Abstract
Perturbations of the endolysosomal pathway have been suggested to play an important role in the pathogenesis of several neurodegenerative diseases, including Parkinson’s disease (PD) and Alzheimer’s disease (AD). Specifically, VPS35 and the retromer complex play an important role in the endolysosomal system and are implicated in the pathophysiology of these diseases. A single missense mutation in VPS35, Asp620Asn (D620N), is known to cause late-onset, autosomal dominant familial PD. In this review, we focus on the emerging role of the PD-linked D620N mutation in causing retromer dysfunction and dissect its implications in neurodegeneration. Additionally, we will discuss how VPS35 and the retromer are linked to AD, amyotrophic lateral sclerosis, and primary tauopathies. Interestingly, reduced levels of VPS35 and other retromer components have been observed in post-mortem brain tissue, suggesting a role for the retromer in the pathophysiology of these diseases. This review will provide a comprehensive dive into the mechanisms of VPS35 dysfunction in neurodegenerative diseases. Furthermore, we will highlight outstanding questions in the field and the retromer as a therapeutic target for neurodegenerative disease at large.
1. Introduction to VPS35 and the Retromer Complex
The vacuolar protein sorting ortholog 35 (VPS35) protein is a key component of the heteropentameric retromer complex, originally identified in yeast [1, 2]. The retromer functions primarily at the endosomal membrane where it selects and binds to transmembrane protein cargo to facilitate endosome-to-trans-Golgi network (TGN) or endosome-to-plasma membrane recycling (Figure 1) [3]. While the initial discovery of the retromer occurred in yeast, it is highly conserved from yeast to mammals, suggesting a fundamental role in cellular health, supported by the fact that VPS35 deletion is embryonic lethal [4]. Initial studies in yeast characterized the endosome-to-TGN recycling of vacuolar protein sorting 10 (VPS10), which is responsible for delivering carboxypeptidase Y, an acid hydrolase, to the lysosome [3]. Studies in yeast described the retromer as a stable heteropentameric complex composed of VPS35, VPS26, VPS29, and a sorting nexin (SNX) protein dimer. The heteropentameric complex can be separated into two distinct associating complexes. The cargo-selective complex (CSC), containing vacuolar protein sorting (VPS) proteins VPS35, VPS26, and VPS29, is responsible for binding to and selecting transmembrane protein cargo. The SNX dimer, composed of VPS5 and VPS17 in yeast, is responsible for binding to the endosomal membrane and aiding in the association of the CSC with the endosomal membrane through a Bin-Amphiphysin-Rvs (BAR) domain and a phox homology (PX) domain [5, 6].
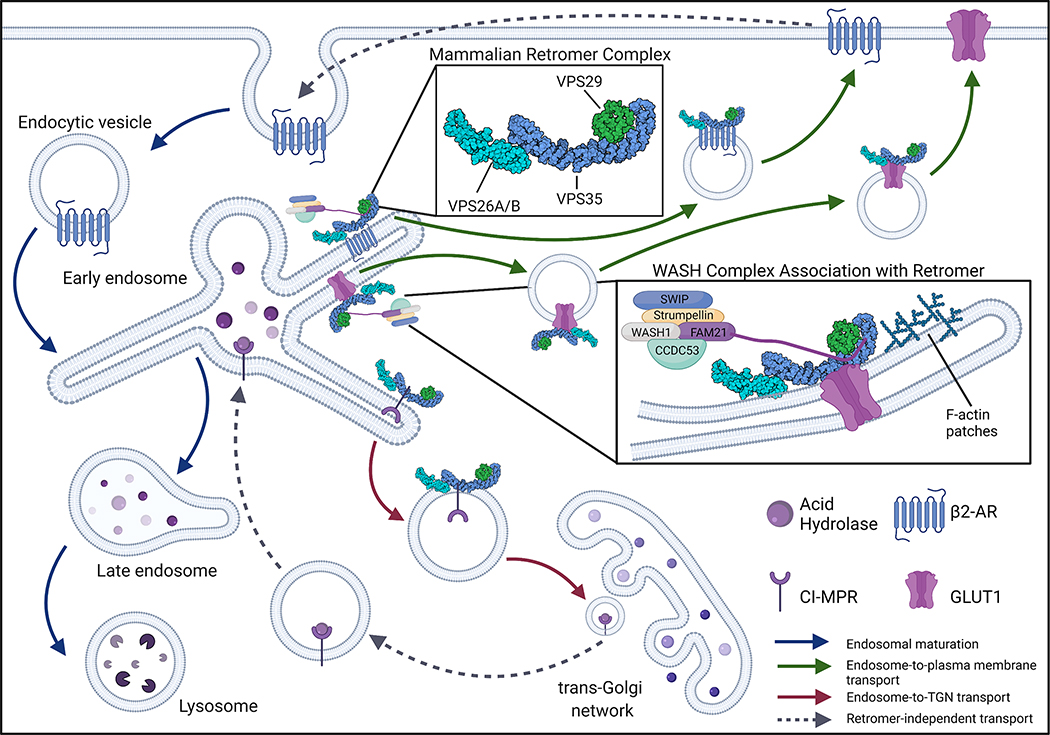
Figure 1: Mammalian retromer complex and retrograde protein sorting.
The mammalian retromer complex, consisting of VPS26, VPS29, and VPS35, functions as a key recycling mechanism for endosomal transmembrane protein cargo [3]. The retromer is depicted here as a single trimer of VPS26, VPS29, and VPS35 (PDB ID: 6VAC), however recent data suggests that the retromer trimer can form dimers of trimers, tetramers of trimers, and oligomers of trimers [16]. The retromer localizes to the cytosolic face of the endosome where it is responsible for selecting and binding to transmembrane proteins. The retromer facilitates two routes of retrograde transport within the cell: endosome-to-trans-Golgi network (TGN) and endosome-to-plasma membrane transport. Retromer-mediated endosome-to-TGN transport was first demonstrated in yeast to be important for the delivery of acid hydrolases to the lysosome [1, 2]. Similarly, in mammalian cells, the retromer is responsible for transporting CI-MPR from the endosome to the TGN whereby acid hydrolases bind to CI-MPR and are delivered back to the endolysosomal system via retromer-independent mechanisms. Additionally, following retromer-independent endocytosis, the retromer has been shown to facilitate the recycling of plasma membrane receptors (i.e. β2-AR, GLUT1) from the endosome to the plasma membrane. In some cases, as with β2-AR and GLUT1, retromer-mediated trafficking can be facilitated through retromer association with the WASH complex. The WASH complex, composed of SWIP, Strumpellin, WASH1, FAM21, and CCDC53, facilitates discrete trafficking pathways by forming F-actin patches along the endosomal tubules [18–21].
While retromer components are largely conserved in mammalian cells, key differences exist. VPS35 and VPS29 remain largely unchanged from yeast to mammals, whereas VPS26 has diverged into two distinct proteins, VPS26A and VPS26B. While VPS26A and VPS26B share a high degree of sequence similarity, the two isoforms of VPS26 can compete to form distinct retromer complexes that can bind to and recycle different protein cargo [5, 7]. Additionally, the mammalian CSC does not form a stable interaction with the SNX dimer [8]. In mammals, the SNX dimer consists of either SNX1 or SNX2 (homologs of yeast VPS5) and SNX5 or SNX6 (homologs of yeast VPS17). Due to the weak interaction of mammalian CSC with the SNX dimer, two additional proteins are required to sustain a sufficiently strong interaction with the endosomal membrane. SNX3 and Ras-related protein Rab-7a (Rab7a) bind directly to the endosomal membrane and are required for the CSC to associate with endosomes [3, 9, 10]. Further, high-resolution microscopy in mammalian cells shows that the SNX dimer and the CSC often exist in distinct regions of the endosome, which would explain the weak interaction between the two complexes [11]. For these reasons, “retromer” in mammalian cells indicates just the CSC and will be referred to as such in the rest of this review [12].
Structurally, VPS35 adopts an α-solenoid structure, where VPS26 binds to the N-terminal end of VPS35 and VPS29 binds to its C-terminus [6, 13, 14]. Interestingly, cryo-electron tomography structural analysis of the yeast retromer reveals VPS26 as the sole point of contact between the SNX dimer and VPS35. VPS35 homodimers form an arch where VPS26 dimers are at the base (N-terminal end of VPS35) and VPS29 dimers bind at the apex of the VPS35 arch (C-terminal end of VPS35) [15]. This structural arrangement is suggested to encourage flexibility of the retromer during endosomal tubule formation. Additionally, a recent study using single-particle cryo-electron microscopy (cryo-EM) revealed a similar configuration of the mammalian retromer whereby retromer dimers form an arch with the C-terminal ends of VPS35 dimerizing [16]. This study also suggests that the retromer can form oligomeric species where retromer trimers can bind each other and form dimers of trimers, tetramers of trimers, and oligomers of trimers. The functional consequences of these different retromer assemblies remain unclear.
