Abstract
Background:
There is controversy regarding the overall value of hepatocellular carcinoma (HCC) surveillance in patients with cirrhosis given a lack of randomized controlled data. To address this issue, we conducted a systematic review and meta-analysis of cohort studies evaluating benefits and harms of HCC surveillance in patients with cirrhosis.
Methods:
We performed a search of the Medline and EMBASE databases and national meeting abstracts from January 2014 through July 2020 for studies reporting early-stage HCC detection, curative treatment receipt, or overall survival, stratified by HCC surveillance status, among patients with cirrhosis. Pooled risk ratios and hazard ratios, according to HCC surveillance status, were calculated for each outcome using the DerSimonian and Laird method for random effects models.
Results:
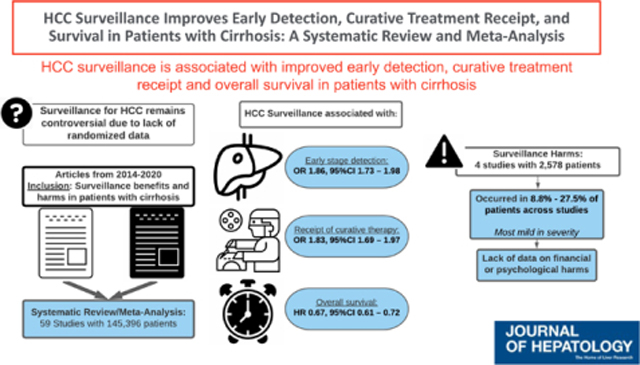
We identified 59 studies with 145,396 patients with HCC, of whom 41,052 (28.2%) were detected by surveillance. HCC surveillance was associated with improved early-stage detection (RR 1.86, 95%CI 1.73 – 1.98; I2=82%), curative treatment receipt (RR 1.83, 95%CI 1.69 – 1.97; I2=75%), and overall survival (HR 0.67, 95%CI 0.61 – 0.72; I2=78%) after adjusting for lead-time bias; however, there was notable heterogeneity in all pooled estimates. Four studies examined surveillance-related physical harms due to false positive or indeterminate surveillance results, but no studies examined potential financial or psychological harms. The proportion of patients experiencing surveillance-related physical harms ranged from 8.8% to 27.5% across studies, although most harms were mild in severity.
Conclusion:
HCC surveillance is associated with improved early detection, curative treatment receipt, and survival in patients with cirrhosis, although there was heterogeneity in pooled estimates. Available data suggest HCC surveillance is of high value in patients with cirrhosis, although continued rigorous studies evaluating benefits and harms are still needed.
Keywords: Screening, cirrhosis, liver cancer, early detection, ultrasound
Graphical Abstract
Lay Summary
There has been ongoing debate about the overall value of hepatocellular carcinoma (HCC) screening in patients with cirrhosis given a lack of randomized controlled data. In a systematic review of contemporary cohort studies, we found HCC screening is associated with improved early detection, curative treatment receipt, and survival in patients with cirrhosis, although there were fewer data quantifying potential screening-related harms. Available data suggest HCC screening is of high value in patients with cirrhosis, although continued studies evaluating benefits and harms are still needed.
INTRODUCTION
Hepatocellular carcinoma (HCC) is a leading cause of death in patients with compensated cirrhosis and one of the few cancers with a rising mortality rate.1 Despite improvements in therapeutic options for HCC, overall prognosis has remained dismal with a 5-year survival below 20%.1 The strongest driver of HCC prognosis is tumor stage, with curative options affording 5-year survival exceeding 60% for patients with early-stage HCC compared to a median survival of 1–2 years for those with more advanced tumor stages.2–4
Considering this association, society guidelines recommend semi-annual HCC surveillance in patients with cirrhosis using abdominal ultrasound, with or without alpha fetoprotein (AFP).3,4 This recommendation is supported by results from a randomized controlled trial in patients with hepatitis B virus (HBV) infection5, but similar level I evidence for surveillance does not exist in those with cirrhosis. Additionally, competing risk of liver-related mortality and impaired visualization due to liver nodularity can impact ultrasound efficacy in patients with cirrhosis, precluding direct extrapolation of data from HBV-infected patients.6,7 Cohort studies have suggested an association between HCC surveillance and improved survival; however, there are notable study limitations including residual confounding and lead- and length time biases.8,9 HCC surveillance benefits also require continued evaluation considering a shifting epidemiology from predominantly active viral hepatitis to an increasing proportion of patients with sustained virological response or nonalcoholic steatohepatitis (NASH), in whom ultrasound-based surveillance may be more prone to failure.10 The need for further data evaluating benefits of HCC surveillance was underscored when a case-control study from the Veterans Affairs health system failed to find an association between surveillance receipt and HCC-related mortality.11
In parallel, there is increasing recognition that the value of cancer screening programs must not only consider benefits but also physical, financial, and psychological harms.12 Data enumerating potential harms of breast and prostate cancer screening have created controversy about guideline recommendations13,14, highlighting a need for early evaluation of HCC surveillance-related harms. To address this need, we conducted a systematic review and meta-analysis of contemporary cohort studies evaluating the benefits and harms of HCC surveillance in patients with cirrhosis.
METHODS
Search Strategy
We conducted a computer-assisted search of the Medline and EMBASE databases to identify relevant articles published between January 1, 2014 through July 1, 2020 using the following keyword combinations: (liver ca$ OR hepatocellular ca$ OR hepatoma) AND (screen$ OR surveillance). We chose to include studies after January 2014 to update prior meta-analyses8,9 and reflect the current status of surveillance effectiveness. We performed manual searches of reference lists to identify citations that may have been missed by the computer-assisted search. Additional searches of AASLD, EASL, DDW, and ACG conference abstracts from 2014–2019 were performed. Finally, consultation with expert hepatologists was performed to identify additional references or unpublished data. This study was conducted in accordance with Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
Study Selection
One investigator (EZ) reviewed citations from the search strategy to generate a list of potentially relevant articles. If the applicability of a study could not be determined by title or abstract alone, the full text was reviewed. Full texts were independently checked for possible inclusion by a second investigator (AGS) and disagreements were resolved through discussion.
Studies were included if they (i) utilized abdominal imaging, with or without AFP, for surveillance; (ii) performed surveillance in a cohort of patients with cirrhosis from any etiology; and (iii) reported the number of HCC detected at an early stage (regardless of staging system), number of HCC patients who received curative therapies, and/or overall survival, stratified by surveillance receipt. If a study included patients with and without cirrhosis, only data regarding patients with cirrhosis were extracted when possible. We excluded studies that only reported outcome measures for patients undergoing surveillance but not for those without surveillance. Additional exclusion criteria included non-human data, lack of original data, non-English studies, and incomplete reports. If duplicate publications used the same cohort of patients, the study with more complete data was included.
Data Extraction and Quality Assessment
Two investigators (EZ and AGS) independently extracted required information from eligible studies using standardized forms. Discrepancies were resolved via discussion, with a third investigator (NR) as needed. The data extraction form included the following: characteristics and size of the cohort, inclusion and exclusion criteria, surveillance tests, surveillance interval, and definition of early-stage HCC. We recorded the following data, stratified by surveillance receipt: number of patients with HCC, proportion of HCC detected at an early stage, proportion of patients who received curative treatments, and overall survival. In most studies, early-stage HCC was defined using the Barcelona Clinic Liver Cancer (BCLC) staging system, and curative treatments included liver transplantation, surgical resection, or local ablative therapy (LAT). Two investigators (EZ and AGS) assessed study quality by a modified checklist based upon the National Institute of Health (NIH) study quality assessment tool for observational cohort studies, with discrepancies resolved by discussion with a third investigator (NR).
