Abstract
Purpose:
Magnetic resonance imaging–guided linear accelerator systems (MR-linacs) can facilitate the daily adaptation of radiotherapy plans. Here, we report our early clinical experience using an MR-linac for adaptive radiotherapy of gynecologic malignancies.
Methods and Materials:
Treatments were planned with an Elekta Monaco v5.4.01 and delivered by a 1.5 Tesla Elekta Unity MR-linac. The system offers a choice of daily adaptation based on either position (ATP) or shape (ATS) of the tumor and surrounding normal structures. The ATS approach has the option of manually editing the contours of tumors and surrounding normal structures before the plan is adapted. Here we documented the duration of each treatment fraction; set-up variability (assessed by isocenter shifts in each plan) between fractions; and, for quality assurance, calculated the percentage of plans meeting the γ-criterion of 3%/3-mm distance to agreement. Deformable accumulated dose calculations were used to compare accumulated versus planned dose for patient treated with exclusively ATP fractions.
Results:
Of the 10 patients treated with 90 fractions on the MR-linac, most received boost doses to recurrence in nodes or isolated tumors. Each treatment fraction lasted a median 32 minutes; fractions were shorter with ATP than with ATS (30 min vs 42 min, P<0.0001). The γ criterion for all fraction plans exceeded >90% (median 99.9%, range 92.4%–100%), i.e., all plans passed quality assurance testing. The average extent of isocenter shift was <0.5 cm in each axis. The accumulated dose to the gross tumor volume was within 5% of the reference plan for all ATP cases. Accumulated doses for lesions in the pelvic periphery were within <1% of the reference plan as opposed to −1.6% to −4.4% for central pelvic tumors.
Conclusions:
The MR-linac is a reliable and clinically feasible tool for treating patients with gynecologic cancer.
Keywords: MR-Linac, Gynecologic Cancer, MRgRT, R-IDEAL
INTRODUCTION
The integration of in-room magnetic resonance (MR) imaging with radiation therapy delivery allows exceptional image-guided treatment that can be readily adapted according to changes in the tumor or surrounding tissues. Because MR imaging provides superior soft tissue characterization over other modalities, treatments can be highly conformal, thereby allowing margin reduction and minimization of inter- and intra-fractional changes in patient anatomy.1,2 Despite these apparent advantages, concerns remain regarding resource utilization and overall implementation of MR-guided systems for radiotherapy.
A 1.5 Tesla MR scanner integrated with a linear accelerator was initially proposed as a proof of concept in 2009.3 In 2018, the 1.5 Tesla Elekta Unity MR-linear accelerator (linac) system (Elekta, Stockholm, Sweden) became the modern commercial incarnation of this design.2 Several reports have described implementation of this system for treating tumors at a variety of disease sites, including prostate, gastrointestinal, thoracic, and head and neck.4–8 However, little has been reported on its use specifically for gynecologic cancers. Gynecologic cancers may prove particularly suitable for treatment with MR-linacs because the primary and nodal targets are difficult to visualize on CT images, are highly mobile and deformable, are subject to substantial regression throughout treatment, and are susceptible to rotational setup error.9
While MR-linac investigations remain relatively new, studies in other disease sites have shown some early potential advances including increased organs at risk (OAR) sparing and improved target coverage. For example in non-small cell lung cancer, MR-linac planning enabled increased skin sparing and target dose escalation;7 PTV margin reduction in prostate cancer planning;10 decreased margins for acceptable GTV coverage in rectal cancer;11 and decreased cardiac dose in gastro-esophageal cancer patients compared to traditional techniques.12
Here we report our initial experiences using a 1.5 Tesla MR-linac to treat gynecologic malignancies, including workflow and feasibility as well as treatment times, quality assurance (QA), and dosimetric variability.
MATERIALS AND METHODS
R-IDEAL Approach
For this pilot study, we leveraged the R-IDEAL conceptual framework for technology development in radiotherapy.13 Using the R-IDEAL approach, we undertook a structured evaluation of a novel radiotherapy technology/approach, namely, adaptive MR-guided radiotherapy for gynecologic cancer, in a single-institution, prospective implementation series. This assessment included both an “idea” stage (R-IDEAL Stage 1; that is, first-in-human implementation) and a “development” stage (R-IDEAL Stage 2a; that is, reporting technical feasibility “when additional modifications are made to further optimize workflow and technology for innovative treatment delivery.”13 The R-IDEAL conceptual schema allows technology development to be substantiated iteratively as parts of a coherent programmatic project8,14–18 and is analogous to the parent IDEAL method for surgery.19
The aim of the current study was to demonstrate the first-in-human implementation of adaptive MR-guided radiotherapy (MRgRT) for gynecologic external-beam application (R-IDEAL Stage 1). We further sought to report the technical feasibility of achieving MR-gRT geometric accuracy and the resultant planning and delivery QA within our standard current clinical workflow by using FDA-approved commercial treatment planning systems, reported as a sequential case series (R-IDEAL Stage 2a).
