Abstract
Background:
Immune checkpoint inhibitors (ICI), combined with hypomethylating agents, can be used to treat acute myeloid leukemia (AML), but this strategy results in a high rate of pneumonitis. We sought to determine risk factors for pneumonitis development, and whether pneumonitis increased mortality.
Methods:
We conducted a retrospective review of 258 AML patients who received ICI-containing regimens from 2016–2018. A multidisciplinary adjudication committee diagnosed pneumonia and pneumonitis by reviewing symptoms, imaging, microbiology, and response to therapies. To measure risk factors for pneumonitis and mortality, we constructed multivariate Cox proportional hazards models. We modeled pneumonia, pneumonitis, and disease progression as time-dependent variable, and incorporated a standard risk set modifying variables into the models.
Results:
Thirty patients developed pneumonitis (12%). Of these, 17 had partial or complete resolution, while 13 patients died from pneumonitis. Increasing age (HR 1.04 per year, 95% CI 1.00–1.08), and baseline shortness of breath increased pneumonitis risk (HR 2.51, 95% CI 1.13–5.55). Female sex (HR 0.33, 95% CI 0.15–0.70) and increasing platelet count (HR 0.52 per log-unit increase, 95% CI 0.30–0.92) decreased pneumonitis risk. In adjusted models, ICI-related pneumonitis significantly increased mortality (HR 2.84, 95% CI 1.84–4.37).
Conclusion:
ICI-related pneumonitis occurs at a high rate in AML patients and increases mortality.
Keywords: immune checkpoint inhibitor, pneumonitis, acute myeloid leukemia, hypomethylating agent, mortality
Lay Summary:
Immune checkpoint inhibitors (ICIs) remove inhibitory signals that reduce T-cell function and allow T-cells to better attack cancer cells. In acute myeloid leukemia (AML), the effectiveness of ICIs is limited in part by inflammation of the lung, called pneumonitis. We reviewed 258 patients with AML who received ICIs and identified thirty patients who developed pneumonitis, nearly half of whom died. Older age and baseline shortness of breath increased pneumonitis risk, while female sex and higher baseline platelet counts decreased pneumonitis risk. Pneumonitis increased mortality by nearly three-fold. This work highlights the significant harm imposed by pneumonitis after ICI therapies.
Precis:
Pneumonitis occurs in 12% of patients with acute myeloid leukemia undergoing immune checkpoint inhibitor therapy and increases mortality by nearly three-fold.
Background
Acute myeloid leukemia (AML) is associated with a high rate of 5-year mortality, particularly in older patients1 and those with adverse cytogenetic or molecular profiles2. The mainstay of therapy in AML has been intensive chemotherapy, sometimes followed by allogeneic hematopoietic transplantation (allo-HCT)3. However, even with allo-HCT, long-term survival remains low, particularly in older patients, and new strategies are needed to improve tolerance of therapies and long-term survival4.
The use of immune checkpoint inhibitors (ICIs) to treat cancers has rapidly grown in the last decade5, and the efficacy of ICIs often improves when given in combination with other active therapies6. Hypomethylating agents (HMA), which are commonly used to treat AML, also demethylate promoter regions involved in PD-1 expression, potentially resulting in PD-1 activation which may blunt T-cell anti-tumor responses7. ICIs may potentially mitigate the effects of PD-1 promoter demethylation by HMAs, and our group has shown that the addition of the PD-1 inhibitor nivolumab to the HMA azacitidine resulted in a 33% response rate in refractory AML8, which is typically associated with poor survival even after allo-HCT9. However, the efficacy of this strategy was limited by high-grade toxicity - particularly pneumonitis, an immune-related adverse event (irAE) that has been associated with high rates of therapy-related mortality10.
Because of the potential for the expanded use of ICIs to treat AML, we sought to quantify the incidence of pneumonitis associated with ICI treatment in this setting. Furthermore, we sought to determine the impact of pneumonitis on survival, and to compare that with the known increase in mortality associated with pneumonia in AML patients11. Therefore, we designed a retrospective study of AML patients who were treated with ICI-containing regimens as part of induction or subsequent therapy between 2016–2018.
Methods
Subjects
We conducted a retrospective review of 258 patients with AML who were started on ICI-containing regimens as induction or subsequent therapy between 2016 and 2018. ICI treatment was either given alone or in combination with azacitidine or idarubicin. We had no pre-specified exclusion criteria. Patient data was collected for at least one year after cessation of ICI therapy, except in cases of death or loss to follow-up. We collected clinical, imaging, and microbiological data from the electronic health record and from a prospectively-maintained database of AML patients. The MD Anderson Institutional Review Board approved the study (PA18–0802).
Study Definitions
The primary goals of this study were to determine: 1) the incidence of pneumonitis after ICI therapy in AML patients; 2) risk factors for the occurrence of pneumonitis after ICI therapy; and 3) the impact of pneumonitis on all-cause mortality. We convened a multidisciplinary adjudication committee to determine whether pulmonary complications were due to pneumonitis, pneumonia, or other etiologies (e.g. pulmonary edema). This committee included expert pulmonologists (A.S., V.S., S.F.), infectious disease specialists (F.K., D.K.), and thoracic radiologists (M.G., G.S.). The committee convened and discussed each case in detail, including clinical features of the pulmonary complication, representative laboratory, imaging, and microbiological data, and treatments or interventions that led to the resolution of the complication.
We classified cases as pneumonia if they had 1) consistent symptoms (e.g. fevers, cough) and consistent imaging (e.g. lobar pneumonia) and 2) had a clear response to antibiotics but not corticosteroids or had microbiological confirmation from a lower respiratory tract specimen of an organism known to cause pneumonia. We classified cases as pneumonitis if they had 1) consistent symptoms (e.g. cough, shortness of breath) and consistent imaging (e.g. diffuse ground-glass opacities) and 2) had a clear response to corticosteroids but not antibiotics or had histopathological confirmation of pneumonitis. We considered pneumonia cases to be definite in cases of microbiological confirmation from a lower respiratory tract source and probable in cases where a clear response to antibiotics was demonstrated. Because biopsies were rarely possible due to the severity of symptoms or contraindications such as thrombocytopenia, we considered pneumonitis cases to be definite in cases of clear response to corticosteroids, and probable in cases with compatible clinical features and imaging with no evidence of lower respiratory infection. For patients with pneumonitis, expert radiologists confirmed whether the initial imaging fit the final adjudicated diagnosis, the primary radiological pattern at the time of pneumonitis12, and whether subsequent imaging changed the radiological opinion on the etiology of the complication. Figure 1 summarizes this process. Pneumonitis was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) 5.013.