Statistical Analysis
For each study, we calculated a risk ratio (RR) with the exposure being surveillance receipt and clinical outcomes being proportion of patients detected at an early stage, proportion who underwent curative treatment, overall survival, and surveillance-related harms. For overall survival, we abstracted an adjusted hazards ratio (HR) for mortality when available; if not reported, we recorded median survival for both groups. For surveillance-related harms, we recorded the proportion of patients with physical, financial, or psychological harms related to surveillance from each study – as defined by an established nomenclature.15 Physical harm is typically defined as any diagnostic testing related to false positive or indeterminate surveillance results, which can be classified as mild (one diagnostic CT or MRI), moderate (repeated diagnostic CT or MRI), or severe (any invasive evaluation such as biopsy). Financial harms include direct costs of screening and diagnostic evaluation plus indirect costs such as missed work. Psychological harms can occur at any step of the screening process and include anticipation or fear of abnormal results, cancer-specific worry, or reactions of depression after positive results.
We calculated a pooled risk ratio estimate with corresponding 95% confidence intervals for early-stage HCC detection and curative treatment receipt and pooled hazard ratio estimate for overall survival, adjusted for lead time bias, using the DerSimonian and Laird method for random effects models. Heterogeneity was evaluated graphically by examination of forest plots and statistically by the chisquared test of heterogeneity and the inconsistency index (I2).16 Values of <25%, 25–75% and >75% were considered as low, moderate, and high heterogeneity, respectively. We performed sensitivity analyses, in which outliers were removed, to determine if this impacted pooled effect estimates. Pre-planned subgroup analyses were performed for: (i) type of publication (full length publication versus conference abstract), (ii) location of study (Asia versus Europe versus United States), (iii) study period (cohort initiation prior to 2000 versus between 2000 – 2005 versus after 2005), (iv) study size (<200 patients versus 200–500 patients versus >500 patients), (v) inclusion of any patients without cirrhosis, (vi) surveillance modality (ultrasound alone versus ultrasound + AFP versus any abdominal imaging), and (vii) length of surveillance interval (semi-annual versus longer intervals versus surveillance-detected).
Thresholds for study period dates (i.e., 2000 and 2005) were selected based on publication dates of prior guidelines.17,18 We also performed a post-hoc subgroup analysis by overall study quality, dichotomized at the median quality score. Publication bias was evaluated graphically using funnel plot analysis and then statistically using Egger’s test. We evaluated the potential effect of publication bias on pooled estimates using the trim-and-fill method.19 All data analysis was conducted using Stata version 11 (StataCorp, College Station TX).
RESULTS
Study Characteristics
The computer-assisted literature search yielded 8872 potentially relevant titles published between January 2014 and July 2020, of which 38 met inclusion criteria after full-text review. A recursive literature search and consultation with experts identified two additional articles and searches of annual meeting abstracts yielded 22 relevant abstracts, resulting in a total of 62 studies for inclusion – 58 studies for HCC surveillance benefits alone, three for HCC harms alone, and one for both (Supplemental Table 1, Supplemental Figure 1).
Characteristics of studies evaluating HCC surveillance benefits are described in Supplemental Table 1. Fifty-nine studies, with 145,396 patients with HCC, assessed the impact of surveillance on at least one outcome of interest. Fifteen studies were conducted in North America, 21 in Europe, 14 in Asia, and 9 elsewhere (four Australia, two New Zealand, two South America, and one Morocco). All but six were retrospective, and most cohorts were diverse in terms of liver disease etiology. Overall, 41,052 (28.2%) patients had HCC detected by surveillance and 104,596 (71.8%) presented symptomatically or incidentally. HCC was detected by surveillance in 14.0% (2692 of 19181) of patients among studies in North America, 40.8% (3033 of 7431) in Europe, 29.2% (33,916 of 116,109) in Asia, and 52.7% (1411 of 2675) of those from other countries.
Early Detection and Curative Treatment Receipt
Forty-nine studies, with a total of 35,104 HCC patients, included data on tumor stage stratified by receipt of HCC surveillance. Most studies (n=27) defined early-stage HCC using BCLC stage 0/A, whereas nine used the Milan criteria and 11 used other staging systems (e.g., tumor node metastases [TNM]); two studies provided data on early-stage detection but did not detail what staging system was used (Supplemental Table 1). Patients who underwent surveillance were more likely to have HCC diagnosed at an early stage (RR 1.86, 95%CI 1.73 – 1.98) (Figure 1); however, there was significant heterogeneity (I2=82%, p<0.001). Although we identified outlier studies on inspection of the forest plots (e.g., Al Hasani, Branch, Eskesen, Sonovane, Wong), we did not find clinical heterogeneity justifying their exclusion. The trim-and-fill method imputed 12 studies to account for publication bias and the pooled estimate of association between surveillance and early detection was unchanged. There was also little change in effect size and heterogeneity when only including studies that defined early-stage as BCLC stage 0/A or within Milan criteria, (RR 1.92, 95%CI 1.74 – 2.09, I2=85%) or those that defined early-stage using BCLC stage 0/A alone (RR 1.99, 95%CI 1.73 – 2.25, I2=87%). The pooled proportion of early-stage detection among patients undergoing surveillance was 66.9% (95% CI 66.0 – 67.8%), compared to only 33.1% (95%CI 32.5 – 33.7%) among those who presented symptomatically or incidentally (Table 1). When restricted to studies that defined early-stage HCC as BCLC 0/A, pooled proportions of early-stage detection were 58.8% (95%CI 57.3 – 60.2%) for surveillance-detected and 27.0% (95%CI 26.0 – 28.1%) for non-surveillance detected. Results were consistent in all pre-planned subgroup analyses according to location of study, study period, type of surveillance tests, surveillance interval, and study size, although high heterogeneity continued to be observed. Improved early tumor detection by surveillance receipt was consistent among studies across study locations: RR 1.85 [95%CI 1.57–2.18] in North America, 1.91 [95%CI 1.67–2.16] in Europe, 2.07 [95%CI 1.83–2.33] in Asia, and 1.63 [95%CI 1.26 −2.09] elsewhere, with I2>70% for all subgroups. Surveillance was associated with early-stage detection among the 17 studies using ultrasound alone (RR 1.87, 95%CI 1.62 – 2.12, I2=88%) and 15 studies using ultrasound with or without AFP (RR 2.21, 95%CI 1.90 – 2.57, I2=81%). Finally, surveillance was associated with early-stage detection among studies classified as low risk of bias (RR 1.92, 95%CI 1.77 – 2.10, I2=87%) and those at higher risk of bias (RR 1.78, 95%CI 1.51 – 2.04, I2=75%).
Figure 1. Association Between HCC Surveillance and Early Tumor Detection.
