Abstract
Double negative (DN) T cells, one of the least studied T lymphocyte subgroups, express T cell receptor (TCRαβ) but lack CD4 and CD8 co-receptors. DN T cells are found in multiple organs including kidney, lung, heart, gastrointestinal tract, liver, genital tract and central nervous system. DN T cells suppress inflammatory responses in different disease models including experimental AKI and significant evidence supports an important role in the pathogenesis of SLE. However, little is known about these cells in other kidney diseases. Therefore, it is important to better understand different functions of DN T cells and their signaling pathways as promising therapeutic targets, particularly with the increasing application of T cell directed therapy in humans. In this review, we aim to summarize studies conducted on DN T cells in normal and diseased organs in the setting of different disease models with a focus on kidney.
Keywords: Double negative T cells, Lymphocytes, Kidney disease, Ischemia-reperfusion, Lupus
Introduction
Among T lymphocytes, CD4+ and CD8+ T cells functions are well known. However, there is an unconventional subset of T cells in which both CD4+ and CD8+ receptors are missing. These cells are termed double negative (DN) T cells and are a subject of increasing research interest over the past decade. DN T cells can be found in lymphoid as well as non-lymphoid tissues and comprise 1–3% of human T cells in the circulating blood of healthy individuals.1 The role of DN T cells in systemic lupus erythematosus (SLE) has been well studied, including in lupus nephropathy, and DN T cells have been identified as a major pathogenic player in various autoimmune conditions.2,3 Protective effects in other diseases, such as acute kidney injury (AKI) and allograft rejection have been described.4,5,6 Additionally, innate-like abilities of DN T cells have been demonstrated in the setting of infectious diseases, e.g., Leishmania major.7
These different roles raise questions about the mechanisms by which DN T cells influence health and disease. Apparent differences have been observed between pro-inflammatory, such as IL-17 producing DN T cells in SLE models, and anti-inflammatory, e.g. IL-10 producing DN T cells with immunosuppressive features.2 Recently, single cell RNA-sequencing analysis of DN T cells identified specific clusters with five DN subsets proposed: resting DN, helper DN, intermediate DN, cytotoxic DN and innate DN.8 Plasticity of DN T cells might also explain observed discrepancies. There are different hypotheses for the origin of DN T cells, which we will describe in more detail, and a redifferentiation to the T cell of origin might be possible.2,9
An important opportunity is to identify a specific positive marker, given that current investigations of DN T cells are challenged by having to use negative selection techniques. The definition and nomenclature of DN T cells has been inconsistent throughout the literature, thus different studies could have been investigating divergent populations. Usage of CD3+ antibody labeling instead of TCRαβ+ for DN T cells will lead to inclusion of TCRγδ+ in addition to TCRαβ+ cells, confounding interpretation. Furthermore, some publications on DN T cells may have included NK T cells, which have pro-inflammatory characteristics and are therefore extremely important to exclude. A homogenous DN T cell population can be best achieved by exclusion of CD1d+ labeled cells targeting NK T cells.10 Importantly, negative selection of NK1.1+ cells does not result in a pure population, since this marker is less specific and not all NK T cells will be targeted. Recent findings described CD1d-unrestricted NK T cells, which might require an additional antibody in the DN T cell isolation process to achieve pure DN T cell populations.11,12 Another type of cells potentially overlapping with DN T cell gating are DN mucosal associated invariant T cells (CD4−CD8− MAIT) cells, but these numbers are considered negligible in kidney and most other organs in mice (Table 1).13,14
Table 1:
Different DN T cell population definitions found in literature and included immune cell subsets
| CD3+ CD4−CD8− | TCRαβ+ CD4− CD8− | TCRαβ+ CD4− CD8− CD1d− (Recommended) | TCRαβ+ CD4− CD8− NK1.1− | |
|---|---|---|---|---|
| TCRαβ+ CD4− CD8− | x | x | x | x |
| NK T cells | x | x | (CD1d-unrestricted NK T cells) | (CD1d+ NK T cells) |
| TCRγδ+ | x | |||
| CD4− CD8− MAIT | (x) | (x) | (x) | (x) |
Differences in technical approaches can have major impact on DN T cell population assessment, with differing flow cytometric gating strategies impacting DN T cell measurements.15 Thus, we recommend that DN T cells be defined as TCRαβ+CD4−CD8−CD1d− cells in mice and TCRαβ+CD4−CD8−CD56− in humans.10
1. Development of DN T Cells
It is important to first review development of αβ T cells. Progenitor lymphocytes originate from the bone marrow before migrating to the thymus where T cell differentiation occurs. There, thymocytes lack CD4 and CD8 co-receptors in early stages of maturation, referred as DN thymocytes.16,17 DN T cell developmental stages are characterized by the expression of cell surface markers such as CD25+ and CD44+. DN thymocyte subsets can be distinguished depending on the expression of these receptors. Murine αβ T cell differentiation includes four stages of DN thymocytes, whereas there are three stages in humans.3,18,19 CD44+ CD25− (DN1) cells lead to the formation of CD44+CD25+ (DN2) cells. Next, DN2 cells lose CD44 expression and become CD44−CD25+ cells (DN3). Here, murine cells undergo TCRβ selection, which promotes rearrangement of TCRα locus to express complete TCRαβ later in development.17,20 Finally, the DN3 T cells lose CD25 and differentiate into CD44−CD25− cells (DN4). DN4 is followed by the CD8+ (CD4+ in humans) immature single positive (ISP) stage, before DN T cells acquire CD4 and CD8 and become DP T cells.21 Following DP state, further differentiation into CD4+CD8− and CD4−CD8+ single positive occurs before these cells exit the thymus and into the periphery.22
There are five main theories regarding the origins of peripheral DN T cells (Figure 1). The first theory claims that DN T cells develop in the periphery. T cells may have committed to either CD4+ or CD8+ T cells in the thymus, but later downregulate their co-receptor. Analysis in lupus-prone MRL-lpr mice, a mouse strain with accumulation of DN T cells, suggests the precursor for DN T cells are CD8+ cells. Cells presenting normal expression of CD8 co-receptor show demethylation of CD8 gene. DN T cells show the same demethylation of this gene, only CD8+ receptors are not present, providing further evidence for the development of DN T cells by downregulation of CD8 receptor of peripheral CD8+ T cells.23 Comparison of adoptive transfer of CD3+ TCRαβ+ CD4+ and CD3+ TCRαβ+ CD8+ thymocytes into Rag1-deficient mice revealed that both led to DN T cell generation. However, CD3+ TCRαβ+ CD8+ thymocytes injection led to significantly higher DN T cell numbers than CD3+ TCRαβ+ CD4+.9
Figure 1. αβT lymphocyte maturation and most common theories about the origin of DN T cells in periphery.
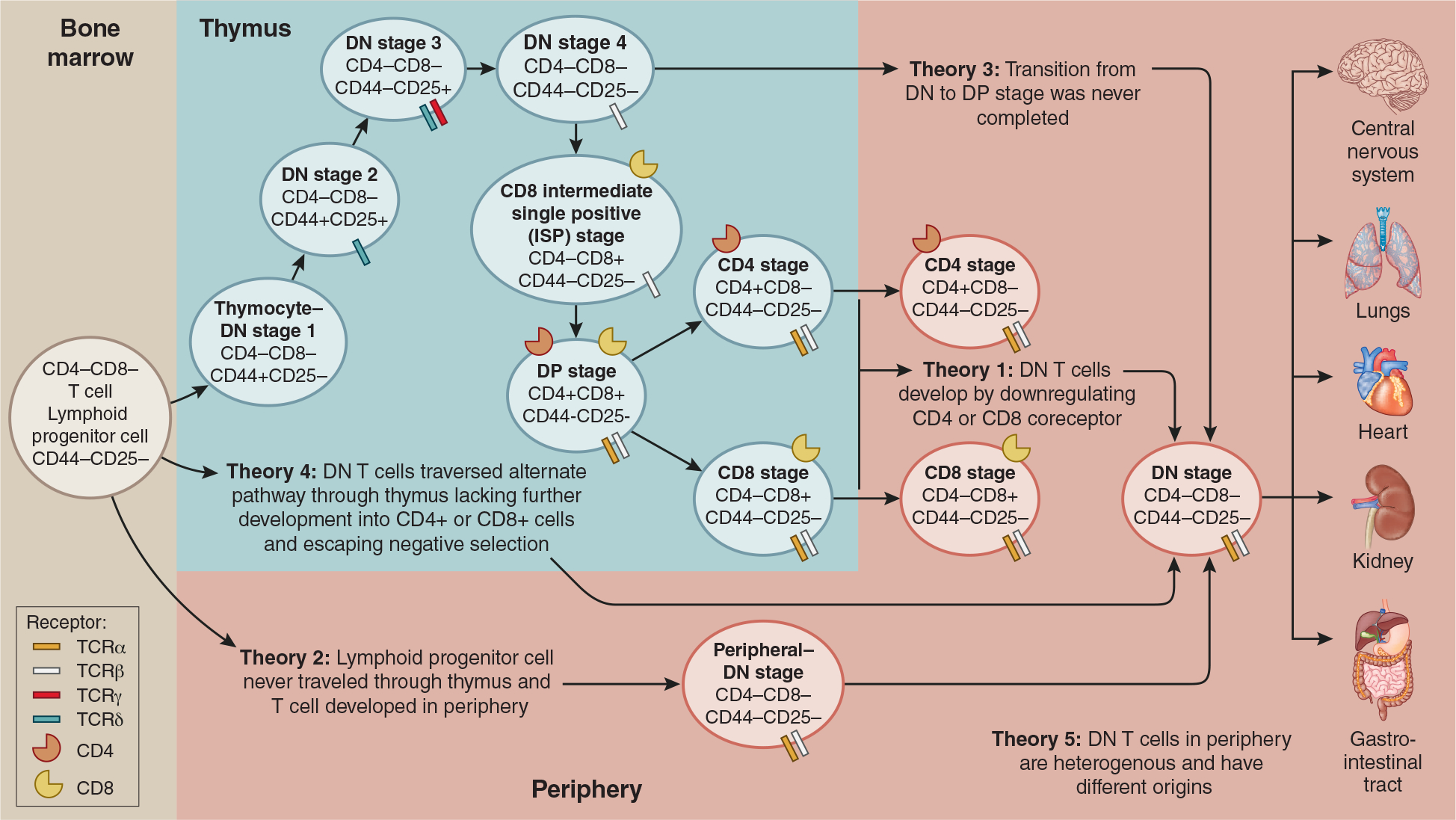
The development of murine αβT cells in thymus physiologically includes DN stages 1–4 of thymocytes that are defined by the expression of CD44+ and CD25+. To describe the development of mature TCRαβ+ double negative (DN) T cells in periphery, there are five theories. According to theory 1 CD4+ or CD8+ down regulate their coreceptor in the periphery to become DN T cells 21,23 Theory 2 hypothesizes hematopoietic stem cells go directly from bone marrow and mature into DN T cells in the periphery.6,24 Theory 3 suggest that DN4 stage to DP transition in thymus was never completed and DN T cells traveled to periphery.25 Theory 4 suggests an alternate pathway through the thymus.6 According to theory 5, peripheral DN T cells are a pool of heterogenous cells with different origins. Peripheral DN T cells have been found in steady-state and diseased organ tissue.4 Of important note, stages of TCR γ and δ selection is currently unclear, however, it is known their arrangement occurs prior to TCR α and β selection during the DN stage.17,20
A second theory supports the notion that early DN T cells, after being synthesized in the bone marrow, were unable to differentiate into either CD4+ or CD8+ co-receptor due to cells passing the thymus completely. This theory is supported by the presence of similar numbers of DN T cells in thymectomized B6 wildtype (WT) mice.6,24 A third theory suggests given that the transition from DN to DP was never completed and T cells left the thymus as DN T cells.2,25 A fourth theory hypothesizes that early T cells made it to the thymus but traversed through an alternate pathway leading to the lack of further development into CD4+ or CD8+ receptors.6,26 Finally, theory five posits that the origin of peripheral DN T cells is variable, and we find a heterogenous pool of cells.2 Testing these different theories represents an opportunity to improve our understanding of the origins of DN T cells.