Figure 1.
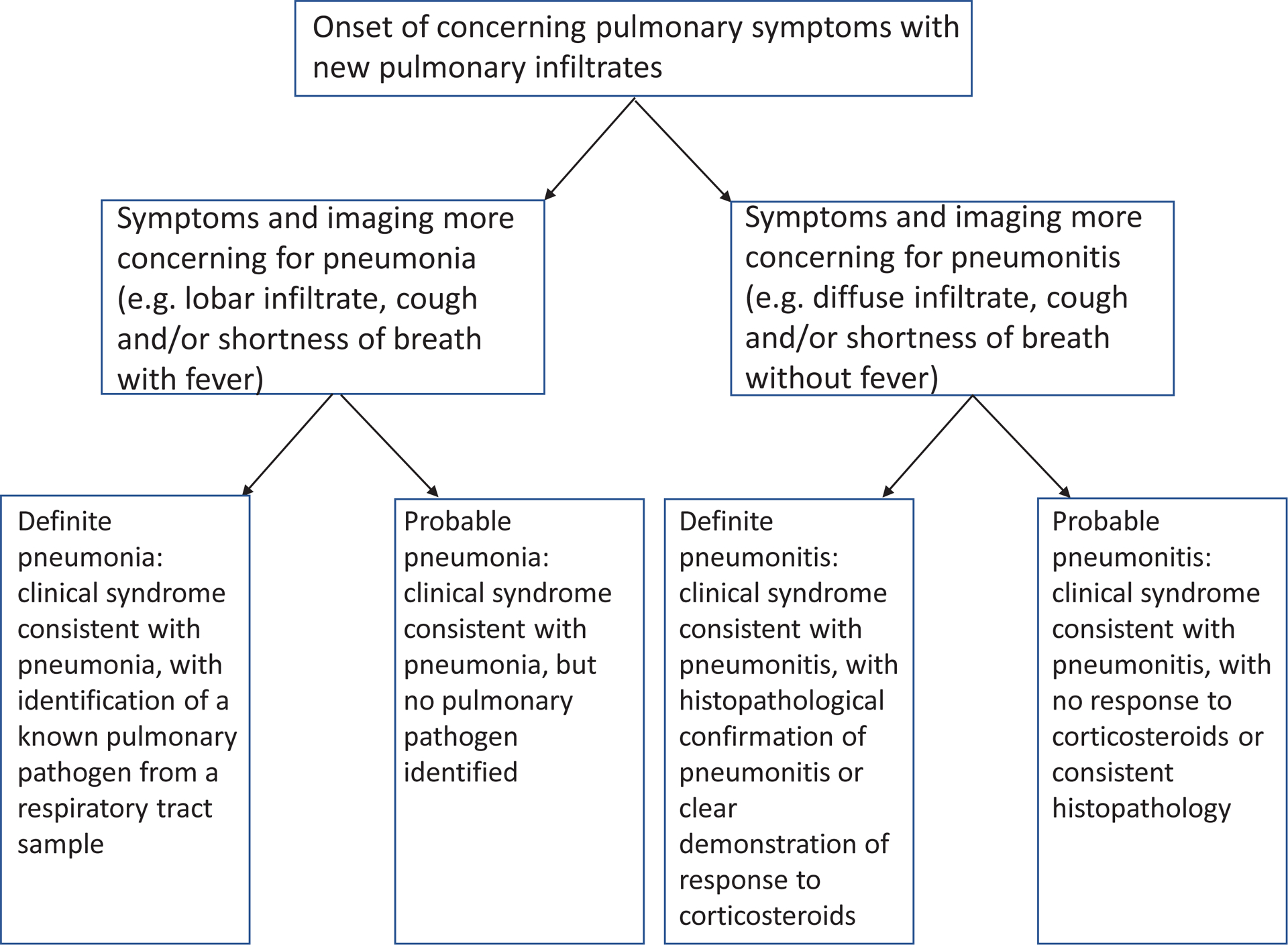
Flowchart for multidisciplinary adjudication to diagnose pneumonitis and pneumonia
Statistical Analysis
Principal statistical analyses were performed by K.J. All authors had access to the complete dataset. All variables were assessed for univariate effects using log-rank tests, simple Cox proportional hazards models, and Kaplan-Meier plots. For modeling of pneumonitis and mortality risk, a multivariate Cox proportional hazards model was fit to the variable set. To reduce the complexity of the model and mitigate collinearity, variable selection was implemented using stepwise regression and the Bayesian Information Criterion (BIC) for complexity selection. For the time-dependent variables of pneumonitis onset, pneumonia onset, and progression, standard risk set modifying variables were incorporated into the models using indicators of status. Variable selection was expanded to incorporate potential interactions after the first-order model was fit. All computations were done using R (version 4.0.0 or later).
Results
Characteristics of the study cohort
Table 1 shows the characteristics of the study cohort (n=258). The median age upon enrollment was 68. 76% of subjects had de novo AML, but ICI was given as frontline therapy in only 39% of cases. All subjects were Eastern Cooperative Oncology Group (ECOG) 0–2. About half of the cohort had adverse cytogenetics by European LeukemiaNet (ELN) criteria. 13% of subjects had pneumonia at the time of ICI initiation. A small minority (n=8) were found to have evidence of prior autoimmune disease; four patients had autoimmune hypothyroidism, two had rheumatoid arthritis, one patient had psoriasis, and one patient had polymyalgia rheumatica.
Table 1.
