Abstract
Native Americans (NAs) have higher pain rates than the general U.S. population. It has been found that increased central sensitization and reduced pain inhibition are pronociceptive processes that increase pain risk; yet, little attention has focused on the influence of psychosocial factors. Discrimination is a psychosocial factor associated with increased pain in other minoritized groups; however, it is unclear whether it also promotes pain in NAs. This study analyzed data from 269 healthy, pain-free participants (N=134 non-Hispanic whites [NHWs], N=135 NAs) from the Oklahoma Study of Native American Pain Risk. Experienced discrimination was measured using the Everyday Discrimination Scale (EDS). Nociceptive processes were measured via static measures of spinal sensitivity (nociceptive flexion reflex [NFR] threshold, 3-stimulation NFR threshold), temporal summation of pain (TS-Pain) and nociceptive flexion reflex (TS-NFR), and conditioned pain modulation of pain (CPM-Pain) and NFR (CPM-NFR). Results demonstrated that greater discrimination was associated with enhanced TS-NFR and impaired CPM-NFR but not static measures of spinal sensitivity or measures of pain modulation (TS-Pain, CPM-Pain). Although the effects of discrimination on outcomes were similar in both groups (not moderated by ethnicity), NAs experienced higher levels of discrimination and therefore discrimination mediated a relationship between ethnicity and impaired CPM-NFR. This indicates experienced discrimination may promote a pain risk phenotype in NAs that involves spinal sensitization resulting from impaired inhibition of spinal nociception without sensitization of pain experience.
Keywords: central sensitization, temporal summation, descending inhibition, conditioned pain modulation, discrimination, ethnic differences, Native Americans
Introduction
Numerous studies have explored pain in minoritized groups, but they infrequently include Native Americans (NAs)1,24. This is a problem because NAs have the highest prevalence rates of disability, migraines, comorbidity with psychiatric disorders, rheumatoid arthritis, juvenile arthritis, chronic joint symptoms, lower back, neck, face, jaw and dental pain than other minoritized groups7,28,38,71,75,88,89.
Pain is influenced by an interaction of biological (e.g., nociception), psychological (e.g., mood), and sociocultural (e.g., context) variables10,40,43, and interactions among these factors also make up a person’s ethnicity30. According to a survey2, 69% of U.S. adults report experiencing discrimination, with 61% reporting everyday discrimination (e.g., harassment). Moreover, minoritized groups report experiencing discrimination at higher rates2 (e.g., 81% of NAs report everyday discrimination). Thus, the experience of discrimination is a psychosocial factor that could contribute to NA pain disparities.
Indeed, discrimination is associated with poor physical and mental health20,49,51,66,76,79,106,108,109,124–127, even after controlling for gender, race, socioeconomic status, and age79, and this relationship is stronger among minoritized groups79. Studies demonstrated an association between discrimination and enhanced experimental and/or clinical pain13,15,17,19,29,31,44,48,54,56,59,116,118, but most focused on differences between NHWs and African Americans (AAs)13,15,17,19,29,44,48,54,56,59,116,118. To our knowledge, only 2 studies have examined this relationship in NAs. One study19 found two-spirit NAs (those identifying as having both masculine and feminine spirits) experiencing discrimination were more likely to report physical pain and impairment. Another found racial discrimination increased the likelihood of smoking among two-spirit NAs, a relationship mediated by higher pain60.
Studies using experimental stimuli are necessary to explore the mechanisms associated with the effects of discrimination (e.g., central sensitization, impaired pain inhibition). For example, temporal summation (TS) of pain is produced by repetitive, constant-intensity suprathreshold stimulation34,68 in which stimulations become increasingly more painful117. This summation is believed to result from CNS hyper-responsivity, a mechanism similar to central sensitization – a key driver of chronic pain5,34,85,111,112,129,130. Pain inhibition is often measured by conditioned pain modulation (CPM), in which the application of a painful conditioning stimulus inhibits pain evoked at another body site32. CPM reflects brain-to-spinal cord mechanisms that inhibit spinal nociception45,103,122. While experimental pain is different from clinical pain, there are significant associations between them (e.g., enhanced TS and impaired CPM are associated with greater clinical pain)4–6,30,34,39,41,67,69,72,81,83,110,131
However, few studies have examined the relationship between discrimination and experimental pain. One study found greater heat pain sensitivity among AAs experiencing symptomatic knee osteoarthritis48,59,116. Another study found discrimination moderated the relationship between greater clinical pain severity and greater TS in a sample with sickle cell disease74. Therefore, experiences of discrimination may promote pronociceptive processes, but studies have not examined this in NAs.
To address this gap in the literature, the current study examined the relationship between experienced discrimination and pronociceptive processes (TS, CPM) in participants from the Oklahoma Study of Native American Pain Risk (OK-SNAP). We assessed perceptual outcomes as well as the nociceptive flexion reflex (NFR), an electrophysiological correlate of spinal nociception91,105,117. Thus, the dependent variables were static measures of spinal sensitivity (NFR threshold, 3-stimulation NFR threshold) and dynamic measures of central sensitization (TS of pain/NFR) and pain inhibition (CPM of pain/NFR). To fully explore the role of discrimination, moderation analyses examined whether the effect of discrimination was similar in NAs and non-Hispanic whites (NHWs), whereas mediation analyses examined whether discrimination promoted a NA disparity in pronociceptive processes. Given we have previously found trauma exposure is associated with pronociception, we controlled for this variable to determine the unique contribution of discrimination. We predicted that: 1) discrimination would be associated with pronociceptive processes, 2) the effect would be stronger in NAs, and 3) discrimination would mediate a NA pain disparity in pronociceptive processes.
Materials and Methods
Participants
The participants in this study were healthy, pain-free individuals primarily from northeastern Oklahoma. Recruitment was through newspaper ads, posted advertisements on the laboratory website, radio and newspaper interviews, posted fliers, personal communications with NA groups, online social media postings (e.g., Facebook), email announcements, and word of mouth. Participants agreed to take part in a larger multi-year study that investigated risk factors for chronic pain in NA populations, the Oklahoma Study of Native American Pain Risks (OK-SNAP). Participants received a $100 honorarium for completion of each testing day (or $10/hour of non-completed days). Data collection occurred between March 2014 and October 2018.
Participants were excluded based on the following self-reported conditions: neurological, cardiovascular, neuroendocrine, musculoskeletal or circulatory problems; chronic or persistent pain; use of over-the-counter pain medication within 2 weeks of participation; current use of antidepressant, stimulant, anxiolytic, or high blood pressure medications; current psychotic symptoms, as screened by the Psychosis Screening Questionnaire9; substance use problems; an inability to read/speak English; and being under 18 years of age. Participants were also excluded for having a body mass index (BMI) of 35 or above (due to difficulties obtaining NFR because of high adiposity). NA status was verified from Certificate of Degree of Indian Blood or tribal membership cards. NA participants in the current study represent tribal nations predominately from the southern plains and eastern Oklahoma tribes, but to respect tribal confidentiality, tribal affiliations are not reported. Participants were given information about the study procedures and provided informed consent prior to testing. Participants were told that they were free to discontinue participation at any time during the study and were compensated for the time they participated in the study regardless of completion of study. All procedures were approved by The University of Tulsa, Cherokee Nation, and the Oklahoma City Area Indian Health Services Institutional Review Boards prior to data collection. Participants were pre-screened over the phone to determine if they met the inclusion criteria and were also screened before testing on their first scheduled testing day session.
Sample Size and Power Analysis
A total of 391 participants attended the first laboratory visit, but 62 did not meet inclusion criteria. Of the 329 who were deemed eligible, 22 were non-NA minorities (n=22) who were not excluded from participation but were excluded from the present analyses. Two participants’ data were lost due to a computer malfunction and 3 participants were later determined to have type 1 or 2 diabetes and were also excluded. This resulted in 302 participants (NA=153, NHW=149) with at least partial data available for analysis. 227 completed both testing days, 46 completed one testing day, and 29 completed part of one testing day. This resulted in 269 participants (NA=135, NHW=134) with data available for analysis in the current study. Table 1 presents differences in study variables for those with and without data, and Table 2 presents ethnic differences in participant characteristics.
Table 1.
Differences between participants with and without data for the current study
|
Participants without Data (n=33)
|
Participants with Data (n=269)
|
95% CI for ES
|
|||||||||
| Continuous Variables | N | M | SD | N | M | SD | t | p-value | Cohen’s d | Lower | Upper |
|
| |||||||||||
| Age (years) | 33 | 33.91 | 14.55 | 269 | 29.07 | 12.86 | 1.85 | 0.072 | 0.41 | 0.04 | 0.77 |
| BMI (kg/m2) | 33 | 25.68 | 3.78 | 264 | 24.95 | 4.30 | 0.95 | 0.341 | 0.18 | −0.19 | 0.54 |
| Blood Pressure (MAP; mmHg) | 31 | 88.63 | 9.18 | 263 | 85.55 | 9.94 | 1.76 | 0.080 | 0.33 | −0.04 | 0.71 |
| General Health Perception (SF-36; 0–100) | 26 | 79.62 | 14.62 | 267 | 79.40 | 13.85 | 0.03 | 0.976 | 0.01 | −0.40 | 0.41 |
| Trauma Exposure (LEC; 0–5) | 33 | 2.00 | 1.64 | 267 | 1.91 | 1.53 | 0.30 | 0.762 | 0.06 | −0.31 | 0.42 |
| Perceived Stress (PSS; 0–40) | 26 | 11.77 | 6.04 | 267 | 14.08 | 6.26 | −1.79 | 0.074 | −0.37 | −0.77 | 0.04 |
| Psychological Distress (GSI; 0–4) | 26 | 0.23 | 0.20 | 267 | 0.39 | 0.38 | −2.19 | 0.029 | −0.45 | −0.86 | −0.05 |
| Experienced Discrimination (EDS; 1–6) | 26 | 1.89 | 0.89 | 267 | 1.89 | 0.89 | 0.00 | 0.997 | 0.00 | −0.40 | 0.40 |
|
| |||||||||||
| Categorical Variables | N | % | N | % | X2 | p-value | |||||
|
| |||||||||||
| Female Sex | 18 | 54.5% | 145 | 53.9% | 0.01 | 0.944 | |||||
| NA Ethnicity | 18 | 54.5% | 135 | 50.2% | 0.22 | 0.636 | |||||
| Income | |||||||||||
| <$10K | 15 | 46.9% | 81 | 30.9% | 5.09 | 0.406 | |||||
| $10K–14.9K | 3 | 9.4% | 33 | 12.6% | |||||||
| $15K–24.5K | 2 | 6.3% | 33 | 12.6% | |||||||
| $25K–34.9K | 1 | 3.1% | 26 | 9.9% | |||||||
| $35K–$49.9K | 5 | 15.6% | 34 | 13.0% | |||||||
| ≥$50K | 6 | 18.8% | 55 | 21.0% | |||||||
| Education | |||||||||||
| <High School / High School | 16 | 48.5% | 41 | 15.4% | 20.96 | <0.001 | |||||
| Partial College | 10 | 30.3% | 129 | 48.3% | |||||||
| College / Professional School Grad | 7 | 21.2% | 97 | 36.3% | |||||||
Note: Ns, means, and standard deviations are from untransformed variables, whereas the t-test, p-values, and Cohen’s d results were from transformed and winsorized variables (as necessary). CI for ES=confidence interval for Cohen’s d effect size.
Table 2.
Differences in background variables by ethnic group.
|
NHW (n=134)
|
NA (n=135)
|
95% CI for ES
|
|||||||||
| Continuous Variables | N | M | SD | N | M | SD | t | p-value | Cohen’s d | Lower | Upper |
|
| |||||||||||
| Age (years) | 134 | 28.31 | 13.39 | 135 | 29.82 | 12.31 | −4.86 | <.0.001 | −0.59 | −0.84 | −0.35 |
| BMI (kg/m2) | 133 | 24.21 | 3.87 | 131 | 25.71 | 4.58 | −2.95 | 0.004 | −0.36 | −0.61 | −0.12 |
| Blood Pressure (MAP; mmHg) | 133 | 82.83 | 8.09 | 130 | 88.34 | 10.87 | −5.17 | <0.001 | −0.64 | −0.89 | −0.39 |
| General Health Perception (SF-36; 0–100) | 134 | 80.56 | 14.10 | 133 | 78.23 | 13.56 | 1.53 | 0.129 | 0.19 | −0.05 | 0.43 |
| Trauma Exposure (LEC; 0–5) | 133 | 1.77 | 1.48 | 134 | 2.06 | 1.57 | −1.57 | 0.118 | −0.19 | −0.43 | 0.05 |
| Perceived Stress (PSS; 0–40) | 134 | 13.42 | 6.11 | 133 | 14.75 | 6.36 | −1.96 | 0.051 | −0.24 | −0.48 | 0.00 |
| Psychological Distress (GSI; 0–4) | 134 | 0.34 | 0.33 | 133 | 0.44 | 0.41 | −2.95 | 0.004 | −0.36 | −0.60 | −0.12 |
| Experienced Discrimination (EDS; 1–6) | 134 | 1.72 | 0.81 | 133 | 2.07 | 0.94 | −3.34 | 0.001 | −0.41 | −0.65 | −0.17 |
| NFR Threshold (mA) | 134 | 16.74 | 10.21 | 135 | 19.67 | 11.14 | −2.25 | 0.025 | −0.27 | −0.51 | −0.03 |
| CPM Stim Intensity (mA) | 134 | 25.20 | 12.53 | 135 | 27.97 | 12.09 | −1.84 | 0.066 | −0.23 | −0.46 | 0.02 |
| TS Stim Intensity (mA) | 134 | 22.05 | 10.79 | 135 | 25.10 | 11.05 | −2.29 | 0.023 | −0.28 | −0.52 | −0.04 |
|
| |||||||||||
| Categorical Variables | N | % | N | % | X2 | p-value | |||||
|
| |||||||||||
| Female Sex | 68 | 50.7% | 77 | 57.0% | 1.07 | 0.301 | |||||
| Income | |||||||||||
| <$10K | 48 | 36.4% | 33 | 25.4% | 9.37 | 0.095 | |||||
| $10K–14.9K | 17 | 12.9% | 16 | 12.3% | |||||||
| $15K–24.5K | 17 | 12.9% | 16 | 12.3% | |||||||
| $25K–34.9K | 11 | 8.3% | 15 | 11.5% | |||||||
| $35K–$49.9K | 10 | 7.6% | 24 | 18.5% | |||||||
| ≥$50K | 29 | 22.0% | 26 | 20.0% | |||||||
| Education | |||||||||||
| <High School / High School | 18 | 13.5% | 23 | 17.2% | 1.49 | 0.474 | |||||
| Partial College | 69 | 51.9% | 60 | 44.8% | |||||||
| College / Professional School Grad | 46 | 34.6% | 51 | 38.1% | |||||||
Note: Ns, means, and standard deviations are from untransformed variables, whereas the t-test, p-values, and Cohen’s d results were from transformed and winsorized variables (as necessary). CI for ES=confidence interval for Cohen’s d effect size.
