Abstract
Purpose
To evaluate retinal thickness fluctuations in patients with diabetic macular oedema (DMO) treated with anti-vascular endothelial growth factor (anti-VEGF) injections.
Methods
Visual acuity (VA) and central subfield thickness (CST) were collected at baseline, 3, 6, 9 and 12 months. Retinal thickness fluctuation was quantified by standard deviation (SD) of CST across 12 months. A mixed effects regression model evaluated the relationship between CST SD and VA at 12 months. Multiple linear regression analysis was performed to investigate predictors of CST SD.
Results
Mean baseline and 12-month VAs were 63.5 ± 15.7 and 69.0 ± 13.8 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters (change = +5.1 ± 16.1 letters, p < 0.001). Mean baseline and 12-month CSTs were 396.9 ± 109.7 and 337.7 ± 100.7 μm (change = −59.2 ± 114.8 μm, p < 0.001). Retinal thickness variability across the first 12 months was 59.4 ± 43.6 μm. Stratification of patient eyes by CST SD demonstrated 9.7 letters difference in 12-month VA between first and fourth quartiles. Significant predictors of CST SD include baseline CST, injection type, laser treatment, and DR stage.
Conclusions
Larger retinal thickness fluctuations are associated with poorer visual outcomes in eyes with DMO treated with anti-VEGF injections. Retinal thickness variability may be an important prognostic biomarker for DMO patients.
Subject terms: Eye diseases, Medical research
Introduction
Diabetic retinopathy (DR) is the leading cause of blindness in middle-aged adults (20–74 years) in developed countries [1–3]. Vision loss in DR is most often secondary to diabetic macular oedema (DMO), which affects ~6.8% of people with diabetes [4]. As diabetes continues to rise in prevalence, DMO has also become a major public health concern.
Primary treatment for DMO involves frequent intravitreal anti-vascular endothelial growth factor (VEGF) injections [5–7]. Despite the efficacy of these treatments, a barrier in clinical management of DMO still exists in that there are currently no dependable methods to predict how individual patients receiving anti-VEGF injection treatment will respond over time, in terms of vision gain or loss. Biomarkers that are predictive of visual outcomes in individual eyes with DMO would allow for better disease management and patient counselling. Hence, identification of these markers is integral.
Optical coherence tomography (OCT) makes it possible to objectively assess and quantify morphological parameters of the retina, such as retinal thickness, intraretinal fluid, and ellipsoid zone integrity, among others [8]. Retinal thickness, in particular, is an important parameter in clinical evaluation of DMO. Because some studies have shown that reduction in retinal thickness is associated with improved visual acuity (VA) in response to intravitreal anti-VEGF therapy in DMO, changes in retinal thickness have been considered representative of disease activity, and retinal thickness has been widely utilized as an outcome parameter in clinical trials for DMO [9, 10].
However, though retinal thickness is important in the clinical evaluation of DMO, it is not a reliable surrogate for VA, with previous investigations showing only a moderate degree of association, at best, between retinal thickness and VA [11–13]. The Diabetic Retinopathy Clinical Research Network’s study investigating the relationship between central subfield thickness (CST) and VA in DMO observed a wide range of visual outcomes for any given degree of retinal oedema, as well as paradoxical decreases in VA accompanying decreases in retinal thickness [11]. Bressler et al.’s study investigated the association between changes in CST and changes in VA in DMO eyes treated with intravitreal anti-VEGF injections, and revealed that changes in CST accounted for only a small fraction of changes in VA [13].
One possible problem with using retinal thickness as a predictor for VA is that single timepoint measurements of retinal thickness fail to account for retinal fluctuations over time. Repeated changes in retinal thickness and retinal deformations may negatively affect the functionality of photoreceptors. Farjood et al.’s 2018 study simulated mechanical stress on retinal pigment epithelium (RPE) cells, and demonstrated that cyclic mechanical stress induces RPE damage and VEGF expression, promoting angiogenesis and the progression of choroidal neovascularization [14]. Ambati and Fowler’s 2012 study showed that photoreceptors rely on the RPE for support, and RPE injury leads to photoreceptor loss and retinal degeneration [15].
Consequently, fluctuations in retinal thickness over time, rather than absolute retinal thickness, may be more reflective of VA, providing clinicians with a more reliable metric with which to predict patients’ outcomes. Evans et al. showed that in eyes with neovascular age-related macular degeneration (nAMD) treated with anti-VEGF injections, larger variation in retinal thickness over 24 months was associated with poorer visual outcome at 24 months [16]. Chen et al. showed that in eyes with retinal vein occlusion (RVO) receiving anti-VEGF treatment, larger macular thickness fluctuations across 12 months were associated with poorer visual outcomes at 12 months [17]. Similar investigations have not been performed in DMO.
