Abstract
Background/Purpose:
To determine the feasibility of training with electromyographically (EMG)-controlled games to improve control of muscle activation patterns in stroke survivors.
Methods:
Twenty chronic stroke survivors (>6 months) with moderate hand impairment were randomized to train either unilaterally (paretic only) or bilaterally over 9 one-hour training sessions. EMG signals from the unilateral or bilateral limbs controlled a cursor location on a computer screen for gameplay. The EMG muscle activation vector was projected onto the plane defined by the first two principal components of the activation workspace for the non-paretic hand. These principal components formed the x- and y-axes of the computer screen.
Results:
The recruitment goal (n=20) was met over 9 months, with no screen failure, no attrition and 97.8% adherence rate. After training, both groups significantly decreased the time to move the cursor to a novel sequence of targets (p=0.006) by reducing normalized path length of the cursor movement (p=0.005), and improved the Wolf Motor Function Test (WMFT) quality score (p=0.01). No significant group difference was observed. No significant change was seen in the WMFT time or Box and Block Test.
Discussion/Conclusion:
Stroke survivors could successfully use the EMG-controlled games to train control of muscle activation patterns. While the non-paretic limb EMG was used in this study to create target EMG patterns, the system supports various means for creating target patterns per user desires. Future studies will employ training with the EMG-controlled games in conjunction with functional task practice for a longer intervention duration to improve overall hand function.
Keywords: stroke, motor impairment, neuro-rehabilitation, upper limb, EMG, serious game
1. Introduction
Almost 800,000 Americans experience a stroke each year.1 While the large majority of these individuals survive the stroke, most face sensorimotor deficits in the upper and lower extremities.2–4 These impairments have profound implications for performance of a variety of everyday tasks, self-care, and employment opportunities. Regaining motor control is a priority for stroke survivors, but one that is difficult to attain.3–5
Current treatment typically focuses on repetitive practice of specific movements or tasks,6 sometimes with assistance,7 strengthening,8 and/or tone reduction.9 Fundamentally, however, the impairments arising after stroke result from altered muscle activation patterns,10–12 which are currently only indirectly addressed in rehabilitation practice. Dexterous movement requires proper muscle activation patterns to be implemented across multiple muscles in a coordinated manner.13,14 For many stroke survivors, achieving proper muscle activation patterns necessary for functional tasks is a challenge.12 Deficits are often apparent in the abilities to fully activate a muscle,15 deactivate a muscle,16 modulate muscle activation with specific tasks,17 and coordinate muscle activation patterns across multiple muscles.18 The inability to fully activate muscles voluntarily, as well as to generate and control a variety of task-appropriate muscle activation patterns, severely limits function. In the hand, these deficits result in diminished finger extension19 and incorrectly directed fingertip forces that preclude stable grip of an object.20,21
Direct training of muscle activation patterns holds promise for improving motor control of the paretic limb. The activation patterns represent underlying control better than other variables such as joint movement or torque, as the latter arise from the net contributions of the different muscles without discernment of different conditions.19,22 For example, lack of movement or torque production at a joint could arise from an inability to activate the agonist muscles, but also could arise from excessive coactivation of agonists and antagonists. Training of activation patterns, such as through the use of electromyography (EMG)-triggered rehabilitation devices like neuromuscular electrical stimulators23 and arm and hand exoskeletons,24 has shown efficacy in stroke rehabilitation. One means of implementing this direct training of muscle activation patterns is through “serious” computer games,25,26 as they can improve participation, motivation, and enjoyment to promote the repetitive practice needed for rehabilitation.27,28
Serious games have been increasingly used to address needs in stroke rehabilitation,29,30 including with utilization of EMG control in an attempt to alleviate excessive coactivation between two proximal arm muscles.31,32 However, these previous studies did not address control of more than two muscles simultaneously, as is needed for most functional tasks. Therefore, we previously developed a therapy platform in which control of activation of up to 8 muscles may be required to play computer games. EMG signals directly control cursor movement, the user input for the games. Mapping of the EMG activation vector to the cursor location can be customized for each individual according to rehabilitation goals. A pilot study involving healthy adults participating in training sessions with this platform verified that individuals could learn to control activation patterns presented implicitly.33
The objective of the present study was to determine the feasibility of training with these EMG-controlled computer games to improve control of the muscle activation patterns in stroke survivors. We focused on muscles of the distal upper extremity, a region commonly impaired after stroke.4,34,35 As a proof-of-concept, we examined whether stroke survivors could use their paretic limb to create target patterns derived from their non-paretic limb. This was analogous to our pilot study in which neurologically intact individuals used their non-dominant limb to create target patterns derived from their dominant limb.33 Also in accordance with that study, we examined a unilateral vs. bilateral training paradigm, in which the participant controlled the game only with the paretic limb, or with the control signal derived from a variably weighted combination of the paretic and non-paretic limbs. Bilateral training of the arms has been shown to have an additive effect on unilateral training,36 possibly due to recruitment of uncrossed corticospinal pathways, bilateral subcortical pathways, or through an interhemispheric mechanism.37,38 As bilateral activities involving the hands are prevalent in daily living,39 bilateral training may also prove beneficial for distal upper extremity muscle control. Additionally, bilateral training involving a gradual shift in relative weighting of input from the non-paretic to the paretic limb could better facilitate maintaining an appropriate level of challenge throughout the training40 than unilateral training. We hypothesized that both groups would improve game performance through better control of muscle activation patterns, but that improvement would be greater for the group using the bilateral paradigm.
2. Methods
Participants
A convenience sample of 20 stroke survivors participated in this pilot study. All participants were recruited from an institutional research database for clinical neuroscience studies that included information about the participant’s stroke including the level of upper limb impairment. Inclusionary criteria required that each participant be an adult (minimum age of 18 years old) who incurred a single, unilateral stroke at least 6 months prior to enrollment in the study, as verified by the participant. Participants were required to have moderate hand impairment as indicated by a rating of Stage 4 or 5 on the Stage of Hand section of the Chedoke-McMaster Stroke Assessment (CMSA-H).41 Stage 4 implies good movement in flexion and some extension; Stage 5 includes movement in flexion, extension, and abduction with slowness. Participants were excluded if they had severe visual deficits that precluded being able to see the game screen or had received botulinum toxin in their upper limb in the past 6 months. This trial was registered on ClinicalTrials.gov (NCT03619772), following the EQUATOR network guidelines for a randomized clinical trial (CONSORT). All participants provided written informed consent as approved by the Northwestern University Institutional Review Board.
