Abstract
Periodontal disease begins as an inflammatory response to a bacterial biofilm deposited around the teeth, which over time leads to the destruction of tooth-supporting structures and consequently tooth loss. Conventional treatment strategies show limited efficacy in promoting regeneration of damaged periodontal tissues. Here, we developed a delivery platform for small extracellular vesicles (sEVs) derived from gingival mesenchymal stem cells (GMSCs) to treat periodontitis. EVs can achieve comparable therapeutic effects to their cells of origin. However, the short half-life of EVs after their administration along with their rapid diffusion away from the delivery site necessitate frequent administration to achieve therapeutic benefits. To address these issues, we engineered ‘dual delivery’ microparticles enabling microenvironment-sensitive release of EVs by metalloproteinases at the affected site along with antibiotics to suppress bacterial biofilm growth. GMSC sEVs were chosen for microparticle decoration as they show superior immunomodulatory properties to sEVs derived from other types of mesenchymal stem cells. GMSC sEVs were able to decrease the secretion of pro-inflammatory cytokines by monocytes/macrophages and T cells, suppress T cell activation and induce the formation of Tregs in vitro and in a rat model of periodontal disease. One-time administration of immunomodulatory GMSC sEV-decorated microparticles led to a significant improvement in regeneration of the damaged periodontal tissue. This approach will have potential clinical applications in the regeneration of a variety of tissues.
Keywords: Periodontitis, Periodontal tissue healing, Immunoengineering, Local drug delivery, Extracellular vesicles
Graphical Abstract
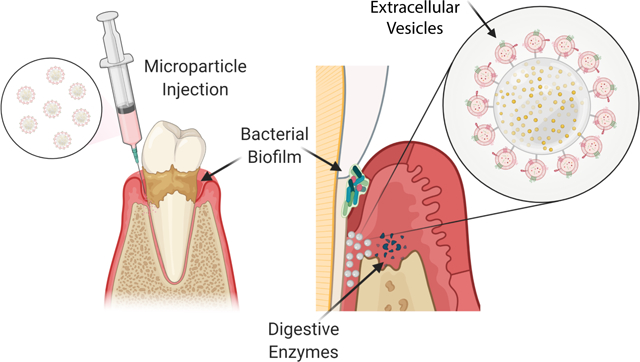
Here we developed multifunctional delivery system to locally present extracellular vesicles derived from dental stem cells to treat periodontal diseases. This immunoengineeering platform could reduce production of inflammatory cytokines and promote regeneration of lost tissue in animal model of periodontitis.
Introduction.
Periodontal disease is one of the most common chronic inflammatory diseases, affecting almost half of the adult population to various extents [1] [2]. It originates from an inflammatory response to a bacterial biofilm deposited around teeth, which in susceptible individuals fails to resolve and over time leads to the destruction of tooth-supporting structures and eventually to tooth loss. Immune cells including monocytes/macrophages and T cells play a central role in the evolution of the disease [3]. Bacterial byproducts such as lipopolysaccharide (LPS) stimulate immune cell activation and differentiation into pro-inflammatory phenotypes. These cells secrete pro-inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukins IL-1β and IL-6, and interferon gamma (INF-γ), which stimulate the production of metalloproteinases (MMPs) [3] that contribute to the tissue degradation. Several studies have documented an increase in MMP expression that correlates with the disease progression [4].
Moreover, the activated immune cells secrete receptor activator of nuclear factor-κβ ligand (RANKL), which orchestrates osteoclast formation and alveolar bone resorption [5]. In contrast, numbers of T regulatory cells (Tregs), which normally control excessive immune responses and ensure tissue homeostasis, are significantly reduced in periodontitis [6] [7].
Over the past two decades, periodontal repair strategies have been mainly based on the physical removal of the bacterial plaque and administration of antibiotics to stop disease progression [8] [9]. Achieving complete periodontal regeneration is, however, challenging mainly because it is not only necessary to suppress the inflammatory immune responses that mediate tissue damage, but also to induce regeneration of a complex set of tissues including bone, cementum, and periodontal ligament.
Mesenchymal stem cells (MSCs) are well known for their immunomodulatory and regenerative potential [10] [11]. For dental applications, gingival mesenchymal stem cells (GMSCs) are particularly interesting. In comparison to bone marrow mesenchymal stem cells (BMSCs), GMSCs can be easily extracted through a minimally invasive process with little patient discomfort [12]. Compared to BMSCs, which exhibit signs of cellular aging at higher passages, GMSCs can withstand twice as many passages while maintaining their characteristics and normal karyotype [13]. Several studies have shown that GMSC delivery alone [14] or through a biomaterial scaffold [15] significantly improved regeneration of diseased tissues. However, therapeutic cell delivery is expensive and can have variable outcomes due to difficulties in maintaining cell viability and achieving differentiation that may be caused by specific factors of the microenvironment. Recently, it has been recognized that the cell therapeutic effect can be, to a great extent, replicated by administration of conditioned media (CM) or extracellular vesicles (EVs) secreted by cells [16]. Most studies on bone and periodontal regeneration have focused on the therapeutic effects of secretome of BMSCs [17] [18], nevertheless, the properties of CM or EVs from other MSC sources such as adipose [19] or dental tissue [20] [21] are also being explored. BMSC secretome contains variety of components including growth factors such as VEGF and TGF-β and was shown to support angiogenesis [22], stem cell mobilization, and also osteoblast proliferation and differentiation [23]. Moreover, BMSC CM or EVs have also powerful immunomodulatory properties, which can ameliorate a variety disease conditions [24]. Studies focusing on regenerative properties of dental stem cell secretome are suggesting that dental stem cell EVs might have similar immunomodulatory function [21] as the most used BMSC EVs, however, direct comparison is missing. We, therefore, tried to compare the properties of BMSC and GMSC EVs to determine the best candidate for the immunomodulatory cell-free therapeutic for periodontal regeneration.
So far, EVs have mainly been administered in a solution, after which they quickly diffuse all over the body and disappear within minutes to hours [25]. We hypothesized that localization, controlled release, and prolonged retention of EVs at the site of tissue damage should have a positive impact on periodontal regeneration.
Here, we developed a dual delivery platform based on polymeric microparticles releasing antibiotics to suppress bacterial biofilm growth. Further, GMSC EVs were immobilized on the surface of the microparticles via an MMP-sensitive linker, enabling the targeted release of the EVs at the site of inflammation for localized immunomodulation. The immunomodulatory properties of GMSC EVs and the whole platform were tested in vitro on macrophages and T cells as well as in vivo in a rat model of periodontitis.
Results and Discussion
EV isolation and characterization.
GMSCs isolated from human gingiva were previously confirmed to be positive for MSC markers such as CD73, CD146, CD166, and Sca-1, and negative for hematopoietic cell markers (CD31, CD34,and CD45) [26] [27]. The growth of GMSCs in a medium containing EV-depleted FBS was significantly greater than that of BMSCs cultured under the same conditions (Fig. 1A), which is in accordance with our previously published results showing that GMSCs have superior growth properties to BMSCs [27]. EVs secreted by cells into the medium were isolated by differential ultracentrifugation and visualized by transmission electron microscopy (Fig. 1B). Here, we decided to focus on small EVs with a diameter less than 220 nm. Unlike larger EVs, small EVs (sEVs) can be easily sterilized by filtration through a 0.22 μm filter, which is more practical for their further clinical translation. The average size of sEVs did not differ between the two cell types and was around 100 nm in both cases. However, GMSCs secreted on average twice as many sEVs per million cells in one day than BMSCs (Fig. 1C), which also corelated with protein concentrations of EV isolations. One million of MSCs produced around 50 ug of sEVs in 24h while one million of GMSCs secreted approximately 100 ug of sEV proteins as determined by Micro BCA protein assay. The specificity of our EV isolation procedure was confirmed by Western blot showing that calnexin, a marker of endoplasmic reticulum, was present only in cell lysates but not in the isolated sEVs (Fig. 1D). Both BMSC and GMSC sEVs were positive for EV markers such as CD63, flotillin 1, CD81, and CD9. However, differences in the levels of expression of CD81 and CD9 were observed, with BMSC sEVs containing lower levels of these two markers. These two tetraspanins were previously shown to localize more to the plasma membrane, whereas CD63 is sorted more into endosomal-derived exosomes [28]. This potentially suggests that GMSCs produce more sEVs by plasma membrane budding than do BMSCs.
