Abstract
Recent advances in computational psychiatry have provided unique insights into the neural and cognitive underpinnings of psychotic symptoms. In particular, a host of new data has demonstrated the utility of computational frameworks for understanding how hallucinations might arise from alterations in typical perceptual processing. Of particular promise are models based in Bayesian inference that link hallucinatory perceptual experiences to latent states that may drive them. In this piece, we move beyond these findings to ask: how and why do these latent states arise, and how might we take advantage of heterogeneity in that process to develop precision approaches to the treatment of hallucinations? We leverage specific models of Bayesian inference to discuss components that might lead to the development of hallucinations. Using the unifying power of our model, we attempt to place disparate findings in the study of psychotic symptoms within a common framework. Finally, we suggest directions for future elaboration of these models in the service of a more refined psychiatric nosology based on predictable, testable, and ultimately treatable information processing derangements.
I. Introduction
Hallucinatory experiences are reported in a wide range of psychiatric (Blom, 2010) and other medical (Sommer et al., 2012a) disorders, contributing significantly to morbidity and health care system burden (Harkavy-Friedman et al., 2003; Waters and Dragovic, 2018). Antipsychotic medications and cognitive behavioral interventions are effective, but their impact is limited by adverse effects, poor adherence, and heterogeneity of response.
New knowledge of the brain circuitry involved in hallucinations has sparked hope of clinical advances in their treatment. Using non-invasive electroencephalography (EEG) and functional MRI (fMRI), researchers have successfully “captured” neural events accompanying the onset of auditory hallucinations (AH). These include heightened 40-Hz gamma-band thalamocortical oscillations and increased activity across a network of cortical regions of the superior temporal gyrus, middle temporal gyrus, anterior insula, frontal operculum, inferior parietal lobule, Broca’s area, and both hippocampal and parahippocampal regions (Blom, 2015). Such gamma-band thalamocortical oscillations in networks that include auditory cortex have long been linked with bottom-up auditory processing and experience more broadly, and thus do not necessarily represent a specifically hallucinatory perceptual signature (Llinás and Ribary 1994). Attempts to link these observed changes in functional networks with structural changes have been less successful. Investigations of brain structure are similarly limited in their ability to address causality, designed primarily as comparisons between groups of voice-hearers and non-voice hearers (Allen and Modinos, 2012).
These discoveries inspired explanatory models that attempted to relate the emergence of AH to known normative or pathological neurobiological or neurocognitive processes. For example, one early model attempted to couch AH as a variant of peduncular hallucinosis, a rare sequela of midbrain, pontine or thalamic lesions causing vivid, colorful visual hallucinations of people and animals (Cascino and Adams, 1986). This model emphasized the role of spontaneous cortical activity with loss of input (as seen in Charles-Bonnet syndrome and related disorders), making the case that AH could arise from unconstrained activity of neural ensembles with loss of meaningful input (Cole et al., 2002). Others highlighted the role of memory and prior experiences in the generation of AH (Copolov et al., 2003), which appealed to the involvement of nodes like the hippocampal complex during AH. Other models propose misattributed inner speech as a causative mechanism for AH (Mechelli et al., 2007), supported by growing evidence of disrupted corollary discharge in schizophrenia (Ford et al., 2001; Ford and Mathalon, 2005).
In recent years, computationally-oriented models have attempted to capture elements of the proposals above within a formalized framework for information processing in the brain (Adams et al., 2013; Powers et al., 2016; Sterzer et al., 2018). In one early computational approach, Hoffman and Dobscha demonstrated that excessive pruning of computational elements in a simulated speech-perception network produced hallucination-like phenomena in the network (Hoffman and Dobscha, 1989). Not only does this suggest a critical role for cortical disinhibition, but also serves to unify deafferentation-based models with histological evidence of excessive synaptic pruning in psychosis (Feinberg, 1982; Keshavan et al., 1994; Sellgren et al., 2019) under a formalized mathematical framework.
More recent efforts echo these findings using graph theory to frame auditory hallucinations as the product of an anomalous attractor state of the auditory processing neural network, able to sustain ongoing activity and network output even once all external input has been removed (Goekoop and Looijestijn, 2012). These anomalous self-reinforcing states are produced in the network models via “sensory deprivation”, instantiated as the degradation of model inputs. Goekoop and Looijestijn argue these degraded external inputs force the model into a more default resting state, wherein internal “noise” derived from the brain itself is now uninhibited and used as new input to sustain further activity and noise, ad infinitum.
Of late, Bayesian approaches to understanding psychotic symptoms have shown particular promise (Adams et al., 2013; K. Friston, 2005; Powers et al., 2016). In these formulations, perception is seen as the process of inferring the causes of sensory events, given both sensory evidence and prior beliefs about those causes, depending on the reliability of this information. Mathematically, this process is typically formalized as a combination of Gaussian probability distributions with a mean expected value and reliability coded as precision (or inverse variance) of the respective distributions (Friston 2005). Such Bayesian precision-weighted combination schemes have proven to be robust simulacra of perceptual processes across a range of contexts, including the ability to differentially weight visual and auditory information as demanded by the task (Alais and Burr, 2019, 2004). Importantly, in different situations and contexts, information coming from sensory evidence and prior beliefs may differ in their relative reliability, and therefore should be weighted accordingly during perception. For example, when navigating your house after a nighttime power outage, your prior beliefs about the relative positions of your furniture will be given more weight than the poor incoming sensory evidence from your eyes, helping you to avoid stubbing your toe on familiar furniture. In contrast, navigating a new friend’s house at a dinner party will require the opposite weighting, as your prior beliefs about their furniture arrangements will be relatively unreliable, having just encountered the novel environment of their dining room; thus, incoming sensory evidence should be weighted more heavily when inferring the environmental causes of that input. This process guides perception through a host of everyday tasks, from understanding speech in noisy environments (Lau et al., 2008; Noppeney et al., 2008) to the interpretation of shading in visual art (Lee and Mumford, 2003). It also occurs automatically and outside the realm of conscious awareness, although careful experimental manipulation has demonstrated its usefulness in explaining the behavior of humans, animals, and neuronal ensembles in sensory cortices during perceptual tasks (Baldeweg, 2006; Dayan, 1998; Petzschner et al., 2015; Schellekens et al., 2014).
If perception is the process of inferring one’s surroundings given information from both the senses and one’s priors, hallucinations--percepts in the absence of a corresponding stimulus--might arise from an aberrant over-weighting of priors during this process (Adams et al., 2013; K. J. Friston, 2005; Powers et al., 2016; Teufel et al., 2013). A confluence of recent studies support this hypothesis. Powers and colleagues used Pavlovian conditioning to produce hallucinations of tones in white noise, demonstrating that participants who hear voices were more susceptible to the conditioned hallucinations elicited by the task (Powers et al., 2017). Additionally, the networks associated with the conditioned hallucinations mirrored those implicated in studies of clinical symptom capture for those who hallucinate in the scanner. A follow-up study (Kafadar et al., 2020) showed that this susceptibility emerges early, during the prodromal or high-risk phase of psychosis. Alderson-Day and colleagues used sine-vocoded speech to show that individuals who report more baseline voice-hearing experiences were also more likely to hear speech at higher levels of degradation, implying a greater weighting of priors in those individuals in favor of perceiving human speech (Alderson-Day et al., 2017). In a novel study linking prior weighting to striatal dopamine, (Cassidy et al., 2018) showed that amphetamine pharmacologically induced hallucination-like phenomena in the form of a perceptual bias on a task identifying the length of an auditory stimulus under differing levels of uncertainty, and that perceptual bias was correlated with striatal dopamine release. Schmack et al later refined this even further, using a combination of mouse and human performance on a signal detection task combined with pharmacological manipulation and invasive imaging techniques (in the mice) to show that prestimulus striatal dopamine release was predictive of high-confidence false alarms, with striatal tail release most predictive of perceptual bias leading to higher false alarm rate, in a manner that coincided with hallucinogenic experience reports in human participants(Schmack et al. 2021). This framework has been applied to visual hallucinations as well, and extended beyond primary psychotic disorders. Zarkali and colleagues used a degraded two-tone image learning paradigm to test individuals with and without visual hallucinations secondary to lewy-body dementia, finding that the hallucinators were better able to integrate prior knowledge about the images to aid in the image discrimination task (Zarkali et al., 2019).
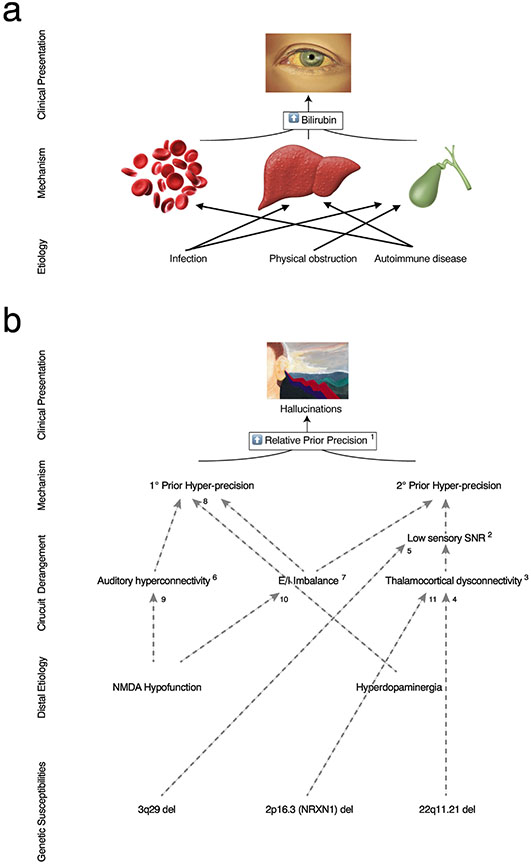
Taken together, available evidence favors a role for relative prior hyper-precision in hallucinations (Corlett et al., 2019). Here we include the modifier relative because the value of the prior precision appears to matter only in its relation to the precision of incoming sensory evidence. This means that, even if relative prior hyper-precision represents a common factor in hallucinations, different groups of people with hallucinations may arrive at this abnormality from a number of different initial derangements (Fig. 1b). For example, either an absolute decrease in sensory evidence precision or an absolute increase in prior precision will result in a relative increase in weighting of the prior (Nu; Fig. 2a) if the other remains constant. While there exists ample evidence to support relative overweighting of prior beliefs as a common pathway toward hallucinogenesis, there exists far less evidence regarding the hallucinogenic potential of aberrations in other parameters in the Hierarchical Gaussian Filter (HGF), a Bayesian model of active inference,which represent factors putatively involved in the process of active inference such as learning rate, belief stability and learner agency, such as those modeled here via the HGF (Fig. 2a). A more thorough review of the latter, including a fuller accounting of the existing gaps in the literature is beyond the scope of the current review and will appear instead in future works.