2. DN T cells in kidney
2.1. Kidney DN T cells in steady state
Unlike the DN T cell population found in lymphoid organs and peripheral blood where they constitute a smaller proportion of T lymphocyte, DN T cells are found in greater quantities in normal kidney tissue (Figure 2).27 20–38% of αβT cells found in normal mouse kidney consists of DN T cells and can vary from 18 to 61% frequency of αβT cells in human kidney biopsy samples.4 In steady state, renal DN T cells tend to express high levels of CD44+ and CD69+ activation subsets, and high levels of CD40L+ and CD28+ co-stimulatory molecules as opposed to CD4+ and CD8+ T cells.4 Kidney DN T cells have also been found to actively divide during steady state when compared to its CD4+ and CD8+ counterparts. It was found that 36% of mouse kidney DN T cells were proliferating, as compared to 1–5% of CD4+ and CD8+ cells by measuring expression of Ki67 nuclear protein.4 This could be due to the lack of co-receptors on DN T cells since these co-receptors have been found to regulate apoptotic signals via the Fas pathway, thus leading to lack of apoptotic signaling within this population.19,28,29 Kidney DN T cells depend on non-classic β2m molecules to maintain their homeostasis in steady-state.10 Moreover, DN T cells in steady state produce high levels of the anti-inflammatory cytokine IL-10, potentially promoted by IL-27, and can suppress CD4+ and CD8+T cells in vitro.4 In mouse kidneys, two key DN T cell subgroups have been identified. The first subgroup is characterized by an MHC-independent programmed cell death protein-1 receptor+ (PD-1+), while the other is an MHC class I–dependent NK1.1+.10,30
Figure 2. Isolation and analysis of kidney DN T cell expansion.
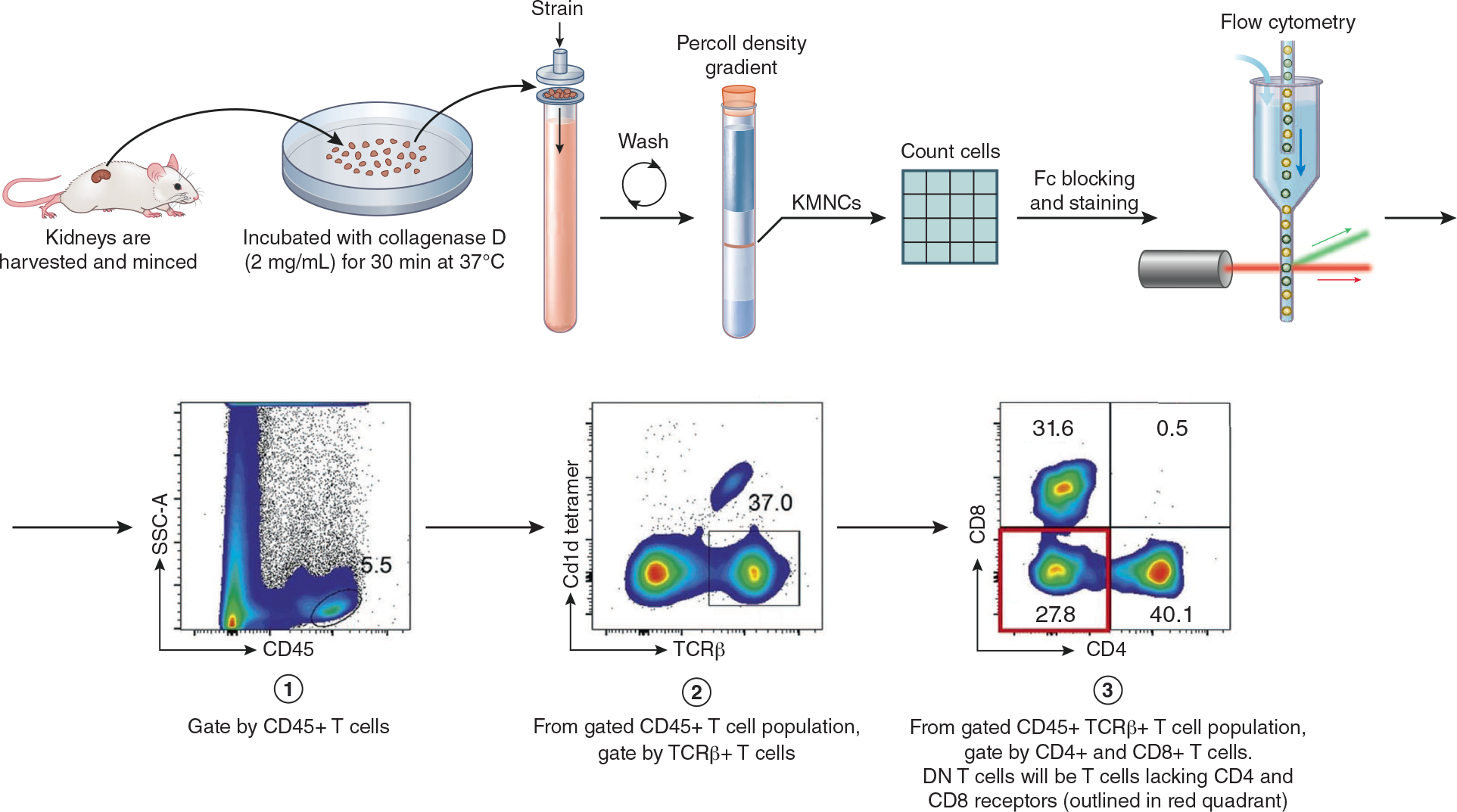
To analyze DN T cell expansion, kidney mononuclear cells (KMNCs) are isolated. After full body exsanguination, kidneys are harvested, minced and incubated with collagenase D (2mg/mL) for 30 min at 37 °C. Kidney tissue is strained to obtain a single cell suspension. After rounds of centrifuging and washing, a Percoll density gradient is obtained. KMNC are then extracted from gradient and counted using a hemocytometer. After Fc-blocking, fluorochrome-conjugated anti-mouse monoclonal antibodies are added and followed by flow cytometric analysis. Cells are gated by CD45+, TCRβ+, Cd1d− and CD4−, CD8− cells. Finally, DN T cell expansion is analyzed. See Martina MN et al. (2014) for full gating strategy.5,27 Gating image obtained from Sadasivam M et al. (2019).10
2.2. DN T cell in acute kidney injury (AKI)
DN T cell subsets in kidney compared to peripheral blood and primary lymphoid organs have been analyzed in AKI mice models (Figure 3).36 An increase of DN T cell number and frequency in mouse kidney 24 hours following ischemic reperfusion injury (IRI) and cisplatin-induced AKI has been described (Table 2).4,5 Concordant increases in the anti-inflammatory cytokine IL-10 and decreases in pro-inflammatory cytokines interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-17A (IL-17A) 24 hours after injury induced by cisplatin supported the hypothesis that the higher DN T cell population could be important for initial AKI recovery.5 The DN T cell population, although increased both in number and frequency, showed a decrease in the programmed cell death protein-1 (PD-1+) DN T cell subset in cisplatin induced AKI.5 CD69+, an important activation marker, has also shown increase among DN T cells post-AKI across studies, also suggesting that these cells may participate in recovery from AKI.5,36
Figure 3. Role of DN T cells in different kidney disease models.