Fig. 1. Comparison and characterization of BMSC and GMSC sEVs.
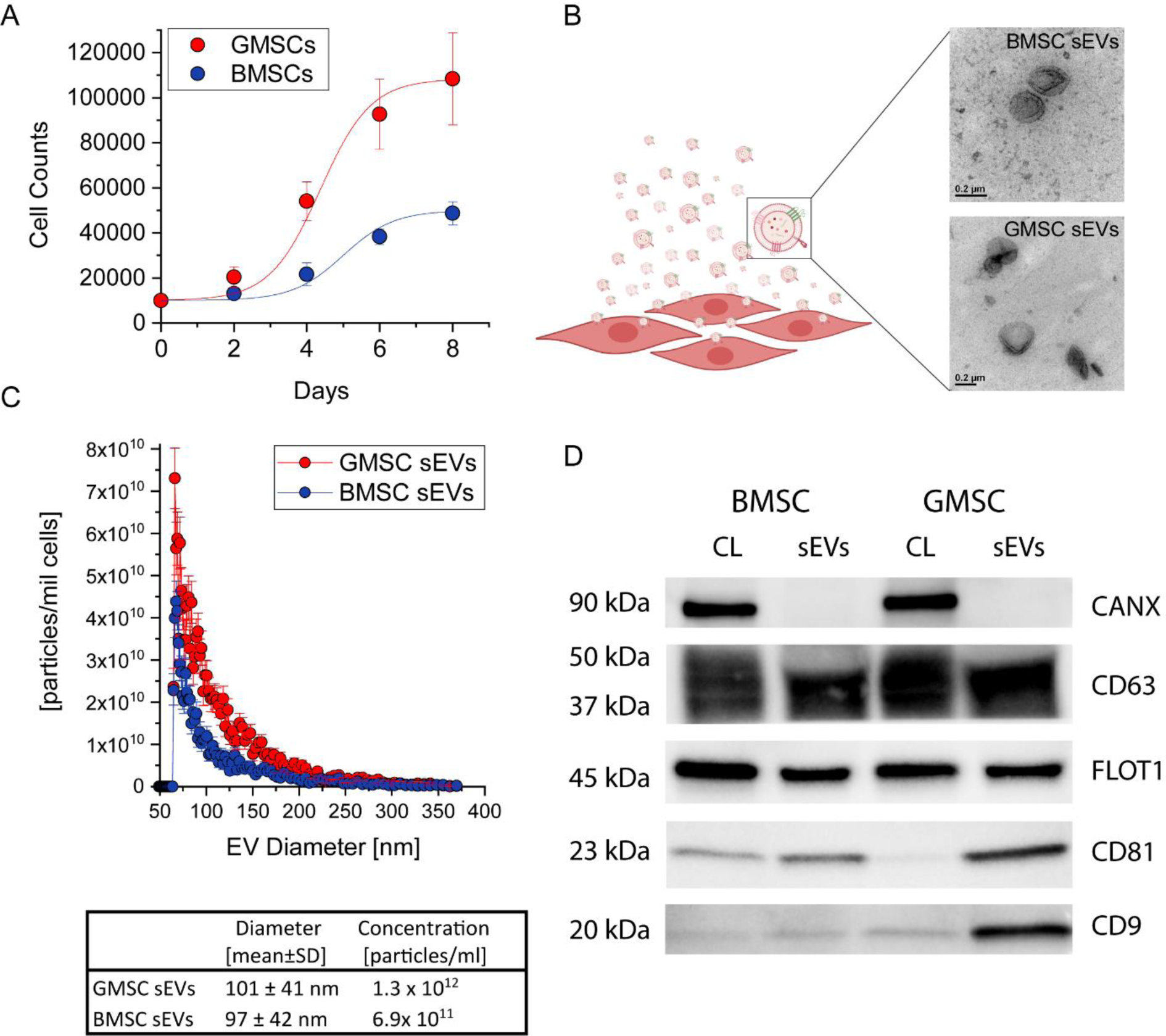
A. Growth of BMSCs and GMSCs in medium containing EV-depleted FBS. B. TEM images of BMSC and GMSC sEVs. C. Comparison of size and concentration of BMSC and GMSC sEVs. D. Western blotting analysis of sEVs. All data are presented as mean ± SD (n = 5).
Comparison of the effects of BMSC and GMSC EVs on immune cells in vitro.
Immune cells including monocytes/macrophages and T cells play an important role in the evolution of periodontitis [29] [30]. Macrophages are also generally reported to be among the first cells to internalize EVs upon administration [31] [32]. Accumulating evidence indicates that MSC EVs have anti-inflammatory and immunosuppressive functions, which can replicate the beneficial effects of MSCs in regenerative therapies [33]. However, direct comparison of immunomodulatory effects of BMSC EVs and GMSC EVs had yet to be performed. To determine the best candidate for EV-based therapeutics to treat periodontal disease, we set out to compare the effect of BMSC and GMSC sEVs on the cytokine secretion and polarization of immune cells. Monocytes were stimulated with LPS in the absence or presence of sEVs. Addition of BMSC or GMSC sEVs to the monocyte culture stimulated with LPS significantly decreased the production of inflammatory cytokine TNF-α (Fig. 2A). In contrast, both EV types increased the secretion of anti-inflammatory IL-10, even in the presence of LPS (Fig. 2B). GMSC sEVs showed a stronger anti-inflammatory effect on monocytes in comparison to BMSC sEVs. M0 macrophages co-incubated with sEVs for 24 h increased expression of markers characteristic of the anti-inflammatory phenotype, namely arginase and CD206, which are expressed at very low levels in pro-inflammatory M1 macrophages (Fig. 2C).
Fig. 2. Effect of sEVs on monocytes and macrophages.
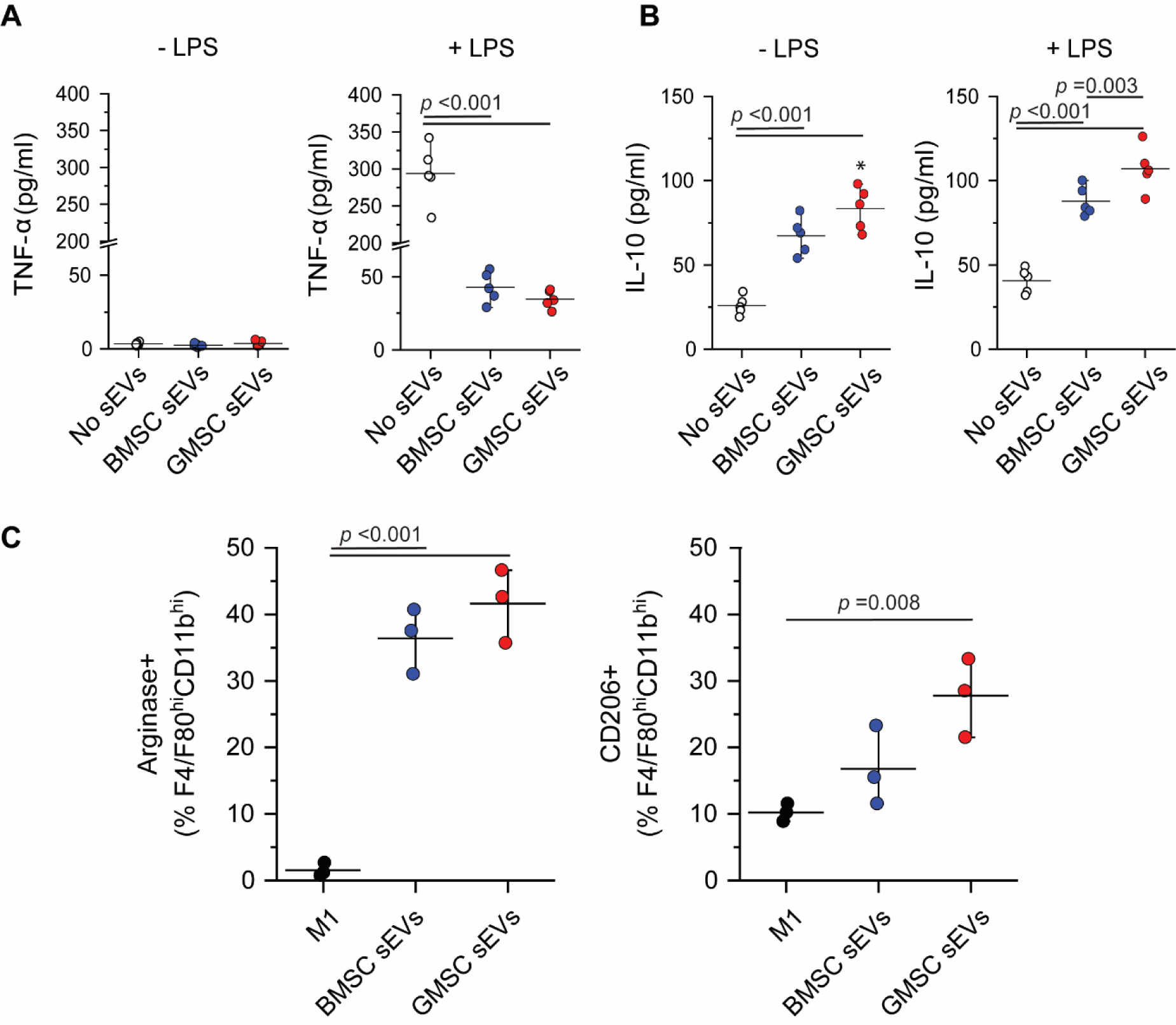
A. Change in secretion of inflammatory cytokine TNF-α after addition of EVs to the monocyte culture. B. Increase in production of anti-inflammatory IL-10 by monocytes treated with sEVs and particularly with GMSC sEVs, even in the presence of LPS. C. BMSC and GMSC sEVs stimulate macrophages to upregulate markers like arginase and CD206, which are characteristic of anti-inflammatory macrophage phenotype. All data are presented as mean ± SD (n = 3).
