Abstract
Background:
Growing evidence supports improved survival with prehospital blood products. Recent trials show a benefit of prehospital tranexamic acid (TXA) administration in select subgroups. Our objective was to determine if receiving prehospital packed red blood cells (pRBC) in addition to TXA improved survival in injured patients at risk of hemorrhage.
Methods:
We performed a secondary analysis of all scene patients from the STAAMP trial. Patients were randomized to prehospital TXA or placebo. Some participating EMS services utilized pRBC. Four resuscitation groups resulted: TXA, pRBC, pRBC+TXA, and neither. Our primary outcome was 30-day mortality and secondary outcome was 24-hour mortality. Cox regression tested the association between resuscitation group and mortality while adjusting for confounders.
Results:
A total of 763 patients were included. Patients receiving prehospital blood had higher injury severity scores in the pRBC (22 [10, 34]) and pRBC+TXA (22 [17, 36]) groups than the TXA (12 [5, 21]) and neither (10 [4, 20]) groups (p<0.01). Mortality at 30 days was greatest in the pRBC+TXA and pRBC groups at 18.2% and 28.6% compared to the TXA only and neither groups at 6.6% and 7.4% respectively. Resuscitation with pRBC+TXA was associated with a 35% reduction in relative hazards of 30-day mortality compared to neither (HR 0.65; 95%CI 0.45–0.94, p=0.02). No survival benefit was observed in 24-hour mortality for pRBC+TXA, but pRBC alone was associated with a 61% reduction in relative hazards of 24h mortality compared to neither (HR 0.39; 95%CI 0.17–0.88, p=0.02).
Conclusions:
For injured patients at risk of hemorrhage, prehospital pRBC+TXA is associated with reduced 30-day mortality. Use of pRBC transfusion alone was associated with a reduction in early mortality. Potential synergy appeared only in longer term mortality and further work to investigate mechanisms of this therapeutic benefit is needed to optimize the prehospital resuscitation of trauma patients.
Level of Evidence:
Therapeutic, Level III
Keywords: Tranexamic acid, Prehospital, Blood, Mortality, Resuscitation
BACKGROUND
Hemorrhage is the leading cause of early preventable death after injury. Minimizing time to resuscitation is critical to curtail early mortality and improve long-term survival.1 Death from hemorrhage often occurs within the first few hours from time of injury and one-third of exsanguinating deaths occur prehospital.1, 2 Damage control resuscitation has become the standard in-hospital strategy; however, prehospital resuscitation is not optimized to mitigate hemorrhage. Prior literature demonstrates inflammation and coagulopathy ensue within minutes of injury underscoring the need for prompt and targeted resuscitation strategies in the field.3, 4
Crystalloid remains the most widely used resuscitation fluid in the prehospital setting due to its availability and durability. Blood product administration in the field is limited by storage challenges and cost. Recent progress adopts the tenets of damage control resuscitation, adding blood products and limiting large volume crystalloids in the field.5–7 The benefits of prehospital blood product resuscitation were demonstrated in military practice8–11 and confirmed to be safe and effective in civilian trauma for patients in hemorrhagic shock.12–18
Important resuscitative and hemostatic adjuncts including anti-fibrinolytics have emerged in advanced resuscitative strategies. Antifibrinolytics aid in hemostasis as demonstrated in elective surgical cases.19 Trauma induced coagulopathy can manifest as a hyperfibrinolytic phenotype in some patients, and is associated with poor outcomes.20, 21 Administration of tranexamic acid (TXA) reduces mortality in bleeding trauma patients when provided at the receiving hospital.22 Extrapolating this data, subsequent guidelines incorporated TXA into prehospital resuscitation algorithms.23, 24 Recent studies further demonstrate safety and efficacy in select prehospital populations and dosing regimens.25, 26
As prehospital resuscitation capabilities become more sophisticated, the impact of combining multiple advanced prehospital resuscitation strategies is unclear. We previously demonstrated combining prehospital pRBC and plasma had a greater mortality benefit than either alone among patients in hemorrhagic shock.16 However, outcomes associated with the combination of prehospital TXA with other prehospital resuscitation products represents a knowledge gap. Our objective was to determine if receiving prehospital pRBC in addition to TXA reduced mortality in injured patients at risk of hemorrhage. We hypothesized the combination of pRBC and TXA will be associated with reduced mortality in this population.
METHODS
Trial Design
We conducted a secondary analysis of the Study of Tranexamic Acid during Air and ground Medical Prehospital transport (STAAMP) trial. The details of the STAAMP trial have been previously published.25 Briefly, the STAAMP trial was a multicenter pragmatic double-blind placebo-controlled trial which randomized patients undergoing emergency medical services (EMS) transport after injury to receive a 1-gram TXA bolus or placebo in the prehospital setting. Patients receiving TXA were further randomized in-hospital to three dosing regimens, including an abbreviated regimen with additional placebo bolus and infusion, a standard regimen with placebo bolus and TXA infusion, and a repeat regimen with additional TXA bolus plus TXA infusion.
In the STAAMP trial, twenty-four EMS bases enrolled and transported patients to four level 1 trauma centers. Among these, 12 EMS bases (all air medical transport agencies), provided pRBC as standard of care for prehospital resuscitation when indications were met (Fig. 1). This configuration of EMS bases led to four prehospital resuscitation groups for purposes of this study: (1) patients receiving prehospital TXA (TXA only group), (2) patients receiving prehospital pRBC and placebo (pRBC only group), (3) patients receiving prehospital pRBC and TXA (pRBC+TXA group), and (4) patients receiving placebo only (Neither group). Of note, all EMS bases provided crystalloid resuscitation to the above four groups.
