Abstract
The effect of stress on reproduction and gonadal function has captivated investigators for nearly 100 years. Following the identification of GnRH 50 years ago, a niche research field emerged fixated on how stress impairs this central node controlling downstream pituitary and gonadal function. It is now clear that both episodic GnRH secretion in males and females, and surge GnRH secretion in females, are inhibited during a variety of stress types. There has been considerable advancement in our understanding of numerous stress-related signaling molecules and their ability to impair reproductive neuroendocrine activity during stress. Recently, much attention has turned to the effects of stress on two populations of kisspeptin neurons—the stimulatory afferents to GnRH neurons that regulate pulsatile and surge-type gonadotropin secretion. Indeed, future work is still required to fully construct the neuroanatomical framework underlying stress effects, directly or indirectly, on GnRH neuron function. The objective of this review is to evaluate and synthesize evidence that stress-related signaling molecules act directly on GnRH neurons. Here, we review the evidence for and against the action of a handful of signaling molecules as inhibitors of GnRH neuron function, including corticotropin-releasing hormone, urocortins, norepinephrine, cortisol/corticosterone, calcitonin gene-related peptide, and arginine-phenylalanine-amide-related peptide-3.
Keywords: GnRH, stress, LH, CRH, urocortins, cortisol, corticosterone, norepinephrine, CGRP, RFRP-3
1. Introduction:
As the GnRH neuron is central to the orchestration of pulsatile luteinizing hormone (LH), surge LH as well as the coordination of reproductive cycles in the female, each of these processes is vulnerable to impairment by stress. We begin by briefly discussing important studies which distinguish effects on pulses versus surge secretion, across stress models and species, and expand to highlight studies of reproductive cyclicity (e.g. menstrual cycles in women or primates and estrous cycles in other mammals). Although addressed separately, we acknowledge that distinguishing the relative importance of impaired pulsatile and surge GnRH secretion is challenging due to the interdependence of these modes of GnRH secretion in manifestation of ovarian cycles. Indeed, pulsatile secretion of LH supports gametogenesis and steroidogenesis in both sexes, and in females, a rise in estradiol (E2) is necessary for triggering the preovulatory LH surge.1 Therefore, stress suppression of LH pulses can have profound effects on the LH surge, ovulation and the reproductive cycle and health in general (E2 and testosterone support musculoskeletal,2 metabolic,3,4 and mental5 health). One important caveat is that much of the evidence supporting our understanding of stress actions on the GnRH neuron is based on the assessment of LH secretion, which does not always directly reflect GnRH secretion, such as in situations of diminished pituitary responsiveness (i.e. during stress or during the surge).6 Despite this limitation, assessment of LH secretion remains a robust and economical method of assessing GnRH secretion indirectly. As such, much of the data discussed below utilize LH concentrations to infer GnRH secretion patterns. Additionally, different stress types impair reproduction via different pathways; thus, identifying the neural systems and central signaling molecules whereby distinct stress types interfere with the secretion of GnRH remains an exciting and expanding field of research. The objective of this review is to examine the effect of stress on GnRH neurons; in particular, the evidence that stress-related signaling molecules act directly on GnRH neurons will be evaluated.
1.1. Pulses:
A variety of stress models have been used to investigate the effects of stress on LH pulsatile secretion including psychosocial, metabolic and immune/inflammatory. For psychosocial stress, physical restraint suppressed LH pulses in monkeys,7 sheep,8 rats,9 and mice.10,11 Various models of metabolic stress including insulin-induced hypoglycema,12–16 glucoprivation,17,18 lipoprivation19 and feed restriction20–22 each suppressed LH pulses. Immune/inflammatory stress modeled with either endotoxin (lipopolysaccharide)23–25 or administration of cytokines26,27 also suppressed LH pulses in many species. Together these data, from a variety of stress models and species, demonstrate potent inhibitory effects of stress on pulsatile LH secretion, in both males and females.
1.2. Surge:
Stress has also been shown to interfere with the generation of the preovulatory GnRH/LH surge in two major manners. First, stress can prevent or delay the rise in E2 necessary for triggering the LH surge. A delay in the rise of E2 and subsequent LH surge, likely reflecting an inhibition of LH pulses, has been demonstrated in sheep during psychosocial (transport stress),28 immune/inflammatory29 and metabolic stress.30 Interestingly, in sheep exposed to metabolic stress in the early follicular phase, although the rise in E2 and LH surge was delayed, timing of estrous behavior was not altered.30 This raises the possibility that if ovulation did occur, it may not have been correctly timed with mating to facilitate fertilization. Second, stress can interfere with the ability of E2 to induce surge-type GnRH and LH secretion. Thus, E2-induced surge models are necessary to isolate and distinguish the effects on the surge generation circuitry from the masking effects on pulsatile LH or ovarian E2 production. Indeed, immune/inflammatory stress also blocked the E2-induced GnRH/LH surge in sheep31 and rats.32,33 In mice, metabolic stress (chronic feed restriction) blocked an E2-induced LH surge.20 Collectively, these data demonstrate multiple central mechanisms whereby stress interferes with generation of the LH surge.
1.3. Reproductive cycles:
In theory, suppression of either pulsatile or surge-type GnRH/LH secretion could inhibit reproductive cycles. Functional hypothalamic amenorrhea is an anovulatory disorder in women, resulting from insufficient GnRH and LH secretion, which is often associated with a variety of life experiences constituting metabolic and/or psychosocial stressors.34 Similarly in monkeys, a combined psychosocial stress, feed restriction and exercise paradigm, inhibited menstrual cycles.35 In mice, both chronic psychosocial stress (daily restraint stress)36 and mild feed restriction20 suppressed estrous cyclicity, as evidenced by persistent diestrus-like vaginal lavage consisting primarily of leukocytes and an absence of cornified cells indicating low E2 levels. However, not all stress paradigms result in suppression of the estrous cycle. For example, altered estrous cyclicity was not identified in either a two-week unpredictable chronic mild stress protocol37 or a daily restraint (homotypic) stress model38 in mice. Determination of estrous cyclicity in rodents is routinely performed by analysis of cells collected from vaginal lavage. Importantly, morphology of these cells is primarily dictated by E2 levels and does not necessarily indicate that ovulation occurred.39 Indeed, normal estrous cyclicity has been observed in mice, as determined by vaginal lavage, that did not produce natural or E2-induced LH surges and had ovaries with few or no corpora lutea, suggesting impairment not revealed by vaginal cells per se.40 In sheep, although some psychosocial stressors (such as 4 hours of transportation stress) delayed and suppressed the LH surge,28 other repeated acute stressors including isolation, restraint and predator sounds did not disrupt follicular phase events.41 Whether application of more intensive stressors would disrupt estrous cycles remains an outstanding question. Overall, these data demonstrate that normal reproductive cycles can be sensitive to the inhibitory effects of stress, though some stress types (immune or metabolic) may be more effective in suppressing cycles than psychosocial stress. These important phenomenological data have provided rationale for investigation of the specific neural substrates and processes responsible for suppression of GnRH secretion and thereby reproductive suppression during stress. In the following sections, the function of a handful of important mediatory molecules with implications for direct vs. indirect actions on GnRH will be reviewed (see Figure 1).
Figure 1:
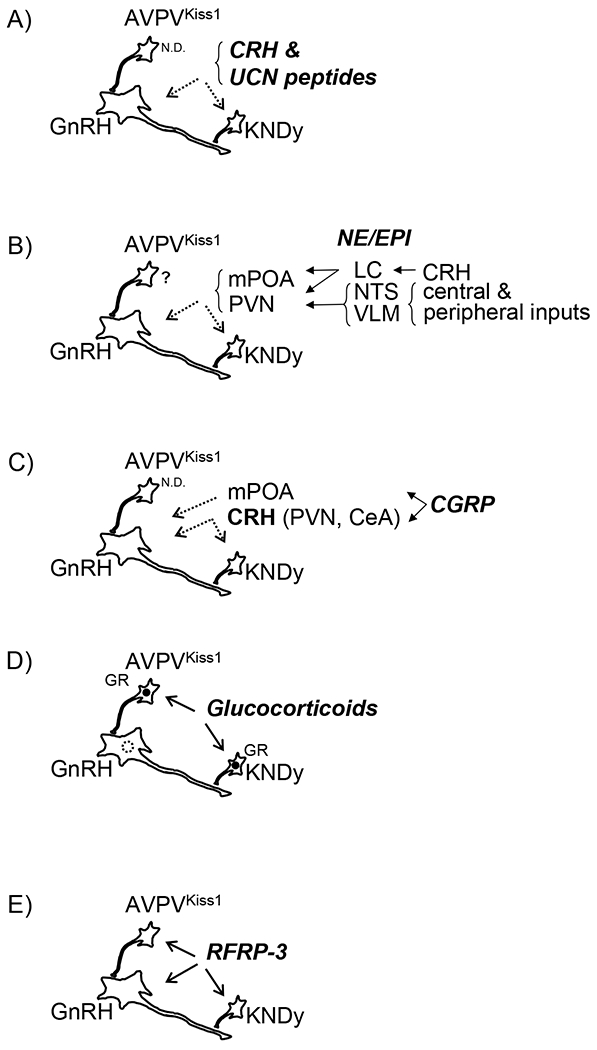
Schematic representation of speculated interactions of key inhibitory stress mediators upon GnRH neurons and/or upstream AVPVKiss1 and KNDy cells. A) CRH & UCN peptides, B) NE/EPI, C) CGRP, D) Glucocorticoids (in sheep and mice, but not rats, see text for details); GR expression is indicated in AVPVKiss1 and KNDy cells (closed circle) and absent in GnRH cells (dashed circle), E) RFRP-3. Solid arrows indicate evidence supporting direct regulation. Dashed arrows indicate evidence supporting indirect regulation. Filled Arrow heads indicated positive regulation. Open arrow heads indicate negative regulation. ND indicates no data available. Question marks indicate evidence supporting regulation of unclear directionality. Note, cartoon schematics largely based on data from rodents.
