Abstract
Non-Hodgkin lymphoma (NHL) is a disease of older adults, with a median age at diagnosis of 67 years. Treatment in older adults with NHL is challenging. The aging process is associated with a decline in functional reserve that varies among individuals, and results in an increasing risk of treatment-related toxicity and mortality. Chronological age and performance status fail to capture the multidimensional and heterogeneous nature of the aging process. A geriatric assessment (GA) screens multiple geriatric domains and provides a more accurate assessment of functional reserve. Several abbreviated GA tools have been developed for use in oncology clinics and help identify patients at high risk for chemotherapy-related toxicity and mortality. In this review, we explore GA tools validated for use in patients with NHL. We discuss the evidence behind GA–guided treatment in NHL and present a suggested approach to assessing frailty in this patient population.
Keywords: Geriatric assessment, Non-Hodgkin lymphoma, Older adults, Lymphoma, Frailty
1. Introduction
Non-Hodgkin lymphoma (NHL) is among the most common hematological malignancies with approximately 77,000 cases diagnosed in the United States in the year 2020 alone [1,2]. NHL, which includes a spectrum of different lymphomas with varied clinical presentations, is essentially a disease of older adults, with a median age at diagnosis of 67 years [1].
Treatment of older adults with NHL presents several challenges. Depending on the specific histology, the treatment goal is often curative, with outcomes dependent on optimal dose delivery [3]. For patients on oral targeted therapies, maintaining adherence without dose interruptions is critical [4]. However, older adults are at increased risk for treatment-related toxicities due to decreased functional reserve and comorbidities [5]. In addition, the presence of chronic illnesses affect life expectancy independent of cancer diagnosis. While increasing age is associated with decreased functional reserve, this process is heterogeneous among older adults and therefore a single approach to treatment based on chronological age alone is fraught with errors. An accurate assessment of individual functional reserve, comorbidities, and goals of treatment is critical to the decision-making process.
Functional reserve is routinely assessed using the Karnofsky and/or Eastern Cooperative Oncology Group (ECOG) performance status (PS) scales [6]. However, these unidimensional assessments are unreliable in older adults, and correlate poorly with outcomes [7]. Subtle geriatric abnormalities (some reversible) are missed, and the considerable physiological heterogeneity in this patient population remains uncaptured, leading to over-treatment in some and under-treatment in other patients [8]. A geriatric assessment (GA) screens older adults for impairments in multiple geriatric domains. In the geriatrician’s office, this assessment accompanied by intervention planning can take up to two hours. However, several GA tools have been developed for use in the oncology clinic that can be completed in under fifteen minutes and add significant information to the PS assessment, predicting both treatment toxicity and mortality [9–11]. In this review, we discuss the various GA tools validated for use in older adults with NHL. We examine the evidence behind GA-guided management in these patients and present a suggested approach for assessment of the older adult with NHL, knowing that many hematology/oncology clinics may not have access to geriatric oncology referral services. A majority of the data is from studies involving patients with aggressive NHL, particularly diffuse large B cell lymphomas (DLBCL). We do not discuss treatment regimens for older adults with aggressive lymphomas and the management of older adults with chronic lymphocytic leukemia (CLL) per se. These have been reviewed in depth previously [12,13].
2. Illustrative Case 1
An 83-year old woman presents to discuss treatment options for newly diagnosed diffuse large B cell lymphoma (DLBCL), stage IV (with bone marrow involvement), with an International Prognostic Index (IPI) score of 3. She presented with worsening bilateral lower extremity swelling. Computed tomography (CT) imaging demonstrated massive splenomegaly and retroperitoneal lymphadenopathy. Pertinent labs include a hemoglobin of 9.9 g/dL and creatinine of 1.92 mg/ dL (creatinine clearance of 24 mL/min). She is independent in her activities of daily living (ADL), but her husband assists with finances and shopping. Her Eastern Cooperative Oncology Group (ECOG) performance status (PS) is “0”. Comorbidities include atrial fibrillation and chronic kidney disease (stage IV). She lives at home with her husband who is 86 years old and has multiple medical problems. Her daughter lives out of town; they have no other local caregivers.
3. Geriatric Assessment
The GA is a multidisciplinary, in-depth assessment of older adults’ overall health and function, and covers multiple domains. These include functional status, nutrition, comorbidities, cognition, psychological status, social support, and medications (Fig. 1) [14]. By taking into account the multidimensional nature of aging, the GA provides a more accurate estimate of functional reserve and life expectancy in older adults. GA helps identify patients at higher risk of chemotherapy toxicity and mortality, guides appropriate cancer management, and addresses geriatric concerns [15,16]. In addition to predicting risk of toxicities, one randomized controlled trial (RCT) showed that GA-guided cancer treatment decreases chemotherapy-related toxicities [17]. Several recent prospective RCTs similarly showed that GA-driven interventions reduced grade 3–5 chemotherapy-related toxicities compared to usual care for older adults with solid tumor malignancies [18–20]. The American Society of Clinical Oncology (ASCO) guidelines for geriatric oncology recommends at a minimum assessing function, comorbidity, falls, depression, cognition, and nutrition using multiple tools: Independent Activities of Daily Living (IADL) for function, a thorough history for comorbidities, question about falls, the Geriatric Depression Scale for depression, the Mini-Cog or the Blessed Orientation-Memory-Concentration for cognition, assessment of unintentional weight loss for nutrition, the Cancer Aging Research Group (CARG) or Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) tools for estimates on chemotherapy toxicity risks, and the Geriatric 8 (G8) or Vulnerable Elders Survey-13 (VES-13) to help predict mortality [16]. Several abbreviated screening tools have been derived to identify which patients should undergo a more complete GA or to predict severe chemotherapy-related complications [21,22]. They include self-administered questionnaires, some of which can be completed in under ten minutes, to more detailed subjective and objective assessments of multiple geriatric domains [11,23–25]. Interestingly, simplified assessments of two or more geriatric domains are able to identify frail patients with inferior survival and at higher risk for toxicity, and have proven to be superior to physician judgment in patients with hematological malignancies [8].
Fig. 1.
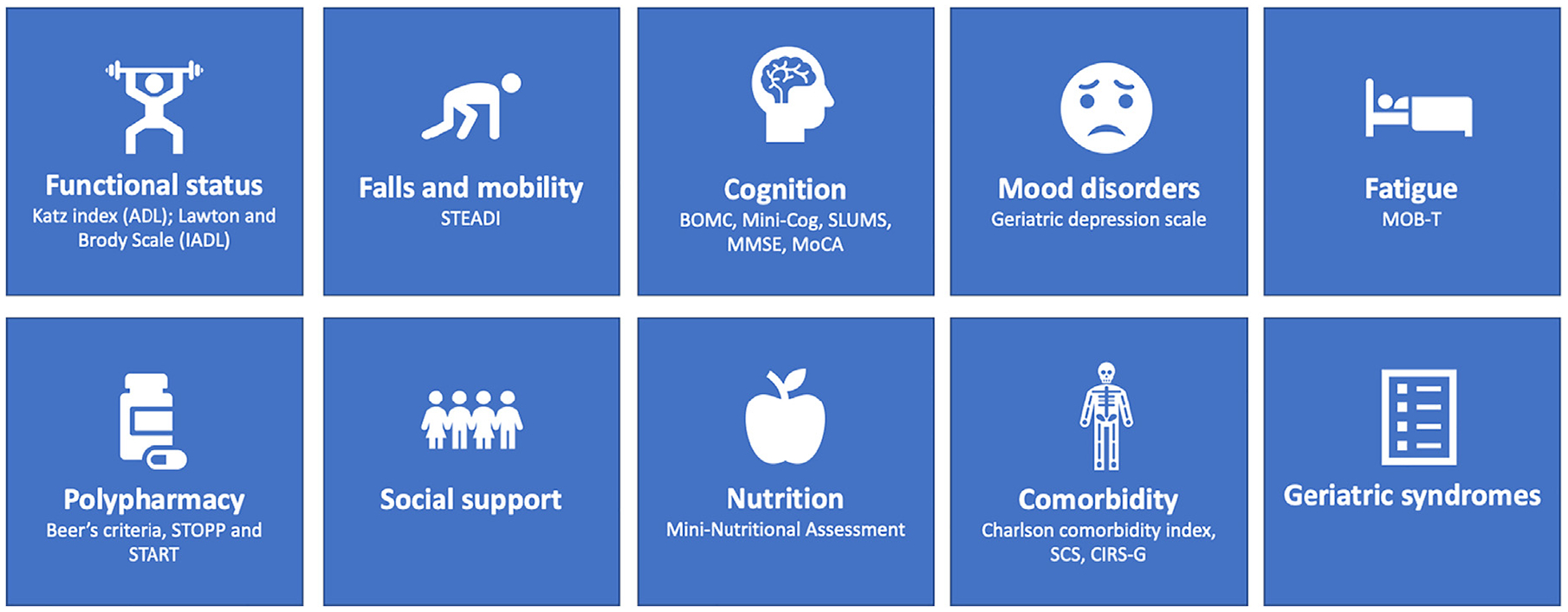
Components of a geriatric assessment and selected screening tools.
ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; STEADI: Stopping Elderly Accidents, Deaths, and Injuries; BOMC: Blessed Orientation Memory Concentration; SLUMS: Saint Louis University Mental Status; MMSE: Mini Mental State Exam; MoCA: Montreal Cognitive Assessment; STOPP: Screening Tool of Older People’s Prescriptions; START: Screening Tool to Alert to Right Treatment; SCS: Simplified Comorbidity Score; CIRS-G: Cumulative Illness Rating Scale-Geriatric; MOB-T: Mobility-Tiredness.
4. Geriatric Assessment Tools in Non-Hodgkin Lymphoma
Before delving into the utility of individual screening tools, it is important to understand the association between individual geriatric impairments and outcomes in patients with NHL. The strength of these associations has made it possible to devise abbreviated tools that function better than conventional performance status evaluations in assessing frailty.
The best defined of these is the association of functional impairment, defined as dependence in one or more activities of daily living (ADL) and/or IADL, on survival outcomes. The ADL questions evaluate impairments in basic activities required for independent functioning (bathing, dressing, toileting, transferring, continence, and eating) (see Table 1) [26]. The IADL is an indirect evaluation of functional abilities necessary for independent living in the community such as managing finances, shopping, food preparation, housekeeping, using the telephone, laundry, transportation, and medication (see Table 1) [27]. In a study of 303 older adults with NHL (age ≥ 80 years), loss of any ADL at presentation was strongly associated with inferior overall survival (OS) in both aggressive (hazard ration [HR] 3.07, p < 0.0001) and indolent (HR 2.06, p < 0.0004) NHL. Apart from stage (III/IV, HR 2.03, p = 0.02) and failure to achieve a complete response (HR 2.41, p = 0.0002), no other factors, including the ECOG PS, were associated with inferior progression free survival (PFS) or OS on multivariate analysis in this cohort [28]. Several other studies have confirmed these findings [7,8,29,30]. In a recent systematic review that included fifteen studies in patients with NHL, impairments in IADL, ADL, physical capacity, and cognition were again associated with mortality in a multivariable analysis [31]. Poor ECOG PS was associated with mortality in univariate analysis but not in multivariate analysis. While only 29% of patients had a PS of ≥2, GA identified at least one geriatric impairment in 51% of patients, again demonstrating the limitations of PS evaluation in older patients with lymphoma.
Table 1.
Commonly used tools for functional assessment.
| A. Katz Index of Independence in Activities of Daily Living (ADL) | ||
|---|---|---|
| Activities | Independence (score = 1 for each) | Dependence (score = 0 for each) |
| Bathing | Bathes self completely or needs help in bathing only a single part of the body such as the back, genital area or disabled extremity. | Needs help with bathing more than one part of the body, getting in or out of the tub or shower. Requires total bathing. |
| Dressing | Get clothes from closets and drawers and puts on clothes and outer garments complete with fasteners. May have help tying shoes. | Needs help with dressing self or needs to be completely dressed. |
| Toileting | Goes to toilet, gets on and off, arranges clothes, cleans genital area without help. | Needs help transferring to the toilet, cleaning self or uses bedpan or commode. |
| Transferring | Moves in and out of bed or chair unassisted. Mechanical transfer aids are acceptable. | Needs help in moving from bed to chair or requires a complete transfer. |
| Continence | Exercises complete self-control over urination and defecation. | Is partially or totally incontinent of bowel or bladder. |
| Feeding | Gets food from plate into mouth without help. Preparation of food may be done by another person. | Needs partial or total help with feeding or requires parenteral feeding. |
| B. Lawton-Brody Instrumental Activities of Daily Living (IADL) [27] | ||
|---|---|---|
| Activity | Level of independence | Score |
| Ability to use telephone | - Operates on own initiative – looks up and dials numbers, etc. | 1 |
| - Dials a few well-known numbers | 1 | |
| - Answers telephone but does not dial | 1 | |
| - Does not use telephone at all | 0 | |
| Shopping | - Takes care of all shopping needs independently | 1 |
| - Shops independently for small purchases | 0 | |
| - Needs to be accompanied on any shopping trip | 0 | |
| - Completely unable to shop | 0 | |
| Food Preparation | - Plans, prepares and serves adequate meals independently | 1 |
| - Prepares adequate meals if supplied with ingredients | 0 | |
| - Heats, serves and prepares meals, or prepares meals, or prepares meals but does not maintain adequate diet | 0 | |
| - Needs to have meals prepared and served | 0 | |
| Housekeeping | - Maintains house alone or with occasional assistance (e.g. “heavy work domestic help”) | 1 |
| - Performs light daily tasks such as dish washing, bed making | 1 | |
| - Performs light daily tasks but cannot maintain acceptable level of cleanliness | 1 | |
| - Needs help with all home maintenance tasks | 1 | |
| - Does not participate in any housekeeping tasks | ||
| Laundry | - Does personal laundry completely | 1 |
| - Launders small items-rinses stockings, etc. | 1 | |
| - All laundry must be done by others | 0 | |
| Mode of transportation | - Travels independently on public transportation or drives own car | 1 |
| - Arranges own travel via taxi, but does not otherwise use public transportation | 1 | |
| - Travels on public transportation when accompanied by another | 1 | |
| - Travel limited to taxi or automobile with assistance of another | 0 | |
| - Does not travel at all | 0 | |
| Responsibility for Own Medications | - Is responsible for taking medication in correct dosages at correct time | 1 |
| - Takes responsibility if medication is prepared in advance in separate dosage | 0 | |
| - Is not capable of dispensing own medication | 0 | |
| Ability to handle finances | - Manages financial matters independently (budgets, writes checks, pays rent, bills, goes to bank), collects and keeps track of income | 1 |
| - Manages day-to-day purchases, but needs help with banking, major purchases, etc. | 1 | |
| - Incapable of handling money | 0 | |
In addition to functional impairment, the role of nutritional status (in particular, albumin levels), comorbidities, and cognition have also been studied [32,33]. A low serum albumin is present in approximately 30% of older patients with NHL, and multiple studies have demonstrated the negative association of hypoalbuminemia with survival outcomes [24,32,34–36]. Most studies are from patients with aggressive B-cell NHL, but data suggest these results can be extrapolated to patients with peripheral T-cell lymphoma and follicular lymphoma [37,38]. It is important to recognize that while hypoalbuminemia is a surrogate marker for malnutrition, it can also reflect an inflammatory state (as a negative inflammatory marker) or disease activity. The clinical relevance of anthropometric measures of malnutrition such as body mass index (BMI), L3 skeletal muscle index, adipopenia, or sarcopenia are not as clearly defined with studies showing mixed results [39–41].
Comorbidity is associated with decreased life expectancy in the general population and is an independent negative prognostic factor in cancer patients [42]. In older adults with aggressive NHL, the presence of “high-impact comorbidity” (heart disease, diabetes, chronic obstructive pulmonary disease, and renal failure) doubles the risk of death, independent of the IPI score, and is associated with a 10–20% decline in 5-year survival [33,43]. In geriatric medicine, comorbidity is often assessed using a scoring system. The two most widely used scores are the cumulative illness rating scale – geriatrics (CIRS-G) and the Charlson Comorbidity Index (CCI). Both require input by the healthcare team and are not self-administered. The CIRS-G is a more comprehensive assessment, while the CCI is simpler to administer; both are independently associated with inferior outcomes in patients with NHL and have been incorporated into GA scoring systems [24,44].
In summary, dependence in ADL or IADL, malnutrition, and the presence of comorbidities independently affect outcomes in older adults with NHL. Screening tools that focus on assessment of these domains have been developed and are better than physician judgment and performance status in identifying frailty in older adults with NHL. In the following subsections, we discuss various abbreviated geriatric assessment and screening tools validated for use in older adults with NHL (Table 2).
Table 2.