Figure 1. Pathways to hallucinogenesis via relative prior hyper-precision.
a. Medical nosology is based on a model of pathogenesis for given signs and symptoms. In this example, scleral icterus is known to be caused by high serum bilirubin. Hyperbilirubinemia itself is likely to be caused by dysfunction in a number of organ systems--increased production of bilirubin by breakdown of red blood cells, dysfunctional processing of bilirubin by the liver, or impaired clearance of bilirubin via the gallbladder. Dysfunction in these organ systems is caused by distal etiologies, including infection, autoimmune disease, or physical obstruction. Treatment is aimed at alteration of these distal causes, and tests allow clinicians to infer the causes of the signs or symptoms observed in order to identify the most appropriate interventions. Credit to Klaas Enno Stephan for the example, b. A map of potential causal pathways to hallucinogenesis with relative prior hyper-precision as a final causal step. In this example map, prior precision may be caused by inability to appropriately decrease precision of priors (primary relative prior hyper-precision) or by decreased precision of incoming sensory evidence (secondary relative prior hyper-precision), itself an adaptive response to low signal-to-noise ratio of sensory afferents. These abnormalities may be causally related to other findings commonly observed in psychosis. Rather than being an exhaustive depiction of pathways to hallucinogenesis, this causal map is meant to illustrate how recent findings may lead to a nosological map akin to that depicted in a. Superscripts denote references relevant to findings and causal pathways depicted. 1. (Cassidy et al., 2018; Corlett et al., 2019; Powers et al., 2017; Schmack et al., 2021; Teufel et al., 2015; Zarkali et al., 2019); 2.(Javitt et al., 1999; Javitt and Freedman, 2015; Rabinowicz et al., 2000); 3. (Li et al., 2017); 4. (Mancini et al., 2020); 5. (Baba et al., 2020); 6. (Hoffman and Hampson, 2011); 7. (Jardri et al., 2016); 8. (Cassidy et al., 2018; Horga and Abi-Dargham, 2014); 9. (Thiebes et al., 2018); 10. (Kehrer et al., 2008); 11. (Hughes et al., 2020; Pak et al., 2021)
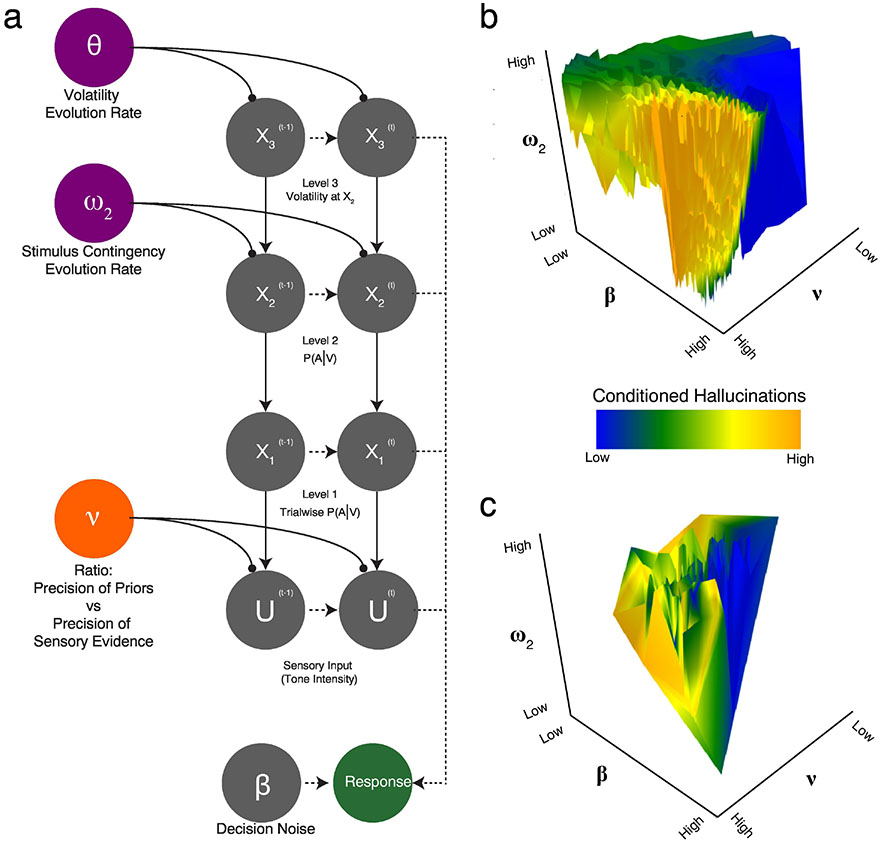
Figure 2. Model schematic for Hierarchical Gaussian Filter (HGF) model.
a. The HGF is a generative model of sensory observations, combining states of the world (X1-3) and sensory evidence (U) into percepts and behavioral responses (in combination with a response model) in a specific experimental context. In this case, the HGF is modeling responses during the Auditory Conditioned Hallucinations task (Powers et al., 2017). Crucially, this model can be inverted based upon data (i.e., actual participant responses and sensory evidence strength) to provide estimates of beliefs about states of the world (μ1-3) are generated from a combination of incoming sensory information and prior beliefs (X1) about those states, which can be differentially weighted (nu) in terms of their contribution to updating these parameters at some time later (t). These prior beliefs are themselves modified by beliefs about those beliefs (x2), and beliefs about those beliefs (x3), each of which is also affected by an evolution rate (w1 and w2, respectively), which can be thought of as the overall volatility of beliefs across time. b-c. Shown here are possible “maps” of hallucination space, or possible combinations of model parameters (from the model detailed in figure 2) that are predicted to lead to more or less hallucinations. Part a shows a hypothetical model space based on simulations generated to be consistent with trends observed in previous work: namely that as beta and nu increase, the chance of hallucinations increases as well. Part b shows a similar plot generated directly from data from a single study, that shows the power of such a mapping approach. What we see in this figure are local pockets of high and low hallucinogenic potential that do not map as neatly onto linear trends of individual model parameters, but rather reflect hallucinogenic “hotspots” that may or may not map onto individuals who are experiencing hallucinations in the moment.
One important consequence of using a framework of relative hyper-precision as a common denominator of hallucinogenesis is its ability to unify the numerous and disparate previously identified models under a single mechanistic umbrella, analogous to how the formulation of the frank-starling mechanism unified mechanisms of heart failure without eliminating the utility for consideration of the different specific causes of dropsy (eg. fluid overload leading to venous stasis as a primary driver of heart failure vs. myocardial infarction leading to decreased cardiac output leading to secondary fluid overload)(Delicce and Makaryus 2021).
Here we consider factors that might lead to hallucinations via alterations in Bayesian inference, deriving either primarily from these alterations or from processes further upstream. We focus primarily on the role of relative prior hyper-precision, caused by either an inability to adjust precision of one’s priors (i.e., primary prior hyper-precision) or as a response to poor-quality sensory evidence and an (adaptive) decrease in weighting on that evidence during perceptual inference (i.e., secondary prior hyper-precision). While these possibilities are discussed as separate paths to a common end, such a separation is artificial and imposed here for the sake of clarity and organization. It is certainly possible for multiple derangements to occur simultaneously, and indeed combinations of primary and secondary hyper-precision may be more hallucinogenic then either one in isolation. We then explore the potential behavioral and neural evidence for the existence of these pathways toward hallucinogenesis and how they may inform differential attempts at prevention and treatment. Lastly, we briefly explore how other, related factors of learning and inference may impact the expression of hallucinations, given relative prior hyper-precision.
II. Primary prior hyper-precision
Behavioral evidence
Because precision of priors is a latent (i.e., not directly observable) property of perceptual systems, it must be inferred from measurable external quantities. Thus, behavioral responses during perceptual tasks are taken as a proxy for what the participant perceives, and the precision of the sensory evidence is either set or varied by experimental design, allowing an estimation of relative--but not absolute--prior precision. One way to work around this limitation would be to manipulate prior precision directly, rather than (or along with), precision of incoming sensory evidence. Two recent studies attempted to address this. The first (Alderson-Day et al., 2017) used a sine-wave speech stimulus that is made intelligible or unintelligible using convolution with sine-wave frequency patterns. The authors then manipulated prior beliefs and expectations by having 2 groups of participants (non-clinical voice hearers, and non-voice hearers) perform a auditory discrimination task with the sine-wave speech being presented to participants as background noise, only asking participants at the end when they first noticed the intelligibility of the background noise. Despite an experimental intervention designed to shift participants’ prior expectations away from voice-hearing, those with a history of voice-hearing experiences identified intelligible speech earlier than those without such a history. The authors found differences in brain activity as measured by fMRI before and after the time at which participants said they first noticed the voices, a difference reasonably attributable to the influence of priors rather than sensory evidence. In the second (Powers et al., 2017), participants (a combination of non-clinical voice hearers, non-voice hearers and clinical voice hearers) were trained to associate an auditory tone with a visual stimulus such that, when the visual stimulus was presented alone, participants often “hallucinated” the tone. As in the first experiment, voice-hearers, regardless of clinical status, were more susceptible to these conditioned hallucinations. Both studies manipulated prior beliefs while attempting to keep sensory evidence fixed, though the latter study did this more explicitly: Powers and colleagues titrated the level of auditory signal-to-noise ratio individually for each subject prior to conditioning and used these values for subsequent stimulus presentations, essentially accounting for individual differences in auditory processing. Rates of stimulus detection were similar across groups, and voice-hearers had lower detection thresholds, which would be inconsistent with an account of the main effects having been driven solely by low precision of sensory evidence (a potential mechanism explored in section III below).
