Abstract
Importance
Antibiotics are commonly used to treat and prevent urinary tract infection (UTI), but resistance is growing. Non-antibiotic prophylaxis such as methenamine hippurate (MH) show clinical promise, but its impact on bladder factors influencing recurrent UTIs (rUTI) is not well-described.
Objectives
To examine the effect of MH on bladder inflammation and barrier function in aged mice and women with rUTI.
Study Design
This study included urine samples from an experimental study involving aged female mice with and without methenamine treatment as well as women with rUTI who received either no prophylaxis; MH alone; vaginal estrogen therapy (VET) and/or d-mannose (DM) alone; or MH in addition to VET and/or DM. We performed comprehensive cytopathological analysis, which included enzyme-linked immunosorbent assay for immunoglobulin A (IgA), interleukin 6 (IL-6, in human samples), and FITC-Dextran permeability assay (in mice) to assess for urothelial permeability.
Results
In the aged mice model, there was a decreased urothelial permeability (as seen by retention of FITC-Dextran fluorescence in superficial cells) and increased urinary IgA in mice treated with MH compared to controls. There was no significant difference in urothelial shedding (p>0.05). In human samples, there was significantly increased urinary IgA in those taking MH alone compared to no prophylaxis (704.1±248.4 vs 469.2±259.6 ng/mL, p=0.04), but no significant difference in IL-6.
Conclusions
MH appears to enhance barrier function as evidenced by decreased urothelial permeability and increased urinary IgA levels, without worsening inflammation. This may reflect another beneficial mechanism by which MH helps to prevent rUTI.
Keywords: UTI, recurrent UTI, non-antibiotic prophylaxis, methenamine hippurate, women
INTRODUCTION
Urinary tract infections (UTIs) are one of the most commonly treated infections that disproportionately affect women.1-3 Furthermore, recurrence occurs in over 25% of women,4,5 with up to 50% of recurrence occurring in postmenopausal women.6 Recurrent UTIs (rUTI) can results in severe morbidity, mortality, and economic burden.2,7 Since women, particularly older women, experience the highest burden of disease, they are also more vulnerable to complications from UTI and antibiotic use. Thus, rUTI prevention strategies are of utmost importance.
Although antibiotics are frequently used to prevent rUTIs, antibiotics expose patients to risk of adverse effects; damage commensal bacteria; promote infections like C. difficile; and induce resistance.8,9 Antibiotic resistance contributes to 3 million illnesses and 23,000 deaths per year.10 Non-antibiotic prophylaxis can avoid such complications. We have previously found that vaginal estrogen decreases risk of rUTI by decreasing bladder inflammation, as evidenced by decreased interleukin-6 (IL-6), leukocyte influx, and urothelial shedding.11 Other promising non-antibiotic prophylaxis include D-mannose (DM) and methenamine hippurate (MH), a promising urinary antiseptic.12 However, inadequate knowledge of their impact on the bladder environment limits our ability to optimize non-antibiotic UTI prophylaxis.
Our objective was to determine the impact of MH on bacteriuria, bladder inflammation, and bladder barrier function: all important factors that impact rUTI. Our primary aim was to examine the impact of MH treatment on bladder inflammation and barrier function markers in an aged female mouse model infected with a uropathogenic E coli isolate. Our parallel primary aim was to examine the impact of MH alone or in combination with other non-antibiotic UTI prophylaxis on bladder inflammation and barrier function correlates in women with rUTI. Our secondary aim was to measure bacterial load, pH, and directly measure barrier function via urothelial permeability in aged, infected mice after MH treatment. We hypothesized that in both models, MH works by dampening inflammation and improving barrier function (decreased urothelial permeability, increased IgA).
MATERIALS AND METHODS
This study involved two models: a prospective cohort study involving aged female mice (18 months old, which represent humans at around 65 years of age) and urine samples from a biorepository of women recruited from Female Pelvic Medicine and Reconstructive Surgery (FPMRS) and general Obstetrics & Gynecology (OBGYN) clinics at Washington University School of Medicine since 2018 (IRB #201712113).
Mouse Model
Our aged mouse model has successfully modeled UTIs seen in older women, with findings significant for delayed bacterial clearance, increased inflammatory response, and impaired urinary barrier function.13,14 Our protocol was approved by Washington University School of Medicine Institutional Animal Care and Use Committee (#20-0056).
Methenamine hippurate (MH) supplementation
Human level dosage of MH (2 grams daily) was converted to murine dosage by the Division of Comparative Medicine. We treated one group of aged female mice with murine dose-adjusted MH and another group with water as control for 4 weeks.