2. Effect of stress mediators on GnRH cells and secretion:
2.1. Corticotropin-Releasing Hormone (CRH) and related peptides:
Since its discovery in 1980, the neuropeptide CRH, which regulates the hypothalamic-pituitary-adrenal (HPA) axis, has been postulated to be the integrator of the reproduction and stress axes. Investigation of CRH as a possible mediator of stress-induced suppression of gonadotropin secretion continues to evolve with understanding of species differences and functional arrangement of neurons that produce CRH receptors and ligands. In ovariectomized (OVX) monkeys, intravenous injection or infusion of CRH suppressed pulsatile LH secretion.42–44 The effects of CRH in sheep, however, are varied. Indeed, CRH delivered intracerebroventricularly (ICV) to ewes in the early follicular phase of the estrous cycle suppressed LH pulse frequency.45 However, in OVX ewes (with or without gonadal steroid replacement) ICV CRH has been reported to have either no effect46 or stimulate LH pulse frequency.46,47 Similarly, in orchidectomized (ORCHX) or testosterone-replaced ORCHX rams, ICV CRH increased mean LH concentrations.48 In rats, ICV but not IV CRH reduced LH in OVX rats and OVX rats treated with estradiol benzoate (an LH surge model).49 Additionally, in anesthetized rats ICV CRH blocked the GnRH surge on the evening of proestrus.50 In OVX mice, chemogenetic activation of CRH neurons in the paraventricular nucleus (PVN) suppressed LH pulses,51 indicating that some molecule(s) released from these neurons is sufficient to inhibit gonadotropin secretion. These varied results likely demonstrate some species differences and highlight the necessity of carefully considering gonadal steroid hormone status of experimental animals; additional experimental details such as dose and route of administration may also influence these varied observations. Importantly, though inhibitory effects of CRH on gonadotropin secretion have been reported, numerous conditions exist in which CRH did not suppress gonadotropin secretion which raises the possibility that other signaling molecules are critical for suppression of reproduction during stress.
2.1.a. Urocortins:
In mammals, there are three urocortin peptides (UCN1, UCN2 and UCN3) that are structurally similar, but distinct from CRH. All three urocortin peptides are produced in the brain and have been investigated for their roles in stress responses. The major site of UCN1 expression is within and adjacent to the Edinger-Westphal nucleus of the midbrain.52,53 UCN2 is produced in the PVN, arcuate (ARC), and supraoptic nuclei of the hypothalamus, as well as the brainstem and spinal cord.54 UCN3 is produced in the medial amygdala and hypothalamus (preoptic area and perifornical region).55 UCN2 injected ICV into E2-replaced OVX rats suppressed LH pulse frequency.56
2.1.b. CRH and urocortin signaling mechanisms:
CRH signals via its receptor corticotropin-releasing hormone receptor 1 (CRHR1). Corticotropin-releasing hormone receptor 2 (CRHR2) shares approximately 70% sequence homology with CRHR1, though it is encoded by a distinct gene. UCN2 and UCN3 have much higher affinity to CRHR2, whereas UCN1 has approximately equal affinity for both receptors. Consideration of both CRHRs and their ligands is important because some commonly used CRHR antagonists (e.g. alpha-helical CRH) are not specific to CRHR1, and thus physiological actions of CRHR2 ligands have been attributed to CRH. Indeed, non-specific CRHR antagonists prevented (or partially reversed) the inhibitory effects of acute metabolic stress on LH in monkeys57 and rats.58 With the advent of specific receptor antagonists the roles of the receptor subtypes have been investigated. For example, CRHR1 blockade prevented the suppression of LH pulses following psychosocial stress, but not metabolic or immune/inflammatory stress in rats.59 In monkeys, a combined psychosocial and metabolic stress paradigm that suppressed pulse frequency was reversed with a specific CRHR1 antagonist.60 Conversely, specific CRHR2 antagonists partially reversed the inhibitory effect of acute metabolic and immune/inflammatory stress on LH pulses in rats.59 Thus, CRH and urocortins are necessary for the suppression of LH during various stress paradigms, though their relative importance varies.
2.1.c. Evidence for direct action at GnRH neurons:
CRHR1 and CRHR2 have heterogenous expression patterns in the brain, including in the vicinity of GnRH neurons. In mice, approximately 30% of GnRH neurons contained immunoreactivity for CRHRs (the antisera could not distinguish type of CRHR).61 Consistent with this finding, ~25% of GnRH neurons in the mouse were found to contain mRNA for Crhr1 via microarray and confirmed with single-cell RT-PCR, yet, no evidence of Crhr2 was found in GnRH cells.61 Additionally, CRH terminals are observed in close contact with GnRH neurons in humans62 and rats,63 and in mice, CRH terminals have been observed in close contact with GnRH fibers in the ARC.51 Inhibitory actions of CRH have been documented in vitro as CRH treatment decreases GnRH transcription in GN11 cells (a model of immature GnRH neurons)64 and decreases GnRH mRNA in GT1-7 cells (model of mature GnRH neurons). Thus, there exists an anatomical framework for actions of CRH directly on GnRH neurons as well as functional evidence for actions of CRH on GnRH cell lines in culture.
2.1.d. Evidence against direct action at GnRH neurons:
Despite reports of CRH terminals in close contact with GnRH neurons in multiple species, retrograde tracing agents delivered to the vicinity of GnRH neurons in the POA did not label CRH neurons in the PVN in rats.65 In sheep, cells located in the PVN that project to the POA were not activated by a psychosocial stress paradigm, despite robust activation of other (non-POA projecting) cells in the PVN.66 Moreover, in contrast to reports of Crhr1 mRNA in GnRH cells, no colocalization was found between GnRH immunoreactivity and CRHR1 using a transgenic mouse with GFP under the CRHR1 promoter67,68 or between mRNA for Gnrh and Crhr1 via dual-label in situ hybridization.68 Moreover, genetic deletion of CRHR1 from GnRH neurons did not prevent suppression of LH following restraint stress or LPS administration.68 Together these anatomical and functional data do not support a major role for CRH acting directly on GnRH neurons.
The effect of CRH on the electrical properties of GnRH neurons are varied but largely support the hypothesis that CRH does not act directly on GnRH neurons. First, in OVX69 and ORCHX68 mice no effect of CRH on GnRH firing rate was observed. In another study, CRH was found to stimulate firing in a sub-set of GnRH neurons in OVX mice.51 Acute brain slices from mice in the diestrous phase of the estrous cycle51 or OVX69 mice treated with a dose of E2 sufficient to induce an LH surge (OVX+E2) showed an increase in firing rate in 20-40% of GnRH neurons. These stimulatory effects of CRH on GnRH neuron firing are likely indirect because CRH treatment did not alter potassium currents nor excitability of GnRH cells but did increase the frequency of GABA post synaptic currents,67 indicating an increase in GABA release from other nearby cells. (Note, often inhibitory elsewhere in the central nervous system, GABA is generally stimulatory upon GnRH cells due to their relatively high intracellular chloride concentrations70). Finally, in OVX+E2 mice treated with a higher dose of CRH, an inhibition of GnRH neurons was observed.69 The inhibitory effect of high doses of CRH was attributed to an action on CRHR2, because application of UCN3 (highly specific to CRHR2) also suppressed GnRH cell firing in OVX+E2 mice.69 It is likely that these CRHR2 mediated inhibitory effects are not directly on GnRH cells, because GnRH cells do not contain Crhr2 mRNA.61 Another caveat pertains to the effects of CRH on GnRH soma described above. Considering evidence that pulsatile secretion of LH (and presumably GnRH) can be induced by activation of GnRH fibers in the median eminence, CRH actions upon the GnRH soma may be applicable only to modulation of surge-type LH secretion.71 Analysis of calcium flux in GnRH fibers in the lateral ARC and median eminence revealed no effect of CRH treatment nor was CRH able to alter the increase in calcium flux (i.e. change in fluorescence) induced by exogenous kisspeptin treatment.51 Based on this collective work, it is likely that CRH and the urocortin peptides act on neurons afferent to GnRH cells to suppress gonadotropin secretion (Figure 1A).
