Abstract
Interactions between the amygdala and prefrontal cortex are fundamental to human emotion. Despite the central role of frontoamygdala communication in adult emotional learning and regulation, little is known about how top-down control emerges during human development. In the present cross-sectional pilot study, we experimentally manipulated prefrontal engagement to test its effects on the amygdala during development. Inducing dorsal anterior cingulate cortex (dACC) activation resulted in developmentally-opposite effects on amygdala reactivity during childhood versus adolescence, such that dACC activation was followed by increased amygdala reactivity in childhood but reduced amygdala reactivity in adolescence. Bayesian network analyses revealed an age-related switch between childhood and adolescence in the nature of amygdala connectivity with the dACC and ventromedial PFC (vmPFC). Whereas adolescence was marked by information flow from dACC and vmPFC to amygdala (consistent with that observed in adults), the reverse information flow, from the amygdala to dACC and vmPFC, was dominant in childhood. The age-related switch in information flow suggests a potential shift from bottom-up co-excitatory to top-down regulatory frontoamygdala connectivity and may indicate profound change in the circuitry supporting maturation of emotional behavior. These findings provide novel insight into the developmental construction of amygdala-cortical connections and implications for the ways in which childhood experiences may influence subsequent prefrontal function.
Keywords: brain development, amygdala, prefrontal cortex, fMRI, connectivity, emotion regulation
INTRODUCTION
Frontoamygdala circuitry is central to emotional learning and regulation (Ochsner et al., 2002; Phelps et al., 2004; Lieberman et al., 2007). The amygdala is a subcortical structure involved in vigilance, attention, and learning about biologically relevant signals, including facial expressions of emotion (Adolphs et al., 1994; Phelps and LeDoux, 2005; Pessoa, 2010; Hadj-Bouziane et al., 2012). In part through bidirectional connections with the amygdala, the ventromedial prefrontal cortex (vmPFC) contributes to a broad array of functions related to valuation and emotion regulation (Roy et al., 2012; Winecoff et al., 2013; Delgado et al., 2016). Particularly in the context of demands for cognitive control (e.g., in the context of conflict, interference, errors, and negative feedback; Shackman et al., 2011; Shenhav et al., 2016; Braem et al., 2017), the dorsal anterior cingulate cortex (dACC) is involved in the broader functional network of regions help to guide emotional behavior (Pessoa et al., 2019). Individual differences in the strength of connectivity between these regions predict behaviors associated with emotion regulation and anxiety in healthy adults (Pezawas et al., 2005; Banks et al., 2007; Kim et al., 2011; Lee et al., 2012). Frontoamygdala circuitry undergoes dynamic changes during development in non-human animals (Cressman et al., 2010; Pattwell et al., 2016; Arruda-Carvalho et al., 2017; Yan et al., 2017) and in humans (Hare et al., 2008; Perlman and Pelphrey, 2011; Gee et al., 2013b; Gabard-Durnam et al., 2014; Swartz et al., 2014; Gabard-Durnam et al., 2016; Jalbrzikowski et al., 2017). However, the mechanisms by which this circuitry is constructed and changes across childhood and adolescence remain unclear in humans.
The majority of empirical research on the development of this circuitry has focused on prefrontal control over the amygdala. Evidence from developmental neuroscience indicates that despite early structural development (Humphrey, 1968; Ulfig et al., 2003), structural and functional maturation of the human amygdala continues into adulthood (Schumann et al., 2004; Østby et al., 2009; Uematsu et al., 2012; Wierenga et al., 2014; Avino et al., 2018; Weir et al., 2018). Importantly though, prefrontal regions including the vmPFC and dACC undergo more protracted maturation than the amygdala (Machado and Bachevalier, 2003; Lenroot and Giedd, 2006; Payne et al., 2010; Chareyron et al., 2012; Gilmore et al., 2012), and the nature of interactions between the amygdala and prefrontal regions appears to be different early in life. Prior work has identified a developmental switch in frontoamygdala functional connectivity around the transition from childhood to adolescence (Gee et al., 2013b; Gabard-Durnam et al., 2014; Silvers et al., 2015; Spielberg et al., 2015; Wu et al., 2016), which parallels the maturation of emotional behavior and reductions in amygdala reactivity with age (Decety et al., 2012; Gee et al., 2013b; Hwang et al., 2014; Swartz et al., 2014; Vink et al., 2014; Silvers et al., 2015). The neural phenotype of frontoamygdala connectivity in adolescence already resembles the adult state (albeit slightly weaker) (Hariri et al., 2003; Kim et al., 2003; Hare et al., 2008). Observation of a dramatic change in the functional connections between the amygdala and vmPFC during development has generated the hypothesis that the nature of communication between the amygdala and vmPFC differs during childhood than at older ages, with the possibility of increasing regulatory control of the vmPFC over the amygdala around this key developmental transition (Gee et al., 2013b). However, existing studies have been primarily limited to observing static correlations between vmPFC and amygdala timeseries, which are limited in terms of elucidating the direction of information flow between these regions. Delineating the directionality of functional interactions between prefrontal regions and the amygdala would provide important insight into how this circuitry changes with development. Moreover, less is known about age-related changes in the nature of connectivity between the amygdala and more dorsal regions of prefrontal cortex (e.g., dACC).
The present cross-sectional pilot study examined directional influences in frontoamygdala circuitry (specifically, interactions between the dACC, vmPFC, and amygdala) throughout childhood and adolescence. First, by experimentally manipulating dACC activation via cognitive conflict, we tested the effects of dACC engagement on subsequent amygdala reactivity during the processing of affective information. We hypothesized that activating the dACC would be followed by lower amygdala activation among adolescents but higher amygdala activation among children. Second, by using Bayesian network analyses, we tested the hypothesis of an age-related switch in frontoamygdala directionality between children (dominated by bottom-up amygdala influence to the dACC and vmPFC) and adolescents (dominated by top-down dACC and vmPFC influence to the amygdala). In an exploratory analysis, we aimed to examine potential associations between frontoamygdala interactions and affective behavior. Specifically, we focused on separation anxiety given prior work linking age-related changes in frontoamygdala circuitry with normative declines in separation anxiety (Gee et al., 2013b, 2013a, 2014), and prior evidence of associations between amygdala responsivity and separation anxiety (Carpenter et al., 2015; Redlich et al., 2015; Green et al., 2016). We hypothesized that participants who exhibited greater reduction in amygdala activation following dACC activation, and those who showed stronger dACC-to-amygdala and vmPFC-to-amygdala connectivity, would display lower separation anxiety.
METHODS
Participants
Participants were 40 healthy children and adolescents (22 females; 18 males), ages 5-19 years (mean age (S.D.) = 12.93 (3.92)) recruited from the local community via flyers and online advertisements. All participants’ caregivers completed a telephone screening. Only participants whose caregivers did not report that the participant had a history of psychiatric disorder were invited to participate in the present study. In-lab assessment with the Child Behavior Checklist (Achenbach, 1991) (available for 28 participants) confirmed that psychiatric symptoms in the current sample fell within the normative range (T-scores for internalizing problems: M = 44.39, S.D. = 9.93; externalizing problems: M = 42.79, S.D. = 8.74; and total problems: M = 42.46, S.D. = 10.23). Participants were from European American (55.0%), African American (12.5%), Asian American (7.5%), and Native Hawaiian or other Pacific Islander (2.5%) backgrounds. Nine participants (22.5%) identified as multiracial, including African American, Asian American, European American, and American Indian or Alaska Native backgrounds. Five participants (12.2%) identified as Hispanic/Latino. Cognitive ability was assessed using the Wechsler Abbreviated Scale of Intelligence for 39 participants. The average full-scale intelligence quotient of the sample was within the high average range (mean (S.D.) = 116.15 (14.85)). Data on the education level of each participant’ primary caregiver were obtained for 40 participants. Of those 40 participants’ primary caregivers, the modal levels of education were a four-year college degree (n=9) and a Master’s degree (n=9). Data on household income were obtained regarding the families of 39 participants. Of the 39 families, the modal income range was $55,001-70,000 (n=7). All participants were right-handed. Individual differences in current normative separation anxiety were assessed using the parent report form of the Revised Child Anxiety and Depression Scale (RCADS) (Chorpita et al., 2005), which was available for 31 participants. Because separation anxiety declines normatively with age and is relevant for understanding risk for anxiety disorders (Gullone and King, 1997; Beesdo et al., 2009), and due to prior findings linking separation anxiety with frontoamygdala circuitry during development (Gee et al., 2013b, 2013a, 2014), our analyses of anxiety focused specifically on the separation anxiety subscale of the RCADS. Pubertal stage was assessed using the Pubertal Development Scale (Petersen et al., 1988) and was available for 39 participants. The protocol was approved by the Institutional Review Board at the University of California, Los Angeles. Participants provided informed consent or assent (parental informed consent for minors). The data that support the findings of this study are available from the corresponding authors upon request.
Procedures
MRI Task Paradigm.