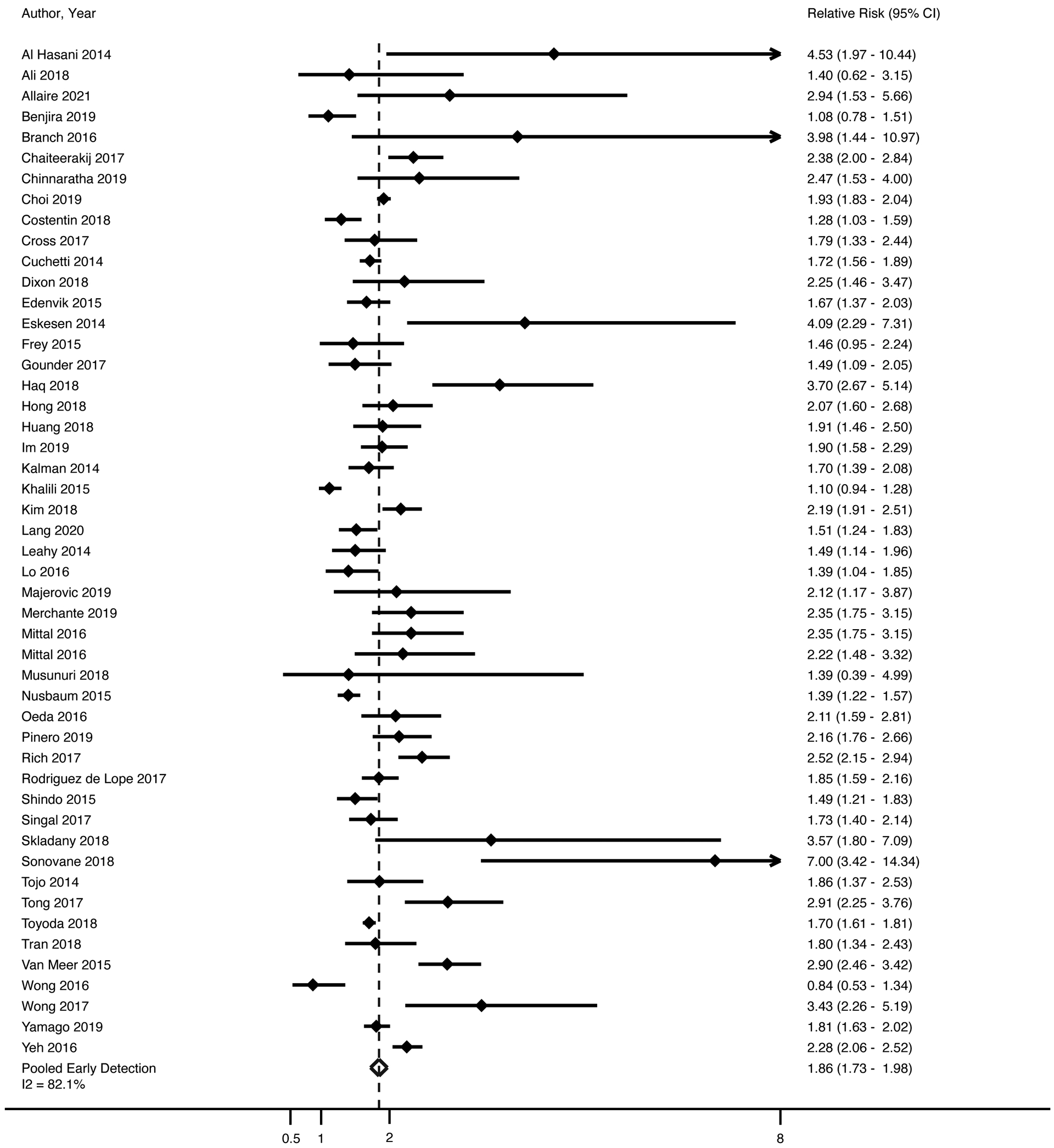
Patients who underwent surveillance were significantly more likely to have HCC diagnosed at an early stage (OR 1.94, 95% CI 1.80 – 2.08); however, there was significant heterogeneity (I2=84%, p<0.001). DerSimonian and Laird method was used for a random effects model.
Table 1:
Clinical outcomes, stratified by surveillance receipt
| Author Year | Proportion of Patients with Early HCC | Proportion of Patients with Curative Treatment | Factors adjusted for in survival analysis | Overall Survival |
|---|---|---|---|---|
| Aby 2019 | NR | 15/29 vs. 131/232 | Not applicable | NR |
| Al Hasani 2014 | 32/48 vs. 5 /34 | NR | Not applicable | NR |
| Ali 2018 | 14/15 vs. 2/3 | NR | Not applicable | NR |
| Allaire 2021 | 39/68 vs. 8/41 | 51/68 vs. 11/41 | None | Median survival 60 vs. 14 months* (p=0.01) |
| Asad 2019 | NR | 5/15 vs. 0/20 | None | 1-year survival 73% vs. 50% (p<0.05) |
| Benjira 2019 | 26/43 vs. 33/59 | 21/43 vs. 9/59 | Not applicable | NR |
| Branch 2016 | 23/27 vs. 3/14 | NR | None | 3-year survival 60% vs. 62% (p=0.30) |
| Chaiteerakij 2017 | 83/103 vs. 116/343 | 76/103 vs. 154/343 | Lead time, demographics, liver disease etiology, BCLC, Child Pugh, MELD, AFP level | HR 0.57 (95%CI 0.43 – 0.76) |
| Chinnaratha 2019 | 14/24 vs. 25/106 | 15/24 vs. 33/106 | BCLC, MELD, AFP level | HR 0.63 (95%CI 0.28 – 1.42) |
| Choi 2019 | 596/937 vs. 4215/12777 | NR | Lead time, demographics, etiology | HR 0.75 (95%CI 0.69 – 0.82) |
| Clegg 2014 | NR | 6/25 vs. 9/121 | None | 3-year survival 20% vs. 8.2% (p<0.05) |
| Costentin 2018 | 92/129 vs. 48/86 | 91/129 vs. 44/86 | Lead time, demographics, liver disease etiology, hepatic decompensation, bilirubin, PT, AFP level | HR 0.46 (95%CI 0.24 – 0.86) |
| Cross 2017 | OR 1.79 (1.33 – 2.44) | OR 1.54 (1.37 – 1.85) | Not applicable | NR |
| Cuchetti 2014 | 689/850 vs. 250/530 | 511/850 vs. 216/530 | Lead time | Median survival 41.6 vs. 28.5 months |
| Debes 2018 | OR 2.22 (1.43 – 3.48) | Lead time, liver disease etiology, cirrhosis, AFP level, curative treatment | HR 0.62 (95%CI 0.48 – 0.78) | |
| Demma 2016 | NR | 55/108 vs. 20/97 | NR | HR 0.45 (95%CI 0.30 – 0.66) |
| Dixon 2018 | 17/25 vs. 23/76 | 6/25 vs. 9/76 | None | 3-year survival 22.2% vs. 8.2% |
| Edenvik 2015 | 92/134 vs. 87/211 | 80/134 vs. 79/211 | None | Median survival 34 vs. 21 months* (p<0.05) |
| Eskesen 2014 | 9/19 vs. 30/259 | 11/19 vs. 36/259 | None | Mean survival 31 vs. 11 months (p<0.001) |
| Frey 2015 | 12/16 vs. 18/35 | 11/16 vs. 12/35 | Not applicable | NR |
| Gounder 2017 | 18/22 vs. 28/51 | NR | Not applicable | NR |
| Haq 2018 | 62/160 vs. 50/478 | 52/160 vs. 41/478 | None | Mean survival 32 vs. 15 months (p<0.001) |
| Hassan 2016 | NR | NR | None | Median survival 12.8 vs. 9.3 months (p=0.001) |
| Hong 2018 | 75/110 vs. 52/158 | 59/110 vs. 40/158 | Demographics, liver disease etiology, Child Pugh, BCLC, AFP level, curative treatment | HR 0.60 (95%CI 0.38 – 0.93) |
| House 2014 | NR | 20/45 vs. 3/9 | Not applicable | NR |
| Huang 2018 | 81/128 vs. 47/142 | 80/128 vs. 43/128 | Demographics, cirrhosis, liver disease etiology | HR 0.52 (95%CI 0.35 – 0.76) |
| Im 2019 | 102/127 vs. 81/192 | Used data per Kwon 2020 | Used data per Kwon 2020 | Used data per Kwon 2020 |
| Kalman 2014 | 48/56 vs. 65/129 | NR | Not applicable | NR |
| Khalili 2015 | 87/109 vs. 67/92 | NR | Not applicable | NR |
| Kim 2018 | 537/834 vs. 167/568 | Used data per Kwon 2020 | Used data per Kwon 2020 | Used data per Kwon 2020 |
| Kwon 2020 | NR | OR 2.58 (2.27 – 2.94) | Lead time, age, sex, cirrhosis, CCI, income | HR 0.76 (95%CI 0.71 – 0.82) |
| Lang 2020 | 71/111 vs. 123/290 | 86/111 vs. 137/290 | Cirrhosis, liver dysfunction, age | HR 0.90 (95%CI 0.69 – 1.19) |
| Leahy 2014 | 61/96 vs 43/101 | 39/96 vs. 15/101 | None | 3-year survival 65% vs. 55%; HR 0.59, p=0.08 |
| Lo 2016 | 16/23 vs. 21/70 | NR | Not applicable | NR |
| Majerovic 2019 | 12/23 vs. 14/57 | NR | Not applicable | NR |
| Merchante 2019 | 79/186 vs. 49/160 | NR | None | Median survival 13 vs. 4 months (p<0.001) |
| Mittal 2016 | 112/412 vs. 55/475 | 86/412 vs. 53/475 | Lead time, age, comorbidity, liver disease etiology, BCLC, MELD, treatment, AFP level | HR 0.92 (95%CI 0.79 – 1.07) |
| Mittal 2016 | 71/94 vs. 17/50 | NR | Lead time | Median survival 72.2 vs. 45 months (p=0.14) |
| Musunuri 2018 | 7/52 vs. 3/31 | NR | None | Median survival 9 vs. 6 months (p=0.001) |
| Nusbaum 2015 | 116/126 vs. 101/162 | 80/131 vs. 57/174 | Age, sex, race, insurance, etiology, stage, treatment | HR 0.66 (95%CI 0.43 – 0.99) |