Figure 1.
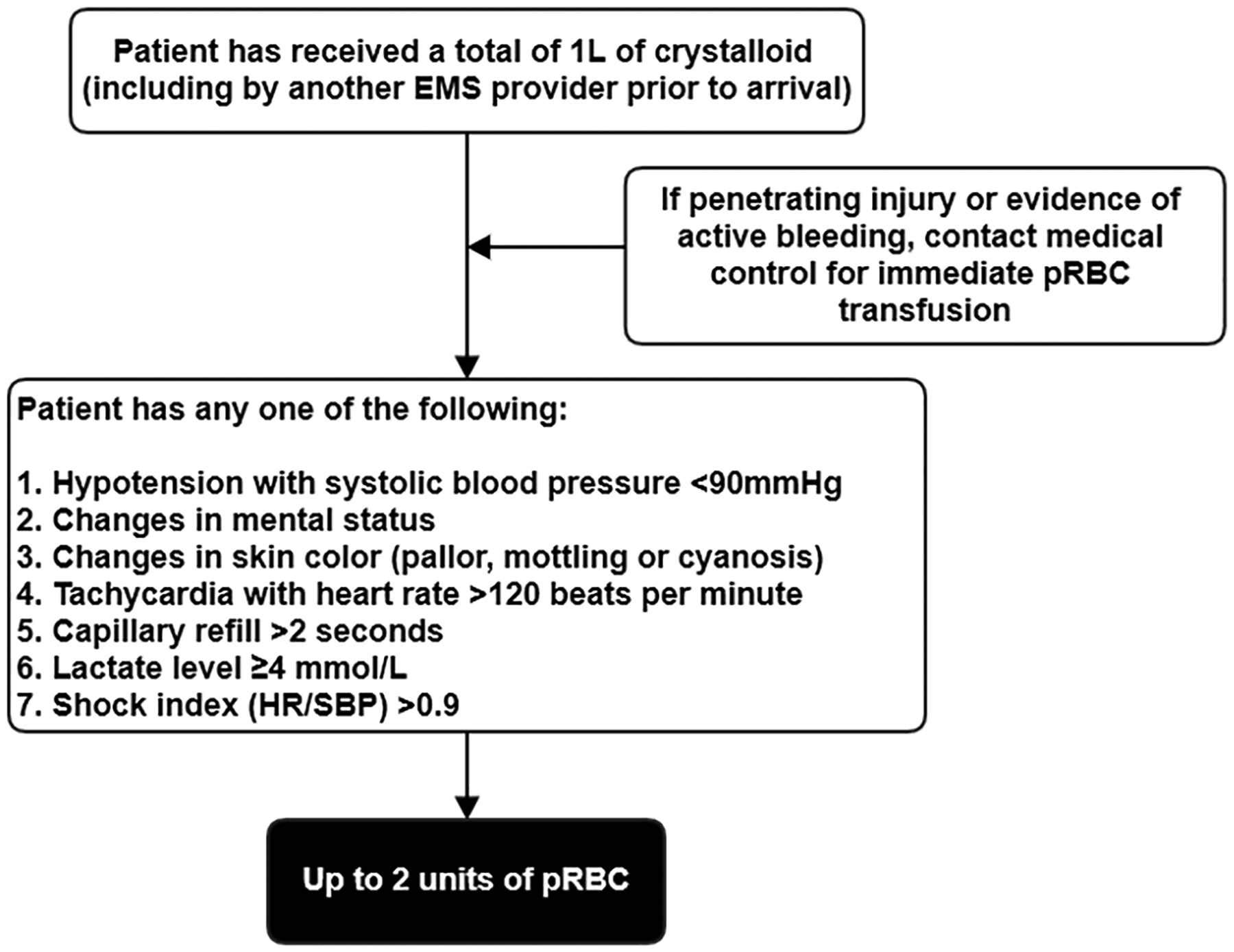
Emergency medical services protocol for prehospital pRBC transfusion on scene.
Study Population
Inclusion criteria for the STAAMP trial were patients with at least one episode of hypotension (systolic blood pressure <90mmHg) or tachycardia (heart rate >110 beats per minute) within 2 hours of injury. Patients were excluded for age older than 90 years or younger than 18 years, lack of intravenous or intraosseous access, isolated fall from standing, documented cervical cord injury, known prisoner or pregnancy, traumatic arrest of more than 5 minutes, penetrating brain injury, isolated drowning or hanging, objection to study voiced at scene, or wearing a STAAMP study opt-out bracelet.
For the current study, we restricted the study population to patients transported directly from the scene of injury. This was done as there was limited data available about resuscitation products and volumes received when initially transported to a referring hospital, which might introduce unmeasured confounding.