During the functional magnetic resonance imaging (fMRI) scan, participants completed a task manipulating cognitive conflict designed to engage the prefrontal cortex and to measure subsequent effects of this manipulation of prefrontal engagement on amygdala activation to emotional faces. Thus, the task consisted of alternating blocks of the cognitive task immediately followed by a block of emotional faces (Figure 1). For the cognitive task, we modified the cognitive Stroop task that was designed for use in the MRI scanner (Bush et al., 1998) for use with our developmental sample. On each trial, participants viewed 1-4 simultaneously-presented words and were asked to press a button to indicate how many words they saw. For the cognitive Stroop task, the number of words on the screen matched the written word on congruent trials (e.g., “two” listed two times; low conflict) and did not match the written word on incongruent trials (e.g., “two” listed four times; high conflict). The words used were “one,” “two,” “three,” and “four.”
Figure 1.
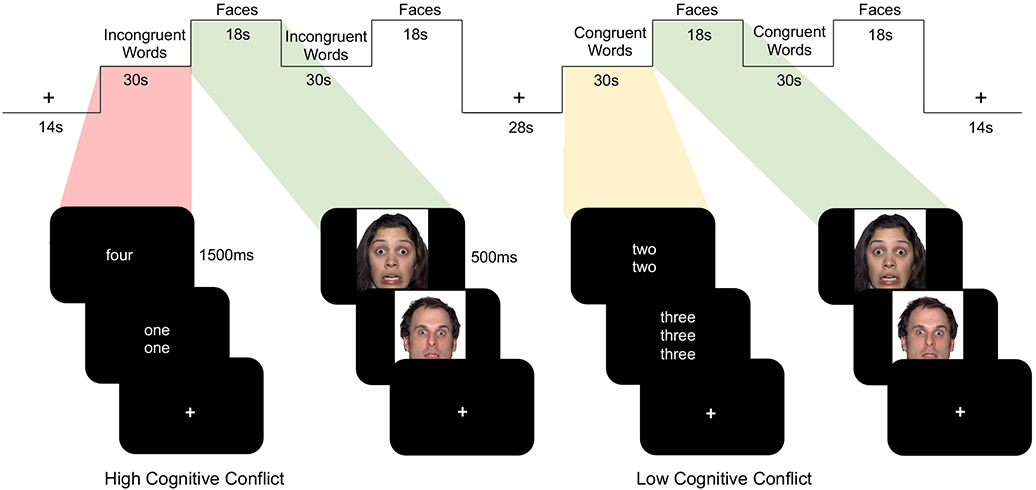
fMRI Paradigm. Participants completed an fMRI task designed to measure the effect of dACC activation (via high cognitive conflict) on subsequent amygdala reactivity to faces. A cognitive Stroop task involved congruent trials (low conflict) and incongruent trials (high conflict). Each block of words was followed by a block of emotional faces.
In order to examine subsequent amygdala activation, each block of words was followed by a block of faces. Faces were selected from the NimStim Set of Facial Expressions (Tottenham et al., 2009). Faces were 50% male and 50% female, and 42% Caucasian, 42% African American, and 16% Asian American. Fear faces comprised 75% of the stimuli in each faces block; neutral faces comprised 25% of the stimuli in each faces block. During the faces blocks, participants were asked to press when they saw a neutral face to ensure they were paying attention. We selected fearful faces as a widely used and developmentally-appropriate stimulus that has been shown to robustly activate the amygdala (Breiter et al., 1996), including in children and adolescents (Baird et al., 1999; Thomas et al., 2001; Hare et al., 2008; Tottenham et al., 2011; Gee et al., 2013b, 2013a). The timing and limited number of neutral faces were not optimized to directly compare responses to fearful versus neutral faces, and the behavioral response and motor demand differed between fearful and neutral faces. Moreover, while the majority of research using task-based fMRI to study frontoamygdala circuitry has focused on activation or functional connectivity to fearful faces (e.g., Costafreda et al., 2008; Sergerie et al., 2008; Fusar-Poli et al., 2009; Di et al., 2017), the amygdala is also responsive to other types of emotional faces and to neutral faces (e.g., Somerville et al., 2004; Marusak et al., 2013). In addition, neutral faces can be perceived as negative (e.g., Somerville et al., 2004; Blasi et al., 2009), especially during development (Tottenham et al., 2013; Marusak et al., 2017) and among more anxious individuals (Somerville et al., 2004; Heuer et al., 2010). These findings underscore challenges in treating neutral faces as a baseline condition in neuroimaging studies focused on development (e.g., Miller et al., 2020). Thus, our analyses focused on activation and effective connectivity to the overall faces block (across fearful and neutral faces, relative to implicit baseline) when it followed incongruent words versus when it followed congruent words.
The fMRI paradigm contained 4 blocks of the Stroop task (2 incongruent, 2 congruent) and 4 blocks of the faces task. Each Stroop block was immediately followed by a faces block. The order of the incongruent and congruent blocks was counterbalanced across participants. The paradigm contained 40 trials of the Stroop task (10 words in each of 4 blocks) and 48 trials of the faces task (12 faces in each of 4 blocks; 9 fearful faces and 3 neutral faces per block). Each word was presented for 1500 msec followed by 1500 msec fixation; each face was presented for 500 msec followed by 1000 msec fixation. Stroop blocks and faces blocks were 30 sec and 18 sec in length, respectively. The stimuli within each block were randomized and fixed across participants.
fMRI Data Acquisition.
Scanning was performed on a Siemens Trio 3.0 Tesla MRI scanner. A standard 12-channel radiofrequency head coil was employed. For each participant, an initial 2D spin echo image (TR=4000ms, TE=40ms, matrix size 256x256, 4mm thick, 0mm gap) in the oblique plane was acquired to allow configuration of slices obtained in the structural and functional scans. A whole-brain high-resolution, T1*weighted anatomical scan (MPRAGE; 256 X 256 in-plane resolution, 256 mm field of view [FOV]; 192 mm X 1 mm sagittal slices) was acquired for each participant for registration and localization of functional data to MNI space. The task was presented using E-Prime (Psychology Software Tools) on a computer screen through MR-compatible goggles. T2*weighted echoplanar images (interleaved) were collected at an oblique angle of approximately 30 degrees (127 volumes, TR=2000, TE=30ms, flip angle=90 degrees, matrix size 64x64, FOV=192, 34 slices, 4mm slice thickness, skip 0mm).
Systematic procedures were implemented to reduce motion, particularly in younger participants, and to ensure that children remained still throughout the duration of the task. Before the MRI scanning session, children participated in a mock scanning session to help them to acclimate to the scanning environment and to feel comfortable with the scanning procedures. In addition, this step provided an opportunity for children to practice and receive feedback on lying still in order to optimize children’s ability to remain still during actual data collection. During data collection, an air vacuum pillow (Siemens Comfort Pack) was used to pad and secure the child’s head in a comfortable, steady position. Additional padding was placed around the child’s head. In addition, all participants were provided with feedback and reminders regarding motion throughout the scanning session.
fMRI Data Analysis.
Task-based Activation Analyses
Functional imaging data were preprocessed and analyzed using the Analysis of Functional NeuroImages (AFNI) software package. One participant’s data was excluded from fMRI analyses due to poor brain coverage (i.e., original sample size = 41; sample size analyzed and reported in current study = 40). Preprocessing of each individual’s images included slice time correction to adjust for temporal differences in slice acquisition within each volume, spatial realignment to correct for head motion, registration to the first volume of the run, and spatial smoothing using a 6-mm Gaussian kernel (FWHM) to increase the signal to noise ratio. Functional data were registered to the participant’s anatomical scan, and the anatomical and functional scans were transformed to the standard coordinate space of MNI152 with align_epi_anat.py. Transformations on the functional scans were combined into a single transformation within align_epi_anat.py to minimize the amount of interpolation applied to the functional data. MNI-transformed images had a resampled resolution of 2mm3. Timeseries were normalized to percent signal change to allow for comparisons across individuals.
Multiple steps were taken to correct for motion. All data were free of motion greater than 0.9 mm in any direction, as recommended by Siegel and colleagues (2014). Volumes with motion greater than 0.9mm in any direction were excluded (mean % of censored volumes across participants=3.20%; S.D.=8.92%; median=0%, mode=0%) via regressors in the GLM in afni_proc_py (Jo et al., 2010, 2013). Mean framewise displacement for the overall sample was 0.079 mm (S.D.=0.050). Preprocessing included standard spatial realignment to correct for motion. Motion regressors were included in our imaging analyses (at the subject level, motion in all six directions at the trial by trial level and the 6 backwards temporal derivatives of those motion regressors, as recommended (Van Dijk et al., 2010; Yan et al., 2013)). In addition, multiple analyses were conducted to rule out potential effects of motion. First, we tested whether motion related to our findings or differed between the two age groups. Average motion across the six directions was not correlated with age, amygdala activation, vmPFC activation, dACC activation, or separation anxiety (r values: −.26 to .34). In addition, we tested whether the number of volumes censored related to our findings or differed between the two age groups. The number of volumes censored was not correlated with age, amygdala activation, dACC activation, or separation anxiety (r values: −.26 to .23) but was correlated with vmPFC activation (r=−.39).
