Abstract
Purpose:
Evaluate association of retinal nonperfusion (NP) on ultrawide field (UWF) fluorescein angiography (FA) with diabetic retinopathy (DR) severity and predominantly peripheral lesions (PPL).
Methods:
Multicenter observational study; 652 eyes (361 participants) having non-proliferative DR (NPDR) without center-involved diabetic macular edema in at least 1 eye. Baseline 200° UWF-color and UWF-FA images were graded by a central reading center for color-PPL and FA-PPL, respectively. UWF-FA were graded for NP index (NPI) within concentric zones: posterior pole (<10mm from fovea), mid-periphery (10–15mm), and far periphery (>15mm).
Results:
Baseline Early Treatment Diabetic Retinopathy Study DR severity was 31.7% no DR/mild NPDR, 24.1% moderate NPDR, 14.0% moderately severe NPDR, 25.6% severe/very severe NPDR, and 4.6% proliferative DR. Worse DR severity was associated with increased NPI overall (P=0.002), in the posterior pole (P<0.001), mid-periphery (P<0.001), and far periphery (P=0.03). On average, 29.6% of imaged retinal NP was in the posterior pole, 33.7% in mid-periphery and 36.7% in far periphery. Increased NPI was associated with FA-PPL (P<0.001) but not with color-PPL (P=0.65).
Conclusions:
Approximately 70% of NP in diabetic eyes is located outside the posterior pole. Increased NP is associated with presence of FA-PPL, suggesting UWF-FA may better predict future DR worsening than UWF-color alone.
Keywords: Diabetic retinopathy, fluorescein angiography nonperfusion, nonperfusion index, predominantly peripheral lesions, ultrawide field fluorescein angiography, ultrawide field retinal imaging
summary statement:
In a multicenter observational study of participants with non-proliferative diabetic retinopathy without center-involved diabetic macular edema in at least one eye, increased retinal nonperfusion was found to be associated with worse diabetic retinopathy severity and presence of predominantly peripheral lesions on ultrawide field fluorescein angiography.
The importance of the retinal mid-periphery has long been recognized in the pathogenesis and progression of diabetic retinopathy (DR).1 The mid-periphery is reportedly the location of the majority of retinal nonperfusion in eyes with DR and is the primary target in panretinal laser photocoagulation.1–3 Increased mid-peripheral nonperfusion is associated with worsening DR severity.3 As shown in the Early Treatment Diabetic Retinopathy Study (ETDRS),4 the use of fluorescein angiographic (FA) risk factors, such as nonperfusion, provides additional information to help stage risk of DR progression, but not to the extent that it provides substantial additional benefit over evaluating color photographs alone. Due to technical limitations, the ETDRS focused on FA evaluation of two 30° fields in the posterior pole only (Figure 1). However, recent preliminary studies suggest that higher risk of DR progression is associated with the presence of predominantly peripheral retinal lesions (PPL), defined as DR lesions with a greater extent outside versus inside standard ETDRS fields.5,6
Figure 1. Early Treatment Diabetic Retinopathy Study (ETDRS) standard fluorescein angiographic fields 1 and 2 (shown for left eye).
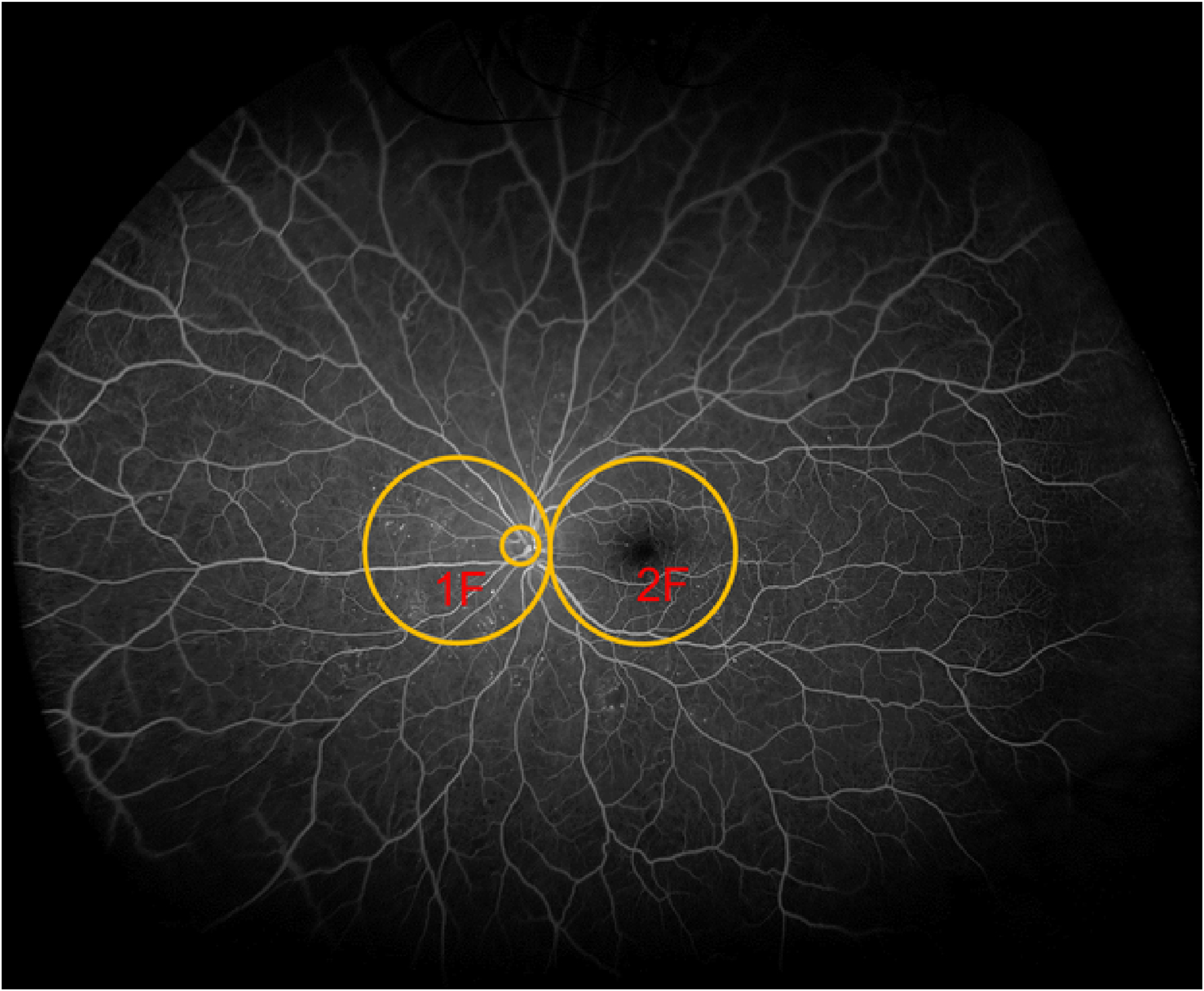
Field 2F is centered one half disc diameter temporal to the center of the macula and field IF is positioned so that the temporal edge of the disc is located one fourth disc diameter from the temporal edge of the field. Background image is a 200-degree ultrawide field fluorescein angiogram. The two 30-degree fields extend along the horizontal meridian from about 15 degrees nasal to the disc to about 10 degrees temporal to the macula. The retinal periphery outside these two fields was not reported in the ETDRS. (Adapted and redrawn based on ETDRS Report 11.9
In a retrospective study, the presence of PPL was associated with greater extent of retinal nonperfusion on UWF-FA, potentially providing a pathophysiologic basis for the association of PPL with increased risk of DR progression.3 DRCR Retina Network Protocol AA evaluated the extent and location of retinal nonperfusion within the retina in diabetic patients using UWF-FA, and assessed the association of nonperfusion with baseline ETDRS DR severity and PPL presence.