| Oeda 2016 | 156/226 vs. 35/107 | 174/226 vs. 44/107 | Lead time | Median survival 56.5 vs. 31.4 months (p=0.011) |
| Pinero 2019 | 244/345 vs. 68/208 | NR | Lead time, age, BCLC, AFP | HR 0.51 (95%CI 0.38 – 0.69) |
| Rich 2017 | 238/359 vs. 151/573 | 255/359 vs. 158/573 | Demographics, Child Pugh, ECOG | HR 0.52 (95%CI 0.43 – 0.62) |
| Rodriguez de Lope 2017 | 221/311 vs. 133/347 | 169/316 vs. 112/356 | Not applicable | NR |
| Schauer 2019 | NR | 147/224 vs. 81/286 | Demographics, treatment | HR 0.70 (95%CI 0.54 – 0.91) |
| Shindo 2015 | 80/93 vs 45/78 | 64/93 vs 34/78 | Liver disease etiology, tumor stage, AFP-L3 and DCP levels, curative treatment | HR 0.22 (95%CI 0.06 – 8.26) |
| Sigurdsson 2019 | NR | NR | None | Median survival 17.1 vs. 4.5 months (HR 0.47, p=0.008) |
| Singal 2017 | 99/157 vs. 79/217 | 48/157 vs. 28/217 | Lead time, demographics, Child Pugh, Milan, ECOG, GI care | HR 0.59 (95%CI 0.37 – 0.93) |
| Skladany 2018 | 15/49 vs. 12/140 | NR | Not applicable | NR |
| Sonovane 2018 | 16/24 vs. 8/84 | None | HR 0.29, p=0.002 | |
| Thein 2015 | NR | 11/17 vs. 677/1466 | Lead time, demographics, residence, comorbidity, Child Pugh, ECOG, treatment | HR 0.76 (95%CI 0.64 – 0.91) |
| Tojo 2014 | 20/24 vs. 34/76 | NR | Not applicable | NR |
| Tong 2017 | 145/175 vs. 45/158 | 110/175 vs. 46/158 | Lead time | Median survival 40.5 vs. 14.5 months (p<0.001) |
| Toyoda 2018 | 1570/2108 vs. 783/1791 | 1408/2108 vs. 836/1791 | Lead time, age, Child Pugh, etiology | HR 0.60 (95%CI 0.55 – 0.66) |
| Tran 2018 | 106/151 vs. 30/77 | 66/151 vs. 18/77 | Demographics, Child Pugh, Milan, curative treatment | HR 0.34 (95%CI 0.16 – 0.72) |
| Van Meer 2015 | 179/295 vs. 163/779 | 167/295 vs. 253/779 | Lead time, age, liver disease etiology, cirrhosis, MELD, ECOG, symptoms | HR 0.51 (95%CI 0.39 – 0.67) |
| Wong 2016 | 6/8 vs. 8/9 | NR | Not applicable | NR |
| Wong 2017 | 54/91 vs. 22/127 | 47/91 vs. 36/127 | None | Median survival 29.2 vs. 14.6 months (p<0.001) |
| Wu 2016 | NR | 2.13 (2.00 – 2.22) | Lead time, demographics, etiology, cirrhosis, comorbidity, GI care | HR 0.66 (95%CI 0.64 – 0.68) |
| Yamago 2019 | 326/398 vs. 214/474 | 332/398 vs. 214/474 | None | Median survival 68.2 vs. 34.1 months (p<0.001) |
| Yeh 2016 | 162/194 vs. 402/1098 | NR | Not applicable | NR |
Median survival estimated from Kaplan Meier curves
AFP – alpha fetoprotein; BCLC – Barcelona Clinic Liver Cancer; CCI – Charlson Comorbidity Index; ECOG – Eastern Cooperative Oncology Group; GI – Gastroenterology; MELD – Model for End-Stage Liver Disease; NR = not reported
Thirty-nine studies, comprised of 86,466 HCC patients, assessed the association between HCC surveillance and receipt of curative therapy. Of included patients, 18,762 (21.7%) were detected by surveillance and 67,704 (78.3%) presented symptomatically or incidentally. Patients diagnosed by surveillance were more likely to undergo curative therapy, with a pooled risk ratio of 1.83 (95%CI 1.69 – 1.97), although there was high heterogeneity (I2=75%, p<0.001) (Figure 2). Similar to early detection analyses, we did not identify clinical heterogeneity justifying exclusion of outlier studies seen on forest plots (e.g., Aby, Asad, Eskesen). The trim-and-fill method imputed 25 studies but the pooled estimate for association between surveillance and curative treatment was unchanged. The pooled rate of curative treatment receipt among patients undergoing surveillance was 58.2% (95%CI 57.1 – 59.3%), compared to 34.0% (95%CI 33.1% – 34.9%) among those who presented outside of surveillance (Table 1). Patients detected by surveillance were significantly more likely to undergo curative treatment across all pre-planned subgroup analyses. The pooled RRs of curative treatment receipt were 1.85 (95%CI 1.37 – 2.33) for studies in North America, 1.69 (95%CI 1.53 – 1.85) in Europe, 1.82 (95%CI 1.51 – 2.12) in Asia and 2.12 (95%CI 1.84 – 2.41) for elsewhere, with I2>70% for all subgroups except elsewhere (I2=0%). Surveillance was associated with curative treatment receipt among the 11 studies using ultrasound alone (RR 1.65, 95%CI 1.49 – 1.81, I2=44%) and the 12 studies using ultrasound with or without AFP (RR 1.99, 95%CI 1.67 – 2.30, I2=84%). Finally, surveillance was associated with curative treatment among studies classified as low risk of bias (RR 1.87, 95%CI 1.71 – 2.04, I2=79%) and those at higher risk of bias (RR 1.75, 95%CI 1.45 – 2.04, I2=63%).
Figure 2. Association Between HCC Surveillance and Curative Treatment Receipt.