In order to examine activation, each participant’s individual-level model included regressors for each of the stimulus conditions (incongruent words, faces following incongruent words, congruent words, faces following congruent words) modeled as a block design. Because the primary goal of the faces condition was to probe amygdala reactivity following the task of cognitive conflict, and given the limited number of neutral faces, we employed a block design to enhance power instead of modeling fearful and neutral faces separately. The regressors were created by convolving the stimulus timing files with the canonical hemodynamic response function. Timecourses for eroded ventricle and eroded white matter masks along with 12 motion regressors (6 rigid-body regressors and their 6 backwards temporal derivatives) were included as physiological nuisance covariates. We employed a general linear model (GLM) to fit the percent signal change timeseries to each regressor. Linear and quadratic trends were modeled for each voxel’s timeseries to control for correlated drift.
The individual-level regression coefficients were submitted to random-effects, group-level whole-brain analyses. We specifically aimed to test the effects of enhanced prefrontal recruitment via cognitive conflict on amygdala processing of emotional faces. Thus, we first examined neural responses to a contrast of incongruent words versus congruent words for the entire sample, including age as an additional regressor in our model using the 3dttest++ program within AFNI. This analysis allowed us to test the prediction that high, relative to low, conflict would engage the dACC. Then we tested for an interaction between conflict and age group on neural responses to faces following incongruent words versus faces following congruent words using the 3dttest++ program within AFNI, which allowed us to test the prediction that children and adolescents would show differential effects of the conflict manipulation on subsequent patterns of prefrontal and amygdala activation. Participants were divided into children (n=16; ages 5-10.4) and adolescents (n=24; ages 10.5-19) based on differences in frontoamygdala connectivity observed in these age groups in prior work1 (Gee et al., 2013b, 2013a; Gabard-Durnam et al., 2014). For the dACC, the initial height threshold of p<.039 was subsequently corrected for multiple comparisons to a corrected level of p<.05 using the most updated version of the 3dClustSim program in AFNI version 18.3.02. This program runs a series of Monte Carlo simulations based on the noise of the residuals of the single subject regression output. Using 3dFWHMx and applying the spatial autocorrelation function (-acf) to account for the non-Gaussian nature of fMRI data, we calculated the average smoothness of the residuals across all participants. Statistical parametric maps were set to a cluster level threshold of k ≥ 1044 voxels (using first nearest neighbor clustering) as determined by 3dClustSim. Given our a priori hypothesis about age-related changes in activation of the amygdala and vmPFC, clusters within these regions were thresholded to achieve a small volume corrected value of p<.05 (k ≥ 30 for the amygdala, k ≥ 118 for the vmPFC), as determined by 3dClustSim. Although anatomically defined regions of interest (ROI) were not used for analyzing activation or connectivity in the current study, masks of the bilateral amygdala from the Talairach atlas in AFNI and vmPFC from the Mackey & Petrides atlas (Mackey and Petrides, 2014) in AFNI were used to approximate cluster size for 3dClustSim in an unbiased manner. Because we employed small volume correction for the amygdala and vmPFC, we did not interpret clusters of activation outside of these regions. A binary mask for each of the dACC (incongruent versus congruent words), vmPFC (faces following incongruent words versus faces following congruent words), and amygdala (faces following incongruent words versus faces following congruent words) clusters was created to extract parameter estimates for visualization purposes only, using 3dmaskave in AFNI to extract the GLM beta value of the condition (averaged across the voxels in the given mask). The specific contrasts of interest were selected based on prior evidence of dACC involvement in cognitive control (and specifically, in tasks of cognitive conflict) (e.g., Bush et al., 1998) and vmPFC and amygdala engagement in processing of emotional faces (e.g., Pessoa et al., 2002b; Phelps and LeDoux, 2005; Zald and Andreotti, 2010; Delgado et al., 2016).
Effective Connectivity Analyses
Analyses of effective connectivity were performed to test for age-related change in the directionality of connections between the amygdala, vmPFC, and dACC and their modulation by the cognitive conflict manipulation. For effective connectivity analyses, a maximum likelihood clustering analysis was conducted to find modal age groups with the highest densities for analysis. This approach was selected to maximize the sample size per age group while also minimizing bias. Connectivity analyses using Bayes networks allow for assessment of the direction of temporal influence among nodes in the network, the strength of the connections between nodes, and the number of connections coming in or out of a node. We employed a Bayesian network algorithm known as IMaGES (Independent Multi-Sample Greedy Equivalence Search) algorithm, which is housed in the larger analysis suite Tetrad (Ramsey et al., 2010, 2011). IMaGES operates on the standard assumption that causes precede effects temporally. Causality in this case refers to the ability of IMaGES, in conjunction with a distributional postprocessing step (LOFS; LiNG Orientation, Fixed Structure), to identify the directionality of connections with high probability (Ramsey et al., 2011). The algorithm takes as inputs the timeseries of selected ROIs and searches forward, starting from an empty graph, one new connection at a time, until it finds the set of connections (i.e., edges, vertices) that optimally represents connectivity among the entire group of subjects, interpolating any missing data. Finding oriented edges requires two steps: first, the estimate of connectivity (provided by GES and individual Bayes Information Criteria (BIC) constraints on time series regressions); and second, a method that systematically investigates conditional dependence/independence and estimates orientation2. The algorithm searches with the restriction of finding only Markov equivalence classes of directed acyclic graphs, and without the option of varying time lags, given that doing so did not improve accuracy in simulation (Ramsey et al., 2010, 2011). The process is penalized to prevent overfitting using the BIC. The reliability of the IMaGES algorithm was found to be higher when using the LOFS search post filter, which determines and assigns the “dominant” direction of the edge (removing bidirectional edges) (Ramsey et al., 2011). Therefore, the current analysis was conducted using this option and thus only unidirectional edges were returned, meaning that the current analysis assigned a dominant direction of influence and determined whether that directionality was statistically significant.
ROIs for the IMaGES analyses were selected and functionally defined based on the whole-brain GLM analysis of activation. We were specifically interested in interactions between the dACC, vmPFC, and amygdala; each of these regions was defined as a functional ROI based on the contrast in which it was activated in the whole-brain GLM analysis of activation (i.e., dACC: incongruent versus congruent words; vmPFC and amygdala: faces following incongruent words versus faces following congruent words). The binary mask for each functional region was used to extract average time series for each condition (incongruent words, congruent words, faces following incongruent words, faces following congruent words) from each participant’s preprocessed functional data. Each mask was binarized so that we did not extract values for any region outside of the ROI. Preprocessing of each individual’s images in AFNI included slice time correction to adjust for temporal differences in slice acquisition within each volume, spatial realignment to correct for head motion, registration to the first volume of the run, and spatial smoothing using a 6-mm Gaussian kernel (FWHM) to increase the signal to noise ratio. Functional data were registered to the participant’s anatomical scan, and the anatomical and functional scans were transformed to the standard coordinate space of MNI152 with align_epi_anat.py. Transformations on the functional scans were combined into a single transformation within align_epi_anat.py to minimize the amount of interpolation applied to the functional data. MNI-transformed images had a resampled resolution of 2mm3. Time series of interest were extracted using FSL’s meanTS module and arranged into a matrix for each participant, with the ROIs as columns and each row representing a single time point. These files were then input into the IMaGES workflow in Tetrad. Graph selection was conducted by choosing the graph with the highest BIC score. Connection weights were exported from Tetrad into LibreOffice Calc (https://www.libreoffice.org). The connection weights from IMaGES/LOFS contain information about directionality by estimating both P(X∣Y) and P(Y∣X). The initial estimate is provided by IMaGES, is oriented again with LOFS, and is tested with structural equation modeling. T statistics were calculated based on the mean and standard error from the time series, averaged across participants within each age group. T statistics were used instead of raw coefficient values because they take into account standard error. The TDIST function was used to calculate significance values.
Behavioral Data Analysis.
For each participant, we calculated accuracy and reaction time for each condition (incongruent words, congruent words) when participants pressed a button to report how many words were on the screen. ANOVA was used to test for a main effect of condition (within subjects), a main effect of age group (between subjects), and their interaction. All statistical analyses were performed as two-tailed tests with alpha=.05. Behavioral data and ROI data were analyzed in SPSS version 24.
RESULTS
Behavioral Performance
We used a cognitive conflict task during fMRI to examine age-related changes in frontoamygdala communication by manipulating conflict prior to presentation of faces. The behavioral manipulation was effective. Specifically, participants performed with lower accuracy (F(1,38)=109.55, p<.001, ηp2=0.74) and slower reaction time (F(1,38)=25.74, p<.001, ηp2=0.40) during the condition of high (incongruent words) versus low (congruent words) conflict in a Stroop task. These differential patterns for accuracy and reaction time were observed across both children and adolescents (i.e., there were no interactions between condition and age for reaction time (F(1,38)=0.94, p=.337) or accuracy (F(1,38)=1.57, p=.218); Figure 2). Relative to children, adolescents performed with faster reaction time overall (F(1,38)=32.52, p<.001, ηp2=0.46), but did not display higher accuracy overall (F(1,38)=2.44, p=.127).
Figure 2.