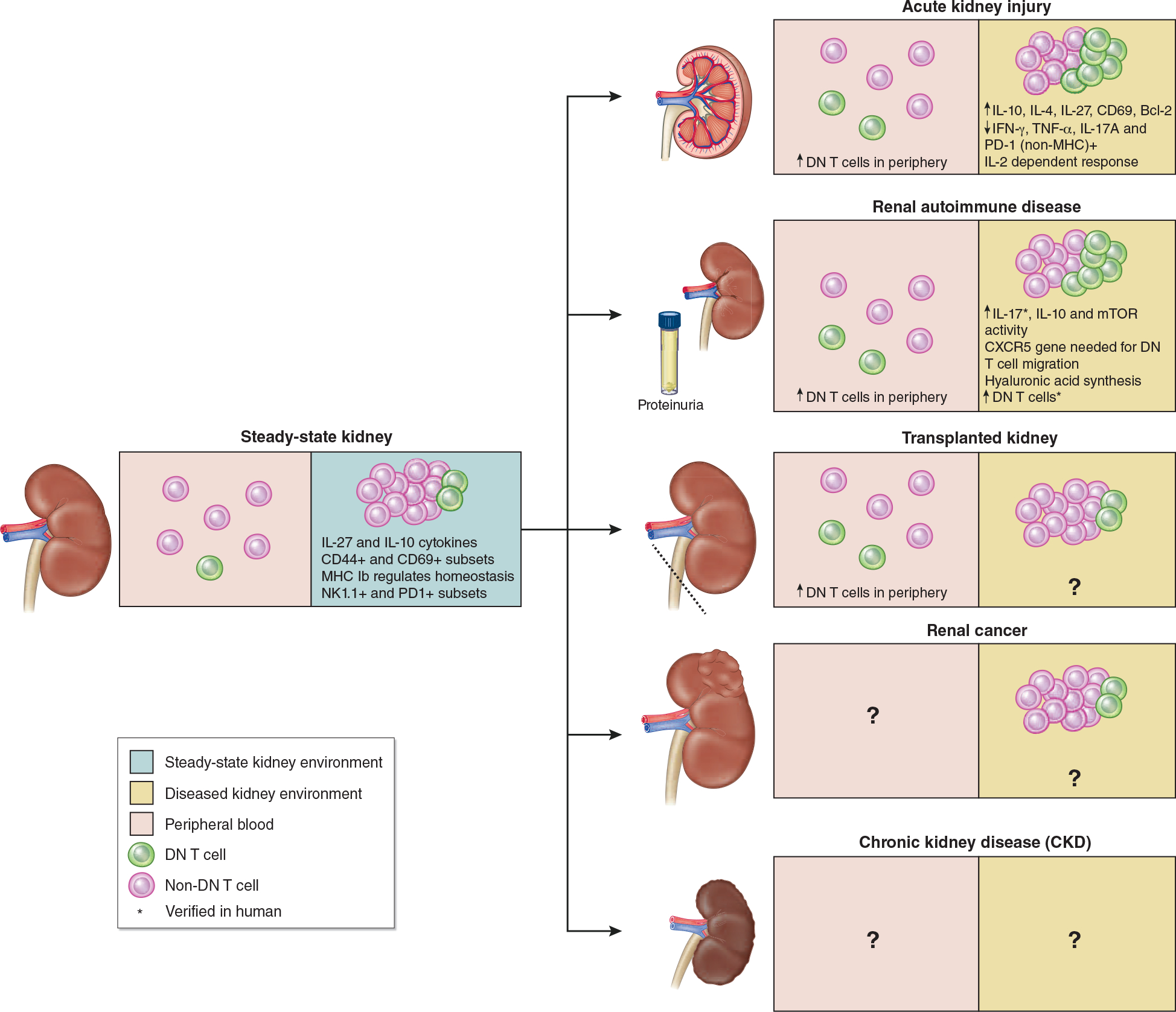
Note: DN T cells are in green and other αβT cells are in red. Proportion between DN T and other αβT cells are not proportional as DN T cells make up a very small percentage of αβT cells. Therefore, the increase in DN T cells is only for the purposes of showing general increases and decreases in DN T cell population percentage.
Steady-state DN T cells in kidney belong to 20–38% of αβT cells in normal murine kidney and show high frequency of IL-27 and IL-10 cytokines and CD44+ and CD69+ subsets.4 MHC Ib regulates homeostasis and two DN T cell subsets have been described – NK1.1+ and PD1+ DN T cells in murine and human kidney. Post-AKI kidney has shown increased DN T cell levels 24hr after injury both in kidney and periphery.4 Autoimmune kidney has shown increase in DN T cell population compared to steady-state kidney, however, DN T cells have been found to further contribute to autoimmune disease progression.31 Following transplanted kidney, peripheral DN T cells have been found to increase, yet the DN T cell population is unknown.19 However, DN T cell adoptive transfer has shown improved survival rates in other cardiac and skin allografts which leads to the hypothesis that this could improve survival rates of kidney transplants.32,33 DN T cell percentage in renal cancer is similar to adjacent tissue, however, the specific role of DN T cells in renal cancer remain unknown.34 We hypothesize treatment of renal cancer with DN T cell adoptive transfer could mitigate growth of cancerous cells.35 The same hypothesis can be made with regards to CKD since adoptive transfer of DN T cells to ischemic kidney has shown decreased kidney injury in mice and can therefore help mitigate AKI to CKD transition.5
Table 2:
Mechanistic studies of DN T cells in AKI
| Experimental Design |
Mechanistic findings | Reference | |
|---|---|---|---|
| Model | Species/strain | ||
|
| |||
| Murine bilateral renal IRI model | Male C57BL/6J WT mice | -Normal mouse kidney of 8-week-old mice showed increased (↑) CD69 on DN T cells compared to 5-week-old mice -3hr post IRI → DN T cell numbers similar to sham-operated -24hr post IRI → decrease (↓) DN T numbers compared to normal and sham-operated → DN T cells had no changes in IFN-γ and TNF-α |
Ascon D et al. (2008)36 |
|
| |||
| Murine bilateral renal IRI model | Male C57BL/6J WT mice | Pre- and post-treatment with mouse anti-thymocyte globulin (mATG) + renal IRI → ↑ DN T percentage and ↓ CD4+ and CD8+ percentage after 72h | Jang HR et al. (2009)37 |
|
| |||
| Murine bilateral renal IRI model | Male C57BL/6J WT mice | -Steady-state kidney → high expression of IL-27 and IL-10 in DN T cells -3 hr and 24hr post IRI → ↑ DN T cell frequency to above baseline levels -72h post IRI → ↓ DN T cell frequency to below baseline levels -3hr post IRI → IL-10 gene expression (16-fold) and ↓ IL-27 gene expression (2-fold) in kidney DN T cells -AT of gld DN T cells into WT → 24 hr → IRI → ↑ protection from AKI compared to PBS treated controls -AT of gld DN T cells into WT + anti-IL-10R neutralizing mAb → 24 hr → IRI → ↓ protection from AKI compared to control In vitro coculture of DN T cells and CD4+ T cells suppressed CD4+ T cell proliferation |
Martina MN et al. (2016)4 |
| Human “normal” kidney adjacent to RCC | Presence of DN T cells with a frequency of 18.3% to 61% | ||
|
| |||
| Murine bilateral renal IRI model | β2m KO (MHC Ia and MHC Ib), MHCII KO and MHC Ia KO C57BL/6J WT mice | -Renal DN T cell homeostasis depends on non-classical MHC Ib: Steady-state β2m KO → ↓ DN T in kidney, potentially due to ↑ apoptosis and ↓ proliferation (no changes in MHCII KO and MHC Ia KO) -Lower activation status at steady-state in β2m KO mice → ↓ CD69 and ↑ CD62L→ maintained high CD44 expression and no change in CD28 expression compared to WT on DN T compared to WT kidney -24h post IRI in WT → ↑ total DN T cell frequency in kidney; ↑ PD-1+ DN T cell subset and ↓ NK1.1+ DN T cell subset -IRI in β2m KO → ↓ total DN T cell frequency in kidney. -DN T cells can be restored by AT of CD8 T cells or IL-2 injection |
Sadasivam M et al. (2019)10 |
| Human “normal” kidney adjacent to RCC | -NK1.1+ (MHC I dependent) and PD-1+ (MHC independent) DN T cell subsets detected -NK1.1+ and PD-1+ DN T cell subsets detected |
||
|
| |||
| Cisplatin-induced AKI model | Male C57BL/6J WT mice | -24hr post cisplatin → ↑ DN T cell frequency and absolute numbers -72hr post cisplatin → ↓ DN T cell frequency and absolute numbers -Cisplatin treatment → ↑ apoptotic DN T cell over time with peak at 72hr post-treatment -24hr post-cisplatin treatment → ↑ CD69 ↑ IL-10; ↓ IFN-γ, TNF-α, and IL-17A in kidney DN T cells -72hr post-cisplatin treatment → CD69 in kidney DN T cells returned to baseline -24hr post-cisplatin treatment → ↓ CD62L in kidney DN T cells but no significant difference from control at 72hr -Cisplatin treatment → ↓ CD44 expression on kidney DN T cells -24hr and 72hr post-cisplatin treatment → ↓ NK1.1+ DN T cells absolute number, but no change in frequency -Adoptive transfer i.v. of gld DN T cells into WT 24hr before cisplatin treatment → renal functional and structural protection after 72hr compared to control - 72hr post-cisplatin treatment in vitro→ ↑ PD-L1 in PTECs -cisplatin treatment of in vitro cocultured PTECs + DN T cells → ↓ PD-L1 protein in PTECs |
Gong J et al. (2020)5 |
Due to evidence of DN T cells correlation with higher anti-inflammatory and lower pro-inflammatory cytokines during AKI recovery, their therapeutic potential was explored in different AKI murine models. DN T cell transfer from lymph nodes of generalized lymphoproliferative disease (gld) mice donors, which have high numbers of DN T cells, to wildtype B6 mice 24 hours before IRI protected against AKI and was IL-10 dependent. In fact, IL-10 increased by 6-fold after IRI in the experimental group.4 The protective effect of adoptively transferred DN T cell was also seen in cisplatin induced AKI.4 When composition of different DN T cell subsets was analyzed in B6 WT mice, an increase of PD-1+ DN T subset at expense of NK1.1+ DN T cell subset, 24h post IRI was found. This might indicate a non-MHC dependent response to ischemia-reperfusion injury of DN T cells in kidney. DN T cells in kidney are also regulated by interleukin (IL)-2 in vivo and in vitro.10 Another study found pre-treatment and post-treatment with mouse anti-thymocyte globulin (mATG) in ischemic murine kidney increased the percentage of DN T cells to become the dominant T cell population, thus decreasing CD4+ and CD8+ T cell percentage within 72 hours after ischemia. However, it is unknown how mATG treatment mechanistically affects DN T cell expansion post-IRI.37
Up to 20% of patients diagnosed with AKI can progress to chronic kidney disease (CKD) within years after hospitalization.38 Increasing data supports the immunomodulatory effect of DN T cells in the recovery phase following AKI.4,5 Hence, novel therapeutic approaches targeting DN T cells could enhance repair and regeneration, which can prevent AKI to CKD transition.