Inoculation with UT189
At the end of 4 weeks, mice were inoculated with UT189, a uropathogenic E.coli clinical cystitis isolate from a frozen glycerol stock grown statically at 37°C for 24hr in 20 mL of Luria-Bertani broth.15 Mice were anesthetized and transurethrally catheterized with 50 uL of 107 CFU of the bacterial suspension diluted in PBS.15 Urine was collected at days 1, 3, 7, 10, and 14 post-inoculation. Mice were sacrificed on day 14 post-inoculation.
Urine Analysis
During treatment, daily urinary pH was measured, as urinary pH of <5.85 produces the optimal bacteriostatic concentration of formaldehyde from MH.16
Urine cytology was obtained by centrifuging 10uL of urine and 40uL sterile PBS onto slides to count exfoliated urothelial cells per high powered field using Papanicolaou staining and light microscopy.
Urine specimens were cultured by serially diluting urine in sterile PBS. Then, 5uL of each dilution was seeded on agar plates for a total of six replicates. Bacterial colonies were counted and bacterial load calculated as colony forming units (CFU)/mL. Urine was considered to be non-infected when log-transformed bacterial colony count were <103.
Urinary levels of secretory IgA were quantified using Invitrogen Mouse IgA Uncoated ELISA Kit (Thermo Scientific, Vienna, Austria) according to manufacturer’s instructions, which has a detection range of 0.39-25 ng/mL.
Assessment of Barrier function
After sacrifice, bladders were fixed in methacarn (60% methanol, 30% chloroform, 10% acetic acid), embedded in paraffin, and 5 μm thick sections cut for further analysis.
Barrier function as reflected by urothelial permeability was measured by injecting 50 uL of 10 mg/mL 10 kD Fluorescein isothiocyanate-conjugated dextran (FITC-Dextran) (D1821, Invitrogen) transurethrally into mouse bladders.17 After sacrifice, frozen bladder tissue sections were made and dipped in 1:1 methanol-acetone and PBS, then cover-slipped with Prolong Diamond Antifade with DAPI (P36971, Invitrogen). Images were acquired using a fixed exposure set to detect fluorescence. Location and intensity of fluorescence permeating through the urothelium was quantified by averaging the mean gray value for FITC channel in 3 random squares from 2 separate images for each mouse using ImageJ software. Higher mean gray values indicated concentration of fluorescence in the superficial cell layers of the urothelium (decreased permeability). Values were combined for each mouse for statistical analysis.
Human urinary samples
Sample Collection
Human urine samples were obtained through an IRB approved biorepository prospectively collected from clinic patients who consented to have samples stored for further study. Informed consent was obtained from all participants. Enrolled women provided baseline medical, surgical, and demographic information; history of rUTI; and any use of UTI prophylaxis with vaginal estrogen (VET), d-mannose (DM), and/or methenamine hippurate (MH) at the time of sample collection. Urine samples were collected via voided midstream clean catch or straight catheterization based on clinical indications. Urine samples were treated with protease inhibitor (EDTA-free, Halt; Thermo Scientific, Waltham, Massachusetts) and stored at −80°C.
Sample Selection
Samples from subjects were included if subjects had a history of rUTI and documentation of any non-antibiotic UTI prophylaxis use. Subjects were excluded if they had no history of rUTI; were on antibiotic prophylaxis or antibiotic treatment; or declined use of their banked samples for other studies. Samples from these women were categorized into four groups:
women who were not on MH, VET, or DM prophylaxis at time of sample collection (NP);
women on MH alone;
women on VET and/or DM alone (VET±DM); and
women on MH in addition to VET and/or DM (MH + VET±DM).
To assess the impact of any MH treatment, we compared samples from group 1 to group 2 or group 4. We also compared samples from group 3 to group 4 to assess the impact of MH to effects already produced by VET and/or d-mannose. Using Gower’s method, we matched samples from comparison groups based on baseline characteristics, medical comorbidities, and medications that could impact local bladder and systemic inflammation (Supplementary Method and Table 1).
Sample Processing
Thawed urine (100 μL) was cyto-centrifuged onto positively charged glass slides. Urothelial sloughing and urinary secretory IgA were measured with the Invitrogen Mouse IgA Uncoated ELISA Kit (Thermo Scientific, Vienna, Austria), which has a detection range of 1.6-100 ng/mL. Because we were able to collect a greater amount of urine from human women than from mice, we were also able to analyze urinary IL-6 to examine urinary inflammation using the R&D Systems DuoSet Human IL-6 ELISA according to manufacturer’s instructions (R&D, Minneapolis, Minnesota, sensitivity 0.6 pg/mL).