Study Design
This study was a single-blind interventional randomized controlled trial. Participants were randomly assigned to either the Unilateral or Bilateral training group (n=10 in each group) by drawling lots, stratified by CMSA-H level. Both groups came to the laboratory (a freestanding rehabilitation hospital) for 11 sessions: a pre-training evaluation, 9 training sessions with the EMG game (approximately 45 minutes each, 3 times per week for 3 weeks), and a post-training evaluation. The Unilateral group played the game with the paretic limb only. The Bilateral group played the game by controlling activation of muscles in both the paretic and non-paretic limbs, gradually shifting from primary control with the non-paretic limb to that of the paretic limb.
EMG Game
For both the Unilateral and Bilateral groups, the purpose of the training with the EMG-controlled games was to improve voluntary control of distal muscles in the paretic hand. For every session, surface active EMG electrodes (19.8 × 5.4 × 35 mm, Bagnoli 2-slot electrode, Delsys, Inc., Natick, MA) were placed on the forearms and hands to record activity from four muscles in each limb for both limbs for both groups. The four muscles were: an extrinsic finger extensor, extensor digitorum communis (EDC), a wrist muscle, extensor carpi ulnaris (ECU), an extrinsic finger flexor, flexor digitorum superficialis (FDS), and an intrinsic muscle, first dorsal interosseous (FDI). The skin was thoroughly cleaned with alcohol swabs before placement of the EMG electrodes. The 8 EMG signals, four from the paretic limb and four from the non-paretic limb, were digitized at 1 kHz (NI-DAQ USB-6443 BNC, National Instruments, Austin TX) and processed in Simulink (Mathworks, Natick MA). Each signal was bandpass filtered between 33 and 439 Hz, then rectified and lowpass filtered with a corner frequency of 1.6 Hz to produce an amplitude envelope of the EMG signal. At the beginning of each training and assessment session, the subject was instructed to produce maximum voluntary contraction (MVC) for each muscle through a variety of guided hand/wrist movements; visual feedback of peak muscle activation was provided to encourage optimum performance. The peak envelope value for each muscle was subsequently used to normalize the processed (rectified and filtered) EMG signal for the corresponding muscle in real time.
The EMG-controlled games translate the muscle activation patterns from multiple surface electrodes onto a two-dimensional game screen.33 The mapping of the EMG vector to cursor location is determined by the projection of the vector onto the plane formed by target muscle activation vectors defining the horizontal and vertical axes of the screen. For this study, the target vectors for both groups were obtained from the patterns employed in the non-paretic limb. At the start of each session, the participants performed a calibration exercise during which they moved the fingers and wrist of the non-paretic limb to create a variety of postures, as directed with a visual display.33 Principal component (PC) analysis was performed on these non-paretic EMG data to identify activation vectors describing the EMG space of the non-paretic hand. Two PCs (named PC1 and PC2) were selected as the target EMG vectors and were mapped to x- and y-axes, respectively, on the computer screen.
In addition to target vector direction, the target vector magnitude was also scaled in a custom manner for each subject in order to provide an appropriate level of challenge. Specifically, calibration data were also collected for the paretic hand for both groups. From these calibration data, an outline of the achievable activation region for the paretic hand was projected onto the plane formed by the target PCs. Study personnel selected the extent of the achievable region for the paretic hand to use for training by drawing a rectangle on a custom graphical user interface (GUI).33 The activation ranges, represented by the lengths of the sides of the rectangle, were mapped to the full ranges of the computer screen. During game play, the EMG vector was projected onto the PC1-PC2 plane through the dot product, and the resulting projection was shown as a cursor on the game screen.
Fundamental cursor control differed for the two groups. For the Unilateral group, the EMG vector from the paretic limb was projected directly onto the PC1-PC2 plane. For the Bilateral group, a linear combination of the EMG vectors for the paretic and non-paretic limbs was projected onto the PC1-PC2 plane for each session. A relative weighting of the two EMG vectors was employed, with weighting of the paretic limb increasing over the 9 sessions in a linear fashion. During the first session, 100% weight was placed on the vector for the non-paretic hand and 0% on the paretic hand. The scaling progressed from 87.5%:12.5% (non-paretic:paretic) for the second session to 75%:25% for the third session, eventually reaching 0% non-paretic and 100% paretic for the final session. In this manner, the challenge of controlling the cursor was systematically increased. The weighting percentage utilized was unknown to the participant.
Cursor movement allowed the participant to play a number of serious computer games, previously described in detail.33 These include a Picture Reveal game, for which movement of the cursor into one of the 5×5 grids causes the tile to disappear, thereby revealing part of a hidden picture (Fig 1A). In the Maze game, participants guide the cursor around the walls to travel from the Start to the Finish (Fig 1B). For an Asteroids-type game, participants move the cursor to collect coins while avoiding asteroids (Fig 1C). A Volume Exploration game encourages thorough exploration of the muscle activation space by displaying the extent to which the participant accesses the 4-dimensional EMG space (Fig 1D). In this game, the activations of the first two muscles control the position of the cursor in the left grid and the activations of the other two muscles control the position of the cursor in the right grid. The color of the tile in the left grid indicates the extent to which the activation region represented by that tile has been explored within the tiles in the right grid. In this way, the 4-dimensional activation space can be represented on the two-dimensional screen.
Figure 1.
