Abstract
Background:
Shared decision making (SDM) is especially important for older adults with cancer given the risks of over- and undertreatment, uncertainty regarding benefits/harms worsened by research underrepresentation, and individual preferences. We aimed to adapt the Best Case/Worst Case (BC/WC) communication tool, which improves SDM in geriatric surgery, to geriatric oncology.
Methods:
We conducted focus groups with 40 stakeholders (fourteen older adults with lung cancer, twelve caregivers, fourteen medical oncologists) to elicit perspectives on using the BC/WC tool for geriatric oncology and to identify components needing refinement. During each focus group, participants viewed a BC/WC demonstration video and answered questions modified from the Decision Aid Acceptability Scale. We analyzed transcripts using deductive and inductive thematic analyses.
Discussion:
Participants believed that the BC/WC tool could help patients understand their cancer care choices, explore tradeoffs and picture potential outcomes, and deliberate about decisions based on their goals, preferences, and values. Oncologists also reported the tool could guide conversations to address points that may frequently be skipped (e.g., alternative options, treatment goals). Participant preferences varied widely regarding discussion of the worst-case scenario and desire for statistical information.
Conclusion:
The BC/WC tool is a promising strategy that may improve SDM in geriatric oncology and patient understanding of alternative options and treatment goals. Based on participant input, adaptations will include framing cancer care as a series of decisions, eliciting patient preferences and asking permission before offering the worst-case scenario, and selection of the two most relevant options to present if multiple exist.
Keywords: geriatric oncology, shared decision making, communication tool, lung cancer
INTRODUCTION
The benefits of shared decision making (SDM) in oncology are well established,1–4 including improved quality of care and physician communication.5 However, implementation of SDM remains challenging given the rapidly changing treatment landscape6,7 and emotional intensity of these discussions.8 For older adults, SDM is especially important given the risks of both over- and undertreatment, uncertainty regarding benefits/harms, and research underrepresentation.9–12 Moreover, older adults may prioritize outcomes other than survival (e.g., quality of life [QOL], function).13–15 Geriatric oncology guidelines recommend geriatric assessments to determine age-related vulnerabilities, estimating the likelihood of treatment benefits/harms, and eliciting patient’s goals, preferences, and values.3,16,17 Unfortunately, these guidelines lack detailed recommendations on how to incorporate risk assessments into treatment discussions and how to best present options to encourage deliberation in the context of individual preferences.
Furthermore, these challenges are compounded by traditional cancer decision making, which focuses on abstract quantitative risks and median outcomes. Consequently, older adults and caregivers frequently do not recall discussing alternatives with oncologists and inaccurate prognostic understanding is common,18–25 which influences decisions.26,27 For example, patients who overestimate their prognosis may be up to 8.5 times more likely to choose life-extending treatment over best supportive care.26 To improve patient-centered decision making to support older adults’ diverse priorities, oncologists need better communication tools.
The Best Case/Worst Case (BC/WC) communication tool may be a promising strategy to address decision-making challenges in geriatric oncology. It is used effectively to improve SDM for older adults making surgical decisions28–30 and has been adapted to support older adults with traumatic injury,31 kidney disease,32 and critical illness.33 BC/WC was developed with stakeholder input from older adults and surgeons28 based on Elwyn’s three-step SDM model.34,35 Elwyn’s model introduces the choice (choice talk), describes options including benefits/harms (option talk), and explores patient preferences to make an informed decision (decision talk).35 The BC/WC framework guides clinicians to present a choice between two options and uses scenario planning36—narrative descriptions of plausible outcomes that acknowledge uncertainty—to describe the best, worst, and most likely case for each option. Scenarios are informed by clinical judgement and knowledge of patient risk factors (e.g., frailty, comorbidities). These scenarios, plus an accompanying graphic aid, help patients formulate and express preferences and concerns about treatment burdens and outcomes. The clinician then provides a goal-concordant recommendation (Figure 1).
Figure 1. Conceptual framework for patient-centered decision making.
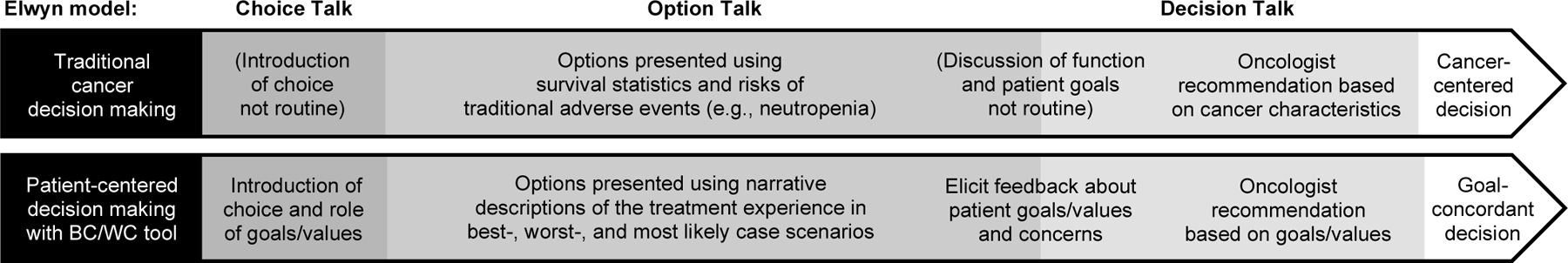
Conceptual framework comparing traditional cancer decision making with an improved patient-centered decision making approach for older adults with cancer using the Best Case/Worst Case (BC/WC) communication tool.