This sample should provide power=.80 to detect associations ≥0.17 (with α=.05, 2-tailed). Therefore, with an α=.05 and a power=.80, this study should be able to detect an indirect (mediated) path ≥ 0.04, as calculated using MedPower65,84.
Procedures
These were secondary analyses of OK-SNAP. The primary OK-SNAP study was conducted over a 2-day period, each lasting 4–6 hours (for a thorough description see98). Order of testing day was counterbalanced, blocking for race and sex. Informed consent and inclusion/exclusion screening were conducted on the first day, followed by a brief semi-structured interview about the meaning of pain (data presented elsewhere33). On one of the testing days the following nociceptive tests were administered and used for the current study: NFR threshold, Pain30 threshold (if necessary), temporal summation of NFR threshold, single electric stimulations (for temporal summation of pain, see description below), temporal summation of NFR and electric pain, and CPM. Tests within each day were partly randomized and breaks were provided between tasks to minimize carryover and sensitization. Questionnaires were administered on each testing day at the beginning and during breaks to assess background characteristics, as well as psychosocial and health predictors.
Background Variables and Physical Characteristics
Age was calculated as the years since birth at the time of study enrollment. Weight and height were assessed from a medical scale to calculate BMI in kg/m2. A medical grade device (Dinamap; Tampa, FL) was used to measure resting systolic and diastolic BP 3 times at the beginning of each testing session (3 min inter-test interval) while the participant sat comfortably in a recliner with their arm resting on the arm of the chair. The average of each variable from the first testing day was used in this study.
Socioeconomic status (SES) was assessed by education level and household income. Education level was assessed by a single variable that asked participants to report their highest completed level of education. Responses were: less than 7th grade, junior high school (9th grade), partial high school (10th or 11th grade), high school graduate, partial college, standard college or university graduation, graduate professional training. Due to limited variability at the lower and upper ends of the scale, the variable was winsorized into 3 levels: less than high school or high school graduate, partial college, college/graduate school graduate. A single item asked participants to report on their annual household income from earnings, unemployment, worker’s compensation, social security, alimony, child support, etc. during the previous year. Ten response categories were provided: <$10K, $10K-14.9K, $15K-24.5K, $25K-34.9K, $35K-$49.9K, $50K-74.9K, $75K-$99.9K, $100K-149.9K, $150K-199.9K, and ≥$200K. However, due to limited variability at the upper end of the scale, all responses above $50K were winsorized into a single category) thus truncating the scale to 6 levels.
The General Health Perceptions subscale of the Medical Outcomes Study 36-item Short Form Health Survey (SF-36)119 was used to assess perceptions of physical health. Scores are standardized to a possible range from 0 to 100, with higher scores indicating better physical health.
Psychosocial Variables
Experienced Discrimination.
The Everyday Discrimination Scale (EDS) is a 9-item measure about experiences of daily discrimination, that measures the frequency of chronic, routine, and relatively minor experiences of unfair treatment in day-to-day life experiences36,128. Participants were asked, “In your day to day life, how often do any of the following things happen to you?” and responded to each of the 9-items on a 6-point Likert-scale ranging from 1 to 6: “1=Almost every day,” “2=At least once a week,” “3=A few times a month,” “4=A few times a year,” “5=Less than once a year,” and “6=Never.” Items were reverse scored and then a mean was computed with a possible range of 1 to 6, with higher scores indicating greater experienced discrimination.
Trauma Exposure.
To assess for exposure to potentially traumatic events, the Life Events Checklist (LEC) for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision, was administered50. The LEC is a self-report measure containing 17 items asking participants whether they have directly experienced, witnessed, or learned about various potentially traumatic events in their lifetime; each item assesses a single stressful/traumatic event (e.g., natural disaster, transportation accident, physical assault, sexual assault). The LEC has been found to be a reliable and valid measure of exposure to potentially traumatic events50. In the current study, trauma exposure was operationally defined as having direct exposure (answering “happened to me”) to any items on the LEC. Thus, the number of traumatic events that happened to the participants was summed to generate a cumulative exposure score63,113, with a possible range of 0–17.
Perceived Stress.
The Perceived Stress Scale (PSS) is a 14-item questionnaire that assesses how much participants appraised life situations as stressful. It has been used extensively to assess perceived stress in research on ethnicity and pain77. The PSS asked about feelings and thoughts for the past month21. Scores were summed such that they ranged from 0 to 40, with higher scores indicating higher perceived stress.
Psychological Distress.
The Symptom Checklist-90-Revised (SCL-90-R) was used to assess general psychological functioning27. The scale consists of 90 items that assess various psychological symptoms (e.g., depression, phobic anxiety, paranoia). The Global Severity Index (GSI) of the SCL-90-R was used to assess overall psychological distress. Total GSI scores had a possible range from 0 to 4 with higher scores indicating greater psychological distress.
Nociceptive Processes
Nociceptive Flexion Reflex (NFR) Threshold.
NFR is a polysynaptic, spinal reflex elicited in response to Aδ fiber activation, so it is used as a measure of spinal nociception105. NFR was measured from left biceps femoris (hamstring) EMG using 2 Ag-AgCl electrodes positioned approximately 10 cm superior to the popliteal fossa42,90. A common reference electrode was placed over the lateral epicondyle of the femur. Sensors were filled with conductive gel (EC60; Grass Technologies). The EMG signal was collected, filtered (10 Hz to 300 Hz), and amplified (×10,000) using a Grass Technologies (West Warwick, RI) Model 15LT amplifier (with AC Module 15A54). The skin was cleaned with alcohol and exfoliated (Nuprep gel; Weaver and Company, Aurora, CO) to achieve impedances <5kΩ. EMG was sampled and digitized at 1000 Hz using a National Instruments analog-to-digital converter (Austin, TX).
NFR threshold (the stimulus intensity necessary to reliably elicit the reflex) was used as a static measure of spinal nociceptive sensitivity (lower thresholds=greater sensitivity). NFR threshold was assessed from 3 ascending-descending staircases of stimulations91. Each stimulus consisted of a train of 5 1-ms pulses with 3-ms interstimulus interval that was experienced as a single stimulus. The first staircase began at 0 mA and increased in 2 mA increments until a reflex was observed (defined as when the averaged, rectified EMG activity of the biceps femoris in the 90 to 150 ms post-stimulus interval was 1.4 SD greater than the averaged, rectified biceps femoris EMG activity during the 60 ms pre-stimulus baseline interval) 91. Once an NFR was obtained, the stimulus intensity decreased in 1 mA steps until a reflex was not observed. The second and third ascending-descending staircases used 1 mA step increments. The interval between electric stimuli varied randomly (8 to 12 s) to minimize predictability and reflex habituation. NFR threshold was defined as the average stimulus intensity (mA) of the two peaks and two troughs of the last 2 ascending-descending staircases.
In the event that stimulations at NFR threshold were not experienced as painful, the stimulation intensity was increased from NFR threshold in 2 mA steps until a rating of 30 was achieved on the VAS (i.e., Pain30; this was only used for stimulation intensity determination for CPM and thus not reported further).
3-Stimulation NFR Threshold.
3-stimulation NFR threshold is a measure of spinal nociceptive sensitization. It was defined as the stimulus intensity required to evoke an NFR on the last stimulus of a series of 3 electric stimulations. Each stimulus consisted of a train of 5 1-ms pulses with 3-ms interstimulus interval. To assess this 3-stimulaton NFR threshold, a series of 3 electric stimulations were delivered with an inter-stimulus interval of 0.5 s (2.0 Hz). The series started at 0 mA and increased in 2 mA steps until an NFR was evoked by the third stimulus in the series. The stimulus intensity at which the NFR was evoked was used as 3-Stimulation NFR threshold. This task is akin to what has been described in the literature as temporal summation of NFR threshold73,80; however, its definition does not require summation of the reflex across the 3-stimulus series (reflexes could decrease across the 3 stimulations, although this is rare).
Conditioned Pain Modulation.
A CPM task was used to assess descending inhibition. In this study, CPM involved the assessment of pain and NFR in response to test stimuli before, during, and after a tonic conditioning stimulus (CS) delivered at a distal body site from the test stimulus. In healthy humans, the CS should inhibit pain evoked by the test stimuli64. The test stimuli were electrical stimulations (intensity = highest of 120% NFR threshold, 120% temporal summation threshold, or 100% electric Pain30) delivered to the left ankle. The CS was exposure to a 2-min long circulating cold-water bath maintained at a temperature of 10±0.1°C. CPM consisted of three 2-min phases: baseline (test stimuli delivered prior to cold water), conditioning (test stimuli delivered while hand/arm is submerged in cold water), and post-test (test stimuli delivered after conditioning). A 2-min rest occurred between baseline and conditioning and a 5-min rest occurred between conditioning and post-test. During conditioning, participants were instructed to submerge their right hand up to their forearm in the painfully cold water and to keep their hand palm down with fingers spread. Each 2-min phase, consisted of a 20 s wait period, followed by 5 electric test stimuli (random 8–12 s inter-stimulus interval). Participants provided pain ratings in response to the electric stimulations verbally using a numerical rating scale (NRS) that was constantly displayed on a computer screen (0 “no pain”, 20 “mild pain”, 40 “moderate pain”, 60 “severe pain”, 80 “very severe pain”, and 100 “worst possible pain”) and an experimenter recorded the ratings. NFR magnitudes in response to electric stimuli were used to assess within-subjects changes in spinal nociception92. NFR magnitudes were calculated as a d-score [NFR d = (mean rectified EMG of 90 to 150 ms post-stimulation interval minus mean of rectified EMG from −60 to 0 ms prestimulation interval) divided by the average standard deviation of the rectified EMG from the two intervals]. Trials with NFR baselines higher than 3.0 μV were excluded from analyses due to excessive muscle tension and/or noise in the recording (3% of trials were excluded). CPM of pain/NFR was conceptualized as the difference in the electric pain/NFR during the CS relative to the electric pain/NFR during the baseline phase.
Temporal Summation.
Temporal summation of the nociceptive flexion reflex (TS-NFR) assesses the degree of spinal neuron hyper-excitability following a series of suprathreshold stimulations117, and is believed to be a correlate of windup in animals78,134,135. The suprathreshold stimulus intensity used for this task was set at 120% of NFR threshold or 120% of 3-Stimulation NFR threshold, whichever was higher (in mA). Each stimulus consisted of a train of 5 1-ms pulses with 3-ms interstimulus interval. In the present study, TS-NFR was defined as the change in reflex magnitude across a series of 3 suprathreshold stimuli. To assess this, 5 trains of 3 suprathreshold stimuli (0.5 s ISI) were used to assess temporal summation. After each train of stimuli, participants were instructed to rate their pain intensity in response to each of the 3 stimulations, using a set of 3 computer-presented VASs ranging from “no pain sensation” to “the most intense pain sensation imaginable.” After the participant completed the ratings, there was an inter-train interval of 8–12 s. The baseline EMG in the 60 ms prior to the third stimulus in the stimulus series was visually inspected in real-time by the experimenter for excessive muscle tension or voluntary movement. If the mean rectified EMG exceeded 5 μV, the train was repeated in order to ensure that EMG activity in the post-stimulus interval was not contaminated by muscle tension unrelated to the NFR117. NFR magnitudes in response to each stimulus in the 3-stimulus train were calculated in d-units by first subtracting the 60 ms baseline prior to the first stimulus in each train from the EMG response 70–150 ms after each stimulus in the train. This difference was then divided by the average of the standard deviations of the rectified EMG from these two intervals. Of note, the post-stimulus interval used here differs from the assessment of NFRs during NFR threshold and CPM (i.e., 90–150 ms post-stimulus) because repeated stimulations with a short (0.5 s) inter-stimulus interval can result in a shorter NFR onset latency3,117.