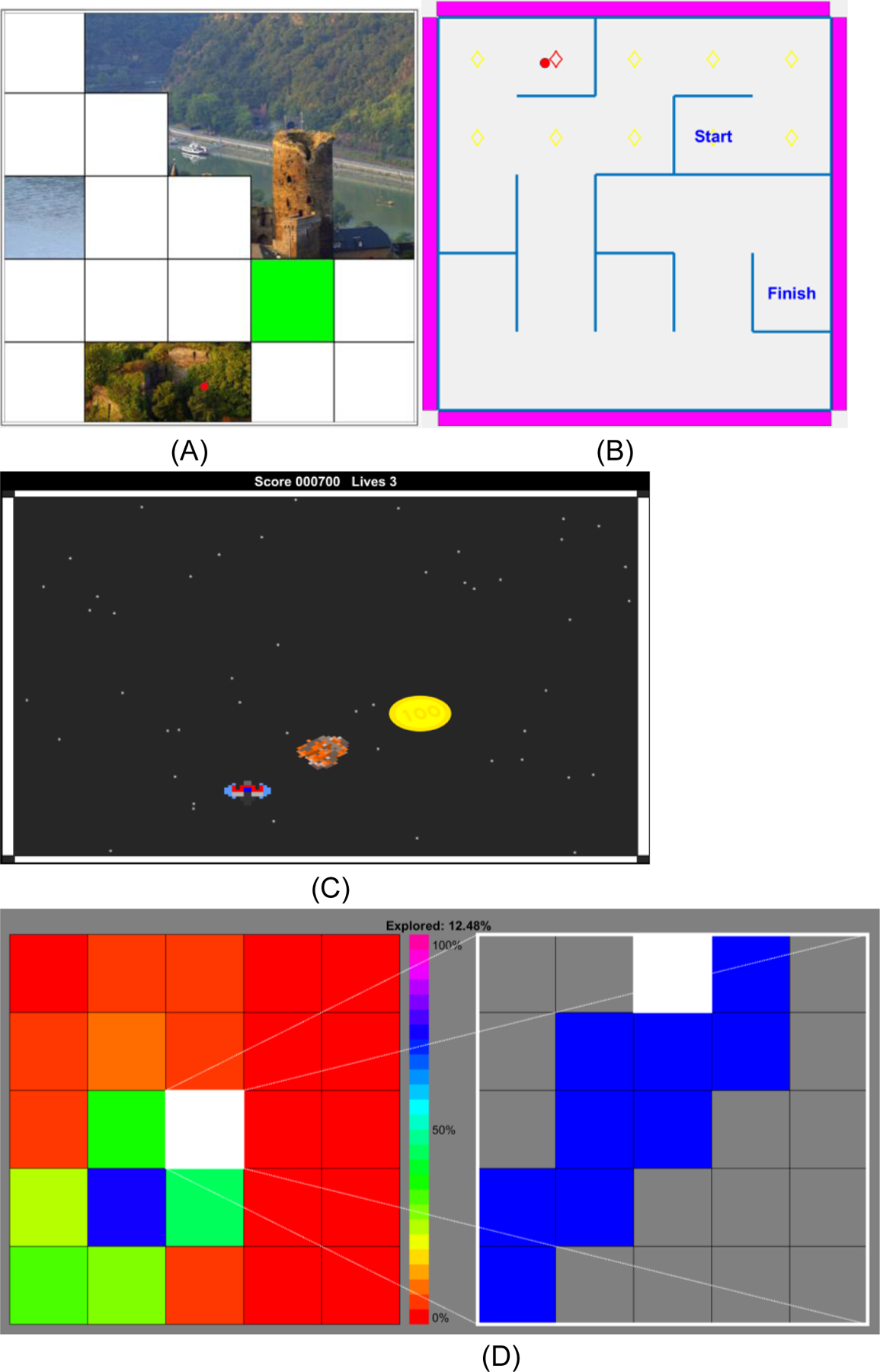
Examples of games: A) Picture Reveal. User controls the location of the cursor (red dot). Maintaining the red dot within the indicated target (green) tile causes the white tile to be removed, revealing part of the hidden picture B) Maze. User attempts to move the red dot from Start to Finish. The walls are barriers to movement. To make the game easier, the game mode can be set to prevent the user from inadvertently moving the cursor backwards and losing forward progress. C) Asteroids. User tries to gather the gold coins without being struck by the asteroids. As performance improves, more asteroids appear. D) Volume Exploration. Activation for two muscles controls the cursor location (white tile) on the left plane and activation for other two muscles controls the cursor location on the right plane. The color represents the extent to which a certain activation region has been explored.
Feasibility
We examined time to recruit the target sample size as well as number of screenings needed. Adherence (measured by attendance and participation in the protocol) as well as attrition were obtained. Adverse events were monitored. Any game equipment malfunctioning was also monitored.
Outcomes
We examined the effect of the EMG game on muscle activation pattern control, as determined by performance on a test that the participants did not practice during the training sessions. Specifically, the control of the muscle activation pattern was assessed by recording the time required to move the cursor into each of four predetermined target squares spanning the 5×5 grid (top center, bottom center, center right, and center left). For both groups, the paretic hand EMG signals were used to control the movement of the cursor while the target PCs were from the EMG vectors of the non-paretic hand. Four repetitions of each target were randomly presented, resulting in a total of 16 targets. This assessment was performed before the initiation of training and after the conclusion of the training sessions. The completion time for this assessment was the primary outcome measure of this study. Additionally, we examined the normalized path length traversed by the cursor during this test by dividing the actual curvilinear distance travelled by the minimal distance (the sum of the Euclidean distances) between consecutive targets.
While not anticipated due to the limited duration of the intervention and lack of integration of functional task practice in the study design, possible translation to upper extremity movement was assessed using standardized clinical assessments. Specifically, the Wolf Motor Function Test (WMFT)42 was administered to assess the duration (time) and quality (score) of prescribed upper limb movements, such as grasping and lifting a pencil. The Box and Blocks Test (BBT)43 assessed the number of blocks moved over the barrier in 60 s. The same rater, blinded to group assignments, administered these clinical assessments for all participants for both Pre and Post evaluations.
Analysis
Repeated measures analysis of variance (ANOVA) was performed to evaluate the effects of training on the primary outcome measures of completion time and normalized path length. The 2×2 ANOVA had the within-subject factor of Session (Pre/Post) and the between-subject factor of Group (Unilateral/Bilateral). Normality of the data distribution was examined using the Shapiro-Wilk’s test. A Bonferroni correction was implemented for the two tests, such that the overall significance level was set to α = 0.025. For the secondary outcomes from the clinical assessments, confidence intervals were created for the change in score from the Pre to Post evaluations, in addition to ANOVA.
3. Results
3.1. Feasibility and Participants
We identified 39 potential participants from the institutional research database. During an initial phone call, 19 declined participation. Twenty potential participants came to a screening visit and all 20 of them met the inclusionary and exclusionary criteria (i.e., no screen fails). The 20 participants were recruited over 9 months with an average accrual rate of 2.2 every month. A consort flow diagram is shown in Fig 2.
