Abstract
Given the substantial cost and low success rate of drug discovery and development, repositioning existing drugs to treat new diseases has gained significant attention in recent years, with potentially lower development costs and shorter time frames. Natural products show great promise in drug repositioning because they have been used for various medical purposes for thousands of years. In this review, we discuss the drug repositioning of six prototypical natural products and their derivatives to reveal new drug–disease associations. We also highlight opportunities and challenges in natural product-based drug repositioning for future reference.
Keywords: drug discovery and development, natural products, drug repositioning, new drug-disease associations
Teaser:
A brief summary on repositioning natural products and their derivatives as an effective approach in drug discovery for diverse diseases.
Introduction
Drug discovery is a high-cost and time-consuming process with high failure rates. In recent years, new technologies, strategies, and approaches, such as drug repositioning, have been developed and applied to accelerate drug discovery. Drug repositioning (also called drug repurposing or drug reprofiling), first proposed by Ashburn and Thor in 2004, is a strategy involving finding new therapeutic potentials for existing drugs (approved or investigational) beyond the scope of their initial indication(s).1 This new strategy has significant advantages over conventional drug discovery, including lower investment needs,2 substantially shortened drug development timelines,3 and potentially higher success rates,4 and has been used to generate ~33% of drugs approved in recent years.5 Therefore, it represents an effective and powerful strategy for modern drug discovery campaigns.
Whereas typical drug repositioning campaigns rely upon synthetic small molecules, natural products can provide an alternative avenue. Natural products, as the source of therapeutic agents, have been used for the treatment of a broad range of diseases for thousands of years.6 The contribution of natural products is indispensable to the drug discovery process. Historically, natural products have had a crucial role in treating diseases, especially cancers and infectious diseases.7 For contemporary drug discovery and development, natural products provide a variety of lead structures and novel medications. David J. Newman and Gordon M. Cragg at the US National Institutes of Health (NIH) and their colleagues have contributed a series of comprehensive reviews summarizing natural products as sources of new drugs.8–13 According to their latest article (2020), natural products and their derivatives comprised 32% (441/1393) of all small-molecule drugs approved from January 1981 to September 2019.13 Another report from Eric Patridge and coworkers, published in 2016, showed that the US Food and Drug Administration (FDA) approved 547 natural products and their derivatives for medication purposes from 1827 to 2013.14 Natural product-based drugs have been applied in the treatment of diverse diseases, such as viral, bacterial, and fungal infections, cancers, allergies, and cardiovascular and metabolic diseases.15 Natural products have unique physicochemical properties, dramatic structural diversities, and broad pharmacological activities. Therefore, they are viewed as privileged molecular entities for drug discovery campaigns, highlighting novel potential therapeutic utilities outside of their original biological space.16
Natural products confer remarkable advantages over conventional synthetic molecules. They are endowed with rich structural diversity and complexity, providing a wider biologically relevant chemical space. They also have various advantageous characteristics, such as a higher molecular weight (> 500 Da), more sp3 hybridized carbon atoms and oxygen atoms but fewer nitrogen and halogen atoms, more hydrogen bond acceptors and donors (HBA and HBD), lower cLogP metrics, and higher molecular rigidity compared with synthetic chemical entities.17,18. For example, the increased saturation determined by the number of sp3 carbon atoms/total carbon count (Fsp3) within a molecule might lead to improved solubility. In addition, the greater rigidity of natural products can be of benefit in drug discovery focusing on protein–protein interactions (PPIs).19 Moreover, natural products contribute significantly to those oral drugs ‘beyond the Rule of 5’ (bRo5), as illustrated by substantially increased cut-offs for molecular weight and HBA of approved oral drugs over the past two decades.20,21 Indeed, conducting drug discovery campaigns in bRo5 chemical space offers the potential for the development of novel therapeutic agents.22 Furthermore, applications of natural products in traditional medicines could help enhance efficacy and safety profiles.17 Taken together, the natural product pool is enriched with a variety of ‘bioactive’ compounds, which will assist in opening alternative and significant avenues for drug discovery and development.
Over the past two decades, increasing numbers of natural products and their derivatives have been exploited in drug-repositioning endeavors, although with few reports of the progress of natural products involved in such campaigns.17 In this review, we discuss the drug repositioning strategy used for six prototypical natural products and their derivatives, to shed light on the basic principles in the natural product repositioning process. Furthermore, we also highlight opportunities and challenges in natural product-based drug repositioning.
Artemisinin and its derivatives
Artemisinin was isolated from Artemisia annua L. (Asteraceae) and structurally elucidated by the Nobel Laureate Tu Youyou and her colleagues during the 1970s.23 Artemisinin and its derivatives are prominent components of antimalarial treatments.24 Currently, such artemisinin derivatives include dihydroartemisinin (DHA, an active metabolite of artemisinin), artemether, arteether, and artesunate (Figure 1).25
Figure 1.