When added to the T cell culture, sEVs were able to suppress the activation of CD4+ T cells, which was determined by evaluating the expression of T cell activation markers CD25 and CD44 by flow cytometry (Fig. 3A). GMSC sEVs showed in this regard superior immunosuppressive properties to those of BMSC sEVs. A slight increase was observed in the numbers of induced Tregs, mainly in the samples where no TGF-β was added (Fig. 3B), which might suggest that GMSC sEVs contain more TGF-β and/or similar regulatory proteins than BMSC sEVs. The reported transcriptomic analysis of GMSC EVs confirms the presence of TGF-β together with other growth factors such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) in these EVs [34]. However, transcripts encoding IL-10 were not found in GMSC EVs. Our result demonstrates, GMSC sEVs were still able to affect the expression profile of T cells. In contrast to BMSC sEVs, GMSC sEVs significantly decreased the production of pro-inflammatory IFN-γ by T cells, while they only slightly increased the production of anti-inflammatory IL-10 (Fig. 3C).
Fig. 3. Effect of sEVs on T cell activation and cytokine production.
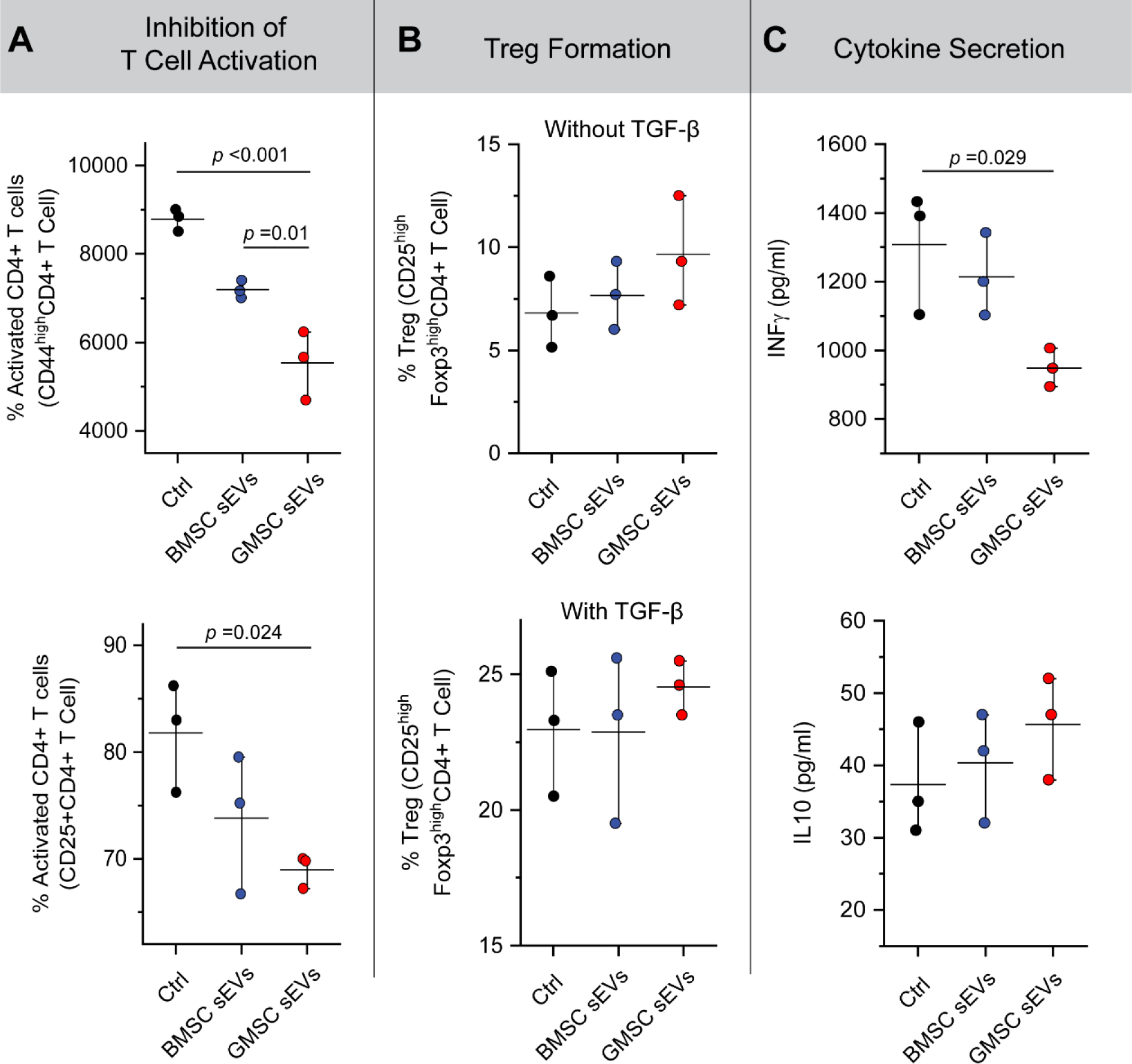
A. Inhibition of T cell activation in the presence of sEVs and particularly GMSC sEVs. B. Effect of BMSC and GMSC sEVs on Treg formation. C. Decrease in production of inflammatory cytokine IFN-γ by CD4+ T cells in the presence of GMSC sEVs and effect of sEVs on the T cell production of anti-inflammatory cytokine IL-10. All data are presented as mean ± SD (n = 3).
To take advantage of the immunomodulatory properties of GMSC sEVs, we designed microparticles for the treatment of periodontitis that would enable improved localization and prolonged presentation of EVs at the site of inflammation as well as release triggered by microenvironmental cues. For this purpose, we immobilized sEVs on the surface of poly(lactic-co-glycolic acid) (PLGA) microparticles with a metalloproteinase-2 (MMP-2)-sensitive linker, which can be cleaved by the metalloproteinases present in the affected tissue at significantly higher concentration than in the normal tissue [35] (Fig. 4A). This bioconjugation increased the size of PLGA microparticles from 4.3+/−1.2 um to 4.5+/− 1.4 um, and also changed the surface charge from −5+/−0.4 mV to −14+/−0.6 mV.