Figure 2.
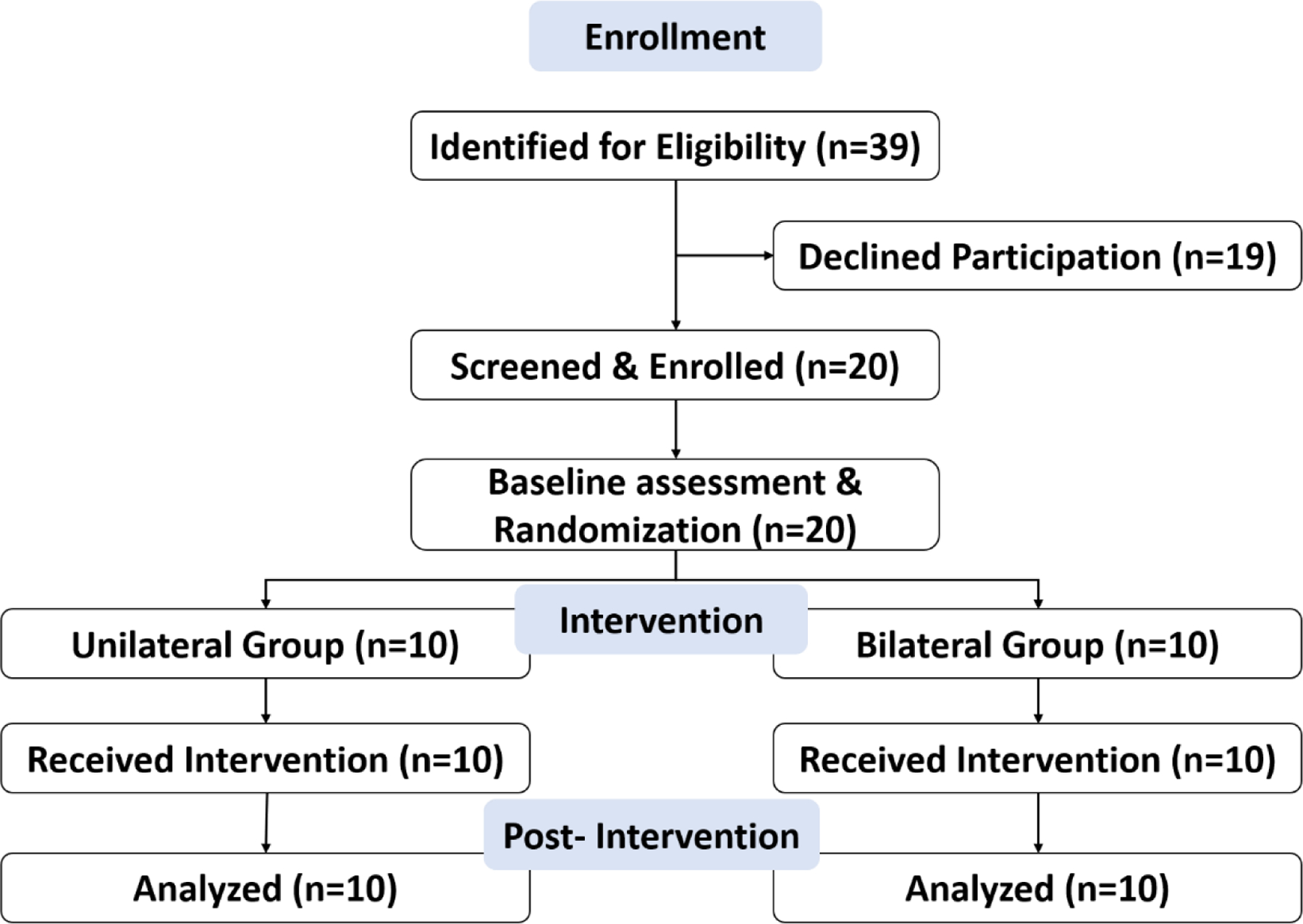
Consort flow diagram.
Participant characteristics are shown in Table 1. There were no significant differences between the groups for the demographic data. Adherence was 97.8%, as only 4 training sessions were missed out of possible 180 across all participants. The attrition rate was 0%. Across all sessions, there were no reported adverse events or equipment problems.
Table 1.
Demographic information
| Unilateral Group (N=10) |
Bilateral Group (N=10) |
p-value | |
|---|---|---|---|
| Age (Years) | 60.4±7.7 | 59.4±8.5 | F(1,18)=0.287, p=0.795 |
| Time Since Stroke (Years) | 9.5±5.0 | 10.5±9.7 | F(1,18)=3.599, p=0.768 |
| Gender | 8M/2F | 4M/6F | χ2=3.333, p=0.068 |
| Chedoke Hand Score (# of participants in score 4 vs. 5) | 7 / 3 | 8 / 2 | χ2=0.267, p=0.606 |
| Affected Hand | 6R/4L | 5R/5L | χ2=0.241, p=0.623 |
| Dominant Hand Before Stroke | 9R/1L | 9R/1L | χ2=0.0, p=1.0 |
3.2. Outcomes
The primary outcome measure, related to control of the cursor to perform the test, improved significantly for both groups. Test completion time to reach the 16 targets on the 5×5 grids decreased significantly after the training across both groups (Fig 3A, p=0.006 for Session). The time dropped by 59% for the Unilateral group and 26% for the Bilateral group (Table 2). We observed no significant differences in outcomes between the two groups (p>0.22 for Group and Group x Session). The normalized path length of the cursor (actual relative to minimum path length) was also significantly reduced from pre-training to post-training (Fig 3B, p=0.005 for Session). One participant in the Bilateral group had a path length that was a significant outlier (37 times greater than the group mean); his/her data were not included in path length analysis. Normalized path length decreased by more than 54% for the Bilateral group and 37% for the Unilateral group. Examination of data for individual subjects confirmed that roughly 75% of the participants exhibited a decrease in normalized path length (Fig 3C). No significant differences in normalized path length were observed between groups (p>0.14 for Group and Group x Session). No group difference at baseline was found for either test completion time or normalized path length (Table 2).
Figure 3.