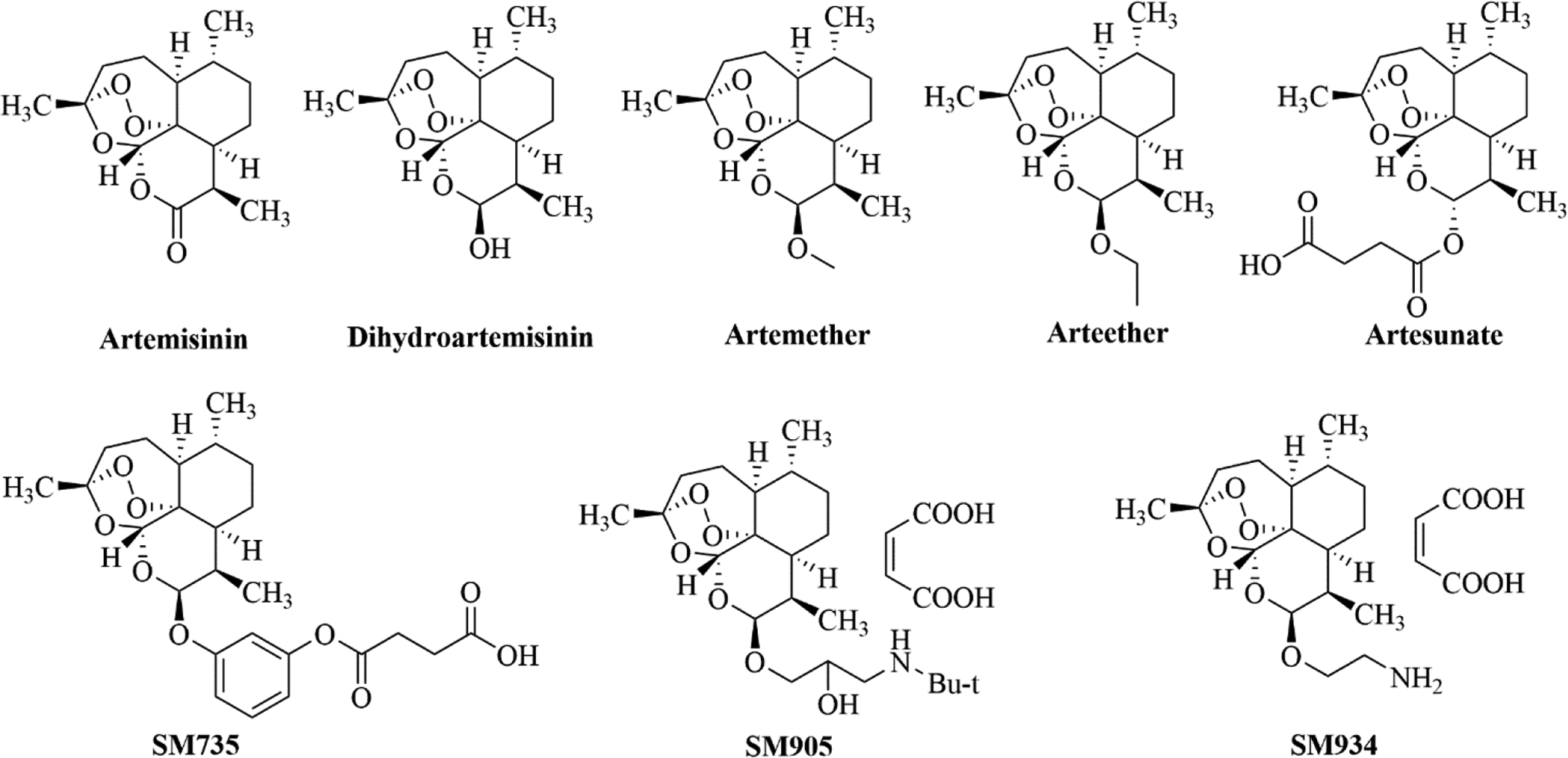
The chemical structures of artemisinin and its derivatives.
Besides their remarkable antimalarial activities, recent studies have also suggested that artemisinin and its derivatives demonstrate potent immunosuppressive efficacy in autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and collagen-induced arthritis (CIA).25 Scientists at the Shanghai Institute of Materia Medica synthesized a variety of novel artemisinin derivatives and evaluated them in cell-based assays for their cytotoxicity to murine lymphocytes (CC50), and their inhibitory activity (IC50) against concanavalin A (ConA)-induced T cell proliferation, lipopolysaccharide (LPS)-induced B cell proliferation, and alloantigen-induced splenocyte proliferation, with artemether, DHA and artesunate as references. SM735, SM905, and SM934 (Figure 1), which were found to be highly soluble and with wide therapeutic window profiles (Table 1), were further characterized in terms of their in vitro and in vivo immunosuppressive activities.26 This study showed that structural modifications facilitate the development of potential candidates derived from original natural products for repositioning purposes. In fact, SM934 was approved by the China Food and Drug Administration (CFDA) in 2015 for clinical trials as a SLE treatment.27,28
Table 1.
Immunosuppressive activities of artemisinin derivatives
| IC50 (uM) | ||||
|---|---|---|---|---|
| ConA | LPS | Alloantigen | ||
| SM735 | 0.33±0.06 | 0.27±0.02 | 0.86±0.18 | 53.1±7.8 |
| SM905 | 1.33±0.37 | 0.31±0.08 | 0.67±0.12 | 79.6±6.0 |
| SM934 | 1.2±0.5 | 2.6±1.4 | 2.11±0.76 | 67.3±32.7 |
| artemether | 6.3±1.9 | 2.4±1.5 | 3.5±0.6 | 80±0.4 |
| DHA | 6.0±1.5 | 1.2±0.3 | 0.82±0.45 | 9.4±1.0 |
| Artesunate | 2.4±0.6 | 0.6±0.1 | 0.83±0.48 | 6.8±2.5 |
Traumatic brain injury (TBI) remains a significant challenge to global public health, impacting patients regardless of age and extensively compromising their quality of life (QoL).29 In 2018, Gugliandolo and colleagues constructed a TBI model in mice, and then assessed the neuroprotective effects of artesunate (treatment dose: 30 mg/kg) via in vitro and in vivo assays. Their results indicated that artesunate exhibited efficacy in diminishing the neuroinflammatory process after TBI injury, and in inhibiting the NLR family pyrin domain containing 3 (NLRP3) inflammasome pathway. Artesunate also demonstrated a neuroprotective effect by promoting neuronal survival and regeneration through modulation of the release of neurotrophic factors (e.g., BDNF, GDNF, and NT-3). This comprehensive study suggested that artesunate could be repositioned as a potential candidate for the treatment of brain trauma.30 Again, this study showed that establishment of appropriate in vivo models to determine the efficacy of natural products is crucial to yield high-quality candidates for repositioning.
Digoxin and its analogs
Cardiac glycosides, such as digoxin and digitoxin (Figure 2a), are a class of compounds commonly derived from the foxglove (Digitalis spp.) that inhibit sodium–potassium (Na+/K+) ATPase. They have been used in the treatment of heart disorders and certain irregular heartbeats for many years.31 The application of Digitalis purpurea extracts comprising cardiac glycosides for treatment of heart failure was first reported by English physician William Withering in 1785.32 More recent discoveries on additional signaling mechanisms of action of Na+/K+ ATPase highlighted potential new therapeutic applications of cardiac glycosides in various diseases.31
Figure 2.