Potential neural correlates
There is emerging experimental evidence to suggest a mechanistic role for dopaminergic neurotransmitter pathway dysfunction in hallucinations, specifically tied to altered prior precision (Cassidy et al., 2018). Such a role has been long implicated clinically in psychosis based on observations of positive symptom amelioration with antidopaminergics (Arnold and Freeman, 1956; Lieberman et al., 1987; Seeman and Lee, 1975; Sommer et al., 2012b) and commensurate symptom induction with pro-dopaminergics (McKetin et al., 2013; Stowe et al., 2008). Under Bayesian models of perceptual inference, wherein the cause of incoming sensory evidence is inferred based on precision-weighted incoming sensory evidence and priors on the causes of those sensations, one hypothesized role of dopamine (and other neuromodulators) is the direct encoding of the precisions of these sources of information in neural circuits (Adams et al., 2013). To test this more directly, Cassidy and colleagues developed a novel perceptual inference task in which prior precision is manipulated directly: participants were asked to reproduce a tone of set length after exposure to a train of tones with either highly variable or more consistent lengths. In order to perform well on the task, participants needed to decrease their own prior precision under the highly variable condition, where the uncertainty of the predictive evidence was high. Both striatal dopamine release and hallucination severity in patients with schizophrenia spectrum disorders correlated with inability to decrease prior precision under high uncertainty. This inability to decrease prior precision could also be induced in healthy controls under the influence of amphetamine (Cassidy et al., 2018). Additionally, work by Katarina Shmack and colleagues(Schmack et al. 2021) linked hallucinatory experiences to striatal dopamine via a signal detection task in mice, with prior beliefs manipulated either via prior expectations or ketamine. Of note, the authors found that elevations in dopamine before stimulus onset predicted false alarms, with ventral striatal dopamine being more predictive of upcoming error probability and striatal tail dopamine reflecting upcoming signal choice probability. Importantly, administration of haloperidol, a d2 antagonist, to the striatal tail reversed these findings. Importantly, the authors backed up these mice studies with analogous human participant task data, finding that such false alarms correlate highly with self-reported hallucinations (ie. the human participants reported actually seeing something during many false-alarm trials, indicative of a hallucination rather than a false alarm due to non-perceptual factors such as accidental button press). These findings generally fit with established literature linking hyper-dopaminergia to risk for schizophrenia more broadly (Egerton et al., 2013; Howes et al., 2020, 2013) and hint at a possible neuro-computational mechanism for this risk.
While a full exploration of the link between hallucinations and various neurotransmitter systems is beyond the scope of the current review, we will briefly touch on some potential roles (and how they fit into primary vs. secondary accounts) here. Neural process models have explicitly linked top-down (prior-related) synaptic transmission to glutamatergic functioning as well via NMDA receptor function (K. Friston, 2005). Interestingly, NMDA receptor blockade with ketamine and phencyclidine (PCP) has been more associated with delusion-like ideas than hallucinations (Powers et al., 2015), whereas lysergic acid (LSD) may enhance NMDA function (Lambe and Aghajanian, 2006) and more reliably produces hallucinations. Intriguingly, recent work by Katrin Preller and colleagues has demonstrated that LSD-related hallucinations are completely blocked by ketanserin (a selective 5-HT2A receptor antagonist), making it unlikely that they function solely via an NMDAR-mediated mechanism (see also serotonin section below)(Preller et al. 2018) . That said, one recent study (Schmack et al. 2021) dosed mice with 30mg/kg of ketamine and had the mice perform a signal detection task. They found increases in false alarm rate, investment-based confidence in false alarms, and the rate of high-confidence false alarms without disrupting perceptual performance on signal-present trials as measured by psychometric curves. The authors also found that the degree of variance in time investment-based confidence explained by statistical confidence (e.g. confidence expected due to trial by trial signal/noise ratio) was not statistically different between mice given ketamine or saline vehicle. Taken together, this is consistent with ketamine producing a shift in perceptual expectations rather than reward expectations, and while the authors ultimately implicate a role for striatal dopamine, they do not directly address a putative mechanism by which ketamine may induce hallucinations.
NMDA-dependent processes are critically involved in synaptic survival during pruning (Tessier and Broadie, 2009), and the pruning process itself may differentially impact glutamatergic pyramidal cells in schizophrenia (Glausier and Lewis, 2013), potentially unifying aberrant pruning (Cannon, 2015; Hoffman and Dobscha, 1989; Hoffman and McGlashan, 2006; Sekar et al., 2016; Sellgren et al., 2019) with theories linking psychosis to NMDA dysfunction (Krystal et al., 2003). As to how a mechanism like pruning could lead to moment to moment fluctuations in perception, one possibility could be a kind of two-hit hypothesis, in which excessive pruning leads to the first hit and subsequent environmental factors including further blockade or disruption via NMDAR blockade providing the second. In this way pruning could predispose to hallucinations later in life. NMDA-receptor functioning aberrations have also been implicated in schizophrenia via genetic analyses (Harrison and Weinberger 2005; Pitcher et al. 2011; Collier and Li 2003) and psychosis (including hallucinations) is inducible via NMDAR blockade/disturbance independent of dopamine(Pitcher et al. 2011; Omdal et al. 2005), though the picture is complicated by links between NMDAR functioning and dopamine excitability (Pereira and Johnson 2003). Pereira and Johnson also proposed alternative signaling pathways for perceptual distortions and frank hallucinations in ketamine, which include increased frontal cortical excitability and AMPA signaling via glutamate-binding-mediated Ca2+ entry through voltage dependent calcium channels.In concert with altered GABAergic signaling, NMDA dysfunction has been implicated in excitatory-inhibitory imbalance, which appears to be an important indicator of brain-region level network health, is measurable with non-invasive imaging techniques in humans including fMRI and EEG (Foss-Feig et al., 2017), and may be linked to alterations in top-down information processing via predictive processing and related frameworks (Jardri et al., 2016; Wilkinson, 2014). Thus, NDMA-dependent signaling could have a role in hallucinogenesis via primary hyper-precision in two ways: the first would be via its interaction with dopaminergic transmission, and the second would be its primary effect on creating an E/I imbalance, thus potentially strengthening top-down signals such as priors.
Lastly, the serotonin system has been implicated in hallucinogenesis primarily via its putative mechanistic role in mediating the effects of hallucinogenic substances like psilocybin and LSD(Vollenweider and Kometer 2010; Geyer and Vollenweider 2008; Winter 2009; Preller et al. 2018), though there remains substantial debate regarding the exact mechanism(Glennon 1990). There also is emerging evidence of serotonin dysfunction in schizophrenia directly(González-Maeso and Sealfon 2009; Baumeister and Hawkins 2004). Finally, because the serotonin system is so strongly implicated in mood(Vollenweider and Kometer 2010), this points to a link between mood/affect and hallucinogenic potential. One possibility is therefore that serotonin system dysfunction leads to alterations in mood and thus mood-associated priors/top down signals are strengthened relative to incoming sensory input. One would therefore expect induced hallucinations to occur more frequently in the context of greater intensity moods/affects, a study that is currently ongoing (Benrimoh and Fisher et al, in prep).
III. Secondary prior hyper-precision (e.g. sensory evidence hypo-precision)
Alternatively, a relative asymmetry in perceptual inference towards prior hyper-precision could be achieved via a primary hypo-precision of incoming sensory evidence. In other words, the proverbial seesaw could be tilted towards a greater relative weighting of priors by decreasing the weight of incoming sensory evidence. Mechanisms and evidence for underlying neural processes that might underlie this shift are considered here.
Behavioral evidence
Secondary prior hyper-precision is an appealing potential mechanism for hallucinogenesis in part because decreased sensory sensitivity is a hallmark of schizophrenia-spectrum illness. A number of psychophysical studies have reported aberrant (often, though not always, decreased) sensitivity to incoming sensory evidence across multiple domains (Adler et al., 1999; Braff et al., 1991; Javitt et al., 1999; Turetsky et al., 2003). In the visual domain, for instance, individuals with schizophrenia have decreased visual processing efficiency (Saccuzzo and Braff, 1986), with findings of increased significantly longer visual registration thresholds correlating with greater negative symptom severity (Weiner et al., 1990). Patients with schizophrenia also have impaired visual detection, motion perception, spatial localization, and gaze (Chen et al., 1999a, 1999b; O’Donnell et al., 1996; Schwartz et al., 1987; Slaghuis, 1998; Stuve et al., 1997), a finding that spans multiple sensory domains (Malaspina et al., 1994). Individuals with schizophrenia also display greater sensitivity to backward masking (Braff et al., 1991; Butler et al., 1996; Slaghuis and Bakker, 1995; Weiner et al., 1990), which has been proposed to reflect hyperactivity within transient visual channels (Cadenhead et al., 1998; Green et al., 1994; Merritt and Balogh, 1989; Schuck and Lee, 1989; Slaghuis and Curran, 1999).
