Abstract
Purpose:
To examine the association between omega-3 polyunsaturated fatty acids (PUFAs), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and age-related macular degeneration (AMD) in the Multi-ethnic Study of Atherosclerosis (MESA) cohort.
Methods:
MESA is a multi-center, prospective cohort study designed to identify risk factors for cardiovascular disease in four ethnic groups. 6,814 participants of white, African American, Hispanic/Latino, and Chinese descent, ages 45 – 84 years, were recruited, with those found to have cardiovascular disease excluded. Our study population included all MESA participants with baseline PUFA measurements and retinal photography at exam 5 (n = 3,772). Fundus photographs were assessed for AMD using a standard grading protocol. Relative risk regression (log-link) determined associations between PUFA levels and AMD.
Results:
There was a significant association between increasing DHA levels and increasing DHA + EPA levels with reduced risk for early AMD (n = 214 participants with early AMD, of which n = 99 (46.3%) are non-white). EPA levels alone were not significantly associated with AMD.
Conclusion:
Our analysis suggests increasing levels of DHA are associated with reduced risk for early AMD in a multi-ethnic cohort. This represents the first racially diverse study demonstrating an association between omega-3 PUFAs and AMD risk.
Keywords: Age-related macular degeneration, Docosahexaenoic acid (DHA), Eicosapentaenoic acid (EPA), Multi-Ethnic Study of Atherosclerosis, Omega-3 fatty acids
Introduction
Age-related macular degeneration (AMD) is the third most common cause of visual impairment worldwide1. AMD can present and progress on a spectrum from “early” to “late”, categorized based on fundus photography findings. Patients with early AMD have a 0.9% risk per annum of late AMD, and a 3.4 – 4.7% risk of progression per annum2. Therefore it is of interest to study the pathophysiology of both early and late AMD to fully understand the mechanisms for disease onset and progression. Over the last two decades, there has been increasing interest in the role of fatty acids in the pathogenesis and prevention of AMD. Docosahexaenoic acid (DHA), a dietary omega-3 long-chain polyunsaturated fatty acid (PUFA), is a major structural component of retinal photoreceptor membranes and retinal vascular tissue3. Fatty acid analysis of ocular tissue from deceased donors with a history of AMD demonstrates a significant reduction in DHA compared to age-matched controls, hypothesized to be secondary to oxidative degradation of DHA and metabolism of DHA to anti-inflammatory molecules in response to AMD4. Another dietary omega-3 long-chain PUFA, eicosapentaenoic acid (EPA), is the metabolic precursor of DHA, and both DHA and EPA have been shown to have anti-angiogenic and anti-vasculogenic properties which could impact the development and progression of AMD3. Both DHA and EPA are found at high levels in fatty fish and certain marine mammals, and are available as dietary supplements such as fish oil capsules3.
Several case-control, cross-sectional, and large cohort studies have reported an association between DHA and EPA from dietary sources5–14 and reduced risk for development and/or progression of AMD. Despite this large body of evidence, the Age-Related Eye Disease Study 2 (AREDS2) administered DHA + EPA supplementation in a prospective clinical trial and found no risk reduction for progression to advanced AMD15. Thus, there is controversy as to the potential impact of DHA and EPA on AMD risk, and further studies are needed.
Additionally, there are gaps and weaknesses to prior studies that should be addressed. To our knowledge, there are only four studies on long-chain PUFAs and AMD that have relied on objective measures of serum/plasma levels in blood10–12, as the majority of studies have relied on dietary intake estimates, which are more subjective and have been shown to underestimate true nutritional component consumption16. Among these, only one was a population-based study, and assessed the association of measured plasma omega-3 PUFAs with late AMD in residents of Bordeaux, France, who were ≥73 years of age10. Therefore, this study covered a limited age range and geographic region, and did not assess association with early AMD. The remaining three studies that measured plasma PUFA levels were case-control studies that assessed association of plasma PUFA levels with neovascular AMD11,12,17, and did not examine association with early AMD. Lastly, the vast majority of studies assessing the relationship between long-chain PUFAs and AMD lack ethnic diversity – AREDS (Age-Related Eye Disease Study)7, AREDS215, the Nurses’ Health Study18 and the Health Professionals Follow-up Study18 were > 95% Caucasian. Additionally, the four studies which relied on measurement of serum/plasma PUFA levels to determine associations with AMD were conducted in specific ethnic cohorts – French10,11, Chinese12, and Japanese17. Therefore, the relationship between long-chain PUFAs and AMD in a multi-ethnic cohort has not been clearly elucidated.