Temporal summation of pain is believed to be the psychophysical correlate of spinal neuron facilitation34,86, but TS-NFR and TS-Pain can diverge99 indicating that the nociceptive signal may undergo additional supraspinal modulation after ascending from the spinal level to impact the degree of pain perception facilitation. Assessing TS-Pain from the 3-stimulus series noted above is not ideal, because the 2 Hz series (i.e., 0.5 ISI) is too fast for participants to make pain ratings immediately following each stimulus. As such, all 3 ratings must be made after the third stimulus, which can be affected by recall bias, especially for the first two stimuli in the series. Therefore we took an approach to define TS-Pain similar to other studies using both electric37 and mechanical47 stimuli. 5 single electric stimuli were delivered using the same suprathreshold intensity as that used during TS-NFR. However, these stimuli had an interstimulus interval that would preclude the evocation of temporal summation (8–12 s ISI; i.e., 0.125 to 0.08 Hz). Participants made pain ratings immediately after each stimulus using the same VAS described above. TS-Pain was defined as the difference in pain rating of these single stimuli (in which no pain summation had occurred) and the rating of the 3rd stimulus in the 3-stimulus train delivered during TS-NFR (in which pain summation had occurred).
Data Analysis
Unless otherwise noted, analyses and data screening were conducted with SPSS (IBM; v27). Prior to primary analyses, variable distributions were screened using boxplots, histograms, and normality statistics. Variables with positive skew were log10 transformed (i.e., psychological distress, discrimination). Next, outliers were identified using Wilcox’s MAD-median procedure (using the recommended 2.24 cutoff) and then winsorized by replacing the outlier value with the next nearest non-outlier value121. The following variables were winsorized: mean arterial blood pressure, health perception, ALEs, discrimination, perceived stress, psychological distress, NFR threshold, 3-Stimulation NFR threshold, CPM-pain, CPM-NFR, TS-pain, TS-NFR. For all analyses, significance was set at p<.05.
To analyze univariate group differences (completers vs. non-completers; NAs vs. NHWs), independent samples t-tests were conducted on continuous-like variables and chi-squared tests were conducted on categorical variables.
Several control variables were used in the analyses because prior or current OK-SNAP analyses have found ethnic group differences that need to be controlled (i.e., general health perception, blood pressure, BMI, age). Further, biological sex was controlled due to well established sex differences in pain and nociceptive processing8,14,100. Analyses also included education level and household income to control for socioeconomic status25,101. To establish that discrimination predicts pain processing above and beyond the effect of other psychological stressors, ALEs, perceived stress, and psychological distress were also included63,113.
Prior to primary analyses, missing values on predictors and control variables were imputed using the expectation maximization algorithm in LISREL v8.8 (Scientific Software International; Lincolnwood, IL)61. Missingness for each variable was: general health perception, stress, psychological distress, education, ALEs, and discrimination had 0.7% each missing; BMI=1.8%, income=2.6%, and MAP=2.2%. All others had complete data. Imputation helped maintain the entire sample because regression and mediation analyses use listwise deletion.
To examine the moderated relationships between discrimination and static markers of spinal sensitization (NFR threshold, 3-Stimulation NFR threshold), multiple regression analyses were conducted in PROCESS and bootstrapped estimates and confidence intervals were created for the unstandardized regression weights.
To analyze TS and CPM variables, multilevel growth models were conducted with pain ratings or NFR magnitudes as the dependent variable. Data for these models were kept in “long form,” meaning that each participant had multiple rows of data that corresponded to each stimulation they received during each task. Participants served as level 2 units in the models and stimulations served as level 1 units. The within-subject variance covariance structure was modeled as a first-order autoregressive matrix (AR1) to account for the significant autocorrelation in the repeated measures. A variable called Stimulus Number was entered into these analyses to model the linear slope of temporal summation (i.e., the change in pain/NFR across the TS stimulations) or the linear slope of descending inhibition (i.e., the change in pain/NFR from baseline and conditioning during CPM). Train Number was also entered as a predictor to account for variance in pain/NFR across the 5 trains (for TS analyses only). Discrimination, ethnicity, and their interactions with Stimulus Number were the primary predictors of interest. All continuous variables were centered by subtracting the grand mean to reduce multicollinearity and aid interpretation. Ethnicity was coded 0=non-Hispanic White, 1=Native American. Sex was contrast coded (Male=−1, Female=1). The models included a random intercept and a random slope for Stimulus Number (to allow summation to vary across participants). Finally, the intercepts and slopes were allowed to covary to control for the law of initial values (i.e., pain/NFR that is higher in response baseline values is less likely to summate but more likely to inhibit for TS and CPM, respectively). The main effects of discrimination and ethnicity examined whether these variables were associated with overall levels (i.e., the intercept) of pain/NFR. The interactions of Discrimination X Stimulus Number and Ethnicity X Stimulus Number tested whether discrimination or ethnicity (respectively) were associated with TS-pain, TS-NFR, CPM-pain, or CPM-NFR. The Discrimination X Ethnicity X Stimulus Number interaction tested whether the relationship between discrimination and TS/CPM differed in NAs. Control variables that were entered included: Age, Sex, Perceived Stress, Psychological Problems, Negative Affect, Positive Affect, and Suprathreshold Stimulus Intensity. In the event of a significant interaction, simple effects for CPM phase or Stimulus Number were calculated for each of the low, medium, and high values of the discrimination variable (i.e., −1 SD, mean, +1SD) to evaluate the effect of discrimination on CPM/TS.
To examine whether discrimination mediated a NA disparity in pronociceptive processes, PROCESS software (v3.3) was used to conduct bootstrapped mediation analyses from 5000 random samples55. Significant mediation occurs when 0 is not contained in the 95% bootstrapped confidence interval for the indirect effect.
For these analyses, CPM of pain/NFR was defined as the difference in the average electric pain/NFR during the CS minus the average electric pain/NFR during the baseline phase. TS-Pain was defined as the difference between average rating of these single stimuli and the average rating of the 3rd stimulus in the 3-stimulus train delivered during TS-NFR.
Results
Differences in Those with and without Data
Table 1 presents differences between those with and without data. As can be seen, those included in the study reported higher psychological distress and higher educational attainment. No other differences were statistically significant.
Ethnic Differences on Study Predictors
Table 2 presents results of ethnic group differences for participants included in the current study. As shown, when compared to NHWs, NAs were slightly older, had higher BMI and blood pressure, reported more psychological distress and discrimination, and had a higher NFR threshold and stimulus intensity used during TS testing.
Is Discrimination Differentially Associated with Nociception for NAs and NHWs?: Tests of Moderation
Static Measures of Spinal Nociception.
Table 3 depicts the results of the regression analyses predicting NFR threshold and 3-Stimulation NFR threshold. The only significant predictor of NFR threshold was age, with higher age being associated with a higher threshold. Discrimination and the Ethnicity x Discrimination interaction were significant predictors of 3-Stimulation NFR Threshold. On average, discrimination was associated with a higher threshold, but the interaction suggests this differs by ethnicity. For NAs, the relationship between discrimination and 3-Stimulation NFR threshold was negative, whereas the relationship for NHWs was positive (Fig 1). However, neither simple effect was significant (NHW: simple slope= 7.1947, p = 0.061; NA: simple slope = −4.9865, p = 0.131); therefore, neither relationship is statistically reliable. As a result, discrimination did not significantly predict static measures of spinal nociception (the significant interaction negates the main effect of discrimination on 3-Stimulation NFR threshold).
Table 3.
Predicting static measures of spinal nociception (N = 269)
| DV = NFR Threshold |
DV = 3-Stimulation NFR Threshold |
|||||||
|---|---|---|---|---|---|---|---|---|
| Boot 95% CI |
Boot 95% CI |
|||||||
| Predictors | Boot B | Boot SE | Lower | Upper | Boot B | Boot SE | Lower | Upper |
| Sex | 0.070 | 0.694 | −1.304 | 1.423 | −0.238 | 0.468 | −1.126 | 0.668 |
| Age | 0.257 | 0.118 | 0.027 | 0.499 | 0.051 | 0.100 | −0.130 | 0.261 |
| Blood Pressure | −0.084 | 0.091 | −0.257 | 0.097 | −0.071 | 0.062 | −0.197 | 0.045 |
| BMI | 0.352 | 0.177 | −0.004 | 0.696 | 0.169 | 0.112 | −0.045 | 0.390 |
| General Health Perception | −0.011 | 0.050 | −0.109 | 0.086 | 0.002 | 0.034 | −0.062 | 0.067 |
| Education Level | −0.001 | 0.957 | −1.875 | 1.852 | 0.125 | 0.749 | −1.355 | 1.554 |
| Income | 0.321 | 0.358 | −0.374 | 1.041 | 0.220 | 0.253 | −0.281 | 0.709 |
| Trauma Exposure | 0.112 | 0.444 | −0.764 | 0.957 | 0.017 | 0.256 | −0.486 | 0.515 |
| Discrimination | 9.001 | 4.816 | −0.749 | 18.420 | 6.980 | 3.311 | 0.444 | 13.266 |
| Ethnicity | 1.259 | 1.480 | −1.653 | 4.187 | 1.164 | 0.927 | −0.665 | 2.954 |
| Ethnicity x Discrimination | −7.715 | 7.016 | −21.197 | 6.142 | −12.043 | 4.655 | −21.140 | −2.800 |
Note: DV=dependent variable; NFR=nociceptive flexion reflex; Boot=bootstrapped; bolded values are associated with significant predictors according to a bootstrapped 95% confidence interval. Ethnicity was coded 0=non-Hispanic White, 1=Native American. Sex was coded 0=male, 1=female.
Figure 1.
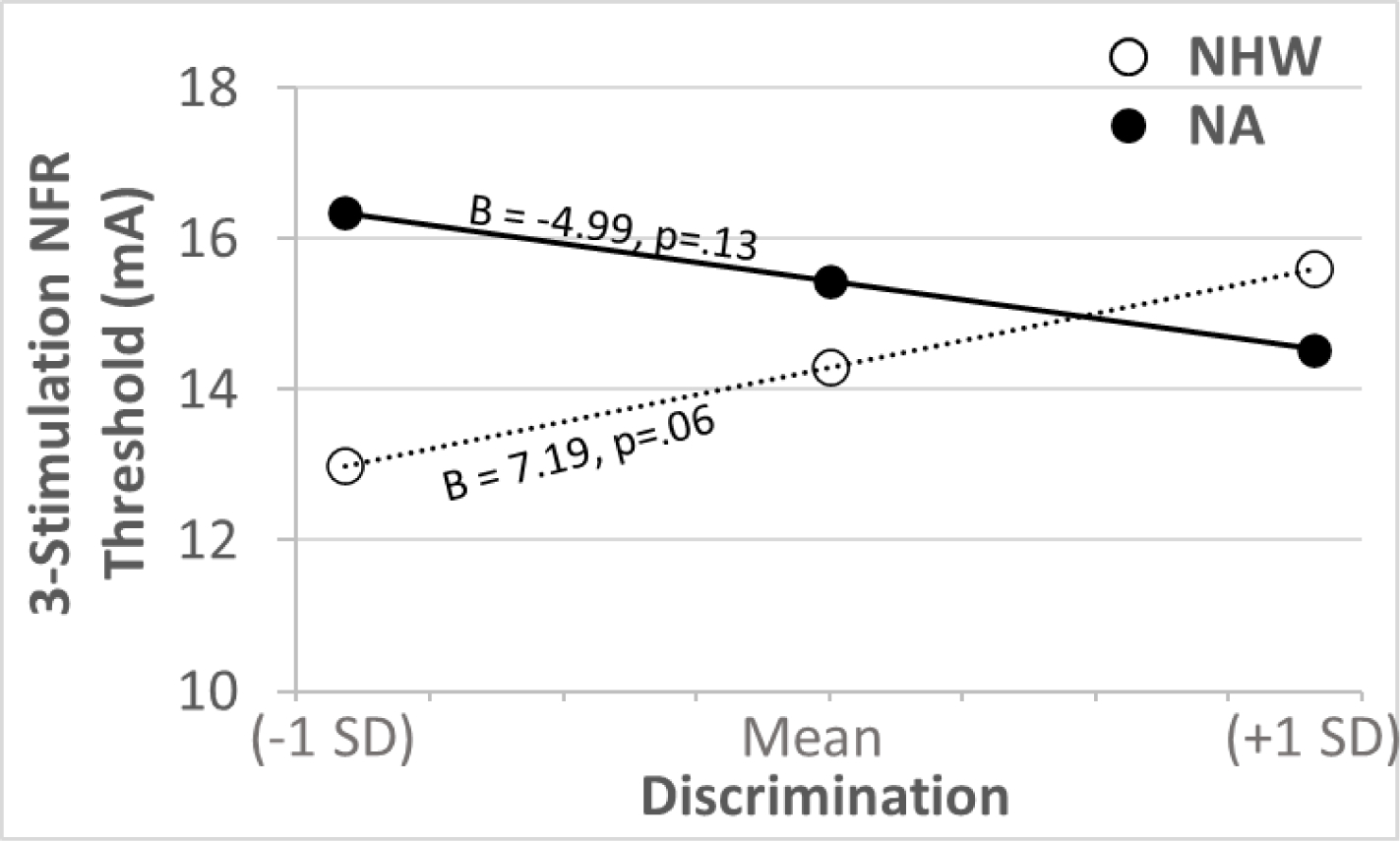
The relationship between experienced discrimination and 3-stimulation nociceptive flexion reflex (NFR) threshold (a static measure of spinal sensitivity). There was a significant Ethnicity × Discrimination interaction; however, the simple regression slope between discrimination and 3-stimulation NFR threshold was non-significant in both ethnic groups. NA=Native American. NHW=non-Hispanic white.