In this study, we elicited feedback from older adults with non-small cell lung cancer (NSCLC), caregivers, and medical oncologists on how to adapt the BC/WC tool for geriatric oncology. We focused on NSCLC, the leading cause of cancer mortality,37 because patients are at high risk for functional decline, cancer symptoms, and treatment toxicity.38–40
METHODS
Study design and participants
We recruited older adults with NSCLC, caregivers, and medical oncologists from the University of California, San Francisco (UCSF) and San Francisco Veterans Affairs Medical Center (SFVAMC). Participants were purposively sampled41 to capture a range of function and treatment decisions (patients), caregiving relationships (caregivers), and years of experience (oncologists). Eligible patients were age ≥65, diagnosed with any stage NSCLC, had made a NSCLC care decision within 12 months, and English speaking. Eligible caregivers were age ≥18, had experience caring for an older adult with NSCLC, participated in a NSCLC care decision within 12 months, and English speaking. Eligible medical oncologists had experience caring for older adults with solid tumors. The UCSF Institutional Review Board and SFVAMC approved this study. For additional details, see Supplemental Table 1.
Data collection
A medical anthropologist (FMN) and research coordinator (VL) conducted focus groups (FGs) for each stakeholder group, which were audio-recorded and transcribed. For caregivers, one-on-one interviews were conducted for those unable to attend a FG. FGs for caregivers and oncologists were in person (9/2019–3/2020). FGs for patients were virtual due to COVID-19 (7/2020–2/2021; Zoom).
Participants watched a demonstration video of a hypothetical 76-year-old woman with metastatic small cell lung cancer (SCLC) with emphysema, a recent fall, and shortness of breath. We chose SCLC to distinguish the example from participants’ actual NSCLC experiences and focus reactions on the tool. In the video, an oncologist used the BC/WC tool and graphic aid (Figure 2) to present two options: chemoimmunotherapy versus comfort-focused care. On the graphic aid, the position of the most likely case on the line represents whether it is more similar to best case (top) or worst case (bottom). We adapted FG questions (Supplemental Materials) from the Decision Aid Acceptability Scale42 to understand suitability of using the tool in geriatric oncology. We asked participants to compare the BC/WC approach to traditional oncology discussions and reflect on the use of narratives to describe options (see Supplemental Materials video script for example scenario narratives). With oncologists, we also explored implementation facilitators and barriers.
Figure 2. Example Best Case/Worst Case (BC/WC) graphic aid used in focus groups.
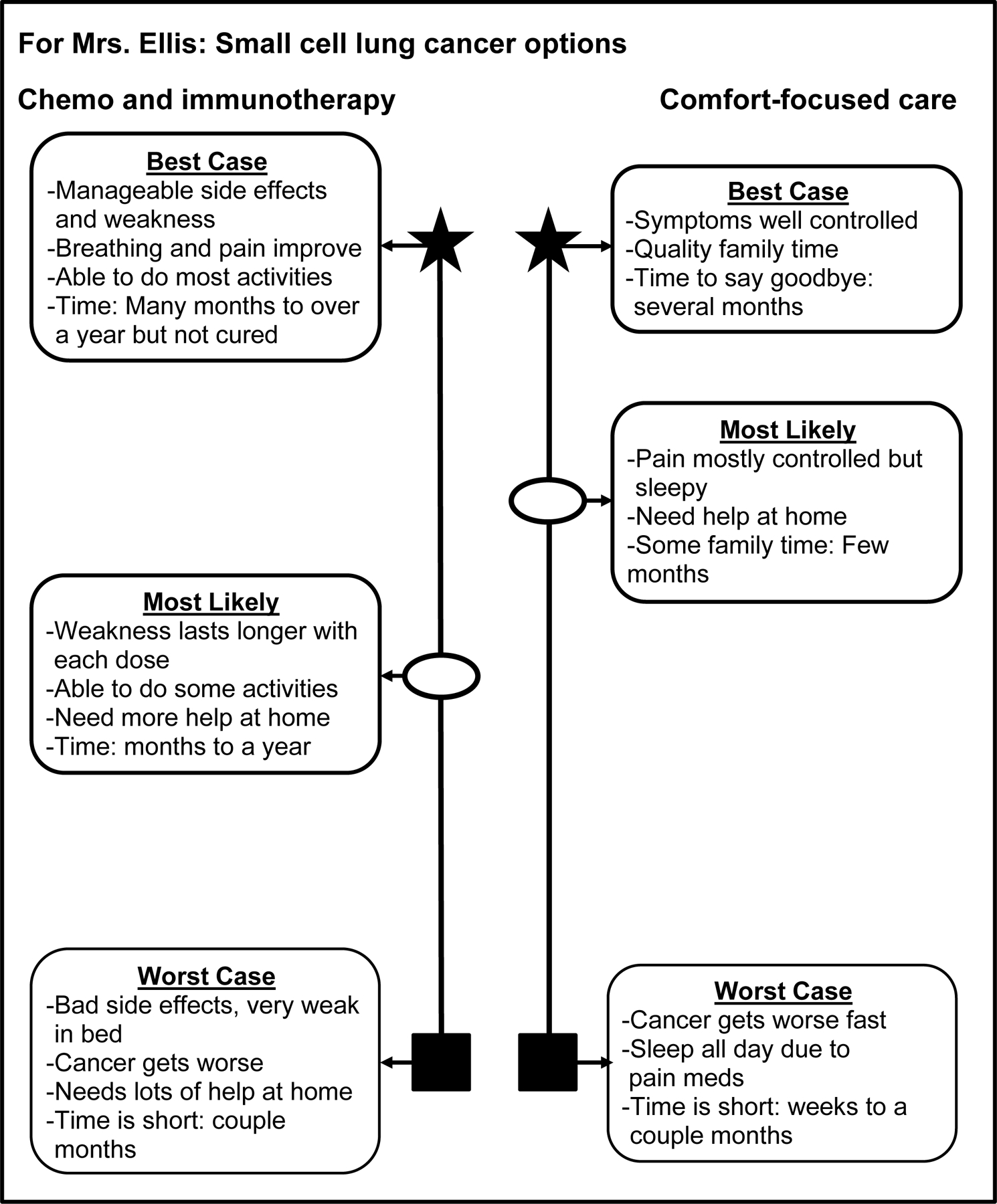
Example BC/WC graphic aid presenting plausible outcomes for 1) chemoimmunotherapy compared with 2) comfort-focused care used in the focus group demonstration video of a hypothetical 76-year-old woman with metastatic small cell lung cancer. In clinical practice, oncologists would create a tailored graphic aid for each patient during their visit starting with a blank template. For each option, oncologists would describe the best, worst, and most likely cases to help patients imagine the range of plausible outcomes and how they might experience each scenario individually. During the conversation, oncologists would add brief notes to the template to summarize key points from each scenario. The oncologist decides where to draw the most likely case (closer to best case at the top or worst case at the bottom) based on their understanding of the patient’s cancer, the option, and the patient’s health status (e.g., frailty, comorbidities). Of note, this example graphic aid depicts the original BC/WC symbols for best (star), worst (box), and most likely case (oval), which will be adapted based on participant feedback.
