Abstract
Nuclear receptor Pregnane X Receptor (PXR; NR1I2) has transcriptional regulation functions for energy homeostasis in the liver. Mouse PXR has a conserved phosphorylation motif at serine 347 (serine 350 in humans) within the ligand-binding domain. PXR phosphorylated at this motif is expressed in mouse livers in response to fasting. Mice with a PXR*Ser347Ala knockin mutation (PXR KI) were generated to block phosphorylation, and utilized to investigate the role of Ser347 phosphorylation in vivo. PXR KI mice had decreased body weight at 8-weeks of age and had much greater weight loss after fasting compared with PXR WT mice. The cDNA microarray analysis of hepatic mRNAs showed that cell death or apoptotic signaling was induced in fasting PXR KI mice. Moreover, increasing hepatic lipids, triglycerides and the development of hypertriglyceridemia were observed in fasting PXR KI mice. These findings are indicative that blocking phosphorylation prevents mice from maintaining hepatic energy homeostasis. Thus, phosphorylated PXR may be an essential factor to prevent the liver from developing damage caused by fasting.
Keywords: Pregnane X receptor, phosphorylation, fasting, energy homeostasis, liver
Introduction
The liver is an essential metabolic organ to controlling energy homeostasis in response to feeding and fasting states, supplying energy sources glucose and ketone bodies to other organs [1,2]. Glucose is catabolized into pyruvate by the glycolysis pathway to produce ATP [3]. Under fasting conditions, the liver breaks down stored glycogen into glucose by the glycogenolysis pathway [3] and transforms non-carbohydrate substrates into glucose through the gluconeogenesis pathway [1]. In addition, fatty acids released from adipose tissue are transported into hepatocytes then converted to ketone bodies by the ketogenesis pathway [2]. Although fasting conditions restrict energy sources, fasting increases lifespan and improves liver health [4,5]. Fasting exerts therapeutic effects on liver lipid accumulation which is developed in patients with type 2 diabetes (T2D) or non-alcoholic fatty liver disease (NAFLD) [6,7]. Under healthy conditions, however, liver lipid accumulation has been reported to be developed by long-term fasting [8]. Liver lipid accumulation causes hepatic steatosis leading to an increase in serum biomarkers related to liver damage, such as aspartate aminotransferase (AST), alkaline phosphatase (ALP), sorbitol dehydrogenase (SDH) or lactate dehydrogenase (LDH); these biomarkers also increase in long-term fasting [9,10]. Under fasting conditions, fatty acid is accumulated in the liver for ketone body production, but a part of the fatty acid is converted to triglyceride which forms lipid droplets in the liver. A recent study has found that one of the precursors of gluconeogenesis inhibits ketogenesis leading to fasting-induced lipid accumulation [11], but the molecular mechanisms of the adverse effects of fasting have not been fully understood.
Pregnane X receptor (PXR: NR1I2), a member of the nuclear receptor superfamily, is highly expressed in the liver [12] and well-characterized as a sensor for xenobiotics, regulating detoxification processes [13]. In addition, it has become clear that PXR has diverse functions not limited to detoxification [14]. Recent studies have demonstrated that PXR plays an essential role in regulating energy metabolisms [15]. PXR activation induces lipid and triglyceride accumulation in the mouse liver [16]. However, a PXR knockout mouse also develops lipid accumulation and hypertriglyceridemia [16,17]. PXR is established as a new regulator for energy homeostasis in the liver, but the molecular mechanisms of PXR for energy regulation have not been fully understood. Recently, a conserved phosphorylation motif located in the ligand-binding domain of nuclear receptors has been reported [18], whose phosphorylation at mouse Ser347 (human Ser350) of PXR or Ser815 of androgen receptor has been confirmed in the mouse [19,20]. Whereas Ser347 of PXR is characterized as a phosphorylation site responding to fasting in the mouse liver [19], the role of PXR phosphorylation at Ser347 in vivo is not evaluated because of the absence of the model.
In this study, we established a novel knock-in mouse model bearing a serine to alanine mutation at PXR Ser347 (PXR KI) that blocks phosphorylation. Subsequently, we utilized PXR KI mice to study the role of phosphorylation at Ser347 of PXR during the fasting responses.
Materials and Methods
Animal:
C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were maintained on a 12 h light/12 h dark cycle and fed with NIH-31 the Open Formula Autoclavable diet (Zeigler, Gardners, PA, USA) and water ad libitum. 8-weeks old PXR WT or PXR S347A KI mice were sacrificed by CO2 inhalation, following feeding or fasting (food withdrawn) for 24 hours. All experiments were performed under the animal protocol approved by the National Institute of Environmental Health Science.