Characteristics of the 258 patients in the study cohort.
| Variable | |
|---|---|
| Median age at enrollment (years) | 68 |
| Female sex (%) | 41 |
| Race (n, %) | |
| White | 217 (84%) |
| Non-white | 41 (16%) |
| Median BMI at ICI initiation | 27.6 |
| AML Diagnosis (n, %) | |
| De novo AML | 196 (76%) |
| Secondary/therapy-related AML | 62 (24%) |
| Line of therapy (n, %) | |
| Induction | 100 (39%) |
| Subsequent | 158 (61%) |
| Prior HCT | 30 (12%) |
| ECOG | |
| 0 | 47 (18%) |
| 1 | 196 (76%) |
| 2 | 15 (6%) |
| ELN risk group | |
| Adverse | 119 (46%) |
| Intermediate | 128 (50%) |
| Favorable | 5 (2%) |
| Unknown | 6 (2%) |
| Median cell counts at ICI initiation | |
| Bone marrow blasts (%) | 15.5 |
| Total WBC (103 cells/μL) | 2.5 |
| ANC (cells/μL) | 586 |
| ALC (cells/μL) | 1002 |
| Platelets (103 cells/μL) | 36 |
| Smoking status | |
| Never | 145 (56%) |
| Former | 102 (40%) |
| Current | 11 (4%) |
| Pneumonia within 30 days of ICI initiation | 33 (13%) |
| Viral URI within 30 days of ICI initiation | 7 (3%) |
| Prior lung disease | |
| COPD | 16 (6%) |
| Asthma | 11 (4%) |
| ILD | 1 (0.3%) |
| Prior autoimmune disease | 8 (3%) |
| Chest radiation prior to ICI | 13 (5%) |
Abbreviations: BMI:, body mass index; ICI: immune checkpoint inhibitor; AML: acute myeloid leukemia; HCT: hematopoietic cell transplant; ECOG: Eastern Cooperative Oncology Group; ELN: European LeukemiaNet; WBC: white blood cell; ANC: absolute neutrophil count; ALC: absolute lymphocyte count; URI: upper respiratory infection; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease
175 patients received nivolumab without ipilimumab, either alone (n=29), or with azacitidine (n=104) or idarubicin (n=42). 40 patients received ipilimumab without nivolumab, either alone (n=17) or with azacitidine (n=23). 43 patients received nivolumab and ipilimumab together, with (n = 32) or without (n=11) azacitidine. The median duration of ICI therapy was 17 weeks (interquartile range, 10–32 weeks). Reasons for stopping ICI therapy included progression (n=167), completion of therapy (n=12), HCT (n=36), physician or patient preference (n=21), and infection or toxicity (n=13). Three patients were still receiving ICI therapy one year after ICI initiation, three patients were lost to follow-up, and three patients died while receiving ICI therapy despite having achieved a complete response without evidence of infection or toxicity. 60 patients were in remission at the last follow-up, and 197 patients died during the observation period.
Clinical features of pneumonitis
During the study period, we diagnosed 167 events consistent with pneumonia; 32 patients developed more than one pneumonia during the study period. Of these, 68 events met criteria for definite pneumonia, while 99 met criteria for probable pneumonia. We also found an overall incidence of pneumonitis of 12% (30/258); one patient had two distinct episodes of pneumonitis. Of these, 17 events met criteria for definite pneumonitis, and 14 met criteria for probable pneumonitis. The median time to pneumonitis was 84 days after ICI initiation (interquartile range 36–160 days, range 1–375 days). 17 patients had a maximum CTCAE 5.0 grade of 2 (n=2) or 3 (n=16) for pneumonitis, while 13 patients died from pneumonitis (5 probable, 8 definite). Four patients with pneumonitis had extrapulmonary irAEs concurrently (skin, nephritis, colitis, mucositis). Pneumonitis rates were similar in patients receiving combination regimens (16%, 7/43), nivolumab-containing regimens (10%, 18/175) or ipilimumab-containing regimens (12.5%, 5/40).
Fifty percent of patients (15/30) had a full recovery after pneumonitis (CTCAE Grade 1 or total resolution), and the median time to resolution was 4 weeks; two patients had permanent scarring and impairment but did not die. BAL was performed in 22/31 instances of pneumonitis and 107/199 instances of pneumonia. BAL leukocyte differentials in patients with pneumonitis showed a median of 2% neutrophils and 12.5% lymphocytes, respectively; in comparison, BAL leukocyte differentials in patients with pneumonia were similar (median 2% neutrophils, 14% lymphocytes). The median initial dose of prednisone or equivalent was 60 mg/day. Four patients required additional therapy with a second agent - two received infliximab, one received cyclophosphamide, and one received rituximab. Of these four patients, only the patient who received rituximab survived. Nine patients resumed ICI after pneumonitis (7 with nivolumab and 2 with ipilimumab), and continued therapy after re-exposure for a median of 5 weeks (range 1–49 weeks). Of these, 8/9 discontinued ICI due to progressive leukemia, while one patient completed one full year of ICI therapy, but developed a second episode of pneumonitis after stopping ICI therapy. Supplemental Table 1 shows detailed patient-level medical information on the 30 patients who developed pneumonitis.
All cases of pneumonia and pneumonitis were independently reviewed by an expert thoracic radiologist who was blinded to the clinical diagnosis. Among these cases, the radiologist determined that the initial imaging pattern agreed with the clinical diagnosis of pneumonia or pneumonitis in 195/198 cases (98%). In the three discordant cases, thoracic radiologists agreed with the clinical diagnosis after reviewing subsequent chest imaging. Among pneumonitis cases, 13/31 episodes had an initial radiological pattern consistent with organizing pneumonia, three had a pattern consistent with widespread alveolar injury, and 15 cases had a mixed or indeterminate radiological pattern. Figure 2 shows representative patterns in cases of pneumonia and pneumonitis.
Figure 2.