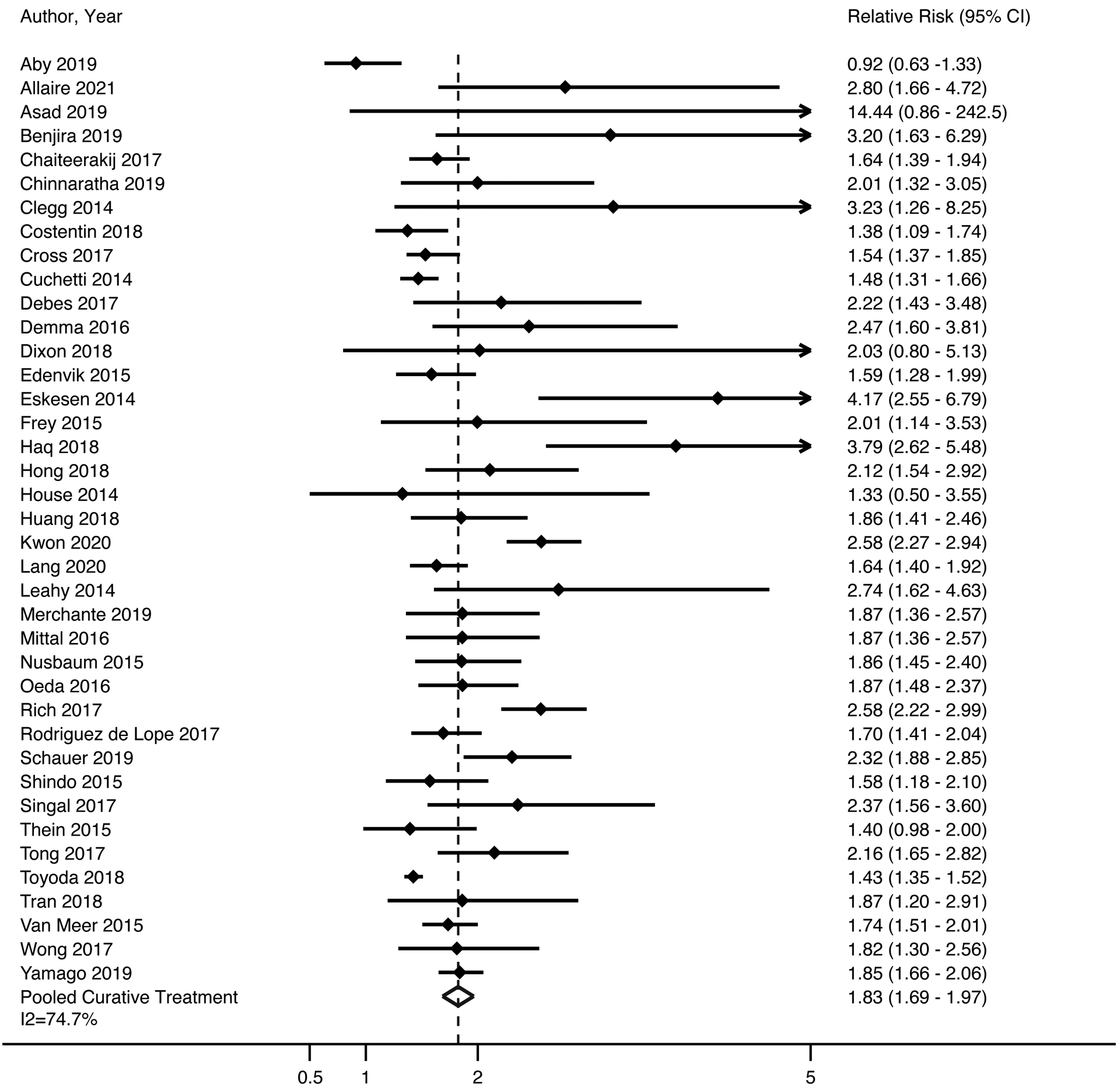
Patients diagnosed by surveillance were significantly more likely to undergo curative therapy, with a pooled odds ratio of 1.83 (95%CI 1.69 – 1.97), although there was high heterogeneity among studies (I2=75%, p<0.001). DerSimonian and Laird method was used for a random effects model.
Overall Survival
Forty-two studies, consisting of 141,522 HCC patients (27.7% [n=39,139] detected via surveillance), included data on survival stratified by receipt of HCC surveillance. There was variability in reporting of survival data, with 22 studies reporting hazard ratios with 95% confidence intervals, 14 reporting median or mean survival, five reporting 1- or 3-year survival, and one reporting hazard ratios without confidence intervals (Supplemental Table 1). All but one study that reported median, 1-, and 3-year survival demonstrated improved survival among surveillance versus non-surveillance patients (Table 1). Of 22 studies with hazard ratios and 95% confidence intervals, seven were from North America, four from Europe, five from Asia, and six from Australia or South America. Among these studies (n=134,345 patients of whom 36,231 were surveillance-detected), HCC surveillance was significantly associated with improved survival, with a pooled hazard ratio of 0.64 (95%CI 0.59 – 0.69); however, we observed high heterogeneity (I2=72%).
Among 12 studies that adjusted for lead time bias when assessing the association between HCC surveillance and survival (Table 1), surveillance remained associated with improved survival (HR 0.67, 95%CI 0.61 – 0.72 I2=78%) (Figure 3). The trim-and-fill method imputed 3 studies but the pooled estimate for the association between surveillance and overall survival was unchanged (HR 0.70, 95%CI 0.63 – 0.77). There was also a consistent association between surveillance and improved survival across all subgroup analyses. Surveillance was associated with improved survival among studies from North America (HR 0.77, 95%CI 0.72 – 0.82, I2=53%), Europe (HR 0.50, 95%CI 0.37 – 0.63, I2=0%), Asia (HR 0.66, 95%CI 0.65 – 0.68, I2=84%), and elsewhere (HR 0.57, 95%CI 0.46 – 0.67, I2=0%). Surveillance was associated with improved survival among the studies using ultrasound alone (HR 0.67, 95%CI 0.65 – 0.68, I2=68%) versus ultrasound with or without AFP (HR 0.74, 95%CI 0.69 – 0.80, I2=66%) as well as studies using shorter (HR 0.66, 95%CI 0.64 – 0.68, I2=61%) versus longer (HR 0.74, 95%CI 0.71 – 0.78, I2=77%) intervals.
Figure 3. Association Between HCC Surveillance and Overall Survival.
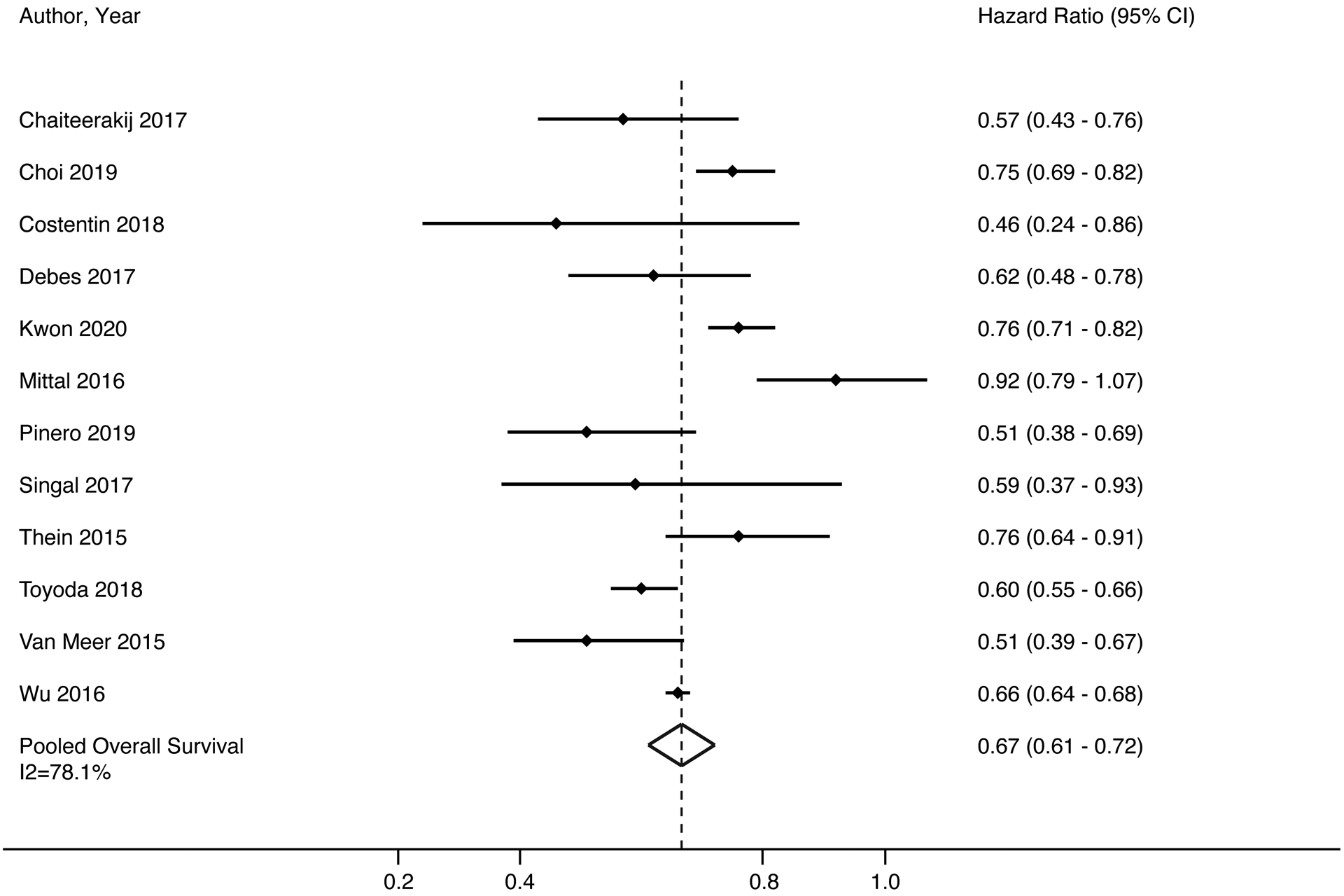
HCC surveillance was significantly associated with improved survival, with a pooled hazard ratio of 0.66 (95%CI 0.61 – 0.71); however, there was high heterogeneity (I2=75%, p<0.001). DerSimonian and Laird method was used for a random effects model.