Within the endolysosomal pathway, the retromer primarily functions in the early to late endosome transition, where Ras-related protein Rab 5 (Rab5) is replaced by Rab7a as a part of normal endosomal maturation [3]. The retromer is responsible for recognizing distinct proteins at this juncture to traffic them away from an eventual lysosomal fate [3]. Canonical retromer cargo sorted from the endosome to the TGN includes cation-independent mannose-6-phosphate receptor (CI-M6PR), Wntless, sortilin, and Sortilin Related Receptor 1 (SORL1). Retromer cargo sorted from the endosome to the plasma membrane includes Solute Carrier Family 2 Member 1 (SLC2A1/GLUT1), β2 adrenergic receptor (β2-AR), and insulin-like growth factor 1 receptor (IGF1R) (Figure 1) [17–19]. The retromer can also interact with accessory proteins that can guide discrete sorting pathways. Particularly of interest, the retromer can bind to the Wiskott-Aldrich syndrome and SCAR homolog (WASH) complex to facilitate the sorting of specific protein cargo from the endosome to the plasma membrane [18]. The WASH complex is a pentameric complex comprised of WASH1, Family With Sequence Similarity 21 Member C (Fam21), strumpellin, Coiled-Coil Domain-Containing Protein 53 (CCDC53), and Strumpellin And WASH-Interacting Protein (SWIP/KIAA1033) (Figure 1). The WASH complex binds to VPS35 through the unstructured tail of Fam21 and guides the sorting of specific transmembrane proteins by forming filamentous (F)-actin patches along the endosomal tubules [18–20]. The WASH complex seems to be involved in primarily endosome-to-plasma membrane retromer sorting of GLUT1 and β2-AR (Figure 1). Interestingly, the sorting of both GLUT1 and β2-AR appears to be further dependent on the association of SNX27 in addition to the WASH complex [19, 21]. SNX27, similar to SNX3, does not contain a BAR domain, however, it seems to have an important function in WASH-dependent endosome-to-plasma membrane retromer sorting [9, 10, 19, 21].
Canonical retromer cargo perform important cellular processes from maintaining lysosomal hydrolases to glucose metabolism and uptake. As such, the retromer plays a critical role in cellular health and is, as expected, ubiquitously expressed throughout the body [22]. Interestingly, VPS35 mRNA is highly expressed in brain tissue and has an important role in the central nervous system (CNS) [4, 22–24]. While VPS35 is found in all anatomical brain regions as indicated in single-cell RNA-sequencing databases, the frontal cortex, hippocampus, striatum, and cerebellum exhibit the highest abundance of VPS35 [23]. Additionally, among CNS cell types, neurons and oligodendrocytes have the highest levels of VPS35 mRNA expression [23]. With this widespread expression of VPS35 across multiple brain regions and cell types, it is expected that VPS35 and the retromer would play important roles within the CNS. Indeed, VPS35 is required for the normal distribution of AMPA and NMDA receptor subunits at post-synaptic sites within dendritic spines, supporting a role for the retromer in regulating excitatory synaptic transmission [25]. Furthermore, as covered in this review, deletion of VPS35 in several different cell types of the brain proves to be deleterious [23, 26, 27].
This review aims to provide a comprehensive overview of the role of VPS35 and the retromer in neurodegenerative disease. Briefly, we will take a deep dive into the genetic contributions of VPS35 mutations to familial Parkinson’s disease (PD) and discuss the various mechanisms put forth to explain the pathogenicity of the heterozygous D620N mutation found in familial PD cases worldwide. Additionally, we will examine the intriguing association of retromer deficiency in affected brain region of several neurodegenerative diseases including Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and primary tauopathies. Furthermore, we will highlight remaining questions in the field, and the retromer as a therapeutic target for neurodegenerative disease at large.
2. VPS35, the Retromer, and Neurodegenerative Disease
Given the essential function of VPS35 and the retromer in the CNS, perhaps it should not be surprising that perturbations in this pathway have been linked to PD and AD. In this section, we will review the discovery of the genetic link between VPS35 and PD, and the intriguing observation of retromer deficiency in patient brains from AD and other neurodegenerative diseases.
2.1. Parkinson’s Disease
PD is a progressive neurodegenerative movement disorder clinically characterized by cardinal motor symptoms, including bradykinesia, rigidity, resting tremor, and in most cases postural instability. PD affects close to 2% of people over the age of 65 years, with incidence increasing to 5% above 85 years of age [28, 29]. Neuropathologically, PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta, leading to a profound loss of dopamine in the caudate putamen. Dopamine loss in the putamen is primarily responsible for the clinical motor symptoms. PD is also defined by the presence of proteinaceous intraneuronal inclusions containing aggregated α-synuclein, termed Lewy bodies and neurites [28, 29]. Currently, no disease-modifying treatments are approved that slow or stop the progression of PD. Additionally, intervention with dopamine replacement therapy is initially effective for a number of years at subduing symptoms, but motor symptoms become less responsive as the disease progresses and patients also develop undesirable side effects such as dyskinesias associated with levodopa usage [30].
While age is considered the largest risk factor in a predominantly sporadic disease, up to 10% of cases display familial inheritance [31]. Familial PD, inherited in an autosomal dominant or autosomal recessive fashion, is associated with mutations in at least 20 distinct genes [32, 33]. Understanding the pathogenesis of these monogenic forms of PD could provide key mechanistic insight into the pathophysiology of sporadic disease, leading to novel drug targets. Interestingly, many genes mutated in monogenic forms of PD (i.e. VPS35, LRRK2, SYNJ1, DNAJC6, ATP13A2, RAB39B, VPS13C) are implicated in common cellular pathways, namely the endolysosomal pathway [34]. Understanding the precise contributions of these PD-linked gene products in the endolysosomal pathway may lead to an improved understanding of the mechanisms contributing to the pathophysiology of familial and sporadic PD, and eventually to the identification of targeted disease-modifying therapies.
One form of monogenic PD is caused by autosomal dominant mutations in the vacuolar protein sorting 35 (VPS35, PARK17) gene [35–37]. Mutations in VPS35 were first discovered using exome sequencing in Swiss and Austrian kindreds in 2011 and have since been found in PD families and subjects worldwide [35, 37, 38]. A single missense mutation in VPS35, Asp620Asn (D620N), segregates with late-onset disease in an autosomal dominant manner. The D620N mutation is the only mutation in VPS35 with proven pathogenicity, however, several putative pathogenic mutations have been identified (i.e. P316S, R524W) [38]. It remains to be established whether these additional VPS35 mutations represent true pathogenic variants, act as risk variants, or represent rare benign genetic polymorphisms.
D620N VPS35 mutation carriers are clinically similar to sporadic PD subjects, typically displaying late-onset disease with cardinal motor symptoms of PD, in addition to mild cognitive impairment in some subjects [36, 39–41]. Mutation carriers respond well to levodopa therapy and exhibit an asymmetric reduction in Fluorodopa-F18 uptake in the caudate putamen upon PET imaging. Overall, clinical observations indicate that D620N VPS35 mutation carriers are indistinguishable from sporadic PD subjects, further supporting the possibility that common mechanisms may underlie both familial and sporadic PD [36, 41]. The neuropathological hallmarks of D620N VPS35 mutation carriers remain to be determined. Only one brain from a PD subject harboring the D620N mutation has been evaluated at autopsy, however, PD-relevant brain regions were not assessed such as the brainstem. Of the regions analyzed, Lewy body pathology or intracellular protein inclusions were not identified, suggesting that if pathology is indeed present in these carriers, it is likely confined to the brainstem [36]. Without additional post-mortem examination on VPS35 mutation carriers, it remains uncertain to what extent VPS35-linked PD recapitulates the neuropathological hallmarks of sporadic PD.
2.2. Alzheimer’s Disease and other Neurodegenerative Diseases
Alzheimer’s disease (AD) is the most frequent neurodegenerative disorder affecting approximately 44 million individuals globally, with AD accounting for 50–75% of dementia cases overall. As with PD, age is a major risk factor for AD. The incidence of AD doubles every 5 years after the age of 65. Clinically, AD patients present with progressive cognitive impairments, memory loss, executive order dysfunction, visual-spatial distortion, and aphasia. While the majority of AD cases are sporadic, autosomal dominant mutations in the amyloid precursor protein (APP) and presenilin 1/2 (PSEN1/2) genes cause early-onset familial forms of AD, whereas the apolipoprotein E4 (APOE ε4) allele is the strongest risk factor for the development of AD [42]. Approximately 5% of all AD cases are caused by mutations in either APP, PSEN1 or PSEN2. However, the APOE ε4 allele may increase susceptibility by 50% [43]. Together, it is estimated that 60–80% of AD involves the inherited genetic mutations or genetic association [44]. The pathological hallmarks of AD include extracellular amyloid-β (Aβ) plaques, intraneuronal neurofibrillary tangles (NFTs) and neuropil threads composed of hyperphosphorylated tau, and dystrophic neurites. While neuronal and synapse loss are closely correlated, whether NFTs are causative is a still a matter of debate. Regardless, the early neuropathological process in AD is characterized by synapse loss and dysfunction [45, 46].
