Abstract
Activation of canonical Wnt signaling has been implicated in podocyte injury and proteinuria. As Wnts are secreted proteins, whether Wnts derived from podocytes are obligatory for promoting proteinuria remains unknown. To address this, we generated conditional knockout mice where Wntless, a cargo receptor protein required for Wnt secretion, was specifically deleted in glomerular podocytes. Mice with podocyte-specific ablation of Wintless (Podo-Wntless−/−) were phenotypically normal. However, after inducing kidney damage with Adriamycin for six days, Podo-Wntless−/− mice developed more severe podocyte injury and albuminuria than their control littermates. Surprisingly, ablation of Wntless resulted in upregulation of β-catenin, accompanied by reduction of nephrin, podocin, podocalyxin, and Wilms tumor 1 proteins. In chronic injury induced by Adriamycin, increased albuminuria, aggravated podocyte lesions and extracellular matrix deposition were evident in Podo-Wntlessl−/− mice, compared to wild type mice. Mechanistically, specific ablation of Wintless in podocytes caused down-regulation of the nuclear factor of activated T cell 1 (NFAT1) and Nemo-like kinase (NLK), key downstream mediators of non-canonical Wnt/calcium signaling. In vitro, knockdown of either NFAT1 or NLK induced β-catenin activation while overexpression of NLK significantly repressed β-catenin induction and largely preserved nephrin in glomerular podocytes. Thus, our results indicate that podocyte-derived Wnts play an important role in protecting podocytes from injury by repressing β-catenin via activating non-canonical Wnt/calcium signaling.
Keywords: Wntless, podocytes, proteinuria, glomerulosclerosis, NFAT1, NLK, Wnt signaling
Graphical Abstract

INTRODUCTION
Podocytes are specialized, terminally differentiated visceral epithelial cells that reside on the glomerular basement membrane outside the glomerular capillaries.1 The foot processes of podocytes and slit diaphragm are an integral component of the glomerular filtration barrier.2 As such, disruption of their integrity plays a critical role in developing proteinuria in a wide variety of glomerular diseases.3,4 Earlier studies demonstrate that activation of β-catenin, the principal intracellular mediator of canonical Wnt signaling, plays a critical role in mediating podocyte dysfunction, proteinuria, and glomerulosclerosis.5–7 As β-catenin activation is also implicated in podocyte injury triggered by TGF-β or oxidative stress, it remains elusive whether the podocyte-derived Wnt ligands are obligatory in the pathogenesis of proteinuric kidney diseases.
Wnt/β-catenin is an evolutionarily conserved, developmental signaling cascade that plays an essential role in kidney development, tissue homeostasis, and the pathogenesis of chronic kidney diseases (CKD).5,8,9 Wnts transmit their signal through both β-catenin-dependent, canonical and β-catenin-independent, non-canonical pathways.10,11 For the canonical route, Wnt ligands bind to the cell membrane receptor Frizzled and co-receptors, which triggers a series of downstream signaling events and causes the stabilization and activation of β-catenin.12 Activated β-catenin then translocates into the nucleus, wherein it binds to T cell factor (TCF)/lymphoid enhancer-binding factor (LEF) to stimulate the transcription of Wnt target genes.13–15 Non-canonical Wnt signaling can be divided into the Wnt/planar cell polarity (PCP) pathway and the Wnt/calcium pathway.16–18 In the Wnt/calcium pathway, the Frizzled receptor interfaces with a trimeric G-protein and results in the release of calcium from the endoplasmic reticulum. This ultimately activates the nuclear factor of activated T cells (NFAT) and Nemo-like kinase (NLK) and regulates gene expression and cell fate.19,20 Several studies suggest that canonical and non-canonical Wnt signaling functionally antagonize each other, and therefore a delicate balance between them is essential for tissue hemostasis.18,21,22 At present, while canonical Wnt/β-catenin signaling in podocyte biology is well established, the role of non-canonical Wnt signaling in physiological and pathological conditions remains poorly understood.
The secretion of Wnt ligands is facilitated by the Wntless (Wl) protein, also known as Evenness interrupted (Evi), which is a conserved multi-span transmembrane cargo receptor dedicated to Wnt trafficking and secretion.23–26 Cell type-specific genetic ablation of the Wl gene blocks Wnt secretion from that particular type of cell.27–30 We previously showed that selective blockade of Wnt secretion from kidney tubular epithelium in mice effectively preserves tubular epithelial cell integrity, inhibits myofibroblast activation and inflammation, and ultimately ameliorates kidney fibrosis after obstructive injury.31 In contrast, conditional deletion of the Wl gene in interstitial fibroblasts does not affect the severity of kidney injury after obstructive injury.31 These observations suggest that by blocking the secretion of Wnts via genetic ablation of Wl, one can illuminate the role and contribution of the podocyte-specific Wnts in podocyte biology under basal and pathological conditions.
In this study, we used conditional knockout mice in which Wl is selectively ablated in glomerular podocytes to understand the roles of podocyte-derived Wnts in the development of proteinuria after kidney injury.
METHODS
Animal models
Wl floxed mutant mice (129S-Wlstm1.1Lan/J) were purchased from The Jackson Laboratory (Stock #012888; Bar Harbor, ME). Transgenic mice expressing Cre recombinase under the control of a 2.5-kb fragment of the human podocin promoter were also obtained from The Jackson Laboratory. Mice with podocyte-specific ablation of Wntless were generated by mating Wl floxed mice with podocin-Cre mice at the animal facility of the University of Pittsburgh Medical Center. The mouse model of podocyte injury was established by intravenous injection of ADR as described in the Detailed Methods. All animal studies were performed according to the NIH Guide for the Care and Use of Laboratory Animals and by use of the procedures approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and the University of Connecticut, School of Medicine.
Cell culture and treatment
Mouse podocytes were cultured according to procedures described previously.32,33 For some studies, podocytes were treated with Wnt1 or transfected with Wl siRNA, NFAT1 siRNA, NLK siRNA, scramble siRNA, pCAGpuro01, or pCAGpuro-NLK using Lipofectamine 2000 reagent as described previously.34
Determination of serum and urine creatinine and urine albumin
Serum creatinine levels were determined by QuantiChrom creatinine assay kits, according to the protocols specified by the manufacturer. Urine albumin was measured by a mouse Albumin ELISA Quantitation Kit according to the manufacturer’s protocol. Urine creatinine was determined by a routine procedure as described previously.7
Quantitative PCR (qPCR)
Total RNA was prepared using the TRIzol RNA isolation system. qPCR was performed on an ABI PRISM 7000 Sequence Detection System. The sequences of the primer pairs are described in Supplementary Table S1.
Histology and immunostaining
Paraffin-embedded mouse kidney sections were prepared by a routine procedure. The sections were stained with Periodic Acid–Schiff (PAS) staining or Masson’s trichrome staining reagents by standard protocol. Immunostaining was performed using a routine protocol. Antibodies used are described in Supplementary Table S2.
Electron microscopy
Transmission electron microscopy (TEM) and scanning EM (SEM) were performed as previously described.32 Ultrathin sections were cut on a Leica EM UC7 ultramicrotome, collected on 200 mesh copper grids, stained with 4% uranyl acetate, followed by 1% lead citrate. Sections were imaged using a JEOL JEM 1011 transmission electron microscope with a side mount digital camera.
Western blot analysis
Kidney tissues were lysed with radioimmunoprecipitation assay (RIPA) buffer on ice. The supernatants were collected after centrifugation at 13,000×g at 4°C for 15 min. Protein expression was analyzed by western blot analysis as described previously.31 The primary antibodies used are described in Supplementary Table S2. .
Enzyme-linked immunosorbent assay (ELISA)
The mouse Wnt1 ELISA Kit was purchased from CUSABIO. This assay employs the quantitative sandwich enzyme immunoassay technique. The levels of secreted Wnt1 in the medium was assessed according to the protocols specified by the manufacturer.
Co-immunoprecipitation
Podocytes transfected with scramble siRNA and Wl siRNA were lysed on ice. After pre-clearing with normal IgG, cell lysates were incubated overnight at 4°C with 2 mg of anti-TCF-4, followed by precipitation with 100 ml of protein A/G Plus-agarose for 3 h at 4°C. The precipitated complexes were separated by SDS–polyacrylamide gel electrophoresis and immunoblotted with active-β-catenin antibody. Antibody information is provided in Supplementary Table S2.
Automated Analysis of Positive Staining Area in Mouse Kidneys
The percentage of the positive staining area in the stained slides was analyzed in Image Pro plus 6.0. An average percentage of the positive area for each glomerulus was calculated.
Laser capture microdissection (LCM)
Laser capture microdissection was performed using the ArcturusXT laser microdissection system from Applied Biosystems. LCM was done using the frozen kidney tissue embedded in optimum cutting temperature medium.
Statistical analyses
All data were expressed as mean ± SEM (standard error of mean). Statistical analysis of the data was performed using GraphPad Prism 9. Comparison between groups was made using one-way ANOVA, followed by the Student-Newman-Keuls test. Comparison between two groups was made using Student’s t-test. For the groups that failed the normality test, a Mann-Whitney test was applied to compare two groups. P < 0.05 was considered significant.
RESULTS
Podocyte-derived Wnts are dispensable for normal glomerular physiology in mice
To investigate the role of podocyte-derived Wnts in glomerular biology, we utilized a genetic model in which Wnt secretion is blocked by deleting Wl, a protein obligatory for Wnt transportation and release.23 As illustrated in Figure 1a, by mating Wl-floxed mice and podocin-Cre mice, we generated conditional knockout mice in which Wl was specifically disrupted in glomerular podocytes using the Cre-LoxP system, designated as Podo-Wl−/−. The Wl floxed mice without Cre expression were used as controls (Podo-Wl+/+) (Supplementary Figure S1a). We found that Podo-Wl−/− mice were phenotypically normal, compared to Podo-Wl+/+ control littermates. There was no significant difference in the ratio of kidney weight/body weight, blood urea nitrogen levels, serum creatinine levels, and urinary albumin levels between 8–10 weeks old Podo-Wl+/+ and Podo-Wl−/− mice under basal conditions (Figure 1b–1e).
Figure 1.
