Abstract
Background:
Pancreatic cancer presents as advanced disease in >80% of patients; yet, appropriate ages to consider prevention and early detection strategies are poorly defined. We investigated age-specific associations and attributable risks of pancreatic cancer for established modifiable and non-modifiable risk factors.
Patients and methods:
We included 167 483 participants from two prospective US cohort studies with 1190 incident cases of pancreatic cancer during >30 years of follow-up; 5107 pancreatic cancer cases and 8845 control participants of European ancestry from a completed multicenter genome-wide association study (GWAS); and 248 893 pancreatic cancer cases documented in the US Surveillance, Epidemiology, and End Results (SEER) Program. Across different age categories, we investigated cigarette smoking, obesity, diabetes, height, and non-O blood group in the prospective cohorts; weighted polygenic risk score of 22 previously identified single nucleotide polymorphisms in the GWAS; and male sex and black race in the SEER Program.
Results:
In the prospective cohorts, all five risk factors were more strongly associated with pancreatic cancer risk among younger participants, with associations attenuated among those aged >70 years. The hazard ratios comparing participants with three to five risk factors with those with no risk factors were 9.24 [95% confidence interval (CI) 4.11–20.77] among those aged ≤60 years, 3.00 (95% CI 1.85–4.86) among those aged 61–70 years, and 1.46 (95% CI 1.10–1.94) among those aged >70 years (Pheterogeneity = 3×10−5). These factors together were related to 65.6%, 49.7%, and 17.2% of incident pancreatic cancers in these age groups, respectively. In the GWAS and the SEER Program, the associations with the polygenic risk score, male sex, and black race were all stronger among younger individuals (Pheterogeneity ≤0.01).
Conclusions:
Established risk factors are more strongly associated with earlier-onset pancreatic cancer, emphasizing the importance of age at initiation for cancer prevention and control programs targeting this highly lethal malignancy.
Keywords: pancreatic cancer, age, risk factor, lifestyle modification, polygenic risk score
INTRODUCTION
Pancreatic cancer is the third leading cause of cancer-related death in the USA, with 5-year survival of only 10%.1 While 50% of pancreatic cancers are diagnosed after age 70 years,2 a rising incidence of pancreatic cancer has been noted particularly in younger populations.3–5 Due to the lethality of pancreatic cancer, younger cases contribute greatly to the societal burden of this disease by causing substantial loss of potential life years.6 In contrast to other major cancers (e.g. colon, lung), only 30%−40% of pancreatic cancers are attributable to genetic predisposition and lifestyle factors,7,8 complicating prevention and early detection of this highly fatal disease.
Cancer development is characterized by a stepwise accumulation of driver gene mutations leading to malignant transformation. Notably, mistakes made during normal stem cell division may contribute a substantial fraction of driver mutations, including for pancreatic cancer.9,10 As people advance in age, an increasing number of these mutations develop proportional to the lifetime number of stem cell divisions within an organ. Consequently, pancreatic cancer that develops at an older age may harbor fewer driver events caused by inherited and lifestyle factors and more from random mutations, such that inherited and lifestyle factors may be more strongly associated with development of earlier-onset versus later-onset pancreatic cancer. Several prior studies have suggested an enrichment of risk factors for pancreatic cancer among younger cases,11–14 which partly supports the differential etiology of pancreatic cancer by age at diagnosis.
In the current study, we comprehensively investigated age-specific associations and attributable risks of pancreatic cancer for inherited and lifestyle risk factors, leveraging detailed epidemiological data from two prospective US cohort studies with repeated exposure measurements over several decades, a completed genome-wide association study (GWAS) of pancreatic cancer, and the Surveillance, Epidemiology, and End Results (SEER) Program. We demonstrate an age-dependent pattern for nearly all pancreatic cancer risk factors, whereby associations with pancreatic cancer were strongest among younger participants and substantially weakened as individuals aged.
METHODS
Study populations
Prospective cohort studies.
We examined risk factors for pancreatic cancer in two prospective US cohorts: the Nurses’ Health Study (NHS)15 that enrolled 121 700 female registered nurses aged 30–55 years in 1976 and the Health Professionals Follow-Up Study (HPFS)16 that enrolled 51 529 male health professionals aged 40–75 years in 1986. Since enrollment, participants were biennially mailed questionnaires inquiring about their medical history and lifestyle factors. We excluded participants with prior history of cancer at enrollment for an analysis population of 167 483 participants. Incident cases of pancreatic cancer were identified from self-report or during follow-up of participant deaths ascertained through reports from the next of kin, the US Postal Service, or the National Death Index.17 Physicians confirmed the diagnosis by reviewing medical records, death certificates, or cancer registry data. Cases with non-adenocarcinoma histology were excluded. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Pancreatic cancer GWAS.
We examined a polygenic risk score (PRS) for pancreatic cancer in 5107 pancreatic cancer cases and 8845 control participants of European ancestry from a completed multicenter GWAS, PanScan/PanC4 I-III, led by the Pancreatic Cancer Cohort Consortium and Pancreatic Cancer Case-Control Consortium (Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2022.03.276)18–20 Each participating study obtained written informed consent from participants and approval from the local institutional review board.
The SEER Program.
We examined incidence rates of pancreatic cancer by sex and race in SEER 21,21 which collected cancer incidence data from 21 population-based cancer registries since 2000, covering 36.7% of the US population. Using SEER*Stat (version 8.3.8), we selected pancreatic cancers diagnosed at ages of ≥30 years without non-adenocarcinoma tumors, resulting in 248 893 cases diagnosed during 2000–2017 (Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2022.03.276).