Fig. 4. Design and testing of microenvironment-sensitive microparticles for GMSC sEV delivery.
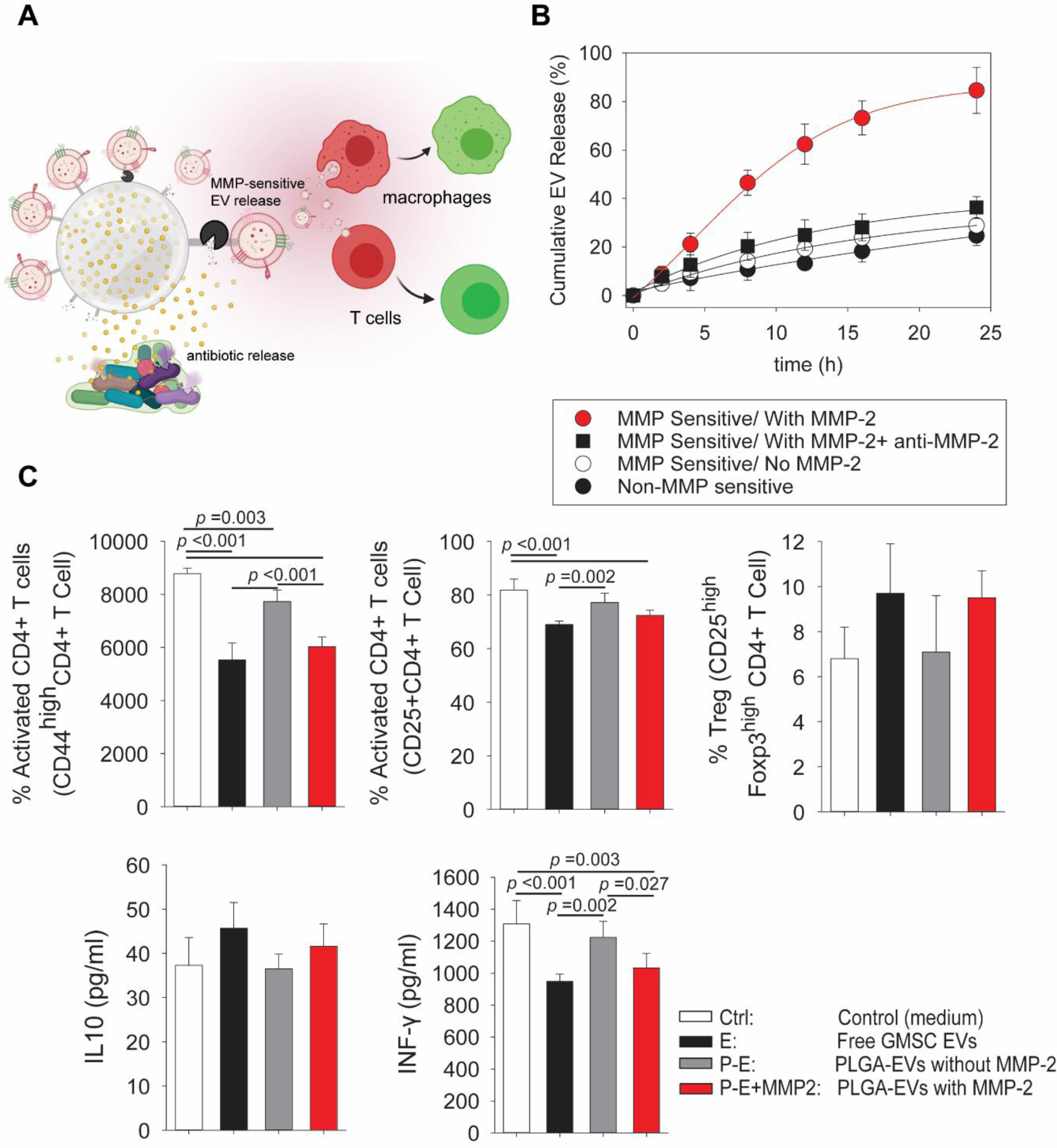
A. Schematics illustrating design of the EV delivery platform. PLGA microparticles release antibiotics to suppress bacterial growth and sEVs are conjugated to the surface of microparticles via an MMP-2-sensitive linker. After their release from the microparticles, sEVs can modulate the behavior of macrophages and T cells present at the site of inflammation. B. Cumulative sEV release from the microparticles in the presence or absence of MMP-2, when MMP-2 is inhibited or when sEVs are conjugated to the particles via a non-MMP-2-sensitive linker. C. Confirmation of GMSC sEV activity on T cells after their release from microparticles by the action of MMP-2 showing a decrease in T cell activation, effect on Treg induction and production of IL-10 by T cells and decrease in the production of pro-inflammatory cytokine IFN-γ by T cells. Ctrl - control medium without addition of microparticles; E – free GMSC sEVs added to the T cell culture; P-E – PLGA microparticles with GMSC sEVs conjugated via MMP-2 sensitive linker without added MMP-2 separated from T cell by 1 μm pore membrane; P-E+MMP2 - PLGA microparticles with GMSC sEVs with MMP-2 added to the culture. All data are presented as mean ± SD (n = 5).
This system was tested in vitro in the presence and absence of soluble MMP-2. We found that in the presence of 10 nM soluble MMP-2, 80% of sEVs bound to the microparticles were released within 24 h, whereas in the samples without MMP-2 or with inhibited MMP-2, only 30% of sEVs were released from the microparticles. When sEVs were conjugated via a non-MMP-2-sensitive linker even less than 30% was released within 24 h (Fig. 4B). These results demonstrate that sEV release rate from microparticles is dependent on the concentration of MMP-2 and the sEV release rate will be therefore regulated by the inflammation stage of the microenvironment and the concentration of MMP-2 in the affected tissue.
The effect of GMSC sEVs released from the microparticles was tested in a co-culture system with microparticles with bound sEVs in the upper chamber separated from T cells by a 1 μm pore membrane. In accordance with the previous results, we sought to see how MMP-responsive release of sEVs from our particles can affect cell fate. sEVs released from the microparticles by the action of MMP-2 were able to suppress CD4+ T cell activation to the same extent as free sEVs, while sEVs bound to the microparticles did not show such powerful T cell suppression (Fig. 4C). Similarly, a slight increase in Treg induction was observed together with enhanced secretion of anti-inflammatory IL-10 after MMP-2-mediated sEV release from microparticles. Moreover, a decrease in IFN-γ production comparable to the effect of free sEVs was seen after MMP-2-mediated sEV release.
In order to suppress the bacterial infection in periodontitis, the microparticles were designed to provide sustained release of antibiotics in addition to sEVs. We found that the prepared PLGA microparticles can encapsulate around 45 ± 6.2 μg of minocycline/mg PLGA. The degradation rate and release profile of the antibiotics, minocycline, were tested for two different microparticle compositions. After 20 days, approximately 20% of the original mass remained from PLGA particles composed of 50% lactide and 50% glycolide, while 40% of the original mass remained from 75:25 (lactide:glycolide) particles (Fig. 5A). Eighty percent of the loaded minocycline was released after two weeks from 50:50 PLGA particles, which was about 15% more than from 75:25 PLGA particles (Fig. 5B).
Fig. 5. Evaluation of microparticle anti-microbial properties.