Herein, the aims of this study are to evaluate retinal thickness fluctuations in DMO patients treated with anti-VEGF agents, and assess the relationship between retinal thickness fluctuations and VA. Findings in this study will further elucidate our understanding of the association between structural retinal changes and visual outcomes, and provide a functional metric that will aid in the evaluation, treatment, and management of DMO.
Methods
Study design and participants
This study is a retrospective, non-comparative, observational cohort study performed following Institutional Review Board approval. All study-related procedures were performed in accordance with good clinical practice (International Conference on Harmonization of Technical Requirements of Pharmaceuticals for Human Use [ICH] E6), applicable FDA regulations, the Health Insurance Portability and Accountability Act, and the Declaration of Helsinki. Patients aged 18 years or older with a documented diagnosis of DMO at the Cleveland Clinic Cole Eye Institute from January 2012 to December 2019 were identified using the electronic medical record. Patients were further screened using the following inclusion criteria: initiation of intravitreal anti-VEGF therapy at the Cleveland Clinic without prior anti-VEGF treatment, follow-up for at least 12 months after first injection, injections at least every 6 months following first injection, and OCT data available every 3 months with no more than one missing data point. The anti-VEGF administration regimen at the Cleveland Clinic was PRN and investigator-determined for all patients, with a focus on treating all fluid until dry with as needed anti-VEGF injections. Eyes were excluded if concomitant maculopathies unrelated to DMO were present, or if concurrent steroid injections or focal laser photocoagulation treatment were given during the study period. Only one eye was selected per patient. In bilateral cases, the first eye to receive anti-VEGF injection was selected. We identified 2503 patients with a documented diagnosis of DMO who were receiving anti-VEGF treatment (Fig. 1). Of these patients, 630 had continuous follow-up for at least 12 months and met the injection frequency criteria (excluded n = 1873). Of these, 266 final patients were selected after exclusion based on OCT frequency criteria, presence of concomitant macular disease, or concurrent administration of steroid or focal laser treatment (excluded n = 364).
Fig. 1. Patient selection workflow.
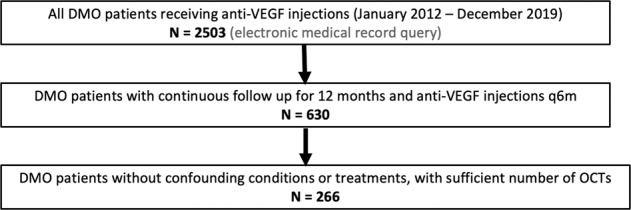
Two thousand five hundred and three patients with a documented diagnosis of DMO who were receiving anti-VEGF treatment were selected via electronic record query. Six hundred and thirty of these patients had continuous follow-up for at least 12 months and met the injection frequency criteria (excluded n = 1873). Two hundred and sixty-six patients were selected after excluding based on OCT frequency criteria, presence of concomitant macular disease, or concurrent administration of steroid or focal laser treatment (excluded n = 364).
Study variables
Baseline data, including demographics and past medical history, and treatment received during the 12-month patient enrolment period were recorded. Best visual acuity (BVA) and CST were collected at baseline, 3-, 6-, 9- and 12-month visits. For each timepoint, BVA and CST were measured during the same encounter. The BVA measurements were a combination of VA without correction, with correction, or pinhole, as per institutional standard. Cirrus High-Definition Spectral Domain-OCT Review (V.9.5.1, Carl Zeiss Meditech, Dublin, CA) was used to calculate macular thickness parameters [18, 19]. CST was defined as the average macular thickness of the 1-mm diameter circle centred around the fovea, using the ETDRS grid [20]. Macular thickness was measured from the internal limiting membrane to the RPE. Macular thickness variability was quantified as the standard deviation (SD) of recorded CST measurements across 12 months.
Statistical methods
R Statistical Software (Version 3.6.1, Vienna, Austria) was used for statistical analysis. Categorical variables were described as frequencies and percentages; continuous variables were described using means ± SD. VA was converted from Snellen to ETDRS via the formula ETDRS = 85 + 50 × log10(Snellen) [21]. Two-sample t-tests were used to compare 12-month visual outcomes and macular thickness parameters from baseline. Mixed effects linear regression analysis was used to identify predictors of VA at 12 months. Standard multiple linear regression was used to identify predictors of macular thickness variability (quantified by CST SD). Regression coefficients were expressed using 95% confidence intervals. For quartile analysis, eyes were stratified by quartiles of macular thickness variability, and one-way analysis of variance was used to compare predicted VA among quartiles, with the Tukey test for pairwise comparisons. p values < 0.05 were considered statistically significant.