Exposure assessment
In the prospective cohorts, we collected data on five established risk factors for pancreatic cancer, cigarette smoking,22 obesity,23 diabetes,24 tall height,23 and non-O blood group,25 as well as three suspected risk factors, inadequate exercise,23 excessive alcohol use,26 and poor diet quality27 (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2022.03.276). Established risk factors were defined as follows: (i) at cohort enrollment, participants were asked detailed information about their current and past smoking. After enrollment, information on smoking status and smoking intensity was reported biennially. Pack-years of smoking were computed to assess cumulative exposure to cigarettes. (ii) Current weight was reported at enrollment and biennially thereafter. Body mass index (BMI) was calculated as weight in kilograms divided by square of height in meters. For main analyses, the cumulative average of BMI was computed by using all available questionnaires until the previous survey cycle. Obesity was defined as a BMI of ≥30 kg/m2. (iii) At enrollment and biennially thereafter, participants were asked if they had diabetes diagnosed by a physician. Because a subset of pancreatic cancer patients develop diabetes before their cancer diagnosis due to a subclinical pancreatic tumor,28 we only considered diabetes for >2 years as a risk factor. (iv) Height was reported at enrollment. Tall height was defined as a height of ≥167 cm for women and ≥181 cm for men, which were the 75th percentiles of height in the National Health and Nutrition Examination Survey 1999–2016.29 (v) In 1996, participants were asked their blood group: O, A, B, AB, or unknown, which has been validated previously.25 Information on assessment of the suspected risk factors is provided in Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2022.03.276.
Genotyping and imputation methods in the PanScan/PanC4 I-III GWAS have been described previously.20 We calculated a PRS for pancreatic cancer by summing risk alleles in 22 previously identified single nucleotide polymorphisms (SNPs) in European populations,18–20,30–32 weighted by reported effect sizes (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2022.03.276)33 In the SEER Program, sex and race were obtained from the registry databases.
Statistical analyses
We categorized participants by age into three groups (≤60, 61–70, and >70 years), or two groups (≤70 and >70 years) when sample size was inadequate, as in the USA, about 20% of pancreatic cancers develop before age 60 years, and 50% before age 70 years.2 Random-effects meta-regression was used to test for heterogeneity of the association magnitude between oldest and youngest age groups.34
In the prospective cohorts, person-years for participants were calculated from when the exposure was first surveyed until pancreatic cancer diagnosis, death, or end of follow-up, whichever came first. During follow-up, participants could contribute person-years to different age groups (Supplementary Figure S2, available at https://doi.org/10.1016/j.annonc.2022.03.276). The Andersen-Gill data structure was used to handle time-varying covariates, which created a record for every 2-year survey cycle at which a participant was at risk, with covariates updated at the time of questionnaire return. Cox proportional hazards regression was used to examine age-stratified associations between risk factors and pancreatic cancer risk. To avoid arbitrary determination of age cut-offs, we also used an alternative to Cox regression with age as the time scale and modeled the association as a smooth function of age approximated by B-spline (R, version 4.0.3)35 Logistic regression and Poisson regression were used to examine associations of pancreatic cancer risk with the PRS in the GWAS and demographic factors in the SEER Program, respectively. Information on covariate adjustment is provided in Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2022.03.276.
In the prospective cohorts, we computed age-specific population attributable fractions (PAFs) for the proportion of incident pancreatic cancers in the study population that theoretically would not have occurred if the risk factor had been absent.36 To provide contemporary estimates for potentially modifiable factors, we also derived PAFs by using contemporary, nationally representative data on exposure prevalence (Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2022.03.276). All analyses were carried out with SAS, version 9.4 (SAS Institute Inc., Cary, NC), unless specified otherwise. A two-sided P < 0.05 indicated statistical significance.
RESULTS
Risk factors in the prospective cohorts
During approximately 5.0 million person-years of follow-up in a study population of 118 148 women from NHS and 49 335 men from HPFS, 1190 incident cases of pancreatic cancer were identified, including 146 cases aged ≤60 years (incidence rate, 6 per 100 000 person-years), 336 aged 61–70 years (24 per 100 000 person-years), and 708 aged >70 years (60 per 100 000 person-years). Table 1 shows age-specific characteristics for study participants.
Table 1.
Age-specific characteristics among participants from two prospective cohorts
| Characteristica | Full study population |
Pancreatic cancer casesb |
|||||
|---|---|---|---|---|---|---|---|
| ≤60 years | 61–70 years | >70 years | ≤60 years | 61–70 years | >70 years | ||
| Women/NHS | |||||||
| No. person-years or cases | 2 002 737 | 1 070 675 | 817 529 | 110 | 223 | 435 | |
| White, % | 96.8 | 96.8 | 96.7 | 97.3 | 96.4 | 96.8 | |
| Ever-smoking, % | 56.1 | 56.0 | 53.9 | 67.3 | 63.8 | 52.1 | |
| Obesityc, % | 10.1 | 13.9 | 14.4 | 17.6 | 20.3 | 18.2 | |
| Diabetes, >2 years, % | 2.1 | 7.3 | 12.8 | 10.8 | 9.5 | 21.6 | |
| Tall heightd, % | 35.1 | 34.1 | 32.4 | 44.5 | 36.9 | 33.6 | |
| Men/HPFS | |||||||
| No. person-years or cases | 364 923 | 357 963 | 363 730 | 36 | 113 | 273 | |
| White, % | 90.8 | 90.7 | 90.7 | 88.9 | 86.7 | 93.0 | |
| Ever-smoking, % | 46.0 | 51.8 | 55.4 | 70.6 | 63.9 | 59.4 | |
| Obesityc, % | 9.2 | 10.6 | 8.9 | 19.4 | 11.8 | 7.1 | |
| Diabetes, >2 years, % | 1.5 | 5.0 | 9.4 | 9.1 | 6.8 | 16.9 | |
| Tall heightd, % | 35.7 | 32.3 | 26.2 | 55.6 | 33.6 | 24.5 | |
|
| |||||||
| ≤70 years | >70 years | ≤70 years | >70 years | ||||
|
| |||||||
| Women/NHS | |||||||
| Non-O blood group, % | 56.9 | 56.9 | 69.7 | 62.1 | |||
| Inadequate exercisee, % | 42.7 | 39.4 | 49.6 | 41.0 | |||
| Excessive alcohol usef, % | 12.8 | 12.6 | 15.3 | 15.9 | |||
| AHEI-2010, mean (SD) | 51 (10) | 53 (9) | 51 (9) | 53 (9) | |||
| Men/HPFS | |||||||
| Non-O blood group, % | 57.6 | 56.4 | 71.2 | 59.0 | |||
| Inadequate exercisee, % | 29.4 | 27.6 | 40.9 | 25.3 | |||
| Excessive alcohol usef, % | 10.8 | 11.7 | 15.5 | 16.0 | |||
| AHEI-2010, mean (SD) | 53 (11) | 56 (10) | 54 (10) | 56 (11) | |||
AHEI, alternate healthy eating index; HPFS, Health Professionals Follow-Up Study; MET, metabolic equivalent; NHS, Nurses’ Health Study; SD, standard deviation.
