Abstract
Background:
Melanoma clinical trials demonstrated that completion lymph node dissection (CLND) is low value for most sentinel lymph node (SLN)-positive patients. Contemporaneous trials of adjuvant systemic immunotherapy and BRAF/MEK targeted therapy showed improved recurrence-free survival in high-risk SLN-positive patients. To better understand how oncologic evidence is incorporated into practice (implementation), we evaluated factors associated with discontinuation of CLND and adoption of systemic treatment at United States (US) Commission on Cancer-accredited centers.
Methods:
In a retrospective cohort study of adults with SLN-positive melanoma treated from 2012-2017 using the National Cancer Database (NCDB), we evaluated use of CLND and adjuvant systemic treatment using mixed effects logistic regression, reporting results as odds ratios (OR) with 95% Confidence Intervals (CI).
Results:
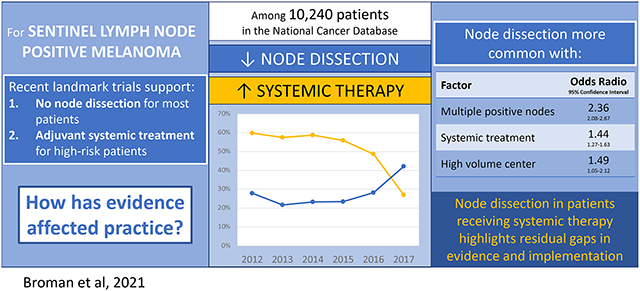
Among 10,240 SLN-positive melanoma patients, performance of CLND declined from 60% to 27%. Adjuvant systemic treatment increased from 29% to 43% (37% in Stage IIIA patients, 46% in IIIB-C). CLND was less common with lower extremity tumors (OR=0.53, 95%CI=0.44-0.64) and more common with multiple positive SLNs (OR=2.36, 95%CI=2.08-2.67), treatment at a high- or moderate-volume center (ORhigh=1.49, 95%CI=1.05-2.12; ORmoderate=1.32, 95%CI=1.05-1.64), and receipt of systemic therapy (OR=1.44, 95%CI=1.27-1.63). The increased likelihood of CLND in patients receiving adjuvant systemic treatment persisted in the most recent study years and in patients with a single positive SLN.
Conclusions:
At a population level, CLND declined, and adjuvant systemic treatment increased, reflecting evidence-responsive care. Variation in persistent use of CLND and in provision of adjuvant treatment for lower risk patients highlights residual gaps in both evidence and implementation.
Graphical Abstract
INTRODUCTION
For melanoma patients with regional lymph node metastases identified on sentinel lymph node biopsy (SLN-positive), nodal observation with ultrasound is a less morbid alternative to routine completion lymph node dissection (CLND), which was shown to have comparable disease-specific survival in two multi-center randomized controlled trials, the German Dermatologic Cooperative Group Trial (DeCOG-SLT) presented in 2015 and published in 2016 and the Second Multicenter Selective Lymphadenectomy Trial (MSLT-II) presented and published in 2017.1-3 At the same time, adjuvant systemic therapies including anti-CTLA-4 and anti-PD1 immunotherapy and BRAF/MEK inhibitors have demonstrated efficacy in patients with SLN-positive melanoma who have a minimum nodal tumor burden of one millimeter. Due to the parallel timing of nodal observation and adjuvant systemic therapy trials, the combined strategy of nodal observation with adjuvant systemic therapy has not been studied in a randomized fashion.
There is an often-cited several year gap in adoption of effective practices such as adjuvant systemic therapies and discontinuation of practices such as CLND.8-10 Further, where innovative management strategies apply to the same patient population but have not been studied together, as is the case with nodal observation and adjuvant systemic therapy, it is unknown how adoption of one treatment impacts discontinuation of another.4-7 A recent study of SLN-positive melanoma patients treated at international melanoma referral centers demonstrated a profound decline in CLND and increased provision of adjuvant systemic therapy, but management varied by treating center and the sample size was insufficient to determine the effect of adjuvant systemic therapy adoption on CLND discontinuation.11 Prior evaluations of practice patterns in US centers, where the organization of healthcare delivery is complex and heterogeneous with multiple payers and provider types, have revealed wide variation in whether and when effective treatments are adopted or low-value procedures are discontinued.12-14 At US healthcare facilities it is currently unknown how management of SLN-positive melanoma has evolved in response to recent evidence or what factors have been influential.
Given this evidence gap, our first objective was to evaluate temporal trends in performance of CLND for SLN-positive melanoma in the US. Second, we sought to understand the relationship between disease-specific and contextual factors and likelihood of CLND. We expected a steep decline in CLND based on observed trends in international melanoma referral centers, but anticipated greater variation based on care delivery factors which could reveal targets for future interventions to increase uptake of evidence-based practices. Our third objective was to evaluate how provision of adjuvant systemic therapy impacted the likelihood that SLN-positive patients received nodal observation versus CLND. We hypothesized that adjuvant systemic therapy would be considered a substitute for CLND, and hence given more often in patients not undergoing CLND.
