. 2012;30:3633–3639. doi: 10.1200/JCO.2011.40.7783. [DOI] [PubMed] [Google Scholar]
- 378.Malekzadeh P, et al. Neoantigen screening identifies broad TP53 mutant immunogenicity in patients with epithelial cancers. J. Clin. Invest. 2019;129:1109–1114. doi: 10.1172/JCI123791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Hsiue, E. H. et al. Targeting a neoantigen derived from a common TP53 mutation. Science371, eabc8697 (2021). [DOI] [PMC free article] [PubMed]
- 380.Jamaspishvili T, et al. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018;15:222–234. doi: 10.1038/nrurol.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 382.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 383.Fruman DA, et al. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Jia S, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Jiang X, Chen S, Asara JM, Balk SP. Phosphoinositide 3-kinase pathway activation in phosphate and tensin homolog (PTEN)-deficient prostate cancer cells is independent of receptor tyrosine kinases and mediated by the p110beta and p110delta catalytic subunits. J. Biol. Chem. 2010;285:14980–14989. doi: 10.1074/jbc.M109.085696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Bendell JC, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 387.Wipf P, et al. Synthesis and biological evaluation of synthetic viridins derived from C(20)-heteroalkylation of the steroidal PI-3-kinase inhibitor wortmannin. Org. Biomol. Chem. 2004;2:1911–1920. doi: 10.1039/b405431h. [DOI] [PubMed] [Google Scholar]
- 388.Hotte SJ, et al. A phase II study of PX-866 in patients with recurrent or metastatic castration-resistant prostate cancer: canadian cancer trials group study IND205. Clin. Genitourin. Cancer. 2019;17:201–208. doi: 10.1016/j.clgc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 389.Risso G, et al. Akt/PKB: one kinase, many modifications. Biochem J. 2015;468:203–214. doi: 10.1042/BJ20150041. [DOI] [PubMed] [Google Scholar]
- 390.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 391.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Crabb SJ, et al. ProCAID: a phase I clinical trial to combine the AKT inhibitor AZD5363 with docetaxel and prednisolone chemotherapy for metastatic castration resistant prostate cancer. Investig. N. Drugs. 2017;35:599–607. doi: 10.1007/s10637-017-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Jhanwar-Uniyal M, et al. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019;72:51–62. doi: 10.1016/j.jbior.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 394.Amato RJ, Jac J, Mohammad T, Saxena S. Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin. Genitourin. Cancer. 2008;6:97–102. doi: 10.3816/CGC.2008.n.015. [DOI] [PubMed] [Google Scholar]
- 395.George DJ, et al. Phase 2 clinical trial of TORC1 inhibition with everolimus in men with metastatic castration-resistant prostate cancer. Urol. Oncol. 2020;38:79.e15–79.e22. doi: 10.1016/j.urolonc.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 396.Rebello RJ, Huglo AV, Furic L. PIM activity in tumours: a key node of therapy resistance. Adv. Biol. Regul. 2018;67:163–169. doi: 10.1016/j.jbior.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 397.Luszczak S, et al. PIM kinase inhibition: co-targeted therapeutic approaches in prostate cancer. Signal Transduct. Target Ther. 2020;5:7. doi: 10.1038/s41392-020-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Eerola SK, et al. Expression and ERG regulation of PIM kinases in prostate cancer. Cancer Med. 2021;10:3427–3436. doi: 10.1002/cam4.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Luszczak S, et al. Co-targeting PIM and PI3K/mTOR using multikinase inhibitor AUM302 and a combination of AZD-1208 and BEZ235 in prostate cancer. Sci. Rep. 2020;10:14380. doi: 10.1038/s41598-020-71263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Kgatle MM, et al. Prostate cancer: epigenetic alterations, risk factors, and therapy. Prostate Cancer. 2016;2016:5653862. doi: 10.1155/2016/5653862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ruggero K, Farran-Matas S, Martinez-Tebar A, Aytes A. Epigenetic regulation in prostate cancer progression. Curr. Mol. Biol. Rep. 2018;4:101–115. doi: 10.1007/s40610-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Sugiura M, et al. Epigenetic modifications in prostate cancer. Int J. Urol. 2021;28:140–149. doi: 10.1111/iju.14406. [DOI] [PubMed] [Google Scholar]
