Abstract
TRP ion channels are sophisticated signaling machines that detect a wide variety of environmental and physiological signals. Every cell in the body expresses one or more members of the extended TRP channel family, which consists of over 30 subtypes, each likely possessing distinct pharmacological, biophysical, and/or structural attributes. While the function of some TRP subtypes remains enigmatic, those involved in sensory signaling are perhaps best characterized and have served as models for understanding how these excitatory ion channels serve as polymodal signal integrators. With the recent ‘resolution revolution’ in electron cryo-microscopy (cryo-EM), these and other TRP channel subtypes are now yielding their secrets to detailed atomic analysis, which is beginning to reveal structural underpinnings of stimulus detection and gating, ion permeation, and allosteric mechanisms governing signal integration. These insights are providing a framework for designing and evaluating modality-specific pharmacological agents for treating sensory and other TRP channel-associated disorders.
Keywords: sensory biology, TRP channels, membrane protein structure, cryo-EM
INTRODUCTION
Transient receptor potential (TRP) channels were first discovered, functionally characterized, and cloned from fruit flies, where genetic and electrophysiological studies identified these non-selective cation channels as drivers of photoreceptor cell depolarization in response to light (1–6). TRP channels are now known to exist in all eukaryotes, where they constitute one of the largest and most diverse ion channel families comprising over 30 subtypes in mammals (7–12). As in flies, vertebrate TRPs play important roles in sensory transduction, as well as other physiological processes such as calcium uptake, neuronal growth cone guidance, and insulin secretion. Moreover, TRP channelopathies are associated with numerous human disorders (e.g., polycystic kidney disease, skeletal dysplasia, and familial episodic pain syndrome) (13–16), further highlighting biological roles and therapeutic opportunities for members of this extensive ion channel family. At the same time, physiologic functions for many TRPs remains unknown, in part reflecting a dearth of pharmacological reagents (natural, endogenous, or synthetic) with which to interrogate these channels in vitro or in vivo.
TRP channels that contribute to our sense of touch and pain (somatosensation) are an exception because they are targeted by natural products from plants or animals that elicit pain for defensive purposes (7, 17). Consequently, these somatosensory channels have led the way in providing insights into functional and structural attributes of TRPs at large, and we have therefore centered this review around three such players. These include TRPV1, TRPM8, and TRPA1, which are receptors for the plant-derived sensorial agents capsaicin, menthol, and mustard oil, respectively (Figure 1A) (7, 18–22). From these channels we have learned that TRPs are especially intriguing in their capacity to function as polymodal signal integrators, as perhaps best exemplified by the capsaicin- and heat-activated receptor, TRPV1, whose ability to detect both physical and chemical stimuli underlies its role in inflammatory and persistent pain (7, 23, 24).
Figure 1. Structural overview of TRPV1, TRPA1 and TRPM8.
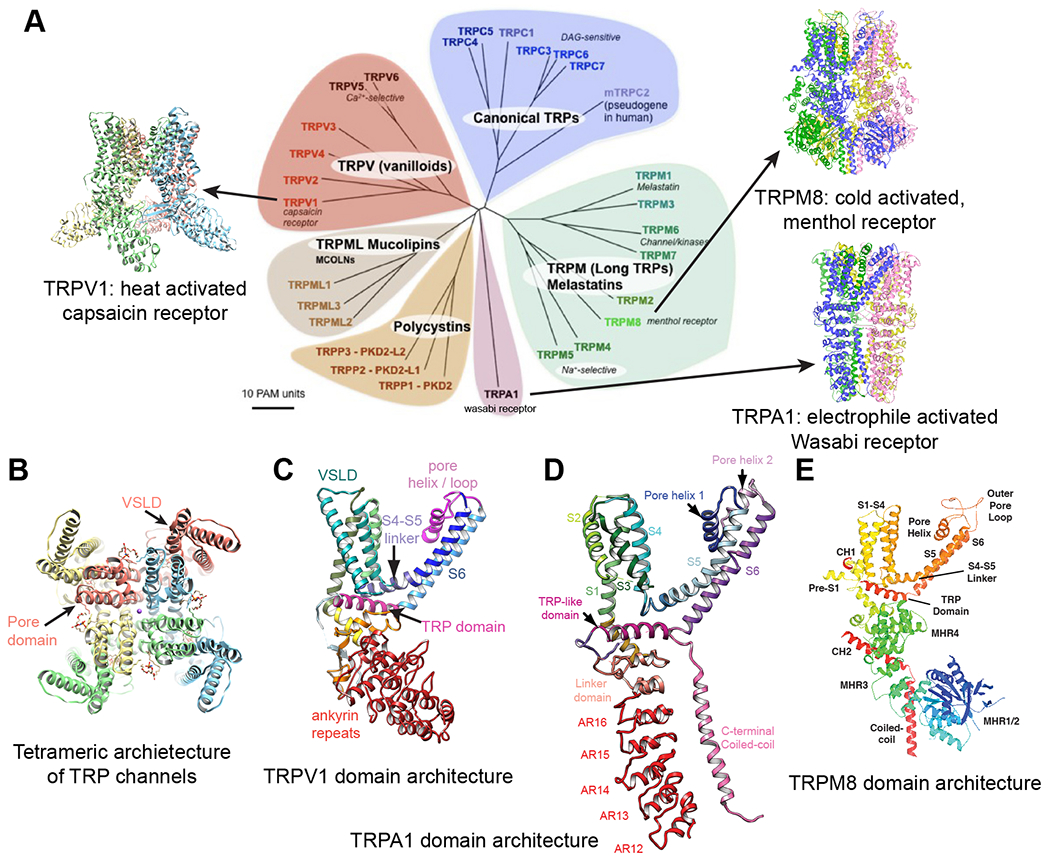
A: Structures of TRPV1, TRPA1 and TRPM8 are illustrated, together with the genetic tree of TRP channel superfamily.
B: Top view of transmembrane domain of TRPV1, illustrating the domain swapped tetrameric architecture of TRP channel in general.
C – E: Details of domain architectures illustrated for a single subunit for TRPV1 (C), TRPA1 (D) and TRPM8 (E).
Indeed, an important goal in ongoing and future studies is to understand the biophysical and structural basis of such phenomena, which will provide critical insights into strategies for developing modality-specific drugs to treat pain and other TRP-associated disorders. There are now many excellent and comprehensive reviews covering specific and general aspects of TRP channel history, function, and pharmacology (7, 8, 25, 26). Our goal here is to focus on recent and exciting advances in TRP channel structural biology that are now revealing detailed mechanisms of ligand binding and stimulus detection, ion permeation, gating, and allosteric regulation. For reasons noted above, we use somatosensory TRPs as the organizing focal point.
In overall structure, TRPs resemble tetrameric voltage-gated ion channels (VGICs) in which the subunits are arranged in four-fold symmetry around a central ion permeation pathway (27, 28). Each monomer is composed of six transmembrane α-helices (S1-S6), where the last two domains (S5-S6) and intervening ‘pore loop’ form the ion selectivity filter and pore (Figure 1C–E). The S1-S4 region associates with the pore region of an adjacent subunit in a ‘domain swap’ organization characteristic of VGICs (Figure 1B), for which this extant domain functions as a voltage sensor that undergo structural movements in response to changes in the membrane electric field. Aside from this general architectural similarity, TRPs differ from other members of the VGIC superfamily in that they exhibit tremendous diversity within their cytoplasmic amino (N) and carboxy (C)-terminal domains (Figure 1C–E), which also differentiates one TRP channel subtype from another but for which physiological roles in ligand recognition, gating, or modulation by cellular factors remain rudimentary (7, 29, 30). Nevertheless, there are some elements that are common to many TRPs and for which mutagenesis and structural studies have begun to shed light on their contributions to subunit interaction and allosteric regulation. Such domains include N-terminal ankyrin repeats, C-terminal coiled-coils, calcium binding sites, and the eponymous C-terminal TRP helix. These and other recent insights are discussed here.
EXPLOITING NATURAL PRODUCTS TO DELINEATE CHANNEL FUNCTION AND STRUCTURE
Natural products from plants and animals are powerful, evolutionarily honed, probes for identifying functionally and conformationally critical control points of proteins and protein complexes. In the context of sensory TRP channels, such agents have presumably evolved to activate sensory nerve fibers that transduce painful signals as a defensive mechanism. Indeed, exploitation of these natural products, both small molecules and peptide toxins, has enabled identification and functional characterization of these biologically important ion channels and their connection to sensory physiology and psychophysics (7, 31, 32).
The capsaicin receptor, TRPV1, functions as a heat thermosensor and is modulated by a range of chemical stimuli, most notably components of the ‘inflammatory soup’ such as extracellular protons, bioactive lipids, neurotrophins, purines, and neuropeptides (7, 20, 33). TRPM8 is a cold-activated channel that is gated by temperatures below 26°C, and by natural and synthetic cooling agents such as menthol, eucalyptol, and icilin (18). TRPM8 channel activity is further modulated by calcium and bioactive lipids (e.g. phosphatidylinositol 4,5-bisphosphate (PIP2)) (34–36). TRPA1 (also known as the ‘wasabi receptor’) functions as a low threshold sensor for reactive electrophiles ranging from small volatile environmental irritants like acrolein or allicin to endogenous reactive lipids, such as 4-hydroxy-2-nonenal and 15-deoxy-Δ(12,14)- prostaglandin J(2) (19, 22, 37, 38).