METHODS
Study Overview
The study cohort was identified from the National Cancer Database (NCDB), a national oncology database developed through a collaboration between the American Cancer Society and the American College of Surgeons Commission on Cancer (CoC). It includes patients treated at over 1,500 accredited sites representing approximately 70% of US cancer patients. The study was deemed exempt by the University of Alabama at Birmingham Institutional Review Board given use of de-identified data and had no designated funding. Reporting is in accordance with EQUATOR guidelines using the STROBE checklist (Supplemental File).
Patient Population
We included adult male and female patients with invasive cutaneous melanoma diagnosed between 2012 and 2017 who had no clinical evidence of regional nodal or distant metastatic disease. Patients underwent primary tumor excision with SLN biopsy and had at least one tumor-involved SLN, identified in the dataset by pathologic N stage and the “Regional Lymph Nodes Positive” variables. Patients were excluded if surgery was performed for palliation, primary tumor excision was not performed, all treatment occurred outside the CoC-participating reporting facility, or the patient died within 90 days of surgery.
Outcomes
Nodal management with observation versus CLND was determined using the “Scope of Regional Lymph Node Surgery” variable which was available starting in 2012. Management was categorized as nodal observation (SLN biopsy only) or CLND performed concurrent with or after positive SLN biopsy. Adjuvant systemic therapy use was determined using the “Systemic/Surgery Sequence” variable and categorized as immunotherapy versus other. Patients were considered to have received adjuvant systemic therapy if treatment was provided after surgery.
Statistical Analysis
We examined temporal trends in the proportion of patients undergoing CLND and receiving systemic therapy. To evaluate variation related to patient and disease-specific factors and to account for clustering by treating facility, we developed a mixed effects logistic regression model with treating facility as the random effect. Year of treatment was included as a categorical variable to allow for nonlinear trends. Model covariates were selected a priori and included patient age, sex, Charlson comorbidity score, tumor location, tumor ulceration, Breslow depth, number of positive SLN, systemic treatment, insurance status, travel distance, location of patient residence (categorized as metropolitan, adjacent to metropolitan, or not adjacent to metropolitan based on USDA Economic Research Service 2013 Urban Rural Continuum Codes), type of treating facility (using CoC determination as academic, comprehensive community cancer center, community cancer center, integrated cancer network), US geographic region (by US Census Divisions), and facility volume of SLN-positive melanoma patients seen per year (averaged over all years of study and categorized in tertiles as low, moderate, or high). We used the final model to estimate the marginal probability of CLND by each factor for an average patient in the cohort. For the multilevel model we calculated an intraclass correlation coefficient to determine the proportion of variation explained by effects at the treating facility level.
Additional subgroup analyses were performed as follows: 1. Patient cohort limited to those diagnosed during the last two years of study which corresponded to timing of presentation and publication of nodal management clinical trials; 2. Including only patients with a single positive SLN; 3. Limited to patients who did not receive systemic therapy; and 4. Limited to patients who did receive systemic therapy; 5. Comparing the least aggressive (nodal observation without adjuvant systemic therapy) versus most aggressive (CLND with adjuvant systemic therapy) strategies.
RESULTS
There were 210,767 patients with clinically node-negative melanoma. Of these, 109,428 (52%) underwent regional nodal surgery. Excluding patients with unknown nodal management or systemic treatment before surgery yielded 85,824 patients, of which 10,240 (12%) had a positive SLN and constituted the analytic cohort. The median age of included patients was 60 years (IQR, 48-71) and patients were predominantly non-Hispanic white (Table 1). Most patients lived in a metropolitan (83%) or metro-adjacent area (12%), were treated at an academic medical center (48%) or comprehensive community cancer program (24%) and had private (53%) or Medicare (37%) insurance. Eighty-five percent had trunk or extremity tumors, median Breslow depth was 2.4 millimeters (IQR, 1.4-4.2), and 72% had a single positive SLN (Table 1).
Table 1.