- 404.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 405.Ruscetti M, et al. HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene. 2016;35:3781–3795. doi: 10.1038/onc.2015.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Pomerantz MM, et al. Prostate cancer reactivates developmental epigenomic programs during metastatic progression. Nat. Genet. 2020;52:790–799. doi: 10.1038/s41588-020-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Ellinger J, et al. Global levels of histone modifications predict prostate cancer recurrence. Prostate. 2010;70:61–69. doi: 10.1002/pros.21038. [DOI] [PubMed] [Google Scholar]
- 408.Chen Z, Wang L, Wang Q, Li W. Histone modifications and chromatin organization in prostate cancer. Epigenomics. 2010;2:551–560. doi: 10.2217/epi.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Varambally S, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 410.Dardenne E, et al. N-Myc induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell. 2016;30:563–577. doi: 10.1016/j.ccell.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Crea F, et al. The emerging role of histone lysine demethylases in prostate cancer. Mol. Cancer. 2012;11:52. doi: 10.1186/1476-4598-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.He Y, et al. LSD1 promotes S-phase entry and tumorigenesis via chromatin co-occupation with E2F1 and selective H3K9 demethylation. Oncogene. 2018;37:534–543. doi: 10.1038/onc.2017.353. [DOI] [PubMed] [Google Scholar]
- 413.Metzger E, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 414.Liang Y, et al. LSD1-mediated epigenetic reprogramming drives CENPE expression and prostate cancer progression. Cancer Res. 2017;77:5479–5490. doi: 10.1158/0008-5472.CAN-17-0496. [DOI] [PubMed] [Google Scholar]
- 415.Kanouni T, et al. Discovery of CC-90011: a potent and selective reversible inhibitor of lysine specific demethylase 1 (LSD1) J. Med Chem. 2020;63:14522–14529. doi: 10.1021/acs.jmedchem.0c00978. [DOI] [PubMed] [Google Scholar]
- 416.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 417.Loven J, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wen S, et al. Aberrant activation of super enhancer and choline metabolism drive antiandrogen therapy resistance in prostate cancer. Oncogene. 2020;39:6556–6571. doi: 10.1038/s41388-020-01456-z. [DOI] [PubMed] [Google Scholar]
- 419.Hnisz D, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. doi: 10.1016/j.cell.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Welti J, et al. Targeting the p300/CBP axis in lethal prostate cancer. Cancer Discov. 2021;11:1118–1137. doi: 10.1158/2159-8290.CD-20-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Lasko LM, et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature. 2017;550:128–132. doi: 10.1038/nature24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.He ZX, et al. Current development of CBP/p300 inhibitors in the last decade. Eur. J. Med. Chem. 2021;209:112861. doi: 10.1016/j.ejmech.2020.112861. [DOI] [PubMed] [Google Scholar]
- 423.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Weichert W, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer. 2008;98:604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Roche J, Bertrand P. Inside HDACs with more selective HDAC inhibitors. Eur. J. Med Chem. 2016;121:451–483. doi: 10.1016/j.ejmech.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 427.Rana, Z., Diermeier, S., Hanif, M. & Rosengren, R. J. Understanding failure and improving treatment using HDAC inhibitors for prostate cancer. Biomedicines8, 22 (2020). [DOI] [PMC free article] [PubMed]
- 428.Stathis A, Bertoni F. BET proteins as targets for anticancer treatment. Cancer Discov. 2018;8:24–36. doi: 10.1158/2159-8290.CD-17-0605. [DOI] [PubMed] [Google Scholar]
- 429.Urbanucci A, et al. Androgen receptor deregulation drives bromodomain-mediated chromatin alterations in prostate cancer. Cell Rep. 2017;19:2045–2059. doi: 10.1016/j.celrep.2017.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Welti J, et al. Targeting Bromodomain and Extra-Terminal (BET) Family Proteins in Castration-Resistant Prostate Cancer (CRPC) Clin. Cancer Res. 2018;24:3149–3162. doi: 10.1158/1078-0432.CCR-17-3571. [DOI] [PubMed] [Google Scholar]
- 431.Asangani IA, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–282. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Dai X, et al. Prostate cancer-associated SPOP mutations confer resistance to BET inhibitors through stabilization of BRD4. Nat. Med. 2017;23:1063–1071. doi: 10.1038/nm.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Zhang P, et al. Intrinsic BET inhibitor resistance in SPOP-mutated prostate cancer is mediated by BET protein stabilization and AKT-mTORC1 activation. Nat. Med. 2017;23:1055–1062. doi: 10.1038/nm.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Piha-Paul SA, et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr. 2020;4:pkz093. doi: 10.1093/jncics/pkz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Kulis M, Esteller M. DNA methylation and cancer. Adv. Genet. 2010;70:27–56. doi: 10.1016/B978-0-12-380866-0.60002-2. [DOI] [PubMed] [Google Scholar]