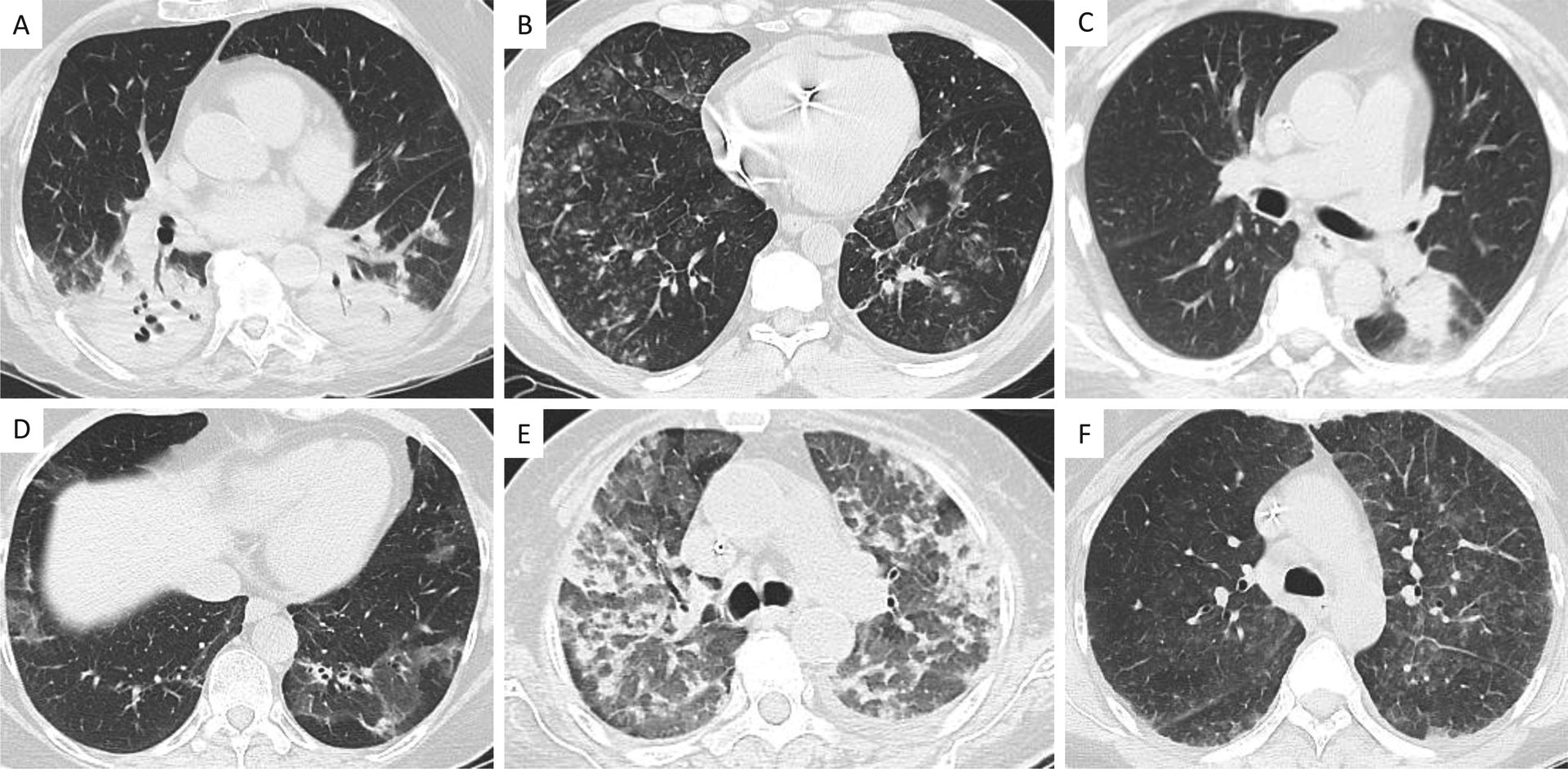
Representative images of pneumonia and pneumonitis. Panels A-C represent examples of pneumonia due to A) cavitary methicillin-resistant Staphylococcus aureus, B) parainfluenza virus, and C) Aspergillus niger. Panels D-F represent examples of pneumonitis, with D) organizing pneumonia pattern, E) widespread alveolar injury pattern, and F) indeterminate pattern (i.e., any pattern not compatible with either organizing pneumonia or widespread alveolar injury).
Risk factors for the development of pneumonitis
Table 2 shows univariate and multivariate risk factors for the development of pneumonitis, as modeled using a Cox regression model with stepwise variable selection. Increasing age (HR 1.04 per year, 95% CI 1.00–1.08), and baseline shortness of breath were associated with pneumonitis risk (HR 2.51, 95% CI 1.13–5.55). Female sex (HR 0.33, 95% CI 0.15–0.70) and increasing platelet count (HR 0.52 per log-unit increase, 95% CI 0.30–0.92) were associated with lower risk for pneumonitis. Furthermore, we found a significant interaction between total WBC and sex (p=0.005); each log-unit increase in WBC in females increased the hazard for developing pneumonitis by 68% (HR 1.68, 95% CI 1.14–2.48, p=0.009), but this effect was not significant in males (p=0.14). Pneumonitis risk did not vary between those who received ICI monotherapy and those who received combination immunotherapy.
Table 2.
Risk for developing pneumonitis
| Variable | Univariate HR (95% CI) | p-value | Multivariate HR (95% CI) | p-value |
|---|---|---|---|---|
| Age (per year) | 1.036 (1.002–1.072) | 0.03 | 1.04 (1.00–1.08) | 0.02 |
| Sex (female to male) | 0.45 (0.22–0.94) | 0.03 | 0.33 (0.15–0.70) | 0.003 |
| BMI (per unit) | 0.99 (0.92–1.05) | 0.66 | ||
| AML Diagnosis (De novo to secondary) | 1.35 (0.62–2.95) | 0.46 | ||
| Prior lines of therapy (per line of treatment) | 0.93 (0.64–1.34) | 0.68 | ||
| Prior HCT | 0.54 (0.13–2.26) | 0.35 | ||
| ECOG | 0.75 | |||
| 0 to 1 | 0.75 (0.32 −1.77) | 0.52 | ||
| 0 to 2 | 1.1 (0.23 – 5.35) | 0.90 | ||
| Adverse to non-adverse ELN | 0.89 (0.44 −1.84) | 0.76 | ||
| Baseline bone marrow blasts (per 1%, n=257) | 1.01 (0.99–1.02) | 0.33 | ||
| Baseline total WBC (per log unit) | 1.06 (0.76 – 1.47) | 0.75 | ||
| Baseline ANC (per 100 cells/μL, n=255) | 0.99 (0.90 – 1.10) | 0.91 | ||
| Baseline ALC (per 100 cells/μL, n=256) | 1.10 (0.85 – 1.44) | 0.49 | ||
| Platelets (per log unit) | 0.63 (0.44–0.89) | 0.01 | 0.52 (0.30 – 0.92) | 0.02 |
| Smoking status (Never vs. former or current) | ||||
| Pneumonia within 30 days of ICI initiation | 1.94 (0.78–4.77) | 0.18 | ||
| Viral URI within 30 days of ICI initiation | 1.17 (0.16–8.58) | 0.88 | ||
| ICI regimen | 0.63 | |||
| Nivolumab and ipilimumab | 1 (reference) | |||
| Ipilimumab only | 1.56 (0.65 −3.73) | |||
| Nivolumab only | 1.41 (0.45 −4.46) | |||
| Prior lung disease | 0.59 (0.14 – 2.50) | 0.96 | ||
| Prior autoimmune disease | 2.12 (0.51–8.93) | 0.35 | ||
| Chest radiation prior to ICI | 1.30 (0.31–5.44) | 0.73 | ||
| Baseline symptoms | ||||
| Cough | 1.70 (0.73 – 4.00) | 0.25 | ||
| Fever | 2.07 (0.92 – 4.66) | 0.10 | ||
| Shortness of breath | 2.26 (1.03–4.94) | 0.06 | 2.51 (1.13–5.55) | 0.03 |
| Baseline WBC × sex interaction | 0.005 |
Abbreviations: HR: hazard ratio; CI: confidence interval; BMI:, body mass index; AML: acute myeloid leukemia; HCT: hematopoietic cell transplant; ECOG: Eastern Cooperative Oncology Group; ELN: European LeukemiaNet; WBC: white blood cell; ANC: absolute neutrophil count; ALC: absolute lymphocyte count; ICI: immune checkpoint inhibitor; URI: upper respiratory infection