Results
Demographics and summary
A total of n = 266 patient eyes with DMO met criteria for inclusion in the study. Of these patients, the average age was 61.0 ± 11.3 years, 125 (47.0%) were female, and 192 (72.2%) were insulin-dependent. Racial distribution was 182 (68.4%) White, 60 (22.6%) Black, 2 (0.8%) Asian/Pacific Islander, 1 (0.4%) American Indian/Alaska Native, 10 (3.8%) Multiracial/Multicultural and undisclosed in 11 (4.1%) patients. In terms of DR stage, 110 (41.4%) patients had proliferative DR (PDR), 67 (25.2%) had severe non-proliferative DR (NPDR) and 89 (33.5%) had mild–moderate NPDR. The average number of anti-VEGF injections over 12 months was 8.2 ± 2.4. In terms of injection type, 151 (56.8%) received only bevacizumab, 19 (7.1%) received only aflibercept, 2 (0.8%) received only ranibizumab and 94 (35.3%) received a combination of bevacizumab and aflibercept. Mean baseline and 12-month VAs were 63.5 ± 15.7 and 69.0 ± 13.8 ETDRS letters, respectively. Change in VA from baseline to 12 months was +5.1 ± 16.1 ETDRS letters (p < 0.001). A summary of this data can be found in Table 1.
Table 1.
Demographics and summary data.
| Variable | DMO patients (n = 266) |
|---|---|
| Age (years) | 61.0 (11.3) |
| Gender | |
| Female | 125 (47.0%) |
| Male | 141 (53.0%) |
| Race | |
| White | 182 (68.4%) |
| Black | 60 (22.6%) |
| Asian/Pacific Islander | 2 (0.8%) |
| American Indian/Alaska Native | 1 (0.4%) |
| Multiracial/multicultural | 10 (3.8%) |
| Declined | 11 (4.1%) |
| Eye laterality | |
| OD | 141 (53.0%) |
| OS | 125 (47.0%) |
| Total anti-VEGF injections | 8.2 (±2.4) |
| Anti-VEGF medication | |
| Aflibercept | 19 (7.1%) |
| Bevacizumab | 151 (56.8%) |
| Mixed (bevacizumab + aflibercept) | 94 (35.3%) |
| Ranibizumab | 2 (0.8%) |
| Diabetic retinopathy stage | |
| Mild–moderate NPDR | 89 (33.5%) |
| Severe NPDR | 67 (25.2%) |
| PDR | 110 (41.4%) |
| Insulin dependence | |
| Insulin-dependent | 192 (72.2%) |
| Insulin-independent | 74 (27.8%) |
| CST (µm) | |
| Baseline | 396.9 (±109.7) |
| 12 monthsa | 337.7 (±100.7) |
| Change | −59.2 (±114.8) |
| Visual acuity (ETDRS letters) | |
| Baseline | 63.5 (±15.7) |
| 12 monthsa | 69.0 (±13.8) |
| Change | +5.1 (±16.1) |
| CST SD across 12 months (µm) | 59.4 (±43.6) |
Categorical variables are expressed as frequency (percentage), numeric variables are expressed as mean (standard deviation).
CST central subfield thickness, SD standard deviation.
aStatistically significant difference found from baseline at p < 0.001.
Macular thickness and variability data
Average CST at baseline and 12 months were 396.9 ± 109.7 and 337.7 ± 100.7 μm, respectively (Table 1). The change in CST from baseline at 12 months was −59.2 ± 114.8 μm (p < 0.001). Variability of macular thickness as quantified by CST SD across the first 12 months was 59.4 ± 43.6 μm.
Relationship between macular thickness variability and visual outcomes
To assess the relationship between macular thickness variability across 12 months and VA at 12 months, a mixed effects linear regression model was utilized, with 12-month VA as the dependent predictor and macular thickness variability as an independent predictor (Fig. 2). Macular thickness variability, quantified by CST SD over 12 months, had a statistically significant negative association with VA at 12 months (Table 2). The regression coefficient quantifying the association between CST SD and 12-month VA was −6.87 [−10.52, −3.23] ETDRS letters/100 μm (p < 0.001). This relationship was independent of other variables in the model including baseline macular thickness, baseline VA, number or type of anti-VEGF injections received, demographics, DR stage, haemoglobin A1c (HbA1c) or insulin dependence.