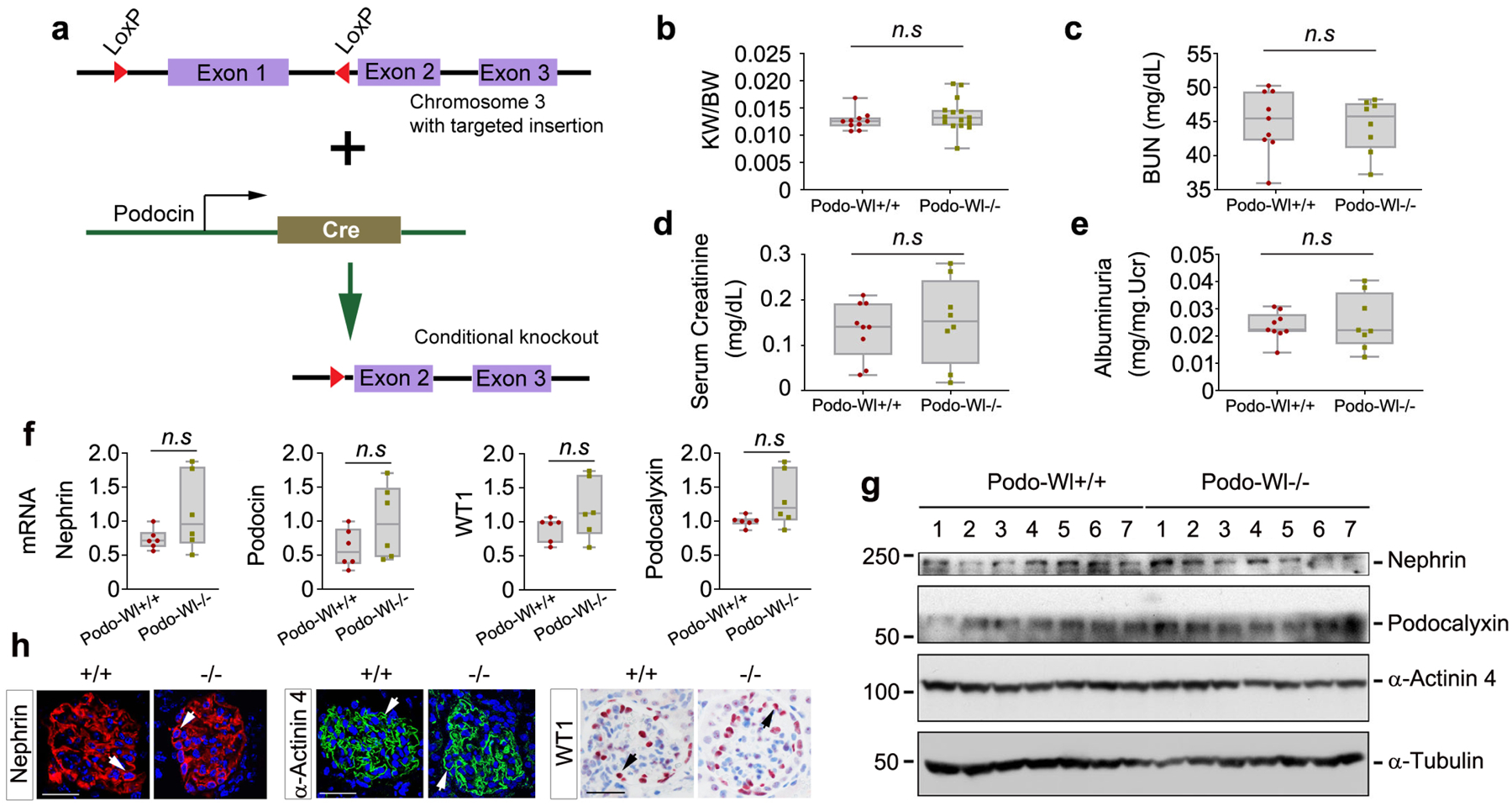
Podocyte-specific deletion of Wntless has no effects on glomerular structure and function. (a) Schematic diagram shows the strategy of crossbreeding Wl floxed mice (129S-Wlstm1.1Lan/J) with podocin-Cre transgenic mice. The Wl floxed mutant mice possess a loxP site before the ATG start site in the 5’ untranslated region and another upstream of exon 2 of the Wl gene. (b-e) Ablation of Wl in podocytes did not affect the ratio of kidney weight (KW)/body weight (BW) (b), blood urea nitrogen (BUN) (c), serum creatinine levels (d), and urinary albumin levels (albuminuria) (e) compared with control mice (n = 8–15). Age (8–10 weeks)- and sex-matched mice littermates were used. (f) qPCR analyses showed that there was little change in renal mRNA levels of nephrin, podocin, podocalyxin, and WT1 between Podo-Wl+/+ and Podo-Wl−/− mice (n = 6). (g) Western blot analyses demonstrated no significant changes in renal expression of nephrin, podocalyxin, and α-actinin 4 proteins between Podo-Wl+/+ and Podo-Wl−/− mice kidneys (n = 7). (h) Representative micrographs showed protein expression of nephrin, α-actinin 4, and WT1 in Podo-Wl+/+ and Podo-Wl−/− kidneys, as indicated. Four mice were assessed (n = 4) with similar results. Scale bar, 25 μm.
To ascertain whether specific deletion of Wl affects glomerular podocyte integrity or functions, we systemically examined the expression of podocyte-specific markers in Podo-Wl+/+ and Podo-Wl−/−mice, respectively. As shown in Figure 1f, qPCR analyses revealed similar mRNA levels of nephrin, podocin, podocalyxin, and Wilms’ tumor 1 (WT1) between Podo-Wl−/− and Podo-Wl+/+ kidneys. Consistently, deletion of Wl in podocytes exhibited little effects on protein expression of nephrin, podocalyxin, and α-actinin 4, as shown in Figure 1g and Supplementary Figure S1c. Also, there was no difference in renal expression of Wnt1, Wnt3a, Wnt5A/B, and Wnt16 as well as their downstream β-catenin, between Podo-Wl−/− and Podo-Wl+/+ kidneys (Supplementary Figure S1b to S1c). Glomerular morphology was normal in Podo-Wl−/− kidneys, compared to Podo-Wl+/+ controls (Supplementary Figure S1d). Immunohistochemical staining revealed that Wl was barely detectable under physiological conditions in both Podo-Wl+/+ and Podo-Wl−/− kidneys, as presented in Supplementary Figure S1e. In Figure 1h, immunostaining confirmed intact podocytes integrity with the characteristic distribution of nephrin and α-actinin 4 along the glomerular basement membrane in Podo-Wl−/− kidneys. Meanwhile, as a pivotal transcription factor that is exclusively expressed in podocytes, WT1-positive cell numbers did not change between Podo-Wl+/+ and Podo-Wl−/− kidneys. Quantification of data in Figure 1h is presented in Supplementary Figure S1f–S1h. Furthermore, deletion of Wl in podocytes also did not impair the integrity of mesangial cells in Podo-Wl−/− glomeruli, as shown by immunostaining of platelet-derived growth factor receptor β (PDGFR-β) (Supplementary Figure S1i).35 Both transmission electron microscopy (TEM) and scanning EM (SEM) revealed no difference in the fine ultrastructure of podocytes between Podo-Wl+/+ and Podo-Wl−/− glomeruli (Supplementary Figure S1j). In short, it appears that podocyte-derived Wnts are dispensable for normal adult kidney physiology.
Blockade of Wnt secretion aggravates adriamycin (ADR)-induced podocyte injury and proteinuria
To investigate the role of podocyte-derived Wnts in podocyte injury and proteinuria under pathological conditions, we challenged Podo-Wl−/− and Podo-Wl+/+ mice with ADR. At 6 days after ADR injection, immunohistochemical staining revealed that the induction of Wntless was largely abolished in Podo-Wl−/− mice diseased podocytes, compared to Podo-Wl+/+ mice. In comparison, the differences of Wntless expression in the tubulointerstitial compartment were less apparent between Podo-Wl+/+ and Podo-Wl−/− mice (Figure 2a and Supplementary Figure S2a). Western blots demonstrated a consistent reduction of Wntless expression in Podo-Wl−/− mice whole kidneys lysates (Figure 2b and Supplementary Figure S2b). To further validate the efficiency of Wl knockout in podocytes, we isolated glomeruli using a laser capture microdissection approach, as illustrated in Supplementary Figure S2c. qPCR analyses demonstrated a marked reduction of Wl mRNA levels in Podo-Wl−/− mouse diseased glomeruli (Figure 2c). Accordingly, immunohistochemical staining further revealed the expression of multiple Wnts (Wnt1, 2, 3, 4, 5A/B, 6, 7A/B, 10A, and 16) were decreased in the injured glomeruli of Podo-Wl−/− mice, compared with Podo-Wl+/+ controls (Figure 2d and Supplementary Figure S2d). However, to our surprise, Podo-Wl−/− mice developed more severe albuminuria than Podo-Wl+/+ controls at 6 days after intravenous injection of ADR (Figure 2e). Analysis of urine samples by SDS-PAGE revealed that albumin was the major constituent of urinary proteins in mice after ADR. Compared with Podo-Wl+/+ mice, more albumin was detected in the urine of Podo-Wl−/− mice (Figure 2f). Consistently, more severe histological changes including glomerular hypertrophy, extensive adhesion of tufts to the capsule, tubular dilation, and proteinaceous cast formation were observed in Podo-Wl−/− kidneys, compared with Podo-Wl+/+ controls (Figure 2g and Supplementary Figure S2e–S2f). Furthermore, we examined the ultrastructure of podocyte foot processes and slit diaphragm by EM. As shown in Figure 2h, foot process effacement was evident and slit diaphragm disappeared in most areas of the glomeruli in Podo-Wl−/− mice after ADR injection, whereas these ultrastructural lesions were mitigated in Podo-Wl+/+ mice. In addition, we did not observe significant changes in the endothelial marker CD31, between Podo-Wl+/+ and Podo-Wl−/− glomeruli at 6 days after ADR injection (Supplementary Figure S2g).
Figure 2.