Sensory TRP channels are also modulated by competitive and noncompetitive antagonists, mainly of synthetic origin. Clinical interest in such compounds originates from the hope that they will be effective for treating chronic inflammatory pain syndromes, acute and chronic itch, or thermal hypersensitivity brought on by nerve injury or treatment with chemotherapeutic agents (39–43). Alongside natural product agonists, these agents have been powerful tools for defining structural mechanisms of activation, inhibition, as well as allosteric regulation.
The determination of cryo-EM structures of sensory TRP channels bound to these pharmacological probes has shown that they target each of the major regions of the channel, including extracellular, intramembrane, and intracellular domains, helping to reveal conformational transitions and functions associated with these sites, as well as interactions among them.
Extracellular Ligand-binding Sites
To-date, this category of ligand-channel interactions pertains largely to TRPV1, whose modulation by extracellular protons underlies its important role as a sensor of local tissue acidosis that accompanies inflammation and drives pain hypersensitivity (23). Thus, delineating the structural basis of proton sensitivity is directly relevant to understanding mechanisms of nociception and analgesia, but may also be of general relevance to assessing the contribution of extracellular domains to TRP channel gating.
Protons activate TRPV1 and enhance its sensitivity to other stimuli through their interactions with two main titratable extracellular sites, namely Glu600 and Glu648 (in the rat channel), respectively (44). Protonation of Glu600, which resides in an extracellular loop that undergoes substantial movement upon gating, disrupts its hydrogen bonding network, causing a widening of the selectivity filter (45, 46). These changes alter the local environment around the other critical protonation site, Glu648, likely driving its subsequent protonation, leading to the open state (46).
Given the important role of extracellular domains in TRPV1 physiology, it is perhaps not surprising that this region has been targeted by natural products, in particular peptide toxins that account for the painful actions of spider venom (Figure 2C, F–G) (47). Like many arachnid toxins, these so-called ‘vanillotoxins’ adopt an inhibitor cysteine knot (ICK) structure in which multiple disulfide bonds scaffold the peptide into a rigid, protease resistant core, thereby facilitating interactions with relevant receptor targets. ICK toxins have been exploited to study a variety of different membrane proteins. For example, Kv gating-modulator toxins such as hanatoxin restrict gating movements through interactions with the voltage-sensing domain (VSD), or more precisely the S3-S4 linker (48, 49). In contrast, the pore-forming domain of TRPV1 specifies sensitivity to vanillotoxins, reflecting the importance of this domain to TRPV1 gating (50–52).
Figure 2. Ligand action on TRPV1.
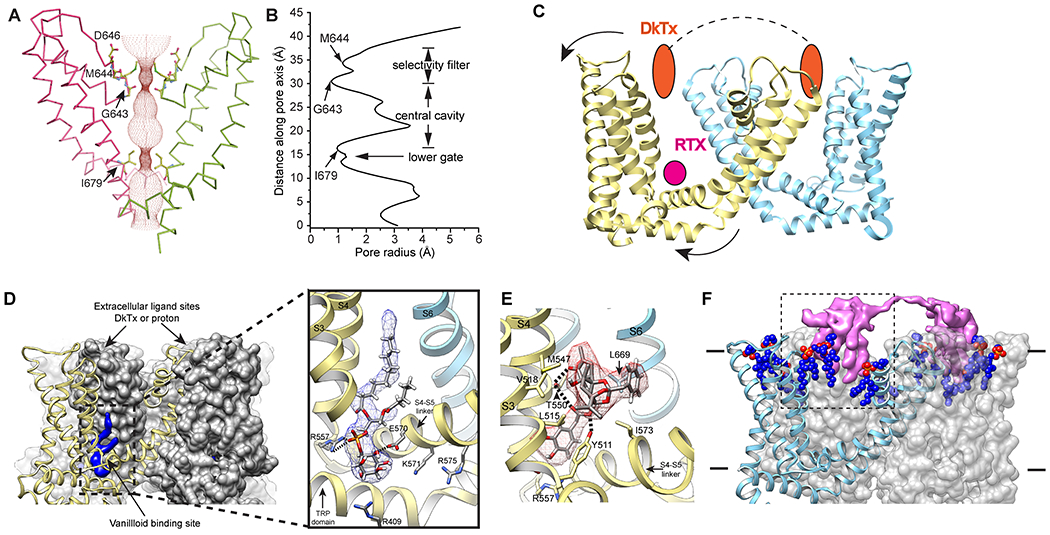
A, B: Solvent accessible pathway (A) and pore radius (B) along the ion permeation pathway calculated from apo structure shows two major restriction sites: the selectivity filter formed by G643 and M644 and the lower gate formed by I679.
C: Extracellular ligand (DkTx) binding site and vanilloid binding site are illustrated. Binding of DkTx pushes VSLD backwards to couple the opening of lower gate. Binding of RTX pulls S5/S6 away from the pore to open the lower gate.
D: transition between π- and α-helix rotates the lower half of S6 helix by one residue, unwinds or reform a helix turn in the joint between S6 and TRP domain.
E: A phosphatidylinositol (PI) lipid resides in the vanilloid binding pocket.
F: Binding of RTX to the vanilloid pocket displays resident PI.
G: Double knot toxin (DkTx) binding to the extracellular surface of the channel.
Among the vanillotoxins described to-date, one from the Chinese bird spider tarantula, Ornithoctonus huwena, is rather unique and has been most useful in probing channel structure. This potent TRPV1-activating toxin, termed ‘double-knot toxin’ (DkTx), consists of two tandemly-repeated ICK motifs joined by a short linker (7 amino acid), a bivalent arrangement that confers high avidity to the toxin-channel interaction, resulting in nearly irreversible channel activation (50). Open structures of TRPV1 bound to DkTx indicate that two bivalent DkTx molecules bind to one TRPV1 tetramer in an antiparallel arrangement such that the toxin exerts its action by binding at the top of the pore helix from one subunit and the outer pore loop proximal to S6 from the neighboring subunit, effectively locking the channel in an activated state (Figure 2G) (45, 46, 53, 54). In this pose, DkTx inserts about 9Å into the bilayer, where it engages in protein-protein interactions with the channel, as well as with membrane phospholipids, thereby forming a tripartite complex that presumably defines the energetics and specificity of toxin-channel engagement (53). Consistent with its role as a gating modifier, DkTx binds preferentially to and stabilizes TRPV1 in its open state by inserting a ‘finger’ into the outer pore that prevents the pore loop and helix from returning to the closed state position. While toxins targeting the extracellular domains of other TRP channels have not yet been well characterized, the hunt for such reagents would be well worthwhile as they could facilitate both physiological and structural studies for other members of this extended family.
Intramembrane binding sites for ligands and regulatory lipids
Whereas extracellular ligand binding sites are thus far unique to TRPV1, cryo-EM studies have elucidated the location of agonist and antagonist sites within the transmembrane core of numerous TRP channels. While the relative location of these sites differs among channel subtypes, they tend to accommodate a wide range of endogenous and exogenous agents, including bioactive lipids and small molecules ligands (natural and synthetic).
Classic natural product agonists (capsaicin and the ultrapotent vanilloid, resiniferatoxin, RTX), synthetic antagonists (capsazepine), as well as regulatory phosphoinositide lipids bind to the ‘vanilloid pocket’ in TRPV1, which is located in a crevice formed by the S4-S5 linker (Figure 2E, F) (45, 46, 53). Accommodation of these structurally and functionally diverse ligands is facilitated by the cavernous space and rearrangement of key amino acid residues. For example, Tyr511 (in the rat channel) assumes distinct rotamers in apo versus liganded TRPV1 structures, reminiscent of an induced fit model for ligand or substrate binding (45). Intriguingly, the vanilloid pocket is not empty in the apo state; rather, a phosphoinositide lipid (likely phosphatidylinositol, PI) is present when the channel is closed (53). Structural studies suggest a mechanism of vanilloid action whereby RTX displaces the resident PI lipid while facilitating movement of the S4-S5 linker away from the central axis to open the ion gate. While this precise mechanism may pertain specifically to vanilloid modulation of TRPV1, the presence of an endogenous lipid at this key regulatory site has now been observed for many TRP subtypes (55–57). Notably, TRPA1 possesses a pocket analogous to the vanilloid pocket in TRPV1, which is seen to bind a synthetic activator, GNE551, as well as phospholipids (58, 59). As the GNE551-bound TRPA1 structure was captured in a non-conducting state, its mechanism of action is yet to be elucidated. The exact identity of the resident lipid (phosphatidyl choline (PC), PI, PIP2 etc.) has not been determined for many channels, nor have their structural or functional relevance been elucidated.