Geriatric assessment and screening tools validated for use in Non-Hodgkin lymphoma patients.
| Screening tool | Items | Time to administer | Correlation with outcomes |
|---|---|---|---|
| Simplified Geriatric Assessment (FIL) | ADL; IADL; Age; CIRS-G; IPI (EPI); Hemoglobin (EPI) | <10 min | Overall survival |
| ACA index and IADL- ACA | Age, Albumin (<3.7 g/dL), CCI IADL (IADL-ACA) | <10 min | Overall survival; Mean chemotherapy dose; Treatment toxicity and treatment-related mortality |
| Geriatric-8 (G8) | Nutritional Status; Polypharmacy; Age; Psychological Status; Health perception | <5 min | Overall Survival; Treatment toxicity |
| Vulnerable Elders Survey (VES-13) | Age; Self-rated health; Physical function; functional disabilities | <5 min | Overall Survival; Response rate |
| fTRST | Cognition; Living situation; Physical function; Polypharmacy | <5 min | Overall Survival; Treatment-related mortality |
| CRASH | Diastolic blood pressure; LDH; Functional status (IADL, Performance status); Nutritional status (MNA); Chemotherapy; Cognition (MMS) | <20 min | Treatment toxicity |
| CARG-TT | Age, Cancer type, Treatment, Hemoglobin, Kidney function, Physical function, IADL (medications), Falls, Hearing, Social activity | <5 min | Treatment toxicity* |
FIL: Italian Lymphoma Foundation; ADL: Activities of daily living; IADL: Independent activities of daily living; CIRS-G – Cumulative Illness Rating Scale-Geriatric; IPI: International Prognostic Index; EPI: Elderly prognostic index; CCI: Charlson Comorbidity Index; ACA: Age, Comorbidities and Albumin Index; fTRST - Flemish version of the triage-screening tool; CRASH: Chemotherapy Risk Assessment Scale for High-Age Patients; MNA: Mini Nutritional Assessment; MMS: Mini Mental Scale; CARG-TT: Cancer Aging and Research Group toxicity tool.
Limited data in patients with NHL.
4.1. Simplified Geriatric Assessment: The Lymphoma Italian Foundation (FIL) Approach
The FIL approach involves the use of a simplified geriatric assessment (sGA) that takes less than ten minutes and includes four items: 1) ADL, 2) IADL, 3) age (>80 years vs <80 years), and 4) comorbidities assessed by CIRS-G (adjusted for hematological comorbidities) [8,23,44,45]. Based on the sGA score, patients are classified into three groups: fit, unfit, and frail (Table 3). Broadly speaking, “fit” patients include those <80 years of age with minimal or no impairments in their ADL (score ≥ 5) and IADL (score ≥ 6), and without significant comorbidities (no comorbidities with a CIRS-G score of 3–4, and < 8 comorbidities with a score of 2). “Unfit” patients include patients >80 years of age, who are independent (ADL = 6, IADL = 8) and without comorbidities or those <80 with significant functional impairment (ADL <5, IADL <6) and comorbidity (≥1 comorbidity with a score of 3–4, >8 comorbidities with a score ≥ 2. “Frail” patients include those age ≥ 80 years with dependence in multiple ADL (score < 6), IADL (score < 8), and/or with significant comorbidities (≥1 comorbidity with a score of 3–4, ≥5 comorbidities with a score of 2).
Table 3.
Lymphoma Italian Foundation Simplified Geriatric Assessment and Elderly Prognostic Index.
| Simplified geriatric assessment | ||||
|---|---|---|---|---|
| Criteria | Fit | Unfit(<80) | Unfit (≥80) | Frail |
| ADL | ≥5 | <5 | 6 | <6 |
| IADL | ≥6 | <6 | 8 | <8 |
| CIRS-G | No comorbidities with score = 3–4, ≤8 with score = 2 | ≥1 with score = 3–4, >8 with score = 2 | No comorbidities with score = 3–4, <5 with score = 2 | ≥1 with score = 3–4, ≥5 with score = 2 |
| Age - years | <80 | <80 | ≥80 | ≥80 |
| Elderly prognostic index | ||||
|---|---|---|---|---|
| Criteria | Score | HR (95% CI) | P-value | |
| sGA | Fit | 0 | 1.00 | |
| Unfit | 3 | 1.93 (1.49 to 2.50) | <0.001 | |
| Frail | 4 | 2.74 (2.07 to 3.62) | <0.001 | |
| IPI | IPI 1 | 0 | 1.00 | |
| IPI 2 | 1 | 1.55 (0.99 to 2.44) | 0.055 | |
| IPI 3–5 | 3 | 2.90 (1.93 to 4.35) | <0.001 | |
| Hemoglobin <12 g/dL | 1 | 1.28 (1.02 to 1.60) | 0.033 | |
| Risk Groups (score) | 3-year OS (95% CI) | HR (95% CI) | P-value |
|---|---|---|---|
| Low (0–1) | 87% (81–91) | 1.00 | |
| Intermediate (3–5) | 69% (63–73) | 2.57 (1.72 to 3.84) | <0.001 |
| High (6–8) | 42% (36–49) | 6.21 (4.17 to 9.25) | <0.001 |
ADL: Activities of Daily Living, IADL: Instrumental Activities of Daily Living; CIRS-G – Comorbidities Illness Rating Scale – Geriatric; sGA: simplified geriatric assessment; IPI: international prognostic index.
The validity of this approach was established in a pivotal multicenter prospective study of 173 adults with DLBCL [23]. There was a significant difference in OS between fit and unfit/frail patients (two-year OS – 84% vs 47%, p < 0.0001). The value of an intermediate group (unfit) could not be assessed due to the small sample size. Around 60% of “unfit” and 27% of “frail” patients still received full-dose therapy with curative intent per physician judgment. Within these two groups, there was no difference in the two-year OS between patients receiving curative therapy versus palliative treatment. Lastly, the difference in OS between fit and unfit/frail patients could not be explained by differences in treatment intensity [23].
More recently, the FIL group have published results of a much larger prospective multicenter study [45]. In this 1353-patient study, the prognostic value of a score combining sGA risk assessment with disease characteristics (international prognostic index and hemoglobin level) was assessed. The Elderly Prognostic Index (EPI) stratifies patients into three main risk groups with a significant difference in outcomes between the low risk, intermediate risk, and high-risk groups (3-year OS – 85%, 65%, and 44%, respectively), see Table 3. The differences in the EPI groups were confirmed when the analysis was limited to patients treated with anthracyclines only.
These data facilitate decision-making on treatment approach and dose intensity. They highlight a group of older adults <80 years of age who may benefit from a curative approach with full-dose therapy with comparable outcomes to younger patients. Alternatively, a group of frail patients with multiple comorbidities and ADL/IADL impairments likely lack the functional reserve to tolerate aggressive treatment, and may have equivalent outcomes with the use of palliative approach [23]. The validity of the EPI and the sGA in patients with NHL other than DLBCL has not been established yet.
4.2. The Age, Comorbidities, and Albumin (ACA) Index and IADL-ACA
Based on the known prognostic impact of comorbidity, serum albumin, and age in older adults with aggressive NHL, Miura et al. developed the age, comorbidities, and albumin (ACA) index [24]. Using an age cut off of >75 years, CCI score ≥ 3 and an albumin level of <3.7 g/dL, each with a weighted score of 1, they were able to categorize 555 patients ≥65-years of age with DLBCL treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) into four groups – excellent (0), good (1), moderate (2), and poor (3). There was a statistically significant difference in 3-year OS between the four groups (86%, 72%, 51%, and 0%, respectively, p < 0.001). In addition, a higher index ACA score was associated with lower mean dose of chemotherapy and higher probability of unanticipated discontinuation, febrile neutropenia, and treatment-related death. For example, in patients with an ACA index score of 0, the probability of treatment discontinuation was 7% vs 61% in patients with a score of 3 (“poor” group).
Subsequently, Liu et al. combined an assessment of functional status (IADL) with the ACA index to create the IADL-ACA (IACA) index in patients ≥65 years of age with DLBCL [36]. Patients were classified into three risk groups – low, intermediate, and poor, with significant differences noted in overall response rate (ORR), cumulative incidence of treatment-related mortality, relapse rate, and OS at two years. For example, the two-year OS in the low, intermediate, and poor risk groups were 96.0%, 70.1%, and 24.1%, respectively, (p = 0.003). The IACA needs to be validated in a larger prospective cohort.
4.3. Geriatric-8 Screening Tool
The geriatric-8 (G8) is a screening tool originally developed to identify potentially frail patients that might benefit from a GA [46]. It has a median sensitivity for detecting frailty of 85% (range, 38% to 97%) and specificity of 64% (range, 28% to 100%) [47]. Due to the high false-positive rate, patients with an abnormal G8 score (≤14) benefit from a more complete GA. Despite these limitations, the utility of the G8 as an independent prognostic tool in patients with cancer has been well described and it remains among the most widely used screening tools in geriatric oncology [46].