Fig. 2. Relationship between macular thickness variability and visual acuity at 12 months.
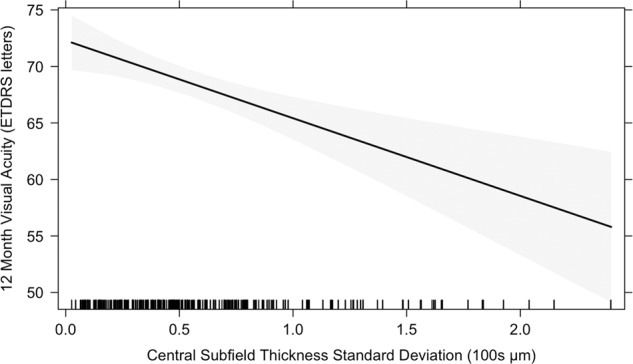
12-month visual acuity predicted by a mixed effects regression model using central subfield thickness standard deviation (CST SD) across 12 months as the predictor, adjusted for demographics, number of treatments and baseline variables. The shaded area represents the 95% confidence band; the rug plot shows the distribution of CST SD values.
Table 2.
Mixed effects linear regression model for 12-month visual acuity in DMO using central subfield.
| Factors | Estimates | CI | p value | |
|---|---|---|---|---|
| (Intercept) | 46.40 | 30.91 | 61.90 | <0.001 |
| CST SD across 12 months (100s µm) | −6.87 | −10.52 | −3.23 | <0.001 |
| Baseline visual acuity (ETDRS letters) | 0.35 | 0.27 | 0.44 | <0.001 |
| Baseline CST (100s µm) | 1.63 | 0.18 | 3.09 | 0.027 |
| Age (years) | −0.03 | −0.15 | 0.10 | 0.682 |
| Gender: male (vs. female) | 2.96 | 0.49 | 5.44 | 0.019 |
| DR stage: PDR (vs. mild–moderate NPDR) | −2.20 | −5.63 | 1.22 | 0.207 |
| DR stage: severe NPDR (vs. mild–moderate NPDR) | −3.95 | −7.35 | −0.56 | 0.023 |
| HbA1c (%) | 0.24 | −0.43 | 0.91 | 0.484 |
| Laser: focala (vs. none) | −1.85 | −7.73 | 4.03 | 0.538 |
| Laser: PRPb (vs. none) | −1.29 | −4.27 | 1.69 | 0.396 |
| Laser: both focal and PRPc (vs. none) | −4.45 | −12.63 | 3.73 | 0.286 |
| Number of anti-VEGF injections | 0.38 | −0.18 | 0.95 | 0.183 |
| Insulin: dependent (vs. non-dependent) | −0.13 | −2.96 | 2.69 | 0.926 |
| Anti-VEGF injection type: bevacizumab (vs. aflibercept) | −5.33 | −10.39 | −0.26 | 0.039 |
| Anti-VEGF injection type: mixedd (vs. aflibercept) | −7.38 | −12.76 | −2.00 | 0.007 |
| Anti-VEGF injection type: ranibizumab (vs. aflibercept) | −4.02 | −25.01 | 16.97 | 0.707 |
| Model characteristics | ||||
| σ2 | 65.86 | |||
| τ00 id | 77.77 | |||
| ICC | 0.54 | |||
| Marginal R2/conditional R2 | 0.273/0.667 | |||
Estimates denote the predicted change in visual acuity at 12 months for a 1 unit increase of a numeric factor, or for that factor level relative to reference level of a categorical factor, with other factors held constant.
CI confidence interval, CST central subfield thickness, SD standard deviation, σ2 variance within subjects, τ00 id variance between subjects, ICC intraclass correlation coefficient, R2 coefficient of determination.
aFocal laser refers to eyes that had received focal laser prior to the study period.
bPRP refers to eyes that had received PRP either prior to or during the study period.
cBoth focal and PRP refers to eyes that had received focal laser prior to the study period in addition to PRP at any time.
dMixed refers to eyes that had received a combination of bevacizumab and aflibercept.
Statistically significant p < 0.05 values are in bold.
Predictors of better VA at 12 months included higher baseline VA (p < 0.001), higher baseline CST (p = 0.027), male gender (p = 0.019), mild–moderate NPDR (as opposed to severe NPDR; p = 0.023) and aflibercept injections only (as opposed to bevacizumab or a mix of both aflibercept and bevacizumab; p = 0.039, p = 0.007, respectively). Age, number of anti-VEGF injections, HbA1c and insulin dependence were not significantly associated with 12-month VA. Whether or not patients had previously received focal laser or PRP was also not associated with 12-month VA.
