Abstract
Purpose:
A nuanced understanding of human papillomavirus (HPV) vaccine hesitancy is key to tailoring public health interventions to reach HPV vaccination goals in the US. We aimed to understand the spectrum of parental vaccine hesitancy and identify reasons for lack of vaccination.
Methods:
Using cross-sectional data from the 2019 National Immunization Survey-Teen, we examined parents of adolescents aged 13-17 years who had not initiated HPV vaccination. Parents who did not intend to vaccinate their child in the next year were classified into three categories: “unsure”, “somewhat hesitant” or “very hesitant”. Survey-weighted multinomial logistic regression was used to identify factors associated with level of vaccine hesitancy.
Results:
Of the 13,090 parents of unvaccinated adolescents, 8,253 (63%) were hesitant. Among those, 63% were very hesitant, 29% were somewhat hesitant, and 8% were unsure. Parents who had received a provider recommendation were less likely to be unsure (adjusted relative risk ratio (aRRR: 0.3, 95%CI:0.2-0.4) or somewhat hesitant (aRRR: 0.8, 95%CI:0.6-0.9). Compared with non-Hispanic White parents, parents of minority race/ethnicity adolescents were more likely to be unsure vs. very hesitant. Safety concerns/side effects was the most common reason for lack of intent to vaccinate among very (30%) and somewhat hesitant parents (20%), whereas lack of provider recommendation was the most common reason among unsure parents (34%).
Conclusions:
We identify three distinct levels of HPV vaccine hesitancy and demonstrate that the characteristics and reasons for lack of vaccination differ amongst these levels.Understanding a parent’s level of hesitancy may help maximize the potential impact of public health interventions to reach HPV vaccination goals.
Keywords: HPV vaccine, vaccine hesitancy, National Immunization Survey-Teen
INTRODUCTION
Human Papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States [1]. Persistent infection with high-risk (HR; oncogenic) HPV types can lead to the development of several types of cancers, including cervical, anal, penile, vaginal, vulvar, and oropharyngeal cancers [2]. Approximately 80 million people are currently infected with HPV and nearly 35,000 people are affected by cancers caused by HPV infection each year in the United States [3]. Timely initiation and completion of the HPV vaccine can provide safe and effective protection against HPV infections that cause these cancers [3]. The Advisory Committee on Immunization Practices (ACIP) recommends a 2-dose HPV vaccination schedule for all pre-adolescents (including girls and boys) at ages 11-12 and a 3-dose schedule for adolescents and young adults who start later at ages 15 through 26 years and for immunocompromised persons [1, 4, 5]. Currently, Gardasil 9 is the HPV vaccine that is distributed in the U.S., which targets HPV types 6, 11 (strains that cause anogenital warts) and seven HR-HPV types (16, 18, 31, 33, 45 52 and 58) [1, 6].
Despite the proven safety and effectiveness of HPV vaccination, vaccine coverage rate in the United States remains below the Healthy People 2020 and 2030 goal of 80% HPV vaccine completion rate among girls [7]. In 2020, approximately 75% of adolescents aged 13-17 years had received at least one dose of HPV vaccine and only 61.4% of adolescent girls and 56.0% of adolescent boys had actually completed the series [8]. Vaccine hesitancy, one of the top ten health threats identified by the World Health Organization (WHO), contributes to suboptimal HPV vaccine coverage in the U.S [9, 10]. The WHO defines vaccine hesitancy as “the reluctance or refusal to vaccinate despite the availability of vaccines”, which may “reverse the progress made in tackling vaccine-preventable diseases” [10]. Adolescent’s vaccination practices are primarily driven by their parent’s decision-making, making parental vaccine hesitancy the primary focus of interventions to improve vaccination rates [11]. Previous studies have identified several factors associated with parental HPV vaccine hesitancy, including adolescent’s sex, race/ethnicity, poverty status, maternal education, and geographical region [9, 12, 13]. Most of these studies defined HPV vaccine hesitancy as a binary outcome (using a cutoff on the response scale) as simply hesitant vs. not hesitant. However, research highlights the heterogeneity of vaccine hesitant individuals [14]. Therefore, a more nuanced understanding of hesitant individuals is needed in order to tailor interventions to maximize the impact on HPV vaccine uptake rates.
We sought to determine if there were differences in characteristics of parents across the HPV vaccine hesitancy spectrum and to identify differences in their reasons for vaccine hesitancy using the 2019 National Immunization Survey-Teen (NIS-Teen) dataset. These data are critical to inform tailored and targeted interventions to address all parents’ concerns and increase HPV vaccine uptake for cancer control, including cervical cancer elimination.
METHODS
Study population and data source
We analyzed data from the 2019 National Immunization Survey-Teen (NIS-Teen), an annual random-digit-dialing survey implemented by the Centers for Disease Control and Prevention (CDC). The NIS-Teen is used to monitor vaccination coverage rates for recommended vaccines among a nationally representative subset of non-institutionalized adolescents aged 13-17 years living in the United States [11]. The NIS-Teen includes two data collection phases: a random digit dialing (RDD) telephone survey of eligible households containing adolescents aged 13-17 years and (in the subset of parents who consent) a mailed survey to the adolescent’s immunization providers that includes vaccine verification. During the telephone survey, the adult respondent (i.e., parent or guardian) who is most knowledgeable about the adolescent’s vaccinations was interviewed after obtaining their consent [11]. Details of NIS-Teen survey sampling, data collection and weighting operations have been described previously [6, 11, 15, 16]. Since the NIS-Teen data in this study were all publicly available, it is considered non-human subjects research and exempt from Institutional Review Board oversight.