Interestingly, AD was the first neurodegenerative disease to be associated with retromer dysfunction by a seminal study in 2005 using model-guided microarray technology to analyze the entorhinal cortex of AD patients. This study revealed a decrease in the expression of VPS35 and VPS26 mRNA, also confirmed using protein analysis [47]. The siRNA-mediated knockdown of VPS35 in cell lines indicated that the retromer can control the levels of Aβ peptides, establishing the first mechanistic relationship between the two proteins [47]. This relationship was further supported in a retrospective study using microarray data from AD patient brain tissue. Notably, a reduction of VPS35 mRNA cross-correlated with a parallel loss of the transmembrane retromer cargo protein SORL1, which can directly bind to APP [48]. Further support was provided by a genetic association study between AD and single nucleotide polymorphisms (SNPs) in retromer-related genes, and in a large genome-wide association study for sporadic AD [49, 50]. More recently, protein levels of VPS26B, but not VPS26A, and the retromer cargo, SORL1, were shown to be enriched in the entorhinal cortex of human brains. This suggests that the brain relies on a distinct retromer conformation where VPS26B is critical. Additionally, protein levels of VPS26B and SORL1 were depleted in the entorhinal cortex of AD brains, further supporting a role of retromer deficiency in AD pathogenesis [52]. Interestingly, common genetic variants SORL1 are associated with risk for late-onset AD [51]. Several additional retromer-related proteins have also been linked to late-onset AD, particularly WASHC4, SNX1, SNX3, and RAB7A, further supporting the notion that widespread depletion of retromer and retromer-related proteins are important for AD pathogenesis [53].
In addition to PD and AD, recent studies have shown that retromer dysfunction is likely involved in the pathogenesis of several other neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS). ALS is characterized by the relatively rapid progressive loss of motor neurons throughout the body, resulting in muscle deterioration, eventual paralysis, and death. Similar to PD, most ALS cases are sporadic, with up to 10% caused by inherited mutations [54]. While no mutations or variants in retromer subunits or retromer-related genes have been associated with ALS, retromer protein depletion has been shown in the ventral horn region of the spinal cord in brains from ALS patients and in induced pluripotent stem cell (iPSC)-derived motor neurons from ALS patients bearing SOD1 mutations [55]. While the molecular contributions of the retromer to ALS pathogenesis remain unclear, the observation of retromer depletion in ALS patient brain tissue may suggest a role for VPS35 and the retromer in the normal maintenance and survival of motor neurons.
Retromer depletion has also been linked to primary tauopathies, a class of heterogeneous neurodegenerative diseases where the primary pathological feature is the accumulation of intracellular inclusions containing abnormal tau [56]. Given the correlation of retromer depletion and AD, a recent study evaluated the protein levels of VPS35, VPS26B, and VPS29 in post-mortem brain tissue from patients with progressive supranuclear palsy (PSP), one of the most common forms of primary tauopathy. Decreased levels of these retromer proteins were observed in forebrain tissue of PSP subjects compared to age-matched controls, with similar findings in Pick’s disease, another primary tauopathy with distinct features compared to PSP [57]. While the clinical symptoms and pathological hallmarks of primary tauopathies display a great deal of heterogeneity, this study suggests that a pathological hallmark of this group of diseases may be retromer depletion.
3. Mechanisms of VPS35 Dysfunction in Neurodegenerative Disease
With a renewed sense of urgency following the 2011 discovery of VPS35 mutations linked to familial PD, several groups set out to determine how mutations in VPS35 and retromer depletion contribute to neurodegenerative disease. Over the past 10 years, several models of both autosomal dominant disease and retromer depletion have been developed to elucidate the function of VPS35 and the retromer in the CNS in health and disease. Several important rodent models have been developed to address these questions, as summarized in Table 1. This section will review and compare the major findings from animal models of both autosomal dominant disease and retromer depletion.
Table 1.
Summary of VPS35 animal models of neurodegenerative disease
| Model | Construct | Age | Neuropathology | Cellular pathology | Behavior | Reference |
|---|---|---|---|---|---|---|
| Mouse KO (germline) | VPS35 +/− | 4, 6, 12, 18 months | ~20% DA neuron loss | ↓ LAMP2a; ↑ α-syn in DA neurons; ↑ mitochondrial fragmentation; ↑ MUL1 in VM and STR | ↓ performance in open field test | [27] |
| Mouse cKO | VPS35 −/− /DAT-Cre | 2–3 months | ↓ DA neurons by 2–3 months | ↓ levels of MFN2; impaired mitochondrial fusion; ↑ α-syn levels in VM | ↓ performance in open field test; weak and unsteady gait; ↓ hind limb stepping | [62] |
| Mouse cKO | VPS35 −/− /Synapsin-1-Cre | Post-natal 1–15 (premature survival ≤P15) | ↑ degeneration of ventral horn motor neurons at P12; widespread reactive gliosis in the spinal cord | ↑ p62-positive inclusions | hind limb tremors; muscle weakness; poor ambulation and righting reflexes; limb paralysis and death | [23] |
| Mouse cKO | VPS35 −/− /CX3CR1-Cre ER | Post-natal 15–30 | ↑ hippocampal neurogenesis; impaired terminal differentiation | ↑ microglial phagocytic activity | Impaired long-term memory; depression-like behavior | [26] |
| Mouse cKO | VPS35 −/− /Neurod6-Cre | Post-natal 0, 7, 14, 21 | impaired terminal differentiation; neocortical atrophy; reactive gliosis | enlarged lysosomes; ↓ lysosomal acidification; ↑ p62 and LC3B-II; ↑ TDP-43 and ubiquitin-conjugated proteins | Unknown | [63] |
| Mouse KI | VPS35 D620N/D620N | 5 months | None (∼5 months) | ↓ evoked striatal dopamine release | None (∼5 months) | [64] |
| Mouse KI | VPS35 D620N/D620N | 3 months | None | ↑ striatal dopamine release and turnover | No gross motor dysfunction | [65] |
| Mouse KI | VPS35 D620N/D620N | 13 months | ~38% DA neuron loss; neurite degeneration in the striatum | ↑ somatodendritic pTau in hippocampus and dopaminergic neurons | None observed | [61] |
| Mouse KI | VPS35 D620N/WT | 12, 16 months | ~30% DA neuron loss | ↑ α-syn and pTau levels in SN; ↓ Wnt1, nuclear β-catenin and survivin; ↑ caspase-8 and caspase-9 in DA neurons; ↑ mitochondrial fragmentation | ↓ performance in open field and pole tests | [66] |
| Mouse KI | VPS35 D620N/D620N | 6, 9–10, 14, 15–16 months | ~12% DA neuron loss at 15–16 months; ↓ dopamine levels | ↑ α-syn; ↑ pTau; ↑ mitochondrial fragmentation; ↑ susceptibility to environmental stressors | ↓ performance in open field at 14 months; ↓ performance in beam walking at 14 months | [67] |
| Mouse KI | VPS35 D620N/D620N | 3 months | Previously reported [65] | ↑ pRab10; ↑ GluR1 surface expression; ↑ glutamate transmission; ↓ presynaptic vGLUT1 cluster intensity | Previously reported [65] | [68] |
| Mouse D620N Tg | VPS35 D620N/D620N Tg (ROSA26) | 20 months | ↑ dopamine content in the striatum | None | No gross motor dysfunction | [69] |
| mouse viral-mediated gene transfer | Lentiviral-VPS35D620N (human) | 3 weeks post-injection (SNpc) | ↓ in DA neurons compared to contralateral side | ↑ mitochondrial fragmentation | Unknown | [70] |
| rat viral-mediated gene transfer | AAV2/6-VPS35D620N (human) | 12 weeks post-injection (SNpc) | ∼30% DA neuron loss | neurite degeneration | None | [24] |
Abbreviations: KO, knockout; cKO, conditional knockout; KI, knockin; Tg, transgenic; DA, dopaminergic; VM, ventral midbrain; SNpc, substantia nigra pars compacta; STR, striatum; α-syn, α-synuclein
3.1. Modeling Autosomal Dominant Disease
One of the most important questions regarding the heterozygous D620N mutation is whether it acts through a gain-of-function or loss-of-function mechanism to cause PD. Although this question has not been fully resolved, several studies demonstrate that D620N VPS35 remains a largely functional protein. First, D620N VPS35 binds to VPS26 and VPS29 normally, indicating that retromer assembly is not disrupted [24, 58]. Additionally, studies in yeast show that wild-type (WT) VPS35 and the analogous D686N mutant, both successfully rescued VPS35 null phenotypes, including resistance to nickel exposure and increased sensitivity to cadmium exposure [24]. Studies in rodent primary cortical neurons showed that D620N VPS35 has normal subcellular distribution within the endolysosomal system (Rab5, Rab7, and LAMP1 compartments) and no disruption to cargo distribution (sortilin, SORL1, and CI-M6PR) [24]. Additionally, two separate studies demonstrated that Drosophila VPS35 (WT and D620N equivalent) could rescue the lethality of VPS35 null flies, further supporting the notion that D620N VPS35 remains largely functional [59, 60]. In addition, compound heterozygous mice bearing one D620N VPS35 knockin (KI) allele and one VPS35 knockout (KO) allele are largely healthy, with a normal lifespan and no early neuronal loss [61], in contrast to homozygous VPS35 KO mice that are embryonic lethal.