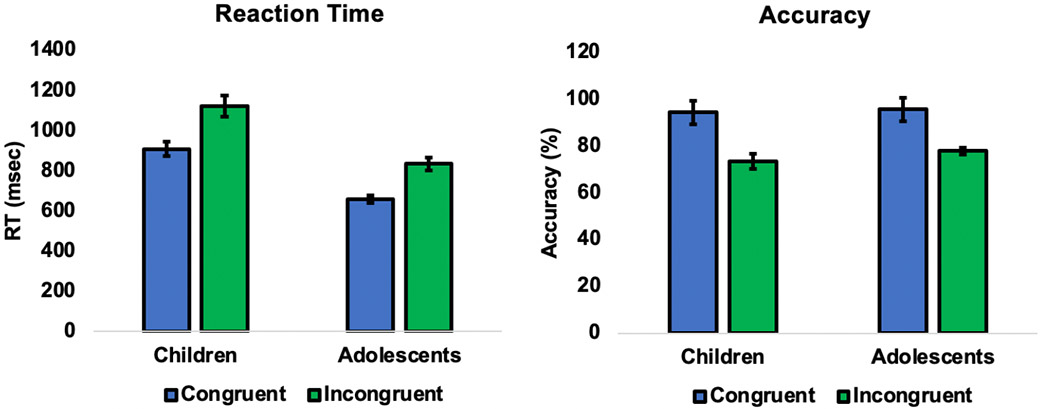
Behavioral performance indicated that the task was effective in increasing conflict across participants. Reaction times were slower (F(1,38)=25.74, p<.001, ηp2=0.40) and accuracy was lower (F(1,38)=109.55, p<.001, ηp2=0.74) for the high (versus low) conflict trials. The effect of conflict was evident in both children and adolescents (plotted separately for visualization purposes only; no interaction between condition and age).
Functional Activation
The high cognitive conflict condition effectively recruited dACC engagement. Results across the entire sample showed elevated dACC (BA 24) activation to high versus low conflict (p<.05, corrected; cluster size: 1381 voxels; peak: 0, 28, 44; Figure 3a; Table S1 for complete table of activated regions). There were no age-related effects in dACC activation. This region of dACC is consistent with prior studies employing the same cognitive conflict task and is thought to mediate response selection or allocate attentional resources in the context of competing information-processing streams (Bush et al., 1998).
Figure 3.
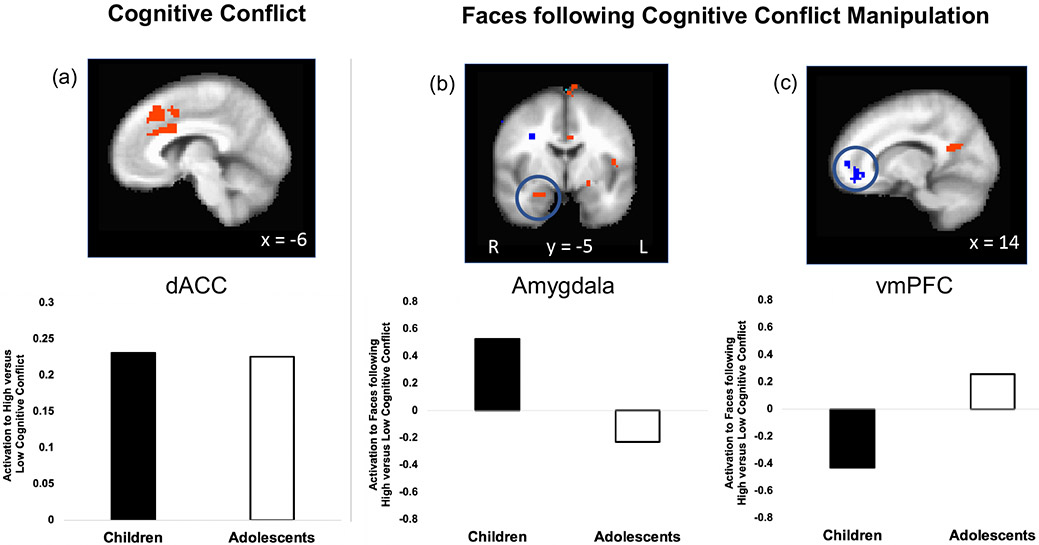
dACC activation (induced via high conflict) differentially relates to subsequent amygdala reactivity in children versus adolescents. A) The task effectively increased activation in the dACC (p<.05, corrected; incongruent versus congruent words) during high conflict compared to low conflict in the overall sample (children and adolescents plotted separately for visualization purposes only; no age difference). (B) The conflict manipulation changed subsequent amygdala reactivity to faces differently for children versus adolescents (p<.05, corrected; faces following incongruent words versus faces following congruent words). Adolescents showed decreased amygdala response after dACC activation, whereas children showed heightened amygdala response to faces after dACC activation. (C) Adolescents showed heightened vmPFC activation, whereas children showed decreased vmPFC activation to faces after dACC activation (p<.05, corrected; faces following incongruent words versus faces following congruent words). For panels A, B, and C, the top image shows the whole-brain GLM for the contrast of interest (for panel A, voxels depicted in orange showed greater activation for high (versus low) cognitive conflict; for panels B and C, voxels depicted in orange showed greater activation to faces following high cognitive conflict (versus faces following low cognitive conflict), whereas voxels depicted in blue showed greater activation to faces following low cognitive conflict (versus faces following high cognitive conflict). The bar plots below show the parameter estimates extracted from the relevant region from the corresponding whole-brain map (for visualization purposes only).
Experimental manipulation of cognitive conflict had differential effects on subsequent amygdala reactivity to faces in children versus adolescents. Specifically, an age group x condition interaction showed that right amygdala activation to faces following high conflict (versus following low conflict) differed between children and adolescents (p<.05, corrected; cluster size: 31 voxels; peak voxel: 26, −4, −22; Figure 3b). Whereas adolescents showed decreased amygdala response, children showed increased amygdala response to the faces following high conflict. Additionally, adolescents showed increased vmPFC activation, whereas children showed decreased vmPFC activation to the faces following high conflict (p<.05, corrected; cluster size: 161 voxels; peak: 14, 48, −10; Figure 3c)3. In summary, the experimental manipulation showed that dACC activation induced via cognitive conflict was followed by reduced amygdala reactivity in adolescents, consistent with evidence of similar patterns in adults (Pessoa et al., 2002a; Etkin et al., 2006; Mitchell et al., 2007; Van Dillen et al., 2009). However, children displayed increased amygdala reactivity following dACC activation, suggesting qualitative differences in the nature of communication between these regions at unique developmental periods.
Effective Connectivity
Next we sought to test age-related changes in the direction of influence between the amygdala and these prefrontal regions using Bayesian network analyses using IMaGES (Ramsey et al., 2010, 2011). A data-driven approach was used to first identify age groups for subsequent effective connectivity analysis (based upon a maximum likelihood clustering). This resulted in three groups (5-9 (n=12), 10-13 (n=10), and 14-19 (n=18) years old). For each age group, a directed connectivity graph was calculated based on the dominant direction of information flow between these regions during the face presentations following the high conflict manipulation (Figure 4; See Supplementary Results for results of all task conditions). The vmPFC and dACC more strongly influenced the amygdala in the late adolescent group (14-19 year olds): dominant connections were observed from the dACC (0.271, t=6.73, p<.0000001) and from the vmPFC (0.803, t=20.13, p<.00000001) to the amygdala (X2=4.15, p<.04; BIC=1.3). In contrast, this approach suggested that the amygdala more strongly influenced the vmPFC and dACC in children (5-9 years old): dominant connections were observed from the amygdala to the vmPFC (0.998, t=60.14, p<.0000001) and from the amygdala to the dACC (0.913, t=11.70, p<.000001) (X2=8.7, p<.003; BIC=6.0). The 10-13 year-olds exhibited an intermediate stage of directional influence: a dominant connection was observed from the amygdala to the dACC (1.078, t=7.83, p<.000001), and non-significant connections were observed from the amygdala to the vmPFC (0.987, t=1.47, p<.10) and from the vmPFC to the dACC (−0.178, t=−0.24, p>.81) (X2=44.0, p<.00001; BIC=46.0).
Figure 4.
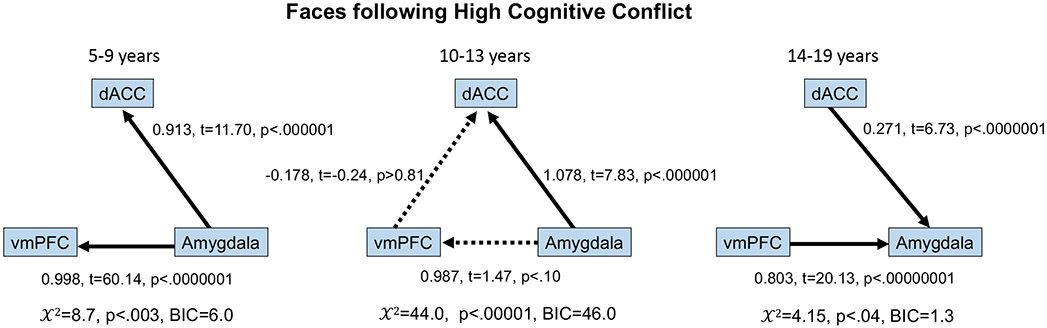
Age-related changes in information flow during faces following high conflict. Whereas connections were dominant from the amygdala to the vmPFC and dACC in children, an age-related switch was observed such that in older adolescents connections were dominant from the vmPFC and dACC to the amygdala, with an intermediate pattern around the transition from childhood to adolescence. Solid lines indicate connections identified by the model that were significant at p<.05. Dashed lines indicate connections identified by the model that were not significant.
