Abstract
Organophosphate esters (OPE) are flame retardants and plasticizers used in a wide range of consumer products. Despite their widespread use, few studies have characterized pediatric exposures. We assessed variability and predictors of OPE exposures in a cohort panel study of 179 predominantly Black school-aged children in Baltimore City, MD. The study design included up to four seasonal week-long in-home study visits with urine sample collection on days 4 and 7 of each visit (nsamples=618). We quantified concentrations of 9 urinary OPE biomarkers: bis(2-chloroethyl) phosphate (BCEtp), bis(1-chloro-2-propyl) phosphate, bis(1,3-dichloro-2-propyl) phosphate (BDCPP), di-benzyl phosphate (DBuP), di-benzyl phosphate, di-o-cresylphosphate, di-p-cresylphosphate (DPCP), di-(2-propylheptyl) phthalate (DPHP), 2,3,4,5-tetrabromo benzoic acid. We assessed potential predictors of exposure, including demographic factors, household characteristics, and cleaning behaviors. We calculated Spearman/tetrachoric correlations and intraclass correlation coefficients (ICCs) to examine within-week and seasonal intra-individual variability, respectively. We assessed OPE predictors using linear models for continuous log2 concentrations (BDCPP and DPHP) and logistic models for odds of detection (BCEtP, DBuP, DPCP), with generalized estimating equations to account for repeated measures. For all OPEs, we observed moderate within-week correlations (rs: 0.31–0.63) and weak to moderate seasonal reliability (ICC: 0.18–0.38). BDCPP and DPHP concentrations were higher in the summer compared to other seasons. DPHP concentrations were lower among males than females (%diff : −53.5%; 95% CI: −62.7, −42.0) and among participants spending >12 hours/day indoors compared to ≤12 hours (%diff : −20.7%; 95% CI: −32.2, −7.3). BDCPP concentrations were lower among children aged 8–10 years compared to 5–7 years (%diff: −39.1%; 95% CI: −55.9, −15.9) and higher among children riding in a vehicle the day of sample collection compared to those who had not (%diff: 28.5%; 95% CI: 3.4, 59.8). This study is the first to characterize within-week and seasonal variability and identify predictors of OPE biomarkers among Black school-aged children with asthma, a historically understudied population.
Keywords: organophosphate esters, flame retardants, school-aged children, urinary biomarkers
INTRODUCTION
Organophosphate esters (OPEs) are an emerging class of contaminants used as replacements for polybrominated diphenyl ethers (PBDEs) in a variety of consumer products including plastics, nail polish, processed foods, clothing, furniture, building materials and electronics [1–8]. They are often physically incorporated into the surfaces of commercial products as opposed to chemically embedded within the product, facilitating their release and volatilization into environmental media including soil, air, and water [3, 4, 6, 9–12]. Sources of indoor exposure to OPEs include house dust and furniture foam [1, 8, 13–20]. Human exposures may occur via ingestion, inhalation, and dermal absorption [2, 9, 16, 21–23], and while the biological half-lives of OPEs remain poorly characterized laboratory studies estimate that they are relatively short-lived and excreted from the body within several hours to days [4, 24, 25]. U.S. national biomonitoring data indicate that OPE exposures are widespread [9], with reports indicating disproportionately higher levels of several OPEs among children compared to adults [3, 26–30]. While studies on the human health effects of OPEs are limited, toxicity testing, risk assessments and epidemiological studies indicate that OPEs may be reproductive toxicants and have carcinogenic and neurotoxic properties [4, 8]. The disparate OPE exposures observed among children and their potential health effects support the need to identify modifiable OPE exposure predictors, particularly among vulnerable populations.
To date, studies characterizing childhood OPE exposures have primarily examined environmental dust samples or urinary OPE biomarkers among predominantly white preschool aged children (i.e., 1 to 6 years) [27–40]. Several of these studies have examined urinary OPE biomarkers in mother-child pairs and have observed BDCPP and DPHP to be the most frequently detected, with significantly higher concentrations found in children compared to their mothers [27–32]. Limited studies among children also report that hand-mouth behavior [27, 31, 40], increased number of infant products owned [30], and attendance of infants in daycare centers [30] are associated with increased biomarker concentrations for several OPEs. Other factors linked to increased OPE exposure via dust or urinary biomarkers in children include, presence of electronics at home or at school [33, 37], use of floor wax or polish in preschools [37], and use of foam mattresses in schools [39]. While one study considered dietary intake as a potential source of OPE exposure among children, no associations were observed [28]. School-aged children have unique behavioral, dietary and time-activity patterns compared to infants and preschool-aged children who have been the main focus of prior studies. For example, infants tend to spend more time closer to floors and have more hand-to-mouth activity than school-aged children and thus may be exposed more frequently to OPEs in dust [24]. Thus, in-depth characterization of OPE exposures and respective predictors among school-aged children are warranted.
Studies examining inter- and intra-individual variability of OPE exposures are also sparse and have only focused on pregnant women or adult men [41–44]. While these studies suggest moderate to high reliability among multiple OPE urinary biomarkers, each was limited by a small sample size, with limited repeat samples, over relatively short periods of time. To accurately assess exposures to OPEs and design studies examining the health effects of these exposures in children, it is critical to elucidate their stability and degree of temporal variability over longer periods of time. Furthermore, characterizing variability of these short-lived OPE biomarkers in children is important to determine the potential for exposure misclassification when single measurements are used [45].
In the present study, we sought to address these exposure data gaps among school-aged children by quantifying concentrations of nine urinary OPE biomarkers, characterizing exposure variability, and identifying exposure predictors. We conducted our analyses using data from a cohort panel study originally designed to examine the role of environmental pollutants on asthma morbidity in a population of predominantly Black children with asthma. This population of children is of particular importance due to their potential for increased vulnerability to the adverse health effects of environmental pollutants.
METHODS
Study population and design
We leveraged data and biobanked samples from the Domestic Indoor PM and Childhood Asthma Morbidity (DISCOVER) cohort panel study. This study has been summarized previously [46] In brief, a total of 180 children (100 atopic asthmatic, 50 non-atopic asthmatic, and 30 non-asthmatic children) between the ages of 5–12 years were recruited from 9 contiguous zip codes in Baltimore City between 2009 and 2013. Inclusion criteria included a previous diagnosis of asthma by a physician with symptoms of asthma and/or reliever medication use in the 6 months prior to enrollment. Children were excluded from the study if they had a current diagnosis of another major pulmonary disease, were planning to relocate residence during the study period, were taking antioxidant supplements, or were unable to follow study protocols. For our study purposes, we focused on examining determinants of OPE exposures.
Each participant was invited to complete four week-long home visits occurring at baseline and at 3, 6, and 9 months after baseline (Figure 1). These visits were intended to capture seasonal variability in exposures and asthma symptoms. Each visit consisted of seven consecutive days (Sunday-Saturday, labeled as days 1–7) and urine samples were collected from participants on days 4 (weekday) and 7 (weekend). Thus, each participant had the possibility of contributing up to a maximum of eight urine samples. All study protocols were approved by the Johns Hopkins Institutional Review Board. Written informed consent was obtained from the parents or legal guardians of all enrolled children.
Figure 1.