While it has become clear that D620N VPS35 does not manifest a complete loss-of-function, the exact mechanism by which this mutation acts remains to be clarified. Understanding how the D620N mutation contributes to neurodegeneration, specifically in PD, has been the focus of several studies since the identification of VPS35 mutations in 2011. Several experimental models of autosomal dominant VPS35-linked PD have been developed with the aim of understanding the molecular underpinnings of D620N VPS35-induced neurodegeneration. Importantly, several pathways and mechanisms, described in this section, are perturbed by the D620N mutation.
3.1.1. Autophagy and lysosomal dysfunction
Autophagy and lysosomal dysfunction have been linked to D620N VPS35 through several proposed mechanisms. Autophagy is the aggregate sum of molecular pathways that result in the sequestration of various intracellular components for delivery to the lysosome for degradation. In the last two decades, several reports point to dysregulated autophagy as a major contributing factor to neurodegenerative disease [71]. The process of autophagy is highly conserved throughout eukaryotes and in mammalian cells, in which there are three primary types of autophagy: microautophagy, chaperone-mediated autophagy, and macroautophagy. As the name implies, macroautophagy or bulk autophagy is the major constituent that participates in protein aggregate and organelle degradation [72].
The retromer, in conjunction with its growing number of accessory proteins, including the WASH complex, has a critical role in endosomal sorting and the autophagic process. The WASH complex was first linked to autophagy after WASH deficiency was observed to suppress autophagy [73]. VPS35 was later connected to this pathway through the observation that the overexpression of D620N VPS35 in HeLa cells resulted in WASH complex binding deficits and impaired endosomal recruitment, resulting in aberrant autophagy through abnormal sorting of the autophagy receptor ATG9A and decreased autophagosome formation [74]. WASH complex binding deficits associated with D620N VPS35 have since been confirmed by other groups, however, this intriguing interaction deficit has yet to be demonstrated specifically in neurons or linked directly to neurodegeneration [61, 75].
The D620N mutation is linked to lysosomal dysfunction, specifically through impaired sorting of cathepsin D. The retromer is responsible for the endosome-to-TGN transport of CI-M6PR, which functions as a critical transporter of acid hydrolases, such as pro-cathepsin D, from the TGN to the pre-lysosomal compartment. The proper delivery of pro-cathepsin D to the late endosomal compartment ensures its maturation to cathepsin D to ensure proper lysosomal function [76]. Many groups have demonstrated normal CI-M6PR sorting in the presence of D620N VPS35, although altered sorting of cathepsin D is observed in cell lines and patient-derived fibroblasts from PD subjects bearing the D620N mutation [58]. Although not directly demonstrated, Follett and colleagues suggest that the impaired delivery of cathepsin D to the lysosome could result in impaired degradation of aggregation-prone proteins, such as α-synuclein. As such, the overexpression of D620N VPS35 in mouse substantia nigra dopaminergic neurons was shown to increase the accumulation of α-synuclein, although cathepsin D sorting was not evaluated in this model [62].
3.1.2. Mitochondrial dysfunction
While a direct role for VPS35 and the retromer in endolysosomal sorting pathways has been proven, recent evidence suggests the retromer may also have roles outside of this main pathway, at mitochondria. The retromer is implicated in a relatively new pathway of localized mitophagy through the formation mitochondrial-derived vesicles (MDVs) [77]. MDVs are suggested to allow for the selective degradation of damaged mitochondrial fragments. In concert with VPS29 and VPS26, VPS35 facilitates MDV formation and their delivery to peroxisomes or lysosomes. Delivery of MDVs to the lysosome promotes degradation of the mitochondrial contents, thereby allowing for removal of damaged portions of mitochondria without removal of the entire organelle [77]. In addition to MDVs, the retromer has also been shown to regulate both mitochondrial fission and fusion events, suggesting a broader role in mitochondrial maintenance.
In a recent study using human cell lines and primary rodent neurons, VPS35 interacts with the dynamin-1-like protein, DLP1 (Drp1), a protein important for mediating mitochondrial fission. This interaction is increased by the D620N mutation, and enhanced mitochondrial fragmentation. The increased interaction between D620N VPS35 and DLP1 is observed in post-mortem brain tissue from sporadic PD subjects, providing further support for this novel interaction. Increased mitochondrial fragmentation has also been observed in the ventral tegmental area (VTA) with the mouse midbrain following delivery of lentiviral vectors expressing WT or D620N VPS35 [70], and in the substantia nigra of heterozygous D620N VPS35 KI mice at 16 months of age [66]. In mice with lentiviral delivery of WT or D620N VPS35, increased mitochondrial fragmentation could be rescued by treatment with mdivi-1, a selective inhibitor of dynamin-related GTPases, suggesting a dependence on DLP1 [70]. Further studies revealed that D620N VPS35 causes deficits in mitochondrial respiration in patient-derived fibroblasts from D620N mutation carriers. These deficits were also rescued by mdivi-1, suggesting that enhanced mitochondrial fission is required for VPS35-induced toxicity [78]. These studies identify a putative mechanism of retromer dysfunction induced by the D620N mutation, however, additional studies are required to establish if altered mitochondrial dynamics directly contribute to D620N VPS35-induced neurotoxicity.
3.1.3. Neurotransmission deficits
Unlike most cells in the body, neurons are postmitotic and terminally differentiated. Therefore, cellular maintenance must be preserved for the duration of the organism’s lifespan. While neurons are highly adaptive to change, maintaining their functional plasticity requires efficient recycling and turnover of cellular components. Given the role of VPS35 and the retromer in excitatory synaptic transmission, it is not surprising that this has been an area of great interest since the discovery of retromer perturbations in neurodegenerative disease [25].
Interestingly, in primary rodent neurons overexpressing WT or D620N VPS35, the D620N mutation was shown to alter the localization and motility of VPS35 at the dendrite [79]. The D620N mutation also increased colocalization between VPS35 and the Glutamate Ionotropic Receptor AMPA Type Subunit 1 (GluR1) and disrupted the recycling of GluR1, suggesting glutamatergic signaling could be dysfunctional. These GluR1 phenotypes were also observed in human iPSC-derived dopaminergic neurons from patients harboring the D620N mutation [79]. While intriguing, these findings do not establish any functional consequences in neurotransmission. While the lack of phenotypic consequence is intriguing, it is not unexpected given that synaptic plasticity is often resistant to manipulation and there may be compensation from other AMPA receptor subunits.
A recent study confirmed the GluR1 association with VPS35 using D620N VPS35 KI mice [68]. Previous studies relied on the exogenous overexpression of VPS35 variants, whereas D620N VPS35 KI mice express VPS35 at endogenous levels, representing a more physiologically relevant model. Notably, this study reported an increase in glutamatergic activity in primary neurons derived from D620N VPS35 KI mice. This was associated with an increase in the surface expression of GluR1 and an increase in glutamate transmission, suggesting a functional consequence of impaired sorting of AMPA receptor subunits. It remains to be determined if glutamatergic transmission directly contributes to D620N VPS35-induced neurodegeneration as observed in PD [68].
The D620N VPS35 KI mouse model has become an important tool in understanding the role of the D620N mutation in neurodegeneration. A common finding in mice expressing D620N VPS35, as well as in a transgenic model of D620N VPS35, is impaired striatal dopamine release, highlighting a fundamental disturbance shared by several independent models [64, 65, 69]. Interestingly, Cataldi and colleagues show that D620N VPS35 KI mice exhibit increased dopamine release in the striatum, while Ishizu and colleagues, using an independent KI model, found a decrease in striatal dopamine release. Additionally, Cataldi and colleagues found a significant reduction in dopamine transporter (DAT) levels in the striatum of mice as young as 3 months old, however another study indicated no change in extracellular dopamine and its metabolites in the striatum of these animals, even at 15 months of age [61, 64, 65]. Questions remain regarding the discrepancies between different D620N VPS35 KI mouse studies, however, genetic background could have a significant contribution.
3.1.4. Dopaminergic cell loss and protein accumulation
To specifically address the mechanisms by which D620N VPS35 induces neurodegeneration in familial PD, rodent models expressing D620N VPS35 have been developed that display progressive neuronal loss [24, 27, 61, 64–68]. To date, studies have been conducted with both overexpression and endogenous rodent models of D620N VPS35. Overexpression of human D620N VPS35 was first reported by Tsika and colleagues whereby intranigral delivery of an AAV2/6 vector expressing human D620N VPS35 induced nigrostriatal pathway damage, including axonal degeneration and dopaminergic neuronal loss in adult rats compared to WT VPS35 [24]. This observation is consistent with a gain-of-function or partial dominant-negative mechanism. This mechanism was also supported by two independent studies in mice using AAV or lentiviral vectors to overexpress D620N VPS35 in the mouse substantia nigra. As in rats, D620N VPS35 induced a marked increase in dopaminergic cell death compared to WT VPS35 [27, 70].