Conditioned Pain Modulation.
Table 4 presents the results of the multilevel model predicting CPM-pain. There was a significant main effect of CPM Phase indicating that pain was inhibited by the cold water CS (Fig 2A). However, this effect was not moderated by discrimination or ethnicity. The significant effect of stimulus intensity indicated that ratings were higher when stimulus intensity was higher. The significant effect of stimulus number indicated that pain ratings increased across the 5-stimulus series in each CPM phase. Higher education and lower health perceptions were associated with higher pain ratings. Results of the random effects indicated that there was still significant variance left in the intercept, slope of stimulus number, and slope of CPM phase to be explained. There were also significant covariances between the intercept and slope of CPM Phase, suggesting that persons with higher pain during the baseline phase were more likely to show greater inhibition during the cold water CS, and vice versa. The significant covariance between the intercept and slope of stimulus number suggests that persons with higher pain ratings during the baseline phase were more likely to show greater pain habituation in the 5-stimulus series in each CPM phase.
Table 4.
Results of multilevel model predicting pain ratings during conditioned pain modulation
| Estimates of Fixed Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | t-test | p-value | Lower | Upper |
|
| ||||||
| Intercept | 31.303 | 2.069 | 15.13 | 0.000 | 27.230 | 35.376 |
| Ethnicity | 0.512 | 1.086 | 0.471 | 0.638 | −1.627 | 2.650 |
| CPM Phase | −6.735 | 0.555 | −12.143 | <0.001 | −7.827 | −5.642 |
| Discrimination | −9.131 | 7.162 | −1.275 | 0.203 | −23.234 | 4.971 |
| CPM Phase x Ethnicity | −0.944 | 0.551 | −1.713 | 0.088 | −2.029 | 0.142 |
| CPM Phase x Discrimination | 1.806 | 3.087 | 0.585 | 0.559 | −4.275 | 7.888 |
| Ethnicity x Discrimination | −1.042 | 5.814 | −0.179 | 0.858 | −12.493 | 10.410 |
| CPM Phase x Ethnicity x Discrimination | 1.978 | 3.087 | 0.641 | 0.522 | −4.103 | 8.060 |
| Stimulus Intensity | 0.195 | 0.076 | 2.569 | 0.011 | 0.046 | 0.345 |
| Stimulus Number | 0.742 | 0.155 | 4.771 | <0.001 | 0.435 | 1.048 |
| Trauma Exposure | 0.506 | 0.670 | 0.756 | 0.450 | −0.813 | 1.826 |
| Sex | 0.493 | 0.978 | 0.504 | 0.615 | −1.434 | 2.419 |
| Education | 3.031 | 1.442 | 2.102 | 0.037 | 0.191 | 5.871 |
| Income | 0.599 | 0.513 | 1.167 | 0.244 | −0.412 | 1.610 |
| General Health Perception | −0.167 | 0.075 | −2.224 | 0.027 | −0.316 | −0.019 |
| Age | 0.024 | 0.174 | 0.138 | 0.890 | −0.319 | 0.367 |
| BMI | 0.134 | 0.254 | 0.525 | 0.600 | −0.367 | 0.634 |
| Stress | −0.099 | 0.236 | −0.418 | 0.676 | −0.563 | 0.366 |
| Psych Distress | 31.438 | 17.837 | 1.763 | 0.079 | −3.693 | 66.568 |
| Blood Pressure | −0.038 | 0.125 | −0.307 | 0.759 | −0.285 | 0.208 |
|
| ||||||
| Estimates of Random Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | z-test | p-value | Lower | Upper |
|
| ||||||
| AR1 diagonal | 31.009 | 2.022 | 15.338 | <0.001 | 27.290 | 35.236 |
| AR1 rho | 0.383 | 0.039 | 9.698 | <0.001 | 0.303 | 0.457 |
| Intercept Variance | 248.385 | 25.618 | 9.696 | <0.001 | 202.925 | 304.031 |
| Intercept, StimNum Covariance | −8.135 | 2.832 | −2.873 | 0.004 | −13.686 | −2.585 |
| StimNum Variance | 4.462 | 0.541 | 8.241 | <0.001 | 3.518 | 5.660 |
| Intercept, CPM Phase Covariance | −46.265 | 10.267 | −4.506 | <0.001 | −66.388 | −26.143 |
| StimNum, CPM Phase Covariance | 1.358 | 1.378 | 0.985 | 0.325 | −1.344 | 4.059 |
| CPM Phase Variance | 53.884 | 6.893 | 7.817 | <0.001 | 41.934 | 69.238 |
Note: CPM=conditioned pain modulation; Phase=codes for modulation during the conditioning stimulus; AR=autoregressive; Bolded values are associated with significant estimates. Ethnicity was coded 0=non-Hispanic White, 1=Native American. Sex was coded 0=male, 1=female.
Figure 2.
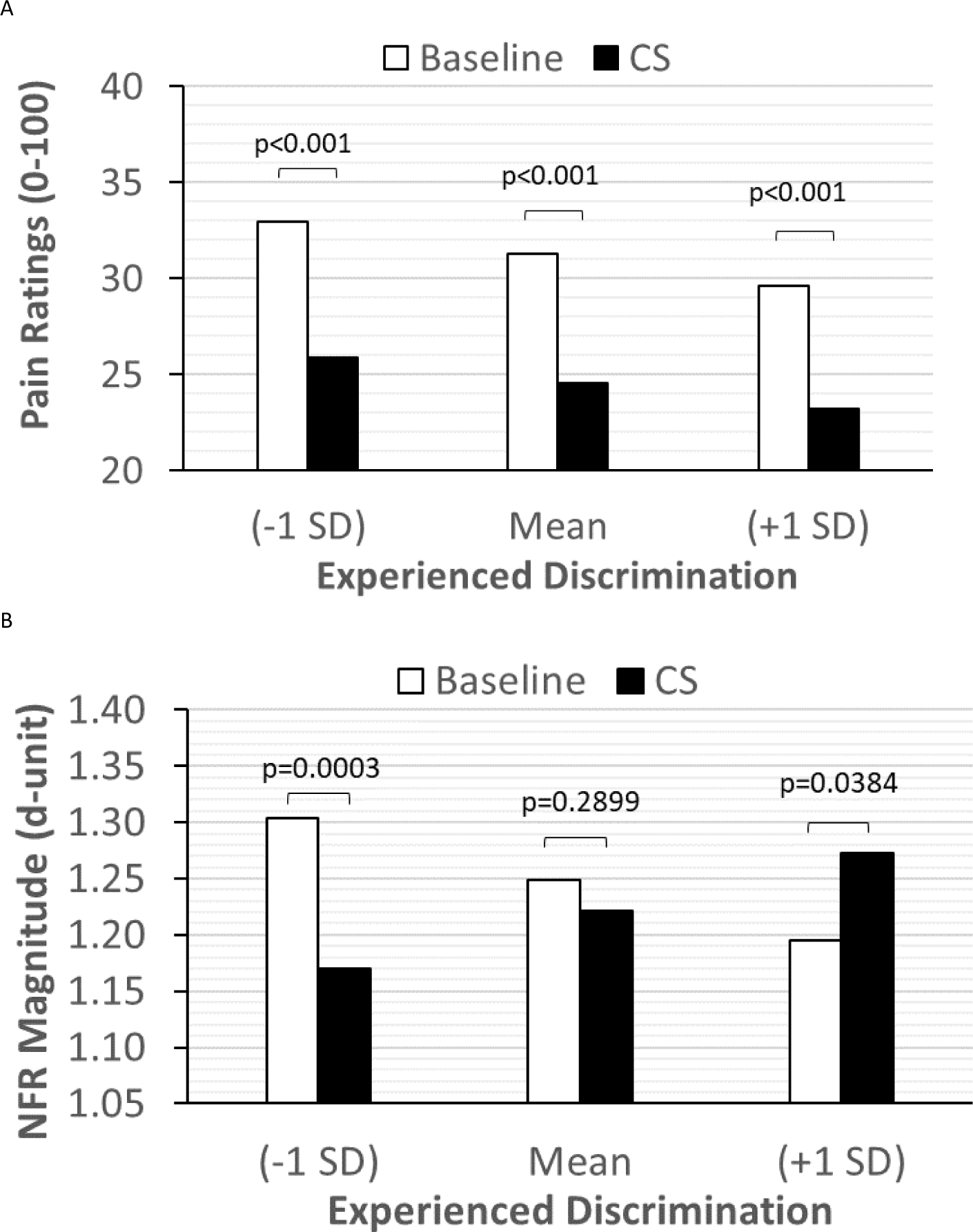
The relationship between experienced discrimination and descending inhibition of pain (panel A) and the nociceptive flexion reflex (NFR; panel B), as measured by the conditioned pain modulation (CPM) task. As shown in panel A, pain inhibition was not moderated by discrimination, but NFR inhibition was (panel B). For those who experienced low discrimination (−1 SD), there was significant NFR inhibition by the cold-water conditioning stimulus (CS). However, NFR inhibition was impaired in those with moderate (mean) levels of discrimination, and NFR was facilitated by the cold-water CS in those experiencing high discrimination (+1 SD).
Table 5 presents the results of the multilevel model predicting CPM-NFR. There was a significant CPM Phase x Discrimination interaction indicating that NFR inhibition due to the cold water CS was strongest for those experiencing lower discrimination, but impaired for those experiencing moderate discrimination, and transitioned to facilitation for those experiencing high discrimination (Fig 2B). This effect was not moderated by ethnicity. The significant effect of stimulus intensity indicated that NFRs were larger when stimulus intensity was higher. The significant effect of stimulus number indicated that NFRs decreased (habituated) across the 5-stimulus series in each CPM phase. Results of the random effects indicated that there was still significant variance left in the intercept, slope of stimulus number, and slope of CPM phase to be explained. There were also significant covariances between the intercept and slope of CPM Phase, suggesting that persons with larger NFRs during the baseline phase were more likely to show greater NFR inhibition during the cold water CS, and vice versa. The significant covariance between the intercept and slope of stimulus number suggests that persons with larger NFRs during the baseline phase were more likely to show greater NFR habituation in the 5-stimulus series in each CPM phase. The significant stimulus number and CPM phase covariance suggests that NFR inhibition during the cold water CS was larger for those showing stronger NFR habituation across the 5 stimulus series in each CPM phase.
Table 5.
Results of multilevel model predicting nociceptive flexion reflexes during conditioned pain modulation
| Estimates of Fixed Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | t-test | p-value | Lower | Upper |
|
| ||||||
| Intercept | 1.249 | 0.078 | 15.925 | 0.000 | 1.095 | 1.404 |
| Ethnicity | 0.010 | 0.040 | 0.255 | 0.799 | −0.069 | 0.089 |
| CPM Phase | −0.028 | 0.026 | −1.047 | 0.296 | −0.080 | 0.024 |
| Discrimination | −0.301 | 0.262 | −1.152 | 0.250 | −0.816 | 0.214 |
| CPM Phase x Ethnicity | 0.015 | 0.026 | 0.582 | 0.561 | −0.036 | 0.066 |
| CPM Phase x Discrimination | 0.587 | 0.145 | 4.038 | <0.001 | 0.301 | 0.874 |
| Ethnicity x Discrimination | 0.062 | 0.214 | 0.289 | 0.773 | −0.360 | 0.484 |
| CPM Phase x Ethnicity x Discrimination | 0.113 | 0.145 | 0.777 | 0.438 | −0.174 | 0.399 |
| Stimulus Intensity | 0.015 | 0.003 | 5.470 | <0.001 | 0.010 | 0.021 |
| Stimulus Number | −0.046 | 0.007 | −6.673 | <0.001 | −0.060 | −0.032 |
| Trauma Exposure | 0.008 | 0.024 | 0.337 | 0.736 | −0.040 | 0.056 |
| Sex | −0.039 | 0.036 | −1.079 | 0.282 | −0.109 | 0.032 |
| Education | −0.026 | 0.053 | −0.493 | 0.622 | −0.130 | 0.078 |
| Income | −0.037 | 0.019 | −1.963 | 0.051 | −0.074 | 0.000 |
| General Health Perception | 0.002 | 0.003 | 0.614 | 0.540 | −0.004 | 0.007 |
| Age | 0.004 | 0.006 | 0.711 | 0.478 | −0.008 | 0.017 |
| BMI | −0.006 | 0.009 | −0.655 | 0.513 | −0.024 | 0.012 |
| Stress | −0.001 | 0.009 | −0.117 | 0.907 | −0.018 | 0.016 |
| Psych Distress | 0.121 | 0.654 | 0.185 | 0.853 | −1.167 | 1.409 |
| Blood Pressure | −0.006 | 0.005 | −1.385 | 0.167 | −0.015 | 0.003 |
|
| ||||||
| Estimates of Random Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | z-test | p-value | Lower | Upper |
|
| ||||||
| AR1 diagonal | 0.141 | 0.005 | 26.032 | <0.001 | 0.131 | 0.152 |
| AR1 rho | 0.020 | 0.030 | 0.669 | 0.503 | −0.039 | 0.079 |
| Intercept Variance | 0.375 | 0.044 | 8.502 | <0.001 | 0.298 | 0.472 |
| Intercept, StimNum Covariance | −0.018 | 0.006 | −3.150 | 0.002 | −0.029 | −0.007 |
| StimNum Variance | 0.004 | 0.001 | 3.818 | <0.001 | 0.003 | 0.007 |
| Intercept, CPM Phase Covariance | −0.093 | 0.020 | −4.669 | <0.001 | −0.132 | −0.054 |
| StimNum, CPM Phase Covariance | 0.007 | 0.003 | 2.556 | 0.011 | 0.002 | 0.013 |
| CPM Phase Variance | 0.102 | 0.015 | 6.745 | <0.001 | 0.076 | 0.137 |
Note: CPM=conditioned pain modulation; NFR=nociceptive flexion reflex; Boot=bootstrapped; AR=autoregressive; Bolded values are associated with significant estimates. Ethnicity was coded 0=non-Hispanic White, 1=Native American. Sex was coded 0=male, 1=female.
