Abstract
BACKGROUND
A 2-gram bolus of tranexamic acid (TXA) has been shown to reduce 28-day mortality in a RCT. This study investigates whether out-of-hospital TXA use is associated with adverse events or unfavorable outcomes in suspected TBI when intracranial hemorrhage (ICH) is absent on initial CT.
METHODS
This study utilized data from a 2015–2017, multicenter, randomized trial studying the effect of the following TXA doses on moderate to severe TBI: 2-gram bolus, 1-gram bolus plus 1-gram infusion over 8 hours, and a placebo bolus with placebo infusion. Of the 966 participants enrolled, 395 with an initial CT negative for ICH were included in this analysis. Fifteen adverse events (28-day incidence) were studied: MI, DVT, seizure, pulmonary embolism, ARDS, cardiac failure, liver failure, renal failure, CVA, cardiac arrest, cerebral vasospasm, “any thromboembolism”, hypernatremia, AKI, and infection. Other unfavorable outcomes analyzed include mortality at 28 days & 6 months, GOSE ≤ 4 at discharge & 6 months, ICU-free days, ventilator-free days, hospital-free days, and combined unfavorable outcomes. In both study drug groups the incidence of dichotomous outcomes and quantity of ordinal outcomes were compared to placebo.
RESULTS
No statistically significant increase in adverse events or unfavorable outcomes was found between either TXA dosing regimen and placebo. Demographics and injury scores were not statistically different other than two methods of injury which were overrepresented in the 1-gram TXA bolus + 1-gram TXA infusion.
CONCLUSIONS
Administration of either a 2-gram TXA bolus or a 1-gram TXA bolus plus 1-gram TXA 8-hour infusion in suspected-TBIs without ICH is not associated with increased adverse events or unfavorable outcomes. Because the out-of-hospital 2-gram bolus is associated with a mortality benefit it should be administered in suspected-TBI.
LEVELS OF EVIDENCE
Level II, Therapeutic
Keywords: Traumatic brain injury, Tranexamic acid, without intracranial hemorrhage, TBI, adverse events
BACKGROUND
The Center for Disease Control and Prevention reports that about 61,000 people in the United States died from traumatic brain injury (TBI) related causes in 2019.1 21,400 or so people over the age of 16 annually receive in-patient rehabilitation for moderate to severe TBI in the U.S. and roughly 20% die within five years of their injury, 35% have a decline in global functioning.2 Prognosis of traumatic brain injuries is multifactorial. Excluding demographic indicators of prognosis, the pathophysiology informing the extent of injury is divided into a primary and secondary injury. The primary injury, or initial focal insult, gives way to the secondary injury at the cellular level resulting in cellular edema, release of excitatory neurotransmitters, cellular hypoxia, inflammation cascades, and cell death.3 Intracranial hemorrhage, if present after TBI, is a component of the primary injury that can also influence the secondary injury. Depending on the location, hemorrhage can increase intracranial pressure contributing to herniation and decreasing cerebral perfusion pressure.4 On the cellular and molecular level, intracranial extravasation contributes to the secondary injury in many ways. Neurons and blood vessels are damaged by free radicals produced by oxyhemoglobin breakdown, the presence of free iron, and reactive oxygen species produced by activated microglia.4 Extravasation results in an increase of leukocyte-endothelial cell interactions thought to contribute to an increased risk of cerebral vasospasm.4 Platelet activation can increase vessel permeability and result in pathological vasoconstriction or dilation depending on the type of vasculature.4 Thus, reducing intracranial hemorrhage and cerebral edema in TBI have the potential to greatly improve mortality and neurological outcomes.
In 2019 the multi-national, multicenter CRASH-3 trial highlighted the safety and benefits tranexamic acid (TXA) provides in treating mild to moderate TBI in terms of reducing head injury-related 28-day mortality.5 CRASH-3 randomized TBI patients within three hours of injury and a GCS less than or equal to 12 or any intracranial hemorrhage on computed tomography (CT) to 1-gram of TXA infused over 10 minutes followed by 1-gram infused over 8 hours or a placebo bolus and infusion. This trial concluded that the dose of TXA they utilized showed no difference in frequency of adverse events as compared to placebo.5 One year later, in 2020, the parent paper to this current study, which was based on data from a randomized, multicenter, trial analyzed the effect of a 2-gram out-of-hospital TXA bolus on 6-month neurological outcomes as well as the same outcomes for a 1-gram out-of-hospital IV bolus followed by a 1-gram IV infusion of TXA within 2 hours of injury.6 The purpose of the trial was “to determine the efficacy of two dosing regimens of TXA initiated [outside of the hospital] in patients with moderate to severe TBI (GCS score ≤ 12)”.7 This parent study did not find a statistical difference in 6-month neurological outcomes or 28-day mortality between either of the treatment groups and placebo in the total study group. Patients who received a 2-gram bolus of TXA had a significant survival benefit (p=0.01) in a predetermined subgroup of patients with proven intracranial hemorrhage (ICH).5,6 Also, 41% of the 966 patients in the parent study had no evidence of ICH on their initial head CT. Since TXA appears to only have a significant benefit in patients with ICH combined with the fact that 41% of patients with suspected TBI and a GCS of 12 or lower did not have ICH on admission, begs the question: was TXA safe in patients without ICH?