Emerging Surveillance Populations
Only seven studies differentiated post-SVR and actively viremic patients when describing patients with hepatitis C infection. One study specifically examined the association between surveillance and clinical outcomes in post-SVR patients with cirrhosis,20 while another included >90% post-SVR patients.21 Branch and colleagues reported a significant association with early-stage detection but no difference in 3-year survival between surveillance-detected patients and those who presented symptomatically.20 In contrast, Costentin reported surveillance was significantly associated with improved early-stage detection, curative treatment receipt and overall survival, even after adjusting for lead-time bias.21 Post-SVR patients accounted for less than 10% of cohorts for the other five studies in which data were available.
While several studies reported the proportion of NAFLD etiology in study demographics, only two studies examined the association between surveillance and clinical outcomes among those with NAFLD. Lo and colleagues reported a significant association with early-stage detection (69.6% vs. 30%, p=0.001)22 whereas Aby et al. failed to find an association with curative treatment receipt (45.5% vs. 51.5%, p=0.72).23 In subgroup analyses by the proportion of NAFLD patients in each study (<10%, 10–20, and >20%), we found similar point estimates for the association between surveillance and early-stage detection (RR 1.86, 2.23, and 2.04, respectively) and curative treatment receipt (RR 1.79, 2.06, and 2.02, respectively). HCC surveillance was also associated with improved survival in studies with <10% NAFLD (HR 0.75, 95%CI 0.61 – 0.89, I2=72%) and 10–20% NAFLD (HR 0.53, 95%CI 0.45 – 0.61, I2=0%). Studies with >20% NAFLD patients did not report survival data using hazard ratios and 95% confidence intervals; however, each study reported improved survival. For example, Clegg and colleagues reported 3-year survival of 20% vs. 8.2% for surveillance-detected vs. others,24 and Sigurdsson reported median survivals of 17.1 and 4.5 months, respectively.25
Differences in Benefits by Surveillance Exposure
Fifteen studies, including 27705 HCC patients, assessed surveillance outcomes, stratified by surveillance exposure, with six studies assessing intervals shorter versus longer than 6–9 months, four assessing intervals shorter versus longer than 12 months, and five comparing semi-annual versus annual surveillance (Supplemental Table 2). There was a consistent association between shorter surveillance intervals and early detection across the nine studies with applicable data (pooled RR 1.38, 95%CI 1.32 – 1.44, I2=84%). However, data were conflicting for curative treatment receipt, with six studies suggesting no significant association and four demonstrating higher curative treatments with shorter intervals (pooled RR 1.11, 95%CI 0.98 – 1.27, I2=75%). Eleven studies assessed overall survival by surveillance exposure, with most demonstrating greater survival benefit with shorter surveillance intervals.
Surveillance-related Harms
We identified four studies, including 2578 patients with cirrhosis, that characterized surveillance-related harms. All studies only reported physical harms, with no studies evaluating potential financial or psychological harms. Atiq et al. evaluated surveillance and benefits and harms in 680 patients with cirrhosis undergoing surveillance over a 3-year period.26 Although surveillance-related physical harms were observed in 187 (27.5%) patients, most cases were mild in severity. Sixty-six (9.7% of the cohort) patients experienced moderate harm, and three (0.4% of the cohort) patients experienced severe harm, such as diagnostic biopsy. The proportion experiencing physical harm increased from 11.9% among those with one surveillance exam to 29.6% among those with two or more exams. Konerman and colleagues evaluated 999 patients in a surveillance program over a median of 2.2 years.27 Of 256 patients with abnormal surveillance ultrasound, 69 were diagnosed with HCC. Of the 187 false positive results, 87 underwent one CT or MRI (mild harm), 77 repeat CT/MRI imaging evaluation (moderate harm), and five underwent biopsy (severe harm). Eighteen patients were followed with ultrasound-based surveillance without evidence of HCC and classified as no surveillance-related harm. Therefore, moderate-severe harm was observed in 8.2% of the cohort. In a cohort of 285 patients undergoing surveillance ultrasound over a 2-year period, Frey and colleagues found 44 patients had a suspicious lesion on ultrasound, of whom nine were diagnosed with HCC.28 The other 35 (12.3%) patients underwent a total of 17 CT exams, 11 contrast-enhanced ultrasounds, nine MRI exams, and two biopsies. An additional 23 (8.1%) patients with indeterminate ultrasounds (i.e., poor visualization) also resulted in 24 CT exams, six MRI exams, and one biopsy. There were insufficient data to determine patient-level severity of harm. Finally, Singal et al. examined outcomes in 614 patients with cirrhosis with at least one surveillance exam over an 18-month period, and surveillance-related physical harms were only observed in 54 (8.8%) patients – most of mild severity and none experiencing severe harm.29
Quality Assessment
Funnel plot analysis revealed potential publication bias (Egger’s test p=0.04), with fewer “negative” small studies reporting a lack of association between surveillance and improved outcomes. Using a modified NIH study quality assessment tool (Supplemental Table 3), we found most studies clearly defined the study objective and eligibility criteria, and all but one selected patients from the same population. Most studies had low risk of bias for exposure measurement; however, 17 studies stratified results as surveillance-detected vs. undetected HCC, which omits possible surveillance failure, or failed to define surveillance regimens so were classified as medium risk of bias. There were also four studies classified as high risk of bias – three which included AFP alone as surveillance exposure and one that relied on patient report for surveillance receipt. Although most studies assessed surveillance receipt as a dichotomous outcome, 15 assessed surveillance benefits across different levels of exposure – either comparing regular vs. irregular surveillance or assessing continuous measures such as proportion time covered by surveillance. Most studies measured objective and guideline-concordant outcomes and classified as low risk of bias; however, 13 studies assessed tumor stage using measures other than the BCLC or Milan Criteria. Several studies (n=28) also failed to report measures of variance, such as 95% confidence intervals, when describing differences in clinical outcomes between groups. The most common limitation was failure to report length of follow (n=30) and/or number lost to follow-up (n=31) for studies assessing treatment receipt or survival after HCC diagnosis. Most studies reporting differences in early detection or curative treatment receipt failed to adjust for potential confounders. Of the 42 studies that reported survival estimates, only half adjusted for demographics and clinical characteristics. Of the other 21 studies which reported unadjusted differences in survival, four statistically accounted for lead-time bias.