The site(s) of action for CRH in the suppression of gonadotropin secretion remains an outstanding question. One possibility is the KNDy cell population in the ARC which forms the GnRH pulse generator and co-expresses kisspeptin (encoded by Kiss1), neurokinin B (encoded by Tac2), and dynorphin,72,73 since these cells express one of the CRHRs in rats (the antisera could not distinguish CRHR1 from CRHR274). CRH inhibited MUA volleys in the MBH of rhesus monkeys42 (an assessment of GnRH pulse generator activity, likely emanating from KNDy cells) and reduced Kiss1 mRNA abundance in rats.75 However, CRH did not alter firing rate in ARC Tac2 cells from female mice67 (highly colocalized with kisspeptin in the ARC, thus KNDy cells), nor did optogenetic activation of CRH terminals in the ARC alter ARC kisspeptin cell firing in female mice,51 which collectively support CRH actions on neurons afferent to the KNDy cells. An alternative site of CRH action on GnRH/LH pulsatility is in the locus coeruleus (LC) as discussed below. In contrast, deletion of CRHR1 or CRHR2 from all neurons and glia did not prevent the suppression of LH secretion following restraint stress or LPS administration, which would support a hypothesis that neither CRH nor the urocortin peptides have a major role in suppression of LH during stress.68 However, these unexpected findings, which are at variance with vast pharmacological data, may be explained by incomplete knockdown of the receptors, potentially spurious effects related to nestin-cre line itself,76 or developmental compensation, and thus should be interpreted cautiously.
2.2. Catecholamines (Norepinephrine and Epinephrine):
The catecholamines, norepinephrine (NE) and epinephrine (EPI), have long been recognized as important mediators of stress responses, both peripherally (released from adrenal medulla) and centrally. In the brain, EPI and NE are primarily produced in the brainstem, largely in three stress-responsive nuclei: ventral lateral medulla (VLM; A1 population), nucleus of the solitary tract (NTS; A2 population), and the LC (A6 population).77 The A1 and A2 populations receive rich interoceptive inputs (e.g. area postrema, vagus nerve), central inputs (e.g. amygdala, hypothalamus), and contain steroid hormone receptors. Anatomically, neurons in the A1 and A2 populations project widely throughout the brain, including the hypothalamus; thus, they are well positioned to survey the brain and body and transmit stress signals to the hypothalamus to regulate neuroendocrine function. Indeed, both the A1 and A2 neuron populations are implicated in the activation of PVN CRH during immune/inflammatory stress.78,79 Neurons in the LC receive input largely from the brain, including from CRH terminals arising from the amygdala, bed nucleus of the stria terminalis, and to a lesser degree the PVN.80 CRH injection in the LC induces ACTH and corticosterone secretion,81 stress-like behaviors82 and suppresses pulsatile LH secretion,83 thus demonstrating the capacity to mediate several stress-related responses.
2.2.a. Evidence for direct action on GnRH cells:
Biosynthesis of catecholamines, NE and EPI, occurs via successive action of enzymes, which serve as markers for the neurons that produce catecholamines. The enzymatic pathway includes, phenylalanine hydroxylase (phenylalanine → L-tyrosine), tyrosine hydroxylase (L-tyrosine → L-dopa), aromatic amino acid decarboxylase (L-dopa → dopamine), dopamine β-hydroxylase (DBH; dopamine → NE), phenylethanolamine N-methyltransferase (NE → EPI). NE and EPI signal via the adrenoreceptor family of G-protein coupled receptors, of which several members are expressed throughout the brain. Low to moderate expression of the α1, α2, and β1 adrenoreceptors have been detected in some pools of mouse GnRH neurons.84,85 DBH immunoreactive terminals have been observed in close contact with GnRH soma86 and dendrites.87 Utilizing a pseudorabies tracing virus to label afferents to GnRH cells, tyrosine hydroxylase-immunoreactive neurons were identified in the NTS and LC at time points corresponding to primary afferents, and in the VLM at a later time point (possibly a secondary afferent).88 It should be noted that timing of pseudorabies spread has been shown not to be a reliable method of distinguishing primary vs. higher order afferents.89 Never-the-less, these data provide an anatomical framework by which catecholamine neurons in the brainstem act on GnRH cells. Consistent with an action on GnRH neurons, NE and adrenoreceptor agonists (α1 and β receptors) suppressed GnRH cell firing in acute brain slices collected from male and female mice.90 Moreover, this suppressive effect occurred in the presence of glutamate, GABA, and voltage-gated sodium channel blockade indicating a direct effect on GnRH neurons.90 Administration of NE into the third ventricle suppressed LH pulses in rats.91 Thus, electrophysiological and pharmacological data raise the possibility of a direct inhibitory action of NE and (possibly EPI) on GnRH cells.
2.2.b. Evidence against direct action on GnRH cells:
Administration of NE or agonists for its receptors into specific brain regions has yielded support against action directly on GnRH neurons during stress. Injection of NE or adrenoreceptor agonists into the POA in E2 replaced OVX rats92 and sheep93 stimulated LH secretion, whereas no effect was observed in the absence of E2.93 These stimulatory effects may be related to the facilitation of LH surge secretion. In contrast, NE and adrenoreceptor agonists injected into the PVN potently suppressed LH pulses in rats.94 Interestingly, the inhibitory effect of adrenoreceptor activation can be blocked by non-specific CRH receptor antagonists,94 which raises the possibility that brainstem catecholamine neurons project to the PVN to suppress LH secretion in an indirect manner. Immunotoxic ablation of DBH terminals in the PVN resulted in depletion of catecholamine neurons in the brainstem (primarily the NTS region), and importantly blocked the suppressive effect on chronic glucoprivation on estrous cyclicity in rats.95 The same DBH ablation technique revealed that decimation of brainstem catecholamine neurons also blocked activation of PVN neurons and attenuated the rise in corticosterone following psychosocial96 and immune/inflammatory79 stress. These data support the hypothesis that brainstem catecholamine neurons project to the PVN to activate the HPA axis and suppress the hypothalamic-pituitary-gonadal axis during stress, thereby suppressing GnRH neurons indirectly (Figure 1B).
2.3. Calcitonin Gene-Related Peptide (CGRP):
CGRP is produced in several brain regions including the stress-responsive parabrachial nucleus (PBN) of the brainstem. CGRP neurons in the PBN are activated during a variety of stress types; moreover, these CGRP neurons are innervated and regulated by NE neurons in the A2. CGRP administration induced HPA axis activation97 and stress-related behaviors.98 Importantly, ICV infusion of CGRP suppressed pulsatile LH secretion in OVX+E2 rats,99 and a CGRP receptor antagonist blocked the inhibitory effect of metabolic stress (hypoglycemia) on LH secretion in rats. CGRP likely has many roles in mediating stress responses, including regulation of gonadotropins during, at least, some types of stress.
2.3.a. Evidence for direct action on GnRH cells:
Although the effects of CGRP on gonadotropin secretion are striking, much is still to be learned about the mechanisms for these effects. CGRP terminals are abundant in the POA. Even though the origin of these fibers is not known, PBN neurons are known to project to the POA. Pharmacological data support a role for this neuropeptide to act in the POA as CGRP microinfused into the POA (vicinity of GnRH neurons), but not other regions, suppressed LH pulses in rats.100 Though it is not known if GnRH neurons in vivo contain the receptor for CGRP, the GT1-7 cell line does,101 and CGRP treatment reduced the abundance of mRNA for Gnrh.101
2.3.b. Evidence against direct action on GnRH cells:
Although CGRP neurons project to and act in the vicinity of GnRH neurons, pharmacological evidence suggests an indirect action of CGRP in vivo. The suppressive effect of ICV CGRP on LH pulses can be blocked by a CRHR1 antagonist,102 indicating that CGRP acts via activation of CRH neurons. CGRP administration increased Crh mRNA in both the PVN and amygdala, supporting a role for either or both populations.102 It is not clear how a CRHR1 dependent action of CGRP might suppress LH during metabolic stress, since a CRHR1 receptor antagonist did not block the suppressive effect of metabolic stress.59 Thus, much is still to be learned about the role of CGRP in mediating the effects of stress and its interactions with GnRH neurons (Figure 1C).
