Abstract
Objective
To assess the outcomes of neonates in a contemporary multi-institutional cohort who receive renal replacement therapy (RRT) for hyperammonemia.
Study design
We performed a retrospective analysis of 51 neonatal patients with confirmed inborn errors of metabolism that were treated at 9 different children’s hospitals in the US between 2000 and 2015.
Results
Twenty-nine patients received hemodialysis (57%), 21 patients received continuous renal replacement therapy (41%), and 1 patient received peritoneal dialysis (2%). The median age at admission of both survivors (n = 33 [65%]) and nonsurvivors (n = 18) was 3 days. Peak ammonia and ammonia at admission were not significantly different between survivors and nonsurvivors. Hemodialysis, having more than 1 indication for RRT in addition to hyperammonemia, and complications during RRT were all risk factors for mortality. After accounting for multiple patient factors by multivariable analyses, hemodialysis was associated with a higher risk of death compared with continuous renal replacement therapy. When clinical factors including evidence of renal dysfunction, number of complications, concurrent extracorporeal membrane oxygenation, vasopressor requirement, and degree of hyperammonemia were held constant in a single Cox regression model, the hazard ratio for death with hemodialysis was 4.07 (95% CI 0.908–18.2, P value = .067). To help providers caring for neonates with hyperammonemia understand their patient’s likelihood of survival, we created a predictive model with input variables known at the start of RRT.
Conclusions
Our large, multicenter retrospective review supports the use of continuous renal replacement therapy for neonatal hyperammonemia.
Neonatal hyperammonemia may result from defects in the primary waste nitrogen excretion pathway, known as urea cycle disorders (UCDs), and organic acidemias (OAs) that result in secondary inhibition of the urea cycle.1 Significantly elevated ammonia levels result in clinical symptoms of hypotonia, cerebral edema, altered mental status, seizures, coma, and ultimately death.2 For patients with neonatal hyperammonemia, overall survival varies between 20%3,4 to over 50% of patients.5–7 The most commonly cited prognostic factors for poor outcomes, such as duration of hyperammonemic coma longer than 3 days, degree of cerebral edema, and ammonia >1000 μmol/L, have been well-described.1,8 However, these data are from an era prior to currently available interventions that have improved outcomes in neonatal hyperammonemia including successful nitrogen scavenger therapy9 and improved renal replacement strategies beyond exchange transfusion and peritoneal dialysis.1,10–12
For an individual neonate with hyperammonemia, factors considered in the choice of renal replacement therapy (RRT) modality are often dependent on available resources and institutional practices rather than evidence-based guidelines. Consensus guidelines were published on timing and preferred modality of RRT.12 Currently, the 2 primary modalities of RRT used for the treatment of hyperammonemia are hemodialysis and continuous renal replacement therapy (CRRT). Each modality has independent risk-benefit profiles. Hemodialysis remains the most efficient modality for ammonia clearance with the highest available flow rates. With rapid ammonia clearance, multiple runs of hemodialysis may be required for rebound hyperammonemia.13 In contrast, CRRT has lower clearance rates but the continuous nature of CRRT has avoided rebound hyperammonemia. Further, CRRT is associated with fewer blood product requirements and fewer cardiovascular complications, given that there are less dramatic fluid and osmotic shifts.12,14–16 Technologic advances in RRT technology have led to the ability for higher clearance rates with CRRT and a biphasic approach to the management of neonatal hyperammonemia has been proposed where high-dose CRRT is initially used to rapidly decrease ammonia levels followed by lower dialysate flow rates to prevent rebound hyperammonemia while long-term medical and dietary interventions are established.12,17
We describe the outcomes of a multicenter, retrospective study in a large contemporary cohort of neonatal patients who received RRT for hyperammonemia because of inborn error of metabolism (IEM). Published guidelines state that RRT should be initiated for patients with ammonia levels >400 μmol/mL and at lower ammonia levels if the patient has signs of encephalopathy or is not responding to nitrogen scavenging therapies.11,12,18 Frequently, institutional preference and equipment resources factor heavily into this decision. What is unknown is if there is a preferred RRT modality for neonatal hyperammonemia based on clinical factors such as diagnosis, clinical status, trend in ammonia, and response to nitrogen scavenger therapy.