Characteristics of sentinel lymph node positive melanoma patient cohort
| Characteristic | N=10,240 | |
|---|---|---|
| PATIENT FACTORS | ||
| Age, years | Median (IQR) | 60 (48-71) |
| Sex, N (%) | Female | 4,161(41) |
| Race, N (%) | White | 9,995 (97) |
| Black | 101 (1) | |
| Other or unknown | 184 (2) | |
| Ethnicity, N (%) | Hispanic | 253 (3) |
| Charlson Deyo score, N (%) | 0 | 8,372 (82) |
| 1 | 1,386 (14) | |
| ≥ 2 | 482 (4) | |
| Insurance status, N (%) | Private | 5,410 (53) |
| Medicare | 3,830 (37) | |
| Medicaid | 509 (5) | |
| Other governmental insurance | 128 (1) | |
| Uninsured | 248 (2) | |
| Unknown | 115 (1) | |
| Location of residence*, N (%) | Metropolitan | 8,211 (83) |
| Urban or rural, adjacent to a metropolitan area | 1,151 (12) | |
| Urban or rural, not adjacent to a metropolitan area | 532 (5) | |
| Travel distance, miles | Median (IQR) | 16.6 (7.5-39.3) |
| TUMOR AND NODAL FACTORS | ||
| Primary site, N (%) | Head and neck | 1,436 (14) |
| Trunk | 3,791(37) | |
| Upper extremity | 2,295 (22) | |
| Lower extremity | 2,667 (26) | |
| Overlapping, other, or unknown | 51 (1) | |
| Breslow depth | Median (IQR) | 2.4 (1.4-4.2) |
| Tumor ulceration, N (%) | Yes | 4,348 (43) |
| No | 5,744 (56) | |
| Unknown | 148 (2) | |
| Number of positive SLN, N (%) | One | 7,362 (72) |
| Two to three | 2,403 (24) | |
| Four or more | 475 (5) | |
| FACILITY FACTORS | ||
| Facility type, N (%) | Academic | 5,114 (48) |
| Community cancer program | 438 (4) | |
| Comprehensive community cancer program | 2,650 (24) | |
| Integrated network cancer program | 1,058 (10) | |
| Unknown | 1,385 (14) | |
| Facility volume, tertile, N (%) | Low (<3 patients/year) | 3,375 (33) |
| Moderate (3-9 patients/year) | 3,411 (33) | |
| High (>9-88 patients/year) | 3,4544 (34) | |
SLN=sentinel lymph node
Urban rural categories determined based on USDA Economic Research Service 2013 Urban Rural Continuum Codes definitions, with adjacency to a metropolitan area defined as a non-metropolitan county physically adjacent to one or more metropolitan areas with at least 2% of its employed labor force commuting to metropolitan counties
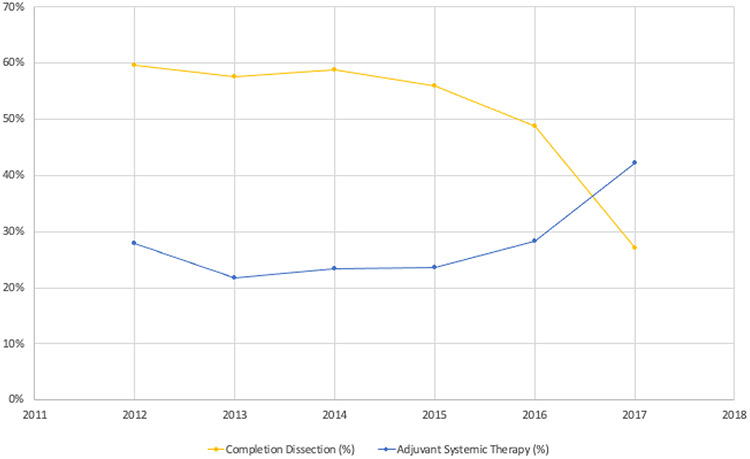
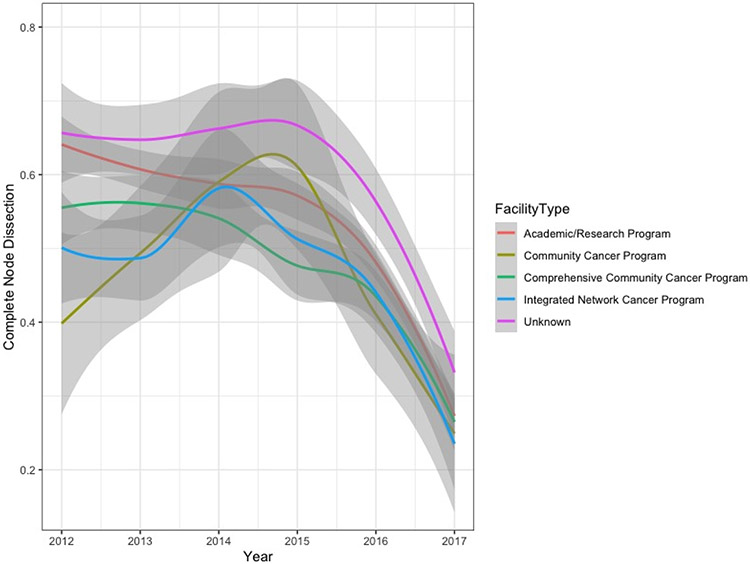
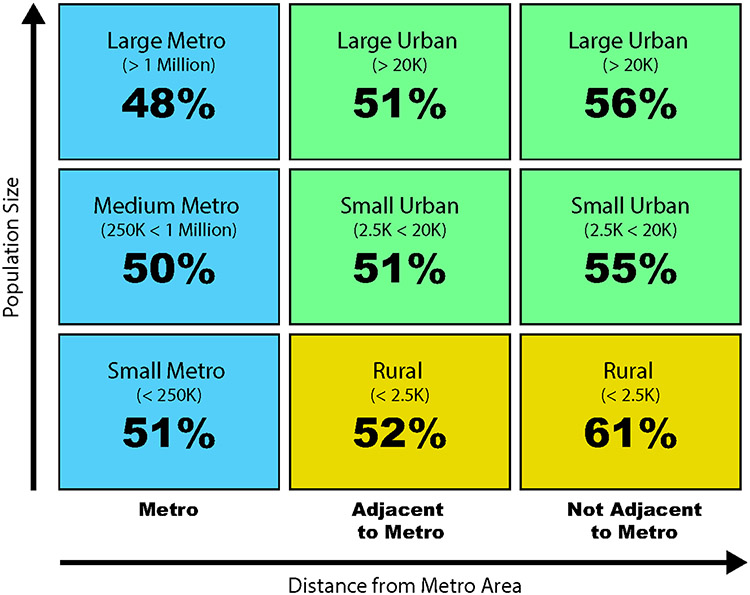
During the study period, 5,178 (51%) SLN-positive patients received nodal observation while 5,062 (49%) underwent CLND. Performance of CLND declined from 60% in 2012 to 27% in 2017 with no differences in temporal trends based on type of treating facility (Figures 1 and 2). Patients living in non-metropolitan areas were more likely to undergo CLND (p=0.002) (Figure 3, Supplemental Table 1). Twenty-nine percent of patients received adjuvant systemic therapy as part of their initial course of treatment, including 25% of stage IIIA patients and 33% of patients with stage IIIB-D disease (Supplemental Table S2). In the most recent year of study, 37% of Stage IIIA patients and 46% of Stage IIIB-D patients received adjuvant systemic therpay. Other factors associated with adjuvant systemic therapy included more sentinel nodes involved by metastatic disease, truncal primary site, younger age, fewer comorbid conditions, private or Medicare insurance, shorter travel distance, treatment at a low volume center, and treatment during later years of study (Figure 1, Supplemental Table S2). Immunotherapy was the most common treatment (85%); other systemic therapies were not reported. CLND was performed in 56% of patients who received systemic therapy versus 47% without systemic treatment (p<0.001).