2.4. Cortisol/Corticosterone:
The adrenal steroid cortisol (or corticosterone in rodents) is a potential mediator of the inhibitory effect of stress on gonadotropin secretion. Hydrocortisone acetate suppressed LH pulses in both OVX pigs103 and ORCHX monkeys104 demonstrating sufficiency of cortisol to inhibit gonadotropin secretion in a E2-independent manner in some species. However, in female sheep105,106 and mice,107 the ability of cortisol or corticosterone, respectively, to suppress LH pulse frequency is dependent on E2. In OVX sheep, a cortisol treatment that achieved a stress-like level of cortisol, suppressed LH pulse amplitude,108 without altering GnRH pulse amplitude, LH pulse frequency or GnRH pulse frequency109 indicating an effect in the pituitary, not hypothalamus. In contrast, in ovary-intact ewes during the early or mid-follicular phase of the estrous cycle (before the LH surge)110 or OVX ewes treated with E2 and progesterone to mimic an estrous cycle,106 cortisol suppressed GnRH and LH pulse frequency, demonstrating a role for E2 to sensitize the hypothalamus to the effect of cortisol.
The molecular mechanisms by which E2 permits the inhibitory effect of glucocorticoids on GnRH pulse frequency remains a significant outstanding question. Intriguingly, glucocorticoids also interfere with the actions of E2 during the LH surge. Corticosterone blocked the E2-induced LH surge in mice,111 and cortisol delayed and blunted the amplitude of the E2-induced LH surge in sheep.112 Since GnRH neurons do not contain glucocorticoid receptors, it is likely that any effects of cortisol or corticosterone are mediated via afferents to GnRH neurons. In sheep113 and mice,107 glucocorticoid receptor is present in the majority of KNDy cells (as well as the AVPV/PeV population in mice) and corticosterone inhibits activation of either kisspeptin population in female mice107,111 supporting the potential for glucocorticoids to act directly upon kisspeptin cells (Figure 1D). Some species differences are also at play, as corticosterone treatment does not alter pulsatile LH secretion in OVX rats with or without gonadal steroid replacement.75 Interestingly, although corticosterone suppressed Kiss1 mRNA in rats,75 kisspeptin neurons do not appear to contain glucocorticoid receptors in this species;74 the relevance of this decrease in transcript levels is unclear since pulsatile LH secretion was not altered. In mice, although corticosterone suppressed KNDy cell activation, the abundance of mRNA for Kiss1 and Tac2 were not altered.107 Interestingly, corticosterone modestly suppressed the abundance of pDyn (mRNA for dynorphin),107 whereas an increase in pDyn would be expected concurrent with decreased LH pulse frequency. In mice, although the majority of ARC kisspeptin cells contain mRNA for dynorphin, there are some non-kisspeptin neurons that contain pDyn, 114 and the method employed in the above work could not resolve which population of neurons was altered by corticosterone. Collectively, these findings support the idea that glucocorticoid-induced inhibition of the pulse generator and the resulting decrease in LH pulse frequency may not be mediated by changes in transcription of KNDy related genes.107 Clearly, future investigation is required to understand how glucocorticoids suppress gonadotropin secretion in many, but not all species, through cells and signaling pathways afferent to GnRH neurons.
2.5. Arginine-Phenylalanine-Amide-Related Peptide-3 (RFRP-3):
RFRP-3 is the mammalian ortholog of the avian neuropeptide, gonadotropin-inhibitory hormone. Unlike the actions of gonadotropin-inhibitory hormone in birds, RFRP-3 appears to act predominantly within the brain to regulate gonadotropin secretion in a variety of physiological contexts, including stress. The RFRP-3 receptor, GPR147 (NPFFR1), is a G-protein coupled receptor that is expressed in GnRH neurons.115 RFRP-3 is a member of the RFamide peptide family which also includes kisspeptin, neuropeptide FF, prolactin-releasing peptide, 26RFa and others.116 These peptides have structural similarity and as a result have some affinity for each other’s receptors.116 A previously used antagonist for GPR147 (RF9) also acts as a partial agonist of the kisspeptin receptor;117 thus, pharmacological approaches to investigating these signaling pathways can be difficult to interpret. Despite these challenges, several lines of evidence support the hypothesis that RFRP-3 is an important regulator of LH secretion during stress. First, the inhibitory effect of fasting on LH secretion was partially reversed in NPFFR1 knock-out mice.118 Second, knockdown of Rfrp3 prevented infertility caused by repeated restraint stress in female rats.119 Third, ablation of RFRP3 neurons prevented restraint stress-induced suppression of LH pulses in female mice.120 Evidence for direct action of RFRP-3 on GnRH neurons include findings that GnRH cells contain the receptor for RFRP-3 (NPFFR1)115,121 and that RFRP-3 terminals are found in close contact with GnRH cell bodies.115,122,123 Additionally, RFRP-3 was shown to inhibit GnRH cell firing, an effect maintained following GABA and glutamate receptor blockade, suggesting a direct effect on GnRH cells.124,125 RFRP-3 may also act on ARC as well as AVPV/PeV kisspeptin cells, since these cells contain GPR147 and also receive close contacts from RFRP-3 neurons.115 Thus, RFRP-3 likely influences GnRH secretion via direct and indirect actions on GnRH cells (Figure 1E).
3. Future Directions and Perspectives:
In this review, we highlighted much of the work performed to address the question: how is GnRH secretion suppressed during stress? Theoretically, the response to stress involves three principal actions: detection of the stressor (the stimuli), transmission of signal(s), and action on some element(s) of the reproductive axis. Here, we focused on the specific matter of whether or not a handful of signaling molecules act directly on GnRH neurons to suppress gonadotropin secretion during stress. We suggest that this is the perfect time to evaluate the evidence supporting direct actions of stress mediators on GnRH neurons as recent advancements have demonstrated the importance of two populations of kisspeptin-containing cells in the hypothalamus that organize pulsatile and surge-type GnRH secretion. With the discovery of these cells, alternative sites of action for stress-related signaling molecules have been revealed yet remain to be fully-tested. The implication is that earlier papers should be read with the understanding that the kisspeptin systems (and subsequent importance of the ARC and rostral hypothalamic [AVPV/PeV in rodents, preoptic area in ruminants and primates] cell populations126) were not known or fully appreciated at the time of publication. Thus, there is still much work to be done to determine the exact pathways, including identifying upstream neural sites, cell types and mediators, by which GnRH cells are inhibited during stress.
Here, we evaluated the evidence for either direct or indirect action of several signaling molecules that have been investigated as mediators of impaired GnRH cell function, including CRH, the urocortin peptides, norepinephrine, CGRP, cortisol/corticosterone and RFRP-3. Though all of these molecules ultimately reduce GnRH cell function, the preponderance of evidence discussed above indicates that most of these signaling molecules do not act directly on GnRH neurons. The exception is RFRP-3, in which data are currently limited. Anatomical and electrophysiological data support the possibility that RFRP-3 acts directly on GnRH neurons,115,121–125 though it is possible that RFRP-3 also acts on kisspeptin neurons to alter GnRH cells indirectly as well.115 Rigorous testing of the hypothesis that RFRP-3 acts directly in GnRH neurons will require generation of animals that lack the RFRP-3 receptor (NPFFR1) in GnRH neurons, which has not been done. Although anatomical and electrophysiological evidence similarly support the hypothesis that catecholamines act directly on GnRH neurons,84–88 functional in vivo data contradict this possibly. First, microinjection of NE into the POA (site of GnRH) neurons does not suppress LH pulses,94 and second, the inhibitory effect of NE is reversed by CRHR antagonists,94 indicating NE acts via CRH or urocortin peptides. The current evidence suggests that the others likely directly or indirectly suppress KNDy cell activity, which ceases to stimulate pulsatile GnRH secretion (summarized in Figure 1).
As the suppression of LH pulses has the potential to blunt or delay the preovulatory rise of E2, any of these mediators, acting directly or indirectly to inhibit pulsatile GnRH secretion, are one potential mechanism whereby stress can also suppress surge GnRH/LH secretion. A second mechanism is interference with the GnRH/LH surge mechanism in the presence of sufficient E2. Indeed, discriminating between direct actions on GnRH cells versus afferent pathways, such as the rostral population of kisspeptin neurons, during stress-induced suppression of the surge remains an open question, with the exception of glucocorticoid-induced suppression of the positive-feedback response to E2 (Figure 1D). It is clear that greater resolution of the upstream circuits controlling GnRH neuron function during either pulsatile or surge modes of secretion will enable clarification of direct versus indirect actions of stress-activated signaling factors on both GnRH neurons as well as the kisspeptin populations afferent to this indispensable cell population.