While valuable information can be gleaned using overexpression models, one must acknowledge that the effects of PD-relevant mutations in the retromer are potentially exaggerated in these models. To circumvent potential issues that overexpression models may present, multiple groups have since developed murine models that express mutated VPS35 at physiological levels. It was consistently shown across studies that endogenous D620N VPS35 expression induces a subtle phenotype compared to the phenotypes observed in crude overexpression experiments. First, homozygous D620N VPS35 expression does not affect mouse viability or survival [61, 64–68], in contrast to homozygous KO mice. Additionally, three independent studies using two different mouse models of endogenous D620N VPS35 expression recapitulate PD-relevant pathology in heterozygous and homozygous mice. PD-relevant pathology included a progressive loss of dopaminergic neurons in the substantia nigra between 13–16 months of age, depending on the study. Neuronal loss was also accompanied by motor deficits, axonal degeneration and, interestingly, the presence of abnormal hyperphosphorylated or conformation-specific tau throughout the brain [61, 66, 67]. Importantly, these phenotypes occurred in both heterozygous and homozygous KI mice, suggesting that a single D620N allele is sufficient to induce neuropathology.
While tau pathology was detected in three independent KI mouse studies, suggesting a potentially PD-relevant phenotype, findings related to α-synuclein pathology in these models are not consistent. Initially, it was shown that endogenous D620N VPS35 expression was not sufficient to induce α-synuclein pathology or alter the lethal neurodegenerative phenotypes exhibited by human A53T α-synuclein transgenic mice [61]. However, recent studies have suggested the accumulation of total α-synuclein in the substantia nigra of two independent mouse models of endogenous D620N VPS35 expression [66, 67]. It remains unclear if pathogenic forms of α-synuclein also accumulate in these mice, as only total α-synuclein levels were evaluated.
Recently, disruption of the Wnt/β-catenin pathway was observed in heterozygous D620N VPS35 KI mice. The retromer has previously been linked to this pathway via the sorting of Wntless, a transmembrane protein important for Wnt secretion, from the endosome to the TGN [9]. Sorting of Wntless by the retromer is an essential step in the eventual delivery of Wnt to the plasma membrane [9]. Given the importance of the Wnt/β-catenin pathway in neuronal survival and the observation that D620N VPS35 expression induces neuronal death, it was hypothesized that VPS35 mutations might disrupt this pathway. Indeed, Chiu and colleagues observed the downregulation of several pro-survival proteins and the upregulation of pro-apoptotic proteins in the substantia nigra of 16-month-old heterozygous D620N VPS35 KI mice, along with dopaminergic cell loss [66]. This could represent a novel pathway through which the retromer induces neurotoxicity in PD, however, it will be important to directly connect the Wnt/β-catenin pathway to D620N VPS35 dysfunction.
3.1.5. Hyperactivation of LRRK2
VPS35 and the retromer have been linked to other PD genes, including α-synuclein, parkin, and LRRK2 [38]. Arguably, the VPS35 interaction with LRRK2 is the most compelling. MacLeod and colleagues first reported a link between the pathogenic G2019S LRRK2 mutation and D620N VPS35. VPS35 and LRRK2 interact in co-immunoprecipitation assays from SH-SY5Y cells, although mutations in either protein do not obviously alter their interaction [80]. Furthermore, D620N VPS35 induces a sorting defect in CI-M6PR that is phenocopied by the overexpression of G2019S LRRK2, suggesting that these two proteins may be involved in a similar pathogenic mechanism [80]. Further support for this interaction was shown in a Drosophila model whereby the overexpression of VPS35 (WT or D620N) could rescue mutant LRRK2-induced retinal degeneration [81]. These studies support a role for VPS35 downstream of LRRK2 in a convergent pathway.
VPS35 and LRRK2 have been connected yet again, although this new mechanism suggests that VPS35 might oppositely lie upstream of LRRK2. The D620N mutation in VPS35 induces a robust induction of LRRK2 kinase activity [82]. Traditionally, LRRK2 kinase activity has been monitored by the detection of autophosphorylated residues, however, it has been consistently shown that a subset of Rab GTPases serve as authentic substrates of LRRK2 kinase activity in cells [83]. Using mouse embryonic fibroblasts (MEFs) isolated from D620N VPS35 KI mice, the LRRK2-mediated phosphorylation of Rab10 was markedly increased in KI MEFs compared to WT MEFs. The enhanced Rab10 phosphorylation is equivalently observed in heterozygous and homozygous KI MEFs, indicating that one copy of the D620N mutation is sufficient for LRRK2 hyperactivation [82], thereby supporting a gain-of-function mechanism for this autosomal dominant mutation. KI MEFs treated with a selective LRRK2 kinase inhibitor (MLi-2) revealed undetectable levels of Rab10 phosphorylation, confirming that the increase in Rab10 phosphorylation is dependent on LRRK2 kinase activity [82]. D620N VPS35 also increased the phosphorylation of Rab8A and Rab12 in a LRRK2-dependent manner in D620N VPS35 KI MEFs. An increase in Rab10 phosphorylation was also observed in lung, kidney, spleen, and brain of D620N VPS35 KI mice that was reversed by MLi-2, suggesting a widespread global effect of this mutation on LRRK2 activity. Rab10 phosphorylation was also increased in patient-derived primary neutrophils and monocytes from subjects harboring a heterozygous D620N mutation compared to sporadic PD and healthy control subjects [82]. While LRRK2 substrate phosphorylation is robustly increased by the D620N VPS35 mutation, the retromer does not possess catalytic activity, implying the existence of an unknown regulator, protein complex or subcellular location of LRRK2 that can be modulated by D620N VPS35 to induce kinase hyperactivation.
3.2. Retromer Depletion Models
Since initial reports of retromer deficiency in AD patient brains, several models of retromer depletion have been developed to better understand the pathological impacts of this observation. Interestingly, germline homozygous KO of VPS35 in rodents induced embryonic lethality, suggesting an important role for VPS35 in development [4]. One important question in the field is understanding how VPS35 depletion affects specific cell types and regions in the brain. With this in mind, several models, reviewed here, explore the overall impact of retromer depletion and cell type-specific roles of VPS35 and the retromer in the brain. New findings suggest that retromer depletion could be a central signaling hub for several pathological features of AD and other neurodegenerative diseases, such as protein aggregation (of Aβ and tau), loss of synapses, neuronal dysfunction, and neuroinflammation [84]. In this section, we will review models of retromer depletion and discuss the similarities and differences to D620N VPS35 models.
3.2.1. Autophagy and lysosomal dysfunction
Chaperone-mediated autophagy (CMA) is a form of autophagy characterized by the selective targeting of substrates to the lysosome through the CMA proteins HSC70 and LAMP2A. The chaperone HSC70 facilitates the transport of selective substrates to LAMP2A-positive lysosomes for degradation [85]. VPS35 deficiency was first linked to defects in CMA by Tang and colleagues who observed that depletion of the core retromer component VPS35 in germline heterozygous VPS35 KO mice resulted in abnormal lysosomal morphology in nigral dopaminergic neurons, including reduced levels of LAMP2-positive vesicles and enlargement of LAMP1-positive vesicles [62]. Furthermore, lysosomal turnover of LAMP2A was significantly increased, leading to an overall decrease in the cellular levels of LAMP2A. The reduction of LAMP2A correlated significantly with increased α-synuclein aggregation of [62]. These findings are in line with previous observations that α-synuclein aggregates interact with LAMP2A and are degraded via the CMA pathway [86, 87]. Conditional deletion of VPS35 in embryonic cortical pyramidal neurons has also been linked to defects in the autophagy-lysosomal pathway [57]. First, LAMP1-positive lysosomes were significantly enlarged and less acidic in KO mice compared to WT controls. This was accompanied by the accumulation of p62/SQSTM1 and LC3B-II, suggesting dysfunctional autophagy. Lysosomal defects were also detected in human P301S-tau transgenic mice, where VPS35 gene silencing led to a significant reduction in the levels of cathepsin D and CI-M6PR that correlated with an increase in tau neuropathology [57].