Similar evidence exists in the auditory domain. Early work showed deficits in some auditory processing tasks in individuals with hallucinations, including elevated discrimination thresholds for tone-matching (Jonsson and Sjöstedt, 1973; Strous et al., 1995) in both pitch (Javitt et al., 2000) and duration (Todd et al., 2003), as well as detection of abnormal tunes (Leitman et al., 2005). Of these, pitch discrimination has proven most robustly associated with hallucinations (McKay et al., 2000), including a positive correlation with severity (McLachlan et al., 2013). Other studies have found lower sensitivity in hallucinating patients with schizophrenia compared to healthy controls (Chhabra et al., 2016), although evidence of improved sensitivity in hallucinating versus non-hallucinating patients has been reported as well (Vercammen et al., 2008). Lastly, studies employing a signal detection framework to study hallucinations (Dolgov and McBeath, 2005) have tended toward findings of shifted criterion bias toward detection (with a corresponding increase in false alarm rate) in addition to sensitivity differences to explain worsened performance on auditory descrimination tasks (Brookwell et al., 2013). This combination may support a secondary prior hyper-precision model, in which sensitivity deficits engender a bias toward detection. Alternatively, they could represent two sub-populations of individuals with hallucinations--one with primary hyper-precision leading to shifted decision criteria (and even increased sensory precision), and the other with reduced sensory precision leading to secondary prior hyper-precision, such as the bimodal distribution found by Donde et al in tone-matching deficits that implicated the presence of discrete schizophrenia subtypes(Dondé et al. 2019).
Lastly, as learning and inference are increasingly recognized as “two sides of the same coin” (Friston, 2018), these two elements may be inseparable within individuals, with linked mechanisms causing altered precision in both pathways simultaneously. In such a formulation, heightened priors cause decreased sensory sensitivity and decreased sensory sensitivity causes heightened priors. Ongoing work on the neural processes encoding these precisions in a normative context could shed light on the separability of these processes: for example, via pharmacological manipulation of incoming sensory evidence or prior precision by altering cholinergic tone, (Marshall et al., 2016) to see if these processes are intrinsically linked.
Potential neural Correlates
The visual detection task deficits described above are consistent with a model of selective deficits in magnocellular over parvocellular visual processing pathways (Butler et al., 2005, 2001). Magnocellular pathways carry low-resolution visual feature information quickly and primarily drive attention capture and salience in downstream higher-level cortical areas, whereas parvocellular streams are thought to be slower and comprise more high-resolution featural information such as fine-grained stimulus configurations that enables operations including object identification in higher-level visual processing cortical areas (Norman, 2002; Steinman et al., 1997; Vidyasagar, 1999; W H Merigan and Maunsell, 2003). In a seminal series of studies, steady-state visual evoked potentials were measured from patients with schizophrenia in response to stimuli varying in luminance contrast, chromatic contrast, and spatial frequency (Butler and Javitt, 2005). The authors found that patients showed greater deficits when stimuli were presented WITHOUT a contrast ‘pedestal’ (a high luminance stimulus designed to saturate magnocellular response and thus, on trials without such a stimulus, bias towards magnocellular processing), consistent with a selective deficit in the magnocellular pathway. The consequences of such dysfunction include deficits in face recognition, especially facial emotion (Edwards et al., 2002), motion detection (Chen et al., 2006) and perceptual closure (Doniger et al., 2002; Kveraga et al., 2007).
Early studies investigating auditory processing deficits in schizophrenia using EEG found differences in multiple components of the event-related potential (ERP), including the P300 (Roth and Cannon, 1972), P50 (Adler et al., 1982; Boutros et al., 1991), N100 (Roach et al., 2020; Thakkar et al., 2021), and MMN (Ford et al., 2016; Thakkar et al., 2021). These correlates are thought to reflect sensory processing pathway deficits at multiple levels, including high-level attention-dependent impairments (P300), low level sensory gating deficiencies (p50; (Braff, 1993)), identification of self vs. other (N100; (Ford et al., 2001)), and pre-attention attribution of salience (MMN; (Javitt, 2000)), any of which could contribute to a lower reliability of sensory evidence with further downstream consequences (Javitt, 2009; Wang et al., 2014).
Multiple studies have demonstrated reduced connectivity between thalamus and frontal cortex (Woodward et al., 2012) accompanied by increased connectivity between thalamus and sensory cortices, including both somatosensory (Woodward et al., 2012) and auditory (Mancini et al., 2020) regions in psychosis. This degree of increased connectivity correlates directly with auditory hallucination severity (Ferri et al., 2018), as does relative sensitivity of auditory cortex to its thalamic afferents (Li et al., 2017). Similarly, hyperconnectivity of thalamus to sensorimotor cortex relates to risk of conversion and total symptom severity in young people at clinical high risk for psychosis (CHR-P) although no relationship to hallucinations was reported (Anticevic et al., 2015). Disrupted thalamus connectivity and functioning has been proposed to underlie symptoms of schizophrenia via aberrant (immature) sensory gating (Faugere et al., 2016; Smith et al., 2013), broadly consistent with a neurodevelopmental model of schizophrenia (Rapoport et al., 2012). Physiologically, several studies have reported decreased auditory cortex activation in patients with AVH in response to external stimuli, and increased auditory cortex activation in the setting of AVH in the absence of external stimuli (Kompus et al., 2011). As mentioned previously, results from hallucination symptom-capture studies have also demonstrated increased gamma band activity in thalamo-cortical networks while participants report experiencing hallucinatory percepts. Sensory-gating models propose a role for the thalamus as a gatekeeper for incoming sensory information, mediated via dynamic use/synchronization of gamma-band thalamocortical oscillations(McCormick and Bal 1994). Consistent with this, these results could suggest a compensatory mechanism for primary decreased sensory precision, wherein the system attempts to pass-through lower quality sensory information that would otherwise be stopped at the gate because better information is not available. Alternatively, the observation that such observations are present during auditory hallucinations could point toward aberrant top-down signals (primary hyper-precision) instructing the thalamus to increase gain, passing forward otherwise subthreshold and noisy auditory input that is more likely to be shaped by the existing priors into a hallucinatory percept. A final possibility is that the gamma-band activity instead reflects primary increased sensory precision, allowing it to pass through the thalamic gate despite being primarily noise and lacking an underlying signal per se, thus unduly affecting perception and influencing perceptual belief updating. Once through the gate, the noisy information would then be treated as signal and potentially misfit by top down prior beliefs, occasionally generating a hallucinatory percept. More work is needed to disentangle these possibilities, but the electrophysiological findings highlighted here may represent one key way of doing so.
In combination, evidence for increased spontaneous activity and relatively decreased evoked activity in the auditory system is broadly consistent with poor-quality incoming sensory evidence, which should optimally be weighted less heavily during perceptual inference.
IV. Other model elements
In addition to considering precision of priors and sensory evidence, we may briefly touch on the role of other elements of learning and inference that could contribute to hallucinogenesis. As illustrated in Figure 2, these elements could also make hallucinations more likely to occur. Depicted in Figure 2a is the Hierarchical Gaussian Filter (HGF), a generative model used to explain behavior across a large number of settings. Of most relevance to our discussion, the HGF has been used to ascertain the latent states driving the occurrence of conditioned hallucinations—false percepts generated by Pavlovian conditioning (Kafadar et al., 2020; Powers et al., 2017). One obvious element of importance in the model is Nu, or the relative weighting of priors relative to incoming sensory evidence. However, the likelihood of reporting a conditioned hallucination may also be influenced by other factors. These include learning rates, the encoding of environmental volatility, and decision noise, all of which may be impacted by other aspects of psychotic and normative cognitive functioning. Because the HGF is a generative model, it can be used to generate synthetic data using a range of parameters (Fig. 2b,c). Doing so clearly demonstrates the importance of Nu in making conditioned hallucinations more likely; however, second order beliefs including task learning rate (ω1), beliefs about the stability of learned environmental associations (ω2), and even decision noise (β−1) may play a clear role, leading to the prediction of “hot spots” of latent states likely to produce conditioned hallucinations. Notably, the last point about decision noise leading to hallucinations is both somewhat controversial and counter-intuitive, as β−1 is a parameter that exits outside of the perceptual model itself, and reflects the stochastity inherent in hallucinatory reports irrespective of percept. That said, there is emerging evidence for a role of policy space and interactions between agent choices/behaviors and prior beliefs/percepts (Benrimoh 2022). And it has long been known that a more structured, low-stress, and predictable environment is beneficial for schizophrenia. The degree to which these factors are represented in individuals with hallucinations remains an unanswered empirical question but may impact our understanding of the details of cognitive and perceptual processing that may work together to produce the constellation of symptoms typically seen in psychosis.
Under our model, various neurotransmitter systems could act via different parameters. As an example, the precision of sensory evidence appears to depend critically upon cholinergic signaling, whereas prior beliefs have been more intrinsically linked with striatal dopamine(Iglesias et al. 2021). NMDA receptor dysfunction or serotonin system dysfunction are less well defined, but could represent deficits at higher level parameters including learning rate (ω2), though further investigation is needed to confirm these predictions.
It is also important to acknowledge that although each factor and parameter above is considered separately by the manuscript, multiple derangements may occur simultaneously and the effects may interact nonlinearly. For instance, that Nu is expressed as a ratio clues us in to the fact that the two factors (sensory and prior precision) may be biologically linked through feedback and maintenance of homeostasis. Thus, changing one factor (prior precision) might influence the other over the course of neurotypical development or psychiatric illness. Following this example further, there may exist a putative tipping point between the factors, the crossing of which could be achieved either with large changes in one or smaller but opposite changes in both. This may mean that in practice, assuming a stochastic random walk, observed individuals in the real world with hallucinations are more likely to land past the tipping point through small changes in both simultaneously, analogous to how flipping a coin 10 times is more likely to result in 5 tails and 5 heads than 10 tails, not because any one specific combination is more likely, but because there are more ways to arrive at 5+5 then 10 and 0. This remains an unanswered empirical question; however, at least theoretically, we can not rule out hallucinogenesis driven by a single derangement.