3.1. Influence of estradiol:
One area of future investigation of particular interest to us is the role of E2 in sensitizing the reproductive axis to the inhibitory effects of stress, which has been demonstrated in many mammalian species. In mice, we (K.M.B. laboratory) have shown that some stimuli (glucocorticoid treatment and immune/inflammatory stress) are E2-dependent; however, other stimuli are not (psychosocial and metabolic stress). Both theoretically and technically this is an important observation. From a technical standpoint, detection of LH pulses in ovary-intact mice is challenging because of low LH pulse frequency and low baseline concentrations. Moreover, although LH pulses and synchronized calcium events in KNDy neurons occur throughout the estrous cycle (except on day of estrus), inter-pulse interval can vary between 20 and 80 min among pulses,127,128 which further complicates identifying a bona fide decrease in LH pulses. Therefore, experimentation on OVX animals is attractive since it permits detection of frequent pulses in the control condition; however, this approach opens critique to a ‘lack of physiological relevance’ and importantly the possibility of missing E2-dependent effects.
An alternative approach is to OVX and replace physiological-like levels of E2 in silastic capsules, as has been performed in other species. We (K.M.B. laboratory) recently published an E2-replacement paradigm that generated diestrous-like levels of E2, using uterine weight as a proxy for circulating E2 concetrations. Moreover, this dose of E2 reduced LH pulse frequency compared to OVX mice and also reversed other physiological effects of OVX including weight gain and loss of circadian corticosterone rhythms, further demonstrating the physiological relevance of this dose.107 Importantly, this dose of E2 permitted the inhibitory effects of immune/inflammatory stress26 and chronic corticosterone treatment107 on LH pulse frequency, demonstrating that this dose of E2 is sufficient to sensitize the neuroendocrine system to stress. Despite the physiological evidence supporting the utility of this dose of E2, it is clear that LH concentrations and pulse frequency in this OVX+E2 model107 are substantially greater than those observed in intact females during diestrus.128 One explanation for this discrepancy is that some ovarian factor other that E2 contributes to the suppression of gonadotropin secretion. Although potentially interesting and illustrative of the many outstanding mysteries of the estrous cycle, the importance of reliable methods to clamp E2 concentrations during experimentation cannot be overstated. In addition to enabling reliable detection of LH pulses, OVX+E2 models offer numerous practical advantages, including overcoming the technical challenge of generating a cohort of animals that can be used for experimentation on the same day, since there are no reliable methods of synchronizing estrus cycles in mice. Furthermore, since E2 regulates GnRH/LH secretion and GnRH/LH secretion in turn regulates E2 concentrations, clamping E2 at a fixed level is necessary for removing the confounding effect of altered E2 whilst studying other regulators of GnRH/LH secretion. Indeed, ovary-intact animals will reveal the full sequalae of stress effects on reproduction with greater physiological relevance; however, OVX+E2 models are necessary for reducing this incredibly complex biologic system into isolated components for detailed analysis of the underlying neural circuits.
The physiological mechanism for E2 to potentiate the inhibitory effects of stress on gonadotropin secretion remains a significant outstanding question. In rats, the observation that E2 delivered into the NTS or PVN (but not the ARC, POA, LC, or VLM) allowed 48 hours of fasting to suppress pulsatile LH secretion, an effect observed in OVX+E2 but not OVX rats,129 offers some clues to sites of action. In mice, E2 treatment did not alter the number of cells that expressed cFos in the brainstem or PVN in response to IL1B.26 Whether this indicates that E2 does not potentiate activity of neurons in these areas or that cFos immunoreactivity is not sufficiently sensitive to detect changes in activity of these cell populations remain outstanding questions. Moreover, although robust suppressive effects of metabolic and psychosocial stress in OVX mice have been documented, it is not known if E2 can heighten the responses to these stress types. Whether E2 would cause suppression of LH pulses in response to more moderate metabolic or psychosocial challenges, or if E2 would prolong the duration of impaired pulsatile LH secretion is unknown. Molecularly, E2 could enable changes in sensitivity to stress in a variety of ways including altered synaptic connectivity of neural circuits, changes in ligand or receptor expression, remodeling epigenetic modifications, altered ion channel abundance or conductivity underlying the excitability of cells or their sensitivity to stimuli. Thus, key in understanding the neurobiology of stress will be deciphering the many actions of E2 (and testosterone) in the brain.
3.2. Influence of species differences:
In reflecting on the past 50 years of literature in this review, some topics are noteworthy for the future. First, as the field of stress effects on reproduction continues to flourish it is clear that differences among species will persist. Hopefully these differences will provide unique and insightful comparisons that enable us to better understand the neurobiology of stress responses. One interesting example of an anatomical-functional correlation is the observation that glucocorticoids suppress LH secretion in OVX and E2 replaced mice107 and sheep,106,110 species in which glucocorticoid receptor has been detected within KNDy cells. In contrast, in rats, which do not express glucocorticoid receptor in KNDy cells, LH secretion is not altered by glucocorticoid treatment.75 As E2 is required for glucocorticoid-induced inhibition of LH in sheep and mice, future work remains in which glucocorticoid levels are clamped in order to tease out the role of other mediators or neuronal populations, potentially influenced by E2. Neuroendocrine research has included many diverse species; this review contained data from humans, monkeys, pigs, sheep, goats, hamsters, rats and mice. Indeed, each bring valuable advantages and physiological contexts, though there has been a trend for increased use of mice in the study of stress on reproduction. Low animal cost, availability of transgenic and molecular approaches, and recent advances in serial blood sampling for analysis of pulsatile LH secretion contribute to the appeal of the mouse model. Application of this species warrants keen understanding of mouse physiology, since this species displays clear differences from other species, including rats.
3.3. Influence of technical advancement:
A second topic is the power of advancing technologies, particularly multi-label immunohistochemistry and in situ hybridization as well as RNA-sequencing, which will provide greater ability to identify and localize important signaling molecules and receptors. These approaches will allow high-throughput screening and targeted analysis of numerous signaling candidates that will accelerate our understanding of stress neural circuits. Hopefully these techniques will permit analysis of heterogenous cell populations, and shine new light on diverse, and at times conflicting roles of neural populations. For example, NE produced in the brainstem is critical for suppression of LH secretion during stress, as discussed above, but is also necessary for the LH surge (i.e. enhanced GnRH outflow).130–132 There is also great heterogeneity in the CRH neurons in the PVN.133 Whether distinct populations regulate circadian rhythms and stress effects or whether different stress types activate different subpopulations of these PVN CRH cells remain to be fully understood. It is likely that sub-populations of neurons will be identified, and refined approaches will allow us to target cell populations with enhanced precision.
3.4. Final thoughts:
A final topic of increased importance will be integrating the effects of the numerous stress-related signaling molecules, of which only a portion are presented here. In the last several decades, several peptides and transmitters have been identified and tested (reviewed above). Though some interactions between signaling molecules have been uncovered, there is still much work to be done in integrating these studies to discover the complete neural pathway between sensing a challenge of or threat to homeostasis all the way to suppression of GnRH neurons. Thus, in the 50 years since the discovery of GnRH, tremendous advancements have been made uncovering how GnRH secretion is regulated, and we are optimistic that future work will continue to expand this theoretically interesting and clinically important field.
Acknowledgements:
This work was supported by NIH grants: R01 HD086100, R01 HD103725, R21 HD105103, and P50 HD012303 and the UCSD Health Sciences Senate. R.B.M. was supported by NIH grants K99104994, F32 HD096811 and T32 HD007203.
Footnotes
Conflict of Interest:
The Authors have nothing to disclose.