Obesity, inadequate exercise, excessive alcohol use, and AHEI-2010 assessed by the cumulative average of body mass index, physical activity, alcohol consumption, and AHEI-2010, respectively.
Time-varying variables assessed at the time of cancer diagnosis.
Defined as a body mass index of ≥30 kg/m2.
Defined as a height of ≥167 cm for women and ≥181 cm for men (i.e. the 75th percentiles of height in the National Health and Nutrition Examination Survey 1999–2016).
Defined as aerobic exercise of <75 vigorous-intensity or 150 moderate-intensity minutes (i.e. 500 MET-minutes/week), according to the Physical Activity Guidelines for Americans, 2nd edition.
Defined as alcohol use of >1 drink/day for women and >2 drinks/day for men, according to the 2015–2020 Dietary Guidelines for Americans.
Association between risk factors and pancreatic cancer
To test the hypothesis that risk factors may be more strongly associated with pancreatic cancer developed at younger ages, we first examined age-stratified associations of pancreatic cancer risk with five established risk factors: cigarette smoking, obesity, diabetes, tall height, and non-O blood group (Table 2).
Table 2.
Age-stratified association of pancreatic cancer risk with established risk factors among participants from two prospective cohorts
| Risk factor | Age ≤60 years |
Age 61–70 years |
Age >70 years |
P heterogeneity b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Person-years | No.cases | Hazard ratio (95% CI)a | Person-years | No.cases | Hazard ratio (95% CI)a | Person-years | No.cases | Hazard ratio (95% CI)a | ||
| Cigarette smoking | ||||||||||
| Never | 1 056 294 | 46 | 1 [Reference] | 629 200 | 119 | 1 [Reference] | 528 219 | 311 | 1 [Reference] | |
| Ever | 1 265 695 | 98 | 1.84 (1.29–2.62) | 764 156 | 210 | 1.45 (1.16–1.82) | 619 032 | 378 | 1.04 (0.89–1.21) | 0.004 |
| Former: ≤39 pack-years | 689 728 | 45 | 1.40 (0.93–2.12) | 500 702 | 109 | 1.12 (0.87–1.46) | 425 156 | 240 | 0.96 (0.81–1.14) | |
| Former: ≥40 pack-years | 39 285 | 5 | 1.79 (0.70–4.55) | 83 192 | 31 | 1.69 (1.13–2.52) | 113 928 | 79 | 1.10 (0.86–1.41) | |
| Current: ≤39 pack-years | 415 911 | 28 | 2.34 (1.44–3.79) | 80 955 | 24 | 1.87 (1.20–2.92) | 28 333 | 15 | 1.04 (0.62–1.76) | |
| Current: ≥40 pack-years | 120 770 | 20 | 3.38 (1.98–5.79) | 99 306 | 46 | 2.83 (2.00–4.01) | 51 615 | 44 | 1.58 (1.14–2.17) | |
| Obesityc | ||||||||||
| No | 1 857 150 | 109 | 1 [Reference] | 1 218 066 | 274 | 1 [Reference] | 1 025 127 | 606 | 1 [Reference] | |
| Yes | 201 523 | 24 | 1.85 (1.19–2.88) | 183 973 | 58 | 1.41 (1.06–1.87) | 146 839 | 98 | 1.18 (0.95–1.46) | 0.07 |
| Diabetes, >2 years | ||||||||||
| No | 2 231 090 | 121 | 1 [Reference] | 1 239 278 | 278 | 1 [Reference] | 948 837 | 514 | 1 [Reference] | |
| Yes | 41 165 | 14 | 3.85 (2.07–7.18) | 88 214 | 26 | 1.12 (0.73–1.72) | 124 844 | 127 | 1.89 (1.54–2.33) | 0.03 |
| Tall heightd | ||||||||||
| No | 1 531 714 | 77 | 1 [Reference] | 946 917 | 215 | 1 [Reference] | 822 601 | 495 | 1 [Reference] | |
| Yes | 833 464 | 69 | 1.68 (1.21–2.33) | 480 195 | 120 | 1.12 (0.90–1.41) | 357 342 | 213 | 1.04 (0.88–1.22) | 0.01 |
|
| ||||||||||
|
Age ≤70 years
|
Age >70 years
|
|||||||||
| Person-years | No. cases | Hazard ratio (95% CI)a | Person-years | No. cases | Hazard ratio (95% CI)a | |||||
|
| ||||||||||
| ABO blood group | ||||||||||
| O | 345 376 | 45 | 1 [Reference] | 307 014 | 160 | 1 [Reference] | ||||
| Non-O | 459 562 | 106 | 1.84 (1.30–2.61) | 402 869 | 251 | 1.18 (0.97–1.44) | 0.03 | |||
| A | 295 242 | 64 | 1.73 (1.18–2.54) | 250 853 | 144 | 1.09 (0.87–1.37) | ||||
| B | 108 326 | 27 | 1.95 (1.20–3.14) | 91 365 | 68 | 1.40 (1.05–1.86) | ||||
| AB | 55 994 | 15 | 2.19 (1.21–3.94) | 60 651 | 39 | 1.22 (0.85–1.73) | ||||
CI, confidence interval.