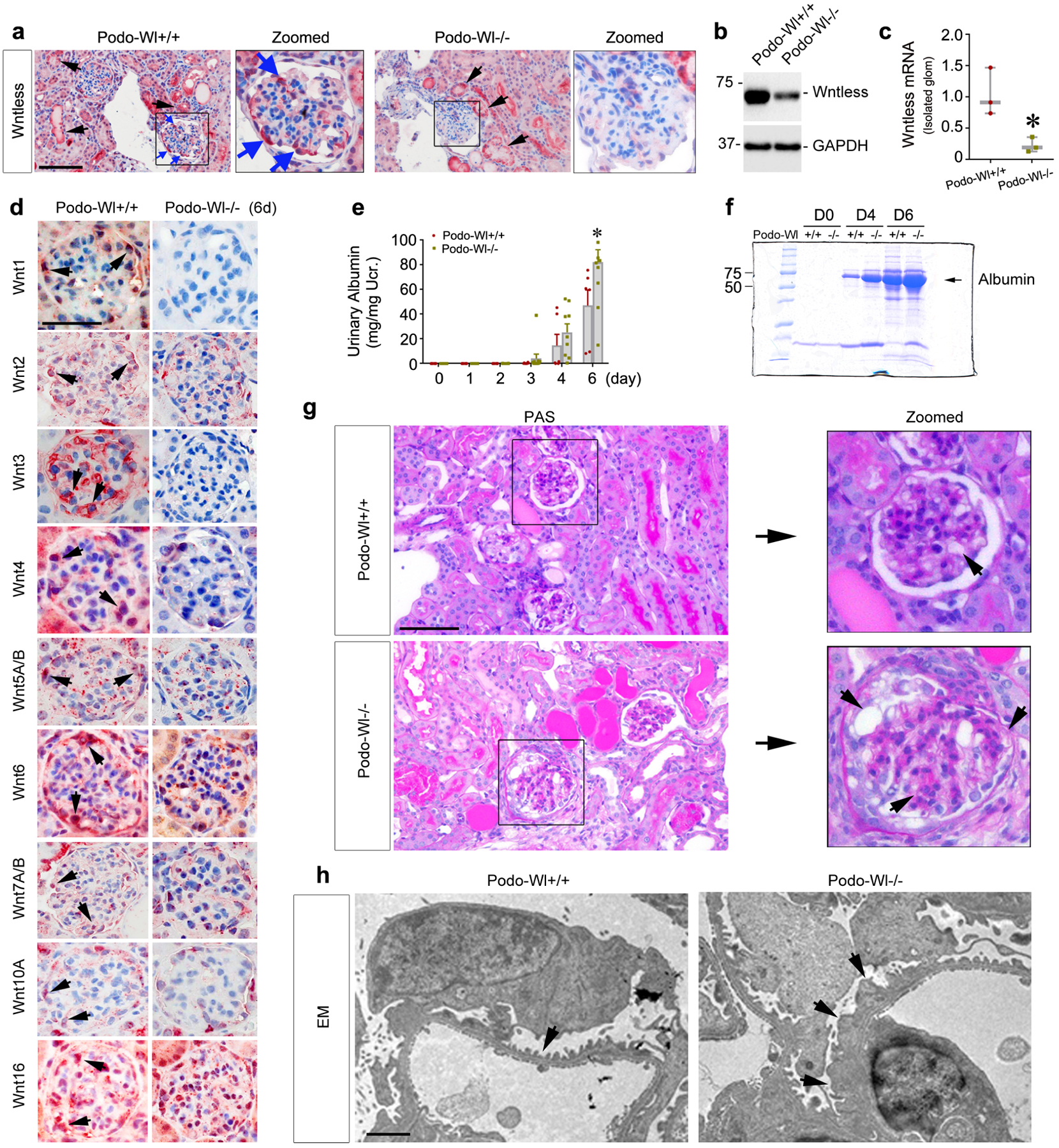
Mice with podocytes-specific ablation of Wntless are more susceptible to glomerular injury induced by Adriamycin (ADR). Podo-Wl+/+ and Podo-Wl−/− mice were treated with ADR and euthanized at 6 days after injection. (a) Immunohistochemical staining showed Wl expression in Podo-Wl+/+ and Podo-Wl−/− mice diseased kidneys. Blue arrows indicate positive staining in podocytes. Black arrows indicate positive staining in tubules. Scale bar, 50 μm. (b) Western blot analyses revealed kidney protein levels of Wl in Podo-Wl+/+ and Podo-Wl−/− mice whole kidney lysates. (c) qPCR analyses of Wl mRNA in isolated glomeruli from Podo-Wl+/+ and Podo-Wl−/− mice. *P < 0.05 (n = 3). (d) Immunohistochemical staining demonstrated multiple Wnts including Wnt1, 2, 3, 4, 5A/B, 6, 7A/B, 10A, and 16 were reduced in Podo-Wl−/− mice diseased glomeruli, compared to Podo-Wl+/+ mice. Arrows indicate positive staining. Scale bar, 25 μm. (e) Urinary albumin levels were increased in Podo-Wl−/− mice, compared with Podo-Wl+/+ mice. *P < 0.05 (n = 6–11). (f) Representative SDS–polyacrylamide gel electrophoresis showed the urinary proteins after normalization to urinary creatinine in Podo-Wl+/+ and Podo-Wl−/− mice as indicated at day (D) 0, 4, and 6. Urine proteins after separation were stained with Coomassie blue R-250. (g) Periodic acid–Schiff (PAS) staining showed kidney histologic changes in Podo-Wl+/+ and Podo-Wl−/− mice at 6 days after ADR. Boxed areas are enlarged. Arrows indicate damaged area. Scale bar, 50 μm. (h) Electron microscopy (EM) shows the ultrastructure of glomerular filtration apparatuses in Podo-Wl+/+ and Podo-Wl−/− kidneys. Representative EM images demonstrated podocyte foot process effacement and altered glomerular basement membrane after ADR injection. Scale bar, 1 μm.
To study the long-term consequence of podocyte-specific deletion of Wl in glomerular injury, we investigated the pathological changes at 3 weeks after ADR injection in Podo-Wl+/+ and Podo-Wl−/− mice (Figure 3a). First, qPCR analysis confirmed Wl mRNA levels were largely abolished in the glomeruli isolated from Podo-Wl−/− kidney, as shown in Supplementary Figure S3a. We then assessed urinary albumin levels in these mice. As presented in Figure 3b and 3c, Podo-Wl−/− mice exhibited worsened albuminuria, compared with Podo-Wl+/+ controls. Of note, because animals have completely different responses to ADR, albuminuria levels vary among mice, whether at 6 days or 3 weeks after injection. We then examined the expression of podocyte-specific markers in Podo-Wl−/− and Podo-Wl+/+ kidneys at both 6 days and 3 weeks after ADR injection. As shown in Figure 3d and 3e, qPCR analyses demonstrated that loss of Wl in podocytes inhibited renal mRNA expression of nephrin, podocin, podocalyxin, and WT1 at 6 days after ADR injection, compared to Podo-Wl+/+ controls. However, at 3 weeks, only podocin mRNA levels exhibited significant differences between the two groups. Western blot analyses confirmed the reductions of nephrin in Podo-Wl−/− kidneys both at 6 days and 3 weeks, compared with Podo-Wl+/+ controls (Figure 3f–3i). Interestingly, little differences in α-actinin 4 expression was observed between the two groups at 6 days but α-actinin 4 was markedly reduced in Podo-Wl−/− mice at 3 weeks after ADR injection (Figure 3f–3i).
Figure 3.
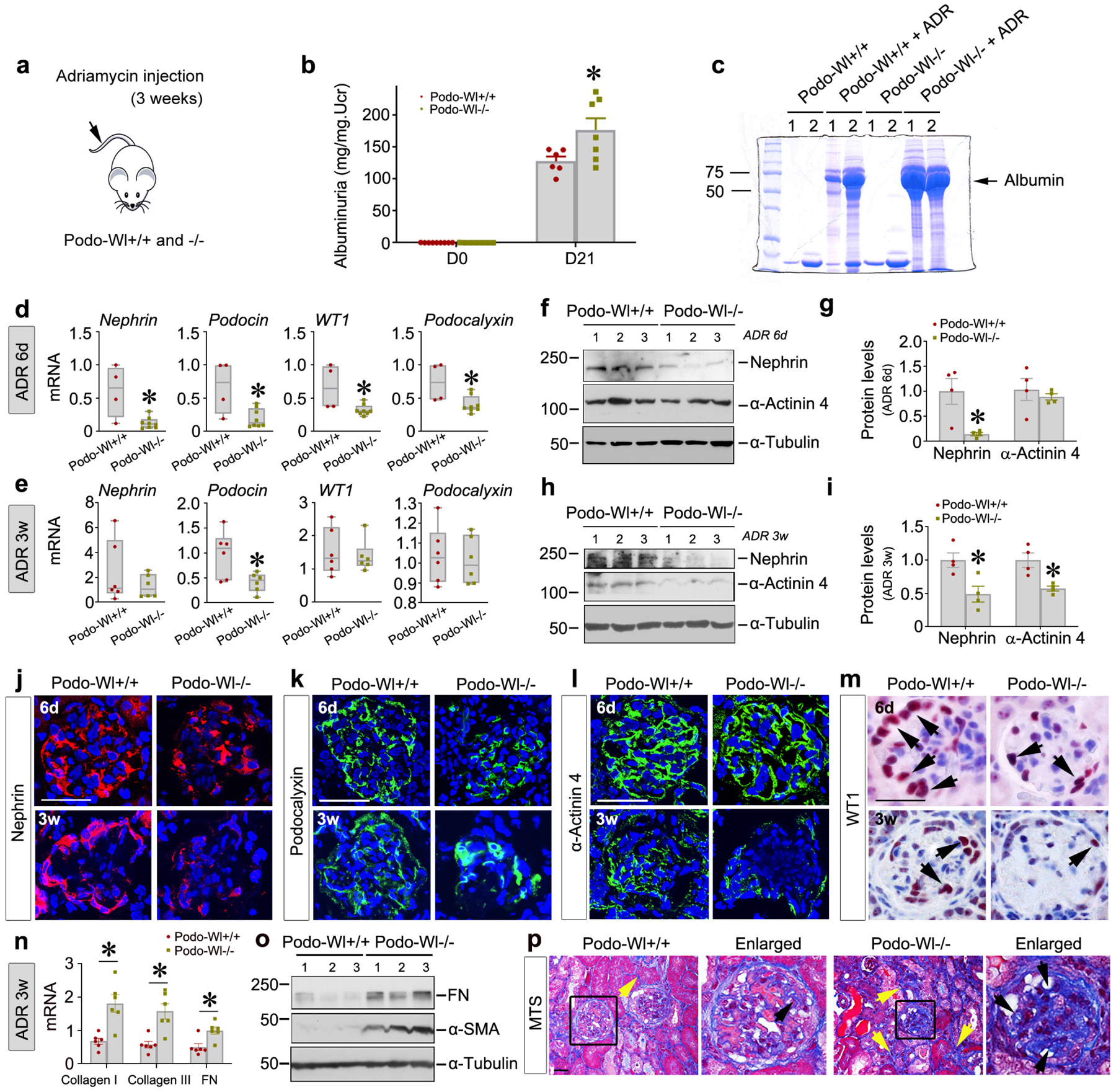
Blockade of Wnt secretion aggravates podocytes injury and glomerulosclerosis induced by Adriamycin. (a) Schematic diagram. (b) Urinary albumin levels were increased in Podo-Wl−/− mice, compared with Podo-Wl+/+ controls, at day (D) 21 after ADR injection. *P < 0.05 (n = 6–7). (c) Representative Coomassie-stained SDS–polyacrylamide gels showing urinary proteins after normalization to urinary creatinine in Podo-Wl+/+ and Podo-Wl−/− mice as indicated. (d-e) qPCR analyses showed renal mRNA expression of podocyte-specific markers nephrin, podocin, WT1, and podocalyxin in Podo-Wl+/+ and Podo-Wl−/− mice at 6 days (d) and 3 weeks (e) after ADR. *P < 0.05 (n = 4–8 for 6 days, n=6–7 for 3 weeks). (f-i) Western blot analyses showed renal protein levels of nephrin and α-actinin 4 in Podo-Wl+/+ and Podo-Wl−/− mice at 6 days and 3 weeks after ADR injection. Representative western blot (f, h) and quantitative data (g, i) are shown. *P < 0.05 (n = 4). (j-m) Representative micrographs show immunostaining of nephrin (j), podocalyxin (k), α-actinin 4 (l), and WT1 (m) in Podo-Wl+/+ and Podo-Wl−/− kidneys at 6 days and 3 weeks after ADR. Scale bar, 25 μm. (n) qPCR revealed increased mRNA expression of collagen I, collagen III, and fibronectin (FN) in Podo-Wl−/− mice, compared with Podo-Wl+/+ mice at 3 weeks after ADR. *P < 0.05 (n = 6). (o) Western blot analyses showed renal FN and α-SMA levels in Podo-Wl+/+ and Podo-Wl−/− mice at weeks after ADR. (p) Masson’s trichrome staining (MTS) showed collagen deposition in Podo-Wl+/+ and Podo-Wl−/− kidneys at 3 weeks after ADR. Scale bar, 25 μm. Yellow arrows indicate collagen deposited in interstitial compartment. Black arrows indicate collagen deposited in glomeruli.