Methods
Study Overview
Protocol AA was a prospective observational study conducted at 37 clinical sites in the United States and Canada. The study adhered to the tenets of the Declaration of Helsinki and was approved by institutional review boards specific to each participating clinical center. Study participants provided written informed consent.
Participants were adults with type 1 or 2 diabetes. Study eyes had non-proliferative DR ([NPDR] ETDRS retinopathy severity levels 35 through 53 based on modified 7-field ETDRS grading), no history of panretinal photocoagulation, and no center-involved diabetic macular edema (CI-DME) on optical coherence tomography based on sex- and machine-based thresholds.6,7 Data from 583 study eyes and 193 non-study fellow eyes were collected.
Image Acquisition
UWF-FA were obtained after pupillary dilation based on DRCR Retina Network image acquisition procedures (www.DRCR.net) using Optos 200Tx (Optos plc, Dunfermline, Scotland, UK). The procedure required 13 images per eye taken in early, mid, and late phases (eTable 1). When both eyes were study eyes, early transit images were obtained of the right eye.
Evaluation of UWF Images and Predominantly Peripheral Lesions
UWF-color images were evaluated for DR severity within the ETDRS fields and assessed for PPL presence.8 For each extended retinal field, a diabetic lesion (hemorrhages and/or microaneurysms, venous beading, intraretinal microvascular abnormalities, and new vessels elsewhere on the retina) was considered predominantly peripheral if more than 50% of the lesion being graded was located outside the standard ETDRS 7-fields.5,8 An eye was graded as having PPL if a DR lesion type was predominantly present in any peripheral field.3,4
Evaluation of UWF-FA and Retinal Nonperfusion
Study methodology for measurement of retinal nonperfusion was described previously.3 In brief, all UWF images were stereographically projected and a template of the combined ETDRS 7-fields and the retinal zones (posterior pole, mid-periphery and far periphery) was digitally overlaid based on foveal and optic nerve head locations. This digital overlay was used to assess the distribution of nonperfusion in the modified extended fields of UWF retinal images (Figure 2A). Each entire UWF image was registered to allow precise pixel-level segmentation and demarcation of each defined extended retinal field and each retinal zone. Using the Fiji distribution of ImageJ (version 1.48), a free-hand line was drawn demarcating the extent of retinal nonperfusion and saved as a projected and registered image mask by graders masked to retinopathy severity and PPL assessment. A similar method was used to demarcate the total gradable retina in each image.
Figure 2.
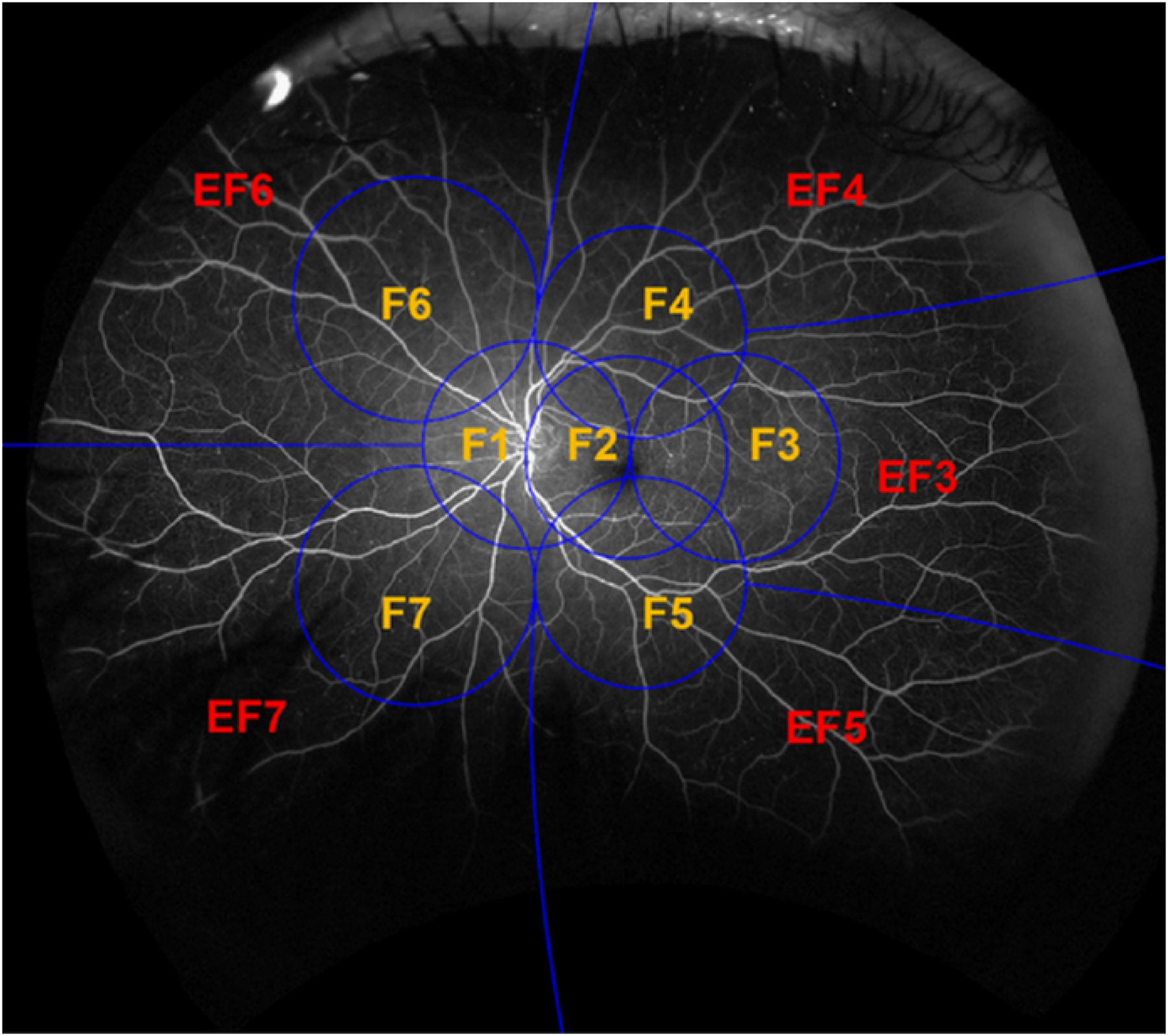
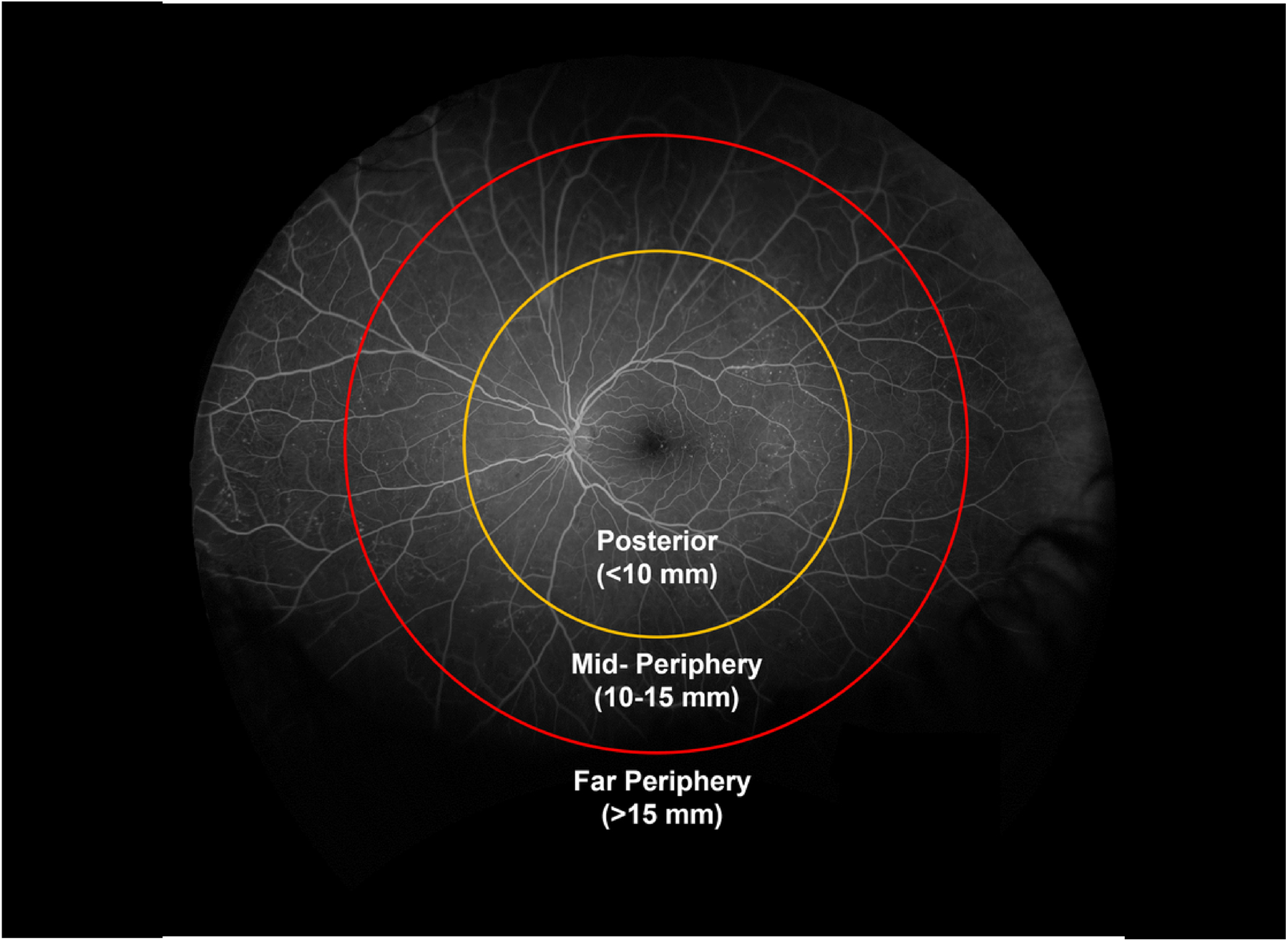
Stereographically projected ultrawide field fluorescein angiogram with (A) grid overlay representing the Early Treatment Diabetic Retinopathy Study 7-standard fields (F) and the peripheral extended fields (EF). (B) concentric zones centered on the fovea for determination of retinal nonperfusion: Posterior (<10 mm), Mid-periphery (10–15 mm) and Far periphery (>15 mm). Adapted and redrawn based on Silva PS, et al.3
The definition of retinal nonperfusion was based on the ETDRS FA grading protocol and Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study.9,10 Shadows were differentiated from nonperfused areas by the presence of retinal vessels and the absence of the stark boundary between the perfused and nonperfused retina (eFigure 1A and 1B). Details on imaging evaluation are reported in eTable 1.
Determining Retinal Nonperfusion Area and Nonperfusion Index