The tool takes about five minutes to complete and consists of an eight-item questionnaire with questions related to nutritional status, weight loss, BMI, motor skills, psychological status, polypharmacy, self-perception of health, and chronological age.
Several studies have confirmed the prognostic impact of G8 in patients with lymphoma. In a study by Sakurai et al. that included 59 older adults with DLBCL, patients with an abnormal G8 score (≤14) had a significantly decreased two-year OS (G8 ≤ 14 vs >14, 66.1% vs 86.8%, p = 0.03) [25]. These results were confirmed by Lee et al. in a cohort of 451 patients with DLBCL [48]. Interestingly, the authors found a linear association between G8 score as a continuous variable and suggest a lower cutoff of ≤9.5 to delineate two prognostic groups rather than the conventionally used score of ≤14. In their study, they found a linear relation between the total average relative dose intensity (tARDI) in patients with a G8 score > 9.5, where a tARDI of >80% was associated with decreased mortality. However, in patients with a score ≤ 9.5, tARDI of both <60% and > 80% were associated with increased mortality. These data suggest that an increased dose intensity was associated with improved OS in patients with a G8 score > 9.5, but not in patients with a score of ≤9.5. The value of the G8 score in predicting treatment-related adverse events (AE) has also been described. A G8 score of ≤11 was predictive of severe AE [9].
These data confirm the utility of G8 in predicting mortality and treatment-related toxicity with a unique approach to scoring the G8 in NHL patients.
4.4. Vulnerable Elders Survey-13 (VES-13)
The VES-13 is a 13-item self-administered screening tool that includes age, self-rated health, limitations in physical function, and functional disabilities, providing a simple method to identify frailty in older adults. It takes about five minutes to complete. An association between low VES-13 scores and treatment toxicity and outcome have been demonstrated in patients with cancer [49].
In older adults with lymphoma, the VES-13 is able to identify vulnerable older adults with a sensitivity of about 72%, and is associated with decreased response rates and higher mortality [10,11,50]. In one study of 2004 patients with DLBCL, the VES-13 was administered within six months of diagnosis to patients prior to or after treatment initiation. Vulnerable status (score of ≥3) was associated with one-year mortality even after adjusting for age, PS, and stage [11].
4.5. CRASH and CARG-TT
The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score was developed to predict chemotherapy-related hematological and non-hematological toxicity [22]. The score combines clinical and laboratory features with assessment of geriatric domains such as cognition, nutrition, and functional status (IADL). The original paper by Extermann et al. included 78 patients out of a total of 518 with a diagnosis of NHL treated with the CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) regimen. Patients were divided into four risk categories – low, medium-low, medium-high, and high. More than 90% of patients in the high-risk category experienced either grade 4 hematological and/or grade 3/4 non-hematological toxicity. It is unclear if these results can be extrapolated to patients treated with rituximab (in combination with chemotherapy or as a single agent), other chemo-immunotherapy regimens, or novel agents since these patients were not included in this study. The Cancer Aging and Research Group toxicity tool (CARG-TT) was developed as a chemotherapy risk stratification tool and includes a combination of disease, treatment, and geriatric assessment factors [21]. This abbreviated tool differs from the CARG cancer-specific geriatric assessment tool which screens multiple domains to assess functional reserve and cancer-independent morbidity and mortality in older adults with cancer [51,52].
In the original study by Hurria et al., patients with NHL were excluded [21]. However, data is emerging that suggests its utility in patients with NHL. In a small study of thirty patients with NHL including DLBCL, CLL, and indolent lymphomas, the CARG-TT risk score was associated with grade 3 or higher adverse events [53]. In another study, however, which included thirty-six patients with lymphoma, CARG scores had a poor correlation with treatment-related toxicity [54]. Further studies are needed to examine the utility of the CRASH and CARG tools in patients with indolent and aggressive NHL, especially since concerns for short treatment-related morbidity are viewed in the context of potential long-term cure in some patients.
4.6. Other Abbreviated GA and Screening Tools
The Flemish version of the triage-screening tool (fTRST) is a screening tool that was originally developed for use in emergency rooms to identify older adults at risk for a failed discharge. Its use in oncology has been validated in a number of studies including in patients with NHL. It includes five simple yes/no questions that assess cognitive impairment, solitary living, difficulty of walking, hospitalization, and polypharmacy; it takes 2 min to complete. In one study including 59 patients at least 65 years with DLBCL, an abnormal fTRST screening test was associated with both increased treatment-related mortality and inferior overall survival [25].
In a study by Soubeyran et al., an abbreviated GA including assessment of functional status using ADL, IADL, Timed Get Up and Go (TUG), nutrition using the Mini Nutritional Assessment (MNA), cognition using the Mini-Mental State Exam, and depression using Geriatric Depression Scale was used to predict early death (at six months) [55]. Out of 348 patients, 105 had NHL. Only four factors were associated with early death in multivariable analysis including age, disease stage, abnormal MNA, and TUG. This study has helped establish the role of objective measures of performance in patients with NHL. In another study involving 75 patients with CLL, an abnormal TUG was associated with inferior OS [56].
5. Geriatric Assessment-Guided Treatment in NHL
In addition to predicting adverse outcomes, GA can be useful for guiding treatment decisions and management to improve clinical outcomes for older adults with lymphoma. Several studies have evaluated older patients with DLBCL with GA and compared outcomes based on fitness category, though treatment choice and intensity were left to clinical judgment of the attending physician without recommendations based on GA results [11,24,30,38,57–59].
A few trials used a GA-guided algorithm to tailor chemotherapy regimen choice and intensity, mostly operationalized with variations of the FIL simplified-GA by groups in Italy. We will summarize response rates and survival data, acknowledging that survival is impacted by fitness level independent of lymphoma diagnosis or treatment (Table 4). In 2003, Bernardi et al. conducted a pilot study for adults at least 70 years of age with aggressive NHL that tailored treatment based on age, ADL, IADL, hepatic/renal function, and comorbidities. Patients with ADL and IADL ≥5, intact hepatic/renal function, and sufficient hematopoietic reserve were treated with curative intent. Patients with IADL <5 or older than 80 were treated with 75% of the planned dose, and frail patients (ADL dependence, ≥3 comorbidities, ≥1 geriatric syndrome) received a palliative approach. They reported promising results (ORR 90%, complete response [CR] 79%) and manageable toxicity, providing support that a patient-tailored approach based on GA is suitable and effective [60]. Spina et al. conducted a multicenter prospective trial in patients aged 70 years or older with DLBCL in which chemotherapy regimens were modified based on comorbidities (no comorbidities: RCHOP/CHOP, mild cardiopathy: RCEOP/CEOP (cyclophosphamide, etoposide, vincristine and prednisone, with or without rituximab), severe cardiopathy: RCVP/CVP (cyclophosphamide, vincristine and prednisone, with or without rituximab), diabetes: RCHO/CHO (cyclophosphamide, doxorubicin, and vincristine with or without rituximab), neuropathy: RCHP/CHP (cyclophosphamide, doxorubicin, and prednisone with or without rituximab). Chemotherapy doses were also modulated based on ADL and IADL scores (100% for ADL 6 and IADL 7–8, 75% for ADL 5 and IADL 5–6, 50% for ADL/IADL <5). The ORR was 87% with similar CR and relapse rates in fit, unfit, and frail subgroups. The five-year OS rates were 76%, 53%, and 29% in fit, unfit, and frail patients, respectively. The authors concluded that GA-guided treatment selection resulted in survival and toxicity outcomes similar to that seen in the overall DLBCL population [61]. Olivieri et al. conducted a multicenter prospective study in patients aged 65 and older with DLBCL demonstrating the feasibility of a simplified GA-tailored treatment approach in the cooperative clinical trial setting, to allow the enrollment of more representative population [62]. They categorized patients as fit (age < 85, no grade 2 comorbidities or geriatric syndromes, ADL independence), with comorbidities, or frail (age ≥ 85, ≥1 ADL dependence, ≥1 geriatric syndrome, ≥3 grade 2 or ≥ 1 CIRS-G grade 3 comorbidity). Treatments were tailored based on GA categorization, with fit patients receiving RCHOP-21, patients with comorbidities receiving RCHOP-21 with liposomal doxorubicin, and frail patients receiving mini-RCHOP-21. The ORR was 79% in all groups; the CR rate was 81.5% in fit patients, 64% in patients with comorbidities, and 60% in frail patients (p > 0.05). Median OS was 37 months, and was statistically significantly better in fit patients than unfit or frail patients, who had comparable OS [62].
Table 4.