- 436.Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733–750. doi: 10.1101/gad.276568.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Antequera F. Structure, function and evolution of CpG island promoters. Cell Mol. Life Sci. 2003;60:1647–1658. doi: 10.1007/s00018-003-3088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Gordevicius J, et al. Cell-free DNA modification dynamics in abiraterone acetate-treated prostate cancer patients. Clin. Cancer Res. 2018;24:3317–3324. doi: 10.1158/1078-0432.CCR-18-0101. [DOI] [PubMed] [Google Scholar]
- 439.Zhao SG, et al. The DNA methylation landscape of advanced prostate cancer. Nat. Genet. 2020;52:778–789. doi: 10.1038/s41588-020-0648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 2018;15:271–286. doi: 10.1038/nrurol.2018.22. [DOI] [PubMed] [Google Scholar]
- 441.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 442.Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 4, a008052 (2012). [DOI] [PMC free article] [PubMed]
- 443.Herbst A, et al. Comprehensive analysis of beta-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/beta-catenin signaling. BMC Genomics. 2014;15:74. doi: 10.1186/1471-2164-15-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Yeh Y, et al. Wnt/Beta-catenin signaling and prostate cancer therapy resistance. Adv. Exp. Med. Biol. 2019;1210:351–378. doi: 10.1007/978-3-030-32656-2_16. [DOI] [PubMed] [Google Scholar]
- 445.Yokoyama NN, et al. Wnt signaling in castration-resistant prostate cancer: implications for therapy. Am. J. Clin. Exp. Urol. 2014;2:27–44. [PMC free article] [PubMed] [Google Scholar]
- 446.Kypta RM, Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nat. Rev. Urol. 2012;9:418–428. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- 447.Thiele S, et al. Expression profile of WNT molecules in prostate cancer and its regulation by aminobisphosphonates. J. Cell Biochem. 2011;112:1593–1600. doi: 10.1002/jcb.23070. [DOI] [PubMed] [Google Scholar]
- 448.Zhu H, et al. Analysis of Wnt gene expression in prostate cancer: mutual inhibition by WNT11 and the androgen receptor. Cancer Res. 2004;64:7918–7926. doi: 10.1158/0008-5472.CAN-04-2704. [DOI] [PubMed] [Google Scholar]
- 449.Chen G, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 450.Wan X, et al. Activation of beta-catenin signaling in androgen receptor-negative prostate cancer cells. Clin. Cancer Res. 2012;18:726–736. doi: 10.1158/1078-0432.CCR-11-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.de la Taille A, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin. Cancer Res. 2003;9:1801–1807. [PubMed] [Google Scholar]
- 452.Zhang Z, et al. Inhibition of the Wnt/beta-catenin pathway overcomes resistance to enzalutamide in castration-resistant prostate cancer. Cancer Res. 2018;78:3147–3162. doi: 10.1158/0008-5472.CAN-17-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Horvath LG, et al. Secreted frizzled-related protein 4 inhibits proliferation and metastatic potential in prostate cancer. Prostate. 2007;67:1081–1090. doi: 10.1002/pros.20607. [DOI] [PubMed] [Google Scholar]
- 454.Yee DS, et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol. Cancer. 2010;9:162. doi: 10.1186/1476-4598-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Zi X, et al. Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 2005;65:9762–9770. doi: 10.1158/0008-5472.CAN-05-0103. [DOI] [PubMed] [Google Scholar]
- 456.Kelsey R. Prostate cancer: Foxy-5 in prostate cancer model. Nat. Rev. Urol. 2017;14:638. doi: 10.1038/nrurol.2017.160. [DOI] [PubMed] [Google Scholar]
- 457.Li HK, et al. alpha-particle therapy for synovial sarcoma in the mouse using an astatine-211-labeled antibody against frizzled homolog 10. Cancer Sci. 2018;109:2302–2309. doi: 10.1111/cas.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Jimeno A, et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin. Cancer Res. 2017;23:7490–7497. doi: 10.1158/1078-0432.CCR-17-2157. [DOI] [PubMed] [Google Scholar]