Sample Size Determination – Of Mice and Women
As there were no prior studies examining MH and IgA (reflecting barrier function) in mice, we based our sample size on our prior work on the impact of VET on bladder inflammation in mice.13 Based on a standard deviation of the log-transformed distribution of IL-6 of 0.75 ng/ml, 10 mice per group would give us 80% power to detect a minimum 2.7-fold difference between MH treated and untreated mice.
We also based our sample size for human urinary samples on IL-6 data from a prior study.11 Assuming that the standard deviation of the log-transformed distribution of change in IL-6 is no greater than 1.7 ng/ml, 10 women per baseline group would give us 91% power to detect a difference between groups with and without MH treatment.
Statistical Analysis
Comparison between groups for both mouse and human samples were analyzed using ANOVA with Tukey’s multiple-comparison posttest for continuous variables, while categorical variables were compared using χ2 test and Fisher exact test. P values of <0.05 were considered statistically significant.
RESULTS
Aged mouse model
pH
During the treatment period, mean daily urinary pH ranged from pH 4.1-4.9 for MH-treated aged mice (N=8) and pH 4.3-5.1 for controls (N=8). There was no significant difference between groups (Overall pH 4.6±0.3 vs 4.8±0.2, mean difference −0.17, p=0.21).
Bacteriuria
There was no difference in bacteriuria between aged mice treated with MH and controls during 14 days of observation post-inoculation (Figure 1A). There was a non-significant trend towards decreased infectivity in aged mice treated with MH compared to control over the course of 14 days (Figure 1B, p=0.06).
Figure 1: Bacteriuria between aged mice with and without methenamine hippurate treatment.
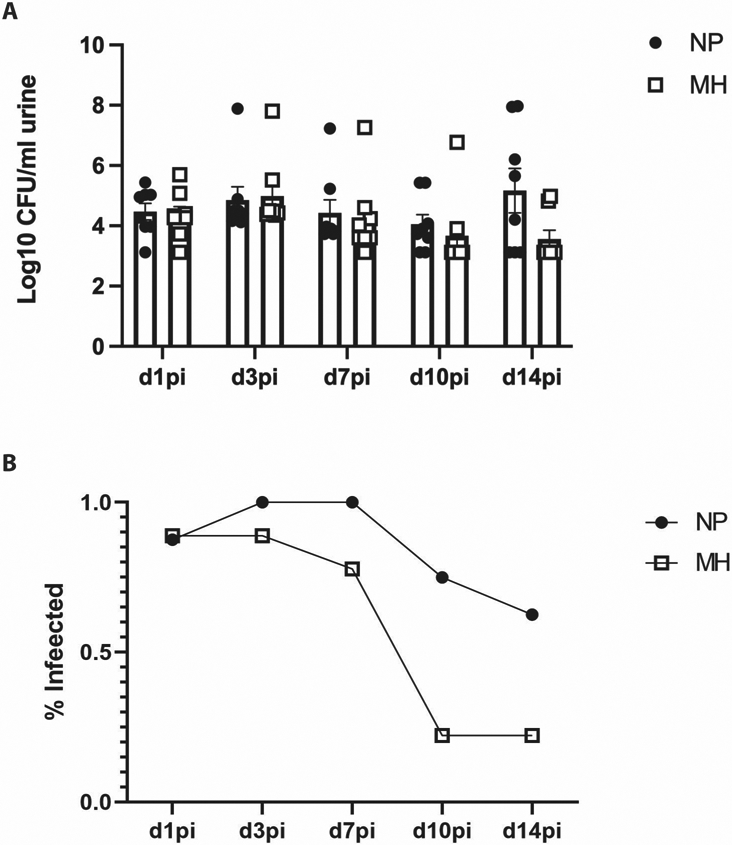
Filled dots: aged mice without MH treatment (NP)
Empty squares: aged mice treated with MH (MH).
A) There was no significant difference between bacterial colony forming units per milliliter of urine between aged mice with and without MH treatment
B) Over time, there was a non-significant trend towards decreased proportion of mice considered infected in aged mice treated MH (p=0.06).
Inflammation: Urothelial shedding
MH treatment did not affect urothelial shedding (56.2±88.4 vs 4.4±8.2 mean urothelial cells/50 uL for control and MH groups respectively, p=0.71, day 1 post-inoculation; 117.5±249.7 vs 25.8±42.1, p=0.9, day 3 post-inoculation).