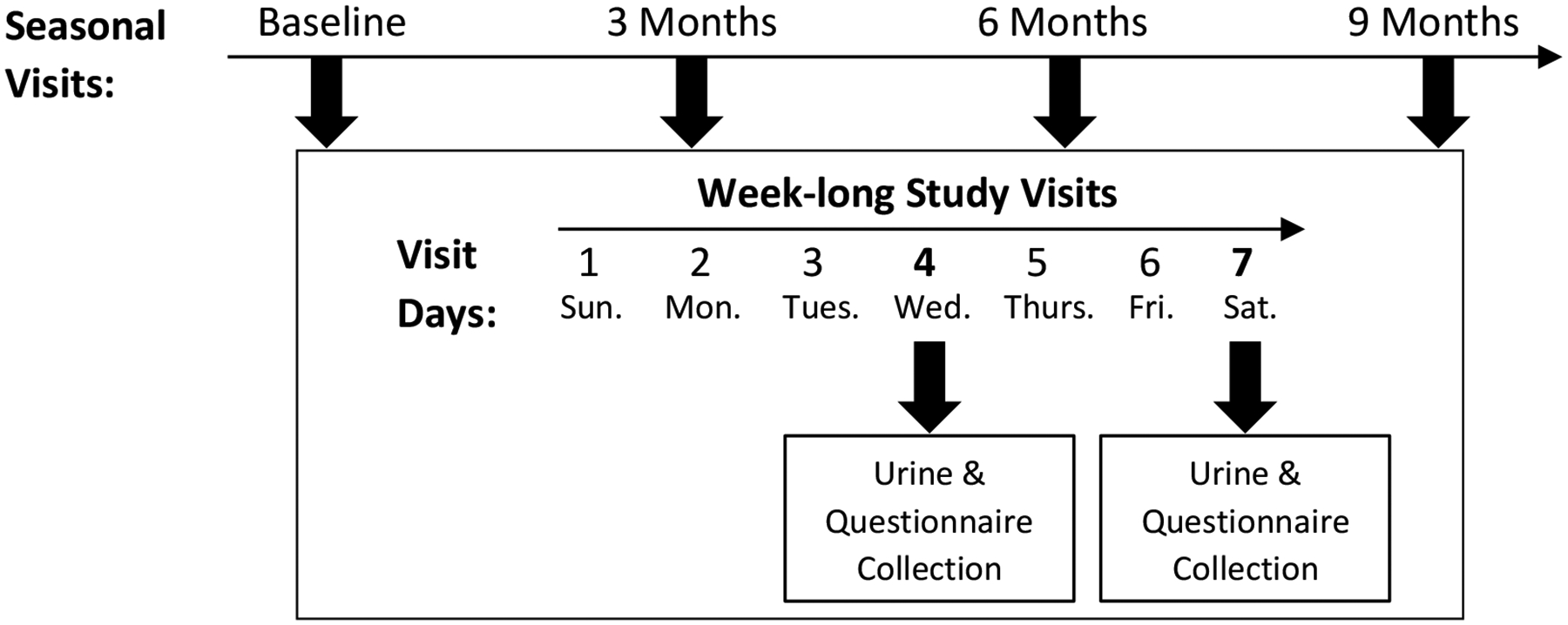
DISCOVER study framework for sample collection.
Questionnaires
During the baseline home visit, trained study staff administered a parental/guardian questionnaire to capture the participating child’s demographic information including race, gender, age at enrollment, body mass index (BMI), as well as the parental/guardian education level, number of household residents. During each week-long visit, parents/guardians reported information on housing characteristics including flooring type in the child’s room (e.g., tile, hardwood floors, wall to wall carpeting etc.). Participants completed an activity questionnaire each day of the week-long study visit to capture information on smoking in the home, location where the child spent his/her time (i.e., hours spent inside the home, outdoors, and indoors in other buildings or vehicles), dietary intake (e.g., fish, meat, juice, soda and fast foods), cleaning practices within the household (e.g., mopping, vacuuming, sweeping, dusting) as well as use of an oven, central air, air conditioning, air purifier, and heater.
OPE exposure assessment
We collected spot urine samples in polypropylene urine collection cups, aliquoted samples into 2mL cryovials, and stored aliquots at −80 °C until shipment on dry ice to NSF International (Ann Arbor, MI). Concentrations of nine urinary OPE biomarkers were quantified including: bis(2-chloroethyl) phosphate (BCEtp), bis(1-chloro-2-propyl) phosphate (BCPP), bis(1,3-dichloro-2-propyl) phosphate (BDCPP), di-benzyl phosphate (DBuP), di-benzyl phosphate (DBzP), di-o-cresylphosphate (DOCP), di-p-cresylphosphate (DPCP), di-(2-propylheptyl) phthalate (DPHP), and 2,3,4,5-tetrabromo benzoic acid (TBBA). Liquid Chromtography-Tandem Mass Spectrometry (LC/MS/MS) analysis was performed using a Thermo Scientific Transcend TXII Turbulent Flow system interfaced with Thermo Scientific Quantiva triple quadrupole mass spectrometer using SRM in negative mode. We used standards of known purity and identity during preparation of the calibration, quality control and internal standards. We analyzed the matrix specific quality control samples in duplicate at three levels throughout the study (n=35). In the low-level quality control (QC), the percent coefficient of variation (CV) ranged from 6.0% to 15.4%, in the mid-level QC, the percent CV ranged from 2.7% to 12.1%, and in the high-level QC, the percent CV ranged from 5.8% to 11.5%. This method was developed to simulate the Centers for Disease Control and Prevention (CDC) analytical method for quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry as reported previously[47]. We evaluated our method against the acceptance criteria established within the CDC methods, and established limits of detection (LOD) by replicate injections of low concentration standards. The reported LOD was also the run limit of quantification, and this level was always included as the low-level standard in the calibration curve. This value was not determined by a statistical evaluation of the data at that point, but rather as an actual standard used as part of the calibration curve, meeting the calibration curve requirements. The limits of detection ranged between 0.05 and 2.0 ng/mL.
Statistical analyses
We used descriptive statistics to summarize participant demographics and household characteristics for our study population at baseline. We also calculated summary statistics of urinary OPE biomarker concentrations (μg/L), including detection frequencies (DF) (%), geometric means (GM) (μg/L), minimums (μg/L), maximums (μg/L) and interquartile ranges (p25, p50, p75) (μg/L), stratified by visit (i.e., denoted by season) and day (i.e., day 4 or day 7) of urine collection. For these summary statistics, we used machine-read values for concentrations below the LOD, and corrected OPE biomarker concentrations for specific gravity (SG) using the formula, Csg= C × [(1.024–1)/(SG-1)] where Csg is the specific-gravity corrected OPE biomarker concentration (μg/L), C is the OPE biomarker concentration (μg/L), SG is the specific gravity for each observed urine sample and 1.024 is the mean SG for the study population [48]. For biomarkers with an overall DF ≥ 70% (BDCPP and DPHP), we log2 transformed concentrations (replacing machine read 0 values with 0.001 before transformation) for our analyses of variability and exposure predictors. For biomarkers with an overall DF between 7 and 31% (BCEtP, DBuP, DPCP) we created binary variables (not detected vs. detected). We excluded biomarkers with an overall DF < 7% (BCPP, DBzP, DOCP, TBBA) from further analyses. Descriptive statistics for these excluded biomarkers are reported in Supplemental Table 1.
We estimated Spearman correlations for BDCPP and DPHP between day 4 (weekday) and day 7 (weekend) samples, by each seasonal visit. For overall correlations, we accounted for the non-independence of samples by first calculating a z-score for each log2-distributed biomarker concentration (adjusted for visit and day), then used a linear mixed effects regression model with a random intercept for each participant to model the association of day 4 with day 7 biomarker z-scores. The resulting regression coefficient is interpreted as the repeated measures Pearson correlation [49]. For BCEtP, DBuP and DPCP, we estimated tetrachoric correlations between day 4 and day 7 samples, by season. For overall tetrachoric correlations, we estimated bivariate probit mixed effects models with a random intercept for participants. Briefly, bivariate probit regression jointly models the latent continuous variables manifested as day 4 and day 7 dichotomous biomarker concentrations, with the Pearson correlation of the latent variables being a direct estimate of the tetrachoric correlation of the dichotomous biomarker concentrations. For each urinary OPE biomarker, intraclass correlations were estimated using mixed effects regression models with random intercepts for participants. For highly detected biomarkers, we used the following formula: , where represents the variance between individuals and γ represents within-individual variance for linear mixed models. For dichotomous exposures we adjusted the formula by using the variance of the standard logistic regression distribution (π2/3) as an estimate of γ.
For our analysis of potential predictors of urinary OPE concentrations, we included baseline demographic characteristics such as participant’s gender, race, age at study enrollment, BMI, type of health insurance (i.e., private/self-pay or public), identity of the participant’s primary caregiver, and the primary caregiver’s highest level of education. We also examined the following household characteristics assessed at baseline: housing type (i.e., detached, row house, apartment) and number of residents living in the household. We examined several time-varying characteristics that were assessed once per week-long study visit including the presence (yes/no) of the following flooring types in the child’s bedroom: hardwood floors, tile/linoleum, wall to wall carpeting, large rugs, and scatter rugs. Finally, we examined time-varying characteristics that were assessed on the day of urine sample collection including smoking in the home (yes/no), time spent outdoors and indoors by the child (i.e., hours per day), riding in a vehicle (yes/no), cleaning practices (yes/no) (i.e., mopping, vacuuming, dusting) and use of oven or specific air systems (yes/no) (i.e., heater, central air, air conditioning, and air purifying) within the household.
