Abstract
Background:
Achalasia is a rare esophageal motility disorder of uncertain etiology. While past studies have indicated that autoimmune conditions and viral infections may be associated with development of achalasia, these associations are yet to be examined in large, population-based studies.
Methods:
A matched case-control study was performed using administrative claims data from the IBM MarketScan Commercial Claims and Encounters Database between 2000-2019. A history of selected autoimmune conditions and viral infections was assessed using past medical claims. Multivariable conditional logistic regression was used to account for the matched nature of the study design and further control for confounding by demographic and clinical characteristics when reporting adjusted odds ratios (aORs).
Key Results:
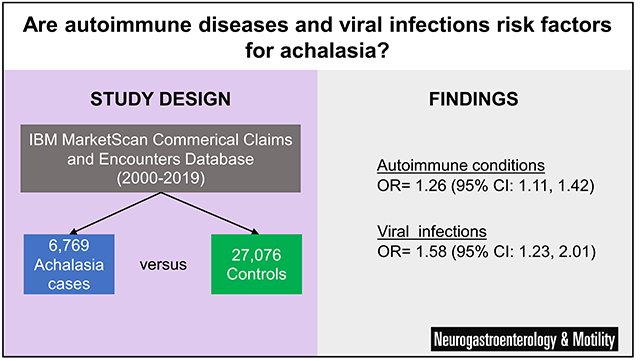
Amongst 6,769 cases and 27,076 controls, presence of any of the autoimmune conditions studied was associated with increased odds of achalasia (aOR= 1.26, 95% CI: 1.11, 1.42). Scleroderma or systemic sclerosis (aOR=8.13, 95% CI: 3.34, 19.80) and Addison’s disease (aOR= 3.83, 95% CI: 1.83, 8.04) had the strongest associations with achalasia. Presence of any of the viral infections studied was also associated with an increased risk of achalasia (aOR= 1.58, 95% CI: 1.23, 2.01). Varicella zoster virus (aOR = 3.84, 95% CI: 1.94, 7.62) and human papillomavirus (aOR = 1.77, 95% CI: 1.15, 2.73) both had strong relationships with achalasia.
Conclusions & Inferences:
These findings suggest that achalasia may have autoimmune and viral components contributing to its etiology. Future mechanistic studies could target specific diseases and agents highlighted by this research.
Keywords: autoimmune diseases, esophageal achalasia, epidemiology, esophagus, infections
GRAPHICAL ABSTRACT
INTRODUCTION
Achalasia, a chronic motility disorder of the esophagus characterized by aperistalsis and loss of relaxation function of the lower esophageal sphincter (LES), causes substantial morbidity.1–3 Epidemiologic estimates report an incidence of 2 to 11 per 100,000 and a prevalence of 11 to 162 per 100,000 in the United States, with considerable increases in burden associated with advancing age.4–6 Symptoms of achalasia are generally severe and include dysphagia, regurgitation, heartburn, and weight loss and the condition has been associated with a substantial decrement to health-related quality of life.7–9 In addition to a high symptom burden, current treatments for achalasia (botulinum toxin injection, pneumatic dilation, surgical myotomy, peroral endoscopic myotomy [POEM], and esophagectomy) are invasive and expensive, may result in complications, and often require re-intervention.10,11 Considering the increasing incidence in older adults4, high morbidity, and treatment burden, research into the causal mechanisms behind development of achalasia is critical.
Currently, the etiology of achalasia is uncertain. Given that neural degeneration in the esophageal myenteric plexus12 some have postulated that an upstream viral insult coupled with an aberrant immune system response may be responsible for causing this damage.13,14 This connection implicates infectious and autoimmune diseases as potential drivers of achalasia pathogenesis.15–17 Existing studies examining the association between these diseases with achalasia have demonstrated a positive relationship, though they are limited by small sample sizes and control selection that is not directly sampled from the underlying source population that gave rise to the cases.18,19 Such designs have high risk of bias. Larger epidemiologic studies have been conducted but were reliant on self-report data for past exposure to viral infections and autoimmune disease.20 There is a need for large-scale studies with well-delineated source populations and uniform exposure measurement to advance the understanding of achalasia etiology.
Therefore, we conducted a large case-control study to quantitatively estimate the associations between select autoimmune conditions and viral infections with development of incident achalasia. Using nationwide employer-sponsored private insurance administrative claims data from the United States, we sought to obtain precise association estimates with uniform exposure measurement. These hypothesis-generating insights will highlight in which conditions future research on the etiology of achalasia may be most fruitful.
MATERIALS AND METHODS
Data source and study population
We conducted a retrospective case-control study using administrative claims data from 2001-2019 in the IBM MarketScan Commercial Claims and Encounters Database (hereafter, “MarketScan”) to examine the association between select autoimmune diseases and viral infections with the risk of incident esophageal achalasia. MarketScan contains insurance enrollment and healthcare claims data on adults with commercial, employer-sponsored insurance and their dependents; these data cover over 39 million individuals.21 The study population consisted of all enrollees ages 18-64 with at least 18 months of continuous medical insurance coverage, with no requirements regarding prescription drug coverage. This served as the source population from which cases arose and matched controls were sampled. While MarketScan contains data on adults 65 years of age and older, their insurance may only be supplemental to Medicare coverage. Thus, they were excluded from the analysis to ensure the full capture of claims. The study was deemed exempt from ongoing review by the Institutional Review Board at UNC-Chapel Hill.