Barrier function
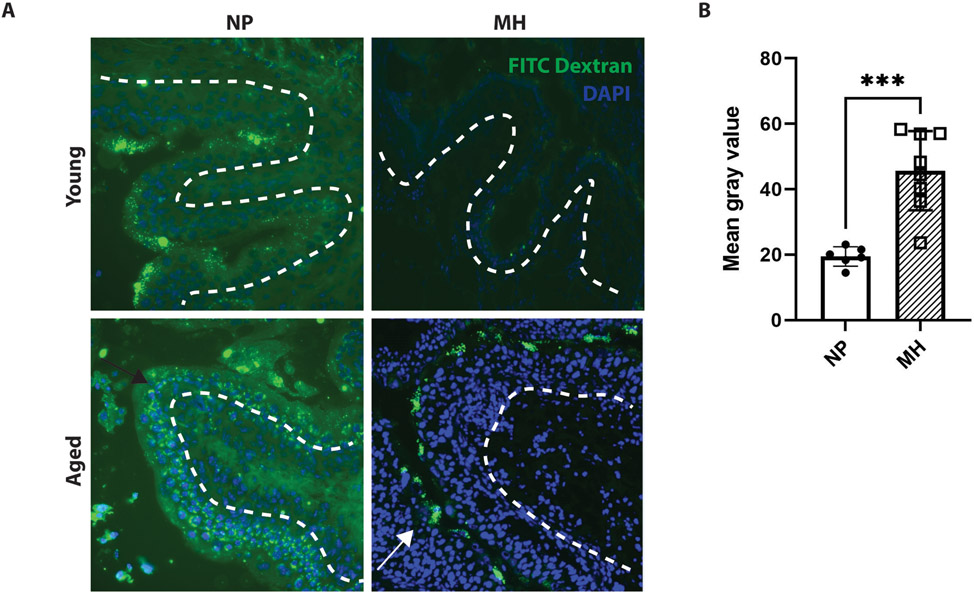
We noted significantly deeper FITC-Dextran permeability through the urothelium in aged mice, indicating worse barrier function (Figure 2A). When comparing aged mice with and without MH treatment, there was decreased permeability in MH-treated aged mice compared to control (Figure 2A), as seen by the FITC-Dextran retention in superficial cells versus deeper permeation in controls. Quantification of FITC-Dextran fluorescence permeability revealed greater mean gray values in MH-treated mice, confirming that there was significantly decreased permeability of FITC-Dextran into the deeper urothelial layers (mean value 19.5±3 vs 45.6±12.1 for control and MH, respectively, Figure 2B, p=0.0007).
Figure 2: FITC-Dextran permeability assay between aged and young mice with and without methenamine hippurate treatment.
The white dashed line indicates the margin of the bladder urothelium. The nuclei stained blue compromise the bladder urothelium that faces the lumen.
*** p ≤0.001
Filled dots: aged mice without MH treatment (NP)
Empty squares: aged mice treated with MH (MH)
A) Aged mice without MH treatment showed greater permeability of fluorescence into deeper layers of the urothelium (as delineated by black arrow), while young mice and aged mice treated with MH showed decreased permeability, with more fluorescence retained in the superficial level of the urothelium (as shown by the white arrow).
B) Greater mean gray values were seen in aged mice treated with MH compared to controls (p≤0.001).
We further evaluated barrier function by measuring urinary IgA post-inoculation. When compared to aged, untreated mice, aged mice treated with MH had greater urinary IgA levels (Figure 3)
Figure 3: Urinary secretory IgA levels in aged mice with and without methenamine hippurate treatment.
