Abstract
Tremor is one of the core symptoms of Parkinson’s disease (PD), but its mechanism is poorly understood. The cerebellum is a growing focus in PD-related researches and is reported to play an important role in tremor in PD. The cerebellum may participate in the modulation of tremor amplitude via cerebello-thalamo-cortical circuits. The cerebellar excitatory projections to the ventral intermediate nucleus of the thalamus may be enhanced due to PD-related changes, including dopaminergic/non-dopaminergic system abnormality, white matter damage, and deep nuclei impairment, which may contribute to dysregulation and resistance to levodopa of tremor. This review summarized the pathological, structural, and functional changes of the cerebellum in PD and discussed the role of the cerebellum in PD-related tremor, aiming to provide an overview of the cerebellum-related mechanism of tremor in PD.
Subject terms: Parkinson's disease, Parkinson's disease, Neurological manifestations
Introduction
Tremor, defined as an involuntary, rhythmic, and oscillatory movement of a body part, is one of the cardinal symptoms of Parkinson’s disease (PD)1,2. Traditional taxonomy of PD introduced several subtypes, including tremor-dominant PD and non-tremor-dominant PD (including postural instability and gait disability-dominant PD, akinesia/rigidity-dominant PD)3. Recent studies demonstrated there might be different biological bases for different subtypes of PD4. Tremor-dominant PD patients tend to have lower proportion of death and disability, slower progression of the disease, better cognitive function, lower burden of nonmotor symptoms, and longer survival time3,4. Thus, tremor-dominant PD is considered a benign subtype of PD5. However, among all the motor symptoms of PD, the mechanism of tremor is still poorly understood6, and its responsiveness to levodopa varies5,7.
The role of the cerebellum in the mechanism of tremor in PD has been increasingly focused8. The cerebellar output has been verified to modulate tremor-related activity, which arises from globus pallidum and propagates to cerebello-thalamo-cortical (CTC) circuits9. Moreover, tremor-related cerebellar activity differs between PD patients with dopamine-responsive tremor and dopamine-resistant tremor5, indicating a role of the cerebellum in the responsiveness of tremor to dopamine in PD.
We reviewed the pathological, structural, and functional changes of the cerebellum in PD and discussed the role of the cerebellum in PD-related tremor, aiming to provide an overview of the cerebellum-related mechanism of tremor in PD.
Pathological changes in the cerebellum in PD
PD is characterized by Lewy body pathology formed by α-synuclein, while cerebellum was thought to be unaffected by Lewy bodies previously8,10. However, recent studies discovered α-synuclein-related pathological changes in the cerebellum in PD patients, which may be associated with tremor symptoms. In PD patients, α-synuclein-formed Lewy bodies, which were speculated to originate in the pre-cerebellar brainstem and spread in a prion-like manner, were identified in the cerebellum11. Lewy bodies were found mainly in the cerebellar nuclei and adjacent white matters, while cerebellar lobules were only affected mildly11. Histologically, in the cerebellum of PD patients, Lewy bodies were found in Bergmann glia in the molecular layer and Purkinje cell axons12,13.
PD patients have longer climbing fiber length, more climbing fibers extending into the molecular layer, more climbing fiber-Purkinje cell synapses, and increased percentage of climbing fiber-Purkinje cell synapses on the thin Purkinje cell dendritic branchlets compared with healthy controls, accompanied by torpedoes/swelling of Purkinje cell axons14,15. Based on cluster analysis, these pathological changes may form a pattern that predicts the presence of resting tremor, PD patients with lower climbing-fiber synaptic density and a higher Purkinje cell count tend to have rest tremor15.
Moreover, iron accumulation has been identified in the deep nuclei of the cerebellum in PD. Using quantitative susceptibility mapping (QSM), iron content in dentate nuclei was found elevated in tremor-dominant PD patients compared with healthy controls and akinesia/rigidity-dominant PD patients, and was proven positively correlated to tremor severity despite subtypes of PD16–18. Because iron accumulation may indicate ferroptosis, a nonapoptotic cell death pathway, increased iron content in dentate nuclei suggests a role of dentate nuclei and cerebellum in the pathophysiological mechanism of tremor in PD16–19. Notably, ferroptosis may also promote α-synuclein aggregation16–18.
Interestingly, both α-synucleinopathies and iron accumulation affect dentate nuclei, which act as the only output nuclei of the cerebellum, indicating a role of the cerebellum in tremor. White matter changes in the cerebellum, especially Purkinje cells and related climbing fiber changes, presented a clinical manifestation-related pattern, suggesting a role of cerebellar network damage in the tremor of PD. Therefore, in PD patients, damage exists in both deep nuclei and white matters of the cerebellum. These pathological changes outline a comprehensive impairment pattern of the cerebellum.
Tremor-related structural and functional changes in the cerebellum in PD
Structural change
In previous studies, several tremor-related structural changes have been identified in the cerebellum of PD. When compared with PD patients without rest tremor, PD patients with rest tremor presented decreased gray matter volume mainly in quadrangular lobe and declive20, and tremor-dominant PD patients had decreased gray matter volume in left cerebellar lobule VIIIa compared with akinesia/rigidity-dominant PD patients21. Besides, larger volume of cerebellar lobule IV is associated with severer resting tremor in all PD patients22. These findings suggest a possible relation between these cerebellar regions and tremor in PD.
Additionally, tremor-dominant PD patients also present decreased gray matter volume in left cerebellar lobule VI, VIIb,VIIIb, and vermal cerebellar lobules VI and VIIIa compared with healthy controls, but such decrease in gray matter volume in these cerebellar regions was not correlated with tremor severity21. It has also been reported that no significant difference in gray matter volume and white matter volume exists between PD patients with tremor and healthy controls23. The relation of volume changes in these cerebellar regions with tremor needs to be further illustrated.
The findings above present controversial relation between tremor and cerebellar gray matter volume changes in PD. Notably, the atrophy of a lobule may cause the neighboring lobule to be volumetrically larger or vice versa. Therefore, volumetric analysis of cerebellar regions separately may be insufficient for outlining the tremor-related volumetric change of cerebellum in PD. A comprehensive pattern of volumetric change of cerebellum may be helpful for a better understanding of the role of the cerebellum in tremor in PD.
More importantly, although possible relevance exists between volumetric changes and tremor in PD, whether the volumetric change is a causal factor, consequence, or concomitant phenomenon of tremor is unclear. Therefore, in the future, histological research may be necessary for further illustration of the relation between cerebellar change and tremor in PD.
Although there is limited Diffusion tensor imaging (DTI) studies investigating the role of the cerebellum in tremor in PD, a DTI study demonstrated white matter abnormality within multiple tracts including middle cerebellar peduncle and superior cerebellar peduncle compared with healthy controls and non-tremor-dominant PD patients24. This study adds a probability of the involvement of the cerebellar white matters in the tremor mechanism in PD. However, another study found no cerebellar white matter change between tremor-dominant PD patients and non-tremor-dominant PD patients25,26 (Table 1). Further DTI study investigating the role of the cerebellar white matter changes in the tremor of PD is needed.
Table 1.
Structural imaging studies reporting on tremor and cerebellum in Parkinson’s disease.
| Authors | Study design | Main finding |
|---|---|---|
| Benninger et al.20 | Comparison of GMV in basal ganglia, thalamus, brainstem and cerebellum in PD patients with rest tremor vs. PD patients without rest tremor | Decreased GMV mainly in quadrangular lobe and declive was found in PD patients with rest tremor |
| Piccinin et al.21 | Comparison of cerebellar GMV in HC vs. ARPD vs. TPD |
(1) Changes in cerebellar GMV seems driven solely by TPD. (2) Decreased GMV in the left cerebellar lobule VIIIa was found in TPD when compared with ARPD. (3) Decreased GMV in multiple cerebellar lobules was found in TPD patients when compared with HC. |
| Lopez et al.22 |
(1) Comparison of cerebellar lobule volumes in PD patients vs essential tremor patients (2) Correlation of severity of symptoms and lobule volume in PD patients and ET separately. |
In PD patients, lobule volume of cerebellar lobule IV was positively correlated with resting tremor and total tremor severity. |
| Choi et al.23 | Comparison of volumes of different brain structures in PD patients with tremor vs. ET vs. healthy controls. | No significant difference in GMV and white matter volume existed between PD patients with tremor and HC. |
| Luo et al.24 | Comparison of white matter integrity in TPDa vs. NTPD vs. HC by tract-based spatial statistics. | White matter integrity differences in the white matter tract, including middle cerebellar peduncle and superior cerebellar peduncle, existed when compared TPD with HC or NTPD. |
ARPD akinetic/rigidity-predominant PD patients, GMV gray matter volume, HC healthy controls, TPD tremor-dominant PD patients.
aTPD in this study is defined by the presence of a severe tremor and NTPD is defined by the absence of tremor at rest.