4. References
- 1.Goodman RL. Neuroendocrine Control of Gonadotropin Secretion: Comparitve Aspects. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology or Reproduction. Vol 2. 4 ed: Elsevier; 2015:1537–1563. [Google Scholar]
- 2.Podfigurna-Stopa A, Pludowski P, Jaworski M, Lorenc R, Genazzani AR, Meczekalski B. Skeletal status and body composition in young women with functional hypothalamic amenorrhea. Gynecol Endocrinol. 2012;28(4):299–304. [DOI] [PubMed] [Google Scholar]
- 3.Rickenlund A, Eriksson MJ, Schenck-Gustafsson K, Hirschberg AL. Amenorrhea in female athletes is associated with endothelial dysfunction and unfavorable lipid profile. J Clin Endocrinol Metab. 2005;90(3):1354–1359. [DOI] [PubMed] [Google Scholar]
- 4.Friday KE, Drinkwater BL, Bruemmer B, Chesnut C, 3rd, Chait A. Elevated plasma low-density lipoprotein and high-density lipoprotein cholesterol levels in amenorrheic athletes: effects of endogenous hormone status and nutrient intake. J Clin Endocrinol Metab. 1993;77(6):1605–1609. [DOI] [PubMed] [Google Scholar]
- 5.Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76(2):310–316. [DOI] [PubMed] [Google Scholar]
- 6.Moenter SM. Leap of Faith: Does Serum Luteinizing Hormone Always Accurately Reflect Central Reproductive Neuroendocrine Activity? Neuroendocrinology. 2015;102(4):256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87(5):850–853. [DOI] [PubMed] [Google Scholar]
- 8.Tilbrook AJ, Canny BJ, Serapiglia MD, Ambrose TJ, Clarke IJ. Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids. J Endocrinol. 1999;160(3):469–481. [DOI] [PubMed] [Google Scholar]
- 9.Li XF, Edward J, Mitchell JC, et al. Differential effects of repeated restraint stress on pulsatile lutenizing hormone secretion in female Fischer, Lewis and Wistar rats. J Neuroendocrinol. 2004;16(7):620–627. [DOI] [PubMed] [Google Scholar]
- 10.Yang JA, Hughes JK, Parra RA, Volk KM, Kauffman AS. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. J Endocrinol. 2018;239(3):339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JA, Song CI, Hughes JK, et al. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology. 2017;158(11):3716–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCosh RB, Kreisman MJ, Tian K, Ho BS, Thackray VG, Breen KM. Insulin-induced hypoglycaemia suppresses pulsatile luteinising hormone secretion and arcuate Kiss1 cell activation in female mice. J Neuroendocrinol. 2019;31(12):e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J, Knobil E. Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992;56(5):666–673. [DOI] [PubMed] [Google Scholar]
- 14.Clarke IJ, Horton RJ, Doughton BW. Investigation of the mechanism by which insulin-induced hypoglycemia decreases luteinizing hormone secretion in ovariectomized ewes. Endocrinology. 1990;127(3):1470–1476. [DOI] [PubMed] [Google Scholar]
- 15.Oltmanns KM, Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hypoglycemia, but not insulin, acutely decreases LH and T secretion in men. J Clin Endocrinol Metab. 2001;86(10):4913–4919. [DOI] [PubMed] [Google Scholar]
- 16.Goubillon ML, Thalabard JC. Insulin-induced hypoglycemia decreases luteinizing hormone secretion in the castrated male rat: involvement of opiate peptides. Neuroendocrinology. 1996;64(1):49–56. [DOI] [PubMed] [Google Scholar]
- 17.Ohkura S, Ichimaru T, Itoh F, Matsuyama S, Okamura H. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology. 2004;145(7):3239–3246. [DOI] [PubMed] [Google Scholar]
- 18.Bucholtz DC, Vidwans NM, Herbosa CG, Schillo KK, Foster DL. Metabolic interfaces between growth and reproduction. V. Pulsatile luteinizing hormone secretion is dependent on glucose availability. Endocrinology. 1996;137(2):601–607. [DOI] [PubMed] [Google Scholar]
- 19.Shahab M, Sajapitak S, Tsukamura H, et al. Acute lipoprivation suppresses pulsatile luteinizing hormone secretion without affecting food intake in female rats. J Reprod Dev. 2006;52(6):763–772. [DOI] [PubMed] [Google Scholar]
- 20.Kreisman MJ, Tadrousse KS, McCosh RB, Breen KM. Neuroendocrine Basis for Disrupted Ovarian Cyclicity in Female Mice During Chronic Undernutrition. Endocrinology. 2021;162(8):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta). Endocrinology. 1991;128(3):1532–1540. [DOI] [PubMed] [Google Scholar]
- 22.Merkley CM, Renwick AN, Shuping SL, Harlow K, Sommer JR, Nestor CC. Undernutrition reduces kisspeptin and neurokinin B expression in castrated male sheep. Reproduction and Fertility. 2020;1(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguie C, Karsch FJ. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology. 1997;138(10):4273–4281. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi Y, Nagabukuro H, Kizumi O, Mori Y. Lipopolysaccharide-induced suppression of the hypothalamic gonadotropin-releasing hormone pulse generator in ovariectomized goats. J Vet Med Sci. 1997;59(2):93–96. [DOI] [PubMed] [Google Scholar]
- 25.Refojo D, Arias P, Moguilevsky JA, Feleder C. Effect of bacterial endotoxin on in vivo pulsatile gonadotropin secretion in adult male rats. Neuroendocrinology. 1998;67(4):275–281. [DOI] [PubMed] [Google Scholar]
- 26.Makowski KN, Kreisman MJ, McCosh RB, Raad AA, Breen KM. Peripheral interleukin-1β inhibits arcuate kiss1 cells and LH pulses in female mice. J Endocrinol. 2020;246(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalra PS, Edwards TG, Xu B, Jain M, Kalra SP. The anti-gonadotropic effects of cytokines: the role of neuropeptides. Domest Anim Endocrinol. 1998;15(5):321–332. [DOI] [PubMed] [Google Scholar]
- 28.Dobson H, Tebble JE, Phogat JB, Smith RF. Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil. 1999;116(1):1–8. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia DF, Beaver AB, Harris TG, Tanhehco E, Viguié C, Karsch FJ. Endotoxin disrupts the estradiol-induced luteinizing hormone surge: interference with estradiol signal reading, not surge release. Endocrinology. 1999;140(6):2471–2479. [DOI] [PubMed] [Google Scholar]
- 30.Fergani C, Saifullizam AK, Routly JE, Smith RF, Dobson H. Estrous behavior, luteinizing hormone and estradiol profiles of intact ewes treated with insulin or endotoxin. Physiol Behav. 2012;105(3):757–765. [DOI] [PubMed] [Google Scholar]
- 31.Breen KM, Billings HJ, Debus N, Karsch FJ. Endotoxin inhibits the surge secretion of gonadotropin-releasing hormone via a prostaglandin-independent pathway. Endocrinology. 2004;145(1):221–227. [DOI] [PubMed] [Google Scholar]
- 32.Rivier C, Vale W. Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology. 1990;127(2):849–856. [DOI] [PubMed] [Google Scholar]
- 33.Kalra PS, Sahu A, Kalra SP. Interleukin-1 inhibits the ovarian steroid-induced luteinizing hormone surge and release of hypothalamic luteinizing hormone-releasing hormone in rats. Endocrinology. 1990;126(4):2145–2152. [DOI] [PubMed] [Google Scholar]
- 34.Fourman LT, Fazeli PK. Neuroendocrine causes of amenorrhea--an update. J Clin Endocrinol Metab. 2015;100(3):812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38(3):199–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL. Stress levels of glucocorticoids inhibit LHbeta-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26(10):1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair BB, Khant Aung Z, Porteous R, et al. Impact of chronic variable stress on neuroendocrine hypothalamus and pituitary in male and female C57BL/6J mice. J Neuroendocrinol. 2021;33(5):e12972. [DOI] [PubMed] [Google Scholar]
- 38.Wagenmaker ER, Moenter SM. Exposure to Acute Psychosocial Stress Disrupts the Luteinizing Hormone Surge Independent of Estrous Cycle Alterations in Female Mice. Endocrinology. 2017;158(8):2593–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Vanacker C, Burger LL, et al. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. The estrous cycle of the ewe is resistant to disruption by repeated, acute psychosocial stress. Biol Reprod. 2010;82(6):1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams CL, Nishihara M, Thalabard JC, Grosser PM, Hotchkiss J, Knobil E. Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology. 1990;52(2):133–137. [DOI] [PubMed] [Google Scholar]
- 43.Olster DH, Ferin M. Corticotropin-releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 1987;65(2):262–267. [DOI] [PubMed] [Google Scholar]
- 44.Gindoff PR, Ferin M. Endogenous opioid peptides modulate the effect of corticotropin-releasing factor on gonadotropin release in the primate. Endocrinology. 1987;121(3):837–842. [DOI] [PubMed] [Google Scholar]
- 45.Ciechanowska M, Łapot M, Malewski T, Mateusiak K, Misztal T, Przekop F. Effects of corticotropin-releasing hormone and its antagonist on the gene expression of gonadotrophin-releasing hormone (GnRH) and GnRH receptor in the hypothalamus and anterior pituitary gland of follicular phase ewes. Reprod Fertil Dev. 2011;23(6):780–787. [DOI] [PubMed] [Google Scholar]