Figure 1. Temporal trends in performance of completion lymph node dissection and provision of systemic therapy in sentinel lymph node positive melanoma.
Proportion of patients with SLN-positive melanoma who underwent completion lymph node dissection (blue solid line) and received systemic therapy (yellow dotted line) at American College of Surgeons Commission on Cancer participating-sites from 2012-2017
Figure 2. Temporal trends in completion lymph node dissection for sentinel lymph node positive melanoma by type of treating facility.
Academic Cancer Program: Accessions at least 500 new cancer cases per year; participates in medical education in at least four program areas including internal medicine and general surgery; Comprehensive Community Cancer Program: Accessions at least 500 new cancer cases per year; no medical education requirement; Community Cancer Program: Diagnoses >100 to <500 new cancer cases per year; no medical education requirement; Integrated Network Cancer Program: Organization owns, operates, leases, or is in a joint venture with multiple facilities providing comprehensive, integrated cancer care; at least one participating facility is CoC-accredited; participates in cancer-related clinical research; no medical education requirement; no minimum caseload requirement
Figure 3. Rural-urban differences in performance of completion lymph node dissection for sentinel lymph node positive melanoma.
Location of residence categories based on USDA Economic Research Service 2013 Urban Rural Continuum Codes, categorized by population and adjacency to a metropolitan area; K=1,000 people
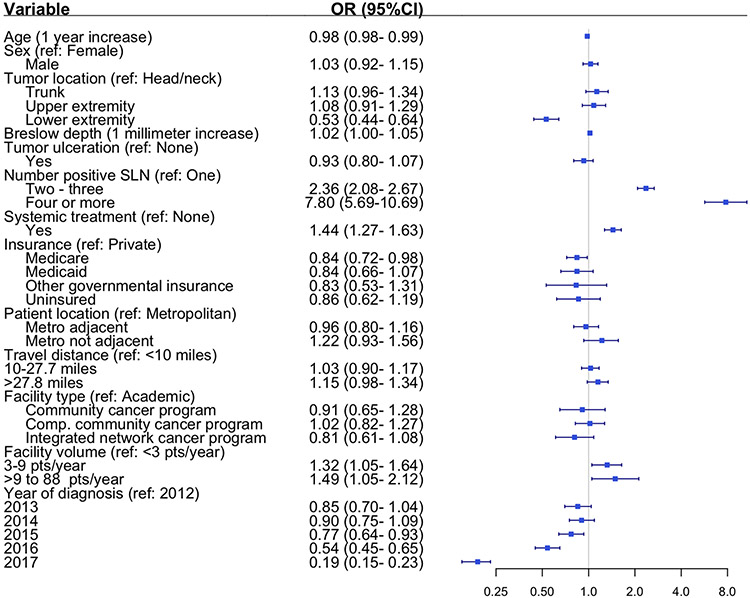
In the multivariable model likelihood of CLND which was adjusted for patient, disease-specific, and contextual factors, the temporal trend in CLND persisted, demonstrating a significant decline during the last three years of study (2015-2017). Diagnosis in the last study year was associated with an 81% lower likelihood of CLND than six years prior (OR 0.19, 95% CI 0.15-0.23) (Figure 4). For an average patient in this cohort, the probability of CLND decreased from 59% (95% CI 56-63%) in 2012 to 27% (95% CI 25-30%) in 2017 (Supplemental Table S3). Other factors associated with lower likelihood of CLND included older age (2% decrease with each one-year increase, OR 0.98, 95% CI 0.98-0.99), lower extremity tumor (relative to head and neck primary, OR 0.53, 95% CI 0.44-0.64), and Medicare insurance (relative to private insurance, OR 0.84, 95% CI 0.72-0.98). While overall trends in the proportion of patients receiving systemic treatment were inversely proportional to CLND, in the multivariable model patients who received systemic treatment were 44% more likely to undergo CLND (OR 1.44, 95% CI 1.27-1.63) (Figure 4). Patients were also more likely to undergo CLND if they had more than one positive SLN (OR 2.36, 95% CI 2.08-2.67 for 2-3 positive SLN; OR 7.80, 95% CI 5.69-10.69 for 4+ positive SLN) or if they received treatment at a moderate or high-volume center relative to a low volume center (ORmoderate 1.32, 95% CI 1.05-1.64, ORhigh 1.49, 95% CI 1.05-2.12). The multilevel model intraclass correlation coefficient was 0.16, indicating residual variation at the treating facility level not explained by included patient and disease-specific factors.