Temporal Summation.
Table 6 presents the results of the multilevel model predicting TS-Pain. The significant main effect of stimulus number/summation indicated that pain ratings summated. This summation was not moderated by ethnicity or discrimination (Fig 3A). The main effect of train number suggests that pain ratings increased across each of the 5 trains of stimuli. The main effect of stimulus intensity indicates that higher stimulus intensity was associated with higher pain ratings. Results of the random effects indicated that there was still significant variance left in the intercept and the summation slopes. There was also significant covariance between the intercept and summation slope suggesting that persons with higher pain ratings in response to the single stimuli showed greater pain summation.
Table 6.
Results of multilevel model predicting pain ratings during temporal summation
| Estimates of Fixed Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | t-test | p-value | Lower | Upper |
|
| ||||||
| Intercept | 45.414 | 3.104 | 14.633 | <0.001 | 39.301 | 51.526 |
| Ethnicity | 0.709 | 1.576 | 0.450 | 0.653 | −2.396 | 3.814 |
| Stimulus Number (Summation) | 3.055 | 0.240 | 12.747 | <0.001 | 2.583 | 3.527 |
| Discrimination | 0.849 | 10.333 | 0.082 | 0.935 | −19.504 | 21.202 |
| Summation x Ethnicity | 0.209 | 0.240 | 0.873 | 0.383 | −0.263 | 0.681 |
| Summation x Discrimination | 1.760 | 1.324 | 1.329 | 0.185 | −0.849 | 4.369 |
| Ethnicity x Discrimination | 2.568 | 8.206 | 0.313 | 0.755 | −13.598 | 18.734 |
| Summation x Ethnicity x Discrimination | 0.719 | 1.324 | 0.543 | 0.588 | −1.890 | 3.328 |
| Train Number | 1.651 | 0.156 | 10.550 | <0.001 | 1.343 | 1.958 |
| Stimulus Intensity | 0.535 | 0.134 | 3.992 | <0.001 | 0.271 | 0.799 |
| Trauma Exposure | 0.736 | 1.012 | 0.728 | 0.467 | −1.257 | 2.729 |
| Sex | −0.340 | 1.475 | −0.231 | 0.818 | −3.245 | 2.564 |
| Education | 0.848 | 2.209 | 0.384 | 0.701 | −3.504 | 5.199 |
| Income | 0.484 | 0.785 | 0.616 | 0.538 | −1.063 | 2.030 |
| General Health Perception | −0.171 | 0.114 | −1.496 | 0.136 | −0.396 | 0.054 |
| Age | −0.261 | 0.258 | −1.012 | 0.312 | −0.769 | 0.247 |
| BMI | 0.746 | 0.390 | 1.915 | 0.057 | −0.021 | 1.514 |
| Stress | −0.639 | 0.347 | −1.845 | 0.066 | −1.322 | 0.043 |
| Psych Distress | 36.897 | 26.272 | 1.404 | 0.161 | −14.855 | 88.649 |
| Blood Pressure | −0.220 | 0.193 | −1.138 | 0.256 | −0.601 | 0.161 |
|
| ||||||
| Estimates of Random Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | z-test | p-value | Lower | Upper |
|
| ||||||
| AR1 diagonal | 83.465 | 3.131 | 26.661 | <0.001 | 77.549 | 89.832 |
| AR1 rho | 0.272 | 0.030 | 9.057 | <0.001 | 0.212 | 0.330 |
| Intercept Variance | 474.538 | 44.641 | 10.630 | <0.001 | 394.635 | 570.618 |
| Intercept, Summation Covariance | 16.361 | 5.504 | 2.972 | 0.003 | 5.573 | 27.148 |
| Summation Variance | 12.092 | 1.217 | 9.933 | <0.001 | 9.927 | 14.730 |
Note: Stimulus Number/Summation=codes for temporal summation; AR=autoregressive; Bolded values are associated with significant estimates. Ethnicity was coded 0=non-Hispanic White, 1=Native American. Sex was coded 0=male, 1=female.
Figure 3.
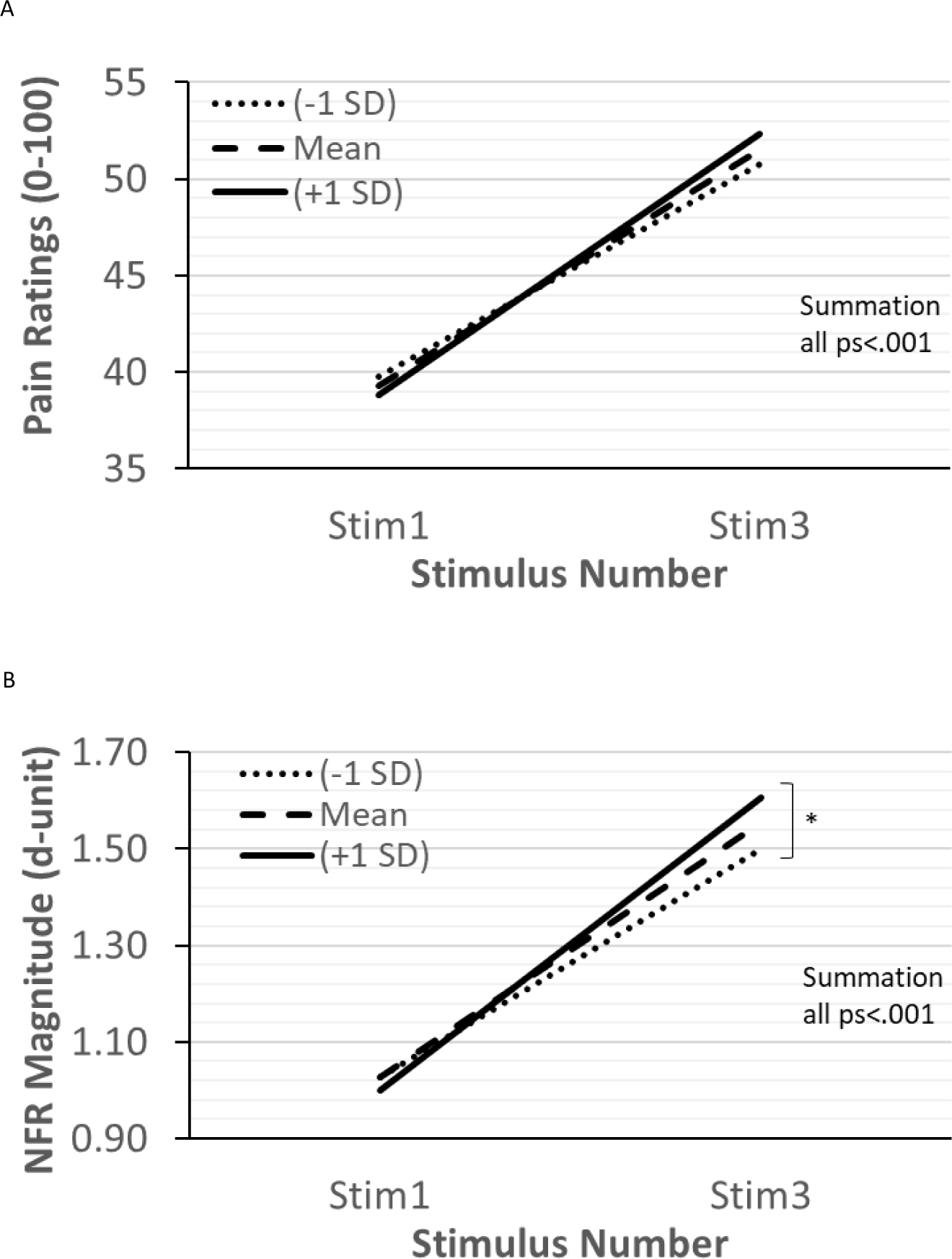
The relationships between experienced discrimination and temporal summation of pain (TS-Pain; panel A) and temporal summation of the nociceptive flexion reflex (TS-NFR; panel B). Discrimination did not impact TS-Pain (persons experiencing low, moderate, and high discrimination all showed similar pain summation), but TS-NFR was moderated by discrimination. Although persons experiencing low, moderate, and high discrimination all showed significant summation of NFR, higher discrimination was associated with greater TS-NFR (panel B).
Table 7 presents the results of the multilevel model predicting TS-NFR. The significant main effect of stimulus number/summation indicated that NFR summated, but this was moderated by discrimination. As shown in Fig 3B, summation of NFR increased with greater experienced discrimination. This was not moderated by ethnicity. The main effect of train number suggests that NFRs decreased across each of the 5 trains of stimuli. The main effect of education indicated that higher education was associated with smaller NFRs. Results of the random effects indicated that there was still significant variance left in the intercept and the summation slopes. There was also significant covariance between the intercept and summation slope suggesting that persons with larger NFRs to the first stimulus in each series showed less summation.
Table 7.
Results of multilevel model predicting nociceptive flexion reflexes during temporal summation
| Estimates of Fixed Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | t-test | p-value | Lower | Upper |
|
| ||||||
| Intercept | 1.013 | 0.053 | 19.038 | <0.001 | 0.908 | 1.117 |
| Ethnicity | −0.033 | 0.028 | −1.163 | 0.246 | −0.088 | 0.023 |
| Stimulus Number (Summation) | 0.270 | 0.017 | 16.323 | <0.001 | 0.237 | 0.302 |
| Discrimination | −0.080 | 0.179 | −0.449 | 0.654 | −0.432 | 0.271 |
| Summation x Ethnicity | 0.015 | 0.017 | 0.933 | 0.352 | −0.017 | 0.048 |
| Summation x Discrimination | 0.186 | 0.091 | 2.034 | 0.043 | 0.006 | 0.366 |
| Ethnicity x Discrimination | −0.096 | 0.147 | −0.654 | 0.514 | −0.386 | 0.194 |
| Summation x Ethnicity x Discrimination | −0.020 | 0.091 | −0.221 | 0.825 | −0.200 | 0.160 |
| Train Number | −0.020 | 0.004 | −1.420 | <0.001 | −0.028 | −0.011 |
| Stimulus Intensity | 0.002 | 0.002 | 0.663 | 0.508 | −0.003 | 0.006 |
| Trauma Exposure | 0.012 | 0.017 | 0.695 | 0.488 | −0.022 | 0.046 |
| Sex | −0.022 | 0.025 | −0.863 | 0.389 | −0.071 | 0.028 |
| Education | −0.079 | 0.037 | −2.116 | 0.035 | −0.152 | −0.005 |
| Income | 0.020 | 0.013 | 1.482 | 0.140 | −0.006 | 0.046 |
| General Health Perception | −0.002 | 0.002 | −0.795 | 0.427 | −0.005 | 0.002 |
| Age | 0.006 | 0.004 | 1.306 | 0.193 | −0.003 | 0.014 |
| BMI | −0.002 | 0.007 | −0.321 | 0.749 | −0.015 | 0.011 |
| Stress | 0.005 | 0.006 | 0.819 | 0.413 | −0.007 | 0.017 |
| Psych Distress | −0.140 | 0.447 | −0.314 | 0.754 | −1.021 | 0.740 |
| Blood Pressure | −0.001 | 0.003 | −0.279 | 0.781 | −0.007 | 0.006 |
|
| ||||||
| Estimates of Random Effects |
95% CI
|
|||||
| Parameter | Estimate | SE | z-test | p-value | Lower | Upper |
|
| ||||||
| AR1 diagonal | 0.128 | 0.003 | 39.247 | <0.001 | 0.122 | 0.134 |
| AR1 rho | 0.089 | 0.019 | 4.634 | <0.001 | 0.051 | 0.126 |
| Intercept Variance | 0.145 | 0.015 | 9.615 | <0.001 | 0.118 | 0.178 |
| Intercept, Summation Covariance | −0.028 | 0.007 | −3.719 | <0.001 | −0.042 | −0.013 |
| Summation Variance | 0.055 | 0.006 | 9.097 | <0.001 | 0.044 | 0.068 |
Note: Stimulus Number/Summation=codes for temporal summation; AR=autoregressive; Bolded values are associated with significant estimates. Ethnicity was coded 0=non-Hispanic White, 1=Native American. Sex was coded 0=male, 1=female.