2.3. DN T cells in autoimmune diseases
DN T cells have been studied in many different autoimmune diseases, though primarily in SLE (Figure 3). Systemic lupus erythematosus involves various organ systems like skin, joints, brain, lungs, heart, and kidneys.39 B cells producing autoantibodies are well recognized mediators, however the role of T cells is less well understood. Among T cell subsets, DN T cells are thought to be important in the pathophysiology of SLE and lupus nephritis, therefore has been investigated as potential treatment targets (Table 3).2,52 In SLE patients, increased numbers of DN T cells with significantly higher expression of activation markers are found in peripheral blood, correlate with disease activity, and therefore might contribute to the pathogenesis of SLE.42 In lupus nephritis, DN T cells are found to increase in kidney.43 DN T cells have an impact on Ab production of autologous B cells.40 There is also evidence for DN T cells inducing isotype switch in an CD1c-restricted manner.41 Additionally, DN T cells of lupus patients have been found to release more IL-4 than control cells, correlating with skewing of B cell compartment and anti-DNA Ab production. This process can be triggered by mTOR activation.46 Additionally, it has been shown that DN T cells from SLE patients constitute a source of proinflammatory IFNγ - and IL17-producing cells when stimulated in vitro.43 A factor leading to increased CD3+ CD8−CD4−CD69+IFN-γ+ in kidney is IFN-α, since IFN-α Tg mice have been shown to develop SLE manifestations.51 In vitro stimulation of DN T cells from MRL/lpr or B6/lpr mice using IL-23 further upregulated IL-17 expression. Adoptive transfer of the stimulated CD3+CD8−CD4− DN T cells caused nephritis in Rag-1−/− mice, which lack T- and B-cells.44 Also, IL-23-Receptor KO B6/lpr mice are protected from SLE development.45 Another negative effect of IL-23 is limiting the production of IL2, which itself is protective by decreasing CD4−CD8−CD17+ cells in an MRL/lpr mouse model.48,50 Not only are IL-17 KO mice protected from pristane induced SLE, but also showed significantly less DN T cells, confirming the pathogenetic role of IL-17 producing DN T cells.47 The chemokine receptor CXCR5 has been shown to play an important role in the DN T cells of lupus prone mice. Rag−/− recipient mice of adoptively transferred CD3+CD8−CD4− T cells of B6/lpr CXCR5−/− presented significantly lower numbers of renal CD3+ cells compared to mice receiving control cells.49
Table 3:
Mechanistic studies of DN T cells in systemic lupus erythematosus and lupus nephritis
| Experimental design |
Mechanistic findings | Reference | |
|---|---|---|---|
| Model | Species/strain | ||
|
| |||
| SLE | Human blood DN T cells from clinically active SLE patients | -In vitro culture of DN T cells from SLE patients → ↑ DN T cell frequency and ↑ IgG-class anti-DNA autoantibodies compared to normal and inactive SLE via IL-2 mechanism -Active SLE → majority (72%) CDw29+ DN T cells subset |
Shivakumar S et al. (1989)40 |
|
| |||
| SLE | Human DN T cells from SLE patients and healthy controls (HC) blood samples | -SLE patients → ↑ DN T cells compared to HC -In vitro coculture of DN T cells from SLE patient + CD11+ APCs → IL-4 and IFN-γ -In vitro coculture of DN T cells from HCs → IFN-γ, no production of IL-4 -In vitro coculture of DN T cells from SLE patient + CD11+ APCs → IL-4 production and ↑ IgG1 compared to HC -IgG production from CD1c+ B cells depends on T cell activation through CD1c → Ab production via IL-4 and CD40L interaction |
Sieling PA et al. (2000)41 |
|
| |||
| SLE | Human DN T cells from SLE patients, RA patients, and HC blood samples | -SLE → ↑ TCRαβ+ DN T cell percentage of total DN T cell population compared to RA and HC -SLE → ↑ HLA-DR+ DN T cells, ↑ CD69+ DN T cells, ↑ CTLA4+ DN T cells, ↑ CD28+ DN T cell number compared to RA and HC |
Anand A et al. (2002)42 |
|
| |||
| SLE In vitro experiments |
Human DN T cells from female SLE patients and HC. Prednisone was paused at least 24h before blood draw | -In vitro culture of DN T cells from SLE patients→ ↑ IL-17+ DN T cells at basal culture conditions and after 5d of culture compared to IL-17+ CD4+ T cells -In vitro anti-CD3 stimulation of DN T cells from SLE patients → 5 days → ↑ DN T frequency -In vitro cultured DN T produce ↓ IL-2, ↑TNF-α, and ↑IFN-γ compared to CD4+ T cells (SLE patients and HC) -In vitro cultured DN T cells from SLE → ↑ IL-17+ DN T compared to DN T cells from HCs |
Crispin JC et al. (2008)43 |
| Kidney biopsies of SLE patients | -SLE patients’ kidney biopsies → DN T cells with positive immunofluorescent staining of IL-17 and IL-23 | ||
|
| |||
| SLE In vivo and in vitro experiments |
MRL/lpr mice | -In vitro IL-23 stimulation of DN T cells → ↑IL-17 expression compared to control cells -AT of stimulated MRL/lpr DN T → nephritis in Rag-1−/− mice (deposition of Ig and C3d in glomeruli) |
Zhang Z et al. (2009)44 |
|
| |||
| SLE | Mice, IL-23R deficient C57BL/6 | -IL-23-R KO B6/lpr mice protected from SLE, ↓ DN T cell number and percentage of IL-17+ DN T cells, ↓ IFN-γ, ↓ IgG and anti-dsDNA Abs compared to B6/lpr mice | Kyttaris VC et al. (2010)45 |
|
| |||
| SLE | Human DN cells from SLE patients and HC | -SLE → ↑ mTOR activity in DN T and ↓ D3+CD4+CD25+ FoxP3+T cells -SLE → ↑IL-4 of DN T cells, correlating with production of anti-DNA Ab -Rapamycin treatment of SLE patients → ↓ necrosis and ↓ IL-4 production of DN T (↓MFI) -No changes in DN IL4+ T cells or DN IL17+ T percentage |
Lai Z et al. (2013)46 |
|
| |||
| SLE | Mice, WT C57BL/6 and double KO IL-17−/−/Stat−/− Pristane lupus induction | -IL-17 KO mice protected from pristane induced SLE, ↓ DN T -IL-17 deficiency → protection from SLE independent of Stat1 activity and ↓ DN T and ↑ Tregs -IL-17 → anti-ssDNA, anti-nRNP and anti-chromatin autoantibody production |
Amarilyo G et al. (2014)47 |
|
| |||
| SLE | Mice, MRL/lpr− | -IL-2 (usage of IL-2-recombinant adeno-associated virus) → ↓ IL-17+ DN T cells by inducing cell death -IL-2/anti–IL-2 Ab complex and targeting of IL-2 to cytotoxic lymphocytes → ↓ DN T cells and INF-γ, no change of anti-dsDNA antibody production -IL-2 → ↑ DN T cell death via CD122 receptor and STAT5 phosphorylation by IL-2 on CD8+ T cells. |
Mizui M et al. (2014)48 |
|
| |||
| SLE | Female MRL-lpr mice | -A77 1726 (active metabolite of leflunomide) treatment → ↓ lupus activation (↓anti-dsDNA, ↓anti-ANA, ↓ inflammation in kidney histology samples) -A77 1726 treatment → Akt dependent ↑ CD3+CD4+CD25+FoxP3 + T cells → ↓ splenic IL17+ DN T |
Qiao G et al. (2015)31 |
|
| |||
| SLE | B6/lpr CXCR5−/− mice | -AT of DN T cells of B6/lpr CXCR5−/− → ↓ CD3+ in recipient Rag−/− mice compared to controls receiving B6/lpr DN T cells | Wiener A et al. (2016)49 |
| In vitro studies | -In vitro trans-well studies → ↓ chemotactic response of splenic DN T cells towards CXCL13 compared to control B6/lpr DN T | ||
|
| |||
| SLE | Human blood sample of SLE patients | -IL-23 treatment → ↑ SLE DN T numbers independent of Tfh markers and ↑ IL-17 production in vitro -IL-23 treatment → ↓ IFN-γ and IL-2 by SLE T cells in vitro via suppression of NFkBp65 (IL-2 enhancer) → ↑ Tfh cells → ↑ anti-dsDNA independent of DN T |
Dai H et al. (2017)50 |
| Mice, IL-23R−/−MRL-lpr | -IL-23R deficiency → ↓ CD45+ DN T infiltration, DN T cells dominant T cell subset, ↓ IL-17 and ↓IFN-γ in SLE kidney model -IL-23 treatment → ↑ IL-17+ T cells and ↑IL-17 production in vitro -IL-23+/+ MRL-lpr → ↑ CD4+ T cells becoming DN T compared to IL-23 KO MRL-lpr and control MRL-MPJ mice -IL-23+/+ MRL-lpr → ↑ DN T proliferation compared to IL-23 KO MRL-lpr in spleen and peripheral lymph nodes -IL-23−/− MRL-lpr → ↑ IL-2 compared to WT MRL-lpr in vitro |
||
|
| |||
| SLE | IFN-α Tg Mice | -IFN-α Tg Mice express ↑ IFN-α and develop SLE manifestations (glomerulonephritis) -↑ IFN-α leads to ↑ CD69+ IFN-γ+ DN T in kidney |
Akiyama C et al. (2019)51 |
Marginal zone macrophages (MZMs) are splenic macrophages at the edge of splenic follicles important for immune tolerance since removing apoptotic cells is important to limit autoimmunity. Both lupus-prone mice and SLE patients have less MZMs.53 Recent work demonstrated a mechanistic link between MZM defects and DN T cells in SLE. DN T cells significantly expanded in spleens and infiltrated kidneys following depletion of MZMs in a lupus-prone mouse model. These DN T cells derived from self-reactive CD8+ T cells, and not CD4+. CD8+ OT-I (or CD4+ OTII) TCR transgenic T cells, designed to recognize ovalbumin peptide residues, and m-OVA Tg-derived apoptotic thymocytes were injected into WT mice with and without depletion of MZM. Autoreactive CD8+ T cells in this model lost CD8+ receptor expression and differentiated into DN T cells after MZM depletion. The authors attributed this mechanism to dead cell debris retention and their associated antigens following MZM depletion. When myeloid cell-specific TGF-β1 KO mice were used as recipients for CD8+ OT-I TCR transgenic T cells and m-OVA Tg- derived apoptotic thymocytes, proliferation of CD8+ OT TCR transgenic T cells was maintained compared to controls. This indicated that tolerogenic cytokine TGF-β1 excreted by MZMs are important for DN T cell suppression and apoptotic cell clearance, which can reduce autoimmunity.54