Figure 4. Factors associated with completion lymph node dissection for sentinel lymph node positive melanoma.
In addition to the variables shown, multivariable model also adjusted for Charlson comorbidity score and geographic region of treating facility (p=non-significant for both)
Limiting the analysis to the last two years of study confirmed observed associations of CLND with more than one positive SLN, primary tumor location other than lower extremity, and receipt of systemic treatment (Supplemental Table S4). Among patients with a single positive SLN, older age and lower extremity primary tumors were associated with a decreased likelihood of CLND while truncal primary, systemic treatment, and longer travel distance were associated with an increased likelihood of CLND (Supplemental Table S4). Separate analyses in patients with and without systemic treatment confirmed main model findings. CLND was associated with more than one positive SLN and treatment at a moderate- or high-volume center, and less likely with older age, lower extremity primary site, and treatment later in the study period (Supplemental Table S4). Additionally, systemic therapy recipients who lived in urban or rural areas that were not metro-adjacent were more likely to undergo CLND while those treated at community cancer centers were less likely to undergo CLND relative to academic centers. In patients who did not receive systemic therapy, longer travel distance was associated with CLND (Supplemental Table S4).
Sixteen percent of patients received the most aggressive treatment strategy, CLND with systemic treatment. Relative to patients receiving nodal observation alone without adjuvant systemic therapy, patients undergoing CLND and receiving systemic therapy more often had had thicker ulcerated tumors with more positive SLNs (Table 2). Factors associated with decreased likelihood of the most aggressive treatment strategy were older age, Medicare insurance, lack of insurance, lower extremity primary site, and treatment later in the study period.
Table 2.
Odds of the most aggressive treatment strategy, completion lymph node dissection with adjuvant treatment, relative to observation alone
| FACTOR | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Age | ||
| per 1 year increase | 0.96 | 0.95-0.97 |
| Patient insurance status | ||
| Private | Ref | |
| Medicare | 0.75 | 0.60-0.92 |
| Medicaid | 0.88 | 0.66-1.16 |
| Other government ins. | 1.09 | 0.64-1.87 |
| Uninsured | 0.47 | 0.30-0.73 |
| Unknown | 0.76 | 0.40-1.45 |
| Tumor location | ||
| Head and neck | Ref | |
| Trunk | 1.06 | 0.86-1.31 |
| Upper extremity | 0.99 | 0.78-1.24 |
| Lower extremity | 0.73 | 0. 58-0.93 |
| Other, unknown | 1.06 | 0.38-3.01 |
| Tumor ulceration | ||
| Present | 1.30 | 1.12-1.50 |
| Breslow depth | ||
| Per 1 millimeter increase | 1.05 | 1.02-1.08 |
| Number of positive SLN | ||
| One | Ref | |
| Two – three | 2.25 | 1.94-2.61 |
| Four or more | 4.52 | 3.49-5.86 |
| Year of diagnosis | ||
| 2012 | Ref | |
| 2013 | 0.67 | 0.53-0.86 |
| 2014 | 0.77 | 0.61-0.97 |
| 2015 | 0.60 | 0.48-0.77 |
| 2016 | 0.82 | 0.65-1.03 |
| 2017 | 0.58 | 0.46-0.73 |
Multivariable model adjusted for patient, disease-specific, and contextual factors; Ref=Reference, SLN=sentinel lymph node; p=not significant for patient sex, location of residence, patient travel distance, Charlson comorbidity score, facility type, facility volume, and geographic region (not shown)
DISCUSSION
Among SLN-positive melanoma patients treated at US CoC-participating centers, there has been a significant decline in performance of CLND and an analogous increase in adjuvant systemic therapy, which aligned temporally with publication of nodal management and adjuvant systemic therapy trial results. Despite significant discontinuation of CLND, approximately one-third of patients still underwent CLND in the latest available year of study, with differences in nodal management being attributable to patient, disease-specific, and contextual factors including higher treating facility volume and receipt of systemic therapy. At the same time, adjuvant systemic treatment increased in all SLN-positive patients including over one-third of patients with stage IIIA disease.
