Abstract
Background:
In mouse skin cancer models, high-dose oral vitamin D3 (VD3; cholecalciferol) combined with photodynamic therapy (PDT) can improve the clearance of squamous precancers (actinic keratoses, AK).
Objective:
To determine whether oral VD3 can improve clinical efficacy of a painless PDT regimen in humans with AK.
Methods:
Baseline lesion counts and serum 25OH-D3 levels were obtained. In one cohort (Group 1), 29 patients received gentle debridement and 15-min ALA preincubation followed by blue light (30 min; 20 J/cm2). In Group 2, 29 patients took oral VD3 (10,000 IU daily, 5 or 14 days) prior to the debridement/PDT. Lesion clearance was assessed at 3–6 months.
Results:
In Group 1, mean clearance rates (CR) of facial AK were lower in patients with VD3 deficiency (25OH-D3 <31 ng/dL; CR 40.9 ± 42%) than in patients with normal 25OH-D3 levels (62.6 ± 14.2%). High dose VD3 supplementation (Group 2) significantly improved overall AK lesion response (72.5 ± 13.6%) compared to Group 1 (54.4 ± 22.8%). No differences in side effects were noted.
Limitations:
Nonrandomized trial design (interventional cohort matched to registry-based controls).
Conclusion:
Oral VD3 pretreatment significantly improves AK clinical responses to PDT. The regimen appears promising and well tolerated.
Keywords: Vitamin D, photodynamic therapy, actinic keratosis, skin cancer, therapeutics, oncology, phototherapy, clinical research
INTRODUCTION:
In photodynamic therapy (PDT), a prodrug 5-aminolevulinic acid (ALA) is selectively taken up by precancerous skin cells which convert it into protoporphyrin IX (PpIX), which is activated by either blue or red light1. For widespread actinic keratoses, PDT compares favorably with cryotherapy2 and topical 5-fluorouracil3, 4 in terms of lesion clearance, shorter treatment duration, and relatively rapid recovery times. Yet nearly twenty years after FDA approval, blue light PDT is still not as widely accepted in the USA as one might expect, given its potential benefits. This may reflect an apparent disconnect between known clinical benefits and negative perceptions (pain for patients, and inconvenience for providers). A major negative factor is the stinging pain associated with the original blue light PDT protocol that specified a 14–18 hour incubation time with ALA5. Practitioners have gradually reduced ALA incubation times to 1 – 4 hours to reduce pain while still maintaining reasonable efficacy6, 7, but fear of intraprocedural pain and post-treatment inflammation continues to keep many patients away8, 9. Also, efficacy of blue light PDT is not totally optimal when performed using 1- to 3-hour ALA incubation; median AK clearance rates on the face and scalp are only 50–60% after a single treatment, which improves to 65–75% if a second treatment is performed 8 weeks after the first6. Similar findings are observed with red light PDT, explaining why European guidelines typically recommend a second PDT session after 3 months10.
Our long-term research goals are to find ways to reduce pain experienced by AK patients, while simultaneously enhancing therapeutic responsiveness to blue light PDT. To achieve the first goal, we recently reported a new blue light regimen in which illumination begins within minutes after applying the photosensitizer, similar to “daylight PDT”11 but instead of sunlight, the protocol uses standardized artificial blue light (30 minutes)12. With this simultaneous incubation/illumination regimen, lesion clearance rates were indistinguishable from a more traditional regimen (1 h ALA incubation), yet the treatment experience was essentially pain free12. With either the new or old regimens, lesion clearance rates at 3 months after treatment were ~55% for the face and ~45% for the scalp after one treatment session12.
The next challenge is to improve the clinical response. Vitamin D3 (VD3) is a steroid hormone that regulates bone and calcium metabolism, and may regulate skin cancer susceptibility13. In preclinical work, we had shown that VD3 increases PpIX production in mitochondria, thereby enhancing PDT-mediated killing of cancer cells in murine skin cancer models14–16. This was true whether Vitamin D3 was delivered topically17 or orally18. Vitamin D3 has an outstanding safety profile19–21, making it a strong candidate as a potential neoadjuvant for PDT. A clinical trial in Brazil showed that topical calcipotriol (synthetic vitamin D analog), when applied to AK lesions on half the scalp for 15 days prior to red light PDT, led to greater PpIX accumulation and lesion clearance compared to the contralateral side22. In the United States, however, topical calcipotriol is FDA-approved only for psoriasis, not for skin cancer/precancer, and is therefore not covered by medical insurers. As an alternative to topical calcipotriol, we report here the results of a clinical trial examining whether oral VD3 (cholecalciferol) might be useful as a neoadjuvant to boost blue light PDT efficacy in patients with AK.