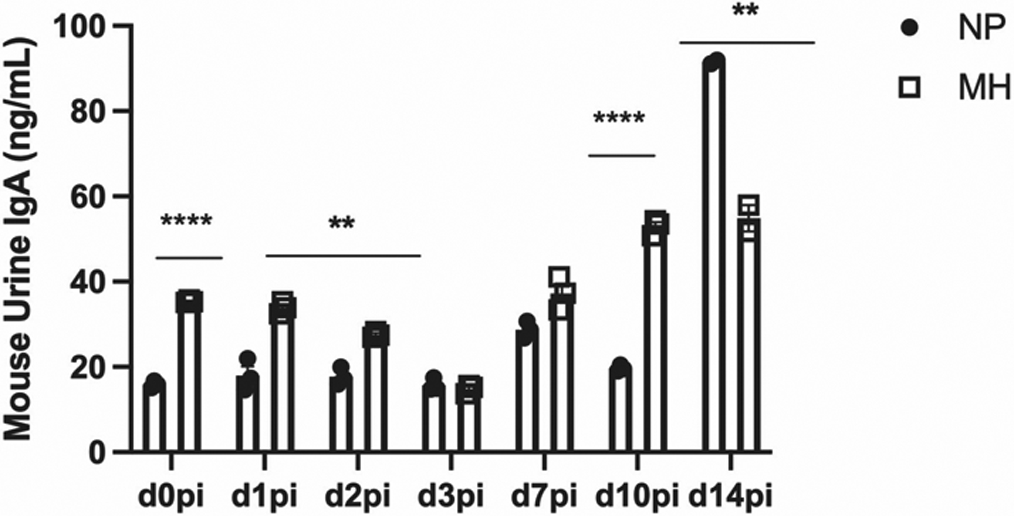
NS Non-significant, p >0.05
* p ≤0.05
** p ≤0.01
*** p ≤0.001
**** p ≤0.0001
Filled dots: aged mice without MH treatment (NP)
Empty squares: aged mice treated with MH (MH)
There was a significant difference between urinary IgA levels between aged mice treated with MH compared to controls at most time points after infection.
Human urinary samples
We identified 8 samples from group 2 (MH) and 29 samples from group 4 (MH + VET±DM). These were matched as described to a total of 78 samples from women categorized as group 1 (NP) and 62 samples from group 3 (VET±DM). Baseline demographics and medical characteristics can be found in Supplementary Table 2.
pH
There was no significant difference in mean urinary pH between NP and MH groups, nor between VET±DM and MH + VET±DM groups. However, mean urinary pH was significantly lower in the NP group compared to the MH + VET±DM group (pH 5.8±0.9 vs 6.3 ±0.9, p=0.04).
Inflammation: IL-6 levels
When comparing urine IL-6 levels between matched groups, there was no significant difference between NP and MH; VET±DM and MH + VET±DM; or NP and MH + VET±DM groups (Figure 4).
Figure 4: Urinary IL-6 levels in women with and without methenamine hippurate treatment.
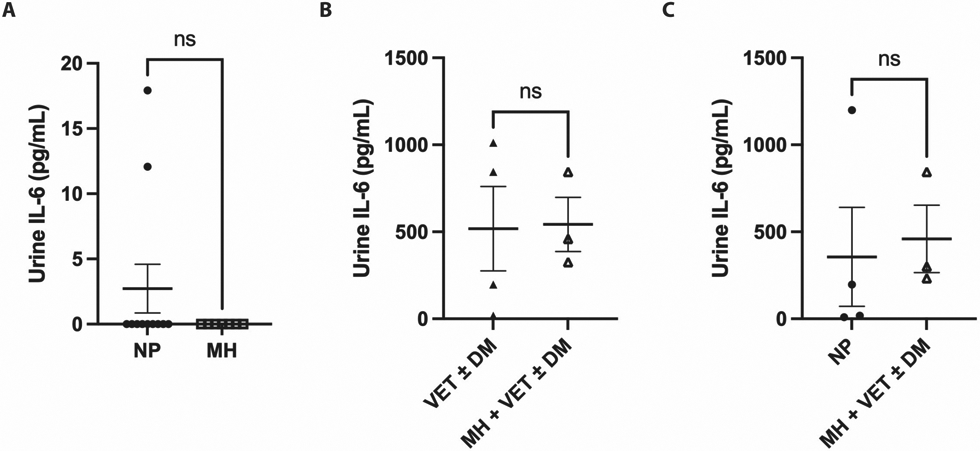
Filled dots: women without MH, VET, or DM prophylaxis (NP)
Empty squares: women receiving MH alone (MH)
Filled triangles: women receiving VET and/or DM (VET±DM)
Empty triangles: women receiving MH in addition to VET and/or DM (MH + VET±DM)
There was no significant difference in urinary IL-6 levels between A) women receiving no prophylaxis with MH, DM, or VET (NP) versus those receiving MH alone (MH); B) women receiving VET and/or DM (VET±DM) versus those receiving MH in addition to VET and/or DM (MH + VET±DM); and C) women receiving no prophylaxis with MH, DM, or VET (NP) versus those MH in addition to VET and/or DM (MH + VET±DM)
Barrier function
There was no difference in IgA levels between VET±DM and MH + VET±DM groups or NP and MH + VET±DM groups (Figure 5B and C). However, there was a statistically significant increase in urinary IgA in the MH group compared to the NP group (704.1±248.4 vs 469.2±259.6 ng/mL, p=0.04, Figure 5A).