Statistical Analysis
The primary outcome was 30-day mortality. Given the potential influence of multiple downstream in-hospital factors that might obscure the impact of prehospital interventions on 30-day mortality, we also assessed a secondary outcome of 24-hour mortality, an endpoint more proximate to the interventions of interest. We used Cox proportional hazard regression to evaluate the association with mortality outcomes and the four prehospital resuscitation groups. The proportional hazards assumption was evaluated by testing for difference from a non-zero slope of Schoenfeld residuals, with a significant testing indicating violation of this assumption. The Neither group, receiving neither prehospital pRBC nor TXA, was used as the reference category for reporting hazard ratios. Based on the results from the original trial, receiving prehospital pRBC was a clear marker of more severe injury and not randomized as the TXA groups were. Thus, we calculated a propensity score that reflected the likelihood of receiving prehospital pRBC based on the protocols for administering prehospital pRBC (Fig. 1) and prehospital variables that potentially could influence this decision. This propensity score included age, injury severity score (ISS), mechanism of injury, prehospital systolic blood pressure, prehospital heart rate, prehospital Glasgow Coma Scale, prehospital time, prehospital intubation, prehospital crystalloid volume, and head, chest, and abdominal abbreviated injury scale (AIS). The propensity score was then used as a covariate in our models. This allowed us to mitigate selection bias, as well as limit the number of variables included in the model to prevent overfitting given the small sample and number of outcome events. Propensity score distributions were examined. We also performed a sensitivity analysis weighted for the maximal overlap in propensity scores (Supplemental Methods).
As the resuscitation groups were not randomized by design, in addition to the propensity to receive prehospital pRBC, we also adjusted the models for intensive care unit admission, emergent/urgent surgical procedures within 24 hours, and TXA in-hospital dose. TXA in-hospital dose was included as it was part of the treatment of interest; however, patients could receive a second bolus plus infusion, infusion alone, or no further in-hospital TXA, representing a potential confounder. Covariates were selected a priori based on clinically relevant predictors of mortality in trauma and retained in the final models if they changed the primary treatment effect of interest coefficient by >10%.27 Multiple organ failure, defined by Denver criteria, was included as a covariate in the 30-day mortality model but not the 24-hour mortality model given that it is calculated after 24 hours. Robust variance estimators were used to account for clustering by site. To evaluate potential differential effects of the resuscitation groups on mortality outcomes, we also tested several interactions, including prehospital systolic blood pressure and heart rate, prehospital time, lactate level, ISS, and AIS for head, chest, and abdominal regions.
Data analysis was conducted using Stata v17MP (StataCorp; College Station, TX). Continuous data are presented as median (interquartile range [IQR]). Continuous data were compared using Wilcoxon rank-sum tests, and categorical data compared using Chi-square. Cox proportional hazard model discrimination was assessed using Harrell’s C-statistic and global goodness-of-fit assessed using the Groennesby and Borgan test. All included variables met the proportional hazards assumption by evaluation with Schoenfeld residuals. The overall fraction of missing data was low at 1% and addressed using multiple imputation as described in the original trial. A two-tailed p value ≤0.05 was considered significant. The University of Pittsburgh Institutional Review Board approved this study. Reporting of this study follows the STROBE guidelines for cohort studies.
RESULTS
A total of 763 patients were included from the 903 patients in the original STAAMP trial. There were 350 (46%) patients in the TXA only group, 35 (5%) patients in the pRBC only group, 22 (3%) patients in the pRBC+TXA group, and 356 (46%) patients in the Neither group (Fig. 2). Patients in the pRBC only and pRBC+TXA groups were sicker with higher ISS, lower systolic blood pressure, and higher unadjusted 24-hour and 30-day mortality than patients in the TXA only or Neither groups (Table 1). The distribution of propensity scores among the four resuscitation treatment groups are shown in eFigure 1.
Figure 2.
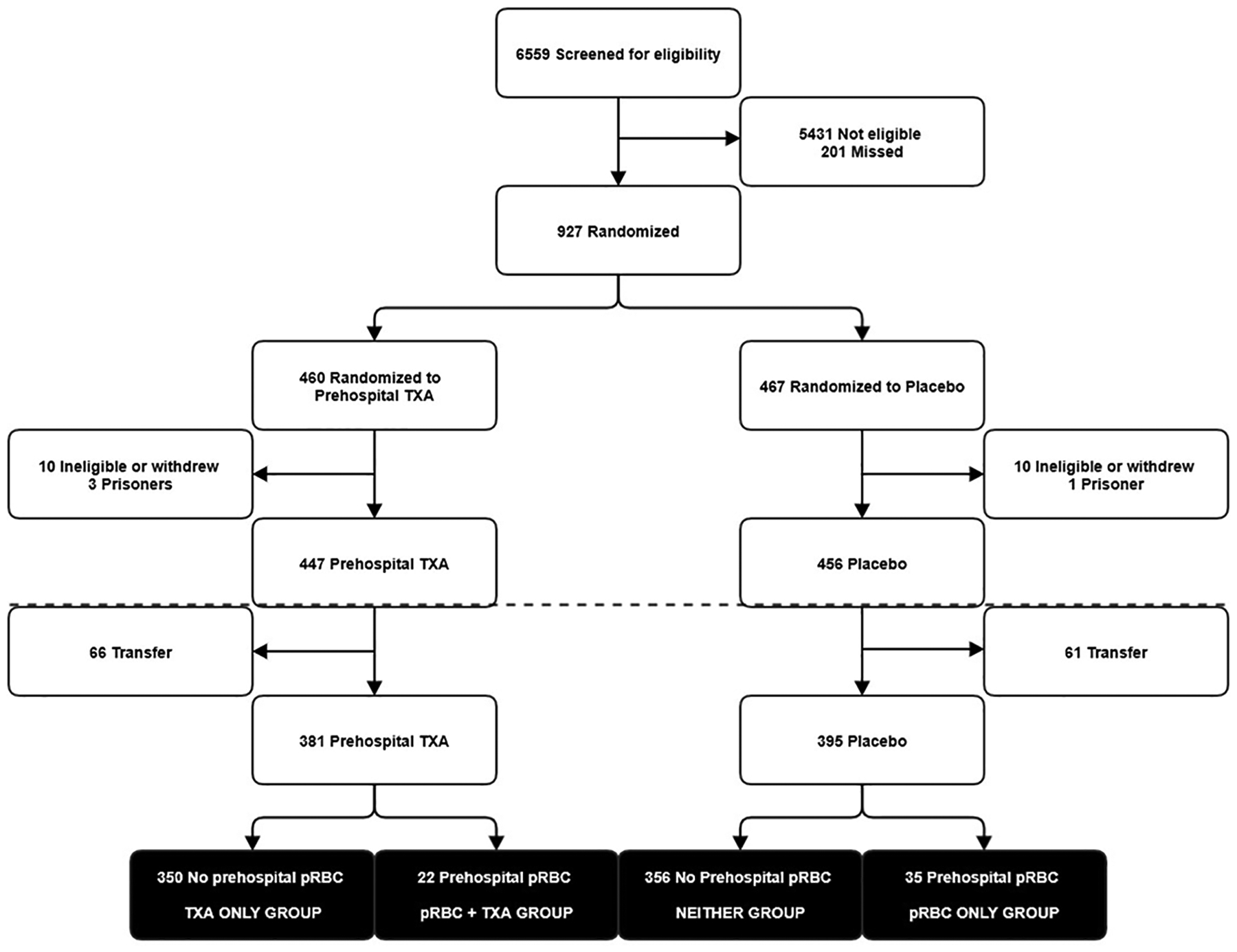
Study participant selection and resuscitation groups from the Study of Tranexamic Acid during Air and ground Medical Prehospital transport (STAAMP) trial. Participant selection below dotted line indicates cohort selection beyond the original trial population for this study.