Given the controversy regarding the association of omega-3 long-chain PUFAs and AMD, the current lack of data on measured plasma PUFA levels and early AMD, and the need to study more ethnically diverse populations, we set forth to analyze the relationship between measured plasma levels of omega-3 fatty acids, DHA and EPA, at baseline in the MESA study and AMD in this large multi-ethnic cohort.
Methods
Study Population
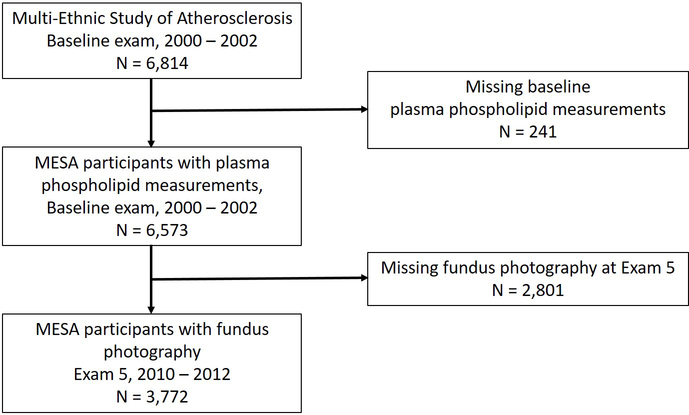
The Multi-Ethnic Study of Atherosclerosis objectives and design have been described previously19. In summary, MESA is a prospective cohort study of 6,814 male and female participants ages 45 – 84 years at baseline from four racial/ethnic groups – Caucasian, Black, Hispanic and Chinese. Participants were recruited at one of six field centers between July 2000 and September 2002, and the primary purpose was to study the development and progression of subclinical cardiovascular disease (CVD) in participants free of known CVD at baseline. The study was approved by institutional review boards at all participating field centers, and all participants provided prospective informed, written consent. Our study population included all MESA participants with baseline plasma phospholipid fatty acid measurements who underwent retinal photography at exam 5 (n = 3,772), which occurred between April 2010 and February 2012 (10 – 12 years after baseline fatty acid measurements) (Figure 1).
Figure 1.
Flowchart of included study participants and exam timeline. MESA: Multi-Ethnic Study of Atherosclerosis.
Laboratory measurement of fatty acids
Measurements of plasma fatty acids (FAs) was performed as previously described by Cao et al.20, on EDTA plasma stored at −70oC. In brief, plasma was diluted in saline and extracted with chloroform:methanol (2:1), with lipid subclasses separated using thin-layer chromatography. The fatty acid subclass was harvested and derivatized to methyl esters. The final products were dissolved in heptane and injected onto a capillary Varian CP7420 100-m column with a Hewlett Packard 5890 gas chromatograph (GC). The GC was equipped with a HP6890A autosampler and interfaced with HP ChemStation software. FAs were expressed as a percent of the total phospholipid FAs. Our primary focus for analysis was on omega-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The following intra-assay CV’s were observed: EPA, 3.3%; DHA, 2.7%.
Fundus photography
Fundus photography was performed as previously described21. Images were assessed for AMD at the University of Wisconsin, Madison, in a semi-quantitative fashion using a modified Wisconsin Age-Related Maculopathy Grading scheme. AMD features evaluated included drusen size, type and area, increased retinal pigmentation, retinal pigment epithelial depigmentation, pure geographic atrophy, and signs of exudative macular degeneration. Graders were masked to participant information, and graded images of both the right and left eyes from the same exam. Early AMD was defined by the presence of either any soft drusen and pigment abnormalities (decreased or increased retinal pigment) or the presence of a large, soft drusen ≥ 125 μm in diameter with a large drusen area > 500 μm in diameter, or large ≥ 125 μm in diameter soft, indistinct drusen, without signs of late AMD21. Late AMD was defined by the presence of geographic atrophy or pigment epithelial detachment, subretinal hemorrhage or visible subretinal new vessel, or subretinal fibrous scar or laser treatment scar for AMD21.
Measurement of other covariates
Standardized questionnaires were administered to participants at baseline to collect demographic and clinical history information, including age, sex, race, lipid lowering medication use, and smoking status19. Body mass index (BMI) was calculated from measured height and weight, as body weight (kg) divided by height (meters) squared. Total cholesterol was measured on the Roche/Hitachi 911 (Roche Diagnostics Corporation, Indianapolis, IN) and triglycerides were measured with a glycerol blanked enzymatic method (Roche Diagnostics Corporation). The Alternate Healthy Eating Index-2010 (AHEI-2010), a metric for dietary quality, was determined at exam 5, as previously described22. In short, the AHEI-2010 is a dietary quality metric determined using a multiple-choice food frequency questionnaire that sums scores for 11 foods and nutrients associated with lower chronic disease risk, including omega-3 fatty acids22.