From Cox proportional hazards regression conditioned on age (in months) and calendar year of the survey cycle and sex/cohort and adjusted for race/ethnicity (white, black, other, or unknown), pack-years of smoking (never, ≤19, 20–39, ≥40, or missing; unadjusted when cigarette smoking was examined), and body mass index in kg/m2 (<25.0, 25.0–29.9, 30.0–34.9, ≥35.0, or missing; unadjusted when obesity was examined).
Heterogeneity between the age groups of ≤60 years (or ≤70 years for non-O blood group) and >70 years tested by using the random-effects meta-regression method.
Defined as a body mass index (cumulative average) of ≥30 kg/m2.
Defined as a height of ≥167 cm for women and ≥181 cm for men (i.e. the 75th percentiles of height in the National Health and Nutrition Examination Survey 1999–2016).
For cigarette smoking, the hazard ratios (HRs) comparing ever smokers to never smokers were 1.84 [95% confidence interval (CI) 1.29–2.62] among participants aged ≤60 years, 1.45 (95% CI 1.16–1.82) among those aged 61–70 years, and 1.04 (95% CI 0.89–1.21) among those aged >70 years (Pheterogeneity = 0.004; Table 2). Among participants aged >70 years, only current smokers who had smoked ≥40 pack-years in their lifetime had an increased risk for pancreatic cancer. To assess cumulative tobacco exposure with different latency periods before cancer diagnosis, we examined lifetime pack-years, as well as pack-years during or beyond the most recent 10 years (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2022.03.276). Regardless of latency period, cigarette smoking was more strongly associated with pancreatic cancer risk among participants aged ≤60 years than those aged >70 years.
As with cigarette smoking, obesity and diabetes were more strongly associated with pancreatic cancer risk at a younger age (Pheterogeneity = 0.07 and 0.03, respectively; Table 2). For obesity and diabetes, the HRs were 1.85 (95% CI 1.19–2.88) and 3.85 (95% CI 2.07–7.18) among participants aged ≤60 years, in contrast to 1.18 (95% CI 0.95–1.46) and 1.89 (95% CI 1.54–2.33) among those aged >70 years. As an individual’s BMI can vary greatly over the life course, we examined obesity at different timepoints in life and restricted the study population to participants who were enrolled before age 60 years (Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2022.03.276). In all these analyses, obesity was more strongly associated with earlier-onset than late-onset pancreatic cancer.
We next examined tall height, which was associated with increased risk of pancreatic cancer among participants aged ≤60 years (HR, 1.68; 95% CI 1.21–2.33), but not among older participants (Pheterogeneity = 0.01; Table 2). Due to an inadequate number of cases aged ≤60 years, the association with ABO blood group was examined by pooling participants aged ≤70 years. Non-O blood group was more strongly associated with pancreatic cancer risk among participants aged ≤70 years (HR, 1.84; 95% CI 1.30–2.61) than those aged >70 years [HR, 1.18 (95% CI 0.97–1.44); Pheterogeneity = 0.03; Table 2].
When considering a combinatorial score that sums the five established risk factors based on presence or absence of each individual factor, we observed a pronounced attenuation with age in the association with pancreatic cancer risk. The HRs comparing participants with three to five risk factors to those with no risk factors were 9.24 (95% CI 4.11–20.77) among those aged ≤60 years, 3.00 (95% CI 1.85–4.86) among those aged 61–70 years, and 1.46 (95% CI 1.10–1.94) among those aged >70 years (Pheterogeneity = 3 × 10−5; Figure 1A). Such attenuation with increasing age was similar by sex/cohort and birth cohort (Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2022.03.276). We also estimated the HR per additional risk factor as a smooth function of age, whereby the association again weakened as individuals aged (Figure 1B).
Figure 1. Age-specific association of pancreatic cancer risk with combinatory risk factors among participants from two prospective cohorts.