Consistently, immunofluorescence staining gave similar results (Figure 3j–l and Supplementary Figure S2f). Immunohistochemical staining revealed a marked reduction of WT1-positive cells in Podo-Wl−/− glomeruli, compared with Podo-Wl+/+ controls (Figure 3m and Supplementary Figure S2h).
Blockade of Wnt secretion from podocytes accelerates glomerulosclerosis
Next, we further examined glomerulosclerosis and renal fibrosis, as they represent the common final outcomes of proteinuric kidney disease. As shown in Figure 3n, renal expression of collagen type I, collagen type III, and fibronectin mRNA was increased in Podo-Wl−/− kidneys, compared with Podo-Wl+/+ controls. Similarly, western blot analyses also showed increased expression of fibronectin and α-smooth muscle actin (α-SMA) proteins in Podo-Wl−/− kidneys, compared to Podo-Wl+/+ controls (Figure 3o and Supplementary Figure S3b). Consistently, Masson’s trichrome staining revealed an increased collagen deposition in Podo-Wl−/− glomeruli as well as tubulointerstitial compartments (Figure 3p and Supplementary Figure S3c). Furthermore, increased α-SMA or desmin-positive cells were observed in Podo-Wl−/− glomeruli, compared to Podo-Wl+/+ controls (Supplementary Figure 3d and 3e). Of particular interest, Podo-Wl−/− kidneys did not exhibit significant differences in CD45+ monocyte infiltration, TNF-α, and MCP-1 secretion, or cell apoptosis including the expression of cleaved caspase 3 and Fas-associating protein with death domain, compared to Podo-Wl+/+ mice after ADR injection (Supplementary Figure S4).
Blockade of Wnt secretion in podocytes induces β-catenin activation
To elucidate the underlying mechanism by which deletion of Wl affects podocyte injury, we first examined the expression of β-catenin. To our surprise, we found that blocking the secretion of Wnts by deleting Wl in podocytes resulted in β-catenin activation in the kidney. This is quite counter-intuitive because we speculated β-catenin activation would be decreased after blocking Wnt secretion. Marked activation of β-catenin was confirmed by western blot analyses at both 6 days and 3 weeks after ADR injection in Podo-Wl−/− kidneys, compared to Podo-Wl+/+ controls (Figure 4a–4d). Furthermore, immunohistochemical staining showed increased β-catenin expression in diseased glomeruli in Podo-Wl−/− mice at both 6 days and 3 weeks after ADR, compared to Podo-Wl+/+ controls (Figure 4e and Supplementary Figure S5a). To corroborate the activation of β-catenin signaling after podocytes-specific deletion of Wl, we examined the renal expression of two β-catenin downstream targets, angiotensin-converting enzyme 1 (ACE1), and sonic hedgehog (Shh),12,35 in Podo-Wl+/+ and Podo-Wl−/− mice. As shown in Figure 4f–4h, expression of ACE1 was induced in Podo-Wl−/− kidneys at both 6 days and 3 weeks after ADR injection. Similarly, renal expression of Shh was induced in Podo-Wl−/− kidneys, compared to Podo-Wl+/+ controls (Figure 4f, 4g, and 4i). Such induction of ACE1 and Shh proteins was mainly localized to glomerular podocytes after ADR injury (Figure 4j–4m). Furthermore, as illustrated by serial section staining (Supplementary Figure S5b), the majority of β-catenin-positive cells were WT1+ podocytes. In addition, 6 days after ADR injection, the glomerular parietal epithelial cell marker Pax8 showed little difference between Podo-Wl+/+ and Podo-Wl−/− mice (Supplementary Figure S5c–5d).
Figure 4.
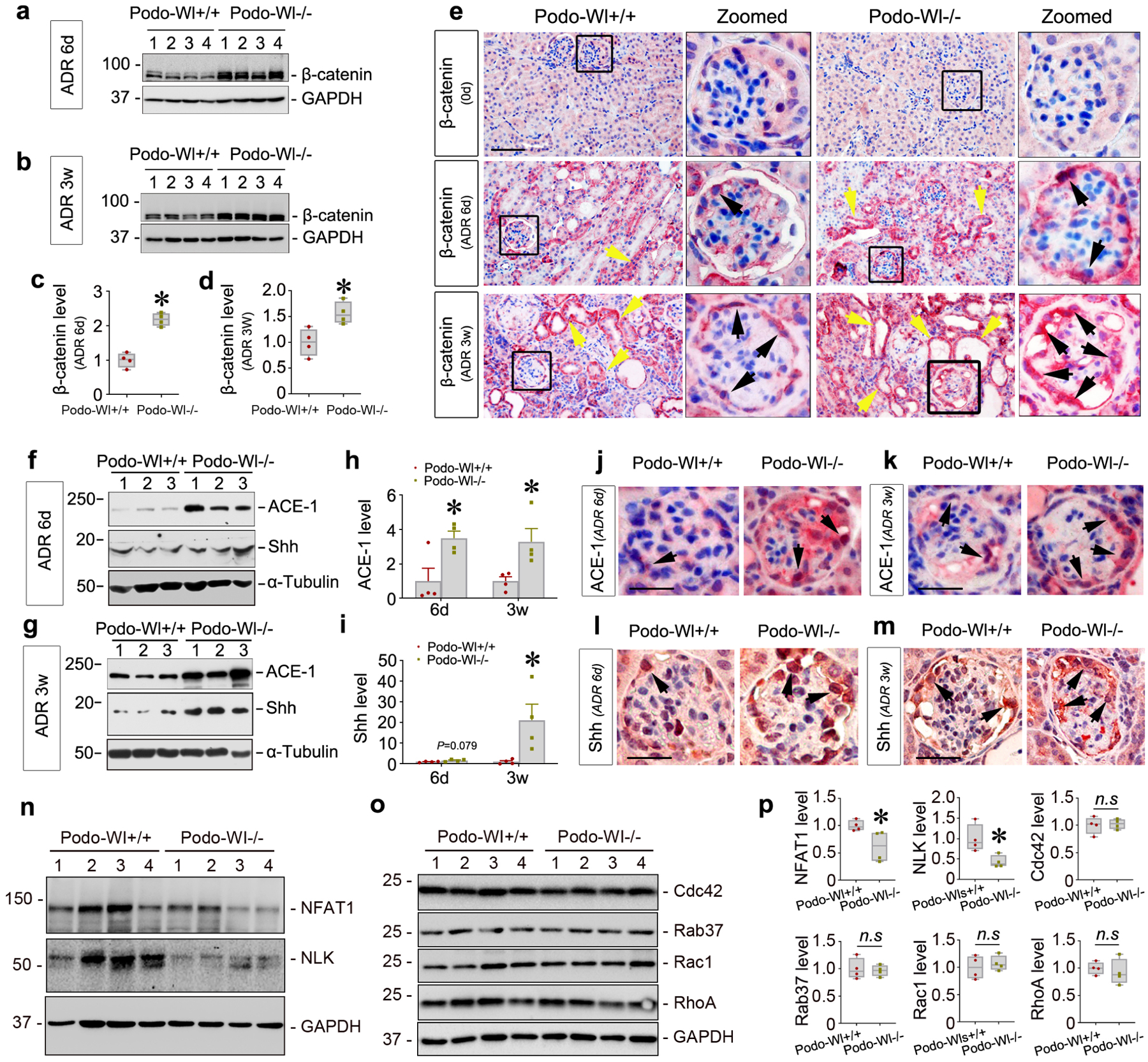
Podocytes-specific ablation of Wntless induces β-catenin activation. (a-d) Western blot analyses show renal β-catenin protein in Podo-Wl−/− mice at 6 days (6d) and 3 weeks (3w) after ADR. Representative western blot (a, b) and quantitative data on β-catenin (c, d) are presented. *P < 0.05 (n = 4). (e) Representative micrographs show β-catenin expression at day 0 (0d), 6 days, and 3 weeks after ADR injection in Podo-Wl+/+ and Podo-Wl−/− mice. Boxed areas are enlarged (Zoomed). Yellow arrows indicate positive staining in tubules. Black arrows indicate positive staining in podocytes. Scale bar, 25 μm. (f, g) Representative western blots show ACE-1 and Shh expression in Podo-Wl+/+ and Podo-Wl−/− kidneys at 6 days or 3 weeks after ADR. (h, i) Graphs of quantitative data for renal ACE-1 (h) and Shh (i) levels after ADR. *P < 0.05 (n = 4). (j, k) Representative micrographs show ACE1 expression at 6 days and 3 weeks after ADR in Podo-Wl+/+ and Podo-Wl−/− glomeruli. Arrows indicate positive staining. Scale bar, 20 μm. (l, m) Representative micrographs show Shh protein expression in Podo-Wl+/+ and Podo-Wl−/− mice diseased glomeruli after ADR at 6 days or 3 weeks. Arrows indicate positive staining. Scale bar, 20 μm. (n-p) Representative western blots (n, o) and quantitative data (p) show NFAT1, NLK, Cdc42, Rab37, Rac1, and RhoA levels in Podo-Wl+/+ and Podo-Wl−/− kidneys after ADR. *P < 0.05 (n = 4).