Analysis
We conducted deductive/inductive thematic analyses43 (Atlas.ti 8, Scientific Software, Germany). Two investigators (MLW, VL) applied initial deductive codes to categorize concepts according to SDM domain (i.e., choice, option, decision talk).35 We generated inductive codes to describe additional constructs gleaned from the data. Transcripts and codes were iteratively discussed in a multidisciplinary team (oncology, geriatrics, palliative medicine, decision making, ethics, medical anthropology) to refine codes and identify common themes across groups. We conducted additional FGs for each stakeholder group until no new themes emerged.44
RESULTS
We enrolled 40 participants (Table 1): fourteen patients (four FGs), twelve caregivers (three FGs, four interviews), and fourteen oncologists (three FGs). Patient median age was 74.5 (range 65–87); 43% were dependent in ≥1 instrumental activity of daily living. Two-thirds of caregivers had a parent with NSCLC; one-third had a partner with NSCLC. Among oncologists, the median years in oncology practice was 4.5 (range 0.5 to 37).
Table 1.
Participant characteristics for older adults with lung cancer, caregivers, and oncologists (N=40).
| Characteristic | n (%) |
|---|---|
| Patients (n = 14) | |
| Age, median (IQR) | 74.5 (68–81) |
| 65–74 | 7 (50) |
| 75–84 | 5 (36) |
| ≥85 | 2 (14) |
| Female | 10 (71) |
| Race | |
| White | 13 (93) |
| Asian | 1 (7) |
| Education | |
| High school or less | 2 (15) |
| College | 6 (46) |
| Graduate level | 5 (38) |
| Partnered | 4 (29) |
| Lives alone | 7 (50) |
| Patient-reported Karnofsky Performance Status | |
| 80–100% | 12 (86) |
| 50–70% | 2 (14) |
| Fall(s) in past 6 months | 4 (29) |
| Dependent in ≥1 instrumental activities of daily living | 6 (43) |
| Caregivers (n = 12) | |
| Age, median (IQR) | 58 (53–67) |
| <55 | 3 (25) |
| 55–64 | 5 (42) |
| ≥65 | 4 (33) |
| Female | 9 (75) |
| Race | |
| White | 8 (73) |
| Asian | 3 (27) |
| Education | |
| College | 4 (33) |
| Graduate level | 8 (67) |
| Caregiver for parent with lung cancer | 8 (67) |
| Caregiver for partner with lung cancer | 4 (33) |
| Medical oncologists (n = 14) | |
| Age, median (IQR) | 40.5 (36–44) |
| <40 | 6 (43) |
| 40–49 | 6 (43) |
| ≥50 | 2 (14) |
| Female | 10 (71) |
| Race | |
| White | 6 (43) |
| Asian | 8 (57) |
| Years in independent oncology practice, median (IQR) | 4.5 (1.5–7) |
Abbreviations: IQR, interquartile range.
Overall, participants appreciated the potential of BC/WC to serve as a step-by-step framework to facilitate all three SDM domains including making choice explicit, supporting option talk with stories of plausible outcomes and a graphic aid, and encouraging deliberation (Tables 2 and 3). Participants also identified implementation challenges to inform our geriatric oncology adaptation.
Table 2.
Older adults with non-small cell lung cancer and cancer caregivers: Support for using the Best Case/Worst Case (BC/WC) communication tool to improve shared decision making (SDM) in geriatric oncology (n = 14 patients, 12 caregivers).