Table 1.
Patient Characteristics by Resuscitation Group
| TXA (n=350) |
pRBC (n=35) |
TXA + pRBC (n=22) |
Neither (n=356) |
p-value | |
|---|---|---|---|---|---|
| Age, years | 39 (26, 52) | 53 (40, 69) | 39 (31, 56) | 37 (25, 55) | <0.001 |
| Sex (male) | 254 (73%) | 23 (66%) | 11 (50%) | 262 (74%) | 0.090 |
| Blunt mechanism | 305 (87%) | 28 (80%) | 18 (82%) | 323 (91%) | 0.130 |
| PH time, min | 37 (28, 46) | 41 (31, 63) | 41 (34, 49) | 36 (30, 45) | 0.068 |
| PH SBP, mmHg | 128 (90, 147) | 84 (74, 95) | 86 (68, 102) | 128 (89, 148) | <0.001 |
| PH HR, bpm | 118 (112, 128) | 112 (90, 120) | 115 (99, 140) | 118 (112, 125) | 0.074 |
| PH GCS | 14 (12, 15) | 13 (3, 15) | 15 (8, 15) | 14 (12, 15) | 0.120 |
| PH crystalloid, mL | 400 (110, 850) | 1000 (700, 2000) | 910 (300, 1400) | 400 (0, 750) | <0.001 |
| PH intubation | 81 (23.1%) | 17 (48.6%) | 9 (40.9%) | 85 (23.9%) | 0.003 |
| ED SBP, mmHg | 130 (110, 144) | 98 (82, 108) | 95 (88, 110) | 132 (110, 150) | <0.001 |
| ED HR, bpm | 105 (91, 117) | 101 (89, 113) | 109.5 (89, 129) | 106 (91, 120) | 0.590 |
| ED GCS | 15 (11, 15) | 11 (3, 15) | 12.5 (3, 15) | 15 (10, 15) | 0.003 |
| ISS | 12 (5, 21) | 22 (10, 34) | 22 (17, 36) | 10 (4, 20) | <0.001 |
| 24-hour pRBC, units | 0 (0, 0) | 5 (1, 11) | 5 (2, 10) | 0 (0, 0) | <0.001 |
| 24-hour plasma, units | 0 (0, 0) | 2 (0, 7) | 0 (0, 5) | 0 (0, 0) | <0.001 |
| 24-hour crystalloid, mL | 2650 (1100, 5000) | 6048 (3900, 7729) | 4663 (2000, 7600) | 2434 (1010, 4925) | <0.001 |
| MOF | 24 (7%) | 9 (26%) | 4 (18%) | 26 (7%) | <0.001 |
| 3-hour mortality | 5 (1%) | 3 (9%) | 1 (5%) | 4 (1%) | 0.007 |
| 6-hour mortality | 8 (2%) | 3 (9%) | 1 (5%) | 6 (2%) | 0.066 |
| 24-hour mortality | 10 (3%) | 5 (14%) | 3 (14%) | 9 (3%) | <0.001 |
| 30-day mortality | 24 (7%) | 10 (30%) | 4 (19%) | 24 (7%) | <0.001 |
Continuous variables presented as median (IQR)
Categorical variables presented as n (%)
TXA, tranexamic acid; pRBC, packed red blood cells; PH, prehospital; SBP, systolic blood pressure; HR, heart rate; ED, emergency department; ISS, injury severity score; MOF, multiple organ failure
In the primary outcome model for 30-day mortality, resuscitation with pRBC+TXA was associated with a 35% relative decrease in the hazards of mortality compared to the Neither group (HR 0.65; 95%CI 0.45–0.94, p=0.02). There was no association between the hazards of 30-day mortality and resuscitation with TXA only or pRBC only when compared to the Neither group (Table 2). Model diagnostics demonstrated excellent discrimination with Harrell’s C-statistic of 0.81, and acceptable calibration with non-significant Groennesby and Borgan test (p=0.324). The proportional hazards assumption was not violated (p=0.259). Analysis weighted towards maximal overlap of the propensity score demonstrated similar overall results (eTable 1).