Blockade of Wnt secretion in podocytes represses Wnt/calcium signaling
To explore the mechanisms of β-catenin activation in Podo-Wl−/− mice kidneys after ADR injection, we examined the effect of blocking the secretion of Wnts on the Frizzled receptors. As presented in Supplementary Figure S5e, qPCR analyses demonstrated that 3 out of 10 Frizzled receptors, including Fzd1, Fzd4, and Fzd6, were repressed in Podo-Wl−/− kidneys after ADR, compared to Podo-Wl+/+ controls. Intriguingly, there were little differences of Wnt1, 2, 3, 5A/B, and 16 expression levels in whole kidney lysates between Podo-Wl+/+ and Podo-Wl−/− mice 3 weeks after ADR injection (Supplementary Figure S5f–5g). Therefore, we speculated that β-catenin activation might be due to changes in the non-canonical Wnt pathway. Indeed, after ADR injection, the expression of proteins in the non-canonical Wnt/calcium pathway including NFAT1 and NLK were substantially repressed in Podo-Wl−/− kidneys, compared to Podo-Wl+/+ controls (Figure 4n and 4p). In comparison, few differences were observed for components of the non-canonical Wnt/PCP pathway, including Cdc42, Rab37, Rac1, and RhoA between Podo-Wl+/+ and Podo−/− mice after ADR injection (Figure 4o and 4p). These results indicate that blockade of Wnt secretion from podocytes specifically inhibits non-canonical Wnt/calcium signaling.
Inhibition of Wnt non-canonical signaling plays a role in β-catenin activation
To validate our in vivo finding, we knocked down the Wl gene in cultured podocytes in vitro. As shown in Supplementary Figure S6a, under basal conditions, loss of Wl had no impact on the expression of nephrin, podocalyxin, and WT1 proteins. Once podocytes with the Wl deletion were incubated with ADR, transforming growth factor-β (TGF-β) or high glucose for 24 hours, the expression of all of the above proteins was inhibited, compared to controls (Figure 5a and Supplementary Figure S6b–S6c). The quantitative data are presented in Supplementary Figure 6d through 6g. Immunofluorescence staining showed reduced levels of podocalyxin after knockdown of Wl under ADR stress (Figure 5b).
Figure 5.
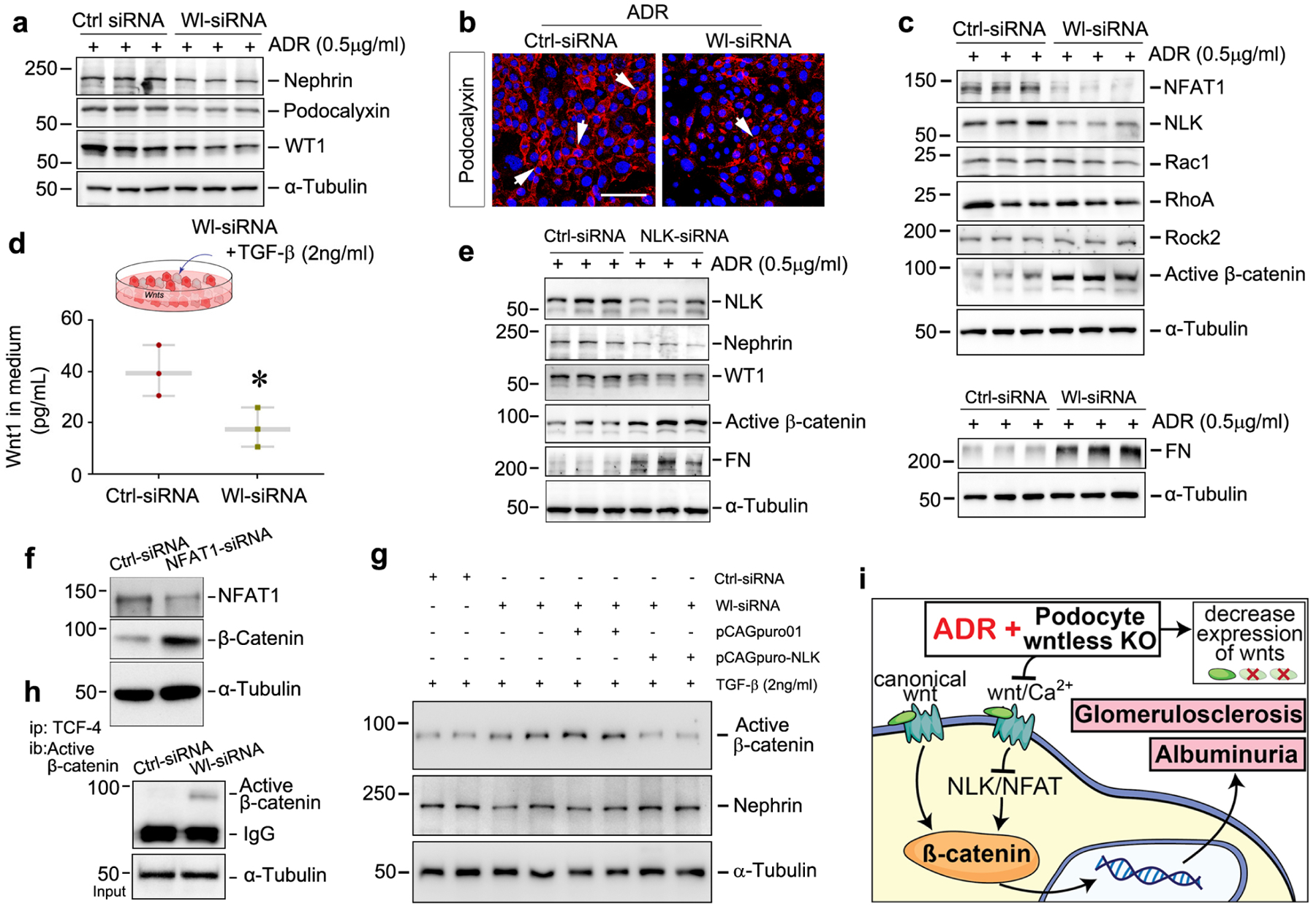
Blockade of Wnt secretion activates β-catenin via repression of non-canonical signaling. (a) Western blot analyses demonstrated knockdown of Wl (Wl-siRNA) in cultured podocytes reduced nephrin, podocalyxin, and WT1 expression under Adriamycin (ADR) stress for 24 hours, compared with control (Ctrl siRNA). (b) Immunofluorescence staining revealed diminished expression of podocalyxin in Wl-knockdown podocytes after stimulation with ADR, compared with control. Arrows indicate positive staining. Scale bar, 25 μm. (c) Blockade of Wnt secretion by knocking down Wl inhibited non-canonical Wnt signaling. Representative western blot analyses show that knockdown of Wl significantly reduced NFAT1 and NLK levels (but not Rac1, RhoA, Rab37, and ROCK2) and activated β-catenin when podocytes were presented with ADR at 0.5μg/ml, compared with control scrambled siRNA. (d) ELISA revealed Wnt1 concentration in the Wl-knockdown podocytes conditioned medium after incubation with TGF-β. (e) Western blot analyses show that knockdown of NLK repressed nephrin and WT1 and induced active β-catenin and fibronectin (FN) in cultured podocytes after incubation with ADR. (f) Western blot analyses showed that knockdown of NFAT1 induced β-catenin activation in cultured podocytes. (g) Western blot analyses demonstrated that overexpression of NLK repressed β-catenin activation and preserved nephrin expression in Wl-knockdown podocytes after incubation with TGF-β in vitro. (h) β-catenin functionally binds to TCF-4 to activate downstream gene transcription. Podocyte Wl knockdown (Wl-siRNA) was immunoprecipitated (IP) with TCF-4 antibody, followed by immunoblotting (IB) with antibody against active β-catenin. (i) Our model shows that ablation of Wl in podocytes represses noncanonical Wnt/calcium signaling, which leads to β-catenin activation, proteinuria, and glomerulosclerosis.
To elucidate the mechanisms that link Wl deficiency-induced β-catenin activation with non-canonical Wnt/calcium signaling in vivo, we performed several experiments using Wl-knockdown and regularly cultured podocytes in vitro. Western blot assays demonstrated that, under basal conditions, transfection of Wl siRNA silenced Wl expression but had no effects on the expression of multiple components of the non-canonical Wnt/calcium or Wnt/PCP signaling pathway, including NFAT1, NLK, Rac1, RhoA, Rab37, and Rock2 in cultured podocytes, compared to scrambled control siRNA (Supplementary Figure S6h). Once podocytes transfected with Wl-siRNA were incubated with ADR, as illustrated in Figure 5c, the levels of NFAT1 and NLK in the Wnt/calcium pathway were substantially reduced, but not the proteins in the Wnt/PCP pathway. As a consequence, the accumulation of fibronectin was increased in the cultured Wl-knockdown podocytes. We also found that β-catenin was simultaneously activated in podocytes transfected with Wl-siRNA, compared to scrambled control. These findings were further validated in TGF-β and high glucose models in vitro (Supplementary Figure S6i–6j). The quantitative data are presented in Supplementary Figure S6k through S6n. Immunofluorescence staining confirmed the enhanced β-catenin nuclear translocation in Wl-knockdown podocytes under TGF-β or high glucose stress (Supplementary Figure S6o). Of note, ELISA results revealed that Wl knockdown did block Wnt1 secretion under TGF-β stress, compared to the scramble controls (Figure 5d). However, exogenous Wnt1 ligand remained able to activate β-catenin after knockdown of Wl in vitro (Supplementary Figure S6p–6q). These data suggest that the blockade of Wnt secretion by knocking down Wl expression is capable of concomitantly repressing Wnt/calcium signaling and activating β-catenin in podocytes.