In this retrospective study we aimed to further elucidate the safety profile of TXA for both dosing profiles from the parent paper. Regardless of which dose is therapeutically advantageous in treating TBI, the goal of this study was to determine whether both doses are equally safe, compared to placebo, to administer in an emergent out-of-hospital environment. Because the presence of intracranial hemorrhage is unknown at the point of injury, TXA must be safe to utilize even in patients who may not benefit from it. This study compares the adverse events and complications in patients without ICH on initial CT in patients who received placebo versus those that received either a 2-gram bolus of TXA or a 1-gram bolus followed by a 1-gram infusion. Other than the parent paper, which reported some adverse events in patients without ICH but did not statistically analyze such data, this study is the first to study adverse events in patients suspected of TBI receiving 2-gram bolus of TXA.
Properties of tranexamic acid
Tranexamic acid is an antifibrinolytic that primarily exerts its effect by competitively inhibiting plasminogen-fibrin binding and consequently fibrinolysis by occupying lysine-binding domains on plasminogen.8 Theoretical areas of concern for therapeutic utilization of TXA include adverse events associated with thrombus formation (cerebral vascular accidents, etc.) due to its mechanism of action as an antifibrinolytic and seizures because of its structural similarity to glycine as it is thought to competitively antagonize inhibitory neurotransmitters in the central nervous system.9 Both concerns have been closely followed in recent studies aimed at determining the utility of TXA in trauma. CRASH-2, which evaluated the association between TXA use and 28-day mortality in extracranial trauma, found no evidence of an increased risk of vascular occlusive events for patients administered a 1-gram infusion of TXA over 10 minutes and then a 1-gram TXA infusion over 8 hours within 8 hours of injury.10 Similarly, the CRASH-3 trial also found no statistically significant association between vascular occlusive events or seizures in mild to moderate TBI patients given the same dosing pattern of TXA as CRASH-2 within 3 hours of injury.5
METHODS
Clinical Trial Data
This study is an analysis of data collected in a phase II randomized, parallel double-blind clinical trial spanning from 2015 to 2017. This multicenter trial included 39 emergency medical service (EMS) agencies and 20 trauma centers in the US and Canada. That dataset consists of 966 participants who were suspected of suffering TBI (967 enrolled). Inclusion criteria for participants included: suspected TBI from a blunt or penetrating mechanism of injury, GCS score ≤ 12 prior to randomization and administration of sedatives or paralytics, systolic blood pressure ≥ 90 mmHg before randomization, IV access before hospital arrival, estimated age ≥ 15, and predetermined EMS transport to participating trauma centers.6,7 Exclusion criteria included a GCS of 3 with nonreactive pupils, estimated time of injury greater than 2 hours from initiating the study drug bolus, unknown time of injury, CPR prior to randomization, burns > 20% of total body surface area, police custody, suspected or known pregnancy, administration of TXA or pro-coagulant prior to randomization, and subjects who chose to “opt-out” based on local regulatory board guidelines.6,7 The dosing regimens of TXA utilized in this trial were as follows: a 2-gram out-of-hospital bolus of TXA “bolus-only,” a 1-gram out-of-hospital bolus of TXA followed by a 1-gram in-hospital infusion of TXA over 8 hours “bolus-maintenance,” and an out-of-hospital placebo bolus followed by an 8-hour placebo infusion “placebo.” These doses were placed in blinded study vials fit into custom study kits for EMS personnel and multiple mixed vial containers for the in-hospital dose by a clinical services company. The randomization was implemented by the ROC Data Coordinating Center in a 1:1:1 proportion of bolus-only, bolus-maintenance, and placebo.
The name of the trial this study gathered its data from for this retrospective analysis is “Prehospital Tranexamic Acid Use for Traumatic Brain Injury (TXA)”. The primary outcome measure originally studied in the trial was “Dichotomized Glasgow Outcome Scale Extended (GOSE) at 6 Months” which categorized scores of 1–4 as unfavorable neurological outcomes and scores of 5–8 as favorable neurological outcomes. Secondary outcomes included: mortality at 28 days, Disability Rating Scale (DRS) at 6 months, number of participants with an unfavorable neurological outcome on dichotomized GOSE at discharge, DRS at discharge, number of participants with intracranial hemorrhage progression (a relative increase of 33%, with at least a 1 ml increase, on any scan as compared to the initial computed tomography scan), Marshall Computed Tomography Score on initial head CT, Rotterdam CT score among subjects with ICH on initial head CT, number of participants with one or more neurosurgical interventions, hospital-free days, intensive care unit-free days, ventilator-free days, number of participants with seizure, number of participants with a cerebral ischemic event, number of participants with myocardial infarction, number of participants with deep vein thrombosis, number of participants with pulmonary embolus, and number of participants with any thromboembolic event. Sample size was determined to ensure 80% power for the primary outcome measure discussed in the parent paper. The primary outcome was statistically analyzed with a modified intention-to-treat analysis (randomized participants with any study drug infused were included) using a logistic regression to determine the strength of association of favorable neurological outcomes with the treatment group.6 An interim futility analysis was also conducted using a Wang-Tsiatis boundary with a parameter of 0.8 based on outcome data from the first 200 subjects. Interventions were quadruple masked (participant, care provider, investigator, outcomes assessor). The full trial protocol, and amendments, can be accessed in supplement 1 of the parent paper or at clinicaltrials.gov: registration number NCT01990768.6,7 CONSORT guidelines were followed when generating this manuscript.11, SDC 1
For the trial, the Human Research Protection Office of the Department of Defense and each site’s institutional review board, approved the study protocol. Trial participation consent was initially waived due to the participants receiving emergency treatment under FDA regulations for Exception from Informed Consent for Emergency Research (21 CFR §50.24) and the TCPS 2 (2014) Canadian Regulation Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. To continue in the trial, participants or their legal surrogate decision maker were informed as soon as reasonably possible and asked to provide written informed consent.