We used generalized estimating equations (GEE) to assess potential OPE predictors using linear regression for continuous log2 concentrations for BDCPP and DPHP, and logistic regression for binary variables (i.e., not detected and detected) for BCEtP, DBuP, and DPCP. These models adjusted for non-independence of repeated observations within children by treating child as a clustering variable and assumed an exchangeable working correlation matrix. From the linear regression models, we estimated the percent difference in urinary OPE biomarker concentrations as (e(β)-1 * 100% with 95% confidence intervals (CIs) estimated as (2(β ± 1.96 × SE) −1), where β and SE are the estimated regression coefficient and standard error, respectively. To account for within-week and within-visit correlation of OPE concentrations, all models included a random effect for person and adjusted for seasonal visit and day. From the logistic regression models, we estimated prevalence odds ratios (PORs, 95% CI) for OPE biomarker detection associated with each predictor. To identify predictors of each OPE biomarker, we first assessed univariate associations with all candidate predictors (Supplemental Table 2). Next, we included all predictors with univariate p-values < 0.20 in fully adjusted biomarker-specific multivariable models. For categorical variables, we calculated the Wald type 3 test statistic.
Finally, we conducted a secondary analysis of potential dietary predictors of OPE concentrations by examining information on weekly food intake (i.e., meat, fish, fast food, soda and juice, etc.). We considered this a secondary analysis due to missing diet questionnaire information for 80 of 179 (45%) study participants, which limited our power to detect associations or conduct multivariable modeling. Therefore, our secondary analyses explored only univariate associations with each OPE, using the same modeling approach described above.
For all adjusted multivariable models, we applied a statistical significance criterion of p<0.05. Correlation analyses were conducted in STATA v17.0 (StataCorp, College Station, TX, USA) and all other analyses were conducted in SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
After excluding observations with missing home visit questionnaire information, our final study sample was comprised of 179 participants with OPE concentrations measured on a total of 618 observation days. Of these 179 children, a little over half were male (53%), most were Black (94%), nearly half were overweight or obese (48%), and the median age at enrollment was 9 years (Table 1). Among characteristics assessed at each study visit (i.e., time-varying) few children’s bedrooms contained hardwood floors (24%), tile/linoleum (25%), wall-to-wall carpeting (42%), large rugs (5%) or scatter rugs (8%). Most participants reported spending greater than half of each collection day indoors (55%). Few participants reported vacuuming (17%), dusting (23%), or mopping (37%) on the day of sample collection while sweeping was more common (58%). Air conditioning, air purification, central air, and heat were not frequently used on the day of sample collection. Stoves were more frequently used on the day of sample collection than ovens (70% versus 29%, respectively).
Table 1.
Baseline and time-varying characteristics for DISCOVER study participants.
| Baseline Characteristics | Na | % |
|---|---|---|
| Gender | ||
| Female | 84 | 46.9 |
| Male | 95 | 53.1 |
| Race | ||
| Black | 168 | 93.9 |
| Other | 11 | 6.1 |
| Age at enrollment (years) | ||
| 5 – 7 | 48 | 26.8 |
| 8 – 10 | 74 | 41.3 |
| 11 – 13 | 57 | 31.8 |
| BMI | ||
| Normal | 13 | 7.3 |
| Underweight | 75 | 41.9 |
| Overweight/Obese | 86 | 48.0 |
| Missing | 5 | 2.8 |
| Type of Health Insurance | ||
| Private or Other (i.e., self-pay) | 26 | 14.5 |
| Public | 153 | 85.5 |
| Primary Caregiver | ||
| Birth mother | 155 | 86.6 |
| Other | 24 | 13.4 |
| Caregiver’s Highest Education Attained | ||
| < High School | 48 | 26.8 |
| High School Graduate | 86 | 48.0 |
| ≥Some College | 44 | 24.6 |
| Missing | 1 | 0.6 |
| Type of House | ||
| Detached | 21 | 11.7 |
| Row House | 132 | 73.7 |
| Apartment | 22 | 12.3 |
| Missing | 4 | 2.2 |
| Number of Household Residents | ||
| 0 to 2 | 47 | 26.3 |
| 3 or 4 | 82 | 45.8 |
| 5 to 12 | 49 | 27.4 |
| Missing | 1 | 0.6 |
| Time Varying Characteristics | nb | % |
| Seasonal Visit | ||
| Fall | 159 | 25.7 |
| Spring | 135 | 21.8 |
| Summer | 171 | 27.7 |
| Winter | 153 | 24.8 |
| Did anyone smoke in the home today? (Yes) | 206 | 33.3 |
| Did the child ride in a vehicle today? (Yes) | 260 | 42.1 |
| Are any of the following present in the child’s bedroom? | ||
| Hardwood Floors (Yes) | 151 | 24.4 |
| Tile/Linoleum (Yes) | 155 | 25.1 |
| Wall to Wall Carpeting (Yes) | 259 | 41.9 |
| Large Rugs (Yes) | 30 | 4.9 |
| Scatter Rugs (Yes) | 50 | 8.1 |
| Hours spent per week | ||
| Indoors | ||
| ≤ 12 hours per day | 280 | 45.3 |
| > 12 hours per day | 338 | 54.7 |
| Outdoors | ||
| 0 hours per day | 334 | 54.1 |
| 1–2 hours per day | 91 | 14.7 |
| ≥ 3 hours per day | 193 | 31.2 |
| On the day of sample collection, were any of the following performed or used in the household? | ||
| Vacuuming (Yes) | 104 | 16.8 |
| Dusting (Yes) | 143 | 23.1 |
| Mopping (Yes) | 228 | 36.9 |
| Sweeping (Yes) | 356 | 57.6 |
| Use of Air conditioning (Yes) | 133 | 21.5 |
| Use of Air Purifier (Yes) | 46 | 7.4 |
| Use of Stove (Yes) | 434 | 70.2 |
| Use of Oven (Yes) | 182 | 29.4 |
| Use of Central Air (Yes) | 94 | 15.2 |
| Use of Heater (Yes) | 27 | 4.4 |
Number of study participants among a total of 179
Number of observation days among a total of 618
Study participants contributed a total of 618 urine samples across all four seasonal visits (i.e., fall, spring, summer, winter) (Table 2). Of the nine urinary OPE biomarkers examined, BDCPP and DPHP consistently exhibited the highest detection frequencies (DFs) (BDCPP DFs: 95.7% – 100%, DPHP DFs: 98.6% – 100%) across seasonal visits and days (i.e., days 4 and 7). GM concentrations for BDCPP and DPHP were highest in the summer visit irrespective of day. BCEtP, DBuP, and DPCP were less widely detected (DF range: 4.6–31.1%) while BCPP, DBzP, DOCP, and TBBA were infrequently detected (DF range: 0.0–6.6%) across seasonal visits and days (Supplemental Table 1).
Table 2.