TRPM8 also binds a bevy of regulatory compounds, including natural and synthetic cooling agents, antagonists, and regulatory lipids. The synthetic antagonists, AMTB and TC-I 2014, and the synthetic super-cooling agonist, icilin, also bind within the transmembrane core, but at a location distinct from the vanilloid pocket in TRPV1. Rather, these compounds occupy a membrane-embedded cleft formed by the lower half of the S1-S4 domain (i.e. voltage-sensor-like domain (VSLD)) near the membrane-cytosol interface and just above the TRP domain (Figure 3A, B, C) (60, 61). Although both inhibitory and activating drugs bind to this same flexible pocket, they dock into unique positions, likely locking the channel in a closed or open configuration, respectively. Indeed, shape complementarity is the major determinant of ligand recognition and architecture of the pocket. This in turn is dictated by the absence or presence of the S4-S5 linker and relative position of the TRP helix, which are markedly different in the closed versus desensitized states (Figure 3A, D) (60). Reminiscent of TRPV1, a discrete density can be observed in this pocket when TRPM8 is in its apo state, although identity of the molecule accounting for this density remains unknown (60). Phosphoinositide lipids also regulate TRPM8 function (as discussed later), however their location with respect to this malleable ligand binding site also remains indeterminant.
Figure 3. Structural attributes of the cold and menthol receptor TRPM8.
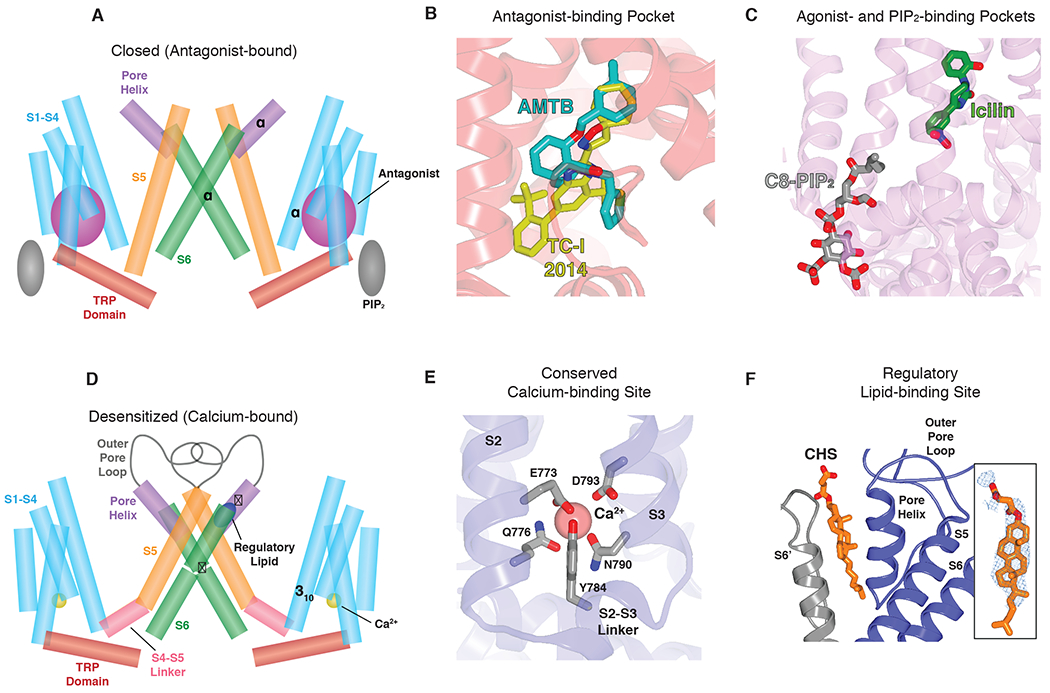
A, D: Structural rearrangements associated with transitioning from closed (A) to desensitized (D) states include a rigid-body tilt of the S1-S4 domain, formation of a canonical S4-S5 linker, shifts of S5, the pore helix, and S6, stabilization of the outer pore loop, and tilting of the TRP domain, such that it is parallel to the membrane bilayer. This transition is accompanied by introduction of a 310-helix in S4 and a π-helix in both the pore helix and S6. Ligand-binding sites are indicated.
B: The antagonists AMTB and TC-I 2014 adopt distinct poses in the S1-S4 ligand-binding pocket.
C: The agonist icilin also adopts a distinct pose in the S1-S4 ligand-binding pocket. PIP2 binds to an interfacial cavity formed by the pre-S1 domain, the S4-S5 linker, the TRP domain, and cytosolic MHR4 domain from an adjacent subunit.
E: Interactions with calcium.
F: In the desensitized state, a stabilizing lipid packs between the pore helix and S6 of the neighboring subunit (modeled as CHS). Inset shows lipid density (blue mesh, 4σ contour).
Intracellular Ligand-binding Sites
Perhaps the best understood example of intracellular ligand action relates to the rather unusual mechanism of TRPA1 activation. As noted above, TRPA1 serves as a detector for a broad range of environmental and endogenous electrophilic irritants of astoundingly diverse structure and size that activate the channel through covalent modification of cytoplasmic cysteine residues (62, 63). Two key cysteines (Cys621 and Cys665 in the human channel) are located within the so-called ‘allosteric nexus’, an intricately folded region situated just below the transmembrane domain of the channel (Figure 4F) (59, 64–67). Current structural and functional data support a model in which stepwise electrophilic addition to Cys621 then Cys665 promotes rearrangement of a nearby loop, thereby positioning a critical lysine within proximity of the C-terminus of the TRP helix, enhancing its dipole moment (65). Enhanced positive electrostatic surface potential of the TRP helix at its N-terminus is proposed to promote repulsion between TRP domains of neighboring subunits and drive expansion of the ion gate (Figure 4F), providing direct structural evidence for the long-standing notion that the TRP helix is a key element contributing to integration of signals that regulate gating. The two-step mechanism proposed for electrophile-mediated activation may enable TRPA1 to effectively balance sensitivity and fidelity in service of detecting small volatile electrophilic irritants in our environment.
Figure 4. Cryo-EM Structures of human TRPA1.
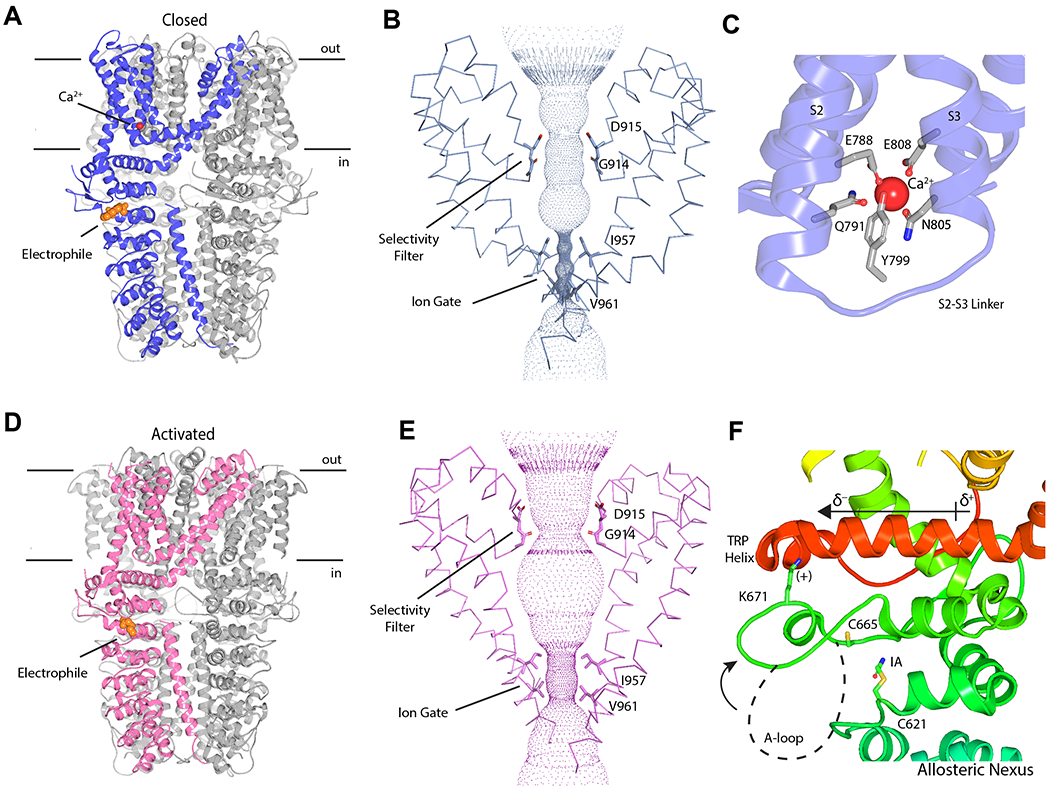
A: Closed structure of TRPA1 with irreversible fluorescent electrophile BODIPY-Iodoacetamide attached to C621 and a bound Ca2+ within the VSLD. TRPA1 was solubilized in LMNG. PDB ID: 6V9V
B: Pore architecture of TRPA1 in a closed (non-conducting) conformation. The ion gate is formed by two hydrophobic residues (I957 and V961) while the selectivity filter is formed by the backbone carbonyl of G914 and the side chain carbonyl of D915.
C: Ca2+ binding site at the base of the VSLD. Ca2+ is coordinated by the indicated polar and negatively charged residues’ side chains.