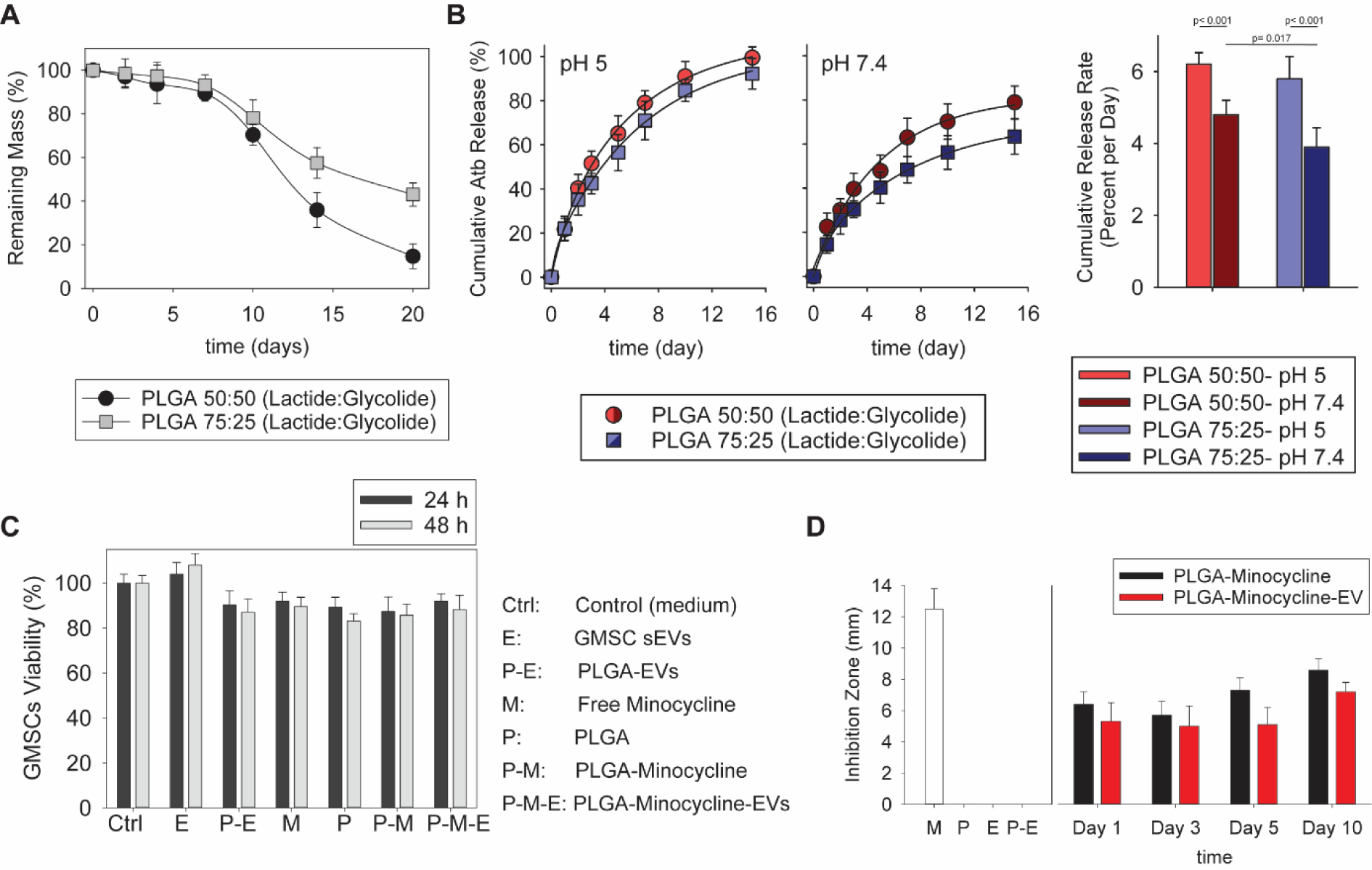
A. Comparison of degradation rate of microparticles with two different ratios of lactide:glycolide. B. Cumulative release of minocycline from microparticles prepared from PLGA with ratios of 50:50 and 75:25 at pH 5 and 7.4. C. Evaluation of microparticle cytotoxicity in the co-culture with GMSCs. D. Comparison of antibacterial properties of PLGA-based microparticles with and without GMSC sEVs against Aggregatibacter actinomycetemcomitans (Aa), Ctrl – control (medium); E – free GMSC sEVs; P-E – PLGA microparticles with GMSC sEVs; M – free minocycline; P – PLGA particles; P-M – PLGA microparticles loaded with minocycline; P-M-E – PLGA microparticles with GMSC sEVs and loaded with minocycline. All data are presented as mean ± SD (n = 5).
There are reports showing that salivary pH slightly decreases in patients with gingivitis and periodontitis in comparison to healthy individuals [36]. However, other studies reported that pH of gingival crevices and periodontal pockets in people with periodontitis does not differ from healthy individuals [37]. It has also been reported that optimal pH for growth of periodontal disease associated microorganisms like P. gingivalis and A. actinomycetemcomitans is 5.0–7.0 [38] [39]. To study the behavior of designed microparticle formulations at disease-relevant pH values, minocycline release experiments were performed at acidic pH 5 and neutral pH 7.4 and release rate was calculated as shown in Fig. 5B. Although, the PLGA particles with a 50:50 lactide:glycolide are showing faster antibiotic release rate at neutral pH (p<0.05), the release rates at acidic pH are quite similar (p>0.05).
For further studies, we decided to use the PLGA particles with a 50:50 lactide:glycolide ratio due to their possible faster degradation rate and release kinetics. The biocompatibility of the loaded microparticles was tested by evaluating the viability of GMSCs co-cultured with the particles, which was above 85% in all the samples (Fig. 5C). The efficiency of the microparticles loaded with minocycline in suppressing bacterial growth was evaluated by a zone of inhibition test with gram-negative Aggregatibacter actinomycetemcomitans (Aa), the bacterium most commonly known to cause periodontitis [40]. Particles alone or particles with sEVs were not able to stop the bacterial proliferation, while particles loaded with antibiotics with or without bound sEVs were able to maintain the inhibition zone for ten days (Fig. 5D).
The functionality of the sEV-modified microparticles was also evaluated in vivo using a rat model of periodontitis. Particles were injected into the created periodontal defect and the effect of the treatment was analyzed after 8 weeks (Fig. 6A). Consequently, buccal and palatal tissues of maxillary molars were isolated and dissociated. The bacterial level at the site of the defect was measured as Aa DNA content. PLGA microparticles alone (blank) did not show any effect on the reduction of bacterial biofilm. Bacterial contamination was significantly reduced in tissue treated with the microparticles loaded with minocycline, and also those that were further modified with GMSC sEVs, here labeled as “Full”. Soluble minocycline and GMSC sEVs were not able to reduce the bacterial contamination so efficiently as the antibiotic-loaded microparticles (Fig. 6B).
Fig. 6. In vivo evaluation of the immunomodulatory platform in a rat model of periodontitis.