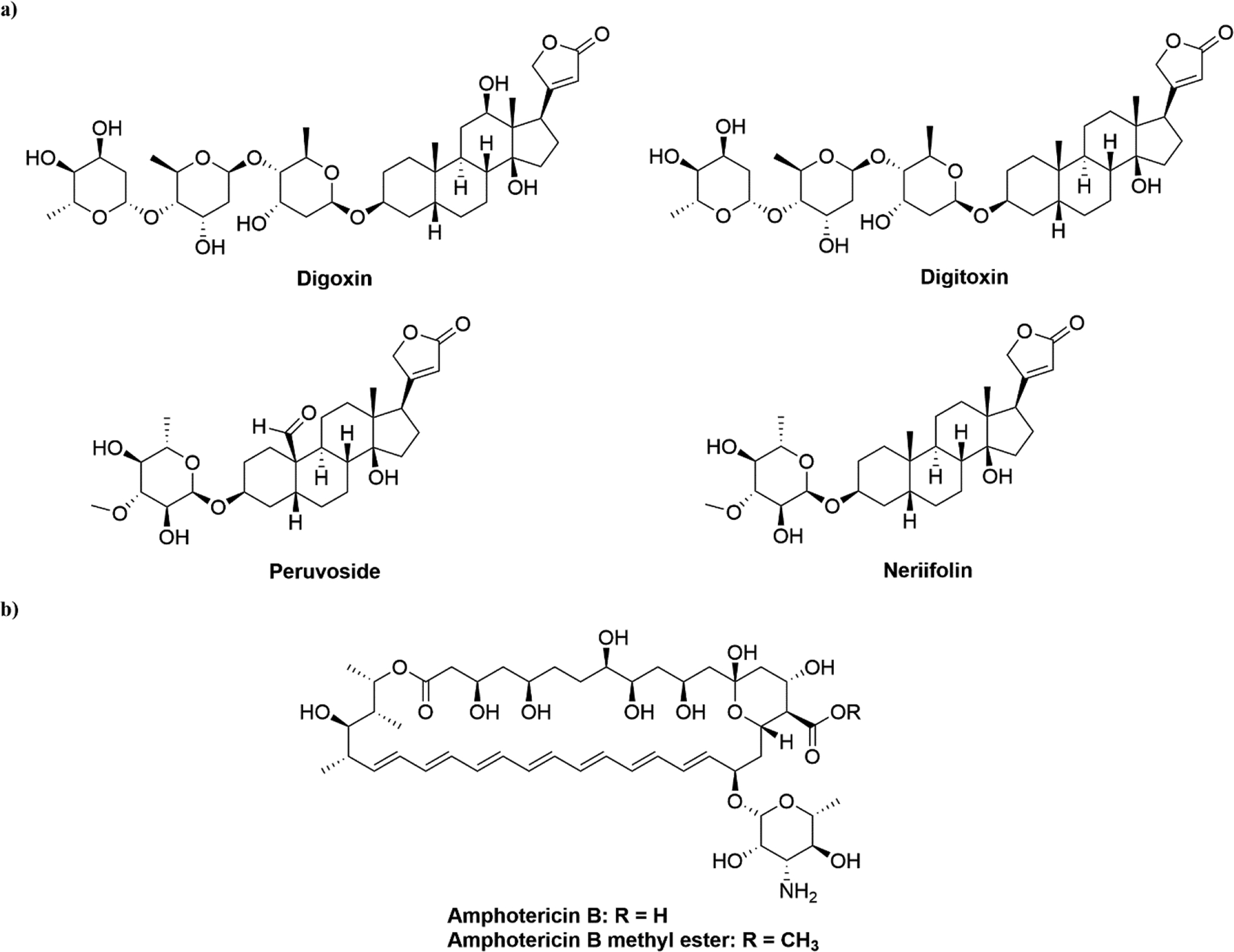
(a) Chemical structures of digoxin, digitoxin, peruvoside, and neriifolin. (b) Chemical structures of amphotericin B and amphotericin B methyl ester.
Wang et al. recently showed that digoxin exhibited potent anticancer activity. In this study, via in vitro assays such as MTT, comet, and flow cytometry assays, digoxin was found to dose-dependently inhibit the proliferation of nonsmall cell lung cancer (NSCLC) A549 and H1299 cells, and to increase cellular DNA damage. Moreover, a mechanism of action (MOA) study of the role of digoxin in DNA damage indicated that it inhibited both double strand break (DSB) and single strand break (SSB) repair pathways and promoted reactive oxygen species (ROS) generation in both cell lines. Furthermore, digoxin was able to significantly inhibit tumor growth in both A549 zebrafish and nude mouse xenograft models. Interestingly, when combined with the chemotherapy agent adriamycin (doxorubicin), digoxin not only enhanced the antitumor effect of adriamycin, but also attenuated its associated cardiotoxicity.33 Taken together, these findings suggested that digoxin could be applied as an antitumor therapy. Meanwhile, the two in vivo models used in this study might be applicable to establish a paradigm for the identification of other natural products to be repurposed as antitumor treatments.
Piccioni et al. revealed a connection between cardiac glycosides and neurodegenerative disorders. They evaluated 1040 drugs for their ability to inhibit polyglutamine-dependent caspase-3 activation in an optimized cell-based screening assay; drugs that exhibited such inhibitory activity were then tested for their ability to protect against polyglutamine-induced cytotoxicity. Interestingly, three (digitoxin, neriifolin, and peruvoside; Figure 2a) out of the four hits identified were digoxin analogs, belonging to the class of cardiac glycosides. This study suggested new therapeutic roles for these digoxin analogs against spinobulbar muscular atrophy and other polyglutamine diseases.34 In addition, it also demonstrated the significance of high-throughput screening in drug repositioning campaigns to identify potential agents both rapidly and effectively.