METHODS:
Study Design
The study comprised two groups. For the interventional group (Group 2), subjects were enrolled in a prospective, nonrandomized trial in which AK patients received painless PDT after pretreatment with high-dose oral VD3 (VDAK; ClinicalTrials.gov NCT04140292). For the control group (Group 1), patients were selected by multivariate matching from a large longitudinal database registry in which patients undergoing ‘painless’ PDT12 had given blood samples for measurement of baseline serum 25OH-D3 levels and Vitamin D receptor status as possible correlates of clinical responsiveness to PDT (BIOMARKERS; ClinicalTrials.gov NCT03319251). Both studies were conducted at a single academic medical center (Cleveland Clinic) after IRB approval. Patients in the BIOMARKERS registry were enrolled from October 2017 to October 2021, and in VDAK from October 2019 to October 2020.
Study Population
Eligible patients were males and nonpregnant females, 18 years or above, with 10 or more Grade 1 or 2 AKs on the face or the scalp (Grade 1: slightly palpable, better felt than seen; Grade 2: moderately thick, easily seen and felt). Use of tetracyclines or topical retinoids, and elevated risk for hypercalcemia were additional criteria for exclusion. More information about study eligibility is provided in Supplementary Materials.
Randomization
Patients were serially recruited. No randomization was required.
Interventions
Procedures used for Group 1 and Group 2 were identical, except that Group 2 patients also received oral VD3 pretreatment. Blood was drawn at Visit #1 to analyze vitamin D in serum (25OH-D3; calcidiol) and to collect leukocytes for vitamin D receptor gene analyses (for reporting at a future date). A vitamin D-relevant history was taken. Nonhypertrophic AK lesions (Olsen grade 1 or 2)23 on the face and/or scalp were identified using visual inspection and palpation by one of two dermatologists (EM or CW) who met initially to calibrate their AK diagnostic criteria. Each lesion was marked with a pen to facilitate accurate recording of counts, backed up by photographs. In balding patients, the original hairline was regarded as the boundary between face and scalp.
Following cleansing of the skin with isopropyl alcohol, all gritty AK lesions were rubbed with moist sandpaper (3M Red Dot Trace Prep tape, Cat. No. 2236). ALA 20% solution (Levulan Kerastick, Sun/DUSA Pharmaceuticals, Wilmington MA) was applied broadly to the entire face and/or scalp and allowed to incubate for 15 min. The patient was placed under blue light (Blu-U, Sun/DUSA Pharmaceuticals, 10 mW/cm2) for 30 min, and sent home with aftercare instructions1. Patients returned for repeat AK counting and photography at 3 to 6 months after treatment.
Group 2 (VDAK) patients had three study visits; see Figure 1. At Visit #1, blood was drawn for serum Vitamin D measurements. After counting of AK lesions, the patient was sent home with 14 vitamin VD3 capsules (10,000 IU; soft gel capsules, Now Foods brand; VD3 content was independently verified by Heartland Assays, Ames, Iowa). If the patient’s serum 25OH-D3 level was normal, instructions were given to take 1 capsule daily for 5 days before PDT treatment. If the 25OH-D3 level was low (< 31 ng/dL), patients were instructed to pretreat for 14 days, based upon literature evidence that doses of VD3 required to normalize Vitamin D deficiency are higher than doses to maintain sufficiency19, 20. Patients received PDT at Visit #2, and follow-up AK counts were performed at Visit #3.
Fig. 1.