Data Collection
In this retrospective study, data on the 395 participants without intracranial hemorrhage on initial CT were isolated from the clinical trial’s 966 participants.SDC 2 Adverse events and other unfavorable outcomes were then compared between those 395 participants without intracranial hemorrhage based on the study drug dosing regimen in which they were randomized. 143 received the bolus-only dose, 124 bolus-maintenance, and 128 received placebo. In the analysis, both TXA treatment groups were compared to placebo outcomes. Adverse events compared between these groups included: myocardial infarction, deep vein thrombosis, seizure, pulmonary embolism, acute respiratory distress syndrome, cardiac failure, liver failure, renal failure, thrombotic cerebrovascular accident, cardiac arrest, cerebral vasospasm, “any thromboembolic event”, hypernatremia, acute kidney injury, and infection. Comparisons made were based on the incidence of each adverse event within the first 28 days of hospitalization. Unfavorable outcomes studied, which did not fall into the category of adverse events, include mortality at 28 days & 6-months, Glasgow Outcome Scale-Extended ≤ 4 at discharge & 6 months, intensive care unit (ICU)-free days, ventilator-free days, hospital-free days, and combined dichotomous unfavorable outcomes (Figure 1). Combined dichotomous unfavorable outcomes are a summation of the incidence of all fifteen adverse events, mortality at 28 days, mortality at 6 months, GOSE ≤ 4 at discharge, and GOSE ≤ 4 at 6 months within each of the three treatment groups.
Figure 1. Patient Population Flow Diagram: Adverse Events and Unfavorable Outcomes.
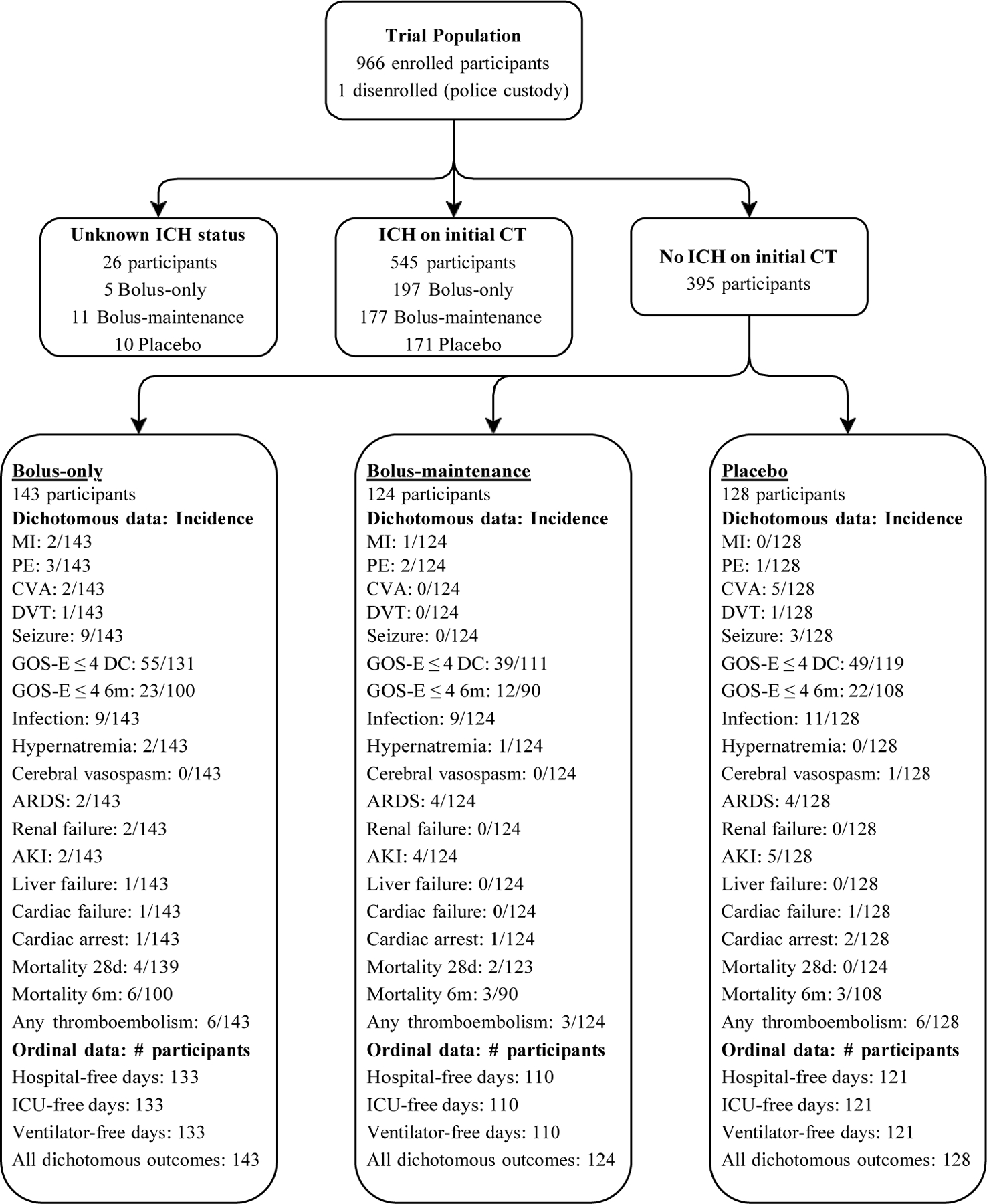
The clinical trial from which this retrospective study is based collected data on 1280 eligible participants. Of these eligible participants 1063 were randomized. The difference is attributable to later determining participants were ineligible due to not meeting inclusion criteria or satisfying exclusion criteria. Of the 1063 participants randomized, 967 received the study drug. A detailed flow diagram of the clinical trial is available in Figure 1 of the parent paper.6 The flow diagram above includes incidence of adverse events and dichotomous unfavorable outcomes within the population. Also listed is the number of participants for each ordinal outcome for the purpose of loss-to-follow up context.