- 459.Moore KN, et al. A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol. Oncol. 2019;154:294–301. doi: 10.1016/j.ygyno.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 461.Katoh M, Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases (Review) Int J. Mol. Med. 2017;40:587–606. doi: 10.3892/ijmm.2017.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Emami KH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc. Natl Acad. Sci. U.S.A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 465.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 466.Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review) Int J. Oncol. 2017;51:1357–1369. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Sarkar, C., Goswami, S., Basu, S. & Chakroborty, D. Angiogenesis inhibition in prostate cancer: an update. Cancers12, 2382 (2020). [DOI] [PMC free article] [PubMed]
- 468.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 469.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 470.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 471.Roberts E, Cossigny DA, Quan GM. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer. 2013;2013:418340. doi: 10.1155/2013/418340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Borre M, Nerstrom B, Overgaard J. Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin. Cancer Res. 2000;6:1882–1890. [PubMed] [Google Scholar]
- 473.Strohmeyer D, et al. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45:216–224. doi: 10.1002/1097-0045(20001101)45:3<216::AID-PROS3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 474.Garcia, J. et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev.86, 102017 (2020). [DOI] [PubMed]
- 475.Kelly WK, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J. Clin. Oncol. 2012;30:1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Bagnato A, Natali PG. Endothelin receptors as novel targets in tumor therapy. J. Transl. Med. 2004;2:16. doi: 10.1186/1479-5876-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat. Rev. Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 478.Yin JJ, et al. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl Acad. Sci. U.S.A. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Drake JM, Danke JR, Henry MD. Bone-specific growth inhibition of prostate cancer metastasis by atrasentan. Cancer Biol. Ther. 2010;9:607–614. doi: 10.4161/cbt.9.8.11112. [DOI] [PubMed] [Google Scholar]
- 480.James ND, et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomised, phase 2 trial. Eur. Urol. 2009;55:1112–1123. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 481.Nelson JB, et al. Phase 3, randomized, placebo-controlled study of zibotentan (ZD4054) in patients with castration-resistant prostate cancer metastatic to bone. Cancer. 2012;118:5709–5718. doi: 10.1002/cncr.27674. [DOI] [PubMed] [Google Scholar]
- 482.Carducci MA, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J. Clin. Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 483.Carducci MA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 484.Lara PN, Jr., et al. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J. Natl Cancer Inst. 2014;106:dju013. doi: 10.1093/jnci/dju013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Hata, A. & Chen, Y. G. TGF-beta Signaling from receptors to Smads. Cold Spring Harb. Perspect. Biol. 8, a022061 (2016). [DOI] [PMC free article] [PubMed]
- 486.Thompson-Elliott B, Johnson R, Khan SA. Alterations in TGFbeta signaling during prostate cancer progression. Am. J. Clin. Exp. Urol. 2021;9:318–328. [PMC free article] [PubMed] [Google Scholar]
- 487.Shariat SF, et al. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin. Cancer Res. 2004;10:1992–1999. doi: 10.1158/1078-0432.CCR-0768-03. [DOI] [PubMed] [Google Scholar]
- 488.Stravodimos K, et al. Immunohistochemical expression of transforming growth factor beta 1 and nm-23 H1 antioncogene in prostate cancer: divergent correlation with clinicopathological parameters. Anticancer Res. 2000;20:3823–3828. [PubMed] [Google Scholar]
- 489.Wikstrom P, Damber J, Bergh A. Role of transforming growth factor-beta1 in prostate cancer. Microsc Res. Tech. 2001;52:411–419. doi: 10.1002/1097-0029(20010215)52:4<411::AID-JEMT1026>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 490.Fournier PG, et al. The TGF-beta signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. Cancer Cell. 2015;27:809–821. doi: 10.1016/j.ccell.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.van der Pluijm G, et al. Interference with the microenvironmental support impairs the de novo formation of bone metastases in vivo. Cancer Res. 2005;65:7682–7690. doi: 10.1158/0008-5472.CAN-04-4188. [DOI] [PubMed] [Google Scholar]