Distribution of specific gravity-corrected urinary organophosphate ester biomarker concentrations (ng/mL) for the 179 study participants (n = 618 samples)a
| Biomarker | LOD | Season | Collection Day | nb | n ≥ LOD | DF (%) | GM | Min | p25 | p50 | p75 | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCEtP | 2.0 | Fall | 4 | 69 | 14 | 20.3 | <LOD | <LOD | <LOD | <LOD | <LOD | 11.81 |
| 7 | 90 | 17 | 18.9 | <LOD | <LOD | <LOD | <LOD | <LOD | 23.90 | |||
| Spring | 4 | 61 | 12 | 19.7 | <LOD | <LOD | <LOD | <LOD | <LOD | 12.22 | ||
| 7 | 74 | 23 | 31.1 | <LOD | <LOD | <LOD | <LOD | 2.90 | 30.84 | |||
| Summer | 4 | 78 | 21 | 26.9 | <LOD | <LOD | <LOD | <LOD | 2.27 | 19.20 | ||
| 7 | 93 | 28 | 30.1 | <LOD | <LOD | <LOD | <LOD | 3.17 | 70.80 | |||
| Winter | 4 | 66 | 8 | 12.1 | <LOD | <LOD | <LOD | <LOD | <LOD | 34.15 | ||
| 7 | 87 | 13 | 14.9 | <LOD | <LOD | <LOD | <LOD | <LOD | 41.22 | |||
| BDCPP | 0.10 | Fall | 4 | 69 | 66 | 95.7 | 1.10 | <LOD | 0.71 | 1.14 | 2.03 | 95.10 |
| 7 | 90 | 89 | 98.9 | 1.24 | <LOD | 0.70 | 1.29 | 2.10 | 20.58 | |||
| Spring | 4 | 61 | 60 | 98.4 | 1.61 | <LOD | 0.85 | 1.68 | 3.41 | 16.10 | ||
| 7 | 74 | 73 | 98.6 | 1.85 | <LOD | 0.99 | 1.59 | 3.67 | 24.00 | |||
| Summer | 4 | 78 | 76 | 97.4 | 2.22 | <LOD | 1.33 | 2.33 | 4.43 | 46.58 | ||
| 7 | 93 | 92 | 98.9 | 2.27 | <LOD | 1.30 | 2.32 | 4.40 | 34.16 | |||
| Winter | 4 | 66 | 65 | 98.5 | 0.88 | <LOD | 0.60 | 1.08 | 1.71 | 4.58 | ||
| 7 | 87 | 87 | 100.0 | 1.01 | 0.23 | 0.60 | 0.96 | 1.53 | 7.29 | |||
| DBuP | 0.50 | Fall | 4 | 69 | 11 | 15.9 | <LOD | <LOD | <LOD | <LOD | <LOD | 3.09 |
| 7 | 90 | 13 | 14.4 | <LOD | <LOD | <LOD | <LOD | <LOD | 13.20 | |||
| Spring | 4 | 61 | 5 | 8.2 | <LOD | <LOD | <LOD | <LOD | <LOD | 5.74 | ||
| 7 | 74 | 11 | 14.9 | <LOD | <LOD | <LOD | <LOD | <LOD | 8.09 | |||
| Summer | 4 | 78 | 10 | 12.8 | <LOD | <LOD | <LOD | <LOD | <LOD | 18.88 | ||
| 7 | 93 | 11 | 11.8 | <LOD | <LOD | <LOD | <LOD | <LOD | 6.19 | |||
| Winter | 4 | 66 | 6 | 9.1 | <LOD | <LOD | <LOD | <LOD | <LOD | 1.78 | ||
| 7 | 87 | 4 | 4.6 | <LOD | <LOD | <LOD | <LOD | <LOD | 0.89 | |||
| DPCP | 0.10 | Fall | 4 | 69 | 16 | 23.2 | <LOD | <LOD | <LOD | <LOD | <LOD | 1.24 |
| 7 | 90 | 11 | 12.2 | <LOD | <LOD | <LOD | <LOD | <LOD | 1.00 | |||
| Spring | 4 | 61 | 11 | 18.0 | <LOD | <LOD | <LOD | <LOD | <LOD | 0.61 | ||
| 7 | 74 | 10 | 13.5 | <LOD | <LOD | <LOD | <LOD | <LOD | 1.72 | |||
| Summer | 4 | 78 | 15 | 19.2 | <LOD | <LOD | <LOD | <LOD | <LOD | 5.38 | ||
| 7 | 93 | 18 | 19.4 | <LOD | <LOD | <LOD | <LOD | <LOD | 1.07 | |||
| Winter | 4 | 66 | 12 | 18.2 | <LOD | <LOD | <LOD | <LOD | <LOD | 2.31 | ||
| 7 | 87 | 9 | 10.3 | <LOD | <LOD | <LOD | <LOD | <LOD | 0.83 | |||
| DPHP | 0.11 | Fall | 4 | 69 | 68 | 98.6 | 1.77 | <LOD | 0.85 | 1.63 | 3.58 | 56.07 |
| 7 | 90 | 90 | 100.0 | 1.69 | 0.23 | 0.91 | 1.46 | 2.96 | 15.13 | |||
| Spring | 4 | 61 | 61 | 100.0 | 2.19 | 0.34 | 1.03 | 1.59 | 3.95 | 104.35 | ||
| 7 | 74 | 73 | 98.6 | 2.01 | <LOD | 1.05 | 1.64 | 2.71 | 211.20 | |||
| Summer | 4 | 78 | 78 | 100.0 | 2.56 | 0.25 | 1.17 | 2.07 | 5.01 | 66.98 | ||
| 7 | 93 | 93 | 100.0 | 2.55 | 0.34 | 1.13 | 2.06 | 3.84 | 314.53 | |||
| Winter | 4 | 66 | 66 | 100.0 | 1.96 | 0.26 | 1.01 | 1.57 | 3.90 | 29.84 | ||
| 7 | 87 | 87 | 100.0 | 1.68 | 0.22 | 0.75 | 1.56 | 3.14 | 22.08 |
Concentrations are based on machine read values. For geometric mean calculations, values of 0 were set to 0.001
Number of samples collected per season and day.
Abbreviations: LOD = Limit of Detection, DF = Detection Frequency, GM = Geometric Mean, BCEtp = bis(2-chloroethyl) phosphate, BCPP= bis(1-chloro-2-propyl) phosphate, BDCPP= bis(1,3-dichloro-2-propyl) phosphate, DBuP= di-benzyl phosphate, DBzP=di-benzyl phosphate, DOCP=di-o-cresylphosphate, DPCP=di-p-cresylphosphate, DPHP= di-(2-propylheptyl) phthalate, TBBA=2,3,4,5-tetrabromo benzoic acid
Spearman and tetrachoric correlation coefficients between day 4 (weekday) and 7 (weekend) concentrations of each OPE biomarker are presented in Table 3. BDCPP and BCEtP had the highest overall correlations of rs=0.50 and rtet=0.63. When stratified by season, the highest within-week correlations were in the summer for both BDCPP (rs=0.72) and BCEtP (rtet=0.79). DPHP had moderate overall correlation (rs=0.40) and season-stratified correlations were similar. Both DBuP and DPCP had lower overall correlations (rtet=0.31). Intra-individual variability of OPE biomarkers over the four seasonal visits are reported in Table 4. In general, we observed greater within person than between person variability in OPE biomarker concentrations (overall ICCs: 0.18–0.38). There were some differences in ICCs for day 4 (weekday) concentrations versus day 7 (weekend) concentrations, but no consistent pattern across OPE biomarkers.
Table 3.
Spearman and tetrachoric correlation coefficients between specific gravity-corrected urinary organophosphate ester biomarker concentrations measured on days 4 and 7 for participating children: overall and stratified by seasonal visit.a
| Biomarker | Overalla | Fall | Spring | Summer | Winter |
|---|---|---|---|---|---|
| BDCPPb | 0.50 | 0.53 | 0.27 | 0.72 | 0.45 |
| DPHPb | 0.40 | 0.42 | 0.49 | 0.31 | 0.45 |
| BCEtPc | 0.63 | 0.32 | 0.67 | 0.79 | 0.62 |
| DBuPc | 0.31 | NA† | 0.23 | 0.65 | 0.61 |
| DPCPc | 0.31 | −0.12 | 0.67 | 0.24 | 0.88 |
Accounted for repeated measures using random intercept for participant
Spearman correlations
Tetrachoric correlations
Not applicable; no participants had a detected concentration at day 4 and day 7
Abbreviations: BDCPP= bis(1,3-dichloro-2-propyl) phosphate, DPHP= di-(2-propylheptyl) phthalate, BCEtp = bis(2-chloroethyl) phosphate, DBuP= di-benzyl phosphate, DPCP=di-p-cresylphosphate
Table 4.