Our findings with respect to CLND are most informative for later years of study which were expected to be most sensitive to the impact of available trial results and for which the decline in CLND was most pronounced.15 The precipitous decline in CLND contrasts with the more gradual curves observed for discontinuation of other low-value clinical practices in oncology including PSA screening in men at average risk of prostate cancer, and nodal staging for women older than 70 years with low-risk breast cancer.8,16 There are several possible reasons why performance of CLND declined so quickly after trial results became available. This study demonstrated that performance of CLND was decreasing before the publication of MSLT-II and DeCOG. In the first three years of study, more than 40% of SLN-positive patients did not undergo CLND despite this still being recommended in national guidelines and designated as a quality measure by the CoC at this time.17 As most CLNDs yielded no additional metastatic disease in non-SLNs, yet the procedure carries a significant risk of lymphedema, the early decline in CLND may have been due to lack of perceived benefit coupled with concern regarding procedural morbidity. Indeed, CLND was performed more often in patients with more than one positive SLN, in whom there is greater risk of residual metastases in non-SLNs.18-21 Further, there was a lower likelihood of CLND in patients with lower extremity tumors who would most often require an inguinal lymph node dissection, a procedure associated with higher rates of long-term lymphedema than for axillary or cervical lymph node dissection.22 This aligns with a recent survey of US melanoma surgeons, who reported greater propensity to offer axillary lymph node dissection for upper extremity tumors than inguinal lymph node dissection for lower extremity tumors.23 Finally, prior non-randomized studies of patients with SLN positive disease who did not undergo CLND had comparable disease-specific survival.24,25 For some providers, this level of evidence may have been sufficient to support practice change.
We found that CLND was performed more often at moderate and high-volume centers than at low volume centers. Additionally, CLND rates were higher in all years of study at academic centers than at community cancer centers or integrated cancer networks. Adjuvant systemic therapy was more common at low volume centers, and among patients who received systemic therapy, treatment at a community cancer program was associated with a decreased likelihood of CLND relative to treatment at an academic center. Given the range of cancers treated by immunotherapy, there may be greater capacity for and experience with systemic therapy than specialized surgical procedures at community cancer centers. Alternatively, practitioners at lower volume and community-based centers may be more open to changing practice, less stringent regarding the level of evidence required to support a change in practice, and/or more comfortable offering lower intensity management strategies than practitioners at high volume and academic centers. Indeed, the association between treatment at a high-volume center and high intensity surgical care is observed in other disease sites. For example, rates of contralateral prophylactic mastectomy for unilateral breast cancer are higher at high-volume centers.13
Patient factors including location of residence and travel distance were also pertinent. In patients who did not receive systemic therapy, longer travel distance was significantly associated with CLND. In those patients who received systemic therapy, living remote from a metropolitan area was associated with increased likelihood of CLND. Clinical trials of nodal observation incorporated an intensive surveillance regimen that required frequent clinical assessment with nodal basin ultrasound, which is technician-dependent and may not be readily available at local centers. Patients who lived further from the treating facility and/or or remote from a major metropolitan area may have been more inclined to undergo CLND to avoid frequent travel for surveillance visits.
Alongside the decline in CLND we observed a contemporaneous increase in adjuvant systemic therapy. This was observed even in stage IIIA patients, for whom evidence supporting adjuvant systemic therapy is most limited.5-7 We hypothesized that the observed inverse relationship between CLND and systemic treatment was due to patients and providers substituting systemic therapy for CLND; however, we found that patients receiving systemic treatment were more likely to undergo CLND. Notably, there were several common disease-specific risk factors which were associated with both CLND and adjuvant treatment including higher stage and more positive sentinel lymph nodes. However, the association between adjuvant systemic therapy and CLND persisted in models adjusted for these risk factors. This may be due to other unmeasured high-risk features that are shared among patients who received adjuvant therapy and undergo CLND. Alternatively, this practice pattern may reflect providers’ strict interpretation of available evidence because all adjuvant systemic therapy trials required CLND prior to starting systemic treatment, highlighting persistent uncertainty regarding the long-term oncologic outcomes for patients managed with adjuvant systemic therapy without CLND.
Another explanation for the positive association between adjuvant systemic therapy and CLND in risk-adjusted models is a maximalist treatment strategy selected by patients, providers, or both in which the most aggressive combination of management options (CLND with systemic treatment) is preferred.24,25 We found that patients with high-risk tumor and nodal features were associated with CLND and adjuvant systemic therapy relative to observation alone. Though a recent retrospective analysis suggested that CLND has even less benefit for such high-risk patients because they are more likely to develop distant metastases than isolated nodal recurrences, this information was not available during the period of study.26 In the model adjusting for high-risk tumor and nodal features, receipt of the most aggressive treatment combination was also more common in patients with private insurance relative to Medicare or no insurance. Whether driven by patients or providers, this variation in treatment intensity based on non-clinical factors may overtreat certain patients, increasing morbidity without commensurate improvement in recurrence-free or melanoma-specific survival.