Generation PXR S347A KI (PxrS347A) mice:
A single CAS9 target site (TGGGGGAAAGCCATCACCTGGGG) was utilized to generate a double-stranded break in the PXR locus near the S347 codon (Supp. Fig. 1A). Complementary oligos were ordered from Integrated DNA Technology (Coralville, IA, USA) and cloned into a T7 sgRNA plasmid, and in vitro transcribed using Epicentre AmpliScribe T7 High Yield Transcription Kit (Madison, WI, USA). A single-stranded 200 bp oligo donor (ssODN) was utilized to generate the S347A mutation with co-insertion of a Pvu I restriction enzyme site in the intron to facilitate founder screening and further disrupt the endogenous CAS9 target site (detailed genome editing design Supp. Fig. 1). C57BL/6J one-cell embryos were microinjected with CAS9 SgRNA (20 ng/ul), 200 bp ssODN (100 ng/ul), and 5’ capped/polyA tailed Cas9 RNA (100 ng/ul) derived from pCAG-T3-hCAS-pA, a gift from Wataru Fujii & Kunihiko Naito [21]. Microinjected embryos were surgically transferred to SWISS pseudo-pregnant females. At weaning, potential founders were screened by PCR amplicon sequencing. Founders of interest were bred to wildtype C57BL/6J mice and F1 offspring were re-screened to confirm germline transmission of the mutant allele. The original genome editing design included the co-introduction of the S347A mutation and a novel intronic Pvu I restriction enzyme site to aid in screening and subsequent genotyping. However, during F0 founder screening, a fortuitous allele was identified where the double-stranded break repair resulted in the introduction of the Ser347Ala codon mutation, but not the intronic Pvu I site (Supp. Fig. 1C). The mouse colony genotyping was done by primer/probe assay by Transnetyx (Cordova, TN, USA) (Supp. Table 1A). Codon 347 is underlined in each probe (Supp. Table 1B). Total RNAs extracted from the liver were used for synthesizing cDNA by MultiScribe Reverse Transcriptase (Thermo Fisher Scientific, Waltham, MA, USA) with oligo dT primer. PCR was performed using PrimeSTAR Max DNA polymerase (TaKaRa, Otsu, Japan).
Western blotting:
Liver from PXR WT or PXR KI mice was homogenized using a Dounce homogenizer in T-PER buffer (Thermo Fisher Scientific). The proteins were separated by SDS-PAGE and transferred onto the PVDF membrane. The membrane was incubated with an anti-PXR antibody (#39589, Active motif, Carlsbad, CA, USA). Protein bands were visualized with WesternBright Sirius chemiluminescent reagents (Advansta, San Jose, CA, USA) and recorded with a western blot scanner (Licor, Lincoln, NE, USA).
Gene arrays and Bioinformatics:
After 24 hours of fasting or feeding, PXR WT or PXR KI mice were sacrificed. Livers were then harvested, the RNAs from which were collected and purified by Trizol reagent (Thermo Fisher Scientific) and RNeasy mini kit (QIAGEN, Valencia, CA, USA). Gene expression analysis was conducted using Agilent Whole Mouse Genome 4×44 multiplex format oligo arrays (014868) (Agilent Technologies, Palo Alto, CA, USA) following the Agilent 1-color microarray-based gene expression analysis protocol. Cy3 labeled cRNA was produced following the manufacturer’s protocol. Cy3 labeled cRNAs were fragmented and hybridized for 17 hours in a rotating hybridization oven. Slides were scanned with an Agilent Scanner. Data were obtained using the Agilent Feature Extraction software (v12). The Agilent Feature Extraction Software performed error modeling, adjusting for additive and multiplicative noise. Genes were considered differentially expressed if they showed a fold-change of at least 1.5 with p-value<0.05 tested by an ANOVA and Benjamini-Hochberg multiple test correction performed using OmicSoft Array Studio (Version 10) software. Gene set enrichment analysis (GSEA, version 4.0.3) was performed with the hallmark gene sets (H) from Molecular Signature Database (MSigDB, v7.1). Gene set with NOM p-val<0.05 was considered as significantly enriched. Ingenuity Pathway Analysis software (IPA, Qiagen) was utilized to search the Canonical pathway and Toxicity function. The pathways with Z-score >2 and <−2 were considered significantly activated and repressed pathways, respectively.
Serum analysis:
Serum isolated with BD Microtainer serum separator tubes (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) were analyzed using an AU480 Chemistry Analyzer (Beckman Coulter, Inc., Brea, CA, USA). Except for the SDH and β-hydroxybutyrate assays, all tests were performed using reagents from Beckman Coulter, Inc. (Brea, CA, USA). Reagents for the SDH assay and the β-hydroxybutyrate assay were obtained from Sekisui Diagnostics, LLC. (Burlington, MA, USA) and Stanbio Laboratory (Boerne, TX, USA), respectively.
Liver triglyceride levels:
Liver triglyceride contents were measured using a triglyceride colorimetric assay kit (Cayman Chemical, Ann Arbor, MI, USA) and following the manufacturer’s instructions.
Statistical analysis:
Statistical analyses were performed using Graphpad Prism (version 8.3.0, Graphpad Software, La Jolla, CA, USA). The distribution normality was checked by the Shapiro Wilk test. One-way ANOVA followed by Sidak’s multiple comparisons and Kruskal-Wallis test followed by Dunn’s multiple comparisons were performed for the group with equal variance and no equal variance, respectively. P < 0.05 was considered statistically significant.
Results
Generation of PXR S347A KI (PxrS347A) mice
To understand the in vivo functions of the conserved PXR mmSer347/hsSer350 phosphorylation site, we established a mouse line with a PXR*Ser347Ala mutation, which blocks phosphorylation at this motif. Genomic DNA of mouse embryos was edited by CRISPR/Cas-9 systems to knockin the mutation of PXR codon 347 from serine to alanine (Fig. 1A). Sequencing analysis of genomic DNA of PXR WT and PXR Ser347Ala knockin (PXR KI) mice confirmed two nucleotide mutations (T>G and C>T) at the Ser347 codon in the mutant locus (Fig. 1B). Since the genomic edit to introduce the Ser347Ala mutation is near the splice donor site of exon 7, canonical splicing was confirmed by sanger sequencing in PXR KI cDNA. Sequencing revealed exon 7 to exon 8 splicing in homozygous PXR KI mice at the exact same sequence as wildtype mice (Fig. 1B). Subsequently, protein expression levels of PXR were analyzed by Western blotting assay. PXR KI mice expressed similar levels of PXR protein as PXR WT mice, indicating PXR KI mutation didn’t affect protein expression levels (Fig. 1C). In contrast, the bodyweight of PXR KI mice was lower than those of PXR WT mice (Fig. 1D).