Geriatric assessment-guided treatment interventions in older adults with NHL.
| Study | Geriatric Assessment Domains | Categorization | Approach | Outcomes | Treatment toxicity |
|---|---|---|---|---|---|
| Bernardi et al. [60] | Age, IADL, IADL, hepatic/renal function | Fit: ADL and IADL score of at least 5, with intact hepatic, renal function and hemopoietic reserve; Intermediate dose group - IADL<5, or age > 80; Frail: dependent on ≥1 ADL, ≥3 comorbidities, or ≥ 1 geriatric syndromes | Curative intent with full dose in “fit” group; 75% of planned dose in intermediate-dose group and palliative approach in “frail” group | Entire cohort: ORR - 90%; CR 79% | Not available |
| Spina et al. [61] | Comorbidities, ADL, IADL | Fit: no grade 3 comorbidities (or < 3 grade 2 comorbidities), an ADL score of 6, and/or an IADL score of 7 or 8; Unfit - no grade 3 comorbidities (or 3–5 grade 2 comorbidities), an ADL score of 5, and/or an IADL score of 5 or 6; Frail: ≥1 grade 3 comorbidities (or > 5 grade 2 comorbidities), an ADL score < 5, or an IADL score < 5. | Two-step approach: Step 1: Regimen based on comorbidities - No Comorbidities: RCHOP; Comorbidities (Cardiac, Neuropathy, Diabetes): R-CEOP/CEOP, R-CVP/CVP, R-CHP/CHP; R-CHO/CHO Step 2: Dosing based on functional status - Full dose (100%): ADL (6), IADL (7–8); Intermediate dose (75%): ADL (5), IADL (5–6); Reduced dose (50%): ADL/IADL <5 | CR rate: 85% in fit versus 72% in unfit and 85% in frail (p = 0.34); 5-year OS: 76% for fit versus 53% for unfit and 29% for frail (p = 0.001) | Severe toxicity reported in 31% in fit versus 48% and 58% in unfit and frail, respectively p =0.11; Treatment-related mortality: (fit - 5%, unfit - 9% and frail - 11%); |
| Olivieri et al. [62] | Age, comorbidities, geriatric syndromes, ADL independence | Fit: Age < 85, no grade 2 comorbidities or geriatric syndromes and ADL independence; With comorbidities: presence of comorbidities; Frail: age ≥ 85, ≥1 ADL dependence, ≥1 geriatric syndrome, ≥3 grade 2 or ≥ 1 CIRS-G grade 3 comorbidity | Fit: RCHOP21 With comorbidities: RCHOP21 (with liposomal doxorubicin) Frail: mini-RCHOP21 | CR Rate was 81.5% in the fit group versus 63.6% in patients with comorbidities (unfit) and 60% in frail; Improved OS in fit vs unfit/frail (p = 0.00933), but no difference between OS between unfit and frail (p = 0.63) | Treatment-related mortality: 1.9% (fit) versus 9.1% (with comorbidities) versus 6.7% (frail). |
| Lastra-German et al. [63] | Unintentional weight loss, physical exhaustion, low physical activity, slowness, weakness | One point for each positive item: fit: 0 points, unfit.: 1–2 points, frail: 3 points | Fit patients: R-CHOP; Unfit patients: R-choP (adjusted to 80% of total R-CHOP dose; Frail patients: R-COP | CR rate was 66.6% in fit, versus 78.3% in unfit and 40% in frail patients; Median OS was 22.5 months in fit, vs 21 months and 11 months in unfit and frail groups respectively. | No significant difference in grade 3/4 hematological toxicity (83.3% in fit versus 65.2% in unfit and 45% in frail, p = 0.192) |
| Bai et al. [20] | ADL, IADL, age, comorbidities (modified CIRS-G) | Fit, unfit and frail groups based on Tucci et al. [23] Study combined unfit and frail into one group for therapeutic interventions. | Fit patients: R-CHOP; Unfit/frail patients: R-CHOP (with 50% anthracycline dose), R-COP or R-miniCHOP | CR rate was 84.4% versus 51.5% (p = 0.002). Three-year OS was 91% versus 69% (statistical significance unknown). | No significant difference in treatment-related toxicity between fit and unfit/frail groups (grade 3/4 hematological toxicity - 51.1% vs 54.5%, p > 0.05) |
ADL: Activities of Daily Living, IADL: Instrumental Activities of Daily Living; CIRS-G – Comorbidities Illness Rating Scale-Geriatrics; ORR – overall response rate; CR- complete response; OS – overall survival; R- rituximab; CEOP - cyclophosphamide, epirubicin, vincristine, and prednisone; CHO - cyclophosphamide, doxorubicin, and vincristine; CHOP - cyclophosphamide, doxorubicin, vincristine, and prednisone; CHP - cyclophosphamide, doxorubicin, and prednisone; CVP - cyclophosphamide, vincristine, and prednisone, COP - cyclophosphamide, vincristine, and prednisone.
The practice of tailoring treatment based on GA categorization is being adapted internationally. In a single-center cohort study in Mexico, the frailty status of patients at least 60 years of age with DLBCL was assessed with the Cardiovascular Health Study criteria (unintentional weight loss, physical exhaustion, low physical activity, slowness, weakness). Fit patients received RCHOP, unfit patients received RchoP (80% dose reduction cyclophosphamide, doxorubicin, vincristine), and frail patients received RCOP (rituximab, cyclophosphamide, vincristine and prednisone). The CR rates were 66.6%, 78.3%, and 40.0% for fit, unfit, and frail patients, respectively. Two-year OS was 87%, 82%, and 59% for fit, unfit, and frail patients. Lastra-German et al. concluded that the encouraging results identified an unfit group that benefited from a dose-reduced regimen [63]. In a single-center study in China, Bai et al. applied the FIL simplified GA to patients with DLBCL aged ≥60 years and tailored therapy based on fitness level. Patients classified as fit received standard dose RCHOP, and patients classified as unfit or frail received RCHOP with 50% doxorubicin, RCOP, or R-miniCHOP. The ORR and CR rates were 97.8% and 84.4% in fit patients, and 60.6% and 51.5% in unfit and frail patients combined (p < 0.001 and p = 0.002, respectively). The two-year OS was 98% for fit patients and 69% for unfit and frail patients (statistical significance not reported). The authors concluded that the fit group had similar outcomes compared to the general DLBCL population, but the unfit and frail had a poorer outcome, with the hypothesis that the level of fitness should be based on baseline status before lymphoma diagnosis in order to minimize the impact of disease on frailty assessment. In addition, the unfit and frail patients were grouped together, which may also have impacted their conclusions [20].
The increasing application of GA-driven treatment algorithms in DLBCL internationally is important. Future studies in other lymphoma histologies would be informative. As these studies were not controlled, the question of whether GA-based treatment algorithms can improve outcomes by minimizing toxicity while optimizing disease control remains difficult to assess, since cross-trial comparisons are fraught with bias. Several recent studies have examined the role of GA-guided interventions in the care of older adults with cancer [18,19,64]. In a recent RCT, 160 older adults with hematological malignancies (including 50 patients with lymphoid malignancies) were randomized to receive standard oncological care with or without geriatric consultation [64]. Geriatric interventions included recommendations on comorbidities, polypharmacy, function/falls, cognition, and depression but not on therapeutic approach or choice of regimen. There was no difference in healthcare utilization or OS between the two groups. It is important to note that treatment recommendations were made by oncologists prior to frailty screening and geriatric consultation. The study was also not powered to assess differences in treatment toxicity or quality of life metrics between the two groups. Two recent studies in patients with solid tumors, one of which included patients with lymphoma, showed that GA-guided interventions reduced toxicity in older adults with cancer without impacting survival. In the Geriatric Assessment for Patients 70+ (GAP70) cluster randomized clinical trial, Mohile et al. compared usual care to an intervention that provided oncologists with a GA summary including management recommendations for identified deficits, with treating oncologists ultimately determining the treatment plan [18]. Included participants were at least 70 years of age, initiating therapy for incurable solid tumors or lymphoma, and having two or more impaired GA domains. Patients in the intervention arm had lower rates of grade 3–5 toxicities in the first 3 months compared to those receiving usual care (50% vs 71%, risk ratio [RR] 0.74, 95% confidence interval [CI] 0.63 = 0.87, p = 0.0002). The decreased toxicity was potentially related to reduced intensity treatment for cycle 1 in the intervention arm (49% vs 35%, RR 0.81, p = 0.01); OS was not impacted (71% vs 74%, p = 0.3). Similarly, in the GA-driven intervention (GAIN) randomized controlled trial, Li et al. randomized patients aged 65 years or older starting a new chemotherapy regimen for a solid tumor to either a GA-driven multidisciplinary intervention or standard of care. In the intervention arm, a multidisciplinary team reviewed the GA results and implemented interventions based on predefined triggers, while in the standard of care arm, GA results were provided to the treating oncologists to use at their discretion. Participants in the intervention arm had less grade 3–5 chemo-related toxicities (50.5% vs 60.4%, 95% CI 53.7–67.1%, p = 0.02) [19]. No difference in health-care utilization, chemotherapy dose modifications or OS was seen. These studies highlight the use of GA-guided interventions to improve outcomes. Future studies, specifically in the lymphoma population, are needed to determine how GA-guided interventions may be applied to patients with lymphoma, especially aggressive lymphomas that are potentially curable.