Neurological outcomes were measured using GOSE scores and dichotomized to those less than or equal to four (unfavorable neurological outcome) and scores greater than four (favorable neurological outcome). ICU-free days, ventilator-free days, and hospital-free days were all calculated over a 28-day period starting from initial hospitalization. Other than 6-month mortality and GOSE ≤ 4, discharge GOSE ≤ 4 is the only individual outcome that was measured at discharge rather than 28 days due to the data not being available. Ideally this limited the extent to which hospitalization influenced differences in neurological outcomes between participants.
In terms of matching the treatment groups based on demographics both the bolus-only group and bolus-maintenance group were compared to placebo to ensure that no statistical difference existed between the following variables: sex, age, race, injury type, cause of injury, out-of-hospital Glasgow Coma Score, maximum head abbreviated injury score, and injury severity score.
Statistical Analysis
Dichotomous outcomes including all studied adverse events, mortality, and GOSE ≤ 4 were analyzed for significance using Chi-square tests and Fisher’s Exact Tests. Chi-square tests were utilized unless there was an expected count of less than five for any of the cells in a cross-tabulation. The four ordinal outcomes: ventilator-free days, hospital-free days, ICU-free days, and combined unfavorable outcomes were analyzed using the non-parametric Mann-Whitney U test. An alpha of 0.05 was utilized for all statistical analyses of unfavorable outcomes and adverse events.
Demographics and injury characteristics were similarly analyzed between each treatment group and placebo. Chi-square tests and Fisher’s Exact Test were used for dichotomous data whereas Mann Whitney U tests were used for ordinal data. A p-value of 0.05 was the limit for significance.
RESULTS
Treatment-group Demographics and Injury Characteristics
The bolus-only and bolus-maintenance treatment groups were largely similar to placebo with respect to demographics and injury characteristics. There was no statistical difference between either group that received TXA and placebo for age (median interquartile range), sex, race, ethnicity, mean out-of-hospital Glasgow Coma Scale scores, injury type (blunt vs. penetrating), maximum head abbreviated injury score, or injury severity score (median interquartile range). In terms of injury characteristics, the only statistically significant differences between either TXA treatment group and the placebo group were that both falls from more than one meter and motor vehicle-bicycle injuries represented a larger proportion of injury causes in the bolus-maintenance group than that of placebo. Many of the patients who were enrolled and subsequently found to have a head AIS of 0 were intoxicated or had TBI without intracranial bleeding commonly known as a concussion (Table 1).
Table 1.
Demographics and Injury Characteristics
| Bolus-only (n=143) | Bolus-maintenance (n=124) | Placebo (n=128) | |
|---|---|---|---|
| Demographics a | |||
| Median age (IQR) | 35 (26.5–52) | 37.5 (25.5–51) | 36 (23–52) |
| Sex- no./no. total (%) | |||
| Female | 37/143 (25.9) | 30/124 (24.2) | 39/128 (30.5) |
| Male | 106/143 (74.1) | 94/124 (75.8) | 89/128 (69.5) |
| Race – no./no. total (%) | |||
| American Indian/Alaska Native | 2/143 (1.4) | 4/124 (3.2) | 1/128 (0.8) |
| Asian | 3/143 (2.1) | 3/124 (2.4) | 3/128 (2.3) |
| Black/African American | 30/143 (21.0) | 28/124 (22.6) | 23/128 (18) |
| Native Hawaiian/Other Pacific Islander | 1/143 (0.7) | 0/124 (0.0) | 0/128 (0.0) |
| White | 87/143 (60.8) | 76/124 (61.3) | 86/128 (67.2) |
| More than one race | 0/143 (0.0) | 0/124 (0.0) | 1/128 (0.8) |
| Unknown | 20/143 (14.0) | 13/124 (10.5) | 14/128 (10.9) |
| Hispanic Ethnicity – no./no. total (%) | 21/143 (14.7) | 22/124 (17.7) | 24/128 (18.8) |
| Injury characteristics b | |||