Amphotericin B and its derivatives
Amphotericin B (AmB; Figure 2b) has been one of the most common first-line treatments of pulmonary mycoses, and a second-line medication for treating visceral leishmaniasis (VL) since the 1950s.35 It was isolated from soil collected near the Orinoco River of Venezuela in 1955 by researchers at the Squibb Institute for Medical Research.36 AmB destroys fungi and single cell parasites, such as Leishmania spp., by preferentially binding to ergosterol rather than to cholesterol because of its high selectivity of the former. In addition to its role as an important antifungal agent, its potential antiviral effects against several enveloped and non-enveloped viruses have been reported.37
After the outbreak of coronavirus disease 2019 (COVID-19), a retrospective observational study was conducted, in which mechanically ventilated patients with COVID-19 in a hospital in Belgium were given inhaled liposomal AmB to assess its effectiveness. It was indicated that a twice-weekly administration of 12.5 mg inhaled liposomal AmB decreased the incidence of COVID-19-associated pulmonary aspergillosis/Aspergillus tracheobronchitis in those patients.38 Therefore, it was concluded that AmB could be a promising candidate drug and prophylactic regimen to treat COVID-19. Thus, retrospective observational studies can provide a fast approach to apply a repositioned drug into alternative clinical uses.
Enterovirus 71 (EV71) infections can lead to cardiopulmonary symptoms and hand–foot–mouth disease (HFMD) in young children. Xu and coworkers demonstrated that AmB significantly reduced the expression of RNA and viral proteins, VP0 and VP2, of EV71 in RD cells and HEK293 cells. In consequence, EV71 replication was inhibited by AmB with EC50 values of 1.75 ± 0.05 μM and 0.32±0.02 μM in RD cells and HEK293 cells, respectively. In addition to EV71, AmB also exhibited a strong inhibitory effect against EV68. MOA studies revealed that AmB acted on the early stage of EV71 infection by impeding both the attachment and internalization of EV71 virions to host cells. Therefore, AmB holds promise as a novel therapeutic agent to treat severe EV71 infection.39 This also suggests that a combination of in vitro screening efforts and MOA studies could prompt the discovery of potent anti-EV71 agents bearing novel scaffolds from natural products.
The antifungal agent, AmB methyl ester (AME, Figure 2b), is a water-soluble derivative of AmB, and has demonstrated anti-HIV-1 activities in previous studies.40,41 Waheed et al. further investigated its potential target proteins and MOA via a variety of cell-based assays. Their results revealed that AME was able to block the replication of a broad panel of HIV-1 isolates in different T cell lines and primary cells regardless of clade or target cell tropism. The MOA study suggested that AME profoundly disrupted both the early stage and late stage of HIV-1 replication, based on impaired viral infectivity and decreased viral particle production.42 This report provided support for AME to be repositioned as an antiretroviral candidate, and showed MOA studies to be essential in establishing a small molecule as a repurposed agent.
Pilocarpine
Pilocarpine (PIL) is an imidazole-type alkaloid found in the leaves of two of species of jaborandi, namely Pilocarpus microphyllus and Pilocarpus jaborandi (Figure 3a) and first described by Hardy and Gerrard in 1875.43 It is a muscarinic cholinergic agonist used to treat elevated intraocular pressure in the eye and to relieve xerostomia (dry mouth) caused by radiation therapy in patients with head and neck cancer.44
Figure 3.
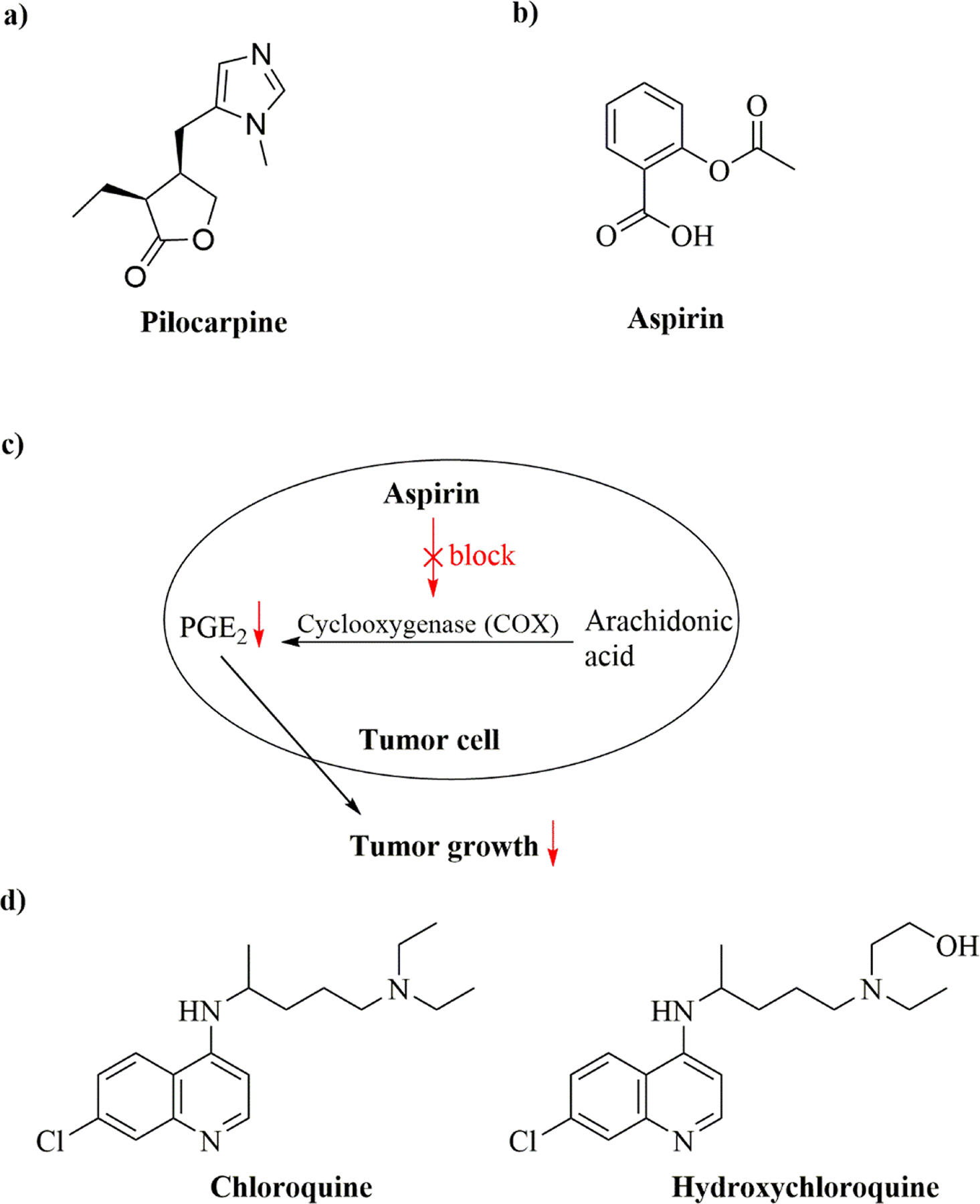
(a) Chemical structure of pilocarpine; (b) Chemical structure of aspirin; (c) Proposed mechanism of action of low-dose aspirin in tumor cells for suppressing tumor growth; (d) Chemical structures of chloroquine and hydroxychloroquine.