Total nonperfusion area (NPA) and total gradable area (TA) for each eye, and the NPA and TA for each individual extended field were calculated in square millimeters by summing the size of all pixels within the appropriate masked area using a proprietary tool (Optos plc, Dunfermline, Scotland, UK) that implements DICOM Supplement 173.11 The size of an individual pixel was individually defined by its location in the image and was calculated using spherical trigonometry after projecting it back onto a spherical surface, allowing an accurate estimation of retinal area (mm2) independent of peripheral image distortion. The nonperfusion index (NPI) for each eye was calculated by dividing NPA by TA, either overall or within each extended field.
The extent and distribution of nonperfusion were determined within concentric rings centered on the fovea. Each image was registered centered on the fovea and concentric zones corresponding to the extent of the ETDRS fields of posterior pole (<10 mm), anatomic mid-periphery (10–15mm) and far periphery (>15mm) were created from the nonperfusion image masks (Figure 2B). Based on measurements made on a Navarro model eye adapted from UWF images, posterior pole, mid-periphery, and far periphery comprised 32%, 35%, and 33% respectively of the total retinal surface area. This distribution is expected to vary in clinical settings based on variations in TA due to imaging artifacts that would primarily affect the superior and inferior far periphery. For each eye, the extent of nonperfusion contributed by each zone to the overall total nonperfusion was determined and averaged across all eyes to estimate the percentage occurring within each zone.
Quality Control Metrics for Grading of Ultrawide Field Fluorescein Angiograms
Following standardized grading protocols at a centralized reading center, the evaluation of UWF-FA for NPA and TA was highly reproducible. All analyses were performed under the direct oversight of a retina specialist experienced in grading DR and retinal nonperfusion. Based on prior studies, grader agreement for NPA was excellent with intragrader correlation of 0.95 and intergrader correlation of 0.86. Jaccard similarity index was 0.72 and 0.93 between repeat grades of the same grader and 0.65 and 0.92 between grades of the two graders for NPA and TA, respectively.3 All image masks for NPA and TA were validated for accuracy with established theoretical limits.
Statistical Analysis
The primary analyses of nonperfusion included study and non-study fellow eyes with gradable baseline UWF-FA and UWF-color images. NPI outcomes were compared among categorical DR severity groups, and between groups with and without PPL using beta regression adjusting for percentage of imaged area (over theoretical maximum).12 A random effect was included in the model to account for the correlation between eyes within the same participant. To accommodate the modeling of zero values, all NPI values were transformed by , where N = 652 (i.e., total sample size).12,13 Descriptive statistics were provided for the NPA outcomes to aid interpretation.
A serial stepwise gatekeeping procedure for multiple testing was applied when exploring whether any observed association differed by retinal location. When the overall analysis demonstrated a significant association (P < 0.05), the analyses were repeated with the same outcome broken into 3 retinal zones (posterior pole, mid-periphery and far periphery), as well as 5 peripheral fields (ETDRS extended fields 3 through 7) and their adjacent fields; otherwise, no testing within individual zones or fields was performed. P < 0.05 was considered significant in all analyses. As type I error is not fully controlled at 5% with this procedure, some significant associations may occur by chance. All analyses were performed using SAS/STAT 15.1 (SAS Institute, Inc, Cary, NC).