A77 1726, an active metabolite of leflunomide, led to reduction of SLE related antibodies, such as anti-dsDNA and anti-ANA and significantly less glomerular and interstitial inflammation in kidney histology samples of MRL/lpr mice. As a potential mechanism, the authors invoked a downregulation of IL17+ DN T cells in spleen of those mice along with an Akt dependent upregulation of CD3+CD4+CD25+FoxP3+ T cells.31
The role of DN T cells in other rheumatic diseases has also been explored. IL-17 producing DN T cells are expanded in the peripheral blood and salivary glands of Sjögren’s syndrome (SS) patients with active disease, further supporting the pathological role of DN T cells in rheumatic diseases.3,55,56 In autoimmune lymphoproliferative syndrome (ALPS), high numbers of DN T cells in peripheral blood and secondary lymphoid organs are found. Clinical manifestations are lymphadenopathy with risk for development of lymphoma, splenomegaly, and autoimmunity.57 The deficiency of Fas or Fas ligand (FasL) in patients with ALPS is thought to lead to a decrease in apoptosis of DN T cells and therefore their accumulation. Mouse strains that lack Fas (lpr) or FasL (gld) mimic ALPS best, and are also used as SLE models as mentioned above.3 Psoriasis can be associated with systemic inflammation. Psoriatic skin lesions are characterized by keratinocyte proliferation, with DN T cells producing IL-17 and IFN-γ identified as pathophysiologic mediators.58,59
2.4. DN T cells in renal and other cancers
DN T cells have been found in many human tumor types including renal cell carcinoma, melanoma, leukemia, multiple myeloma, non-small cell lung cancers (NSCLC), among others (Figure 3). Although the role of DN T cells specifically in cancer development is still largely unknown, quantification of these cells in different kinds of cancer tissue and treatment options has been analyzed. In renal cell carcinoma (RCC), there are approximately 6% of DN T cells. Similar frequencies were found in adjacent normal kidney tissues. However, this was despite an overall increase of T cells in RCCs. The presence of DN T cells, which have also been found to express certain immune co-inhibitory receptors, such as PD-1, might make them a potential target for immunotherapy.34
Much like the population of DN T cells in primary lymphoid organs, those present in lung cancer tissue make up only a small fraction of total T cells (1.4%).60 Additionally, patient lung adenocarcinoma tissue shows a reduced frequency of DN T cell population compared to that of normal lung tissue and DN T cells from lung cancer patients have high cytotoxicity against lung cancer cell lines.61,62 Similarly, DN T cell populations in human multiple myeloma (MM) and monoclonal gammopathy of uncertain significance (MGUS) peripheral blood samples were significantly lower than healthy controls, which might contribute to progression.63 In contrast, DN T cell percentage of T cell population was doubled in melanoma tumors compared to healthy lymph nodes (7.23% ± 17.18% and 3.57% ± 1.52% respectively).64 MHC class-I restricted human DN T cell isolated from the peripheral blood of a melanoma patient secreted IFN-γ and TNF cytokines and strong TCR-dependent Ag-specific cytotoxic activity, thus possible anti-tumor activity.65
Other reports have used human DN T cells isolated from the peripheral blood and cocultured pancreatic cancer cells (Panc-1). Panc-1 cells cocultured with DN T cells showed reduced cell migration compared to control.66 Similarly in mice, DN T cells had a 35.5% inhibitory rate on pancreatic cancer cells in vitro.67 Moreover, human DN T cells promoted an increase in IFN-γ and FasL levels when cocultured with Panc-1 cells.66 Decreased MHCI chain-related molecules (MICA) mRNA and protein levels, with NKG2D mRNA and protein levels were increased in pancreatic cancer cells with DN T cell injection i.v. than those without an intervention in a mouse model.67
The immunotherapy potential of DN T cells has been explored in different disease models. DN T cell therapy has shown clinical promise for the treatment of acute myeloid leukemia (AML). Expanded ex-vivo DN T cells have also been found to eliminate allogeneic and autologous primary CD34+ leukemic blasts in vitro following chemotherapy therapy which can lead less frequent relapse among AML patients.68 Their ability to target AML cells is due primarily to cognate ligands expressed on AML cells binding to the high levels of NKG2D and DNAM-1 receptors on DN T cells.69 Moreover, IFN-γ and TNF-α were found at high levels in DN T cells expanded during chemotherapy, suggesting an effective ability of DN T cells in tumor immunity. Furthermore, when used as effectors in cytotoxicity assays in vitro, DN T cells were able to eliminate leukemic cells and higher doses of DN T cells resulted in higher eradication rates.68 Healthy donor derived allogeneic ex-vivo expanded DN T cells have been found to have anticancer properties when targeting myeloid leukemia in-vitro and in immunodeficient mouse AML xenograft model. This was evidenced by cytotoxic activity against leukemic blast markers and decreased AML engraftment levels in the bone marrow by 17.1-fold, respectively.70 DN T cells can prevent local tumor development in animals when DN T cells were co-infused locally with A20 lymphoma cell line.71 The mechanisms of DN T cell anti-tumor activity is relatively unknown.
Anti-PD-1 has been shown to increase the ability of DN T cells to attack cancerous lung tissue, therefore leading to greater chances of survival for mice affected.61 Moreover, IL-15 cytokine has been demonstrated to increase in cytotoxicity against non-small-cell lung cancer.62 It has also been suggested, due to DN T cells promoting increased FasL levels when cocultured with Panc-1 cells, the increasing inhibition of cancer cell proliferation occurs via Fas/FasL pathway, an apoptosis mediating pathway.71
3. DN T cells in organ transplant
Accumulation of DN T cells has been found in the peripheral blood following organ transplantation, suggesting a possible role in alloimmunity.19 The correlation between high DN T cell percentage and increased graft survival in humans can be modeled using C57BL/6.lpr or C57BL/6 gld mice, which accumulate high numbers of DN T cells due to homozygous lymphoproliferation spontaneous mutation in Faslpr and Fasgld respectively. These models have shown high tolerance to skin allografts via antigen-specific syngeneic T cell suppression and via the Fas-FasL pathway.72,73 In patients who received hematopoietic stem cell allografts, higher DN T cell percentage was correlated with lower graft rejection risk.74 In lpr and gld mice, the natural presentation of heightened DN T cell levels, and thus correlated protective behavior against skin allografts, demonstrates the protective role of DN T cell for allograft transplantation. DN T cell adoptive transfer from normal mice could promote skin allograft, cardiac allograft, and xenograft survival in mice.75,76 Moreover, DN T adoptive transfer to lethally irradiated mice after bone marrow transplant can prevent allogeneic T cell-induced graft versus host disease (GvHD).77 The possible mechanisms by which DN T cells provide graft protection depend on the model. Bone marrow engraftment survival by DN T cells occurs due to perforin (PFN) expression on DN T cells and their subsequent inhibition of NK cells in murine models. The NK cell suppression by DN T cells is via PFN/FasL-dependent mechanism as evidenced by DN T cells from C57BL/6 and gld mice displaying a stronger ability to kill NK cells than DN T cells from PFN−/− mice in vitro.78 Likewise, the PFN/granzyme-dependent process of NK cell inhibition via DN T cells was also found in rat to mouse cardiac xenograft survival. In this study, adoptive transfer of DN T cells was also found to elicit B-cell apoptosis through PFN dependent pathway while also inhibiting anti-donor antibody production.79 Moreover, DN T cell suppression of syngeneic CD4+ and CD8+ T cells can occur via FasL-Fas dependent and independent mechanisms, antigen-specific, and DN T to effector T cell contact and cytotoxicity via TCR-MHC mechanisms in mice.6 However, these mechanisms of action differ in human DN T cells, where their dependence relies on reversible effector T cell interactions independent of FasL-Fas pathway and perforin expression.80 Data on kidney transplant patients revealed a correlation between peripheral DN T cells and graft survival. As such, patients with stable transplant function in the first year had increased DN T cells in peripheral blood and late graft dysfunction correlated with a drop in DN T cells in blood.81 Thus, the role of DN T cells after kidney transplantation presents an opportunity for further investigation.