| Injury type– no. (%) | |||
| Blunt | 142/143 (99.3) | 123/124 (99.2) | 126/128 (98.4) |
| Penetrating | 1/143 (0.7) | 2/124 (1.6) | 2/128 (1.6) |
| Cause of Injury – no./no. total (%) | |||
| Motor Vehicle Occupant | 50/143 (35) | 42/124 (33.9) | 54/128 (42.2) |
| Motor Vehicle Pedestrian | 18/143 (15.4) | 11/124 (8.9) | 22/128 (17.2) |
| Motor Vehicle Bicycle | 8/143 (5.6) | 8/124 (6.5) | 2/128 (1.6) |
| Motor Vehicle Motorcycle | 13/143 (9.1) | 5/124 (4.0) | 9/128 (7.0) |
| Suicide | 2/143 (1.4) | 1/124 (0.8) | 0/128 (0.0) |
| Assault | 11/143 (7.7) | 12/124 (9.7) | 9/128 (7.0) |
| Fall at ground level | 24/143 (16.8) | 21/124 (16.9) | 19/128 (14.8) |
| Fall at more than 1 meter | 13/143 (9.1) | 22/124 (17.7) | 11/128 (8.6) |
| Other | 2/143 (1.4) | 2/124 (1.6) | 1/128 (0.8) |
| Mean out-of-hospital Glasgow Coma Score (SD) | 9.0 (3.2) | 9.0 (3.3) | 8.6 (3.1) |
| Categories – no. (%) | |||
| 3–4 | 22 (15.4) | 19 (15.3) | 13 (10.1) |
| 5–6 | 13 (9.1) | 16 (12.9) | 23 (18) |
| 7–8 | 14 (9.8) | 8 (6.4) | 29 (22.7) |
| 9–10 | 38 (26.6) | 30 (24.2) | 17 (13.30) |
| 11–12 | 48 (33.6) | 41 (33.1) | 40 (31.3) |
| 13–15 | 8 (5.6) | 10 (8) | 6 (4.6) |
| Max Head Abbreviated Injury Scored – no./total no. (%) | |||
| 0 – none | 81/142 (57.0) | 65/123 (52.8) | 62/122 (50.8) |
| 1 – minor | 19/142 (13.4) | 13/123 (10.6) | 11/122 (9.0) |
| 2 – moderate | 28/142 (19.7) | 26/123 (21.1) | 26/122 (21.3) |
| 3 – serious | 11/142 (7.7) | 15/123 (12.2) | 14/122 (11.5) |
| 4 – severe | 3/142 (2.1) | 4/123 (3.3) | 8/122 (6.6) |
| 5 – critical | 0/142 (0.0) | 0/123 (0.0) | 1/122 (0.8) |
| 6 – unsurvivable | 0/142 (0.0) | 0/123 (0.0) | 0/122 (0.0) |
| Median Injury Severity Score (IQR) | 5 (1–14) | 6.78 (1–14) | 8.5 (2–17) |
SD: standard deviation; IQR: interquartile range
Statistical analysis comparing demographics and injury characteristics between each TXA treatment group and placebo was conducted for age (median interquartile range), sex, race, ethnicity, mean out-of-hospital Glasgow Coma Scale scores, injury type (blunt vs. penetrating), cause of injury, maximum head abbreviated injury score, and injury severity score (median interquartile range). No statistical difference was found between the bolus-only treatment group and placebo for any of the demographics or characteristics. Significant differences were only found between bolus-maintenance and placebo for cause of injury: motor-vehicle bicycle (p=0.046) and fall from greater than 1 meter (p=0.031).
Race and ethnicity data was collected via electronic health record review for each participant at US sites included in the original randomized controlled trial. This data was not recorded at participating Canadian sites.
One participant in the bolus-maintenance group had both a blunt and penetrating injury.
Combined Unfavorable Outcomes
Total dichotomous adverse event and unfavorable outcome incidence (combined unfavorable outcomes) were not statistically different between bolus-only and placebo. Similarly, combined unfavorable outcomes in the bolus-maintenance group were also not statistically different compared to placebo. Of the 143 participants in the bolus-only group, there were 130 total combined unfavorable outcomes, whereas there were 81 in the bolus-maintenance group of 124 participants. In the placebo group 114 unfavorable outcomes were recorded amongst 128 participants.
Adverse Events
Of the fifteen adverse events individually compared in both TXA treatment groups none were found to statistically differ from the placebo population in terms of 28-day incidence. Relative risk of adverse event or unfavorable outcome incidence was not significant for any outcome in either treatment group. Relative risk was only calculated in populations where there was at least one adverse event in both the TXA group and placebo group being studied (Figure 2).
Figure 2. Relative Risk of Adverse Events and Unfavorable Outcomes for Out-of-hospital TXA v Placebo.