The MR-linac system
The Elekta Unity MR-linac system (Elekta AB, Stockholm, Sweden) consists of a 7-megavolt (MV) flattening filter free (FFF) beam linac (Elekta AB) integrated with a 1.5 Tesla MR scanner (Philips, Best, The Netherlands).20 The linac is mounted on a ring gantry to allow continuous rotation with the 1.5 Tesla magnetic field perpendicular to the entrance of the bore. The source-to-axis distance is 143.5 cm. The maximum field size is 22 cm longitudinally and 57.4 cm laterally. The couch moves only in the longitudinal direction, preventing any non-coplanar beams in treatment planning. The multi-leaf collimator consists of 80 leaf pairs, each with a projected width of approximately 0.72 cm at isocenter.
Patient selection
This report is a part of the *** study on behalf of the *** (NCT***),21 and was approved by the appropriate institutional review board (IRB ***). All patients aged 18 years or older who were being treated on the MR-Linac for a primary or recurrent gynecologic malignancy at a single institution were eligible for data extraction. Suitability for treatment on the MR-linac was initially considered by the treating physician and discussed with a multidisciplinary team consisting of physicists, dosimetrists, therapists, and radiation oncologists. Only those patients with gross disease being treated to high doses (>60 Gy EQD2) were considered; other considerations included body size (with regard to MR bore width and field-size limitations),22 overall mobility (ability to walk to and from the vault), ability to maintain a consistent position over prolonged treatments, and lack of claustrophobia or contraindications to MR imaging (e.g., implanted ferrous metal objects or pacemakers).
Simulation and treatment planning
Treatments were simulated first on a CT scan followed by an MR scan (ideally obtained on the same day) to facilitate rigid registration (Supplementary Tables E1–E2). Patients were immobilized with a lower-body Vac-Lok system (CIVCO Medical Solutions, Coralville, Iowa, USA) individualized for reproducibility of scan length and treatment site (Supplementary Table E1). The CT scans were obtained with a Philips Brilliance 16-slice CT scanner with ≤2.5 cm slices. The MR scans were obtained with the MR-linac, with 2-minute and 6-minute 3D T1- and T2-weighted sequences acquired via scanning protocols identical to those used during daily MR imaging (Supplementary Table E2). 18F-FDG PET/CT images were obtained as described in published guidelines.23
Treatment planning was done with the Monaco treatment planning system, which uses a Monte Carlo–based dose calculation engine (Elekta, Inc., Maryland Heights, MO, USA). Target volumes were contoured on a multi-modality planning image composed of fused scans of CT simulation images and T1- and T2-weighted MR and PET/CT images (Figure 1). Planning target volume (PTV) margins were typically 3–5 mm around the gross tumor volume (GTV), but were modified as needed for each patient to account for factors such as tumor location and size and receipt of prior radiotherapy. As appropriate, internal target volume (ITV) was generated to take into account tumor motion. For plans created with the adapt-to-shape (ATS) approach, margins were often reduced to <5 mm (at the physician’s discretion) based on anatomy, proximity to critical structures, and perceived motion. Organs at risk (OARs), that is, bladder, bowel, rectum, pelvic bones, kidneys, and spinal cord, were contoured for each patient. The step-and-shoot intensity-modulated radiation therapy (IMRT) planning technique was used to create MR-linac reference plan. A backup plan for treatment on a conventional linac was also created as a backup for potential machine downtime.
Figure 1.

Magnetic resonance linear accelerator workflow: Pretreatment planning, daily treatment setup, and adaptation and treatment. Central workflow in black, backup workflow in grey. Abbreviations: ATP, Adapt-to-point; ATS, Adapt-to-shape; MRI, magnetic resonance image; MRL, Magnetic resonance linear accelerator; sim, simulation.
Pretreatment QA involved verification of monitor units (MU) by using RadCalc (Version 63, Lifeline Software Inc., Austin, TX, USA) and dose measurements by using ArcCheck MR (Sun Nuclear Corp., Melbourne, FL, USA).
Of note, three physicians were involved in the planning and treatment of these patients. Each patient had a central physician that worked to plan and contour an individual plan, however, all three physicians cross-covered each other depending on the day to assist in daily treatment.
Treatment set-up and delivery
Patients were positioned for treatment delivery according to an index value recorded at simulation to determine their longitudinal position. No external lasers are present within the MR-linac vault.
A 2-minute T2 3D MR imaging scan (Supplementary Table E2) was acquired and subsequently fused via rigid registration with the reference plan image to facilitate verification of daily setup and the need for plan adaptation. Two adaptation workflows were used: adapt to position (ATP) and adapt to shape (ATS) (Figure 1).2 Briefly, ATP is a workflow wherein an isocenter shift is made on the simulation CT plan based on rigid registration followed by either a dose recalculation or plan reoptimization. The ATS workflow, on the other hand, uses deformable image registration to reproduce contours onto the daily MR setup image followed by full-plan re-optimization according to daily changes in anatomy. The decision to use ATP vs ATS was left to the physician’s discretion and was generally determined based on extent of tumor shrinkage (by more or less than 3 mm), the need to reduce PTV margins, movement of OARs into the treatment area leading to violation of predefined dosimetric limits, and individual patient tolerance on the day of treatment.
All adaptive plans were then independently checked for MUs by using RadCalc before beam delivery. Real-time motion monitoring was done with orthogonal cine MR imaging during beam-on. After treatment completion, a final quality check was performed, with the stipulation that IMRT QA measurements of the adaptive plan must be within 3% dosimetric difference and 3 mm distance to agreement γ criteria. Gating was not utilized as part of treatment.