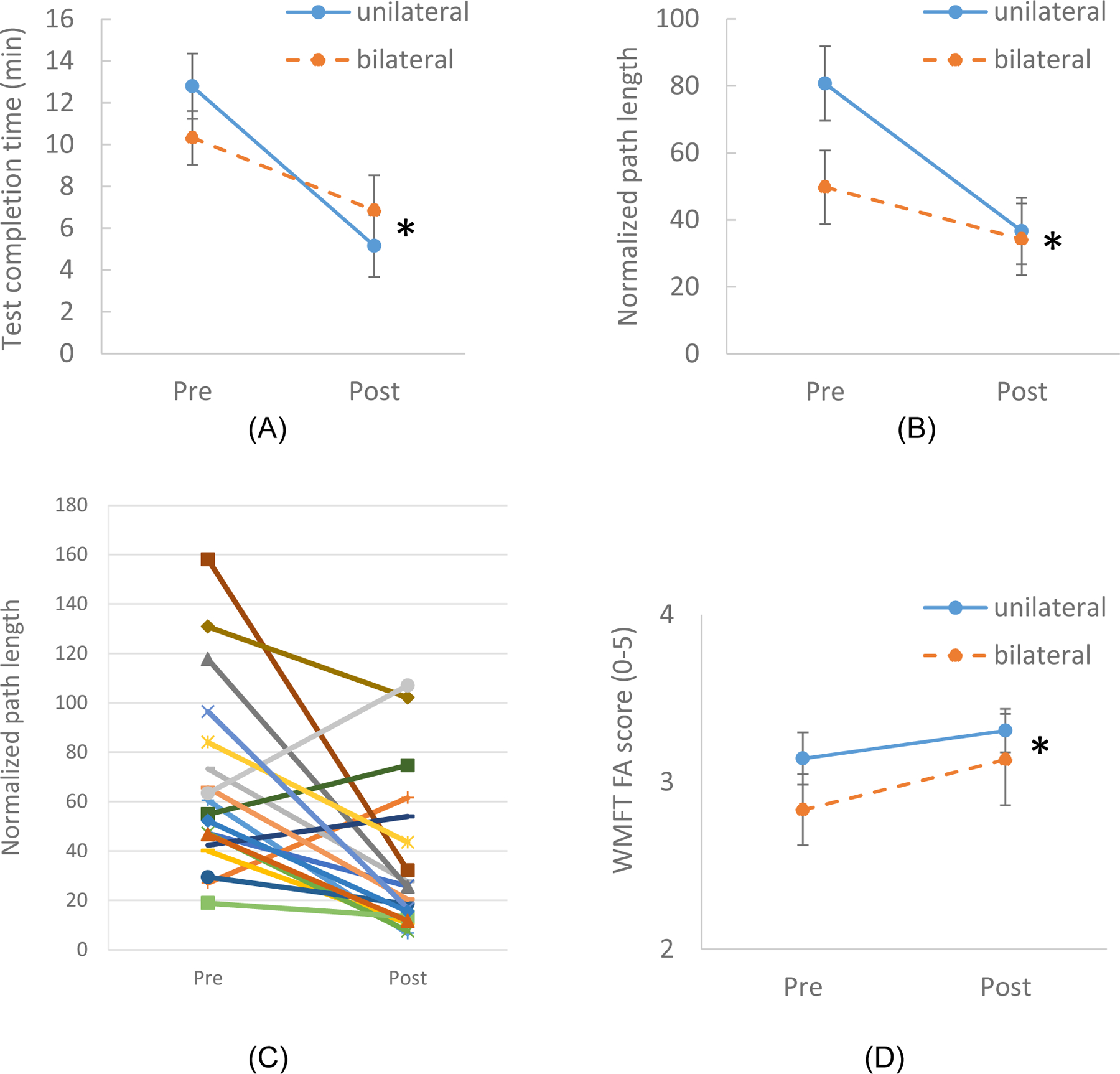
The effect of the EMG game on the control of the muscle activation patterns as assessed by Test Completion Time (A) and normalized path length (B). Normalized path length during the test for individual subjects (C). The effect of the EMG game on the secondary outcome measure, WMFT score (D). Error bars indicate standard errors. The star signs indicate statistically significant difference between Pre and Post (for both groups).
Table 2.
Outcome measures at Pre-training and Post-training. Mean ± standard error (95% CI) are shown. P-values are for the change from pre to post (for both groups). No significant group difference was found for any of the outcomes.
| Outcome | Unilateral (n=10) | Bilateral (n=10) | F-value | p-value | ||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| Test Completion Time (min) | 12.6 ± 1.3 (9.6, 15.6) |
5.1 ± 1.3 (2.2, 8) |
10.6 ± 1.0 (8.4, 12.8) |
7.8 ± 1.9 (3.6, 12) |
F(1,17)=9.703 | p=0.006 |
| Normalized Path Length | 80.7 ± 9.6 (58.9, 102.5) |
36.7 ± 8.6 (17.3, 56.1) |
55.0 ± 9.6 (33.2, 76.8) |
34.2 ± 9.2 (13.3, 55.1) |
F(1,17)=10.273 | p=0.005 |
| WMFT (time) | 17.8 ± 0.5 (16.6, 19.0) |
15.5 ± 2.1 (10.7, 20.3) |
24.6 ± 1.0 (22.3, 26.9) |
26.0 ± 1.0 (23.7, 28.3) |
F(1,17)=0.076 | p=0.786 |
| WMFT (score) | 3.1 ± 0.1 (2.8, 3.4) |
3.3 ± 0.1 (3.1, 3.5) |
2.8 ± 0.2 (2.4, 3.2) |
3.1 ± 0.3 (2.5, 3.7) |
F(1,17)=8.429 | p=0.010 |
| BBT (blocks) | 17.6 ± 1.3 (14.6, 20.6) |
17.4 ± 1.7 (13.5, 21.3) |
19.0 ± 3.3 (11.5, 26.5) |
19.6 ± 3.3 (12.1, 27.1) |
F(1,17)=0.007 | p=0.935 |
WMFT: Wolf Motor Function Test, BBT: Box and Block Test
For the secondary outcome measures, the WMFT Functional Abilities score did increase across both groups: the average score of both groups increased from 3.0 ± 0.3 at Pre to 3.2 ± 0.3 at Post (p = 0.01 for Session, Fig 3D). The mean increase was greater than the minimum detectable difference of 0.144 but less than the minimum clinically important difference of 1.0–1.2.45 We did not observe any statistically significant effect of Session or Group on the time to complete the WMFT or BBT (p > 0.26 for Session, Group, and Group x Session for both WMFT time and BBT), although there was a group difference at baseline for WMFT time.