Risk for mortality
Table 3 shows risk factors for death after ICI initiation, which are based on a variable-selected Cox regression model, treating status variable as time-dependent variables. Age (HR 1.02 per year, 95% CI 1.01–1.04), number of prior lines of chemotherapy (HR 1.17, 95% CI 1.01–1.34), baseline lung disease (HR 1.87, 95% CI 1.36–2.58), ECOG (ECOG 0 to 2, HR 2.45, 95% CI 1.26–4.76), leukemia progression (HR 2.21, 95% CI 1.80–2.71), pneumonia after ICI (HR 1.99, 95% CI 1.57–2.51) and pneumonitis after ICI (HR 2.84, 95% CI 1.84–4.37) increased mortality risk, while higher baseline total WBC (HR 0.91 per log unit, 95% CI 0.79–1.05), higher baseline platelets (HR 0.81, 95% CI 0.69–0.95), non-adverse ELN (HR 0.50, 95% CI 0.38–0.67) and ipilimumab monotherapy (HR 0.66, 95% CI 0.48–0.90) were associated with decreased mortality. Figure 3 shows the Kaplan-Meier survival estimates for the entire cohort.
Table 3.
Risk for mortality
| Variable | Univariate HR (95% CI) | p-value | Multivariate HR (95% CI) | p-value |
|---|---|---|---|---|
| Age (per year) | 1.02 (1.01–1.04) | <0.001 | 1.02 (1.01 – 1.04) | <0.001 |
| Sex (female to male) | 0.88 (0.67–1.17) | 0.39 | ||
| BMI (per unit) | 1.02 (0.997–1.05) | 0.09 | ||
| AML Diagnosis (De novo to secondary) | 1.17 (0.84–1.60) | 0.36 | ||
| Prior lines of therapy (per line) | 1.32 (1.17–1.49) | <0.001 | 1.17 (1.01–1.34) | 0.003 |
| Prior HCT | 1.29 (0.86–1.93) | 0.23 | ||
| ECOG | 0.003 | <0.001 | ||
| 0 to 1 | 1.73 (1.15–2.61) | 0.008 | 1.51 (0.96–2.37) | 0.07 |
| 0 to 2 | 2.90 (1.52–5.55) | 0.001 | 2.45 (1.26–4.76) | 0.008 |
| Non-adverse ELN (compared to adverse) | 0.50 (0.38 −0.67) | <0.001 | 0.57 (0.43–0.77) | <0.001 |
| Baseline bone marrow blasts (per 1%, n=257) | 1.00 (0.996–1.007) | 0.51 | ||
| Baseline total WBC (per log unit) | 0.82 (0.72 – 0.95) | 0.07 | 0.67 (0.49–0.93) | <0.001 |
| Baseline ANC (per 100 cells/μL, n=255) | 0.96 (0.92–1.01) | 0.10 | ||
| Baseline ALC (per 100 cells/μL, n=256) | 0.95 (0.83–1.09) | 0.48 | ||
| Platelets (per unit) | 0.68 (0.59–0.79) | <0.001 | 0.80 (0.69–0.94) | <0.001 |
| Smoking status (Never vs. former or current) | 1.20 (0.91 – 1.59) | 0.20 | ||
| Pneumonia within 30 days of ICI initiation | 1.81 (1.22–2.68) | 0.005 | ||
| Viral URI within 30 days of ICI initiation | 1.09 (0.48–2.45) | 0.84 | ||
| Prior lung disease | 1.50 (0.98–2.29) | 0.06 | 1.87 (1.36–2.58) | <0.001 |
| Prior autoimmune disease | 1.05 (0.49–2.23) | 0.90 | ||
| Chest radiation prior to ICI | 1.04 (0.55–1.97) | 0.90 | ||
| Baseline symptoms | ||||
| Cough | 1.26 (0.88 – 1.80) | 0.21 | ||
| Fever | 1.45 (1.02 – 2.07) | 0.05 | ||
| Shortness of breath | 1.51 (1.07–2.13) | 0.02 | ||
| Treatment regimen | ||||
| Nivolumab and ipilimumab | 1 (reference) | |||
| Ipilimumab only | 0.99 (0.68–1.46) | 0.99 | 0.66 (0.48–0.90) | <0.001 |
| Nivolumab only | 0.82 (0.50–1.35) | 0.82 | ||
| Pneumonitis | 1.61 (0.95–2.71) | 0.07 | 2.84 (1.84–4.37) | <0.001 |
| Pneumonia after ICI initiation | 1.89 (1.48–2.39) | <0.001 | 1.99 (1.57–2.51) | <0.001 |
| Progression of disease | 2.29 (1.85–2.84) | <0.001 | 2.21 (1.80–2.71) | <0.001 |
Abbreviations: HR: hazard ratio; CI: confidence interval; BMI:, body mass index; AML: acute myeloid leukemia; HCT: hematopoietic cell transplant; ECOG: Eastern Cooperative Oncology Group; ELN: European LeukemiaNet; WBC: white blood cell; ANC: absolute neutrophil count; ALC: absolute lymphocyte count; ICI: immune checkpoint inhibitor; URI: upper respiratory infection
Figure 3.