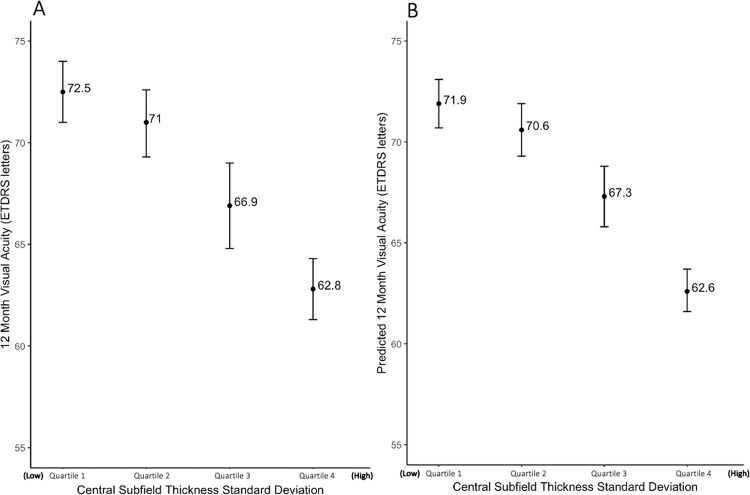
To further visualize the relationship between macular thickness variability and visual outcomes at 12 months, the data were stratified into quartiles based on CST SD across 12 months, and VA at 12 months was predicted using the mixed effects linear regression model to control for baseline, demographic and treatment variables. The mean CST SD for each quartile, in order of increasing CST SD, was 17.1 ± 7.3, 40.5 ± 5.9, 62.9 ± 8.7, and 121.4 ± 38.5 μm. The VAs at 12 months for the first and fourth quartiles were 72.5 and 62.8 ETDRS letters, respectively, for a statistically significant difference of 9.7 ETDRS letters (p < 0.001) (Fig. 3A). The predicted VAs at 12 months for the first and fourth quartiles were 71.9 and 62.6 ETDRS letters, respectively, for a statistically significant difference of 9.3 ETDRS letters (p < 0.001) (Fig. 3B).
Fig. 3. Visual acuity by macular thickness variability quartiles.
A Actual 12-month visual acuity stratified by quartiles of central subfield thickness standard deviation (CST SD) across 12 months. B Predicted 12-month visual acuity using a mixed effects regression model, stratified by quartiles of central subfield thickness standard deviation (CST SD) across 12 months, adjusted for demographics, number of treatments and baseline variables. Error bars represent the standard error of each quartile.
Predictors of macular thickness variability
Because macular thickness variability was found to be an independent predictor of VA at 12 months, we performed multiple regression analyses to identify predictors of macular thickness variability (Supplementary Table 1). Baseline CST was a significant positive predictor of CST SD (p < 0.001). PDR (as opposed to mild–moderate NPDR), severe NPDR (as opposed to mild–moderate NPDR), PRP laser treatment (as opposed to no laser treatment) and ranibizumab (as opposed to aflibercept) were also significant positive predictors of CST SD (p = 0.007, p < 0.001, p = 0.018, p < 0.001, respectively). Male gender, prior focal laser treatment (as opposed to no laser treatment) and both focal and PRP laser treatment (as opposed to no laser treatment) were significant negative predictors of CST SD (p = 0.004, p = 0.001, p = 0.006, respectively). Age, number of anti-VEGF treatments and insulin dependence were not associated with CST SD.
Discussion
This study evaluated macular thickness fluctuations in DMO patients treated with intravitreal anti-VEGF injections, and investigated the association between macular thickness fluctuation and visual outcomes. The study demonstrated that larger fluctuations in macular thickness were associated with poorer visual outcomes at 12 months. On mixed effects regression analysis, there was a mean decrease in 12-month VA of 6.87 letters per 100-μm CST SD. In addition, on quartile stratification of eyes based on CST SD, there was a significant difference of 9.7 letters in 12-month VA between the least variable to most variable quartiles. These findings suggest that macular thickness fluctuations correlate with retinal functional outcomes, and thus can potentially serve as a prognosticator for visual outcomes in patients with DMO treated with anti-VEGF injections.