Case ascertainment and control sampling
Incident achalasia cases were identified using the ICD-9 (530.0) and ICD-10 (K22.0) diagnosis codes for achalasia and CPT codes for achalasia treatments (supplemental materials). For our primary case definition, we required at least one inpatient achalasia diagnosis or two outpatient diagnoses on different dates (at least 30 days apart), consistent with our prior work.4 A single outpatient diagnosis did not meet the case definition to protect against false positives from rule-out codes potentially listed during a diagnostic evaluation. In addition, all diagnosis codes had to be in the primary diagnosis position on the claim. This definition emphasizes specificity and positive predictive value to increase the likelihood that the cases included are true cases. We conducted a sensitivity analysis of this case definition that additionally required a procedure code for pneumatic dilation, botulinum toxin injection, POEM (using the code for unlisted procedure of the esophagus as a proxy), myotomy, or esophagectomy within 180 days of diagnosis.
To be considered an incident case, a patient had to have their first claim containing an achalasia diagnosis in the patient’s full medical claims history, with at least 18 months of continuous prior insurance enrollment during which there were no claims with a diagnosis code for achalasia. The earliest date listed on a case-meeting sequence of claims was defined as the index date. Controls were selected at the index date using incidence-density sampling and matched on length of continuous insurance enrollment, age, and sex.22 In this control sampling design, controls can become future cases and are only required to be disease-free at the matched index date.23 Four randomly selected eligible controls were required for each case. The case-control odds ratio (OR) in this design estimates the incidence rate ratio (IRR) from the underlying cohort.24
Exposure assessment
Exposure to pre-selected autoimmune conditions and viral infections was assessed by examining the claims history of each patient prior to the index date using ICD-9 and ICD-10 diagnosis codes. All available past claims were used for exposure assessment, except for the recent time window six months prior to the index date. These six months were excluded from the exposure assessment period to reduce the chance of differential exposure misclassification due to increased healthcare utilization around the time of diagnosis during clinical workup of achalasia cases. A study schematic is provided in Figure 1 that provides a visualization of the study design and variable measurement. Autoimmune conditions were selected based on a literature review and included Grave’s disease, Addison’s disease, Crohn’s disease, Hashimoto’s disease, Sjogren’s syndrome, Raynaud’s syndrome, rheumatoid arthritis, psoriatic arthritis, scleroderma or systemic sclerosis, psoriasis, sarcoidosis, lupus, ulcerative colitis, multiple sclerosis, uveitis, celiac disease, alopecia areata, vitiligo, and type-1 diabetes. Viral infections included cytomegalovirus (CMV), herpes simplex virus (HSV), varicella zoster virus (VZV), Epstein-Barr virus (EBV), JC virus (JCV), human immunodeficiency virus (HIV), human papillomavirus (HPV), and measles (all codes are listed in supplemental materials). These conditions were considered to exist in a patient if 1 inpatient or 2 outpatient diagnosis codes (on separate dates) occurred in the medical claims history.
Figure 1. Study design schematic depicting case ascertainment, control selection, and exposure assessment periods for four cases and their matched controls.
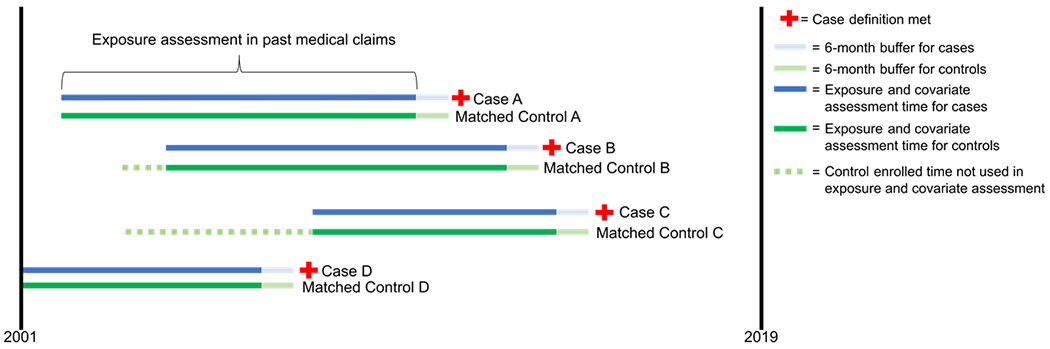
The red cross symbol indicates when a case definition was met. Dark blue and light green sections of lines represent exposure assessment periods for cases and matched controls, respectively. Light blue and light green sections of lines represent the 6-month buffer that was not used in assessing exposures for cases and matched controls, respectively. Dashed green line indicates control insurance-enrolled time that was not used in exposure assessment because it was during a period where the matched case was not enrolled.