While we identified some factors associated with variation in CLND and systemic treatment including tumor location, nodal tumor burden, locations of residence and treatment, and insurance status, there was persistent unexplained variation which may be due to unmeasured factors. One unmeasured clinical factor was nodal tumor size, which is not available in the NCDB. The median nodal tumor size in MSLT-II was 1 mm. One millimeter was also the minimum nodal tumor size for inclusion in most adjuvant therapy trials so this variable may further explain observed variation in nodal management and adjuvant systemic therapy.
Further, other unmeasured non-clinical factors may have been influential. The characteristics of treating providers and the organizations in which they work have been strongly associated with whether low value treatments are discontinued and effective treatments adopted.27 The present analysis is limited in its exploration of detailed care delivery factors such as the structure and culture of the organizations where patients are treated, treating providers’ access to and incorporation of evidence into practice, and their engagement in inter-facility and/or multi-disciplinary care discussions. Understanding these factors, which may significantly influence how SLN-positive melanoma patients are managed, is critical to future efforts aimed at increasing evidence-based care. Further, while the National Cancer Database is an incredible resource for obtaining detailed information on large populations US cancer patients, it does not include patients treated at sites that are not CoC-accredited where quality of care may differ. Also, because of its retrospective and de-identified nature we are unable to reconcile inherent data inconsistencies or address missing data, which could result in loss of informative patient records and may introduce bias.
CONCLUSIONS
There has been a decline in performance of routine CLND and an increase in adjuvant systemic therapy for SLN-positive melanoma at United States Commission on Cancer-participating sites which was most pronounced during later years of study and corresponded to the timing of publication and presentation of landmark trials of nodal observation and adjuvant systemic therapy. Despite rapid integration of evidence into care of many SLN-positive patients, the observed variation in CLND based on nodal tumor burden, primary tumor location, and concurrent adjuvant systemic therapy reveal a need to refine our understanding of which management strategies are most appropriate for which patients. Beyond clinical indications, our finding of variation in performance of CLND and use of systemic therapy based on treating facility type and volume, patient location of residence and travel distance, and insurance status highlight opportunities to understand and optimize the care delivery factors that influence whether low value treatments are discontinued and effective treatments adopted.
Supplementary Material
Article Summary.
Among 10,240 sentinel lymph node-positive melanoma patients treated at United States Commission on Cancer sites from 2012 to 2017 there was a dramatic decline in completion lymph node dissection and concurrent increase in adjuvant systemic treatment at the population level, yet patients receiving adjuvant treatment were still more likely to have node dissection and there was high adoption of adjuvant treatment including in lower-risk stage IIIA patients. The importance of these findings is that there remain persistent gaps in knowledge and implementation related to nodal management and adjuvant treatment for sentinel node-positive melanoma.
ACKNOWLEDGEMENTS
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
FUNDING/FINANCIAL SUPPORT
There was no designated funding for this study
Abbreviations
- SLN
sentinel lymph node
- CLND
completion lymph node dissection
- NCDB
National Cancer Database
- US
United States
- OR
odds ratio
- CI
confidence interval
- DeCOG-SLT
German Dermatologic Cooperative Group Trial
- MSLT-II
Second Multicenter Selective Lymphadenectomy Trial
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- PD-1
programmed cell death protein 1
- CoC
Commission on Cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST/DISCLOSURES
The authors declare no potential conflicts of interest.