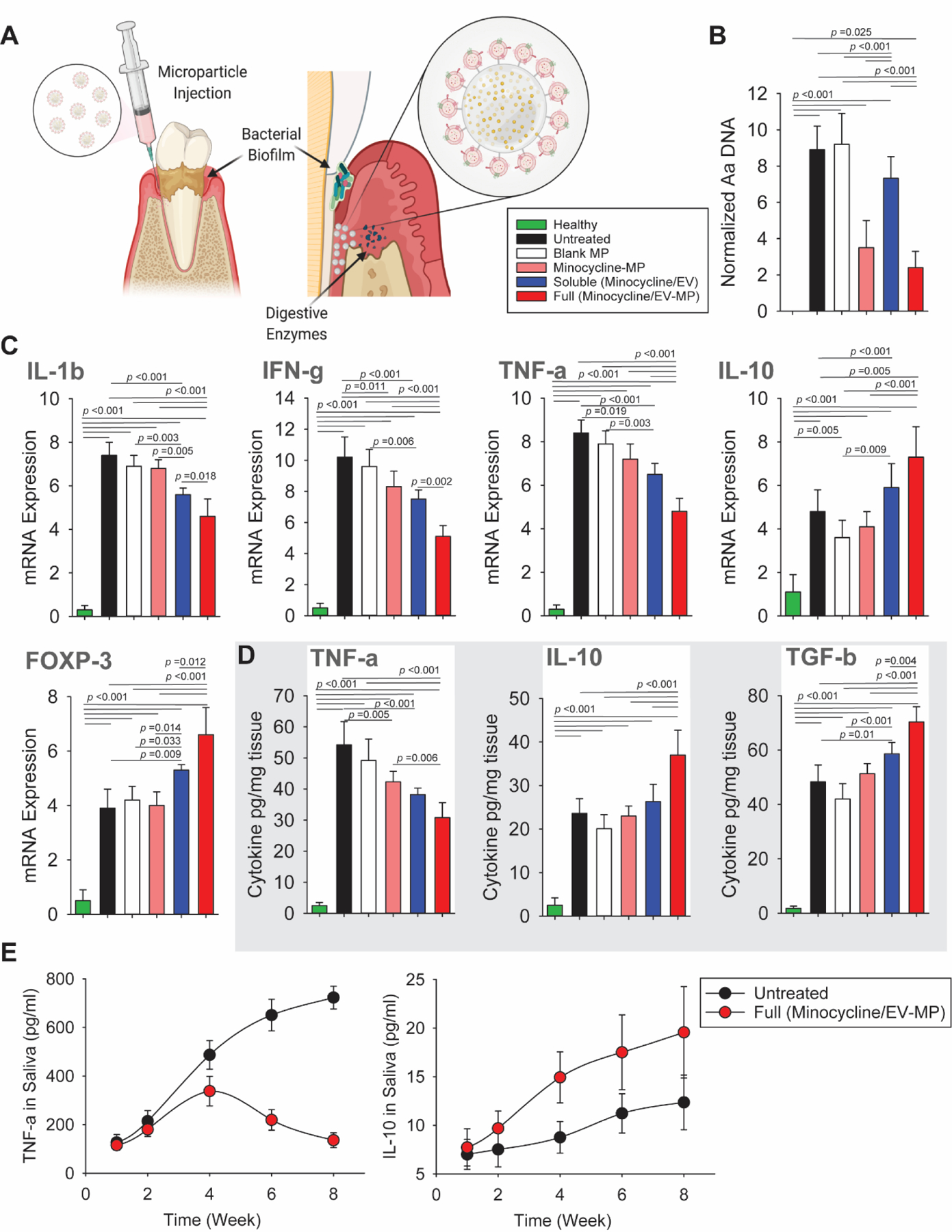
A. Schematics of administration of microparticles loaded with antibiotics and GMSC sEVs connected to the particles via an MMP-2-sensitive linker. B. Comparison of the effect of sEV-microparticles on the suppression of bacterial growth evaluated 8 weeks after microparticle administration. C. Relative mRNA expression levels of pro-inflammatory (IL-1β, IFN-γ, TNF-α) and anti-inflammatory (IL-10) cytokines in the periodontal tissue. FOXP3 mRNA level increased the most significantly in the group treated with the full microparticles containing both GMSC sEVs and minocycline. D. Evaluation of cytokine concentration in the periodontal tissue. Healthy – healthy rats; Untreated – untreated periodontitis; Blank – PLGA microparticles; Minocycline-MP – minocycline-loaded microparticles; Soluble –minocycline and GMSC sEVs administered in solution; Full – PLGA microparticles with GMSC sEVs and loaded with minocycline. All data are presented as mean ± SD (n = 5). E. Concentration of TNF-α and IL-10 in the saliva during 8 weeks after the GMSC sEV-microparticle administration.
The expression of several genes related to immune response was evaluated. In the untreated group or in the group treated just with PLGA microparticles, the pro-inflammatory cytokines such as IL-1β, IFN-γ and TNF-α were significantly upregulated. Application of minocycline-loaded microparticles led only to a slight decrease in expression of these cytokines. Treatment with soluble minocycline and GMSC sEVs further decreased the mRNA expression of the pro-inflammatory cytokines in the periodontal tissue, but the best results were obtained when full particles containing both the antibiotics and GMSC sEVs were used (Fig. 6C). Full particles were also able to upregulate the expression of anti-inflammatory IL-10 and FOXP3 more significantly than the other treatment groups. The elevated FOXP3 expression indicates increased induction of Tregs at the site of the defect by full particles compared to soluble GMSC sEVs and antibiotics. These results were also confirmed by ELISA, which showed a decrease in the inflammatory cytokine TNF-α in the group treated with full microparticles as compared to the untreated group and the groups treated with blank microparticles and microparticles loaded with minocycline (Fig. 6D). We also confirmed an increased concentration of IL-10 and TGF-β, which is necessary for Treg induction, in the periodontal tissue of animals treated with full particles. The concentration per mg of tissue was higher than in a group treated with a soluble minocycline and GMSC sEVs and other treatment groups. The regeneration process was also monitored by measuring TNF-α and IL-10 levels in rat saliva over the course of 8 weeks (Fig. 6E). The level of TNF-α peaked around week 4 after the GMSC sEV-microparticles administration and then gradually decreased while in the untreated group the concentration of this inflammatory cytokine kept on increasing. On the contrary, the level of IL-10 in rat saliva increased significantly more in the group treated with full particles then in the untreated group.
Microcomputed tomography (μCT) was performed to assess the effect of treatment on the amount of regenerated bone at the defect site. Fig. 7A shows μCT images after 8 weeks of treatment. The alveolar bone gain at each defect site was determined from the μCT scans as bone area relative to the healthy control. The alveolar bone area was significantly increased in the group treated with full microparticles containing GMSC sEVs, which didn’t differ from the healthy control. On the contrary, the other treatment groups including blank group treated with only PLGA microparticles, group treated with minocycline-loaded microparticles, or soluble minocycline and GMSC sEVs did not show significant improvement (Fig. 7B). Moreover, the group treated with full microparticles containing antibiotics and GMSC sEVs also showed significant reduction in the distance between the alveolar bone crest and cementoenamel junction (CEJ), which was not significantly different from the healthy control (Fig. 7C). The other treatment groups did not show such a significant improvement. Furthermore, increased expression of osteogenic markers BMP2 and RUNX2 together with elevated levels of late osteogenic marker osteocalcin (OCN) in the group with full microparticles containing GMSC sEVs indicates increased osteoblast differentiation and bone formation (Fig. 7D). This also correlated with elevated type 1 collagen expression, suggesting deposition of new extracellular matrix mainly in the group treated with full microparticles followed by the group treated with soluble minocycline and GMSC sEVs. No significant increase in COL1 expression was observed in tissues treated with blank microparticles or microparticles loaded with minocycline compared to untreated group.
Fig. 7. Evaluation of the effect of GMSC sEV microparticles on the regeneration of periodontal tissue.