3.2.2. Mitochondrial dysfunction
Mitochondrial dysfunction has been reported in several age-related diseases, including several neurodegenerative diseases [88]. Given the role of VPS35 at the mitochondria, understanding the exact contribution of the retromer to mitochondrial health is essential. Conditional deletion of VPS35 in midbrain dopaminergic neurons has been associated with mitochondrial dysfunction through the observation of increased mitochondrial fragmentation and abnormal mitochondrial morphology [27]. Interestingly, these KO neurons display a marked increase in the levels of Mul1 (MAPL), a mitochondrial E3 ubiquitin-protein ligase which results in decreased levels of mitofusin 2 (Mfn2), which is important for mitochondrial fusion. These changes were accompanied by impaired mitochondrial respiration and function. Mitochondrial defects in KO neurons were rescued by the exogenous expression of WT VPS35, further supporting a role for VPS35 in mitochondrial health and dynamics in dopaminergic neurons [27]. However, the exogenous expression of D620N VPS35 failed to rescue these phenotypes, suggesting that this mutation may confer a loss-of-function in the context of mitochondrial dynamics [27].
3.2.3. Neurotransmission deficits
Similar to D620N VPS35, the depletion of VPS35 has also been shown to have profound impacts on neurotransmission. Similar to the overexpression of D620N VPS35, depletion of VPS35 in germline heterozygous VPS35 KO mice causes a decrease in the surface levels of the AMPA receptor subunits GluR1 and GluR2 [89]. This was accompanied by impaired dendritic spine maturation. An additional study showed that knockdown of VPS35 via lentiviral delivery of shRNAs induced significant blockage of long-term potentiation (LTP) in mature hippocampal neurons. Although no reduction in AMPA receptors was detected, localization defects were observed that could underlie the LTP impairment [90]. Embryonic cortical pyramidal neurons are also sensitive to retromer depletion, with the selective deletion of VPS35 in this population of neurons resulting in profound defects in terminal differentiation, a defect also seen in hippocampal neurons of germline heterozygous VPS35 KO mice [63, 89].
Initial characterization of the D620N VPS35 KI and transgenic mice presented some evidence for dysregulation of striatal dopamine signaling [64, 65, 69]. In retromer depletion models, endocytic recycling of DAT was shown to be retromer-dependent, whereby shRNA-mediated knockdown of VPS35 leads to reduced total and surface expression levels of DAT in neuronal cell lines [91]. The retromer was also shown to be important for the sorting of dopamine receptor D1 (DRD1). Upon shRNA-mediated retromer depletion in HEK-293 cells, levels of exogenous DRD1 were significantly decreased at the cell surface, resulting in downstream signaling deficits [70]. Dopamine signaling deficits are also seen in the striatum and substantia nigra of germline heterozygous VPS35 KO mice at 6 months. Dopamine levels were diminished in both regions and dopamine metabolite ratios were elevated, suggesting dopamine transmission deficits [62]. Taken together, these studies suggest a fundamental role of VPS35 and the retromer in synaptic transmission and may represent a commonly disrupted pathway in neurodegenerative diseases upon retromer depletion. However, a remaining question is whether the retromer has unique neuron-specific cargo or functions compared to other cell types, or if perturbations to its more universal cellular functions are the root cause of neuronal vulnerability in neurodegenerative disease.
3.2.4. Cell-specific neurotoxicity and protein accumulation
Several studies suggest that VPS35 depletion can cause pathology similar to that observed in neurodegenerative disease, and that retromer function is critically required for the normal maintenance and survival of certain neuronal populations. While homozygous deletion of VPS35 (and VPS26) in mice results in early embryonic lethality, heterozygous VPS35 KO mice exhibit normal development and lifespan [4, 61]. Heterozygous VPS35 KO mice also reproduce some aspects of neuropathology with advanced age, including progressive dopaminergic neuronal loss, striatal dopamine loss, motor deficits, and the accumulation of insoluble α-synuclein in the substantia nigra [62]. Furthermore, the conditional deletion of VPS35 specifically in dopaminergic neurons using DAT-Cre mice results in rapid dopaminergic neuronal loss by 2–3 months of age, accompanied by motor deficits. VPS35 KODAT-Cre mice also display a marked accumulation of total and pathogenic phospho-S129-α-synuclein, recapitulating PD-relevant phenotypes [62]. It is important to note that deletion of VPS35 in dopaminergic neurons does not necessarily recreate PD pathology but may instead suggest a fundamental role for VPS35 in the maturation and maintenance of these neurons.
Conditional deletion of VPS35 has also been evaluated selectively in cortical pyramidal neurons of mice, where VPS35 is deleted early during embryogenesis using Neurod6-Cre mice. Embryonic deletion of VPS35 in pyramidal neurons leaves neurons with substantial terminal differentiation deficits, accompanied by neocortical atrophy and reactive gliosis. Interestingly, the accumulation of TDP-43 and ubiquitin-conjugated proteins, pathological hallmarks associated with neurodegenerative disease, were also observed [63]. These findings suggest that selective deletion of VPS35 in mouse cortical pyramidal neurons may represent a model of frontotemporal dementia (FTD), whose hallmark features are recapitulated in this model. However, it should be noted that retromer deficiency has not previously been documented in brains of FTD patients. Most recently, VPS35 was shown to be critical for the normal maintenance and survival of motor neurons during postnatal development [23]. Using synapsin-1-Cre mice, a conditional KO mouse model was created to delete VPS35 pan-neuronally. This model showed early post-natal lethality, accompanied by profound motor deficits and selective degeneration of motor neurons in the ventral horn region of the spinal cord. Additionally, reactive gliosis and p62-positive inclusions were observed in the affected region of the spinal cord [23]. Interestingly, these phenotypes suggest the VPS35 deletion in motor neurons could provide a novel model for studying ALS, which has previously been suggested to exhibit retromer depletion in vulnerable neurons.
Taken together, these studies support the idea that VPS35 is essential for the development and survival of several different neuronal populations. However, VPS35 is widely expressed throughout the brain and not limited to neurons. VPS35 has high levels of expression in glial cells, specifically oligodendrocytes and oligodendrocyte progenitor cells [23]. Therefore, it would be important to understand the impact of VPS35 deletion in other cell types outside of neurons. As such, a recent study looked at the impact of selective VPS35 deletion in microglia using CX3CR1-Cre mice. Interestingly, these conditional KO mice display increased microglia density and activity in the dentate gyrus region of the hippocampus and show an increase in the proliferation of neuronal progenitors. Strikingly, neuronal differentiation is significantly decreased and is accompanied by dendritic spine defects. Behaviorally, these mice also display depressive-like behavior and memory impairment [26]. In addition, these mice show an increased microglial phagocytic activity through the observed increased levels of PSD95, a postsynaptic marker, which may underlie the dendritic spine defects. Further work needs to be done to understand the cell type-specific role of VPS35 in other glial cells throughout the brain.
4. Conclusions and Future Perspectives
Since the initial discovery linking VPS35 and the retromer to neurodegenerative disease nearly 17 years ago, the field has advanced its understanding of the role of these proteins in health and disease. The discovery in 2011 of VPS35 mutations linked to PD solidifies this relationship and has since fueled the publication of numerous studies aiming at dissecting the mechanisms of VPS35-related neurodegenerative disease. While the exact pathophysiological role of retromer-mediated endosomal sorting in neurodegenerative disease remains elusive, here we reviewed the growing body of work aimed at answering this important question. The autosomal dominant D620N mutation in VPS35 has been shown to segregate with disease in families worldwide, thus spurring mechanistic studies in a variety of models [38]. Conversely, retromer depletion in AD, ALS, and primary tauopathies have revealed a non-genetic role for VPS35 in neurodegeneration, suggesting that defects in retromer-mediated endosomal sorting could be a fundamental feature of these neurodegenerative diseases.
While some features of autosomal dominant disease resemble the phenotypes of retromer depletion in animal models, several key differences exist. Most importantly, the D620N mutation has only been associated with PD where no conclusive retromer depletion has been reported in vulnerable brain regions [24]. Additionally, the D620N mutation seems to have a relatively subtle effect on retromer function where it can often rescue VPS35 null phenotypes, whereas retromer depletion induces severe phenotypes that do not always recapitulate PD-related pathology. Also, retromer depletion induces different phenotypes in distinct cell types, suggesting that some cells may be more susceptible to retromer deficiency than others, leading to different disease phenotypes [23, 26, 63]. The D620N VPS35 protein has been reported to be largely functional in many assays and models, suggesting the mutation does not confer a complete loss-of-function effect, as seen with retromer depletion [24, 58–61]. As such, it remains unclear whether the D620N mutation induces its pathogenic effects through a toxic gain-of-function or partial loss-of-function (i.e. dominant-negative) mechanism. Additionally, it is not well understood through which cellular pathways D620N VPS35 exerts neurotoxicity in D620N VPS35 rodent models. Further studies are required to elucidate such key mechanisms.
Given the identification of retromer perturbations in a wide range of neurodegenerative diseases, the retromer is an emerging therapeutic target. Since VPS35 and the core subunits of the retromer complex do not exhibit any enzymatic activity and instead play a role as protein scaffolds, targeting their functions pharmacologically is challenging. Additionally, as described in this review, animal models of D620N VPS35 generally are phenotypically distinct from animal models of retromer depletion. A further difficulty in targeting VPS35 in neurodegenerative disease at large is that the mechanism-of-action of the D620N mutation is still poorly understood, although many studies support a subtle or partial effect of the D620N mutation on overall retromer function. Furthermore, overexpression of WT VPS35 has been shown to induce neuronal degeneration, albeit to a lower extent than D620N VPS35, in the rat nigrostriatal pathway, suggesting that perturbing the physiological expression levels of VPS35 is poorly tolerated [24].