Intraclass correlation coefficients (95% confidence intervals) of specific gravity-corrected urinary organophosphate ester biomarker concentrations measured at four seasonal visits for participating children: overall and stratified by day.a
| Biomarker | Overall | Day 4 | Day 7 |
|---|---|---|---|
| BDCPP | 0.28 (0.19, 0.39) | 0.43 (0.29, 0.57) | 0.24 (0.12, 0.42) |
| DPHP | 0.25 (0.17, 0.36) | 0.24 (0.11, 0.44) | 0.29 (0.17, 0.45) |
| BCEtP | 0.38 (0.23, 0.56) | 0.19 (0.03, 0.65) | 0.34 (0.15, 0.61) |
| DBuP | 0.18 (0.05, 0.46) | NA† | 0.09 (0.00, 0.90) |
| DPCP | 0.33 (0.17, 0.54) | 0.31 (0.10, 0.66) | 0.31 (0.11, 0.62) |
Number of samples: Day 4 = 201; Day 7=237; All = 270
Not applicable; model did not converge
Abbreviations: BDCPP= bis(1,3-dichloro-2-propyl) phosphate, DPHP= di-(2-propylheptyl) phthalate, BCEtp = bis(2-chloroethyl) phosphate, DBuP= di-benzyl phosphate, DPCP=di-p-cresylphosphate
Results from our multivariable predictor analysis examining baseline and time-varying characteristics are reported in Table 5 (univariate model results are shown in Supplemental Table 2). For those OPE biomarkers examined continuously, we found that DPHP concentrations were significantly higher in the summer compared to the fall (%diff: 28.3%; 95% CI: 0.1, 64.6), lower among males compared to females (%diff: −53.5%; 95% CI: −62.7, −42.0), and lower among those spending more than 12 hours per day indoors on the day of sample collection compared to less than 12 hours (%diff: −20.7%; 95% CI: −32.2, −7.3). BDCPP concentrations were significantly higher in the summer compared to the fall (%diff: 62.5%; 95% CI: 12.1, 133.7) and lower among children aged 8–10 years (%diff: −39.1%; 95% CI: −55.9, −15.9) or 11–13 years (%diff: −51.1%; 95% CI: −65.4, −31.1%) compared to children aged 5–7 years. In addition, BDCPP concentrations were significantly higher among children who reported riding in a vehicle on the day of sample collection compared to those who did not (%diff: 28.5%; 95% CI: 3.4, 59.8), as well as among those households reporting use of central air on the day of sample collection (%diff: 33.5%; 95% CI: 0.1, 78.0).
Table 5.
Percent differences or prevalence odds ratios (95% confidence interval) of OPE-specific multivariable associations between predictors and specific gravity-corrected urinary organophosphate ester biomarker concentrations.a
| Characteristic | nb | % | DPHPc %Diff (95% CI) |
BDCPPc %Diff (95% CI) |
BCEtPd POR (95% CI) |
DBuPd POR (95% CI) |
DPCPd POR (95% CI) |
|
|---|---|---|---|---|---|---|---|---|
| Season | Spring (vs Fall) | 135 | 21.8 | 19.0(−8.1,54.1) | 34.9(−0.6,83.0)† | 1.72(0.95,3.12)† | 1.12(0.44,2.86) | 0.63(0.21,1.84) |
| Summer (vs Fall) | 171 | 27.7 | 28.3(0.1,64.6)* | 62.5(12.9,133.7)**,† | 0.98(0.33,2.91)† | 1.39(0.26,7.33) | 1.34(0.74,2.44) | |
| Winter (vs Fall) | 153 | 24.8 | 11.2(−10.3,37.8) | −12.5(−34.4,16.7)† | 0.64(0.32,1.27)† | 0.76(0.26,2.19) | 0.72(0.39,1.33) | |
| Day | 7 (vs 4) | 344 | 55.7 | −7.4(−20.4,7.8) | 12.8(−7.0,36.8) | 1.35(0.95,1.90) | 1.01(0.67,1.54) | 0.56(0.37,0.85)** |
| BASELINE | ||||||||
| Gender | Male (vs Female) | 349 | 56.5 | −53.5(−62.7,−42.0) ** | 0.73(0.41,1.30) | |||
| Race | Black (vs Other) | 575 | 93.0 | |||||
| Age at Enrollment | 8–10 yrs (vs 5–7) | 281 | 45.5 | −39.1(−55.9,−15.9) **, † | 0.70(0.37,1.32) | |||
| 11–13 yrs (vs 5–7) | 181 | 29.3 | −51.1(−65.4,−31.1) **, † | 0.46(0.21,1.04) | ||||
| BMI | Normal (vs Underweight) | 42 | 43.5 | 2.04(0.88,4.70) | ||||
| Overweight/Obese (vs Underweight) | 286 | 46.3 | 1.49(0.80,2.78) | |||||
| Type of Health Insurance | ||||||||
| Public (vs Private or self-pay) | 512 | 82.9 | −0.4(−25.2,32.6) | −21.9(−42.0,5.0) | ||||
| Caregiver’s Highest Education | ||||||||
| High School Graduate (vs < High School) | 316 | 51.1 | 18.4(−8.1,52.6) | 2.54(1.21,5.33)**, † | ||||
| ≥ Some College (vs < High School) | 156 | 25.2 | −11.5(−34.8,20.1) | 1.74(0.74,4.11)† | ||||
| Type of House | Row House (vs Detached) | 468 | 75.7 | 1.35(0.55,3.33) | ||||
| Apartment (vs Detached) | 66 | 10.7 | 1.78 (0.47,6.74) | |||||
| # of Residents | 3 or 4 (vs 0 to 2) | 281 | 45.5 | 1.53(0.83,2.84) | 1.13(0.52,2.46) | |||
| 5 to 12 (vs 0 to 2) | 165 | 26.7 | 0.95(0.45,1.97) | 1.70(0.83,3.47) | ||||
| TIME-VARYING | ||||||||
| Did anyone smoke in the home today? | ||||||||
| Yes (vs No) | 206 | 33.3 | 6.2(−15.8,34.0) | 0.55(0.31,0.97)* | ||||
| Did child ride in a vehicle today? | ||||||||
| Yes (vs No) | 260 | 42.1 | 28.5(3.4,59.8)* | 1.31(0.83,2.07) | ||||
| Are any of the following present in the child’s bedroom? | ||||||||
| Hardwood Floors | Yes (vs No) | 219 | 35.4 | 1.20(0.65,2.21) | ||||
| Tile/Linoleum | Yes (vs No) | 155 | 25.1 | −10.1(−29.3,14.2) | 0.54(0.27,1.09) | |||
| Wall to Wall Carpeting | Yes (vs No) | 259 | 41.9 | 0.5(−20.8,27.5) | ||||
| Large Rug | Yes (vs No) | 30 | 4.8 | −7.3(−36.1,34.5) | −18.0(−49.9,34.1) | 0.44(0.07,2.76) | ||
| Time spent per week: | ||||||||
| Indoors | > 12 hrs per day (vs ≤ 12) | 283 | 45.8 | −20.7(−32.2,−7.3)** | −6.3(−23.2,14.3) | |||
| Outdoors | 1–2 hrs per day (vs 0) | 56 | 9.1 | −23.1(−41.0,0.1) | −12.5(−35,17.9) | 0.92(0.34,2.47) | 0.30(0.10,0.95)*, † | |
| ≥ 3 hrs per day (vs 0) | 283 | 45.8 | 10.1(−8.3,32.1) | 23.3(−6.5,62.8) | 1.59(0.88,2.87) | 0.97(0.56,1.66)† | ||
| On the day of sample collection, were any of the following performed or used in the household? | ||||||||
| Vacuuming | Yes (vs No) | 104 | 16.8 | 20.4(−6.0,54.2) | 3.7(−16.2,28.3) | |||
| Dusting | Yes (vs No) | 143 | 23.1 | 0.59(0.34,1.03) | ||||
| Mopping | Yes (vs No) | 228 | 36.9 | 1.17(0.64,2.13) | 1.71(1.09,2.69)* | |||
| Sweeping | Yes (vs No) | 356 | 57.6 | 1.45(0.83,2.52) | ||||
| Use of Air Purifier | Yes (vs No) | 46 | 7.4 | 0.48(0.17,1.38) | ||||
| Use of Stove | Yes (vs No) | 434 | 70.2 | 1.33(0.84,2.12) | ||||
| Use of Oven | Yes (vs No) | 182 | 29.5 | 15.3(−3.1,37.1) | ||||
| Use of Central Air | Yes (vs No) | 94 | 15.2 | 33.0(−0.6,77.9) | 33.5(0.1,78.0)* | 1.65(0.69,3.96) | ||
| Use of Heater | Yes (vs No) | 27 | 4.40 | 1.92(0.82,4.53) | ||||
All models were adjusted for season and day of sample collection to account for the cohort panel study design. For each OPE, all predictors were included in a single multivariable model and thus estimates are co-adjusted. Variables with no estimate were not included in the model for that OPE
Number of observation days out of 618 total observation days among 179 study participants
BDCPP and DPHP were assessed using linear regression for continuous log2 biomarker concentrations
BCEtP, DBuP and DPCP were assessed using logistic regression and modeled as detect vs. non-detect
Abbreviations: CI= Confidence interval, POR= Prevalence odds ratio; BCEtp = bis(2-chloroethyl) phosphate, BDCPP= bis(1,3-dichloro-2-propyl) phosphate, DBuP= di-benzyl phosphate, DPCP=di-p-cresylphosphate, DPHP= di-(2-propylheptyl) phthalate
p < 0.05,
p < 0.01,
Type-3 p < 0.05
For those OPE biomarkers examined as detect/non-detect we found detectable BCEtP concentrations were significantly associated with a child’s primary caregiver having graduated from high school compared to not having graduated from high school (POR: 2.54, 95% CI: 1.21, 5.33). DPCP detection was positively associated with mopping on the day of sample collection (POR: 1.71; 95% CI: 1.09, 2.69) but inversely associated with smoking in the household (POR: 0.55; 95% CI: 0.31, 0.97) and spending between 1–2 hours compared to no time outdoors (POR: 0.30; 95% CI: 0.10, 0.95). DPCP detection was also inversely associated with weekend (day 7) vs. weekday (day 4) urine specimen collection. No statistically significant associations were found between any potential exposure predictors and DBuP detection.