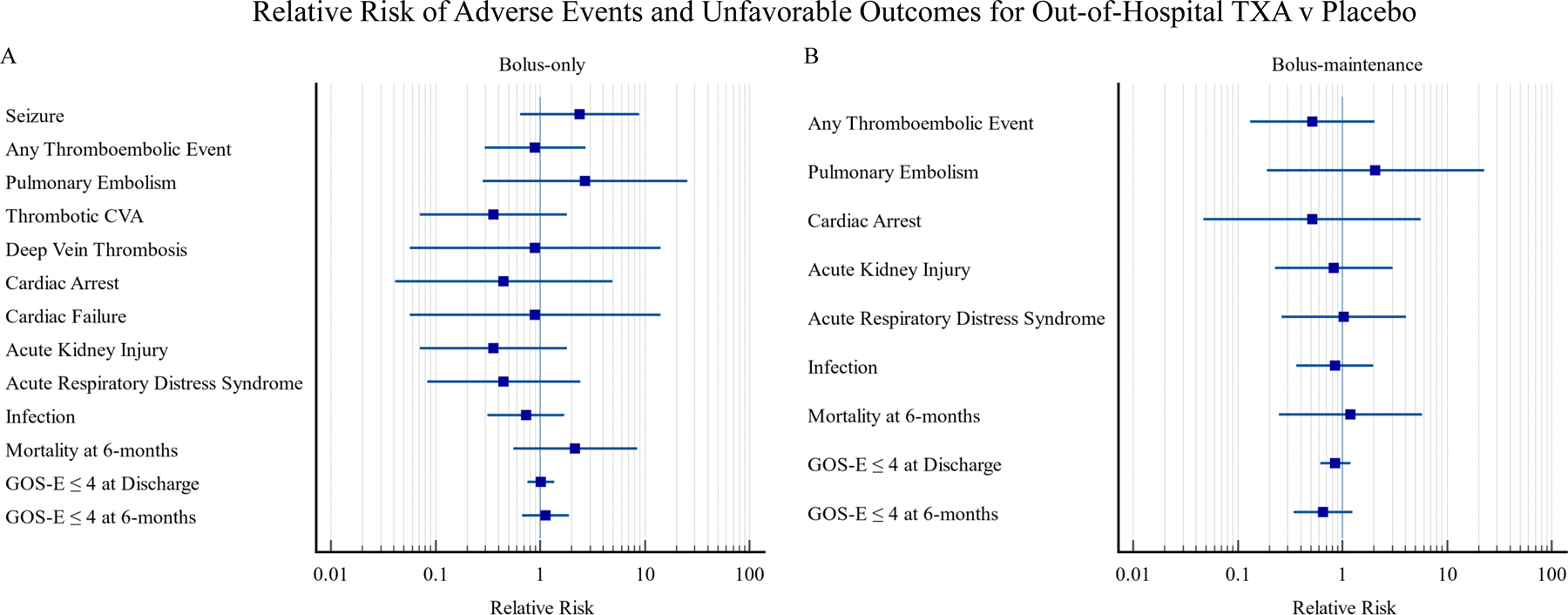
A. Relative risk with 95% confidence intervals for adverse events and negative outcomes in patients administered a 2-gram TXA bolus (bolus-only) as compared to a 1-gram placebo bolus + 1-gram placebo infusion over 8 hours (placebo). All participants represented in these forest plots had a CT negative for intracranial hemorrhage when they first arrived at the hospital after their suspected TBI. Not all of the 19 dichotomous outcomes in this study are represented on the above RR forest plots as some study drug groups had an incidence of zero for certain outcomes.
B. Relative risk with 95% confidence intervals for adverse events and negative outcomes in patients administered a 1-gram TXA bolus + 1-gram infusion over 8 hours (bolus-maintenance) as compared to a 1-gram placebo bolus + 1-gram placebo infusion over 8 hours (placebo).
The incidence of all adverse events possibly related to thrombus formation was similar between the study drug groups and placebo. Myocardial infarctions occurred only in the TXA treatment groups, but this did not result in a statistical difference as compared to placebo. Deep vein thrombosis and thrombotic cerebrovascular accidents occurred exclusively in the bolus-only and placebo populations. The absence of deep vein thrombosis and thrombotic cerebrovascular event in the bolus-maintenance group were not statistically significant findings. Pulmonary embolism and acute kidney injury were recorded in all three treatment groups. Overall, thromboembolic events were observed in each study group (Figure 1).
Outside of adverse events potentially related to thrombus formation, the risk of lowering the seizure threshold was the other primary concern for TXA utilization due to the previously mentioned theoretical mechanism. Seizures did not occur in the bolus-maintenance group but did in the bolus-only and placebo groups. No statistical relationship was identified between either treatment group and seizure occurrence.
Other theoretically less-related adverse events include acute respiratory distress syndrome, cardiac failure, liver failure, renal failure, cardiac arrest, cerebral vasospasm, hypernatremia, and infection. None of which were statistically significant when comparing either TXA dosing group to placebo. Acute respiratory distress syndrome, cardiac arrest, and all cause infection occurred to some extent in all three groups. Whereas cardiac failure was only present in the placebo and bolus-only groups. Lack of cardiac failure in the bolus-maintenance group did not result in a statistical difference. Both liver failure and renal failure were only present in the bolus-only group. Cerebral vasospasm did not occur in either TXA treatment group. Whereas hypernatremia was only found in the TXA treatment groups.
Mortality and Neurological Outcomes
No statistically significant relationship was observed between 28-day or 6-month mortality incidence and TXA use. Some mortality at 28 days after hospitalization was present in both TXA treatment groups but not placebo. 6-month mortality, was seen in all three populations. Neurological outcomes dichotomously measured as incidence of GOSE ≤ 4 at discharge and 6 months were not statistically different from placebo neurological outcomes in either TXA treatment group. 28-day mortality, 6-month mortality, discharge GOSE ≤ 4, and 6-month GOSE ≤ 4 were studied in smaller populations within each treatment group due to loss-to-follow-up. Population sizes are available in Figure 1.
Illness Severity
Hospital-free days, ICU-free days, and ventilator-free days were not found to statistically differ between either TXA treatment groups and placebo.