Figure 5: Urinary secretory IgA levels in women with and without methenamine hippurate treatment.
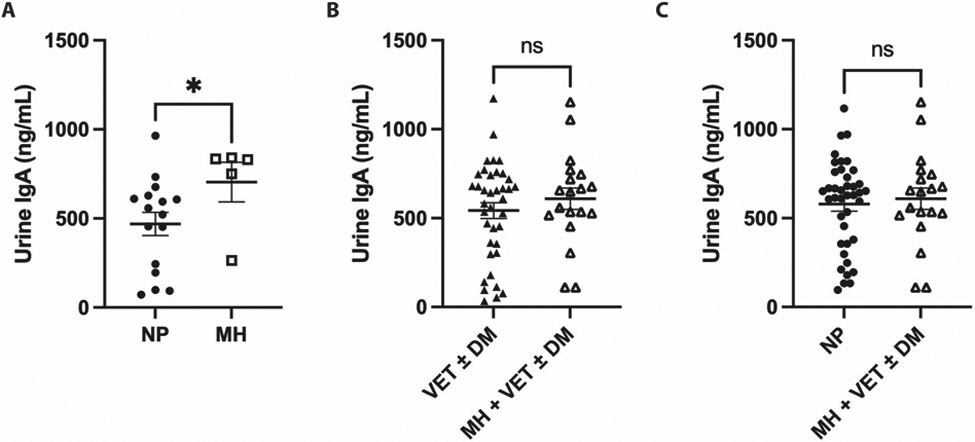
NS Non-significant, p >0.05
* p ≤0.05
Filled dots: women without MH, VET, or DM prophylaxis (NP)
Empty squares: women receiving MH alone (MH)
Filled triangles: women receiving VET and/or DM (VET±DM)
Empty triangles: women receiving MH in addition to VET and/or DM (MH + VET±DM)
A) Women receiving MH alone (MH) had significantly higher levels of urinary IgA compared to women not receiving MH, VET, or DM prophylaxis (NP). However, there was no significant difference in urinary IgA levels between B) women receiving no prophylaxis with MH, DM, or VET (NP) versus those receiving MH alone (MH) or C) women receiving VET and/or DM (VET±DM) versus those receiving MH in addition to VET and/or DM (MH + VET±DM).
DISCUSSION
In our study, MH appears to increase IgA levels without increasing bladder inflammation in aged mice and humans. In aged mice, while MH did not affect pH or bacteriuria, it appeared to decrease urothelial permeability, as evidenced by decreased infiltration of FITC-Dextran in MH-treated mice versus controls. While we were limited by our sample size and heterogeneity of human samples, we also observed increased urinary IgA in the MH group compared to NP. The agreement between these two models of UTI suggests that MH may work by improving urothelial barrier function.
Methenamine hippurate is bacteriostatic in human urine.16 It is hydrolyzed to formaldehyde in acidic urine, but does not generate significant levels of formaldehyde in the gut or other tissues.16 MH is overall well tolerated and does not induce bacterial resistance. Thus, MH is a promising bladder antiseptic for rUTI prophylaxis. In metaanalysis, MH was associated with a reduced risk of UTI in patients with normal urinary tracts (RR 0.24, 95% CI 0.07-0.89).18 An upcoming RCT may shed further light on its clinical efficacy for rUTI prevention.19
While MH appears to be effective clinically, few studies have examined its impact on other factors associated with rUTI. The observation of potential barrier function improvement in our study may be due to the effect of formaldehyde, which “fixes” cells by denaturing proteins and cross-linking amino groups. However, the mechanism by which MH improves urothelial barrier function requires further investigation. Urothelial barrier function is important to rUTIs development, as UPEC can establish quiescent intracellular reservoirs within the urinary bladder epithelium through invasion of the superficial facet cells, and thus evade eradication and enable reemergence and recurrent infection.20 If MH does reinforce the bladder mucosal barrier, it may also decrease rUTIs by limiting UPEC’s ability to invade the urothelium.