4. Discussion
Stroke survivors with moderate hand impairment were able to use the system of EMG-controlled serious games. Twenty participants were successfully recruited, with a 97.8% adherence rate and no attrition. Lack of screen fails is attributable to the research registry that included upper limb impairment information for each participant, thereby allowing for efficient recruitment. Although we did not use satisfaction measures, individuals anecdotally reported that they liked the study, potentially due to the ability within the game to use their muscles to achieve a desired end even when they did not have the capability to use their hand during activities of daily living outside the game.40 Additionally, the system is customizable to the abilities of the user on the given day. Game challenge can be matched to the user on the GUI through selection of the scaling of the EMG space to be mapped to the screen and adjustment of game parameters, such as number of game tiles or the inclusion of walls to prevent loss of forward progress in the Maze game. It is possible that the high adherence rate and high retention may be related to participants’ enjoyment of the challenge inherent in the games. This may have positively affected motivation.27,28
After the training, the stroke survivors in both groups improved the ability to control the cursor on the screen through the creation of muscle activation patterns in the paretic limb. Across all participants, Test Completion Time (16 targets) decreased by 5.3 minutes, a 46% reduction. This was achieved primarily by reducing undesired cursor movement. The normalized path length across all of the targets was reduced by 45%. These findings indicate that participants were able to move the cursor and achieve the target muscle activation pattern in a much more efficient manner, as we observed previously with neurologically intact subjects.33 An improved control of activation patterns might explain these results.
While not anticipated, participants had a significant improvement in the WMFT Functional Abilities score after training. For both groups combined, the WMFT Functional Abilities score increased by 0.23 out of 5. This increase is above the minimum detectable change of 0.1,44 indicating that this increase is above the level of the measurement error. The WMFT Functional Abilities score assesses proper postural control, the presence of abnormal synergies, the involvement of other body parts to assist task performance, movement precision, fine coordination, and movement fluidity during prescribed tasks.46 Thus, the training with the EMG game may have a potential to impact quality of movement. However, time to complete the WMFT and number of blocks transported in the BBT did not change significantly. Overall, the result is consistent with the previous two-muscle EMG-based training in which impairment was reduced after training.31
It should be noted that the target activation patterns for this study were drawn from the EMG signals recorded in the non-paretic limb. It is not clear that this is the optimal choice for stroke rehabilitation, at least for all individuals. The system is sufficiently flexible to allow the selection of other target vectors. For example, PCs can be fit to the EMG space of the paretic hand to work on improving control within and expanding this space. Alternatively, arbitrary desired target vectors can be read into the GUI. The clinician or user can choose the target activation patterns according to their specific therapy goals.
We did not observe any statistical differences in outcomes between the Unilateral and Bilateral groups. For these participants with moderate hand impairment, muscle control may have been adequate at the beginning of the study to directly work with the PCs from the non-paretic activation workspace. For more impaired subjects, it is possible that the bilateral approach may be beneficial. Alternatively, due to the ability to customize the training to the individual, it may be just as effective to immediately focus on unilateral training and incorporate non-paretic only if needed. The influence of motor impairment severity on efficacy of bilateral vs. unilateral training is an area worthy of further study.47
Limitations
This initial study of the system with stroke survivors was limited in scope. First, the sample size was small. Thus, the effect should be interpreted with caution. Second, a group receiving usual care or no treatment was not included as a control. While upper limb function is stable in the chronic stage,48 the effect of psychosocial factors from participating in the study cannot be excluded. Third, no bilateral function was assessed. Thus, the effect of the bilateral training on bilateral function is unknown. Fourth, stroke survivors participated in 3 weeks of training. The effects of longer training periods are unknown. Fifth, as the primary goal of this study was to determine if stroke survivors, particularly in the chronic phase of recovery, could learn to improve control of their muscle activation patterns, no functional task performance was included in the therapy. A future study could implement training with the EMG-controlled game in conjunction with repetitive practice of upper extremity tasks to examine whether this improves control of the muscle activation pattern, quality of movement, and task performance.
Conclusions
In summary, EMG game training for improving control of muscle activation patterns in stroke survivors could be performed and participant compliance was high. Future directions may include investigation of utility of the bilateral approach in persons with greater upper limb impairments and expanding the activation workspace, while including bilateral functional outcome measures. Future studies may also examine the effect of the training with the EMG-controlled games in conjunction with repetitive practice of functional tasks, for a longer intervention duration, against a control group, while monitoring user satisfaction. Incorporation of proximal upper limb muscles may also be explored to target overall upper limb function.31
Supplementary Material
Acknowledgment:
This work was supported by NIH (NCMRR) grant 1R01HD075813–01A1. The authors want to thank Drs. Rajiv Ranganathan and Sandro Mussa-Ivaldi for their efforts in developing the human-machine interface for the serious games.
Sources of funding:
NIH/NICHD 1R01HD075813–01A1
Footnotes
Preliminary results with a smaller sample size were presented at a conference: Seo NJ, Ghassemi M, Barry A, Triandafilou K, Vidakovic L, Roth E, Kamper D (2017). Use of an EMG-controlled game as a therapeutic tool to retrain hand muscle activation patterns following stroke, American Society of Neurorehabilitation, Baltimore, MD.
Clinical trials number: NCT03619772
Conflict of Interest: All authors report no conflict of interest.
Contributor Information
Na Jin Seo, Department of Rehabilitation Sciences, Department of Health Science and Research, Medical University of South Carolina, Charleston, SC, USA. Ralph H. Johnson VA Medical Center, Charleston, SC, USA., 151B Rutledge Ave, MSC 962 Charleston SC 29425. 843-792-0084.
Alex Barry, Arms + Hands Lab, Shirley Ryan Ability Lab, Chicago, IL, USA..
Mohammad Ghassemi, Joint Department of Biomedical Engineering, North Carolina State University/ University of North Carolina at Chapel Hill, Raleigh/ Chapel Hill, NC, USA..