A negative control exposure of superficial injury was used as a test of bias, wherein a null association should be expected between superficial injury and achalasia if confounding by healthcare utilization is appropriately addressed.25,26 Specific ICD diagnosis codes for superficial injury are listed in the supplementary table. Broadly, superficial injury diagnosis codes are used to reimburse for medical encounters to treat skin cuts, wounds, and abrasions that do not impact deeper layers of tissue. This negative control was selected because we theorized that it will not be a cause of achalasia but may still be subject to the same confounding factor of healthcare utilization, wherein a positive (but erroneous) relationship between superficial injury and achalasia would be documented if this confounding factor were not adequately handled.
Covariate measurement
Potential confounders included age, sex, time spent continuously enrolled in insurance, comorbidities, frailty, and degree of healthcare utilization. Age was ascertained at the index date. Comorbidities were measured with the Gagne combined comorbidity index using all available past claims history. The Gagne combined comorbidity index combines the Elixhauser and Charlson comorbidity indices and has been demonstrated to have improved predictive performance of patient mortality.27 We similarly calculated the Kim claims-based frailty index for each patient.28 Though unlikely to be a strong confounder in this younger cohort, frailty was included because it may serve as proxy for degree of healthcare utilization and subsequent likelihood of both exposure and achalasia being documented via claims. All available past claims were used in characterizing comorbidities and healthcare utilization, as this is known to increase the validity of the measurements compared to short lookback periods. Given that the comorbidities were measured in the same window as exposure assessment, the assumption is that these covariates are not causal intermediaries affected by prior autoimmune disease. Healthcare utilization was measured as three separate variables: the number of hospitalizations, number of outpatient visits, and use of preventive services in the patients claims history prior to the index date.
Statistical analysis
Descriptive statistics of patient demographics and clinical characteristics were calculated for cases and controls. Multivariable conditional logistic regression was used to obtain adjusted odds ratios relating exposure with achalasia development and account for the matched design used in control selection. A separate model was built for each exposure of interest. In each model, case status served as the outcome with predictor variables consisting of exposure status, geographic region, indicator variables for level of Gagne Comorbidity Index and Kim Frailty Index, number of inpatient visits in the past year, number of outpatient visits in the past year, and a dichotomous variable for use of preventive services. Adjusted odds ratios (aORs) and 95% confidence intervals (CI) were reported for each exposure. All analyses were performed using SAS 9.4 (Cary, NC).
RESULTS
Achalasia case and control characteristics
The study population consisted of 6,769 achalasia cases and 27,076 matched controls. Matching factors (age, sex, length of enrollment) resulted in identical distributions of these variables in cases and controls (Table 1). The median age of cases and controls was nearly 52 (IQR: 41, 59) and 52.2% of cases and controls were females. The median length of enrollment prior to achalasia diagnosis or control selection date was 3.4 years. Regarding factors that were not matched on, cases had a higher burden of comorbidities (12% in the highest Combined Comorbidity Index category compared to 6% of controls), were less likely to be in the most robust category of the frailty index (68.3% vs 80.9%), had more extensive health care utilization prior to the index date, and were slightly more likely to have received preventive services prior to the index date. Cases were slightly more likely to reside in the Northeast or South geographic regions compared to controls. There were 2,359 (35%) cases treated with either pneumatic dilation, myotomy, unlisted procedure of the esophagus (a proxy code for POEM), or an esophagectomy within 180 days of diagnosis. This treated subset of diagnosis-based cases constitutes the sensitivity analysis sample of cases.
Table 1.
Study population characteristics of achalasia and age, sex, enrollment-length matched controls, 2001-2019.
| Achalasia cases N= 6,769 |
Incidence-density controls N= 27,076 |
|
|---|---|---|
| Age, median (IQR) | 51.6 (41.3, 58.5) | 51.5 (41.3, 58.5) |
| Sex | ||
| Male | 3,233 (47.8) | 12,932 (47.8) |
| Female | 3,536 (52.2) | 14,144 (52.2) |
| Region | ||
| Northeast | 1,266 (18.7) | 4,269 (15.8) |
| North Central | 1,416 (20.9) | 6,380 (23.6) |
| South | 2,889 (42.7) | 11,263 (41.6) |
| West | 1,133 (16.7) | 4,860 (18.0) |
| Unknown | 65 (1.0) | 304 (1.1) |
| Years enrolled prior to achalasia diagnosis, median (IQR) | 3.4 (2.2, 5.7) | 3.4 (2.2, 5.7) |
| Achalasia treatments within 180 days of diagnosis | ||
| Pneumatic dilation | 362 (5.3) | -- |
| Myotomy | 1,829 (27.0) | -- |
| Unlisted procedure of esophagus | 188 (2.8) | -- |
| Esophagectomy | 42 (0.6) | -- |
| No treatment | 4,410 (65.2) | -- |
| Gagne comorbidity score, n (%) | ||
| −1 | 858 (12.7) | 3,853 (14.2) |
| 0 | 3,295 (48.7) | 15,758 (58.2) |
| 1 | 1,266 (18.7) | 4,491 (16.6) |
| 2 | 541 (8.0) | 1,489 (5.5) |
| ≥3 | 809 (12.0) | 1,485 (5.5) |
| Kim Frailty Index, n (%) | ||
| Robust, <0.15 | 4,626 (68.3) | 21,902 (80.9) |
| Prefrail, 0.15-0.24 | 1,700 (25.1) | 4,718 (17.4) |
| Mildly frail, 0.25-0.34 | 360 (5.3) | 381 (1.4) |
| Moderate-to-severely frail, ≥0.35 | 83 (1.2) | 75 (0.3) |
| Preventive services in past year | ||
| Yes | 2,390 (35.3) | 8,686 (31.1) |
| No | 4,379 (64.7) | 18,390 (67.9) |
| Number of outpatient visits during past year | ||
| 0 | 423 (6.3) | 3,731 (13.8) |
| 1 | 365 (5.4) | 2,317 (8.6) |
| 2-10 | 2,924 (43.2) | 13,782 (50.9) |
| >=11 | 3,057 (45.2) | 7,246 (26.8) |
| Number of hospitalizations during past year | ||
| 0 | 6,042 (89.3) | 25,762 (95.2) |
| 1 | 482 (7.1) | 1,068 (3.9) |
| 2-4 | 212 (3.1) | 226 (0.8) |
| >=5 | 33 (0.5) | 20 (0.1) |
Abbreviations: IQR, interquartile range.