- 492.Yuen HF, et al. Id proteins expression in prostate cancer: high-level expression of Id-4 in primary prostate cancer is associated with development of metastases. Mod. Pathol. 2006;19:931–941. doi: 10.1038/modpathol.3800602. [DOI] [PubMed] [Google Scholar]
- 493.Lee HM, Timme TL, Thompson TC. Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res. 2000;60:1927–1933. [PubMed] [Google Scholar]
- 494.Matthews E, et al. Down-regulation of TGF-beta1 production restores immunogenicity in prostate cancer cells. Br. J. Cancer. 2000;83:519–525. doi: 10.1054/bjoc.2000.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Herbertz S, et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Devel Ther. 2015;9:4479–4499. doi: 10.2147/DDDT.S86621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Knudson KM, et al. M7824, a novel bifunctional anti-PD-L1/TGFbeta Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018;7:e1426519. doi: 10.1080/2162402X.2018.1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.George DJ. Receptor tyrosine kinases as rational targets for prostate cancer treatment: platelet-derived growth factor receptor and imatinib mesylate. Urology. 2002;60:115–121. doi: 10.1016/S0090-4295(02)01589-3. [DOI] [PubMed] [Google Scholar]
- 498.Chang YM, Kung HJ, Evans CP. Nonreceptor tyrosine kinases in prostate cancer. Neoplasia. 2007;9:90–100. doi: 10.1593/neo.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Paul MD, Hristova K. The transition model of RTK activation: a quantitative framework for understanding RTK signaling and RTK modulator activity. Cytokine Growth Factor Rev. 2019;49:23–31. doi: 10.1016/j.cytogfr.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Sahadevan K, et al. Selective over-expression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. J. Pathol. 2007;213:82–90. doi: 10.1002/path.2205. [DOI] [PubMed] [Google Scholar]
- 502.Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin. Cancer Res. 1999;5:1063–1071. [PubMed] [Google Scholar]
- 503.Heer R, et al. Fibroblast growth factor 17 is over-expressed in human prostate cancer. J. Pathol. 2004;204:578–586. doi: 10.1002/path.1668. [DOI] [PubMed] [Google Scholar]
- 504.Gnanapragasam VJ, et al. FGF8 isoform b expression in human prostate cancer. Br. J. Cancer. 2003;88:1432–1438. doi: 10.1038/sj.bjc.6600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Memarzadeh S, et al. Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 2007;12:572–585. doi: 10.1016/j.ccr.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Acevedo VD, et al. Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell. 2007;12:559–571. doi: 10.1016/j.ccr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 507.Johansson A, et al. Mast cells are novel independent prognostic markers in prostate cancer and represent a target for therapy. Am. J. Pathol. 2010;177:1031–1041. doi: 10.2353/ajpath.2010.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Bluemn EG, et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32:474–489. doi: 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Perera TPS, et al. Discovery and pharmacological characterization of JNJ-42756493 (Erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol. Cancer Ther. 2017;16:1010–1020. doi: 10.1158/1535-7163.MCT-16-0589. [DOI] [PubMed] [Google Scholar]
- 510.Canil CM, et al. Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group. J. Clin. Oncol. 2005;23:455–460. doi: 10.1200/JCO.2005.02.129. [DOI] [PubMed] [Google Scholar]
- 511.Di Lorenzo G, et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 2002;8:3438–3444. [PubMed] [Google Scholar]
- 512.Festuccia C, et al. Epidermal growth factor modulates prostate cancer cell invasiveness regulating urokinase-type plasminogen activator activity. EGF-receptor inhibition may prevent tumor cell dissemination. Thromb. Haemost. 2005;93:964–975. doi: 10.1160/TH04-09-0637. [DOI] [PubMed] [Google Scholar]
- 513.Cathomas R, et al. Efficacy of cetuximab in metastatic castration-resistant prostate cancer might depend on EGFR and PTEN expression: results from a phase II trial (SAKK 08/07) Clin. Cancer Res. 2012;18:6049–6057. doi: 10.1158/1078-0432.CCR-12-2219. [DOI] [PubMed] [Google Scholar]
- 514.Fizazi K. The role of Src in prostate cancer. Ann. Oncol. 2007;18:1765–1773. doi: 10.1093/annonc/mdm086. [DOI] [PubMed] [Google Scholar]
- 515.Vlaeminck-Guillem V, Gillet G, Rimokh R. SRC: marker or actor in prostate cancer aggressiveness. Front Oncol. 2014;4:222. doi: 10.3389/fonc.2014.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Saad F. Src as a therapeutic target in men with prostate cancer and bone metastases. BJU Int. 2009;103:434–440. doi: 10.1111/j.1464-410X.2008.08249.x. [DOI] [PubMed] [Google Scholar]