Associations with Anxiety
The observed age-related changes in frontoamygdala communication were associated with affective behavior, providing insight into their functional significance. As anticipated, normative parent-reported levels of children’s current separation anxiety decreased with age (r(29)=−.42, p=.018). However, controlling for age, individuals for whom dACC activation was followed by reduced amygdala reactivity (i.e., the “adolescent pattern,” n=17) had lower separation anxiety than those participants who exhibited increased amygdala reactivity following dACC activation (i.e., the “child pattern,” n=14) (F(1,28)=17.92, p<.001, ηp2=0.39) (Figure 5), consistent with individual differences that would be expected based on the functional role of frontoamygdala connectivity. Separation anxiety was not significantly correlated with vmPFC-amygdala (r(29)=.12, p=.519) or dACC-amygdala (r(29)=.02, p=.909) effective connectivity during faces following high cognitive conflict.
Figure 5.
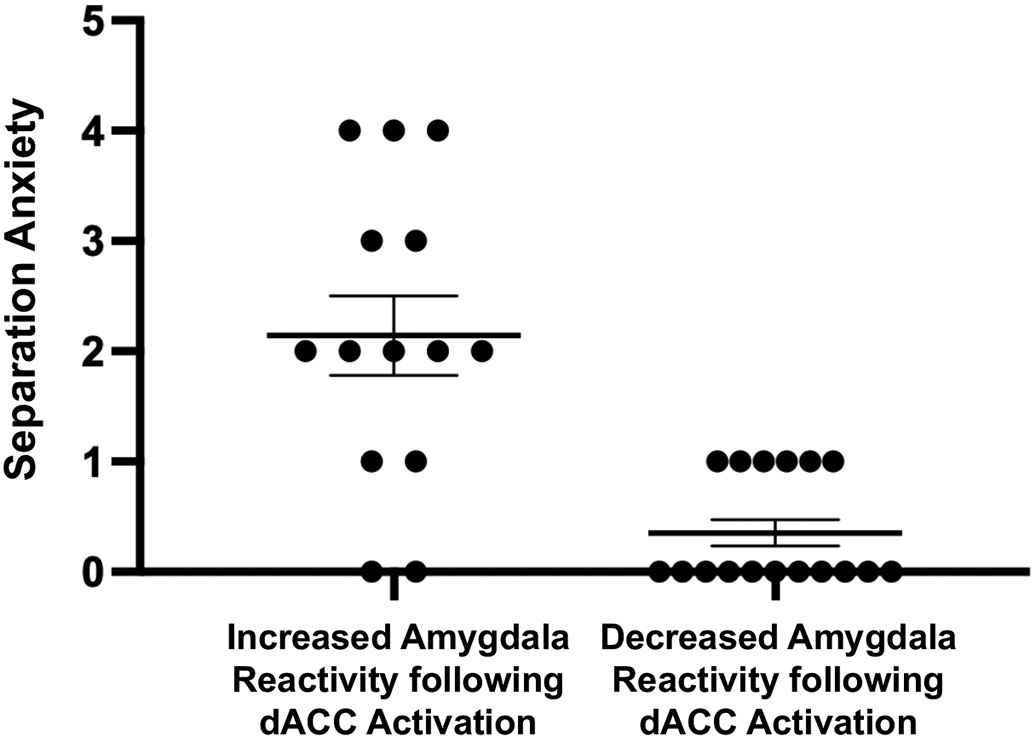
Individual differences in separation anxiety. Controlling for age, individuals who showed decreased amygdala reactivity after dACC activation (via cognitive conflict; i.e., decreased amygdala activation to faces immediately after the high conflict versus the low conflict manipulation) were those with lower normative separation anxiety (F(1,28)=17.92, p<.001, ηp2=0.39), relative to individuals who showed increased amygdala reactivity after dACC activation induced via cognitive conflict (i.e., increased amygdala activation to faces immediately after the high conflict versus low conflict conditions). Data presented as mean +/− 1 standard error of the mean.
DISCUSSION
Taken together, the identification of an age-related switch in frontoamygdala communication in the current cross-sectional pilot study suggests a shift from bottom-up to top-down frontoamygdala connectivity in human development, whereby the direction of information flow shifted from amygdala-to-prefrontal (vmPFC and dACC) in childhood to prefrontal-to-amygdala by late adolescence, with a transitional period demarcating this shift. This pattern of early bottom-up dominance is consistent with the increased amygdala reactivity (Gee et al., 2013b; Hwang et al., 2014; Silvers et al., 2015) and heightened normative anxiety (Gullone et al., 2001) that children typically exhibit. The obtained results add support to the hypothesis that amygdala influences during childhood identified in the present study may play an active role in shaping the development of and future function of frontoamygdala circuitry (Gabard-Durnam et al., 2016; Tottenham and Gabard-Durnam, 2017), consistent with prior evidence of bottom-up input shaping higher-order cortical development (Powell et al., 2018; Casey et al., 2019). Indeed, bottom-up projections from the amygdala to the vmPFC and dACC (Klavir et al., 2013) have been shown to be important for learning in the affective domain, which is especially relevant during early development. Predominance of amygdala influences in early life has vast implications for how environmental factors such as early-life stress may have cascading effects on the long-term regulatory function of prefrontal regions.
The period from 10-13 years of age was characterized by a less stable network of connections between the dACC, vmPFC, and amygdala, which may signal an important transition point characterized by reorganization in this circuit. Prior studies of frontoamygdala development in humans (Perlman and Pelphrey, 2011; Gee et al., 2013b; Gabard-Durnam et al., 2014, 2016; Silvers et al., 2015; Wu et al., 2016) have relied on correlations of BOLD timecourses and lacked experimental manipulation, precluding a more nuanced understanding of the circuitry’s development in humans. However, it is notable that the switch observed here occurred at a similar age (10 years old) as prior observations of changes in functional connectivity (Gee et al., 2013b; Gabard-Durnam et al., 2014, 2016; Hwang et al., 2014) both during task and at rest. Sensitive periods are posited to occur during times of substantial change in a network (Lupien et al., 2009); thus it may be that this period is marked by greater plasticity and openness to environmental influences.
Evidence from cross-species research provides important context for the observed age-related switch in frontoamygdala communication. The shift in directed influence from bottom-up to top-down connectivity in humans may be consistent with evidence from tracing studies in rodents that projections from the amygdala to the medial prefrontal cortex (mPFC; including regions encompassing both vmPFC and dACC) emerge earlier in development than the reciprocal projections from the mPFC to the amygdala (Bouwmeester et al., 2002b, 2002a; Cressman et al., 2010). Though fMRI research in humans cannot dissociate excitatory from inhibitory connections, recent evidence suggests that modulating prefrontal-amygdala functional connectivity is related to changes in GABA concentrations in the prefrontal cortex and anterior cingulate cortex (Zich et al., 2020), and optogenetic work in mice suggests that the age-related switch observed here may index a shift toward stronger prefrontal inhibition of the amygdala (Arruda-Carvalho et al., 2017). However, with regard to timing, the shift from bottom-up to top-down connectivity in humans appears to occur much later in development than in rodents. Whereas rodent models (Bouwmeester et al., 2002a, 2002b) suggest the switch would occur around the prenatal or infant period in humans, the identified switch around the transition from childhood to adolescence is consistent with prior evidence of protracted brain development in humans (Thompson-Schill et al., 2009; Callaghan and Tottenham, 2016) and may serve to extend a unique period of learning and openness to environmental influence in childhood. Similar mechanisms of protracted development have been theorized to contribute to experiential shaping in other domains. For example, protracted changes in the mPFC and its connectivity to subcortical regions might be important for developing face perception and shaping responses to social interactions early in life (Powell et al., 2018). Moreover, consistent with the current findings in the faces condition, prior work suggests that the spatial organization of circuitry involved in processing faces is present (Deen et al., 2017) and already right-lateralized early in life (Otsuka et al., 2007; de Heering and Rossion, 2015; Adibpour et al., 2018), though the developmental mapping of bottom-up versus top-down connectivity related to face perception remains unknown.