Does Discrimination Promote Pronociceptive Processes in NAs?: Indirect (Mediated) Effect Tests
Table 8 presents the results of PROCESS bootstrapped indirect effect tests.
Table 8.
Results of bootstrapped indirect effects tests predicting nociceptive outcomes
| Boot 95% CI |
|||||
|---|---|---|---|---|---|
| Dependent Variable | Indirect Effect | Boot SE | Lower | Upper | R2 |
| NFR Threshold | 0.282 | 0.259 | −0.172 | 0.869 | 0.088 |
| 3-Stimulation NFR Threshold | 0.010 | 0.172 | −0.372 | 0.341 | 0.027 |
| CPM-Pain | 0.203 | 0.181 | −0.102 | 0.633 | 0.097 |
| CPM-NFR | 0.033 | 0.017 | 0.006 | 0.073 | 0.126 |
| TS-Pain | 0.548 | 0.371 | −0.014 | 1.406 | 0.079 |
| TS-NFR | 0.019 | 0.014 | −0.003 | 0.052 | 0.152 |
Note: All models included the following covariates: sex, age, MAP, BMI, general health perception, education, income, and trauma exposure. CPM/TS models controlled for the stimulation intensity of the electric stimulations delivered. CPM=conditioned pain modulation. NFR=nociceptive flexion reflex. TS=temporal summation. R-squared values represent the proportion of variance explained in the nociceptive outcome by the model.
Static Measures of Spinal Nociception.
Discrimination did not contribute to a NA disparity in NFR threshold or 3-Stimulation NFR threshold.
Conditioned Pain Modulation.
Discrimination did not contribute to a NA disparity in in pain inhibition (CPM-pain) but did contribute to a NA disparity in impaired inhibition of spinal nociception (CPM-NFR). The positive sign of the indirect effect indicates that NAs experienced higher discrimination that in turn impaired descending inhibition of spinal nociception.
Temporal Summation.
Discrimination did not contribute to a NA disparity in TS-pain or TS-NFR.
Discussion
This study examined the effects of discrimination on pronociceptive processes in NAs and NHWs. Discrimination was associated with greater TS-NFR (central sensitization) and impaired CPM-NFR (impaired inhibition of spinal nociception), even after controlling for exposure to traumatic events, perceived stress, and psychological distress. The strength of the relationships did not differ between ethnic groups, but discrimination served as a mediator between NA ethnicity and CPM-NFR, suggesting it promotes impaired descending inhibition of spinal nociception in NAs. Discrimination was not related to static measures of spinal sensitivity (NFR threshold, 3-Stimulation NFR threshold; suggesting results are not due to a general sensitization of motoneurons), TS-Pain, or CPM-Pain.
Discrimination is Associated with Modulation of Spinal Nociception
To our knowledge, this is the first study to find that discrimination is associated with enhanced spinal nociception (TS-NFR, CPM-NFR). Prior studies have found a positive relationship between discrimination and chronic pain conditions13,54, including back pain31, osteoarthritis48,116, knee pain118, and frequent/severe headaches44. Although it is rarely studied in experimental settings, experienced discrimination is associated with greater heat pain sensitivity48 and greater mechanical TS-Pain74 in chronic pain samples.
Our finding that discrimination led to spinal sensitization, but not pain sensitization (TS-Pain) or impaired pain inhibition (CPM-Pain), is somewhat surprising, as enhanced spinal nociception should contribute to greater pain. This discrepancy between spinal nociception and subjective pain ratings may result from separate modulatory processes occurring at spinal and supraspinal levels, respectively. Indeed, an imaging study82 found that CPM-Pain and CPM-NFR are mediated by distinct cerebral and cerebrospinal mechanisms, respectively. Similarly, TS-NFR and TS-Pain are also likely modulated by separate circuits and have been found to diverge (e.g., pain catastrophizing influences TS-Pain but not TS-NFR99). Consistent with this interpretation, the correlation between CPM-pain and CPM-NFR was r=−.009 (p=.88) and between TS-pain and TS-NFR was r=.195 (p=.001).
Although discrimination predicted enhanced TS-NFR, it was not related to static measures of spinal sensitivity (i.e., NFR threshold, 3-stimulation NFR threshold). This implies discrimination may contribute to spinal sensitization via increased N-methyl-D-aspartate receptor activity, which is believed to be responsible for TS-NFR53. Together, our results indicate that discrimination may contribute to impaired descending inhibition of spinal nociception as well as spinal facilitation without similarly impairing pain inhibition or pain facilitation.
Does Discrimination Lead to Latent Sensitization?
Since discrimination predicted enhanced spinal nociception without predicting enhanced pain, discrimination may promote latent sensitization (LS; see Fig 4), a chronic pain vulnerability observed in animal models defined as the presence of spinal sensitization in “silent form” without signs of pain hypersensitivity115. LS can be initiated after injuries (e.g., paw incision) or non-nociceptive environmental stressors70,87,102 that cause initial hyperalgesia. However, hyperalgesia is brief because it is offset by compensatory endogenous inhibitory processes, which resolve the hyperalgesia. Yet, spinal sensitization remains23. The sensitization is “latent” because it occurs without pain behaviors or hyperalgesia. According to the LS model, subsequent injuries/stressors can then trigger a re-emergence of hyperalgesia, promoting chronic pain onset. Correspondingly, psychosocial stressors, like discrimination, may serve as triggering events for LS-like states in humans87,102,115. Together, the cumulative effects of discrimination and other psychosocial stressors may cause fatigue in compensatory inhibitory systems, leading to hyperalgesia and chronic pain. LS may help explain the novel findings in the current study and serves as a potential phenotype for chronic pain risk in NAs. Importantly, these findings add to a growing body of evidence that a number of stress-related factors (e.g., sexual assault, cumulative trauma exposure, cardiometabolic allostatic load) may contribute to spinal sensitization without impacting pain perception57,95,97. Therefore, discrimination seems to also promote chronic pain risk through a LS-like mechanism.
Figure 4.
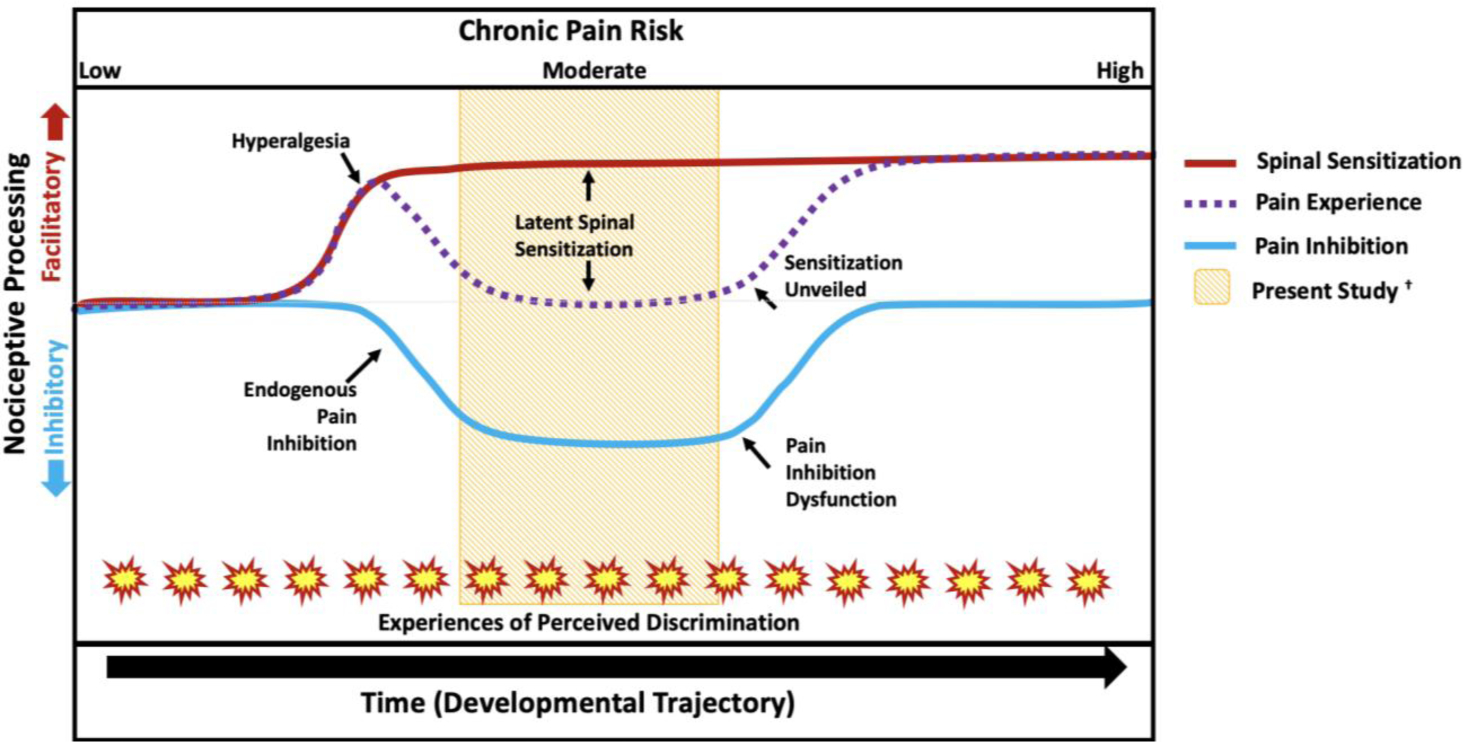
Hypothetical developmental trajectory for the effect of discrimination on latent sensitization and chronic pain risk, adapted from Rhudy and Hellman93 and Taylor and Corder115. Together, OK-SNAP studies suggest that psychosocial stressors increase pain risk, which can occur via a process akin to latent sensitization. Under low chronic pain risk, it is expected that pain regulatory systems function adequately. However, when an injury/stressor is encountered this can produce spinal sensitization and a temporary hyperalgesia that is offset via endogenous pain inhibition. Risk increases as time progresses and with an increase of stressors (e.g., experiences of discrimination). The added burden could lead to an eventual dysfunction of pain inhibition, allowing pain facilitation, and an “unveiling” of latent spinal sensitization. The yellow band in the middle represents the time in the trajectory in which the present sample was likely assessed (after hyperalgesia had been resolved).
Discrimination Hurts, Regardless of Ethnicity
The current and prior13,48,74,118 studies support the argument that discrimination is associated with chronic pain risk. But the present study extends the body of research on the pronociceptive effects of discrimination by assessing healthy, pain-free individuals without chronic pain. Importantly, this difference in our sample may explain why studies with chronic pain patients found a relationship between discrimination and hyperalgesia while the present study did not48,74 (Fig 4). Indeed, discrimination would be expected to promote hyperalgesia once endogenous pain inhibition fails and chronic pain ensues. By contrast, the model in Figure 4 hypothesizes that discrimination would only lead to a temporary hyperalgesia in individuals who are currently pain-free, because compensatory pain inhibitory mechanisms would activate to offset it.
Contrary to our hypothesis that the effect of discrimination would be stronger in NAs than in NHWs, discrimination was equally associated with enhanced spinal sensitization and impaired descending inhibition of spinal nociception in both groups. However, discrimination may still lead to increased pain risk in NAs because they report higher experienced discrimination. Consistent with this, we found that discrimination was a mediator for impaired descending inhibition of spinal nociception (CPM-NFR) for NAs. Indeed, recent analyses of longitudinal follow-up data from OK-SNAP found that impaired CPM-NFR prospectively predicted individuals who developed chronic pain, that NAs developed chronic pain at higher rates, and that impaired CPM-NFR mediates this NA pain disparity96. Therefore, experienced discrimination appears to contribute to chronic pain risk in NAs given that NAs experience higher rates of discrimination and discrimination is associated with impaired CPM-NFR.
Treatment and Clinical Implications
Health inequities are often explained by innate or biological deficits in minoritized groups in comparison to NHWs, when in reality, there are larger, systemic factors not considered or insufficiently measured11,12,62. Indeed, public health experts recommend that in order to reduce health inequities, it is vital to better define, understand, and address societal factors that perpetuate them123. Unfortunately, developing interventions that specifically target the intersectional effects of discrimination is challenging due to the nature of discrimination as a structural and systematic psychosocial construct.
Additionally, research shows that disease trajectories may often start early and persist throughout the lifespan unless addressed26,62. Exposure to early life adversity during sensitive developmental periods may be associated with pronociceptive effects in the future107, such as pain amplification132 and spinal sensitization52,132,133. It is recommended to develop and implement interventions that address early life adversity (perhaps through a traumatic-stress perspective18) and focus on early-childhood to foster protective factors and encourage adaptation to persistent stress62. For instance, researchers have found that intergenerational communication between youth, adults, and elders about the meaning of cultural identity and group membership can foster resiliency in young NAs120. The pain research community has also long-considered how to address pain inequities by increasing advocacy and enacting policy to increase the reach of relevant findings16.