Table 2.
Cox proportional hazard regression resuscitation group results for 30-day mortality
| Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Resuscitation group | |||
| Neither | Reference | - | - |
| TXA only | 0.99 | 0.39 – 2.51 | 0.986 |
| pRBC only | 1.05 | 0.57 – 2.00 | 0.842 |
| pRBC+TXA | 0.65 | 0.45 – 0.94 | 0.022 |
| ICU admission | 1.13 | 0.75 – 1.69 | 0.552 |
| MOF | 1.95 | 1.38 – 2.75 | <0.001 |
| Urgent procedure | 1.24 | 1.17 – 1.33 | <0.001 |
| TXA dose | 0.96 | 0.74 – 1.24 | 0.768 |
| Propensity Score | 76.67 | 50.43 – 122.72 | <0.001 |
TXA, tranexamic acid; pRBC, packed red blood cells; ICU, intensive care unit; MOF, multiple organ failure
In the secondary outcome model for 24-hour mortality, resuscitation with pRBC-only was associated with a 61% relative reduction in the hazards of mortality compared to the Neither group (HR 0.39; 95%CI 0.17–0.88, p=0.02). There was no association between the hazards of 24-hour mortality and resuscitation with TXA only or pRBC+TXA when compared to the Neither group (Table 3), although the pRBC+TXA group was associated with a strong reduction in the point estimate of 24-hour mortality hazards with a p-value of 0.06. Model diagnostics demonstrated good discrimination with Harrell’s C-statistic of 0.72, and acceptable calibration with non-significant Groennesby and Borgan test (p=0.472). The proportional hazards assumption was not violated (p=0.181). Analysis weighted towards maximal overlap of the propensity score demonstrated similar overall results, although the pRBC+TXA group did reach statistical significance in the association with reduced hazards of 24-hour mortality (eTable 2).
Table 3.
Cox proportional hazard regression resuscitation group results for 24-hour mortality
| Hazard Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Resuscitation group | |||
| Neither | Reference | - | - |
| TXA only | 1.00 | 0.71 – 1.40 | 0.990 |
| pRBC only | 0.39 | 0.17 – 0.88 | 0.024 |
| pRBC+TXA | 0.33 | 0.10 – 1.08 | 0.068 |
| ICU admission | 0.11 | 0.06 – 0.20 | <0.001 |
| Urgent procedure | 1.58 | 0.89 – 2.81 | 0.081 |
| TXA dose | 0.97 | 0.60 – 1.58 | 0.916 |
| Propensity Score | 991.57 | 475.57 – 2067.46 | <0.001 |
TXA, tranexamic acid; pRBC, packed red blood cells; ICU, intensive care unit
Several variables were significant in interaction testing. The pRBC+TXA group had significant interactions with prehospital systolic blood pressure (p<0.001), lactate level (p<0.001), and AIS abdomen (p<0.001). All resuscitation groups had significant interactions with prehospital heart rate (p≤0.001), AIS head (p<0.001), and AIS chest (p≤0.01). Finally, the pRBC only and pRBC+TXA groups had significant interactions with prehospital time (p<0.01) and ISS (p<0.001). Overall, for the pRBC only and pRBC+TXA groups, patients with lower systolic blood pressure, higher heart rate, higher lactate, more severe anatomic injuries, and longer prehospital time derived greater mortality benefit than less severely injured patients receiving similar prehospital resuscitation.
DISCUSSION
We found that prehospital resuscitation with the combination of pRBC and TXA reduces the hazards of mortality at 30 days for injured patients at risk for hemorrhagic shock. Notably, there was no 30-day mortality benefit for patients that received either product in isolation. Additionally, resuscitation with pRBC alone was associated with reduced mortality at 24 hours, consistent with prior literature,15 while no early benefit was shown for TXA alone. There was a strong point estimate association of reduced 24-hour hazards for the pRBC+TXA; however, the p-value was 0.06. This may reflect an underpowered analysis given the low numbers in this group, especially as our weighted sensitivity analysis showed the pRBC+TXA group to be significantly associated with a reduction in the hazards of 24-hour mortality.