Autoimmune and viral risk factors for achalasia
The results of the multivariable conditional logistic regression models are contained in Figures 2 and 3, presenting adjusted relationships between autoimmune conditions (Figure 2) and viral infections (Figure 3) with achalasia. Overall, adjusted associations varied across conditions from null relationships to increased risk associations. Presence of any of the autoimmune conditions studied was associated with increased odds of achalasia (aOR= 1.26, 95% CI: 1.11, 1.42). Among specific autoimmune conditions, scleroderma or systemic sclerosis (aOR=8.13, 95% CI: 3.34, 19.80) and Addison’s disease (aOR= 3.83, 95% CI: 1.83, 8.04) had the strongest associations with achalasia. Sarcoidosis (aOR= 1.91, 95% CI: 1.19, 3.05), Crohn’s disease (aOR= 1.69, 95% CI: 1.14, 2.50), and ulcerative colitis (aOR= 1.50, 95% CI: 1.01, 2.23) were also associated with increased odds of developing achalasia. Positive associations with limited precision (wide confidence intervals) were seen for Raynaud’s syndrome, Sjogren’s syndrome, alopecia areata, Hashimoto’s disease, lupus, psoriatic arthritis, and uveitis. Null associations were seen for celiac disease, psoriasis, rheumatoid arthritis, and vitiligo. Negative associations with limited precision were seen for type I diabetes, multiple sclerosis, and Grave’s disease.
Figure 2.
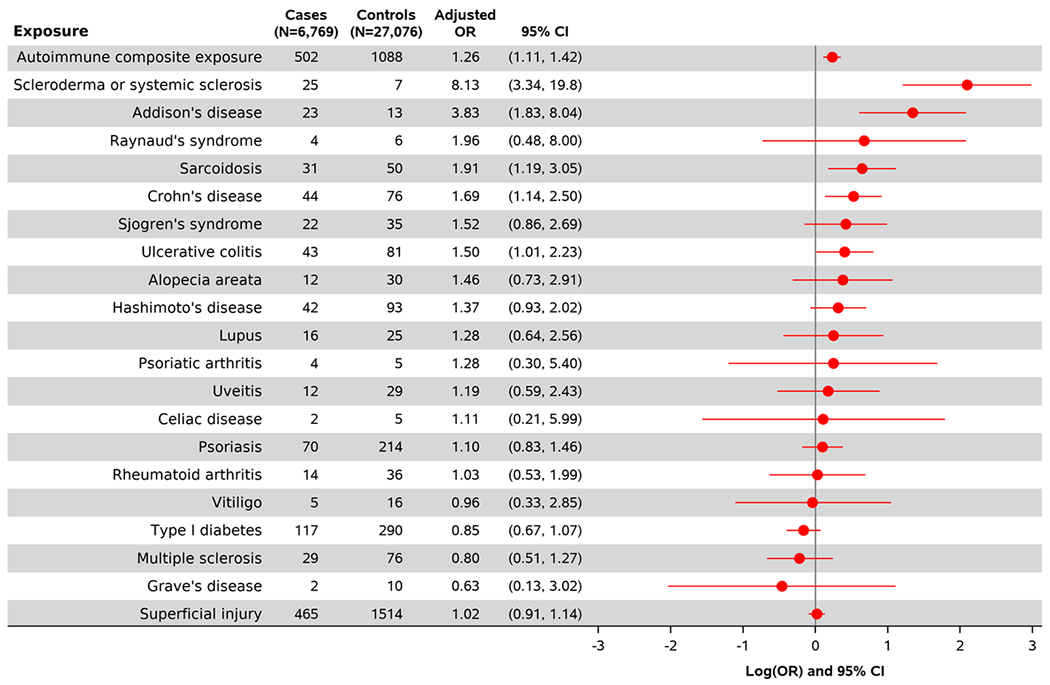
Multivariable adjusted association between autoimmune exposures with the odds of developing incident achalasia.
Figure 3.