The focus of this study was on childhood and adolescence due to the marked changes in subcortical-cortical interactions during these stages of development (Casey et al., 2016, 2019; Heller et al., 2016; Murty et al., 2016; Silvers et al., 2017; Gee et al., 2018), with the transition from childhood to adolescence potentially having particular significance for frontoamygdala connectivity (Gee et al., 2013b, 2013a, 2016; Gabard-Durnam et al., 2016). However, future research that extends into adulthood and examines age continuously will also provide important information about frontoamygdala trajectories and whether the identified age-related changes are linear or non-linear. For example, though some prior studies suggest linear decreases in amygdala reactivity from childhood to adolescence and from adolescence to adulthood (Decety et al., 2012; Gee et al., 2013b; Hwang et al., 2014; Swartz et al., 2014; Vink et al., 2014; Silvers et al., 2015), we cannot infer from the current data that amygdala reactivity following high conflict would continue to decline into adulthood. Of note, the pattern of reduced amygdala reactivity following high conflict in adolescents (but not children) observed here is consistent with prior evidence of lower amygdala reactivity in the context of high (relative to low) conflict in adults (Etkin et al., 2006), suggesting that the adolescents but not children in our study exhibit more adult-like patterns of amygdala reactivity in the context of prefrontal engagement during cognitive conflict. Regarding differences in amygdala activation following high (relative to low) conflict between children and adolescents, it is possible that the high conflict task was more anxiety-provoking in children than adolescents. However, this possibility seems less likely given that the conflict manipulation operated similarly across children and adolescents (i.e., there was no condition x age interaction for accuracy, reaction time, or dACC activation to incongruent versus congruent words).
The complex nature of cognition-emotion interactions has been widely studied in adults, and cognitive engagement that recruits the dACC has been associated with reduced amygdala reactivity in adulthood (Pessoa et al., 2002a, 2002b; Mitchell et al., 2007, 2008), even after emotional stimuli have activated the amygdala (Van Dillen et al., 2009). Similar to these prior studies of adults, adolescents demonstrated decreased amygdala reactivity following dACC activation with high cognitive conflict. Despite similar dACC engagement, children showed increased amygdala reactivity. Though the relationship between cognitive load or conflict and emotional responding likely differs by task (e.g., Wagner and Heatherton, 2013), the present findings suggest that the nature of this relationship changes with age. In addition to the shift in connectivity between the dACC and amygdala, changing interactions between the vmPFC and amygdala with age may contribute to the difference in children’s amygdala reactivity following the cognitive conflict manipulation. In the present study, adolescents recruited the vmPFC when viewing emotional faces following high cognitive conflict, whereas children deactivated the vmPFC. In adulthood the vmPFC regulates amygdala reactivity during threat (Banks et al., 2007; Stein et al., 2007; Gold et al., 2015) and has dense connections with the amygdala (Aggleton et al., 1980; Barbas, 1995; Ghashghaei et al., 2007). These connections may emerge prior to adulthood, contributing to downregulation of the amygdala later in development. Consistent with this idea, we observed a developmental shift in information flow between the vmPFC and amygdala, such that children showed greater amygdala-to-vmPFC connectivity, whereas late adolescents exhibited greater vmPFC-to-amygdala connectivity.
Because of our specific hypothesis about the changing nature of interactions between prefrontal regions and the amygdala during development, the current study focused on functional interactions. The use of effective connectivity analyses provided unique insight into a possible transition from stronger bottom-up to stronger top-down influences within frontoamygdala circuitry. It is important to note that the use of fMRI precludes directly mapping bottom-up versus top-down projections between the amygdala and vmPFC (Ray and Zald, 2012), as has been performed in anatomical studies with rodents and non-human primates (Bouwmeester et al., 2002b, 2002a; Ghashghaei et al., 2007). However, the IMaGES method shows strong concordance with anatomical connectivity (Sun et al., 2012), and the developmental patterns observed here are highly consistent with findings in rodents (Bouwmeester et al., 2002b, 2002a; Arruda-Carvalho et al., 2017). Studies in humans that can examine covariation between changes in functional and structural connectivity will also provide valuable insight into frontoamygdala development. Moreover, our hypotheses focused on interactions between the amygdala with the vmPFC and with the dACC based on prior work (Bouwmeester et al., 2002b, 2002a; Gee et al., 2013b; Klavir et al., 2013; Gabard-Durnam et al., 2014), whereas we did not have specific hypotheses about connections between the vmPFC and dACC. Future work will be important for better understanding age-related changes in vmPFC-dACC connections and for clarifying the precise mechanisms that may drive developmental changes in top-down connections to the amygdala. As one example, tasks without a predetermined temporal order (i.e., in which one condition is not intended to influence responses to another condition) may lend further insight into the directionality of information flow between regions.
Our connectivity analyses employed a data-driven approach that was specifically designed for BOLD data, which has several methodological advantages. IMaGES is an empirically-validated (Mill et al., 2017) search-based algorithm that performs well in model simulations, does not require a pre-specified model, and reduces the risk of false positives by penalizing for overfitting (Ramsey et al., 2010, 2011). Importantly, the models are limited to unidirectional influence, and the present study cannot dissociate whether information flow in a specific connection is solely unidirectional or simply stronger in one of the directions. However, prior evidence suggests that studies of effective connectivity during human development can map changes in directed information flow (acyclic) associated with emotion regulation (Kadosh et al., 2016; Jiang et al., 2019; Jamieson et al., 2021), and we note that the discovery of an age-related switch in the dominance of information flow provides critical insight into frontoamygdala development regardless of whether that dominance reflects an underlying unidirectional or bidirectional connection. Though the sample size in the present pilot study is limited, the IMaGES approach is the only effective connectivity method to aggregate across participants, thus maximizing statistical power (i.e., the effective sample size is the number of timepoints x participants). Unlike dynamic causal modeling, IMaGES can fail to detect a graph and to produce significant edge directions. The identification of graphs, in combination with a modest sample size which could increase the risk of missing a true effect if it exists, may suggest that the approach here was sufficiently powered. The current sample size falls within, but on the lower end, of the range of sample sizes typical of past functional neuroimaging studies on frontoamygdala circuitry development (e.g., 18-273; Thomas et al., 2001; Hare et al., 2008; Todd et al., 2011; Decety et al., 2012; Qin et al., 2012; Gee et al., 2013b; Gabard-Durnam et al., 2014; Hwang et al., 2014; Swartz et al., 2014; Vink et al., 2014; Silvers et al., 2015, 2017; Spielberg et al., 2015; Heller et al., 2016; Wu et al., 2016; Jalbrzikowski et al., 2017; van Duijvenvoorde et al., 2019; Zich et al., 2020), and at the same time, testing these hypotheses in larger samples will be important for assessing the robustness of findings (e.g., Poldrack et al., 2017).
Although the current findings may provide novel insight into the dynamic nature of frontoamygdala circuitry during the transition from childhood to adolescence, various future directions will be essential to further testing an age-related switch in this circuit and its functional implications. The cross-sectional design precludes modeling within-subject trajectories. In addition, examinations of age-related effects focused on age groups instead of treating age continuously in the present study, and the connectivity and activation analyses examined distinct age groups, making it difficult to directly align the findings across functional activation and connectivity. Given that variance within individuals can be much higher than within groups (Fisher et al., 2018), longitudinal research will be necessary to confirm developmental changes in frontoamygdala connectivity. Future work employing this task would benefit from incorporating psychophysiological measures of arousal and self-reported ratings of anxiety to better understand possible age-related patterns in task effects, particularly as the number of trials with neutral faces was insufficient to examine behavioral responses during the faces blocks or to compare responses to fearful faces versus neutral faces. Given that analyses focused on affective stimuli more generally (i.e., aggregating across fearful and neutral faces), future work that examines age-related differences during tasks more specifically targeting affect learning or regulation will provide further insight into the role of frontoamygdala changes in emotional development. In addition, as examination of normative anxiety in the current study was limited to separation anxiety, additional research will be needed to clarify to what extent developmental changes in frontoamygdala circuitry relate to separation anxiety specifically or to other domains of anxiety. Lastly, given known sex differences in brain development (Giedd et al., 1997; Sowell et al., 2002; Lenroot et al., 2007) and potential influences of puberty on frontoamygdala development, a future study powered to test for sex differences in age-related changes and to dissociate age-related versus puberty-related changes (van Duijvenvoorde et al., 2019) would be beneficial for better understanding whether developmental changes in frontoamygdala connectivity differ between girls and boys and how hormonal changes might play a role in circuit maturation.
Frontoamygdala circuitry supports fundamental processes of human emotion that undergo dynamic changes across childhood and adolescence. Although the ontogeny of frontoamygdala circuitry has remained elusive, understanding the developmental construction of this circuitry is essential for identifying sensitive periods in socioemotional development and elucidating the neurobiological sequelae of early-life stress. Here we discovered evidence for an age-related switch in the nature of communication between the amygdala, vmPFC, and dACC during the transition from childhood to adolescence. The age-related shift from dominant amygdala-to-prefrontal (vmPFC and dACC) information flow in childhood to dominant prefrontal-to-amygdala information flow in adolescence suggests profound change that could shape the nature of emotional reactivity and regulation during human development.
Supplementary Material
RESEARCH HIGHLIGHTS.