Calculations of accumulated dose
Doses of daily adaptive plans were accumulated for six patients treated with ATP for every fraction. The ATP plan was adapted from the MR-linac reference plan with re-optimization based on the simulation CT with a new isocenter shifted from the MR-linac reference plan using the fusion of daily MR to the simulation CT (Figure 1). As each ATP plan has the total treatment plan dose but only one fraction of the dose was delivered with each treatment, summation of each fractional dose was used for dose accumulation. Two types of dose accumulation were performed: a summed planned dose (Dsum) to assess the planned dose for the treatment and the accumulated deformed dose (Ddef) to approximate the actual delivered dose to the target and OARS (Figure 2). The summed planned dose was calculated by direct summation of each fractional dose of each ATP treatment (Figure 2A). The accumulated deformed dose was calculated by having each fractional dose of every ATP plan rigidly shifted to the daily MR coordinate space, using the isocenter shift obtained from CT-MR fusion, and then deformably mapping back to the simulation CT coordinate space by using an in-house deformable registration tool4 for dose accumulation [Figure 2B)]. Thus as the accumulated deformed dose (Ddef) overlays the daily MR image this more accurately represents the actual dose to daily anatomy and more closely approximates the actual delivered dose to the target and OARs.
Figure 2.

Dose Accumulation Methods: Illustration of dose accumulation methods in adapt-to-position (ATP) plans. (A) ATP dose summation: direct summation of fractions doses (DFx1, DFx2, …, DFxn) to create summed planned dose (Dsum); (B) ATP dose deformation and accumulation: in each fraction (Fx1 for example), the fraction dose (DFx1) was first shifted to the corresponding daily MR coordinate space, creating a dose matrix , using the iso-center shift obtained from MR-CT fusion, and then the deformable registration (DIR) between daily MR and simulation CT was used to deform the dose matrix to simulation CT space, creating dose matrix , for accumulation. Summation of DFx1, DFx2, …, DFxn, creates the accumulated deformed dose (Ddef).
The accumulated deformed dose was compared with the summed planned dose by calculating the percentage difference of each plan quality metric with the following equation:
Analyses of targets and nearby OARs were based on the anatomic location of the irradiated lesion and included the GTV and one or more of the following OARs: bladder, femoral heads, bowel, rectum, and sigmoid colon. Doses to the GTV were compared by the average dose to the GTV volume Daverage, and doses to the OARs were compared by using a surrogate of max dose, D1% (1% of the volume received at least this dose or higher). When a conventional linac system was used to deliver a fraction, only the MR-linac plans were included, and the reference plan was scaled to the number of fractions treated on the MR-linac before calculating the percentage difference.
Data analysis
Descriptive statistics were used for patient characteristics such as age, body mass index, and performance status (scored according to the Eastern Cooperative Oncology Group [ECOG] criteria).24 Extracted treatment characteristics included daily treatment duration, method of adaptation, isocenter shift, and number of beams. Treatment duration was measured from time stamps in Mosaiq, with the total duration measured from the patient entering the vault to beam-off; treatment durations were compared with t tests. Daily setup variability was measured from isocenter shift data, which were measured along each X, Y, and Z axis (left-right, superior-inferior, anterior-posterior, respectively) for all adapted fractions.
RESULTS
Patients
Ten patients with gynecologic malignancies were treated on the MR-linac system from May 2019 through January 2020 (Table 1). The median age was 67 years (range 45–87) and the median body mass index was 26.5 (range 16.5–43.1). All but one patient had an ECOG performance status score of ≤1. Primary gynecologic tumors were cervical (n=4), endometrial (n=3), vaginal (n=1), ovarian (n=1), and peritoneal (n=1); most patients (7 of 10) were being treated for isolated recurrent disease.
Table 1.
Patient and Treatment Characteristics
| Patient | Age at Treatment Start | Race | BMI | ECOG PS Score | Primary Tumor Location | FIGO Disease Stage | Tumor Location | Chemotherapy | MRL Use | Total MRL dose, Gy | No. of Fractions | Adaptation Method | No. of Beams |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | Black | 20.6 | 0 | Cervix | R | Vaginal cuff | Cisplatin | Boost | 16 | 8 | ATP | 9 |
| 2 | 77 | White | 32.8 | 1 | Uterus | R | Inferior splenic node | Carboplatin + paclitaxel | Full plan | 61.6 | 28 | ATP/ATS | 7 |
| 3 | 67 | Unknown | 43.1 | 1 | Uterus | R | Left external iliac node | Carboplatin + paclitaxel | Full plan | 40 | 5 | ATP | 6 |
| 4 | 56 | White | 16.5 | 1 | Cervix | IIIB | Cervical | Cisplatin | Boost | 22 | 8 | ATP/ATS | 8 |
| 5 | 49 | Asian | 22.3 | 0 | Cervix | R | Left pelvic sidewall | None | Full plan | 42.5 | 5 | ATP | 7 |
| 6 | 67 | White | 31.9 | 1 | Ovary | R | LEI and left pelvic mass | None | Boost | 16 | 8 | ATP/ATS | 7 |
| 7 | 46 | White | 21.3 | 0 | Cervix | R | Left Ischium | None | Full plan | 27 | 3 | ATP | 8 |
| 8 | 69 | White | 18.9 | 2 | Peritoneum | R | Left pelvic/abdomen | Cisplatin | Boost | 30 | 15 | ATP/ATS | 9 |
| 9 | 70 | Hispanic | 30.7 | 1 | Vagina | I | Vaginal cuff | Cisplatin | Boost | 10 | 5 | ATP | 9 |
| 10 | 87 | Black | 30.6 | 1 | Uterus | IIIC | Uterine fundus | None | Boost | 12 | 5 | ATP | 11 |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status; R, recurrent; ATP, adapt-to-tumor-position; ATS, adapt-to-tumor-shape; LEI, left external iliac node; MRL, Magnetic resonance imaging linear accelerator.