Given the expected subtle effect of the D620N mutation on VPS35 and retromer function, drugs stabilizing the retromer complex could potentially serve as a therapeutic strategy, assuming that the primary mechanism of retromer-induced neurotoxicity is caused by assembly or protein binding deficits, such as reduced Fam21 binding. For example, would restoring the interaction between D620N VPS35 and the WASH complex protect against neurodegeneration? Additionally, would stabilizing the retromer in brains with retromer depletion restore the function of VPS35 to sufficient levels to provide neuroprotection? While the answer to these questions are unknown, two chemical chaperones, R33 and R55, have been developed that stabilize the retromer through strengthening the interaction between VPS35 and VPS29 [92]. Indeed, these chaperones have been shown to provide neuroprotection in human iPSC-derived cellular models from sporadic AD patients, however, it is unclear if these iPSC models displayed retromer depletion to begin with [93]. The effects of R33 and R55 on D620N VPS35 protein function has not yet been explored but is warranted. Additionally, novel macrocyclic peptides have recently been developed that stabilize the VPS35-VPS26 interface and are suggested to provide a higher level of retromer stabilization compared to R33 and R55 [94]. It will be important for future studies to determine the most effective molecular chaperone for retromer stabilization and whether these molecules can provide neuroprotection in animal models of D620N VPS35. It should be noted that retromer stabilization could be detrimental if the neurotoxic mechanism of D620N VPS35 involves stronger binding to a given cargo, as seen with DLP1 [70].
Retromer stabilization could have undesired effects on LRRK2 kinase activity that may further enhance its hyperactivity induced by D620N VPS35. However, one intriguing idea is whether LRRK2 kinase activity could be targeted in D620N VPS35 patients, considering that D620N VPS35 robustly induces LRRK2 hyperactivation [82]. LRRK2 kinase inhibitors are already widely studied as potential therapies for both sporadic and familial PD [95]. Studies have demonstrated in mouse and human iPSC-derived cell models of D620N VPS35 that treatment with the LRRK2 kinase inhibitor MLi-2 could successfully reduce Rab substrate phosphorylation [82]. Whether LRRK2 kinase inhibitors or deletion could protect against D620N VPS35-induced neurodegeneration in animal models would be the first step to establish if LRRK2 inhibition could provide a potential therapeutic option for VPS35-linked PD.
Acknowledgements
The authors work on VPS35 is supported in part by grants from the National Institutes of Health (R01 NS105432 and R01 NS117137 to D.J.M.) and Van Andel Institute.
References
- 1.Seaman MNM, E. G.; Cereghino JL; & Emr SD, Endosome to Golgi Retrieval of the Vacuolar Protein Sorting Receptor, Vps10p, Requires the Function of the VPS29, VPS30, and VPS35 Gene Products. The Journal of Cell Biology, 1997. 137(1): p. 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seaman MNM, J. M.; & Emr SD, A Membrane Coat Complex Essential for Endosome-to-Golgi Retrograde Transport in Yeast. The Journal of Cell Biology, 1998. 142(3): p. 665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaman MN, The retromer complex - endosomal protein recycling and beyond. Journal of Cell Science, 2012. 125(Pt 20): p. 4693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen L, et al. , VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. The Journal of Cell Biology, 2011. 195(5): p. 765–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaman MNJ, The Retromer Complex: From Genesis to Revelations. Trends Biochem Sci, 2021. 46(7): p. 608–620. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino JS and Hurley JH, Retromer. Current Opinion in Cell Biology, 2008. 20(4): p. 427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugarcic A, et al. , Vps26A and Vps26B subunits define distinct retromer complexes. Traffic, 2011. 12(12): p. 1759–73. [DOI] [PubMed] [Google Scholar]
- 8.Harbour ME, et al. , The cargo-selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci, 2010. 123(Pt 21): p. 3703–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harterink M, et al. , A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nature Cell Biology, 2011. 13(8): p. 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, et al. , SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nature Cell Biology, 2001. 3(7): p. 658–66. [DOI] [PubMed] [Google Scholar]
- 11.Kvainickas A, et al. , Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J Cell Biol, 2017. 216(11): p. 3677–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tu Y and Seaman MNJ, Navigating the Controversies of Retromer-Mediated Endosomal Protein Sorting. Front Cell Dev Biol, 2021. 9: p. 658741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokool S, et al. , Identification of a conserved motif required for Vps35p/Vps26p interaction and assembly of the retromer complex. Biochemical Journal, 2007. 408(2): p. 287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hierro A, et al. , Functional architecture of the retromer cargo-recognition complex. Nature, 2007. 449(7165): p. 1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovtun O, et al. , Structure of the membrane-assembled retromer coat determined by cryo-electron tomography. Nature, 2018. 561(7724): p. 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendall AK, et al. , Mammalian Retromer Is an Adaptable Scaffold for Cargo Sorting from Endosomes. Structure, 2020. 28(4): p. 393–405.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y, Yang Z, and Teasdale RD, The functional roles of retromer in Parkinson’s disease. FEBS Lett, 2018. 592(7): p. 1096–1112. [DOI] [PubMed] [Google Scholar]
- 18.Seaman MN, Gautreau A, and Billadeau DD, Retromer-mediated endosomal protein sorting: all WASHed up! Trends in Cell Biology, 2013. 23(11): p. 522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg F, et al. , A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nature Cell Biology, 2013. 15(5): p. 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez TS and Billadeau DD, A FAM21-containing WASH complex regulates retromer-dependent sorting. Developmental Cell, 2009. 17(5): p. 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullen PJ and Korswagen HC, Sorting nexins provide diversity for retromer-dependent trafficking events. Nature Cell Biology, 2012. 14(1): p. 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sargent D and Moore DJ, Chapter Five - Mechanisms of VPS35-mediated neurodegeneration in Parkinson’s disease, in International Review of Movement Disorders, Dehay B and Bezard E, Editors. 2021, Academic Press. p. 221–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sargent D, et al. , Neuronal VPS35 deletion induces spinal cord motor neuron degeneration and early post-natal lethality. Brain Communications, 2021. 3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsika E, et al. , Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Human Molecular Genetics, 2014. 23(17): p. 4621–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choy RW, et al. , Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron, 2014. 82(1): p. 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel JR, et al. , Increased Microglial Activity, Impaired Adult Hippocampal Neurogenesis, and Depressive-like Behavior in Microglial VPS35-Depleted Mice. J Neurosci, 2018. 38(26): p. 5949–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang FL, et al. , VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function. Cell Reports, 2015. 12(10): p. 1631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang AE and Lozano AM, Parkinson’s disease. Second of two parts. The New England Journal of Medicine, 1998. 339(16): p. 1130–43. [DOI] [PubMed] [Google Scholar]
- 29.Lang AE and Lozano AM, Parkinson’s disease. First of two parts. The New England Journal of Medicine, 1998. 339(15): p. 1044–53. [DOI] [PubMed] [Google Scholar]
- 30.Poewe W, et al. , Parkinson disease. Nature Reviews Disease Primers, 2017. 3(1): p. 17013. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez DG, Reed X, and Singleton AB, Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. Journal of Neurochemistry, 2016. 139 Suppl 1: p. 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blauwendraat C, Nalls MA, and Singleton AB, The genetic architecture of Parkinson’s disease. Lancet Neurol, 2020. 19(2): p. 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Przedborski S, The two-century journey of Parkinson disease research. Nat Rev Neurosci, 2017. 18(4): p. 251–259. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham LA and Moore DJ, Endosomal sorting pathways in the pathogenesis of Parkinson’s disease. Prog Brain Res, 2020. 252: p. 271–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vilarino-Guell C, et al. , VPS35 mutations in Parkinson disease. American Journal of Human Genetics, 2011. 89(1): p. 162 – 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wider C, et al. , Autosomal dominant dopa-responsive parkinsonism in a multigenerational Swiss family. Parkinsonism & Related Disorders, 2008. 14(6): p. 465–470. [DOI] [PubMed] [Google Scholar]
- 37.Zimprich A, et al. , A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. American Journal of Human Genetics, 2011. 89(1): p. 168 – 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams ET, Chen X, and Moore DJ, VPS35, the Retromer Complex and Parkinson’s Disease. Journal of Parkinson’s Disease, 2017. 7(2): p. 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishiguro M, et al. , Clinical manifestations of Parkinson’s disease harboring VPS35 retromer complex component p.D620N with long-term follow-up. Parkinsonism Relat Disord, 2021. 84: p. 139–143. [DOI] [PubMed] [Google Scholar]
- 40.Kumar KR, et al. , Frequency of the D620N mutation in VPS35 in Parkinson disease. Archives of Neurology, 2012. 69(10): p. 1360–4. [DOI] [PubMed] [Google Scholar]
- 41.Struhal W, et al. , VPS35 Parkinson’s disease phenotype resembles the sporadic disease. J Neural Transm (Vienna), 2014. 121(7): p. 755–9. [DOI] [PubMed] [Google Scholar]