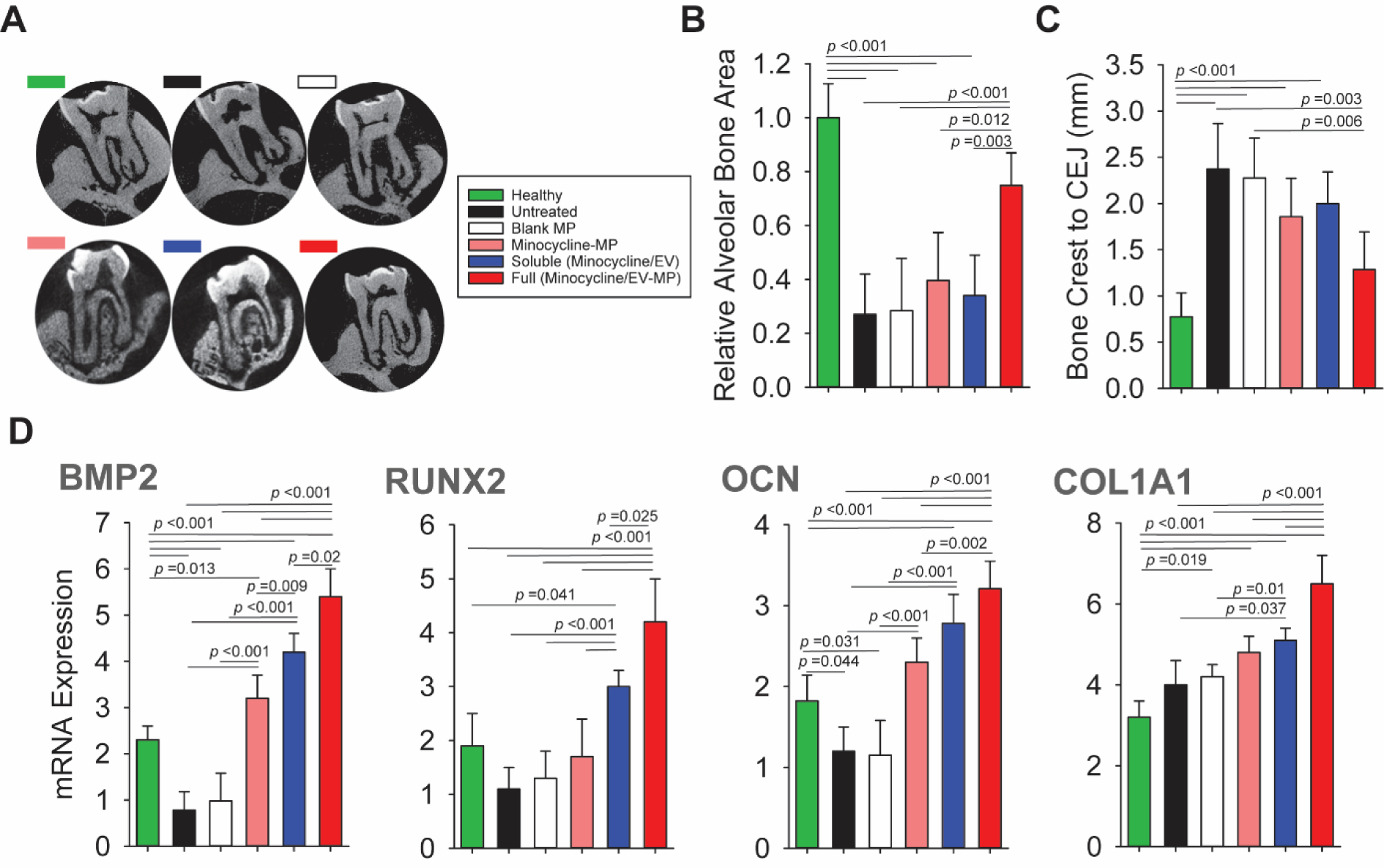
A. μCT images after 8 weeks of treatment. B. Evaluation of relative alveolar bone area in different treatment groups. C. Comparison of the distance between the alveolar bone crest and cementoenamel junction (CEJ) D. Relative mRNA expression levels of BMP2, RUNX2, OCN and COL1A1 in the periodontal tissue. Healthy – healthy rats; Untreated – untreated periodontitis; Blank – PLGA microparticles; Minocycline-MP – minocycline-loaded microparticles; Soluble –minocycline and GMSC sEVs administered in solution; Full – PLGA microparticles with GMSC sEVs and minocycline. All data are presented as mean ± SD (n = 5).
The short-term half-life of therapeutic EVs in the body is recognized as one of major hurdles impeding their clinical translation. EVs delivered in a solution by injection disappear quickly from the circulation [25] [41]. Higher dosage and frequent administration are generally necessary to compensate for the rapid clearance of EVs from the body [42] [43]. Biomaterial-assisted delivery of EVs can help to solve this problem and to achieve enhanced therapeutic outcomes. Biomaterials can localize EVs to the affected area and enable their prolonged presentation and controlled release, which should allow a reduction in the amount of EVs necessary to produce the intended therapeutic effect while preventing off-target side effects. So far, mainly simple delivery methods such as EV encapsulation in different types of hydrogels [21] [24] have been used to address this issue. However, in the hydrogel-delivery systems, EVs need to be premixed with a hydrogel immediately before use otherwise they might be released from the hydrogel already during the storage. Moreover, the hydrogel might mask the surface of EVs and affect their bioactivity. We tried to address these issues by covalently conjugating EVs to microparticles using an enzyme-sensitive linker. Unless the enzyme is present in the solution, EVs are bound to the microparticles. EVs conjugated to the particle surface are also better accessible to cells. The biological activity of GMSC sEVs after their release from the microparticle delivery platform was confirmed by functional tests with T cells that showed that their immunomodulatory properties were not affected by the conjugation via MMP-2-sensitive linker.
So far it is not clear what is the optimal period during which the therapeutic EVs should be released to induce tissue regeneration. In this study, we engineered microparticles enabling microenvironment-sensitive release of GMSC sEVs and controlled immunomodulation based on the concentration of MMP-2 in the damaged tissue. With this approach, the kinetics of EV release is adjusted to the inflammatory state of the affected tissue that might differ from patient to patient. We were able to achieve enhanced regeneration of periodontal tissue after a single administration of EV-decorated microparticles.
In vivo data on the use of EV-based therapeutics in the field of periodontal and dental diseases are scarce. Conditioned media (CM) from GMSCs and periodontal ligament-derived mesenchymal stem cells (PDLSCs) were shown to support periodontal regeneration by decreasing the levels of proinflammatory cytokines (TNF-α and IL-1β) in the tissue and increasing the expression of anti-inflammatory IL-10, which was significantly elevated mainly in the GMSC CM-treated group [44]. However, concentrated medium conditioned by cells is even more complex mixture of bioactive factors than purified EVs. CM might also contain many other components such as free growth factors or ECM proteins and therefore it might be harder to determine the key bioactive components that should be measured during the quality testing of the cell-free therapeutics. We demonstrated that purified GMSC sEVs can alleviate inflammation and promote anti-inflammatory and immunosuppressive macrophages and T cells, thus helping to re-establish the tissue homeostasis. In combination with antibiotic delivery, this immunomodulatory dual delivery platform showed promising capabilities to facilitate regeneration of tissue affected by periodontitis.
Conclusion
EV-based therapeutics offer the complexity of bioactive factors contained in cell secretome while being easier to handle and control than viable cells. Here, we showed that GMSC sEVs can be obtained in larger quantities than BMSC sEVs and that they have powerful immunomodulatory properties. GMSC sEVs can polarize both innate and adaptive immune cells into anti-inflammatory phenotypes. To harness the immunomodulatory power of GMSC sEVs to treat periodontal disease, we engineered PLGA microparticles for dual delivery of GMSC sEVs and antibiotics. The immobilization of sEVs to the microparticles via MMP2-sensitive linker enabled localization and prolonged presentation of sEVs at the site of tissue damage, which led to the enhanced periodontal tissue regeneration.
Supplementary Material
Acknowledgments
Funding: This work was supported by grants from the National Institute of Dental and Craniofacial Research (DE029876 to A.M. and 1R56DE029157 to S.L.).
Footnotes
Competing interests: The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Jana Zarubova, Department of Bioengineering, University of California, 420 Westwood Plaza, 5121 Engineering V, Los Angeles, CA 90095-1600, United States; Department of Biomaterials and Tissue Engineering, Institute of Physiology of the Czech Academy of Sciences, Prague, 14220, Czech Republic.
Mohammad Mahdi Hasani-Sadrabadi, Department of Bioengineering, University of California, 420 Westwood Plaza, 5121 Engineering V, Los Angeles, CA 90095-1600, United States.
Erfan Dashtimighadam, Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC, 27599-3290 United States.
Xuexiang Zhang, Department of Bioengineering, University of California, 420 Westwood Plaza, 5121 Engineering V, Los Angeles, CA 90095-1600, United States.
Sahar Ansari, Weintraub Center for Reconstructive Biotechnology, Division of Advanced Prosthodontics, School of Dentistry, University of California, Los Angeles, California 90095, United States.
Song Li, Department of Bioengineering, University of California, 420 Westwood Plaza, 5121 Engineering V, Los Angeles, CA 90095-1600, United States.
Alireza Moshaverinia, Weintraub Center for Reconstructive Biotechnology, Division of Advanced Prosthodontics, School of Dentistry, University of California, Los Angeles, California 90095, United States.