D: Activated structure of TRPA1 with irreversible electrophile Iodoacetamide attached to C621. TRPA1 was solubilized in PMAL-C8. PDB ID: 6V9X
E: Pore architecture of TRPA1 in an activated state. In an activated state, the ion gate expands and the selectivity filter undergoes upward rotation and translation, which enhances exposure of the D915 to cytoplasm.
F: Model for activation of TRPA1 by electrophiles. Electrophilic addition to C621 triggers stabilization of the Activation (A-) loop in an ‘up’ conformation, which repositions K671 to the C-terminus of the TRP helix, enhancing its N-terminus dipole moment. This promotes repulsion between the symmetrically opposed TRP helices and, ultimately, opening of the ion gate. In the ‘up’ conformation, the C665 within the A-loop rotates into the ligand-binding pocket, becoming available for electrophilic modification, which supports stabilization of the A-loop and full channel activation. Panels (B) and (E) are modified and reproduced with permission from (Zhao et al., Nature, 2020)
The functional importance of the allosteric nexus is further highlighted by a striking example of convergent evolution in which a scorpion toxin also targets this domain to activate TRPA1. This so-called wasabi receptor toxin (WaTx) is a disulfide-stabilized helical hairpin that acts as a cell-penetrating peptide, enabling it to access the allosteric nexus and stabilize the channel open state (68). While the allosteric nexus specifies WaTx sensitivity, its precise mechanism of binding and channel activation remains unknown and awaits structural analysis of a toxin-channel complex.
Mysterious Intracellular Domains
The allosteric nexus of TRPA1 constitutes a cytoplasmic element for which a now well-defined function has been elucidated. Yet this region represents but a tiny fraction of the intracellular mass of the channel. Indeed, this is true for many TRP channels, where N- and C-termini form large intracellular domains that differ extensively between subfamilies and constitute half or more of the mass of any given channel (29, 30). These intracellular regions often contain a variety of canonical structural motifs, such as ankyrin repeats, Nudix and PDZ domains, kinases, and coiled-coils (Figure 1C–E), suggesting that they bind endogenous cellular factors or protein partners that contribute to unique aspects of function, regulation, or pharmacology (8). One of the main challenges in this field is to better understand what these and other structured motifs are doing, and whether and how they foster interaction of TRP channels with cytoplasmic partners. Indeed, aside from structures of calmodulin bound to TRPV5 or TRPV6 (discussed below) and polycystin complexes involving PKD subtypes (69–72), structures of TRPs in complex with other membrane or cytoplasmic partners have not been reported.
Intracellular Domains Contributing to Subunit Interaction
N-terminal ankyrin repeat (ARDs) and C-terminal coiled-coil domains are two motifs that have been shown to play critical roles in assembly and stabilization of TRP channel tetramers (Figure 1). For ARDs, the clearest illustration is seen in members of the TRPV subfamily, where the first two of six repeats engage in interactions with a 3-stranded beta-sheet from the C-terminus of a neighboring subunit (Figure 1A) (28). Interestingly, mutations in this interaction zone of the human TRPV4 channel are associated with a multitude of neural and skeletal developmental disorders, possibly resulting from instability at the lower gate manifesting as a higher spontaneous channel open probability (73).
A well-defined functional role of a given intracellular motif in one TRP channel subtype does not necessarily illuminate its function in other family members, limiting the utility of generalization. For example, while ankyrin repeats engage in subunit-subunit interactions in TRPV channels, they likely subserve distinct functions in TRPA1 (e.g., specifying chemical versus thermal sensitivity across species) and other channel subtypes (74, 75). TRPA1 contains an exceptionally large array of at least 16 ARDs that accounts for ~80% of the total channel mass and which forms an extensive cytoplasmic skirt (64). The majority of TRPA1’s ARDs are unresolved in present structures, which suggests that interaction with co-factors or other cytoplasmic ligands are required to scaffold these repeats into a stable configuration.
Coiled-coil interactions facilitate assembly of the channel tetramer in several subtypes including TRPA1, TRPM, and TRPC family members (64, 76, 77). These structures show characteristic interlocking of subunits through formation of a 4-stranded α-helical coiled-coil structures along a four-fold axis. For TRPA1, the coiled-coil is non-canonical and apparently requires stabilization through interactions with a polyanion, such as inositol hexakisphosphate (InsP6) (64). This is consistent with physiologic studies suggesting that inositol polyphosphates help stabilize channel function (78), but the biological significance of this interaction within the cell remains unknown, in particular whether such interactions are only structural in nature or reflect a mechanism of regulation.
A REGULATED, DYNAMIC ION PERMEATION PATHWAY
Many TRP channels are non-selective for cations, allowing both mono- and divalent ions to transverse the permeation pathway, although some are highly selective for one or the other. It is also notable that some non-selective TRP channels, like TRPV1, TRPA1, and TRPM8, demonstrate a relatively high permeability for calcium ions (TRPV1: PCa2+/PNa+ ~10,TRPA1: PCa2+/PNa+ ~8, TRPM8: PCa2+/PNa+ ~3), which may be relevant to their involvement in calcium-mediated proinflammatory neuropeptide release (18, 20, 68, 79). Some TRPs can also permeate large organic cations, such as NMDG, YO-PRO-1, or QX-134 and other lidocaine derivatives (80–84). The ion permeation pathway in TRP channels, starting from the extracellular side, consists of an outer pore region that forms the selectivity filter, a central cavity, and a cytoplasmic gate formed by hydrophobic side chains. Structures of TRPV1 and TRPA1 have shown that the cytoplasmic ion gate and selectivity filter constitute two highly dynamic constrictions along their ion permeation pathway (Figure 2A, B, 3B, E), which are functionally coupled to regulate channel opening under different physiologic conditions (45, 65).
Dynamic selectivity filter
The selectivity filter is comprised of elements within the outer pore region of the channel, consisting of one or two short pore helices and an extracellular facing loop connecting transmembrane helices S5 and S6 (28, 60, 64). In most TRP channels, the filter sits below a shallow, negatively charged bowl that attracts cations without a need for direct coordination. While this can account for cation selectivity, our understanding of how preference for mono- versus divalent cations is specified by structure is limited. In some channels, such as TRPM8, existing structures do not allow for an accurate description of how ions permeate the selectivity filter (60, 61, 76). In contrast, for TRPV6, which is highly selective for calcium, structures show a rigid scaffold of aspartate side chains that coordinate permeating ions, akin to that observed for voltage-gated potassium channels (85).
In between these examples are channels such as TRPV1 and TRPA1, in which multiple conformations of the selectivity filter clearly reveal transitions across a range of widths (45, 46, 53, 65). The dynamic nature of TRPV1’s selectivity filter has been a topic of much analysis and discussion, in particular, whether it constitutes a true ‘open/close’ gate or otherwise controls ion selectivity or contributes to activation of the cytoplasmic gate (86). In any case, the outer pore domain of TRPV1 is a locus for modulation by pharmacologic agents, including spider toxins and extracellular proteins (as discussed in detail above), attesting to the functional importance of this conformationally dynamic region in regulating channel gating. Transitions between apo and liganded states are defined by outward movements of the entire outer pore domain, including a restrictive glycine, as well as associated rotational movements of a hydrophobic methionine side chain, ultimately increasing the diameter of this region of the permeation pathway to allow entry of partially- or fully-hydrated cations or large organic cations (45, 46).
In TRPA1, upon electrophilic addition, the selectivity filter undergoes an upward rotation and translation, increasing its diameter and negative electrostatic surface potential through repositioning of acidic residues that are critical for calcium selectivity (Figure 4B,E) (65, 79). Structural and pharmacological evidence support the notion that this transition underlies the dynamic ion selectivity of TRPA1, whereby the channel displays an enhanced preference (~25%) for calcium upon activation by electrophilic agonists. Intriguingly, selectivity is dependent on what activates the channel (PCa2+/PNa+ ~8 for electrophile versus ~3 for WaTx) (68). Taken together, the visualization of distinct selectivity filter states in TRPM8, TRPV1, TRPA1, and structures of other TRP channels suggests that the conformationally dynamic nature of this region is of general functional import, and that determining mechanisms of communication between upper and lower restrictions is relevant to understanding the polymodality of numerous TRP subtypes.
Another notable feature within the outer pore region of TRP channels and other members of the VGIC superfamily is the pore helix or helices. In some TRPs, pore helices undergo substantial movement during gating. In TRPV1, mutations in this region dramatically increase basal channel activity and responsiveness to chemical and thermal stimuli (87), attesting to its functional import. The pore helix forms a negative dipole at its carboxyl-terminal end, which may facilitate its movement during membrane depolarization, thereby enhancing open probability at positive membrane potentials (45). It is also an important scaffolding region that may be critical for allosteric coupling between the selectivity filter and cytoplasmic gate. Interestingly, structures of TRPM8 and TRPA1 have shown that regulatory molecules, including drugs and lipids, wedge into this region to stabilize or limit motion (Figure 3F) (60, 64). For example, the TRPA1 antagonists, A-967079 and GDC-0334, bind the channel at a distal membrane-embedded pocket formed by S5, S6, and the first pore helix (64, 65, 88). Presumably, these antagonists hinder channel activity by acting as molecular wedges, thereby impeding pore movements required for opening of the ion gate.