Kristen M Triandafilou, Arms + Hands Lab, Shirley Ryan Ability Lab, Chicago, IL, USA..
Mary Ellen Stoykov, OTR/L, Department of Physical Medicine and Rehabilitation, Northwestern University Feinberg School of Medicine, Shirley Ryan Ability Lab, Chicago, IL, USA..
Lynn Vidakovic, Department of Physical Medicine and Rehabiliation, Northwestern University Feinberg School of Medicine, Shirley Ryan AbilityLab, Chicago, IL, USA.
Elliot Roth, Department of Physical Medicine and Rehabiliation, Northwestern University Feinberg School of Medicine, Shirley Ryan AbilityLab, Chicago, IL, USA..
Derek G Kamper, Joint Department of Biomedical Engineering, North Carolina State University/ University of North Carolina at Chapel Hill, Raleigh/ Chapel Hill, NC, USA..
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation Mar 20 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 2.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch Phys Med Rehabil Aug 1994;75(8):852–7. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil Apr 1994;75(4):394–8. [DOI] [PubMed] [Google Scholar]
- 4.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke Apr 2013;44(4):1111–6. doi: 10.1161/STROKEAHA.111.674671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwakkel G, Kollen BJ, Wagenaar RC. Therapy impact on functional recovery in stroke rehabilitation: a critical review of the literature. Physiotherapy 1999;85(7):377–391. [Google Scholar]
- 6.Waddell KJ, Birkenmeier RL, Moore JL, Hornby TG, Lang CE. Feasibility of high-repetition, task-specific training for individuals with upper-extremity paresis. Am J Occup Ther Jul-Aug 2014;68(4):444–53. doi: 10.5014/ajot.2014.011619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knutson JS, Gunzler DD, Wilson RD, Chae J. Contralaterally Controlled Functional Electrical Stimulation Improves Hand Dexterity in Chronic Hemiparesis: A Randomized Trial. Stroke Oct 2016;47(10):2596–602. doi: 10.1161/STROKEAHA.116.013791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pak S, Patten C. Strengthening to promote functional recovery poststroke: an evidence-based review. Top Stroke Rehabil May-Jun 2008;15(3):177–99. doi: 10.1310/tsr1503-177 [DOI] [PubMed] [Google Scholar]
- 9.Raghavan P The nature of hand motor impairment after stroke and its treatment. Curr Treat Options Cardiovasc Med Jun 2007;9(3):221–8. doi: 10.1007/s11936-007-0016-3 [DOI] [PubMed] [Google Scholar]
- 10.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain Apr 1995;118 ( Pt 2):495–510. [DOI] [PubMed] [Google Scholar]
- 11.Ellis MD, Schut I, Dewald JPA. Flexion synergy overshadows flexor spasticity during reaching in chronic moderate to severe hemiparetic stroke. Clin Neurophysiol Jul 2017;128(7):1308–1314. doi: 10.1016/j.clinph.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SW, Wilson KM, Lock BA, Kamper DG. Subject-specific myoelectric pattern classification of functional hand movements for stroke survivors. IEEE Trans Neural Syst Rehabil Eng Oct 2011;19(5):558–66. doi: 10.1109/TNSRE.2010.2079334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearlman JL, Roach SS, Valero-Cuevas FJ. The fundamental thumb-tip force vectors produced by the muscles of the thumb. J Orthop Res Mar 2004;22(2):306–12. doi: 10.1016/j.orthres.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 14.Johanson ME, Valero-Cuevas FJ, Hentz VR. Activation patterns of the thumb muscles during stable and unstable pinch tasks. J Hand Surg Am Jul 2001;26(4):698–705. doi: 10.1053/jhsu.2001.26188 [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann G, Conrad MO, Qiu D, Kamper DG. Contributions of voluntary activation deficits to hand weakness after stroke. Top Stroke Rehabil Dec 2016;23(6):384–392. doi: 10.1179/1945511915Y.0000000023 [DOI] [PubMed] [Google Scholar]
- 16.Seo NJ, Rymer WZ, Kamper DG. Delays in grip initiation and termination in persons with stroke: effects of arm support and active muscle stretch exercise. J Neurophysiol Jun 2009;101(6):3108–15. doi: 10.1152/jn.91108.2008 [DOI] [PubMed] [Google Scholar]
- 17.Cruz EG, Waldinger HC, Kamper DG. Kinetic and kinematic workspaces of the index finger following stroke. Brain May 2005;128(Pt 5):1112–21. doi: 10.1093/brain/awh432 [DOI] [PubMed] [Google Scholar]
- 18.Triandafilou KM, Fischer HC, Towles JD, Kamper DG, Rymer WZ. Diminished capacity to modulate motor activation patterns according to task contributes to thumb deficits following stroke. J Neurophysiol Oct 2011;106(4):1644–51. doi: 10.1152/jn.00936.2010 [DOI] [PubMed] [Google Scholar]
- 19.Kamper DG, Rymer WZ. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle Nerve May 2001;24(5):673–81. doi: 10.1002/mus.1054 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Seo NJ, Rymer WZ, Kamper DG. Altered digit force direction during pinch grip following stroke. Exp Brain Res May 2010;202(4):891–901. doi: 10.1007/s00221-010-2193-7 [DOI] [PubMed] [Google Scholar]
- 21.Seo NJ, Enders LR, Motawar B, Kosmopoulos ML, Fathi-Firoozabad M. The extent of altered digit force direction correlates with clinical upper extremity impairment in chronic stroke survivors. J Biomech Jan 21 2015;48(2):383–7. doi: 10.1016/j.jbiomech.2014.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve Sep 2003;28(3):309–18. doi: 10.1002/mus.10443 [DOI] [PubMed] [Google Scholar]
- 23.Monte-Silva K, Piscitelli D, Norouzi-Gheidari N, Batalla MAP, Archambault P, Levin MF. Electromyogram-Related Neuromuscular Electrical Stimulation for Restoring Wrist and Hand Movement in Poststroke Hemiplegia: A Systematic Review and Meta-Analysis. Neurorehabil Neural Repair Feb 2019;33(2):96–111. doi: 10.1177/1545968319826053 [DOI] [PubMed] [Google Scholar]