Functional change
Studies found functional changes in the cerebellum in tremor-dominant PD patients. PD patients presented a tremor-related metabolic pattern in 18F-deoxyglucose positron emission tomography (FDG-PET), glucose metabolism in their dentate nuclei and anterior cerebellar lobule (IV and V) was increased in resting state. This increase in glucose metabolism was positively correlated with tremor amplitude and could be suppressed by both VIM and subthalamic nucleus (STN) deep brain stimulation (DBS)27.
In resting-state functional MRI (fMRI), tremor-dominant PD patients showed decreased voxel-mirrored homotopic connectivity (VMHC) in the cerebellar posterior lobe. Moreover, VMHC in the cerebellar posterior lobe was reported negatively correlated with tremor severity in PD patients, while the amplitude of low-frequency fluctuations (ALFF) in this region was reported positively correlated with tremor severity28,29. Besides, increased local synchronization of activity in cerebellar crus I and cerebellar lobule VI and decreased local synchronization of activity in cerebellar vermis III, cerebellar lobule IV, and cerebellar lobule V in resting-state was also found in tremor-dominant PD patients. However, no correlation between local synchronization of activity in those regions and clinical manifestations was identified30. fMRI studies also found changes in functional connectivity between different cerebellar regions. Functional connectivity between cerebellar cortex and dentate nuclei in tremor-dominant PD patients is increased when compared with non-tremor-dominant PD patients31. Interestingly, among these functional connectivities, the functional connectivity between dentate nuclei and the cerebellar posterior lobe is positively correlated with tremor severity, while the connectivity between the anterior cerebellar lobules and dentate nuclei presents no correlation with clinical manifestations31. Additionally, tremor-dominant PD patients presented decreased connectivity between bilateral cerebellar hemispheres compared with healthy controls32.
Functional connectivity between the cerebellum and other structures was also altered. Functional connectivity in tremor-dominant PD patients between cerebellar lobule VI and basal ganglia, between the cerebellum and supplementary motor areas/insula, were found to be increased compared with healthy controls32. Connectivity between bilateral dentate nuclei and prefrontal cortex in tremor-dominant PD patients was decreased compared with healthy controls and non-tremor-dominant PD patients31. Furthermore, connectivity between these regions was negatively correlated with tremor severity, while connectivity between a region comprising cerebellar lobules V, VI, VII, and VIII, and supplementary motor areas was positively correlated with tremor severity in all PD patients31,32 (Table 2).
Table 2.
Functional imaging studies reporting on tremor and cerebellum in Parkinson’s disease.
| Authors | Study design | Main findings |
|---|---|---|
| Mure et al.27 |
(1) Identify the metabolic network in TPD. (2) Identify the correlation between the metabolic network (above) and clinical manifestations/ characters and interventions directed at tremor. |
(1) Tremor-related metabolic pattern was characterized by increases in cerebellum/dentate nucleus, primary motor cortex, and caudate/putamen. (2) VIM and STN DBS lead to reduced expression of the tremor-related metabolic pattern. (3) Pattern expression values correlated with tremor amplitude. |
| Hu et al.28 |
(1) Comparison of homotopic resting-state functional connectivity patterns (revealed by VMHC) in akinetic-rigid PD (ARPD) vs. tremor-dominant PD (TPD) vs. healthy controls (2) Identify the correlation between VMHC values and clinical characters. |
(1) TPD exhibited lower VMHC in the posterior lobe of the cerebellum when compared with ARPD and HC. (2) Tremor scores are negatively correlated with VMHC in the posterior lobe of the cerebellum (only) in TPD. |
| Chen et al.29 |
(1) Comparison of spontaneous neural activity (revealed by ALFF) in resting-state in PIGD vs. TPD vs. HC. (2) Identify the correlation between ALFF values and clinical characters. |
(1) TPD exhibited higher ALFF in the cerebellar posterior lobe when compared with PIGD and HC. (2) Tremor scores are positively correlated with ALFF in the cerebellar posterior lobe in all PD patients. |
| Ma et al.31 |
Comparison of functional connectivity of DN (with other brain structures) in TD vs. PIGD. Identify the correlation between functional connectivity of DN and the tremor severity |
(1) In TPD, DN exhibited higher connectivity with the cerebellar anterior lobe and lower connectivity with the prefrontal cortex when compared with HC and PIGD. (2) In TPD, DN exhibited higher connectivity with the cerebellar posterior lobe when compared with PIGD. (3) Connectivity between DN and cerebellar posterior lobe correlated with tremor positively in all PD patients. (4) Connectivity between DN and prefrontal cortex correlated with tremor severity negatively in all PD patients. |
| Hou et al.32 |
(1) Comparison of functional connectivity (focusing on the basal ganglia (BG) and cerebellum) in TPD vs. PIGD vs. HC (2) Identify the correlation between functional connectivity and tremor severity. |
(1) Higher functional connectivity between the cerebellum and paracentral lobule, sensorimotor areas was identified when compared PIGD or TPD with healthy controls. (2) Higher functional connectivity between the BG and cerebellar lobule VI, between the cerebellum and supplementary motor areas (SMA)/insula and lower FC within the cerebellum circuit was found when compared TPD with HC. (3) In all PD patients, functional connectivity between a region comprising cerebellar lobules V, VI, VII, and VIII and supplementary motor areas positively correlated with tremor scores in all PD patients. |
ARPD akinetic/rigidity-predominant PD patients, HC healthy controls, TPD tremor-dominant PD patients, PIGD postural instability and gait difficulty PD patients, VIM ventral intermediate nucleus of the thalamus, STN subthalamic nucleus of the thalamus, DBS deep brain stimulation, VMHC voxel-mirrored homotopic connectivity, ALFF increased amplitude of low-frequency fluctuations, DN dentate nucleus of the cerebellum.
According to these previous reports, it seems that dentate nuclei plays a key role in cerebellum-related tremor regulation in tremor-dominant PD patients. It is possible that the activation of dentate nuclei or increase of dentate nuclei-cerebellar cortex interaction may enhance tremor, while the prefrontal cortex may suppress tremor via regulating the activity of dentate nuclei. These findings suggest that direct or indirect intervention on dentate nuclei may be a potential target of alleviating tremor in PD. As dentate nuclei lies deep in the cerebellum, it is inconvenient for intervention therapy such as DBS. However, the prefrontal cortex may be a potential target to regulate dentate nuclei more accessibly. Moreover, cerebellar lobules may be involved in the mechanism of tremor in PD via its influence on dentate nuclei activity. The interaction between the bilateral cerebellar hemisphere, cerebellar lobules, and dentate nuclei may also play a role in tremor activity, but the definite mechanism requires further study.
The role of the cerebellum in tremor in PD
Cerebellum may participate in tremor mechanism via cerebello-thalamo-cortical circuit
The basic of recent research on rest tremor and its responsiveness to dopaminergic treatment is the “dimmer switch model”, which is described in a systematic/circuit-level, and this model is also a foundation for the role of the cerebellum in tremor in PD1. This model focus on activity within two important tremor-related circuits, the basal ganglia circuits and the cerebello-thalamo-cortical circuits, and the interaction between these two important circuits. According to the “dimmer-switch model”, transient tremor-related activity first arises in the basal ganglia and, more precisely, internal pallidal globus (GPi), possibly as a consequence of pathological activity due to dopamine depletion in striato-pallidal circuit1,9,33. This tremor-related activity propagates to cerebello-thalamo-cortical (CTC) circuits via the connection between GPi and motor cortex5,9,33–35. Then, tremor-related activity may propagate to the thalamus and cerebellum via cortico-thalamic and cortico-cerebellar connectivity, within the CTC circuits9,36,37. And tremor-related activity may continue to exist in the CTC circuits and thus maintain the tremor until another signal that interacts with tremor-related firing in the CTC circuits is generated1.