Figure 1: Generation of PXR S347A KI (PxrS347A) mice.
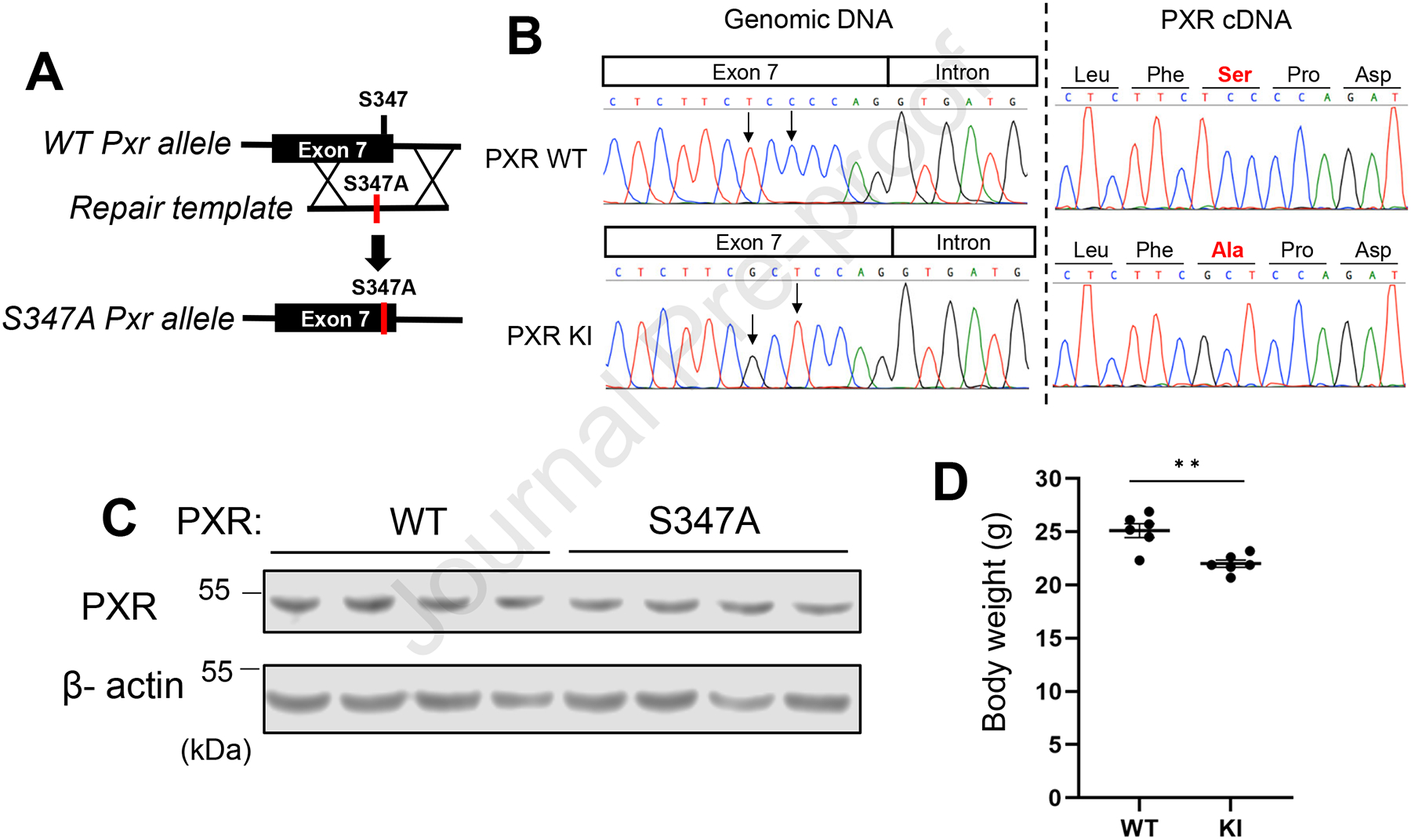
(A) General schematic diagram showing the strategy for producing PxrS347A mice. (B) Sequence chromatograms for the target site of genomic DNA (left) or cDNA (Right) of wild-type mice (Top) and PXR KI mice (Bottom). (C) Western blotting was performed using liver lysate from PXR WT or KI. Beta-actin was used as an internal control. (D) The bodyweight of PXR WT or PXR KI mice was evaluated. Values are presented as means ± S.E.M.
Gene Expression changed by fasting in PXR KI
Next, we evaluated the impact of fasting on bodyweight and RNA expression profiles in the liver among PXR WT or PXR KI mice. In addition to significant bodyweight loss by fasting in both PXR WT and PXR KI, the bodyweight loss of PXR KI by fasting was markedly greater than those of PXR WT (Fig. 2A). cDNA microarray analysis showed expression levels of 1154 and 1690 genes were changed significantly by fasting in PXR WT mice and PXR KI, respectively (Fig. 2B). Among these genes, 120 and 621 genes were up-regulated by fasting in PXR WT and PXR KI mice, respectively (Fig. 2C). Similarly, 326 and 351 genes were down-regulated by fasting in PXR WT and PXR KI mice, respectively (Fig. 2C). Gene set enrichment analysis (GSEA) with hallmark gene sets was employed with differentially expressed genes (DEGs) between feeding and fasting conditions in only PXR WT or PXR KI to determine how these genes regulated biological functions. Although a significant pathway was not identified in DEGs in PXR WT, glycolysis was found to be up-regulated in the fasted PXR KI mice (Fig. 2D and Supp. Table 2).