| SDM Domain | Theme | Description | Exemplar patient quotes | Exemplar caregiver quotes |
|---|---|---|---|---|
| Choice talk | BC/WC framework makes choice explicit | Participants appreciated how the BC/WC tool clearly laid out a choice between more than one option. | “[Dr. Artz*] spelled out the options very clearly so that you had pretty good ideas.” | “[Dr. Artz’s] showing all the options in very honest, very open terms.” |
| Option talk | Range of plausible outcomes helps patients/caregivers understand tradeoffs | Participants praised the balanced discussion of potential positive and negative outcomes including how treatment might affect different aspects of their daily life. | “I think it’s practical because you probably will fall in the middle somewhere. You hopefully won’t be worst case. You may not be the best case, but barring that, it is good to know what is the blend of all that. I think you need to know as much as possible to be well-informed to make a well-informed decision, so I would find that helpful, definitely.” | “I thought it was balanced. [Dr. Artz] talked about best case and worst case. He talked about the things that were important to the patient, quality of life versus time.” |
| Stories help patients/caregivers imagine plausible outcomes | Comprehensive BC/WC stories can help patients/caregivers imagine how treatment might be experienced in real life. | “If the story is, ‘You’re going to be weak and probably bed-ridden and unable to do this, that and the other,’ well, you can picture that in your mind. So, I think that is helpful.” | “[Dr. Artz] described the options very clearly. He described what it consisted of, and he then described the impact on how [Mrs. Ellis*] would go under comfort care clearly and realistically.” | |
| Graphic aid as a guide and durable reference | Participants appreciated how the visual reference can help patients and families understand options and different possible outcomes. | “Just being able to see the two [options], the top and bottom and most likely. I think that’s very helpful.” | “If I had received this or something like this, it would have helped me. Especially my brother who couldn’t—he came to one but he just couldn’t come to all the appointments. I think it would have helped him understand a little bit better what [our mother] was going through.” | |
| Decision talk | Encourages deliberation | The BC/WC tool can encourage oncologists to ask and patients to reflect upon how each possible outcome scenario fits within their goals and values. | “I did think that [Dr. Artz] spelling out, when he came to the place where he said, ‘Probably your treatment is somewhere between, midway between best and worst case,’ I think that that’s probably really important to be able to say. That the symptoms, spelling out what [Mrs. Ellis] needs to consider about how important it is for her not to be in a nursing care facility, to be able to stay at home, and so forth. Those were not choices that were discussed with me, and I think that’s worth, really worth including.” | “A very good thing in this communication that often doctors don’t ask and don’t talk about is, ‘What is your situation at home and what do you want? What is most important to you?’ In this kind of decision, that is key to ask. It is one of the most important things that could be asked because then the doctor can emphasize certain things that will reach those goals. So I thought that was really good about it.” |
In the BC/WC example demonstration video, Dr. Artz was the oncologist and Mrs. Ellis was a 76-year-old woman with metastatic small cell lung cancer.
Table 3.
Medical oncologists: Support for using the Best Case/Worst Case (BC/WC) communication tool to facilitate shared decision making (SDM) in geriatric oncology (n = 14 oncologists).
| SDM Domain | Theme | Description | Exemplar oncologist quotes |
|---|---|---|---|
| Choice talk | BC/WC framework makes choice explicit | BC/WC tool guides oncologists to routinely present more than one option to help patients understand that they have a choice, including best supportive care. | “The only time I’m more supportive of treatment is if there’s a curative possibility. But, if there’s not, then you’re really just prolonging someone’s life. Then you’ve got to say that from the get go because people need to know that they don’t have to have treatment.” |
| Option talk | Range of plausible outcomes helps patients/caregivers understand tradeoffs |
BC/WC tool provides structure to communicate both the best potential benefit, worst potential harm, and most likely outcome to help patients weigh tradeoffs. | “It’s really important as a prop for the doctor to remember that there is this worst case scenario. There are oncologists who are consistent about sort of painting that picture, but then there are also ones that the natural tendency is to focus on the best-case scenario.” |
| Stories help patients/caregivers imagine plausible outcomes | The narrative approach can helps oncologists translate statistical outcome information for patients/caregivers to improve understanding. | “I think rather than giving them just the median survival or median progression free survival, this kind of approach might work better.” | |
| Graphic aid as a guide and durable reference | Oncologists valued how the graphic aid can guide the discussion and visually communicate where the estimated most likely case falls relative to best and worst case. | “I think that having a prop, [Dr. Artz’s*] flowsheet helped him kind of direct things and guide the conversation. It seems like that could be good strategy to have that, the tool to use to do that, and I think that he by using best case/worst case, it allowed [Mrs. Ellis*] to see the possibilities.” | |
| Decision talk | Encourages deliberation | Oncologists appreciated how the BC/WC tool can help them elicit and understand patients’ goals and preferences as each scenario is described. | “At one point, [Dr. Artz and Mrs. Ellis] were talking about the best case and worst case when [Mrs. Ellis] would chime in about, ‘Oh that sounds like that’s what I want’ or they say, ‘That is what I want to do with my time.’ It helps sort of for them to mention their goals more organically. They are responding to what you’re telling them about the benefits or the side effects. I think they can just actually tell you what makes sense to them, what they like or don’t like about these options, which I think thaťs pretty nice.” |
In the BC/WC example demonstration video, Dr. Artz was the oncologist and Mrs. Ellis was a 76-year-old woman with metastatic small cell lung cancer.
Support for using BC/WC to facilitate SDM in geriatric oncology
Choice talk: BC/WC framework makes choice explicit
Participants valued how the BC/WC framework made the cancer treatment choice explicit by routinely presenting more than one option. A caregiver commented that BC/WC could help guide oncologists to show “all the options in very honest, very open terms.” Oncologists found the framework helpful in approaching the overarching decision to pursue cancer-directed treatment because they could use it to present comfort-focused care as the second option when appropriate. One oncologist explained, “You have to be sure that the patient has the full picture, right? We first need to decide what your goals are and whether you want treatment. They don’t understand that they have an option.”
The importance of making choice explicit was reinforced when several patients and caregivers described trusting their oncologists and not asking follow-up questions about alternatives. A 74-year-old patient shared, “My doctor talked about one option. I have very limited knowledge as to what any options are so I just accepted that.” In one instance, a caregiver did not recognize that they had made any decisions for his wife’s care—despite his wife having undergone surgery and chemotherapy.
Option talk: Range of plausible outcomes helps patients/caregivers understand tradeoffs
Participants supported using BC/WC to help patients/caregivers understand tradeoffs between options based on the range of plausible outcomes. One oncologist explained, “I like how they can see it side-by-side what you’re thinking as an expert. What is the best possible benefit? What am I really risking?” Oncologists acknowledged their “tremendous power in framing the message right” and both oncologists and patients/caregivers noted that BC/WC can help balance the discussion in the setting of uncertainty. An 81-year-old patient said, “I think it’s important to have the different scenarios put forth. Every patient has a different reaction.” After watching the demonstration video, a caregiver reflected, “What the patient needs and deserves is getting that information so they can make informed decisions. If the information is not correct, the decision will not be well informed. I thought the balance was difficult to achieve but successful.”