Experimentally manipulating prefrontal engagement (specifically, dorsal anterior cingulate cortex activation) resulted in developmentally-opposite effects on amygdala reactivity during childhood versus adolescence
Bayesian network analyses revealed a shift from bottom-up to top-down connectivity around the transition from childhood (dominant amygdala-to-PFC connectivity) to adolescence (dominant PFC-to-amygdala connectivity)
Frontoamygdala connectivity related to differences in developmentally-normative anxiety, pointing to relevant behavioral consequences of the balance between bottom-up and top-down processes during development
We show an age-related switch for the first time in humans, which occurs much later in human development than a parallel switch in rodents
Funding statement:
This project was supported by grants from the Dana Foundation and the National Institute of Mental Health (R01MH091864) to NT, and grants from the Brain and Behavior Research Foundation (NARSAD Young Investigator Award), National Institutes of Health (NIH Director’s Early Independence Award; DP5OD021370), and American Psychological Foundation (Elizabeth Munsterberg Koppitz Child Psychology Fellowship, Harry and Miriam Levinson Scholarship) to DGG.
Footnotes
Conflict of interest disclosure: The authors have no conflicts of interest to disclose.
Ethics approval statement: All procedures were approved by the Institutional Review Board at the University of California, Los Angeles. Participants provided informed consent or assent (parental informed consent for minors).
Half of the participants (n=20) in the current study also participated in the Gee et al., 2013b study.
The complete absence of a connection between two nodes in a given graph indicates that no adjacency/connection was detected between those two regions in the first step; a connection represented with a dashed line indicates that an adjacency/connection was detected in the first step, but that no significant oriented edge was detected in the second step. Within the IMaGES framework, comparisons between task conditions can be made by comparing the resulting graphs. That is, if a connection is present versus absent or the directionality of a connection differs between two graphs, there is a significant difference (Ramsey et al., 2011)
Because the number of volumes censored correlated with vmPFC activation, we conducted a post-hoc analysis testing whether vmPFC activation to faces following high cognitive conflict versus faces following low cognitive conflict differed between children and adolescents while controlling for number of volumes censored. This age-related difference held, over and above the effect of number of volumes censored (F(1,37)=12.45, p=.001, ηp2=0.25). The finding was highly similar when non-linear motion terms were included in the model (with quadratic term for volumes censored in the model: F(1,36)=12.18, p=.001, ηp2=0.25; with cubic term for volumes censored in the model: F(1,35)=12.68, p=.001, ηp2=0.27).
References
- Achenbach TM (1991) Manual for Child Behavior Checklist 4-18, 1991 Profile. Univ Vermont/Dept Psychiatry. [Google Scholar]
- Adibpour P, Dubois J, Dehaene-Lambertz G (2018) Right but not left hemispheric discrimination of faces in infancy. Nat Hum Behav 2:67. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A (1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669–672. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE (1980) Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Res 190:347–368. [DOI] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Wu W-C, Cummings KA, Clem RL (2017) Optogenetic examination of prefrontal-amygdala synaptic development. J Neurosci:3097–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avino TA, Barger N, Vargas MV, Carlson EL, Amaral DG, Bauman MD, Schumann CM (2018) Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc Natl Acad Sci 115:3710–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Maas LC, Steingard RJ, Renshaw PF, Cohen BM, Yurgelun-Todd DA (1999) Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J Am Acad Child Adolesc Psychiatry 38:195–199. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL (2007) Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H (1995) Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev 19:499–510. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS (2009) Anxiety and Anxiety Disorders in Children and Adolescents: Developmental Issues and Implications for DSM-V. Psychiatr Clin North Am 32:483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Hariri AR, Alce G, Taurisano P, Sambataro F, Das S, Bertolino A, Weinberger DR, Mattay VS (2009) Preferential amygdala reactivity to the negative assessment of neutral faces. Biol Psychiatry 66:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H, Smits K, Van Ree JM (2002a) Neonatal development of projections to the basolateral amygdala from prefrontal and thalamic structures in rat. J Comp Neurol 450:241–255. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Wolterink G, van Ree JM (2002b) Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol 442:239–249. [DOI] [PubMed] [Google Scholar]
- Braem S, King JA, Korb FM, Krebs RM, Notebaert W, Egner T (2017) The Role of Anterior Cingulate Cortex in the Affective Evaluation of Conflict. J Cogn Neurosci 29:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR (1996) Response and Habituation of the Human Amygdala during Visual Processing of Facial Expression. Neuron 17:875–887. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998) The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum Brain Mapp 6:270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N (2016) The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology 41:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KLH, Angold A, Chen N-K, Copeland WE, Gaur P, Pelphrey K, Song AW, Egger HL (2015) Preschool Anxiety Disorders Predict Different Patterns of Amygdala-Prefrontal Connectivity at School-Age. PLOS ONE 10:e0116854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galván A, Somerville LH (2016) Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Dev Cogn Neurosci 17:128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Heller AS, Gee DG, Cohen AO (2019) Development of the emotional brain. Neurosci Lett 693:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Amaral DG, Lavenex P (2012) Postnatal development of the amygdala: A stereological study in macaque monkeys. J Comp Neurol 520:1965–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Moffitt CE, Gray J (2005) Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther 43:309–322. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY (2008) Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res Rev 58:57–70. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H (2010) Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol 518:2693–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Heering A, Rossion B (2015) Rapid categorization of natural face images in the infant right hemisphere Culham JC, ed. eLife 4:e06564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD (2012) The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cereb Cortex N Y N 1991 22:209–220. [DOI] [PubMed] [Google Scholar]
- Deen B, Richardson H, Dilks DD, Takahashi A, Keil B, Wald LL, Kanwisher N, Saxe R (2017) Organization of high-level visual cortex in human infants. Nat Commun 8:13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Beer JS, Fellows LK, Huettel SA, Platt ML, Quirk GJ, Schiller D (2016) Viewpoints: Dialogues on the functional role of the ventromedial prefrontal cortex. Nat Neurosci 19:1545–1552. [DOI] [PubMed] [Google Scholar]
- Di X, Huang J, Biswal BB (2017) Task modulated brain connectivity of the amygdala: a meta-analysis of psychophysiological interactions. Brain Struct Funct 222:619–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006) Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron 51:871–882. [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Medaglia JD, Jeronimus BF (2018) Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci U S A 115:E6106–E6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, Barale F, Perez J, McGuire P, Politi PL (2009) Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neurosci Lett 452:262–267. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N (2014) The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage 95:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Gee DG, Goff B, Flannery J, Telzer E, Humphreys KL, Lumian DS, Fareri DS, Caldera C, Tottenham N (2016) Stimulus-Elicited Connectivity Influences Resting-State Connectivity Years Later in Human Development: A Prospective Study. J Neurosci Off J Soc Neurosci 36:4771–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Bath KG, Johnson CM, Meyer HC, Murty VP, van den Bos W, Hartley CA (2018) Neurocognitive Development of Motivated Behavior: Dynamic Changes across Childhood and Adolescence. J Neurosci Off J Soc Neurosci 38:9433–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Fetcho RN, Jing D, Li A, Glatt CE, Drysdale AT, Cohen AO, Dellarco DV, Yang RR, Dale AM, Jernigan TL, Lee FS, Casey BJ, PING Consortium (2016) Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proc Natl Acad Sci U S A 113:4500–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, Tottenham N (2014) Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci 25:2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N (2013a) Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A 110:15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N (2013b) A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci Off J Soc Neurosci 33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H (2007) Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage 34:905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL (1997) Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry 21:1185–1201. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, Hamer RM, Styner M, Shen D (2012) Longitudinal Development of Cortical and Subcortical Gray Matter from Birth to 2 Years. Cereb Cortex 22:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Morey RA, McCarthy G (2015) Amygdala–Prefrontal Cortex Functional Connectivity During Threat-Induced Anxiety and Goal Distraction. Biol Psychiatry 77:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Goff B, Gee DG, Gabard-Durnam L, Flannery J, Telzer EH, Humphreys KL, Louie J, Tottenham N (2016) Discrimination of amygdala response predicts future separation anxiety in youth with early deprivation. J Child Psychol Psychiatry 57:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullone E, King NJ (1997) Three-year follow-up of normal fear in children and adolescents aged 7 to 18 years. Br J Dev Psychol 15:97–111. [Google Scholar]
- Gullone E, King NJ, Ollendick TH (2001) Self-reported anxiety in children and adolescents: a three-year follow-up study. J Genet Psychol 162:5–19. [DOI] [PubMed] [Google Scholar]
- Hadj-Bouziane F, Liu N, Bell AH, Gothard KM, Luh W-M, Tootell RBH, Murray EA, Ungerleider LG (2012) Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proc Natl Acad Sci 109:E3640–E3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ (2008) Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry 63:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR (2003) Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53:494–501. [DOI] [PubMed] [Google Scholar]
- Heller AS, Cohen AO, Dreyfuss MFW, Casey BJ (2016) Changes in cortico-subcortical and subcortico-subcortical connectivity impact cognitive control to emotional cues across development. Soc Cogn Affect Neurosci 11:1910–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer K, Lange W-G, Isaac L, Rinck M, Becker ES (2010) Morphed emotional faces: Emotion detection and misinterpretation in social anxiety. J Behav Ther Exp Psychiatry 41:418–425. [DOI] [PubMed] [Google Scholar]
- Humphrey T (1968) The development of the human amygdala during early embryonic life. J Comp Neurol 132:135–165. [DOI] [PubMed] [Google Scholar]