DISCUSSION
The goal of HCC surveillance is to reduce HCC-related mortality by promoting very-early tumor detection and facilitating curative treatments. Our meta-analysis highlights a consistent association between receipt of surveillance and improved clinical outcomes, including overall survival, across cohort studies, although high heterogeneity precluded precise point estimates. Additionally, we found semi-annual surveillance intervals were associated with improved early detection and overall survival compared to longer surveillance intervals. It is therefore noteworthy that less than one-third of HCC cases were detected by surveillance. To inform discussions regarding the overall value of surveillance, we also summarized data for surveillance-related harms; however, few studies characterized surveillance-related harms, with available data focusing only on physical harms and no studies reporting psychological or financial harms. Although there was variation in the magnitude of physical harms experienced by patients, most harms appeared mild and consistent with guideline-concordant follow-up of abnormal surveillance results.
We found HCC surveillance was associated with significant improvements in early HCC detection, with two-thirds of surveillance-detected HCC identified at an early stage. This proportion parallels the sensitivity of current surveillance tools, ultrasound with or without AFP.7 With an aim of increasing sensitivity for early HCC detection, there has been increased interest in alternative imaging (e.g., MRI) and blood-based biomarkers (e.g., GALAD).30,31 We did not find any difference in clinical benefits of various surveillance strategies in subgroup analyses, although these were conducted at the study-instead of patient-level. Therefore, continued evaluation of screening benefits and harms of novel surveillance strategies in prospective cohort studies is still needed.
Improving early detection only addresses one step in the cancer care continuum, as survival is also dependent on the receipt of curative treatment.32 Although HCC surveillance was associated with increased curative treatment receipt, only 58% of surveillance-detected patients received curative therapies. These data are consistent with studies demonstrating underuse of curative treatments, including in patients with early-stage HCC. Despite this issue, surveillance was associated with a reduction in mortality, which was consistent across examined subgroups, including in those that statistically adjusted for lead time bias. It is likely the potential association between HCC surveillance and reduced mortality is underestimated across studies given downstream process failures among those detected at an early stage.
Notably, we observed heterogeneity across pooled analyses, which we were unable to eliminate across study-level subgroup analyses. Unfortunately, we were unable to explore other reasons for heterogeneity given a lack of patient-level data. For example, heterogeneity in early HCC detection may be related to several factors including variations in operator experience and technique, patient body habitus, and liver nodularity, which we were unable to explore. Similarly, we were unable to perform subgroup analyses by patient characteristics such as liver disease etiology and degree of liver dysfunction. Heterogeneity in the pooled estimate for the association with survival may be exacerbated by differences in confounders included in multivariable models. This high heterogeneity precludes precise estimates for the magnitudes of association, although the consistency of association with improved clinical outcomes across studies provides can provide some reassurance that the associations are likely true.
Although the efficacy and value of HCC surveillance would be best evaluated by a randomized clinical trial, a prior attempt suggested this may not be feasible.33 As such, we are dependent on data from available cohort studies. Modeling and cost-effectiveness studies incorporating these data may also aid in informing important nuances of HCC surveillance, such as subgroups who have worse risk-benefit ratio, stopping rules, and optimal surveillance intervals.34 In the interim, our data highlight the clear need for strategies to increase surveillance uptake.35
Notably, some data have suggested HCC surveillance may not be associated with improved clinical outcomes. For example, a case-control study with 238 patients who died of HCC and 238 matched controls from the Veterans Affairs health system failed to find an association between surveillance and reduced HCC-related mortality.11 As above, this lack of mortality benefit may not have been related to surveillance failure but instead downstream process failures, such as underuse of HCC treatment or application of surveillance in patients who are not candidates for any HCC treatment. These conflicting data highlight the need for continued evaluation of HCC surveillance, particularly considering inherent limitations of cohort studies such as residual confounding and length time bias. For instance, few studies adjusted for hepatology subspecialty care and lower medical comorbidity, which are often associated with receipt of HCC surveillance.35 Similarly, HCC has historically been considered a uniformly aggressive cancer although data suggest one-third of HCC may have indolent growth patterns.36,37 Continued evaluation of HCC surveillance is also critical considering the changing at-risk population, with a shift from a viral-mediated disease to one related to alcohol and NAFLD. Studies have suggested lower recognition of cirrhosis in patients with NAFLD, resulting in lower surveillance utilization.38,39 Further, non-viral liver disease predisposes ultrasound to poorer visualization and AFP to impaired test performance.40 Finally, a higher prevalence of comorbid conditions including cardiovascular disease or worse performance status may preclude surgical therapies and mitigate a survival benefit among those detected at an early stage.41,42 Although we did not see a difference in surveillance benefits across subgroups, including study period, most study populations still largely consisted of active viral liver disease. Few studies specifically examined post-SVR or NAFLD patient populations, highlighting this as an area of future research.
It is critical that future studies evaluate overall surveillance value, by assessing not just benefits but also potential harms. While we identified 59 studies evaluating surveillance benefits, only four quantified potential harms due to false positive or indeterminate results. Furthermore, all four only examined physical harms, with no studies quantifying financial or psychological harms. These data are important to evaluate, particularly considering screening-related harms observed in other cancer types.14 Notably, measures of specificity may not equate to screening-related harms when surveillance tests are applied in clinical practice. For example, Atiq and colleagues reported higher screening-related harms with ultrasound than AFP, despite higher specificity, due to differences in how providers interpreted and managed abnormal results for both.26 This same principle may apply to emerging surveillance modalities, given how providers interpret longitudinal changes in biomarker values and mitigate potential harms. In contrast, ultrasound-related harms were increased by providers often performing diagnostic evaluation for subcentimeter lesions, despite most guidelines recommending short-interval ultrasound surveillance.3,4 Studies reported a wide variation in the proportion of patients experiencing physical harms from ultrasound and AFP-based surveillance. Two studies reported less than 10% of patients experienced harm, whereas two others reported over 25% experienced harm. It is unclear if these differences relate to differences in patient populations, variation in provider practice patterns, or differences in study design including study duration. While AFP is prone to false positive results in patients with viral hepatitis, ultrasound has lower specificity in those with non-viral liver disease.40 With a shift in cirrhosis epidemiology from viral to non-viral etiologies, biomarkers such as AFP may start to have higher specificity and lower risk of harms than ultrasound. Rigorous evaluation of benefits and harms in a single population, ideally multi-center and diverse in terms of liver disease etiology, will provide a better understanding of surveillance value.
We acknowledge limitations of our study, which should be considered when interpreting results. We observed heterogeneity across pooled analyses and, which we were unable to eliminate across study-level subgroup analyses. Unfortunately, we were unable to explore other reasons for heterogeneity given a lack of patient-level data. For example, heterogeneity in early HCC detection may be related to several factors including variations in operator experience and technique, patient body habitus, and liver nodularity, which we were unable to explore. Similarly, we were unable to perform subgroup analyses by patient characteristics such as liver disease etiology and degree of liver dysfunction. Second, non-surveillance groups were comprised of patients with incidental and symptomatic presentations, who have distinct prognosis; however, most studies did not report data separately for these two subgroups. Third, we were able to summarize physical harms of surveillance but did not find data characterizing psychological or financial harms. Finally, interpretation of results from our meta-analysis is limited by the quality of included studies. We were pleased to observe improvement in study quality compared to a prior meta-analysis8, including most assessing outcomes by surveillance exposure instead of surveillance detection, using BCLC or Milan Criteria to define early-stage HCC, reporting continuous measures of survival benefit (i.e., hazard ratios), and adjusting for liver dysfunction and lead time bias. There has also been increased recognition of surveillance harms contributing to the overall value of HCC surveillance. Future studies should address remaining limitations such as adjusting for potential confounders and reporting measures of variance for all outcomes, median length of follow-up, and number of patients lost to follow-up.