The clinical presentations in this analysis varied. Patients 1, 4, 6, 8, 9, and 10 received central pelvic irradiation on the MR-linac to either the whole pelvis or to centrally located lesions such as the uterine fundus; patients 3 and 5 were treated to peripheral pelvic lesions such as the external iliac node and pelvic sidewall lesion, and the others were treated to extrapelvic areas such as an infrasplenic node (patient 2) and the left ischium (patient 7). In 6 patients, the MR-linac was used to deliver boost doses in a hybrid conventional linac–MR-linac treatment regimen; the other 4 patients were treated exclusively with the MR-linac.
Treatment characteristics
A total of 92 fractions were planned, 90 of which were delivered on the MR-linac; the 2 missed fractions were delivered with a conventional linac owing to unexpected MR-linac system downtime. Six patients received concurrent platinum-based chemotherapy (40 mg/m2 cisplatin for four, carboplatin-paclitaxel for two).
The median total dose delivered on the MR-linac was 22.5 Gy (range 10.2–60.5 Gy), in a median of 6 fractions (range 3–28); the median dose per fraction was 2.2 Gy (range 2–9 Gy). The median number of beams per plan was 8 (range 6–11). All fractions passed IMRT QA with a γ-value in excess of 90% (median 99.9%; range 92.4%–100%), indicating a high level of agreement between expected and delivered dose distributions (Supplementary Figure E1).
The average isocenter shift for daily patient setup was <0.5 cm in the X, Y, and Z axes (left-right, superior-inferior, and anterior-posterior, respectively). The absolute maximum isocenter shift in any fraction was 3.5 cm for the X axis, 3.3 cm for the Y axis, and 1.9 cm cm for the Z axis (Supplementary Figure E2). A large (>3 cm) shift was considered necessary for one patient because of the laterality of disease and limits on the MR field of view.
Of the total 90 fractions, 73 were delivered with the ATP method and 17 were delivered with the ATS method; no patient was treated solely with the ATS method. The median duration of treatment was 32 minutes overall and was significantly shorter with ATP (median 30 min, range 17–66 min) than with ATS (median 42 min, range 30–65 min), P<0.0001 (Supplementary Figure E3). Importantly, this does not take into account the time for plan development for MR-linac treatments nor the time necessary to create a secondary backup plan.
Dose accumulation analysis
The accumulated deformed dose for the GTV was within 5% of the summed planned dose for all patients treated with the ATP method (Figure 3). For patients whose irradiated target lesions were within the periphery of the pelvis or a fixed bony lesion, the relative GTV dose was within <1%; by contrast, the relative GTV dose for centrally located lesions ranged from −1.6% (vaginal cuff boost) to −2.8 (uterine fundus boost) to −4.4% (vaginal cuff boost) of the summed planned dose. The relative dose calculations for OARs varied, with that of the femoral heads and rectum all being within 5% and that of the bowel, bladder and sigmoid being within 20%. Overall, of the 21 OAR comparisons in our analysis, the accumulated deformed dose was at or below the summed planned dose for all but 4 examples with the majority, 17, showing a decrease in dose received.
Figure 3.