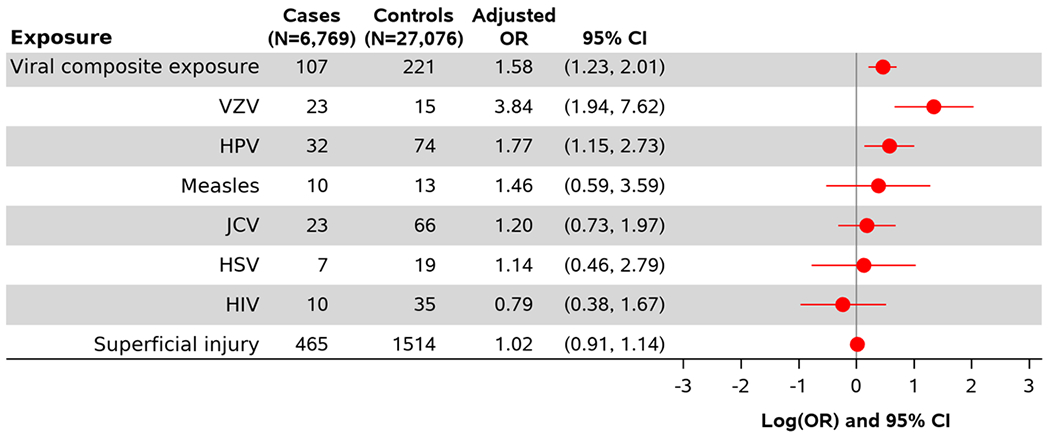
Multivariable adjusted association between viral infections with the odds of developing incident achalasia.
Presence of any of the viral infections studied was associated with an increased odds of achalasia (Figure 3, aOR= 1.58, 95% CI: 1.23, 2.01). Among specific viral infections, varicella zoster virus (aOR = 3.84, 95% CI: 1.94, 7.62) and HPV (aOR = 1.77, 95% CI: 1.15, 2.73) both had strong relationships with achalasia. Measles and JC virus also displayed positive relationships with increased odds of achalasia, though confidence intervals were wide and meaningfully encompassed negative and null association values. No association was seen for HIV.
Our negative control exposure, superficial injury, demonstrated a null relationship with the outcome of achalasia in the adjusted model. The odds of developing incident achalasia among those with a history of superficial injury were equal in those without such a history (aOR= 1.02, 95% CI: 0.91, 1.15).
A sensitivity analysis that re-estimated these associations restricted in a cohort of patients who were treated for achalasia within 180 days (Table 2) did not meaningfully change the estimates for the composite exposure measure for autoimmune disease (aOR=1.31, 95% CI: 1.06, 1.62). However, the association for viral infection was attenuated (aOR=1.12, 95% CI: 0.71, 1.76). Associations for specific autoimmune diseases and viral infections are additionally presented in Table 2.
Table 2.
Sensitivity analysis using only cases that were treated for achalasia within 180 days of diagnosis, and their matched controls.
| Exposure | Treated Cases (N= 2,359) |
Controls N = 9,436 |
Adjusted OR (95% CI) |
|---|---|---|---|
| Autoimmune condition composite exposure | 143 | 350 | 1.31 (1.06, 1.62) |
| Scleroderma or systemic sclerosis | 1 | 3 | 0.78 (0.07, 8.16) |
| Lupus | 4 | 7 | 1.72 (0.48, 6.12) |
| Rheumatoid arthritis | 2 | 10 | 0.57 (0.12, 2.69) |
| Psoriatic arthritis | 2 | 1 | 2.40 (0.21, 27.68) |
| Sjogren’s syndrome | 5 | 11 | 1.24 (0.41, 3.72) |
| Sarcoidosis | 7 | 13 | 2.02 (0.79, 5.14) |
| Multiple sclerosis | 8 | 19 | 0.96 (0.41, 2.28) |
| Ulcerative Colitis | 12 | 29 | 1.37 (0.69, 2.72) |
| Crohn’s Disease | 9 | 26 | 1.12 (0.52, 2.42) |
| Uveitis | 5 | 6 | 2.99 (0.90, 9.95) |
| Psoriasis | 24 | 77 | 1.05 (0.65, 1.68) |
| Grave’s disease | 1 | 2 | 1.85 (0.17, 20.49) |
| Hashimoto’s disease | 15 | 33 | 1.55 (0.82, 2.93) |
| Addison’s disease | 4 | 3 | 3.63 (0.74, 17.84) |
| Raynaud’s syndrome | 2 | 2 | 2.88 (0.32, 26.17) |
| Celiac disease | 2 | 1 | 4.46 (0.37, 53.64) |
| Vitiligo | 1 | 3 | 1.23 (0.13, 12.11) |
| Alopecia areata | 5 | 12 | 1.56 (0.54, 4.48) |
| Type I diabetes | 37 | 95 | 1.14 (0.76, 1.69) |
| Viral infection composite exposure | 26 | 82 | 1.12 (0.71, 1.76) |
| HSV | 2 | 8 | 0.88 (0.19, 4.19) |
| VZV | 3 | 6 | 1.53 (0.37, 6.23) |
| JCV | 6 | 24 | 1.00 (0.40, 2.45) |
| HIV | 2 | 16 | 0.46 (0.11, 2.03) |
| HPV | 12 | 24 | 1.86 (0.92, 3.75) |
| Measles | 1 | 5 | 0.28 (0.03, 2.68) |
| Superficial injury | 158 | 550 | 1.03 (0.85, 1.25) |
Abbreviations: VZV, varicella zoster virus; HPV, human papillomavirus; JCV, John Cunningham virus; HSV, herpes simplex virus; HIV, human immunodeficiency virus.