GA’s have been shown to be useful in other ways, as well. In a cross-sectional study of adults aged 65 years or older with hematological malignancies (36.1% lymphoma), a GA with recommendations performed by a oncogeriatric consulting team resulted in a change of hematological treatment plan in 21.7% of patients, including exclusive palliative care, protocol change, dose reduction, or close reassessment [65]. Factors associated with a change in treatment plan were functional impairment, mobility impairment, comorbidities, and age [65]. Other benefits of a GA-guided intervention include increased discussion of comorbidities and increased advance directive completion. [20,66]
6. Illustrative Case 1 Continued
After discussion with the patient and her family, the decision is made to initiate treatment with a reduced-dose chemo-immunotherapy regimen (R-miniCHOP with growth factor support). Following initiation of therapy, she has multiple hospital admissions for complications including acute on chronic congestive heart failure, atrial fibrillation with rapid ventricular response (requiring admission to the intensive care unit), and deconditioning, resulting in significant functional status impairment. PET-CT after two cycles of therapy shows evidence of a complete response; further treatment is held due to concerns for therapy-related toxicities. One year later, she presents in clinic for follow up. She has had a good recovery of her functional status and her disease remains in remission.
7. Our Suggested Approach
This case demonstrates the limits of unidimensional assessments of frailty. Our 83-year old patient had no symptoms from her disease per se (except for mild lower extremity edema) and was subjectively determined to have an ECOG PS of “0”. Given her age (>80 years) and comorbidities, the decision was taken to initiate her on a steroid pre-phase followed by dose-reduced R-CHOP – unfortunately, she tolerated this very poorly with significant decline in her functional status. If we were to use the FIL-simplified GA approach in this patient, we would immediately note that based on her age (≥80 years), presence of comorbidities (score of 5), and dependence in 1 or more IADL, she would fall into the “frail” category. This patient represents a group of patients with significantly decreased functional reserve, at high risk for chemotherapy-related toxicities, who are likely to have equal benefit from palliative therapy as from a curative approach with conventional regimens. Specifically, the presence of cardiac and renal dysfunction puts her at risk for volume overload, which resulted in atrial fibrillation with rapid ventricular response and worsening congestive heart failure. Her anemia further contributed to her cardiac decompensation and functional deterioration. Based on her IPI of 3, presence of anemia, and frail status on the sGA, she would be classified in the “high-risk” EPI group. As discussed earlier, these patients have a 3-year OS of 42%.
In new patients, ≥70 years of age with NHL, we suggest performing a GA as per ASCO guidelines or at minimum, a simplified-GA (Fig. 2). Currently, the FIL approach is backed by evidence from a large multi-institutional study and is feasible to implement in busy oncology clinics. We recommend treatment with a curative approach in patients within the “fit” category. When resources are available, we recommend a geriatric oncology or geriatrics referral for patients in the unfit/frail category. Patients in the “unfit” category might still benefit from a curative approach with reduced-intensity regimens with or without a steroid pre-phase (+/− vincristine/cyclophosphamide) [23]. However, patients identified as “frail” are likely to have a similar benefit from a curative approach using conventional regimens or symptomatic treatment [23]. Shared-decision making is imperative, keeping in view the cancer-independent mortality, availability of social support, and individual preferences, including the desire for quality of life (QoL) versus quantity of life [67]. For those wishing to avoid treatment-related adverse effects, a palliative approach is reasonable [68]. For patients desiring a curative approach, as our patient above, it is important to discuss the uncertainty of benefit and the potential for treatment-related morbidity and mortality [68]. Early engagement with multidisciplinary teams may help minimize loss of function and QoL. Ultimately, randomized studies are needed to determine the optimal treatment approach and dose intensity for patients within this category including the role for novel agents.
Fig. 2.
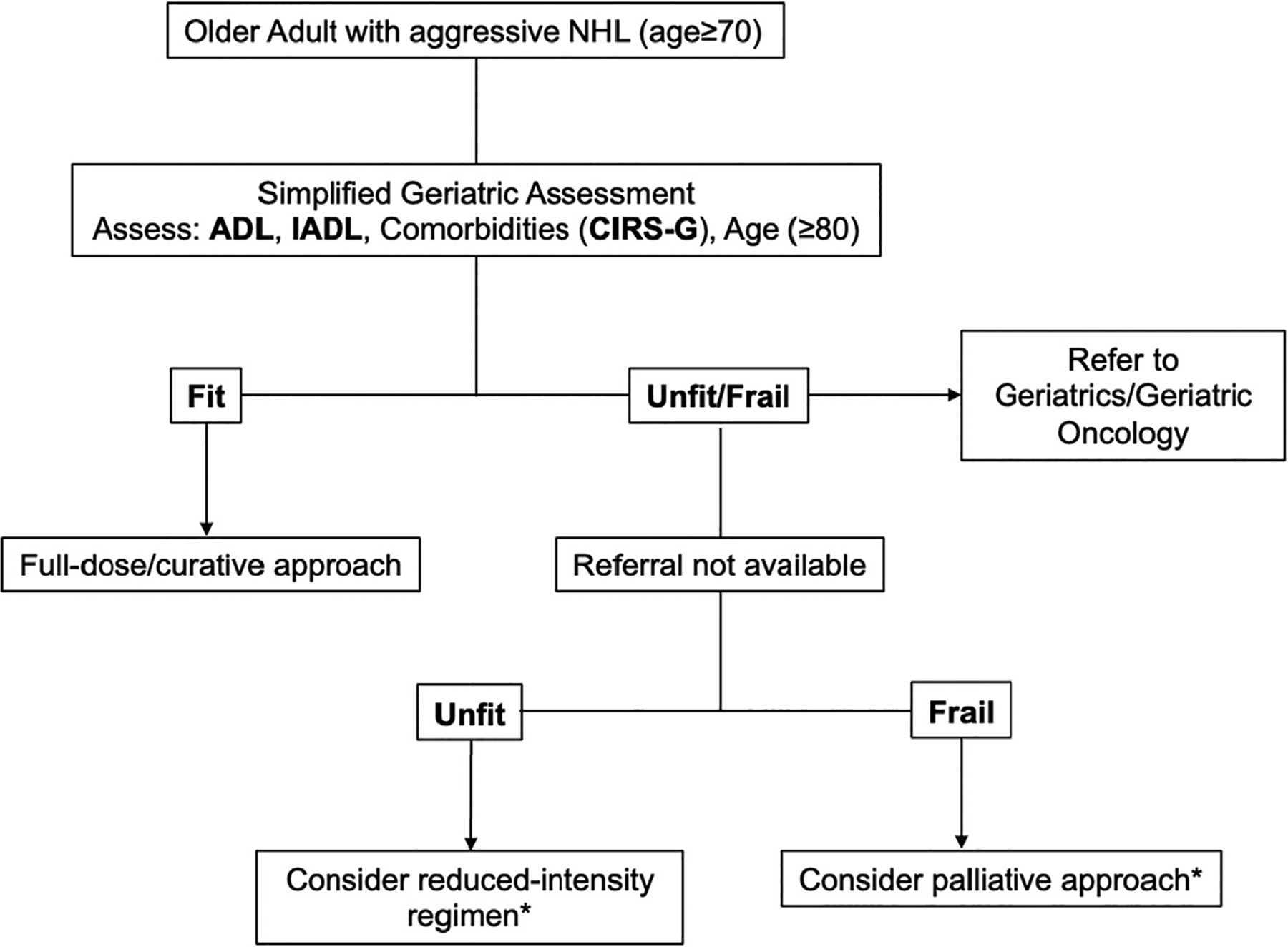
Our approach to assessing frailty in patients with aggressive NHL.
ADL: Activities of Daily Living, IADL: Instrumental Activities of Daily Living; CIRS-G – Comorbidities Illness Rating Scale- Geriatric; NHL: Non-Hodgkin lymphoma.
*Treatment plan individualized based on comorbidities, social support, individual preferences.