Active Inference Models
Recently, Parr and colleagues utilized an active inference model to test behavioral predictions under various model perturbations to more directly probe the relationship between cognitive model parameters and behavior/symptoms (Parr et al., 2018). Specifically, they modeled the process of Active Inference by simulating visual scene construction using a Markov Decision Process model, wherein beliefs about the putative visual scene were updated in real time through a combination of prior evidence (previous beliefs), incoming sensory evidence (modeled as observations constructed from a combination of latent variables representing the “true” identity of the scene modified by a precision term), and a set of policies, or actions, that the agent was allowed to pursue in order to optimally construct the scene (such as where in the visual scene to direct gaze next). Crucially, this model was informed by known physiologic constraints surrounding regional connectivity and the visual processing hierarchy, enabling them to explicitly model and test what effects making modifications to various model parameter strengths (such as prior belief precision) had on model behavior, belief updating, and final output, affording them the chance to demonstrate which factors most directly contributed to the generation of visual hallucinations. As a result, they found a clear relationship between reduced policy space (agent decision actions) and hallucinogenic potential, though policy space was not manipulated directly. Namely, in the discussion section of the cited paper, the authors conclude that more abstract or complex behaviors (policies) may require the integration of prior beliefs across multiple sensory modalities, therefore requiring a minimization of free energy across multiple domains. One implication of this is that a reduced policy space relative to an agent’s environment can cause phenomena such as hallucinations to develop, as a selected policy that minimizes total free energy, primarily driven by a reduction in one sensory modality (say an interocepted elevated heart rate) implies an exteroceptive state (something dangerous is around), which may not fit actual incoming sensory information. The HGF model used in this review notably does not include a policy space parameter (i.e. the model does not allow itself to consider actions it could take to benefit future perceptual inferences). The model described above therefore exhibits a potential extension of the HGF; inclusion of a parameter to model policy space allows for yet more of the possible hallucinogenic space to map, and may even lead to novel treatments or evidence for the use of existing treatments, such as described in another article in this issue (Benrimoh et al, 2021), which links findings related to the hallucinatory potential of altered policy spaces and real world treatments for schizophrenia, including environmental structuring.
V. Model predictions
The preceding text has concerned itself primarily with outlining the evidence for a model of hallucinogenesis based upon a unifying neurocomputational mechanism of relatively increased influence (precision) of prior beliefs. The major utility of such a model is its explanatory power, and the benchmark for its success is its ability to make testable and falsifiable predictions. In the next section, we consider the latter, focusing on predictions of novel neural and behavioral phenomena that may predispose to hallucinogenesis across the lifespan.
Some of the predictions made by the model can be tested with existing technology, such as Laminar fMRI, while others first require further technological advances. Laminar fMRI is a recently developed technique which leverages ultra-high-field (7-Tesla and above) MRI scanners to interrogate functional changes in vascular activity at sufficient submillimetre resolutions to observe activity in previously difficult-to-image layers of the cortex in awake humans(Lawrence et al. 2019). The key factor in terms of testing competing ideas of precision in hallucinogenesis is that different layers have different interregional patterns of connectivity, with this concept discussed expertly by Joost Haarsma and colleagues (Haarsma et al. 2020) taking into account ideas of canonical microcircuits(Bastos et al. 2012). Bottom-up signals largely flow from superficial Layers 2/3 to the granular Layer 4 of downstream regions. By contrast, feedback arises from the deep layers 5/6 and targets agranular Layers 1 and 5/6 of upstream regions(Kok et al. 2016). If we logically assume that prior information travels via feedback channels, this circuitry allows us to form hypotheses about the likely outcome of previous experiments that observed experimentally controlled or conditioned hallucinations or visual illusions, thus offering the possibility to explore the neural circuitry underlying hallucinogenesis.
If hallucinations are a result of decreased weighting towards sensory input, then this might be assessed by examining laminar activity patterns caused by direct sensory input. Here, we might expect to observe decreased activity in middle layers receiving thalamic input and also potentially in superficial layers thought to process prediction errors due to a weaker comparison between sensory inputs and prior beliefs. Alternatively, if hallucinations are a product of overly precise or strong priors, then this could be reflected in increased deep layer activity in patients prone to hallucinations. Previous work has shown that it is possible to record stimulus specific representations of expected/cued visual stimuli in deep layers of V1 even when no stimulus is actually present (Aitken et al. 2020) providing support for this idea. However, it is important to note here that simply finding differences in laminar activity does not necessarily support accounts of alterations of prior or sensory precision during hallucinogenesis. In order to tease these apart, it would be helpful to manipulate both components directly and independently, a rarity in the current literature. One such attempt by Karvelis et al (Karvelis et al. 2018) used a statistical learning task in which participants were instructed to estimate the direction of a patch of coherently moving dots. Over the course of the task, participants were shown to rapidly and implicitly learn the most frequently presented directions, allowing the experimenters to manipulate their prior beliefs via direction presentation frequencies independent of trial by trial sensory precision (as indexed by dot cohesion). One challenge of this method however is that an individual with low static sensory precision may learn more poorly or slowly than another individual, because the incoming frequency information is less well internalized due to imprecise sensory priors. However, this possibility is mitigated somewhat by using high coherence trials for learning and low coherence trials for testing. Another method might be to use serial dependence, an illusion in which percepts of low-level stimulus features such as orientation and position are highly influenced by past presentations (Cicchini, Mikellidou, and Burr 2011.; Corbett, Fischer, and Whitney 2011; Kahneman, Slovic, and Tversky 1982) with this idea also touched upon in Haarsma et al 2020. Serial dependence is inversely related to sensory noise such that the impact of previous stimulus values increases as stimuli become harder to see and sensitive to correlations in previous stimulus presentations (Cicchini et al. 2016; Cicchini, Mikellidou, and Burr 2018; Taubert et al. 2016). If participants prone to hallucination exhibit more serial dependence than controls even when inappropriate (i.e., when prior stimulus presentation was random) and its magnitude is inversely correlated with their sensory thresholds, then this would indicate that disruptions in sensory precision are responsible for the misperception of stimuli. In terms of laminar activation patterns, we would expect corresponding decreased activity in middle layers receiving sensory input and superficial layers coding prediction errors. Alternatively, if participants that are more prone to hallucinations exhibit serial dependence related to the correlation levels of prior stimuli and more than controls, this would indicate that disruptions in prior precision are responsible for the misperception of stimuli. Here we would expect increased activity in deep layers receiving predictive feedback input from higher regions.
Characterizing the activity of feedforward and feedback perceptual circuits is a logical next step in the study of hallucination formation. Extension of investigations into the role of less-studied, sensory-distal regions will also be crucial. One relevant brain system strongly implicated in higher-order modeling and integration of prior beliefs across time and sensory domains is the hippocampal formation (Felleman and Van Essen 1991; Duncan et al. 2012; Kok and Turk-Browne 2018; Lavenex et al 2000 ; Schapiro et al. 2014, 2017). Previous work has assessed the role of the hippocampus in learning higher order sequence structures (Chen et al. 2011.; Lavenex and Amaral, 2000 ; Schapiro et al. 2014; Schapiro, Kustner, and Turk-Browne 2012) and in some cases has related learning to temporally suppressive effects in early sensory regions (Kok and Turk-Browne 2018a, 2018b) consistent with predictive coding accounts of repetition suppression (Grill-Spector, Henson, and Martin 2006; Rao and Ballard 1999). In line with these findings, Kok and Turk-Browne (2018a, 2018b) also reported that the hippocampus represents shapes predicted by stimulus associations (tone predicts a shape) whereas visual cortex represented what was shown to the eyes with suppressive effects in V1 commensurate to the strength of hippocampal representation. Application of similar designs in the study would allow testing of a number of hypotheses designed to assess the hyper-precise prior vs hypo-precise sensory evidence accounts of hallucinogenesis.
For example, if hallucinations are a result of an increased weighting of prior beliefs then we might predict stronger predicted shape representations within the hippocampus producing a faster and increased suppression of activity in early sensory regions (from an initially normal intensity) in those prone to hallucinations. Conversely, if hallucinations are a product of hypo-precise sensory evidence, we could predict that we will see overall lower activity in early sensory regions and a slower and weaker build-up of predicted shape representation in the hippocampus in those more likely to experience hallucinations due to weaker prediction error signaling. Assessing potential differences in the temporal evolution of prior beliefs and their integration across sensory domains in individuals would also benefit from further research at a higher temporal resolution made possible by the use of Magnetoencephalography (MEG) or electroencephalogram(EEG)
Previous MEG studies have shown that the strength of representations of expected stimuli proportionately modulate the effects of such expectations on early sensory processing (~150 ms) (Aitken et al., 2020; Kok et al., 2017). Analysis has revealed that subjects with a stronger perceptual bias towards previously cued orientations can also show a stronger reflection of the predicted direction in the MEG signal. Here, in some subjects exhibiting such a bias, a representation of the expected orientation emerged in the visual cortex before the stimulus was actually presented. These paradigms present a further means to gauge both the relative influence of hyper-precise priors and hypo-precise sensory evidence in hallucinogenesis and also any potential differences in temporal representation of prior beliefs at the stimulus-specific level. If hallucinogenesis is a result of overly precise priors, we would observe a stronger representation of cued stimuli in the MEG signal relative to presented stimuli in those at risk for hallucinations as opposed to controls. We may also observe a very strong influence of priors on perceptual processing in such individuals, as indicated by an earlier representation of the expected stimulus. Alternatively, if hallucinations arise from an underweighting of current sensory inputs then we should observe a weaker representation of presented stimuli relative to expected stimuli and no pre-stimulus modulation of visual cortex.
Finally, at the level of brain regional networks, via a combination of computational modeling approaches (such as Dynamic Causal Modeling, DCM (David et al., 2006)), we predict that different hallucinogenic phenotypes will display different network properties. For instance, an individual with hallucinations driven by primary low learning rate (ω2, Fig. 2) represents an individual with broad hyper-precision relative to an individual with average ω2 but high Nu, whose hyper-precise priors are limited to beliefs about a specific task or sensory modality. We might therefore expect the first individual to display a more uniformly strengthened default mode network, or attentional network, regardless of task engagement. Additionally, we would expect deficits across a wide range of sensory inference tasks, including across different sensory modalities. In contrast, the second individual may show a marked increase in network connectivity associated with a specific task or modality, reflecting their more specific prior.