- 46.Caraty A, Miller DW, Delaleu B, Martin GB. Stimulation of LH secretion in sheep by central administration of corticotrophin-releasing hormone. J Reprod Fertil. 1997;111(2):249–257. [DOI] [PubMed] [Google Scholar]
- 47.Naylor AM, Porter DW, Lincoln DW. Central administration of corticotrophin-releasing factor in the sheep: effects on secretion of gonadotrophins, prolactin and cortisol. J Endocrinol. 1990;124(1):117–125. [DOI] [PubMed] [Google Scholar]
- 48.Tilbrook AJ, Canny BJ, Stewart BJ, Serapiglia MD, Clarke IJ. Central administration of corticotrophin releasing hormone but not arginine vasopressin stimulates the secretion of luteinizing hormone in rams in the presence and absence of testosterone. J Endocrinol. 1999;162(2):301–311. [DOI] [PubMed] [Google Scholar]
- 49.Rivier C, Vale W. Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology. 1984;114(3):914–921. [DOI] [PubMed] [Google Scholar]
- 50.Petraglia F, Sutton S, Vale W, Plotsky P. Corticotropin-releasing factor decreases plasma luteinizing hormone levels in female rats by inhibiting gonadotropin-releasing hormone release into hypophysial-portal circulation. Endocrinology. 1987;120(3):1083–1088. [DOI] [PubMed] [Google Scholar]
- 51.Yip SH, Liu X, Hessler S, Cheong I, Porteous R, Herbison AE. Indirect Suppression of Pulsatile LH Secretion by CRH Neurons in the Female Mouse. Endocrinology. 2021;162(3). [DOI] [PubMed] [Google Scholar]
- 52.Shah NS, Pugh PC, Nam H, et al. A subset of presympathetic-premotor neurons within the centrally projecting Edinger-Westphal nucleus expresses urocortin-1. J Chem Neuroanat. 2013;52:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morin SM, Ling N, Liu XJ, Kahl SD, Gehlert DR. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience. 1999;92(1):281–291. [DOI] [PubMed] [Google Scholar]
- 54.Reyes TM, Lewis K, Perrin MH, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98(5):2843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22(3):991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li XF, Bowe JE, Lightman SL, O’Byrne KT. Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile luteinizing hormone secretion in the rat. Endocrinology. 2005;146(1):318–322. [DOI] [PubMed] [Google Scholar]
- 57.Chen MD, Ordog T, O’Byrne KT, Goldsmith JR, Connaughton MA, Knobil E. The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology. 1996;137(5):2012–2021. [DOI] [PubMed] [Google Scholar]
- 58.Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Effect of corticotropin-releasing hormone antagonist on oestrogen-dependent glucoprivic suppression of luteinizing hormone secretion in female rats. J Neuroendocrinol. 1999;11(2):101–105. [DOI] [PubMed] [Google Scholar]
- 59.Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol. 2006;18(8):602–610. [DOI] [PubMed] [Google Scholar]
- 60.Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011;300(1):E19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jasoni CL, Todman MG, Han SK, Herbison AE. Expression of mRNAs encoding receptors that mediate stress signals in gonadotropin-releasing hormone neurons of the mouse. Neuroendocrinology. 2005;82(5-6):320–328. [DOI] [PubMed] [Google Scholar]
- 62.Dudás B, Merchenthaler I. Close juxtapositions between luteinizing hormone-releasing hormone-immunoreactive neurons and corticotropin-releasing factor-immunoreactive axons in the human diencephalon. J Clin Endocrinol Metab. 2002;87(12):5778–5784. [DOI] [PubMed] [Google Scholar]
- 63.MacLusky NJ, Naftolin F, Leranth C. Immunocytochemical evidence for direct synaptic connections between corticotrophin-releasing factor (CRF) and gonadotrophin-releasing hormone (GnRH)-containing neurons in the preoptic area of the rat. Brain Res. 1988;439(1–2):391–395. [DOI] [PubMed] [Google Scholar]
- 64.Tellam DJ, Perone MJ, Dunn IC, et al. Direct regulation of GnRH transcription by CRF-like peptides in an immortalized neuronal cell line. Neuroreport. 1998;9(14):3135–3140. [DOI] [PubMed] [Google Scholar]
- 65.Hahn JD, Coen CW. Comparative study of the sources of neuronal projections to the site of gonadotrophin-releasing hormone perikarya and to the anteroventral periventricular nucleus in female rats. J Comp Neurol. 2006;494(1):190–214. [DOI] [PubMed] [Google Scholar]
- 66.Rivalland ET, Tilbrook AJ, Turner AI, Iqbal J, Pompolo S, Clarke IJ. Projections to the preoptic area from the paraventricular nucleus, arcuate nucleus and the bed nucleus of the stria terminalis are unlikely to be involved in stress-induced suppression of GnRH secretion in sheep. Neuroendocrinology. 2006;84(1):1–13. [DOI] [PubMed] [Google Scholar]
- 67.Phumsatitpong C, De Guzman RM, Zuloaga DG, Moenter SM. A CRH Receptor Type 1 Agonist Increases GABA Transmission to GnRH Neurons in a Circulating-Estradiol-Dependent Manner. Endocrinology. 2020;161(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raftogianni A, Roth LC, García-González D, et al. Deciphering the Contributions of CRH Receptors in the Brain and Pituitary to Stress-Induced Inhibition of the Reproductive Axis. Front Mol Neurosci. 2018;11:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phumsatitpong C, Moenter SM. Estradiol-Dependent Stimulation and Suppression of Gonadotropin-Releasing Hormone Neuron Firing Activity by Corticotropin-Releasing Hormone in Female Mice. Endocrinology. 2018;159(1):414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Front Neurosci. 2014;8:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Guo W, Shen X, et al. Different dendritic domains of the GnRH neuron underlie the pulse and surge modes of GnRH secretion in female mice. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy Cells Revisited. Endocrinology. 2018;159(9):3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herbison AE. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology. 2018;159(11):3723–3736. [DOI] [PubMed] [Google Scholar]
- 74.Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett. 2012;531(1):40–45. [DOI] [PubMed] [Google Scholar]
- 75.Kinsey-Jones JS, Li XF, Knox AM, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21(1):20–29. [DOI] [PubMed] [Google Scholar]
- 76.Harno E, Cottrell EC, White A. Metabolic pitfalls of CNS Cre-based technology. Cell Metab. 2013;18(1):21–28. [DOI] [PubMed] [Google Scholar]
- 77.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89(2):535–606. [DOI] [PubMed] [Google Scholar]
- 78.Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121(3):924–930. [DOI] [PubMed] [Google Scholar]
- 79.Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156(4):1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poe GR, Foote S, Eschenko O, et al. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci. 2020;21(11):644–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rassnick S, Sved AF, Rabin BS. Locus coeruleus stimulation by corticotropin-releasing hormone suppresses in vitro cellular immune responses. J Neurosci. 1994;14(10):6033–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Butler PD, Weiss JM, Stout JC, Nemeroff CB. Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci. 1990;10(1):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell JC, Li XF, Breen L, Thalabard JC, O’Byrne KT. The role of the locus coeruleus in corticotropin-releasing hormone and stress-induced suppression of pulsatile luteinizing hormone secretion in the female rat. Endocrinology. 2005;146(1):323–331. [DOI] [PubMed] [Google Scholar]
- 84.Vastagh C, Rodolosse A, Solymosi N, Liposits Z. Altered Expression of Genes Encoding Neurotransmitter Receptors in GnRH Neurons of Proestrous Mice. Front Cell Neurosci. 2016;10:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132(3):703–712. [DOI] [PubMed] [Google Scholar]
- 86.Turi GF, Liposits Z, Moenter SM, Fekete C, Hrabovszky E. Origin of neuropeptide Y-containing afferents to gonadotropin-releasing hormone neurons in male mice. Endocrinology. 2003;144(11):4967–4974. [DOI] [PubMed] [Google Scholar]
- 87.Miller MM, Zhu L. Ovariectomy and age alter gonadotropin hormone releasing hormone-noradrenergic interactions. Neurobiol Aging. 1995;16(4):613–625. [DOI] [PubMed] [Google Scholar]
- 88.Campbell RE, Herbison AE. Definition of brainstem afferents to gonadotropin-releasing hormone neurons in the mouse using conditional viral tract tracing. Endocrinology. 2007;148(12):5884–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saleeba C, Dempsey B, Le S, Goodchild A, McMullan S. A Student’s Guide to Neural Circuit Tracing. Front Neurosci. 2019;13:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han SK, Herbison AE. Norepinephrine suppresses gonadotropin-releasing hormone neuron excitability in the adult mouse. Endocrinology. 2008;149(3):1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergen H, Leung PC. Norepinephrine inhibition of pulsatile LH release: receptor specificity. Am J Physiol. 1986;250(2 Pt 1):E205–211. [DOI] [PubMed] [Google Scholar]