In addition to macular thickness variability, other predictors of better VA at 12 months included higher baseline VA (p < 0.001), higher baseline CST (p = 0.027), male gender of the patient (p = 0.019), mild–moderate NPDR (as opposed to severe NPDR; p = 0.023) and aflibercept injections (versus bevacizumab only or a mix of both aflibercept and bevacizumab; p = 0.039, p = 0.007, respectively). Though higher baseline VA was associated with better 12-month VA, it was associated with smaller gains in VA from baseline to 12 months. This result is expected, as patients with better baseline VA have less room for improvement compared to patients with lower baseline VA due to a ceiling effect. In this study, male gender was associated with better 12-month VA. To the best of our knowledge, previous studies have not investigated correlation between gender and visual outcome in patients with DMO. However, studies have shown that among patients with DMO, on average, men had greater central macular thickness than did women [18, 22].
In this study, higher baseline CST was associated with better 12-month VA. Though higher baseline macular thickness can reflect active disease at baseline and indicate that a patient may be receptive to treatment, macular thickness has been shown to be an unreliable reflection of retinal function. Existing literature on associations between macular thickness and VA have reported conflicting results [11–14, 23, 24]. This may be because macular thickness reflects a combination of retinal features, such as loss of neurons, disruption of anatomical connections, disorganization of the retinal inner layers and presence of extracellular fluid in the retina [25]. Hence, CST inadequately reflects changes occurring in the retina at a molecular level. Importantly, although baseline CST was associated with 12-month VA, the association between CST SD and 12-month VA was independent of baseline CST. Furthermore, as per the mixed effects linear regression model, 100-μm change in CST SD had a larger effect size on 12-month VA (−6.87 letters per 100-μm CST SD) compared to 100-μm change in baseline CST (1.63 letters per 100-μm CST). In terms of the types of intravitreal anti-VEGF agents patients received, our analysis showed that in comparison to receiving aflibercept injections only, receiving either bevacizumab or a combination of bevacizumab and aflibercept were correlated with worse BCVA at 12 months, which supports the findings from Protocol T.
In this analysis, age, number of anti-VEGF injections, insulin dependence, HbA1c, DR stage and laser treatment status were not significantly associated with 12-month VA. Though baseline HbA1c was included in our analysis, other factors relating to glycaemic control, such as length of diabetes, were not included in our analysis because the information was only available for a small percentage of the cohort. Our results were consistent with previous studies, which have shown that VA improvement with anti-VEGF injections are not dependent on baseline HbA1c levels [26].
To investigate the predictors of macular thickness variability itself, we performed multiple linear regression analysis and found that increased baseline CST was associated with increased CST SD. This was expected, as patients with higher CST at baseline had more room for thickness reduction, and patients with lower CST at baseline had limited potential to become even thinner. Hence patients with higher baseline CST had more potential for CST fluctuation. Having PDR or severe NPDR (versus mild–moderate NPDR), PRP (versus no laser treatment) or high HbA1c were also positive predictors of CST SD. Having prior focal or both focal and PRP laser treatment was a negative predictor of CST SD, which is expected, as focal laser has been hypothesized to reduce retinal thickness changes by photocoagulating the retina thus not allowing it to swell to previous thickness levels. Of note, other factors studied, such as age, insulin dependence and number of injections, were not associated with CST SD.
OCT has become an increasingly important tool for evaluating and managing vitreoretinal disorders, including DMO. Because OCT allows for the objective quantification of morphological features of the retina, many studies have focused on investigating the prognostic value of OCT parameters such as subfoveal choroidal thickness, ellipsoid zone status, subfoveal neuroretinal detachment and disorganization of the retinal inner layers etc. [27–29]. To the best of our knowledge, no previous studies have investigated macular thickness variability as a prognosticator for visual outcomes in DMO. Prior studies have investigated circadian variations of macular thickness in DMO eyes. Larsen et al. reported an overnight increase in mean macular thickness from 316 to 336 μm, accompanied by an overnight decline in mean VA from 41 to 36 letters in DMO patients [30]. Frank et al. observed declines in mean macular thickness throughout the day in DMO eyes [31]. However, macular thickness variability has not been studied longitudinally in DMO patients in a manner similar to this study.
There are studies that have investigated the association between retinal thickness variability and visual outcomes in eyes with nAMD and RVO receiving anti-VEGF treatment [16, 17]. Both studies reported that larger fluctuations in retinal thickness over time were associated with worse final visual outcome, which is consistent with our results.