Methods
This study was conducted as a multisite retrospective review at 9 children’s hospitals within the US. The University of Michigan Health System institutional review board approved a retrospective chart review of patients who received RRT for hyperammonemia for all sites contributing to this study. This study met all STROBE criteria for cohort studies as outlined on equator-network.org. Individually, each site obtained institutional review board approval for data collection at their own institution. The patients included in this study were neonatal patients (28 days of age or less) with confirmed IEM (UCD or OA) between 2000 and 2015 who received RRT for treatment of hyperammonemia. Patients were identified by internal review at each individual institution’s RRT records. A total of 69 patients were identified. Seven patients were excluded based on age or date of birth outside of inclusion criteria. Five patients were excluded for having a diagnosis not consistent with a known IEM (such as ‘idiopathic hyperammonemia’ or ‘hemophagocytic lymphohistiocytosis’). IEM diagnoses were obtained from medical records based upon either biochemical or molecular confirmation of a specific IEM. Outcomes were categorized based on the reason for RRT discontinuation; survival was defined as no further need for RRT because of improved ammonia levels and nonsurvival was defined by death, redirection toward comfort care, or discharge to hospice. Plasma ammonia levels were recorded on admission, at initiation of RRT, and at discontinuation of RRT. Peak ammonia was the highest ammonia level between the values noted at admission or RRT initiation. If only 1 value was collected/recorded, this value was considered the peak ammonia level. Peak ammonia was used rather than duration of hyperammonemic coma because the timing of symptoms was not clearly documented in the medical records to allow for statistical analysis. Review of medical records for each patient determined basic demographic information (sex, age, weight, length, IEM diagnosis) as well as specifics of clinical status and management (creatinine at admission, change in creatinine from admission to RRT initiation, use of nitrogen-scavenging medications [sodium phenylacetate, sodium benzoate, and or carglumic acid]), use of vasopressors, and other indications for RRT or evidence of renal dysfunction (acute kidney injury [AKI], electrolyte disturbance, or fluid overload). Body surface area (BSA) was calculated using the Meeh-type equation (BSA = 0.09395*weight0.7032).19 RRT records were reviewed to determine RRT modality (hemodialysis, CRRT: continuous venovenous hemodialysis [CVVHD] or continuous venovenous hemodiafiltration [CVVHDF], or peritoneal dialysis) and RRT-associated complications (hypotension, bleeding, thrombosis, line malfunction, infection, or electrolyte disturbance). Ammonia clearance was estimated by calculating dialysate flow rate when available in mL/hour divided by BSA and multiplied by 1.73. Hypotension at RRT initiation was defined as blood pressure lower than the age-adjusted fifth percentile. The primary outcome of this study was survival to hospital discharge. Mortality rates at 6, 12, and 24 months after hospital discharge were secondary outcomes in the Cox model. Date of liver transplantation was documented if known. Neurologic impairment was determined by review of medical records by medical providers using diagnostic codes associated with neurologic deficits, developmental delays, or descriptive terms in medical records of examinations and patient assessments.
Descriptive statistics were presented as frequencies with percentages for categorical variables, means with SDs for symmetric continuous variables, and medians with IQRs for asymmetric continuous variables. All analyses were performed using RStudio (RStudio, PBC). For univariable analyses, continuous variables were compared with independent sample t tests for parametric data and Mann-Whitney-Wilcoxon U tests were used for nonparametric data. Two-sided P values were calculated. The level of significance was defined as P < .05. Fisher exact tests of independence were performed for comparisons of categorical variables. Survival analyses are based on Cox proportional hazards models implemented with the following variables: RRT modality (CRRT vs hemodialysis), number of symptoms of renal dysfunction, number of complications, extracorporeal membrane oxygenation status (ECMO or not), ammonia level, and use of vasopressor medications or not. The nonsurvival prediction model used a logistic regression based on peak ammonia prior to RRT, number of indications for RRT, and hypotension at RRT initiation to produce a predicted probability of non-survival for each case. The model predicted nonsurvival in each case where the predicted probability was larger than the threshold of 36% predicted probability of death that maximized the objective function 0.6*sensitivity + 0.4*specificity. Sensitivity and specificity are the percentages of correct predictions for deaths and survival, respectively. This objective function reflected the desire to correctly predict nonsurvival with the potential cost of additional survivors predicted as deaths.