Statistical analysis
Statistical analysis was conducted using STATA 16.0 (College Station, Texas). Omega-3 fatty acid measurements at baseline (2000 – 2002) for DHA, EPA, and DHA + EPA were examined in quartiles to determine association with early AMD at exam 5 (2010 – 2012), which included both prevalent and incident cases (n = 214 cases). For MESA, prevalent cases are defined as participants who had evidence of AMD at exam 2, when the first fundus photographs of participants were taken. Incident cases have been defined as those that were determined to have AMD at exam 5 that did not have evidence of AMD at exam 2. Late AMD cases (n = 42) were at insufficient numbers for adequate statistical power and were not analyzed. Risk ratios were estimated using relative risk regression (generalized linear model with log link function and robust standard errors) for associations between early AMD and omega-3 fatty acids with model 1 adjusted for gender, age, and race. Model 2 included adjustments in model 1, plus additional adjustment for cigarette smoking history, total cholesterol, triglycerides, BMI, and statin use. Model 2 covariates were selected based on their known role as risk factors for AMD, and/or due to being previously identified as significantly associated with AMD in the MESA cohort21,23. Model 3 included adjustments in model 2, plus adjustment for the AHEI-2010 dietary quality score.
Results
Baseline characteristics were stratified by the presence or absence of early age-related macular degeneration (Table 1), DHA quartile (Table 2) and EPA quartile (Table 3). Baseline characteristic comparison of MESA participants excluded due to missing exam 5 fundus photography (n = 2,801) to participants with exam 5 fundus photography included in our analysis (n = 3,772) is shown in Supplemental Table 2; differences noted between included and excluded participants are unlikely to significantly impact the findings of our study. Among the 3,772 MESA participants who underwent fundus photography at exam 5, 214 (5.7%) were determined to have early AMD; of the 214 with early AMD, 99 are non-white (46.3%) (Table 1). Early AMD prevalence at exam 5 in MESA participants is in line with a previously published meta-analysis of U.S. population-based study data, which found an estimated prevalence of 6.12% for early AMD24. Individuals with early AMD in the MESA cohort were more likely to be older and White, with lower triglycerides.
Table 1.
Baseline characteristics of 3,772 MESA participants stratified by the presence or absence of early age-related macular degeneration (AMD), as determined by visit 5 fundus photography
| Variable | No AMD n=3558 | Early AMD n=214 | p-value |
|---|---|---|---|
| Age, mean (SD) | 58.7 (9.0) | 65.8 (9.8) | <0.001* |
| Age categories, n (% Female) | <0.001* | ||
| 40–49 | 622 (17.5%) | 16 (7.5%) | |
| 50–59 | 1388 (39.0%) | 40 (18.7%) | |
| 60–69 | 1072 (30.1%) | 69 (32.2%) | |
| 70–79 | 442 (12.4%) | 79 (36.9%) | |
| 80+ | 34 (1.0%) | 10 (4.7%) | |
| Sex, n (% Female) | 1659 (46.6) | 93 (43.5) | 0.37 |
| Race, n (%) | <0.001* | ||
| White | 1410 (39.6) | 115 (53.7) | |
| Chinese | 437 (12.3) | 27 (12.6) | |
| Black | 912 (25.6) | 29 (13.6) | |
| Hispanic | 799 (22.5) | 43 (20.1) | |
| Cigarette smoking status, n (%) | 0.14 | ||
| Never | 1847 (51.9) | 100 (46.7) | |
| Former | 1260 (35.4) | 90 (42.1) | |
| Current | 451 (12.7) | 24 (11.2) | |
| Total cholesterol, mg/dL, mean (SD) | 195.2 (35.7) | 192.0 (32.1) | 0.21 |
| Triglycerides, mg/dL, mean (SD) | 131.8 (87.2) | 118.0 (61.8) | 0.022* |
| Body mass index, kg/m2, mean (SD) | 28.3 (5.3) | 27.7 (5.8) | 0.088 |
| Lipid lowering medication, n (%) | 1365 (38.4) | 84 (39.3) | 0.80 |
| DHA, % of total, mean (SD) | 3.8 (1.5) | 3.7 (1.5) | 0.15 |
| EPA, % of total, mean (SD) | 0.9 (0.8) | 0.9 (0.7) | 0.69 |
| DHA + EPA, % of total, mean (SD) | 4.7 (2.1) | 4.6 (2.0) | 0.24 |
AMD: age-related macular degeneration; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid;
Significant p-values (<0.05)
Table 2.