Recent studies suggested new pharmacological functions of PIL as a potential treatment of inflammatory diseases. For example, Nile and coworkers showed that different cholinergic receptors might promote favorable outcomes of Candida albicans systemic infection, and that a muscarinic-type receptor might be involved in the modulation of C. albicans by inhibiting filamentous growth and biofilm formation. Correspondingly, PIL was able to specifically inhibit C. albicans biofilm formation and pathogenicity. Using a Galleria mellonella killing assay, it was also found that PIL could specifically modulate the pathogenesis of C. albicans infection in vivo.45 These data indicated that targeting muscarinic cholinergic receptors by repurposing PIL could provide new therapeutic options for the prevention of fatal fungal infections.
Aspirin
Aspirin (acetylsalicylic acid, Figure 3b), a nonsteroidal anti-inflammatory drug (NSAID), is widely used in the treatment of pain and fever to reduce common cold and flu symptoms.46 In 1897, the German chemist Felix Hoffman at Bayer prepared aspirin from salicylic acid, which can be generated from salicin isolated from willow bark.47 Recent years, aspirin has shown great promise for repositioning applications in prevention and/or treatment of various diseases.
Cardiovascular disease (CVD) and colorectal cancer (CRC) are leading causes of death among US adults, with heart diseases, cancer, and stroke accounting for more than 50% of all deaths in the USA in 2011.48 In April 2016, based on retrospective clinical data analysis, the US Preventive Services Task Force (USPSTF) made recommendations on the use of aspirin to prevent CRC and CVDs. The USPSTF recommended initiating low-dose aspirin use for the primary prevention of CVD and CRC in adults aged 50 to 59 years who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take low-dose aspirin daily for at least 10 years.49 A follow-up meta-analysis of individual patient data from randomized trials indicated a substantial benefit of preventing vascular events in patients weighing less than 70 kg (50–69 kg) taking low doses of aspirin (75–100 mg).50 Moreover, another retrospective study showed that daily aspirin was able to reduce deaths caused by several common cancers during and after the trials. Benefits increased during the treatment period and were consistent in the different trial populations.51 Taken together, these results highlighted retrospective clinical analysis, as a systematic analysis of clinical data obtained from available sources, as an increasingly important approach to identify drug repurposing opportunities.
Using various cell-based assays, such as three different NSCLC cell lines and two CRC cell lines, systemic Prostaglandin E2 (PGE2) biosynthesis was found to be inhibited by low-dose aspirin (81 mg per day for 14 days) in healthy volunteers.52 PGE2 is thought to be important in accelerating cell proliferation and promoting tumor growth.53 Aspirin also demonstrated inhibitory effects against cyclooxygenase (COX) activity and PGE2 production in lung and colon adenocarcinoma cells which were as great or greater than that for COX-1 in platelets. This suggested that low-dose aspirin could partially inhibit tumor COX activity, leading to a reduction in the synthesis of PGE2 in these cells, eventually suppressing tumor growth.52,53 A proposed MOA of low-dose aspirin in suppressing tumor growth is depicted in Figure 3c. This study also indicated that multiple cell-based assays might be crucial for uncovering the MOA of the repurposed drug for new therapeutic applications.
A more recent example further supports the important role of aspirin in the treatment of cancers. In this study, using transcriptional gene signatures collected from the public database Gene Expression Omnibus (GEO) to query Connectivity Map (CMap) database, followed by in vitro cell-based screening and in vivo examination of 12 FDA-approved drugs, Li et al. found that aspirin not only exhibited inhibitory effects on the proliferation of lung and breast cancer cells as well as dose-dependently promoting their apoptosis, but also delayed and overcame acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in lung cancer and to tamoxifen in breast cancer when subjected to combination therapies.54 This study was also an example of signature matching by CMap for drug repositioning, which is based on drug–drug and drug–disease comparisons in terms of the ‘signature’/unique characteristic of a drug.55 In other words, CMap can be used to identify small-molecule compounds that induce either a similar or an opposite gene-expression signature to a variety of diseases. There have been more than 100 publications describing CMap-related studies.56 However, because the CMap database primarily comprises cancer cell information, there is a significant need to collect data from other cell lines to enrich this database.
In 2017, Ahmed et al. evaluated the inhibitory effects of several NSAIDs, including aspirin, on a collection of urinary tract pathogens, and determined the combined effect of these NSAIDs with some antibiotics on isolated bacterial strains. It was found aspirin had a minimum inhibitory concentration (MIC90) value of 4 μg/ml against bacilli, 512 μg/ml against Enterococcus faecalis, Proteus, and Pseudomonas spp., and 1024 μg/ml against Escherichia coli, Klebsiella, Staphylococcus aureus, staphylococci, and streptococci. Furthermore, aspirin showed a synergistic effect when combined with β-lactam antibiotics in inhibiting Klebsiella pneumoniae (ATCC 10031) and Pseudomonas aeruginosa (ATCC 10145) strains.57 Thus, the results from this study suggested the potential use of aspirin as an antibiotic.