Results
A total of 51 eligible patients were identified (Table I). Overall survival rate was 65% (33 survivors and 18 nonsurvivors). Within the patient cohort, 40 patients were male (78%) and 11 were female (22%). The high prevalence of male patients (78%) in our cohort reflects the prevalence of X-linked ornithine transcarbamylase deficiency as the most common IEM associated with hyperammonemia. There were 43 patients with UCDs (ornithine transcarbamylase deficiency [n = 26], carbamoyl phosphate synthetase deficiency [n = 7], citrullinemia [n = 6], argininosuccinic lyase deficiency [n = 4]) and 8 patients with OA (methylmalonic acidemia [n = 3], propionic acidemia [n = 5]). Age at admission ranged from 0 days to 11 days, with a median age of 3 days. Gestational age when known ranged from 29 to 41 weeks. Peak plasma ammonia levels varied greatly from 233–3869 μmol/L (median 1102, IQR 745–1848 μmol/L). Most patients (41 of 51 patients, 80%) received nitrogen-scavenging medications (ammonul [sodium phenylacetate and sodium benzoate] or carbaglu [carglumic acid]) prior to dialysis initiation. Regarding initial RRT modality, 29 patients (57%) received hemodialysis, 21 patients (41%) received CRRT, and 1 patient (2%) received peritoneal dialysis. Although our intention was to capture all forms of RRT, the only one patient who received peritoneal dialysis was removed from analyses given the low sample size. This patient had a birth weight of 2.95 kg and presented within the first year of our study period (2000–2001) with a peak ammonia of 1057 μmol because of methylmalonic acidemia. The ammonia improved to 115 μmol/L after 17 hours of peritoneal dialysis with no second RRT modality. After 2 years of follow-up, the patient had neurologic impairment but survived to liver transplantation. Of the patients who received CRRT, 5 underwent CVVHDF (24%) and 16 underwent CVVHD (76%). Of the 29 patients who received hemodialysis, 14 patients (48%) required additional RRT beyond the initial hemodialysis session. Three patients received a second hemodialysis session, and 11 patients were transitioned to CRRT (6 patients received CVVHDF and 5 patients received CVVHD). Twenty-seven patients (53%) had evidence of renal dysfunction (14 patients [27%] had AKI, 14 patients [27%] had electrolyte disturbances, and 4 patients [8%] had fluid overload). Six patients (12%) were simultaneously receiving RRT and extracorporeal membrane oxygenation (ECMO, 3 of these 6 patients survived). Twenty-six patients (51%) were on vasopressors at RRT initiation.
Table I.
Demographic characteristics of infants with IEM who received dialysis, 2000–2015
| Demographics of patient cohort (n = 51 patients) | |
|---|---|
| Age (d) at presentation | 3 (2–4) |
| Gestational age (wk) | 39 (37–40) |
| Ammonia at admission (umol/L) | 993 (652–1482) |
| Creatinine at admission (mg/dL) | 0.8 (0.6–0.9) |
| Peak ammonia (umol/L) | 1102 (745–1848) |
| Sex | |
| Male | 40 (78%) |
| Female | 11 (22%) |
| Medical therapy* prior to RRT (yes/no) | |
| Yes | 41 (80%) |
| No | 10 (20%) |
| Type of RRT (hemodialysis/CRRT/peritoneal dialysis) | |
| Hemodialysis | 29 (57%) |
| CRRT | 21 (41%) |
| Peritoneal dialysis | 1 (2%) |
| Other indications for RRT | |
| Yes | 24 (47%) |
| No | 27 (53%) |
| Complications during RRT | |
| Yes | 36 (71%) |
| No | 15 (29%) |
Data presented as median (25th-75th percentiles) or as total number of patients.
Indications for RRT include AKI, electrolyte disturbance, or fluid overload. Complications during RRT include hypotension, bleeding, line malfunction, electrolyte disturbances, thrombosis, vital sign instability, cerebral edema, and line-associated infection; patients noted to have complications had one or more complication but counted as one individual.
Ammonia-reducing medications, intravenous ammonul/arginine, or carbaglu.
Of the 50 patients who received hemodialysis or CRRT, the choice of anticoagulation, when known, was divided between 21 patients receiving heparin, 17 receiving citrate, and one receiving alteplase. Type of dialysis access was also divided within our cohort: 33 patients had nontunneled catheters, 16 were tunneled catheters, and 1 was unknown. One patient had double lumen catheter and the remaining 49 patients had single lumen catheters. Lumen size was also equally distributed: 14 patients had 8 French, 29 had 7 French, and 3 had 5 French catheters. Eight patients had femoral catheters, 36 had internal jugular catheters, 1 had a subclavian catheter, and 5 did not have location documented in the medical record.
Thirty-six patients (71%) had complications during RRT: hypotension (26 patients, 51%), bleeding (11, 22%), access malfunction (8, 16%), electrolyte disturbances (8, 16%), thrombosis (5, 10%), vital sign instability (mainly brady-cardia or other arrhythmias, 3 patients, 6%), clinically significant cerebral edema (2, 4%), and line-associated infection (1, 2%).
The survival rate during initial hospitalization for infants with hyperammonemia receiving RRT in our cohort was 65% (33 survivors and 18 nonsurvivors). Overall, there were 2 patients (4%) lost to follow-up between 12 and 24 months following initial hospital admission. Three additional patients (6%) died within the first 2 years after hospital discharge. At 1 year after hospital discharge, 21 of 33 (64%) survivors with available clinical data on neurologic status, had neurological impairment, 6 were reported to be neurologically normal, 5 had no documentation of neurologic impairment, and 1 patient passed in the first year of life. Importantly, this long-term outcome data does not differentiate between patients that had additional metabolic decompensations or episodes of hyperammonemia and those that did not. Of those who survived their initial hospitalization for hyperammonemia, 24 patients (73%) underwent liver transplantation within the first 2 years of life.