- 24.Ockenfeld C, Tong RK, Susanto EA, Ho SK, Hu XL. Fine finger motor skill training with exoskeleton robotic hand in chronic stroke: stroke rehabilitation. IEEE Int Conf Rehabil Robot Jun 2013;2013:6650392. doi: 10.1109/ICORR.2013.6650392 [DOI] [PubMed] [Google Scholar]
- 25.Mubin O, Alnajjar F, Al Mahmud A, Jishtu N, Alsinglawi B. Exploring serious games for stroke rehabilitation: a scoping review. Disabil Rehabil Assist Technol Jun 8 2020:1–7. doi: 10.1080/17483107.2020.1768309 [DOI] [PubMed]
- 26.Doumas I, Everard G, Dehem S, Lejeune T. Serious games for upper limb rehabilitation after stroke: a meta-analysis. J Neuroeng Rehabil Jun 15 2021;18(1):100. doi: 10.1186/s12984-021-00889-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieira C, Ferreira da Silva Pais-Vieira C, Novais J, Perrotta A. Serious Game Design and Clinical Improvement in Physical Rehabilitation: Systematic Review. JMIR Serious Games Sep 23 2021;9(3):e20066. doi: 10.2196/20066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis GN, Woods C, Rosie JA, McPherson KM. Virtual reality games for rehabilitation of people with stroke: perspectives from the users. Disabil Rehabil Assist Technol 2011;6(5):453–63. doi: 10.3109/17483107.2011.574310 [DOI] [PubMed] [Google Scholar]
- 29.Cargnin DJ, Cordeiro d’Ornellas M, Cervi Prado AL. A Serious Game for Upper Limb Stroke Rehabilitation Using Biofeedback and Mirror-Neurons Based Training. Stud Health Technol Inform 2015;216:348–52. [PubMed] [Google Scholar]
- 30.Noveletto F, Soares AV, Eichinger FLF, Domenech SC, Hounsell MDS, Bertemes-Filho P. Biomedical Serious Game System for Lower Limb Motor Rehabilitation of Hemiparetic Stroke Patients. IEEE Trans Neural Syst Rehabil Eng Apr 16 2020;doi: 10.1109/TNSRE.2020.2988362 [DOI] [PubMed]
- 31.Mugler EM, Tomic G, Singh A, et al. Myoelectric Computer Interface Training for Reducing Co-Activation and Enhancing Arm Movement in Chronic Stroke Survivors: A Randomized Trial. Neurorehabil Neural Repair Apr 2019;33(4):284–295. doi: 10.1177/1545968319834903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright ZA, Rymer WZ, Slutzky MW. Reducing Abnormal Muscle Coactivation After Stroke Using a Myoelectric-Computer Interface: A Pilot Study. Neurorehabil Neural Repair Jun 2014;28(5):443–51. doi: 10.1177/1545968313517751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghassemi M, Triandafilou K, Barry A, et al. Development of an EMG-Controlled Serious Game for Rehabilitation. IEEE Trans Neural Syst Rehabil Eng Feb 2019;27(2):283–292. doi: 10.1109/TNSRE.2019.2894102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghavan P, Petra E, Krakauer JW, Gordon AM. Patterns of impairment in digit independence after subcortical stroke. J Neurophysiol Jan 2006;95(1):369–78. doi: 10.1152/jn.00873.2005 [DOI] [PubMed] [Google Scholar]
- 35.Lang CE, Wagner JM, Edwards DF, Sahrmann SA, Dromerick AW. Recovery of grasp versus reach in people with hemiparesis poststroke. Neurorehabil Neural Repair Dec 2006;20(4):444–54. doi: 10.1177/1545968306289299 [DOI] [PubMed] [Google Scholar]
- 36.McCombe Waller S, Whitall J, Jenkins T, et al. Sequencing bilateral and unilateral task-oriented training versus task oriented training alone to improve arm function in individuals with chronic stroke. BMC Neurol Dec 14 2014;14:236. doi: 10.1186/s12883-014-0236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitall J, Waller SM, Sorkin JD, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair Feb 2011;25(2):118–29. doi: 10.1177/1545968310380685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Cheng H, Zhang J, Bai Z, Cai S. The modulatory effects of bilateral arm training (BAT) on the brain in stroke patients: a systematic review. Neurol Sci Feb 2021;42(2):501–511. doi: 10.1007/s10072-020-04854-z [DOI] [PubMed] [Google Scholar]
- 39.McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits? NeuroRehabilitation 2008;23(1):29–41. [PMC free article] [PubMed] [Google Scholar]
- 40.Kim WS, Cho S, Ku J, et al. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J Clin Med Oct 21 2020;9(10)doi: 10.3390/jcm9103369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 1993;24:58–63. [DOI] [PubMed] [Google Scholar]
- 42.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke Jul 2001;32(7):1635–9. doi: 10.1161/01.str.32.7.1635 [DOI] [PubMed] [Google Scholar]
- 43.Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L. Validation of the Box and Block Test as a measure of dexterity of elderly people: reliability, validity, and norms studies. Arch Phys Med Rehabil Jul 1994;75(7):751–5. doi:0003-9993(94)90130-9 [pii] [PubMed] [Google Scholar]
- 44.Fritz SL, Blanton S, Uswatte G, Taub E, Wolf SL. Minimal detectable change scores for the Wolf Motor Function Test. Neurorehabil Neural Repair Sep 2009;23(7):662–7. doi: 10.1177/1545968309335975 [DOI] [PubMed] [Google Scholar]
- 45.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Arch Phys Med Rehabil Sep 2008;89(9):1693–700. doi: 10.1016/j.apmr.2008.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris DM, Uswatte G, Crago JE, Cook EW 3rd, Taub E. The reliability of the wolf motor function test for assessing upper extremity function after stroke. Arch Phys Med Rehabil Jun 2001;82(6):750–5. doi: 10.1053/apmr.2001.23183 [DOI] [PubMed] [Google Scholar]
- 47.Chen PM, Kwong PWH, Lai CKY, Ng SSM. Comparison of bilateral and unilateral upper limb training in people with stroke: A systematic review and meta-analysis. PLoS One 2019;14(5):e0216357. doi: 10.1371/journal.pone.0216357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil Aug 1999;21(8):357–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