R2 values for each of our final adjusted multivariable predictor models were low (i.e., DPHP, R2=0.20; BDCPP, R2=0.16; BCEtP, R2=0.07; DBuP, R2=0.04; DPCP, R2=0.13).
In univariate secondary analyses, we found no significant associations of any urinary OPE biomarker concentrations with weekly food intake (i.e., meat, fish, fast food, soda and juice, etc.) (Supplemental Table 3).
DISCUSSION
In this study we characterized OPE exposures among school-aged children, we found nearly universal detection of BDCPP and DPHP with higher concentrations during the summer months. Despite low DFs for DBzP, DOCP, TBBA and BCPP, we are the first to our knowledge to report detectable levels of DBzP in human urine samples [3, 50, 51]. We observed moderate correlation of within-week OPE biomarker concentrations (i.e., visit-days 4 and 7) and low to moderate reproducibility of concentrations over nine months. Predictors of higher OPE concentrations in our sample of school-aged children included female gender, younger age, more education of the primary caregiver, more time spent indoors, and some home characteristics. However, these variables explained little of the variation in OPE biomarker concentrations in our population.
Consistent with results from previous biomonitoring studies among children, concentrations of BDCPP and DPHP were highest among the OPE urinary biomarkers [3, 26, 28, 30, 33, 52]. Moreover, GM concentrations of DPHP and BDCPP were similar to those reported in a recent biomonitoring report of NHANES data (2013–2014) by the U.S. Centers for Disease Control and Prevention [52]. Among NHANES children ages 6–11 years, GMs were 2.25 ng/mL for BDCPP and 1.69 ng/mL for DPHP, comparable to our GMs of 1.43 ng/mL for BDCPP and 1.84 ng/mL for DPHP. Concentrations of DPHP and BDCPP were two to three times higher among children in our study and among NHANES children aged 6–11 years compared to NHANES adults [3, 26]. Several biomonitoring studies among mother-child pairs have found BDCPP levels to be significantly greater in children than those of their mothers [27–29, 31, 32]. Metabolic differences and greater body surface area to body size ratios in children may contribute to their higher OPE biomarker concentrations than when compared to adults [24].
We found DPHP and BDCPP concentrations to be higher in the summer compared to other seasons. A study of OPE residential exposures among children (ages 3–6 years) found outdoor air temperature to be a significant predictor of urinary BDCPP concentrations with a 1°C increase in temperature corresponding to a 4% increase in urinary BDCPP [35]. Another study in a pooled analysis of children aged ≤10 years also examined seasonal trends in OPE exposures similarly reporting significantly higher concentrations of DPHP and BDCPP in the summer compared to winter [9]. Increased temperature may increase emissions of flame retardants from consumer products and their subsequent volatilization in indoor environments [53]. Our findings lend additional evidence that season of sample collection may be an important factor to consider in assessing environmental exposures to OPEs among children.
We generally found moderate Spearman and tetrachoric correlation coefficients of within visit-week samples (i.e., days 4 and 7), suggesting that OPE exposure sources and patterns may vary within week and specifically differ between weekdays (day 4) and weekends (day 7). Given the relatively short biological half-life of most OPE biomarkers reported [4, 25], multiple samples per time period of interest may be necessary to fully capture OPE exposures. Some exceptions where we found high correlations include the summer visit for BDCPP (rs = 0.72), summer visit for BCEtP (rtet = 0.79), and winter visit for DPCP (rtet = 0.88). Higher correlation of some OPE biomarkers in the summer may be due to children being in the same environment on weekdays and weekends, compared to other seasons when children attend school on weekdays.
In addition, we found low to moderate ICCs for all OPE biomarkers across the four seasonal visits (overall and when stratified by day), indicating low reproducibility of OPE biomarker concentrations over nine months. While no studies have examined urinary OPE biomarker variability among children, one study of seven men with nine repeat measurements of OPE biomarkers over a three month period reported moderate to strong intra-individual reliability for BDCPP (ICC range: 0.55–0.72) [43]. Other studies examining intra-individual variability in OPE biomarker concentrations have focused on pregnant women with ICCs ranging between 0.1 and 0.8 depending on the biomarker analyzed and time period of interest [41, 42, 44]. Several explanations may contribute to the variability differences between studies including OPE exposure sources, biological half-lives of individual OPEs, timing and number of samples measured, and metabolic differences between study populations.
We found associations of certain baseline demographic characteristics with OPE biomarker concentrations. Compared to females, males had lower concentrations of most OPE biomarkers which remained statistically significant in multivariable models for DPHP. Similar to our findings, Ospina et al. reported higher urinary biomarker concentrations for DPHP among females compared to males aged 6–80 years in the U.S. general population, however the authors did not assess sex differences specifically among children [3]. In our study, age was inversely associated with concentrations of most OPE biomarkers, remaining significantly associated with BDCPP concentrations in multivariable models. Several studies have consistently reported that age is inversely associated with urinary BDCPP biomarker concentrations [3, 26, 29, 33], including NHANES analyses that reported BDCPP concentrations to decrease with increasing childhood age [3, 26]. Greater inhalation and metabolic rate, different breathing zone, and increased hand-to-mouth behavior may explain increased OPE exposure differences between children and adults [29]. Finally, higher primary caregiver educational attainment was associated with detection of BCEtP. To our knowledge, socioeconomic factors such as caregiver educational attainment have not been previously assessed as an OPE exposure predictor among children. However, due to the increasing use of OPEs as replacements for PBDEs, higher educational attainment may be related to the purchasing of newer-OPE containing products [8].
Determinants related to where children spent their time were significantly associated with several OPE biomarkers. Spending more time indoors (i.e., > 12 hours per day) and less time outdoors (i.e., 1–2 hours per day) were significantly associated with higher concentrations of DPHP and detectable concentrations of DPCP respectively. While no studies have examined time spent indoors or outdoors as a predictor for OPE biomarker concentrations, ventilation may be an important factor contributing to OPE exposures [54]. Previous studies have found OPEs are widely incorporated into construction materials and electrical cable equipment [4]. Thus, it is possible that more time spent indoors and less time spent outdoors may increase exposures to these OPE sources. We also found that riding in a vehicle was significantly associated with higher concentrations of BDCPP, which is commonly found in upholstery and plastics used in the manufacturing of vehicle interiors [4, 55–57]. Previous studies have suggested that the volatilization and subsequent off-gassing of TDCIPP (parent compound to BDCPP) in these materials may contribute to increased exposures when riding or commuting in a vehicle, particularly within densely populated traffic-congested areas [55, 57].