VI. Impact of Knowledge of Trajectories
Beyond predictions this model makes with respect to fully-formed hallucinations, even more exciting is the prospect of testing predictions made about the earlier stages of hallucinogenesis, including those with first episode psychosis (FEP) and CHR (Kafadar et al., 2020), as well as those with known genetic mutations that confer advanced risk but who show no symptoms of psychosis, or genetic high risk (GHR). The dynamics inherent in the development of psychotic symptoms are crucial not only for the testing and elaboration of the models we propose here, but also for the identification of differential trajectories toward hallucinogenesis and worsening of psychopathology. The power of a framework that links derangements of different aspects of perceptual functioning in explaining the emergence of a psychotic symptom is that it can form the basis of biomarkers meant to identify distinct mechanisms leading to a common phenotype. The mapping of these different subtypes can also aid in prospective clinical identification. For instance, individuals with a primary sensory precision derangement may show different early symptoms than individuals with a primary prior derangement, leading to an enhancement in their clinical identification and, ultimately, to tailored intervention.
These interventions may certainly be biologically-based, using medications, neurofeedback, or brain stimulation to directly alter circuits involved in the development of hallucinations. However, this need not be the case: more comprehensive models capable of capturing not only hallucinogenesis but also the impact of psychosocial interventions may be critical for development of personalized interventions. For example, recent evidence highlights the ability of some non-clinical voice-hearers to exert voluntary control over their voice-hearing experiences (Mourgues et al., 2020; Swyer and Powers, 2020). Current models are not structured to take into account voluntary action in the process of hallucinogenesis; their modification may lead to further insight not only into how this might be possible, but also into other pathways toward their development in the first place. Similarly, model elaboration to take into account the impact of mood and affect may elucidate whether and how affective psychoses may take different pathways toward the ultimate development of hallucinations, and how those hallucinations are often informed by affective state, despite violation of strict perceptual modularity (Powers et al., 2016). These findings may also extend to the role of other stressors in hallucinogenesis as well as relapse of psychotic symptoms over the course of treatment.
VII. Conclusions
In summary, the current work has reviewed the literature on potential neural mechanisms underlying hallucinogenesis, organized through the lens of an explanatory model of perceptual inference. The act of placing what is known in the current literature into a rational structure allows one to not only more easily conceptualize, remember, and draw together a cohesive body of knowledge, but also to more easily visualize the negative space: in highlighting what is not known, the field can more easily address these gaps, filling in the corpus overall. Lastly, asserting and testing a well-delineated pathophysiology of hallucinations should have the added benefit of limiting the stigma associated with these experiences. It is clear that the suffering of our patients is compounded by the stigma society places on their symptoms; we hope that the identification of well-defined biological processes leading to hallucinogenesis may place these otherwise othering phenomena more solidly into the realm of medical science.
Acknowledgments
ARP is supported by a K23 Career Development Award and R21 from the National Institute of Mental Health (K23 MH115252-01A1; 5R21 MH122940-02), by a Career Award for Medical Scientists from the Burroughs-Wellcome Fund, and by the Yale Department of Psychiatry and the Yale School of Medicine. EK receives support from the Yale Science, Technology, and Research Scholars II (STARS II) program, itself supported by the Yale College Dean’s Office and Yale University. AMN received support through the Veterans Affairs Office of Academic Achievement postdoctoral fellowship program. This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
Role of the funding source
The research presented in this manuscript was supported by the funding sources named in the Acknowledgments section. These funding sources played no role in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
References
- Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ, 2013. The computational anatomy of psychosis. Front. Psychiatry 4, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler LE, Freedman R, Ross RG, Olincy A, Waldo MC, 1999. Elementary phenotypes in the neurobiological and genetic study of schizophrenia. Biol. Psychiatry 46, 8–18. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R, 1982. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol. Psychiatry 17, 639–654. [PubMed] [Google Scholar]
- Aitken F, Menelaou G, Warrington O, Koolschijn RS, Corbin N, Callaghan MF, Kok P, 2020. Prior expectations evoke stimulus templates in the deep layers of V1. Cold Spring Harbor Laboratory, 10.1101/2020.02.13.947622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alais D, Burr D, 2019. Cue Combination Within a Bayesian Framework. Multisensory Processes. 10.1007/978-3-030-10461-0_2 [DOI] [Google Scholar]
- Alais D, Burr D, 2004. The ventriloquist effect results from near-optimal bimodal integration. Curr. Biol 14, 257–262. [DOI] [PubMed] [Google Scholar]
- Alderson-Day B, Lima CF, Evans S, Krishnan S, Shanmugalingam P, Fernyhough C, Scott SK, 2017. Distinct processing of ambiguous speech in people with non-clinical auditory verbal hallucinations. Brain 140, 2475–2489. [DOI] [PubMed] [Google Scholar]
- Allen P, Modinos G, 2012. Structural Neuroimaging in Psychotic Patients with Auditory Verbal Hallucinations, in: Blom JD, Sommer IEC (Eds.), Hallucinations: Research and Practice. Springer New York, New York, NY, pp. 251–265. [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet D, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD, 2015. Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk. JAMA Psychiatry 72, 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AL, Freeman H, 1956. Reserpine in hospitalized psychotics; a controlled study on chronically disturbed women. AMA Arch. Neurol. Psychiatry 76, 281–285. [DOI] [PubMed] [Google Scholar]
- Baba M, Yokoyama K, Seiriki K, Matsumura K, Kondo M, Yamamoto K, Kasai A, Ago Y, Nagayasu K, Hayata A, Yamaguchi S, Mori D, Ozaki N, Yamamoto T, Takuma K, Hashimoto R, Hashimoto H, Nakazawa T, 2020. Psychiatric-disorder-related behavioral phenotypes and cortical hyperactivity in a mouse model of 3q29 deletion syndrome. Proceedings for Annual Meeting of The Japanese Pharmacological Society. 10.1254/jpssuppl.93.0_1-ss-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeweg T, 2006. Repetition effects to sounds: evidence for predictive coding in the auditory system. Trends Cogn. Sci 10, 93–94. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ, 2012. Canonical microcircuits for predictive coding. Neuron 76, 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom JD, 2015. Chapter 24 - Auditory hallucinations, in: Aminoff MJ, Boiler F, Swaab F (Eds.), Handbook of Clinical Neurology. Elsevier, pp. 433–455. [DOI] [PubMed] [Google Scholar]
- Blom JD, 2010. A Dictionary of Hallucinations. Springer, New York, NY. [Google Scholar]
- Boutros NN, Zouridakis G, Overall J, 1991. Replication and extension of P50 findings in schizophrenia. Clin. Electroencephalogr 22, 40–45. [DOI] [PubMed] [Google Scholar]
- Braff DL, 1993. Information processing and attention dysfunctions in schizophrenia. Schizophr. Bull 19, 233–259. [DOI] [PubMed] [Google Scholar]
- Braff DL, Saccuzzo DP, Geyer MA, 1991. Information processing dysfunctions in schizophrenia: Studies of visual backward masking, sensorimotor gating, and habituation. Neuropsychology, psychophysiology, and information processing. 687, 303–334. [Google Scholar]
- Brookwell ML, Bentall RP, Varese F, 2013. Externalizing biases and hallucinations in source-monitoring, self-monitoring and signal detection studies: a meta-analytic review. Psychol. Med 43, 2465–2475. [DOI] [PubMed] [Google Scholar]
- Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM, 1996. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol. Psychiatry 40, 295–298. [DOI] [PubMed] [Google Scholar]
- Butler PD, Javitt DC, 2005. Early-stage visual processing deficits in schizophrenia. Curr. Opin. Psychiatry 18, 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC, 2001. Dysfunction of early-stage visual processing in schizophrenia. Am. J. Psychiatry 158, 1126–1133. [DOI] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC, 2005. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry 62, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Serper Y, Braff DL, 1998. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol. Psychiatry 43, 132–138. [DOI] [PubMed] [Google Scholar]
- Cannon TD, 2015. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn. Sci 19, 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascino GD, Adams RD, 1986. Brainstem auditory hallucinosis. Neurology 36, 1042–1047. [DOI] [PubMed] [Google Scholar]
- Cassidy CM, Balsam PD, Weinstein JJ, Rosengard RJ, Slifstein M, Daw ND, Abi-Dargham A, Horga G, 2018. A Perceptual Inference Mechanism for Hallucinations Linked to Striatal Dopamine. Curr. Biol 28, 503–514 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Nakayama K, Matthysse S, Palafox G, Holzman PS, 1999a. Dependence of impaired eye tracking on deficient velocity discrimination in schizophrenia. Arch. Gen. Psychiatry 56, 155–161. [DOI] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Sheremata S, Holzman PS, 2006. Bipolar and schizophrenic patients differ in patterns of visual motion discrimination. Schizophr. Res 88, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS, 1999b. Motion perception in schizophrenia. Arch. Gen. Psychiatry 56, 149–154. [DOI] [PubMed] [Google Scholar]
- Chhabra H, Sowmya S, Sreeraj VS, Kalmady SV, Shivakumar V, Amaresha AC, Narayanaswamy JC, Venkatasubramanian G, 2016. Auditory false perception in schizophrenia: Development and validation of auditory signal detection task. Asian J. Psychiatr 24, 23–27. [DOI] [PubMed] [Google Scholar]
- Cole MG, Dowson L, Dendukuri N, Belzile E, 2002. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int. J. Geriatr. Psychiatry 17, 444–452. [DOI] [PubMed] [Google Scholar]