The median age of presentation for both survivors and nonsurvivors was 3 days (Table II, P value = .13). The median weight at admission was the same for both survivors and nonsurvivors (3.1 kg, P value = .88). Ammonia and creatinine at admission were not significantly different between survivors and nonsurvivors. There was no statistically significant difference between IEM type (UCD vs OA) and survival. Nonsurvivors had significantly more symptoms of renal dysfunction in addition to hyperammonemia and had more complications compared with survivors. A significantly higher percentage (78%) of nonsurvivors received hemodialysis compared with survivors (45% of survivors received hemodialysis). Age at time of RRT initiation did not differ significantly between those that received hemodialysis and CRRT (median age for CRRT was 3 days [IQR 2–5 days] and median age for hemodialysis was 3 days [IQR 2–4 days], P value .95). Ammonia clearance rates also did not differ significantly between survivors and nonsurvivors. The use of vasoactive medications at RRT initiation was significantly higher in nonsurvivors.
Table II.
Comparison of survival and nonsurvival to hospital discharge for infants with IEM who received dialysis for hyperammonemia
| Comparison of survivors and nonsurvivors | |||
|---|---|---|---|
| Survivors (n = 32) | Nonsurvivors (n = 18) | P value | |
| Age (d) at admission | 3 (3–5) | 3 (2–4) | .11 |
| Weight (kg) at admission | 3.1 (2.7–3.4) | 2.8 (2.5–3.6) | .51 |
| Peak ammonia (umol/L) | 1100 (703–1345) | 1556 (880–2473) | .097 |
| Creatinine at admission (mg/dL) | 0.8 (0.6–0.99) | 0.8 (0.8–0.9) | .82 |
| Ammonia clearance rate (mL/h/1.73 m2) | 4670 (3814–6235) | 4455 (3390–5103) | .58 |
| Number of symptoms of kidney dysfunction per patient (ie, indication for RRT) | 1 (1–1) | 1 (1–2) | .0031* |
| Number of complications per patient | 1 (0–1) | 2 (1–3) | .000033* |
| IEM type | |||
| UCD | 26 (81%) | 17 (94%) | .4 |
| OA | 6 (19%) | 1 (6%) | |
| Initial RRT modality (hemodialysis/CRRT/peritoneal dialysis) | |||
| Hemodialysis | 15 (47%) | 14 (78%) | .042* |
| CRRT | 17 (53%) | 4 (22%) | |
| CRRT modality (CVVHD/CVVHDF) | |||
| CVVHD | 12 (75%) | 4 (25%) | .53 |
| CVVHDF | 5 (100%) | 0 (0%) | |
| Vasoactive medications at RRT initiation (yes/no)† | |||
| Yes | 13 (45%) | 13 (81%) | .027* |
| No | 16 (55%) | 5 (19%) | |
| 1-y neurologic impairment documented (yes/no/unknown) | |||
| Yes | 20 (63%) | ||
| No | 6 (19%) | ||
| Death | 1 (3%) | ||
| Unknown | 5 (16%) | ||
| 2-y survival (yes/no/unknown) | |||
| Yes | 27 (84%) | ||
| No | 3 (9%) | ||
| Unknown | 2 (6%) | ||
| Liver transplant (yes/no) | |||
| Yes | 23 (72%) | ||
| No | 9 (28%) | ||
Data presented as medians (IQR) or as total number of patients.
Indications for RRT include AKI, electrolyte disturbance, or fluid overload. Complications during RRT include hypotension, bleeding, line malfunction, electrolyte disturbances, thrombosis, vital sign instability, cerebral edema, and line-associated infection.
P value < .05.
Three patients excluded due to uncertainty when vasopressors were initiated.
In a multivariable Cox regression model including RRT modality, number of symptoms of kidney dysfunction, number of complications, ECMO vs no ECMO, each 100 μmol/L increase in peak ammonia level, and use of vasopressors, hemodialysis was associated with a higher risk of death (hazard ratio [HR] 4.07, 95% CI 0.91–18.2, P value = .067) relative to CRRT, but this did not reach statistical significance (Table III; available at www.jpeds.com). Increasing number of complications (HR 3.83 95% CI 1.86–7.90, P value = .00027) and additional elevations in ammonia level (HR 1.09, 95% CI 1.03–1.15, P value = .0016) were significantly associated with death in the multivariable model. Figure 1 shows the outcomes of neonatal hyperammonemia throughout the study period. In the first 5 years of the study period, hemodialysis was more commonly used, however, these neonates also had higher ammonia levels. The overall mortality for this period (2000–2005, 11 patients) was 64% (7 nonsurvivors). The following 5-year period (2006–2010, 16 patients), the mortality was 31% (5 nonsurvivors). In the last 5-year period (2011–2015, 24 patients), the mortality was 25% (6 nonsurvivors). As our data suggest, nonsurvivors were more likely to have received hemodialysis compared with CRRT.