- 517.Spetsieris N, et al. A Phase 2 trial of abiraterone followed by randomization to addition of dasatinib or sunitinib in men with metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer. 2021;19:22–31.e25. doi: 10.1016/j.clgc.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Araujo JC, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–1316. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Posadas EM, et al. Saracatinib as a metastasis inhibitor in metastatic castration-resistant prostate cancer: a University of Chicago Phase 2 Consortium and DOD/PCF Prostate Cancer Clinical Trials Consortium Study. Prostate. 2016;76:286–293. doi: 10.1002/pros.23119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Lara PN, Jr., et al. A phase II trial of the Src-kinase inhibitor AZD0530 in patients with advanced castration-resistant prostate cancer: a California Cancer Consortium study. Anticancer Drugs. 2009;20:179–184. doi: 10.1097/CAD.0b013e328325a867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Dorff TB, et al. Randomized phase II trial of abiraterone alone or with dasatinib in men with metastatic castration-resistant prostate cancer (mCRPC) Clin. Genitourin. Cancer. 2019;17:241–247. doi: 10.1016/j.clgc.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Choi YJ, et al. Phase II study of dovitinib in patients with castration-resistant prostate cancer (KCSG-GU11-05) Cancer Res Treat. 2018;50:1252–1259. doi: 10.4143/crt.2017.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J. Clin. Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 524.Michaelson MD, et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J. Clin. Oncol. 2014;32:76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 525.Yakes FM, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. [DOI] [PubMed] [Google Scholar]
- 526.Dubreuil P, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Basch EM, et al. Cabozantinib versus mitoxantrone-prednisone in symptomatic metastatic castration-resistant prostate cancer: a randomized phase 3 trial with a primary pain endpoint. Eur. Urol. 2019;75:929–937. doi: 10.1016/j.eururo.2018.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Dueck AC, et al. Assessment of adverse events from the patient perspective in a phase 3 metastatic castration-resistant prostate cancer clinical trial. JAMA Oncol. 2020;6:e193332. doi: 10.1001/jamaoncol.2019.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Sonpavde GP, et al. Cabozantinib for progressive metastatic castration-resistant prostate cancer following docetaxel: combined analysis of two phase 3 trials. Eur. Urol. Oncol. 2020;3:540–543. doi: 10.1016/j.euo.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Mukherjee R, et al. Raf-1 expression may influence progression to androgen insensitive prostate cancer. Prostate. 2005;64:101–107. doi: 10.1002/pros.20211. [DOI] [PubMed] [Google Scholar]
- 531.McCubrey JA, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Mulholland DJ, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012;72:1878–1889. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Taylor BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Gioeli D, et al. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–284. [PubMed] [Google Scholar]
- 535.Mukherjee R, et al. Upregulation of MAPK pathway is associated with survival in castrate-resistant prostate cancer. Br. J. Cancer. 2011;104:1920–1928. doi: 10.1038/bjc.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Antonopoulou, E. & Ladomery, M. Targeting splicing in prostate cancer. Int. J. Mol. Sci. 19, 1287 (2018). [DOI] [PMC free article] [PubMed]
- 537.Oltean S, Febbo PG, Garcia-Blanco MA. Dunning rat prostate adenocarcinomas and alternative splicing reporters: powerful tools to study epithelial plasticity in prostate tumors in vivo. Clin. Exp. Metastasis. 2008;25:611–619. doi: 10.1007/s10585-008-9186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Li L, Zheng J, Stevens M, Oltean S. A repositioning screen using an FGFR2 splicing reporter reveals compounds that regulate epithelial-mesenchymal transitions and inhibit growth of prostate cancer xenografts. Mol. Ther. Methods Clin. Dev. 2022;25:147–157. doi: 10.1016/j.omtm.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Adamo P, Ladomery MR. The oncogene ERG: a key factor in prostate cancer. Oncogene. 2016;35:403–414. doi: 10.1038/onc.2015.109. [DOI] [PubMed] [Google Scholar]