Permeability of TRP channels to large organic cations has been proposed to occur through a controversial process known as ‘pore dilation’ that is initiated by repetitive or prolonged channel activation (89, 90). Irrespective of whether this involves a temporal mechanism, recent high resolution TRPV1 structures show that dynamic changes in the selectivity filter do occur and accommodate permeation by large organic cations, thus supporting at least one tenet of the pore dilation hypothesis - that pore size is not limited to one fixed open state (46). Clearly an important future goal will be to visualize channels in the act of permeating a range of different cation species to understand whether and how these solutes transverse the ion permeation pathway and stabilize the selectivity filter and/or cytoplasmic gate in one or more open states.
Cytoplasmic gate and relationship to the S4-S5 linker and TRP domain
The cytoplasmic gate is formed by one or two hydrophobic residues, which represent the narrowest constriction along the ion permeation pathway and the key control point for opening and closing of the channel (45, 60, 64). A relatively short constriction allows for diverse structural solutions to achieve channel gating, but the hydrophobic nature of the gate likely specifies permeation by either fully or partially hydrated ions. Reminiscent of VGICs, TRP channel gating involves movement of the S4-S5 linker, a short amphipathic helix that runs almost parallel to the inner membrane and connects the S1-S4 region to the pore domain (S5-S6). The TRP domain, a conserved α-helical motif of ~25 residues that also lies parallel to the inner membrane leaflet just below the S4-S5 linker and cytoplasmic gate, has been implicated in subunit assembly, interaction with membrane phospholipids or scaffolding proteins, and/or regulation of the channel gate (7, 8, 25). For most channel subtypes, the TRP domain is readily recognizable from its primary sequence, whereas for others, such as TRPA1, the existence of this structurally conserved motif became apparent only once their 3D structures were elucidated (64). While mechanisms impinging on the TRP helix are surprisingly distinct for different channel subtypes, the main conclusion from mutagenesis and more recent structural studies is that this domain modulates gating. This is further supported by discovery of a novel TRPA1 antagonist that lodges into a crevice formed at the interface of S4, the S4-S5 linker and the TRP helix, where it presumably inhibits conformational transitions in this region that accompany gating (91).
Broadly speaking, one can divide TRP channels into two classes, those exemplified by TRPV channels where rather subtle movements lead to gating, and another exemplified by TRPA1 and TRPM channels where large, concerted movements throughout the protein occur during the gating cycle. For TRPV1, where ligands bind adjacent to the cytoplasmic gate, observed movements of the TRP helix are relatively subtle: it resides more-or-less parallel to the inner leaflet both when the channel is open or closed (45, 46). Rigid-body outward rotation of the S1-S4 domain and/or local movement of the S4-S5 linker and S6 are accompanied by lateral displacement of the TRP domain and sequential channel opening. In contrast, for TRPM8, where ligands bind to the S1-S4 domain, substantial concerted movements in the transmembrane domain result in a more significant movement of the TRP domain, namely tilting that reorients it from an angled position in the closed state to one in which this domain is rendered parallel to the membrane bilayer in its desensitized state (Figure 3A, D) (60). Ultimately, this motion alters the status of the lower gate. In TRPA1, the role of the TRP helix is especially interesting and perhaps mechanistically best understood owing to its role coupling ligand binding at the intracellular ‘allosteric nexus’ to the overlying gate, as described above (65).
Role of helical transitions in gating
Recent structural studies have revealed that helical transitions within the transmembrane domain, including S4, S6, and pore helices, are integral to TRP gating mechanisms. One prominent example, which pertains to numerous TRP channel subgroups, concerns a π-helix in the middle of S6, which introduces a bulge that shifts the register of the helix downstream by one residue (Figure 2D) (92). In many cases, alternative conformations containing an α-helix in this position are also seen for the same channel, sometimes under distinct pharmacological conditions. Transition between the two helical forms has direct effects on the configuration of the cytoplasmic gate by changing the residue(s) that form the most restrictive part of the ion permeation pathway. To date, the meaning of this transition is unclear because the helical state does not strictly align with a particular functional state. Is the π- to α-transition reversible? Is it a post-opening state that leads to a pre-activated or desensitized state?
In TRPV1, S6 contains a π-helix when the ion conduction pathway is either closed or opened by simultaneous binding of two ligands, one that modulates the selectivity filter and one that modulates the cytoplasmic gate (e.g., DkTx plus RTX) (46). Intriguingly, a transition from π- to α- is observed when the channel is activated by only one ligand that stabilizes either the cytoplasmic gate or selectivity filter (e.g., DkTx or RTX). In TRPM8, S6 contains an α-helix when the ion conduction pathway is closed, but a π-helix in the desensitized state (Figure 3A, D) (60). Thus, one cogent model is that transition between π and α is associated with desensitization or accounts for the characteristic (but enigmatic) ‘flickery’ nature of single channel openings observed for numerous TRP channels.
In TRPA1, π- to α-transitions have not been observed, but the π -helix in S6 shifts its position by ~1 helical turn in the activated channel. Now located closer to the selectivity filter, the π-helix supports a pronounced bending of S6 that repositions the outer pore loop, including the pore helices and selectivity filter (65). As previously discussed, these structural rearrangements may help tune cation selectivity.
TRPM8 structures also reveal that π to α transitions can occur in other locals, such as the pore helix, where the α configuration is associate with the closed state and π with the desensitized state (Figure 3A, D) (60). The functional significance of this transition to ion permeation and/or gating is not yet understood, however given the importance of the pore helix is intriguing.
Other types of helical transitions also play a role in TRP channel gating. In comparing apo to agonist-bound or desensitized TRPM8 structures, an α to 310 helical transition is seen in S4 that is associated with a dramatic restructuring of two elements, the S4-S5 linker and TRP domain, that are key to gating, as discussed further below (Figure 3A, D) (60, 61).
Structural insights into temperature-induced gating
Mutagenesis studies have identified numerous residues and regions that contribute to heat or cold sensitivity of thermosensitive TRP channels, including the outer pore region and cytoplasmic N- or C-termini (75, 93–95). Failure to collectively pinpoint one specific domain suggests that thermal gating reflects a distributed mechanism in which temperature alters channel structure globally, involving conformational transitions affecting numerous domains (96). As such, elucidating mechanisms of thermal gating will likely require structural methods that can visualize a trajectory of conformational substates – which may now be possible using cryo-EM. A first step in this direction has recently been reported for TRPV1 and TRPV3, in which structures of these channels are described at elevated temperatures (97, 98). Consistent with the notion of a distributed activation mechanism, these studies suggest that heat-evoked channel activation promotes movements throughout the channel, including within the cytoplasmic domains, which generally remain static following activation by chemical ligands. Moreover, analysis of TRPV3 shows that the resident lipid in the vanilloid pocket-like region is absent in the heated sample (97), consistent with the hypothesis that ejection of this regulatory lipid is a key step in channel activation by either chemical or thermal stimuli (53).
Repurposing the Voltage Sensor-like Domain
VGICs and TRPs consist of two topologically distinct transmembrane domains; namely the voltage sensor (VSD) or voltage sensor-like domain (VSLD), and the central ion permeation pathway (27, 28). For VGICs, structural rearrangement of the VSD in response to changes in membrane potential results in closing or opening of the ion gate. This response originates from the charge transfer center within the VSD, which facilitates movement of positively charged amino acids (an arginine or lysine at every third position in S4) across the membrane field (99).
Some TRP channel subtypes, including TRPV1, TRPM8, and TRPA1, exhibit a degree of voltage dependence characterized by outward rectification (100), however, they are only weakly voltage sensitive and the critical basic residues in S4 that constitute the charge transfer center of VGICs are not present in TRPs. If this domain is not responsible for voltage sensing, then what is its physiological role? Structures of several sensory TRP channels, most notably TRPM8, illustrate that the S1-S4 domain has been repurposed to bind a variety of ligands of physiological import, as noted above, and that these binding events are coupled to channel gating (60, 61). Specifically, upon agonist binding, the VSLD undergoes a rigid-body tilt away from the central axis that is accompanied by transition from an α-helical turn to a 310 helix in S4 (Thr830 to Arg832 in Parus major). This leads to an unusual rearrangement in which the distal regions of S4 and S5 take on a configuration of a canonical S4-S5 linker (Figure 3A, D). Though an open conformation of TRPM8 has yet to be captured, from the desensitized state, it is apparent that these structural rearrangements are accompanied by large shifts in S5, the pore helix, the outer pore domain, and S6, which alter the configuration of the lower gate (60). Unique features of the closed state, including absence of the S4-S5 linker and the relative position of the TRP domain, create an antagonist-selective binding pocket that, when occupied, locks the channel in a closed conformation, further attesting to the importance of conformational changes within the VSLD.
In the case of TRPV1, we see a different situation wherein the VSLD has a rigid structure supported by a hydrophobic core and is not noticeably different when comparing distinct channel states (45). Instead, the VSLD undergoes subtle rigid body tilting that transduces stimulus-evoked conformational changes in the outer pore to the cytoplasmic gate via the S4-S5 linker (Figure 2C). For TRPA1, rigid body movement of the VSLD upon electrophile-evoked activation is somewhat more exuberant, although the mechanistic link to gating is currently less clear (65). While the specific structural movements for TRPs versus VGICs are quite different, the common connection to gating perhaps reflects their shared evolutionary roots and conserved overall architecture.