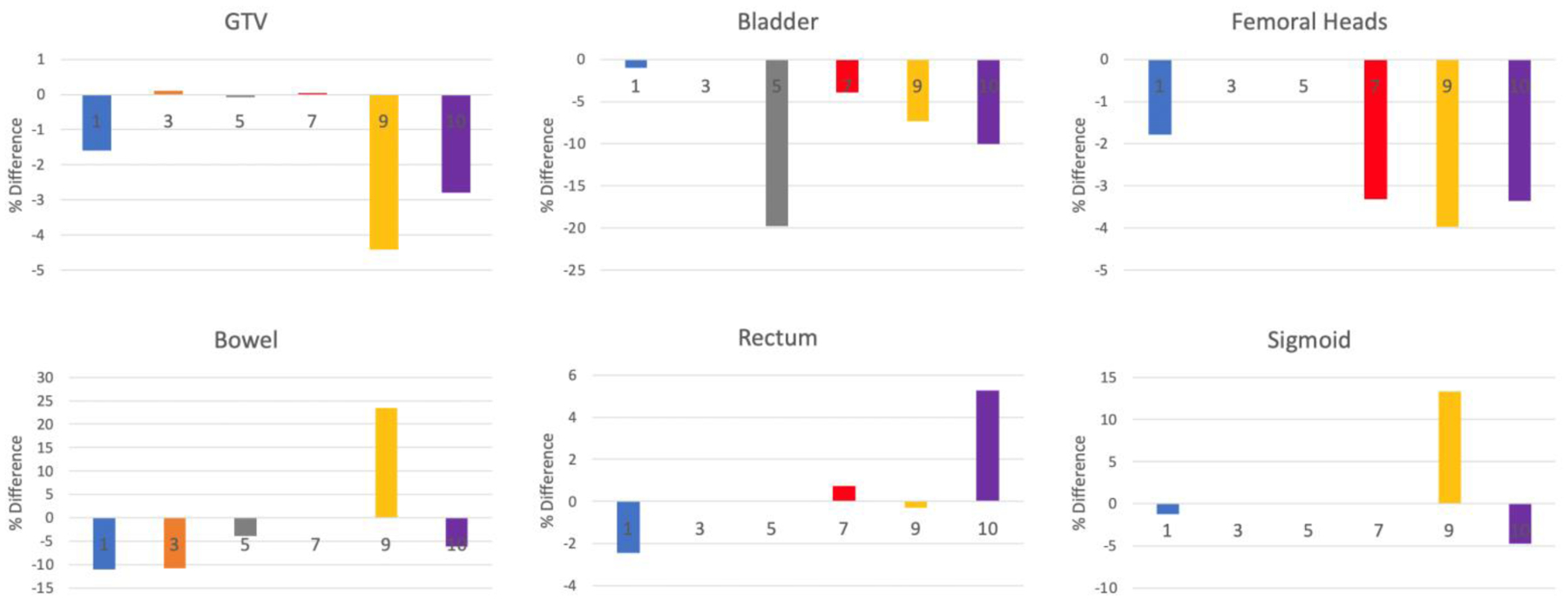
Percent difference between accumulated deformed dose and summed adaptive plan dose to GTV & OARs. Differences between the accumulated deformed dose and the summed adaptive plan dose, shown for the Dave (average dose to the volume) of thegross tumor volume (GTV) and the D1% (surrogate of max dose: 1% of the volume received at least this dose or higher) of various organs at risk (OARs) for 6 patients treated with solely ATP plans. Positive values indicate that the accumulated deformed dose exceeded the summed plan dose. The numbers on each bar refer to the patients as shown in Table 1. The various GTV targets for each patient were as follows: Patient 1, vaginal cuff; Patient 3, external iliac node; Patient 5, pelvic sidewall; Patient 7, left ischium; Patient 9, vaginal cuff boost; and Patient 10, uterine fundus. If an OAR was not considered in a particular patient, then no bar appears on the panel (e.g., for Patient 3, the bladder, femoral heads, rectum, and sigmoid colon were not considered as OARs).
DISCUSSION
Advances in radiation therapy for gynecologic malignancies have led to increasingly conformal treatments and image-guided adaptive targeting over time, from the widespread adoption of CT-planned external-beam methods25,26 to the development of MR imaging–based image-guided adaptive brachytherapy.27,28 MR-guided adaptive external-beam therapy represents the next step in this advancement. Rather than relying on a single CT data set that may not reflect the geometry of the target and OARs at the time of treatment delivery, the MR-linac system allows daily adaptation to plans that account for changes in the highly mobile deformable structures within the pelvis (Figure 4). This report is among the first to provide details on the feasibility and initial clinical experience with using a 1.5 Tesla MR-linac specifically for patients with gynecologic cancer.
Figure 4. CT reference plan and adapt-to-shape plans throughout treatment:

Comparisons of CT-based reference scans (left column) with magnetic resonance (MR) images of daily adapt-to-tumor-shape plans at fractions 5, 7, and 8 for Patient 6, a 67-year-old woman with recurrent ovarian cancer treated to 50 Gy with a conventional linear accelerator (linac) (not shown) and given a sequential 16-Gy pelvic boost with the MR-linac. Top row, axial views; middle row, coronal views; and bottom row, transverse views. The gross tumor volume (GTV) is outlined in in red; the planning target volume (PTV; i.e., GTV + a 2-mm expansion) in blue; the bladder in yellow; and the rectum in green. The GTV on the reference CT scan was 275 cm3; volumes on the daily adaptation scans were 264 cm3 at fraction 5, 266 cm3 at fraction 7, and 223 cm3 at fraction 8. These scans illustrate the high level of conformality possible with the ability of the MR-linac system to account for differences in bowel and rectal filling between daily fractions.
The best basis for identifying patients who would derive the greatest benefit from MR-linac treatment remains unclear. In this study, patients were selected for MR-linac treatment if they had gross nodal or primary disease that was either mobile or closely approximating OARs such as the bladder, rectum, or bowel; in such cases, daily imaging would help to reduce treatment margins and reduce the dose to OARs. The Elekta Unity system used for this study has a structural limit of a 22-cm longitudinal field size, thus precluding treatment for patients with extensive nodal or metastatic disease requiring an extended field. However, methods for treating larger fields are being actively investigated. Most of the patients in our study were given isolated nodal or tumor boost doses with the MR-linac system.