To develop new drugs to combat drug resistance in fungal infections, Ogundeji et al. repurposed aspirin and ibuprofen as alternatives to inhibit the growth of cryptococcal cells. Aspirin was able to control the growth of five Cryptococcus neoformans strains and five Cryptococcus gattii strains in a dose-dependent manner. Moreover, aspirin enhanced the ability of macrophages to phagocytose cryptococcal cells. Most importantly, aspirin treatment in cryptococcal cells led to stress induction by activating the high-osmolarity glycerol pathway, and cell death was ultimately achieved because of membrane damage mediated by reactive oxygen species (ROS). Thus, this study highlighted the potential application of aspirin as a candidate anti-Cryptococcus agent in various assays.58
Chloroquine/hydroxychloroquine
Chloroquine phosphate has long been used to treat malaria and amebiasis. Hydroxychloroquine sulfate, a more soluble chloroquine analog, was obtained by introduction of a hydroxyl group into chloroquine and was shown to be remarkably less toxic than chloroquine in animal studies.59 Chloroquine and hydroxychloroquine (Figure 3d) were discovered during efforts to synthesize alternatives of quinine as antimalarials, whereas quinine was naturally derived from cinchona bark.60 In recent years, chloroquine and hydroxychloroquine have been repurposed for the treatment of SLE, RA, and related inflammatory rheumatic diseases.61,62
Growing evidence has suggested that chloroquine could also be repurposed for the treatment of cancers.63–65 For example, Levy and colleagues demonstrated via various in vitro and ex vivo assays that, by targeting autophagy, chloroquine could circumvent multiple BRAF inhibitor resistance mechanisms in brain tumors. It was observed that tumor cell growth was inhibited and cell death was increased by genetic and pharmacological autophagy inhibition.66 Thus, this study provided a valid approach to overcome multiple mechanisms of kinase inhibitor resistance.
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to pose a significant risk to global public health. Cell-based antiviral screening assays testing the inhibitory effects of multiple small molecules identified chloroquine and hydroxychloroquine as effective anti-SARS-CoV-2 agents in vitro.59,67,68 Furthermore, over 80 clinical trials of chloroquine and/or hydroxychloroquine, sometimes in combination with other drugs, have been registered worldwide.69 However, based on examination of emerging data, the US FDA revoked the Emergency Use Authorization for chloroquine and hydroxychloroquine as a treatment of certain patients hospitalized with COVID-19.70 Moreover, through a combination of structural biology and molecular modeling approaches, Fantini and coworkers demonstrated that chloroquine bound to sialic acids and gangliosides with a high affinity so that the viral Spike protein was unable to bind to gangliosides on the host cell membrane.71 The possible MOA of both drugs against the virus could be explained as follows: as weak bases, they might increase the endosomal/lysosomal pH of acidic host intracellular organelles, inhibit autophagosome–lysosome fusion, and inactivate enzymes essential for virus replication.69 In summary, these studies revealed that experimental and computational approaches could be useful for identifying new anti-SARS-CoV-2 agents (i.e., chloroquine and hydroxychloroquine). Thus, a combination of these approaches could help accelerate the discovery of potential therapeutic agents to treat COVID-19.
Concluding remarks and prospects
Significant progress in natural product-derived drug repositioning has been achieved in recent years. Natural products remain a promising pool for the discovery of diverse scaffolds and various bioactivities that can often be directly repositioned into novel drugs targeting new and existing diseases. There are numerous advantages associated with natural product-based drug repositioning. First, natural products have been the key source of new drugs to combat various diseases for thousands of years. Thus, they can offer a rapid and efficient way to fight new diseases, as exemplified by COVID-19, because all their preclinical, efficacy, and safety data are readily available. Second, natural products have been structurally ‘optimized’ by evolution to serve specific pharmacological applications, which might not alter the normal functions of the human body significantly. Natural products, as secondary metabolites from living organisms, are often found to be unique or can be regarded as ‘biomarkers’ to show the individuality of a species. Third, natural products are endowed with high structural diversity, thus providing more space for optimization during drug development. Fourth, natural products, similar to synthetic small molecules, can also have good drug-like properties because they commonly have privileged scaffolds.
Meanwhile, several limitations and barriers to the implementation of the repositioning of natural products remain to be addressed. First, for all the prototypes we discussed, phenotypic screening combined with relevant in vitro and in vivo assays appears to be the most commonly adopted approach for natural product-based drug repositioning (also for synthetic small molecules). However, this approach can be time and labor consuming, with a low success rate. Second, when a natural product molecule is established as a lead or candidate drug targeting a specific disease, it is usually more difficult to synthesize its derivatives and explore their structure–activity relationships to further optimize their drug-like properties because of their common structural complexity. In addition, if they are not amenable to production in nature (many would be impossible if starting from scratch), it might be difficult to investigate the in vivo efficacy of natural products because large quantities are required for such studies. Third, there can be sourcing issues for some natural products, including issues surrounding national resource exploitation. Finally, because drug repositioning requires a thorough understanding of biological and molecular pathways, our limited knowledge of the potentially relevant MOAs of natural products might hinder preclinical studies to establish the fully validated efficacy of such compounds.
We believe that chemical structure similarity comparisons and use of ‘privileged structures’ could be valid ways to identify potential candidates from the pool of natural products. Additionally, a comprehensive understanding of the disease and therapeutic target(s) will help guide structure-based optimization. Moreover, chemical syntheses adopting natural products as a starting point for analog generation and biosynthetic engineering technologies for the production of analogs will be vital for overcoming hurdles to obtaining enough candidates for repurposing efforts. Most importantly, systematic biological and pharmacological evaluations through diversified approaches are crucial for obtaining high-quality leads or candidate compounds derived from natural products. Given that artificial intelligence (AI) and big-data-driven techniques have accelerated drug development pipelines, we expect that drug repositioning campaigns from natural products could benefit from such advances in the near future.
Highlights.
Natural products show great promises in the drug repositioning campaign.
New drug-disease associations are uncovered and discussed.
Pros and cons in natural product-based drug repositioning are summarized.