Baseline characteristics of 3,772 MESA participants stratified by DHA quartile
| Variable | Q1 (0.57 – 2.70) n=967 | Q2 (2.71 – 3.57) n=933 | Q3 (3.58 – 4.69) n=932 | Q4 (4.70 – 10.41) n=940 | p-value |
|---|---|---|---|---|---|
| Age, mean (SD) | 57.5 (8.9) | 58.8 (9.0) | 60.0 (9.2) | 60.1 (9.3) | <0.001* |
| Age categories, n (% Female) | <0.001* | ||||
| 40–49 | 217 (22.4%) | 167 (17.9%) | 122 (13.1%) | 132 (14.0%) | |
| 50–59 | 390 (40.3%) | 344 (36.9%) | 355 (38.1%) | 339 (36.1%) | |
| 60–69 | 246 (25.4%) | 290 (31.1%) | 299 (32.1%) | 306 (32.6%) | |
| 70–79 | 105 (10.9%) | 127 (13.6%) | 139 (14.9%) | 150 (16.0%) | |
| 80+ | 9 (0.9%) | 5 (0.5%) | 17 (1.8%) | 13 (1.4%) | |
| Sex, n (% Female) | 510 (52.7%) | 433 (46.4%) | 416 (44.6%) | 393 (41.8%) | <0.001* |
| Race, n (%) | <0.001* | ||||
| White | 497 (51.4%) | 418 (44.8%) | 339 (36.4%) | 271 (28.8%) | |
| Chinese | 18 (1.9%) | 49 (5.3%) | 122 (13.1%) | 275 (29.3%) | |
| Black | 102 (10.5%) | 236 (25.3%) | 311 (33.4%) | 292 (31.1%) | |
| Hispanic | 350 (36.2%) | 230 (24.7%) | 160 (17.2%) | 102 (10.9%) | |
| Cigarette smoking status, n (%) | <0.001* | ||||
| Never | 420 (43.4%) | 452 (48.4%) | 508 (54.5%) | 567 (60.3%) | |
| Former | 374 (38.7%) | 363 (38.9%) | 313 (33.6%) | 300 (31.9%) | |
| Current | 173 (17.9%) | 118 (12.6%) | 111 (11.9%) | 73 (7.8%) | |
| Total cholesterol, mg/dL, mean (SD) | 198.8 (38.6) | 195.2 (35.5) | 191.7 (33.9) | 194.0 (33.5) | <0.001* |
| Triglycerides, mg/dL, mean (SD) | 148.1 (102.8) | 136.1 (96.9) | 122.2 (66.9) | 117.2 (67.1) | <0.001* |
| Body mass index, kg/m2, mean (SD) | 29.0 (5.4) | 29.0 (5.6) | 28.3 (5.3) | 26.7 (4.7) | <0.001* |
| Lipid lowering medication, n (%) | 342 (35.4%) | 371 (39.8%) | 382 (41.0%) | 354 (37.7%) | 0.059 |
DHA = docosahexaenoic acid; SD = standard deviation
Significant p-values (<0.05)
Table 3.
Baseline characteristics of 3,772 MESA participants stratified by EPA quartile
| Variable | Q1 (0.11 – 0.49) n=906 | Q2 (2.71 – 3.57) n=959 | Q3 (3.58 – 4.69) n=952 | Q4 (4.70 – 10.41) n=955 | p-value |
|---|---|---|---|---|---|
| Age, mean (SD) | 58.1 (9.2) | 58.9 (9.3) | 59.0 (9.0) | 60.3 (9.1) | <0.001* |
| Age categories, n (% Female) | <0.001* | ||||
| 40–49 | 198 (21.9%) | 169 (17.6%) | 149 (15.7%) | 122 (12.8%) | |
| 50–59 | 335 (37.0%) | 365 (38.1%) | 377 (39.6%) | 351 (36.8%) | |
| 60–69 | 260 (28.7%) | 282 (29.4%) | 282 (29.6%) | 317 (33.2%) | |
| 70–79 | 105 (11.6%) | 128 (13.3%) | 133 (14.0%) | 155 (16.2%) | |
| 80+ | 8 (0.9%) | 15 (1.6%) | 11 (1.2%) | 10 (1.0%) | |
| Sex, n (% Female) | 451 (49.8%) | 466 (48.6%) | 403 (42.3%) | 432 (45.2%) | 0.005* |
| Race, n (%) | <0.001* | ||||
| White | 322 (35.5%) | 388 (40.5%) | 399 (41.9%) | 416 (43.6%) | |
| Chinese | 63 (7.0%) | 87 (9.1%) | 117 (12.3%) | 197 (20.6%) | |
| Black | 196 (21.6%) | 259 (27.0%) | 258 (27.1%) | 228 (23.9%) | |
| Hispanic | 325 (35.9%) | 225 (23.5%) | 178 (18.7%) | 114 (11.9%) | |
| Cigarette smoking status, n (%) | <0.001* | ||||