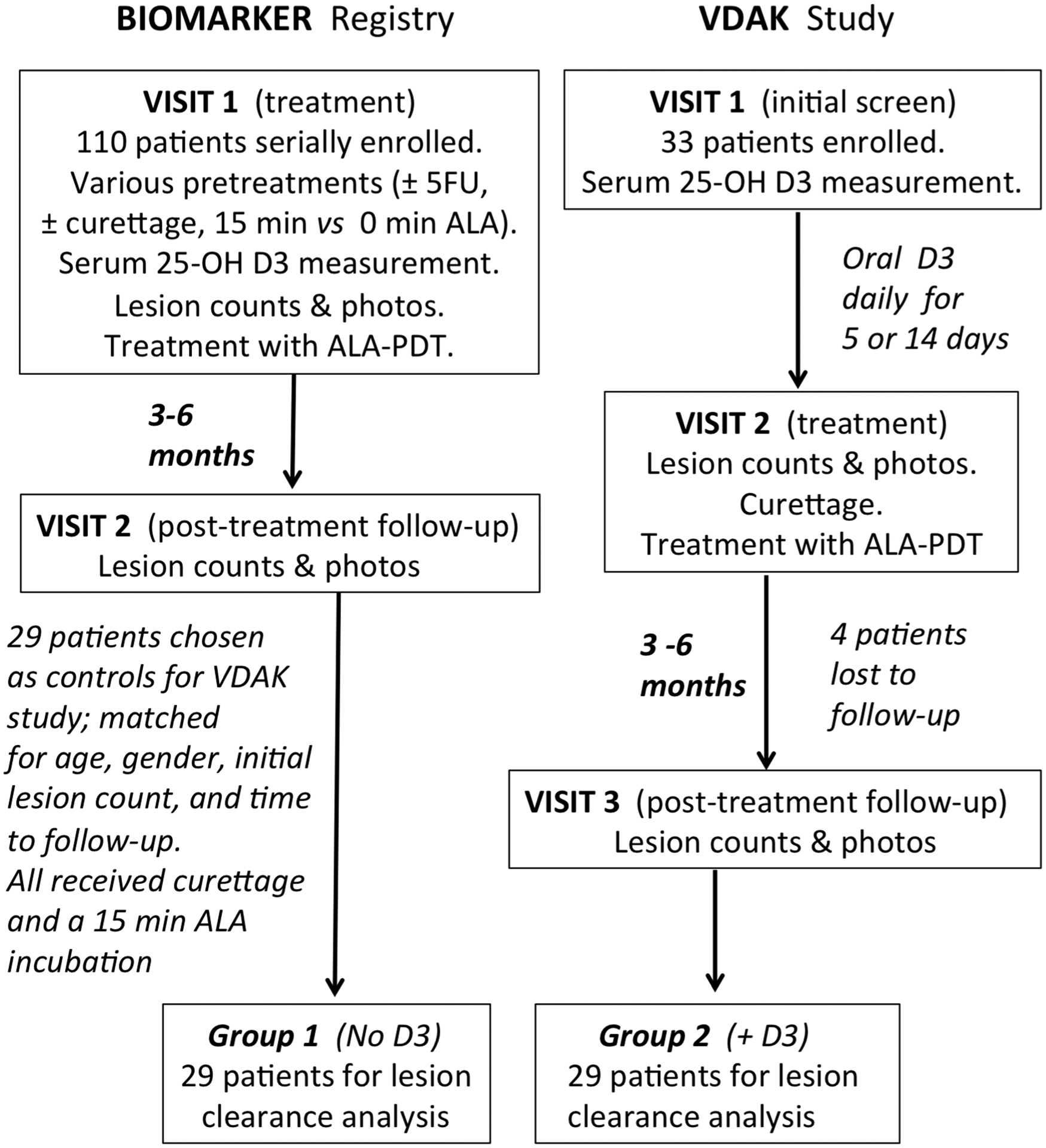
Patient enrollment in the registry study and the interventional study.
Outcome Measures
The primary endpoint was therapeutic efficacy (AK lesion clearance), defined as percent decrease in AK lesion counts between the first and last visit. Secondary endpoints were pain during treatment, and side effects experienced during and after treatment. During PDT illumination, patients reported their pain on a 0 to 10 visual-analog (VAS) scale. At the final follow-up visit, patients were asked (Yes/No) whether they had experienced each of the side effects listed in Supplemental Figure 1 during the week after PDT. The ‘Yes’ answers were added to yield a Side Effects Score (SES) for each patient.