Table III.
Cox regression model of clinical variables and outcome at 24 months of age
| Clinical variables | Adjusted nonsurvival HR (95% CI) | P value |
|---|---|---|
| Hemodialysis vs RRT | 4.07 (0.91–18.20) | .067 |
| Number of symptoms of kidney dysfunction | 0.94 (0.34–2.65) | .91 |
| Number of complications | 3.83 (1.86–7.90) | .00027* |
| ECMO vs no ECMO | 1.09 (0.28–4.22) | .91 |
| Additional 100 μmol/L of ammonia | 1.09 (1.03–1.15) | .0016* |
| Vasopressors vs no vasopressors | 1.87 (0.46–7.64) | .38 |
Symptoms of kidney dysfunction include AKI, electrolyte disturbance, or fluid overload. Complications during RRT include hypotension, line-associated bleeding, line malfunction, electrolyte disturbances, thrombosis, vital sign instability, cerebral edema, and line-associated infection.
P value < .05.
Figure 1.
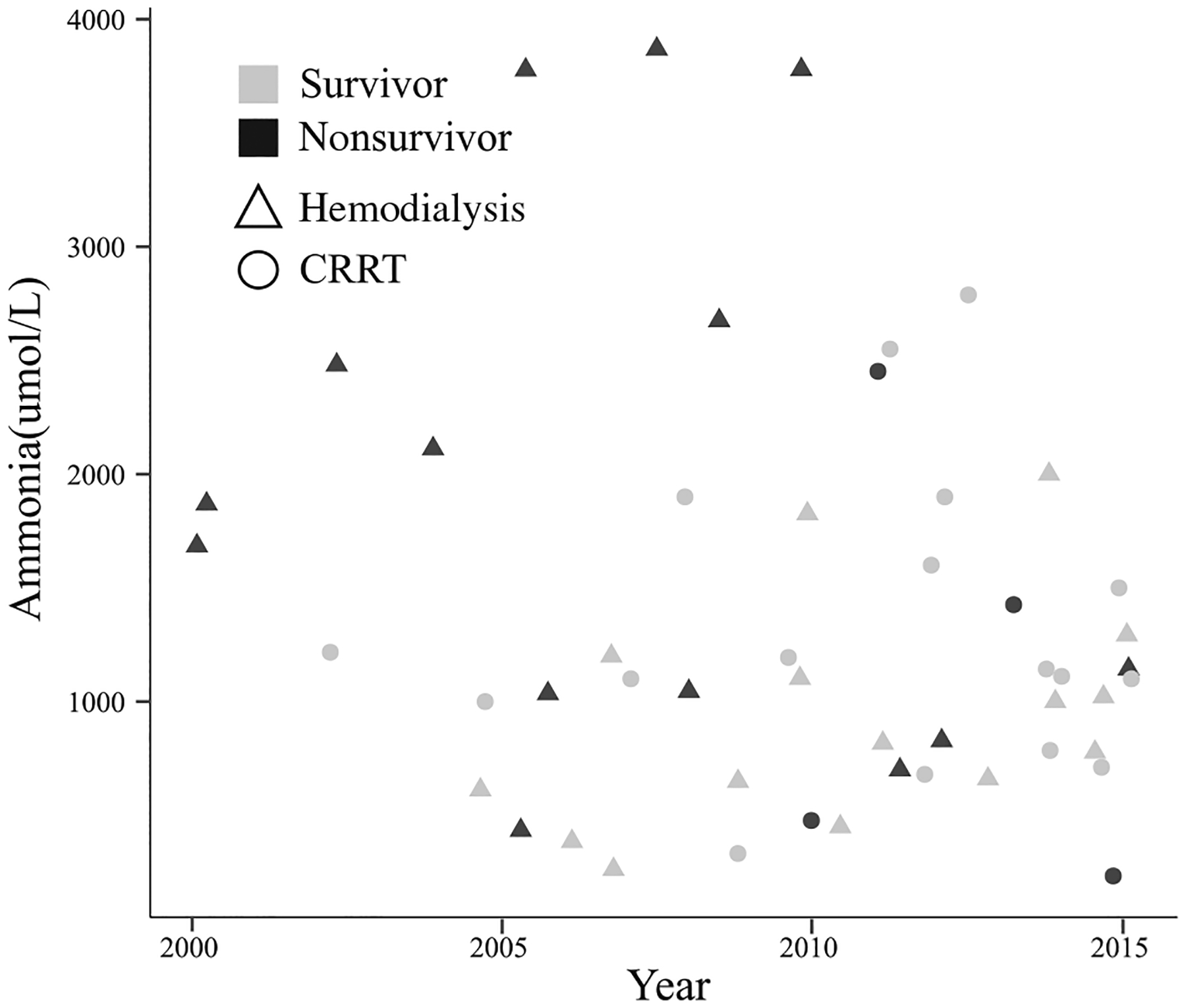
Outcomes over time based on peak ammonia level and RRT modality. X-axis plots time in years. Y-axis plots peak ammonia level (μmol/L). Shapes of data points highlight individuals who received CRRT vs hemodialysis as an initial RRT modality (Δ represents those that received hemodialysis and ○ represents CRRT). Shaded shapes denote nonsurvival whereas open shapes represent those that survived.