- 42.Escott-Price V, et al. , Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain, 2015. 138(12): p. 3673–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raber J, Huang Y, and Ashford JW, ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging, 2004. 25(5): p. 641–50. [DOI] [PubMed] [Google Scholar]
- 44.Gatz M, et al. , Role of Genes and Environments for Explaining Alzheimer Disease. Archives of General Psychiatry, 2006. 63(2): p. 168–174. [DOI] [PubMed] [Google Scholar]
- 45.DeKosky ST and Scheff SW, Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Annals of Neurology, 1990. 27(5): p. 457–464. [DOI] [PubMed] [Google Scholar]
- 46.Terry RD, et al. , Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology, 1991. 30(4): p. 572–580. [DOI] [PubMed] [Google Scholar]
- 47.Small SA, et al. , Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol, 2005. 58(6): p. 909–19. [DOI] [PubMed] [Google Scholar]
- 48.Small SA and Gandy S, Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron, 2006. 52(1): p. 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambert JC, et al. , Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet, 2013. 45(12): p. 1452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogaeva E, et al. , The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet, 2007. 39(2): p. 168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reitz C, et al. , Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4,and the risk of late-onset Alzheimer disease in African Americans. Jama, 2013. 309(14): p. 1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simoes S, et al. , Alzheimer’s vulnerable brain region relies on a distinct retromer core dedicated to endosomal recycling. Cell Reports, 2021. 37(13): p. 110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vardarajan BN, et al. , Identification of Alzheimer disease-associated variants in genes that regulate retromer function. Neurobiol Aging, 2012. 33(9): p. 2231.e15–2231.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masrori P and Van Damme P, Amyotrophic lateral sclerosis: a clinical review. European Journal of Neurology, 2020. 27(10): p. 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muzio L, et al. , Retromer stabilization results in neuroprotection in a model of Amyotrophic Lateral Sclerosis. Nature Communications, 2020. 11(1): p. 3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganguly J and Jog M, Tauopathy and Movement Disorders-Unveiling the Chameleons and Mimics. Front Neurol, 2020. 11: p. 599384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vagnozzi AN, et al. , VPS35 regulates tau phosphorylation and neuropathology in tauopathy. Mol Psychiatry, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Follett J, et al. , The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic, 2014. 15(2): p. 230–44. [DOI] [PubMed] [Google Scholar]
- 59.Inoshita T, et al. , Vps35 in cooperation with LRRK2 regulates synaptic vesicle endocytosis through the endosomal pathway in Drosophila. Hum Mol Genet, 2017. 26(15): p. 2933–2948. [DOI] [PubMed] [Google Scholar]
- 60.Malik BR, Godena VK, and Whitworth AJ, VPS35 pathogenic mutations confer no dominant toxicity but partial loss of function in Drosophila and genetically interact with parkin. Human Molecular Genetics, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, et al. , Parkinson’s disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration. Proceedings of the National Academy of Sciences, 2019. 116(12): p. 5765–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang FL, et al. , VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for alpha-Synuclein Degradation and Prevention of Pathogenesis of Parkinson’s Disease. The Journal of Neuroscience, 2015. 35(29): p. 10613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang F-L, et al. , Coupling of terminal differentiation deficit with neurodegenerative pathology in Vps35-deficient pyramidal neurons. Cell Death & Differentiation, 2020. 27(7): p. 2099–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishizu N, et al. , Impaired striatal dopamine release in homozygous Vps35 D620N knock-in mice. Human Molecular Genetics, 2016. [DOI] [PubMed] [Google Scholar]
- 65.Cataldi S, et al. , Altered dopamine release and monoamine transporters in Vps35 p.D620N knock-in mice. npj Parkinson’s Disease, 2018. 4(1): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiu CC, et al. , (D620N) VPS35 causes the impairment of Wnt/β-catenin signaling cascade and mitochondrial dysfunction in a PARK17 knockin mouse model. Cell Death Dis, 2020. 11(11): p. 1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niu M, et al. , VPS35 D620N knockin mice recapitulate cardinal features of Parkinson’s disease. Aging Cell, 2021. 20(5): p. e13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadgien CA, Kamesh A, and Milnerwood AJ, Endosomal traffic and glutamate synapse activity are increased in VPS35 D620N mutant knock-in mouse neurons, and resistant to LRRK2 kinase inhibition. Molecular Brain, 2021. 14(1): p. 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanan S, et al. , Altered striatal dopamine levels in Parkinson’s disease VPS35 D620N mutant transgenic aged mice. Molecular Brain, 2020. 13(1): p. 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, et al. , Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nature Medicine, 2016. 22(1): p. 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo F, et al. , Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol, 2018. 28(1): p. 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aman Y, et al. , Autophagy in healthy aging and disease. Nature Aging, 2021. 1(8): p. 634–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia P, et al. , WASH inhibits autophagy through suppression of Beclin 1 ubiquitination. Embo j, 2013. 32(20): p. 2685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zavodszky E, et al. , Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nature Communications, 2014. 5: p. 3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGough IJ, et al. , Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Current Biology, 2014. 24(14): p. 1670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miura E, et al. , VPS35 dysfunction impairs lysosomal degradation of alpha-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson’s disease. Neurobiology of Disease, 2014. 71: p. 1–13. [DOI] [PubMed] [Google Scholar]
- 77.Braschi E, et al. , Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Current Biology, 2010. 20(14): p. 1310–5. [DOI] [PubMed] [Google Scholar]
- 78.Wang W, et al. , A conserved retromer sorting motif is essential for mitochondrial DLP1 recycling by VPS35 in Parkinson’s disease model. Hum Mol Genet, 2017. 26(4): p. 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munsie LN, et al. , Retromer-dependent neurotransmitter receptor trafficking to synapses is altered by the Parkinson’s Disease VPS35 mutation p.D620N. Human Molecular Genetics, 2014. [DOI] [PubMed] [Google Scholar]
- 80.MacLeod DA, et al. , RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron, 2013. 77(3): p. 425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Linhart R, et al. , Vacuolar protein sorting 35 (Vps35) rescues locomotor deficits and shortened lifespan in Drosophila expressing a Parkinson’s disease mutant of Leucine-Rich Repeat Kinase 2 (LRRK2). Molecular Neurobiology, 2014. 9: p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mir R, et al. , The Parkinson’s disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem J, 2018. 475(11): p. 1861–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steger M, et al. , Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife, 2016. 5: p. e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Small SA and Petsko GA, Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nature Reviews Neuroscience, 2015. 16(3): p. 126–132. [DOI] [PubMed] [Google Scholar]
- 85.Sala G, et al. , Role of Chaperone-Mediated Autophagy Dysfunctions in the Pathogenesis of Parkinson’s Disease. Frontiers in Molecular Neuroscience, 2016. 9(157). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cuervo AM, et al. , Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science, 2004. 305(5688): p. 1292–5. [DOI] [PubMed] [Google Scholar]
- 87.Mak SK, et al. , Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem, 2010. 285(18): p. 13621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monzio Compagnoni G, et al. , The Role of Mitochondria in Neurodegenerative Diseases: the Lesson from Alzheimer’s Disease and Parkinson’s Disease. Molecular Neurobiology, 2020. 57(7): p. 2959–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian Y, et al. , VPS35-deficiency results in an impaired AMPA receptor trafficking and decreased dendritic spine maturation. Molecular Brain, 2015. 8(1): p. 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Temkin P, et al. , The Retromer Supports AMPA Receptor Trafficking During LTP. Neuron, 2017. 94(1): p. 74–82.e5. [DOI] [PubMed] [Google Scholar]
- 91.Wu S, et al. , The Dopamine Transporter Recycles via a Retromer-Dependent Postendocytic Mechanism: Tracking Studies Using a Novel Fluorophore-Coupling Approach. J Neurosci, 2017. 37(39): p. 9438–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mecozzi VJ, et al. , Pharmacological chaperones stabilize retromer to limit APP processing. Nature Chemical Biology, 2014. 10(6): p. 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Young JE, et al. , Stabilizing the Retromer Complex in a Human Stem Cell Model of Alzheimer’s Disease Reduces TAU Phosphorylation Independently of Amyloid Precursor Protein. Stem Cell Reports, 2018. 10(3): p. 1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen K-E, et al. , De novo macrocyclic peptides for inhibiting, stabilizing, and probing the function of the retromer endosomal trafficking complex. Science Advances, 2021. 7(49): p. eabg4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taymans JM and Greggio E, LRRK2 Kinase Inhibition as a Therapeutic Strategy for Parkinson’s Disease, Where Do We Stand? Curr Neuropharmacol, 2016. 14(3): p. 214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]