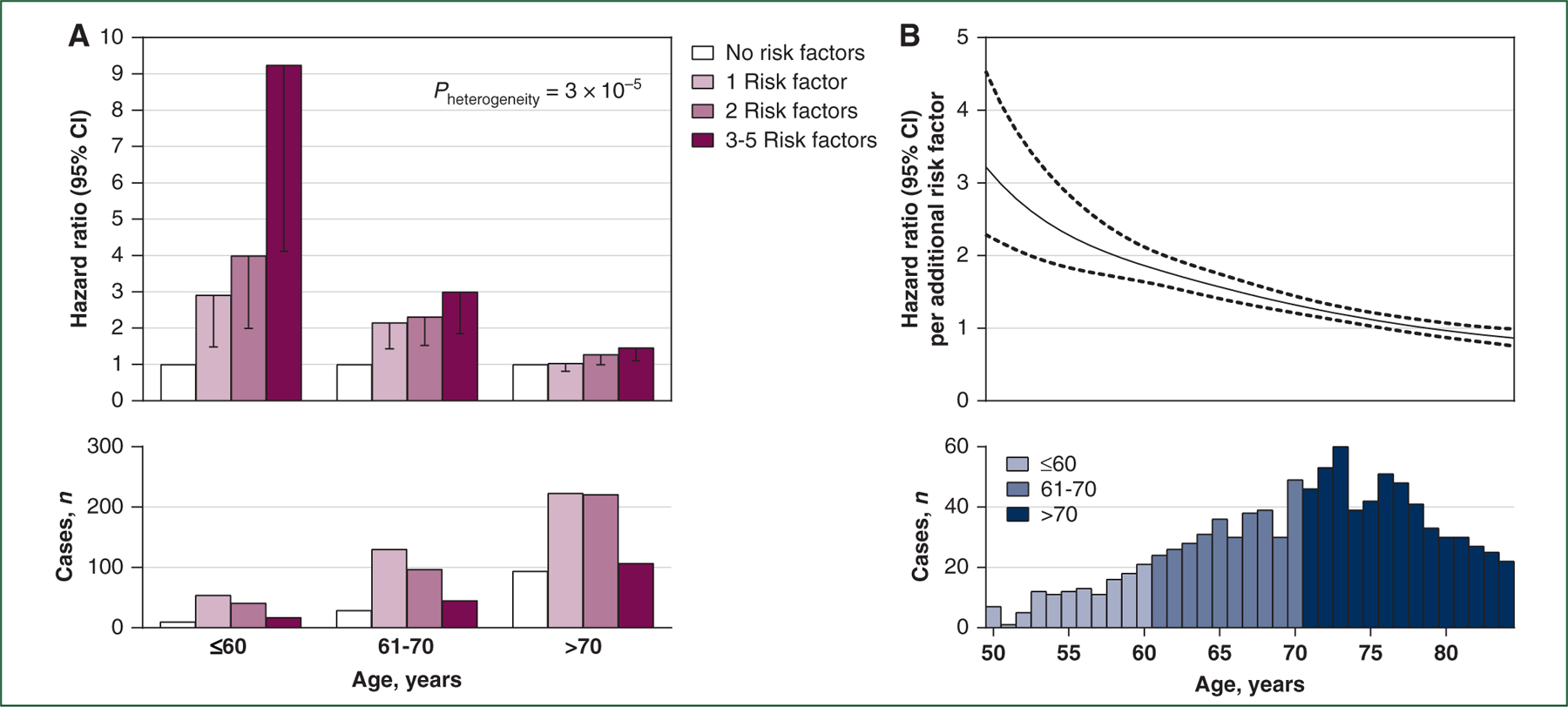
Five established risk factors for pancreatic cancer were considered: ever-smoking, obesity, >2-year diabetes, tall height, and non-O blood group. Participants with missing information on more than one of the factors were excluded. (A) Age-stratified hazard ratios by number of risk factors from Cox proportional hazards regression conditioned on age (in months) and calendar year of the survey cycle and sex/cohort and adjusted for race/ethnicity (white, black, other, or unknown). Only lower limits of 95% CIs are shown. Heterogeneity between the age groups of ≤60 years and >70 years was tested by using the random-effects meta-regression method, comparing participants with 3–5 risk factors to those with no risk factors. (B) Age-varying hazard ratios per additional risk factor from Cox B-spline piecewise regression with age as the time scale and conditioned on sex/cohort and calendar year of the survey cycle and adjusted for race/ethnicity (white, black, other, or unknown). Solid curve represents point estimates and dashed curves represent 95% CIs.
CI, confidence interval.
In exploratory analyses, we examined several suspected risk factors for pancreatic cancer (Supplementary Table S5, available at https://doi.org/10.1016/j.annonc.2022.03.276). Notably, inadequate exercise was associated with pancreatic cancer risk among participants aged ≤70 years (HR, 1.37; 95% CI 1.11–1.69) but not among those aged >70 years [HR, 0.98 (95% CI 0.83–1.15); Pheterogeneity = 0.01]. In contrast, excessive alcohol use was not differentially associated with pancreatic cancer risk by age, and diet quality was not associated with pancreatic cancer risk in either age group.
Attributable risk of pancreatic cancer for established risk factors
We next examined the proportion of incident pancreatic cancers by age that could be attributed to the five established risk factors individually and in combination (Figure 2 and Supplementary Table S6, available at https://doi.org/10.1016/j.annonc.2022.03.276). PAFs were greater for earlier-onset versus late-onset pancreatic cancer for each of the risk factors, aside from diabetes, which increased in prevalence as individuals aged. The PAFs for combinatory risk factors were 65.6%, 49.7%, and 17.2% among participants aged ≤60 years, 61–70 years, and >70 years, respectively, indicating a greater proportion of earlier-onset pancreatic cancers that were attributable to these risk factors.
Figure 2. Age-specific proportion of incident pancreatic cancers attributable to established risk factors among participants from two prospective cohorts.