We created a model that could predict nonsurvival for patients who are being considered to receive RRT. This model used information that would be available at the start of RRT, such as ammonia at admission, evidence of renal dysfunction (AKI, electrolyte disturbance, or fluid overload), and presence of hypotension before initiation of RRT (defined as blood pressure lower than the age-adjusted fifth percentile). The most predictive variable in the model was the additional evidence for renal dysfunction. The odds of nonsurvival went up by 542% for each additional problem (95% CI 50%−4573% increase, P value = .028). Children with hypotension had 117% higher odds of death, which was not statistically significant (95% CI −47% to +833%, P value = .28). A 100-unit change in ammonia was associated with a 3% increase in the odds of death, and this was not statistically significant (P value = .57). Figure 2, A shows the receiver operating characteristic curve: area under this curve for the nonsurvival model was 0.73 (95% CI 0.57–0.89). Figure 2, B gives predicted and actual values for death based on the 36% predicted probability threshold above. The resulting sensitivity and specificity values were 61% and 81%, respectively.
Figure 2.
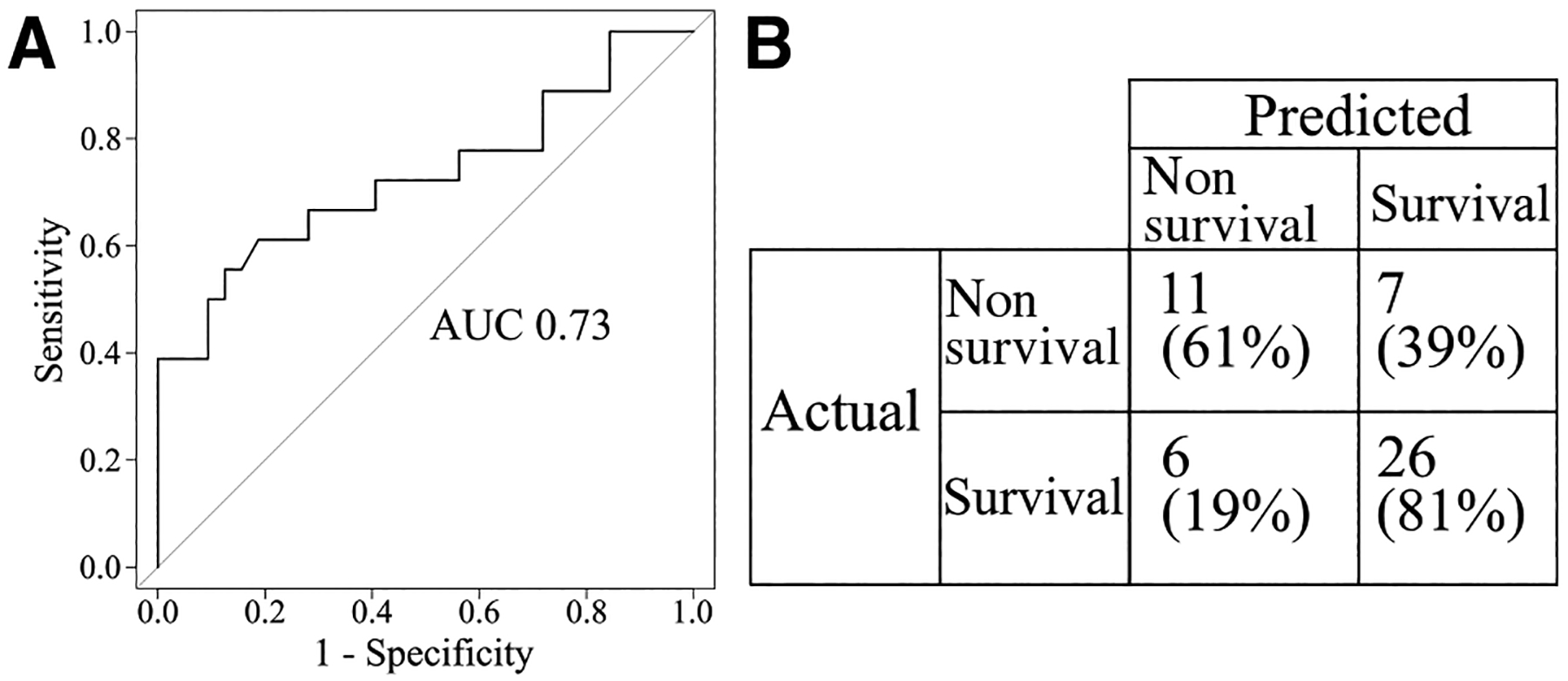
Predictive model for nonsurvival. A, Receiver operating characteristic curve, where the x-axis plots the false positive rate (1-specificity) and the y-axis plots the true positive rate (sensitivity) for predicted probability thresholds varying from 0 to 1. The area under this curve (AUC) is a value between 0 and 1 representing the performance of the model. The diagonal line represents the results from a model making random predictions and has an AUC value of 0.5. B, Performance of model prediction for nonsurvival.