DISCUSSION
Out-of-hospital administration of a 2-gram bolus of TXA or 1-gram bolus of TXA followed by an 8-hour in-hospital 1-gram infusion of TXA is not associated with a statistically significant change in adverse event incidence in patients with suspected TBI, a GCS of 12 or less at the point of injury, who are not in shock, and who do not have intracranial hemorrhage on initial CT. No statistical difference was observed in 28-day mortality, 6-month mortality, discharge GOSE ≤ 4, 6-month GOSE ≤ 4, ventilator-free days, hospital-free days, or ICU-free days between either TXA dosing regimen and placebo.
The retrospectively analyzed data in this study was originally gathered to study the relationship between out-of-hospital TXA use in moderate to severe TBI and 6-month neurological outcomes.6 After the collaborators in the trial determined that a 2-gram bolus of TXA resulted in reduced 28-day mortality and 6 month disability rating scores in patients with intracranial hemorrhage, the effects on patients without intracranial hemorrhage became the next logical assessment considering that they made up 41% of suspected TBIs with a GCS of 12 or lower transported to definitive care at a participating center in the parent study.6 This is especially true as this is the first large-scale trial of out-of-hospital TXA for suspected TBI to incorporate a 2-gram bolus dosing regimen rather than solely a 1-gram bolus followed by a 1-gram in-hospital 8-hour infusion. There was reasonable concern to investigate certain outcomes. For example, in the total population of participants with moderate-to-severe TBI in the trial, there was a trend demonstrating that seizures were more likely to occur in the bolus-only group than either bolus-maintenance or placebo.6 To inform the true safety profile of both dosing regimens for out-of-hospital use, this retrospective study compared adverse events and other unfavorable outcomes in the trial participants who potentially had the least theoretical need for an antifibrinolytic agent like TXA: suspected TBI participants with no ICH on initial CT.
Although the doses of tranexamic acid analyzed in this trial appear to be safe in terms of not increasing one’s risk for the parameters studied, future studies should continue to test tranexamic acid’s safety in patients without intracranial hemorrhage as this study may be underpowered. This is because this study analyzed many outcomes in a subset of data from a trial powered to 80% to detect a single outcome: 6-month neurological outcomes. We did not conduct post-hoc power analyses as they are not generally meaningful in retrospective studies. Data from this study are useful for powering future prospective studies. Specifically, it can be utilized to calculate sample sizes necessary to conduct large prospective studies to prove or disprove safety in widespread use. For example, based on the data from this study, one adverse event that would require a very large study is pulmonary embolism. Specifically, 2,568 individuals meeting all the same criteria of this study would be needed to power a prospective study observing the incidence of pulmonary embolism in patients receiving either a 2-gram bolus of TXA or placebo. The population would likely need to be closer to 6,264 individuals since the presence of ICH currently cannot feasibly be known at the point of injury. This example assumes such a study is powered to 80%, the population sizes for each intervention are identical, and that an alpha of 0.05 is being utilized by those interpreting the results.
The impetus for conducting this study was the mortality benefit observed in patients with proven intracranial hemorrhage receiving the 2-gram bolus of TXA in the parent study (p=0.01).6 The number needed to prevent one death (NNT) in this population is 12, which we believe represents the most meaningful NNT in patients with suspected traumatic brain injury as there is minimal opportunity for improvement in uninjured patients.6 This assertion that the NNT based only on patients with ICH is more relevant than a NNT based on all patients receiving TXA is further substantiated by the lack of complications observed in uninjured patients, evidenced by this study’s findings. Additionally, TXA’s cost effectiveness, demonstrated in a study of the CRASH-2 trial, showed that its use in the setting of trauma is cost effective.12
We thus assert that a number needed to prevent one death of 12 is low enough to support widespread use of the 2-gram bolus considering that there are no other proven therapeutics to improve outcomes after TBI.
Limitations
This study is a retrospective review of a prospective randomized trial that was not predetermined at the time of the study. There were no standardized protocols for collection of some of the complication data. For instance, some institutions have standardized screening protocols for deep vein thrombosis and others do not. Similarly, seizures were defined by observation of activity that could be consistent with a seizure and evidence that this activity was medically treated with an anti-seizure medication which was also not standardized. Adverse events and unfavorable outcomes were prospectively assessed throughout hospitalization. They were not prospectively assessed when participants were discharged prior to 28 days. Participants were only assessed for these outcomes through discharge or 28 days, whichever occurred first. Participants notified directly (rather than through family members) who were lost to follow-up after discharge or withdrew from the study prior to 28 days were assumed to survive through 28 days. Future studies may consider prospectively following patients who discharge or self-withdraw as adverse events and unfavorable outcomes may have occurred in these participants.
The randomized controlled trial on which this study was based, was powered to detect differences in 6-month GOSE ≤ 4. This study used data from that trial but studied adverse events and unfavorable outcomes in a subgroup of the studied population. The rates of adverse events were low in each studied group, it is likely that some of the data is underpowered in this retrospective study. Some patients were also lost to follow-up for certain outcomes including: GOSE ≤ 4 (discharge & 6 months), mortality (28 days & 6 months), ventilator-free days, hospital-free days, and ICU-free days (Figure 1).