To study whether β-catenin activation after Wl deletion is linked to inhibition of the non-canonical Wnt/calcium signaling, we assessed β-catenin expression in cultured podocytes after knockdown of NLK and NFAT1, respectively. As shown in Figure 5e and Supplementary Figure S7a–7c, active-β-catenin protein was markedly induced in podocytes when the cells were incubated with ADR, TGF-β or high glucose after transfection with NLK-specific siRNA, compared to scrambled siRNA. Additionally, nephrin, WT1, and podocalyxin levels were diminished while fibronectin was increased in the NLK-knockdown podocytes. Similarly, knockdown of NFAT1 also induced β-catenin in cultured podocytes (Figure 5f and Supplementary Figure S7d). Conversely, overexpression of NLK in Wl-knockdown or regularly cultured podocytes significantly repressed β-catenin activation and largely preserved nephrin in vitro (Figure 5g and Supplementary Figure S7e–7g). These results suggest that loss of NLK or NFAT1 is associated with β-catenin activation in podocytes. Furthermore, immunoprecipitation confirmed that knockdown of Wl promoted β-catenin binding to TCF-4 (Figure 5h). Notably, knockdown of Wl or NLK had no effects on cell apoptosis of cultured podocytes (Supplementary Figure S8). Collectively, our data suggest that the non-canonical Wnt/calcium signaling plays a predominant role in regulating β-catenin activation in podocytes.
DISCUSSION
Dysregulation of Wnt/β-catenin signaling has been shown to play a critical role in mediating podocyte injury and proteinuria in glomerular diseases.5,36–38 In this study, we investigated the role of podocyte-derived Wnts in the pathogenesis of proteinuric kidney disease by creating mice with podocyte-specific ablation of Wl, a protein required for the secretion of all Wnt ligands23. Surprisingly, we found that blocking the secretion of Wnts from podocytes activates β-catenin and aggravates podocyte injury, proteinuria, and glomerulosclerosis after ADR insult. This counter-intuitive outcome was accompanied by the inhibition of NLK and NFAT1, the key intermediate mediators of non-canonical Wnt/calcium signaling.19 In vitro, knockdown of either Wl, NLK, or NFAT1 induces β-catenin activation in podocytes, while overexpression of NLK exhibited enhanced capacity to preserve nephrin. These studies uncover an important role for non-canonical Wnt/calcium signaling in protecting podocytes against injury possibly through constraining β-catenin activation. Our findings also provide insights into differential effects and unique mechanisms of canonical versus non-canonical Wnt signaling in podocyte biology.
Wnt/β-catenin has emerged as a key signal cascade in regulating kidney development, injury repair and regeneration, tissue remodeling, and fibrosis.8,15 This signaling is generally quiescent in adult kidneys, which could account for the observation in the present study that blockade of Wnt secretion via genetic ablation of Wl in Podo-Wl−/− mice do not exhibit any abnormality under normal conditions (Figure 1). Consistently, earlier studies indicated that inhibition of Wnt secretion from tubular epithelial cells or interstitial fibroblasts also has no influence on kidney structure and function.31 Similarly, knockout of β-catenin in glomerular podocytes, tubular epithelial cells, or interstitial fibroblasts also does not cause any histologic lesions or kidney dysfunction in normal physiologic settings.6,39 Taken together, all these studies illustrate that, despite the crucial role of Wnt/β-catenin in nephron formation, this signaling is commonly dispensable in maintaining the structural and functional integrity of adult kidneys.
One of the interesting and unexpected findings in the present study is that blockade of Wnt secretion by podocyte-specific deletion of the Wl gene causes β-catenin activation, and aggravates podocyte damage, proteinuria, and glomerulosclerosis after ADR injury. In theory, blockade of Wnt secretion in Podo-Wl−/− mice should lead to an eradication of the autocrine action of Wnt signaling in podocytes and cause less β-catenin activation, because Wl is obligatory for the secretion of all Wnt ligands. However, we observed an opposite result in which loss of Wl in podocytes activates β-catenin signaling and induces the expression of its downstream target genes. Notably, such activation of β-catenin was associated with the repression of non-canonical Wnt/calcium signaling, as reflected by the inactivation of NFAT1 and NLK. These findings led us to speculate an intricate connection between β-catenin activation and repression of non-canonical Wnt signaling, as earlier reports indicate that there is a mutual antagonism between canonical and non-canonical Wnt signaling.21,22,40 Indeed, knockdown of Wl in podocytes in vitro induces β-catenin and concomitantly represses NFAT1 and NLK, the readouts of the Wnt/calcium pathway (Figure 5).18 Furthermore, knockdown of NFAT1 or NLK alone activates β-catenin in podocytes, which directly confirms a causative role of depressed NFAT1 or NLK in triggering β-catenin activation.
Inhibition of Wnt secretion from podocytes by disrupting the Wl gene also aggravated glomerulosclerosis in an advanced stage of kidney injury after ADR injection (Figure 3), in addition to worsening podocytes injury and proteinuria. Such increased glomerulosclerosis in Podo-Wl−/− mice is most likely caused by enhanced β-catenin activation in podocytes as well. Extensive studies have shown that activation of β-catenin in podocytes represses WT1 and induces Snail1, matrix metalloproteinase-7 (MMP-7), transient receptor potential cation channel 6 (TRPC6), renin-angiotensin system (RAS) components, and Shh.5,12,35 These actions of β-catenin not only directly impair podocyte integrity but also trigger mesangial activation leading to matrix overproduction and glomerulosclerosis, as well as tubulointerstitial lesions. In this regard, RAS activation as manifested by ACE1 induction would have broad deleterious effects not only on podocytes but also on mesangial cells, as well as on tubular cells and interstitial fibroblasts.12,41 Recent studies also indicate that although podocytes-originated Shh, as a secreted factor, does not impair podocytes per se, it specifically activates mesangial cells to produce excessive amounts of extracellular matrix.35 Therefore, it is conceivable that Shh induced in the Podo-Wl−/− kidney could play a key role in linking podocytes injury to mesangial activation and glomerulosclerosis. Our current study has not drawn a complete picture to reflect how the loss of Wnts in podocytes influences various cellular components in glomeruli or other compartments of the kidney. This deserves to be fully explored in the future.
The present study has some limitations. For example, we only characterized the podo-Wl−/− mice at 8–10 weeks of age under physiological conditions. It is possible that specific deletion of Wl in podocytes could cause phenotypes at later time points when mice age. In addition, in both 6-day and 3-week models, the albuminuria levels caused by ADR vary among mice, therefore, more mice or a more reliable model would strengthen our findings. Furthermore, we isolated glomeruli from frozen kidneys to confirm the efficiency of Wl knockout at the level of gene expression. Collecting sufficient freshly isolated glomeruli may be needed to fully assess glomerular injury and lesions based on protein levels, instead of using whole kidney lysates.
In summary, by generating conditional knockout mice in which Wl is selectively ablated in podocytes, we demonstrated that blockade of Wnt secretion from podocytes leads to decreased Wnt/calcium signaling. This caused β-catenin activation, leading to worsened ADR-induced podocytes injury, proteinuria, and glomerulosclerosis. These results highlight a protective role for non-canonical Wnt/calcium signaling in the pathogenesis of proteinuric kidney diseases. Future studies are warranted to develop strategies to boost Wnt/calcium signaling for the treatment of proteinuric kidney diseases.
Supplementary Material
Table S1. Nucleotide sequences of the primers used for real-time polymerase chain reaction.
Table S2. Details for applied primary antibodies.
Supplementary Figure S1. Blockade of Wnt secretion from podocytes does not affect glomerular structure and integrity under normal physiological conditions. In the normal Podo-Wl+/+ and Podo-Wl−/− mice, (a) qRT-PCR revealed Cre recombinase expression in the kidneys (n=6). * P < 0.05. (b) Western blot assay demonstrated no differences in Wnt1, Wnt3a, Wnt5A/B, Wnt16, and β-catenin proteins expression between Podo-Wl+/+ and Podo-Wl−/− mice (n = 7). (c) Quantitative data. (d, e) Representative micrographs showed renal glomerular histology (d) (Periodic acid–Schiff [PAS] staining) and protein expression of Wntless (e) in Podo-Wl+/+ and Podo-Wl−/− kidneys. Four mice were assessed (n = 4) with similar results. Scale bar, 25 μm. (f-h) The quantitative data of nephrin (f) and α-Actinin 4 (g) % area, and WT1+ cell number (h) in glomeruli (n=4). In each mouse, 5 glomeruli were randomly selected. (i) Immunofluorescence staining showed PDGFR-β expression in glomeruli. Scale bar, 25 μm. (j) Transmission and scanning electron microscopy (EM) showed the ultrastructure of glomeruli in Podo-Wl+/+ and Podo-Wl−/− kidneys. Scale bar in TEM, 0.5 μm. Scale bar in SEM, 5 μm.
Supplementary Figure S2. Podocyte-specific deletion of Wntless affects glomerular expression of Wnt ligands and glomerular injury after ADR nephropathy. At 6 days after ADR injection in Podo-Wl+/+ and Podo-Wl−/− mice, (a) Quantitative data for Wl expression in glomeruli. * P < 0.05 (n=4, 5 glomeruli were randomly selected in each kidney). (b) Quantitative data for Wl protein expression. * P < 0.05 (n=3). (c) Representative images showed glomeruli isolation by Laser-capture microdissection approach. G, glomeruli. (d) Area (%) of positive staining of Wnt1, Wnt2, Wnt3, Wnt4, Wnt5a/b, Wnt6, Wnt7a/b, Wnt10a, and Wnt16 in the diseased glomeruli. * P < 0.05, (n=4). (e) Kidney injury score. * P < 0.05, (n=4). (f) H&E staining showed kidney histological changes in Podo-Wl+/+ and Podo-Wl−/− mice at 6 days after ADR injection. Boxes indicate “Zoomed” area. Scale bar, 50 μm. (g) Immunofluorescence staining showed no differences of CD31 expression in diseased glomeruli between Podo-Wl+/+ and Podo-Wl−/− mice. Scale bar, 25 μm. (h) Area (%) of positive staining of nephrin, podocalyxin, α-Actinin 4, and WT1in the diseased glomeruli after ADR injection at 6 days (6d) or 3 weeks (3w). * P < 0.05, (n=4, 5 glomeruli/mouse). ADR, Adriamycin.