Oncologists appreciated how BC/WC could provide structure to communicate both the best plausible benefit and worst plausible harm. Oncologists viewed the best-case scenario as an opportunity to discuss the overall treatment goal (i.e., curative versus palliative). For incurable cancers, one oncologist imagined sharing with a patient, that even in the best case, “this disease sadly is not going to allow us to cure you.” At the opposite end of the spectrum, oncologists viewed the worst-case scenario as an opportunity to discuss the potential for treatment to cause more symptoms than the cancer itself, or worse yet, “that the treatments I’m giving them could cause their death.”
Option talk: Stories help patients/caregivers imagine plausible outcomes
Patients/caregivers praised the use of stories to describe plausible outcomes, which could help them imagine how treatment might be experienced individually. A 68-year-old patient said, “A story form is something that you can relate to. You can kind of put yourself in that situation and you can see yourself.” Participants viewed this information as necessary to understand and compare options. They appreciated how the narrative approach could prompt oncologists to tell more comprehensive stories about treatment effects on QOL, function, and cognition.
Oncologists also viewed the narrative approach as a useful strategy to translate statistics to improve understanding. For example, “This might be better than just giving out the median survival because median survival can be misleading. [Patients] tend to stick to that median number and they believe that they’re going to live up to that time.” In contrast to presenting summary statistics, some oncologists felt that BC/WC stories could better capture patient heterogenity and emphasize the range of possible outcomes. One oncologist said, “Statistics are from a group of people. The patient’s an individual patient. I don’t even use statistics. I talk about a range.”
Option talk: Graphic aid as a guide and durable reference
Participants valued the accompanying graphic aid as a discussion guide and durable reference to share with family. The graphic aid could reinforce their ability to visualize each option and recall the discussion. A 76-year-old patient explained, “I like to see things in front of me so I can go back to it and take it in.” A caregiver highlighted the importance of having written notes since people can be overwhelmed during the appointment: “It’s hard to absorb what’s being said. You’re listening, you’re looking, but also [to] have something in a written format.”
Oncologists agreed that the graphic aid could help compare options side-by-side and show where the most likely case would fall based on everything they know about the patient’s cancer, function, and comorbidities. One oncologist described that showing where the most likely case lay relative to best and worst case communicates his individualized clinical assessment: “Visually tell the patient, ‘Wow, I’m closer to bad side effects, being weak and in bed, and the cancer getting worse.’ Or if you think they’re close to the top, ‘Hey, I’m up here.’”
Decision talk: Encourages deliberation
Participants believed BC/WC could encourage deliberation and reflection on patients’ goals, preferences, and values. Oncologists envisioned using it to elicit and understand patients’ goals in the context of different plausible outcomes: “While talking about each scenario, asking if it aligns with the patient’s wishes. Or if what I’m talking about is important to the patient or if that is what they were hoping for.” A caregiver agreed that discussing how options affect important aspects of daily life beyond what is typically discussed might change decisions. She reflected, “If someone had told my mom there is a possibility that you’ll have some brain dysfunction or you may need to live in an assisted living facility, she might have foregone treatment.” Similarly, a 74-year-old patient felt the scenarios could provide helpful anticipatory guidance for symptom management: “If I had this laid out at the very beginning, it probably would’ve benefited my decision, or it would’ve made me a little more comfortable with my decision. There is a lot of this that you have to do for yourself. And managing your symptoms are part of it.”
Challenges with using BC/WC for geriatric oncology
Choice talk: Cancer care as a series of decisions
Participants suggested that for use in oncology, the BC/WC tool needs to accommodate cancer care as a series of decisions with the ability to modify/stop treatment if needed. One oncologist explained, “Unlike a surgery, where once you get in there, you sort of got to finish the job. With chemotherapy, you can always reassure a patient that, ‘If we can’t get it right, you always can stop treatment.’” Participants suggested adding a discussion of how and when decisions might change the framework. For example, a caregiver wanted to understand, “These choices, when do they have to be made? Are they reversible? Can you try one for a while?”
Option talk: Avoidance of worst-case information
Some patients valued hearing the worst-case scenario to help them understand tradeoffs between options: “What is the worst thing that could happen and what can we do about it? That’s how I need to know.” However, others were concerned that hearing the worst case could be upsetting and wanted to avoid this information. A 68-year-old patient said, “I don’t need [my oncologist] to personally tell me the worst-case scenario. I know what that is and I don’t really need all the details.” One caregiver recommended careful word choice to improve acceptability: “I wouldn’t call it worst case. Worst case is to me a very negative connotation.” Additionally, some participants wanted oncologists to maintain a positive outlook and focus on potential benefits. Oncologists agreed that some patients might find the worst-case scenario overwhelming. Offered one oncologist, “You don’t want to give them unnecessary hopes or expectations, but then some of them don’t want, ‘This is the worst-case scenario.’” Instead, she suggested asking patients, “How much do you want to know about your prognosis?”
Option talk: Desire for statistical information
Some participants wanted to hear statistics along with stories to understand the likelihood of each scenario. One caregiver asked, “Does ‘most likely’ mean 40% likely and the extremes are 30% likely, or does ‘most likely’ mean 90% likely and the extremes are 5% likely?” Similarly, an oncologist commented, “I thought this was a pretty effective way of communicating, but what’s missing here is the probability of each scenario.” Given the emphasis on stories and not statistics, one oncologist explained that BC/WC might not be the best fit for some patients (e.g., data engineer).