Acknowledgments
This work was partially supported by NIH/NIDA Grants DA044855 and DA050311 (Y.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing financial interest
References
- 1.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004; 3(8): 673–683. [DOI] [PubMed] [Google Scholar]
- 2.Nosengo N Can you teach old drugs new tricks? Nature. 2016; 534(7607): 314–316. [DOI] [PubMed] [Google Scholar]
- 3.Wu G, Zhao T, Kang D, Zhang J, Song Y, Namasivayam V, et al. Overview of recent strategic advances in medicinal chemistry. J Med Chem. 2019; 62(21): 9375–9414. [DOI] [PubMed] [Google Scholar]
- 4.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019; 18(1): 41–58. [DOI] [PubMed] [Google Scholar]
- 5.Talevi A, Bellera CL. Challenges and opportunities with drug repurposing: finding strategies to find alternative uses of therapeutics. Expert Opin Drug Discov. 2020; 15(4): 397–401. [DOI] [PubMed] [Google Scholar]
- 6.Romano JD, Tatonetti NP. Informatics and computational methods in natural product drug discovery: a review and perspectives. Front Genet. 2019; 10: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015; 14(2): 111–129. [DOI] [PubMed] [Google Scholar]
- 8.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997; 60(1): 52–60. [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981−2002. J Nat Prod. 2003; 66(7): 1022–1037. [DOI] [PubMed] [Google Scholar]
- 10.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007; 70(3): 461–477. [DOI] [PubMed] [Google Scholar]
- 11.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012; 75(3): 311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016; 79(3): 629–661. [DOI] [PubMed] [Google Scholar]
- 13.Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020; 83(3): 770–803. [DOI] [PubMed] [Google Scholar]
- 14.Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016; 21(2): 204–207. [DOI] [PubMed] [Google Scholar]
- 15.Koehn FE. Biosynthetic medicinal chemistry of natural product drugs. MedChemComm. 2012; 3(8): 854–865. [Google Scholar]
- 16.Rastelli G, Pellati F, Pinzi L, Gamberini MC. Repositioning natural products in drug discovery. Molecules. 2020; 25(5): 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atanasov AG, Zotchev SB, Dirsch VM; International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021; 20(3): 200–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clardy J, Walsh C. Lessons from natural molecules. Nature. 2004; 432(7019): 829–837. [DOI] [PubMed] [Google Scholar]
- 19.Lawson ADG, MacCoss M, Heer JP. Importance of rigidity in designing small molecule drugs to tackle protein–protein interactions (PPIs) through stabilization of desired conformers. J Med Chem. 2018; 61(10): 4283–4289. [DOI] [PubMed] [Google Scholar]
- 20.Doak Bradley C, Over B, Giordanetto F, Kihlberg J. Oral druggable space beyond the Rule of 5: insights from drugs and clinical candidates. Chem Biol. 2014; 21(9): 1115–1142. [DOI] [PubMed] [Google Scholar]
- 21.Shultz MD. Two Decades under the influence of the Rule of Five and the changing properties of approved oral drugs. J Med Chem. 2019; 62(4): 1701–1714. [DOI] [PubMed] [Google Scholar]
- 22.DeGoey DA, Chen H-J, Cox PB, Wendt MD. Beyond the Rule of 5: lessons learned from AbbVie’s drugs and compound collection. J Med Chem. 2018; 61(7): 2636–2651. [DOI] [PubMed] [Google Scholar]
- 23.Su X-Z, Miller LH. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci China Life Sci. 2015; 58(11): 1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008; 320(5874): 330–334. [DOI] [PubMed] [Google Scholar]
- 25.Shi C, Li H, Yang Y, Hou L. Anti-inflammatory and immunoregulatory functions of artemisinin and Its derivatives. Mediators Inflamm. 2015; 2015: 435713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Tang W, Zuo J. Development of artemisinin drugs in the treatment of autoimmune diseases. Sci Bull. 2016; 61(1): 37–41. [Google Scholar]
- 27.Wu Y, He S, Bai B, Zhang L, Xue L, Lin Z, et al. Therapeutic effects of the artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition of TLR-triggered B-cell activation and plasma cell formation. Cell Mol Immunol. 2016; 13(3): 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Zuo J, Tang W. Water-soluble artemisinin derivatives as promising therapeutic immunosuppressants of autoimmune diseases. Cell Mol Immunol. 2017; 14(11): 887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polinder S, Haagsma JA, van Klaveren D, Steyerberg EW, van Beeck EF. Health-related quality of life after TBI: a systematic review of study design, instruments, measurement properties, and outcome. Popul Health Metr. 2015; 13(1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gugliandolo E, D’Amico R, Cordaro M, Fusco R, Siracusa R, Crupi R, et al. Neuroprotective effect of artesunate in experimental model of traumatic brain injury. Front Neurol. 2018; 9: 590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008; 7(11): 926–935. [DOI] [PubMed] [Google Scholar]
- 32.Bessen HA. Therapeutic and toxic effects of digitalis: William Withering, 1785. J Emerg Med. 1986; 4(3): 243–248. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Ma Q, Zhang S, Liu H, Zhao B, Du B, et al. Digoxin enhances the anticancer effect on non-small cell lung cancer while reducing the cardiotoxicity of adriamycin. Front Pharmacol. 2020; 11: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccioni F, Roman BR, Fischbeck KH, Taylor JP. A screen for drugs that protect against the cytotoxicity of polyglutamine-expanded androgen receptor. Hum Mol Genet. 2004; 13(4): 437–446. [DOI] [PubMed] [Google Scholar]
- 35.Al-Khikani FHO. Amphotericin B from antifungal to antiviral therapy: promising modern therapeutic branch. Res Results Pharmacol. 2020; 6(2): 57–65. [Google Scholar]
- 36.ACS. Molecule of the Week Archive Amphotericin B. www.acs.org/content/acs/en/molecule-of-the-week/archive/a/amphotericin-b.html [accessed February 14, 2022].