Amidst the push of advanced resuscitative techniques into the prehospital setting, pRBCs are the most commonly available blood products, and almost exclusively through air medical transport agencies.28 Prior reports demonstrate feasibility, safety29 and a growing body of evidence supports a mortality benefit.9, 14, 15, 30–32 This was initially demonstrated out of literature from recent military conflicts wherein improved survival was observed for severely injured patients that received advanced prehospital care including pRBC transfusion.9, 30 More recent civilian-based studies demonstrated a reduction in early mortality as well as biochemical improvements in coagulopathy for those that received prehospital pRBC.14 A large propensity-matched, single-center study matched severely injured patients that received prehospital pRBC to those that did not and, similarly, found a reduction in early mortality at 24 hours.15
Given the logistical challenges and relatively limited availability of prehospital blood products, it is important that we continue to pursue adjunctive hemostatic therapies. Tranexamic acid has greater shelf life than blood products and can be widely deployed in ground and air EMS agencies. Tranexamic acid impairs the biochemical conversion of plasminogen to plasmin, and at higher concentrations, inhibits plasmin, serving as potent adjunct in resuscitation efforts as an inhibitor of fibrinolysis.33 Our finding of 30-day but not 24-hour survival benefit of adding prehospital TXA to pRBC in the main analysis could suggest its anti-fibrinolytic mechanism may not be the only property responsible for its effects in recent trials. Notably, plasmin carries proinflammatory effects by activating endothelium and platelets, and also has a role in innate immune signaling.34, 35 Plasmin induces chemotaxis of monocytes and dendritic cells with release of proinflammatory cytokines.35, 36 Thus, the benefit of TXA observed in the current study may be through inhibition of plasmin and attenuation of inflammation, ischemia-reperfusion, and complement activation.37–40
TXA effectively reduces surgical bleeding19 and following the CRASH-2 trial22, is increasingly utilized in prehospital care. This trial demonstrated a reduction in mortality from bleeding with early administration from time of injury at the receiving hospital, as compared to placebo, with the greatest benefit observed for those that received TXA within one hour of injury. Subsequently, the CRASH-3 trial compared TBI patients and in-hospital head-related mortality at 28 days for those that received in-hospital TXA within three hours of injury vs placebo. Although a negative trial for the primary outcome, head-related mortality was reduced in the TXA arm at 24-hours and in sensitivity analysis for those with mild to moderate TBI, without observed differences in vaso-occlusive or seizure events.41 Morrison and colleagues thereafter conducted a retrospective military study comparing hospital TXA versus no TXA in combat injuries for patients requiring pRBCs. They also demonstrated improved survival for the most severely injured patients with an associated improvement in coagulopathy.42
Extrapolating this evidence to the field, EMS systems implemented TXA protocols43, 44 and retrospective studies demonstrate reduced fibrinolysis, a safety profile marked by venous thromboembolism, mixed results on early outcomes, and a paucity of long-term data.23, 45, 46 The STAAMP trial addressed this evidence gap and showed a reduction in mortality in patients with TXA ≤ 1 hour, 3-gram total dose, and those with SBP ≤ 70 mmHg, with no differences in seizure or thrombotic complications.25 In another secondary analysis of STAAMP, for patients at greatest risk of hemorrhage, receipt of early TXA ≤ 1 hour was associated with a reduction in 30-day mortality risk.47 A prehospital trial by Rowell et al. investigated the impact of prehospital TXA on long term neurologic function for patients with TBI.26 The authors found no difference in 6-month neurologic outcomes; however, there was lower 28-day mortality among patients that received a 2-gram bolus only dosing regimen.
Taken together, the current body of literature indicates that TXA is safe and effective but should be administered in appropriately selected patient cohorts as evidenced by the subgroup analyses of several of these trials.22, 25, 41 In the present study, we demonstrate the best outcomes for patients that received combined therapy with pRBC and TXA transfusion and similar to the original trial, the greatest benefit is observed for those most severely injured with severe shock and high ISS. A secondary analysis of the PAMPer trial also corroborates best outcomes for patients receiving combination prehospital therapies.13 Severely injured patients in hemorrhagic shock who received combined prehospital pRBC and plasma showed the greatest mortality benefit.16
Trauma and severe shock cause early coagulopathy, endotheliopathy, and systemic inflammation that is difficult to reverse.48 Our findings have important implications for improving the prehospital phase of trauma care. Optimal management requires prehospital, early, and multi-modal resuscitation to ameliorate the complex pathophysiologic insult of injury. Early prehospital use of TXA may benefit patients who require prehospital pRBC resuscitation. Future investigations should aim to delineate the mechanisms responsible for combined resuscitation strategy benefits, as well as further define the injured patient populations most likely to benefit from prehospital TXA. The PATCH multicenter trial is currently underway and evaluates prehospital TXA in severe trauma.49, 50 Results of this trial will supplement the available evidence as we work to improve prehospital care of trauma patients at risk of exsanguination.
This study has several important limitations to acknowledge. First, this was a secondary analysis of a randomized trial with a hypothesis that differed from that of the original design. This resulted in relatively small sample sizes within our defined resuscitation groups. In particular, there were only 22 patients in the pRBC+TXA group, potentially making some of our analyses underpowered to find an effect, again such as no association with 24-hour mortality. Efforts to explore sensitivity analyses and better elucidate significant interactions led to unstable models. Additionally, the majority of our patients sustained blunt trauma and were treated in mature trauma systems of four participating trauma centers by trial protocol. This may limit the generalizability of our findings for those with penetrating injury or patients served in differing trauma systems without access to advanced prehospital resuscitation. As noted, prehospital pRBC are limited in availability currently, although advances such as freeze-dried products may make prehospital blood products more readily available.
CONCLUSION
For injured patients at risk of hemorrhage, prehospital resuscitation with a combination of pRBC and TXA is associated with a reduction in the risk of 30-day mortality. Prehospital pRBC transfusion alone was associated with a reduction in the risk of early mortality. Prehospital professionals with the capability to provide both pRBC and TXA should consider administering TXA to any patient meeting criteria for prehospital pRBC transfusion. Potential synergy appeared most robustly in longer term mortality and further work to investigate mechanisms of this therapeutic benefit is needed to optimize the prehospital resuscitation of trauma patients.
Supplementary Material
Funding:
The original trial was funded by grant W81XWH 13-2-0080 from the US Army Medical Research and Material Command. No funding or support was directly received to perform the current study.
Footnotes
This paper was presented as a podium presentation at the Eastern Association for the Surgery of Trauma’s 35th Annual Scientific Assembly, January 11–15, 2022, Austin, TX
There are no conflicts of interest for the current study.
SUPPLEMENTAL DIGITAL CONTENT
Contributor Information
Andrew-Paul Deeb, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, Pittsburgh, Pennsylvania.
Lara Hoteit, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, Pittsburgh, Pennsylvania.