In this study, eligible study participants included parents of adolescents who self-reported that they had not initiated the HPV vaccine series (adolescents who had received zero HPV vaccine doses) and whose parents responded to questions on HPV vaccination intent (Figure 1). To maximize the generalizability of our findings, we focused on the full eligible population, not just those with provider verified data, as our interest was in understanding parent-reported hesitancy based on their understanding of their adolescent’s vaccination status. We compared the baseline characteristics of participants with provider-verified data and those without; the majority of characteristics were similar between the two groups, but there were some qualitative differences noted in the distributions of race, poverty level, and receipt of provider recommendation that could have potentially biased our results if we had limited to only those with provider verified vaccination data (see Online Appendix 1).
Figure 1. Flow diagram of exclusion criteria and sample sizes.
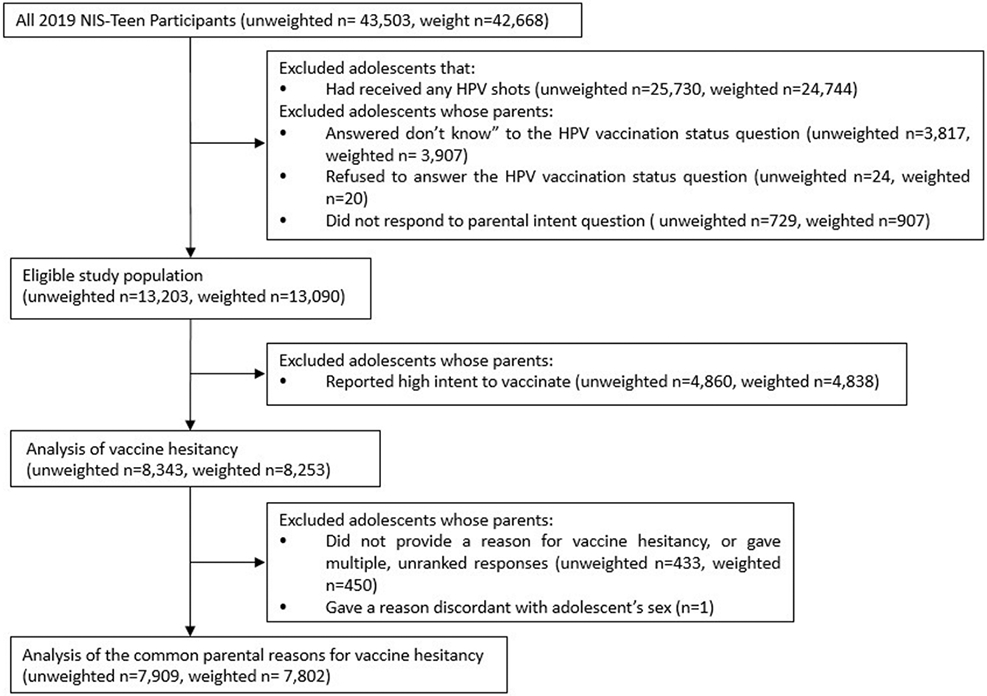
Abbreviations: HPV, human papillomavirus; NIS-Teen, National Immunization Survey-Teen
HPV vaccine hesitancy and covariates
Adolescent’s HPV vaccination status was recalled by a parent. Adolescents were categorized as unvaccinated (not initiated) if they had not received any HPV vaccine doses. For those who had not initiated the vaccine, parents were asked: “How likely it is that [adolescent’s name] will receive HPV shots in the next 12 months?” Response options were “very likely”, “somewhat likely”, “not too likely”, “not likely at all”, and “not sure/don’t know”. In this study, parents who responded “not likely at all” to vaccinate their child against HPV were classified as being “very hesitant”, and those who responded “not too likely” were classified as being “somewhat hesitant.” Parents who chose “unsure/don’t know” were classified as being “unsure” about vaccinating their child. These three groups of parents with HPV vaccine hesitancy were further asked: “What is the MAIN reason [adolescent’s name] will not receive any HPV shots in the next 12 months?” Parents selected the main reasons from a list of 29 pre-coded reasons in rank order. If unlisted, the response was elicited in an open-ended response and then was collapsed into one of the existing 29 categories [11].
The NIS-Teen survey collected data including sociodemographic characteristics and general vaccination history. An a priori set of covariates potentially related to HPV vaccine practices and HPV vaccine hesitancy was identified based on previous NIS-Teen studies [11, 15-17]. Covariates included adolescent’s sex (female, male), age of the adolescent at time of interview (13, 14-15, 16-17 years), race/ethnicity of adolescent (Hispanic, non-Hispanic White, non-Hispanic Black, Non-Hispanic other and multiple race), mother’s age ( ≤34, 35-44, ≥45), mother’s marital status (married, not married), mother’s education attainment (<12 y of education, ≥12y (non-college graduate), college graduate), poverty status (below poverty, above poverty and below $75,000, over $75,000, Unknown), provider recommendation to receive HPV shots (yes, no, unknown), parent-reported meningitis vaccination status of the adolescent (yes, no), geographic region (Northeast, Midwest, South, and West) and well-child checkup at 11-12 years old (yes, no, unknown).
Statistical methods
To account for the complex survey design of the NIS-Teen survey, all estimates were computed using the RDD sampling weight. The weighted RDD variable was normalized by dividing each weight value by the mean weight. Weighted estimates are presented throughout this study. Descriptive statistics were calculated to characterize the overall population of parents of unvaccinated adolescents who answered the HPV vaccine intent question. Descriptive statistics were then used to compare characteristics amongst the three HPV vaccine hesitant group levels (VH, SH, Unsure). Multinomial logistic regression models were fitted to estimate the adjusted relative risk ratio (aRRR) of being (a) “somewhat hesitant” vs. “very hesitant” and (b) “unsure” vs. “very hesitant”, adjusting for covariates chosen a priori.