4. DN T cells in non-renal organs
4.1. CNS
The role of DN T cells following ischemic stroke and other pathological conditions of the central nervous system is being increasingly studied. DN T cells have shown to significantly increase to 17% of lymphocytes in post-ischemic cerebral hemisphere in mice.82 Numbers of DN T cells in ischemic brain were elevated by 3–4-fold following ischemia in mouse brain via permanent and transient middle cerebral artery occlusion model.83 Similar results were found in human postmortem brain sections following stroke. In both ischemic mice and human samples, DN T cells were found to accumulate near activated microglia over time, suggesting both spatial and temporal cross-talk between these cells.84 The same study concluded DN T cells contribute to a proinflammatory response by TNF-α secretion via the FasL pathway which led to increase in proinflammatory microglia (CD86+), and a decrease in anti-inflammatory microglia (CD206+) following coculture with DN T cells.84 When analyzing mononuclear cells in the subarachnoid space of rats with experimental autoimmune encephalomyelitis (EAE), more than 50% of the TCRαβ cells were DN T cells. This was similar to findings in extrathymic T cells in autoimmune mice.85,86
4.2. Lung
DN T cells have shown promising outcomes during various types of lung infection models in mice. In a study observing lungs of mice with pulmonary Francisella tularensis live vaccine strain (LVS) intranasal infection, DN T cells were major responders contributing to 11–15% of all lung T cells two weeks after infection. Pulmonary DN T cells were responsible for high IFN-γ production during acute phase of infection and IL-17A a week after infection.87 However, DN T cells alone are hypothesized to lack the function required for clearance of LVS infection in vivo due to prolonged infection in mice depleted of all other T cells.87 DN T cells in murine lungs have also been found to produce IL-5 cytokines following infection with Toxocara canis.88 Similar results were found in mice infected with cilia-associated respiratory (CAR) bacillus, with a 12% increase in DN T cells of bronchiolar lymph nodes compared to uninfected mice.89 Additionally, frequency of pulmonary DN T cells showed a significant increase in M. tuberculosis infected mice.90 In murine M. tuberculosis challenge model, vaccination against tuberculosis (TB) using BCGA4 mutant strain (BCG-A4) prepared with DDA/TDB adjuvant (A4/Adj) showed higher stimulation of DN T cells, higher frequency of IFN-γ production by DN T cells and enhanced protection against TB infection compared to non-vaccinated controls.91 However, in an other infectious pulmonary model, DN T cells administered to SCID/beige mice infected with Rhodococcus equi were unable to clear the infection, unlike CD4+ and CD8+ administration.92 Following influenza A virus (IAV) infection in mice, NK1.1− DN T cells expand 20-fold. Yet, the capability of these DN T cells to produce IFN-γ cytokines decreased. Moreover, NK1.1− DN T cell role in the lung has shown to stimulate the survival of CD11chi dendritic cells in coculture, pointing out their potential role as immunoregulatory cells.93
Given the promise of DN T cell therapy in ischemic AKI, the effects in lung IRI were studied. Using a murine lung ischemia model, DN T cells were found to expand post-injury and increase production of anti-inflammatory cytokines IL-10 and IFN-γ. Moreover, mice treated with DN T cell adoptive transfer showed significant protection from lung IRI.94 DN T cells have also shown promise for treating asthma, with adoptive transfer of DN T cells primed with ovalbumin (OVA) in an OVA-induced allergic airway disease model showed reduced inflammatory cell infiltration of lungs and reduced proportion of lung DCs.95
4.3. Heart
Recent studies have examined the efficacy of DN T cell therapy on cardiac graft-versus-host disease survival and autoimmune myocarditis. A study in mice found adoptive transfer of donor-lymphocyte infusion (DLI) activated DN Treg cells pre-transplant prevented CD4+ cells from rejecting cardiac xenografts of Lewis rats. Moreover, DN T cells isolated from DLI treated heart graft recipient mice were able to suppress proliferation of CD4+ cells in vitro.32 A similar study showed that infusion of DN T cell clones prior to MHC class I-mismatched allogenic heart-grafts in mice showed survival of over 100 days compared to infusion of DN T cell mutants that had acquired CD8+ expression, which had a survival average of 19.5 days post-transplantation.96 Rats with experimental autoimmune myocarditis (EAM) were found to have greater concentration of DN T cells in mononuclear cells isolated from the pericardial effusion (16.1%) compared to in the heart (1.7%).97 It is important to note that work on the heart demonstrates the paradox of the protective role of DN T cells in alloimmunity, but deleterious role in autoimmunity.
4.4. Gastrointestinal Tract
About 15–20% of CD3+ cells are DN in the mucous membranes of the lamina propria of small and large intestine in euthymic and athymic mice, suggesting that DN T cells may be resident in the gut epithelium and not derived from DP T cells.98 Additionally, the percentages of TCRαβ DN T cells were higher in the cecum and colon compared to the duodenum, jejunum and ileum, and was significantly high in the cecum of female mice.99 In steady-state intestine, DN and DP T cells were found in similar distributions.100 In the epithelium of 6-week-old WT mouse colon, duodenum, jejunum and ileum, TCRαβ DN T cells expressing syndecan-1 (sdc-1), a surface marker for intestinal epithelium, have been found and frequency increases with age. By the time WT are 24 weeks old, DN T cells form the majority of the TCRαβ T cells.101 The same study found gld DN T cell proliferation in the gut epithelium is independent of the Fas pathway and occurring rapidly compared to the gld DN T cell proliferation in the periphery which has allowed DN T cells to be highly resident in the gut epithelium. The authors suggested that the Fas pathway plays a key role in confining gld DN T cells to the gut epithelium and preventing lymphoproliferation through Fas-mediated apoptosis. This provides evidence for the importance of the Fas-pathway when distributing DN T cells to different organs, including the gut.101
When analyzing duodenal biopsies from children with celiac disease, DN T cells were increased 6-fold compared to controls, however 80% of these DN T cells were TCRγδ and not TCRαβ.102 Additionally, there was an increase of the general DN T cell population in microscopic colitis and a reduction of DN T cells in Crohn’s disease, while showing no differences in the peripheral blood compared to healthy individuals.100 Whereas homeostasis of DN T population in lymphoid tissue has been shown to depend on Fas-mediated apoptosis of DN T cells in the periphery, homeostasis of intraepithelial tissue DN T cells is independent of peripheral Fas-regulated apoptosis.101
Conclusion
There is increasing data from experimental studies and limited human samples that DN T cells are important mediators in many diseases. Research in the field has been hampered by challenges in identifying a specific positive surface marker on these cells. In addition, it is important that researchers on DN T cells use uniform methodology to characterize these cells. Understanding how DN T cells engage in non-renal diseases can aid in understanding how they could be involved in kidney diseases. DN T cells are increasingly felt to be a heterogenous T cell subset, which could explain the broad range of activities attributed to them.
Kidney DN T cells are found in relatively high numbers and have unique subsets which are closely regulated. DN T cells play a pathophysiologic role in lupus nephritis, protect from ischemic AKI in murine models, correlate with stable kidney transplant function, can be found in human RCCs. With the tremendous recent success and promise for T cell based human therapeutics such as immune checkpoint inhibitors and CAR T cells, the DN T cells are a promising target in many different kidney diseases. Recent advances in gene editing technology, such as CRISPR-Cas9, could allow scientists to engineer proinflammatory DN T cells in patients with SLE to slow disease progression. Alternatively, patients at risk for or with AKI could be administered in vitro expanded DN T lymphocytes for prevention or to improve recovery.
Acknowledgements
The authors are grateful for the support of Dr. Werner Jackstädt Foundation scholarship (project number S 134-10.117 to JTK), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK104662 (to HR).