Shimena Li, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, Pittsburgh, Pennsylvania.
Francis X. Guyette, Department of Emergency Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Brian J. Eastridge, Department of Surgery, University of Texas Health San Antonio, San Antonio, TX.
Raminder Nirula, Department of Surgery, University of Utah, Salt Lake City, UT.
Gary A. Vercruysse, Department of Surgery, University of Arizona, Tucson, AZ.
Terence O’Keeffe, Department of Surgery, University of Arizona, Tucson, AZ.
Bellal Joseph, Department of Surgery, University of Arizona, Tucson, AZ.
Matthew D. Neal, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Jason L. Sperry, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Joshua B. Brown, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, Pittsburgh, Pennsylvania.
REFERENCES
- 1.Drake SA, Holcomb JB, Yang Y, Thetford C, Myers L, Brock M, et al. Establishing a Regional Trauma Preventable/Potentially Preventable Death Rate. Ann Surg. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB. Transport Time and Preoperating Room Hemostatic Interventions Are Important: Improving Outcomes After Severe Truncal Injury. Crit Care Med. 2018;46:447–453. [DOI] [PubMed] [Google Scholar]
- 3.Floccard B, Rugeri L, Faure A, Saint Denis M, Boyle EM, Peguet O, et al. Early coagulopathy in trauma patients: an on-scene and hospital admission study. Injury. 2012;43:26–32. [DOI] [PubMed] [Google Scholar]
- 4.Spielmann S, Kerner T, Ahlers O, Keh D, Gerlach M, Gerlach H. Early detection of increased tumour necrosis factor alpha (TNFα) and soluble TNF receptor protein plasma levels after trauma reveals associations with the clinical course. Acta Anaesthesiol Scand. 2001;45:364–370. [DOI] [PubMed] [Google Scholar]
- 5.Bickell WH, Wall MJ Jr., Pepe PE, Martin RR, Ginger VF, Allen MK, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–1109. [DOI] [PubMed] [Google Scholar]
- 6.Cotton BA, Guy JS, Morris JA Jr., Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. [DOI] [PubMed] [Google Scholar]
- 7.Kasotakis G, Sideris A, Yang Y, de Moya M, Alam H, King DR, et al. Aggressive early crystalloid resuscitation adversely affects outcomes in adult blunt trauma patients: an analysis of the Glue Grant database. J Trauma Acute Care Surg. 2013;74:1215–1221; discussion 1221–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malsby RF 3rd, Quesada J, Powell-Dunford N, Kinoshita R, Kurtz J, Gehlen W, et al. Prehospital blood product transfusion by U.S. army MEDEVAC during combat operations in Afghanistan: a process improvement initiative. Mil Med. 2013;178:785–791. [DOI] [PubMed] [Google Scholar]
- 9.Morrison JJ, Oh J, DuBose JJ, O’Reilly DJ, Russell RJ, Blackbourne LH, et al. En-route care capability from point of injury impacts mortality after severe wartime injury. Ann Surg. 2013;257:330–334. [DOI] [PubMed] [Google Scholar]
- 10.O’Reilly DJ, Morrison JJ, Jansen JO, Apodaca AN, Rasmussen TE, Midwinter MJ. Prehospital blood transfusion in the en route management of severe combat trauma: a matched cohort study. J Trauma Acute Care Surg. 2014;77:S114–120. [DOI] [PubMed] [Google Scholar]
- 11.Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, et al. Association of Prehospital Blood Product Transfusion During Medical Evacuation of Combat Casualties in Afghanistan With Acute and 30-Day Survival. JAMA. 2017;318:1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold-stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg. 2016;81:21–26. [DOI] [PubMed] [Google Scholar]
- 13.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med. 2018;379:315–326. [DOI] [PubMed] [Google Scholar]
- 14.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, et al. Pretrauma Center Red Blood Cell Transfusion Is Associated With Reduced Mortality and Coagulopathy in Severely Injured Patients With Blunt Trauma. Ann Surg. 2014;261:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX. Pre-Trauma Center Red Blood Cell Transfusion Is Associated with Improved Early Outcomes in Air Medical Trauma Patients. J Am Coll Surg. 2015:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyette FX, Sperry JL, Peitzman AB, Billiar TR, Daley BJ, Miller RS, et al. Prehospital Blood Product and Crystalloid Resuscitation in the Severely Injured Patient: A Secondary Analysis of the Prehospital Air Medical Plasma Trial. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 17.Holcomb JB, Donathan DP, Cotton BA, Del Junco DJ, Brown G, Wenckstern TV, et al. Prehospital Transfusion of Plasma and Red Blood Cells in Trauma Patients. Prehosp Emerg Care. 2014;19:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Rijnhout TWH, Wever KE, Marinus R, Hoogerwerf N, Geeraedts LMG Jr., Tan E. Is prehospital blood transfusion effective and safe in haemorrhagic trauma patients? A systematic review and meta-analysis. Injury. 2019;50:1017–1027. [DOI] [PubMed] [Google Scholar]
- 19.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011:CD001886. [DOI] [PubMed] [Google Scholar]