Furthermore, our study indicated that urinary IgA levels may be elevated with MH treatment. This may reflect improved barrier function, as secretory IgA is normally found in the healthy bladder. On the other hand, promoting IgA may be another way by which MH improves barrier function. In the gut, secretory IgA recognizes and cross-links antigenic epitopes on surfaces, preventing epithelial penetration. In a study involving probiotics bound to IgA, secretory IgA was associated with improved probiotic adhesion and increased phosphorylation of tight junction proteins;21 thus, IgA may improve barrier function by promoting tight junction function. Secretory IgA increases with acute UTI and is higher in aged mice, potentially in response to chronic, age-related inflammation.14, 22 Lastly, in other mucosal surfaces, secretory IgA also helps to control commensal bacteria. While we did not study MH’s impact on the bladder microbiome, if methenamine does increase secretory IgA, this may help to promote normalization of the urinary microbiota.
In older clinical practice, concentrated formaldehyde was used to superficially necrose and coagulate the bladder to stop severe hemorrhage, which resulted in significant fibrosis on pathological analysis.23 Animal studies have also noted initial urothelial necrosis, edema, and leukocytic infiltration after concentrated formaldehyde exposure.24 Thus, our concern was that MH, though resulting in significantly lower levels of formaldehyde, could adversely affect bladder inflammation. However, we found no significant difference in IL-6 between women receiving MH treatment versus other groups. Likewise, urothelial sloughing, a correlate of bladder inflammation, was not different between aged mice with and without MH treatment. While our findings are limited by our small sample size, this is encouraging preliminary evidence that MH does not adversely impact inflammation in the bladder environment.
MH’s potential mechanism of action is contrasted with that of vaginal estrogen. Studies suggest that VET may prevent rUTI in older women by dampening their enhanced urinary inflammatory response, as excessive inflammation may impair barrier function and promote rUTI.11 This suggests that MH could be used synergistically with other non-antibiotic prophylaxis to increase efficacy of rUTI prophylaxis as different therapies would act through multiple mechanisms. Our results did not suggest further benefit to inflammation and barrier function with the addition of MH to VET and/or DM. However, this may reflect our small sample size. Furthermore, effect size may have been dampened by the inclusion of women with either VET, DM, or both into the same group. Larger clinical studies would be more useful to study the efficacy of a multimodal, non-antibiotic regimen compared to monotherapy.
This is one of the first studies to explore the impact of MH on the bladder factors that may lead to rUTI. Our translational approach allowed us to compare our findings in both mice and human models. We further matched patients for factors that could influence systemic inflammation to decrease bias.
Our small sample size may have limited our ability to detect statistically significant differences between groups, particularly in the MH only group. Our human samples were also limited by collection from women with and without UTI at the time of collection, as well as unknown UTI symptom status at time of collection. This limits our ability to compare these results with the results from mice, which were all infected prior to sample collection. Our human samples were also impacted by lack of therapeutic randomization and clinical prescription practices, as most women were offered VET and/or DM prior to MH. Thus, women who opted for prophylaxis with MH alone may represent a different population of women or have characteristics that we were unable to control through matching.
Overall, this study provides new insight into mechanisms by which MH may prevent UTIs. MH’s impact on urothelial barrier function and urine IgA suggests that it may improve barrier function and possibly play a role in regulating urinary microbiota, important factors in rUTI prevention. Our findings also suggest that MH may work best in synergy with other non-antibiotic prophylaxis that work through other distinct mechanisms, such as VET. Though our evidence is preliminary, methenamine hippurate appears to have therapeutic advantages for UTI prophylaxis beyond bacteriostasis.
Supplementary Material
Sources of support
This work was supported by NIH grants R01 AG052494, P20 DK119840, and R56 AG064634 (to IUM); and Foundation for Barnes-Jewish Hospital ICTS Pilot Award, Grant #4806 (to CMC). Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Contributor Information
Jessica L. Sawhill, Department of Medicine, Section of Infectious Diseases, Baylor College of Medicine, Houston, Texas
Amy Mora, Department of Obstetrics & Gynecology, Washington University School of Medicine, St. Louis, Missouri.
Kendall McDaniel, Tulane University School of Medicine, New Orleans, Louisiana.
Marianne M Ligon, Department of Obstetrics & Gynecology, Washington University School of Medicine, St. Louis, Missouri.
Jerry L Lowder, Department of Obstetrics & Gynecology, Division of Female Pelvic Medicine and Reconstructive Surgery, Washington University School of Medicine, St. Louis, Missouri.
Indira U Mysorekar, Department of Medicine, Section of Infectious Diseases and Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas.
Christine M Chu, Department of Obstetrics & Gynecology, Division of Female Pelvic Medicine and Reconstructive Surgery, University of North Carolina School of Medicine, Chapel Hill, North Carolina.