Footnotes
Disclosure Statement
The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fischer K, Voelkl S, Heymann J, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8− double-negative regulatory T cells. Blood. 2005;105(7):2828–35. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Tsokos GC. Double-negative T cells in autoimmune diseases. Current Opinion in Rheumatology. 2021;33(2):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt D, Hedrich CM. TCRαβ+CD3+CD4−CD8− (double negative) T cells in autoimmunity. Autoimmun Rev. 2018;17(4):422–430. [DOI] [PubMed] [Google Scholar]
- 4.Martina MN, Noel S, Saxena A, et al. Double-negative αβ T cells are early responders to AKI and are found in human kidney. J Am Soc Nephrol. 2016;27(4):1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong J, Noel S, Hsu J, et al. TCR+CD4−CD8− (double negative) T cells protect from cisplatin-induced renal epithelial cell apoptosis and acute kidney injury. Am J Physiol Renal Physiol. 2020;318(6):F1500–F1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juvet SC, Zhang L. Double negative regulatory T cells in transplantation and autoimmunity: Recent progress and future directions. J Mol Cell Biol. 2012;4(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mou Z, Liu D, Okwor I, Jia P, Orihara K, Uzonna JE. MHC class II restricted innate-like double negative T cells contribute to optimal primary and secondary immunity to leishmania major. 2014;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Zhu Y, Tian D, et al. Transcriptome landscape of double negative T cells by single-cell RNA sequencing. J Autoimmun. 2021;121:102653. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Rodríguez N, Flores-Mendoza G, Apostolidis SA, Rosetti F, Tsokos GC, Crispín JC. TCR-α/β CD4(−) CD8(−) double negative T cells arise from CD8(+) T cells. J Leukoc Biol. 2020;108(3):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadasivam M, Noel S, Lee SA, et al. Activation and proliferation of PD-1+ kidney double-negative T cells is dependent on nonclassical MHC proteins and IL-2. J Am Soc Nephrol. 2019;30(2):277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farr AR, Wu W, Choi B, Cavalcoli JD, Laouar Y. CD1d-unrestricted NKT cells are endowed with a hybrid function far superior than that of iNKT cells. Proc Natl Acad Sci U S A. 2014;111(35):12841–12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda M, Shadeo A, MacFadyen AM, Takei F. CD1d-independent NKT cells in beta 2-microglobulin-deficient mice have hybrid phenotype and function of NK and T cells. J Immunol. 2004;172(10):6115–6122. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Chen Z, McCluskey J, Corbett AJ. Mouse models illuminate MAIT cell biology. 2021;130:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murayama G, Chiba A, Suzuki H, et al. A critical role for mucosal-associated invariant T cells as regulators and therapeutic targets in systemic lupus erythematosus. Front Immunol. 2019;10:2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemenoff RA, Kleczko EK, Hopp K. Renal double negative T cells: Unconventional cells in search of a function. Ann Transl Med. 2019;7(8):S342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1(1):31–40. [DOI] [PubMed] [Google Scholar]
- 17.Spits H Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2(10):760–772. [DOI] [PubMed] [Google Scholar]
- 18.Dik WA, Pike-Overzet K, Weerkamp F, et al. New insights on human T cell development by quantitative T cell receptor gene rearrangement studies and gene expression profiling. J Exp Med. 2005;201(11):1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martina MN, Noel S, Bandapalle S, Hamad AR, Rabb H. T lymphocytes and acute kidney injury: Update. Nephron Clin Pract. 2014;127(1–4):51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livák F, Tourigny M, Schatz DG, Petrie HT. Characterization of TCR gene rearrangements during adult murine T cell development. J Immunol. 1999;162(5):2575–2580. [PubMed] [Google Scholar]
- 21.D’Acquisto F, Crompton T. CD3+CD4−CD8− (double negative) T cells: Saviours or villains of the immune response? Biochemical Pharmacology. 2011;82(4):333–340. [DOI] [PubMed] [Google Scholar]
- 22.Burt R, Verda L. Immune reconstitution. Handbook of Stem Cells. 2004;2:745–761. [Google Scholar]
- 23.Mehal WZ, Crispe IN. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol. 1998;161(4):1686–1693. [PubMed] [Google Scholar]
- 24.Ford MS, Zhang Z, Chen W, Zhang L. Double-negative T regulatory cells can develop outside the thymus and do not mature from CD8+ T cell precursors. J Immunol. 2006;177(5):2803–2809. [DOI] [PubMed] [Google Scholar]
- 25.Takahama Y, Kosugi A, Singer A. Phenotype, ontogeny, and repertoire of CD4−CD8− T cell receptor alpha beta + thymocytes. variable influence of self-antigens on T cell receptor V beta usage. J Immunol. 1991;146:1134–1141. [PubMed] [Google Scholar]
- 26.Egerton M, Scollay R. Intrathymic selection of murine TCR alpha beta+CD4−CD8− thymocytes. Int Immunol. 1990;2(2):157–163. [DOI] [PubMed] [Google Scholar]
- 27.Martina MN, Bandapalle S, Rabb H, Hamad AR. Isolation of double negative αβ T cells from the kidney. J Vis Exp. 2014(87). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Dudhane A, Orlikowsky T, et al. CD4 engagement induces fas antigen-dependent apoptosis of T cells in vivo. European Journal of Immunology. 1994;24(7):1549–1552. [DOI] [PubMed] [Google Scholar]
- 29.McConkey DJ, Fosdick L, D’Adamio L, Jondal M, Orrenius S. Co-receptor (CD4/CD8) engagement enhances CD3-induced apoptosis in thymocytes. implications for negative selection. J Immunol. 1994;153(6):2436–2443. [PubMed] [Google Scholar]
- 30.D’Alessio FR, Kurzhagen JT, Rabb H. Reparative T lymphocytes in organ injury. J Clin Invest. 2019;129(7):2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao G, Yang L, Li Z, Williams JW, Zhang J. A77 1726, the active metabolite of leflunomide, attenuates lupus nephritis by promoting the development of regulatory T cells and inhibiting IL-17-producing double negative T cells. Clin Immunol. 2015;157(2):166–74. [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Zhou D, Torrealba JR, Waddell TK, Grant D, Zhang L. Donor lymphocyte infusion induces long-term donor-specific cardiac xenograft survival through activation of recipient double-negative regulatory T cells. J Immunol. 2005;175(5):3409–16. [DOI] [PubMed] [Google Scholar]
- 33.Hillhouse EE, Delisle J, Lesage S. Immunoregulatory CD4−CD8− T cells as a potential therapeutic tool for transplantation, autoimmunity, and cancer. Frontiers in immunology. 2013;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurzhagen JT, Noel S, Sadasivam M, et al. Double-negative T cells in human renal cell carcinoma – potential target for immune checkpoint inhibition. J Am Soc Nephrol. 2018;29. [Google Scholar]
- 35.Chen J, Hu P, Wu G, Zhou H. Antipancreatic cancer effect of DNT cells and the underlying mechanism. Pancreatology. 2019;19(1):105–113. [DOI] [PubMed] [Google Scholar]
- 36.Ascon DB, Ascon M, Satpute S, et al. Normal mouse kidneys contain activated and CD3+CD4−CD8− double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol. 2008;84(6):1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang HR, Gandolfo MT, Ko GJ, Racusen L, Rabb H. The effect of murine anti-thymocyte globulin on experimental kidney warm ischemia-reperfusion injury in mice. Transpl Immunol. 2009;22(1–2):44–54. [DOI] [PubMed] [Google Scholar]
- 38.Belayev LY, Palevsky PM. The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23(2):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6(1):7–9. [DOI] [PubMed] [Google Scholar]
- 40.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143(1):103–112. [PubMed] [Google Scholar]
- 41.Sieling PA, Porcelli SA, Duong BT, et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J Immunol. 2000;165(9):5338–5344. [DOI] [PubMed] [Google Scholar]
- 42.Anand A, Dean GS, Quereshi K, Isenberg DA, Lydyard PM. Characterization of CD3+ CD4−CD8− (double negative) T cells in patients with systemic lupus erythematosus: Activation markers. Lupus. 2002;11(8):493–500. [DOI] [PubMed] [Google Scholar]
- 43.Crispín JC, Oukka M, Bayliss G, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183(5):3160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184(9):4605–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai ZW, Borsuk R, Shadakshari A, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol. 2013;191(5):2236–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amarilyo G, Lourenço EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193(2):540–543. [DOI] [PubMed] [Google Scholar]
- 48.Mizui M, Koga T, Lieberman LA, et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4−CD8− IL-17-producing T cells. J Immunol. 2014;193(5):2168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiener A, Schippers A, Wagner N, et al. CXCR5 is critically involved in progression of lupus through regulation of B cell and double-negative T cell trafficking. Clin Exp Immunol. 2016;185(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai H, He F, Tsokos GC, Kyttaris VC. IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J Immunol. 2017;199(3):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akiyama C, Tsumiyama K, Uchimura C, et al. Conditional upregulation of IFN-α alone is sufficient to induce systemic lupus erythematosus. J Immunol. 2019;203(4):835–843. [DOI] [PubMed] [Google Scholar]
- 52.Katsuyama T, Tsokos GC, Moulton VR. Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front Immunol. 2018;9:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGaha TL, Karlsson MC. Apoptotic cell responses in the splenic marginal zone: A paradigm for immunologic reactions to apoptotic antigens with implications for autoimmunity. Immunol Rev. 2016;269(1):26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Adamopoulos IE, Moulton VR, et al. Systemic lupus erythematosus favors the generation of IL-17 producing double negative T cells. Nat Commun. 2020;11(1):2859–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alunno A, Carubbi F, Bistoni O, et al. CD4(−)CD8(−) T-cells in primary sjögren’s syndrome: Association with the extent of glandular involvement. J Autoimmun. 2014;51:38–43. [DOI] [PubMed] [Google Scholar]
- 56.Alunno A, Bistoni O, Bartoloni E, et al. IL-17-producing CD4−CD8− T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary sjogren’s syndrome. Ann Rheum Dis. 2013;72(2):286–292. [DOI] [PubMed] [Google Scholar]
- 57.Bleesing JJ, Brown MR, Novicio C, et al. A composite picture of TcR alpha/beta(+) CD4(−)CD8(−) T cells (alpha/beta-DNTCs) in humans with autoimmune lymphoproliferative syndrome. Clin Immunol. 2002;104(1):21–30. [DOI] [PubMed] [Google Scholar]