To explore the reasons for HPV vaccine hesitancy, we compared the proportions of the top 10 most common reasons among the three different HPV vaccine hesitancy group levels. This analysis of reasons for vaccine hesitancy further excluded adolescents whose parents did not provide a reason for hesitancy, gave multiple, unranked reasons or gave a reason discordant with sex. The corresponding 95% confidence limits (CIs) and P-values were derived from Wald chi-square statistics. All analyses were performed using Stata version 15.1 (StataCorp, TX) Statistical significance was set at an alpha level of 0.05.
RESULTS
Patient characteristics
Of the 42,668 parents of adolescents 13-17 years old who participated in the NIS-Teen survey, 13,090 (30.7%) had an adolescent who had not initiated the vaccine and responded to the parental intent question (Figure 1). Approximately half (55%) were parents of a male adolescent (Table 1). Fifty-seven percent of adolescents were non-Hispanic white, 22% were Hispanic, and 13% were non-Hispanic black. The largest portion of adolescents (41%) were from the Southern region of the US.
Table 1.
Characteristics of adolescents aged 13 to 17 not vaccinated against human papillomavirus (Weighted total N=13090).
| Variable | Weighted n (%) |
|---|---|
| Adolescent’s sex | |
| Female | 5900 (45) |
| Male | 7190 (55) |
| Adolescent’s current age in years | |
| 13 | 2952 (23) |
| 14-15 | 5373 (41) |
| 16-17 | 4765 (36) |
| Race/ethnicity of adolescent | |
| Non-Hispanic White | 7425 (57) |
| Non-Hispanic Black | 1718 (13) |
| Hispanic | 2880 (22) |
| Non-Hispanic other and multiple race | 1067 (8) |
| Mother’s age | |
| ≤34 | 987 (7) |
| 35-44 | 5979 (46) |
| ≥45 | 6124 (47) |
| Mother’s marital status | |
| Married | 8500 (65) |
| Not married | 4590 (35) |
| Mother’s education | |
| <12 y | 1289 (10) |
| ⩾ 12y, non-college graduate | 6329 (48) |
| College graduate | 5472 (42) |
| Poverty status | |
| Below poverty | 2149 (16) |
| Above poverty, ≤$75,000 | 3948 (30) |
| Above poverty, >$75,000 | 6121 (47) |
| Unknown poverty status | 872 (7) |
| Received provider recommendation to get HPV vaccine | |
| Yes | 5713 (44) |
| No | 6821 (52) |
| Unknownb | 556 (4) |
| Other vaccinationsc | |
| Ever received any meningitis shots | 6708 (51) |
| Ever received any tetanus booster shots | 11341 (87) |
| Ever received “any” vaccination | 13090 (100) |
| Geographic region | |
| Midwest | 2829 (22) |
| Northeast | 1894 (15) |
| South | 5375 (41) |
| West | 2993 (22) |
| Well child check-up at 11-12 years old | |
| Yes | 9862 (75) |
| No | 486 (4) |
| Unknownd | 2742 (21) |
Note:
Two participants refused to answer this question and 121 participants did not know the number of visits to doctor in the past year.
Three participants refused to answer this question and 553 participants did not know the provider recommendation information.
Meningitis shots, tetanus booster shots, “any” vaccination were self-reported data.
A total of 514 participants did not know the well child check-up status at 11-years old and 2228 participants had missing data.
All parents (100%) of children not vaccinated against HPV reported that their adolescent had received at least one vaccination of any kind in their lifetime, with 87% reporting their adolescent was vaccinated against Tdap and 51% against meningitis. Most parents (75%) reported their adolescent had had an 11-12 year old well-child check-up, and 44% reported they had received a provider recommendation for the HPV vaccine in the past. Of the 13,090 non-initiators, 4,838 (37%) reported they planned to have their adolescent vaccinated against HPV in the next year and were thus not considered HPV vaccine hesitant.
Levels of hesitancy
A total of 8,253 (63%) parents of non-initiators were hesitant. Of those, 5,182 (63%) were very hesitant (VH), 2,354 (29%) were somewhat hesitant (SH), and 717 (8%) were unsure (Table 2). Amongst parents of females, 66% were very hesitant (VH), 25% somewhat hesitant (SH) and 9% unsure. Amongst parents of males, 60% were very hesitant, 32% were somewhat hesitant, and 8% were unsure. The distribution of levels of hesitancy also differed amongst race/ethnicity. non-Hispanic White parents were more often very hesitant (67% VH), compared to parents of adolescents of minority race/ethnicity (non-Hispanic Black: 56% VH; Hispanic 56% VH). Interestingly, more parents who had already received a provider recommendation were very hesitant (70%) compared to those who had not (58%).
Table 2.