Moreover, cortico-thalamic excitatory projections from the motor cortex to VIM may lead to low-frequency oscillations within the thalamocortical network37. On the other hand, the cerebellum influences the thalamus via glutamatergic excitatory projections from cerebellar deep nuclei to VIM38–41.
Conclusionally, tremor-related activity origins at GPi and propagates to the motor cortex, where the CTC circuit is activated. Activation of CTC circuits forms cortex-thalamus oscillations, which act as the base of tremor in PD, while the cerebellum modulates tremor amplitude by modulating the activity of VIM in the thalamus. Moreover, the modulation of the cerebellum on VIM is regulated by the motor cortex9,33 (Fig. 1).
Fig. 1. Dimmer switch model of tremor in Parkinson’s disease (PD).
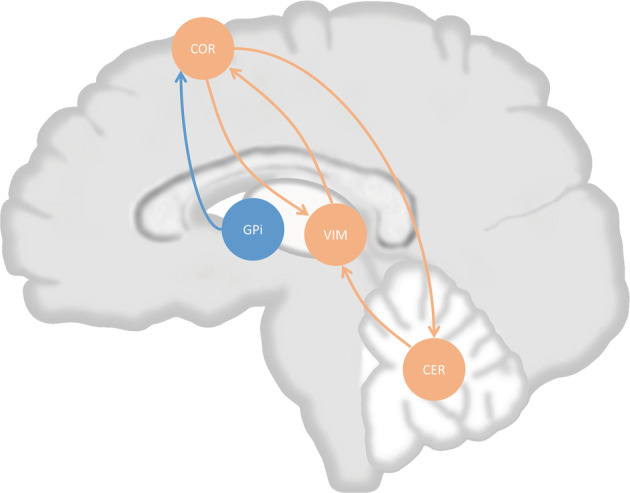
Tremor-related activity originates at internal pallidal globus (GPi), which propagate to cortex. Cortex and ventral intermediate nucleus of thalamus (VIM) form a circuit, which is possible the base of tremor-related oscillation. Cerebellum also projects to VIM, this projection possibly modulate amplitude of tremor, while cerebellum was modulated by cerebral cortex. COR cortex, CER cerebellum. Orange arrows indicate projections within cerebello-thalamo-cortical circuit, blue arrow indicates projection from GPi to cortex.
Before the “dimmer-switch model” was proposed, some previous studies focus on single oscillator (or pacemaker) in tremor mechanism, and localized tremor pacemaker in the basal ganglia or the thalamus according to the ability to oscillate at the same or double frequency of tremor in PD1. However, studies found multiple nodes, such as VIM, subthalamic nucleus, and pallidum might serve as a pacemaker, while the modulation of tremor frequency and tremor amplitude seemed independent9. Therefore, the “dimmer-switch model” was proposed for a better explanation of the mechanism of tremor in PD.
The “dimmer-switch model” attributes different contributions to different networks (or network nodes), and provide us with a comprehensive view of the tremor mechanism in PD. However, due to the spatial resolution limit of the methods used in current studies, some small nucleus that has been recognized to play a role in tremor mechanism, such as the subthalamic nucleus, is not involved in this model. The absence of these small but important nuclei can make the model incomplete and may lead to errors, especially when speculating the pathways in which tremor-related activity propagates through the brain using dynamic causal modeling (DCM) approaches that require a priori model1,9,34. Therefore, it is necessary to be cautious when making anatomical-level speculations on activity propagating pathways based on the “dimmer-switch model”.
In the dimmer switch model, the cerebellum may act as a modulator of tremor-related activity in PD mainly based on the following evidence: (1) there is bidirectional connectivity between the motor cortex and thalamus, forming a circuit that could maintain the tremor-related oscillation independently; (2) cerebellum participates in tremor-related circuit with a unidirectional manner, more specifically cortico→cerebello→thalamic connectivity9; (3) cerebellar stimulation could not reset tremor1 (Fig. 1).
Additionally, previous studies found that the cerebellum may be involved in processing tremor-related afferents from periphery42, while the cortex drives limb tremor43, and VIM is involved in both processing tremor-related afferents and driving of limb tremor44. Therefore, the cerebellum may be a key structure in the feedback of tremor. Thus, impaired cerebellar function due to PD may contribute to the tremor mechanism in PD via modulating tremor amplitude and feedback of peripheral tremor-related afferents.
Moreover, recent studies found there were bidirectional anatomical connectivities between the cerebellum and basal ganglia, that was: (1) cerebellum nuclei (dentate nuclei) → thalamus → striatum (2) subthalamic nuclei→ pontine nuclei→ cerebellum cortex45–47, but whether this bidirectional connectivity has a role in the mechanism of tremor in PD requires further investigation.
Neurotransmitters, cerebellum, and tremor in PD
Dopaminergic dysfunction is traditionally regarded as the cause of PD48. More specifically, there may be a pallidal and thalamic dopamine depletion in PD patients, subsequent to dopaminergic degeneration in substantia nigra, ventral tegmental area, and mesencephalic retrorubral area1,49. According to the “dimmer switch model”, dopamine depletion in pallidum may be the trigger of tremor-related activity in PD33. Dopamine depletion may also cause thalamic excitation, which contributes to tremor-related circuit activity34. Additionally, although the cerebellum is traditionally regarded as a non-dopaminergic brain area, recent studies demonstrated dopaminergic neurotransmission in the cerebellum50. These projections may arise from basal ganglia based on the anatomical connectivity between the cerebellum and basal ganglia45–47 and may be influenced by PD-related pathology in the cerebellum50. Unfortunately, few studies directly investigated the relevance of the cerebellar dopaminergic system to tremor in PD.
Although dopaminergic dysfunction may contribute to tremor, it seems not the unique cause of tremor in PD. Levodopa treatment presents various effects on tremor in PD patients. In some PD patients, levodopa may present a poor effect on tremor, even though their other symptoms, including bradykinesia and rigidity, are alleviated5,7,51,52. This phenomenon persists even at a high dose of levodopa treatment5,7, and this tremor is known as dopamine-resistant tremor, which we discussed in the section “Dopamine-resistant Parkinson’s tremor and its relation with cerebellum” below.
Serotonergic neurons in raphe nuclei act as the main source of serotonin in the brain, they gradually degenerate in PD patients as PD pathology progresses53–55, leading to serotonin depletion in structures that receive serotonergic projections, such as cortex, thalamus, and basal ganglia56–59.
Serotonin system damage plays an important role in the mechanism of tremor in PD. Polymorphism of the SLC6A4 gene encoding the serotonin reuptake transporter is associated with rest tremor in PD60. The serotonin-transporter availability in raphe nuclei is decreased in both early stage and advanced tremor-dominant PD patients compared with akinetic-rigidity-dominant PD patients. Moreover, this availability was negatively correlated with tremor severity in all subtypes of PD patients57,61. Importantly, serotonergic system degeneration contributes more to tremor than striatal dopaminergic degeneration, and its severity is negatively correlated with responsiveness to levodopa61.
Serotonergic neurons influence the cerebellum directly by projection to cerebellar cortex62–65 and indirectly via multiple structures45–47. Serotonergic neurons in raphe nuclei project to basal ganglia66, which connects with the cerebellum bidirectionally45–47. Besides, serotonergic neurons also project to structures such as the cortex, several brainstem nuclei, and spinal cord that may influence cerebellar activity59,67. Thus, serotonergic system impairment may result in abnormal input into the cerebellum and, consequently, affect tremor-related circuits1.
Although a few single photon emission computed tomography (SPECT) studies investigate the state and role of the serotoninergic system in the tremor of PD57,61, they did not investigate the role of the serotoninergic system in the cerebellum, possibly due to that 123I-FP-CIT in SPECT could act as a tracer for serotonin only in serotonin-transporter-rich region such as raphe nuclei, and cerebellum may not meet the requirement68. Besides, in PET and SPECT studies, the cerebellum is traditionally considered as a region with mainly nonspecific binding and is usually used as a reference region for calculating binding ratio68–70. Thus, the status and role of the serotonergic system in the cerebellum in PD may be ignored. Radioligand that could selectively reflect serotonin distribution may help investigate the role of the cerebellar serotonergic system in PD tremor.
Locus coeruleus is affected in the early stage of PD pathology spread, which leads to noradrenergic content loss of up to 70% in the brain71–76, resulting in decreased noradrenergic projection to cerebellum77, thalamus78, and motor cortex78 in PD. In other words, all structures involved in CTC circuits suffer the loss of noradrenergic input in PD.