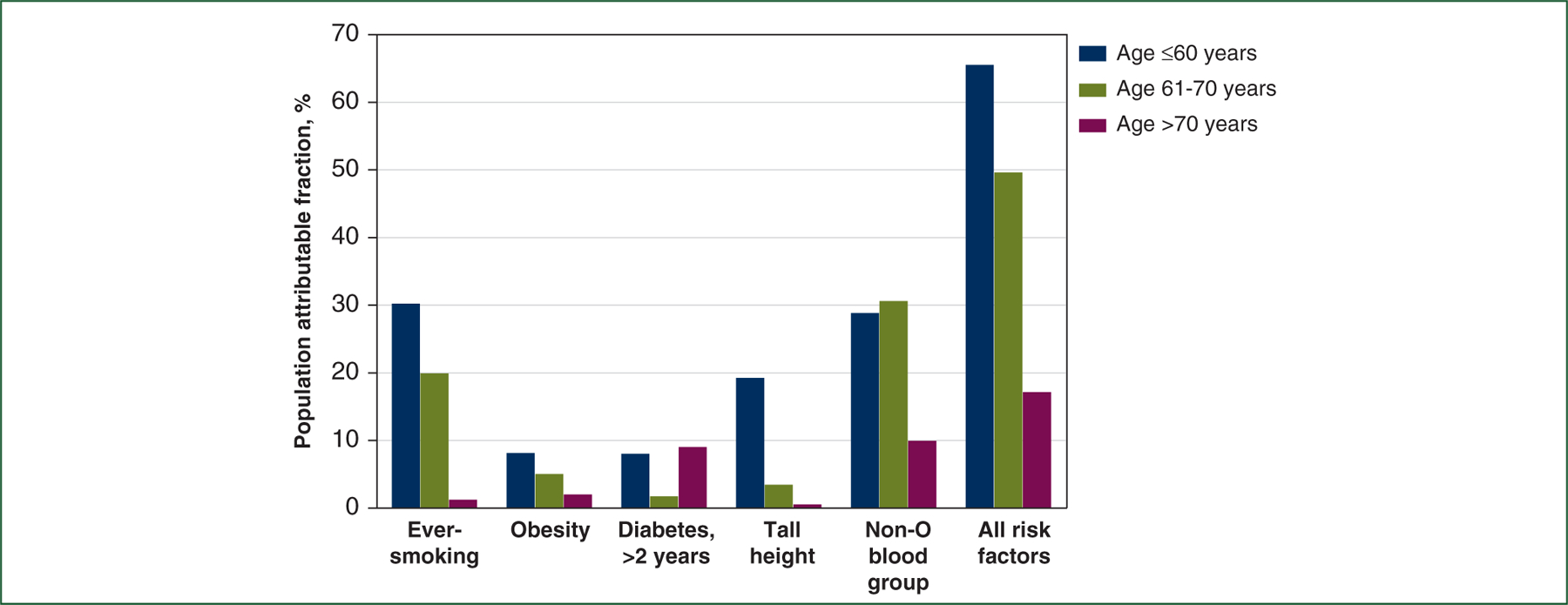
Population attributable fraction estimates the proportion of incident pancreatic cancers in the study population that theoretically would not have occurred if the risk factor had been absent and demographic factors (age, sex, and race/ethnicity) remained unchanged.
Prevention potential of pancreatic cancer
We next considered attributable risks by age for potentially modifiable factors, namely, ever-smoking, obesity, inadequate exercise, and excessive alcohol use (Supplementary Table S7, available at https://doi.org/10.1016/j.annonc.2022.03.276). Together, PAFs for these factors were 37.7% and 6.5% among those aged ≤70 years and >70 years, respectively. Estimates were similar when national prevalence of exposure was considered (Supplementary Table S8, available at https://doi.org/10.1016/j.annonc.2022.03.276). Given this finding, we also examined the ages at which individuals quit smoking or became obese in relation to pancreatic cancer risk (Supplementary Table S9, available at https://doi.org/10.1016/j.annonc.2022.03.276). Notably, smoking cessation at earlier ages was associated with more pronounced risk reduction for pancreatic cancer (Ptrend = 0.001), and individuals who quit smoking before age 50 years had the same risk reduction as individuals who never smoked. Similarly, obesity onset before age 50 years rather than at later ages was associated with increased risk of pancreatic cancer. Furthermore, weight gain between 18–21 years and 50 years of age rather than between 50 and 65 years was associated with increased pancreatic cancer risk.
PRS in the PanScan/PanC4 I-III GWAS
We next examined whether a PRS that summarized the cumulative effect of 22 independent genome-wide significant SNPs would have differential associations with pancreatic cancer risk by age (Table 3). As with the risk factors described in the preceding text, the strongest association of the PRS with pancreatic cancer risk was noted among participants aged ≤60 years, with lesser associations identified in older participants (Pheterogeneity = 0.01). Comparing participants in the top versus bottom 10% of the PRS, the odds ratios for pancreatic cancer were 6.91 (95% CI 4.60–10.40) among those aged ≤60 years and 4.12 (95% CI 3.08–5.52) among those aged >70 years.
Table 3.
Age-stratified association of pancreatic cancer risk with polygenic risk score among cases and controls from the PanScan/PanC4 I-III genome-wide association study
| Polygenic risk scorea | Age ≤60 years |
Age 61–70 years |
Age >70 years |
P heterogeneity c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. cases | No. controls | Odds ratio (95% CI)b | No. cases | No. controls | Odds ratio (95% CI)b | No. cases | No. controls | Odds ratio (95% CI)b | ||
| Bottom 10% (low-risk) | 46 | 189 | 1 [Reference] | 75 | 405 | 1 [Reference] | 100 | 290 | 1 [Reference] | |
| >10%−50% | 324 | 746 | 1.75 (1.20–2.54) | 509 | 1577 | 1.48 (1.11–1.98) | 608 | 1215 | 1.50 (1.15–1.96) | |
| >50%−90% | 567 | 726 | 3.35 (2.32–4.84 | 802 | 1610 | 2.20 (1.65–2.92) | 979 | 1203 | 2.43 (1.88–3.16) | |
| Top 10% (high-risk) | 292 | 161 | 6.91 (4.60–10.40) | 371 | 399 | 4.13 (3.02–5.66) | 434 | 324 | 4.12 (3.08–5.52) | |
| Per SD increase | 1229 | 1822 | 1.71 (1.57–1.87) | 1757 | 3991 | 1.50 (1.41–1.60) | 2121 | 3032 | 1.50 (1.41–1.60) | 0.01 |
CI, confidence interval; SD, standard deviation.
Categorization and standardization based on distribution among all control participants (score range: bottom 10%, 1.949–3.491; >10–50%, 3.492–4.121; >50–90%, 4.122–4.751; top 10%, 4.751–6.454).
From logistic regression adjusted for age subgroups (<51 or 51–60 years among those aged ≤60 years; 71–80 or >80 years among those aged >70 years), sex, and study site.