This study is limited by its retrospective nature, which prevents us from establishing causality. Although our results demonstrated an association between higher macular thickness fluctuations and worse visual outcomes at 12 months, we cannot infer causality. We also do not know if there are other unaccounted-for factors that are mediating the results. For example, hyperglycaemia is a well-established determinant of DR progression [32, 33]. Hence it would be interesting to include factors reflecting long term glycaemic control (such as HbA1c levels early in the course of diabetes and fluctuations in HbA1c levels throughout the length of diabetes) to our analysis to assess if they are influencing the variables studied. The presence of diabetic macular ischaemia has also been associated with reduced VA in DMO, so it could be another unaccounted-for factor mediating our analysis [34]. Another limitation common to retrospective studies is that the collected BVA was not standardized; the BVA collected in our study was a combination of pinhole-corrected, spectacle-corrected and uncorrected VA. Similarly, because of the retrospective nature of this study, the types of intravitreal anti-VEGF agents patients received were not standardized; patients were treated PRN and based on physicians’ clinical judgement, with differing anti-VEGF agents, treatment intervals and total number of injections over a 12-month period.
Another limitation of this study is its generalizability. Due to the strict inclusion and exclusion criteria implemented during patient selection, selection bias may limit the applicability of our analysis to the general DMO patient population. For example, because receiving injections every 6 months or more frequently was an inclusion criterion to isolate a population of patients requiring re-treatment, DMO patients whose eyes quickly stabilized in response to injections and did not require further treatment were excluded. In addition, patients who had poor follow-up were excluded (<4 OCTs in a 12-month period). Patients who received concurrent steroid or focal laser treatment were excluded to minimize confounding, as it is difficult to quantify these treatments’ effects on macular thickness fluctuations; this makes it difficult to infer conclusions for these populations. Although our patient selection criterion may have made our findings less generalizable, they were necessary to investigate our hypothesis and ensure the integrity of our results. Finally, our sample size was 266, which, though adequate for our purposes, was not large enough for us to perform analysis on stratified subgroups of patients. Future studies that are prospective, have more inclusive criterion, have larger sample sizes or investigate longer term outcomes, would provide more insight.
Overall, this study investigates the association between macular thickness variability and visual outcomes in patients with DMO receiving anti-VEGF injections. As pointed out in the previous paragraph, future investigations should study patients prospectively in routine clinical practice to attain more generalizable results, as there are often discrepancies between carefully selected patients in studies versus real world patient populations. Despite its limitations, this study helps establish macular thickness variability as a novel biomarker that may change the monitoring of DMO patients’ progress in clinical practice. Future studies on specific morphological features that affect macular thickness fluctuations, such as change in fluid compartments, could identify the specific contributors of CST that influence visual outcomes, and further elucidate our understanding.
Summary
What was known before
Retinal thickness fluctuations was not directly related to DMO.
What this study adds
In DMO eyes treated with anti-VEGF agents, large retinal thickness fluctuations are associated with poorer visual outcomes. Retinal thickness fluctuation may be an important prognostic biomarker for patients with DMO.
Supplementary information
Multiple Regression Model for Central Subfield Thickness Standard Deviation across 12 Months in DMO
Author contributions
VYW: research design, data acquisition/research execution, data analysis/interpretation, manuscript preparation. BLK: data acquisition/research execution, manuscript preparation. AXC: research design, data analysis/interpretation. KW: data acquisition/research execution. TEG: research design, data analysis/interpretation, manuscript preparation. TFC: research design, data analysis/interpretation, manuscript preparation. RPS: research design, data analysis/interpretation, manuscript preparation.
Competing interests
RPS: Genentech/Roche (personal fees), Alcon/Novartis (personal fees), Apellis (grant), Graybug (grant), Zeiss (personal fees), Bausch + Lomb (personal fees), Regeneron Pharmaceuticals, Inc. (personal fees). The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-021-01672-1.