Statistical Analysis
The primary endpoint was AK lesion reduction (% decrease from initial counts). Patients receiving neoadjuvant VD3/PDT (Group 2) were matched to those receiving PDT alone (Group 1) in a 1:1 ratio based upon the baseline 25OH-D3 values, then stratified into two groups (normal, >31 ng/dL; or deficient, <31 ng/dL). Within each stratum, optimal matching was performed by considering 25OH-D3 as a continuous variable. Within each matched dataset, linear regression was used to compare the percent decrease of lesion numbers on face, scalp and both areas, respectively, while adjusting for age, sex, pre-treatment lesion counts, and length of time to follow-up. Statistical significance was established at two-sided p-value < 0.05.
Sample Size Justification
The study was powered to evaluate AK lesion clearance. We estimated the variance within our study population by using data from prior clinical studies that examined PDT for AK of the face and scalp12, 24. Assuming the mean clearance rate (CR) was 45%, with SD of 20% three months after PDT, n=17 subjects per group will have 80% power to detect a difference of 20% CR (i.e., 65% CR with neoadjuvant VD3) at two-sided alpha of 0.05. To detect a 15% difference, n=29 subjects per group are needed.
RESULTS
In the interventional study (VDAK, Group 2), 29 out of 33 enrolled patients completed all visits (Figure 1). Four were lost to follow-up due to Covid-19 related issues. Those 29 patients were matched to an equal number of patients from the BIOMARKER registry (Group 1) by multivariate matching as described in Methods. Demographic values were similar between the groups, with no significant differences noted for age (mean > 70 years), gender (predominantly male), average serum 25OH-D3 levels, or initial number of AK lesions (Table 1); see Supplementary Table 1 for all the raw data. Differences in individual follow-up times after PDT could be important because maximal AK clearances are typically observed at 3 months and then begin to climb back to baseline levels by 9–12 months24, 25; we accounted for this in our study using multivariate analysis (see Methods).
Table 1.
Patient demographics, Vitamin D3 status, and AK counts at baseline
| Group 1 | Group 2 | |
|---|---|---|
| (PDT only) | (VD3 + PDT) | |
| Values shown are Mean ± SD | ||
| Age (years) | 71.1 ± 8.2 | 71.1 ± 8.5 |
| Sex (number of males, females) | 26 M, 3 F | 27 M, 2 F |
| Serum 25OH-D3 values (mg/dL) | 42.6 ± 14.9 | 39.3 ± 17.4 |
| Time between PDT and final visit (days) | 111 ± 31 | 124 ± 30 |
| Initial number of AK lesions (face) | 26 ± 18 | 34 ± 21 |
| Initial number of AK lesions (scalp) | 40 ± 32 | 30 ± 21 |
The primary endpoint was clinical efficacy, defined as % reduction in AK lesion counts at 3 to 6 months after a single PDT treatment. Our overall hypothesis was that Vitamin D status is important for the AK lesion clearance response after PDT. We first looked for a relationship between Vitamin D3 serum levels and clearance rate (CR) for facial AK lesions, by arranging patients in order of increasing 25OH-D3 serum concentrations. Results presented in Figure 2 and Table 2 show that VD3 deficient patients had a lower mean CR (40.9 %) than did patients with normal VD3 levels (62.6 %), a difference that is statistically significant (Table 2, Group 1). This finding indicates that normal Vitamin D levels may be required for an optimal therapeutic response. Next, we investigated the effect of transient VD3 supplementation using high dose oral cholecalciferol. As apparent from Table 2, Group 2, administration of high-dose oral VD3 increased the mean CR to 72.5 %, up from 54.4% overall for Group 1. This 18% increase in treatment response is highly significant. We also evaluated AK of the scalp (Supplem Table 2). The results suggest that scalp lesions may not benefit from high-dose VD3 prior to PDT to the same degree as facial AK lesions, although too few patients with scalp AK were available to provide adequate statistical power. Overall, when AK counts from all patients with facial or scalp lesions are combined, the change associated with high-dose VD3/PDT represents an ~11% increase in lesion clearance and is statistically significant (Supplem Table 1).
Fig. 2.