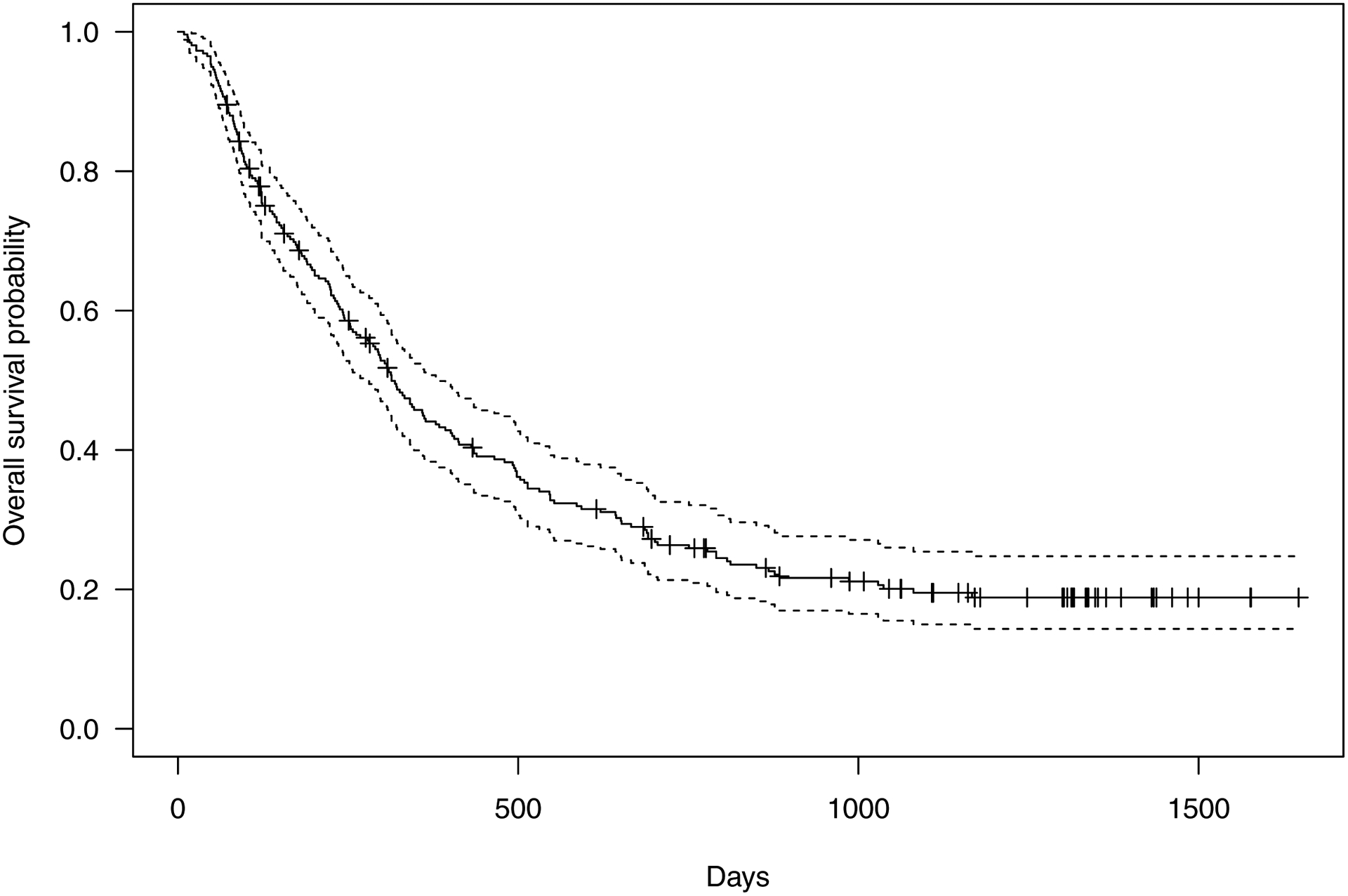
Kaplan-Meier estimates of survival for the overall cohort (n=258).
Discussion
In this work, we show that in a large, novel, high-risk immunodeficient cohort of patients with AML undergoing treatment ICI agents, ICI-related pneumonitis occurs at a high rate and increases mortality. We demonstrate that over 10% of patients with AML who undergo ICI therapy will develop pneumonitis. Older age and shortness of breath at the time of ICI initiation increased the risk for developing pneumonitis, while female sex and increasing platelet counts were protective. Nearly half of patients who developed pneumonitis died shortly after developing pneumonitis. Using novel multi-state modeling, we show that developing pneumonitis increased the risk of death over two-fold, with a similar impact on mortality as the development of pneumonia. This work highlights the toxicities of transformative therapies like ICIs, and the need for multidisciplinary collaboration to identify and treat these toxicities promptly.
ICI-related pneumonitis is a serious complication of checkpoint inhibitor immunotherapy, and the observed case-fatality rate in non-small cell lung cancer (NSCLC) may be as high as 20%14–16. By understanding who is at high risk for ICI-related pneumonitis prior to initiating therapy, it may be possible to inform patient selection for ICI-based therapies. This study offers insights into factors that affect the risk for pneumonitis. To our knowledge, our study is the first to report an association with increased age and the risk for ICI-related pneumonitis, with a 2% increase in the risk for pneumonitis with each additional year of age at enrollment. A review of the United States Food and Drug Administration pharmacovigilance database showed that younger age may be associated with a higher risk for pneumonitis, while retrospective studies have found no association between age and ICI-related toxicities17,18. Studies of radiation pneumonitis show conflicting results, with some suggesting no effect of age but a strong effect of prior ILD19,20, and others suggesting an effect of age in addition to prior ILD16,21. Most patients in our study had asthma or chronic obstructive pulmonary disease (COPD), and only one patient had ILD. Therefore, we cannot properly evaluate whether ILD affects pneumonitis risk. However, we note patients who perceived dyspnea at enrollment had over a twofold higher risk for pneumonitis. It is possible that the perception of dyspnea was a stronger indicator of active underlying lung disease than a clinical diagnosis of lung disease, but because the evaluation of pulmonary function tests at enrollment was sporadic, we cannot speculate further on the etiology of dyspnea. Our finding that age increases the risk for ICI-related pneumonitis in addition to inherent lung disease is novel.