- 540.Li L, et al. Targeting the ERG oncogene with splice-switching oligonucleotides as a novel therapeutic strategy in prostate cancer. Br. J. Cancer. 2020;123:1024–1032. doi: 10.1038/s41416-020-0951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Luo J, et al. Role of androgen receptor variants in prostate cancer: report from the 2017 mission androgen receptor variants meeting. Eur. Urol. 2018;73:715–723. doi: 10.1016/j.eururo.2017.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Karantanos T, et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur. Urol. 2015;67:470–479. doi: 10.1016/j.eururo.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.He Y, et al. Androgen receptor splice variants bind to constitutively open chromatin and promote abiraterone-resistant growth of prostate cancer. Nucleic Acids Res. 2018;46:1895–1911. doi: 10.1093/nar/gkx1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.He Y, et al. Ailanthone targets p23 to overcome MDV3100 resistance in castration-resistant prostate cancer. Nat. Commun. 2016;7:13122. doi: 10.1038/ncomms13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Luo J. Development of AR-V7 as a putative treatment selection marker for metastatic castration-resistant prostate cancer. Asian J. Androl. 2016;18:580–585. doi: 10.4103/1008-682X.178490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Lu C, Luo J. Decoding the androgen receptor splice variants. Transl. Androl. Urol. 2013;2:178–186. doi: 10.3978/j.issn.2223-4683.2013.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71:1656–1667. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Kohli M, et al. Androgen receptor variant AR-V9 is coexpressed with AR-V7 in prostate cancer metastases and predicts abiraterone resistance. Clin. Cancer Res. 2017;23:4704–4715. doi: 10.1158/1078-0432.CCR-17-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Li H, et al. Discovery of small-molecule inhibitors selectively targeting the DNA-binding domain of the human androgen receptor. J. Med Chem. 2014;57:6458–6467. doi: 10.1021/jm500802j. [DOI] [PubMed] [Google Scholar]
- 551.Peng S, et al. Regression of castration-resistant prostate cancer by a novel compound QW07 targeting androgen receptor N-terminal domain. Cell Biol. Toxicol. 2020;36:399–416. doi: 10.1007/s10565-020-09511-x. [DOI] [PubMed] [Google Scholar]
- 552.Roubaud G, Liaw BC, Oh WK, Mulholland DJ. Strategies to avoid treatment-induced lineage crisis in advanced prostate cancer. Nat. Rev. Clin. Oncol. 2017;14:269–283. doi: 10.1038/nrclinonc.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Huang J, et al. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate. 2006;66:1399–1406. doi: 10.1002/pros.20434. [DOI] [PubMed] [Google Scholar]
- 554.Tan HL, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin. Cancer Res. 2014;20:890–903. doi: 10.1158/1078-0432.CCR-13-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Grasso CS, et al. Integrative molecular profiling of routine clinical prostate cancer specimens. Ann. Oncol. 2015;26:1110–1118. doi: 10.1093/annonc/mdv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.He Y, et al. A noncanonical AR addiction drives enzalutamide resistance in prostate cancer. Nat. Commun. 2021;12:1521. doi: 10.1038/s41467-021-21860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Arora VK, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–1322. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Vidal SJ, et al. A targetable GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer. Cancer Cell. 2015;27:223–239. doi: 10.1016/j.ccell.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Guo H, et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat. Commun. 2019;10:278. doi: 10.1038/s41467-018-08133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Rodriguez-Bravo V, et al. Nuclear pores promote lethal prostate cancer by increasing POM121-driven E2F1, MYC, and AR nuclear import. Cell. 2018;174:1200–1215.e1220. doi: 10.1016/j.cell.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Lee JK, et al. N-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell. 2016;29:536–547. doi: 10.1016/j.ccell.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Ci X, et al. Heterochromatin protein 1alpha mediates development and aggressiveness of neuroendocrine prostate cancer. Cancer Res. 2018;78:2691–2704. doi: 10.1158/0008-5472.CAN-17-3677. [DOI] [PubMed] [Google Scholar]