Figure 2: Gene Expression change due to fasting in PXR KI.
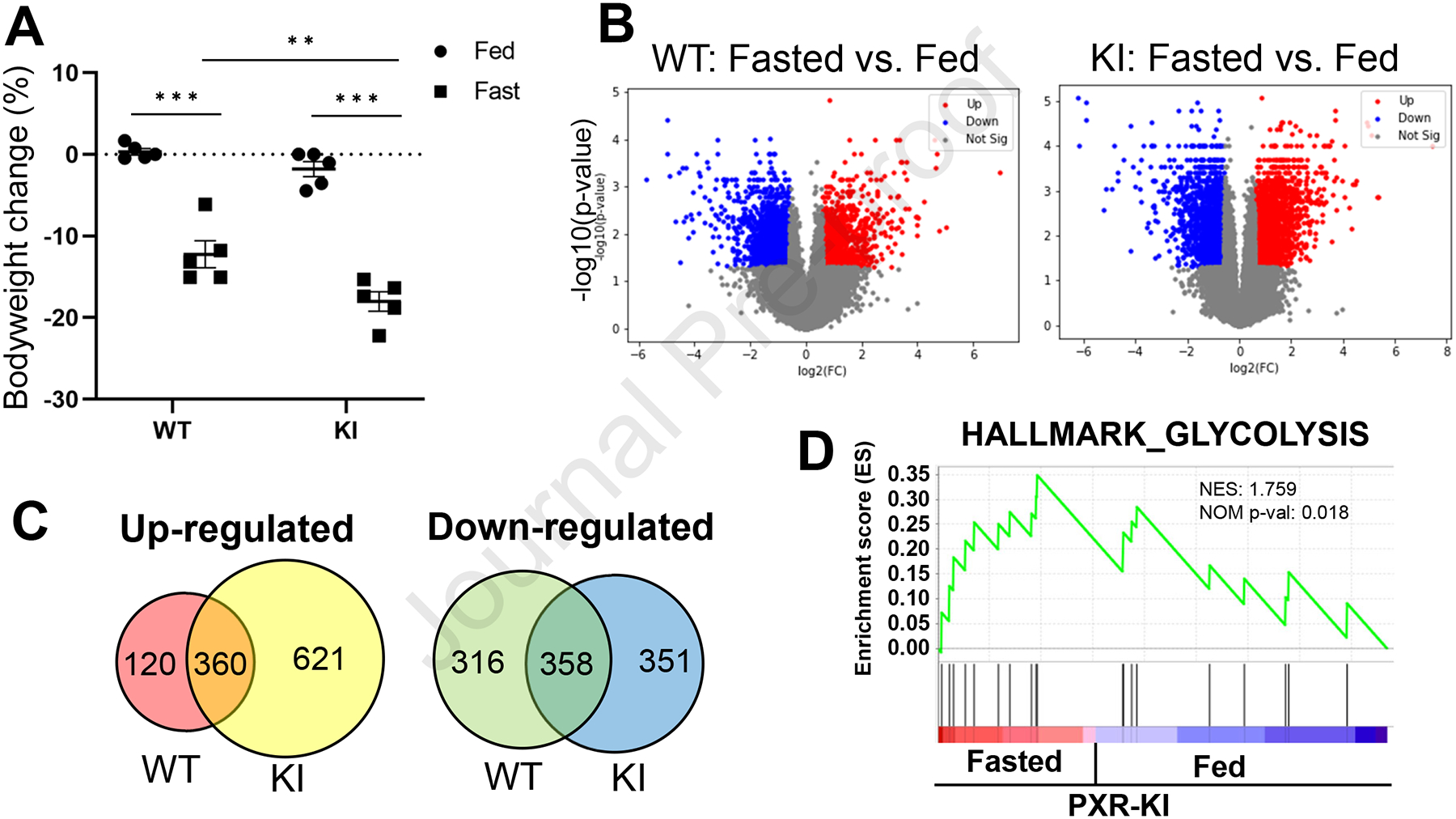
(A) Bodyweight changes from before to after feeding or fasting was evaluated in PXR WT or PXR KI mice. Values are presented as means ± S.E.M. (B) Volcano plot showing differentially expressed genes (DEGs) between fed and fasted PXR WT (left) or PXR KI (right) mice. (C) Venn diagram analyses for up-or down-regulated genes by fasting in PXR WT or PXR KI mice were performed. (D) Enrichment plots of genes, a hallmark of glycolysis, in DEGs of PXR KI are presented by GSEA. NES: normalized enrichment score. NOM p-val: nominal p-value. The p-value was calculated by GSEA.
The levels of liver toxicity marker in the serum after fasting
Subsequently, ingenuity pathway analysis (IPA) identified significant activated or inactivated canonical pathways or toxicity functions. Canonical pathway analysis showed estrogen biosynthesis, nicotine degradation, melatonin degradation and glutathione-mediated detoxification were induced by fasting in PXR KI mice (Supp. Fig. 2). Toxicity function analysis showed function related to cell death or apoptosis was induced by fasting in PXR KI mice, whereas inflammation was attenuated (Fig. 3A). Next, serum analyses determined the levels of biomarkers of liver functions and damage (Fig. 3B). The serum levels of AST were significantly increased by fasting in both PXR WT and PXR KI mice. In contrast, alanine aminotransferase (ALT) levels were not significantly changed by fasting. On the other hand, the serum levels of ALP, SDH or LDH were significantly increased by fasting in PXR KI mice, but not PXR WT. These results suggested that fasting had increased liver toxicity in PXR KI mice as compared to PXR WT mice.