Our risk model for pneumonitis suggests that female sex reduces the risk for pneumonitis. Females are thought to mount stronger innate and adaptive immune responses to antigens compared to males, though males may have certain specific responses which are on average stronger (e.g. tumor necrosis factor [TNF] release after lipopolysaccharide exposure)22. However, in a study of 140 patients who underwent ipilimumab therapy for metastatic melanoma, interleukin-6 levels and female sex were associated with a 50% higher risk for toxicity23. Another study of 245 patients with NSCLC or metastatic melanoma showed higher rates of irAEs among women, including pneumonitis in females with NSCLC24. Others have found no association between sex and irAE risk25. In contrast, regardless of treatment regimen, we found a lower risk for pneumonitis with females. To our knowledge, this is the first report to show that females experience a lower rate of pneumonitis than males after ICI therapy. This protective effect for females diminished as the total leukocyte count increased. This variation between the strength of immune response and clinical outcomes by sex has been observed in other settings. After SARS-CoV-2 infection, females tended to generate stronger T-cell responses to viral infection, while males tended to have stronger innate immune responses26. Females with stronger innate immune responses fared worse than those with weaker responses, but this trend was not observed in males. Our work adds to prior studies by showing the complexity of sex differences in immune responses after ICI therapy, and further showing that female sex is not necessarily associated with a higher risk for irAE.
We found that a higher baseline platelet count was associated with a lower risk for pneumonitis. Platelets are involved in antigen presentation and express co-stimulatory molecules such as CD86 to stimulate T-cell responses27, but may also inhibit T-cell responses when they express PD-L1 at the cell surface28,29. Conversely, platelet depletion results in higher CD8+T-cell production of TNF-alpha and interferon-gamma29. Platelets can directly bind lymphocytes and diminish T-cell activity30, and in the setting of sepsis, platelets may have a critical role in immunosuppression by diminishing CD8+ T-cell count and function through the expression of major histocompatibility complex class 1 at the cell surface31. Higher platelet counts have also been associated with poorer survival in patients with cancer treated with ICIs, potentially suggesting diminished immune response32. Our finding that higher baseline platelet counts reduce the risk for pneumonitis adds to the observations that platelets diminish CD8+ cell activity and therefore may reduce off-target immune responses. Our cohort is novel in that we observed high variability in baseline platelet counts and a high degree of baseline thrombocytopenia. It is possible that the protection against irAEs conferred by platelets is better appreciated in this context, as opposed to the less variable platelet counts seen in many cohorts of solid tumor malignancies. Our work adds to the field showing the immunomodulatory role for platelets, but mechanistic studies are needed to confirm this phenomenon.
Though pneumonitis and pneumonia both impact the care of patients in our cohort, pneumonia occurred at a fivefold higher rate. Though we did not observe an increased risk for pneumonitis with combined nivolumab and ipilimumab therapy, we observed an overall incidence of 12%, which is higher than the rate reported from most clinical trials33, but lower than reported rates in NSCLC10,14. Blinded qualitative assessment of chest imaging by expert thoracic radiologists at the time of pulmonary injury, whether infectious or ICI-related, was concordant with clinical assessments in most cases. Most cases were consistent with organizing pneumonia or a mixed pattern. When pneumonitis occurred in our cohort, most patients required oxygen supplementation (CTCAE grade 3), and nearly half died due to pneumonitis. Those who survived the initial episode of pneumonitis had minimal residual scarring, and several were re-challenged successfully with ICI therapy.
The high rate of mortality among patients who developed pneumonitis is striking, and developing pneumonitis increased the hazard for death by nearly threefold. The impact of pneumonitis on mortality was similar to pneumonia34 and cancer progression, both of which are well known to increase mortality. Higher baseline platelet counts and white-blood cell counts were associated with better survival, perhaps indicating less aggressive disease, while more extensive treatment prior to ICI initiation predicted mortality. Similarly, adverse ELN characteristics were associated with higher mortality, as was worse performance status and older age, as has been previously demonstrated35. Prior lung disease, primarily COPD and asthma, increased mortality, consistent with prior reports that patients with obstructive lung diseases have a high rate of respiratory complications and failure36. Although we found a protective effect for ipilimumab in our final model, we would caution that our study design was not appropriate to evaluate the efficacy of ipilimumab on overall survival in AML, and this finding may be due to chance or other unmeasured confounders that were not included in the model. In summary, we found that pneumonitis increased mortality risk to a degree that was similar to pneumonia or cancer progression.
Our study has several strengths. This is a large, novel cohort of prospectively enrolled AML patients treated with ICI. All outcomes were reviewed by an expert multidisciplinary committee in detail. Our model is potentially more flexible than prior state-change models because it does not rely on the assumption that the transition state is stable15. Our state-change model allowed us to predict the hazard for mortality with several predictors within the same model. However, our study has notable weaknesses. The study was performed at a single center which may limit generalizability, and our findings will benefit from validation in other studies of AML patients treated with ICIs. The retrospective design raises the probability for unmeasured confounders, perhaps best highlighted by the lack of granular pulmonary function data at baseline. We could not confirm the diagnosis of pneumonitis with histopathology in nearly all cases due to the concern for bleeding due to thrombocytopenia or the concern for pulmonary deterioration after a biopsy procedure. Furthermore, steroids and antibiotics were often administered together in most (28/31) cases of suspected pneumonitis, which made the adjudication of definite pneumonitis challenging. Finally, while our experienced radiology team was able to determine in most cases whether lung infiltrates were more consistent with pneumonitis or pneumonia, in actual clinical practice, multifocal pneumonia may appear radiologically similar to pneumonitis. These limitations highlight the need for specific biomarkers that can distinguish pneumonia and pneumonitis. Although baseline dyspnea increased pneumonitis risk, we could not determine whether this was due to underlying lung disease, anemia, or for other reasons. We did not include time-varying hematological data to precisely measure whether pneumonitis risk and mortality varied based upon leukocyte or platelet recovery, or if only the baseline assessments were pertinent. Though we observed a high rate of mortality among patients who developed pneumonitis, we could not definitively determine whether pneumonitis or cancer progression in the setting of treatment cessation was the primary cause for death, though all deaths attributed to pneumonitis occurred in the setting of respiratory failure shortly after the onset of pneumonitis. While we could not statistically demonstrate a link between pneumonitis and higher rates of progression among pneumonitis survivors, our ability to find such an association was limited by the small sample size of pneumonitis survivors (n=17). Finally, ICIs are not approved to treat AML, limiting the current impact of our study.
In conclusion, we highlight the incidence, risk factors and clinical features of pneumonitis in a novel cohort of AML patients treated with ICI-containing regimens. Pneumonitis is a common irAE and must be promptly distinguished from pneumonia due to its attendant risk for death and distinct treatment requirements. The association of sex and platelet counts with the risk for ICI-related pneumonitis are novel, and these should be explored as predictors of interest in other cohorts. Our study highlights the important role of multidisciplinary evaluation of toxicities in high-risk cohorts of cancer patients treated with ICIs.