One significant concern regarding the use of MR-linac systems is the time required to deliver each treatment fraction; in this study, the median fraction time was 32 minutes, with a range of 17 to 66 minutes; notably, the ATS plans took longer, at a median 42 minutes. Although these values are similar to the fraction times for treating head and neck cancer (median 41 minutes) or pelvic nodal disease and prostate (30–40 minutes) with the Elekta Unity,4,5,29 they are much longer than the typical 3–4 minutes of “beam-on” time within 15-minute scheduled blocks for patients receiving conventional IMRT. That said, all adaptive plans in the current study passed QA checks, with exceeding 90% (median 99.9%, range 92.4%–100%), indicating a high level of agreement between expected and delivered dose distributions. The high pass rate indicates good agreement between expected and delivered dose distributions; indeed, this dosimetric stability is probably attributable to the comprehensive series of safety checks that are implemented at each level.
The average isocenter shift was <0.5 cm in each axis. Although the magnitude of isocenter shifts was larger in this study than in another of patients with head and neck cancer,8 this could be explained by the use of a stabilizing mask used for head and neck cases and the greater degree of inter- and intra-fractional movement of pelvic and abdominal lesions in the current study. Notably, these isocenter shifts should be interpreted as couch shift corrections and should not be used to derive margins, for the following reasons. For one, the set-up accuracy of the MR-linac for these patients is typically not as accurate as regular IGRT because external lasers are not used in MR systems. For another, the daily adaptation possible with the MR-linac accounts for the isocenter shift during the online adaptive planning. Further studies of marginal status would consider the residual match due to anatomic change or deformation set-up error, with individual evaluations of different target locations. Given the small number of patients and the heterogeneity in target locations in the current study, we did not have enough data to draw any conclusions regarding appropriate margins.
ATP plan adaptation re-optimizes the reference plan using a new iso-center based on daily setup. It is similar to traditional IGRT in that the adaptation does not take into account the daily anatomy variations. Compared to ATS plan adaptation, ATP workflow is faster, more predictable, and requires less direct human input. The dose accumulation analysis in this study was used to identify cases that ATP workflow could be applied with sufficient dosimetric accuracy, similar to a previous head and neck study.30 Our analysis showed that for peripheral lesions, i.e. lesions close to the pelvic wall or pelvic bony lesions themselves that are relatively fixed with little mobility, had only minor differences, <1%, between summed planned dose and accumulated deformed dose relative to central pelvic lesions, i.e soft tissue lesions located within the center of the pelvic including the bladder or rectum, for which the dose difference approximated 5%. For example, for patient 9, who was treated with a vaginal cuff boost, the difference between planned and deformed dose was −4.6%. Importantly, however, this difference may not reflect the actual dosimetric difference of the target but rather the limitations of our deformation calculations. For patient 9 in particular we observed large anatomical changes in her target lesion resulting in potentially inaccurate deformable registrations and possibly inaccurate differences in dosimetric comparison. In a similar study for prostate cancer patients in which the same in-house tools as described herem, a <0.5% difference was found in prostate GTVs, likely reflecting the relatively immobility of prostate volumes relative to pelvic and abdominal targets.4
While the inexactness described above regarding deformation registration and the limited number of patients in this study limit any establishment of strict guidelines, our study does suggest some broad findings. We found that more fixed or immobile lesions lend themselves well to the ATP workflow with negligible differences in accumulated deformed dose over summed planned dose. For plans with more mobile lesions or large anatomic variation, ATP workflow may not be optimal and ATS adaptation may be needed to to achieve optimal dosimetric goals. And finally, our study suggests that the MR-linac may serve to increase overall OAR sparing. Nevertheless, our observations should be confirmed in larger studies. Additional research is needed to further delineate what targets lend themselves to ATP/ATS workflows, appropriate margins for varying clinical scenarios, and how this system affects departmental resources and patient experience.
In summary, we found that the Elekta Unity 1.5 Tesla MR-linac is a versatile system that can be used to treat gynecologic disease at a variety of sites, alone or in combination with other radiation techniques in a reliable, effective manner. Treatment duration is acceptable when compared with that of conventional radiation modalities, especially when ATP is used. Although our study was limited by the small cohort and the heterogeneity of treatments, it does show the initial feasibility of using this novel modality in gynecologic oncology. Further exploration is required to assess how the MR-linac might be implemented for patients requiring extended-field treatments; for patients for whom brachytherapy is unavailable, and how the MR-linac might replicate similar doses; and for how current margin standards could be safely attenuated given the enhanced adaptability of the system. Thus, even though the MR-linac is a novel treatment modality, we found that MR-linac treatment plans were clinically similar to the current standard of care and allow greater precision and individualization in the treatment of gynecologic malignancies.
Supplementary Material
Acknowledgments:
The authors thank Christine F. Wogan, MS, ELS, from MD Anderson’s Division of Radiation Oncology for editorial assistance.