Results
UWF-FA images and UWF-color images, masked to show only the ETDRS 7-fields, were evaluated from 769 eyes of 388 participants (eTable 2).8 Among the 741 eyes with gradable DR severity, nonperfusion was gradable on UWF-FA in 652 eyes (88.0%). Images were ungradable for nonperfusion due to missing images (3 eyes, 0.4%), poor image quality (51 eyes, 6.9%) or presence of retinal laser photocoagulation scars interfering with nonperfusion grading (35 eyes [all fellow eyes], 4.7%). In this cohort, 207 (31.7%) eyes had no DR or mild NPDR, 157 (24.1%) had moderate NPDR, 91 (14.0%) had moderately severe NPDR, 167 (25.6%) had severe to very severe NPDR and 30 (4.6%) had PDR (Table 1). Other baseline characteristics are shown in Table 1.
Table 1.
Baseline participant characteristics among eyes with gradable UWF-FA images and color photographs
| Participant Characteristics | ||
|---|---|---|
| (N = 361 participants) | ||
| Female, N (%) | 183 | (50.7) |
| Age (yr.), Median (IQR) | 62 | (53, 69) |
| Race/Ethnicity, N (%) | ||
| White | 242 | (67.0) |
| Black/African American | 68 | (18.8) |
| Hispanic or Latino | 33 | (9.1) |
| Asian | 10 | (2.8) |
| Native Hawaiian/Other Pacific Islander | 1 | (0.3) |
| More than one race | 3 | (0.8) |
| Unknown/not reported | 4 | (1.1) |
| Diabetes type, N (%) | ||
| Type 1 | 49 | (13.6) |
| Type 2 | 307 | (85.0) |
| Uncertain | 5 | (1.4) |
| Duration of diabetes (yr.), Median (IQR) | 20 | (13, 28) |
| HbA1c (%), Median (IQR) * | 7.9 | (7.0, 9.0) |
| Mean arterial pressure (mmHg), Median (IQR) | 97 | (90, 106) |
| Bilateral participant, N (%) | 186 | (51.5) |
| Ocular Characteristics | ||
| (N = 652 eyes) | ||
| Study eyes, N (%) | 529 | (81.1) |
| Visual acuity (letter score), Median (IQR) | 85 | (81, 89) |
| ~Snellen equivalent, Median (IQR) | 20/20 | (20/25, 20/16) |
| DR severity assessed within the ETDRS 7-fields on UWF-color, N (%) | ||
| DR Absent (Level 10/12) | 3 | (0.5) |
| DR Questionable or Microaneurysms Only (Level 14/15/20) | 4 | (0.6) |
| Mild NPDR (Level 35) | 200 | (30.7) |
| Moderate NPDR (Level 43) | 157 | (24.1) |
| Moderately Severe NPDR (Level 47) | 91 | (14.0) |
| Severe and Very Severe NPDR (Level 53) | 167 | (25.6) |
| Inactive PDR or Mild PDR (Level 60/61) | 12 | (1.8) |
| Moderate PDR (Level 65) | 12 | (1.8) |
| High-risk PDR (Level 71/75) | 6 | (0.9) |
| DME severity based on UWF-color, N (%) | ||
| No DME | 401 | (61.5) |
| Questionable DME | 4 | (0.6) |
| Less than clinically significant macular edema | 175 | (26.8) |
| Clinically significant macular edema | 61 | (9.4) |
| Ungradable | 11 | (1.7) |
| OCT central subfield thickness (Stratus equivalent, μm), Median (IQR)† | 213 | (195, 234) |
| Phakic lens status on clinical examination, N (%) | 479 | (73.5) |
HbA1c unavailable for 6 participants
Central subfield thickness unavailable for 1 eye
IQR, interquartile range; DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; UWF, ultrawide field; NPDR, non-proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; DME, diabetic macular edema; OCT, optical coherence tomography.
Extent of Retinal Area Imaged on Ultrawide Field Fluorescein Angiography
The median (interquartile range, IQR) total retinal area imaged was 799.8 (759.5, 829.1) mm2, representing an average (±standard deviation, SD) of 85.3% (±6.1%) of the theoretical maximal area (based on the Navarro model eye) that can be imaged. The mean (± SD) percentage of theoretical maximal area imaged in the ETDRS fields was 99.5 ± 1.7%, in posterior pole (<10 mm) was 99.9 ± 0.7%, in mid-periphery (10–15 mm) was 94.8 ± 6.9%, and in far periphery (>15 mm) was 61.2 ± 13.1%. The extent of retinal area imaged on UWF-FA for each of the five ETDRS extended fields and four regions is shown in eTable 3.
Association of Diabetic Retinopathy Severity with Retinal Nonperfusion
Worse DR severity was associated with increased NPI (P = 0.002; Figure 3), with mean NPI increased more than 2.0-fold between eyes with no DR and those with PDR. There was a general trend of increased NPI with worse DR severity in each retinal zone (P < 0.001 for posterior pole and mid-periphery and P = 0.03 for far periphery; Table 2). Results were similar in the sensitivity analysis excluding eyes with gradable area in the far periphery below the overall mean value (eTable 4). In addition to worse DR severity, greater NPI was associated with longer duration of diabetes (eTable 5).
Figure 3. Relationship of diabetic retinopathy severity with retinal nonperfused area and nonperfusion index.