Interestingly, tremor-dominant PD patients present less neuronal loss in locus coeruleus79, which may indicate a relatively preserved noradrenergic system. Consistently, activating the noradrenergic system (by acute cognitive stress72) in human could enhance the activity of the CTC circuit, exacerbate tremor, and suppress the effect of levodopa on tremor in PD patients72,80. Activation of the noradrenergic system stimulates both the bottom-up arousal and top-down cognitive control networks, enhances the thalamic activity and CTC circuit activity80. On the other hand, inactivating the noradrenergic system by sleep, β-blockers, or placebo, may ameliorate tremor in PD patients81–85. These findings support that the noradrenergic system exacerbates tremor in PD.
Interestingly, noradrenergic neurons in locus coeruleus project directly to cerebellum71,72,86–94, and may indirectly influence the cerebellum by affecting the thalamus and cortex. It is possible that the direct and indirect effect of noradrenergic system on the cerebellum may influence its modulation on tremor amplitude in PD1. Notably, current studies focused on the short-time effect of noradrenergic system change in PD. However, the noradrenergic system is persistently preserved in tremor-dominant PD patients compared with non-tremor dominant PD patients79, suggesting a potential background of the continuous noradrenergic system activation, which may lead to a different cerebellar activity status in tremor-dominant PD patients from in non-tremor dominant PD patients. It is a pity that, to our knowledge, there is an absence of direct measurements of noradrenergic activity in vivo, making it difficult to investigate the relation between cerebellar noradrenergic status and tremor in PD patients. Further study is needed to illustrate the role of the noradrenergic system in cerebellum-related tremor activity.
Abnormal output of cerebellum may contribute to tremor in PD, possibly via CTC circuit
Cerebellum itself has a complex information processing system. The Glutamatergic granule cell in the cerebellum receives multiple inputs, including dopaminergic input from mesencephalon, serotonergic input from raphe nuclei, and noradrenergic input from locus coeruleus, while all these structures are affected in PD95–97 (Fig. 2). For cerebellar projection, GABAergic Purkinje cells form a network in the cerebellum and integrate input from parallel fibers (from granule cell in the cerebellum) and climbing fibers (from inferior olive nuclei), and connect with dentate nuclei76,95,96, which project to thalamus and other structures8,45–47,98,99. This integrating process could also be damaged by PD-induced Purkinje cell loss, axonal/white matter abnormality, and altered fiber character11–15. Moreover, iron accumulation in dentate nuclei in PD patients may also indicate damage of cerebellar output16–19, more specifically, the output from cerebellar dentate nuclei to the thalamus via its excitatory glutamatergic projection on VIM8,45–47,98,99. Therefore, the external input into the cerebellum, the integrating process in the cerebellum, and the output nuclei are all damaged in PD.
Fig. 2. Neurotransmitters modulate tremor activity in Parkinson’s disease (PD) via influencing cerebellar output.
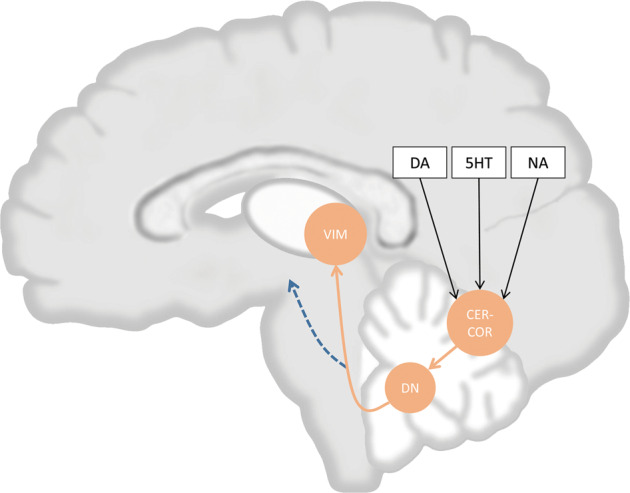
Various neurotransmitters including dopamine (DA), serotonin (5HT), and noradrenaline (NA), affect the cerebellar cortex (CER-COR), which modulate the output activity of dentate nuclei (DN). DN project to ventral intermediate nucleus of the thalamus (VIM) and modulate tremor activity. Orange arrows indicate projections between CER-COR, DN, and VIM. The Blue arrows indicate cerebellar output to other brain regions. Black arrows indicate the effect of neurotransmitters.
VIM receives glutamatergic projections from cortex and cerebellum, and GABAnergic self-inhibitory projection98, and it may be a key modulator of tremor in PD patients, according to recent studies reporting the following findings: (1) high-frequency DBS stimulation on VIM may induce synaptic fatigue at excitatory glutamatergic synapses after a transient excitation of these synapses, thereby suppresses tremor in PD patients8,98; (2) low-frequency DBS stimulation on VIM that can excite glutamatergic synapses while allowing glutamatergic synaptic vesicle to be replenished worsens tremor in PD patients98; (3) excitation of VIM induced by cognitive load was reported to exacerbate tremor in PD patients80 (Fig. 3).
Fig. 3. Ventral intermediate nucleus of the thalamus (VIM) plays a central role in tremor activity and may be a target of tremor interference.
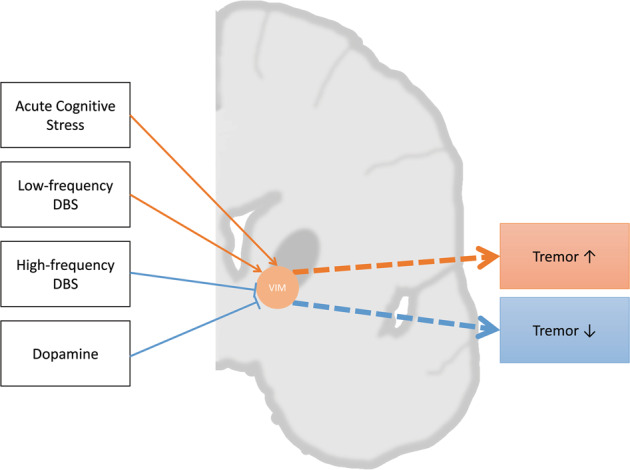
Acute cognitive stress, deep brain stimulation (DBS), and dopamine could influence VIM and thus regulate tremor. DBS with different frequency present a different effect on tremor activity. Orange arrows indicate excitatory effect, blue arrows indicate inhibitory effect.
Notably, excitatory glutamatergic synapses on VIM are from the cerebellum, at least partially5,98, indicating an important role of the cerebellum in the modulation of tremor in PD patients, possibly by its glutamatergic projection. Consistently, levodopa-resistant tremor-dominant PD patients present relatively higher activity in the cerebellum, including dentate nuclei, lobule IV, lobule V, vermis IX, and interposed nuclei5. Therefore, the cerebellum may also play an important role in the modulation of tremor in PD via regulating the activity of VIM by its glutamatergic projection. Thus, suppressing cerebellar glutamatergic projection to VIM may be a potential strategy for treating PD tremor.
Additionally, it has been demonstrated in essential tremor that GABAergic projection abruption15,100–104, as well as its possible influence on deep nuclei105,106, may lead to disinhibition of dentate nuclei, which may serve as the pacemaker and drive the CTC circuit to generate tremor15,99. Therefore, it is also possible in PD that abnormal output of dentate nuclei may contribute to disinhibition of the CTC circuit and lead to tremor, but direct evidence is required for this hypothesis.
Conclusionally, in PD, altered input from the dopaminergic, serotonergic and noradrenergic system into the cerebellum, damage of cerebellar integrating process, and dentate nuclei damage may alter cerebellar (excitatory) output at three different levels, and thereby, may possibly influence the modulation of tremor via affecting VIM and then CTC circuit, as indicated by the “dimmer switch model”1.
Cerebellum-related mechanism of therapy for tremor in PD
As the most commonly used drug therapy for PD, levodopa could ameliorate tremor in PD patients by suppressing tremor-onset-related activity in GPi and inhibiting tremor-amplitude-related activity in VIM (where D2 receptor was identified recently)34. The latter mechanism possibly decreases/normalizes functional coupling between the thalamus and motor cortical areas5,107. The mechanism of VIM inhibiting exists in both dopamine-responsive and dopamine-resistant PD patients, but is more prominent in dopamine-responsive PD patients5,34.