Figure 3: The levels of liver toxicity marker in the serum after fasting.
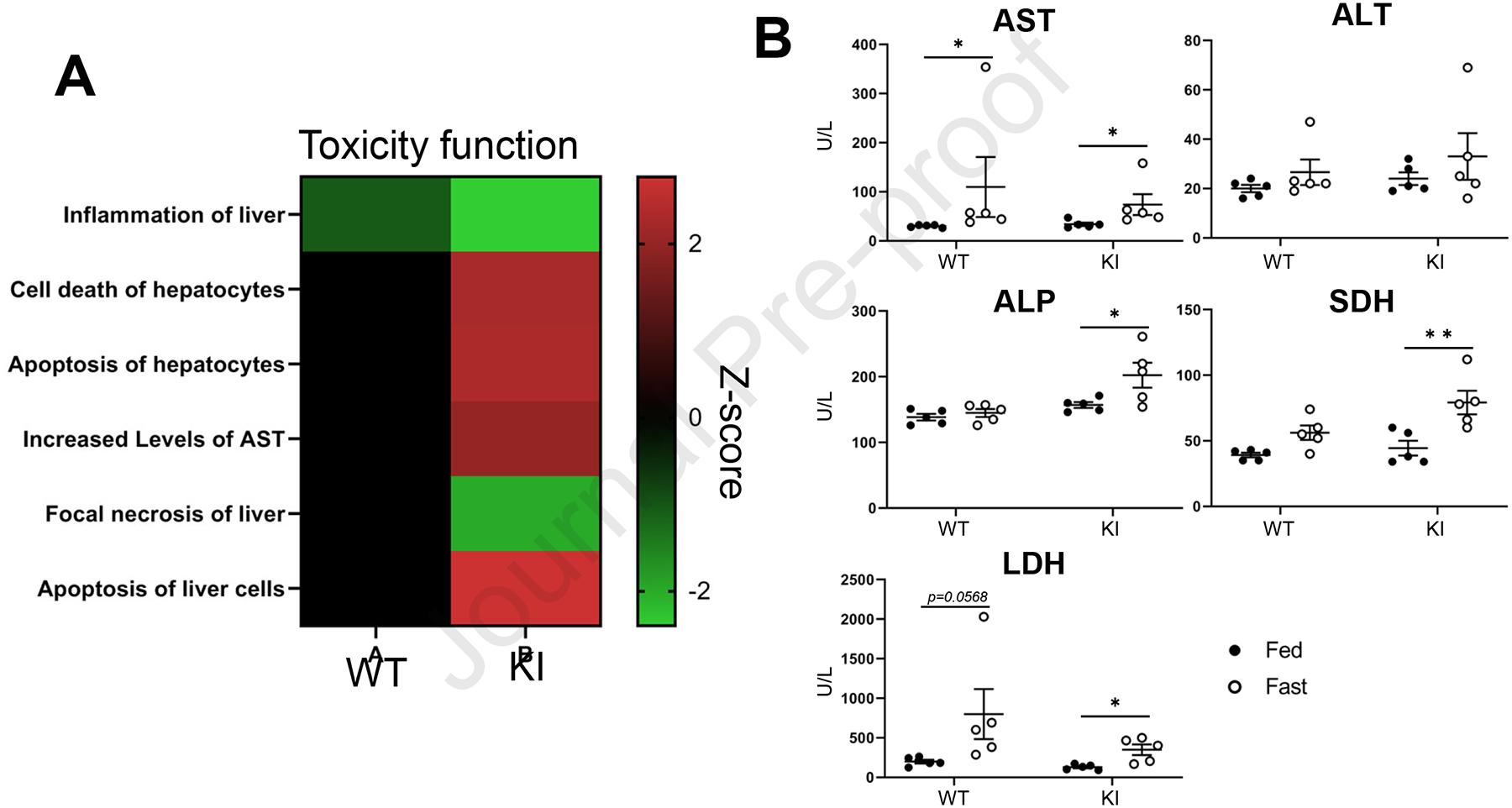
(A) DEGs between feeding and fasting of PXR WT or PXR KI were analyzed by IPA for Toxicity function. (B) Following feeding or fasting, the serum was prepared from PXR WT or PXR KI. Serum levels of Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP), Aspartate Aminotransferase (AST), Lactate Dehydrogenase (LDH) and Sorbitol Dehydrogenase (SDH) were evaluated. Values are presented as means ± S.E.M.
Effect of PXR KI on Glucose, Ketone, and Triglyceride levels during fasting
Next, we assessed the glucose, beta-hydroxybutyrate (the most abundant ketone body), and triglyceride levels in the serum from PXR WT and PXR KI mice (Fig. 4A). The serum glucose and beta-hydroxybutyrate levels were decreased and increased, respectively, by fasting in both PXR WT and PXR KI mice. On the other hand, triglyceride levels were significantly increased by fasting in PXR KI, but not in PXR WT mice. Liver sections from PXR WT or PXR KI mice were prepared following feeding or fasting for 24 hours and subjected to histological analysis by HE staining. Whereas lipid droplets in the liver sections of PXR WT mice were decreased by fasting, they were increased by fasting in PXR KI mice (Fig. 4B). Liver triglyceride levels were increased by fasting in PXR WT and PXR KI mice. Moreover, liver triglyceride levels in the livers of fasted PXR KI were higher than those of fasted PXR WT mice (Fig. 4C). These results indicated that fasting-induced lipid accumulation was augmented in PXR KI mice.