Characteristics of adolescents aged 13 to 17 years not vaccinated against human papillomavirus (HPV) by parental HPV vaccine hesitancy (Weighted total N=8253)
| Very hesitant Weighted n (%) |
Somewhat hesitant Weighted n (%) |
Unsure Weighted n (%) |
|
|---|---|---|---|
| Weighted total | 5182 (63) | 2354 (29) | 717 (8) |
| Adolescent’s sex | |||
| Female | 2356 (66) | 877 (25) | 338 (9) |
| Male | 2826 (60) | 1477 (32) | 379 (8) |
| Adolescent’s current age in years | |||
| 13 | 1041 (63) | 446 (27) | 160 (10) |
| 14-15 | 2159 (63) | 966 (28) | 297 (9) |
| 16-17 | 1982 (62) | 942 (30) | 261 (8) |
| Race/ethnicity of adolescent | |||
| Non-Hispanic White | 3358 (67) | 1394 (28) | 243 (5) |
| Non-Hispanic Black | 536 (56) | 287 (30) | 129 (14) |
| Hispanic | 899 (56) | 465 (29) | 254 (16) |
| Non-Hispanic other and multiple race | 388 (57) | 208 (30) | 91 (13) |
| Mother’s age | |||
| ≤34 | 317 (55) | 177 (31) | 83 (14) |
| 35-44 | 2409 (64) | 1053 (28) | 328 (9) |
| ≥45 | 2455 (63) | 1124 (29) | 306 (8) |
| Mother’s marital status | |||
| Married | 3694 (65) | 1600 (28) | 386 (7) |
| Not married | 1488 (58) | 754 (29) | 331 (13) |
| Mother’s education | |||
| <12 y | 310 (48) | 201 (31) | 129 (20) |
| ⩾ 12y, non-college graduate | 2626 (64) | 1132 (28) | 336 (8) |
| College graduate | 2246 (64) | 1021 (29) | 252 (7) |
| Poverty status | |||
| Below poverty | 625 (53) | 333 (28) | 213 (18) |
| Above poverty, ≤$75,000 | 1534 (61) | 750 (30) | 222 (9) |
| Above poverty, >$75,000 | 2673 (67) | 1134 (28) | 199 (5) |
| Unknown poverty status | 349 (61) | 137 (24) | 83 (15) |
| Received provider recommendation to get HPV vaccine | |||
| Yes | 2496 (70) | 932 (26) | 131 (4) |
| No | 2520 (58) | 1318 (30) | 516 (12) |
| Unknownb | 166 (49) | 103 (31) | 69 (21) |
| Other vaccinations | |||
| Ever received any meningitis shots | 2575 (63) | 1217 (30) | 312 (7) |
| Ever received any tetanus booster shots | 4501 (64) | 2023 (29) | 553 (7) |
| Ever received “any” vaccination | 5182 (63) | 2354 (29) | 717 (8) |
| Geographic region | |||
| Midwest | 1219 (68) | 448 (25) | 131 (7) |
| Northeast | 721 (61) | 353 (30) | 110 (9) |
| South | 2045 (61) | 995 (30) | 303 (9) |
| West | 1197 (62) | 558 (29) | 174 (9) |
| Well child check-up at 11-12 years old | |||
| Yes | 4051 (64) | 1799 (28) | 518 (8) |
| No | 175 (57) | 103 (33) | 31 (10) |
| Unknownd | 955 (61) | 452 (29) | 131 (7) |
Note:
Two participants refused to answer this question and 71 participants did not know the number of visits to doctor in the past year.
Three participants refused to answer this question and 335 participants did not know the provider recommendation information.
Meningitis shots, tetanus booster shots, “any” vaccination were self-reported data.
A total of 308 participants did not know the well child check-up status at 11-years old and 1266 participants had missing data.
Comparing somewhat hesitant to very hesitant parents, independent associations with adolescent’s sex, provider recommendation, and geographic region were found (Table 3). Parents of male adolescents compared to female adolescents were 30% as likely to be somewhat hesitant vs. very hesitant (aRRR 1.3, 95%CI: 1.1-1.6). Compared to those parents living in the Midwest, parents living in the Northeast (aRRR: 1.3, 95%CI 1.0-1.7) or South (aRRR 1.3, 95%CI 1.1-1.6) were more likely to be somewhat vs. very hesitant. Compared to parents who had not received a provider recommendation, parents who had received a provider recommendation were less likely to be somewhat vs. very hesitant (aRRR: 0.8, 95%CI: 0.6-0.9). We did not identify any independent associations of parental vaccine hesitancy with adolescent’s age, race/ethnicity, mother’s age, marital status, education, income, or if the adolescent had attended a well-child check-up at 11-12 years of age between somewhat and very hesitant parents.
Table 3.
Characteristics of adolescents aged 13 to 17 years not vaccinated against human papillomavirus (HPV) associated with 3 levels of vaccine hesitancy (Weighted total N=8253).