DISCUSSION
Achalasia is a debilitating esophageal disease and its cause is not completely understood. Prior studies have suggested that viral infections and autoimmune disease may play a role in the risk of developing achalasia, but whether these associations persisted in large, population-based datasets with rigorous procedures to mitigate bias was unknown. We conducted a large case-control study using administrative claims data to examine differences in past exposure to autoimmune diseases and viral infections in achalasia patients compared to population-based controls that were selected from the underlying insurance-enrolled source population that gave rise to the cases. Of the conditions considered, both positive associations of varying precision and null associations were found. Scleroderma or systemic sclerosis was the autoimmune condition that had the strongest association with achalasia, though positive associations were also seen with Addison’s disease, sarcoidosis, Sjogren’s syndrome, and inflammatory bowel disease. The strong association seen with scleroderma or systemic sclerosis may be partly explained by the fact that achalasia and scleroderma both result in esophageal motility abnormalities characterized by severely impaired bolus transit. Thus, it is possible that some achalasia cases may have been previously misdiagnosed as scleroderma, contributing to the strong association demonstrated. With the development of high-resolution manometry in the 2010s and the Chicago classification, it may have become easier to distinguish between these conditions in cases diagnosed after 2010. Varicella zoster virus was the viral condition with the strongest association, but a positive association was also seen with HPV. These hypothesis-generating results may help refine the focus of subsequent etiologic studies of achalasia.
Our findings were mostly harmonious with that of prior published studies. Becker et al. demonstrated in a large case-control study that viral infections (OR = 4.07, 95% CI: 2.33, 7.10) and autoimmune conditions (OR = 1.78, 95% CI: 1.27, 2.50) were both associated with increased risk of achalasia. Through primary data collection and requiring a gastroenterologist’s diagnosis, the achalasia case-definition in Becker et al. was stronger than our claims-based definition, although exposure measurement relied on self-report which may be subject to recall bias. Our finding of no association between psoriasis and achalasia (OR = 1.10, 95% CI: 0.83, 1.46) was divergent from Becker et al. who reported a positive association (OR = 2.70, 95% CI: 1.34, 5.42). In a case-control study of 114 cases and age and sex matched controls with gastroesophageal reflux disease (GERD), Romero-Hernández et al. found that achalasia patients were more likely to have an autoimmune disease documented in their medical records (OR = 3.8, 95% CI: 1.47, 9.83).19 Booy et al. also found a positive relationship between autoimmune disease and achalasia (OR = 3.6, 95% CI: 2.5, 5.3); however, the controls were derived external to the study from the epidemiologic literature and did not represent the source population which gave rise to the cases. To the extent that the controls from the literature have a different exposure level than the source population, the reported associations may be biased towards or away from the null.
In particular, our finding of a strong positive association between VZV infection and achalasia is congruent with evidence from prior studies that have examined this association. Nearly 30 years ago, Robertson et al. found a significantly higher proportion of VZV serum antibody titers in 9 achalasia cases (33%) compared to 20 age and sex matched controls (0%).29 Most recently, Naik et. al. found a strikingly high prevalence (80%) of salivary VZV DNA in fifteen achalasia cases compared to five controls (0%); laboratory examination of surgically resected tissue from Heller myotomies found that 87% of the cases contained transcripts that encoded late gene products of VZV.30
The existing literature has presented potential mechanisms through which autoimmune conditions and viral infections could function as risk factors for achalasia development. The predominant theme from these proposed mechanisms is that a viral or environmental insult coupled with dysfunction of immune-mediated processes leads to achalasia.13,15,16,31 A specific molecular risk factor of eight-residue insertion in the HLA-D Q β1 gene has been presented in other studies as a risk factor for achalasia.20,32 Mutations in the same gene have been associated with Addison’s disease, inflammatory bowel disease, and scleroderma, which may suggest shared genetic predisposition or other shared immunologic mechanisms for these associations observed in our data.33–36 However, inflammatory bowel disease and sarcoidosis are not unequivocally autoimmune conditions, so another possible explanation for the positive relationship found between these diseases and achalasia could be the inflammatory nature of the diseases.
This study contains numerous strengths including the substantial sample size facilitated by the use of a large, nationwide administrative claims database, and the strategies taken to address the potential for bias. With over 6,500 cases, this study represents one of the largest cohorts of achalasia patients assembled and the most precise study of autoimmune disease and viral illness as risk factors. In terms of bias reduction, three study design techniques were used to decrease the chance of differential healthcare utilization leading to a spurious association between disease exposures and achalasia. First, an a priori decision was made to exclude the six months closest to achalasia diagnosis from the exposure assessment window. We hypothesized that an achalasia case may have more medical visits in general around the time of achalasia diagnosis, leading to an artificially greater opportunity for autoimmune disease and viral infections to be diagnosed and documented in claims compared to controls. Second, we measured proxies of health care use intensity by measuring and adjusting for the count of past inpatient hospitalizations, outpatient visits, and use of preventive services. Third, we used a negative control exposure of superficial injury (diagnosis codes for cuts, abrasions, and wounds that impact skin tissue but not deeper layers) that has no conceivable association with documented achalasia diagnosis other than through a common cause of high healthcare utilization. Our finding that superficial injury had a null association with achalasia (OR =1.02) after adjustment supports the notion that differential intensity of healthcare utilization between cases and controls was adequately controlled for by design and statistical adjustment.