Figure 4: Effect of PXR KI on Glucose, Ketone, and Triglyceride levels by fasting.
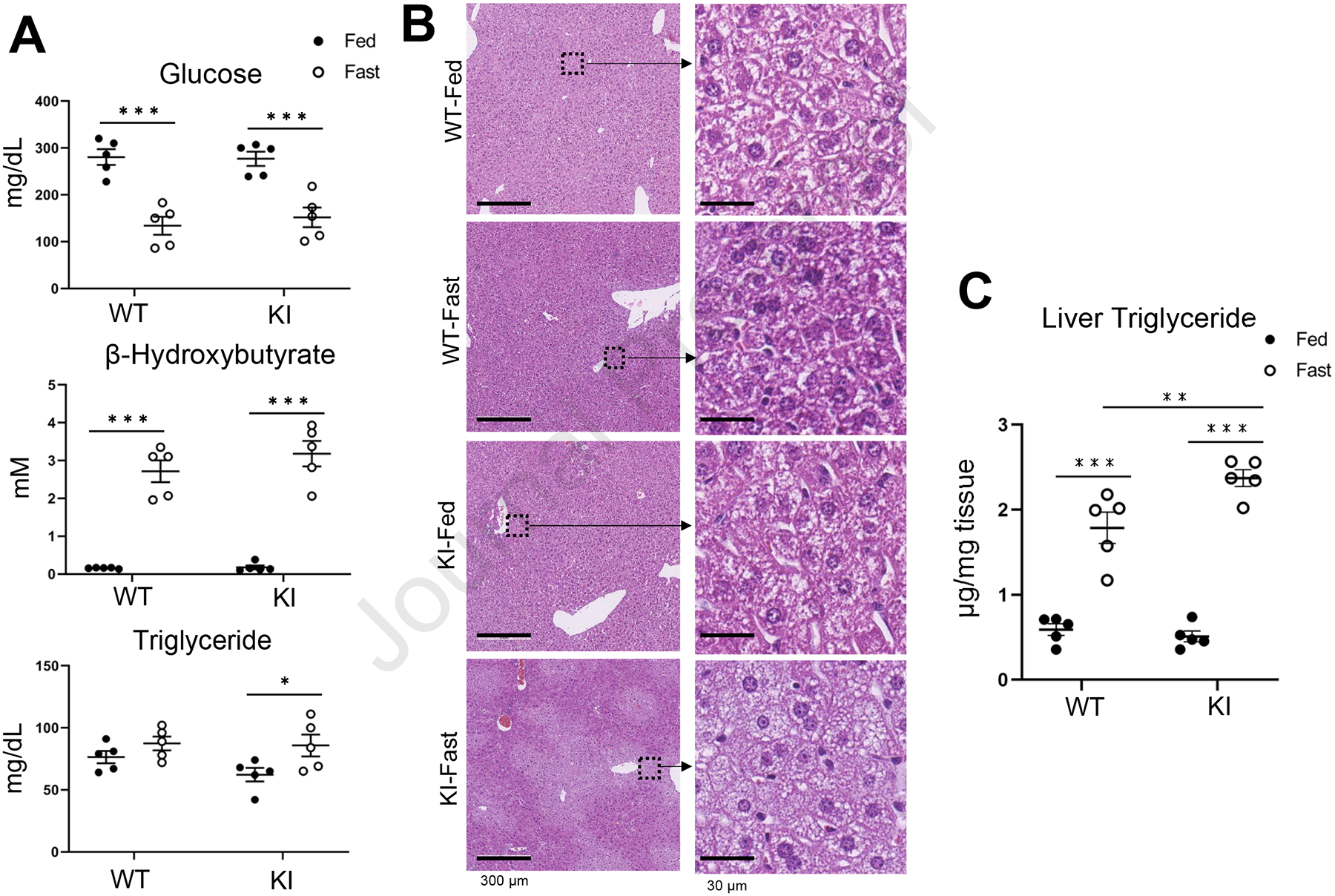
(A) The levels of glucose, β-Hydroxybutyrate and Triglyceride were assessed using serum from PXR WT or PXR KI following feeding or fasting. Values are presented as means ± S.E.M. (B) H&E staining was performed using liver sections from PXR WT or PXR KI mice following feeding or fasting. (C) The triglyceride levels of the liver from PXR WT or PXR KI mice following feeding or fasting were evaluated. Values are presented as means ± S.E.M.
Discussion
In this study, we generated a PXR*Ser347Ala mouse line to study the role of PXR phosphorylation at the Ser347 residue (equivalent to human Ser350). We subsequently characterized the role of mouse PXR Ser347 phosphorylation in response to fasting conditions in vivo. The PXR KI mice displayed decreasing bodyweight and enhanced bodyweight loss during fasting. In addition, fasting-induced liver toxicity, high triglycerides in either serum or liver and liver lipid accumulation were augmented in PXR KI mice. These results suggest that hepatic triglyceride accumulation causes lipid accumulation resulting in hepatic steatosis in fasting PXR KI mice. Thus, PXR phosphorylation plays an essential role in regulating energy homeostasis in response to fasting.