| Characteristics | Somewhat hesitant (N=2354) Vs. Very hesitant (N=5182) |
Unsure (N=717) Vs. Very hesitant (N=5182) |
|---|---|---|
| aRRR(95%CI)a | aRRR (95% CI)a | |
| Adolescent’s sex | ||
| Female | ref | ref |
| Male | 1.3 (1.1-1.6) | 0.8 (0.6-1.1) |
| Adolescent’s current age in years | ||
| 13 | ref | ref |
| 14-15 | 1.1 (0.8-1.4) | 1.0 (0.6-1.4) |
| 16-17 | 1.1 (0.9-1.5) | 0.9 (0.6-1.4) |
| Race/ethnicity of adolescent | ||
| Non-Hispanic White | ref | ref |
| Non-Hispanic Black | 1.2 (0.9-1.6) | 2.2 (1.4-3.6) |
| Hispanic | 1.1 (0.8-1.5) | 2.5 (1.7-3.7) |
| Non-Hispanic other and multiple races | 1.2 (0.9-1.7) | 2.7 (1.6-4.5) |
| Mother’s age | ||
| ≤34 | ref | ref |
| 35-44 | 0.8 (0.6-1.3) | 0.8 (0.5-1.4) |
| ≥45 | 0.9 (0.6-1.4) | 0.9 (0.5-1. 6) |
| Mother’s marital status | ||
| Married | ref | ref |
| Not married | 1.1 (0.9-1.4) | 1.3 (0.9-1.9) |
| Mother’s education | ||
| <12 y | ref | ref |
| ⩾ 12y, non-college graduate | 0.7 (0.5-1.1) | 0.6 (0.3-1.0) |
| College graduate | 0.8 (0.6-1.2) | 0.7 (0.4-1.3) |
| Poverty status | ||
| Below poverty | (ref) | ref |
| Above poverty, ≤$75,000 | 1.0 (0.7-1.5) | 0.6 (0.4-1.0) |
| Above poverty, >$75,000 | 0.9 (0.6-1.3) | 0.4 (0.2-0.6) |
| Unknown poverty status | 0.8 (0.5-1.3) | 1.0 (0.6-1.8) |
| Received provider recommendation to get HPV vaccine | ||
| No | ref | ref |
| Yes | 0.8 (0.6-0.9) | 0.3 (0.2-04) |
| Don’t know | 1.2 (0.8-1.9) | 2.0 (1.1-3.6) |
| Having vaccinated against meningitis | 1.2 (1.0-1.4) | 1.0 (0.7-1.4) |
| Geographic region | ||
| Midwest | ref | ref |
| Northeast | 1.3 (1.0-1.7) | 1.5 (1.0-2.2) |
| South | 1.3 (1.1-1.6) | 1.2 (0.8-1.7) |
| West | 1.2 (0.9-1.7) | 1.1 (0.7-1.8) |
| Well-child checkup at 11-12 years old | ||
| No | ref (−) | ref |
| Yes | 0.8 (0.5-1.2) | 0.9 (0.5-1.6) |
| Unknown | 0.8 (0.5-1.3) | 1.0 (0.5-2.0) |
aRRR= adjusted relative risk ratio
Comparing unsure parents to very hesitant parents, independent associations with level of hesitancy were identified for race/ethnicity, mother’s education level, poverty status, geographic region, and provider recommendation (Table 3). Compared to non-Hispanic White parents, non-Hispanic Black parents (aRRR: 2.2, 95%CI: 1.4-3.6), Hispanic parents (aRRR: 2.5, 95%CI: 1.7-3.7) and other race parents (aRRR: 2.7, 95%CI: 1.6-4.5) all had higher odds of being unsure vs. very hesitant. Mothers who had graduated high school but not college compared to mothers who had not graduated high school were 40% less likely to be unsure compared to very hesitant (aRRR:0.6, 95%CI 0.3-1.0). Parents with incomes at least $75,000 above the poverty line were 60% less likely to be unsure vs. very hesitant (aRRR: 0.4, 95%CI: 0.2-0.6). Parents with a provider recommendation were 70% less likely to be unsure vs. very hesitant (aRRR: 0.3, 95% CI 0.2-0.4). Parents living in the Northeast compared to those in the Midwest were more likely to be unsure vs. very hesitant (aRRR 1.5, 95%CI: 1.0-2.2). There were no associations with being unsure vs. very hesitant with regards to adolescent’s age, sex, mother’s age, or if the adolescent had undergone a well-child 11-12 year old check-up.
Reasons for hesitancy
The most common reasons for lack of intent to vaccinate in very hesitant parents were, in rank order: 1) safety concern/side effects, 2) lack of necessity, 3) reporting the adolescent is already up-to-date, 4) reporting the adolescent is not sexually active, and 5) lack of provider recommendation (Figure 2). The most common reasons reported by somewhat hesitant parents were: 1) safety concern/side effects, 2) lack of provider recommendation, 3) lack of necessity, 4) lack of knowledge and 5) reporting the adolescent is already up-to-date. The most common reasons in unsure parents were: 1) lack of provider recommendation, 2) lack of knowledge, 3) safety concern/side effects, 4) reporting the adolescent is already up-to-date and 5) lack of necessity.
Figure 2. The top 10 most common reasons for lack of intent to vaccinate (overall).
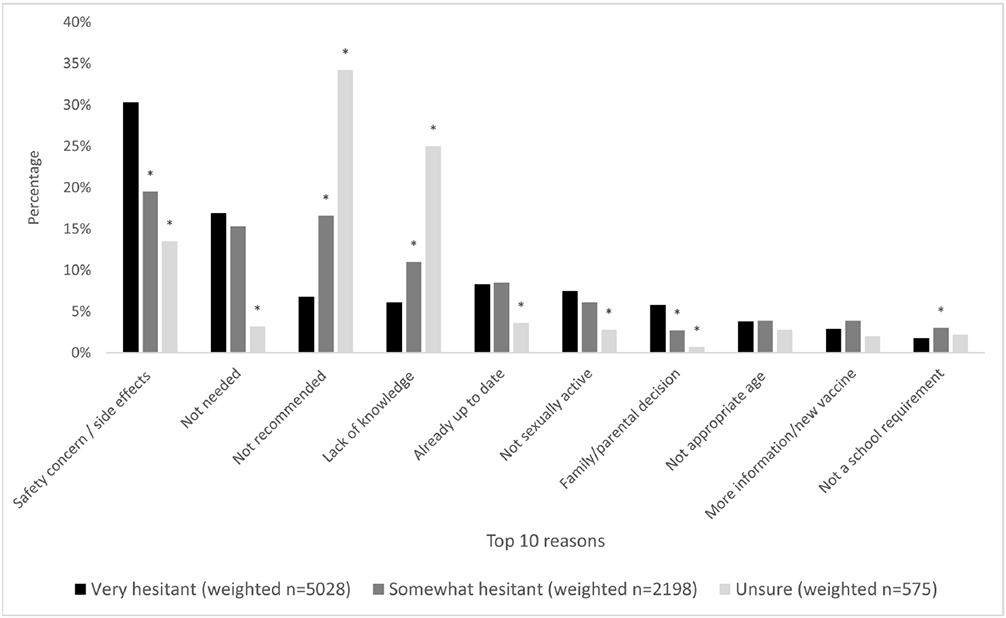
Note: We compared the proportions of each of the top 10 reasons among 3 vaccine hesitancy levels, using “very hesitant” as the reference group. Any P values less than 0.05 were designated with one bolded asterisk (*).