STRUCTURES REVEAL CONSERVED SITES FOR SECOND MESSENGERS
TRP channels in the fly eye function as ‘receptor-operated’ channels that depolarize the photoreceptor cell following light activation of G protein-coupled rhodopsin (2–4, 6). Genetic and biophysical studies have shown that rhodopsin-dependent activation of phospholipase C (PLC) is required to activate Drosophila TRP. Although the underlying mechanism remains unclear, both PIP2 depletion and generation of PIP2-derived second messengers are likely important for channel activation, attributes that have been retained by many members of the vertebrate TRP channel family (101). Indeed, some vertebrate TRPs likewise serve as receptor-operated channels that are activated or sensitized downstream of PLC-coupled metabotropic receptors. Recent structural studies provide new insights into how second messengers, namely calcium and lipids, interact with sensory TRP channels to mediate receptor-operation or other aspects of cellular regulation.
Structural mechanisms for modulation by cytoplasmic calcium
Some TRP channels are modulated by calcium (via intracellular store release or plasma membrane entry) through calmodulin (CaM)-dependent pathways. Indeed, TRPV5 and TRPV6 have been shown to form a tight complex with CaM, which binds to non-canonical binding sites within channel cytosolic domains leading to calcium-dependent channel inactivation (70–72). In contrast, recent structural studies of TRPM8 and TRPA1 (as well as a subset of other TRPM and TRPC channels) have shown that calcium can also interact directly with TRP channels, independent of CaM or other calcium sensors (61, 65, 102, 103). Taken together, these studies have identified a conserved calcium binding site within the VSLD domain, where the calcium ion is coordinated by four negatively charged residues (glutamate, glutamine, asparagine, and aspartate or glutamate) from the S2 and S3 helices, as well as a tyrosine from the S2-S3 linker (Figure 3E, 4C).
TRPM8 shows pronounced calcium-dependent desensitization during continuous agonist application, and the synthetic ‘super cooling’ agent icilin activates the channel in a calcium-dependent manner (35). Indeed, calcium binding to this single site in TRPM8 accounts for all these calcium-mediated actions (60). TRPA1 functions as a receptor-operated channel downstream of PLC-coupled GPCRs that detect inflammatory and pruritic (itch-inducing) signals, such as bradykinin and chloroquine (38). Electrophysiological studies have shown that elevated intracellular calcium first activates and/or potentiates TRPA1 currents, followed by desensitization (19, 79). Here, again, the conserved calcium-binding site described above accounts for each of these actions (65). While identification of this key site represents an important step forward in elucidating mechanisms of calcium modulation, we still do not understand the structural mechanisms whereby calcium binding affects gating. Interestingly, calcium-mediated desensitization of TRPV1 was described many years ago, but the mechanism(s) remains elusive, suggesting that other partners or calcium sites remain to be identified in this and other TRP channels, as recently shown for TRPM5 (104).
Structural mechanisms for modulation by lipids
Many TRP channels require phosphoinositide (PI) lipids at some point in their gating cycle (105–107). The structural basis and physiological consequences of these important regulatory mechanisms remain controversial and incompletely understood, but there has been some recent progress in visualizing PI lipid binding sites in several TRP subtypes, which is now critical for bringing mechanistic clarity to these important questions.
Studies of TRPM8 provide insight into the class of channels for which PIP2 binding contributes to channel opening. Zheng et al. were the first to suggest involvement of the pre-S1 domain in the binding of PIP2 (108). This has now been corroborated by PIP2-bound structures of TRPM8, which show that the inositol 1,4,5-trisphosphate head group of this lipid is positioned in an interfacial cavity where it forms direct interactions with basic amino acid residues contributed by the pre-S1 domain, the S4-S5 linker, the TRP domain, and cytosolic MHR4 domain from an adjacent subunit, and has been proposed to act by stabilizing the 310-configuration of S4 associated with the open state (Figure 3C) (61). Presumably, the lipid acyl chains extend upwards into the transmembrane region, although these tails aren’t sufficiently resolved to enable their placement within the protein. In the absence of PIP2, a mixture of lipids packs into the pre-S1 lipid-binding domain, whose size changes dramatically in different conformational states (60).
Studies of TRPV channels provide insight into the class of channels for which PI hydrolysis or displacement facilitates gating (Figure 2E, F) (109, 110). Physiologic experiments showed that activation of PLC-coupled receptors enhances the sensitivity of TRPV1 to capsaicin or heat (111–114). Consistent with this, reconstitution of purified TRPV1 into proteoliposomes showed that inclusion of numerous PI lipid species diminishes channel sensitivity to chemical and thermal stimuli (115). However, other findings have suggested that PI lipids are required for TRPV1 activation (116). As described above, a binding site for phosphoinositides has been identified in TRPV1’s vanilloid binding pocket. Displacement of this lipid by vanilloid agonists, such as capsaicin or RTX, promotes conformational changes that lead to the opening of the cytoplamsic gate (46, 53). Some classic competitive antagonists, most notably capsazepine, also bind to this site, displacing the resident PI lipid while blocking the conformational changes that promote channel gating (53). These observations provide an explanation for how PI lipids may serve as both positive and negative regulators of TRPV1 function (117, 118): occupancy of this site in the closed state may be required for stability or to prime the channel for activation, which is followed by lipid displacement during gating in response to chemical or thermal stimuli. Mutagenesis studies of TRPV1 suggest that additional sites may contribute to PLC / phosphatidylinositide regulation, but this has not yet been confirmed by structural studies (111, 119).
An alternate PLC-derived second messenger, diacylglycerol (DAG), is also capable of directly activating several TRP channel subtypes, including TRPC3 and TRPC6 (101, 120). A recent structure revealed that in TRPC6, the DAG binds within an extracellular membrane-facing cavity formed by S6 and the pore helix of an adjacent subunit (121). The analogous site in TRPM8 was occupied by a CHS molecule in the desensitized structure (the only structure in which the outer pore loop is visible), suggesting that occupancy of lipids at this site may modulate channel activity in sensory TRP channels as well (Figure 3F) (60).
CONCLUDING REMARKS
The last few years have seen incredible progress in the structural analysis of TRP channels, including not only sensory TRPs, but those of other physiological import. The studies have revealed both common and unique elements of channel architecture that are providing fresh insight into what makes these channels different, and why. Still, further advances in pharmacological, biochemical, and structural tools are needed to fully bridge gaps between physiological and biophysical understanding of channel function.
For example, a molecular understanding of gating mechanisms requires high-resolution structure determination, but to confidently assign functional states (e.g., open, closed, or inactivated/desensitized pore configurations) to structural ‘snapshots’, it is also essential to capture multiple conformational states, ideally encompassing a trajectory. This is still a challenge for many TRP channels, particularly those that lack adequate pharmacological reagents with which to stabilize discrete physiological states. In all cases, the ability to visualize transient states will improve with advances in cryo-EM technology that facilitate the acquisition and analysis of large datasets of heterogenous protein populations. Also critical will be the continued development of biochemical methods and reagents for solubilizing and stabilizing membrane proteins, while maintaining or recapitulating interactions with key structural or regulatory lipids. In this regard, the identification of lipids and other non-protein components has greatly improved with increased resolution and computations methods, but other approaches facilitating unambiguous identification and modeling of such factors represent another important goal for the field.
As noted above, cytoplasmic domains of TRP channels are large and complex and yet we still know relatively little about whether or with whom these channels interact. Thus, another important future goal is to identify such interactions physiologically and biochemically, and ultimately to use structural methods to define the nature of such interactions and their effects on stimulus detection, gating, subcellular localization, or turnover. Such complexes may also reveal additional ‘druggable’ sites for modulating TRP function outside of those that directly target the channel per se.
ACKNOWLEDGMENTS
A special thanks to all members of our groups, past and present, and the many collaborators who have contributed to the studies discussed in this review. Work from our laboratories is supported by grants from the National Institutes of Health (K99AT010478 to M.M.D., R35NS105038 to D.J. and R01GM098672 and 1R35GM140847 to Y.C.). Y.C. is an Investigator of the Howard Hughes Medical Institute. J.L.K. is a fellow of the Jane Coffin Childs Memorial Fund.