- 20.Kutcher ME, Cripps MW, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Criteria for empiric treatment of hyperfibrinolysis after trauma. J Trauma Acute Care Surg. 2012;73:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore HB, Moore EE, Liras IN, Gonzalez E, Harvin JA, Holcomb JB, et al. Acute Fibrinolysis Shutdown after Injury Occurs Frequently and Increases Mortality: A Multicenter Evaluation of 2,540 Severely Injured Patients. J Am Coll Surg. 2016;222:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 23.Boudreau RM, Deshpande KK, Day GM, Hinckley WR, Harger N, Pritts TA, et al. Prehospital Tranexamic Acid Administration During Aeromedical Transport After Injury. J Surg Res. 2019;233:132–138. [DOI] [PubMed] [Google Scholar]
- 24.Napolitano LM. Prehospital tranexamic acid: what is the current evidence? Trauma Surg Acute Care Open. 2017;2:e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, et al. Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial. JAMA Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA. 2020;324:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camazine MN, Hemmila MR, Leonard JC, Jacobs RA, Horst JA, Kozar RA, et al. Massive transfusion policies at trauma centers participating in the American College of Surgeons Trauma Quality Improvement Program. J Trauma Acute Care Surg. 2015;78:S48–53. [DOI] [PubMed] [Google Scholar]
- 29.Higgins GL 3rd, Baumann MR, Kendall KM, Watts MA, Strout TD. Red blood cell transfusion: experience in a rural aeromedical transport service. Prehosp Disaster Med. 2012;27:231–234. [DOI] [PubMed] [Google Scholar]
- 30.Apodaca A, Olson CM Jr., Bailey J, Butler F, Eastridge BJ, Kuncir E. Performance improvement evaluation of forward aeromedical evacuation platforms in Operation Enduring Freedom. J Trauma Acute Care Surg. 2013;75:S157–163. [DOI] [PubMed] [Google Scholar]
- 31.Smith IM, James RH, Dretzke J, Midwinter MJ. Prehospital Blood Product Resuscitation for Trauma: A Systematic Review. Shock. 2016;46:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumida MP, Quinn K, Lewis PL, Jones Y, Barker DE, Ciraulo DL, et al. Prehospital blood transfusion versus crystalloid alone in the air medical transport of trauma patients. Air Med J. 2000;19:140–143. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Sugiura J. The effect of a new potent antifibrinolytic agent, tranexamic acid. J Jpn Obstet Gynecol Soc. 1966;13:158–167. [PubMed] [Google Scholar]
- 34.Medcalf RL. Fibrinolysis, inflammation, and regulation of the plasminogen activating system. J Thromb Haemost. 2007;5 Suppl 1:132–142. [DOI] [PubMed] [Google Scholar]
- 35.Syrovets T, Lunov O, Simmet T. Plasmin as a proinflammatory cell activator. J Leukoc Biol. 2012;92:509–519. [DOI] [PubMed] [Google Scholar]
- 36.Levy JH. Antifibrinolytic therapy: new data and new concepts. Lancet. 2010;376:3–4. [DOI] [PubMed] [Google Scholar]
- 37.Chang M, Kistler EB, Schmid-Schonbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez JJ, Iribarren JL, Lorente L, Rodriguez JM, Hernandez D, Nassar I, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care. 2007;11:R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burk AM, Martin M, Flierl MA, Rittirsch D, Helm M, Lampl L, et al. Early complementopathy after multiple injuries in humans. Shock. 2012;37:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalle Lucca JJ, Li Y, Simovic M, Pusateri AE, Falabella M, Dubick MA, et al. Effects of C1 inhibitor on tissue damage in a porcine model of controlled hemorrhage. Shock. 2012;38:82–91. [DOI] [PubMed] [Google Scholar]
- 41.collaborators C-t. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147:113–119. [DOI] [PubMed] [Google Scholar]
- 43.Strosberg DS, Nguyen MC, Mostafavifar L, Mell H, Evans DC. Development of a Prehospital Tranexamic Acid Administration Protocol. Prehosp Emerg Care. 2016;20:462–466. [DOI] [PubMed] [Google Scholar]
- 44.Vu EN, Schlamp RS, Wand RT, Kleine-Deters GA, Vu MP, Tallon JM. Prehospital use of tranexamic acid for hemorrhagic shock in primary and secondary air medical evacuation. Air Med J. 2013;32:289–292. [DOI] [PubMed] [Google Scholar]
- 45.Neeki MM, Dong F, Toy J, Vaezazizi R, Powell J, Wong D, et al. Tranexamic Acid in Civilian Trauma Care in the California Prehospital Antifibrinolytic Therapy Study. West J Emerg Med. 2018;19:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein P, Studt JD, Albrecht R, Muller S, von Ow D, Fischer S, et al. The Impact of Prehospital Tranexamic Acid on Blood Coagulation in Trauma Patients. Anesth Analg. 2018;126:522–529. [DOI] [PubMed] [Google Scholar]
- 47.Li SR, Guyette F, Brown J, Zenati M, Reitz KM, Eastridge B, et al. Early Prehospital Tranexamic Acid Following Injury Is Associated With a 30-day Survival Benefit: A Secondary Analysis of a Randomized Clinical Trial. Ann Surg. 2021;274:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Namas R, Ghuma A, Hermus L, Zamora R, Okonkwo DO, Billiar TR, et al. The acute inflammatory response in trauma / hemorrhage and traumatic brain injury: current state and emerging prospects. Libyan J Med. 2009;4:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitra B, Bernard S, Gantner D, Burns B, Reade MC, Murray L, et al. Protocol for a multicentre prehospital randomised controlled trial investigating tranexamic acid in severe trauma: the PATCH-Trauma trial. BMJ Open. 2021;11:e046522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pre-hospital Anti-fibrinolytics for Traumatic Coagulopathy and Haemorrhage (The PATCH Study). NCT02187120. Available at: https://clinicaltrials.gov/ct2/show/NCT02187120. Accessed: June 13, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