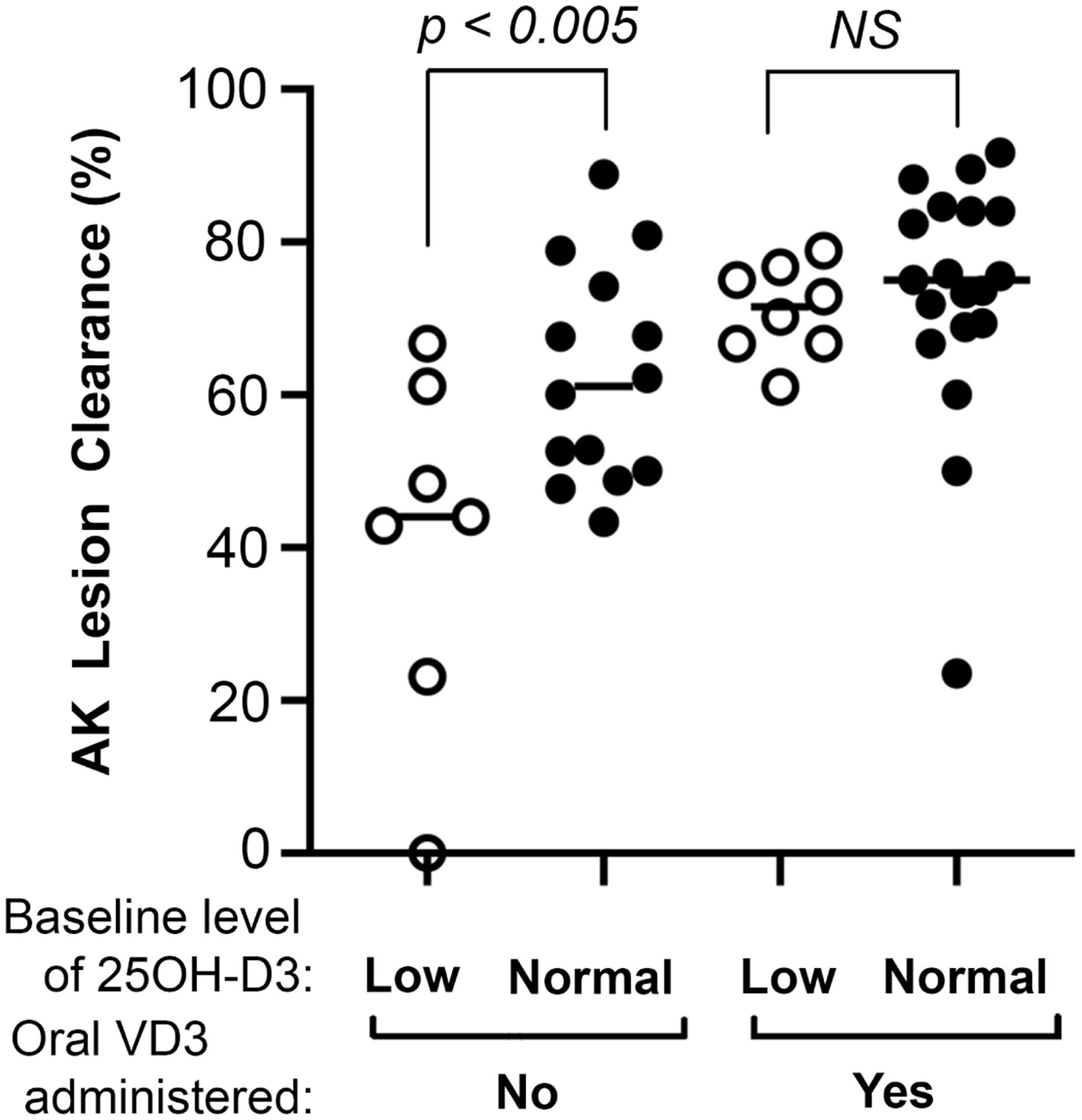
Lesion clearance rates for patients with facial AK. Clearance rates are dependent upon serum 25OH-D3 levels, and improve with the addition of neoadjuvant oral VD3. NS, not significant.
Table 2.