- Copolov DL, Seal ML, Maruff P, Ulusoy R, Wong MTH, Tochon-Danguy HJ, Egan GF, 2003. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res. 122, 139–152. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR, 2019. Hallucinations and Strong Priors. Trends Cogn. Sci 23. 10.1016/j.tics.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Kiebel SJ, Harrison LM, Mattout J, Kilner JM, Friston KJ, 2006. Dynamic causal modeling of evoked responses in EEG and MEG. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Dayan P, 1998. A hierarchical model of binocular rivalry. Neural Comput. 10, 1119–1135. [DOI] [PubMed] [Google Scholar]
- Dolgov I, McBeath MK, 2005. A signal-detection-theory representation of normal and hallucinatory perception. Behav. Brain Sci 28, 761–762. [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC, 2002. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch. Gen. Psychiatry 59, 1011–1020. [DOI] [PubMed] [Google Scholar]
- Edwards J, Jackson HJ, Pattison PE, 2002. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin. Psychol. Rev 22, 789–832. [DOI] [PubMed] [Google Scholar]
- Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MAP, Bhattacharyya S, Allen P, McGuire PK, Howes OD, 2013. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol. Psychiatry 74, 106–112. [DOI] [PubMed] [Google Scholar]
- Faugere M, Micoulaud-Franchi J-A, Boyer L, Cermolacce M, Richieri R, Faget C, Vion-Dury J, Lançon C, 2016. Does sensory gating have a protective effect against hallucinatory behavior in schizophrenia? Clin. Neurophysiol 127, 1746–1748. [DOI] [PubMed] [Google Scholar]
- Feinberg I, 1982. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res 17, 319–334. [DOI] [PubMed] [Google Scholar]
- Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, Voyvodic J, Preda A, Belger A, Bustillo J, O’Leary D, Mueller BA, Lim KO, McEwen SC, Calhoun VD, Diaz M, Glover G, Greve D, Wible CG, Vaidya JG, Potkin SG, Mathalon DH, 2018. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol. Med 48, 2492–2499. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, 2005. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int. J. Psychophysiol 58, 179–189. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Heinks T, Kalba S, Faustman WO, Roth WT, 2001. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am. J. Psychiatry 158, 2069–2071. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Palzes VA, Mathalon DH, 2016. Using concurrent EEG and fMRI to probe the state of the brain in schizophrenia. Neuroimage Clin 12, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, Krystal JH, Murray JD, Anticevic A, 2017. Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biol. Psychiatry 81, 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, 2018. Does predictive coding have a future? Nature Neuroscience. 10.1038/S41593-018-0200-7 [DOI] [PubMed] [Google Scholar]
- Friston K, 2005. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci 360, 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, 2005. Hallucinations and perceptual inference. Behav. Brain Sci 28, 764–766. [Google Scholar]
- Glausier JR, Lewis DA, 2013. Dendritic spine pathology in schizophrenia. Neuroscience 251, 90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goekoop R, Looijestijn J, 2012. A Network Model of Hallucinations, in: Blom JD, Sommer IEC (Eds.), Hallucinations: Research and Practice. Springer New York, New York, NY, pp. 33–54. [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J, 1994. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch. Gen. Psychiatry 51, 945–951. [DOI] [PubMed] [Google Scholar]
- Harkavy-Friedman JM, Kimhy D, Nelson EA, Venarde DF, John Mann J, 2003. Suicide Attempts in Schizophrenia: The Role of Command Auditory Hallucinations for Suicide. J. Clin. Psychiatry 64, 871–874. [PubMed] [Google Scholar]
- Hoffman RE, Dobscha SK, 1989. Cortical Pruning and the Development of Schizophrenia: A Computer Model. Schizophrenia Bulletin. 10.1093/schbul/15.3.477 [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hampson M, 2011. Functional connectivity studies of patients with auditory verbal hallucinations. Front. Hum. Neurosci 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH, 2006. Using a speech perception neural network computer simulation to contrast neuroanatomic versus neuromodulatory models of auditory hallucinations. Pharmacopsychiatry 39, S54–S64. [DOI] [PubMed] [Google Scholar]
- Horga G, Abi-Dargham A, 2014. The striatum and dopamine: a crossroad of risk for schizophrenia. JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- Howes OD, Hird EJ, Adams RA, Corlett PR, McGuire P, 2020. Aberrant Salience, Information Processing, and Dopaminergic Signaling in People at Clinical High Risk for Psychosis. Biol. Psychiatry 88, 304–314. [DOI] [PubMed] [Google Scholar]
- Howes OD, Shotbolt P, Bloomfield M, Daalman K, Demjaha A, Diederen KMJ, Ibrahim K, Kim E, McGuire P, Kahn RS, Others, 2013. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr. Bull 39, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RB, Whittingham-Dowd J, Simmons RE, Clapcote SJ, Broughton SJ, Dawson N, 2020. Ketamine Restores Thalamic-Prefrontal Cortex Functional Connectivity in a Mouse Model of Neurodevelopmental Disorder-Associated 2p16.3 Deletion. Cerebral Cortex, 10.1093/cercor/bhz244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R, Hugdahl K, Hughes M, Brunelin J, Waters F, Alderson-Day B, Smailes D, Sterzer P, Corlett PR, Leptourgos P, Debbane M, Cachia A, Deneve S, 2016. Are Hallucinations Due to an Imbalance Between Excitatory and Inhibitory Influences on the Brain? Schizophr. Bull 42, 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2009. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol 5, 249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, 2000. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol. Neurootol 5, 207–215. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Freedman R, 2015. Sensory Processing Dysfunction in the Personal Experience and Neuronal Machinery of Schizophrenia. Am. J. Psychiatry 172, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Liederman E, Cienfuegos A, Shelley AM, 1999. Panmodal processing imprecision as a basis for dysfunction of transient memory storage systems in schizophrenia. Schizophr. Bull 25, 763–775. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W, 2000. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin. Neurophysiol 111, 1733–1737. [DOI] [PubMed] [Google Scholar]
- Jonsson CO, Sjöstedt A, 1973. Auditory perceaption in schizophrenia: a second study of the Intonation test. Acta Psychiatr. Scand 49, 588–600. [DOI] [PubMed] [Google Scholar]
- Kafadar E, Mittal VA, Strauss GP, Chapman HC, Ellman LM, Bansal S, Gold JM, Alderson-Day B, Evans S, Moffatt J, Silverstein SM, Walker EF, Woods SW, Corlett PR, Powers AR, 2020. Modeling perception and behavior in individuals at clinical high risk for psychosis: Support for the predictive processing framework. Schizophr. Res 10.1016/j.schres.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer C, Maziashvili N, Dugladze T, Gloveli T, 2008. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front. Mol. Neurosci 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW, 1994. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J. Psychiatr. Res 28, 239–265. [DOI] [PubMed] [Google Scholar]
- Kok P, Mostert P, de Lange FP, 2017. Prior expectations induce prestimulus sensory templates. Proc. Natl. Acad. Sci. U. S. A 114, 10473–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K, Westerhausen R, Hugdahl K, 2011. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: A meta-analysis of functional neuroimaging studies. Neuropsychologia 49, 3361–3369. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R, 2003. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology 169, 215–233. [DOI] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M, 2007. Magnocellular projections as the trigger of top-down facilitation in recognition. J. Neurosci 27, 13232–13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK, 2006. Hallucinogen-induced UP states in the brain slice of rat prefrontal cortex: role of glutamate spillover and NR2B-NMDA receptors. Neuropsychopharmacology 31, 1682–1689. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D, 2008. A cortical network for semantics: (de)constructing the N400. Nat. Rev. Neurosci 9, 920–933. [DOI] [PubMed] [Google Scholar]
- Lee TS, Mumford D, 2003. Hierarchical Bayesian inference in the visual cortex. J. Opt. Soc. Am. A Opt. Image Sci. Vis 20, 1434–1448. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC, 2005. Sensory contributions to impaired prosodic processing in schizophrenia. Biol. Psychiatry 58, 56–61. [DOI] [PubMed] [Google Scholar]
- Li B, Cui L-B, Xi Y-B, Friston KJ, Guo F, Wang H-N, Zhang L-C, Bai Y-H, Tan Q-R, Yin H, Lu H, 2017. Abnormal Effective Connectivity in the Brain is Involved in Auditory Verbal Hallucinations in Schizophrenia. Neurosci. Bull 33, 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Kane JM, Alvir J, 1987. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology 91,415–433. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Wray AD, Friedman JH, Amador X, Yale S, Hasan A, Gorman JM, Kaufmann CA, 1994. Odor discrimination deficits in schizophrenia: association with eye movement dysfunction. J. Neuropsychiatry Clin. Neurosci 6, 273–278. [DOI] [PubMed] [Google Scholar]
- Mancini V, Zöller D, Schneider M, Schaer M, Eliez S, 2020. Abnormal Development and Dysconnectivity of Distinct Thalamic Nuclei in Patients With 22q11.2 Deletion Syndrome Experiencing Auditory Hallucinations. Biol Psychiatry Cogn Neurosci Neuroimaging 5, 875–890. [DOI] [PubMed] [Google Scholar]
- Marr D, Poggio T, 1976. From Understanding Computation to Understanding Neural Circuitry. [Google Scholar]
- Marshall L, Mathys C, Ruge D, de Berker AO, Dayan P, Stephan KE, Bestmann S, 2016. Pharmacological Fingerprints of Contextual Uncertainty. PLoS Biol. 14, e1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys CD, Lomakina EI, Daunizeau J, Iglesias S, Brodersen KH, Friston KJ, Stephan KE, 2014. Uncertainty in perception and the Hierarchical Gaussian Filter. Front. Hum. Neurosci 8, 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, Headlam DM, Copolov DL, 2000. Central auditory processing in patients with auditory hallucinations. Am. J. Psychiatry 157, 759–766. [DOI] [PubMed] [Google Scholar]
- McKetin R, Lubman DI, Baker AL, Dawe S, Ali RL, 2013. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry 70, 319–324. [DOI] [PubMed] [Google Scholar]