| Never | 450 (49.7%) | 466 (48.6%) | 510 (53.6%) | 521 (54.6%) | |
| Former | 321 (35.4%) | 343 (35.8%) | 330 (34.7%) | 356 (37.3%) | |
| Current | 135 (14.9%) | 150 (15.6%) | 112 (11.8%) | 78 (8.2%) | |
| Total cholesterol, mg/dL, mean (SD) | 190.5 (36.8) | 195.6 (36.0) | 197.3 (35.1) | 196.2 (33.9) | <0.001* |
| Triglycerides, mg/dL, mean (SD) | 132.5 (91.4) | 136.3 (96.1) | 135.3 (82.8) | 120.2 (71.2) | <0.001* |
| Body mass index, kg/m2, mean (SD) | 28.6 (5.4) | 28.7 (5.5) | 28.6 (5.5) | 27.1 (4.7) | <0.001* |
| Lipid lowering medication, n (%) | 299 (33.0%) | 369 (38.5%) | 388 (40.8%) | 393 (41.2%) | <0.001* |
EPA = eicosapentaenoic acid; SD = standard deviation
Significant p-values (<0.05)
Omega-3 fatty acid-related risk ratios for early AMD are reported in Table 4 (Model 1 and Model 2). Early AMD cases for this analysis included both prevalent and incident cases detected at exam 5 of MESA. In both models, DHA and DHA+EPA were inversely associated with early AMD risk, with statistically significant p-values for trend in Model 1 (ptrend = 0.03 for DHA, ptrend = 0.034 for DHA+EPA) and in Model 2 (ptrend = 0.014 for DHA, ptrend = 0.014 for DHA+EPA). Specifically, individuals in the two highest quartiles for DHA levels had a statistically significant 40 – 50% risk reduction for early AMD, and AMD risk declined with increasing quartiles of DHA in Model 1 (Q3 vs. Q1 risk ratio = 0.592, 95% CI 0.371 – 0.947, p = 0.029; Q4 vs. Q1 risk ratio = 0.557, 95% CI 0.333 – 0.931, p = 0.026) and Model 2 (Q3 vs. Q1 risk ratio = 0.500, 95% CI 0.287 – 0.871, p = 0.014; Q4 vs. Q1 risk ratio = 0.535, 95% CI 0.320 – 0.893, p = 0.017). Similarly, for DHA+EPA, the highest two quartiles showed a risk reduction ranging from 41 – 53% for early AMD in both models with significant p-values for Model 1 (Q3 vs. Q1 risk ratio = 0.508, 95% CI 0.297 – 0.870, p = 0.014; Q4 vs. Q1 risk ratio = 0.587, 95% CI 0.350 – 0.985, p = 0.044) and for Model 2 (Q3 vs. Q1 risk ratio = 0.472, 95% CI 0.261 – 0.854, p = 0.013; Q4 vs. Q1 risk ratio = 0.562, 95% CI = 0.334 – 0.945, p = 0.030). By contrast, EPA levels alone were not associated with early AMD risk. Since dietary omega-3 fatty acid intake is generally correlated with healthy eating habits, we further adjusted Model 2 with the AHEI-2010 score (Model 3, Supplemental Table 2) to determine whether the associations between omega-3 fatty acids and AMD risk could be attributed to general dietary quality. Model 3 had similar risk ratios and p-values to Model 1 and Model 2 and thus adjustment for dietary quality did not impact our findings, providing further support that AMD risk is specifically related to omega-3 fatty acid intake. Separate analyses of incident cases only (individuals without AMD at exam 2 who developed AMD by exam 5) demonstrated comparable risk reductions but were not statistically significant (data not shown). Lastly, we tested for interaction between each fatty acid and race, and there was no evidence of race interaction (data not shown).
Table 4.