Option talk: Challenge of more than two options
Oncologists felt that BC/WC would work best with two treatment options, potentially each with subchoices (e.g., dose reductions). They viewed discussions of ≥3 options to be too complex due to time constraints. One oncologist asked, “What if you have the option of surgery, chemoradiation, and also chemo by itself? I can’t imagine going [through] BC/WC with every one of those options. I suppose you could maybe make it work by saying the best case for what you think you’re going to recommend and then go from there.” This example also highlighted the challenge of presenting options that involve multiple modalities requiring multidisciplinary expertise.
Option Talk: Recommendations to improve BC/WC graphic aid
Suggestions to improve the graphic aid focused on increasing clarity and thoughtful use of symbols. Participants recommended displaying each option on a separate page. One caregiver described the black box currently used for worst case as a “death box,” and recommended a more neutral symbol. Lastly, participants recommended using the star to draw attention to the most likely case, rather than best case.
DISCUSSION
Our qualitative study of older adults with NSCLC, caregivers, and oncologists identified multiple components of the BC/WC communication tool that could facilitate SDM in geriatric oncology. Participants believed that BC/WC could help patients understand cancer care choices, explore tradeoffs and picture potential outcomes, and deliberate about decisions based on their goals, preferences, and values. Oncologists found that the tool could guide conversations to address commonly omitted points including alternatives and treatment goals. Implementation challenges included heterogeneous preferences regarding the worst-case presentation and statistical information, highlighting the importance of tailoring the discussion to each patient.
As noted by oncologist participants, the BC/WC tool has the potential to improve patient/caregiver prognostic understanding by guiding oncologists to describe treatment goals (i.e., curative versus palliative) as part of best-case scenarios. For incurable cases, oncologists can share that even in the best case, treatment can manage the cancer for some time, but it will eventually progress. Additionally, based on prior literature,45 we hypothesize that descriptions of worst-case scenarios may further support accurate prognostic understanding. In a study of oncologist-patient communication, patients were more likely to agree with their oncologist’s assessment of curability if the oncologist made ≥1 statement of concern/worry about the cancer.45 Statements of optimism or uncertainty did not improve prognostic understanding.45 In future studies, we will explore whether statements of concern/worry shared during the tool’s worst-case scenarios improve patient/caregiver prognostic understanding.
Participants highlighted important challenges that will inform our geriatric oncology adaptation. In contrast to non-oncological surgery, oncology decisions are often a series of choices and discussions. If an initial decision needs reevaluation due to toxicity, the original graphic aid can be helpful in discussing where the patient’s actual experience landed between best/worst case. If the initial treatment is tolerable but the cancer progresses/recurs, the framework can be used again to discuss new options.
We found diverse patient/caregiver information preferences, with some wanting to hear the worst case first and others not wanting to know at all. To address the patient’s emotional readiness to learn about both positive and negative potential outcomes, oncologists will need to assess the patient’s current illness understanding and information preferences, then ask permission before offering the worst-case scenario. Framing worst case as “this is what we are worried about” might also increase acceptability.
Although BC/WC was developed for use with two options, participants identified cases in geriatric oncology where there may be multiple options (e.g., different combinations of multimodality treatment) or only one obvious, well-tolerated cancer-directed treatment option (e.g., osimertinib for EGFR-mutated lung adenocarcinoma). In situations with multiple reasonable options, adaptations may include presenting the two most goal-concordant options or two options that represent opposite ends of the spectrum (i.e., most and least aggressive) to stimulate discussion about alternatives in between. When cases require multidisciplinary input, the BC/WC conversation may need an iterative, well-coordinated approach as patients meet with different specialists to discuss options. Scanning graphic aids into the medical record may facilitate this multidisciplinary communication. When oncologists recommend only one cancer-directed treatment option with a high likelihood of benefit and low likelihood of harm, the tool can still be used to describe the range of plausible outcomes to clarify the treatment goal and provide anticipatory guidance. In these cases, a thorough discussion of what treatment might look like for that particular patient can slow the strong clinical momentum to start treatment immediately and skip important details of possible downstream outcomes.
While several participants described not finding statistical information helpful during discussions, others desired information about the likelihood of each scenario. The challenges of low health numeracy in oncology are well documented,26,46–48 especially among older adults.49,50 In geriatric oncology, data on treatment benefits and specific harms (e.g., impact on cognition) are often unavailable or extrapolated from clinical trials of younger patients. Accordingly, BC/WC acknowledges this uncertainty through descriptions of a range of plausible outcomes. Of note, only one oncologist shared that estimating the most likely case might be difficult. We hypothesize that the difficulty of describing plausible outcomes was not a common theme because BC/WC focuses on narrative descriptions of what might happen rather than precise statistical estimates. The tool also allows the oncologist to translate information about multiple important outcomes (e.g., QOL, function, cognition), which may be informed by geriatric assessment results, into integrated stories to help patients visualize options holistically. This contrasts with traditional cancer discussions focused on statistics that typically represent outcomes separately. Nonetheless, if patients request quantitative information to help them better understand different options, oncologists can add this complementary information (if available) to their scenarios.
Our study has several limitations. We recruited participants from two cancer clinics within one Comprehensive Cancer Center in the San Francisco Bay Area, resulting in a predominately white, well-educated sample. All participants spoke English, so we did not explore unique aspects of using BC/WC with an interpreter. Participants were predominately women, so there may be differences in decision-making preferences by gender that we did not explore. We focused on cancer decision making between older adults with NSCLC and medical oncologists. Further investigation is needed to understand SDM across different cancer types as well as surgical and radiation oncology decisions. Lastly, the majority of oncologists have practiced oncology independently for ≤5 years; there may be differences in how more experienced oncologists might use BC/WC clinically.