- 92.Szawka RE, Poletini MO, Leite CM, et al. Release of norepinephrine in the preoptic area activates anteroventral periventricular nucleus neurons and stimulates the surge of luteinizing hormone. Endocrinology. 2013;154(1):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scott CJ, Cumminst JT, Clarke IJ. Effects on plasma luteinizing hormone levels of microinjection of noradrenaline and adrenaline into the septo-preoptic area of the brain of the ovariectomized ewe: changes with season and chronic oestrogen treatment. J Neuroendocrinol. 1992;4(1):131–141. [DOI] [PubMed] [Google Scholar]
- 94.Tsukamura H, Nagatani S, Cagampang FR, Kawakami S, Maeda K. Corticotropin-releasing hormone mediates suppression of pulsatile luteinizing hormone secretion induced by activation of alpha-adrenergic receptors in the paraventricular nucleus in female rats. Endocrinology. 1994;134(3):1460–1466. [DOI] [PubMed] [Google Scholar]
- 95.I’Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology. 2003;144(10):4325–4331. [DOI] [PubMed] [Google Scholar]
- 96.Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci. 2014;39(11):1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kovács A, Biró E, Szeleczky I, Telegdy G. Role of endogenous CRF in the mediation of neuroendocrine and behavioral responses to calcitonin gene-related peptide in rats. Neuroendocrinology. 1995;62(4):418–424. [DOI] [PubMed] [Google Scholar]
- 98.Poore LH, Helmstetter FJ. The effects of central injections of calcitonin gene-related peptide on fear-related behavior. Neurobiol Learn Mem. 1996;66(2):241–245. [DOI] [PubMed] [Google Scholar]
- 99.Li XF, Bowe JE, Mitchell JC, Brain SD, Lightman SL, O’Byrne KT. Stress-induced suppression of the gonadotropin-releasing hormone pulse generator in the female rat: a novel neural action for calcitonin gene-related peptide. Endocrinology. 2004;145(4):1556–1563. [DOI] [PubMed] [Google Scholar]
- 100.Li XF, Kinsey-Jones JS, Bowe JE, et al. A role for the medial preoptic area in CGRP-induced suppression of pulsatile LH secretion in the female rat. Stress. 2009;12(3):259–267. [DOI] [PubMed] [Google Scholar]
- 101.Kinsey-Jones JS, Li XF, Bowe JE, Brain SD, Lightman SL, O’Byrne KT. Effect of calcitonin gene-related peptide on gonadotrophin-releasing hormone mRNA expression in GT1-7 cells. J Neuroendocrinol. 2005;17(9):541–544. [DOI] [PubMed] [Google Scholar]
- 102.Bowe JE, Li XF, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT. The role of corticotrophin-releasing hormone receptors in the calcitonin gene-related peptide-induced suppression of pulsatile luteinising hormone secretion in the female rat. Stress. 2008;11(4):312–319. [DOI] [PubMed] [Google Scholar]
- 103.Estienne MJ, Barb CR, Kesner JS, Kraeling RR, Rampacek GB. Luteinizing hormone secretion in hypophysial stalk-transected gilts given hydrocortisone acetate and pulsatile gonadotropin-releasing hormone. Domest Anim Endocrinol. 1991;8(3):407–414. [DOI] [PubMed] [Google Scholar]
- 104.Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod. 1985;33(2):423–431. [DOI] [PubMed] [Google Scholar]
- 105.Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150(1):341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oakley AE, Breen KM, Tilbrook AJ, Wagenmaker ER, Karsch FJ. Role of estradiol in cortisol-induced reduction of luteinizing hormone pulse frequency. Endocrinology. 2009;150(6):2775–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kreisman M, McCosh R, Tian K, Song C, Breen K. Estradiol enables chronic corticosterone to inhibit pulsatile LH secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology. 2019;110(6):501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Debus N, Breen KM, Barrell GK, et al. Does cortisol mediate endotoxin-induced inhibition of pulsatile luteinizing hormone and gonadotropin-releasing hormone secretion? Endocrinology. 2002;143(10):3748–3758. [DOI] [PubMed] [Google Scholar]
- 109.Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145(2):692–698. [DOI] [PubMed] [Google Scholar]
- 110.Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ. Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology. 2005;146(4):2107–2115. [DOI] [PubMed] [Google Scholar]
- 111.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone Blocks Ovarian Cyclicity and the LH Surge via Decreased Kisspeptin Neuron Activation in Female Mice. Endocrinology. 2016;157(3):1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wagenmaker ER, Breen KM, Oakley AE, et al. Cortisol interferes with the estradiol-induced surge of luteinizing hormone in the ewe. Biol Reprod. 2009;80(3):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oakley AE. Central Inhibitory Actions of Glucocorticoids on Reproductive Function: Permissive Role of Estradiol: Molecular and Integrative Physiology, University of Michagan; 2008. [Google Scholar]
- 114.Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal Androgen Exposure Alters KNDy Neurons and Their Afferent Network in a Model of Polycystic Ovarian Syndrome. Endocrinology. 2021;162(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rizwan MZ, Poling MC, Corr M, et al. RFamide-related peptide-3 receptor gene expression in GnRH and kisspeptin neurons and GnRH-dependent mechanism of action. Endocrinology. 2012;153(8):3770–3779. [DOI] [PubMed] [Google Scholar]
- 116.Findeisen M, Rathmann D, Beck-Sickinger AG. Structure-activity studies of RFamide peptides reveal subtype-selective activation of neuropeptide FF1 and FF2 receptors. ChemMedChem. 2011;6(6):1081–1093. [DOI] [PubMed] [Google Scholar]
- 117.Angelopoulou E, Quignon C, Kriegsfeld LJ, Simonneaux V. Functional Implications of RFRP-3 in the Central Control of Daily and Seasonal Rhythms in Reproduction. Front Endocrinol (Lausanne). 2019;10:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.León S, García-Galiano D, Ruiz-Pino F, et al. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: studies in the NPFF1 receptor null mouse. Endocrinology. 2014;155(8):2953–2965. [DOI] [PubMed] [Google Scholar]
- 119.Geraghty AC, Muroy SE, Zhao S, Bentley GE, Kriegsfeld LJ, Kaufer D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. Elife. 2015;12(4):e04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mamgain A, Sawyer IL, Timajo DAM, et al. RFamide-Related Peptide Neurons Modulate Reproductive Function and Stress Responses. J Neurosci. 2021;41(3):474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poling MC, Kim J, Dhamija S, Kauffman AS. Development, sex steroid regulation, and phenotypic characterization of RFamide-related peptide (Rfrp) gene expression and RFamide receptors in the mouse hypothalamus. Endocrinology. 2012;153(4):1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ubuka T, Inoue K, Fukuda Y, et al. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153(1):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ubuka T, Lai H, Kitani M, et al. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol. 2009;517(6):841–855. [DOI] [PubMed] [Google Scholar]
- 124.Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150(6):2799–2804. [DOI] [PubMed] [Google Scholar]
- 125.Constantin S, Pizano K, Matson K, Shan Y, Reynolds D, Wray S. An Inhibitory Circuit From Brainstem to GnRH Neurons in Male Mice: A New Role for the RFRP Receptor. Endocrinology. 2021;162(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Res. 2010;1364:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH Pulse Generator Activity Across the Estrous Cycle of Female Mice. Endocrinology. 2019;160(6):1480–1491. [DOI] [PubMed] [Google Scholar]
- 128.Czieselsky K, Prescott M, Porteous R, et al. Pulse and Surge Profiles of Luteinizing Hormone Secretion in the Mouse. Endocrinology. 2016;157(12):4794–4802. [DOI] [PubMed] [Google Scholar]
- 129.Nagatani S, Tsukamura H, Maeda K. Estrogen feedback needed at the paraventricular nucleus or A2 to suppress pulsatile luteinizing hormone release in fasting female rats. Endocrinology. 1994;135(3):870–875. [DOI] [PubMed] [Google Scholar]
- 130.Everett JW, Sawyer CH, Markee JE. A neurogenic timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology. 1949;44(3):234–250. [DOI] [PubMed] [Google Scholar]
- 131.Drouva SV, Laplante E, Kordon C. alpha 1-adrenergic receptor involvement in the LH surge in ovariectomized estrogen-primed rats. Eur J Pharmacol. 1982;81(2):341–344. [DOI] [PubMed] [Google Scholar]
- 132.Clarke IJ, Scott CJ, Pereira A, Pompolo S. The role of noradrenaline in the generation of the preovulatory LH surge in the ewe. Domest Anim Endocrinol. 2006;30(4):260–275. [DOI] [PubMed] [Google Scholar]
- 133.Romanov RA, Alpár A, Hökfelt T, Harkany T. Molecular diversity of corticotropin-releasing hormone mRNA-containing neurons in the hypothalamus. J Endocrinol. 2017;232(3):161–172. [DOI] [PubMed] [Google Scholar]