Supplementary Figure S3. Loss of Wntless in podocytes accelerates glomerulosclerosis in ADR nephropathy. At 3 weeks after ADR injection, (a) qPCR revealed Wntless mRNA levels in isolated glomeruli. * P < 0.05 (n = 3). (b) Quantitative data of renal FN and α-SMA levels in Podo-Wl+/+ and Podo-Wl−/− mice. * P < 0.05 (n = 4). (c) Quantitative data for Masson’s Trichrome staining. * P < 0.05 (n = 4, 5 tubulointerstitium sections or glomeruli /mouse). (d) Representative micrographs show α-SMA and desmin expression in Podo-Wl+/+ and Podo-Wl−/− kidneys. Scale bar, 25μm. (e) Quantitative data of α-SMA and desmin expression. * P < 0.05 (n = 4, 5 glomeruli/mouse). Glom, glomeruli.
Supplementary Figure S4. Loss of Wntless in podocytes has little effect on inflammatory cell infiltration and podocyte apoptosis after ADR nephropathy. (a) Immunohistochemical staining showed CD45 expression in Podo-Wl+/+ and Podo-Wl−/− mice kidneys at 6 days (6d) or 3 weeks (3w) after ADR. Black arrows indicate positive staining in interstitium. Blue arrows indicate positive staining in the glomeruli. Scale bar, 25 μm. (b,c) qPCR analyses revealed TNF-α and MCP1expression in the kidneys of Podo-Wl+/+ and Podo-Wl−/− mice after ADR at 3 weeks (n=6; n.s=not significant). (d) Immunofluorescence staining showed cleaved caspase 3 expression in Podo-Wl+/+ and Podo-Wl−/− mice diseased glomeruli after ADR at 6 days. Scale bar, 25 μm. (e) Western blot analyses demonstrated FADD expression in whole kidney lysates of Podo-Wl+/+ and Podo-Wl−/− mice after ADR (n=4). TNF-α, tumor necrosis factor-1; MCP-1, monocyte chemoattractant protein-1; FADD, Fas-associated death domain.
Supplementary Figure S5. Loss of Wntless in podocytes induces β-catenin but not Wnt members in podocytes and the whole kidneys after ADR nephropathy. (a) The quantitative data for kidney β-catenin staining at 6 days (6d) or 3 weeks (3w) in the glomeruli and tubulointerstitial compartment after ADR injection. *P < 0.05, n=4. (b) Kidney serial sections were stained for WT1 and β-catenin after ADR injection at 3 weeks. Black arrows indicated WT1+/β-catenin+ cells. Scale bar, 25 μm. (c) The expression of the marker for parietal epithelial cells, Pax8, after ADR injection at 6 days or 3 weeks. Black arrows indicated Pax8+ cells. Scale bar, 25 μm. (d) Quantitative data of Pax8 expression. (e) qPCR analyses show frizzled receptor levels in Podo-Wl+/+ and Podo-Wl−/− mice kidneys after ADR at 6 days (n=4–8). (f, g) Western blot analyses demonstrated Wnt1, Wnt2, Wnt3, Wnt5A/B, and Wnt16 expression in whole kidney lysates of Podo-Wl+/+ and Podo-Wl−/− mice after ADR injection at 3 weeks (n=4). Representative band (f) and the quantitative data (g) are presented. *P < 0.05, n.s=not significant. ADR, Adriamycin.
Supplementary Figure S6. Knockdown of Wntless damages podocytes by influencing non-canonical Wnt/Ca2+ signaling pathway in vitro. (a) Western blot assay demonstrated the expression of Wntless, neprhin, podocalyxin, and WT1 in Wl-knockdown podocytes (Wl-siRNA) without treatment, compared to controls (Ctrl-siRNA). (b, c) Western blot assay demonstrated the expression of neprhin, WT1, and podocalyxin in Wl-knockdown podocytes under TGF-β (b) or high glucose (HG) (c) stress. (d-g) Quantitative data. (h-j) Western blot assay demonstrated the levels of NFAT1, NLK, Rac1, RhoA, Rab37, Rock2, and active β-catenin proteins in cultured podocytes without treatment (h) or incubated with TGF-β (i) or HG (j) after knockdown of Wl, compared to controls. (k-n) Quantitative data. (o) Immunofluorescence staining revealed β-catenin nuclear translocation in Wl-knockdown podocytes under baseline or TGF-β or HG stress. Arrows indicate positive staining. Scale bar, 25 μm. (p) Western blot assay demonstrated that β-catenin was activated by Wnt1 recombinant protein in the Wl-knockdown podocytes after treatment with TGF-β. (q) Quantitative data. n=3, * P < 0.05. Wl, Wntless.
Supplementary Figure S7. Knockdown of NLK aggravates podocytes injury and accelerates extracellular matrix accumulation in vitro. (a, b) Western blot analyses showed that knockdown of NLK induced active β-catenin and fibronectin while reduced nephrin, podocalyxin, or WT1 in cultured podocytes under TGF-β (a) or high glucose (HG) (b) stress. (c) Quantitative data for NLK, active β-catenin, fibronectin, nephrin, podocalyxin, or WT1 proteins in cultured podocytes incubated with ADR, TGF-β, or high glucose after NLK knockdown (NLK-siRNA), compared with controls (Ctrl-siRNA). (d) Quantitative data of NFAT1 and β-catenin expression after knockdown of NFAT1 in cultured podocytes. (e) Quantitative data for active-β-catenin and nephrin levels after overexpression of NLK in Wl-knockdown podocytes under TGF-β stress. (f, g) Western blot assay demonstrated the expression of active β-catenin and nephrin in NLK-overexpressed podocytes after TGF-β stimulation (f) and quantitative data are presented in (g). n=3, * P < 0.05.
Supplementary Figure S8. Loss of Wntless in podocytes has little effect on podocyte apoptosis in vitro. In cultured podocytes, knockdown of Wntless (a-d) or NLK (e-g) has no effects on cell apoptosis-related protein expression including Bcl-2 and Bax no matter whether podocytes were treated with TGF-β, HG, ADR or not (untreated). The quantitative data are presented (h) (n=3). HG, high glucose; ADR, Adriamycin.
Translational Statement.
Activation of β-catenin-dependent, canonical Wnt signaling is implicated in podocyte injury and proteinuria in glomerular diseases. However, whether Wnt ligands derived from podocytes are required in this process is unknown. The present study tackles this question by creating conditional knockout mice in which Wnt secretion is blocked specifically from podocytes. Surprisingly, blockade of Wnt secretion aggravates podocyte damage and proteinuria by inducing β-catenin activation. Such effects are mediated by the inhibition of non-canonical Wnt/calcium signaling. These findings uncover a protective role for non-canonical Wnt signaling in podocytes injury. Therefore, strategies to boost non-canonical Wnt signaling may be conceivable in developing therapeutics against proteinuric kidney diseases.
ACKNOWLEDGEMENT
We are grateful to the Center for Biologic Imaging at the University of Pittsburgh for the use of their core facilities. This work was supported by the National Institutes of Health (NIH) grants DK064005, DK106049 (to Liu Y), DK116816, and DK128529 (to Zhou D).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
All the authors declared no competing interests.
SUPPLEMENTARY MATERIAL
Supplementary File (PDF)
REFERENCES
- 1.Tan RJ, et al. Tubular injury triggers podocyte dysfunction by beta-catenin-driven release of MMP-7. JCI Insight 4; e122399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greka A & Mundel P Cell biology and pathology of podocytes. Annu Rev Physiol 74, 299–323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torban E, et al. From podocyte biology to novel cures for glomerular disease. Kidney Int 96, 850–861 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Assady S, Wanner N, Skorecki KL & Huber TB New Insights into Podocyte Biology in Glomerular Health and Disease. J Am Soc Nephrol 28, 1707–1715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L & Liu Y Wnt/beta-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol 11, 535–545 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai C, et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20, 1997–2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, et al. Wnt/beta-catenin links oxidative stress to podocyte injury and proteinuria. Kidney Int 95, 830–845 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou D, Tan RJ, Fu H & Liu Y Wnt/beta-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest 96, 156–167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinhart Z & Angers S Wnt signaling in development and tissue homeostasis. Development 145(2018). [DOI] [PubMed] [Google Scholar]
- 10.Galli LM, Zebarjadi N, Li L, Lingappa VR & Burrus LW Divergent effects of Porcupine and Wntless on WNT1 trafficking, secretion, and signaling. Exp Cell Res 347, 171–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angers S & Moon RT Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10, 468–477 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/beta-catenin signaling. J Am Soc Nephrol 26, 107–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusse R & Clevers H Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169, 985–999 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Clevers H & Nusse R Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Zuo Y & Liu Y New insights into the role and mechanism of Wnt/beta-catenin signalling in kidney fibrosis. Nephrology (Carlton) 23 Suppl 4, 38–43 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Alfred V & Vaccari T Mechanisms of Non-canonical Signaling in Health and Disease: Diversity to Take Therapy up a Notch? Adv Exp Med Biol 1066, 187–204 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Corda G & Sala A Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis 6, e364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae WJ & Bothwell ALM Canonical and Non-Canonical Wnt Signaling in Immune Cells. Trends Immunol 39, 830–847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromigue O, Hay E, Barbara A & Marie PJ Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. J Biol Chem 285, 25251–25258 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De A Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai) 43, 745–756 (2011). [DOI] [PubMed] [Google Scholar]
- 21.McCoy KE, Zhou X & Vize PD Non-canonical wnt signals antagonize and canonical wnt signals promote cell proliferation in early kidney development. Dev Dyn 240, 1558–1566 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuzugullu H, et al. Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer 8, 90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das S, Yu S, Sakamori R, Stypulkowski E & Gao N Wntless in Wnt secretion: molecular, cellular and genetic aspects. Front Biol (Beijing) 7, 587–593 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J, Morse M, Frey C, Petko J & Levenson R Expression of GPR177 (Wntless/Evi/Sprinter), a highly conserved Wnt-transport protein, in rat tissues, zebrafish embryos, and cultured human cells. Dev Dyn 239, 2426–2434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franch-Marro X, et al. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 10, 170–177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banziger C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Zhong Z, et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A 109, E2197–2204 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenter AC, Rao S, Wells JM, Campbell K & Lang RA Generation of mice with a conditional null allele for Wntless. Genesis 48, 554–558 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irvine KM, et al. Deletion of Wntless in myeloid cells exacerbates liver fibrosis and the ductular reaction in chronic liver injury. Fibrogenesis Tissue Repair 8, 19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornett B, et al. Wntless is required for peripheral lung differentiation and pulmonary vascular development. Dev Biol 379, 38–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou D, et al. Tubule-Derived Wnts Are Required for Fibroblast Activation and Kidney Fibrosis. J Am Soc Nephrol 28, 2322–2336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan RJ, et al. Tubular injury triggers podocyte dysfunction by beta-catenin-driven release of MMP-7. JCI Insight 4, e122399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D, et al. Early activation of fibroblasts is required for kidney repair and regeneration after injury. FASEB J 33, 12576–12587 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int (2019). [DOI] [PubMed] [Google Scholar]
- 35.Zhou D, et al. Sonic hedgehog connects podocyte injury to mesangial activation and glomerulosclerosis. JCI Insight 4, e130515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li SY, et al. Four-and-a-Half LIM Domains Protein 2 Is a Coactivator of Wnt Signaling in Diabetic Kidney Disease. J Am Soc Nephrol 26, 3072–3084 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons M & Huber TB Old friends form alliance against podocytes. Kidney Int 80, 1117–1119 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Kato H, et al. Wnt/beta-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem 286, 26003–26015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou D, et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int 82, 537–547 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzotta S, et al. Distinctive Roles of Canonical and Noncanonical Wnt Signaling in Human Embryonic Cardiomyocyte Development. Stem Cell Reports 7, 764–776 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang T & Xu C Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J Am Soc Nephrol 28, 1040–1049 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Nucleotide sequences of the primers used for real-time polymerase chain reaction.