LITERATURE CITED
- 1.Cosens DJ, Manning A. 1969. Abnormal Electroretinogram from a Drosophila Mutant. Nature 224: 285–87 [DOI] [PubMed] [Google Scholar]
- 2.Hardie RC, Minke B. 1992. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8: 653–51 [DOI] [PubMed] [Google Scholar]
- 3.Montell C, Jones K, Hafen E, Rubin G. 1985. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230: 1040–43 [DOI] [PubMed] [Google Scholar]
- 4.Montell C, Rubin GM. 1989. Molecular characterization of the Drosophila trp locus: putative integral membrane protein required for phototransduction. Neuron 2: 1313–23 [DOI] [PubMed] [Google Scholar]
- 5.Hardie RC. 2001. Phototransduction in Drosophila melanogaster. Journal of Experimental Biology 204: 3403–09 [DOI] [PubMed] [Google Scholar]
- 6.Smith DP, Stamnes MA, Zucker CS. 1991. Signal transduction in the visual system of Drosophila. Annual Reviews of Cell Biology 7: 161–90 [DOI] [PubMed] [Google Scholar]
- 7.Julius D 2013. TRP channels and pain. Annual review of cell and developmental biology 29: 355–84 [DOI] [PubMed] [Google Scholar]
- 8.Clapham DE. 2003. TRP channels as cellular sensors. Nature 426: 517–24 [DOI] [PubMed] [Google Scholar]
- 9.Ramsey IS, Delling M, Clapham DE. 2006. AN INTRODUCTION TO TRP CHANNELS. Annual Review of Physiology 68: 619–47 [DOI] [PubMed] [Google Scholar]
- 10.Nilius B, Owsianik G. 2011. The transient receptor potential family of ion channels. Genome Biology 12: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatachalam K, Montell C. 2007. TRP Channels. Annual Review of Biochemistry 76: 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vay L, Gu C, McNaughton PA. 2012. The thermo-TRP ion channel family: properties and therapeutic implications. British Journal of Pharmacology 165: 787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mochizuki T, Wu G, Hayashki T, Xenophontos SL, Veldhuisen B, et al. 1996. PKD2, a Gene for Polycystic Kidney Disease That Encodes an Integral Membrane Protein. Science 272: 1339–42 [DOI] [PubMed] [Google Scholar]
- 14.Nilius B, Owsianik G, Voets T, Peters JA. 2007. Transient Receptor Potential Cation Channels in Disease. Physiological Reviews 87: 165–217 [DOI] [PubMed] [Google Scholar]
- 15.Nilius B, Voets T. 2013. The puzzle of TRPV4 channelopathies. EMBO reports 14: 152–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, et al. 2010. A Gain-of-Function Mutation in TRPA1 Causes Familial Episodic Pain Syndrome. Neuron 66: 671–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sexton JE, Vernon J, Wood JN. 2014. In Mammalian Transient Receptor Potential (TRP) Cation Channels : Volume II, ed. Nilius B, Flockerzi V, pp. 873–97. Cham: Springer International Publishing [Google Scholar]
- 18.McKemy DD, Neuhausser WM, Julius D. 2002. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58 [DOI] [PubMed] [Google Scholar]
- 19.Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, et al. 2004. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–65 [DOI] [PubMed] [Google Scholar]
- 20.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–24 [DOI] [PubMed] [Google Scholar]
- 21.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, et al. 2002. A TRP Channel that Senses Cold Stimuli and Menthol. Cell: 705–15 [DOI] [PubMed] [Google Scholar]
- 22.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, et al. 2004. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–57 [DOI] [PubMed] [Google Scholar]
- 23.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, et al. 1998. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 21: 531–43 [DOI] [PubMed] [Google Scholar]
- 24.Laing RJ, Dhaka A. 2016. ThermoTRPs and Pain. The Neuroscientist 22: 171–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montell C 2005. The TRP Superfamily of Cation Channels. Science’s STKE: 1–26 [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, McVeigh BM, Moiseenkova-Bell VY. 2021. Structural pharmacology of TRP channels. Journal of Molecular Biology 433: 166914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long SB, Campbell EB, Mackinnon R. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309: 897–903 [DOI] [PubMed] [Google Scholar]
- 28.Liao M, Cao E, Julius D, Cheng Y. 2013. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504: 107–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao E 2020. Structural mechanisms of transient receptor potential ion channels. The Journal of general physiology 152: 32–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madej MG, Ziegler CM. 2018. Dawning of a new era in TRP channel structural biology by cryo-electron microscopy. 1–13 [DOI] [PubMed] [Google Scholar]
- 31.Herzig V, Cristofori-Armstrong B, Israel MR, Nixon SA, Vetter I, King GF. 2020. Animal toxins — Nature’s evolutionary-refined toolkit for basic research and drug discovery. Biochemical Pharmacology 181: 114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vetter I, Lewis RJ. 2011. In Transient Receptor Potential Channels, ed. Islam MS, pp. 41–85. Dordrecht: Springer Netherlands [Google Scholar]
- 33.Geppetti P, Nassini R, Materazzi S, Benemei S. 2008. The concept of neurogenic inflammation. British Journal of Urology International [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Qin F. 2005. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. The Journal of neuroscience 25: 1674–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuang H-H, Neuhausser WM, Julius D. 2004. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 43: 859–69 [DOI] [PubMed] [Google Scholar]
- 36.Rohacs T, Lopes CMB, Michailidis I, Logothetis DE. 2005. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nature Neuroscience 8: 626–34 [DOI] [PubMed] [Google Scholar]
- 37.Bautista DM, Jordt S-E, Nikai T, Tsuruda PR, Read AJ, et al. 2006. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 124: 1269–82 [DOI] [PubMed] [Google Scholar]
- 38.Bautista DM, Pellegrino M, Tsunozaki M. 2013. TRPA1: A Gatekeeper for Inflammation. Annual Review of Physiology 75: 181–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xing H, Chen M, Ling J, Tan W, Gu JG. 2007. TRPM8 Mechanism of Cold Allodynia after Chronic Nerve Injury. Journal of Neuroscience 27: 13680–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, et al. 2011. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Molecular Medicine 3: 266–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araujo DSMd, Nassini R, Geppetti P, Logu FD. 2020. TRPA1 as a therapeutic target for nociceptive pain. Expert Opinion on Therapeutic Targets 24: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunthorpe MJ, Chizh BA. 2009. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discovery Today 14: 56–67 [DOI] [PubMed] [Google Scholar]
- 43.Moran MM, Szallasi A. 2018. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. British Journal of Pharmacology 175: 2185–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jord S-E, Tominaga M, Julius D. 2000. Acid potentiation of the capsaicin receptor determined by a key extracellular site. PNAS 97: 8134–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao E, Liao M, Cheng Y, Julius D. 2013. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504: 113–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang K, Julius D, Cheng Y. 2021. Structural snapshots of TRPV1 reveal mechanism of polymodal functionality. Cell In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geron M, Hazan A, Priel A. 2017. Animal Toxins Providing Insights into TRPV1 Activation Mechanism. Toxins 9: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swartz KJ, MacKinnon R. 1997. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 18: 675–82 [DOI] [PubMed] [Google Scholar]
- 49.Swartz KJ. 2007. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon 49: 213–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. 2010. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141: 834–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, et al. 2006. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature 444: 208–12 [DOI] [PubMed] [Google Scholar]
- 52.Bohlen CJ, Julius D. 2012. Receptor-targeting mechanisms of pain-causing toxins: How ow? Toxicon 60: 254–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y, Cao E, Julius D, Cheng Y. 2016. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534: 347–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bae C, Swartz KJ. 2016. Structural insights into the mechanism of activation of the TRPV1 channel by a membrane-bound tarantula toxin. eLife: 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes TET, Lodowski DT, Huynh KW, Yazici A, Rosario JD, et al. 2018. Structural basis of TRPV5 channel inhibition by econazole revealed by cryo-EM. Nature structural & molecular biology: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, Hedger G, Aryal P, Grieben M, Nasrallah C, et al. 2019. Lipid Interactions of a Ciliary Membrane TRP Channel: Simulation and Structural Studies of Polycystin-2 (PC2). eLIFE 121: 3364–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zubcevic L, Hsu AL, Borgnia MJ, Lee S-Y. 2019. Symmetry transitions during gating of the TRPV2 ion channel in lipid membranes. eLife 8: e45779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C, Reese R, Vu S, Rougé L, Shields SD, et al. 2020. A Non-covalent Ligand Reveals Biased Agonism of the TRPA1 Ion Channel. Neuron: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suo Y, Wang Z, Zubcevic L, Hsu AL, He Q, et al. 2020. Structural Insights into Electrophile Irritant Sensing by the Human TRPA1 Channel. Neuron 105: 882–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diver MM, Cheng Y, Julius D. 2019. Structural insights into TRPM8 inhibition and desensitization. Science 365: 1434–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin Y, Le SC, Hsu AL, Borgnia MJ, Yang H, Lee SY. 2019. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 363: eaav9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hinman A, Chuang H-H, Bautista DM, Julius D. 2006. TRP channel activation by reversible covalent modification. Proceedings of the National Academy of Sciences 103: 19564–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, et al. 2007. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–45 [DOI] [PubMed] [Google Scholar]
- 64.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. 2015. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 525: 552. [DOI] [PubMed] [Google Scholar]