CONCLUSION
Administration of either a 2-gram tranexamic acid bolus or a 1-gram tranexamic acid bolus followed by a 1-gram tranexamic acid 8-hour infusion to patients without intracranial hemorrhage is not associated with an increase in adverse events or unfavorable outcomes. This has important implications for out-of-hospital protocols and supports the safety of administration of tranexamic acid to patients suspected of TBI prior to imaging. Considering that TXA does not appear to be associated with complications in an uninjured population and the proven mortality benefit associated with a out-of-hospital 2-gram bolus of TXA in patients with ICH, we recommend that all patients with a GCS of 12 or less with suspected traumatic brain injury receive a 2-gram bolus of TXA within 2-hours of injury.
Supplementary Material
SDC 1. CONSORT 2010 checklist of information to include when reporting a randomised trial
Consort guideline checklist for reporting clinical trials. As this is a retrospective study of a clinical trial some parts of the checklist are only available within the parent paper.6 Additional information about the trial can be found at clinicaltrials.gov.7
SDC 2. Consort 2010 Flow Diagram
Consort diagram of the clinical trial data utilized in this retrospective study. Additional information can be found within the parent paper or at clinicaltrials.gov.6,7
Acknowledgments
The data represented in this paper were collected in a trial sponsored by institutions of the Resuscitation Outcomes Consortium for the purpose of studying the use of tranexamic acid in treating traumatic brain injury. The trial was supported by the following cooperative agreements from the National Heart, Lung, and Blood institute: U01 HL077863 (University of Washington Data Coordinating Center), U01 HL077866 (Medical College of Wisconsin), U01 HL077871 (University of Pittsburgh), U01 HL077873 (Oregon Health and Science University), U01 HL077881 (University of Alabama at Birmingham), U01 HL077887 (University of Texas Southwestern Medical Center/Dallas). The contents of this manuscript do not necessarily represent the official views of the National Institute of Health or National Heart, Lung, and Blood Institute. The listed authors are solely responsible for its contents.
Clinical Trial Funding
The data utilized in this retrospective study was from a clinical trial made possible by a cooperative agreement awarded and administered by the U.S. Army Medical Research & Material Command (W81XWH-13–2-0090) and was conducted by the Resuscitation Outcomes Consortium (ROC). The ROC is supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077867-University of Washington, HL077871-University of Pittsburgh, HL077872-St. Michael’s Hospital, HL077873-Oregon Health and Science University, HL077881-University of Alabama at Birmingham, HL077885-Ottawa Hospital Research Institute, HL077887-University of Texas SW Medical Ctr/Dallas) from the National Heart, Lung and Blood Institute in partnership with the The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Defense, National Heart, Lung and Blood Institute or the National Institutes of Health and should not be construed as an official DoD/Army policy unless designated by other documentation. No official endorsement should be made.
Study Funding
No funding was provided or solicited to conduct this study.
Footnotes
Conflicts of Interest
No conflicts of interest were reported by any of the authors.
Presentations
Eastern Association for the Surgery of Trauma annual scientific assembly: January 13th, 2022
REFERENCES
- 1.Centers for Disease Control and Prevention. Get the Facts About TBI – Traumatic Brain Injury & Concussion. Injury Prevention & Control: Preventing Injury https://www.cdc.gov/traumaticbraininjury/get_the_facts.html. Last reviewed May 12, 2021. Accessed September 19, 2021.
- 2.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. One and five year outcomes after moderate-to-severe traumatic brain injury requiring inpatient rehabilitation: traumatic brain injury report https://stacks.cdc.gov/view/cdc/59524. Published March 22, 2018. Accessed September 20, 2021.
- 3.Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med Clin North Am 2020;104(2):213–238. [DOI] [PubMed] [Google Scholar]
- 4.Lok J, Leung W, Murphy S, Butler W, Noviski N, Lo EH. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta Neurochir Suppl 2011;111:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial [published correction appears in Lancet. 2019 Nov 9;394(10210):1712]. Lancet 2019;394(10210):1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury [published correction appears in JAMA. 2020 Oct 27;324(16):1683]. JAMA 2020;324(10):961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prehospital Tranexamic Acid Use for Traumatic Brain Injury (TXA). ClinicalTrials.gov identifier: NCT01990768. Updated January 14, 2019. Accessed September 21, 2021. https://clinicaltrials.gov/ct2/show/NCT01990768
- 8.Hunt BJ. The current place of tranexamic acid in the management of bleeding. Anaesthesia 2015;70 Suppl 1:50-e18. [DOI] [PubMed] [Google Scholar]
- 9.Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: Causes and treatment. Ann Neurol 2016;79(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2) A randomised, placebo-controlled trial. West Indian Med J 2010;59(6):612–624. [PubMed] [Google Scholar]
- 11.Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152(11):726–732. [DOI] [PubMed] [Google Scholar]
- 12.Guerriero C, Cairns J, Perel P, Shakur H, Roberts I; CRASH 2 trial collaborators. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One 2011;6(5):e18987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 1. CONSORT 2010 checklist of information to include when reporting a randomised trial
Consort guideline checklist for reporting clinical trials. As this is a retrospective study of a clinical trial some parts of the checklist are only available within the parent paper.6 Additional information about the trial can be found at clinicaltrials.gov.7
SDC 2. Consort 2010 Flow Diagram
Consort diagram of the clinical trial data utilized in this retrospective study. Additional information can be found within the parent paper or at clinicaltrials.gov.6,7