Lastly, several household characteristics and cleaning behaviors were associated with some OPE biomarkers. Specifically, we found mopping on the day of sample collection to be significantly associated with a lower prevalence of DPCP detection. While this finding is consistent with some studies suggesting that increased cleaning frequency reduces indoor OPE exposures [58], other studies have suggested that increasing the frequency of cleaning practices may resuspend OPEs in the indoor environment, increasing indoor OPE exposures [59]. In addition, the presence of smoking in the household was significantly associated with a lower likelihood of detectable DPCP concentrations. To our knowledge, no studies have examined associations of urinary OPE concentrations among children with household smoking. While this may be a spurious finding it may also reflect increased ventilation in the home while smoking is present, however we were unable to examine this in our study. Further exposure studies examining predictors of urinary OPE biomarker concentrations in children are warranted to corroborate and expand upon these results.
In sensitivity analyses assessing dietary factors, we did not find strong evidence that meat, fish, fast food, soda, or juice consumption was associated with OPE biomarker concentrations. OPEs are used as plasticizers in many products that treat, process and package foodstuffs [4, 51, 60–62]. Research on potential dietary sources of OPE exposure remain sparse. Some studies have reported high concentrations of OPEs in rice [21], cereal products [63], fish [34, 64], meat [34, 64, 65], and packaged foodstuffs [61–63]. However, a recent NHANES analysis found no significant associations between urinary OPE concentrations and intake of ultra or minimally processed foods [62]. Understanding the potential for OPE exposure in food packaging is an important consideration for future studies examining OPE exposure among children.
Our study has multiple notable strengths. To our knowledge, this is the first study to examine OPE urinary biomarkers and exposure predictors among school-aged children. Previous analyses among children have focused predominantly on infants and preschool-aged children who exhibit different behavioral and time-activity patterns than school-aged children [27–39]. To accurately characterize exposures to these short-lived biomarkers, it is important to assess their stability and temporal variability. Our cohort panel study design and repeat urine specimen collection allowed us for to examine both seasonal and within-week intraindividual variability of OPE biomarkers in a pediatric population. Characterizing this variability is important to understanding potential exposure misclassification when single measurements of exposure are used [45]. Our questionnaires also collected detailed information on many possible predictors including baseline demographics and time-varying characteristics such as household characteristics, lifestyle behaviors and cleaning practices. Importantly, our questionnaire information was also captured on the same day of urine sample collection thereby decreasing the likelihood of recall bias.
We also note several limitations of our study. We adjusted our models for season as a confounder to account for seasonal differences in OPE exposures and predictors, but we were unable to assess season as a potential effect modifier due to small samples sizes in stratified analyses. Additionally, we did not have information on use of specific OPE-containing products such as electronics, building materials, and furniture foam [1, 4, 66], thereby limiting our ability to characterize sources of OPE exposures more specifically among our study participants. Our analyses included many possible determinants of OPE exposures, and therefore we conducted multiple tests and it is possible some associations were due to chance. Another limitation includes the possible lack of generalizability of our findings due to our focus on a pediatric population of urban Black asthmatic children. It is possible that OPE exposure levels may vary between households with and without an asthmatic child or between those in urban versus non-urban areas. For example, cleaning practices, flooring type, ventilation, or smoking behaviors may vary between these households, thereby impacting OPE exposures. Despite these potential differences, our findings provide an important contribution given the need for additional studies among school-aged children as well as historically understudied and underrepresented populations.
In summary, we found associations of certain OPE biomarkers with demographic characteristics, household practices, and time activity patterns, though our comprehensive questionnaire data explained little of the variability in urinary OPE concentrations in our population. We also found low to moderate within-week correlation and seasonal intra-individual variability of OPE concentrations among a population of predominantly Black school-aged children. With the increased use of OPEs as replacement chemicals for PBDEs in a wide range of consumer products [1, 8, 13, 51] and the rising health concerns linked with exposures to OPEs [67–71], it is important that we understand sources and routes of exposures among populations vulnerable to the potential adverse health effects of OPE exposures, including children with chronic health conditions or those from marginalized groups. Further studies are warranted to replicate and expand upon our findings among different study populations, while assessing additional potential predictors of OPE exposures such as diet and consumer products.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by an NIEHS/NIH Award (U2CES026553). Lydia M. Louis was supported by an NIEHS Training Grant (T32ES007141). Lesliam Quirós-Alcalá was supported by an NHLBI Career Development Award (K01HL138124). Nadia N. Hansel was supported by an NIEHS Award (P50ES018176). Meredith C. McCormack was supported by an NIEHS Career Development Award (K23ES016819). Gregory Diette was supported by an NIEHS Award (P50 ES015903). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Stapleton HM, et al. , Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol, 2009. 43(19): p. 7490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Environmental Protection Agency, U.S., Furniture Flame Retardancy Partnership: Envoronmental Profiles of Chemical Flame-Retardant Alternatives for Low-Density Polyurethane Foam. September 2005.
- 3.Ospina M, et al. , Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int, 2018. 110: p. 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, et al. , A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants (OPFRs). Int J Mol Sci, 2019. 20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendelsohn E, et al. , Nail polish as a source of exposure to triphenyl phosphate. Environ Int, 2016. 86: p. 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Veen I and de Boer J, Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere, 2012. 88(10): p. 1119–53. [DOI] [PubMed] [Google Scholar]
- 7.Hou R, Xu Y, and Wang Z, Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere, 2016. 153: p. 78–90. [DOI] [PubMed] [Google Scholar]
- 8.Blum A, et al. , Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ Sci Technol Lett, 2019. 6(11): p. 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman K, et al. , Temporal Trends in Exposure to Organophosphate Flame Retardants in the United States. Environ Sci Technol Lett, 2017. 4(3): p. 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim UJ and Kannan K, Occurrence and Distribution of Organophosphate Flame Retardants/Plasticizers in Surface Waters, Tap Water, and Rainwater: Implications for Human Exposure. Environ Sci Technol, 2018. 52(10): p. 5625–5633. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, et al. , Occurrence of organophosphate flame retardants in farmland soils from Northern China: Primary source analysis and risk assessment. Environ Pollut, 2019. 247: p. 832–838. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton HM, et al. , Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol, 2012. 46(24): p. 13432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapleton HM, et al. , Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol, 2008. 42(18): p. 6910–6. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman K, et al. , Monitoring indoor exposure to organophosphate flame retardants: hand wipes and house dust. Environ Health Perspect, 2015. 123(2): p. 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim UJ, et al. , Occurrence of and human exposure to organophosphate flame retardants/plasticizers in indoor air and dust from various microenvironments in the United States. Environ Int, 2019. 125: p. 342–349. [DOI] [PubMed] [Google Scholar]
- 16.Wu M, et al. , Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere, 2016. 150: p. 465–471. [DOI] [PubMed] [Google Scholar]
- 17.Tajima S, et al. , Detection and intake assessment of organophosphate flame retardants in house dust in Japanese dwellings. Sci Total Environ, 2014. 478: p. 190–9. [DOI] [PubMed] [Google Scholar]
- 18.Ali N, et al. , Occurrence of alternative flame retardants in indoor dust from New Zealand: indoor sources and human exposure assessment. Chemosphere, 2012. 88(11): p. 1276–82. [DOI] [PubMed] [Google Scholar]
- 19.Ali N, et al. , Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: implication for human exposure via dust ingestion. Environ Int, 2013. 55: p. 62–70. [DOI] [PubMed] [Google Scholar]
- 20.Yadav IC, et al. , Occurrence and fate of organophosphate ester flame retardants and plasticizers in indoor air and dust of Nepal: Implication for human exposure. Environ Pollut, 2017. 229: p. 668–678. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, et al. , Rice ingestion is a major pathway for human exposure to organophosphate flame retardants (OPFRs) in China. J Hazard Mater, 2016. 318: p. 686–693. [DOI] [PubMed] [Google Scholar]