- McLachlan NM, Phillips DS, Rossell SL, Wilson SJ, 2013. Auditory processing and hallucinations in schizophrenia. Schizophr. Res 150, 380–385. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Allen P, Amaro E Jr, Fu CHY, Williams SCR, Brammer MJ, Johns LC, McGuire PK, 2007. Misattribution of speech and impaired connectivity in patients with auditory verbal hallucinations. Hum. Brain Mapp 28, 1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RD, Balogh DW, 1989. Backward masking spatial frequency effects among hypothetically schizotypal individuals. Schizophr. Bull 15, 573–583. [DOI] [PubMed] [Google Scholar]
- Mourgues C, Negreira AM, Guagan B, Mercan NE, Niles H, Kafadar E, Bien C, Kamal F, Powers AR 3rd, 2020. Development of Voluntary Control Over Voice-Hearing Experiences: Evidence From Treatment-Seeking and Non-Treatment-Seeking Voice-Hearers. Schizophr Bull Open 1, sgaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Josephs O, Hocking J, Price CJ, Friston KJ, 2008. The effect of prior visual information on recognition of speech and sounds. Cereb. Cortex 18, 598–609. [DOI] [PubMed] [Google Scholar]
- Norman J, 2002. Two visual systems and two theories of perception: An attempt to reconcile the constructivist and ecological approaches. Behav. Brain Sci 25, 73–96; discussion 96–144. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW, 1996. Selective deficits in visual perception and recognition in schizophrenia. Am. J. Psychiatry 153, 687–692. [DOI] [PubMed] [Google Scholar]
- Pak C, Danko T, Mirabella VR, Wang J, Liu Y, Vangipuram M, Grieder S, Zhang X, Ward T, Huang Y-WA, Jin K, Dexheimer P, Bardes E, Mitelpunkt A, Ma J, McLachlan M, Moore JC, Qu P, Purmann C, Dage JL, Swanson BJ, Urban AE, Aronow BJ, Pang ZP, Levinson DF, Wernig M, Südhof TC, 2021. Cross-platform validation of neurotransmitter release impairments in schizophrenia patient-derived -mutant neurons. Proc. Natl. Acad. Sci. U. S. A 118. 10.1073/pnas.2025598118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T, Benrimoh DA, Vincent P, Friston KJ, 2018. Precision and False Perceptual Inference. Front. Integr. Neurosci 12, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzschner FH, Glasauer S, Stephan KE, 2015. A Bayesian perspective on magnitude estimation. Trends Cogn. Sci 10.1016/j.tics.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Powers AR 3rd, Gancsos MG, Finn ES, Morgan PT, Corlett PR, 2015. Ketamine-Induced Hallucinations. Psychopathology 48, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AR lii, Kelley M, Corlett PR, 2016. Hallucinations as Top-Down Effects on Perception. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 1, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AR, Mathys C, Corlett PR, 2017. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 357, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC, 2000. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch. Gen. Psychiatry 57, 1149–1155. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N, 2012. Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry 17, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Ford JM, Loewy RL, Stuart BK, Mathalon DH, 2020. Theta Phase Synchrony Is Sensitive to Corollary Discharge Abnormalities in Early Illness Schizophrenia but Not in the Psychosis Risk Syndrome. Schizophr. Bull 10.1093/schbul/sbaa110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Cannon EH, 1972. Some features of the auditory evoked response in schizophrenics. Arch. Gen. Psychiatry 27, 466–471. [DOI] [PubMed] [Google Scholar]
- Saccuzzo DP, Braff DL, 1986. Information-processing abnormalities: trait- and state-dependent components. Schizophr. Bull 12, 447–459. [DOI] [PubMed] [Google Scholar]
- Schellekens W, van Wezel RJ, Petridou N, Ramsey NF, Raemaekers M, 2014. Predictive coding for motion stimuli in human early visual cortex. Brain Struct. Funct 10.1007/S00429-014-0942-2 [DOI] [PubMed] [Google Scholar]
- Schmack K, Bose M, Ott T, Sturgill JF, Kepecs A, 2021. Striatal dopamine mediates hallucination-like perception in mice. Science 372. 10.1126/science.abf4740 [DOI] [PubMed] [Google Scholar]
- Schuck JR, Lee RG, 1989. Backward masking, information processing, and schizophrenia. Schizophr. Bull 15, 491–500. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, McGinn T, Winstead DK, 1987. Disordered spatiotemporal processing in schizophrenics. Biol. Psychiatry 22, 688–698. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, 1975. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 188, 1217–1219. [DOI] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Schizophrenia Working Group of the Psychiatric Genomics Consortium, Daly MJ, Carroll MC, Stevens B, McCarroll SA, 2016. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, Fu T, Worringer K, Brown HE, Wang J, Kaykas A, Karmacharya R, Goold CP, Sheridan SD, Perlis RH, 2019. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci 22, 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, 2016. Neural Elements for Predictive Coding. Front. Psychol 7, 1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaghuis WL, 1998. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol 107, 49–62. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Bakker VJ, 1995. Forward and backward visual masking of contour by light in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol 104, 41–54. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Curran CE, 1999. Spatial frequency masking in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol 108, 42–50. [DOI] [PubMed] [Google Scholar]
- Smith DM, Grant B, Fisher DJ, Borracci G, Labelle A, Knott VJ, 2013. Auditory verbal hallucinations in schizophrenia correlate with P50 gating. Clin. Neurophysiol 124, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Koops S, Blom JD, 2012a. Comparison of auditory hallucinations across different disorders and syndromes. Neuropsychiatry 2, 57. [Google Scholar]
- Sommer IEC, Slotema CW, Daskalakis ZJ, Derks EM, Blom JD, van der Gaag M, 2012b. The treatment of hallucinations in schizophrenia spectrum disorders. Schizophr. Bull 38, 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman BA, Steinman SB, Lehmkuhle S, 1997. Research Note Transient Visual Attention is Dominated by the Magnocellular Stream. Vision Res. 37, 17–23. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Adams RA, Fletcher P, Frith C, Lawrie SM, Muckli L, Petrovic P, Uhlhaas P, Voss M, Corlett PR, 2018. The Predictive Coding Account of Psychosis. Biol. Psychiatry 84, 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RL, Ives NJ, Clarke C, van Hilten J, Ferreira J, Hawker RJ, Shah L, Wheatley K, Gray R, 2008. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst. Rev CD006564. [DOI] [PubMed] [Google Scholar]
- Strous RD, Cowan N, Ritter W, Javitt DC, 1995. Auditory sensory (“echoic”) memory dysfunction in schizophrenia. Am. J. Psychiatry 152, 1517–1519. [DOI] [PubMed] [Google Scholar]
- Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY, 1997. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol. Med 27, 143–152. [DOI] [PubMed] [Google Scholar]
- Swyer A, Powers AR 3rd, 2020. Voluntary control of auditory hallucinations: phenomenology to therapeutic implications. NPJ Schizophr 6, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier CR, Broadie K, 2009. Activity-dependent modulation of neural circuit synaptic connectivity. Front. Mol. Neurosci 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C, Subramaniam N, Dobler V, Perez J, Finnemann J, Mehta PR, Goodyer IM, Fletcher PC, 2015. Shift toward prior knowledge confers a perceptual advantage in early psychosis and psychosis-prone healthy individuals. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1503916112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel C, Subramaniam N, Fletcher PC, 2013. The role of priors in Bayesian models of perception. Front. Comput. Neurosci 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Mathalon DH, Ford JM, 2021. Reconciling competing mechanisms posited to underlie auditory verbal hallucinations. Philos. Trans. R. Soc. Lond. B Biol. Sci 376, 20190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebes S, Steinmann S, Curie S, Polomac N, Andreou C, Eichler I-C, Eichler L, Zöllner C, Gallinat J, Leicht G, Mulert C, 2018. Alterations in interhemispheric gamma-band connectivity are related to the emergence of auditory verbal hallucinations in healthy subjects during NMDA-receptor blockade. Neuropsychopharmacology 43, 1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J, Michie PT, Jablensky AV, 2003. Association between reduced duration mismatch negativity (MMN) and raised temporal discrimination thresholds in schizophrenia. Clin. Neurophysiol 114, 2061–2070. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE, 2003. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol. Psychiatry 53, 403–411. [DOI] [PubMed] [Google Scholar]
- Vercammen A, de Haan EHF, Aleman A, 2008. Hearing a voice in the noise: auditory hallucinations and speech perception. Psychol. Med 38, 1177–1184. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, 1999. A neuronal model of attentional spotlight: parietal guiding the temporal. Brain Res. Brain Res. Rev 30, 66–76. [DOI] [PubMed] [Google Scholar]
- Wang J, Mathalon DH, Roach BJ, Reilly J, Keedy SK, Sweeney JA, Ford JM, 2014. Action planning and predictive coding when speaking. Neuroimage 91, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters F, Dragovic M, 2018. Hallucinations as a presenting complaint in emergency departments: Prevalence, diagnosis, and costs. Psychiatry Res. 261, 220–224. [DOI] [PubMed] [Google Scholar]
- Weiner RU, Opler LA, Kay SR, Merriam AE, Papouchis N, 1990. Visual information processing in positive, mixed, and negative schizophrenic syndromes. J. Nerv. Ment. Dis 178, 616–626. [DOI] [PubMed] [Google Scholar]
- Merigan WHA, Maunsell JHR, 2003. How Parallel are the Primate Visual Pathways? 10.1146/annurev.ne.16.030193.002101 [DOI] [PubMed] [Google Scholar]
- Wilkinson S, 2014. Accounting for the phenomenology and varieties of auditory verbal hallucination within a predictive processing framework. Conscious. Cogn 30, 142–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S, 2012. Thalamocortical dysconnectivity in schizophrenia. Am. J. Psychiatry 169, 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkali A, Adams RA, Psarras S, Leyland L-A, Rees G, Weil RS, 2019. Increased weighting on prior knowledge in Lewy body-associated visual hallucinations. Brain Commun 1, fcz007. [DOI] [PMC free article] [PubMed] [Google Scholar]