REFERENCES
- 1.Santo L, Okeyode T. National Ambulatory Medical Care Survey: 2018 National Summary Tables. Available from: Urinary tract infection in postmenopausal women. Korean J Urol. 2011;52(12):801–808. doi: 10.4111/kju.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998-2011. Open Forum Infect Dis. 2017;4(1):ofw281. Published 2017 Feb 24. doi: 10.1093/ofid/ofw281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000;10(8):509–515. doi: 10.1016/s1047-2797(00)00072-7 [DOI] [PubMed] [Google Scholar]
- 4.Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17(4):259–268. doi: 10.1016/s0924-8579(00)00350-2 [DOI] [PubMed] [Google Scholar]
- 5.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28(1):1–13. doi: 10.1016/j.idc.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 6.Ikäheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22(1):91–99. [DOI] [PubMed] [Google Scholar]
- 7.Medina M, Castillo-Pino E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol. 2019;11:1756287219832172. Published 2019 May 2. doi: 10.1177/1756287219832172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;2004(3):CD001209. doi: 10.1002/14651858.CD001209.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001;1(2):101–114. doi: 10.1016/S1473-3099(01)00066-4 [DOI] [PubMed] [Google Scholar]
- 10.CDC. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, CDC; 2019 [Google Scholar]
- 11.Meister MR, Wang C, Lowder JL, Mysorekar IU. Vaginal Estrogen Therapy Is Associated With Decreased Inflammatory Response in Postmenopausal Women With Recurrent Urinary Tract Infections. Female Pelvic Medicine & Reconstructive Surgery. 2021; 27 (1): e39–e44. doi: 10.1097/SPV.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenger SM, Bradley MS, Thomas DA, Bertolet MH, Lowder JL, Sutcliffe S. D-mannose vs other agents for recurrent urinary tract infection prevention in adult women: a systematic review and meta-analysis. Am J Obstet Gynecol. 2020;223(2):265.e1–265.e13. doi: 10.1016/j.ajog.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Symington JW, Ma E, Cao B, Mysorekar IU. Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect Immun. 2013;81(3):733–739. doi: 10.1128/IAI.01234-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligon MM, Wang C, DeJong EN, Schulz C, Bowdish DME, Mysorekar IU. Single cell and tissue-transcriptomic analysis of murine bladders reveals age- and TNFα-dependent but microbiota-independent tertiary lymphoid tissue formation. Mucosal Immunol. 2020;13(6):908–918. doi: 10.1038/s41385-020-0290-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4(8):1230–1243. doi: 10.1038/nprot.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musher DM, Griffith DP. Generation of formaldehyde from methenamine: effect of pH and concentration, and antibacterial effect. Antimicrob Agents Chemother. 1974;6(6):708–711. doi: 10.1128/AAC.6.6.708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin K, Lee J, Guo N, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472(7341):110–114. doi: 10.1038/nature09851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BS, Bhuta T, Simpson JM, Craig JC. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10(10):CD003265. Published 2012 Oct 17. doi: 10.1002/14651858.CD003265.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes R, Ali A, Abouhajar A, et al. ALternatives To prophylactic Antibiotics for the treatment of Recurrent urinary tract infection in women (ALTAR): study protocol for a multicentre, pragmatic, patient-randomised, non-inferiority trial. Trials. 2018;19(1):616. Published 2018 Nov 9. doi: 10.1186/s13063-018-2998-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103(38):14170–14175. doi: 10.1073/pnas.0602136103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathias A, Pais B, Favre L, Benyacoub J, Corthésy B. Role of secretory IgA in the mucosal sensing of commensal bacteria. Gut Microbes. 2014;5(6):688–695. doi: 10.4161/19490976.2014.983763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacerda Mariano L, Ingersoll MA. The immune response to infection in the bladder. Nat Rev Urol. 2020;17(8):439–458. doi: 10.1038/s41585-020-0350-8 [DOI] [PubMed] [Google Scholar]
- 23.Fair WR. Formalin in the treatment of massive bladder hemorrhage. Techniques, results, and complications. Urology. 1974;3(5):573–576. doi: 10.1016/s0090-4295(74)80250-5 [DOI] [PubMed] [Google Scholar]
- 24.Pust R, Butz M, Rost A, Ogbuihi S, Riedel B. Denudation of the urinary bladder mucosa in the cat by formaldehyde. Urol Res. 1976;4(2):55–61. doi: 10.1007/BF00256318 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.