Facial AK clearance rates after PDT in relation to Vitamin D status prior to treatment, and with or without oral VD3 pretreatment
| Group 1 (PDT only) |
Group 2 (VD3 + PDT) |
|
|---|---|---|
| Vitamin D deficient (< 31 mg/dL) | ||
| No. of patients (n) | 7 | 8 |
| 25OH-D3 values (mg/dL), range | 14.9 to 29.3 | 14.2 to 28.7 |
| AK clearance (%, mean ± SD) | 40.9 ± 48 | 71.0 ± 6.0 |
| Vitamin D sufficient (> 31 mg/dL) | ||
| No. of patients (n) | 22 | 21 |
| 25OH-D3 values (mg/dL), range | 32.0 to 83.3 | 31.0 to 100 |
| AK clearance (%, mean ± SD) | 62.6 ± 14.2 | 73.1 ± 15.9 |
| Are the Vitamin D deficient and replete groups statistically different? * | Yes, p<0.014 | No, p = 0.71 |
| All patients | 29 | 29 |
| AK clearance (%, mean ± SD) | 54.4 ± 22.8 | 72.5 ± 13.6 |
| Statistical comparison of AK clearance | ||
| between Groups 1 and 2 ** | ----- | p < 0.002 |
FOOTNOTES:
P-value, Student t-test
P-value, multivariate regression analysis which includes the % decrease in number of lesions on the face while adjusting for age, sex, pre-treatment lesion counts, and length of time to follow-up.
In terms of side effects, the consequences of adding high-dose VD3 pretreatment appears to be negligible. Some patients reported warmth and a mild tickling sensation during illumination, but none reported a pain level greater than 1/10. All patients developed inflammatory side effects typically seen after PDT (i.e., the 12 parameters documented in Supplemental Figure 1). The sum of all ‘yes’ answers, when an individual patient was asked about these 12 parameters, was defined as the Side Effects Score (mean ± SEM); this score was statistically identical for patients who received VD3 supplementation (3.72 ± 0.34; Group 2) and those who did not (3.65 ± 0.44; Group 1). All patients described side effects as tolerable, and almost all patients who had received traditional PDT (long incubation) in the past expressed a preference for the new regimen.
DISCUSSION
We have shown that Vitamin D can affect the outcome of blue light PDT for facial actinic keratoses in two ways: (1) Vitamin D deficiency is associated with a lower AK lesion clearance rate, and (2) pretreatment with high-dose oral VD3 significantly improves lesion clearance. These findings offer a way to simultaneously address two major problems with traditional blue light PDT regimens, i.e., relatively low efficacy and unacceptable pain. Both issues were addressed by adding high-dose VD3 supplementation to our previously reported painless blue light protocol12, with slight modifications. When blue light PDT is performed exactly as described here with a 5-day pretreatment with high dose VD3, mild debridement of lesions with fine sandpaper, brief incubation with ALA (≤15 min), and 30 min of blue light, a mean clearance rate of 73% is achievable as compared to 55% in the original painless PDT study12. With addition of high-dose VD3, intraprocedural pain remains low and cutaneous side effect profiles remain unchanged (i.e., reported by patients as being quite tolerable).
Oral VD3 supplemented PDT appears to be safe and convenient. Vitamin D3 has a wide safety margin; in fact, many physicians recommend large amounts (e.g., 50,000 IU in a single monthly dose) for supplementation without any evidence of Vitamin D toxicity19, 21. Over-the-counter VD3 capsules are cheap, widely available, and do not require a prescription. The modified VD3/PDT regimen adds improved efficacy (CR ~ 18% higher than before) to other positive features (low pain, high tolerability, and shorter total in-office treatment time). Patients who are not confronted with PDT-associated pain will undergo multiple treatments and enjoy better outcomes, making the therapy more desirable for both patients and physicians.
This study has several limitations. The study design was not randomized, and patients in the control group (BIOMARKER registry) were accrued over a longer period of time (3 years) than those in the interventional VDAK study (1 year). However, we wish to point out that patients in the BIOMARKER and VDAK studies received exactly the same treatment except for oral VD3, and matching algorithms were used to select and compare patients between the two groups. Having two different physician evaluators may have led to some inter-observer variance. Finally, understanding how high dose VD3 works to affect PDT responsiveness (mechanism of action) remains a work in progress. In preclinical studies, VD3 increased protoporphyrin IX accumulation in various epithelial cancer cells14, 26 by affecting heme-synthetic enzymes27. Also, high-dose Vitamin D stimulated protoporphyrin IX production in a greater proportion of cancer cells (all, not just some), leading to a more uniform therapeutic response28. Data in Table 2 (smaller standard deviation for patients in Group 2 versus Group 1) reflect this increased homogeneity of response. However, other mechanisms may also be at play. Recent literature suggests that PDT stimulates strong anti-tumor immune responses29, reinforced by our recent work in a mouse model of AK28. VD3 exerts profound effects on PDT tumor responses16, and this might influence PDT-induced immunomodulatory activities. The fact that neoadjuvant VD3 affects PDT responses on the face but not the scalp could reflect differential effects of VD3 on pilosebaceous units, which contain hormonally-responsive stem cells. Despite remaining biological unknowns, the practical value of this new VD3/PDT regimen is that practitioners can use it immediately to benefit patients with widespread facial AK.