- 37.Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020; 79(6): 713. [DOI] [PubMed] [Google Scholar]
- 38.Van Ackerbroeck S, Rutsaert L, Roelant E, Dillen K, Wauters J, Van Regenmortel N. Inhaled liposomal amphotericin-B as a prophylactic treatment for COVID-19-associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care. 2021; 25(1): 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu F, Zhao X, Hu S, Li J, Yin L, Mei S, et al. Amphotericin B inhibits enterovirus 71 replication by impeding viral entry. Sci Rep. 2016; 6(1): 33150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen JE, Witzke NM, Nielsen C, Mathiesen LR, Teglbjaerg LS, Nielsen CM, et al. Derivatives of amphotericin inhibit infection with human immunodeficiency virus in vitro by different modes of action. Antiviral Res. 1990; 14(3): 149–159. [DOI] [PubMed] [Google Scholar]
- 41.Pontani D, Plescia OJ, Schaffner CP, Sun D, Shahied SI, Sarin PS. Targets of amphotericin B methyl ester (AME) in the inhibition of infection of different cell lines by HIV-1. Antiviral Chem Chemother. 1990; 1(1): 67–72. [Google Scholar]
- 42.Waheed AA, Ablan SD, Mankowski MK, Cummins JE, Ptak RG, Schaffner CP, et al. Inhibition of HIV-1 replication by amphotericin B methyl ester: selection for resistant variants. J Biol Chem. 2006; 281(39): 28699–28711. [DOI] [PubMed] [Google Scholar]
- 43.Holmstedt B, Wassén SH, Schultes RE. Jaborandi: an interdisciplinary appraisal. J Ethnopharmacol. 1979; 1(1): 3–21. [DOI] [PubMed] [Google Scholar]
- 44.Cifuentes M, Del Barrio-Díaz P, Vera-Kellet C. Pilocarpine and artificial saliva for the treatment of xerostomia and xerophthalmia in Sjögren syndrome: a double-blind randomized controlled trial. Br J Dermatol. 2018; 179(5): 1056–1061. [DOI] [PubMed] [Google Scholar]
- 45.Nile C, Falleni M, Cirasola D, Alghamdi A, Anderson OF, Delaney C, et al. Repurposing pilocarpine hydrochloride for treatment of Candida albicans infections. mSphere.4(1): e00689–00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eccles R Efficacy and safety of over-the-counter analgesics in the treatment of common cold and flu. J Clin Pharm Ther. 2006; 31(4): 309–319. [DOI] [PubMed] [Google Scholar]
- 47.Miner J, Hoffhines A. The discovery of aspirin’s antithrombotic effects. Tex Heart Inst J. 2007; 34(2): 179–186. [PMC free article] [PubMed] [Google Scholar]
- 48.Heron M Deaths: leading causes for 2011. Natl Vital Stat Rep. 2015; 64(7): 1–96. [PubMed] [Google Scholar]
- 49.US Preventive Services Taskforce. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Preventive Medication. www.uspreventiveservicestaskforce.org/uspstf/recommendation/aspirin-to-prevent-cardiovascular-disease-and-cancer [Accessed February 14, 2022].
- 50.Rothwell PM, Cook NR, Gaziano JM, Price JF, Belch JFF, Roncaglioni MC, et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. The Lancet. 2018; 392(10145): 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. The Lancet. 2011; 377(9759): 31–41. [DOI] [PubMed] [Google Scholar]
- 52.Boutaud O, Sosa IR, Amin T, Oram D, Adler D, Hwang HS, et al. Inhibition of the biosynthesis of prostaglandin E2 by low-dose aspirin: implications for adenocarcinoma metastasis. Cancer Prev Res. 2016; 9(11): 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishida J, Konishi M, Ebner N, Springer J. Repurposing of approved cardiovascular drugs. J Transl Med. 2016; 14(1): 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L, Hu M, Wang T, Chen H, Xu L. Repositioning aspirin to treat lung and breast cancers and overcome acquired resistance to targeted therapy. Front Oncol. 2020; 9: 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Low ZY, Farouk IA, Lal SK. Drug repositioning: new approaches and future prospects for life-debilitating diseases and the COVID-19 pandemic outbreak. Viruses. 2020; 12(9): 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell. 2017; 171(6): 1437–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed EF, El-Baky RMA, Ahmed ABF, Waly NG, Gad GFM. Antibacterial activity of some non-steroidal anti-inflammatory drugs against bacteria causing urinary tract infection. Am J Infect Dis. 2017; 5(1): 66–73. [Google Scholar]
- 58.Ogundeji Adepemi O, Pohl Carolina H, Sebolai Olihile M. Repurposing of aspirin and ibuprofen as candidate anti-Cryptococcus drugs. Antimicrob Agents Chemother. 60(8): 4799–4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020; 6(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Browning DJ. Pharmacology of chloroquine and hydroxychloroquine. Hydroxychloroquine and Chloroquine Retinopathy. 2014: 35–63. [Google Scholar]
- 61.Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016; 123(6): 1386–1394. [DOI] [PubMed] [Google Scholar]
- 62.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020; 16(3): 155–166. [DOI] [PubMed] [Google Scholar]
- 63.Weyerhäuser P, Kantelhardt SR, Kim EL. Re-purposing chloroquine for glioblastoma: potential merits and confounding variables. Front Oncol. 2018; 8: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye H, Chen M, Cao F, Huang H, Zhan R, Zheng X. Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis. BMC Neurol. 2016; 16(1): 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verbaanderd C, Maes H, Schaaf MB, Sukhatme VP, Pantziarka P, Sukhatme V, et al. Repurposing Drugs in Oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience. 2017; 11: 781–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mulcahy Levy JM, Zahedi S, Griesinger AM, Morin A, Davies KD, Aisner DL, et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. eLife. 2017; 6: e19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30(3): 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P,et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020; 71(15): 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020; 369: m1432. [DOI] [PubMed] [Google Scholar]
- 70.FDA. FDA Cautions against Use of Hydroxychloroquine or Chloroquine for COVID-19 Outside of the Hospital Setting or a Clinical Trial due to Risk of Heart Rhythm Problems. www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or [Accessed February 14, 2022].
- 71.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020; 55(5): 105960. [DOI] [PMC free article] [PubMed] [Google Scholar]