Providers may also benefit from routine assessment of discrimination and its potential effect on the patient’s well-being114, particularly for those who are already at high-risk for chronic pain118. Importantly, practitioners are urged to continuously seek understanding of the cultures of the communities they serve22,58. Additionally, brief cognitive-behavioral interventions have been found to reduce spinal sensitization104, and relaxation strategies increase inhibition of spinal nociception35,94 Finally, it is vital to consider these implications within a social justice framework, as this may encourage the advocacy necessary to bring forth systemic changes that are long overdue.
Limitations and Future Directions
Since only healthy and pain-free individuals were recruited, the generalizability of our findings to other populations is limited. Further, variances for the discrimination variable were unequal. Results should be interpreted with caution given this might have impacted the magnitude of the group-level relationships. Although multiple tribes were represented, results may not generalize to NAs from other regions. In fact, NA scholars often lament that the heterogeneity of tribal nations is overlooked, and instead NAs are considered one monolithic group46. Additionally, requiring CDIB/tribal membership cards as proof of ethnic identity can limit inclusion of participants that are unable to obtain such documentation. Given that pain risk is influenced by the experience of discrimination, extending our findings to others that self-identify as NA (but without CDIB/tribal membership) may be important. Next, the current study was cross-sectional and correlational which does not allow for determination of causal effects. Also, no family-wise Type I error correction was conducted, thus results should be interpreted with caution until replicated. Further, our assessment of TS-pain and TS-NFR involved different procedures that might have led to divergent relationships with discrimination, although this cannot explain the divergent findings with CPM-pain and CPM-NFR.
The EDS is a widely-used instrument, but it does not measure several important aspects of discrimination (e.g., frequency, chronicity, distress caused) thus, assessment of discrimination could be improved. It might also be important to assess the developmental period during which discrimination occurred, given that early life adversity can be related to pain risk132,133. Finally, participants in this study reported more psychological distress and higher education than participants who did not contribute data, which may limit the generalizability of the results.
Summary
Discrimination was associated with spinal sensitization (enhanced TS-NFR, impaired CPM-NFR) in NAs and NHWs, but produced a NA disparity in impaired descending inhibition of spinal nociception. These findings provide preliminary evidence that discrimination promotes latent sensitization (spinal sensitization in the absence of hyperalgesia), which might increase chronic pain risk.
Perspective:
This study found that discrimination was associated with spinal sensitization and impaired descending inhibition of spinal nociception. These findings bolster our understanding of how social stressors experienced disproportionately by minoritized groups can contribute to pain outcomes.
Highlights.
Native Americans experience higher rates of chronic pain than other U.S. groups
Native Americans reported greater experienced discrimination
Discrimination mediated an inequity in impaired inhibition of spinal nociception
Thus, experienced discrimination may contribute to Native American pain inequities
Acknowledgments:
The authors would like to express gratitude to the Tribal Nations and Indigenous Peoples for their contributions to our ongoing research. The authors would also like to thank Burkhart Hahn, Heather Coleman, Kathryn Thompson, Jessica Fisher, Samuel Herbig, Ky’Lee Barnoski, Grey Howard, Garrett Newsom, Michael Payne, and Lucinda Chee for their help with data collection, as well as Dr. John Chaney for his consultation on the project.
Disclosures: This research was supported by the National Institute on Minority Health and Health Disparities of the National Institute of Health under Award Number R01MD007807. Yvette Güereca (DGE-1009425), Edward Lannon (DGE-1546597), and Shreela Palit (DGE-1546597) were supported by the National Science Foundation Graduate Research Fellowship Program. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health, National Science Foundation, Indian Health Service, or the Cherokee Nation. Aspects of this research have been presented at the 2019 American Pain Society conference. The authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman BM, Rasch EK: Disability among Native Americans. In: Using Survey Data to Study Disability: Results from the National Health Survey on Disability, Emerald Group Publishing Limited, 2003, pp. 299–326. [Google Scholar]
- 2.American Psychological Association: Stress in America: The impact of discrimination. In: Stress in America™ Survey, APA, 2016. [Google Scholar]
- 3.Arendt-Nielsen L, Brennum J, Sindrup S, Bak P. Electrophysiological and psychophysical quantification of temporal summation in the human nociceptive system. European Journal of Applied Physiology and Occupational Physiology. 68:266–273, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Petersen-Felix S. Wind-up and neuroplasticity: is there a correlation to clinical pain? European journal of anaesthesiology. Supplement. 10:1–7, 1995 [PubMed] [Google Scholar]
- 5.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. The Journal of Pain. 10:556–572, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, Curatolo M. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 107:7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Barnes PM, Adams PF, Powell-Griner E: Health characteristics of the American Indian or Alaska Native adult population: United States, 2004–2008.(Services, U.S.D.o.H.a.H., Ed.), National Center for Health Statistics, Hyattesville, MD, 2010. [PubMed] [Google Scholar]
- 8.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. British journal of anaesthesia. 111:52–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bebbington PE, Nayani T. The Psychosis Screening Questionnaire. International Journal of Methods in Psychiatric Research. 5:11–19, 1995 [Google Scholar]
- 10.Blyth FM, Macfarlane GJ, Nicholas MK. The contribution of psychosocial factors to the development of chronic pain: the key to better outcomes for patients? Pain. 129:8–11, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Bronfenbrenner U Toward an experimental ecology of human development. American psychologist. 32:513, 1977 [Google Scholar]
- 12.Bronfenbrenner U: Ecological systems theory, Jessica Kingsley Publishers, 1992. [Google Scholar]
- 13.Brown TT, Partanen J, Chuong L, Villaverde V, Griffin AC, Mendelson A. Discrimination hurts: The effect of discrimination on the development of chronic pain. Social Science & Medicine. 204:1–8, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Bulls HW, Freeman EL, Anderson AJ, Robbins MT, Ness TJ, Goodin BR. Sex differences in experimental measures of pain sensitivity and endogenous pain inhibition. Journal of pain research. 8:311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess DJ, Grill J, Noorbaloochi S, Griffin JM, Ricards J, van Ryn M, Partin MR. The effect of perceived racial discrimination on bodily pain among older African American men. Pain Medicine. 10:1341–1352, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Campbell LC, Robinson K, Meghani SH, Vallerand A, Schatman M, Sonty N. Challenges and opportunities in pain management disparities research: Implications for clinical practice, advocacy, and policy. The Journal of Pain. 13:611–619, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlisle SK. Perceived discrimination and chronic health in adults from nine ethnic subgroups in the USA. Ethnicity & health. 20:309–326, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Carter RT, Pieterse AL: Measuring the Effects of Racism Guidelines for the Assessment and Treatment of Race-Based Traumatic Stress Injury, Columbia University Press, New York, 2020. [Google Scholar]
- 19.Chae DH, Walters KL. Racial discrimination and racial identity attitudes in relation to self-rated health and physical pain and impairment among two-spirit American Indians/Alaska Natives. American Journal of Public Health. 99:S144–S151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi K-H, Paul J, Ayala G, Boylan R, Gregorich SE. Experiences of discrimination and their impact on the mental health among African American, Asian and Pacific Islander, and Latino men who have sex with men. American Journal of Public Health. 103:868–874, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 24:385–396, 1983 [PubMed] [Google Scholar]
- 22.Comas-Díaz L, Hall GN, Neville HA. Racial trauma: Theory, research, and healing: Introduction to the special issue. American Psychologist. 74:1, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf J, Mogil J, Storm D. Constitutive μ-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 341:1394–1399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross SL, Day AG. American Indians’ response to physical pain: functional limitations and help-seeking behaviors. Journal of social work in disability & rehabilitation. 14:176–191, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report. 67:1001, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & behavior. 106:29–39, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Derogatis LR: SCL-90-R : symptom checklist-90-R : administration, scoring & procedures manual, [National Computer Systems, Inc.], [Minneapolis, Minn.], 1994. [Google Scholar]
- 28.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine. 31:2724–2727, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Dugan SA, Lewis TT, Everson-Rose SA, Jacobs EA, Harlow SD, Janssen I. Chronic discrimination and bodily pain in a multiethnic cohort of midlife women in the Study of Women’s Health Across the Nation. Pain. 158:1656–1665, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 94:133–137, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Edwards RR. The association of perceived discrimination with low back pain. Journal of Behavioral Medicine. 31:379–389, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): Association with clinical variables. Pain. 106:427–437, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Ehrhardt MD, Gray KN, Kuhn BL, Lannon EW, Palit S, Sturycz CA, Güereca YM, Payne MF, Hellman N, Toledo TA, Hahn BJ, Rhudy JL, Shadlow JO. A qualitative analysis of pain meaning: Results from the Oklahoma Study of Native American Pain Risk (OK-SNAP). Ethnicity & Health. 20:1–12, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Eide PK. Wind-up and the NMDA receptor complex from a clinical perspective. European Journal of Pain. 4:5–15, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Emery CF, Keefe FJ, France CR, Affleck G, Waters S, Fondow MD, McKee DC, France JL, Hackshaw KV, Caldwell DS. Effects of a brief coping skills training intervention on nociceptive flexion reflex threshold in patients having osteoarthritic knee pain: a preliminary laboratory study of sex differences. Journal of Radiology Nursing. 25:128, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Essed P: Understanding everyday racism: An interdisciplinary theory, Sage, 1991. [Google Scholar]
- 37.Farrell M, Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Medicine. 8:514–520, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Ferucci ED, Templin DW, Lanier AP. Rheumatoid arthritis in American Indians and Alaska Natives: a review of the literature. Seminars in Arthritis and Rheumatism. 34:662–667, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Fillingim RB: Sex, gender and pain, 2001. [Google Scholar]
- 40.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL III. Sex, gender, and pain: A review of recent clinical and experimental findings. The Journal of Pain. 10:447–485, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fillingim RB, Maixner W. The influence of resting blood pressure and gender on pain responses. Psychosomatic Medicine. 58:326–332, 1996 [DOI] [PubMed] [Google Scholar]
- 42.France CR, Rhudy JL, McGlone S. Using normalized EMG to define the Nociceptive Flexion Reflex (NFR) threshold: Further evaluation of standardized scoring criteria. Pain. 145:211–218, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological bulletin. 133:581, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Gee GC, Spencer MS, Chen J, Takeuchi D. A nationwide study of discrimination and chronic health conditions among Asian Americans. American journal of public health. 97:1275–1282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia--when the spine echoes what the brain expects. Pain. 130:137, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Gone JP. A community-based treatment for Native American historical trauma: Prospects for evidence-based practice. Journal of Consulting and Clinical Psychology. 77:751–762, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, Cruz-Almeida Y, Staud R. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and above with knee osteoarthritis: ethnic differences. Psychosomatic medicine. 76:302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodin BR, Pham QT, Glover TL, Sotolongo A, King CD, Sibille KT, Herbert MS, Cruz-Almeida Y, Sanden SH, Staud R. Perceived racial discrimination, but not mistrust of medical researchers, predicts the heat pain tolerance of African Americans with symptomatic knee osteoarthritis. Health Psychology. 32:1117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grandner MA, Hale L, Jackson N, Patel NP, Gooneratne NS, Troxel WM. Perceived racial discrimination as an independent predictor of sleep disturbance and daytime fatigue. Behavioral sleep medicine. 10:235–249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric Properties of the Life Events Checklist. Assessment. 11:330–341, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Grollman EA. Multiple forms of perceived discrimination and health among adolescents and young adults. Journal of Health and Social Behavior. 53:199–214, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Güereca Y, Kuhn BL, Lannon E, Palit S, Sturycz C, Payne M, Hellman N, Toledo T, Huber F, Demuth M. (265) The Relationship between Discrimination and Pain Tolerance and its Potential Mediation by Stress: Results from the Oklahoma Study of Native American Pain Risk (OK-SNAP). The Journal of Pain. 20:S40–S41, 2019 [Google Scholar]
- 53.Guirimand F, Dupont X, Brasseur L, Chauvin M, Bouhassira D. The effects of ketamine on the temporal summation (wind-up) of the R(III) nociceptive flexion reflex and pain in humans. Anesthesia and Analgesia. 90:408–414, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Hansen KL. Ethnic discrimination and health: the relationship between experienced ethnic discrimination and multiple health domains in Norway’s rural Sami population. International journal of circumpolar health. 74:25125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayes AF: Introduction to mediation, moderation, and conditional process analysis: A regression-based approach, Guilford Publications, 2017. [Google Scholar]