Discussion
Here we present a retrospective analysis of neonatal patients with IEMs who received RRT for hyperammonemia at multiple children’s hospitals across the US from 2000 to 2015. Nonsurvival was more frequently observed in patients who had multiple symptoms of kidney dysfunction, hypotension at the start of RRT requiring vasoactive medications, and those who had more complications during RRT. There was a trend toward nonsurvival in patients who received hemodialysis compared with CRRT. To explore the relationship between risk of death and different clinical variables, our multivariable analyses found that number of complications, increasing levels of ammonia, and to a lesser extent hemodialysis, was associated with nonsurvival after accounting for other clinical variables during RRT.
In the past, hyperammonemic coma duration greater than 3 days, evidence of cerebral edema, and a peak ammonia >1000 μmol/L have all been widely discussed as having prognostic value for poor outcomes.1,5,8,15 However, with the current practice of medicine, hyperammonemic coma for >72 hours is less frequently encountered and there has been evidence to suggest that the peak ammonia level may not be as good of a prognostic factor15 and new measures are needed. Using pre-RRT patient details, we attempted to identify specific prognostic indicators that would help clinicians interpret an individual patient’s risk for nonsurvival. Our model identified additional prognostic factors such as hypotension requiring vasoactive medications before initiation of RRT and symptoms of renal dysfunction, which were relatively accurate in prediction of nonsurvival. Although both prognostic factors seem intuitively associated with poor prognosis, these factors were more statistically significant than peak ammonia level alone. It also highlights the importance of considering the whole patient’s clinical status (multi-organ dysfunction, evidence of poor perfusion) rather than simply focusing on the absolute value of ammonia. Our study found that non-survivors were likely sicker on admission than survivors given that they had more symptoms of renal dysfunction, more need for vasoactive medications, and had more complications during RRT.
Limitations to our study include, as with any rare IEM, a small sample size. However, our sample size of 51 neonatal patients represents a robust sample of patients cared for at 9 large, midrange, and small children’s hospitals within the US. Within our cohort of neonatal hyperammonemia, there were numerous biochemical diagnoses including both urea cycle disorders and organic acidemias. Due to the rarity of each diagnosis, we were unable to compare outcomes between individual causes of hyperammonemia. Other reports of RRT outcomes in patients with IEM are case reports or single center case series.12 Our patient cohort also spans a period where there were significant developments in RRT technology and the ability of CRRT to provide clearance rates closer to hemodialysis. The expansion of newborn screening to include more disorders detected by tandem mass spectrometry also occurred during our study period. However, based on the comparable age at admission between survivors and non-survivors, it does not appear as though patients who survived were identified earlier. It does not appear that expanded newborn screening had a significant impact on the outcome of neonatal onset metabolic diseases that present with severe hyperammonemia as our patient cohort presented on average at 3 days of life before newborn screening results are typically reported.
Our findings support physician teams caring for neonates with all but the most extreme hyperammonemia to consider CRRT as the initial modality for RRT. Although hemodialysis does offer higher clearance rates, it is associated with a significantly higher risk of complications. Biphasic CRRT is likely a better and safer option for neonatal patients, providing higher initial clearance rates and minimizing complications. Our findings also provide valuable prognostic information for biochemical geneticists, pediatric nephrologists, and pediatric intensivists about how to approach a neonate with hyperammonemia. Although our findings support increased used of CRRT and a biphasic strategy for management of neonatal hyperammonemia, prospective clinical trials while challenging to perform will provide the most definitive evidence for overall safety and neurologic outcomes.
Acknowledgments
Supported by the Gerber Foundation, Fremont, MI (N026404-391375 to E.A). The authors declare no conflicts of interest.