Supplementary Material
Funding:
This work was supported by the NIH/NIAID (K23 AI117024; to A.S.) and the National Cancer Institute (Cancer Center Support Grant, P30 CA016672) at the National Institutes of Health.
Abbreviations
- AML
Acute myeloid leukemia
- allo-HCT
Allogeneic hematopoietic cell transplantation
- COPD
Chronic obstructive pulmonary disease
- CTCAE
Common Terminology Criteria for Adverse Events
- CI
Confidence interval
- ECOG
Eastern Cooperative Oncology Group
- ELN
European LeukemiaNet
- HR
Hazard ratio
- HMA
Hypomethylating agent
- irAE
Immune-related adverse event
- ILD
Interstitial Lung Disease
- NSCLC
Non-small cell lung cancer
- TNF
Tumor necrosis factor
Footnotes
Ethics approval: This study was approved by the MD Anderson Institutional Review Board approved (PA18–0802).
Consent for publication: No patient identifiers are included in this document
Competing interests: No relevant conflicts of interest.
Availability of data:
De-identified data will be made available upon reasonable request. Please address requests to asheshadri@mdanderson.org
References
- 1.Shah A, Andersson TM, Rachet B, Bjorkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971–2006: a population-based study. Br J Haematol. 2013;162(4):509–516. [DOI] [PubMed] [Google Scholar]
- 2.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knipp S, Hildebrand B, Kundgen A, et al. Intensive chemotherapy is not recommended for patients aged >60 years who have myelodysplastic syndromes or acute myeloid leukemia with high-risk karyotypes. Cancer. 2007;110(2):345–352. [DOI] [PubMed] [Google Scholar]
- 4.Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biol Blood Marrow Transplant. 2016;22(4):651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers (Basel). 2020;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. The Lancet Oncology. 2014;15(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orskov AD, Treppendahl MB, Skovbo A, et al. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget. 2015;6(11):9612–9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daver N, Garcia-Manero G, Basu S, et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019;9(3):370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid C, Schleuning M, Schwerdtfeger R, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–1099. [DOI] [PubMed] [Google Scholar]
- 10.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2018;13(12):1930–1939. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JB, Lei X, Wierda W, et al. Pneumonia during remission induction chemotherapy in patients with acute leukemia. Ann Am Thorac Soc. 2013;10(5):432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. [DOI] [PubMed] [Google Scholar]
- 13.program Cte. Common Terminology Criteria for Adverse Events (CTCAE). 2018; 5.0:https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 01/30, 2018.
- 14.Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands). 2018;125:150–156. [DOI] [PubMed] [Google Scholar]
- 15.Suresh K, Naidoo J. Lower Survival in Patients Who Develop Pneumonitis Following Immunotherapy for Lung Cancer. Clin Lung Cancer. 2020;21(3):e169–e170. [DOI] [PubMed] [Google Scholar]
- 16.Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer. 2019;10(10):2006–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbaux P, Maillet D, Boespflug A, et al. Older and younger patients treated with immune checkpoint inhibitors have similar outcomes in real-life setting. European journal of cancer (Oxford, England : 1990). 2019;121:192–201. [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein MRL, Nipp RD, Muzikansky A, et al. Impact of Age on Outcomes with Immunotherapy in Patients with Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2019;14(3):547–552. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Liu H, Wu H, Liang S, Xu Y. Risk factors for radiation pneumonitis in lung cancer patients with subclinical interstitial lung disease after thoracic radiation therapy. Radiat Oncol. 2021;16(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okubo M, Itonaga T, Saito T, et al. Predicting risk factors for radiation pneumonitis after stereotactic body radiation therapy for primary or metastatic lung tumours. The British Journal of Radiology. 2017;90(1073):20160508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang J, Li G, Zang S, Zhang S, Yao L. Risk and predictors for early radiation pneumonitis in patients with stage III non-small cell lung cancer treated with concurrent or sequential chemoradiotherapy. Radiat Oncol. 2014;9:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. [DOI] [PubMed] [Google Scholar]
- 23.Valpione S, Pasquali S, Campana LG, et al. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J Transl Med. 2018;16(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duma N, Abdel-Ghani A, Yadav S, et al. Sex Differences in Tolerability to Anti-Programmed Cell Death Protein 1 Therapy in Patients with Metastatic Melanoma and Non-Small Cell Lung Cancer: Are We All Equal? The Oncologist. 2019;24(11):E1148–E1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing Y, Zhang Y, Wang J, et al. Association Between Sex and Immune-Related Adverse Events During Immune Checkpoint Inhibitor Therapy. J Natl Cancer Inst. 2021. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman LM, Aggrey AA, Field DJ, et al. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189(2):916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolfes V, Idel C, Pries R, et al. PD-L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget. 2018;9(44):27460–27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaslavsky AB, Adams MP, Cao X, et al. Platelet PD-L1 suppresses anti-cancer immune cell activity in PD-L1 negative tumors. Sci Rep. 2020;10(1):19296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zamora C, Canto E, Nieto JC, et al. Binding of Platelets to Lymphocytes: A Potential Anti-Inflammatory Therapy in Rheumatoid Arthritis. J Immunol. 2017;198(8):3099–3108. [DOI] [PubMed] [Google Scholar]
- 31.Guo L, Shen S, Rowley JW, et al. Platelet MHC class I mediates CD8+ T-cell suppression during sepsis. Blood. 2021;138(5):401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, He A, Liu A, Tong W, Cao D. Evaluation of the prognostic role of platelet-lymphocyte ratio in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int Immunopharmacol. 2019;77:105957. [DOI] [PubMed] [Google Scholar]
- 33.Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest. 2017;152(2):271–281. [DOI] [PubMed] [Google Scholar]
- 34.Sheshadri A, Godoy M, Erasmus JJ, et al. Progression of the Radiologic Severity Index is associated with increased mortality and healthcare resource utilisation in acute leukaemia patients with pneumonia. BMJ Open Respir Res. 2019;6(1):e000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantarjian H, Kadia T, DiNardo C, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11(2):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ofran Y, Tallman MS, Rowe JM. How I treat acute myeloid leukemia presenting with preexisting comorbidities. Blood. 2016;128(4):488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data will be made available upon reasonable request. Please address requests to asheshadri@mdanderson.org