Omega-3 fatty acid-related risk for early age-related macular degeneration among participants of the Multi-Ethnic Study of Atherosclerosis, Model 1* and Model 2†.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| Quartiles (range) | # cases/n | Risk (95% CI) p-value | Trend test p-value | Risk (95% CI) p-value | Trend test p-value |
| DHA | |||||
| Q1 (0.57 – 2.70) | 66/967 | ref | 0.03‡ | ref | 0.014‡ |
| Q2 (2.71 – 3.57) | 50/933 | 0.707 (0.470 – 1.064) 0.096 | 0.723 (0.439 – 1.190) 0.202 | ||
| Q3 (3.58 – 4.69) | 50/932 | 0.592 (0.371 – 0.947) 0.029‡ | 0.500 (0.287 – 0.871) 0.014‡ | ||
| Q4 (4.70 – 10.41) | 48/940 | 0.557 (0.333 – 0.931) 0.026‡ | 0.535 (0.320 – 0.893) 0.017‡ | ||
| EPA | |||||
| Q1 (0.11 – 0.49) | 62/906 | ref | 0.54 | ref | 0.28 |
| Q2 (0.50 – 0.67) | 40/959 | 0.639 (0.400 – 1.021) 0.061 | 0.680 (0.408 – 1.133) 0.139 | ||
| Q3 (0.68 – 1.00) | 54/952 | 0.889 (0.582 – 1.359) 0.587 | 0.841 (0.524 – 1.349) 0.472 | ||
| Q4 (1.01 – 14.46) | 58/955 | 0.836 (0.542 – 1.288) 0.416 | 0.777 (0.498 – 1.214) 0.268 | ||
| DHA + EPA | |||||
| Q1 (0.80 – 3.26) | 66/962 | ref | 0.034c | ref | 0.014‡ |
| Q2 (3.27 – 4.26) | 53/926 | 0.731 (0.486 – 1.098) 0.131 | 0.803 (0.512 – 1.259) 0.338 | ||
| Q3 (4.27 – 5.63) | 44/953 | 0.508 (0.297 – 0.870) 0.014‡ | 0.472 (0.261 – 0.854) 0.013‡ | ||
| Q4 (5.64 – 22.10) | 51/931 | 0.587 (0.350 – 0.985) 0.044‡ | 0.562 (0.334 – 0.945) 0.030‡ | ||
CI = confidence interval; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid;
Model 1 adjusted for gender, age, and race.
Model 2 adjusted for Model 1 variables, plus cigarette smoking, total cholesterol, triglycerides, BMI, and statin use.
Significant p-values (< 0.05)
Discussion
Our results support prior studies that have found an inverse correlation between DHA and DHA+EPA and AMD risk. Ours is the first study to demonstrate this association in a multi-ethnic cohort, and is also the first study to demonstrate an association between measured plasma PUFAs and early AMD risk. Most prior studies on long-chain PUFAs and AMD have relied on dietary intake estimates, rather than direct plasma measurements, of PUFAs; dietary intake estimates have been shown to be less accurate than plasma measurements, as participants tend to underestimate their actual food intake16. Our study found increasing levels of EPA alone did not confer a consistent, statistically significant reduced risk for AMD. While most studies have focused on the potential impact of combined DHA+EPA supplementation on AMD risk, some prior studies have also noted a greater risk reduction associated with DHA alone compared to EPA alone18. Specifically, retrospective analyses of 75,889 women from the Nurses’ Health Study and 38,961 men from the Health Professional Follow-Up Study demonstrated significant associations between DHA intake and reduced risk for intermediate AMD, with no significant associations between intermediate AMD risk and EPA intake alone18. It is well-established that DHA comprises the majority of the total fatty acid content within the outer segments of retinal photoreceptors, and therefore our findings are consistent with DHA’s known, prominent role in retinal structure and function. Therefore, the statistically significant association seen in our study with increasing levels of DHA+EPA may simply reflect the association with DHA alone.
Recently, the beneficial effect of omega-3 fatty acids on AMD risk has been called into question, based on the findings of the AREDS2 study, which demonstrated supplementation with DHA and EPA did not further decrease risk for AMD15. However, there are several potential explanations for failure of the omega-3 fatty acid arm of AREDS2. A recent review article by van Leeuwen et al. points out that AREDS2 participants were taking AREDS1 formula, and thus the study design required a beneficial effect of omega-3 fatty acids to exceed that of the AREDS1 formula25. Therefore omega-3 fatty acid supplementation may have a beneficial effect that was not detected due to this study design. Additionally, van Leeuwen et al. point out that the control group in AREDS2 was not representative of the general population, in that a high percentage (11.1%) took omega-3 fatty acid supplements, and the majority were well-educated and well-nourished based on baseline nutritional data25.