Interestingly, levodopa suppresses tremor in PD via inhibiting tremor-amplitude-related activity in VIM, supporting VIM as a key target of levodopa treatment in suppressing tremor in PD5,34,98,107. Thereby, abnormally enhanced glutamatergic projection from the cerebellum on VIM may decrease the susceptibility of VIM to dopamine, and thus, results in levodopa resistance5,34. Therefore, if further investigated, the cerebellum may be a promising alternative therapeutic target in PD patients with dopamine-resistant tremor.
Dopamine agonists (such as pramipexole)85,108–110, monoamine oxidase B (MAOB) inhibitors (such as selegiline, rasagiline)111–113, and catechol-O-methyltransferase (COMT) inhibitors (such as entacapone, tolcapone)85,114 were also reported to alleviate tremor via different mechanisms. It is easy to understand that COMT inhibitors alleviate tremor by promoting the effect of levodopa85,114. Pramipexole has been reported to be effective in some tremor resistant to antiparkinsonian drugs other than pramipexole110. However, the effect of pramipexole on tremor-related circuits and cerebellum is unclear. Similarly, the effect of MAOB inhibitors on tremor-related circuits and cerebellum is also unclear, and further investigations are needed.
Additionally, as classical anti-tremor drugs, anticholinergic drugs, including benzhexol, could alleviate tremor by modulating the balance between the dopaminergic and cholinergic systems. However, the specific mechanism and its effect on tremor-related circuits and cerebellum are also poorly understood115–117.
Notably, dopamine receptors and choline receptors are expressed in the cerebellum, although at relatively low level50,118, it is possible that these drugs (except levodopa) may modulate cerebellar activity and thus, alleviate tremor via CTC circuits. However, no existing direct evidence support this hypothesis; further study is needed.
In addition to drug therapy, non-pharmacological treatment is another approach to alleviate tremor, particularly dopamine-resistant tremor, in PD119. Thalamotomy is historically an invasive method of relieving drug-resistant tremor in PD120,121. The mechanism may be direct damage to the VIM, which plays an essential role in the tremor mechanism120. However, thalamotomy is irreversible and uncontrollable, and may bring complications such as paresthesia and gait disturbance122. Even with modification of surgical approach, complications related to surgery itself may occur122,123.
DBS is the preferred method for relieving PD tremor compared to thalamotomy currently122. VIM-DBS effectively alleviates both dopamine-responsive and dopamine-resistant tremor, but has limited effect on other parkinsonian symptoms such as rigidity and bradykinesia124. This puts VIM at a disadvantage compared with other targets for DBS, such as the subthalamic nucleus, which could alleviate multiple symptoms and possibly slow the progression of PD125. Anyway, the effectiveness of VIM-DBS provides us with better insight into the tremor mechanism in PD. VIM-DBS was reported to ameliorate tremor by inhibiting neuronal firing in the VIM, and this inhibition occurs after transient neuronal firing in the VIM, which leads to transient worsening of tremor, and finally lead to fatigue of VIM excitatory afferents, and inhibition of VIM activity98. Indeed, VIM is nucleus that receives glutamatergic excitatory afferents from the cerebellum38,39. Thus, VIM-DBS may alleviate tremor by modulating cerebellum-regulated VIM activity, and thus may change the activity in the CTC circuit. However, the specific mechanism that VIM-DBS ameliorate tremor in PD is still unclear and needs further research.
Dopamine-resistant Parkinson’s tremor and its relation with cerebellum
The effect of dopamine on tremor in PD is variable between individuals. Zach, et al. Categorized PD patients into three clusters (the dopamine-responsive, intermediate, and dopamine-resistant rest tremor) based on the change of tremor amplitude and the change of tremor power after levodopa challenge7. The PD patients with dopamine-responsive rest tremor (PD-RP) display a higher disease severity, longer disease duration, and a higher frequency of accompanying dyskinesia when compared with PD patients with intermediate and dopamine-resistant rest tremor7. As mentioned above, VIM inhibition is an essential mechanism for dopamine to alleviate tremor34, this inhibition is more significant in PD-RP when compared with dopamine-resistant rest tremor (PD-RS)5,34. Furthermore, several brain regions in the cortex, thalamus, and cerebellum present different tremor-related activity between PR-RS and PR-RP5. Besides, although not statistically significant, the score for rest tremor severity is lower in PD-RS when compared with PD-RP5,7. These studies indicate that there may be a different mechanism underlying the two/three phenotypes as they represent different clinical and pathophysiological characters.
A possible explanation for the above differences in responsiveness of tremor to dopamine between PD-RS and PD-RP may be as follows: Because different brain regions may be affected as the PD pathology progresses11,53–56,73–78. At different disease stages, brain regions responsible for tremor may be affected by PD pathology to different degrees. In other words, many brain regions may participate in tremor mechanisms, but at a certain disease stage, one of these regions may account most for tremor, which may result in altered responsiveness of rest tremor to dopamine as the disease progresses7. As the cerebellum is mainly affected by various non-dopaminergic neurotransmitters rather than dopaminergic neurotransmitters5, resistance to dopamine may occur when the cerebellum accounts most for tremor at a certain disease stage. This speculation is supported by the study of Dirkx, et al., which found increased tremor-related activity in cerebellar lobules IV/V/IX and nuclei5.
Further longitudinal researches on the change of tremor-related activity in different brain regions (e.g., cerebellum, thalamus, and cortex), tremor responsiveness to dopamine, and tremor-related activity-dopamine responsiveness relationships as disease progressing are needed for a better understanding of the mechanisms of dopamine-resistant rest tremor and the role of the cerebellum in it.
In conclusion, the cerebellum is affected by α-synuclein-formed Lewy bodies and by iron accumulation in PD, which may induce tremor-related white matter alteration and tremor-specific structural and functional changes in the cerebellum. This damage in the cerebellum, together with damage of other structures, including mesencephalon, raphe nuclei, and locus coeruleus, may alter cerebellar (excitatory) output at three different levels (the input, the integrating process, and the output nuclei). The altered output of the cerebellum to VIM could excite the CTC circuit and enhance the tremor amplitude in PD. The dysregulation of cerebellum-modulated VIM activity may also decrease the susceptibility of VIM to levodopa, thus leading to dopamine-resistant tremor. Currently, few studies investigated the role of the cerebellum in PD-related tremor and its therapy, but still indicated an important role of the cerebellum in the mechanism of PD-related tremor. If further researched, the cerebellum may be a promising target of understanding and treatment of tremor in PD.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (grant number: 81771391, receiver: Lifen Chen; grant number: 82001367, receiver: Xi Liu), Natural Science Foundation of Chongqing (grant number: cstc2021jcyj-msxmX0180, receiver: Xi Liu), and Kuanren Talent Program of The Second Affiliated Hospital of Chongqing Medical University (receiver: Xi Liu).
Author contributions
L.C., F.D., and X.L.: conception and design; Y.Z., H.L., and G.L.: literature search; Y.Z., L.Z., C.D., and Y.L.: literature screening; Y.Z., J.D., and X.Z.: first draft; Y.Z., L.M., C.T., X.T., and X.L.: review and editing. All authors reviewed and approved the manuscript.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xi Liu, Email: tianwailiuxi@cqmu.edu.cn.
Lifen Chen, Email: lifen_chen@cqmu.edu.cn.