Safety concern/side effects was the primary concern for very hesitant parents (30%) and for somewhat hesitant parents (20%) but was less frequently reported by unsure parents (14%) (p<0.01; Online Appendix Table 2). Lack of necessity (17% VH, 15% SH and 3% unsure; p<0.01), belief the adolescent is already up-to-date (8%, 8% and 4%; p<0.01), and reporting the adolescent is not sexually active (8% VH, 6% SH and 3% unsure; p<0.01) were more commonly reported among very hesitant and somewhat hesitant parents compared to unsure parents. In contrast, lack of provider recommendation (7%VH, 16% SH, and 34% unsure; p<0.01) and lack of knowledge (6% VH, 11% SH, and 25% unsure; p<0.01) were less frequently reported as primary reasons for lack of intent to vaccinate by very or somewhat hesitant parents compared to unsure parents. More unsure parents (vs. somewhat or very hesitant parents) reported their primary reasons for hesitancy as “lack of provider recommendation”.
Fewer than 1% of all hesitant groups reported male sex, concern for increased sexual activity, or lack of vaccine availability or transportation to appointments as reasons for lack of intent to vaccinate (Online Appendix Table 2). Anti-vaccination beliefs were very uncommon, reported in only 2% of very hesitant parents compared to 1% of somewhat hesitant parents and 0.1% of unsure parents (p<0.01).
DISCUSSION
In this nationally representative analysis of HPV vaccine non-initiators, we examined the characteristics of current non-initiators and reasons for lack of intent to vaccinate across the spectrum of parental HPV vaccine hesitancy. We demonstrated that the majority of non-initiators are very or somewhat hesitant about HPV vaccination, while only a small proportion are unsure. There were more differences in parent characteristics between unsure and very hesitant parents whereas somewhat and very hesitant parents were quite similar to each other. Reasons for lack of intent to vaccinate varied by level of hesitancy: both very and somewhat hesitant parents reported safety concerns most frequently, whereas unsure parents more often cited lack of provider recommendation or lack of knowledge as their primary reason. Understanding the degree of hesitancy expressed by parents is an important step in tailoring public health campaigns to maximize HPV vaccine uptake amongst hesitant parents and achieving the Health People 2030 HPV vaccination goal of 80% vaccine completion [7].
While the proportion of adolescents initiating the HPV vaccine has increased each year since the vaccine was first approved, many non-initiators remain very hesitant towards HPV vaccination. From 2010-2018, parents who were ‘not likely at all’ to vaccinate their child in the next 12 months (i.e. very hesitant in our study) consistently made up the largest subgroup of non-initiators in the NIS-Teen survey [11, 17]. This is consistent with our findings in the most recent 2019 dataset. We identified that parents of non-Hispanic white adolescents, females, and those with higher income and education levels have higher odds of being very hesitant. This reflects the general trend in HPV vaccine uptake disparities: parents with higher income and education, of non-Hispanic white race/ethnicity, and who have private insurance are less likely to initiate the HPV vaccine [8, 11]. Additionally, we found that non-initiating parents who received a provider recommendation were more likely be very hesitant as opposed to somewhat hesitant or unsure. This is notable since a large fraction of non-initiating parents is very hesitant. Additionally, amongst those with a recommendation, the proportion of parents who are hesitant has increased from 2012-2018 [18, 19]. In an analysis of general vaccine hesitancy amongst a subgroup of NIS-Teen 2018-2019 respondents, 18% of parents self-identified as being ‘somewhat’ or ‘very’ vaccine hesitant, and 13% of all parents did not feel their child’s doctor as their most trusted source for information about childhood vaccines [20]. While a high-quality provider recommendation has been repeatedly demonstrated to be the strongest factor associated with HPV vaccine uptake [21], our findings suggest this strategy may not be effective in the most hesitant parents.
We found that unsure parents had distinct characteristics from very and somewhat hesitant parents. Unsure parents make up fewer than 10% of non-initiators, a proportion that has not substantially changed since the vaccine was first introduced [11, 15, 17]. While most studies exclude the unsure population from their analyses of HPV vaccination intent [11, 17, 22], a subgroup analysis of 2014 NIS-Teen survey data found that unsure parents were more likely to be parents of males, parents of non-White race/ethnicity adolescents (compared to non-Hispanic White), and living below the poverty level when compared to parents who had clear intent (either for or against vaccination) [15]. While we did not identify the same association with adolescent’s sex when comparing unsure vs. very hesitant parents (perhaps because of the changing attitudes and understanding of male vaccination since 2014), we otherwise demonstrate similar findings. Importantly, both that and the present study demonstrate that parents who had not received a provider recommendation are more likely to be unsure about their intent to vaccinate their child [15]. Unsure parents’ reasons for hesitancy are thus reflective of this: lack of provider recommendation and lack of knowledge made up more than half of the reasons for lack of intent to vaccinate in this group. In contrast to very hesitant parents, this suggests that improved provider recommendation, possibly including information to address parents’ knowledge gaps, could achieve meaningful change. Moreover, as lower income is associated with being unsure, interventions that impact policy on a larger scale such as Medicaid expansion and the Vaccines for Children (VFC) Program could improve access to care and increase HPV vaccine uptake in this population [23-24].