Heterogeneity between the age groups of ≤60 years and >70 years tested by using the random-effects meta-regression method.
Sex and race in the SEER Program
Male sex and black race are known risk factors for pancreatic cancer in the USA.37,38 Thus, we examined whether these risk factors had differential associations with pancreatic cancer incidence by age (Supplementary Table S10, available at https://doi.org/10.1016/j.annonc.2022.03.276). As with the aforementioned exposures, the male-to-female incidence rate ratio (IRR) was greater in the age group of <60 years (1.41; 95% CI 1.39–1.44) than in the age group of ≥70 years (1.14; 95% CI 1.12–1.15; Pheterogeneity < 1 × 10−8). Similarly, black-to-white IRRs declined monotonically with age (Pheterogeneity < 1 × 10−8), as did joint associations when considering sex and race together.
DISCUSSION
More than 80% of patients with pancreatic cancer present with advanced disease, when cure is uncommon and the disease rapidly acquires resistance to available treatments. To make significant progress in patient outcomes, pancreatic cancer prevention and earlier detection will be critical. Nevertheless, how risk factors for pancreatic cancer change with age has not been systematically explored. In the current study, we show a remarkably consistent pattern across known pancreatic cancer risk factors, whereby risk factors play a relatively large role in development of earlier-onset pancreatic cancer and a limited role in late-onset disease. Furthermore, behavioral changes earlier in life appear to be required to reduce cancer risk from those factors that are modifiable, such as cigarette smoking and obesity. These results demonstrate the value of understanding how risk factors over a lifetime impact disease development, while emphasizing the importance of timing in public health measures for prevention and early detection.
Previous studies have suggested that pancreatic cancer cases with a predisposing family history or germline mutations,11,13,14 cigarette smoking,13 or excess body weight,12 had earlier age of onset than those without these factors. By utilizing four distinct, large, population-based studies, we examined more than 10 risk factors to estimate the association and attributable risk across a range of ages. In two prospective cohorts, we found stronger associations and greater attributable risk for pancreatic cancer among younger participants for each of the established risk factors assessed, including cigarette smoking, obesity, diabetes, tall height, and non-O blood group. In the GWAS, we utilized a large multi-institutional dataset to examine a weighted genetic risk score and again identified a stronger association among younger individuals. Finally, we examined male sex and black race in relation to pancreatic cancer incidence in a nationwide cancer database. Across these different patient populations and considering a multitude of different exposures, a similar pattern emerged, whereby risk factors were more strongly associated with pancreatic cancer risk at younger ages; that is, the relative risk conferred by a risk factor for a younger adult was greater than that for an older adult, although incidence rates for pancreatic cancer are higher in older adults.
These findings have several important implications. First, to facilitate disease prevention, interventions for modifiable risk factors will likely require implementation at younger ages, as these factors were not strongly associated with risk after age 70 years. Conversely, early detection programs may have greater potential to reduce pancreatic cancer mortality in older populations, as 50% of pancreatic cancer cases are diagnosed after age 70 years. Second, future studies to investigate risk factors and prediction models for pancreatic cancer will need to consider age not only as a risk marker, but also as a stratification variable that may modify the association or predictive ability of other factors. Third, given the relatively weak associations between traditional risk factors and late-onset pancreatic cancer, detection strategies that include early identification of tumor-induced symptoms or signs may be needed, such as with machine learning tools applied to patient records39 or regular blood-based assessments.40–42
This study has important strengths. In the two prospective cohorts, participants were followed longitudinally starting in mid-life for >30 years, minimizing selection bias and reducing the impact of exposures differing by birth cohort. Furthermore, modifiable factors were assessed not only at enrollment, but in a repeated manner throughout follow-up. Thus, we were able to assess participants’ exposure every 2 years and model-specific risk factors over their lifetime, rather than at a single timepoint. Because all data were collected prospectively and before disease development, misclassification and recall bias were minimized.
The current study also has limitations. Although we examined many of the known risk factors for pancreatic cancer, several were not available in our patient populations, including family history of pancreatic cancer and personal history of chronic pancreatitis. Although we examined race in the SEER dataset, the prospective cohorts and GWAS included predominantly white participants. In the SEER Program, we were unable to control for potential confounders as data were unavailable, such as cigarette smoking or BMI.
In conclusion, inherited and lifestyle factors are more strongly associated with earlier-onset pancreatic cancer, emphasizing the importance of considering age in design of cancer prevention and control programs for this highly lethal malignancy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their valuable contributions and the cancer registries of the following states for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY,NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
FUNDING
The Nurses’ Health Study was supported by the National Institutes of Health (NIH) [grant numbers UM1 CA186107,P01 CA87969]. The Health Professionals Follow-Up Study was supported by the NIH [grant number U01 CA167552]. The PanScan/PanC4 I-III GWAS was supported by the Lustgarten Foundation and the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH. The authors acknowledge the research contributions of the Cancer Genomics Research Laboratory for their expertise, execution, and support of this research in the areas of project planning, wet laboratory processing of specimens, and bioinformatics analysis of generated data. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, NIH, under NCI Contract No. 75N910D00024. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, and mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. A full list of funding acknowledgments for each participating study is provided in Supplementary Material, available at https://doi.org/10.1016/j.annonc.2022.03.276. Additional support was received from the NIH [grant number K07 CA222159 to AB], Broman Family Fund (to KN), and the Hale Family Center for Pancreatic Cancer Research, Lustgarten Foundation Dedicated Laboratory program, NIH [grant number U01 CA210171], NIH [grant number P50 CA127003], Stand Up To Cancer, Pancreatic Cancer Action Network, Noble Effort Fund, Wexler Family Fund, Promises for Purple, and Bob Parsons Fund (to BMW).