References
- 1.Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain. 2012;135:3206–3226. doi: 10.1093/brain/aws023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia KP, et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018;33:75–87. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson’s disease: clinical and prognostic implications. Neurology. 1985;35:522–526. doi: 10.1212/WNL.35.4.522. [DOI] [PubMed] [Google Scholar]
- 4.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov. Disord. 2016;31:1095–1102. doi: 10.1002/mds.26510. [DOI] [PubMed] [Google Scholar]
- 5.Dirkx MF, et al. Cerebral differences between dopamine-resistant and dopamine-responsive Parkinson’s tremor. Brain. 2019;142:3144–3157. doi: 10.1093/brain/awz261. [DOI] [PubMed] [Google Scholar]
- 6.Hallett M. Parkinson’s disease tremor: pathophysiology. Parkinsonism Relat. Disord. 2012;18:S85–S86. doi: 10.1016/S1353-8020(11)70027-X. [DOI] [PubMed] [Google Scholar]
- 7.Zach H, et al. Dopamine-responsive and dopamine-resistant resting tremor in Parkinson disease. Neurology. 2020;95:e1461–e1470. doi: 10.1212/WNL.0000000000010316. [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirkx MF, et al. The cerebral network of Parkinson's tremor: an effective connectivity fMRI study. J. Neurosci. 2016;36:5362–5372. doi: 10.1523/JNEUROSCI.3634-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis MM, et al. The role of the cerebellum in the pathophysiology of Parkinson’s disease. Can. J. Neurol. Sci. 2013;40:299–306. doi: 10.1017/S0317167100014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidel K, et al. Involvement of the cerebellum in Parkinson disease and dementia with Lewy bodies. Ann. Neurol. 2017;81:898–903. doi: 10.1002/ana.24937. [DOI] [PubMed] [Google Scholar]
- 12.Piao YS, et al. Alpha-synuclein pathology affecting Bergmann glia of the cerebellum in patients with alpha-synucleinopathies. Acta Neuropathol. 2003;105:403–409. doi: 10.1007/s00401-002-0655-0. [DOI] [PubMed] [Google Scholar]
- 13.Mori F, et al. Alpha-synuclein accumulates in Purkinje cells in Lewy body disease but not in multiple system atrophy. J. Neuropathol. Exp. Neurol. 2003;62:812–819. doi: 10.1093/jnen/62.8.812. [DOI] [PubMed] [Google Scholar]
- 14.Louis ED, et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov. Disord. 2009;24:1600–1605. doi: 10.1002/mds.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo SH, et al. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol. 2017;133:121–138. doi: 10.1007/s00401-016-1626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan X, et al. Influence of regional iron on the motor impairments of Parkinson’s disease: A quantitative susceptibility mapping study. J. Magn. Reson Imaging. 2017;45:1335–1342. doi: 10.1002/jmri.25434. [DOI] [PubMed] [Google Scholar]
- 17.Acosta-Cabronero J, et al. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson’s disease. Brain. 2017;140:118–131. doi: 10.1093/brain/aww278. [DOI] [PubMed] [Google Scholar]
- 18.He, N. et al. Dentate nucleus iron deposition is a potential biomarker for tremor-dominant Parkinson’s disease. NMR Biomed.30, 10.1002/nbm.3554 (2017). [DOI] [PMC free article] [PubMed]
- 19.Magtanong L, Dixon SJ. Ferroptosis and brain injury. Dev. Neurosci. 2018;40:382–395. doi: 10.1159/000496922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benninger DH, Thees S, Kollias SS, Bassetti CL, Waldvogel D. Morphological differences in Parkinson’s disease with and without rest tremor. J. Neurol. 2009;256:256–263. doi: 10.1007/s00415-009-0092-2. [DOI] [PubMed] [Google Scholar]
- 21.Piccinin CC, et al. Differential pattern of cerebellar atrophy in tremor-predominant and akinetic/rigidity-predominant Parkinson's disease. Cerebellum. 2017;16:623–628. doi: 10.1007/s12311-016-0834-5. [DOI] [PubMed] [Google Scholar]
- 22.Lopez AM, et al. Structural correlates of the sensorimotor cerebellum in Parkinson's disease and essential tremor. Mov. Disord. 2020;35:1181–1188. doi: 10.1002/mds.28044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi SM, et al. Comparison of the brain volume in essential tremor and Parkinson's disease tremor using an automated segmentation method. Eur. Neurol. 2015;73:303–309. doi: 10.1159/000381708. [DOI] [PubMed] [Google Scholar]
- 24.Luo C, et al. White matter microstructure damage in tremor-dominant Parkinson’s disease patients. Neuroradiology. 2017;59:691–698. doi: 10.1007/s00234-017-1846-7. [DOI] [PubMed] [Google Scholar]
- 25.Barbagallo G, et al. Structural connectivity differences in motor network between tremor-dominant and nontremor Parkinson’s disease. Hum. Brain Mapp. 2017;38:4716–4729. doi: 10.1002/hbm.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haghshomar, M. et al. Cerebellar microstructural abnormalities in parkinson’s disease: a systematic review of diffusion tensor imaging studies. Cerebellum, 10.1007/s12311-021-01355-3 (2022). [DOI] [PubMed]
- 27.Mure H, et al. Parkinson’s disease tremor-related metabolic network: characterization, progression, and treatment effects. Neuroimage. 2011;54:1244–1253. doi: 10.1016/j.neuroimage.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X, et al. Decreased interhemispheric functional connectivity in subtypes of Parkinson’s disease. J. Neurol. 2015;262:760–767. doi: 10.1007/s00415-014-7627-x. [DOI] [PubMed] [Google Scholar]
- 29.Chen HM, et al. Different patterns of spontaneous brain activity between tremor-dominant and postural instability/gait difficulty subtypes of Parkinson’s disease: a resting-state fMRI study. CNS Neurosci. Ther. 2015;21:855–866. doi: 10.1111/cns.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, et al. Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of intrinsic brain activity. Parkinsonism Relat. Disord. 2015;21:23–30. doi: 10.1016/j.parkreldis.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Ma H, et al. Resting-state functional connectivity of dentate nucleus is associated with tremor in Parkinson’s disease. J. Neurol. 2015;262:2247–2256. doi: 10.1007/s00415-015-7835-z. [DOI] [PubMed] [Google Scholar]
- 32.Hou Y, et al. Patterns of striatal and cerebellar functional connectivity in early-stage drug-naïve patients with Parkinson’s disease subtypes. Neuroradiology. 2018;60:1323–1333. doi: 10.1007/s00234-018-2101-6. [DOI] [PubMed] [Google Scholar]
- 33.Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I. Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann. Neurol. 2011;69:269–281. doi: 10.1002/ana.22361. [DOI] [PubMed] [Google Scholar]
- 34.Dirkx MF, et al. Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain. 2017;140:721–734. doi: 10.1093/brain/aww331. [DOI] [PubMed] [Google Scholar]
- 35.Helmich RC, Bloem BR, Toni I. Motor imagery evokes increased somatosensory activity in Parkinson’s disease patients with tremor. Hum. Brain Mapp. 2012;33:1763–1779. doi: 10.1002/hbm.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J. Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kultas-Ilinsky K, Sivan-Loukianova E, Ilinsky IA. Reevaluation of the primary motor cortex connections with the thalamus in primates. J. Comp. Neurol. 2003;457:133–158. doi: 10.1002/cne.10539. [DOI] [PubMed] [Google Scholar]
- 38.Kuramoto, E. et al. Complementary distribution of glutamatergic cerebellar and GABAergic basal ganglia afferents to the rat motor thalamic nuclei. Eur. J. Neurosci.33, 10.1111/j.1460-9568.2010.07481.x (2011). [DOI] [PubMed]
- 39.Kultas-Ilinsky K, Ilinsky IA. Fine structure of the ventral lateral nucleus (VL) of the Macaca mulatta thalamus: cell types and synaptology. J. Comp. Neurol. 1991;314:319–349. doi: 10.1002/cne.903140209. [DOI] [PubMed] [Google Scholar]
- 40.Anderson ME, Turner RS. Activity of neurons in cerebellar-receiving and pallidal-receiving areas of the thalamus of the behaving monkey. J. Neurophysiol. 1991;66:879–893. doi: 10.1152/jn.1991.66.3.879. [DOI] [PubMed] [Google Scholar]
- 41.Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983;286:237–265. doi: 10.1016/0165-0173(83)90015-2. [DOI] [PubMed] [Google Scholar]
- 42.Deuschl G, et al. The pathophysiology of parkinsonian tremor: a review. J. Neurol. 2000;247:V33–V48. doi: 10.1007/PL00007781. [DOI] [PubMed] [Google Scholar]