8. Future Scope
Moving forward, studies incorporating GA into the prognostication and management of older adults with lymphoma are needed. In addition, future research must focus on exploring outcomes that are relevant to older adults, such as quality of life and function. This is especially important with use of novel therapies, such as chimeric antigen receptor (CAR) T-cell therapy, which are increasingly being applied to older adults outside of clinical trials [57]. Because of the intensity of CAR T-cell therapy, it is imperative to evaluate how it impacts geriatric issues. This is being addressed in a current pilot study that assesses the longitudinal geriatric and neurocognitive effects of CAR T-cell therapy in older patients with lymphoma (NCT04300998). Lastly, the management of older adults with lymphomas is evolving with the advent of novel therapies. For patients on oral targeted therapies, long-term adherence is an important issue. Studies exploring the role of GA in predicting treatment adherence and quality of life in patients on oral therapies are ongoing [69]. Clinical trials evaluating modified treatment regimens in older patients with DLBCL are also ongoing. This includes a phase II/III trial comparing oral azacitidine in combination with R-miniCHOP vs. R-miniCHOP alone in adults 75 years and older with newly diagnosed DLBCL (NCT04799275). Another is a phase II trial assessing tolerability of split-dose R-CHOP in adults >70 years with DLBCL (NCT03943901). Studies looking at the value of incorporating GA-based risk assessments within the electronic health record are also ongoing with promising results [70]. There is a paucity of data on the role of GA and GA-guided treatment approach in older adults with indolent NHL - future studies are needed to fill this knowledge gap.
9. Conclusion
In conclusion, several GA tools have been developed and validated for use in the management of older adults with NHL. These add significant information to conventional PS evaluation, and aid in addressing the multidimensional and heterogeneous nature of the aging process. These tools are feasible in oncology clinic usage, most taking <10 min to perform, and correlate well with outcomes. Future randomized studies are needed to examine the optimal approach of GA-directed treatment strategies.
Acknowledgement
We thank Stuart M. Lichtman, MD, Jessica L. Krok-Schoen, PhD, MA, the SIOG Publication Committee, and the Young SIOG Governance for their thoughtful reviews and insightful comments on this paper.
Financial Support
Dr. Loh is supported by the National Cancer Institute at the National Institute of Health (R00CA237744 to KPL) and the Wilmot Research Fellowship Award. Dr. Huang is supported by the National Institute on Aging of the National Institutes of Health under Award Number R03AG067935 and the Hellman Fellows Award.
Footnotes
Disclosures
OSA: none; LWH: none; MT: none; PT: none; KPL: consultant for Pfizer and Seattle Genetics; honorarium from Pfizer; RC: advisory for Janssen, Abbvie, Astra Zeneca, Beigene, Lilly, Kite, Celgene/BMS, Takeda, Kyowa-Kiri, Incyte, ADC Therapeutics, and grant research from Pfizer. VAM-consultant for Merck; honorarium from BMS.
References
- [1].Surveillance, Epidemiology, and End Results (SEER). Program Populations (1969–2019). www.seer.cancer.gov/popdata. released February 2021.
- [2].Thandra KC, et al. Epidemiology of Non-Hodgkin’s lymphoma. Med Sci (Basel). 2021;9. 10.3390/medsci9010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bataillard EJ, et al. Impact of R-CHOP dose intensity on survival outcomes in diffuse large B-cell lymphoma: a systematic review. Blood Adv. 2021;5:2426–37. 10.1182/bloodadvances.2021004665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Parikh SA, et al. The impact of dose modification and temporary interruption of ibrutinib on outcomes of chronic lymphocytic leukemia patients in routine clinical practice. Cancer Med. 2020;9:3390–9. 10.1002/cam4.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goede V, et al. Frailty assessment in the care of older people with haematological malignancies. Lancet Healthy Longev. 2021. 10.1016/s2666-7568(21)00184-7. [DOI] [PubMed] [Google Scholar]
- [6].Zubrod CG, et al. Appraisal of methods for the study of chemotherapy of cancer in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- [7].Repetto L, et al. Comprehensive geriatric assessment adds information to eastern cooperative oncology group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20:494–502. 10.1200/jco.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- [8].Tucci A, et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115:4547–53. 10.1002/cncr.24490. [DOI] [PubMed] [Google Scholar]
- [9].Oiwa K, et al. Utility of the geriatric 8 for the prediction of therapy-related toxicity in older adults with diffuse large B-cell lymphoma. Oncologist. 2021;26:215–23. 10.1002/onco.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ribi K, et al. Cancer-specific geriatric assessment and quality of life: important factors in caring for older patients with aggressive B-cell lymphoma. Support Care Cancer. 2017;25:2833–42. 10.1007/s00520-017-3698-4. [DOI] [PubMed] [Google Scholar]
- [11].Fama A, et al. Vulnerable elders Survey-13 (VES-13) predicts 1-year mortality risk in newly diagnosed non-Hodgkin lymphoma (NHL). Blood. 2019;134:69. 10.1182/blood-2019-129499. [DOI] [Google Scholar]
- [12].Cordoba R, Luminari S, Eyre TA. The use of frailty assessments in treating older adults with aggressive lymphomas. Br J Haematol. 2021;194:677–85. 10.1111/bjh.17384. [DOI] [PubMed] [Google Scholar]
- [13].Stauder R, et al. Management of chronic lymphocytic leukemia (CLL) in the elderly: a position paper from an international Society of Geriatric Oncology (SIOG) task force. Ann Oncol. 2017;28:218–27. 10.1093/annonc/mdw547. [DOI] [PubMed] [Google Scholar]
- [14].Fusco D, et al. Comprehensive geriatric assessment in older adults with cancer: recommendations by the Italian Society of Geriatrics and Gerontology (SIGG). Eur J Clin Invest. 2021.;51:e13347. 10.1111/eci.13347. [DOI] [PubMed] [Google Scholar]
- [15].Decoster L, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendationsdagger. Ann Oncol. 2015;26:288–300. 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- [16].Mohile SG, et al. Practical assessment and Management of Vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36:2326–47. 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Corre R, et al. Use of a comprehensive geriatric assessment for the Management of Elderly Patients with advanced non-small-cell lung cancer: the phase III randomized ESOGIA-GFPC-GECP 08–02 study. J Clin Oncol. 2016;34:1476–83. 10.1200/JCO.2015.63.5839. [DOI] [PubMed] [Google Scholar]
- [18].Mohile SG, et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: a University of Rochester Cancer Center NCI community oncology research program cluster randomized clinical trial (CRCT). J Clin Oncol. 2020;38:12009. 10.1200/JCO.2020.38.15_suppl.12009. [DOI] [Google Scholar]
- [19].Li D, et al. Geriatric assessment–driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021. 10.1001/jamaoncol.2021.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bai JF, et al. Comprehensive geriatric assessment (CGA): a simple tool for guiding the treatment of older adults with diffuse large B-cell lymphoma in China. Oncologist. 2020;25:e1202–8. 10.1634/theoncologist.2019-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hurria A, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–65. 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Extermann M, et al. Predicting the risk of chemotherapy toxicity in older patients: the chemotherapy risk assessment scale for high-age patients (CRASH) score. Cancer. 2012;118:3377–86. 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- [23].Tucci A, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the lymphoma Italian foundation (FIL). Leuk Lymphoma. 2015;56:921–6. 10.3109/10428194.2014.953142. [DOI] [PubMed] [Google Scholar]
- [24].Miura K, et al. A host-dependent prognostic model for elderly patients with diffuse large B-cell lymphoma. Oncologist. 2017;22:554–60. 10.1634/theoncologist.2016-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sakurai M, et al. Geriatric screening tools predict survival outcomes in older patients with diffuse large B cell lymphoma. Ann Hematol. 2019;98:669–78. 10.1007/s00277-018-3551-y. [DOI] [PubMed] [Google Scholar]
- [26].Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- [27].Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- [28].Nabhan C, et al. Analysis of very elderly (>/=80 years) non-hodgkin lymphoma: impact of functional status and co-morbidities on outcome. Br J Haematol. 2012; 156:196–204. 10.1111/j.1365-2141.2011.08934.x. [DOI] [PubMed] [Google Scholar]
- [29].Chou WC, et al. Application of comprehensive geriatric assessment in predicting early mortality among elder patients with B-cell lymphoma receiving immunochemotherapy. Eur J Haematol. 2020;105:399–407. 10.1111/ejh.13457. [DOI] [PubMed] [Google Scholar]