- 563.Akamatsu S, et al. The placental gene PEG10 promotes progression of neuroendocrine prostate cancer. Cell Rep. 2015;12:922–936. doi: 10.1016/j.celrep.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 564.Li Y, et al. SRRM4 drives neuroendocrine transdifferentiation of prostate adenocarcinoma under androgen receptor pathway inhibition. Eur. Urol. 2017;71:68–78. doi: 10.1016/j.eururo.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 565.Bishop JL, et al. The master neural transcription factor BRN2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov. 2017;7:54–71. doi: 10.1158/2159-8290.CD-15-1263. [DOI] [PubMed] [Google Scholar]
- 566.Beketova E, Owens JL, Asberry AM, Hu CD. PRMT5: a putative oncogene and therapeutic target in prostate cancer. Cancer Gene Ther. 2022;29:264–276. doi: 10.1038/s41417-021-00327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Teng M, et al. Pioneer of prostate cancer: past, present and the future of FOXA1. Protein Cell. 2021;12:29–38. doi: 10.1007/s13238-020-00786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Lupien M, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Grasso CS, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Barbieri CE, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 2012;44:685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Gerhardt J, et al. FOXA1 promotes tumor progression in prostate cancer and represents a novel hallmark of castration-resistant prostate cancer. Am. J. Pathol. 2012;180:848–861. doi: 10.1016/j.ajpath.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 572.Parolia A, et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature. 2019;571:413–418. doi: 10.1038/s41586-019-1347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Adams EJ, et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature. 2019;571:408–412. doi: 10.1038/s41586-019-1318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Beltran H, et al. Circulating tumor DNA profile recognizes transformation to castration-resistant neuroendocrine prostate cancer. J. Clin. Investig. 2020;130:1653–1668. doi: 10.1172/JCI131041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Shao J, et al. Destruction of DNA-binding proteins by programmable oligonucleotide PROTAC (O’PROTAC): effective targeting of LEF1 and ERG. Adv. Sci. 2021;8:e2102555. doi: 10.1002/advs.202102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Khalaf DJ, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20:1730–1739. doi: 10.1016/S1470-2045(19)30688-6. [DOI] [PubMed] [Google Scholar]
- 577.Agarwal, N. et al. Cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): results of Cohort 6 of the COSMIC-021 Study. J. Clin. Oncol. 38, 6_suppl.139 (2020).
- 578.Peng S, et al. Single-cell analysis reveals EP4 as a target for restoring t-cell infiltration and sensitizing prostate cancer to immunotherapy. Clin. Cancer Res. 2022;28:552–567. doi: 10.1158/1078-0432.CCR-21-0299. [DOI] [PubMed] [Google Scholar]
- 579.Molgora M, et al. TREM2 modulation remodels the tumor myeloid landscape enhancing anti-PD-1 immunotherapy. Cell. 2020;182:886–900. doi: 10.1016/j.cell.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Koikawa K, et al. Targeting Pin1 renders pancreatic cancer eradicable by synergizing with immunochemotherapy. Cell. 2021;184:4753–4771.e4727. doi: 10.1016/j.cell.2021.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Jayaprakash P, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Investig. 2018;128:5137–5149. doi: 10.1172/JCI96268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Zhang SM, et al. KDM5B promotes immune evasion by recruiting SETDB1 to silence retroelements. Nature. 2021;598:682–687. doi: 10.1038/s41586-021-03994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Bagati A, et al. Integrin alphavbeta6-TGFbeta-SOX4 pathway drives immune evasion in triple-negative breast cancer. Cancer Cell. 2021;39:54–67.e59. doi: 10.1016/j.ccell.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Manguso RT, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547:413–418. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Lawson KA, et al. Functional genomic landscape of cancer-intrinsic evasion of killing by T cells. Nature. 2020;586:120–126. doi: 10.1038/s41586-020-2746-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Ishizuka JJ, et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature. 2019;565:43–48. doi: 10.1038/s41586-018-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Han H, et al. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36:483–497e415.. doi: 10.1016/j.ccell.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]