Our study demonstrates another persistent and troubling trend in reasons for vaccine hesitancy: concern about safety and side effects continue to be the most frequently reported reason for lack of vaccination by the most hesitant parents. Since the vaccine’s inception, the proportion of parents reporting reasons related to sexual disinhibition and adolescent’s sex has decreased to below 1%, while the proportion reporting safety concerns has increased [22, 25]. One study found that parents of non-Hispanic White adolescents were more likely to report safety concerns compared to parents of minority race/ethnicity adolescents, consistent with our characterization of unsure vs very hesitant parents. As noted above, provider recommendation may not assuage concerns about safety and side effects for the most hesitant parents. In one study of hesitant parents, 34% of those with a provider recommendation cited safety/side effects as their main concern, compared to 23% without a provider recommendation [11]. One educational intervention that included both safety information and varying strengths of provider recommendation found that willingness to vaccinate was influenced primarily by the presence of safety information in the materials presented [26]. Future studies are needed to identify the most trusted source of safety information amongst hesitant parents, and leverage that to increase their confidence in the vaccine’s safety.
The results presented here suggest that tailoring interventions to the parents’ level of hesitancy could provide a greater impact compared to a one size fits all approach. While we demonstrate that the unsure parents have reasons that may be more easily addressed (provider recommendation, lack of knowledge) – this group makes up the minority of hesitant parents. In contrast, most hesitant parents are primarily concerned with safety, a persistent concern which continues to plague the HPV vaccine despite over 15 years of safety data [27]. The question thus becomes, where do we find the most leverage and get the biggest return on our investment: the larger proportion of hesitant parents with more difficult reasons to address, or the smaller group of unsure parents in whom standard interventions such as provider recommendation should have significant impact?
The strengths of our study include the nationally representative, contemporary dataset. We were able to differentiate parental characteristics and reasons amongst three distinct levels of hesitancy based on one simple question about level of intent. This question could be posed to parents in advance of provider counseling and provide valuable information on parents’ level of hesitancy to enable tailoring of vaccine messaging. This analysis is limited by the cross-sectional nature of the NIS-Teen survey, and as such we are unable examine temporal relationships of associations, for example between provider recommendation and level of hesitancy. The varying influence provider recommendation may have at different levels of hesitancy warrants investigation. Moreover, the hesitancy issue may be broader than is represented in this study. The NIS-Teen does not ask parents of fully vaccinated children about hesitancy, and so parents who overcame their hesitancy to vaccinate their child, for example through a strong and trusted provider recommendation, are not represented here. Other limitations included the relatively small sample size of the unsure group, which may have limited our ability to detect differences between the groups. We were also unable to assess the influence of insurance on vaccination intent due to missingness of this variable in the dataset, though this was an uncommon parent-reported reason for lack of intent to vaccinate. Lastly, vaccine decision-making is complex and reasons for lack of intent to vaccinate are likely multifactorial so we are only able report on the primary, most prominent reason from each parent, while interventions may need to address multiple reasons concurrently. Moreover, similar to other studies, we found that “already-up-to-date” was a common reason for lack of intent to vaccinate even though the adolescents were reportedly not vaccinated, which indicating a lack of understanding or miscommunication with the parent.
Conclusion
In conclusion, we demonstrate that amongst parents who have not initiated HPV vaccination, there is a spectrum of hesitancy. Public health campaigns will need to consider this when tailoring their interventions to various groups. Understanding what to address with each level of hesitancy will help providers individualize their counseling. Thus far, a one-size fits-all approach to improving HPV vaccination rates has resulted in over 70% of adolescents initiating vaccination. However, closing the gap on the remaining 28% of US adolescents will require tailored approaches to vaccine hesitancy, and merely increasing provider recommendation will likely not affect the most hesitant parents. Future implementation studies are needed to test interventions specifically tailored varying levels of hesitancy. These studies could then help determine in which hesitant group the biggest impact could be made to improve HPV vaccination rates and reach the Healthy People 2030 goals.
Supplementary Material
IMPLICATIONS AND CONTRIBUTIONS.
Understanding the continuum of parental HPV vaccine hesitancy provides opportunities to develop targeted communication strategies to different group of people and support large-scale HPV vaccine uptake. This study observed distinctions in characteristics and reasons associated with three levels of vaccine hesitancy, suggesting the potential to tailor interventions to vaccine-hesitant subgroups.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA006973 (PI Nelson) awarded to AF Rositch and AL Beavis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ACIP
Advisory Committee on Immunization Practices
- aRRR
adjusted relative risk ratio
- CDC
Centers for Disease Control and Prevention
- HPV
Human papilloma virus
- RDD
Random digit dialing
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Anne F. Rositch: Conceptualization, Methodology, Investigation, Writing-Original draft, Writing - Review & Editing, Supervision, Funding acquisition. Tanxin Liu: Data Curation, Formal analysis, Writing - Original Draft, Writing - Review & Editing, Visualization. Christina Chao: Project administration, Writing - Review & Editing. Meghan Moran: Resources, Writing –Review & Editing. Anna L. Beavis: Conceptualization, Methodology, Investigation, Writing-Original draft, Writing - Review & Editing, Supervision, Funding acquisition. All authors attest they meet the ICMJE criteria for authorship.