- 58.Ueyama A, Imura C, Fusamae Y, et al. Potential role of IL-17-producing CD4/CD8 double negative αβ T cells in psoriatic skin inflammation in a TPA-induced STAT3C transgenic mouse model. J Dermatol Sci. 2017;85(1):27–35. [DOI] [PubMed] [Google Scholar]
- 59.Brandt D, Sergon M, Abraham S, Mäbert K, Hedrich CM. TCR(+)CD3(+)CD4(−)CD8(−) effector T cells in psoriasis. Clin Immunol. 2017;181:51–59. [DOI] [PubMed] [Google Scholar]
- 60.Stankovic B, Bjørhovde HAK, Skarshaug R, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol. 2019;9:3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang L, Ly D, Wang SS, et al. Targeting late-stage non-small cell lung cancer with a combination of DNT cellular therapy and PD-1 checkpoint blockade. J Exp Clin Cancer Res. 2019;38(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao J, Ly D, Dervovic D, et al. Human double negative T cells target lung cancer via ligand-dependent mechanisms that can be enhanced by IL-15. J Immunother Cancer. 2019;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feyler S, von Lilienfeld-Toal M, Jarmin S, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(−)CD8(−)alphabetaTCR(+) double negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol. 2009;144(5):686–95. [DOI] [PubMed] [Google Scholar]
- 64.Greenplate AR, McClanahan DD, Oberholtzer BK, et al. Computational immune monitoring reveals abnormal double-negative T cells present across human tumor types. Cancer Immunol Res. 2019;7(1):86–99. Accessed Apr 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voelkl S, Moore TV, Rehli M, Nishimura MI, Mackensen A, Fischer K. Characterization of MHC class-I restricted TCRalphabeta+ CD4− CD8− double negative T cells recognizing the gp100 antigen from a melanoma patient after gp100 vaccination. Cancer Immunol Immunother. 2009;58(5):709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu Y, Hu P, Zhou H, et al. Double-negative T cells inhibit proliferation and invasion of human pancreatic cancer cells in co-culture. Anticancer Res. 2019;39(11):5911–5918. [DOI] [PubMed] [Google Scholar]
- 67.Xu H, Zhu X, Chen J. DNT cell inhibits the growth of pancreatic carcinoma via abnormal expressions of NKG2D and MICA in vivo. Biochemical and Biophysical Research Communications. 2016;469(2):145–150. [DOI] [PubMed] [Google Scholar]
- 68.Merims S, Li X, Joe B, et al. Anti-leukemia effect of ex vivo expanded DNT cells from AML patients: A potential novel autologous T-cell adoptive immunotherapy. Leukemia. 2011;25(9):1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J, Minden MD, Chen WC, et al. Allogeneic human double negative T cells as a novel immunotherapy for acute myeloid leukemia and its underlying mechanisms. Clin Cancer Res. 2018;24(2):370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee JB, Kang H, Fang L, D’Souza C, Adeyi O, Zhang L. Developing allogeneic double-negative T cells as a novel off-the-shelf adoptive cellular therapy for cancer. Clin Cancer Res. 2019;25(7):2241–2253. [DOI] [PubMed] [Google Scholar]
- 71.Young KJ, Kay LS, Phillips MJ, Zhang L. Antitumor activity mediated by double-negative T cells. Cancer Res. 2003;63(22):8014–21. [PubMed] [Google Scholar]
- 72.Ford MS, Young KJ, Zhang Z, Ohashi PS, Zhang L. The immune regulatory function of lymphoproliferative double negative T cells in vitro and in vivo. J Exp Med. 2002;196(2):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamad AR, Mohamood AS, Trujillo CJ, Huang CT, Yuan E, Schneck JP. B220+ double-negative T cells suppress polyclonal T cell activation by a fas-independent mechanism that involves inhibition of IL-2 production. J Immunol. 2003;171(5):2421–2426. [DOI] [PubMed] [Google Scholar]
- 74.McIver Z, Serio B, Dunbar A, et al. Double-negative regulatory T cells induce allotolerance when expanded after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2008;141(2):170–178. [DOI] [PubMed] [Google Scholar]
- 75.Zhang ZX, Lian D, Huang X, et al. Adoptive transfer of DNT cells induces long-term cardiac allograft survival and augments recipient CD4(+)Foxp3(+) treg cell accumulation. Transpl Immunol. 2011;24(2):119–126. [DOI] [PubMed] [Google Scholar]
- 76.Chen W, Ford MS, Young KJ, Cybulsky MI, Zhang L. Role of double-negative regulatory T cells in long-term cardiac xenograft survival. J Immunol. 2003;170(4):1846–53. [DOI] [PubMed] [Google Scholar]
- 77.Young KJ, DuTemple B, Phillips MJ, Zhang L. Inhibition of graft-versus-host disease by double-negative regulatory T cells. J Immunol. 2003;171(1):134–41 [DOI] [PubMed] [Google Scholar]
- 78.He KM, Ma Y, Wang S, et al. Donor double-negative treg promote allogeneic mixed chimerism and tolerance. Eur J Immunol. 2007;37(12):3455–3466. [DOI] [PubMed] [Google Scholar]
- 79.Ma Y, He KM, Garcia B, Min W, Jevnikar A, Zhang ZX. Adoptive transfer of double negative T regulatory cells induces B-cell death in vivo and alters rejection pattern of rat-to-mouse heart transplantation. Xenotransplantation. 2008;15(1):56–63. [DOI] [PubMed] [Google Scholar]
- 80.Ligocki AJ, Niederkorn JY. Advances on non-CD4 + Foxp3+ T regulatory cells: CD8+, type 1, and double negative T regulatory cells in organ transplantation. Transplantation. 2015;99(8):1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zybleva SV, Zyblev SL. Research of subpopulation CD3+ CD4− CD8− double-negative T lymphocytes in kidney transplant recipients. Transplantologiya. The Russian Journal of Transplantation. 2020;12(1):20–27. [Google Scholar]
- 82.Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. [DOI] [PubMed] [Google Scholar]
- 83.Chu HX, Kim HA, Lee S, et al. Immune cell infiltration in malignant middle cerebral artery infarction: Comparison with transient cerebral ischemia. J Cereb Blood Flow Metab. 2014;34(3):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng H, Zhao H, Cao X, et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci U S A. 2019;116(12):5558–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsuchida M, Hanawa H, Hirahara H, et al. Identification of CD4− CD8− alpha beta T cells in the subarachnoid space of rats with experimental autoimmune encephalomyelitis. A possible route by which effector cells invade the lesions. Immunology. 1994;81(3):420–7. [PMC free article] [PubMed] [Google Scholar]
- 86.James WG, Hutchinson P, Bullard DC, Hickey MJ. Cerebral leucocyte infiltration in lupus-prone MRL/MpJ-fas lpr mice--roles of intercellular adhesion molecule-1 and P-selectin. Clin Exp Immunol. 2006;144(2):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cowley SC, Meierovics AI, Frelinger JA, Iwakura Y, Elkins KL. Lung CD4−CD8− double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with francisella tularensis live vaccine strain. J Immunol. 2010;184(10):5791–801. [DOI] [PubMed] [Google Scholar]
- 88.Takamoto M, Kusama Y, Takatsu K, Nariuchi H, Sugane K. Occurrence of interleukin-5 production by CD4− CD8− (double-negative) T cells in lungs of both normal and congenitally athymic nude mice infected with toxocara canis. Immunology. 1995;85(2):285–91. [PMC free article] [PubMed] [Google Scholar]
- 89.Kendall LV, Riley LK, Hook R Jr, Besch-Williford CL, Franklin CL. Characterization of lymphocyte subsets in the bronchiolar lymph nodes of BALB/c mice infected with cilia-associated respiratory bacillus. Comp Med. 2002;52(4):322–7. [PubMed] [Google Scholar]
- 90.Phyu S, Sornes S, Mustafa T, Tadesse A, Jonsson R, Bjune G. Changes in T-lymphocyte subsets in lungs and spleens of mice with slowly progressive primary mycobacterium tuberculosis infection: Involvement of unconventional T-cell subsets. Scand J Immunol. 1999;50(2):137–44. [DOI] [PubMed] [Google Scholar]
- 91.Derrick SC, Yabe I, Morris S, Cowley S. Induction of unconventional T cells by a mutant mycobacterium bovis BCG strain formulated in cationic liposomes correlates with protection against mycobacterium tuberculosis infections of immunocompromised mice. Clin Vaccine Immunol. 2016;23(7):638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ross TL, Balson GA, Miners JS, et al. Role of CD4+, CD8+ and double negative T-cells in the protection of SCID/beige mice against respiratory challenge with rhodococcus equi. Can J Vet Res. 1996;60(3):186–92. [PMC free article] [PubMed] [Google Scholar]
- 93.Neyt K, GeurtsvanKessel CH, Lambrecht BN. Double-negative T resident memory cells of the lung react to influenza virus infection via CD11c(hi) dendritic cells. Mucosal Immunol. 2016;9(4):999–1014. [DOI] [PubMed] [Google Scholar]
- 94.Hsu J, Krishnan A, Lee SA, et al. CD3(+)CD4(−)CD8(−) double-negative αβ T cells attenuate lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian D, Yang L, Wang S, et al. Double negative T cells mediate Lag3-dependent antigen-specific protection in allergic asthma. Nat Commun. 2019;10(1):4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee BP, Mansfield E, Hsieh SC, et al. Expression profiling of murine double-negative regulatory T cells suggest mechanisms for prolonged cardiac allograft survival. J Immunol. 2005;174(8):4535–44. [DOI] [PubMed] [Google Scholar]
- 97.Hanawa H, Tsuchida M, Matsumoto Y, et al. Characterization of T cells infiltrating the heart in rats with experimental autoimmune myocarditis. their similarity to extrathymic T cells in mice and the site of proliferation. J Immunol. 1993;150(12):5682–95. [PubMed] [Google Scholar]
- 98.Boll G, Reimann J. Lamina propria T cell subsets in the small and large intestine of euthymic and athymic mice. Scand J Immunol. 1995;42(2):191–201. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki H Differences in intraepithelial lymphocytes in the proximal, middle, distal parts of small intestine, cecum, and colon of mice. Immunol Invest. 2009;38(8):780–96. [DOI] [PubMed] [Google Scholar]
- 100.Carrasco A, Fernández-Bañares F, Pedrosa E, et al. Regional specialisation of T cell subsets and apoptosis in the human gut mucosa: Differences between ileum and colon in healthy intestine and inflammatory bowel diseases. J Crohns Colitis. 2016;10(9):1042–54. [DOI] [PubMed] [Google Scholar]
- 101.Hamad AR. Analysis of gene profile, steady state proliferation and apoptosis of double-negative T cells in the periphery and gut epithelium provides new insights into the biological functions of the fas pathway. Immunol Res. 2010;47(1–3):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steenholt JV, Nielsen C, Baudewijn L, et al. The composition of T cell subtypes in duodenal biopsies are altered in coeliac disease patients. PLoS One. 2017;12(2):e0170270. [DOI] [PMC free article] [PubMed] [Google Scholar]