CONCLUSION
Participants believed that the BC/WC communication tool is a promising intervention that can provide an accessible framework to facilitate SDM for older adults with cancer that addresses their unique needs. Next steps include oncologist training development to effectively use scenario planning, testing feasibility and acceptability of implementing this approach in geriatric oncology clinical practice, and, ultimately, evaluating whether the BC/WC tool improves SDM, prognostic understanding, and goal-concordant decisions.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Cancer and Aging Research Group patient advocate Beverly Canin for her feedback on the video script, actress Vivian Pisano of Stagebridge (the nation’s oldest performing arts organization of older adults) for playing the part of Mrs. Ellis in the focus group demonstration video, and videographer Beatrice Hackman for filming and editing the video. We also thank thoracic medical oncologists Carolyn J. Presley, MD, MHS and Julia Rotow, MD for their clinical review of the example small cell lung cancer case used in the focus groups and interviews. Lastly, we thank Amy J. Markowitz, JD (scientific writing specialist for the University of California, San Francisco Clinical & Translational Science Institute K Scholars Program) for her copyediting assistance with this manuscript.
Funding:
This work was supported by the National Institutes of Health National Institute on Aging (K76AG064431 [MLW], K24AG068312 [AKS], K24AG068312 [LCW], K24AG056589 [SGM], R33AG059206 [SGM]), National Cancer Institute (K99CA237744 [KPL]), University of California, San Francisco Helen Diller Family Comprehensive Cancer Center [MLW], and Wilmot Fellowship Award [KPL]. Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
MLW, KPL, and MLS reported conflicts of interest outside of the submitted work (MLW: receives royalties from UpToDate, immediate family member is an employee of Genentech with stock ownership; KPL: consultant for Pfizer and Seattle Genetics, receipt of honorarium from Pfizer; MLS: immediate family member has ownership interest in MezLight LLC). The remaining authors have no conflicts to report.
Prior presentation: This study was presented as an oral presentation at the 2021 International Society of Geriatric Oncology Annual Meeting (virtual).
REFERENCES
- 1.Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15(1):9–19. [DOI] [PubMed] [Google Scholar]
- 2.Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52(12):1865–1878. [DOI] [PubMed] [Google Scholar]
- 3.DuMontier C, Loh KP, Soto-Perez-de-Celis E, Dale W. Decision Making in Older Adults With Cancer. Journal of Clinical Oncology. 2021;39(19):2164–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaston CM, Mitchell G. Information giving and decision-making in patients with advanced cancer: a systematic review. Soc Sci Med. 2005;61(10):2252–2264. [DOI] [PubMed] [Google Scholar]
- 5.Kehl KL, Landrum MB, Arora NK, Ganz PA, van Ryn M, Mack JW, et al. Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care. JAMA Oncol. 2015;1(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Upadhaya S, Hubbard-Lucey VM, Yu JX. Immuno-oncology drug development forges on despite COVID-19. Nat Rev Drug Discov. 2020;19(11):751–752. [DOI] [PubMed] [Google Scholar]
- 7.Melosky B, Wheatley-Price P, Juergens RA, Sacher A, Leighl NB, Tsao MS, et al. The rapidly evolving landscape of novel targeted therapies in advanced non-small cell lung cancer. Lung Cancer. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Mazzocco K, Masiero M, Carriero MC, Pravettoni G. The role of emotions in cancer patients’ decision-making. Ecancermedicalscience. 2019;13:914–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuMontier C, Loh KP, Bain PA, Silliman RA, Hshieh T, Abel GA, et al. Defining Undertreatment and Overtreatment in Older Adults With Cancer: A Scoping Literature Review. Journal of Clinical Oncology.0(0):JCO.19.02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions. CA Cancer J Clin. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson DR, Loh KP. Improving personalized treatment decision-making for older adults with cancer: The necessity of eliciting patient preferences. Journal of Geriatric Oncology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rostoft S, van den Bos F, Pedersen R, Hamaker ME. Shared decision-making in older patients with cancer - What does the patient want? Journal of Geriatric Oncology. 2021;12(3):339–342. [DOI] [PubMed] [Google Scholar]
- 13.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066. [DOI] [PubMed] [Google Scholar]
- 14.Celis ESPD, Li D, Sun C-L, Kim H, Twardowski P, Fakih M, et al. Patient-defined goals and preferences among older adults with cancer starting chemotherapy (CT). Journal of Clinical Oncology. 2018;36(15_suppl):10009–10009. [Google Scholar]
- 15.Loh KP, Mohile SG, Epstein RM, McHugh C, Flannery M, Culakova E, et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer. 2019;125(14):2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Older Adult Oncology. Version 1.2021; https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed 29 July 2021.