- 43.Timmermann L, et al. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- 44.Lenz FA, et al. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain. 1994;117:531–543. doi: 10.1093/brain/117.3.531. [DOI] [PubMed] [Google Scholar]
- 45.Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc. Natl. Acad. Sci. USA. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweet JA, et al. Fiber tractography of the axonal pathways linking the basal ganglia and cerebellum in Parkinson disease: implications for targeting in deep brain stimulation. J. Neurosurg. 2014;120:988–996. doi: 10.3171/2013.12.JNS131537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. #N./A. 2018;19:338–350. doi: 10.1038/s41583-018-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roa B, et al. Initial clinical manifestations of Parkinson's disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8:1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- 49.Freeman A, et al. Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann. Neurol. 2001;50:321–329. doi: 10.1002/ana.1119. [DOI] [PubMed] [Google Scholar]
- 50.Hurley MJ, Mash DC, Jenner P. Markers for dopaminergic neurotransmission in the cerebellum in normal individuals and patients with Parkinson’s disease examined by RT-PCR. Eur. J. Neurosci. 2003;18:2668–2672. doi: 10.1046/j.1460-9568.2003.02963.x. [DOI] [PubMed] [Google Scholar]
- 51.Koller WC, Hubble JP. Levodopa therapy in Parkinson’s disease. Neurology. 1990;40:47–9. doi: 10.1212/WNL.40.8.1218. [DOI] [PubMed] [Google Scholar]
- 52.Vingerhoets F. Tremor revisited: treatment of PD tremor. Parkinsonism Relat. Disord. 2012;18:S81–S81. doi: 10.1016/S1353-8020(11)70030-X. [DOI] [PubMed] [Google Scholar]
- 53.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 54.Pasquini J, Ceravolo R, Brooks DJ, Bonuccelli U, Pavese N. Progressive loss of raphe nuclei serotonin transporter in early Parkinson’s disease: A longitudinal (123)I-FP-CIT SPECT study. Parkinsonism Relat. Disord. 2020;77:170–175. doi: 10.1016/j.parkreldis.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 55.Halliday GM, Blumbergs PC, Cotton RGH, Blessing WW, Geffen LB. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res. 1990;510:104–107. doi: 10.1016/0006-8993(90)90733-R. [DOI] [PubMed] [Google Scholar]
- 56.Cheshire P, et al. Serotonergic markers in Parkinson’s disease and levodopa-induced dyskinesias. Mov. Disord. 2015;30:796–804. doi: 10.1002/mds.26144. [DOI] [PubMed] [Google Scholar]
- 57.Qamhawi Z, et al. Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain. 2015;138:2964–2973. doi: 10.1093/brain/awv215. [DOI] [PubMed] [Google Scholar]
- 58.Bohlhalter S, Kaegi G. Parkinsonism: heterogeneity of a common neurological syndrome. Swiss Med. Wkly. 2011;141:w13293. doi: 10.4414/smw.2011.13293. [DOI] [PubMed] [Google Scholar]
- 59.Politis M, et al. Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol. Dis. 2010;40:216–221. doi: 10.1016/j.nbd.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 60.Wang JY, et al. SLC6A4 repeat and single-nucleotide polymorphisms are associated with depression and rest tremor in Parkinson's disease: an exploratory study. Front Neurol. 2019;10:333. doi: 10.3389/fneur.2019.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pasquini J, et al. Progression of tremor in early stages of Parkinson’s disease: a clinical and neuroimaging study. Brain. 2018;141:811–821. doi: 10.1093/brain/awx376. [DOI] [PubMed] [Google Scholar]
- 62.Lavoie B, Parent A. Immunohistochemical study of the serotoninergic innervation of the basal ganglia in the squirrel monkey. J. Comp. Neurol. 1990;299:1–16. doi: 10.1002/cne.902990102. [DOI] [PubMed] [Google Scholar]
- 63.Beas-Zarate C, Sandoval ME, Feria-Velasco A. Serotonin uptake and release from rat cerebellum in vitro. J. Neurosci. Res. 1984;12:129–136. doi: 10.1002/jnr.490120112. [DOI] [PubMed] [Google Scholar]
- 64.Walker JJ, Bishop GA, Ho RH, King JS. Brainstem origin of serotonin- and enkephalin-immunoreactive afferents to the opossum’s cerebellum. J. Comp. Neurol. 1988;276:481–497. doi: 10.1002/cne.902760403. [DOI] [PubMed] [Google Scholar]
- 65.Andrée B, et al. The PET radioligand [carbonyl-(11)C]desmethyl-WAY-100635 binds to 5-HT(1A) receptors and provides a higher radioactive signal than [carbonyl-(11)C]WAY-100635 in the human brain. J. Nucl. Med. 2002;43:292–303. [PubMed] [Google Scholar]
- 66.Miguelez C, Morera-Herreras T, Torrecilla M, Ruiz-Ortega JA, Ugedo L. Interaction between the 5-HT system and the basal ganglia: functional implication and therapeutic perspective in Parkinson’s disease. Front Neural Circuits. 2014;8:21. doi: 10.3389/fncir.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hornung JP. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 68.de Win MM, et al. Validation of [(123)I]beta-CIT SPECT to assess serotonin transporters in vivo in humans: a double-blind, placebo-controlled, crossover study with the selective serotonin reuptake inhibitor citalopram. Neuropsychopharmacology. 2005;30:996–1005. doi: 10.1038/sj.npp.1300683. [DOI] [PubMed] [Google Scholar]
- 69.Parsey RV, et al. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J. Cereb. Blood Flow. Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 70.Ganz M, et al. Cerebellar heterogeneity and its impact on PET data quantification of 5-HT receptor radioligands. J. Cereb. Blood Flow. Metab. 2017;37:3243–3252. doi: 10.1177/0271678X16686092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devos D, et al. New pharmacological options for treating advanced Parkinson’s disease. Clin. Ther. 2013;35:1640–1652. doi: 10.1016/j.clinthera.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Zach H, Dirkx MF, Pasman JW, Bloem BR, Helmich RC. Cognitive stress reduces the effect of levodopa on Parkinson's resting tremor. CNS Neurosci. Ther. 2017;23:209–215. doi: 10.1111/cns.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ulusoy A, et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol. Med. 2013;5:1119–1127. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–641. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 76.Paredes-Rodriguez E, Vegas-Suarez S, Morera-Herreras T, De Deurwaerdere P, Miguelez C. The noradrenergic system in Parkinson’s disease. Front Pharm. 2020;11:435. doi: 10.3389/fphar.2020.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kish SJ, Shannak KS, Rajput AH, Gilbert JJ, Hornykiewicz O. Cerebellar norepinephrine in patients with Parkinson’s disease and control subjects. Arch. Neurol. 1984;41:612–614. doi: 10.1001/archneur.1984.04210080020007. [DOI] [PubMed] [Google Scholar]
- 78.Pifl C, Kish SJ, Hornykiewicz O. Thalamic noradrenaline in Parkinson’s disease: deficits suggest role in motor and non-motor symptoms. Mov. Disord. 2012;27:1618–1624. doi: 10.1002/mds.25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Dirkx MF, et al. Cognitive load amplifies Parkinson’s tremor through excitatory network influences onto the thalamus. Brain. 2020;143:1498–1511. doi: 10.1093/brain/awaa083. [DOI] [PubMed] [Google Scholar]
- 81.Askenasy JJ, Yahr MD. Parkinsonian tremor loses its alternating aspect during non-REM sleep and is inhibited by REM sleep. J. Neurol. Neurosurg. Psychiatry. 1990;53:749–753. doi: 10.1136/jnnp.53.9.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbagallo G, et al. The placebo effect on resting tremor in Parkinson’s disease: an electrophysiological study. Parkinsonism Relat. Disord. 2018;52:17–23. doi: 10.1016/j.parkreldis.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Marsden CD, Owen DA. Mechanisms underlying emotional variation in parkinsonian tremor. Neurology. 1967;17:711–715. doi: 10.1212/WNL.17.7.711. [DOI] [PubMed] [Google Scholar]
- 84.Barcroft H, Peterson E, Schwab RS. Action of adrenaline and noradrenaline on the tremor in Parkinson’s disease. Neurology. 1952;2:154–160. doi: 10.1212/WNL.2.5-6.154. [DOI] [PubMed] [Google Scholar]
- 85.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. Jama. 2014;311:1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 86.Hoffer BJ, Siggins GR, Bloom FE. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. II. Sensitivity of Purkinje cells to norepinephrine and related substances administered by microiontophoresis. Brain Res. 1971;25:523–534. doi: 10.1016/0006-8993(71)90458-6. [DOI] [PubMed] [Google Scholar]