References
- 1.Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. doi: 10.1016/S1470-2045(16)00141-8 [DOI] [PubMed] [Google Scholar]
- 2.Leiter U, Stadler R, Mauch C, et al. Final Analysis of DeCOG-SLT Trial: No Survival Benefit for Complete Lymph Node Dissection in Patients With Melanoma With Positive Sentinel Node. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(32):3000–3008. doi: 10.1200/JCO.18.02306 [DOI] [PubMed] [Google Scholar]
- 3.Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1 [DOI] [PubMed] [Google Scholar]
- 5.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539 [DOI] [PubMed] [Google Scholar]
- 7.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 8.Ubel PA, Asch DA. Creating value in health by understanding and overcoming resistance to de-innovation. Health Aff Proj Hope. 2015;34(2):239–244. doi: 10.1377/hlthaff.2014.0983 [DOI] [PubMed] [Google Scholar]
- 9.van Dulmen SA, Naaktgeboren CA, Heus P, et al. Barriers and facilitators to reduce low-value care: a qualitative evidence synthesis. BMJ Open. 2020;10(10):e040025. doi: 10.1136/bmjopen-2020-040025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsen. Theories, models, and frameworks for de-implementation of low value care: A scoping review of the literature. Implement Res Pract. 2020;1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broman K, Hughes T, Dossett L, et al. Active Surveillance of Melanoma Patients with Sentinel Node Metastasis: An International Multi-Institution Evaluation of Post-MSLT-2 Adoption and Early Outcomes. Cancer. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard DH, Soulos PR, Chagpar AB, Mougalian S, Killelea B, Gross CP. Contrary To Conventional Wisdom, Physicians Abandoned A Breast Cancer Treatment After A Trial Concluded It Was Ineffective. Health Aff Proj Hope. 2016;35(7):1309–1315. doi: 10.1377/hlthaff.2015.1490 [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Bredbeck BC, Sinco B, et al. Variations in Persistent Use of Low-Value Breast Cancer Surgery. JAMA Surg. Published online February 3, 2021. doi: 10.1001/jamasurg.2020.6942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Balch CM, Wayne JD, et al. Health care system and socioeconomic factors associated with variance in use of sentinel lymph node biopsy for melanoma in the United States. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(11):1857–1863. doi: 10.1200/JCO.2008.18.7567 [DOI] [PubMed] [Google Scholar]
- 15.Herb JN, Dunham LN, Ollila DW, Stitzenberg KB, Meyers MO. Use of Completion Lymph Node Dissection for Sentinel Lymph Node-Positive Melanoma. J Am Coll Surg. 2020;230(4):515–524. doi: 10.1016/j.jamcollsurg.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Baskin AS, Dossett LA. Deimplementation of the Choosing Wisely Recommendations for Low-Value Breast Cancer Surgery: A Systematic Review. JAMA Surg. Published online June 3, 2020. doi: 10.1001/jamasurg.2020.0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minami CA, Wayne JD, Yang AD, et al. National Evaluation of Hospital Performance on the New Commission on Cancer Melanoma Quality Measures. Ann Surg Oncol. 2016;23(11):3548–3557. doi: 10.1245/s10434-016-5302-4 [DOI] [PubMed] [Google Scholar]
- 18.MacDonald S, Siever J, Baliski C. Performance of models predicting residual lymph node disease in melanoma patients following sentinel lymph node biopsy. Am J Surg. 2020;219(5):750–755. doi: 10.1016/j.amjsurg.2020.02.059 [DOI] [PubMed] [Google Scholar]
- 19.Bertolli E, Franke V, Calsavara VF, et al. Validation of a Nomogram for Non-sentinel Node Positivity in Melanoma Patients, and Its Clinical Implications: A Brazilian-Dutch Study. Ann Surg Oncol. 2019;26(2):395–405. doi: 10.1245/s10434-018-7038-9 [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Essner R, Torisu-Itakura H, Wanek L, Wang H, Morton DL. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(18):3677–3684. doi: 10.1200/JCO.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 21.Rossi CR, Mocellin S, Campana LG, et al. Prediction of Non-sentinel Node Status in Patients with Melanoma and Positive Sentinel Node Biopsy: An Italian Melanoma Intergroup (IMI) Study. Ann Surg Oncol. 2018;25(1):271–279. doi: 10.1245/s10434-017-6143-5 [DOI] [PubMed] [Google Scholar]
- 22.Moody JA, Botham SJ, Dahill KE, Wallace DL, Hardwicke JT. Complications following completion lymphadenectomy versus therapeutic lymphadenectomy for melanoma - A systematic review of the literature. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2017;43(9):1760–1767. doi: 10.1016/j.ejso.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 23.Hui JYC, Burke E, Broman KK, et al. Surgeon decision-making for management of positive sentinel lymph nodes in the post-Multicenter Selective Lymphadenectomy Trial II era: A survey study. J Surg Oncol. Published online December 1, 2020. doi: 10.1002/jso.26302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong SL, Morton DL, Thompson JF, et al. Melanoma patients with positive sentinel nodes who did not undergo completion lymphadenectomy: a multi-institutional study. Ann Surg Oncol. 2006;13(6):809–816. doi: 10.1245/ASO.2006.03.058 [DOI] [PubMed] [Google Scholar]
- 25.Bamboat ZM, Konstantinidis IT, Kuk D, Ariyan CE, Brady MS, Coit DG. Observation after a positive sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol. 2014;21(9):3117–3123. doi: 10.1245/s10434-014-3758-7 [DOI] [PubMed] [Google Scholar]
- 26.Mott N, Bredback B, Ellsworth B, Miller J, Broman K, Hughes T, Dossett L. Decisional Conflict in Patients with Different Maximizing-Minimizing Preferences following MSLT-II. Oral presentation at Academic Surgical Congress, February 2021 [Google Scholar]
- 27.Mott N, Wang T, Miller J, et al. Medical Maximizing-Minimizing Preferences in Relation to Low-Value Services for Older Women with Hormone Receptor-Positive Breast Cancer: A Qualitative Study. Ann Surg Oncol. 2021;28(2):941–949. doi: 10.1245/s10434-020-08924-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broman KK, Hughes TM, Dossett LA, et al. Surveillance of sentinel node-positive melanoma patients with reasons for exclusion from MLST-II: Multi-institutional propensity score matched analysis. J Am Coll Surg. 2021;232(4):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci IS. 2020;15(1):2. doi: 10.1186/s13012-019-0960-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.