- Hwang S, White SF, Nolan ZT, Sinclair S, Blair RJR (2014) Neurodevelopmental changes in the responsiveness of systems involved in top down attention and emotional responding. Neuropsychologia 62:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Larsen B, Hallquist MN, Foran W, Calabro F, Luna B (2017) Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biol Psychiatry 82:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson AJ, Davey CG, Harrison BJ (2021) Differential Modulation of Effective Connectivity in the Brain’s Extended Face Processing System by Fearful and Sad Facial Expressions. eNeuro 8:ENEURO.0380-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Tian Y, Wang Z (2019) Causal Interactions in Human Amygdala Cortical Networks across the Lifespan. Sci Rep 9:5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS (2013) Effective Preprocessing Procedures Virtually Eliminate Distance-Dependent Motion Artifacts in Resting State FMRI. J Appl Math 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW (2010) Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage 52:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh KC, Luo Q, de Burca C, Sokunbi MO, Feng J, Linden DEJ, Lau JYF (2016) Using real-time fMRI to influence effective connectivity in the developing emotion regulation network. NeuroImage 125:616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ (2003) Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14:2317–2322. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ (2011) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O, Genud-Gabai R, Paz R (2013) Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron 80:1290–1300. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ (2012) Amygdala-prefrontal coupling underlies individual differences in emotion regulation. NeuroImage 62:1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN (2006) Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30:718–729. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN (2007) Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM (2007) Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci 18:421–428. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J (2003) Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry 44:64–87. [DOI] [PubMed] [Google Scholar]
- Mackey S, Petrides M (2014) Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci 40:2777–2796. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Carré JM, Thomason ME (2013) The stimuli drive the response: An fMRI study of youth processing adult or child emotional face stimuli. NeuroImage 83:679–689. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Zundel CG, Brown S, Rabinak CA, Thomason ME (2017) Convergent behavioral and corticolimbic connectivity evidence of a negativity bias in children and adolescents. Soc Cogn Affect Neurosci 12:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill RD, Bagic A, Bostan A, Schneider W, Cole MW (2017) Empirical validation of directed functional connectivity. NeuroImage 146:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JG, Ho TC, Humphreys KL, King LS, Foland-Ross LC, Colich NL, Ordaz SJ, Lin J, Gotlib IH (2020) Early Life Stress, Frontoamygdala Connectivity, and Biological Aging in Adolescence: A Longitudinal Investigation. Cereb Cortex N Y N 1991 30:4269–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DGV, Luo Q, Mondillo K, Vythilingam M, Finger EC, Blair RJR (2008) The interference of operant task performance by emotional distracters: An antagonistic relationship between the amygdala and frontoparietal cortices. NeuroImage 40:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DGV, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJR (2007) The impact of processing load on emotion. NeuroImage 34:1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Calabro F, Luna B (2016) The role of experience in adolescent cognitive development: Integration of executive, memory, and mesolimbic systems. Neurosci Biobehav Rev 70:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE (2002) Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14:1215–1229. [DOI] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB (2009) Heterogeneity in Subcortical Brain Development: A Structural Magnetic Resonance Imaging Study of Brain Maturation from 8 to 30 Years. J Neurosci 29:11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Y, Nakato E, Kanazawa S, Yamaguchi MK, Watanabe S, Kakigi R (2007) Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. NeuroImage 34:399–406. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, Deisseroth K, Lee FS (2016) Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun 7:11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J (2010) Maturation of the Hippocampal Formation and Amygdala in Macaca mulatta: A Volumetric Magnetic Resonance Imaging Study. Hippocampus 20:922–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA (2011) Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol 108:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2010) Emotion and Cognition and the Amygdala: From “what is it?” to “what’s to be done?” Neuropsychologia 48:3416–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG (2002a) Attentional control of the processing of neutral and emotional stimuli. Cogn Brain Res 15:31–45. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG (2002b) Neural processing of emotional faces requires attention. Proc Natl Acad Sci 99:11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Medina L, Hof PR, Desfilis E (2019) Neural architecture of the vertebrate brain: implications for the interaction between emotion and cognition. Neurosci Biobehav Rev 107:296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A (1988) A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 17:117–133. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR (2005) 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8:828–834. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004) Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48:175–187. [DOI] [PubMed] [Google Scholar]
- Powell LJ, Kosakowski HL, Saxe R (2018) Social Origins of Cortical Face Areas. Trends Cogn Sci 22:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, Menon V (2012) Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci 109:7941–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JD, Hanson SJ, Glymour C (2011) Multi-subject search correctly identifies causal connections and most causal directions in the DCM models of the Smith et al. simulation study. NeuroImage 58:838–848. [DOI] [PubMed] [Google Scholar]
- Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C (2010) Six problems for causal inference from fMRI. NeuroImage 49:1545–1558. [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH (2012) Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev 36:479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Grotegerd D, Opel N, Kaufmann C, Zwitserlood P, Kugel H, Heindel W, Donges U-S, Suslow T, Arolt V, Dannlowski U (2015) Are you gonna leave me? Separation anxiety is associated with increased amygdala responsiveness and volume. Soc Cogn Affect Neurosci 10:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD (2012) Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG (2004) The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci Off J Soc Neurosci 24:6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL (2008) The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32:811–830. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM (2016) Dorsal anterior cingulate cortex and the value of control. Nat Neurosci 19:1286–1291. [DOI] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, Petersen SE (2014) Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Hum Brain Mapp 35:1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, Weber J, Mischel W, Casey BJ, Ochsner KN (2017) vlPFC–vmPFC–Amygdala Interactions Underlie Age-Related Differences in Cognitive Regulation of Emotion. Cereb Cortex 27:3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN (2015) Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev Sci 18:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ (2004) Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry 55:897–903. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL (2002) Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol 44:4–16. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Jarcho JM, Dahl RE, Pine DS, Ernst M, Nelson EE (2015) Anticipation of peer evaluation in anxious adolescents: divergence in neural activation and maturation. Soc Cogn Affect Neurosci 10:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A (2007) A validated network of effective amygdala connectivity. NeuroImage 36:736–745. [DOI] [PubMed] [Google Scholar]
- Sun J, Hu X, Huang X, Liu Y, Li K, Li X, Han J, Guo L, Liu T, Zhang J (2012) Inferring consistent functional interaction patterns from natural stimulus FMRI data. NeuroImage 61:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS (2014) Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. NeuroImage 86:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ (2001) Amygdala response to facial expressions in children and adults. Biol Psychiatry 49:309–316. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Ramscar M, Chrysikou EG (2009) Cognition without control: When a little frontal lobe goes a long way. Curr Dir Psychol Sci 18:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RM, Evans JW, Morris D, Lewis MD, Taylor MJ (2011) The changing face of emotion: age-related patterns of amygdala activation to salient faces. Soc Cogn Affect Neurosci 6:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Gabard-Durnam L (2017) The developing amygdala: a student of the world and a teacher of the cortex. Curr Opin Psychol 17:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ (2011) Elevated amygdala response to faces following early deprivation. Dev Sci 14:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Phuong J, Flannery J, Gabard-Durnam L, Goff B (2013) A negativity bias for ambiguous facial-expression valence during childhood: Converging evidence from behavior and facial corrugator muscle responses. Emotion 13:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C (2009) The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H (2012) Developmental Trajectories of Amygdala and Hippocampus from Infancy to Early Adulthood in Healthy Individuals. PLOS ONE 7:e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J (2003) Ontogeny of the human amygdala. Ann N Y Acad Sci 985:22–33. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010) Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LF, Heslenfeld DJ, Koole SL (2009) Tuning down the emotional brain: an fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage 45:1212–1219. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Westhoff B, de Vos F, Wierenga LM, Crone EA (2019) A three-wave longitudinal study of subcortical-cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum Brain Mapp 40:3769–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS (2014) Functional differences in emotion processing during adolescence and early adulthood. NeuroImage 91:70–76. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF (2013) Self-regulatory depletion increases emotional reactivity in the amygdala. Soc Cogn Affect Neurosci 8:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir RK, Bauman MD, Jacobs B, Schumann CM (2018) Protracted dendritic growth in the typically developing human amygdala and increased spine density in young ASD brains. J Comp Neurol 526:262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S (2014) Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage 96:67–72. [DOI] [PubMed] [Google Scholar]
- Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA (2013) Ventromedial Prefrontal Cortex Encodes Emotional Value. J Neurosci 33:11032–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS, Phan KL (2016) Age-related changes in amygdala–frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp 37:1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N, Castellanos FX, Milham MP (2013) A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Rincón-Cortés M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, Yang Z, Gerum S, Biswal BB, Milham MP, Sullivan RM, Castellanos FX (2017) Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl Psychiatry 7:e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Andreotti C (2010) Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia 48:3377–3391. [DOI] [PubMed] [Google Scholar]
- Zich C, Johnstone N, Lührs M, Lisk S, Haller SP, Lipp A, Lau JY, Kadosh KC (2020) Modulatory effects of dynamic fMRI-based neurofeedback on emotion regulation networks in adolescent females. NeuroImage 220:117053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