The effect of fasting on the liver is like a double-edged sword: it is an effective non-medical treatment for T2D or NAFLD [22,23], but in normal health conditions, it induces liver lipid accumulation and steatosis [24]. The molecular mechanisms of these diverse effects of fasting are poorly understood. In this study, we showed that PXR Ser347 non-phosphorylation mutation augmented fasting-induced lipid and triglyceride accumulation in the liver, suggesting that fastingassociated liver toxicity is prevented by PXR phosphorylation. In addition, PXR Ser347 has been reported as a phosphorylation site in response to fasting conditions [19]. Therefore, the phosphorylation of PXR Ser347 acts as a sensor for the fasting response and modifies the PXR functions to maintain liver health. The molecular mechanisms of how PXR phosphorylation regulates its function are not fully understood. PXR is known to form protein complexes for the transactivation of target genes. Retinoid X receptor (RXR) α is a well-characterized PXR protein partner to form a heterodimer, but PXR phosphorylation at Ser347 has been reported to interrupt heterodimerization [25]. PXR interacts with not only RXRα but also FOXO1, FOXA2, PPARα, CREB and PGC-1α to co-regulate hepatic genes, including glucose or lipid metabolizing enzymes [16,26–29]. Therefore, PXR phosphorylation at Ser347 has a potential role in controlling interacting protein partners for regulating target gene expressions in response to energy stress.
One of the adverse effects of fasting is hepatic steatosis which is associated with an increase in liver toxicity biomarkers in serum. In this study, the serum levels of ALP, SDH, and LDH were significantly increased by fasting in only PXR KI mice. The microarray analysis showed the PXR KI specific gene expression changed by fasting was correlated with apoptosis or liver damage-marker induction. In addition, GSEA suggested fasting in PXR KI mice leads to activation of the glycolysis pathway which is reported as an activated pathway in injured livers [30]. Therefore, the liver damage caused by fasting was augmented in PXR KI mice, suggesting PXR phosphorylation helps prevent liver damage caused by fasting. PXR ablation has been reported to develop hepatic steatosis associated with lipid and triglyceride accumulation by fasting [16]. Although further studies will be required to clarify the mechanisms, those observations in PXR ablation should be caused by the loss of phosphorylated PXR expression. The microarray analysis also showed that fasted PXR KI mice have altered activation of estrogen biosynthesis, nicotine degradation, melatonin degradation and glutathione-mediated detoxification. The activation of these pathways has been reported in mice with PXR ligand treatment [31]. Among them, nicotine degradation and glutathione-mediated detoxification relate to liver detoxification systems. Estrogen has a protective effect on liver lipid accumulation [32]. These results suggested that PXR and its Ser347 phosphorylation have a role in the protection of the liver from not only toxic but also energy stresses.
In conclusion, PXR phosphorylation at Ser347 is a crucial post-translational modification to maintain energy homeostasis as well as liver health under fasting conditions in vivo. The blocking of PXR phosphorylation enhances fasting-induced liver lipid and triglyceride accumulation and hypertriglyceridemia, suggesting PXR plays a role through Ser347 phosphorylation to prevent developing severe liver damage by fasting. Thus, PXR S347A KI mice will be a unique mouse model for studies to clarify the molecular mechanisms of how energy homeostasis is regulated during fasting conditions in the liver.
Supplementary Material
Highlight.
PXR phosphorylation at Ser347 (P-PXR) deficient mice (PXR KI) was established.
PXR KI exhibited bodyweight reduction.
Fasting-induced liver lipid and triglyceride accumulation were enhanced in PXR KI.
P-PXR plays an essential role in protecting from fasting-induced liver toxicity.
Acknowledgments
The following cores at NIEHS are appreciated for their support: DNA sequence core, Microarray core, histology core, pathology image analysis core, clinical pathology group and Comparative Medicine branch. This work was supported by an Intramural project Z01ES1005-01 at NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Petersen MC, Vatner DF, Shulman GI, Regulation of hepatic glucose metabolism in health and disease, Nat Rev Endocrinol 13 (2017) 572–587. 10.1038/nrendo.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kolb H, Kempf K, Rohling M, Lenzen-Schulte M, Schloot NC, Martin S, Ketone bodies: from enemy to friend and guardian angel, BMC Med 19 (2021) 313. 10.1186/s12916-021-02185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiang G, Zhang BB, Glucagon and regulation of glucose metabolism, Am J Physiol Endocrinol Metab 284 (2003) E671–678. 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- [4].Longo VD, Mattson MP, Fasting: molecular mechanisms and clinical applications, Cell Metab 19 (2014) 181–192. 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N, Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age, Mech Ageing Dev 55 (1990) 69–87. 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- [6].Longo VD, Di Tano M, Mattson MP, Guidi N, Intermittent and periodic fasting, longevity and disease, Nature Aging 1 (2021) 47–59. 10.1038/s43587-020-00013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hodson L, Gunn PJ, The regulation of hepatic fatty acid synthesis and partitioning: the effect of nutritional state, Nat Rev Endocrinol 15 (2019) 689–700. 10.1038/s41574-019-0256-9. [DOI] [PubMed] [Google Scholar]
- [8].Nishikawa S, Doi K, Nakayama H, Uetsuka K, The effect of fasting on hepatic lipid accumulation and transcriptional regulation of lipid metabolism differs between C57BL/6J and BALB/cA mice fed a high-fat diet, Toxicol Pathol 36 (2008) 850–857. 10.1177/0192623308323920. [DOI] [PubMed] [Google Scholar]