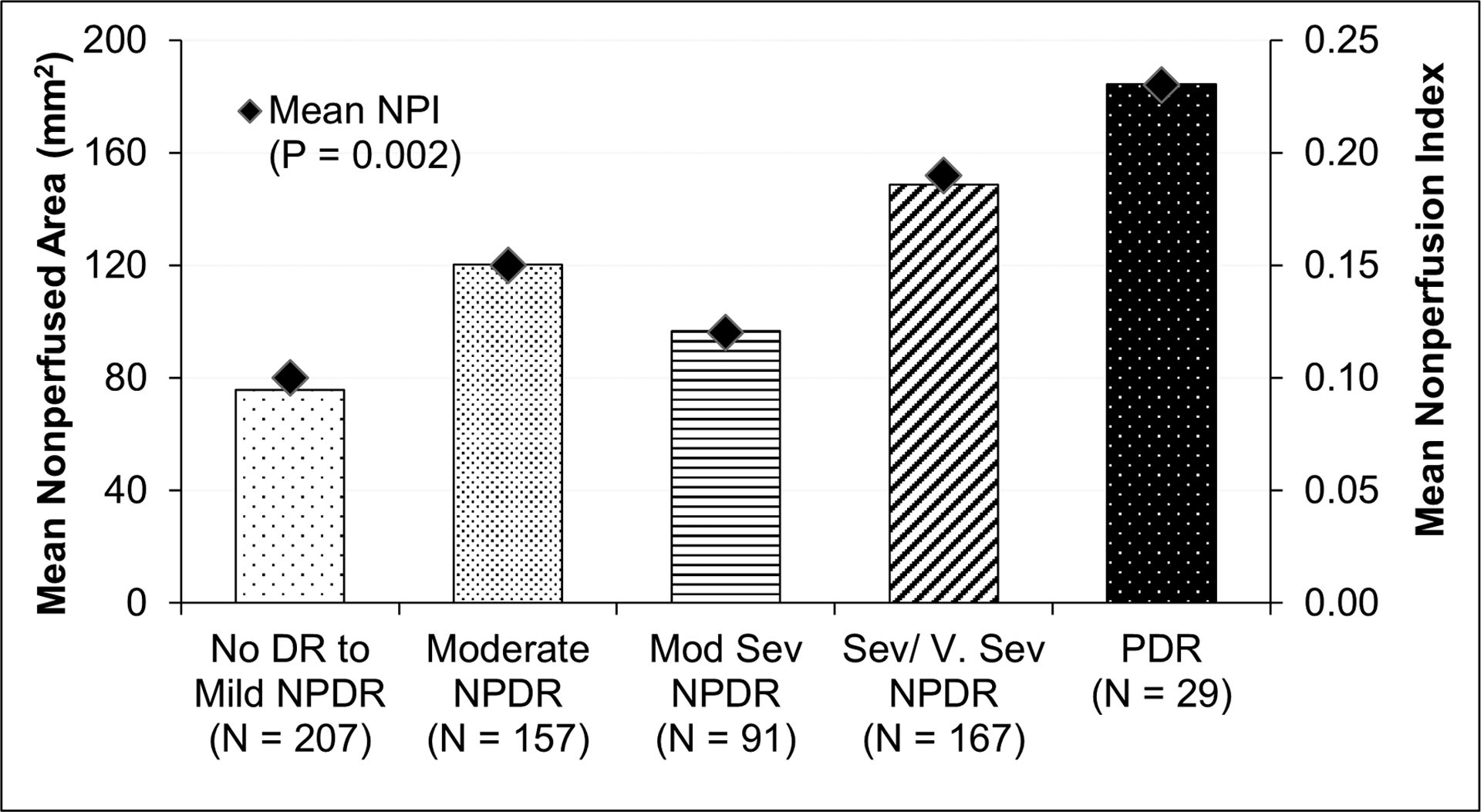
Bars represent the average total retinal nonperfused area on UWF-FA in each DR severity group. Mean overall NPI for each DR severity group are shown as diamond symbols. NPI, nonperfusion index; DR, diabetic retinopathy; NPDR, non-proliferative DR; Mod Sev, moderately severe; Sev/V.Sev, severe or very severe; PDR, proliferative DR.
Table 2.
Association of retinal nonperfusion index and nonperfused area with DR severity
| Statistics Mean ± SD Median (IQR) |
No DR to Mild NPDR | Mod NPDR | Mod Sev NPDR | Sev/V.Sev NPDR | PDR | P Value* |
|---|---|---|---|---|---|---|
| No. of Eyes | 207 | 157 | 91 | 167 | 30 | |
| Nonperfusion Index | ||||||
| Overall | 0.10 ± 0.13 | 0.15 ± 0.17 | 0.12 ± 0.17 | 0.19 ± 0.20 | 0.23 ± 0.20 | 0.002 |
| 0.02 (0.00, 0.15) | 0.11 (0.01, 0.23) | 0.04 (0.01, 0.20) | 0.11 (0.03, 0.32) | 0.23 (0.02, 0.35) | ||
| By Zones of the Retina | ||||||
| Posterior pole (<10 mm) | 0.02 ± 0.04 | 0.04 ± 0.08 | 0.05 ± 0.07 | 0.08 ± 0.10 | 0.09 ± 0.09 | <0.001 |
| 0.00 (0.00, 0.01) | 0.01 (0.00, 0.04) | 0.02 (0.00, 0.07) | 0.04 (0.01, 0.10) | 0.04 (0.01, 0.14) | ||
| Mid-periphery (10–15 mm) | 0.09 ± 0.16 | 0.17 ± 0.21 | 0.13 ± 0.22 | 0.22 ± 0.25 | 0.27 ± 0.26 | <0.001 |
| 0.01 (0.00, 0.10) | 0.05 (0.00, 0.27) | 0.02 (0.00, 0.19) | 0.11 (0.01, 0.37) | 0.25 (0.02, 0.45) | ||
| Far periphery (>15 mm) | 0.21 ± 0.29 | 0.32 ± 0.32 | 0.22 ± 0.31 | 0.33 ± 0.35 | 0.40 ± 0.39 | 0.03 |
| 0.01 (0.00, 0.44) | 0.27 (0.00, 0.56) | 0.01 (0.00, 0.40) | 0.19 (0.00, 0.62) | 0.42 (0.00, 0.79) | ||
| Nonperfused Area (mm2) | ||||||
| Overall | 75.74 ± 105.00 | 120.19 ± 135.08 | 96.65 ± 132.95 | 148.75 ± 155.74 | 184.43 ± 162.91 | -- |
| 15.74 (0.29, 126.99) | 81.06 (7.12, 194.97) | 32.76 (4.15, 155.92) | 89.84 (22.65, 233.66) | 164.93 (19.46, 296.85) | ||
| By Zones of the Retina | ||||||
| Posterior pole (<10 mm) | 5.36 ± 13.01 | 10.58 ± 23.00 | 14.01 ± 19.99 | 22.75 ± 28.93 | 25.43 ± 27.63 | -- |
| 0.13 (0.00, 3.23) | 1.61 (0.00, 11.64) | 5.23 (0.68, 20.36) | 11.87 (2.34, 30.57) | 12.78 (3.18, 42.34) | ||
| Mid-periphery (10–15 mm) | 28.68 ± 49.66 | 50.62 ± 66.12 | 40.62 ± 68.53 | 65.42 ± 76.93 | 84.50 ± 81.09 | -- |
| 2.74 (0.00, 33.09) | 16.30 (0.40, 83.44) | 6.72 (0.27, 52.42) | 34.67 (2.74, 112.01) | 75.94 (5.07, 139.87) | ||
| Far periphery (>15 mm) | 41.69 ± 57.06 | 58.99 ± 61.78 | 42.03 ± 59.02 | 60.58 ± 67.21 | 74.50 ± 77.67 | -- |
| 2.05 (0.00, 84.56) | 42.63 (0.00, 103.28) | 0.79 (0.00, 88.80) | 34.29 (0.02, 119.55) | 63.84 (0.06, 132.57) | ||
P value obtained from a beta regression model with adjustment for the percentage of imaged area on UWF-FA and the correlation between the two eyes from the same participant.
SD, standard deviation; IQR, interquartile range; DR, diabetic retinopathy; NPDR, non-proliferative DR; Mod NPDR, moderate NPDR; Mod Sev NPDR, moderately severe NPDR; Sev/V.Sev NPDR, severe to very severe NPDR; PDR, proliferative DR.
In the overall cohort, NPI (median [IQR]) appeared to increase from posterior pole (0.01 [0.00, 0.05]) to mid-periphery (0.04 [0.00, 0.25]) to far periphery (0.11 [0.00, 0.54]), and similar trends were observed within each DR severity group (P < 0.001; eTable 6). The distribution of NPI within each retinal zone by DR severity category is shown in Supplemental Figure 2. Among 588 eyes with nonperfusion, the mean (±SD) percentage of nonperfusion from each zone was 29.6 ± 34.4% in the posterior pole, 33.7 ± 21.7% in the mid-periphery, and 36.7 ± 32.2% in the far periphery.