- 56.Haywood C, Diener-West M, Strouse J, Carroll CP, Bediako S, Lanzkron S, Haythornthwaite J, Onojobi G, Beach MC, Woodson T. Perceived discrimination in health care is associated with a greater burden of pain in sickle cell disease. Journal of pain and symptom management. 48:934–943, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hellman N, Sturycz C, Lannon E, Kuhn BL, Güereca Y, Toledo T, Payne M, Huber F, Demuth M, Palit S, Shadlow JO, Rhudy JL. Conditioned pain modulation in sexual assault survivors. The Journal of Pain. 20:1027–1039, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Helms JE, Nicolas G, Green CE. Racism and ethnoviolence as trauma: Enhancing professional and research training. Traumatology. 18:65–74, 2012 [Google Scholar]
- 59.Herbert MS, Goodin BR, Bulls HW, Sotolongo A, Petrov ME, Edberg JC, Bradley LA, Fillingim RB. Ethnicity, Cortisol, and Experimental Pain Responses Among Persons With Symptomatic Knee Osteoarthritis. The Clinical journal of pain. 33:820–826, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson-Jennings MD, Belcourt A, Town M, Walls ML, Walters KL. Racial discrimination’s influence on smoking rates among American Indian Alaska Native two-spirit individuals: does pain play a role? Journal of health care for the poor and underserved. 25:1667–1678, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jöreskog KG, Sörbom D. LISREL 8.80. Chicago: Scientific Software International. 2006 [Google Scholar]
- 62.Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews. 35:2–16, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Kell PA, Hellman N, Huber FA, Lannon EW, Kuhn BL, Sturycz CA, Toledo TA, Demuth MJ, Hahn BJ, Shadlow JO, Rhudy JL. The relationship between adverse life events and endogenous inhibition of pain and spinal nociception: Findings from the Oklahoma Study of Native American Pain Risk (OK-SNAP). The Journal of Pain. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC. Reliability of conditioned pain modulation: A systematic review. PAIN. 157:2410–2419, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kenny D: Power and N Computations for Mediation Available at: https://davidakenny.shinyapps.io/MedPower/
- 66.Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. Journal of health and social behavior.208–230, 1999 [PubMed] [Google Scholar]
- 67.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 70:41, 1997 [DOI] [PubMed] [Google Scholar]
- 68.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The Journal of Pain. 10:895–926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. The Clinical Journal Of Pain. 13:189, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin J-P, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. The Journal of Pain. 12:1069–1079, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Leake J, Jozzy S, Uswak G. Severe dental caries, impacts and determinants among children 2–6 years of age in Inuvik Region, Northwest Territories, Canada. Journal (Canadian Dental Association). 74:519–519, 2008 [PubMed] [Google Scholar]
- 72.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 63:341–351, 1995 [DOI] [PubMed] [Google Scholar]
- 73.Manresa JAB, Neziri AY, Curatolo M, Arendt-Nielsen L, Andersen OK. Test–retest reliability of the nociceptive withdrawal reflex and electrical pain thresholds after single and repeated stimulation in patients with chronic low back pain. European journal of applied physiology. 111:83–92, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Mathur VA, Kiley KB, Haywood C Jr, Bediako SM, Lanzkron S, Carroll CP, Buenaver LF, Pejsa M, Edwards RR, Haythornthwaite JA. Multiple levels of suffering: discrimination in health-care settings is associated with enhanced laboratory pain sensitivity in sickle cell disease. The Clinical journal of pain. 32:1076, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mauldin J, Cameron HD, Jeanotte D, Solomon G, Jarvis JN. Chronic arthritis in children and adolescents in two Indian health service user populations. BMC Musculoskeletal Disorders. 5:30–30, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu. Rev. Psychol. 58:201–225, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mechlin MB, Heymen S, Edwards CL, Girdler SS. Ethnic differences in cardiovascular-somatosensory interactions and in the central processing of noxious stimuli. Psychophysiology. 48:762–773, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature. 206:97, 1965 [DOI] [PubMed] [Google Scholar]
- 79.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychological bulletin. 135:531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perrotta A, Bolla M, Anastasio MG, Serrao M, Sandrini G, Pierelli F. Modulation of temporal summation threshold of the nociceptive withdrawal reflex by transcutaneous spinal direct current stimulation in humans. Clinical Neurophysiology. 127:755–761, 2016 [DOI] [PubMed] [Google Scholar]
- 81.Peters ML, Schmidt AJ, Van den Hout MA, Koopmans R. Chronic back pain, acute postoperative pain and the activation of diffuse noxious inhibitory controls (DNIC). Pain. 50:177, 1992 [DOI] [PubMed] [Google Scholar]
- 82.Piché M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. Journal of Neuroscience. 29:14236–14246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibition in chronic tension-type headache. Pain. 118:215–223, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 36:717–731, 2004 [DOI] [PubMed] [Google Scholar]
- 85.Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Experimental Neurology. 37:371–387, 1972 [DOI] [PubMed] [Google Scholar]
- 86.Price DD, Hayes RL, Ruda M, Dubner R. Neural representation of cutaneous aftersensations by spinothalamic tract neurons. Federation Proceedings. 37:2237, 1978 [PubMed] [Google Scholar]
- 87.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends in neurosciences. 32:611–618, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rhee H Prevalence and predictors of headaches in US adolescents. Headache: The Journal of Head and Face Pain. 40:528–538, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Rhee H Racial/ethnic differences in adolescents’ physical symptoms. Journal of Pediatric Nursing. 20:153–162, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain. 128:244–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: a comparison of different scoring criteria. Pain. 128:244–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhudy JL, France CR, Bartley EJ, McCabe KM, Williams AE. Psychophysiological responses to pain: Further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiology. 46:939–948, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Rhudy JL, Hellman N: Adverse life events, spinal sensitization, and chronic pain risk. In: Pain, Anesthetics and Analgesics: Book 3. Neurobiology, Physiology and Behaviour of Pain.(Preedy VR, Rajendram R, Patel VB, Martin C, Eds.), Elsevier, San Diego, CA, 2020. [Google Scholar]
- 94.Rhudy JL, Hellman N, Sturycz C, Toledo T, Palit S. Modified biofeedback (Conditioned Biofeedback) promotes antinociception by increasing the nociceptive flexion reflex threshold and reducing temporal summation of pain: a controlled trial. The Journal of Pain. 2019 [DOI] [PubMed] [Google Scholar]
- 95.Rhudy JL, Huber F, Kuhn BL, Lannon E, Palit S, Payne M, Hellman N, Sturycz C, Güereca Y, Toledo T. Pain-related anxiety promotes pronociceptive processes in Native Americans: bootstrapped mediation analyses from the Oklahoma Study of Native American Pain Risk. Pain Reports. 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rhudy JL, Huber F, Toledo TA, Kell PA, Street EN, Shadlow JO. Psychosocial and cardiometabolic predictors of chronic pain onset in Native Americans: Serial mediation analyses of 2-year prospective data from the Oklahoma Study of Native American Pain Risk. PAIN. 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rhudy JL, Kuhn BL, Demuth M, Huber F, Hellman N, Toledo T, Lannon E, Palit S, Payne M, Sturycz C, Kell P, Güereca Y, Shadlow JO. Are Cardiometabolic Markers of Allostatic Load Associated With Pronociceptive Processes in Native Americans?: A Structural Equation Modeling Analysis From the Oklahoma Study of Native American Pain Risk. Journal of Pain. 22:1429–1451, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rhudy JL, Lannon EW, Kuhn BL, Palit S, Payne MF, Sturycz CA, Hellman N, Guereca YM, Toledo TA, Huber F, Demuth MJ, Hahn BJ, Chaney JM, Shadlow JO. Assessing peripheral fibers, pain sensitivity, central sensitization, and descending inhibition in Native Americans: Main findings from the Oklahoma Study of Native American Pain Risk. PAIN. 161:388–404, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rhudy JL, Martin SL, Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL. Pain catastrophizing is related to temporal summation of pain, but not temporal summation of the nociceptive flexion reflex. Pain. 152:794–801, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Rhudy JL, Williams AE: Sex differences in affective modulation of pain and the nociceptive flexion reflex. In: Society for Psychophysiological Research Abstracts, Savannah, GA, 2007. [Google Scholar]
- 101.Rios R, Zautra AJ. Socioeconomic disparities in pain: The role of economic hardship and daily financial worry. Health Psychology. 30:58–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rivat C, Laboureyras E, Laulin J-P, Le Roy C, Richebé P, Simonnet G. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 32:2217–2228, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Roby-Brami A, Bussel B. Long-latency spinal reflex in man after flexor reflex afferent stimulation. Brain. 110:707–725, 1987 [DOI] [PubMed] [Google Scholar]
- 104.Salomons TV, Moayedi M, Erpelding N, Davis KD. A brief cognitive-behavioural intervention for pain reduces secondary hyperalgesia. PAIN®. 155:1446–1452, 2014 [DOI] [PubMed] [Google Scholar]
- 105.Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Progress in Neurobiology. 77:353–395, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Schmitt MT, Branscombe NR, Postmes T, Garcia A. The consequences of perceived discrimination for psychological well-being: a meta-analytic review. Psychological bulletin. 140:921, 2014 [DOI] [PubMed] [Google Scholar]
- 107.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. Jama. 301:2252–2259, 2009 [DOI] [PubMed] [Google Scholar]
- 108.Sohn L, Harada ND. Effects of racial/ethnic discrimination on the health status of minority veterans. Military medicine. 173:331–338, 2008 [DOI] [PubMed] [Google Scholar]
- 109.Sorkin DH, Ngo-Metzger Q, De Alba I. Racial/ethnic discrimination in health care: impact on perceived quality of care. Journal of General Internal Medicine. 25:390–396, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ Jr. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 102:87–95, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. The Journal Of Pain: Official Journal Of The American Pain Society. 8:893–901, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 91:165–175, 2001 [DOI] [PubMed] [Google Scholar]
- 113.Sturycz CA, Hellman N, Payne MF, Kuhn BL, Hahn B, Lannon EW, Palit S, Güereca YM, Toledo TA, Shadlow JO, Rhudy JL. Race/Ethnicity Does Not Moderate the Relationship Between Adverse Life Experiences and Temporal Summation of the Nociceptive Flexion Reflex and Pain: Results From the Oklahoma Study of Native American Pain Risk. The Journal of Pain. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sue DW, Sue D, Neville HA, Smith L: Counseling the culturally diverse: Theory and practice, John Wiley & Sons, 2019. [Google Scholar]
- 115.Taylor BK, Corder G: Endogenous analgesia, dependence, and latent pain sensitization. In: Behavioral Neurobiology of Chronic Pain, Springer, 2014, pp. 283–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taylor JLW, Campbell CM, Thorpe RJ, Whitfield KE, Nkimbeng M, Szanton SL. Pain, Racial Discrimination, and Depressive Symptoms among African American Women. Pain Management Nursing. 19:79–87, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL, Vincent AL, Rhudy JL. Standardizing procedures to study sensitization of human spinal nociceptive processes: Comparing parameters for temporal summation of the nociceptive flexion reflex (TS-NFR). International Journal of Psychophysiology. 81:263–274, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Terry EL, Fullwood MD, Booker SQ, Cardoso JS, Sibille KT, Glover TL, Thompson KA, Addison AS, Goodin BR, Staud R. Everyday Discrimination in Adults with Knee Pain: The Role of Perceived Stress and Pain Catastrophizing. Journal of Pain Research. 13:883, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ware JE, Snow KK, Kosinski M, Gandek B: SF-36 Health Survey manual and interpretation guide, The Health Institute New England Medical Center, Boston, 1993. [Google Scholar]
- 120.Wexler L Looking across three generations of Alaska Natives to explore how culture fosters indigenous resilience. Transcultural psychiatry. 51:73–92, 2014 [DOI] [PubMed] [Google Scholar]
- 121.Wilcox RR: Understanding and applying basic statistical methods using R, John Wiley & Sons, Hoboken, N.J., 2016. [Google Scholar]
- 122.Willer JC, Roby A, Le Bars D. Psychophysical and electrophysiological approaches to the pain-relieving effects of heterotopic nociceptive stimuli. Brain: A Journal Of Neurology. 107 ( Pt 4):1095, 1984 [DOI] [PubMed] [Google Scholar]
- 123.Williams DR, Cooper LA. Reducing racial inequities in health: Using what we already know to take action. International journal of environmental research and public health. 16:606, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williams DR, Costa MV, Odunlami AO, Mohammed SA. Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. Journal of public health management and practice: JPHMP. 14:S8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williams DR, Gonzalez HM, Williams S, Mohammed SA, Moomal H, Stein DJ. Perceived discrimination, race and health in South Africa. Social science & medicine. 67:441–452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Williams DR, Mohammed S: Racial Harassment/Discrimination. 2nd edition, Oxford, Academic Press, 2007. [Google Scholar]
- 127.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. Journal of behavioral medicine. 32:20–47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of health psychology. 2:335–351, 1997 [DOI] [PubMed] [Google Scholar]
- 129.Woolf CJ. Windup and central sensitization are not equivalent. Pain. 66:105–108, 1996 [PubMed] [Google Scholar]
- 130.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 44:293, 1991 [DOI] [PubMed] [Google Scholar]
- 131.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y. Prediction of chronic post-operative pain: preoperative DNIC testing identifies patients at risk. Pain. 2008 [DOI] [PubMed] [Google Scholar]
- 132.You DS, Meagher MW. Childhood adversity and pain sensitization. Psychosomatic medicine. 78:1084–1093, 2016 [DOI] [PubMed] [Google Scholar]
- 133.You DS, Meagher MW. Childhood adversity and pain facilitation. Psychosomatic Medicine. 80:869–879, 2018 [DOI] [PubMed] [Google Scholar]
- 134.You HJ, Dahl Morch C, Chen J, Arendt-Nielsen L. Simultaneous recordings of wind-up of paired spinal dorsal horn nociceptive neuron and nociceptive flexion reflex in rats. Brain Research. 960:235–245, 2003 [DOI] [PubMed] [Google Scholar]
- 135.You HJ, Morch CD, Arendt-Nielsen L. Electrophysiological characterization of facilitated spinal withdrawal reflex to repetitive electrical stimuli and its modulation by central glutamate receptor in spinal anesthetized rats. Brain Research. 1009:110–119, 2004 [DOI] [PubMed] [Google Scholar]