Supplementary Material
Suppl. Figure 1. Side effect frequencies after PDT with or without neoadjuvant VD3
Suppl. Table 1. Patient demographics, Vitamin D levels, and actinic keratosis lesion counts.
Suppl. Table 2. Scalp AK counts, with or without neoadjuvant VD3
CAPSULE SUMMARY.
This study demonstrates that patients with actinic keratosis of the face who are pretreated with high-dose oral vitamin D3 supplements prior to blue light PDT show significantly increased lesion clearance.
A combination approach using oral Vitamin D3 pretreatment and an ultra-short 5-ALA incubation/blue light regimen is nearly painless and provides clearance rates of ~75% after a single treatment session.
Acknowledgements:
We thank Dr. Sanjay Anand for his helpful advice, and all the nurses in the Clinical Research Unit at Cleveland Clinic who provided logistical support for the study.
Funding sources:
Grant numbers R01 CA204158 (E. Maytin) and P01 CA084203 (T. Hasan, E. Maytin) from the National Cancer Institute of the National Institutes of Health
ABBREVIATIONS
- AK
Actinic keratosis
- ALA
Aminolevulinic acid
- D3
Vitamin D3; cholecalciferol
- PDT
Photodynamic therapy
- SES
Side effect score
- VD
Vitamin D (generic term)
- VDR
Vitamin D receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None declared.
Clinicaltrials.gov (or equivalent) listing (if applicable):
Biomarkers registry: NCT03319251 VDAK: NCT04140292
REFERENCES
- 1.Maytin EV, Warren CB. Photodynamic Therapy. Maytin EV, Warren CB Photodynamic therapy Post TW, ed UpToDate Waltham, MA: UpToDate Inc; http://www.uptodate.com Last accessed February 15, 2022. [Google Scholar]
- 2.Patel G, Armstrong AW, Eisen DB. Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses: a systematic review and meta-analysis. JAMA Dermatol 2014;150:1281–8. [DOI] [PubMed] [Google Scholar]
- 3.Kurwa HA, Yong-Gee SA, Seed PT, Markey AC, Barlow RJ. A randomized paired comparison of photodynamic therapy and topical 5-fluorouracil in the treatment of actinic keratoses. J Am Acad Dermatol 1999;41:414–8. [DOI] [PubMed] [Google Scholar]
- 4.Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M et al. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or Fluorouracil for treatment of squamous cell carcinoma in situ: Results of a multicenter randomized trial. Arch Dermatol 2006;142:729–35. [DOI] [PubMed] [Google Scholar]
- 5.Piacquadio DJ, Chen DM, Farber HF, Fowler JF Jr., Glazer SD, Goodman JJ et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol 2004;140:41–6. [DOI] [PubMed] [Google Scholar]
- 6.Pariser DM, Houlihan A, Ferdon MB, Berg JE, Group P-AI. Randomized Vehicle-Controlled Study of Short Drug Incubation Aminolevulinic Acid Photodynamic Therapy for Actinic Keratoses of the Face or Scalp. Dermatol Surg 2016;42:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touma D, Yaar M, Whitehead S, Konnikov N, Gilchrest BA. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol 2004;140:33–40. [DOI] [PubMed] [Google Scholar]
- 8.Warren CB, Karai LJ, Vidimos A, Maytin EV. Pain associated with aminolevulinic acid-photodynamic therapy of skin disease. J Am Acad Dermatol 2009;61:1033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ang JM, Riaz IB, Kamal MU, Paragh G, Zeitouni NC. Photodynamic therapy and pain: A systematic review. Photodiagnosis Photodyn Ther 2017;19:308–44. [DOI] [PubMed] [Google Scholar]
- 10.Braathen LR, Szeimies RM, Basset-Seguin N, Bissonnette R, Foley P, Pariser D et al. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus. International Society for Photodynamic Therapy in Dermatology, 2005. J Am Acad Dermatol 2007;56:125–43. [DOI] [PubMed] [Google Scholar]
- 11.Wiegell SR, Haedersdal M, Philipsen PA, Eriksen P, Enk CD, Wulf HC. Continuous activation of PpIX by daylight is as effective as and less painful than conventional photodynamic therapy for actinic keratoses; a randomized, controlled, single-blinded study. Br J Dermatol 2008;158:740–6. [DOI] [PubMed] [Google Scholar]