We evaluated regional differences in NPI (eTable 6) and found that greater NPI was present in the temporal compared with nasal fields, a finding evident for eyes with no DR to moderate NPDR levels. The difference in NPI between superior and inferior fields in the overall cohort was not significant (P = 0.18). The heat map showing the distribution of nonperfusion across all eyes (Figure 4) highlights that nonperfusion across all UWF-FA images in this study was most frequently present in the temporal and superotemporal periphery. Nonperfusion was present in over 40% of eyes within the temporal and superotemporal far periphery.
Figure 4. Nonperfusion heat map summarizing the distribution of nonperfusion in all eyes in this cohort.
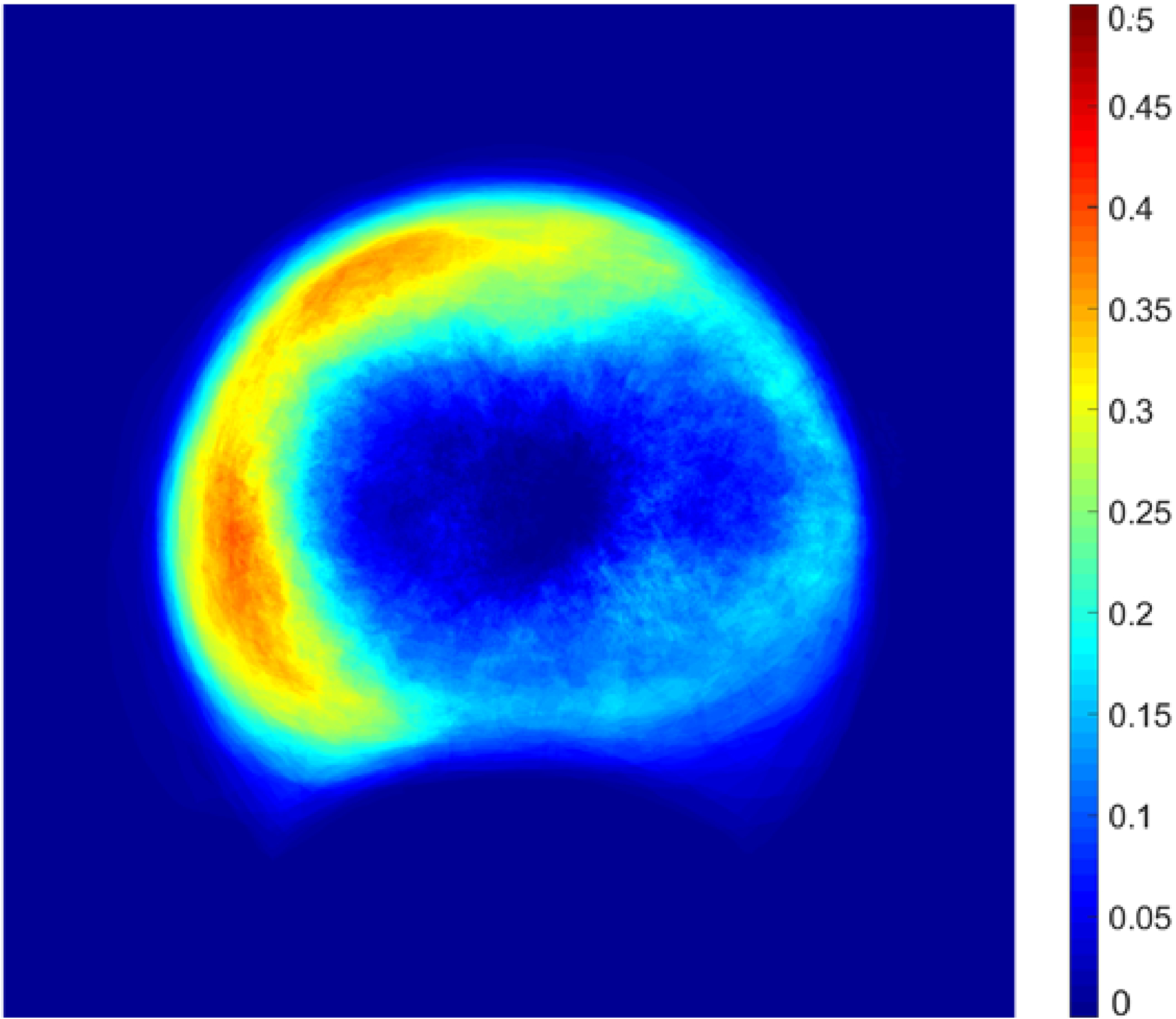
The colors in the heat map represent the percentage of eyes in which nonperfusion was found for that specific retinal location.
Global NPI measurements were highly correlated with NPI in individual fields and retinal zones. Strong to very strong correlation was found between NPI in ETDRS fields 3–7 and global NPI (r = 0.78 to 0.93), with the highest correlations found in field 4 (superotemporal); and between NPI in each of the regions and global NPI (r = 0.91 to 0.97; eTable 7), with higher correlations for superior (vs. inferior: P < 0.001) and for temporal (vs. nasal: P < 0.001).
Association of Nonperfusion with Predominantly Peripheral Lesions
Based on UWF-color images, PPL were present in 256 (39.3%) eyes. No significant association between overall NPI and presence of PPL on UWF-color images (color-PPL) was identified (P = 0.65; Table 3). Summary statistics of NPI and NPA by color-PPL and DR severity are provided in eTables 8A and 9.
Table 3.
Association of retinal nonperfusion index and nonperfused area with the presence of predominantly peripheral lesions on UWF-color image and on UWF-FA
| Statistics Mean ± SD Median (IQR) |
Presence of Predominantly Peripheral Lesions on UWF-Color |
Presence of Predominantly Peripheral Lesions on UWF-FA |
||||
|---|---|---|---|---|---|---|
| Color-PPL Absent | Color-PPL Present | P Value * | FA-PPL Absent | FA-PPL Present | P Value * | |
| No. of Eyes | 396 | 256 | 370 | 282 | ||
| Nonperfusion Index | ||||||
| Overall | 0.13 ± 0.17 | 0.16 ± 0.17 | 0.65 | 0.12 ± 0.17 | 0.18 ± 0.17 | <0.001 |
| 0.05 (0.00, 0.22) | 0.09 (0.01, 0.27) | 0.03 (0.00, 0.19) | 0.13 (0.03, 0.29) | |||
| By Zones of the Retina | ||||||
| Posterior pole (<10 mm) | 0.05 ± 0.08 | 0.04 ± 0.07 | -- | 0.04 ± 0.08 | 0.04 ± 0.07 | 0.38 |
| 0.01 (0.00, 0.06) | 0.00 (0.00, 0.05) | 0.01 (0.00, 0.05) | 0.01 (0.00, 0.06) | |||
| Mid-periphery (10–15 mm) | 0.15 ± 0.22 | 0.17 ± 0.22 | -- | 0.13 ± 0.21 | 0.19 ± 0.22 | <0.001 |
| 0.02 (0.00, 0.21) | 0.07 (0.00, 0.29) | 0.01 (0.00, 0.18) | 0.09 (0.01, 0.32) | |||
| Far periphery (>15 mm) | 0.25 ± 0.32 | 0.32 ± 0.33 | -- | 0.22 ± 0.31 | 0.36 ± 0.33 | <0.001 |
| 0.04 (0.00, 0.48) | 0.22 (0.00, 0.59) | 0.00 (0.00, 0.41) | 0.30 (0.01, 0.64) | |||
| Nonperfused Area (mm2) | ||||||
| Overall | 105.75 ± 138.01 | 124.37 ± 134.51 | -- | 93.00 ± 133.62 | 139.38 ± 136.79 | -- |
| 38.73 (3.76, 167.93) | 75.40 (4.93, 207.26) | 23.79 (1.96, 155.92) | 98.11 (21.33, 222.59) | |||
| By Zones of the Retina | ||||||
| Posterior pole (<10 mm) | 14.14 ± 24.79 | 11.75 ± 20.22 | -- | 13.14 ± 24.08 | 13.29 ± 21.83 | -- |
| 3.18 (0.19, 16.44) | 1.44 (0.00, 14.68) | 2.74 (0.16, 14.07) | 2.04 (0.00, 16.81) | |||
| Mid-periphery (10–15 mm) | 44.30 ± 67.89 | 52.72 ± 66.50 | -- | 39.05 ± 64.79 | 58.84 ± 69.26 | -- |
| 6.60 (0.26, 65.03) | 21.13 (0.73, 87.23) | 2.93 (0.00, 53.99) | 28.89 (3.99, 95.51) | |||
| Far periphery (>15 mm) | 47.31 ± 61.79 | 59.90 ± 63.81 | -- | 40.82 ± 58.84 | 67.26 ± 64.84 | -- |
| 5.89 (0.00, 93.69) | 38.17 (0.00, 111.99) | 0.76 (0.00, 77.48) | 52.04 (1.21, 120.19) | |||
P value obtained from a beta regression model with adjustment for the percentage of imaged area on UWF-FA and the correlation between the two eyes from the same participant. Analyses of nonperfusion index for each retinal zone were performed only when the overall analysis demonstrated a significant association (P < 0.05).