An additional explanation for the failure of the omega-3 arm in the AREDS2 study is the relatively low doses of DHA (350 mg) and EPA (650 mg) used in that trial. For example, data from multiple studies utilizing lower doses of fatty acid supplements demonstrated no association of omega-3 fatty acid supplementation with reduced risk for fatal or nonfatal coronary heart disease or other vascular events26. Additionally, the Cardiovascular Outcome Study (COS), an ancillary study on the AREDS2 cohort, demonstrated no reduction of CVD risk at the AREDS2 omega-3 fatty acid dose of 1 g/day27. However, recent findings published by the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) place the AREDS2 results in a new light28,29. Specifically, REDUCE-IT utilized a synthetic derivative of the omega-3 fatty acid EPA, icosapent ethyl, at a significantly higher dose (4 g/day) than what was utilized in other studies, and demonstrated that with a high dose, significant reductions in vascular events and death could be achieved29. Given these findings, it may be premature to discount the impact of omega-3 fatty acids on AMD risk until the effect of higher doses is examined. Additionally, given the lack of association between EPA levels and AMD risk, future research on the impact of high dose DHA alone should be considered. To clarify the impact of EPA alone, ancillary analysis of AMD risk in the REDUCE-IT cohort is recommended.
There are several limitations to our current study. First, the exam 5 AMD diagnosis data includes both prevalent and incident cases. Fundus photography was also performed at exam 2, to establish prevalence of AMD in MESA. However the number of prevalent cases (at exam 2) or incident cases (exam 5 cases, excluding prevalent exam 2 cases) were too low for adequate statistical power to detect associations between fatty acids and AMD prevalence or incidence. Additionally, while the number of prevalent and incident cases at exam 5 allowed for detection of statistically significant associations between DHA and DHA + EPA and decreased AMD risk, the number of AMD cases at exam 5 may still be underpowered to detect weak associations, or to examine association stratified by race. Thus, our findings need to be further validated in other cohorts. Additionally, the plasma fatty acid measurements were performed at a single time-point, on baseline samples collected in 2000 – 2002, with AMD assessed via fundus photography ten years later (2010 – 2012). However prior studies assessing repeated versus single measurements of plasma omega-3 fatty acids to assess variability over time have concluded that single measurements correlate with repeat measures, and that single measures can be reasonably used to establish associations with future outcomes30. Lastly, while of interest, the number of late AMD cases was insufficient to analyze due to inadequate statistical power.
In conclusion, our results suggest a significant association between DHA and DHA+EPA levels and reduced risk for early AMD in a multi-ethnic cohort. Given recent findings of the REDUCE-IT trial, additional studies are warranted to determine whether high doses of omega-3 fatty acid supplementation can decrease risk for AMD. In addition, future studies on the association between omega-3 fatty acids and late AMD risk in the multi-ethnic MESA cohort will be of value, once the number of late AMD events are of a sufficient number to achieve adequate statistical power.
Supplementary Material
Summary Statement:
In this prospective multi-ethnic cohort study, there was a significant association between increasing plasma docosahexaenoic acid (DHA), and increasing DHA + eicosapentaenoic acid (EPA), with reduced risk for early AMD. Our study represents the first racially diverse study to demonstrate an association between omega-3 fatty acids and AMD risk.
Acknowledgments/Disclosure:
a) Funding/Support: This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
b) Competing Interests: The authors have no competing or proprietary interests related to this manuscript. Dr. Amy Karger reports research funding unrelated to this manuscript from Siemens Healthcare Diagnostics (Tarrytown, New York) and Kyowa Kirin Pharmaceutical Development Inc. (Tokyo, Japan). Dr. Karger also serves as an external consultant to Roche Diagnostics, unrelated to this manuscript. There are no additional financial disclosures.
c) This manuscript is dedicated to Dr. Ronald Klein, who passed away on August 31, 2019. Dr. Klein served as a co-principal investigator for the MESA study, providing instrumental expertise on the epidemiology of eye disease.