- 65.Zhao J, Lin King JV, Paulsen CE, Cheng Y, Julius D. 2020. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 585: 141–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parks TA, Bahia PK, Taylor-Clark TE. 2021. Functional evidence of distinct electrophile-induced activation states of the ion channel TRPA1. Biochemistry and Biophysics Reports 27: 101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bahia PK, Parks TA, Stanford KR, Mitchell DA, Varma S, et al. 2016. The exceptionally high reactivity of Cys 621 is critical for electrophilic activation of the sensory nerve ion channel TRPA1. Journal of General Physiology 147: 451–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin King JV, Emrick JJ, Kelly MJS, Herzig V, King GF, et al. 2019. A Cell-Penetrating Scorpion Toxin Enables Mode-Specific Modulation of TRPA1 and Pain. Cell 178: 1362–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su Q, Hu F, Ge X, Lei J, Yu S, et al. 2018. Structure of the human PKD1/PKD2 complex. Science 361: eaat9819–14 [DOI] [PubMed] [Google Scholar]
- 70.Dang S, van Goor MK, Asarnow D, Wang Y, Julius D, et al. 2019. Structural insight into TRPV5 channel function and modulation. PNAS 116: 8869–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hughes TET, Pumroy RA, Yazici AT, Kasimova MA, Fluck EC, et al. 2018. Structural insights on TRPV5 gating by endogenous modulators. Nature Communications: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh AK, McGoldrick LL, Sobolevsky AI. 2018. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nature structural & molecular biology 25: 805–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng Z, Paknejad N, Maksaev G, Sala-Rabanal M, Nichols CG, et al. 2018. Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nature structural & molecular biology: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, et al. 2010. Molecular basis of infrared detection by snakes. Nature 464: 1006–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cordero-Morales JF, Gracheva EO, Julius D. 2011. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proceedings of the National Academy of Sciences 108: E1184–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin Y, Wu M, Zubcevic L, Borschel WF, Lander GC, Lee S-Y. 2018. Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359: 237–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang Q, Guo W, Zheng L, Wu J-X, Liu M, et al. 2018. Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Research: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim D, Cavanaugh EJ. 2007. Requirement of a Soluble Intracellular Factor for Activation of Transient Receptor Potential A1 by Pungent Chemicals: Role of Inorganic Polyphosphates. The Journal of Neuroscience 27: 6500–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. 2008. The Nociceptor Ion Channel TRPA1 Is Potentiated and Inactivated by Permeating Calcium Ions. Journal of Biological Chemistry 283: 32691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung M-K, Güler AD, Caterina MJ. 2008. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nature Neuroscience 11: 555–64 [DOI] [PubMed] [Google Scholar]
- 81.Chen J, Kim D, Bianchi BR, Cavanaugh EJ, Faltynek CR, et al. 2009. Pore Dilation Occurs in TRPA1 but Not in TRPM8 Channels. Molecular Pain 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McCoy DD, Palkar R, Yang Y, Ongun S, McKemy DD. 2017. Cellular permeation of large molecules mediated by TRPM8 channels. Neuroscience Letters 639: 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Binshtok AM, Bean BP, Woolf CJ. 2007. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449: 607–10 [DOI] [PubMed] [Google Scholar]
- 84.Puopolo M, Binshtok AM, Yao G-L, Oh SB, Woolf CJ, Bean BP. 2013. Permeation and block of TRPV1 channels by the cationic lidocaine derivative QX-314. Journal of neurophysiology 109: 1704–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGoldrick LL, Singh AK, Saotome K, Yelshanskaya MV, Twomey EC, et al. 2017. Opening of the human epithelial calcium channel TRPV6. Nature Publishing Group 553: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jara-Oseguera A, Huffer KE, Swartz KJ. 2019. The ion selectivity filter is not an activation gate in TRPV1–3 channels. eLife 8: e51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Myers BR, Bohlen CJ, Julius D. 2008. A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating. Neuron 58: 362–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balestrini A, Joseph V, Dourado M, Reese RM, Shields SD, et al. 2021. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. Journal of Experimental Medicine 218: e20201637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung MK, Guler AD, Caterina MJ. 2008. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci 11: 555–64 [DOI] [PubMed] [Google Scholar]
- 90.Bean BP. 2015. Pore dilation reconsidered. Nature Neuroscience 18: 1534–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Terrett JA, Chen H, Shore DG, Villemure E, Larouche-Gauthier R, et al. 2021. Tetrahydrofuran-Based Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonists: Ligand-Based Discovery, Activity in a Rodent Asthma Model, and Mechanism-of-Action via Cryogenic Electron Microscopy. Journal of Medicinal Chemistry 64: 3843–69 [DOI] [PubMed] [Google Scholar]
- 92.Zubcevic L, Lee S-Y. 2019. The role of π-helices in TRP channel gating. Current Opinion in Structural Biology 58: 314–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matos-Cruz V, Schneider ER, Mastrotto M, Merriman DK, Bagriantsev SN, Gracheva EO. 2017. Molecular Prerequisites for Diminished Cold Sensitivity in Ground Squirrels and Hamsters. Cell reports 21: 3329–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang F, Jara-Oseguera A, Chang T-H, Bae C, Hanson SM, Swartz KJ. 2018. Heat activation is intrinsic to the pore domain of TRPV1. Proceedings of the National Academy of Sciences 115: E317–E24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. 2006. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. Journal of Neuroscience 26: 4835–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clapham DE, Miller C. 2011. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proceedings of the National Academy of Sciences 108: 19492–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nadezhdin KD, Neuberger A, Trofimov YA, Krylov NA, Sinica V, et al. 2021. Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Nature Structural & Molecular Biology: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwon DH, Zhang F, Suo Y, Bouvette J, Borgnia MJ, Lee S-Y. 2021. Heat-dependent opening of TRPV1 in the presence of capsaicin. Nature Structural & Molecular Biology: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao X, Lee A, Limapichat W, Dougherty DA, MacKinnon R. 2010. A gating charge transfer center in voltage sensors. Science 328: 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. 2005. Gating of TRP channels: a voltage connection? J Physiol 567: 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardie RC. 2007. TRP channels and lipids: from Drosophila to mammalian physiology. The Journal of Physiology 578: 9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Autzen HE, Myasnikov AG, Campbell MG, Asarnow D, Julius D, Cheng Y. 2018. Structure of the human TRPM4 ion channel in a lipid nanodisc. Science 359: 228–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duan J, Li J, Bo Z, Chen G-L, Peng X, et al. 2018. Structure of the mouse TRPC4 ion channel. Nature Communications 9:3102: 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruan Z, Haley E, Orozco IJ, Sabat M, Myers R, et al. 2021. Structures of the TRPM5 channel elucidate mechanisms of activation and inhibition. Nature Structural & Molecular Biology: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suh B-C, Hille B 2008. PIP2 Is a Necessary Cofactor for Ion Channel Function: How and Why? Biophysics 37: 175–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rohacs T 2014. In Mammalian Transient Receptor Potential (TRP) Cation Channels : Volume II, ed. Nilius B, Flockerzi V, pp. 1143–76. Cham: Springer International Publishing; [PubMed] [Google Scholar]
- 107.Qin F 2007. In Transient Receptor Potential (TRP) Channels, ed. Flockerzi V, Nilius B, pp. 509–25. Berlin, Heidelberg: Springer Berlin Heidelberg [Google Scholar]
- 108.Zheng W, Cai R, Hofmann L, Nesin V, Hu Q, et al. 2018. Direct Binding between Pre-S1 and TRP-like Domains in TRPP Channels Mediates Gating and Functional Regulation by PIP2. Cell Rep 22: 1560–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harraz OF, Longden TA, Hill-Eubanks D, Nelson MT. 2018. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. eLife 7: e38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chuang H-h, Prescott ED, Kong H, Shields S, Jordt S-E, et al. 2001. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–62 [DOI] [PubMed] [Google Scholar]
- 111.Prescott ED, Julius D. 2003. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300: 1284–88 [DOI] [PubMed] [Google Scholar]
- 112.Zhang X, Huang J, McNaughton PA. 2005. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. The EMBO Journal 24: 4211–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shu X-Q, Llinas A, Mendell LM. 1999. Effects of trkB and trkC neurotrophin receptor agonists on thermal nociception: a behavioral and electrophysiological study. PAIN 80: 463–70 [DOI] [PubMed] [Google Scholar]
- 114.Bonnington JK, McNaughton PA. 2003. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. The Journal of Physiology 551: 433–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. 2013. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron 77: 667–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ufret-Vincenty CA, Klein RM, Collins MD, Rosasco MG, Martinez GQ, Gordon SE. 2015. Mechanism for phosphoinositide selectivity and activation of TRPV1 ion channels. Journal of General Physiology 145: 431–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu B, Zhang C, Qin F. 2005. Functional Recovery from Desensitization of Vanilloid Receptor TRPV1 Requires Resynthesis of Phosphatidylinositol 4,5-Bisphosphate. The Journal of Neuroscience 25: 4835–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rohacs T 2015. Phosphoinositide regulation of TRPV1 revisited. Pflügers Archiv - European Journal of Physiology 467: 1851–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ufret-Vincenty CA, Klein RM, Hua L, Angueyra J, Gordon SE. 2011. Localization of the PIP2 Sensor of TRPV1 Ion Channels. Journal of Biological Chemistry 286: 9688–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. 1999. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–63 [DOI] [PubMed] [Google Scholar]
- 121.Bai Y, Yu X, Chen H, Horne D, White R, et al. 2020. Structural basis for pharmacological modulation of the TRPC6 channel. Elife 9 [DOI] [PMC free article] [PubMed] [Google Scholar]