Table S2. Details for applied primary antibodies.
Supplementary Figure S1. Blockade of Wnt secretion from podocytes does not affect glomerular structure and integrity under normal physiological conditions. In the normal Podo-Wl+/+ and Podo-Wl−/− mice, (a) qRT-PCR revealed Cre recombinase expression in the kidneys (n=6). * P < 0.05. (b) Western blot assay demonstrated no differences in Wnt1, Wnt3a, Wnt5A/B, Wnt16, and β-catenin proteins expression between Podo-Wl+/+ and Podo-Wl−/− mice (n = 7). (c) Quantitative data. (d, e) Representative micrographs showed renal glomerular histology (d) (Periodic acid–Schiff [PAS] staining) and protein expression of Wntless (e) in Podo-Wl+/+ and Podo-Wl−/− kidneys. Four mice were assessed (n = 4) with similar results. Scale bar, 25 μm. (f-h) The quantitative data of nephrin (f) and α-Actinin 4 (g) % area, and WT1+ cell number (h) in glomeruli (n=4). In each mouse, 5 glomeruli were randomly selected. (i) Immunofluorescence staining showed PDGFR-β expression in glomeruli. Scale bar, 25 μm. (j) Transmission and scanning electron microscopy (EM) showed the ultrastructure of glomeruli in Podo-Wl+/+ and Podo-Wl−/− kidneys. Scale bar in TEM, 0.5 μm. Scale bar in SEM, 5 μm.
Supplementary Figure S2. Podocyte-specific deletion of Wntless affects glomerular expression of Wnt ligands and glomerular injury after ADR nephropathy. At 6 days after ADR injection in Podo-Wl+/+ and Podo-Wl−/− mice, (a) Quantitative data for Wl expression in glomeruli. * P < 0.05 (n=4, 5 glomeruli were randomly selected in each kidney). (b) Quantitative data for Wl protein expression. * P < 0.05 (n=3). (c) Representative images showed glomeruli isolation by Laser-capture microdissection approach. G, glomeruli. (d) Area (%) of positive staining of Wnt1, Wnt2, Wnt3, Wnt4, Wnt5a/b, Wnt6, Wnt7a/b, Wnt10a, and Wnt16 in the diseased glomeruli. * P < 0.05, (n=4). (e) Kidney injury score. * P < 0.05, (n=4). (f) H&E staining showed kidney histological changes in Podo-Wl+/+ and Podo-Wl−/− mice at 6 days after ADR injection. Boxes indicate “Zoomed” area. Scale bar, 50 μm. (g) Immunofluorescence staining showed no differences of CD31 expression in diseased glomeruli between Podo-Wl+/+ and Podo-Wl−/− mice. Scale bar, 25 μm. (h) Area (%) of positive staining of nephrin, podocalyxin, α-Actinin 4, and WT1in the diseased glomeruli after ADR injection at 6 days (6d) or 3 weeks (3w). * P < 0.05, (n=4, 5 glomeruli/mouse). ADR, Adriamycin.
Supplementary Figure S3. Loss of Wntless in podocytes accelerates glomerulosclerosis in ADR nephropathy. At 3 weeks after ADR injection, (a) qPCR revealed Wntless mRNA levels in isolated glomeruli. * P < 0.05 (n = 3). (b) Quantitative data of renal FN and α-SMA levels in Podo-Wl+/+ and Podo-Wl−/− mice. * P < 0.05 (n = 4). (c) Quantitative data for Masson’s Trichrome staining. * P < 0.05 (n = 4, 5 tubulointerstitium sections or glomeruli /mouse). (d) Representative micrographs show α-SMA and desmin expression in Podo-Wl+/+ and Podo-Wl−/− kidneys. Scale bar, 25μm. (e) Quantitative data of α-SMA and desmin expression. * P < 0.05 (n = 4, 5 glomeruli/mouse). Glom, glomeruli.
Supplementary Figure S4. Loss of Wntless in podocytes has little effect on inflammatory cell infiltration and podocyte apoptosis after ADR nephropathy. (a) Immunohistochemical staining showed CD45 expression in Podo-Wl+/+ and Podo-Wl−/− mice kidneys at 6 days (6d) or 3 weeks (3w) after ADR. Black arrows indicate positive staining in interstitium. Blue arrows indicate positive staining in the glomeruli. Scale bar, 25 μm. (b,c) qPCR analyses revealed TNF-α and MCP1expression in the kidneys of Podo-Wl+/+ and Podo-Wl−/− mice after ADR at 3 weeks (n=6; n.s=not significant). (d) Immunofluorescence staining showed cleaved caspase 3 expression in Podo-Wl+/+ and Podo-Wl−/− mice diseased glomeruli after ADR at 6 days. Scale bar, 25 μm. (e) Western blot analyses demonstrated FADD expression in whole kidney lysates of Podo-Wl+/+ and Podo-Wl−/− mice after ADR (n=4). TNF-α, tumor necrosis factor-1; MCP-1, monocyte chemoattractant protein-1; FADD, Fas-associated death domain.
Supplementary Figure S5. Loss of Wntless in podocytes induces β-catenin but not Wnt members in podocytes and the whole kidneys after ADR nephropathy. (a) The quantitative data for kidney β-catenin staining at 6 days (6d) or 3 weeks (3w) in the glomeruli and tubulointerstitial compartment after ADR injection. *P < 0.05, n=4. (b) Kidney serial sections were stained for WT1 and β-catenin after ADR injection at 3 weeks. Black arrows indicated WT1+/β-catenin+ cells. Scale bar, 25 μm. (c) The expression of the marker for parietal epithelial cells, Pax8, after ADR injection at 6 days or 3 weeks. Black arrows indicated Pax8+ cells. Scale bar, 25 μm. (d) Quantitative data of Pax8 expression. (e) qPCR analyses show frizzled receptor levels in Podo-Wl+/+ and Podo-Wl−/− mice kidneys after ADR at 6 days (n=4–8). (f, g) Western blot analyses demonstrated Wnt1, Wnt2, Wnt3, Wnt5A/B, and Wnt16 expression in whole kidney lysates of Podo-Wl+/+ and Podo-Wl−/− mice after ADR injection at 3 weeks (n=4). Representative band (f) and the quantitative data (g) are presented. *P < 0.05, n.s=not significant. ADR, Adriamycin.
Supplementary Figure S6. Knockdown of Wntless damages podocytes by influencing non-canonical Wnt/Ca2+ signaling pathway in vitro. (a) Western blot assay demonstrated the expression of Wntless, neprhin, podocalyxin, and WT1 in Wl-knockdown podocytes (Wl-siRNA) without treatment, compared to controls (Ctrl-siRNA). (b, c) Western blot assay demonstrated the expression of neprhin, WT1, and podocalyxin in Wl-knockdown podocytes under TGF-β (b) or high glucose (HG) (c) stress. (d-g) Quantitative data. (h-j) Western blot assay demonstrated the levels of NFAT1, NLK, Rac1, RhoA, Rab37, Rock2, and active β-catenin proteins in cultured podocytes without treatment (h) or incubated with TGF-β (i) or HG (j) after knockdown of Wl, compared to controls. (k-n) Quantitative data. (o) Immunofluorescence staining revealed β-catenin nuclear translocation in Wl-knockdown podocytes under baseline or TGF-β or HG stress. Arrows indicate positive staining. Scale bar, 25 μm. (p) Western blot assay demonstrated that β-catenin was activated by Wnt1 recombinant protein in the Wl-knockdown podocytes after treatment with TGF-β. (q) Quantitative data. n=3, * P < 0.05. Wl, Wntless.
Supplementary Figure S7. Knockdown of NLK aggravates podocytes injury and accelerates extracellular matrix accumulation in vitro. (a, b) Western blot analyses showed that knockdown of NLK induced active β-catenin and fibronectin while reduced nephrin, podocalyxin, or WT1 in cultured podocytes under TGF-β (a) or high glucose (HG) (b) stress. (c) Quantitative data for NLK, active β-catenin, fibronectin, nephrin, podocalyxin, or WT1 proteins in cultured podocytes incubated with ADR, TGF-β, or high glucose after NLK knockdown (NLK-siRNA), compared with controls (Ctrl-siRNA). (d) Quantitative data of NFAT1 and β-catenin expression after knockdown of NFAT1 in cultured podocytes. (e) Quantitative data for active-β-catenin and nephrin levels after overexpression of NLK in Wl-knockdown podocytes under TGF-β stress. (f, g) Western blot assay demonstrated the expression of active β-catenin and nephrin in NLK-overexpressed podocytes after TGF-β stimulation (f) and quantitative data are presented in (g). n=3, * P < 0.05.
Supplementary Figure S8. Loss of Wntless in podocytes has little effect on podocyte apoptosis in vitro. In cultured podocytes, knockdown of Wntless (a-d) or NLK (e-g) has no effects on cell apoptosis-related protein expression including Bcl-2 and Bax no matter whether podocytes were treated with TGF-β, HG, ADR or not (untreated). The quantitative data are presented (h) (n=3). HG, high glucose; ADR, Adriamycin.