- [30].Yamasaki S, et al. Clinical impact of comprehensive geriatric assessment in patients aged 80 years and older with diffuse large B-cell lymphoma receiving rituximab-mini-CHOP: a single-institute retrospective study. Eur Geriatr Med. 2021. 10.1007/s41999-021-00539-8. [DOI] [PubMed] [Google Scholar]
- [31].Scheepers ERM, et al. Geriatric assessment in older patients with a hematologic malignancy: a systematic review. Haematologica. 2020;105:1484–93. 10.3324/haematol.2019.245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bairey O, Shacham-Abulafia A, Shpilberg O, Gurion R. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: an important simple prognostic factor. Hematol Oncol. 2016;34:184–92. 10.1002/hon.2233. [DOI] [PubMed] [Google Scholar]
- [33].Janssen-Heijnen ML, et al. A population-based study of severity of comorbidity among patients with non-Hodgkin’s lymphoma: prognostic impact independent of international prognostic index. Br J Haematol. 2005;129:597–606. 10.1111/j.1365-2141.2005.05508.x. [DOI] [PubMed] [Google Scholar]
- [34].Hohloch K, et al. Low serum albumin is an independent risk factor in elderly patients with aggressive B-cell lymphoma: results from prospective trials of the German high-grade Non-Hodgkin’s lymphoma study group. eJHaem. 2020;1:181–7. 10.1002/jha2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim SH, et al. Prognostic impact of pretreatment albumin to globulin ratio in patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk Res. 2018; 71:100–5. 10.1016/j.leukres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- [36].Liu H, et al. Validation and refinement of the age, comorbidities, and albumin index in elderly patients with diffuse large B-cell lymphoma: an effective tool for comprehensive geriatric assessment. Oncologist. 2018;23:722–9. 10.1634/theoncologist.2017-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chihara D, et al. Analysis of prognostic factors in peripheral T-cell lymphoma: prognostic value of serum albumin and mediastinal lymphadenopathy. Leuk Lymphoma. 2009;50:1999–2004. 10.3109/10428190903318311. [DOI] [PubMed] [Google Scholar]
- [38].Gebauer N, et al. Prognostic impact of nutritional and inflammation-based risk scores in follicular lymphoma in the era of anti-CD20 targeted treatment strategies. J Cancer Res Clin Oncol. 2021. 10.1007/s00432-021-03758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Camus V, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol. 2014;93:9–18. 10.1111/ejh.12285. [DOI] [PubMed] [Google Scholar]
- [40].Hong F, et al. The role of body mass index in survival outcome for lymphoma patients: US intergroup experience. Ann Oncol. 2014;25:669–74. 10.1093/annonc/mdt594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lanic H, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. 2014;55:817–23. 10.3109/10428194.2013.816421. [DOI] [PubMed] [Google Scholar]
- [42].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [43].van Spronsen DJ, Janssen-Heijnen ML, Lemmens VE, Peters WG, Coebergh JW. Independent prognostic effect of co-morbidity in lymphoma patients: results of the population-based Eindhoven cancer registry. Eur J Cancer. 2005;41:1051–7. 10.1016/j.ejca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- [44].Merli F, et al. The elderly project by the Fondazione Italiana Linfomi (FIL): a prospective multidimensional assessment of elderly patients with diffuse large B-cell lymphoma. Blood. 2016;128:3049. 10.1182/blood.V128.22.3049.3049. [DOI] [Google Scholar]
- [45].Merli F, et al. Simplified geriatric assessment in older patients with diffuse large B-cell lymphoma: the prospective elderly project of the Fondazione Italiana Linfomi. J Clin Oncol. 2021;39:1214–22. 10.1200/jco.20.02465. [DOI] [PubMed] [Google Scholar]
- [46].Bellera CA, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–72. 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- [47].van Walree IC, et al. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol. 2019;10:847–58. 10.1016/j.jgo.2019.04.016. [DOI] [PubMed] [Google Scholar]
- [48].Lee S, et al. Association of the Geriatric 8 with treatment intensity and prognosis in older patients with diffuse large B-cell lymphoma. Br J Haematol. 2021;194:325–35. 10.1111/bjh.17554. [DOI] [PubMed] [Google Scholar]
- [49].Luciani A, et al. Estimating the risk of chemotherapy toxicity in older patients with cancer: the role of the vulnerable elders Survey-13 (VES-13). J Geriatr Oncol. 2015;6:272–9. 10.1016/j.jgo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- [50].Okuyama T, et al. Screening performance for frailty among older patients with cancer: a cross-sectional observational study of two approaches. J Natl Compr Canc Netw. 2015;13:1525–31. 10.6004/jnccn.2015.0180. [DOI] [PubMed] [Google Scholar]
- [51].Hurria A, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998–2005. 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- [52].Hurria A, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29:1290–6. 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Prica A, et al. Functional predictors of chemotherapy toxicity in elderly lymphoma patients: a prospective pilot study. Blood. 2018;132:4843. 10.1182/blood-2018-99-115400. [DOI] [Google Scholar]
- [54].Mariano C, et al. Utility of a chemotherapy toxicity prediction tool for older patients in a community setting. Curr Oncol. 2019;26:234–9. 10.3747/co.26.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Soubeyran P, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–34. 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- [56].Goede V, et al. Evaluation of geriatric assessment in patients with chronic lymphocytic leukemia: results of the CLL9 trial of the German CLL study group. Leuk Lymphoma. 2016;57:789–96. 10.3109/10428194.2015.1091933. [DOI] [PubMed] [Google Scholar]
- [57].Boccomini C, et al. A brief rituximab, bendamustine, mitoxantrone (R-BM) induction followed by rituximab consolidation in elderly patients with advanced follicular lymphoma: a phase II study by the Fondazione Italiana Linfomi (FIL). Br J Haematol. 2021;193:280–9. 10.1111/bjh.17283. [DOI] [PubMed] [Google Scholar]
- [58].Marchesi F, et al. A retrospective study on 73 elderly patients (≥75years) with aggressive B-cell non Hodgkin lymphoma: clinical significance of treatment intensity and comprehensive geriatric assessment. J Geriatr Oncol. 2013;4:242–8. 10.1016/j.jgo.2013.04.003. [DOI] [PubMed] [Google Scholar]
- [59].Spina M, et al. Definition and validation of the new elderly prognostic index (EPI) for elderly patients with diffuse large B-cell lymphoma integrating geriatric and clinical assessment: results of the prospective “elderly project” on 1353 patients by the Fondazione Italiana Linfomi. Blood. 2019;134:398. 10.1182/blood-2019-123027. [DOI] [Google Scholar]
- [60].Bernardi D, et al. Comprehensive geriatric evaluation in elderly patients with lymphoma: feasibility of a patient-tailored treatment plan. J Clin Oncol. 2003;21:754. 10.1200/jco.2003.99.210. [DOI] [PubMed] [Google Scholar]
- [61].Spina M, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist. 2012;17:838–46. 10.1634/theoncologist.2011-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Olivieri A, et al. Tailored therapy in an unselected population of 91 elderly patients with DLBCL prospectively evaluated using a simplified CGA. Oncologist. 2012;17: 663–72. 10.1634/theoncologist.2011-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lastra-German IK, Navarrete-Reyes AP, Mejía-Domínguez NR, Agreda-Vásquez GP. Adjusted chemotherapy according to frailty status in elderly patients with diffuse large B-cell lymphoma: experience from a single referral Center in Mexico City. Clin Lymphoma Myeloma Leuk. 2019;19:e98–106. 10.1016/j.clml.2018.11.013. [DOI] [PubMed] [Google Scholar]
- [64].DuMontier C, et al. Randomized controlled trial of geriatric consultation versus standard care in older adults with hematologic malignancies. Haematologica. 2021. 10.3324/haematol.2021.278802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Garric M, et al. Impact of a comprehensive geriatric assessment on decision-making in older patients with hematological malignancies. Eur J Haematol. 2021;106: 616–26. 10.1111/ejh.13570. [DOI] [PubMed] [Google Scholar]
- [66].Kleckner AS, et al. Using geriatric assessment to guide conversations regarding comorbidities among older patients with advanced cancer. JCO Oncol Pract. 2021; Op2100196. 10.1200/op.21.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].DuMontier C, Loh KP, Soto-Perez-de-Celis E, Dale W. Decision making in older adults with cancer. J Clin Oncol. 2021;39:2164–74. 10.1200/jco.21.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rosko AE, et al. Advances in management for older adults with hematologic malignancies. J Clin Oncol. 2021;39:2102–14. 10.1200/jco.21.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Akhtar OS, et al. Evaluating the role of baseline geriatric assessment in predicting adherence to oral targeted therapies and outcomes in older adults with non-Hodgkin lymphoma. J Clin Oncol. 2021;39:12043. 10.1200/JCO.2021.39.15_suppl.12043. [DOI] [Google Scholar]
- [70].Chan H, et al. Electronic FRAIL score may predict treatment outcomes in older adults with myeloma. J Geriatr Oncol. 2021;12:515–20. 10.1016/j.jgo.2020.09.031. [DOI] [PubMed] [Google Scholar]