Declaration of competing interest
The authors declare that they have no competing interest.
References
- [1].Meites E, Szilagyi PG, Chesson HW, et al. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Center for Disease Control and Prevention. Gardasil 9 Vaccine Protects against Additional HPV Types. Available at: https://www.cancer.gov/types/cervical/research/gardasil9-prevents-more-hpv-types. Accessed July 27, 2021.
- [3].Center for Disease Control and Prevention. Human Papillomavirus (HPV) Vaccination & Cancer Prevention. Available at: https://www.cdc.gov/vaccines/vpd/hpv/index.html. Accessed July 14, 2021.
- [4].Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–8. [DOI] [PubMed] [Google Scholar]
- [5].Center for Disease Control and Prevention. Human Papillomavirus (HPV) Vaccination: What Everyone Should Know. Available at https://www.cdc.gov/vaccines/vpd/hpv/public/index.html. Accessed July 14, 2021.
- [6].Yoo W, Koskan A, Scotch M, et al. Patterns and Disparities in Human Papillomavirus (HPV) Vaccine Uptake for Young Female Adolescents among U.S. States: NIS-Teen (2008-2016). Cancer Epidemiol Biomarkers Prev. 2020;29:1458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Office of Disease Prevention and Health Promotion. Increase the proportion of adolescents who get recommended doses of the HPV vaccine-IID-08. Available at: https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08/. Accessed July 26, 2021.
- [8].Pingali C, Yankey D, Elam-Evans LD, Markowitz LE, Williams CL, Fredua B, McNamara LA, Stokley S, Singleton JA. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021; 70(35): 1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Szilagyi PG, Albertin CS, Gurfinkel D, et al. Prevalence and characteristics of HPV vaccine hesitancy among parents of adolescents across the US. Vaccine. 2020;38:6027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].World Health Organization. Ten threats to global health in 2019. Available at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019. Accessed July 27, 2021.
- [11].Sonawane K, Zhu Y, Montealegre JR, et al. Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: a nationwide, cross-sectional survey. The Lancet Public Health. 2020;5:e484–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bocquier A, Fressard L, Cortaredona S, et al. Social differentiation of vaccine hesitancy among French parents and the mediating role of trust and commitment to health: A nationwide cross-sectional study. Vaccine. 2018;36:7666–73. [DOI] [PubMed] [Google Scholar]
- [13].Patel PR, Berenson AB. Sources of HPV vaccine hesitancy in parents. Hum Vaccin Immunother. 2013; 9:2649–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].The Strategic Advisory Group of Experts (SAGE). Report of the SAGE working group on vaccine hesitancy. Available at: https://www.who.int/immunization/sage/meetings/2014/october/SAGE_working_group_revised_report_vaccine_hesitancy.pdf?ua=1/. Accessed July 26, 2021.
- [15].Mohammed KA, Vivian E, Loux TM, et al. Factors Associated With Parents' Intent to Vaccinate Adolescents for Human Papillomavirus: Findings From the 2014 National Immunization Survey-Teen. Prev Chronic Dis. 2017;14:E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krakow M, Beavis A, Cosides O, et al. Characteristics of Adolescents Lacking Provider-Recommended Human Papillomavirus Vaccination. J Adolesc Health. 2017;60:619–22. [DOI] [PubMed] [Google Scholar]
- [17].Hanson KE, Koch B, Bonner K, et al. National Trends in Parental Human Papillomavirus Vaccination Intentions and Reasons for Hesitancy, 2010-2015. Clin Infect Dis. 2018;67:1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sonawane K, Zhu Y, Lin YY, et al. HPV Vaccine Recommendations and Parental Intent. Pediatrics. 2021;147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brewer NT, Gilkey MB, Thompson P. RE: Progress in HPV Vaccine Hesitancy. Pediatrics. 2021;147. [DOI] [PubMed] [Google Scholar]
- [20].Nguyen KH, Santibanez TA, Stokley S, et al. Parental vaccine hesitancy and its association with adolescent HPV vaccination. Vaccine. 2021;39:2416–23. 10.1016/j.vaccine.2021.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gilkey MB, Calo WA, Moss JL, et al. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine. 2016;34:1187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hirth JM, Fuchs EL, Chang M, et al. Variations in reason for intention not to vaccinate across time, region, and by race/ethnicity, NIS-Teen (2008-2016). Vaccine. 2019;37:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoff BM, Livingston MD 3rd, Thompson EL. The association between state Medicaid expansion and human papillomavirus vaccination. Vaccine. 2020;38:5963–5. [DOI] [PubMed] [Google Scholar]
- [24].Center for Disease Control and Prevention. HPV vaccine. 2021. Available at: https://www.cdc.gov/hpv/parents/vaccine-for-hpv.html. Accessed Nov 30, 2021.
- [25].Beavis A, Krakow M, Levinson K, et al. Reasons for Lack of HPV Vaccine Initiation in NIS-Teen Over Time: Shifting the Focus From Gender and Sexuality to Necessity and Safety. J Adolesc Health. 2018;63:652–6. [DOI] [PubMed] [Google Scholar]
- [26].Donahue K, Hendrix K, Sturm L, et al. Provider Communication and Mothers' Willingness to Vaccinate Against Human Papillomavirus and Influenza: A Randomized Health Messaging Trial. Acad Pediatr. 2018;18:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rositch AF, Krakow M. Invited Commentary: Moving From Evidence to Impact for Human Papillomavirus Vaccination-The Critical Role of Translation and Communication in Epidemiology. Am J Epidemiol. 2018;187:1277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