PanScan/PanC4 I-III Consortium:
The members are L. T. Amundadottir; E. Ardanaz; A. A. Arslan; L. E. Beane-Freeman; P. M. Bracci; B. Bueno-de-Mesquita; M. Du; S. Gallinger; G. G. Giles; P. J. Goodman; V. A. Katzke; A. P. Klein; C. Kooperberg; P. Kraft; D. Li; N. Malats; L. L. Marchand; M. L. McCullough; R. L. Milne; J. P. Neoptolemos; S. Perdomo; G. M. Petersen; H. A. Risch; X. O. Shu; R. Z. Stolzenberg-Solomon; S. K. Van Den Eeden; K. Visvanathan; E. White; B. M. Wolpin; and W. Zheng. Their affiliations appear in Supplementary Material, available at https://doi.org/10.1016/j.annonc.2022.03.276.
Footnotes
DISCLOSURE
KP declares serving on advisory panel for Eisai and Helsinn/QED. BMW declares research funding from Celgene and Eli Lilly and consulting for BioLineRx, Celgene, and GRAIL. All other authors have declared no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017 Bethesda, MD: National Cancer Institute; 2020. Available at https://seer.cancer.gov/csr/1975_2017/. Accessed December 1, 2020. [Google Scholar]
- 3.Tavakkoli A, Singal AG, Waljee AK, et al. Racial disparities and trends in pancreatic cancer incidence and mortality in the United States. Clin Gastroenterol Hepatol 2020;18:171–178 e110. [DOI] [PubMed] [Google Scholar]
- 4.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019;4:e137–e147. [DOI] [PubMed] [Google Scholar]
- 5.Gaddam S, Abboud Y, Oh J, et al. Incidence of pancreatic cancer by age and sex in the US, 2000–2018. JAMA 2021;326:2075–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yabroff KR, Bradley CJ, Mariotto AB, Brown ML, Feuer EJ. Estimates and projections of value of life lost from cancer deaths in the United States. J Natl Cancer Inst 2008;100:1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amundadottir LT. Pancreatic cancer genetics. Int J Biol Sci 2016;12: 314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islami F, Sauer AG, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31–54. [DOI] [PubMed] [Google Scholar]
- 9.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015;347:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017;355:1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu C, Hart SN, Polley EC, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018;319:2401–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McWilliams RR, Bamlet WR, Rabe KG, Olson JE, de Andrade M, Petersen GM. Association of family history of specific cancers with a younger age of onset of pancreatic adenocarcinoma. Clin Gastroenterol Hepatol 2006;4:1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James TA, Sheldon DG, Rajput A, et al. Risk factors associated with earlier age of onset in familial pancreatic carcinoma. Cancer 2004;101: 2722–2726. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–468. [DOI] [PubMed] [Google Scholar]
- 17.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140: 1016–1019. [DOI] [PubMed] [Google Scholar]
- 18.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet 2009;41:986–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet 2010;42:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolpin BM, Rizzato C, Kraft P, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet 2014;46:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER Research Limited-Field Data, 21 Registries, Nov 2019 Sub (2000–2017). In: National Cancer Institute, DCCPS, Surveillance Research Program released April 2020, based on the November 2019 submission Available at www.seer.cancer.gov. Accessed December 1, 2020. [Google Scholar]
- 22.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med 1996;156:2255–2260. [PubMed] [Google Scholar]
- 23.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–929. [DOI] [PubMed] [Google Scholar]
- 24.Yuan C, Babic A, Khalaf N, et al. Diabetes, weight change, and pancreatic cancer risk. JAMA Oncol 2020;6:e202948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst 2009;101:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol Biomarkers Prev 2001;10:429–437. [PubMed] [Google Scholar]
- 27.Arem H, Reedy J, Sampson J, et al. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP study. J Natl Cancer Inst 2013;105:1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flegal KM, Ogden CL, Fryar C, Afful O, Klein R, Huang DT. Comparisons of self-reported and measured height and weight, BMI, and obesity prevalence from national surveys: 1999–2016. Obesity (Silver Spring) 2019;27:1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein AP, Wolpin BM, Risch HA, et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun 2018;9:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Childs EJ, Mocci E, Campa D, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet 2015;47:911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Wang Z, Obazee O, et al. Three new pancreatic cancer susceptibility signals identified on chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget 2016;7:66328–66343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Yuan C, Babic A, et al. Genetic and circulating biomarker data improve risk prediction for pancreatic cancer in the general population. Cancer Epidemiol Biomarkers Prev 2020;29:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 35.Abrahamowicz M, Mackenzie T, Esdaile JM. Time-dependent hazard ratio: modeling and hypothesis testing with application in lupus nephritis. J Am Stat Assoc 1996;91:1432–1439. [Google Scholar]
- 36.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control 2007;18:571–579. [DOI] [PubMed] [Google Scholar]
- 37.Gordon-Dseagu VL, Devesa SS, Goggins M, Stolzenberg-Solomon R. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol 2018;47:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raimondi S, Maisonneuve P, Lohr JM, Lowenfels AB. Early onset pancreatic cancer: evidence of a major role for smoking and genetic factors. Cancer Epidemiol Biomarkers Prev 2007;16:1894–1897. [DOI] [PubMed] [Google Scholar]
- 39.Kenner B, Chari ST, Kelsen D, et al. Artificial intelligence and early detection of pancreatic cancer: 2020 summative review. Pancreas 2021;50:251–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalaf N, Wolpin BM. Metabolic alterations as a signpost to early pancreatic cancer. Gastroenterology 2019;156:1560–1563. [DOI] [PubMed] [Google Scholar]
- 41.Cohen JD, Javed AA, Thoburn C, et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A 2017;114: 10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.. Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol 2020;31:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