Glossary
- AKI
Acute kidney injury
- BSA
Body surface area
- CRRT
Continuous renal replacement therapy
- CVVHD
Continuous venovenous hemodialysis
- CVVHDF
Continuous venovenous hemodiafiltration
- ECMO
Extracorporeal membrane oxygenation
- HR
Hazard ratio
- IEM
Inborn error of metabolism
- OA
Organic acidemia
- RRT
Renal replacement therapy
- UCD
Urea cycle disorder
Footnotes
Portions of this study were presented as a oral presentation during the Neonatal Kidney Collaborative meeting, May 4, 2020, Virtual.
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.Burton BK. Inborn errors of metabolism in infancy: a guide to diagnosis. Pediatrics 1998;102:E69. [DOI] [PubMed] [Google Scholar]
- 2.Auron A, Brophy PD. Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr Nephrol 2012;27:207–22. [DOI] [PubMed] [Google Scholar]
- 3.Kido J, Nakamura K, Mitsubuchi H, Ohura T, Takayanagi M, Matsuo M, et al. Long-term outcome and intervention of urea cycle disorders in Japan. J Inherit Metab Dis 2012;35:777–85. [DOI] [PubMed] [Google Scholar]
- 4.Unsinn C, Das A, Valayannopoulos V, Thimm E, Beblo S, Burlina A, et al. Clinical course of 63 patients with neonatal onset urea cycle disorders in the years 2001–2013. Orphanet J Rare Dis 2016;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames EG, Luckritz KE, Ahmad A. A retrospective review of outcomes in the treatment of hyperammonemia with renal replacement therapy due to inborn errors of metabolism. Pediatr Nephrol 2020;35:1761–9. [DOI] [PubMed] [Google Scholar]
- 6.McBryde KD, Kershaw DB, Bunchman TE, Maxvold NJ, Mottes TA, Kudelka TL, et al. Renal replacement therapy in the treatment of confirmed or suspected inborn errors of metabolism. J Pediatr 2006;148:770–8. [DOI] [PubMed] [Google Scholar]
- 7.Burgard P, Kölker S, Haege G, Lindner M, Hoffmann GF. Neonatal mortality and outcome at the end of the first year of life in early onset urea cycle disorders—review and meta-analysis of observational studies published over more than 35 years. J Inherit Metab Dis 2016;39:219–29. [DOI] [PubMed] [Google Scholar]
- 8.Msall M, Batshaw M, Suss R, Brusilow S, Mellits ED. Neurologic outcome in children with inborn error of urea synthesis. N Engl J Med 1984;310:1500–5. [DOI] [PubMed] [Google Scholar]
- 9.Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A. Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med 2007;356:2282–92. [DOI] [PubMed] [Google Scholar]
- 10.Donn SM, Swartz RD, Thoene JG. Comparison of exchange transfusion, peritoneal dialysis, and hemodialysis for the treatment of hyperammonemia in an anuric newborn infant. J Pediatr 1979;95:67–70. [DOI] [PubMed] [Google Scholar]
- 11.Häberle J, Burlina A, Chakrapani A, Dixon M, Karall D, Lindner M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: first revision. J Inherit Metab Dis 2019;42:1192–230. [DOI] [PubMed] [Google Scholar]
- 12.Raina R, Bedoyan JK, Lichter-Konecki U, Jouvet P, Picca S, Mew NA, et al. Consensus guidelines for management of hyperammonaemia in paediatric patients receiving continuous kidney replacement therapy. Nat Rev Nephrol 2020;16:471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajpoot DK, Gargus JJ. Acute hemodialysis for hyperammonemia in small neonates. Pediatr Nephrol 2004;19:390–5. [DOI] [PubMed] [Google Scholar]
- 14.Lai YC, Huang HP, Tsai IJ, Tsau YK. High-volume continuous venovenous hemofiltration as an effective therapy for acute management of inborn errors of metabolism in young children. Blood Purif 2007;25: 303–8. [DOI] [PubMed] [Google Scholar]
- 15.Picca S, Dionisi-Vici C, Abeni D, Pastore A, Rizzo C, Orzalesi M, et al. Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol 2001;16:862–7. [DOI] [PubMed] [Google Scholar]
- 16.Hiroma T, Nakamura T, Tamura M, Kaneko T, Komiyama A. Continuous venovenous hemodiafiltration in neonatal onset hyperammonemia. Am J Perinatol 2002;19:221–4. [DOI] [PubMed] [Google Scholar]
- 17.Hanudel M, Avasare S, Tsai E, Yadin O, Zaritsky J. A biphasic dialytic strategy for the treatment of neonatal hyperammonemia. Pediatr Nephrol 2014;29:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapman KA, Gropman A, MacLeod E, Stagni K, Summar ML, Ueda K, et al. Acute management of propionic acidemia. Mol Genet Metab 2012;105:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkawi El Edelbi R, Lindemalm S, Nydert P, Eksborg S. Estimation of body surface area in neonates, infants, and children using body weight alone. Int J Pediatr Adolesc Med 2021;8:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing statement available at www.jpeds.com.