References
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 12 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 2.Tikellis G, Robman LD, Dimitrov P, Nicolas C, McCarty CA, Guymer RH. Characteristics of progression of early age-related macular degeneration: the cardiovascular health and age-related maculopathy study. Eye (Lond). Feb 2007;21(2):169–76. doi: 10.1038/sj.eye.6702151 [DOI] [PubMed] [Google Scholar]
- 3.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. Jan 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Liu A, Chang J, Lin Y, Shen Z, Bernstein PS. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J Lipid Res. Nov 2010;51(11):3217–29. doi: 10.1194/jlr.M007518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augood C, Chakravarthy U, Young I, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. Aug 2008;88(2):398–406. doi: 10.1093/ajcn/88.2.398 [DOI] [PubMed] [Google Scholar]
- 6.Christen WG, Schaumberg DA, Glynn RJ, Buring JE. Dietary ω−3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch Ophthalmol. Jul 2011;129(7):921–9. doi: 10.1001/archophthalmol.2011.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangiovanni JP, Agrón E, Meleth AD, et al. {omega}−3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. Dec 2009;90(6):1601–7. doi: 10.3945/ajcn.2009.27594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho L, van Leeuwen R, Witteman JC, et al. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and ω−3 fatty acids: the Rotterdam study. Arch Ophthalmol. Jun 2011;129(6):758–66. doi: 10.1001/archophthalmol.2011.141 [DOI] [PubMed] [Google Scholar]
- 9.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. May 2009;127(5):656–65. doi: 10.1001/archophthalmol.2009.76 [DOI] [PubMed] [Google Scholar]
- 10.Merle BM, Delyfer MN, Korobelnik JF, et al. High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J Nutr. Apr 2013;143(4):505–11. doi: 10.3945/jn.112.171033 [DOI] [PubMed] [Google Scholar]
- 11.Merle BM, Benlian P, Puche N, et al. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. Mar 2014;55(3):2010–9. doi: 10.1167/iovs.14-13916 [DOI] [PubMed] [Google Scholar]
- 12.Ng AL, Leung HH, Kawasaki R, et al. Dietary Habits, Fatty Acids and Carotenoid Levels Are Associated with Neovascular Age-Related Macular Degeneration in Chinese. Nutrients. Jul 2019;11(8)doi: 10.3390/nu11081720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agrón E, Mares J, Clemons TE, et al. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology. Mar 2021;128(3):425–442. doi: 10.1016/j.ophtha.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds R, Rosner B, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. May 2013;120(5):1020–8. doi: 10.1016/j.ophtha.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group A-REDSR. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. May 2013;309(19):2005–15. doi: 10.1001/jama.2013.4997 [DOI] [PubMed] [Google Scholar]
- 16.Serra-Majem L, Nissensohn M, Øverby NC, Fekete K. Dietary methods and biomarkers of omega 3 fatty acids: a systematic review. Br J Nutr. Jun 2012;107 Suppl 2:S64–76. doi: 10.1017/S000711451200147X [DOI] [PubMed] [Google Scholar]
- 17.Orban T, Johnson WM, Dong Z, et al. Serum levels of lipid metabolites in age-related macular degeneration. FASEB J. Nov 2015;29(11):4579–88. doi: 10.1096/fj.15-275289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Cho E, Giovannucci EL, et al. Dietary Intakes of Eicosapentaenoic Acid and Docosahexaenoic Acid and Risk of Age-Related Macular Degeneration. Ophthalmology. May 2017;124(5):634–643. doi: 10.1016/j.ophtha.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. Nov 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 20.Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. Dec 2006;52(12):2265–72. doi: 10.1373/clinchem.2006.072322 [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. Mar 2006;113(3):373–80. doi: 10.1016/j.ophtha.2005.12.013 [DOI] [PubMed] [Google Scholar]
- 22.Reid M, Maras JE, Shea S, et al. Association between diet quality and sleep apnea in the Multi-Ethnic Study of Atherosclerosis. Sleep. 01 January 2019;42(1)doi: 10.1093/sleep/zsy194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher DE, Klein BE, Wong TY, et al. Incidence of Age-Related Macular Degeneration in a Multi-Ethnic United States Population: The Multi-Ethnic Study of Atherosclerosis. Ophthalmology. 06 2016;123(6):1297–308. doi: 10.1016/j.ophtha.2015.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prevalence of Age-Related Macular Degeneration in the United States. Archives of Ophthalmology. 2004;122(4):564. doi: 10.1001/archopht.122.4.564 [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen EM, Emri E, Merle BMJ, et al. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res. 11 2018;67:56–86. doi: 10.1016/j.preteyeres.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Aung T, Halsey J, Kromhout D, et al. Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77 917 Individuals. JAMA Cardiol. 03 2018;3(3):225–234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonds D E, Molly Harrington B Worrall B, et al. Effect of Long-Chain ω−3 Fatty Acids and Lutein + Zeaxanthin Supplements on Cardiovascular Outcomes. JAMA Internal Medicine. 2014–05-01 2014;174(5):763. doi: 10.1001/jamainternmed.2014.328 [DOI] [PubMed] [Google Scholar]
- 28.Bhatt DL, Steg PG, Brinton EA, et al. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin Cardiol. Mar 2017;40(3):138–148. doi: 10.1002/clc.22692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 01 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 30.Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. Sep 2014;53(6):1403–8. doi: 10.1007/s00394-013-0642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.