Funding:
This work is partially supported by an academic-industrial grant by Elekta AB. Dr. Fuller received/receives unrelated direct funding and salary support during the period of study execution from: the National Institutes of Health (NIH) NIBIB Research Education Programs for Residents and Clinical Fellows Grant (R25EB025787-01); the NCI Early Phase Clinical Trials in Imaging and Image-Guided Interventions Program (1R01CA218148); the NSF/NIH Joint Smart Connected Health Program (R01CA257814); the NCI Parent RPG mechanism (R01CA258827); NIDCR Academic Industrial Partnership Grant (R01DE028290); NCI Parent Research Project Grant (R01CA258827); the NIH/NCI Cancer Center Support Grant (CCSG) Pilot Research Program Award from the UT MD Anderson CCSG Radiation Oncology and Cancer Imaging Program (P30CA016672); and an NSF Division of Civil, Mechanical, and Manufacturing Innovation (CMMI) grant (NSF 1933369). Dr. Fuller has received federal NIH sub-awards from Oncospace, Inc (R43CA254559) and Carnegie-Mellon University (OT2OD026675) for efforts unrelated to the present work. Dr. Fuller has received direct industry grant support, honoraria, and travel funding from Elekta AB related to this project; Elekta had no oversight regarding the writing, analysis and formulation of this manuscript, nor in the decision to submit. Direct infrastructure support is provided by the multidisciplinary Radiation Oncology and Cancer Imaging Program of the MD Anderson Cancer Center’s Support (Core) Grant P30CA016672 and the MD Anderson Program in Image-guided Cancer Therapy.
Dr. Lin received/receives unrelated direct funding and/or salary support during the period of study execution from: Astrazeneca and Pfizer for the conduct of multiple investigator-initiated therapeutic clinical studies as well as grants from the NCI (R01CA249329, R01CA258717).
Conflicts of Interests:
This work is partially supported by an academic-industrial grant by Elekta AB. Dr. Fuller has received direct industry grant support, honoraria, and travel funding from Elekta AB related to this project. Elekta had no oversight regarding the writing, analysis, and formulation of this manuscript, nor in the decision to submit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.
References
- 1.Brock KK. Adaptive Radiotherapy: Moving Into the Future. Semin Radiat Oncol. 2019;29(3):181–184. doi: 10.1016/j.semradonc.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winkel D, Bol GH, Kroon PS, et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clinical and Translational Radiation Oncology. 2019/September/01/ 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raaymakers BW, Lagendijk JJ, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. Jun 21 2009;54(12):N229–37. doi: 10.1088/0031-9155/54/12/n01 [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Vedam S, Lee B, et al. Online adaptive planning for prostate stereotactic body radiotherapy using a 1.5 Tesla magnetic resonance imaging-guided linear accelerator. Physics and Imaging in Radiation Oncology. 2021/January/01/ 2021;17:20–24. doi: 10.1016/j.phro.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werensteijn-Honingh AM, Kroon PS, Winkel D, et al. Feasibility of stereotactic radiotherapy using a 1.5 T MR-linac: Multi-fraction treatment of pelvic lymph node oligometastases. Radiotherapy and Oncology. 2019;134:50–54. doi: 10.1016/j.radonc.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 6.Intven MPW, de Mol van Otterloo SR, Mook S, et al. Online adaptive MR-guided radiotherapy for rectal cancer; feasibility of the workflow on a 1.5T MR-linac: clinical implementation and initial experience. Radiotherapy and Oncology. 2021;154:172–178. doi: 10.1016/j.radonc.2020.09.024 [DOI] [PubMed] [Google Scholar]
- 7.Bainbridge HE, Menten MJ, Fast MF, Nill S, Oelfke U, McDonald F. Treating locally advanced lung cancer with a 1.5 T MR-Linac – Effects of the magnetic field and irradiation geometry on conventionally fractionated and isotoxic dose-escalated radiotherapy. Radiotherapy and Oncology. 2017;125(2):280–285. doi: 10.1016/j.radonc.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald BA, Vedam S, Yang J, et al. Initial Feasibility and Clinical Implementation of Daily MR-Guided Adaptive Head and Neck Cancer Radiation Therapy on a 1.5T MR-Linac System: Prospective R-IDEAL 2a/2b Systematic Clinical Evaluation of Technical Innovation. Int J Radiat Oncol Biol Phys. Dec 16 2020;doi: 10.1016/j.ijrobp.2020.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White IM, Scurr E, Wetscherek A, et al. Realizing the potential of magnetic resonance image guided radiotherapy in gynaecological and rectal cancer. Br J Radiol. 2019;92(1098):20180670–20180670. doi: 10.1259/bjr.20180670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Silva Mendes V, Nierer L, Li M, et al. Dosimetric comparison of MR-linac-based IMRT and conventional VMAT treatment plans for prostate cancer. Radiat Oncol. Jul 21 2021;16(1):133. doi: 10.1186/s13014-021-01858-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eijkelenkamp H, Boekhoff MR, Verweij ME, Peters FP, Meijer GJ, Intven MPW. Planning target volume margin assessment for online adaptive MR-guided dose-escalation in rectal cancer on a 1.5 T MR-Linac. Radiother Oncol. Sep 2021;162:150–155. doi: 10.1016/j.radonc.2021.07.011 [DOI] [PubMed] [Google Scholar]