References
- 1.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132:1334–40. doi: 10.1001/jamaophthalmol.2014.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic retinopathy preferred practice pattern(R) Ophthalmology. 2020;127:P66–145. doi: 10.1016/j.ophtha.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–54. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Sheu SJ, Lee YY, Horng YH, Lin HS, Lai WY, Tsen CL. Characteristics of diabetic macular edema on optical coherence tomography may change over time or after treatment. Clin Ophthalmol. 2018;12:1887–93. doi: 10.2147/OPTH.S173956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou WC, Brown DM, Payne JF, Wykoff CC. Relationship between visual acuity and retinal thickness during anti-vascular endothelial growth factor therapy for retinal diseases. Am J Ophthalmol. 2017;180:8–17. doi: 10.1016/j.ajo.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130:1153–61. doi: 10.1001/archophthalmol.2012.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browning DJ, Glassman AR, Diabetic Retinopathy Clinical Research N, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam F. Retinal thickness and visual acuity in diabetic macular edema: an optical coherence tomography-based study. J Coll Physicians Surg Pak. 2016;26:598–601. [PubMed] [Google Scholar]
- 13.Bressler NM, Odia I, Maguire M, Glassman AR, Jampol LM, MacCumber MW, et al. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the Protocol T Randomized Clinical Trial. JAMA Ophthalmol. 2019;137:977–985. [DOI] [PMC free article] [PubMed]
- 14.Farjood F, Vargis E. Novel devices for studying acute and chronic mechanical stress in retinal pigment epithelial cells. Lab Chip. 2018;18:3413–24. doi: 10.1039/C8LC00659H. [DOI] [PubMed] [Google Scholar]
- 15.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans RN, Chakravarthy U, Reeves B. Associations between variation in retinal thickness and visual function. Investig Ophthalmol Vis Sci. 2019;60:3456. doi: 10.1167/iovs.18-26533. [DOI] [PubMed] [Google Scholar]
- 17.Chen AX, Greenlee TE, Conti TF, Briskin IN, Singh RP. Fluctuations in macular thickness in patients with retinal vein occlusion treated with anti-vascular endothelial growth factor agents. Ophthalmol Retin. 2020;4:1158–1169. [DOI] [PubMed]
- 18.Gupta P, Sidhartha E, Tham YC, et al. Determinants of macular thickness using spectral domain optical coherence tomography in healthy eyes: the Singapore Chinese Eye study. Invest Ophthalmol Vis Sci. 2013;54:7968–76. doi: 10.1167/iovs.13-12436. [DOI] [PubMed] [Google Scholar]
- 19.Keane PA, Mand PS, Liakopoulos S, Walsh AC, Sadda SR. Accuracy of retinal thickness measurements obtained with Cirrus optical coherence tomography. Br J Ophthalmol. 2009;93:1461–7. doi: 10.1136/bjo.2008.155846. [DOI] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Research G. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie house classification: ETDRS report number 10. Ophthalmology. 2020;127:S99–119. doi: 10.1016/j.ophtha.2020.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30:1046–50. doi: 10.1097/IAE.0b013e3181d87e04. [DOI] [PubMed] [Google Scholar]
- 22.Arthur E, Young SB, Elsner AE, et al. Central macular thickness in diabetic patients: a sex-based analysis. Optom Vis Sci. 2019;96:266–75. doi: 10.1097/OPX.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannouche RZ, Avila MP, Isaac DL, Silva RS, Rassi AR. Correlation between central subfield thickness, visual acuity and structural changes in diabetic macular edema. Arq Bras Oftalmol. 2012;75:183–7. doi: 10.1590/S0004-27492012000300007. [DOI] [PubMed] [Google Scholar]
- 24.Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142:405–12. doi: 10.1016/j.ajo.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Deak GG, Schmidt-Erfurth UM, Jampol LM. Correlation of central retinal thickness and visual acuity in diabetic macular edema. JAMA Ophthalmol. 2018;136:1215–6. doi: 10.1001/jamaophthalmol.2018.3848. [DOI] [PubMed] [Google Scholar]
- 26.Singh RP, Wykoff CC, Brown DM, Larsen M, Terasaki H, Silva FQ, et al. Outcomes of diabetic macular edema patients by baseline hemoglobin A1c: analyses from VISTA and VIVID. Opthalmol Retin. 2017;1:382–8. doi: 10.1016/j.oret.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Campos A, Campos EJ, do Carmo A, et al. Evaluation of markers of outcome in real-world treatment of diabetic macular edema. Eye Vis. 2018;5:27. doi: 10.1186/s40662-018-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–16. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 29.Gerendas BS, Bogunovic H, Sadeghipour A, et al. Computational image analysis for prognosis determination in DME. Vis Res. 2017;139:204–10. doi: 10.1016/j.visres.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Larsen M, Wang M, Sander B. Overnight thickness variation in diabetic macular edema. Invest Ophthalmol Vis Sci. 2005;46:2313–6. doi: 10.1167/iovs.04-0893. [DOI] [PubMed] [Google Scholar]
- 31.Frank RN, Schulz L, Abe K, Iezzi R. Temporal variation in diabetic macular edema measured by optical coherence tomography. Ophthalmology. 2004;111:211–7. doi: 10.1016/j.ophtha.2003.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443–51. doi: 10.1016/j.ophtha.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, et al. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim DA, Keane PA, Zarranz-Ventura J, et al. The effects of macular ischemia on visual acuity in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:2353–60. doi: 10.1167/iovs.12-11103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple Regression Model for Central Subfield Thickness Standard Deviation across 12 Months in DMO