- 12.Kaw U, Ilyas M, Bullock T, Rittwage L, Riha M, Vidimos A et al. A regimen to minimize pain during blue light photodynamic therapy of actinic keratoses: Bilaterally controlled, randomized trial of simultaneous versus conventional illumination. J Am Acad Dermatol 2020;82:862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang JY, Fu T, Lau C, Oh DH, Bikle DD, Asgari MM. Vitamin D in cutaneous carcinogenesis: part I. J Am Acad Dermatol 2012;67:803 e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer Res 2011;71:6040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand S, Ortel BJ, Pereira SP, Hasan T, Maytin EV. Biomodulatory approaches to photodynamic therapy for solid tumors. Cancer Lett 2012;326:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maytin EV, Hasan T. Vitamin D and Other Differentiation-promoting Agents as Neoadjuvants for Photodynamic Therapy of Cancer. Photochem Photobiol 2020;96:529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollakanti KR, Anand S, Davis SC, Pogue BW, Maytin EV. Noninvasive Optical Imaging of UV-Induced Squamous Cell Carcinoma in Murine Skin: Studies of Early Tumor Development and Vitamin D Enhancement of Protoporphyrin IX Production. Photochem Photobiol 2015;91:1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand S, Rollakanti KR, Horst RL, Hasan T, Maytin EV. Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem Photobiol 2014;90:1126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieth R The pharmacology of Vitamin D, including fortification strategies. in Vitamin D, 2nd edition. Feldman D, Pike WJ, Glorieux FH, series editors. London: Elsevier Academic Press; 2005, pages 995–1015. [Google Scholar]
- 20.Stechschulte SA, Kirsner RS, Federman DG. Vitamin D: bone and beyond, rationale and recommendations for supplementation. Am J Med 2009;122:793–802. [DOI] [PubMed] [Google Scholar]
- 21.Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc 2010;85:752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torezan L, Grinblat B, Haedersdal M, Valente N, Festa-Neto C, Szeimies RM. A randomized split-scalp study comparing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br J Dermatol 2018;179:829–35. [DOI] [PubMed] [Google Scholar]
- 23.Olsen EA, Abernethy ML, Kulp-Shorten C, Callen JP, Glazer SD, Huntley A et al. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J Am Acad Dermatol 1991;24:738–43. [DOI] [PubMed] [Google Scholar]
- 24.Maytin EV, Anand S, Riha M, Lohser S, Tellez A, Ishak R et al. 5-Fluorouracil Enhances Protoporphyrin IX Accumulation and Lesion Clearance during Photodynamic Therapy of Actinic Keratoses: A Mechanism-Based Clinical Trial. Clin Cancer Res 2018;24:3026–35. [DOI] [PubMed] [Google Scholar]
- 25.Apalla Z, Sotiriou E, Chovarda E, Lefaki I, Devliotou-Panagiotidou D, Ioannides D. Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study. Br J Dermatol 2010;162:171–5. [DOI] [PubMed] [Google Scholar]
- 26.Rollakanti KR, Anand S, Maytin EV. Vitamin D enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med 2015;4:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand S, Hasan T, Maytin EV. Mechanism of differentiation-enhanced photodynamic therapy for cancer: upregulation of coproporphyrinogen oxidase by C/EBP transcription factors. Mol Cancer Ther 2013;12:1638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand S, Govande M, Yasinchak A, Heusinkveld L, Shakya S, Fairchild RL et al. Painless Photodynamic Therapy Triggers Innate and Adaptive Immune Responses in a Murine Model of UV-induced Squamous Skin Pre-cancer. Photochem Photobiol 2021;97:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand S, Chan TA, Hasan T, Maytin EV. Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review). Pharmaceuticals (Basel) 2021;14:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. Side effect frequencies after PDT with or without neoadjuvant VD3
Suppl. Table 1. Patient demographics, Vitamin D levels, and actinic keratosis lesion counts.
Suppl. Table 2. Scalp AK counts, with or without neoadjuvant VD3