Limitations of our study include the lack of clinical detail found in claims data and the associational nature of the results; thus, causality cannot be determined from this study design. We also acknowledge that there are no validated coding case definitions for achalasia, but we have previously used the present one4 and coding decisions were justified to increase specificity of diagnosis, a desirable feature in etiologic risk factor studies as it minimizes the inclusion of false-negative cases. Additionally, the sensitivity analysis wherein cases were restricted to those treated within 6 months warrant cautious interpretation as the stricter case definition in this sensitivity analysis substantially reduced the sample size.
In summary, our study documented that select autoimmune exposures and viral infections, particularly Addison’s disease, scleroderma, varicella virus, and HPV are associated with an increased risk of achalasia. These hypothesis generating results help inform directions of future etiologic research, serving as a screening mechanism to reveal which exposures may warrant the collection of more clinically-detailed data. Further work anchored on the exposure of varicella virus should investigate whether the presence of underlying autoimmune disease at the time of exposure to viral infections may modify the effect of such infections on long-term risk of achalasia development. Our findings have the potential to provide insight into specific mechanisms of disease, which may guide further work and preventive strategies considering the molecular pathways in which nerve function and motility may be compromised in the esophagus of achalasia patients.
Supplementary Material
Funding:
The project described was supported by the National Institutes of Health, through Grant Award Number T32DK007634 (CEG and CCC) and P30DK034987. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Grant Support:
The project described was supported by the National Institutes of Health, through Grant Award Number T32DK007634 (CEG and CCC) and P30DK034987. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data sharing:
The data that support the findings of this study are available IBM MarketScan Commercial Claims and Encounters Database. Restrictions apply to the availability of these data, which were used under license for this study.
Footnotes
Competing Interests: the authors have no competing interests.
REFERENCES
- 1.Pandolfino JE, Gawron AJ. Achalasia: A Systematic Review. Jama. 2015;313(18):1841–1852. doi: 10.1001/jama.2015.2996 [DOI] [PubMed] [Google Scholar]
- 2.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet Lond Engl. 2013;383(9911):83–93. doi: 10.1016/s0140-6736(13)60651-0 [DOI] [PubMed] [Google Scholar]
- 3.Gyawali CP. Achalasia: new perspectives on an old disease. Neurogastroenterol Motil. 2016;28(1):4–11. doi: 10.1111/nmo.12750 [DOI] [PubMed] [Google Scholar]
- 4.Gaber CE, Eluri S, Cotton CC, et al. Epidemiologic and Economic Burden of Achalasia in the United States. Clin Gastroenterol H. Published online 2021. doi: 10.1016/j.cgh.2021.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samo S, Carlson DA, Gregory DL, Gawel SH, Pandolfino JE, Kahrilas PJ. Incidence and Prevalence of Achalasia in Central Chicago, 2004–2014, Since the Widespread Use of High-Resolution Manometry. Clin Gastroenterol H. 2017;15(3):366–373. doi: 10.1016/j.cgh.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22(9):e256–e261. doi: 10.1111/j.1365-2982.2010.01511.x [DOI] [PubMed] [Google Scholar]
- 7.Nenshi R, Takata J, Stegienko S, et al. The Cost of Achalasia: Quantifying the Effect of Symptomatic Disease on Patient Cost Burden, Treatment Time, and Work Productivity. Surg Innov. 2010;17(4):291–294. doi: 10.1177/1553350610376392 [DOI] [PubMed] [Google Scholar]
- 8.Loosen SH, Kandler J, Luedde T, Kostev K, Roderburg C. Achalasia is associated with a higher incidence of depression in outpatients in Germany. Plos One. 2021;16(4):e0250503. doi: 10.1371/journal.pone.0250503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalantari M, Hollywood A, Lim R, Hashemi M. Mapping the experiences of people with achalasia from initial symptoms to long-term management. Health Expect. 2021;24(1):131–139. doi: 10.1111/hex.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung Y-C, Westfal ML, Chang DC, Kelleher CM. Heller myotomy is the optimal index procedure for esophageal achalasia in adolescents and young adults. Surg Endosc. 2018;33(10):3355–3360. doi: 10.1007/s00464-018-06625-6 [DOI] [PubMed] [Google Scholar]
- 11.Gupta K, Khan A, Chalhoub J, Groudan K, Desilets D. Rehospitalization, Treatment, and Resource Use After Inpatient Admission for Achalasia in the USA. Digest Dis Sci. Published online 2021:1–10. doi: 10.1007/s10620-020-06775-5 [DOI] [PubMed] [Google Scholar]