In summary, we observed a consistent association between HCC surveillance and improved clinical outcomes, including overall survival, across contemporary cohort studies, although high heterogeneity precluded precise point estimates. There are fewer data evaluating surveillance-related harms, although available studies found that most harms were mild in severity. Therefore, current data suggest HCC surveillance is of high value and should be promoted in patients with cirrhosis, particularly given the low proportion of surveillance-detected patients across studies.
Supplementary Material
Highlights.
HCC surveillance was associated with significantly improved early-stage detection, curative treatment receipt, and prolonged survival across contemporary cohort studies.
Semi-annual surveillance intervals were associated with improved early HCC detection and overall survival compared to longer surveillance intervals.
Few studies evaluated surveillance outcomes in post-SVR or NAFLD patient populations, highlighting this as an area of future research.
Few studies characterized surveillance-related harms, although available data suggests surveillance harms are mild in severity.
Financial Source:
This study was conducted with support from National Cancer Institute U01 CA230694, U01 CA230669, R01 CA222900, and R01 CA212008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Conflict of Interest:
Amit Singal has served as a consultant or on advisory boards for Bayer, Wako Diagnostics, Exact Sciences, Roche, Glycotest, and GRAIL.
Jorge Marrero has served as a consultant for Glycotest.
Neehar Parikh has served as a consultant or on advisory boards for Bayer, Wako Diagnostics, Exact Sciences, Glycotest, and Freenome.
Maria Reig has served as consulant or advisory boards for Bayer-Shering Pharma, BMS, Roche, Ipsen, AstraZeneca, Lilly, BTG/Paid conferences: Bayer-Shering Pharma, BMS, Gilead, Lilly and is a principal investigator of research Grants of Bayer-Shering Pharma, Ipsen.
Giuseppe Cabibbo has served as a consultant or on advisory boards for Bayer, Eisai, and Ipsen.
Ju Dong Yang has served as a consultant or on advisory boards for Exact Sciences and Gilead Sciences and Eisai.
None of the other authors have any relevant conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing: All available data used for the meta-analysis have been included in Table 1 and Supplemental Tables 1–3.
REFERENCES
- 1.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol 2020;18:2650–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet JM, Kelley RK, Villanueva A, et al. Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 3.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis M, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 5.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–22. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberger H, Chong N, Fetzer DT, Rich NE, Yokoo T, Khatri G, et al. Dynamic Changes in Ultrasound Quality for Hepatocellular Carcinoma Screening in Patients With Cirrhosis. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero J, Yopp A, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018;154:1706–1718 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kansagara D, Papak J, Pasha AS, O’Neil M, Freeman M, Relevo R, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med 2014;161:261–9. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Kanwal F, Feng Z, Marrero J, Khaderi S, Singal AG. Risk Factors for Cirrhosis in Contemporary Hepatology Practices-Findings From the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology 2020;159:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin G, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology 2018;155:1128–1139 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RP, Wilt TJ, Qaseem A, High Value Task Force of the American College of Physicians. A value framework for cancer screening: advice for high-value care from the American College of Physicians. Ann Intern Med 2015;162:712–7. [DOI] [PubMed] [Google Scholar]
- 13.Brewer NT, Salz T, Lillie SE. Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med 2007;146:502–10. [DOI] [PubMed] [Google Scholar]
- 14.Heleno B, Thomsen MF, Rodrigues DS, Jorgensen K, Brodersen J. Quantification of harms in cancer screening trials: literature review. BMJ 2013;347:f5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris RP, Sheridan SL, Lewis CL, Barclay C, Vu M, Kistler C, et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med 2014;174:281–5. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M, Llovet J, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL Conference. J. Hepatology 2001; 35: 421–430. [DOI] [PubMed] [Google Scholar]
- 18.Bruix J and Sherman M. Management of Hepatocellular Carcinoma. Hepatology 2005; 42(5): 1208–1236. [DOI] [PubMed] [Google Scholar]
- 19.Shi L and Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019; 98:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branch A, Rocha C, Fiel M, Doyle E, Goosens N, Hoshida Y, et al. Liver cancer after Hep C cure: Less cirrhosis and less fat than expected. International Antiviral Society 2016;24:245. [Google Scholar]
- 21.Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology 2018;155:431–442 e10. [DOI] [PubMed] [Google Scholar]
- 22.Lo S, Gane E, Bartlett A, Orr Dl. Clinical features and survival of non-alcoholic fatty liver disease-related hepatocellular carcinoma. Hepatology International 2016;10:S437. [Google Scholar]
- 23.Aby E, Phan J, Truong E, Grotts J, Saab S. Inadequate Hepatocellular Carcinoma Screening in Patients With Nonalcoholic Steatohepatitis Cirrhosis. J Clin Gastroenterol 2019;53:142–146. [DOI] [PubMed] [Google Scholar]
- 24.Clegg F, Bailey L, Ramachandran P, Dundas P, English S, McLeman L, et al. Hepatocellular carcinoma detected in the cirrhosis surveillance programme have better outcomes than those diagnosed symptomatically. Gut 2014;63:A184. [Google Scholar]
- 25.Sigurdsson B, Arnardottir M, Sigurdardottir R, Jonasson J, Bjornsson E. Incidence, etiology, and outcome of patients with hepatocellular cancer in Iceland 1998–2017: A population-based study. Gastroenterology 2019;156:S1311. [Google Scholar]
- 26.Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konerman MA, Verma A, Zhao B, Singal AG, Lok AS, Parikh ND. Frequency and Outcomes of Abnormal Imaging in Patients With Cirrhosis Enrolled in a Hepatocellular Carcinoma Surveillance Program. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey RS, Boldanova T, Heim M. Ultrasound surveillance for hepatocellular carcinoma: real-life performance in a hepatology outpatient clinic. Swiss Med Wkly 2015;145:w14200. [DOI] [PubMed] [Google Scholar]
- 29.Singal AG, Patibandla S, Obi J, Fullington H, Parikh ND, Yopp AC, et al. Benefits and Harms of Hepatocellular Carcinoma Surveillance in a Prospective Cohort of Patients With Cirrhosis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh ND, Mehta AS, Singal AG, Block T, Marrero JA, Lok AS. Biomarkers for the Early Detection of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev 2020;29:2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khatri G, Pedrosa I, Ananthakrishnan L, Diaz de Leon A, Fetzer D, Leyendecker J, et al. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. J Magn Reson Imaging 2020;51:415–425. [DOI] [PubMed] [Google Scholar]
- 32.Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poustchi H, Farrell GC, Strasser SI, Lee A, McCaughlin GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 2011;54:1998–2004. [DOI] [PubMed] [Google Scholar]
- 34.Taylor EJ, Jones RL, Guthrie JA, Rowe I. Modeling the benefits and harms of surveillance for hepatocellular carcinoma: Information to support informed choices. Hepatology 2017;66:1546–1555. [DOI] [PubMed] [Google Scholar]
- 35.Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology 2020;72:1654–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, et al. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut 2021;70:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singal AG, Yopp A, Gupta S, Skinner CS, Halm E, Okolo E, et al. Failure rates in the hepatocellular carcinoma surveillance process. Cancer Prevention Research 2012; 5(9): 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertot L, Jeffrey G, Wallace M, MacQuillan G, Garas G, Ching H, et al. Nonalcoholic fatty liver disease-related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatology Communications 2017; 1(1): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hester C, Rich NE, Singal AG, Yopp AC. Comparative analysis of nonalcoholic steatohepatitis-versus viral hepatitis- and alcohol-related liver disease-related hepatocellular carcinoma. J Natl Compr Canc Netw 2019; 17(4): 322–329. [DOI] [PubMed] [Google Scholar]
- 42.Ganne-Carrie N, Nahon P, Chaffaut C, K’Nontchou G, Layese R, Audureau E, et al. Impact of cirrhosis aetiology on incidence and prognosis of hepatocellular carcinoma diagnosed during surveillance JHEP Reports 2021; 3(3): 100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