References
- [1].Nazir MA, Int J Health Sci (Qassim) 2017, 11, 72. [PMC free article] [PubMed] [Google Scholar]
- [2].Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W, Journal of dental research 2014, 93, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cekici A, Kantarci A, Hasturk H, Van Dyke TE, Periodontol 2000 2014, 64, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bostanci N, Mitsakakis K, Afacan B, Bao K, Johannsen B, Baumgartner D, Müller L, Kotolová H, Emingil G, Karpíšek M, Scientific Reports 2021, 11, 6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Di Benedetto A, Gigante I, Colucci S, Grano M, Clinical and Developmental Immunology 2013, 2013, 503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Campbell L, Millhouse E, Malcolm J, Culshaw S, Molecular Oral Microbiology 2016, 31, 445. [DOI] [PubMed] [Google Scholar]
- [7].Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, Iwakura Y, Nakashima T, Okamoto K, Takayanagi H, Nature Communications 2018, 9, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Haffajee AD, Socransky SS, Gunsolley JC, Annals of periodontology 2003, 8, 115. [DOI] [PubMed] [Google Scholar]
- [9].Mombelli A, Samaranayake LP, International Dental Journal 2004, 54, 3. [DOI] [PubMed] [Google Scholar]
- [10].Zhang QZ, Nguyen AL, Yu WH, Le AD, J Dent Res 2012, 91, 1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang M, Xie J, Wang C, Zhong D, Xie L, Fang H, Stem Cells International 2020, 2020, 9836518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fawzy El-Sayed KM, Dörfer CE, Stem cells international 2016, 2016, 7154327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC, Wani MR, Biochemical and Biophysical Research Communications 2010, 393, 377. [DOI] [PubMed] [Google Scholar]
- [14].Yu X, Ge S, Chen S, Xu Q, Zhang J, Guo H, Yang P, Cells Tissues Organs 2013, 198, 428. [DOI] [PubMed] [Google Scholar]
- [15].Hasani-Sadrabadi MM, Sarrion P, Pouraghaei S, Chau Y, Ansari S, Li S, Aghaloo T, Moshaverinia A, Science Translational Medicine 2020, 12, eaay6853. [DOI] [PubMed] [Google Scholar]
- [16].Wiklander OPB, Brennan MÁ, Lötvall J, Breakefield XO, EL Andaloussi S, Science Translational Medicine 2019, 11, eaav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gholami L, Nooshabadi VT, Shahabi S, Jazayeri M, Tarzemany R, Afsartala Z, Khorsandi K, Cell & Bioscience 2021, 11, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawai T, Katagiri W, Osugi M, Sugimura Y, Hibi H, Ueda M, Cytotherapy 2015, 17, 369. [DOI] [PubMed] [Google Scholar]
- [19].Mohammed E, Khalil E, Sabry D, Biomolecules 2018, 8, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hua S, Bartold PM, Gulati K, Moran CS, Ivanovski S, Han P, Nanomaterials (Basel) 2021, 11, 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, Lin Z, Bioactive materials 2020, 5, 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M, Tissue Engineering Part A 2012, 18, 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chu C, Wei S, Wang Y, Wang Y, Man Y, Qu Y, Journal of Biomedical Materials Research Part A 2019, 107, 243. [DOI] [PubMed] [Google Scholar]
- [24].Brennan MÁ, Layrolle P, Mooney DJ, Advanced Functional Materials 2020, 30, 1909125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gupta D, Liang X, Pavlova S, Wiklander OPB, Corso G, Zhao Y, Saher O, Bost J, Zickler AM, Piffko A, Maire CL, Ricklefs FL, Gustafsson O, Llorente VC, Gustafsson MO, Bostancioglu RB, Mamand DR, Hagey DW, Görgens A, Nordin JZ, El Andaloussi S, Journal of Extracellular Vesicles 2020, 9, 1800222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moshaverinia A, Chen C, Akiyama K, Xu X, Chee WW, Schricker SR, Shi S, J Biomed Mater Res A 2013, 101, 3285. [DOI] [PubMed] [Google Scholar]
- [27].Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, Shi S, Tissue Eng Part A 2014, 20, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fordjour FK, Daaboul GG, Gould SJ, bioRxiv 2019, DOI: 10.1101/545228545228. [DOI] [Google Scholar]
- [29].Graves DT, Oates T, Garlet GP, Journal of Oral Microbiology 2011, 3, 5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Figueredo CM, Lira-Junior R, Love RM, Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y, Journal of Extracellular Vesicles 2015, 4, 26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, Harlepp S, Mary B, Bauer J, Mercier L, Busnelli I, Lefebvre O, Fekonja N, Garcia-Leon MJ, Machado P, Delalande F, López AA, Silva SG, Verweij FJ, van Niel G, Djouad F, Peinado H, Carapito C, Klymchenko AS, Goetz JG, Developmental Cell 2019, 48, 554. [DOI] [PubMed] [Google Scholar]
- [33].Ha DH, Kim H-K, Lee J, Kwon HH, Park G-H, Yang SH, Jung JY, Choi H, Lee JH, Sung S, Yi YW, Cho BS, Cells 2020, 9, 1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Silvestro S, Chiricosta L, Gugliandolo A, Pizzicannella J, Diomede F, Bramanti P, Trubiani O, Mazzon E, Genes (Basel) 2020, 11, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mäkelä M, Salo T, Uitto VJ, Larjava H, J Dent Res 1994, 73, 1397. [DOI] [PubMed] [Google Scholar]
- [36].Vprb RR, Thamaraiselvan M, Journal of Research in Medical and Dental Science 2020, 8, 423. [Google Scholar]
- [37].Eggert FM, Drewell L, Bigelow JA, Speck JE, Goldner M, Archives of Oral Biology 1991, 36, 233. [DOI] [PubMed] [Google Scholar]
- [38].Takahashi N, Schachtele CF, J Dent Res 1990, 69, 1266. [DOI] [PubMed] [Google Scholar]
- [39].Takahashi N, Saito K, Schachtele CF, Yamada T, Oral microbiology and immunology 1997, 12, 323. [DOI] [PubMed] [Google Scholar]
- [40].Fine DH, Patil AG, Velusamy SK, Frontiers in Immunology 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO, ACS Nano 2014, 8, 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Porzionato A, Zaramella P, Dedja A, Guidolin D, Wemmel KV, Macchi V, Jurga M, Perilongo G, Caro RD, Baraldi E, Muraca M, American Journal of Physiology-Lung Cellular and Molecular Physiology 2019, 316, L6. [DOI] [PubMed] [Google Scholar]
- [43].Bonafede R, Turano E, Scambi I, Busato A, Bontempi P, Virla F, Schiaffino L, Marzola P, Bonetti B, Mariotti R, International journal of molecular sciences 2020, 21, 3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Qiu J, Wang X, Zhou H, Zhang C, Wang Y, Huang J, Liu M, Yang P, Song A, Stem cell research & therapy 2020, 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ansari S, Sarrion P, Hasani-Sadrabadi MM, Aghaloo T, Wu BM, Moshaverinia A, Journal of biomedical materials research. Part A 2017, 105, 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lutolf M, Lauer-Fields J, Schmoekel H, Metters AT, Weber F, Fields G, Hubbell JA, Proceedings of the National Academy of Sciences 2003, 100, 5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wade RJ, Bassin EJ, Rodell CB, Burdick JA, Nature communications 2015, 6, 6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Malek-Khatabi A, Javar HA, Dashtimoghadam E, Ansari S, Hasani-Sadrabadi MM, Moshaverinia A, Acta Biomaterialia 2020, 108, 326. [DOI] [PubMed] [Google Scholar]
- [49].Yao W, Xu P, Pang Z, Zhao J, Chai Z, Li X, Li H, Jiang M, Cheng H, Zhang B, International journal of nanomedicine 2014, 9, 3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hasani-Sadrabadi MM, Majedi FS, Bensinger SJ, Wu BM, Bouchard LS, Weiss PS, Moshaverinia A, Adv Mater 2018, 30, e1706780. [DOI] [PubMed] [Google Scholar]
- [51].Hasani-Sadrabadi MM, Sarrion P, Nakatsuka N, Young TD, Taghdiri N, Ansari S, Aghaloo T, Li S, Khademhosseini A, Weiss PS, ACS nano 2019, 13, 3830. [DOI] [PubMed] [Google Scholar]
- [52].Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, Little SR, Proceedings of the National Academy of Sciences 2013, 110, 18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, Iwakura Y, Nakashima T, Okamoto K, Takayanagi H, Nature communications 2018, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Glowacki AJ, Yoshizawa S, Jhunjhunwala S, Vieira AE, Garlet GP, Sfeir C, Little SR, Proc Natl Acad Sci U S A 2013, 110, 18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