- 12.Lee SL, Mahler PA, Olson SJ, et al. Comparison of Cardiac Substructure Dosimetry in Gastro-Esophageal Junction Cancer: Respiratory-Gated MR-Linac Plans Versus 4DCT VMAT Plans. International Journal of Radiation Oncology, Biology, Physics. 2020;108(3):e319–e320. doi: 10.1016/j.ijrobp.2020.07.763 [DOI] [Google Scholar]
- 13.Verkooijen HM, Kerkmeijer LGW, Fuller CD, et al. R-IDEAL: A Framework for Systematic Clinical Evaluation of Technical Innovations in Radiation Oncology. Front Oncol. 2017;7:59. doi: 10.3389/fonc.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsai S, Qiu RLJ, Qi P, et al. In vivo assessment of the safety of standard fractionation Temporally Feathered Radiation Therapy (TFRT) for head and neck squamous cell carcinoma: An R-IDEAL Stage 1/2a first-in-humans/feasibility demonstration of new technology implementation. Radiother Oncol. Jul 29 2021;163:39–45. doi: 10.1016/j.radonc.2021.07.023 [DOI] [PubMed] [Google Scholar]
- 15.Kong V, Hansen VN, Hafeez S. Image-guided Adaptive Radiotherapy for Bladder Cancer. Clin Oncol (R Coll Radiol). Jun 2021;33(6):350–368. doi: 10.1016/j.clon.2021.03.023 [DOI] [PubMed] [Google Scholar]
- 16.Bartels M, Verpalen IM, Ferrer CJ, et al. Combining radiotherapy and focused ultrasound for pain palliation of cancer induced bone pain; a stage I/IIa study according to the IDEAL framework. Clin Transl Radiat Oncol. Mar 2021;27:57–63. doi: 10.1016/j.ctro.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkel D, Bol GH, Werensteijn-Honingh AM, et al. Evaluation of plan adaptation strategies for stereotactic radiotherapy of lymph node oligometastases using online magnetic resonance image guidance. Phys Imaging Radiat Oncol. Jan 2019;9:58–64. doi: 10.1016/j.phro.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahig H, Yuan Y, Mohamed ASR, et al. Magnetic Resonance-based Response Assessment and Dose Adaptation in Human Papilloma Virus Positive Tumors of the Oropharynx treated with Radiotherapy (MR-ADAPTOR): An R-IDEAL stage 2a-2b/Bayesian phase II trial. Clin Transl Radiat Oncol. Nov 2018;13:19–23. doi: 10.1016/j.ctro.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. Sep 26 2009;374(9695):1105–12. doi: 10.1016/s0140-6736(09)61116-8 [DOI] [PubMed] [Google Scholar]
- 20.Woodings SJ, Bluemink JJ, de Vries JHW, et al. Beam characterisation of the 1.5 T MRI-linac. Phys Med Biol. Apr 19 2018;63(8):085015. doi: 10.1088/1361-6560/aab566 [DOI] [PubMed] [Google Scholar]
- 21.de Mol van Otterloo SR, Christodouleas JP, Blezer ELA, et al. The MOMENTUM Study: An International Registry for the Evidence-Based Introduction of MR-Guided Adaptive Therapy. Study Protocol. Frontiers in Oncology. 2020-September-07 2020;10(1328)doi: 10.3389/fonc.2020.01328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuter RW, Whitehurst P, Choudhury A, van Herk M, McWilliam A. Technical Note: Investigating the impact of field size on patient selection for the 1.5T MR-Linac. Med Phys. Nov 2017;44(11):5667–5671. doi: 10.1002/mp.12557 [DOI] [PubMed] [Google Scholar]
- 23.Adam JA, Loft A, Chargari C, et al. EANM/SNMMI practice guideline for [(18)F]FDG PET/CT external beam radiotherapy treatment planning in uterine cervical cancer v1.0. Eur J Nucl Med Mol Imaging. 2021;48(4):1188–1199. doi: 10.1007/s00259-020-05112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. Dec 1982;5(6):649–55. [PubMed] [Google Scholar]
- 25.D’Souza DP, Rumble RB, Fyles A, Yaremko B, Warde P. Intensity-modulated Radiotherapy in the Treatment of Gynaecological Cancers. Clinical Oncology. 2012/September/01/ 2012;24(7):499–507. doi: 10.1016/j.clon.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 26.Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. Nov 2011;84(1007):967–96. doi: 10.1259/bjr/22373346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haie-Meder C, Pötter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group☆ (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiotherapy and Oncology. 2005/March/01/ 2005;74(3):235–245. doi: 10.1016/j.radonc.2004.12.015 [DOI] [PubMed] [Google Scholar]
- 28.Pötter R, Haie-Meder C, Limbergen EV, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiotherapy and Oncology. 2006/January/01/ 2006;78(1):67–77. doi: 10.1016/j.radonc.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 29.Bertelsen AS, Schytte T, Møller PK, et al. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol. Oct 2019;58(10):1352–1357. doi: 10.1080/0284186x.2019.1627417 [DOI] [PubMed] [Google Scholar]
- 30.Lim SY, Tran A, Tran ANK, et al. Dose accumulation of daily adaptive plans to decide optimal plan adaptation strategy for head-and-neck patients treated with MR-Linac. Med Dosim. Spring 2022;47(1):103–109. doi: 10.1016/j.meddos.2021.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon reasonable request to the corresponding author.