- 12.Tłottrup A, Forman A, Funch-Jensen P, Raundahl U, Andersson KE. Effects of postganglionic nerve stimulation in oesophageal achalasia: an in vitro study. Gut. 1990;31(1):17. doi: 10.1136/gut.31.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park W, Vaezi MF. Etiology and Pathogenesis of Achalasia: The Current Understanding. Am J Gastroenterology. 2005;100(6):1404–1414. doi: 10.1111/j.1572-0241.2005.41775.x [DOI] [PubMed] [Google Scholar]
- 14.Furuzawa-Carballeda J, Torres-Landa S, Valdovinos MÁ, Coss-Adame E, Campo LAM del, Torres-Villalobos G. New insights into the pathophysiology of achalasia and implications for future treatment. World J Gastroentero. 2016;22(35):7892–7907. doi: 10.3748/wjg.v22.i35.7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pressman A, Behar J. Etiology and Pathogenesis of Idiopathic Achalasia. J Clin Gastroenterol. 2017;51(3):195–202. doi: 10.1097/mcg.0000000000000780 [DOI] [PubMed] [Google Scholar]
- 16.O’Neill OM, Johnston BT, Coleman HG. Achalasia: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroentero. 2013;19(35):5806–5812. doi: 10.3748/wjg.v19.i35.5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu XY, Liu ZQ, Wang Y, et al. The etiology of achalasia: An immune-dominant disease. J Digest Dis. 2021;22(3):126–135. doi: 10.1111/1751-2980.12973 [DOI] [PubMed] [Google Scholar]
- 18.Booy JD, Takata J, Tomlinson G, Urbach DR. The prevalence of autoimmune disease in patients with esophageal achalasia. Dis Esophagus. 2012;25(3):209–213. doi: 10.1111/j.1442-2050.2011.01249.x [DOI] [PubMed] [Google Scholar]
- 19.Romero-Hernández F, Furuzawa-Carballeda J, Hernández-Molina G, et al. Autoimmune comorbidity in achalasia patients. J Gastroen Hepatol. 2018;33(1):203–208. doi: 10.1111/jgh.13839 [DOI] [PubMed] [Google Scholar]
- 20.Becker J, Niebisch S, Ricchiuto A, et al. Comprehensive epidemiological and genotype–phenotype analyses in a large European sample with idiopathic achalasia. Eur J Gastroen Hepat. 2016;28(6):689–695. doi: 10.1097/meg.0000000000000602 [DOI] [PubMed] [Google Scholar]
- 21.IBM MarketScan Research Databases for life sciences researchers. Published 2020. Accessed May 22, 2021. https://www.ibm.com/downloads/cas/OWZWJ0QO
- 22.Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004;61(12):e59. doi: 10.1136/oem.2004.014472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, Pearce N. Case–control studies: basic concepts. Int J Epidemiol. 2012;41(5):1480–1489. doi: 10.1093/ije/dys147 [DOI] [PubMed] [Google Scholar]
- 24.Pearce N What Does the Odds Ratio Estimate in a Case-Control Study? Int J Epidemiol. 1993;22(6):1189–1192. doi: 10.1093/ije/22.6.1189 [DOI] [PubMed] [Google Scholar]
- 25.Dusetzina SB, Brookhart MA, Maciejewski ML. Control Outcomes and Exposures for Improving Internal Validity of Nonrandomized Studies. Health Serv Res. 2015;50(5):1432–1451. doi: 10.1111/1475-6773.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipsitch M, Tchetgen ET, Cohen T. Negative Controls. Epidemiology. 2010;21(3):383–388. doi: 10.1097/ede.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. Journals Gerontology Ser. 2017;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson CS, Martin BA, Atkinson M. Varicella-zoster virus DNA in the oesophageal myenteric plexus in achalasia. Gut. 1993;34(3):299. doi: 10.1136/gut.34.3.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik RD, Vaezi MF, Gershon AA, et al. Association of Achalasia with Active Varicella Zoster Virus Infection of the Esophagus. Gastroenterology. Published online 2021. doi: 10.1053/j.gastro.2021.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ates F, Vaezi MF. The Pathogenesis and Management of Achalasia: Current Status and Future Directions. Gut Liver. 2015;9(4):449. doi: 10.5009/gnl14446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gockel I, Becker J, Wouters MM, et al. Common variants in the HLA-DQ region confer susceptibility to idiopathic achalasia. Nat Genet. 2014;46(8):901–904. doi: 10.1038/ng.3029 [DOI] [PubMed] [Google Scholar]
- 33.Pani MA, Seidl C, Bieda K, et al. Preliminary evidence that an endogenous retroviral long-terminal repeat (LTR13) at the HLA-DQB1 gene locus confers susceptibility to Addison’s disease. Clin Endocrinol. 2002;56(6):773–777. doi: 10.1046/j.1365-2265.2002.t01-1-01548.x [DOI] [PubMed] [Google Scholar]
- 34.Reinshagen M, Loeliger C, Kuehnl P, et al. HLA class II gene frequencies in Crohn’s disease: a population based analysis in Germany. Gut. 1996;38(4):538. doi: 10.1136/gut.38.4.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato H, Grutters JC, Pantelidis P, et al. HLA-DQB1*0201. Am J Resp Cell Mol. 2002;27(4):406–412. doi: 10.1165/rcmb.4782 [DOI] [PubMed] [Google Scholar]
- 36.Gladman DD, Kung TN, Siannis F, Pellett F, Farewell VT, Lee P. HLA markers for susceptibility and expression in scleroderma. J Rheumatology. 2005;32(8):1481–1487 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