- 22.Li J, et al. , Occurrence of organophosphate flame retardants in drinking water from China. Water Res, 2014. 54: p. 53–61. [DOI] [PubMed] [Google Scholar]
- 23.Cequier E, et al. , Occurrence of a broad range of legacy and emerging flame retardants in indoor environments in Norway. Environ Sci Technol, 2014. 48(12): p. 6827–35. [DOI] [PubMed] [Google Scholar]
- 24.Doherty BT, et al. , Organophosphate Esters: Are These Flame Retardants and Plasticizers Affecting Children’s Health? Curr Environ Health Rep, 2019. 6(4): p. 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tongue ADW, et al. , Flame retardant concentrations and profiles in wild birds associated with landfill: A critical review. Environ Pollut, 2019. 248: p. 646–658. [DOI] [PubMed] [Google Scholar]
- 26.Boyle M, Buckley JP, and Quiros-Alcala L, Associations between urinary organophosphate ester metabolites and measures of adiposity among U.S. children and adults: NHANES 2013–2014. Environ Int, 2019. 127: p. 754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butt CM, et al. , Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol, 2014. 48(17): p. 10432–8. [DOI] [PubMed] [Google Scholar]
- 28.Cequier E, et al. , Human exposure pathways to organophosphate triesters - a biomonitoring study of mother-child pairs. Environ Int, 2015. 75: p. 159–65. [DOI] [PubMed] [Google Scholar]
- 29.Van den Eede N, et al. , Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ Int, 2015. 74: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman K, et al. , High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environ Sci Technol, 2015. 49(24): p. 14554–9. [DOI] [PubMed] [Google Scholar]
- 31.Butt CM, et al. , Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int, 2016. 94: p. 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson EA, et al. , Differential exposure to organophosphate flame retardants in mother-child pairs. Chemosphere, 2019. 219: p. 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He C, et al. , Concentrations of organophosphate flame retardants and plasticizers in urine from young children in Queensland, Australia and associations with environmental and behavioural factors. Environ Res, 2018. 164: p. 262–270. [DOI] [PubMed] [Google Scholar]
- 34.Thomas MB, et al. , Demographic and dietary risk factors in relation to urinary metabolites of organophosphate flame retardants in toddlers. Chemosphere, 2017. 185: p. 918–925. [DOI] [PubMed] [Google Scholar]
- 35.Phillips AL, et al. , Children’s residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ Int, 2018. 116: p. 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammel SC, et al. , Comparing the Use of Silicone Wristbands, Hand Wipes, And Dust to Evaluate Children’s Exposure to Flame Retardants and Plasticizers. Environ Sci Technol, 2020. 54(7): p. 4484–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson K, et al. , Brominated Flame Retardants and Organophosphate Esters in Preschool Dust and Children’s Hand Wipes. Environ Sci Technol, 2018. 52(8): p. 4878–4888. [DOI] [PubMed] [Google Scholar]
- 38.Hammel SC, et al. , Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ Sci Technol, 2016. 50(8): p. 4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradman A, et al. , Flame retardant exposures in California early childhood education environments. Chemosphere, 2014. 116: p. 61–6. [DOI] [PubMed] [Google Scholar]
- 40.Sugeng EJ, et al. , Toddler behavior, the home environment, and flame retardant exposure. Chemosphere, 2020. 252: p. 126588. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman K, Daniels JL, and Stapleton HM, Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ Int, 2014. 63: p. 169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romano ME, et al. , Variability and predictors of urinary concentrations of organophosphate flame retardant metabolites among pregnant women in Rhode Island. Environ Health, 2017. 16(1): p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meeker JD, et al. , Urinary metabolites of organophosphate flame retardants: temporal variability and correlations with house dust concentrations. Environ Health Perspect, 2013. 121(5): p. 580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuiper JR, et al. , Predictors and reproducibility of urinary organophosphate ester metabolite concentrations during pregnancy and associations with birth outcomes in an urban population. Environ Health, 2020. 19(1): p. 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrier F, et al. , Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology, 2016. 27(3): p. 378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bose S, et al. , Vitamin D Status Modifies the Response to Indoor Particulate Matter in Obese Urban Children with Asthma. J Allergy Clin Immunol Pract, 2019. 7(6): p. 1815–1822 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayatilaka NK, et al. , Quantification of three chlorinated dialkyl phosphates, diphenyl phosphate, 2,3,4,5-tetrabromobenzoic acid, and four other organophosphates in human urine by solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem, 2017. 409(5): p. 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boeniger MF, Lowry LK, and Rosenberg J, Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J, 1993. 54(10): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 49.Bakdash JZ and Marusich LR, Repeated Measures Correlation. Front Psychol, 2017. 8: p. 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingle ME, et al. , The association of urinary organophosphate ester metabolites and self-reported personal care and household product use among pregnant women in Puerto Rico. Environ Res, 2019. 179(Pt A): p. 108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, et al. , Organophosphate (OP) diesters and a review of sources, chemical properties, environmental occurrence, adverse effects, and future directions. Environ Int, 2021. 155: p. 106691. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals: Updated Tables. March 2021, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- 53.Cao Z, et al. , Differences in the seasonal variation of brominated and phosphorus flame retardants in office dust. Environ Int, 2014. 65: p. 100–6. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, et al. , A review on organophosphate flame retardants in indoor dust from China: Implications for human exposure. Chemosphere, 2020. 260: p. 127633. [DOI] [PubMed] [Google Scholar]
- 55.Reddam A, et al. , Longer commutes are associated with increased human exposure to tris(1,3-dichloro-2-propyl) phosphate. Environ Int, 2020. 136: p. 105499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brommer S and Harrad S, Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ Int, 2015. 83: p. 202–7. [DOI] [PubMed] [Google Scholar]
- 57.Christia C, et al. , Legacy and emerging organophosphomicronrus flame retardants in car dust from Greece: Implications for human exposure. Chemosphere, 2018. 196: p. 231–239. [DOI] [PubMed] [Google Scholar]
- 58.Gibson EA, et al. , Flame retardant exposure assessment: findings from a behavioral intervention study. J Expo Sci Environ Epidemiol, 2019. 29(1): p. 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bi C, et al. , Phthalates and organophosphates in settled dust and HVAC filter dust of U.S. low-income homes: Association with season, building characteristics, and childhood asthma. Environ Int, 2018. 121(Pt 1): p. 916–930. [DOI] [PubMed] [Google Scholar]
- 60.Gbadamosi MR, Abdallah MA, and Harrad S, A critical review of human exposure to organophosphate esters with a focus on dietary intake. Sci Total Environ, 2021. 771: p. 144752. [DOI] [PubMed] [Google Scholar]
- 61.Li J, et al. , A review on organophosphate Ester (OPE) flame retardants and plasticizers in foodstuffs: Levels, distribution, human dietary exposure, and future directions. Environ Int, 2019. 127: p. 35–51. [DOI] [PubMed] [Google Scholar]
- 62.Kim H, et al. , Urinary organophosphate ester concentrations in relation to ultra-processed food consumption in the general US population. Environ Res, 2020. 182: p. 109070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poma G, et al. , Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem Toxicol, 2017. 100: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 64.Han L, Sapozhnikova Y, and Nunez A, Analysis and Occurrence of Organophosphate Esters in Meats and Fish Consumed in the United States. J Agric Food Chem, 2019. 67(46): p. 12652–12662. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y and Kannan K, Concentrations and Dietary Exposure to Organophosphate Esters in Foodstuffs from Albany, New York, United States. J Agric Food Chem, 2018. 66(51): p. 13525–13532. [DOI] [PubMed] [Google Scholar]
- 66.Stapleton HM, et al. , Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol, 2011. 45(12): p. 5323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Canbaz D, et al. , Exposure to organophosphate and polybrominated diphenyl ether flame retardants via indoor dust and childhood asthma. Indoor Air, 2016. 26(3): p. 403–13. [DOI] [PubMed] [Google Scholar]
- 68.Araki A, et al. , Combined exposure to phthalate esters and phosphate flame retardants and plasticizers and their associations with wheeze and allergy symptoms among school children. Environ Res, 2020. 183: p. 109212. [DOI] [PubMed] [Google Scholar]
- 69.Doherty BT, et al. , Prenatal exposure to organophosphate esters and cognitive development in young children in the Pregnancy, Infection, and Nutrition Study. Environ Res, 2019. 169: p. 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Araki A, et al. , Associations between allergic symptoms and phosphate flame retardants in dust and their urinary metabolites among school children. Environ Int, 2018. 119: p. 438–446. [DOI] [PubMed] [Google Scholar]
- 71.Castorina R, et al. , Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere, 2017. 189: p. 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