SD, standard deviation; IQR, interquartile range; PPL, predominantly peripheral lesions; UWF, ultrawide field
Based on UWF-FA, PPL were present in 282 (43.3%) eyes. Presence of PPL on FA (FA-PPL) was associated with greater overall NPI (P < 0.001), and greater nonperfusion in mid-periphery (P < 0.001) and far periphery (P < 0.001; Table 3). Results were similar in the sensitivity analysis excluding eyes with gradable area in the far periphery below the overall mean value (eTable 4). Greater nonperfusion was identified in eyes with FA-PPL compared with eyes without FA-PPL in fields 3–5, and in fields 3–7 when combined with adjacent fields (eTable 9). FA-PPL were associated with longer duration of diabetes and less severe DR severity (eTable 10).
Association of Extent of Nonperfusion with Diabetic Macular Edema
No association between overall peripheral nonperfusion was identified with either the presence of any DME (P = 0.74) or CI-DME (P = 0.51) on UWF-color images (eTable 11). However, increased macular (5 mm × 5 mm square centered on the macula) NPI was associated with the presence of any DME (median [IQR] macular NPI: No DME: 0.000 [0.000, 0.002], any DME present: 0.000 [0.000, 0.020], P = 0.002; eTable 12). Stratified by prior treatment for DME, a significant difference was only observed in eyes without prior treatment (P < 0.001).
Discussion
In this subset of Protocol AA participants, a diverse multisite cohort of 652 eyes across the full spectrum of DR severity, worse DR severity was strongly associated with increases in nonperfusion. Nonperfusion identified on UWF-FA was located primarily in the mid- and far- periphery, areas that are not visualized by standard ETDRS FA imaging, with non-uniformity in the retinal distribution of nonperfusion and higher NPI in the temporal compared to the nasal fields.3 The correlation between overall and regional NPI was greatest in the superotemporal ETDRS field and in the temporal and superior regions. Additionally, there was an association between PPL identified on UWF-FA and increased NPI and NPA; the former co-localized with the areas of retinal nonperfusion.
Baseline association between both peripheral and posterior pole nonperfusion, with increased DR severity, supports continued evaluation of nonperfusion on UWF-FA as a possible prognostic marker of DR worsening. The ETDRS reported significant associations of FA findings with DR severity, however, the authors felt the FA findings did not provide significant clinical benefit compared to evaluating color photography alone.14 Thus, FA variables were not incorporated as part of the ETDRS severity scales. However, the ETDRS evaluated only a small portion of the central retina using two posterior pole (disc and macula centered) images (Figure 1). The peripheral retina, demonstrated by several groups to contribute the majority of total nonperfusion across all DR severity levels, is not completely visualized with ETDRS standard 7-field photography.3
A retrospective single center study of 68 eyes found a significant association between presence of PPL and extent of nonperfusion with co-localization of nonperfusion with PPL on UWF-color images.3 In Protocol AA, no significant association between overall nonperfusion and presence of color-PPL was identified but FA-PPL was associated with NPI and NPA and there was co-localization of nonperfusion with areas of PPL. Similar UWF fluorescein studies support an association between PPL and peripheral nonperfusion in diabetic eyes.3,15–30 UWF-FA detects significantly more DR lesions as compared to UWF-color imaging, which may explain discrepancy in color-PPL findings. This discrepancy is evident in studies evaluating microaneurysm counts on UWF-color imaging and UWF-FA, with UWF-FA identifying 3- to 5-fold more microaneurysms and 37.6% of eyes having more severe DR on UWF-FA than on UWF-color imaging.31 The association between FA-PPL, but not color-PPL and retinal nonperfusion, raises the question of whether FA-PPL may prove to be more strongly associated than color-PPL with risk of DR worsening over time. This issue will be addressed in subsequent longitudinal analyses from Protocol AA.
There can be significant variability in extent of visible retinal area between UWF images. Imaging of the retinal far periphery is affected by artifacts resulting from the patient’s eyelashes and eyelids. The reported ungradable rates are 2- to 3-fold higher in inferior fields compared to temporal and superior fields.5 Given the importance of PPL and identification of DR lesions for determining risk of DR progression, imaging artifacts need to be minimized and extent of retinal area imaged maximized for accurate risk assessment in clinical trials and teleophthalmology programs. In the future, noninvasive methods of assessing retinal nonperfusion, such as widefield optical coherence tomography angiography, may also allow more frequent evaluation of the retinal vasculature across a wider, more diverse patient population.
Strengths of this study include the use of standardized and rigorous masked evaluation of UWF images and angiograms, masked graders and a reading center environment optimized for evaluating UWF images. The methodology used in this study was previously described and excellent agreement rate between graders was shown.3 Limitations include, only an average 85% of the theoretically visualized retinal area was imaged on UWF-FA with an average 61% of the theoretical far-peripheral area available for grading, and only the cross-sectional association of retinal nonperfusion with DR severity and PPL at baseline was addressed. Future analyses of Protocol AA will address the extent to which peripheral DR findings on UWF images can predict the risk of DR progression. It should be noted, the measurement of retinal NPA is a surrogate marker for retinal ischemia and tissue hypoxia. Future studies utilizing novel devices that accurately measure retinal oximetry, retinal/tissue ischemia, or provide direct mapping of metabolic activity will be necessary to determine the effect of retinal ischemia and metabolic activity on DR worsening.
These findings demonstrate a strong association between increased retinal nonperfusion and worse DR severity and PPL presence on UWF-FA, suggesting that evaluation of nonperfusion in the mid and far retinal periphery may be important in understanding disease evolution in eyes with DR. The data presented and described in this prospective observational study provide clinical information and matched UWF imaging that reflects the natural history of a contemporary cohort of diabetes eyes. Future data from this study will determine whether UWF-FA, through assessment of retinal nonperfusion and/or PPL, enables more sensitive and specific methods to predict future DR worsening than are currently available with standard FA or color fundus photography.