- 17.Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh KP, Soto Pérez de Celis E, Duberstein PR, Culakova E, Epstein RM, Xu H, et al. Patient and caregiver agreement on prognosis estimates for older adults with advanced cancer. Cancer. 2020;127(1):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh KP, Mohile SG, Lund JL, Epstein R, Lei L, Culakova E, et al. Beliefs About Advanced Cancer Curability in Older Patients, Their Caregivers, and Oncologists. Oncologist. 2019;24(6):e292–e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson LL, Temel B, Fuh CX, Server C, Kay P, Landay S, et al. Perceptions of medical status and treatment goal among older adults with advanced cancer. J Geriatr Oncol. 2020;11(6):937–943. [DOI] [PubMed] [Google Scholar]
- 21.El-Jawahri A, Nelson-Lowe M, VanDusen H, Traeger L, Abel GA, Greer JA, et al. Patient-Clinician Discordance in Perceptions of Treatment Risks and Benefits in Older Patients with Acute Myeloid Leukemia. Oncologist. 2019;24(2):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loh KP, Xu H, Back A, Duberstein PR, Gupta Mohile S, Epstein R, et al. Patient-hematologist discordance in perceived chance of cure in hematologic malignancies: A multicenter study. Cancer. 2020;126(6):1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh KP, Abdallah M, Kadambi S, Wells M, Kumar AJ, Mendler JH, et al. Treatment decision-making in acute myeloid leukemia: a qualitative study of older adults and community oncologists. Leuk Lymphoma. 2021;62(2):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattellari M, Voigt KJ, Butow PN, Tattersall MH. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol. 2002;20(2):503–513. [DOI] [PubMed] [Google Scholar]
- 25.Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weeks JC, Cook EF, O’Day SJ, Peterson LM, Wenger N, Reding D, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. Jama. 1998;279(21):1709–1714. [DOI] [PubMed] [Google Scholar]
- 27.Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319–2326. [DOI] [PubMed] [Google Scholar]
- 28.Kruser JM, Nabozny MJ, Steffens NM, Brasel KJ, Campbell TC, Gaines ME, et al. “Best Case/Worst Case”: Qualitative Evaluation of a Novel Communication Tool for Difficult in-the-Moment Surgical Decisions. J Am Geriatr Soc. 2015;63(9):1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruser JM, Taylor LJ, Campbell TC, Zelenski A, Johnson SK, Nabozny MJ, et al. “Best Case/Worst Case”: Training Surgeons to Use a Novel Communication Tool for High-Risk Acute Surgical Problems. J Pain Symptom Manage. 2017;53(4):711–719 e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor LJ, Nabozny MJ, Steffens NM, Tucholka JL, Brasel KJ, Johnson SK, et al. A Framework to Improve Surgeon Communication in High-Stakes Surgical Decisions: Best Case/Worst Case. JAMA Surg. 2017;152(6):531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann CJ, Zelenski AB, Buffington A, Baggett ND, Tucholka JL, Weis HB, et al. Best Case/Worst Case for the Trauma ICU: Development and Pilot Testing of a Communication Tool for Older Adults with Traumatic Injury. J Trauma Acute Care Surg. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann CJ, Jhagroo RA, Wakeen M, Schueller K, Zelenski A, Tucholka JL, et al. Opportunities to Improve Shared Decision Making in Dialysis Decisions for Older Adults with Life-Limiting Kidney Disease: A Pilot Study. J Palliat Med. 2020;23(5):627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarze ML, Zelenski A, Baggett ND, Kalbfell E, Ljumani F, Silverman E, et al. Best Case/Worst Case: ICU (COVID-19)-A Tool to Communicate with Families of Critically Ill Patients with COVID-19. Palliat Med Rep. 2020;1(1):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elwyn G, Durand MA, Song J, Aarts J, Barr PJ, Berger Z, et al. A three-talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarze ML, Taylor LJ. Managing Uncertainty - Harnessing the Power of Scenario Planning. N Engl J Med. 2017;377(3):206–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021. [Google Scholar]
- 38.Loh KP, Lam V, Webber K, Padam S, Sedrak MS, Musinipally V, et al. Characteristics Associated With Functional Changes During Systemic Cancer Treatments: A Systematic Review Focused on Older Adults. J Natl Compr Canc Netw. 2021;19(9):1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Presley CJ, Arrato NA, Janse S, Shields PG, Carbone DP, Wong ML, et al. Functional disability among older vs. younger adults with advanced non-small cell lung cancer. JCO Oncol Pract. 2021:(In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong ML, Paul SM, Cooper BA, Dunn LB, Hammer MJ, Conley YP, et al. Predictors of the multidimensional symptom experience of lung cancer patients receiving chemotherapy. Support Care Cancer. 2017;25(6):1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm Policy Ment Health. 2015;42(5):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor A, Cranney A. User Manual - Acceptability [document on the Internet]. 1996. [modified 2002]; http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Acceptability.pdf. Accessed June 6, 2018.
- 43.Fereday J, Muir-Cochrane E. Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development. International Journal of Qualitative Methods. 2006;5(1):80–92. [Google Scholar]
- 44.Braun V, Clarke V. To saturate or not to saturate? Questioning data saturation as a useful concept for thematic analysis and sample-size rationales. Qualitative Research in Sport, Exercise and Health. 2021;13(2):201–216. [Google Scholar]
- 45.Robinson TM, Alexander SC, Hays M, Jeffreys AS, Olsen MK, Rodriguez KL, et al. Patient–oncologist communication in advanced cancer: predictors of patient perception of prognosis. Supportive Care in Cancer. 2008;16(9):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donelle L, Arocha JF, Hoffman-Goetz L. Health literacy and numeracy: key factors in cancer risk comprehension. Chronic Dis Can. 2008;29(1):1–8. [PubMed] [Google Scholar]
- 47.Wegwarth O, Gigerenzer G. The Barrier to Informed Choice in Cancer Screening: Statistical Illiteracy in Physicians and Patients. Recent Results Cancer Res. 2018;210:207–221. [DOI] [PubMed] [Google Scholar]
- 48.Davids SL, Schapira MM, McAuliffe TL, Nattinger AB. Predictors of pessimistic breast cancer risk perceptions in a primary care population. J Gen Intern Med. 2004;19(4):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCleary NJ, Cleveland J, Zhang S, Lepisto EM, Lee S, Hassett MJ, et al. Patient-reported health literacy and numeracy among new patients seeking consultation at a comprehensive cancer center. Journal of Clinical Oncology. 2020;38(15_suppl):7038–7038. [Google Scholar]
- 50.Amalraj S, Starkweather C, Nguyen C, Naeim A. Health literacy, communication, and treatment decision-making in older cancer patients. Oncology (Williston Park). 2009;23(4):369–375. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