- 87.Siggins GR, Hoffer BJ, Bloom FE. Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. 3. Evidence for mediation of norepinephrine effects by cyclic 3′,5′-adenosine monophosphate. Brain Res. 1971;25:535–553. doi: 10.1016/0006-8993(71)90459-8. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto T, Ishikawa M, Tanaka C. Catecholaminergic terminals in the developing and adult rat cerebellum. Brain Res. 1977;132:355–361. doi: 10.1016/0006-8993(77)90428-0. [DOI] [PubMed] [Google Scholar]
- 89.Palacios JM, Hoyer D, Cortés R. alpha 1-Adrenoceptors in the mammalian brain: similar pharmacology but different distribution in rodents and primates. Brain Res. 1987;419:65–75. doi: 10.1016/0006-8993(87)90569-5. [DOI] [PubMed] [Google Scholar]
- 90.Pazos A, Probst A, Palacios JM. Beta-adrenoceptor subtypes in the human brain: autoradiographic localization. Brain Res. 1985;358:324–328. doi: 10.1016/0006-8993(85)90977-1. [DOI] [PubMed] [Google Scholar]
- 91.Cash R, Raisman R, Ploska A, Agid Y. High and low affinity [3H]imipramine binding sites in control and parkinsonian brains. Eur. J. Pharm. 1985;117:71–80. doi: 10.1016/0014-2999(85)90473-X. [DOI] [PubMed] [Google Scholar]
- 92.Kobayashi RM, Palkovits M, Kopin IJ, Jacobowitz DM. Biochemical mapping of noradrenergic nerves arising from the rat locus coeruleus. Brain Res. 1974;77:269–279. doi: 10.1016/0006-8993(74)90790-2. [DOI] [PubMed] [Google Scholar]
- 93.Olson L, Fuxe K. On the projections from the locus coeruleus noradrealine neurons: the cerebellar innervation. Brain Res. 1971;28:165–171. doi: 10.1016/0006-8993(71)90533-6. [DOI] [PubMed] [Google Scholar]
- 94.Powers RE, O’Connor DT, Price DL. Noradrenergic systems in human cerebellum. Brain Res. 1989;481:194–199. doi: 10.1016/0006-8993(89)90504-0. [DOI] [PubMed] [Google Scholar]
- 95.Hori K, Hoshino M. GABAergic neuron specification in the spinal cord, the cerebellum, and the cochlear nucleus. Neural Plast. 2012;2012:921732. doi: 10.1155/2012/921732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hibi M, Shimizu T. Development of the cerebellum and cerebellar neural circuits. Dev. Neurobiol. 2012;72:282–301. doi: 10.1002/dneu.20875. [DOI] [PubMed] [Google Scholar]
- 97.Flace P, et al. The cerebellar dopaminergic system. Front Syst. Neurosci. 2021;15:650614. doi: 10.3389/fnsys.2021.650614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Milosevic L, et al. Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain. 2018;141:2142–2155. doi: 10.1093/brain/awy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paris-Robidas S, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135:105–116. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 100.Louis ED, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 101.Louis ED, Babij R, Lee M, Cortés E, Vonsattel JP. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov. Disord. 2013;28:1854–1859. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Babij R, et al. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–3061. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Louis ED, et al. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum. 2011;10:812–819. doi: 10.1007/s12311-011-0291-0. [DOI] [PubMed] [Google Scholar]
- 104.Kuo SH, et al. Increased number of heterotopic Purkinje cells in essential tremor. J. Neurol. Neurosurg. Psychiatry. 2011;82:1038–1040. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nicoletti G, et al. Diffusion tensor MRI changes in cerebellar structures of patients with familial essential tremor. Neurology. 2010;74:988–994. doi: 10.1212/WNL.0b013e3181d5a460. [DOI] [PubMed] [Google Scholar]
- 106.Novellino F, et al. Brain iron deposition in essential tremor: a quantitative 3-Tesla magnetic resonance imaging study. Mov. Disord. 2013;28:196–200. doi: 10.1002/mds.25263. [DOI] [PubMed] [Google Scholar]
- 107.Pollok B, et al. Levodopa affects functional brain networks in Parkinsonian resting tremor. Mov. Disord. 2010;24:91–98. doi: 10.1002/mds.22318. [DOI] [PubMed] [Google Scholar]
- 108.Titova NV. [A current view on dopamine receptor agonists in the treatment of Parkinson’s disease] Zh . Nevrol. Psikhiatr Im. SS Korsakova. 2015;115:76–83. doi: 10.17116/jnevro20151159176-83. [DOI] [PubMed] [Google Scholar]
- 109.Künig G, Pogarell O, Möller JC, Delf M, Oertel WH. Pramipexole, a nonergot dopamine agonist, is effective against rest tremor in intermediate to advanced Parkinson’s disease. Clin. Neuropharmacol. 1999;22:301–305. [PubMed] [Google Scholar]
- 110.Möller JC, Oertel WH. Pramipexole in the treatment of Parkinson’s disease: new developments. Expert Rev. Neurother. 2005;5:581–586. doi: 10.1586/14737175.5.5.581. [DOI] [PubMed] [Google Scholar]
- 111.Lew MF. Rasagiline treatment effects on parkinsonian tremor. Int J. Neurosci. 2013;123:859–865. doi: 10.3109/00207454.2013.812085. [DOI] [PubMed] [Google Scholar]
- 112.Sivertsen B, et al. Selegiline and levodopa in early or moderately advanced Parkinson’s disease: a double-blind controlled short- and long-term study. Acta Neurol. Scand. Suppl. 1989;126:147–152. doi: 10.1111/j.1600-0404.1989.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 113.Brannan T, Yahr MD. Comparative study of selegiline plus L-dopa-carbidopa versus L-dopa-carbidopa alone in the treatment of Parkinson’s disease. Ann. Neurol. 1995;37:95–98. doi: 10.1002/ana.410370117. [DOI] [PubMed] [Google Scholar]
- 114.Khan ST, Ahmed S, Gul S, Khan A, Al-Harrasi A. Search for safer and potent natural inhibitors of Parkinson’s disease. Neurochem Int. 2021;149:105135. doi: 10.1016/j.neuint.2021.105135. [DOI] [PubMed] [Google Scholar]
- 115.Katzenschlager, R., Sampaio, C., Costa, J. & Lees, A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev, Cd003735, 10.1002/14651858.Cd003735 (2003). [DOI] [PMC free article] [PubMed]
- 116.Sahoo LK, et al. Comparison of effectiveness of trihexyphenidyl and levodopa on motor symptoms in Parkinson’s disease. J. Neural Transm. (Vienna) 2020;127:1599–1606. doi: 10.1007/s00702-020-02257-0. [DOI] [PubMed] [Google Scholar]
- 117.Lester DB, Rogers TD, Blaha CD. Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci. Ther. 2010;16:137–162. doi: 10.1111/j.1755-5949.2010.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Naganawa M, et al. First-in-human assessment of (11)C-LSN3172176, an M1 muscarinic acetylcholine receptor PET radiotracer. J. Nucl. Med. 2021;62:553–560. doi: 10.2967/jnumed.120.246967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kremer, N. I. et al. Deep brain stimulation for tremor: update on long-term outcomes, target considerations and future directions. J. Clin. Med.10, 10.3390/jcm10163468 (2021). [DOI] [PMC free article] [PubMed]
- 120.Fox MW, Ahlskog JE, Kelly PJ. Stereotactic ventrolateralis thalamotomy for medically refractory tremor in post-levodopa era Parkinson’s disease patients. J. Neurosurg. 1991;75:723–730. doi: 10.3171/jns.1991.75.5.0723. [DOI] [PubMed] [Google Scholar]
- 121.Nagaseki Y, et al. Long-term follow-up results of selective VIM-thalamotomy. J. Neurosurg. 1986;65:296–302. doi: 10.3171/jns.1986.65.3.0296. [DOI] [PubMed] [Google Scholar]
- 122.Fasano A, Lozano AM, Cubo E. New neurosurgical approaches for tremor and Parkinson’s disease. Curr. Opin. Neurol. 2017;30:435–446. doi: 10.1097/WCO.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 123.Alterman RL. One step backward: Magnetic resonance guided focused ultrasound thalamotomy for the treatment of medically refractory tremor. Ann. Neurol. 2017;81:348–350. doi: 10.1002/ana.24893. [DOI] [PubMed] [Google Scholar]
- 124.Ondo W, Jankovic J, Schwartz K, Almaguer M, Simpson RK. Unilateral thalamic deep brain stimulation for refractory essential tremor and Parkinson’s disease tremor. Neurology. 1998;51:1063–1069. doi: 10.1212/WNL.51.4.1063. [DOI] [PubMed] [Google Scholar]
- 125.Benabid AL, Torres N. New targets for DBS. Parkinsonism Relat. Disord. 2012;18:S21–S23. doi: 10.1016/S1353-8020(11)70009-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