- [9].Jensen TL, Kiersgaard MK, Sorensen DB, Mikkelsen LF, Fasting of mice: a review, Lab Anim 47 (2013) 225–240. 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- [10].Ma J, Cheng Y, Su Q, Ai W, Gong L, Wang Y, Li L, Ma Z, Pan Q, Qiao Z, Chen K, Effects of intermittent fasting on liver physiology and metabolism in mice, Exp Ther Med 22 (2021) 950. 10.3892/etm.2021.10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang X, Gao T, Deng S, Shang L, Chen X, Chen K, Li P, Cui X, Zeng J, Fasting induces hepatic lipid accumulation by stimulating peroxisomal dicarboxylic acid oxidation, J Biol Chem 296 (2021) 100622. 10.1016/j.jbc.2021.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM, An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway, Cell 92 (1998) 73–82. 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- [13].Kliewer SA, Goodwin B, Willson TM, The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism, Endocr Rev 23 (2002) 687–702. 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- [14].Oladimeji PO, Chen T, PXR: More Than Just a Master Xenobiotic Receptor, Mol Pharmacol 93 (2018) 119–127. 10.1124/mol.117.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wada T, Gao J, Xie W, PXR and CAR in energy metabolism, Trends Endocrinol Metab 20 (2009) 273–279. 10.1016/j.tem.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [16].Nakamura K, Moore R, Negishi M, Sueyoshi T, Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver, J Biol Chem 282 (2007) 9768–9776. 10.1074/jbc.M610072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barretto SA, Lasserre F, Fougerat A, Smith L, Fougeray T, Lukowicz C, Polizzi A, Smati S, Regnier M, Naylies C, Betoulieres C, Lippi Y, Guillou H, Loiseau N, Gamet-Payrastre L, Mselli-Lakhal L, Ellero-Simatos S, Gene Expression Profiling Reveals that PXR Activation Inhibits Hepatic PPARalpha Activity and Decreases FGF21 Secretion in Male C57Bl6/J Mice, Int J Mol Sci 20 (2019). 10.3390/ijms20153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Negishi M, Kobayashi K, Sakuma T, Sueyoshi T, Nuclear receptor phosphorylation in xenobiotic signal transduction, J Biol Chem 295 (2020) 15210–15225. 10.1074/jbc.REV120.007933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gotoh S, Miyauchi Y, Moore R, Negishi M, Glucose elicits serine/threonine kinase VRK1 to phosphorylate nuclear pregnane X receptor as a novel hepatic gluconeogenic signal, Cell Signal 40 (2017) 200–209. 10.1016/j.cellsig.2017.09.003. [DOI] [PubMed] [Google Scholar]
- [20].Yokobori K, Kawasaki Y, Sekine Y, Nobusawa S, Sakaki T, Negishi M, Kakizaki S, Androgen receptor phosphorylated at Ser815: The expression and function in the prostate and tumor-derived cells, Biochem Pharmacol 194 (2021) 114794. 10.1016/j.bcp.2021.114794. [DOI] [PubMed] [Google Scholar]
- [21].Fujii W, Kawasaki K, Sugiura K, Naito K, Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease, Nucleic Acids Res 41 (2013) e187. 10.1093/nar/gkt772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Albosta M, Bakke J, Intermittent fasting: is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians, Clin Diabetes Endocrinol 7 (2021) 3. 10.1186/s40842-020-00116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yin C, Li Z, Xiang Y, Peng H, Yang P, Yuan S, Zhang X, Wu Y, Huang M, Li J, Effect of Intermittent Fasting on Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-Analysis, Front Nutr 8 (2021) 709683. 10.3389/fnut.2021.709683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guan HP, Goldstein JL, Brown MS, Liang G, Accelerated fatty acid oxidation in muscle averts fasting-induced hepatic steatosis in SJL/J mice, J Biol Chem 284 (2009) 24644–24652. 10.1074/jbc.M109.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang YM, Chai SC, Lin W, Chai X, Elias A, Wu J, Ong SS, Pondugula SR, Beard JA, Schuetz EG, Zeng S, Xie W, Chen T, Serine 350 of human pregnane X receptor is crucial for its heterodimerization with retinoid X receptor alpha and transactivation of target genes in vitro and in vivo, Biochem Pharmacol 96 (2015) 357–368. 10.1016/j.bcp.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kodama S, Koike C, Negishi M, Yamamoto Y, Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes, Mol Cell Biol 24 (2004) 7931–7940. 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shizu R, Ezaki K, Sato T, Sugawara A, Hosaka T, Sasaki T, Yoshinari K, PXR Suppresses PPARalpha-Dependent HMGCS2 Gene Transcription by Inhibiting the Interaction between PPARalpha and PGC1alpha, Cells 10 (2021). 10.3390/cells10123550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kodama S, Moore R, Yamamoto Y, Negishi M, Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene, Biochem J 407 (2007) 373–381. 10.1042/BJ20070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bhalla S, Ozalp C, Fang S, Xiang L, Kemper JK, Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism, J Biol Chem 279 (2004) 45139–45147. 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- [30].Nishikawa T, Bellance N, Damm A, Bing H, Zhu Z, Handa K, Yovchev MI, Sehgal V, Moss TJ, Oertel M, Ram PT, Pipinos II, Soto-Gutierrez A, Fox IJ, Nagrath D, A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease, J Hepatol 60 (2014) 1203–1211. 10.1016/j.jhep.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hassani-Nezhad-Gashti F, Kummu O, Karpale M, Rysa J, Hakkola J, Nutritional status modifies pregnane X receptor regulated transcriptome, Sci Rep 9 (2019) 16728. 10.1038/s41598-019-53101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Palmisano BT, Zhu L, Stafford JM, Role of Estrogens in the Regulation of Liver Lipid Metabolism, Adv Exp Med Biol 1043 (2017) 227–256. 10.1007/978-3-319-70178-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