Supplementary Material
Footnotes
Supplemental Digital Content 1. docx
Presented in part at the Annual Meeting of the Retina Society, London, UK, September 10, 2019
Contributor Information
Paolo S. Silva, Joslin Diabetes Center, Beetham Eye Institute, Harvard Department of Ophthalmology, Boston, Massachusetts.
Danni Liu, Jaeb Center for Health Research.
Adam R. Glassman, Jaeb Center for Health Research.
Lloyd P. Aiello, Joslin Diabetes Center, Beetham Eye Institute, Harvard Department of Ophthalmology, Boston, Massachusetts.
Sandeep Grover, University of Florida – Jacksonville.
Ronald M. Kingsley, Dean A. McGee Eye Institute.
Michele Melia, Jaeb Center for Health Research.
Jennifer K. Sun, Joslin Diabetes Center, Beetham Eye Institute, Harvard Department of Ophthalmology, Boston, Massachusetts.
References
- 1.Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88(7):601–612. [DOI] [PubMed] [Google Scholar]
- 2.Niki T, Muraoka K, Shimizu K. Distribution of capillary nonperfusion in early-stage diabetic retinopathy. Ophthalmology. 1984;91(12):1431–1439. [DOI] [PubMed] [Google Scholar]
- 3.Silva PS, Dela Cruz AJ, Ledesma MG, et al. Diabetic retinopathy severity and peripheral lesions are associated with nonperfusion on ultrawide field angiography. Ophthalmology. 2015;122(12):2465–2472. [DOI] [PubMed] [Google Scholar]
- 4.Silva PS, Cavallerano JD, Haddad NM, et al. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology. 2015;122(5):949–956. [DOI] [PubMed] [Google Scholar]
- 5.Silva PS, Cavallerano JD, Sun JK, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120(12):2587–2595. [DOI] [PubMed] [Google Scholar]
- 6.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015;314(20):2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalam KV, Bressler SB, Edwards AR, et al. Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(13):8154–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiello LP, Odia I, Glassman AR, et al. Comparison of Early Treatment Diabetic Retinopathy Study Standard 7-Field Imaging With Ultrawide-Field Imaging for Determining Severity of Diabetic Retinopathy. JAMA Ophthalmol. 2019;137(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Ophthalmology. 1991;98:807–822. [PubMed] [Google Scholar]
- 10.Blodi BA, Domalpally A, Scott IU, et al. Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study system for evaluation of stereoscopic color fundus photographs and fluorescein angiograms: SCORE Study Report 9. Arch Ophthalmol. 2010;128(9):1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DICOM Standards Committee. Digital imaging and communications in medicine (DICOM): Supplement 173: Wide field ophthalmic photography image storage SOP Classes. https://www.dicomstandard.org/News/ftsup/docs/sups/sup173.pdf. Accessed Accessed 6 December 2021.
- 12.Ferrari S, Cribari-Neto F. Beta Regression or Modeling Rates and Proportions. J Appl Statist. 2004;31(7):799–815. [Google Scholar]
- 13.Smithson M, Verkuilen J. A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods. 2006;11(1):54–71. [DOI] [PubMed] [Google Scholar]
- 14.Early Treatment Diabetic Retinopathy Study Research Group. Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS report number 13. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:834–840. [PubMed] [Google Scholar]
- 15.Wessel MM, Aaker GD, Parlitsis G, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012;32(4):785–791. [DOI] [PubMed] [Google Scholar]
- 16.Sim DA, Keane PA, Rajendram R, et al. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2014;158(1):144–153 e141. [DOI] [PubMed] [Google Scholar]
- 17.Oliver SC, Schwartz SD. Peripheral vessel leakage (PVL): a new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin Ophthalmol. 2010;25(1–2):27–33. [DOI] [PubMed] [Google Scholar]
- 18.Wessel MM, Nair N, Aaker GD, et al. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell JF, Al-Khersan H, Shi Y, et al. Retinal Nonperfusion in Proliferative Diabetic Retinopathy Before and After Panretinal Photocoagulation Assessed by Widefield OCT Angiography. Am J Ophthalmol. 2020;213:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couturier A, Rey PA, Erginay A, et al. Widefield OCT-Angiography and Fluorescein Angiography Assessments of Nonperfusion in Diabetic Retinopathy and Edema Treated with Anti-Vascular Endothelial Growth Factor. Ophthalmology. 2019;126(12):1685–1694. [DOI] [PubMed] [Google Scholar]
- 21.Pichi F, Smith SD, Abboud EB, et al. Wide-field optical coherence tomography angiography for the detection of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258(9):1901–1909. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa T, Tabuchi H, Masumoto H, et al. Accuracy of Diabetic Retinopathy Staging with a Deep Convolutional Neural Network Using Ultra-Wide-Field Fundus Ophthalmoscopy and Optical Coherence Tomography Angiography. J Ophthalmol. 2021;2021:6651175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan W, Wang K, Ghasemi Falavarjani K, et al. Distribution of Nonperfusion Area on Ultra-widefield Fluorescein Angiography in Eyes With Diabetic Macular Edema: DAVE Study. Am J Ophthalmol. 2017;180:110–116. [DOI] [PubMed] [Google Scholar]
- 24.Fan W, Uji A, Nittala M, et al. Retinal vascular bed area on ultra-wide field fluorescein angiography indicates the severity of diabetic retinopathy. Br J Ophthalmol. 2021. [DOI] [PubMed] [Google Scholar]
- 25.Reddy S, Hu A, Schwartz SD. Ultra Wide Field Fluorescein Angiography Guided Targeted Retinal Photocoagulation (TRP). Semin Ophthalmol. 2009;24(1):9–14. [DOI] [PubMed] [Google Scholar]
- 26.Patel RD, Messner LV, Teitelbaum B, et al. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013;155(6):1038–1044 e1032. [DOI] [PubMed] [Google Scholar]
- 27.Wykoff CC, Nittala MG, Zhou B, et al. Intravitreal Aflibercept for Retinal Nonperfusion in Proliferative Diabetic Retinopathy: Outcomes from the Randomized RECOVERY Trial. Ophthalmol Retina. 2019;3(12):1076–1086. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson L, Ramu J, Chan EW, et al. Retinal Nonperfusion Characteristics on Ultra-Widefield Angiography in Eyes With Severe Nonproliferative Diabetic Retinopathy and Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2019;137(6):626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Querques L, Parravano M, Sacconi R, et al. Ischemic index changes in diabetic retinopathy after intravitreal dexamethasone implant using ultra-widefield fluorescein angiography: a pilot study. Acta Diabetol. 2017;54(8):769–773. [DOI] [PubMed] [Google Scholar]
- 30.Levin AM, Rusu I, Orlin A, et al. Retinal reperfusion in diabetic retinopathy following treatment with anti-VEGF intravitreal injections. Clin Ophthalmol. 2017;11:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdelal OMAM, Shokrollahi S, Rocha JT. Comparison of diabetic retinopathy severity identified on ultrawide field retinal images and ultrawide fils fluorescein angiograms ARVO; 2019; Vancouver, Canada. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


